Abstract
Background
Hypertrophic cardiomyopathy (HCM) is a common inherited cardiac disease characterized by varying degrees of left ventricular outflow tract obstruction. In a large cohort, we compare the outcomes among 3 different hemodynamic groups.
Methods and Results
We prospectively enrolled patients fulfilling standard diagnostic criteria for HCM from January 2005 to June 2015. Detailed phenotypic characterization, including peak left ventricular outflow tract pressure gradients at rest and after provocation, was measured by echocardiography. The primary outcome was a composite cardiovascular end point, which included new‐onset atrial fibrillation, new sustained ventricular tachycardia/ventricular fibrillation, new or worsening heart failure, and death. The mean follow‐up was 3.4±2.8 years. Among the 705 patients with HCM (mean age, 52±15 years; 62% men), 230 with obstructive HCM were older and had a higher body mass index and New York Heart Association class. The 214 patients with nonobstructive HCM were more likely to have a history of sustained ventricular tachycardia/ventricular fibrillation and implantable cardioverter defibrillator implantation. During follow‐up, 121 patients experienced a composite cardiovascular end point. Atrial fibrillation occurred most frequently in the obstructive group. Patients with nonobstructive HCM had more frequent sustained ventricular tachycardia/ventricular fibrillation events. In multivariate analysis, obstructive (hazard ratio, 2.80; 95% confidence interval, 1.64–4.80) and nonobstructive (hazard ratio, 1.94; 95% confidence interval, 1.09–3.45) HCM were associated with more adverse events compared with labile HCM.
Conclusions
Nonobstructive HCM carries notable morbidity, including a higher arrhythmic risk than the other HCM groups. Patients with labile HCM have a relatively benign clinical course. Our data suggest detailed sudden cardiac death risk stratification in nonobstructive HCM and monitoring with less aggressive management in labile HCM.
Keywords: classification, hypertrophic cardiomyopathy, outcome
Subject Categories: Cardiomyopathy
Clinical Perspective
What Is New?
Although all clinicians follow a new hemodynamic classification for hypertrophic cardiomyopathy (HCM), there are no long‐term data of prognosis using this new classification.
The study provides clinical outcomes, not just mortality, among patients with different hemodynamic classifications.
Our results from a large cohort suggest that patients with nonobstructive HCM have a high risk of arrhythmic and sudden death events.
Conversely, patients with labile HCM have a relatively benign clinical course.
What Are the Clinical Implications?
Despite low annual mortality rates, patients with nonobstructive HCM have high rates of adverse clinical events, almost equivalent to obstructive HCM.
Patients with nonobstructive HCM warrant thorough vetting, with a focus on the need for defibrillator therapy.
Patients with labile HCM have the best prognosis and may need less aggressive management.
Routine exercise stress echocardiography in all patients with HCM and particularly in those with resting gradients <30 mm Hg may be suggested, regardless of symptoms.
Introduction
Hypertrophic cardiomyopathy (HCM) is one of the most common inherited cardiac diseases, characterized by ventricular hypertrophy, myofiber disarray, and fibrosis.1, 2, 3 Clinical manifestations include exercise intolerance, heart failure (HF), and cardiac arrhythmias, including sudden death.4 Approximately two thirds of patients with HCM demonstrate a dynamic left ventricular outflow tract (LVOT) gradient at rest or with provocation. This obstruction is thought to be the primary driver of symptoms.5, 6 A resting LVOT gradient ≥30 mm Hg is a strong independent predictor for progression of HF and death.7, 8 Conversely, nonobstructive HCM (rest/stress gradient <30 mm Hg) is generally managed conservatively. These concepts were reiterated in recent guidelines and expert reviews, which summarized that a “majority of non‐obstructive HCM patients experience a relatively stable clinical course without significant symptoms, high‐risk profile, or the necessity of major treatment options (p. 94).”4
Novel imaging methods have documented that, in addition to LVOT gradients, HCM is associated with myocardial fibrosis,2 microvascular ischemia,3, 9 and abnormal cardiac mechanics,10 which may be important contributors to clinical adverse events.2, 11 We recently demonstrated that more patients with nonobstructive HCM had a large scar burden (on magnetic resonance imaging) and higher rates of microvascular ischemia (by positron emission tomography).12 Thus, there are lines of evidence suggesting that nonobstructive HCM may not be pathophysiologically benign. The wider question that has not yet been well addressed is whether the current hemodynamics‐based 3 HCM classification (nonobstructive, labile, and obstructive) confers any distinctive clinical risk.
In this study, we examined the long‐term outcomes in patients with HCM, stratified by rest/stress outflow tract hemodynamics, in a relatively large, well‐characterized, single‐center cohort.
Methods
Study Population and Data Collection
The data, analytic methods, and study materials have been made available to other researchers for purposes of reproducing the results or replicating the procedure.12 This HCM Registry study was approved by the Institutional Review Board of the Johns Hopkins Hospital, and patients signed informed consent for any procedures performed only for research purposes. Patients were prospectively enrolled in the Johns Hopkins HCM Registry from January 2005 to June 2015 at their first visit if they met the standard diagnostic criteria for HCM, which was unexplained LV hypertrophy with maximal wall thickness ≥15 mm in the absence of other systemic or explainable cause. Patients with a previous history of septal reduction therapy (septal myectomy or alcohol septal ablation) were excluded. Patients with reduced (<50%) ejection fraction were also excluded, because end‐stage cardiomyopathy is associated with higher risk. Retrospective analysis of data was performed in this study. The latest clinical assessment was obtained by clinic visit, mail, or telephone contact up to June 30, 2016. Patients were censored at development of any of the preidentified end points or 1 day before septal reduction therapy. Clinical information was collected, as previously reported.13
Conventional and Stress Echocardiography
Transthoracic echocardiography was performed using a GE Vivid 7 or E‐9 ultrasound machine (GE Ultrasound, Milwaukee, WI) with a multifrequency phased‐array transducer. Conventional biplane LV volume and LV ejection fraction were measured by the modified Simpson rule, according to previously published guidelines.14 Doppler measurements consisted of mitral inflow early diastole (E) and atrial contraction waves. LVOT pressure gradients were measured in the apical views by continuous‐wave Doppler echocardiography under resting conditions and during provocative maneuvers, including Valsalva, treadmill exercise, and/or amyl nitrite inhalation, to elicit latent obstruction, as previously reported.13, 15, 16 Tissue Doppler peak early diastolic wave (e′) was derived from the apical 4‐chamber view at the basal level of the septal wall and was used to calculate E/e′ ratio.17 After measuring peak resting and stress pressure gradients, classification of HCM was established as nonobstructive (<30 mm Hg at rest and stress), labile (<30 mm Hg at rest and ≥30 mm Hg with stress), and obstructive (≥30 mm Hg at rest and stress).18, 19
After completion of conventional echocardiography, patients without contraindications underwent a treadmill exercise test. Those with active angina, decompensated HF, uncontrolled arrhythmias, hemodynamic instability, severe hypertension/hypotension, and inability to walk on a treadmill were excluded. Standard Bruce protocol was implemented in all subjects, except those with a history of poor functional status, in which case we used a modified Bruce or Naughton protocol. All subjects were monitored for symptoms, heart rate, blood pressure, and continuous 12‐lead electrocardiography. Abnormal blood pressure response was considered as an increase of <20 mm Hg in systolic blood pressure (SBP) from resting state to peak exercise, an initial increase in SBP with a subsequent decrease of >20 mm Hg compared with the SBP value at peak exercise, or a continuous decrease in SBP throughout the exercise test of >20 mm Hg compared with SBP at rest.20, 21 After exercise, patients were immediately placed in the left lateral decubitus position, and peak instantaneous LV outflow tract velocities were measured again, as previously mentioned, in the apical view.
Definition of Cardiovascular Events and Follow‐Up
A composite cardiovascular end point was prespecified as the primary outcome variable. The components of this composite end point were the following common complications of HCM: new‐onset atrial fibrillation (AF); new sustained (≥30 seconds) ventricular tachycardia/ventricular fibrillation (VT/VF), with or without appropriate implantable cardioverter defibrillator (ICD) discharge; new‐onset or worsening HF (defined as worsening of New York Heart Association [NYHA] functional class to class III or IV) requiring hospitalization; and all‐cause mortality. If an outcome event was experienced before enrollment and recurred during follow‐up, that particular event was not considered as an outcome. Arrhythmic outcomes (AF and VT/VF) were recorded by reviewing clinical visit documents, Holter monitoring, and ICD interrogation reports. New‐onset or worsening HF at NYHA class III or IV had to be documented in outpatient visits or in‐hospital medical records. All‐cause mortality statistics for the study populations were obtained by linking our database to the Social Security Death Index, with a follow‐up duration of up to 10 years. All events were clinically adjudicated by 1 of 2 HCM clinical experts (T.P.A. and M.R.A.), who also reviewed raw data and electronic documentation of all arrhythmic events. Any conflict was resolved by repeated review of the documentation and consensus between the experts. During follow‐up, patients who underwent septal reduction therapy before any adverse event were considered as censored 1 day before septal reduction therapy. Patients who remained event free were censored on June 30, 2016, with the longest duration being 10 years.
Statistical Analysis
Descriptive statistics were performed on patient demographics, hemodynamics, conventional echocardiographic parameters, and outcomes, stratified by each category of HCM. Data distribution was evaluated with kernel density plots and the Shapiro‐Wilk test for normality. Continuous variables are presented as mean±SD, and categorical variables are presented as the total number and percentage. Comparison of continuous variables across groups was performed using ANOVA if normally distributed or Kruskal‐Wallis test if not normally distributed, and comparison of categorical variables was performed using the Fisher exact test. Kaplan‐Meier analysis of the primary end point was analyzed for the time of enrollment to the first composite cardiovascular event, with the significance based on the log‐rank test. The Cox proportional hazards model was used to adjust for possible confounders of composite outcomes. Variables that were statistically significant in the univariate analysis were enrolled in a multivariate model, which included age, sex, baseline NYHA functional class, history of sustained VT/VF, left atrial (LA) diameter, and E/e′. P<0.05 was considered statistically significant. The analyses were performed using STATA software, version 14 (StataCorp LP, College Station, TX).
Results
After excluding 13 patients for previous myectomy and 16 for LV ejection fraction <50% (9 nonobstructive, 4 labile, and 3 obstructive), we analyzed 705 patients (mean age, 52.7±15.1 years at study enrollment; 62% men) with complete follow‐up data (mean duration of follow‐up, 3.4±2.8 years).
Clinical Features
Among the 705 patients, 214 (30%) had nonobstructive HCM, whereas 261 (37%) had labile and 230 (33%) had obstructive HCM. The clinical and echocardiographic characteristics are summarized in Table 1. All continuous variables were normally distributed. Patients with obstructive HCM were older, had a higher body mass index, and were associated with higher frequencies of female sex and comorbidities (hypertension, dyslipidemia, and dyspnea), reflecting a more advanced NYHA class. Among risk factors for sudden cardiac death, patients with obstructive HCM had more prevalent abnormal blood pressure response during exercise. Patients with nonobstructive HCM were associated with a higher frequency of history of nonsustained VT/VF and ICD implantation.
Table 1.
Baseline Characteristics in Patients Stratified by HCM Classification
| Characteristics | Nonobstructive HCM (N=214) | Labile HCM (N=261) | Obstructive HCM (N=230) | P Value |
|---|---|---|---|---|
| Age, y | 49.6±15.9 | 52.0±15.3 | 56.3±13.3 | <0.001 |
| Male sex, n (%) | 134 (63) | 181 (69) | 121 (53) | 0.001 |
| BMI, kg/m2 | 28.7±5.4 | 29.7±6.6 | 30.1±6.6 | 0.039 |
| NYHA functional class, n (%) | <0.001 | |||
| I | 138 (65) | 157 (60) | 94 (41) | |
| II–III | 76 (35) | 104 (40) | 136 (59) | |
| Risk factor for SCD | ||||
| Syncope, n (%) | 37 (17) | 53 (20) | 42 (18) | 0.687 |
| Family history of SCD, n (%) | 56 (26) | 65 (25) | 54 (24) | 0.790 |
| NSVT, n (%) | 36 (17) | 26 (10) | 17 (7) | 0.005 |
| ABPR, n (%) | 50 (25) | 82 (33) | 87 (43) | 0.001 |
| IVS ≥30 mm, n (%) | 23 (11) | 15 (6) | 15 (7) | 0.097 |
| No. of risk factors | 1.0±0.9 | 0.9±0.9 | 1.0±0.9 | 0.641 |
| Comorbidity, n (%) | ||||
| Atrial fibrillation | 38 (18) | 38 (15) | 36 (16) | 0.633 |
| Hypertension | 83 (39) | 139 (53) | 125 (55) | 0.001 |
| Diabetes mellitus | 21 (10) | 35 (13) | 19 (8) | 0.165 |
| Dyslipidemia | 85 (40) | 124 (48) | 126 (55) | 0.007 |
| Coronary artery disease | 17 (8) | 26 (10) | 22 (10) | 0.743 |
| Stroke | 5 (2) | 6 (2) | 11 (5) | 0.212 |
| ICD implantation | 26 (12) | 13 (5) | 16 (7) | 0.012 |
| Family history of HCM, n (%) | 65 (31) | 38 (15) | 30 (13) | <0.001 |
| Medications, n (%) | ||||
| β Blocker | 145 (68) | 179 (69) | 182 (79) | 0.012 |
| Calcium channel blocker | 45 (21) | 74 (28) | 79 (34) | 0.008 |
| RAS blockade | 38 (18) | 50 (19) | 38 (17) | 0.749 |
| Disopyramide | 3 (1) | 11 (4) | 8 (1) | 0.203 |
Data are given as mean±SD unless otherwise indicated. ABPR indicates abnormal blood pressure response; BMI, body mass index; HCM, hypertrophic cardiomyopathy; ICD, implantable cardioverter defibrillator; IVS, interventricular septum; NSVT, nonsustained ventricular tachycardia; NYHA, New York Heart Association; RAS, angiotensin‐converting enzyme inhibitor and angiotensin II receptor blocker; and SCD, sudden cardiac death.
Echocardiography
Echocardiographic features are summarized in Table 2. By definition, patients with obstructive HCM had the highest rest and stress LVOT pressure gradients. Mean rest gradient in the obstructive group was 66±31 mm Hg, and stress gradient was 118±45 mm Hg. Although LV ejection fraction was similar across the 3 groups, patients with obstructive HCM had more advanced diastolic dysfunction, as demonstrated by a larger LA diameter, a lower E/atrial contraction ratio, and a higher E/e′ ratio. Of the study patients, 644 (91%) underwent stress echo with treadmill exercise. Patients with obstructive HCM were associated with worst exercise capacity and least increment in peak exercise heart rate.
Table 2.
Echocardiographic and Treadmill Exercise Parameters in Patients Stratified by HCM Classification
| Parameters | Nonobstructive HCM (N=214) | Labile HCM (N=261) | Obstructive HCM (N=230) | P Value |
|---|---|---|---|---|
| Echocardiography | ||||
| Left atrium diameter, mm | 40±7 | 41±7 | 44±7 | <0.001 |
| Septal thickness, mm | 21±6 | 20±5 | 22±5 | 0.007a |
| Posterior wall, mm | 11±3 | 12±4 | 13±3 | <0.001a |
| LVEF, % | 65±8 | 66±7 | 66±8 | 0.128 |
| E/A | 1.5±0.9 | 1.3±0.7 | 1.3±0.7 | 0.039a |
| E/e′ | 15.4±9.1 | 16.7±8.5 | 24.0±12.7 | <0.001a |
| LVOT gradient at rest, mm Hg | 8±4 | 16±10 | 66±31 | <0.001a |
| LVOT gradient at stress, mm Hg | 17±6 | 72±41 | 118±45 | <0.001a |
| Treadmill exercise | ||||
| Bruce protocol, n (%) | 177 (83) | 215 (82) | 143 (62) | <0.001 |
| Exercise time, sb | 560±207 | 563±220 | 490±171 | <0.001a |
| METs | 10.7±4.0 | 10.4±4.4 | 8.3±3.6 | <0.001a |
| Resting SBP, mm Hg | 129±27 | 135±18 | 133±18 | <0.001a |
| Resting DBP, mm Hg | 77±11 | 79±12 | 76±11 | 0.033 |
| Resting heart rate, bpm | 67±14 | 65±13 | 67±14 | 0.050a |
| Peak SBP, mm Hg | 159±36 | 165±36 | 152±35 | <0.001a |
| Peak DBP, mm Hg | 80±18 | 82±18 | 79±19 | 0.059a |
| Peak heart rate, bpm | 148±30 | 145±28 | 133±27 | <0.001 |
Data are given as mean±SD unless otherwise indicated. Bpm indicates beats/min; DBP, diastolic blood pressure; E/A, ratio of early diastolic mitral flow velocity/late diastolic mitral flow velocity; E/e′, ratio of early diastolic mitral flow velocity/early diastolic mitral septal annulus motion velocity; HCM, hypertrophic cardiomyopathy; LVEF, left ventricular ejection fraction; LVOT, left ventricle outflow tract; MET, metabolic equivalent; and SBP, systolic blood pressure.
Analysis was performed using a Kruskal‐Wallis test.
Data from 535 patients who performed a treadmill test using the Bruce protocol.
Composite and Specific Outcomes
During a 2407 person‐year follow‐up, 121 patients (17%) experienced a composite cardiovascular end point, including 38 new cases of AF, 27 new cases of sustained VT/VF (10 were resuscitated successfully, and 17 had appropriate ICD shock), 38 cases of HF, and 18 deaths. Nonobstructive and obstructive groups had similar cumulative incidence of composite events, whereas the labile group had the lowest cumulative incidence (nonobstructive:labile:obstructive, 20%:8%:24%; P<0.001).
There were differences in the incidence of individual end points when segregated by hemodynamic subgroups of HCM and by the specific outcome. When analyzing by HCM subgroups, sustained VT/VF was the most common event in nonobstructive HCM (37%), followed by HF and death (Figure 1A). In the labile group, HF, AF, and sustained VT/VF were the most common events (Figure 1B). In contrast, AF accounted for almost half of overall events in obstructive group, whereas sustained VT/VF incidence was only 13% (Figure 1C).
Figure 1.
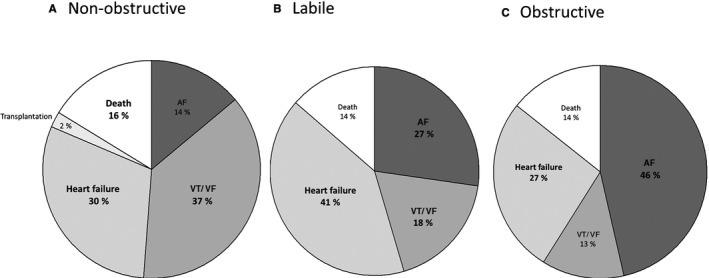
Cumulative incidence of individual cardiovascular outcomes in nonobstructive (A), labile (B), and obstructive (C) hypertrophic cardiomyopathy (HCM) groups. AF indicates atrial fibrillation; and VT/VF, ventricular tachycardia/ventricular fibrillation.
When we focused on specific outcomes, obstructive HCM had the highest risk of developing AF (log‐rank P<0.001) (Figure 2A and Table 3). For sustained VT/VF, patients with labile obstruction had the best event‐free survival, followed by the obstructive group (log‐rank P=0.004) (Figure 2B). Labile and obstructive HCM subgroups experienced most of their sustained VT/VF events within 5 years of enrollment, with significantly lower rates of sustained VT/VF beyond 5 years, suggesting some clinical stabilization. In contrast, the nonobstructive group continued to experience frequent malignant arrhythmic events; the sustained VT/VF‐free survival tracing declined progressively over time, resulting in the overall worst VT/VF‐free survival across the 3 subgroups. Event‐free survival rates for HF and all‐cause death were similar across the 3 subgroups (Figure 2C and 2D).
Figure 2.
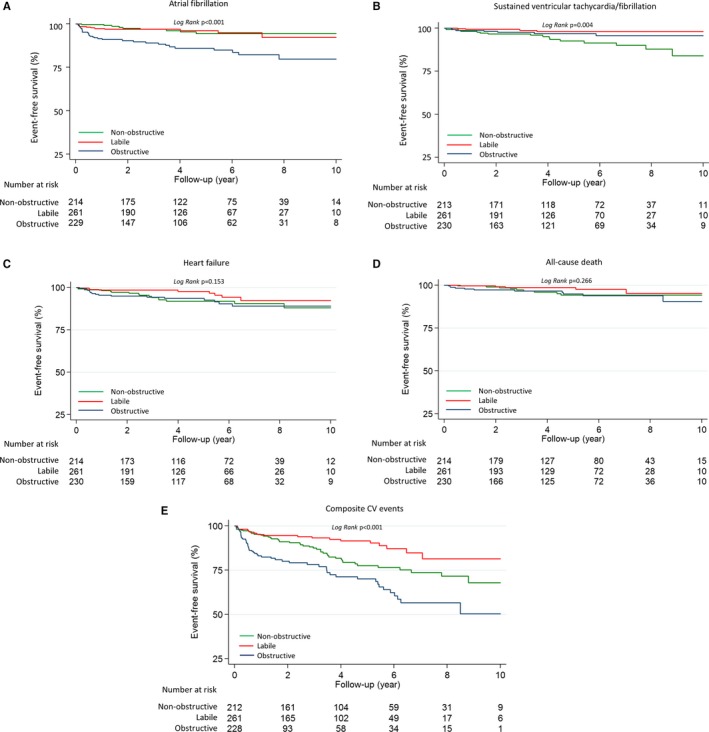
Kaplan‐Meier 10‐year event‐free survival analysis for atrial fibrillation (A), sustained ventricular tachycardia/fibrillation (B), heart failure (C), all‐cause death (D), and composite cardiovascular events (E) stratified by nonobstructive, labile, and obstructive hypertrophic cardiomyopathy (HCM) groups.
Table 3.
HRs and 95% CIs for Individual Cardiovascular Events During 10 Years of Follow‐Up by Classification of HCM
| Classification | Model 1 | Model 2 | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Atrial fibrillation | ||||
| Labile HCM | 1 | 1 | ||
| Nonobstructive HCM | 0.90 (0.37–2.17) | 0.815 | ··· | ··· |
| Obstructive HCM | 3.43 (1.73–6.81) | <0.001 | 3.22 (1.61–6.47) | 0.001 |
| Sustained ventricular tachycardia/ventricular fibrillation | ||||
| Labile HCM | 1 | 1 | ||
| Nonobstructive HCM | 4.64 (1.56–13.82) | 0.006 | 4.69 (1.57–13.97) | 0.006 |
| Obstructive HCM | 1.89 (0.55–6.46) | 0.310 | ··· | ··· |
| Heart failure | ||||
| Labile HCM | 1 | 1 | ||
| Nonobstructive HCM | 1.97 (0.87–4.47) | 0.103 | ··· | ··· |
| Obstructive HCM | 2.10 (0.94–4.71) | 0.072 | ··· | ··· |
| All‐cause death | ||||
| Labile HCM | 1 | 1 | ||
| Nonobstructive HCM | 1.89 (0.63–5.66) | 0.254 | ··· | ··· |
| Obstructive HCM | 235 (0.82–6.78) | 0.113 | ··· | ··· |
The labile HCM was set as a reference group. Model 1, crude ratio; and model 2, adjusted for age and sex. CI indicates confidence interval; HCM, hypertrophic cardiomyopathy; and HR, hazard ratio.
We performed additional analysis of the labile HCM group that demonstrated the best event‐free survival. Of 261 patients with labile HCM, 103 (39%) had NYHA class II to III symptoms, and 158 (61%) were asymptomatic (NYHA class I). There were 14 composite events in symptomatic patients, including 5 cases of new‐onset AF, 8 cases of new‐onset or worsening HF, and 1 death. On the other hand, asymptomatic patients had 8 composite events, with 1 case of new‐onset AF, 4 cases of new‐onset VT/VF, 1 case of new‐onset or worsening HF, and 2 deaths. Symptomatic patients had significantly higher risks of HF (P=0.006) and composite end points (P=0.028). Examining only the 391 asymptomatic patients in our study, labile HCM still carried a better prognosis than the other 2 groups (nonobstructive versus labile versus obstructive, 15% versus 5% versus 26%; P<0.001).
For composite cardiovascular outcomes, patients with labile HCM had the best, and patients with obstructive HCM had the worst, event‐free survival at the end of follow‐up (Figure 2E). Both obstructive and nonobstructive HCM were independent predictors of cardiovascular events compared with the labile group, after adjusting for age and sex. The hazard ratios and 95% confidence intervals were 3.41 (2.07–5.63) for obstructive HCM and 1.99 (1.19–3.33) for nonobstructive HCM (model 2; Table 4). In univariate Cox regression, age, male sex, NYHA class, history of sustained VT/VF, LA diameter, E/e′, and metabolic equivalents were predictive of future composite events (Table 5). After additional adjustments for these potential confounding parameters, the HCM subgroups remained independently associated with clinical outcomes (model 3; Table 4).
Table 4.
HRs and 95% CIs for Composite Cardiovascular Events During 10 Years of Follow‐Up by Classification of HCM
| Classification | Events/Cases | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| Composite outcome | |||||||
| Nonobstructive HCM | 43/214 | 2.00 (1.19–3.34) | 0.008 | 1.99 (1.19–3.33) | 0.009 | 1.94 (1.09–3.45) | 0.024 |
| Labile HCM | 22/261 | 1 | 1 | 1 | |||
| Obstructive HCM | 56/230 | 3.73 (2.27–6.11) | <0.001 | 3.41 (2.07–5.63) | <0.001 | 2.80 (1.64–4.80) | <0.001 |
Model 1, crude ratio; model 2, adjusted for age and sex; and model 3, adjusted for age, sex, New York Heart Association functional class, history of sustained ventricular tachycardia/ventricular fibrillation, left atrial diameter, ratio of early diastolic mitral flow velocity/early diastolic mitral septal annulus motion velocity, and metabolic equivalents. CI indicates confidence interval; HCM, hypertrophic cardiomyopathy; and HR, hazard ratio.
Table 5.
Determinants of Cardiovascular Events During 10 Years of Follow‐Up: Univariate Cox Regression Analysis
| Univariate Factors | HR (95% CI) | P Value |
|---|---|---|
| Age, per y | 1.02 (1.00–1.03) | 0.017 |
| Male sex | 0.65 (0.45–0.93) | 0.017 |
| Body mass index, per unit | 1.00 (0.98–1.03) | 0.954 |
| NYHA functional class | 1.52 (1.21–1.91) | <0.001 |
| History of hypertension | 1.00 (0.70–1.43) | 0.985 |
| History of hyperlipidemia | 0.96 (0.67–1.37) | 0.810 |
| History of sustained VT/VF | 2.90 (1.52–5.54) | 0.001 |
| Abnormal blood pressure response | 0.97 (0.64–1.49) | 0.896 |
| Left atrial diameter, per mm | 1.05 (1.03–1.07) | <0.001 |
| Septal thickness, per mm | 1.02 (0.99–1.06) | 0.126 |
| Posterior wall thickness, per mm | 1.03 (0.99–1.07) | 0.149 |
| E/A, per unit | 1.16 (0.94–1.42) | 0.160 |
| E/e′, per unit | 1.03 (1.02–1.04) | <0.001 |
| Metabolic equivalents, per unit | 0.90 (0.86–0.95) | <0.001 |
| Resting systolic blood pressure, per mm Hg | 0.99 (0.99–1.00) | 0.220 |
| Resting diastolic blood pressure, per mm Hg | 0.99 (0.97–1.00) | 0.074 |
| Peak systolic blood pressure, per mm Hg | 1.00 (0.99–1.00) | 0.161 |
CI indicates confidence interval; E/A, ratio of early diastolic mitral flow velocity/late diastolic mitral flow velocity; E/e′, ratio of early diastolic mitral flow velocity/early diastolic mitral septal annulus motion velocity; HR, hazard ratio; NYHA, New York Heart Association; and VT/VF, ventricular tachycardia/ventricular fibrillation.
Discussion
In this large single‐center HCM cohort, we present several novel and clinically important insights into the most common inherited cardiac disease. To our knowledge, there are no clinical outcome data using the modern hemodynamic classification of HCM into nonobstructive, labile, and obstructive HCM.
A recent study in 2016 described HCM‐related mortality among patients with nonobstructive HCM as low (0.5% per year), with a 10‐year survival rate of 97%, which was similar to expected all‐cause mortality in an age‐ and sex‐matched US population. However, focusing on mortality rate alone may underestimate the substantial morbidity burden in a subset of patients with nonobstructive HCM.22 In contrast with most literature to date, we find that patients with nonobstructive HCM are at significantly higher risk than previously suspected. Conversely, despite the obstructive hemodynamics, patients with labile‐obstructive HCM experience a generally benign clinical course. Concordant with previous studies, resting LVOT obstruction (LVOTO) is associated with the highest risk of developing our composite end point. Specifically, we found an increased risk of arrhythmia, HF, and death. Our findings may stimulate a critical rethinking of how best to manage nonobstructive and labile‐obstructive HCM. In addition, our study provides some granularity on the nature of specific clinical outcomes across the 3 HCM groups. Patients with obstructive HCM with significant LVOT pressure gradients predominantly experienced AF, HF, and death. Patients with nonobstructive HCM had more ventricular arrhythmic (sustained VT/VF) events.
Dynamic LVOTO has long been considered the primary driver of symptoms and complications in HCM. Landmark publications indicated that resting LVOT gradient ≥30 mm Hg is a strong independent predictor for symptoms, progression of HF, and death.7 Our data further support the high‐risk nature of obstructive HCM, and this finding validates that our cohort is generally similar to previously published large HCM cohorts. Obstructive HCM was associated with the worst diastolic function and the largest LA size. The relationship between diastolic dysfunction and LA size has been demonstrated in various studies,23, 24 and LA volume may be taken as a reflection of severity and chronicity of diastolic dysfunction.25 In the Framingham study, LA enlargement was demonstrated as a predictor for nonrheumatic AF in the general population,26 and was also the most sensitive and specific parameter associated with paroxysmal AF in patients with HCM.27
Given the higher rates of symptoms and complications, it is understandable that clinicians pay most attention to patients with obstructive HCM. However, this focus on high gradients seems to have inexorably led to an underappreciation of the adverse outcomes in patients with nonobstructive HCM.28 A recent study with 573 patients reported the clinical course was largely favorable in nonobstructive HCM, with low risk of worsening HF and low mortality.22 Although the annual mortality for nonobstructive HCM was <1%, the rate of disease‐related events, many of them potentially life threatening, was high.29 Thus, the data in that study corroborate our data on the high rates of ventricular arrhythmia in patients with nonobstructive HCM. In a report by Hebl et al, the prevalence of ICD placed preceding the index visit was also up to 11% of the cohort, reflecting the heavy burden of ventricular arrhythmia.30 Our observation that particular patients with nonobstructive HCM are at high risk is the key message of this article, and hopefully it stimulates the wider HCM community to examine their own databases to either confirm or validate our observation in this large cohort. Furthermore, our findings suggest that evaluation of nonobstructive HCM should be more nuanced and deliberative. More important, lack of high gradients should not lull clinicians into reassuring patients with nonobstructive hemodynamics without further investigation.
Our clinical outcome data in nonobstructive HCM have a substantial biological and pathophysiologic basis. Myocardial fibrosis, resulting from recurrent microvascular ischemia, is believed to be the substrate for potentially life‐threatening ventricular arrhythmias in patients with HCM.31 Previous studies have described a strong association between nonsustained VT on ambulatory Holter monitoring and the presence of late gadolinium enhancement on cardiac magnetic resonance imaging that represents fibrosis.32, 33 In addition, late gadolinium enhancement was associated with HF symptoms and was a strong determinant of LV dysfunction in HCM.34 In our previous report, we connected these pathological characteristics with nonobstructive HCM by demonstrating that more patients with nonobstructive HCM had a large fibrosis burden (late gadolinium enhancement, >20% of LV mass) and microvascular ischemia.12 In that study, even with a substantially smaller cohort size, we already saw a pattern of worse arrhythmic events in nonobstructive HCM. Others have shown that the prevalence and extent of scar (late gadolinium enhancement) were larger in nonobstructive HCM.22 Thus, our data and other published evidence suggest that myocardial fibrosis, microvascular ischemia, and a host of unidentified factors likely mediate the generation of malignant ventricular arrhythmias in nonobstructive HCM.
Our data lay out, for the first time, that all obstructive hemodynamics are not bad. Those with resting obstruction (fixed obstructive HCM) have poor long‐term outcomes. However, those with obstruction only on provocation (labile HCM) do extremely well, with low rates of adverse outcomes. Each of the 3 HCM subgroups has distinctive clinical outcomes. The clinical characteristics and distribution of outcomes are summarized in Table 6.
Table 6.
Summary of Clinical Outcomes of the 3 HCM Hemodynamic Spectrums
| Outcomes | Nonobstructive HCM | Labile HCM | Obstructive HCM |
|---|---|---|---|
| Need for SRT | − | + | ++ |
| Clinical outcomes | |||
| AF | + | + | +++ |
| VT/VF | +++ | ++ | + |
| Heart failure | ++ | + | ++ |
| Death | + | +/− | + |
| Overall risk | Intermediate | Low | High |
The left ventricular outflow tract pressure gradients were as follows: nonobstructive HCM, at rest, <30 mm Hg, and provoked, <30 mm Hg; labile HCM, at rest, <30 mm Hg, and provoked, ≥30 mm Hg; and obstructive HCM, at rest, ≥30 mm Hg, and provoked, ≥30 mm Hg. + indicates low risk; ++, intermediate risk; +++, high risk; −, not applicable; AF, atrial fibrillation; HCM, hypertrophic cardiomyopathy; SRT, septal reduction therapy; and VT/VF, ventricular tachycardia/ventricular fibrillation.
Maron et al reported that more than half of patients with HCM without resting gradients developed LVOTO on exercise.5 Of this labile‐obstruction subset, 43% were symptomatic (NYHA class II or III), but 57% were asymptomatic. The observation that most patients (ie, 60%) who developed moderate‐to‐severe HF symptoms did, in fact, generate hemodynamically significant gradients only with exercise brought the hypothesis that mechanical outflow obstruction related to exercise in such patients could prove to be of pathophysiological significance over time.5 On the basis of this finding, the 2011 American College of Cardiology/American Heart Association guidelines give exercise echocardiography a class IIa recommendation for detection and quantification of dynamic LVOTO in patients with HCM without resting gradients, irrespective of symptoms.19 In contrast, the 2014 European Society of Cardiology guidelines only recommend exercise stress echocardiography in symptomatic patients if bedside maneuvers fail to induce an LVOTO ≥50 mm Hg, but they do not recommend exercise stress echocardiography in asymptomatic patients unless the presence of an LVOT gradient is relevant to lifestyle advice and decisions on medical treatment.18 The present study may help place in perspective the discrepancy between the HCM recommendations offered by the American College of Cardiology/American Heart Association versus the European Society of Cardiology. In our data, the overall risks among 3 different hemodynamic classifications did not change, whether they were symptomatic or not. Even among asymptomatic patients in our study, labile HCM still carried a better prognosis than the other 2 groups. The difference in adverse outcomes between nonobstructive and labile HCM, regardless of symptoms, underscores the importance of identifying provoked LVOT pressure gradients, which may actually indicate a favorable outcome.
Our data highlight 2 important points that may affect how both HCM guidelines are interpreted. First, on the basis of our data, there is no evidence that labile HCM is associated with adverse outcomes (within a mean follow‐up of ≈3 years). This is contrary to the current guideline premise that labile LVOTO might generally lead to clinical deterioration. In the present study, the patients with labile LVOTO had more favorable outcomes than those with nonobstructive disease. Second, if this association is corroborated by subsequent studies, then there would be a strong argument for integration of routine exercise stress echocardiography into risk stratification protocols to identify latent LVOTO. The caveat, however, is critical: latent LVOTO must first be identified as a significant independent predictor of favorable outcome in additional, large‐scale studies with longer‐term follow‐up. In the present study, we found that patients with HCM with labile LVOTO had more favorable outcomes than those with nonobstructive disease. If this association is corroborated by subsequent studies, there would be an argument for routine exercise stress echocardiography in all patients with HCM and particularly in those with resting gradients <30 mm Hg, regardless of symptoms. However, labile HCM will need to be identified as a significant independent predictor of favorable outcome in additional large‐scale studies with longer‐term follow‐up.
Limitations of the Study
This was a single, tertiary, referral center cohort. Hence, a referral bias might exist. However, this cohort is no different than those reported in most HCM‐related publications.7, 35 There is a chance that asymptomatic patients were not referred to us. However, this bias should uniformly affect all groups; thus, we believe it should not unduly affect the results. This is an observational study, and we were not equipped to address why patients with nonobstructive HCM may develop more ventricular arrhythmias and why patients with obstructive HCM have more atrial arrhythmias. Our previous work and the work of others led us to speculate on potential mechanisms that may underlie the development of these complications in particular HCM subgroups.12, 22 We did not include any genetics data in our analysis because genotyping is not considered for clinical diagnosis of HCM.19 There is an abundance of literature on the lack of a clear connection between genotype and phenotype or clinical outcomes in HCM.36 Although we had a relatively large number of patients with 10 years of follow‐up, the median follow‐up was ≈3 years. There is still a need for replication of our findings with large‐scale studies with longer follow‐up. We used a composite clinical end point, like other seminal articles on HCM.37, 38 Several articles document the low rates of death and adverse events in HCM.22, 39 Moreover, using death as the only end point ignores the serious nonfatal complications in HCM that affect quality of life. Also, as astutely pointed out in a recent editorial,29 if high‐risk patients were not identified and intervened on in a timely manner, there would be significantly more deaths. We humbly submit that a composite clinical end point is clinically meaningful and useful in the population with HCM. The high rates of appropriate defibrillator discharges in our and other studies underscore this latter point.
Conclusion
Despite low annual mortality rates, patients with nonobstructive HCM have high rates of adverse clinical events, almost equivalent to those with obstructive HCM. Patients with nonobstructive HCM warrant thorough vetting, with a focus on the need for defibrillator therapy. Patients with obstructive HCM have the worst symptoms and high rates of adverse events. They merit aggressive treatment. Patients with labile HCM have the best prognosis and may need less aggressive management, with the recognition that all patients with HCM should be objectively risk stratified. Further large‐scale studies with longer follow‐up are needed to support our results.
Sources of Funding
This work was partially supported by National Institutes of Health (grant HL 98046). Lu was supported by Taipei Veterans General Hospital–National Yang‐Ming University Excellent Physician Scientists Cultivation Program, No. 104‐V‐A‐005. Ventoulis and Pozios were supported by fellowship grants from the Hellenic Cardiologic Society.
Disclosures
None.
Acknowledgments
We thank all our study subjects for their participation. We thank the sonographers and nurses of the Johns Hopkins Hospital Echocardiography Laboratory for their expertise and assistance. We thank Glenn Lie and Gunnar Hansen (General Electric Ultrasound, Horten, Norway) for loaning us the EchoPAC analysis software. We thank the Dr Lawrence and Sheila Pakula Foundation for their kind and generous support.
(J Am Heart Assoc. 2018;7:e006657 DOI: 10.1161/JAHA.117.006657.)29478967
References
- 1. Moon JC, Reed E, Sheppard MN, Elkington AG, Ho SY, Burke M, Petrou M, Pennell DJ. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2004;43:2260–2264. [DOI] [PubMed] [Google Scholar]
- 2. O'Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, Webb J, Kulkarni M, Dawson D, Sulaibeekh L, Chandrasekaran B, Bucciarelli‐Ducci C, Pasquale F, Cowie MR, McKenna WJ, Sheppard MN, Elliott PM, Pennell DJ, Prasad SK. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:867–874. [DOI] [PubMed] [Google Scholar]
- 3. Cecchi F, Olivotto I, Gistri R, Lorenzoni R, Chiriatti G, Camici PG. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med. 2003;349:1027–1035. [DOI] [PubMed] [Google Scholar]
- 4. Maron BJ, Ommen SR, Semsarian C, Spirito P, Olivotto I, Maron MS. Hypertrophic cardiomyopathy: present and future, with translation into contemporary cardiovascular medicine. J Am Coll Cardiol. 2014;64:83–99. [DOI] [PubMed] [Google Scholar]
- 5. Maron MS, Olivotto I, Zenovich AG, Link MS, Pandian NG, Kuvin JT, Nistri S, Cecchi F, Udelson JE, Maron BJ. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114:2232–2239. [DOI] [PubMed] [Google Scholar]
- 6. Finocchiaro G, Haddad F, Pavlovic A, Sinagra G, Schnittger I, Knowles JW, Perez M, Magavern E, Myers J, Ashley E. Latent obstruction and left atrial size are predictors of clinical deterioration leading to septal reduction in hypertrophic cardiomyopathy. J Card Fail. 2014;20:236–243. [DOI] [PubMed] [Google Scholar]
- 7. Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, Cecchi F, Maron BJ. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348:295–303. [DOI] [PubMed] [Google Scholar]
- 8. Elliott PM, Gimeno JR, Tome MT, Shah J, Ward D, Thaman R, Mogensen J, McKenna WJ. Left ventricular outflow tract obstruction and sudden death risk in patients with hypertrophic cardiomyopathy. Eur Heart J. 2006;27:1933–1941. [DOI] [PubMed] [Google Scholar]
- 9. Bravo PE, Pinheiro A, Higuchi T, Rischpler C, Merrill J, Santaularia‐Tomas M, Abraham MR, Wahl RL, Abraham TP, Bengel FM. PET/CT assessment of symptomatic individuals with obstructive and nonobstructive hypertrophic cardiomyopathy. J Nucl Med. 2012;53:407–414. [DOI] [PubMed] [Google Scholar]
- 10. Abraham TP, Dimaano VL, Liang HY. Role of tissue Doppler and strain echocardiography in current clinical practice. Circulation. 2007;116:2597–2609. [DOI] [PubMed] [Google Scholar]
- 11. Rubinshtein R, Glockner JF, Ommen SR, Araoz PA, Ackerman MJ, Sorajja P, Bos JM, Tajik AJ, Valeti US, Nishimura RA, Gersh BJ. Characteristics and clinical significance of late gadolinium enhancement by contrast‐enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ Heart Fail. 2010;3:51–58. [DOI] [PubMed] [Google Scholar]
- 12. Pozios I, Corona‐Villalobos C, Sorensen LL, Bravo PE, Canepa M, Pisanello C, Pinheiro A, Dimaano VL, Luo H, Dardari Z, Zhou X, Kamel I, Zimmerman SL, Bluemke DA, Abraham MR, Abraham TP. Comparison of outcomes in patients with nonobstructive, labile‐obstructive, and chronically obstructive hypertrophic cardiomyopathy. Am J Cardiol. 2015;116:938–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Canepa M, Sorensen LL, Pozios I, Dimaano VL, Luo HC, Pinheiro AC, Strait JB, Brunelli C, Abraham MR, Ferrucci L, Abraham TP. Comparison of clinical presentation, left ventricular morphology, hemodynamics, and exercise tolerance in obese versus nonobese patients with hypertrophic cardiomyopathy. Am J Cardiol. 2013;112:1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nagueh SF, Bierig SM, Budoff MJ, Desai M, Dilsizian V, Eidem B, Goldstein SA, Hung J, Maron MS, Ommen SR, Woo A; American Society of Echocardiography; American Society of Nuclear Cardiology; Society for Cardiovascular Magnetic Resonance; Society of Cardiovascular Computed Tomography . American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with hypertrophic cardiomyopathy: endorsed by the American Society of Nuclear Cardiology, Society for Cardiovascular Magnetic Resonance, and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr. 2011;24:473–498. [DOI] [PubMed] [Google Scholar]
- 15. Marwick TH, Nakatani S, Haluska B, Thomas JD, Lever HM. Provocation of latent left ventricular outflow tract gradients with amyl nitrite and exercise in hypertrophic cardiomyopathy. Am J Cardiol. 1995;75:805–809. [DOI] [PubMed] [Google Scholar]
- 16. Lancellotti P, Pellikka PA, Budts W, Chaudhry FA, Donal E, Dulgheru R, Edvardsen T, Garbi M, Ha JW, Kane GC, Kreeger J, Mertens L, Pibarot P, Picano E, Ryan T, Tsutsui JM, Varga A. The clinical use of stress echocardiography in non‐ischaemic heart disease: recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30:101–138. [DOI] [PubMed] [Google Scholar]
- 17. Yu CM, Sanderson JE, Marwick TH, Oh JK. Tissue Doppler imaging a new prognosticator for cardiovascular diseases. J Am Coll Cardiol. 2007;49:1903–1914. [DOI] [PubMed] [Google Scholar]
- 18. Authors/Task Force members ; Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733–2779. [DOI] [PubMed] [Google Scholar]
- 19. Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Failure Society of America; Heart Rhythm Society; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons . 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:e783–e831. [DOI] [PubMed] [Google Scholar]
- 20. Sadoul N, Prasad K, Elliott PM, Bannerjee S, Frenneaux MP, McKenna WJ. Prospective prognostic assessment of blood pressure response during exercise in patients with hypertrophic cardiomyopathy. Circulation. 1997;96:2987–2991. [DOI] [PubMed] [Google Scholar]
- 21. Olivotto I, Maron BJ, Montereggi A, Mazzuoli F, Dolara A, Cecchi F. Prognostic value of systemic blood pressure response during exercise in a community‐based patient population with hypertrophic cardiomyopathy. J Am Coll Cardiol. 1999;33:2044–2051. [DOI] [PubMed] [Google Scholar]
- 22. Maron MS, Rowin EJ, Olivotto I, Casey SA, Arretini A, Tomberli B, Garberich RF, Link MS, Chan RH, Lesser JR, Maron BJ. Contemporary natural history and management of nonobstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2016;67:1399–1409. [DOI] [PubMed] [Google Scholar]
- 23. Pritchett AM, Mahoney DW, Jacobsen SJ, Rodeheffer RJ, Karon BL, Redfield MM. Diastolic dysfunction and left atrial volume: a population‐based study. J Am Coll Cardiol. 2005;45:87–92. [DOI] [PubMed] [Google Scholar]
- 24. Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol. 2002;90:1284–1289. [DOI] [PubMed] [Google Scholar]
- 25. Geske JB, Sorajja P, Nishimura RA, Ommen SR. The relationship of left atrial volume and left atrial pressure in patients with hypertrophic cardiomyopathy: an echocardiographic and cardiac catheterization study. J Am Soc Echocardiogr. 2009;22:961–966. [DOI] [PubMed] [Google Scholar]
- 26. Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation: the Framingham Heart Study. Circulation. 1994;89:724–730. [DOI] [PubMed] [Google Scholar]
- 27. Tani T, Tanabe K, Ono M, Yamaguchi K, Okada M, Sumida T, Konda T, Fujii Y, Kawai J, Yagi T, Sato M, Ibuki M, Katayama M, Tamita K, Yamabe K, Yamamuro A, Nagai K, Shiratori K, Morioka S. Left atrial volume and the risk of paroxysmal atrial fibrillation in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2004;17:644–648. [DOI] [PubMed] [Google Scholar]
- 28. Aron LA, Hertzeanu HL, Fisman EZ, Nosrati IS, Kellermann JJ. Prognosis of nonobstructive hypertrophic cardiomyopathy. Am J Cardiol. 1991;67:215–217. [DOI] [PubMed] [Google Scholar]
- 29. Elliott PM. Hypertrophic cardiomyopathy: job done or work in progress? J Am Coll Cardiol. 2016;67:1410–1411. [DOI] [PubMed] [Google Scholar]
- 30. Hebl VB, Miranda WR, Ong KC, Hodge DO, Bos JM, Gentile F, Klarich KW, Nishimura RA, Ackerman MJ, Gersh BJ, Ommen SR, Geske JB. The natural history of nonobstructive hypertrophic cardiomyopathy. Mayo Clin Proc. 2016;91:279–287. [DOI] [PubMed] [Google Scholar]
- 31. Basso C, Thiene G, Corrado D, Buja G, Melacini P, Nava A. Hypertrophic cardiomyopathy and sudden death in the young: pathologic evidence of myocardial ischemia. Hum Pathol. 2000;31:988–998. [DOI] [PubMed] [Google Scholar]
- 32. Adabag AS, Maron BJ, Appelbaum E, Harrigan CJ, Buros JL, Gibson CM, Lesser JR, Hanna CA, Udelson JE, Manning WJ, Maron MS. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51:1369–1374. [DOI] [PubMed] [Google Scholar]
- 33. Kwon DH, Setser RM, Popovic ZB, Thamilarasan M, Sola S, Schoenhagen P, Garcia MJ, Flamm SD, Lever HM, Desai MY. Association of myocardial fibrosis, electrocardiography and ventricular tachyarrhythmia in hypertrophic cardiomyopathy: a delayed contrast enhanced MRI study. Int J Cardiovasc Imaging. 2008;24:617–625. [DOI] [PubMed] [Google Scholar]
- 34. Maron MS, Appelbaum E, Harrigan CJ, Buros J, Gibson CM, Hanna C, Lesser JR, Udelson JE, Manning WJ, Maron BJ. Clinical profile and significance of delayed enhancement in hypertrophic cardiomyopathy. Circ Heart Fail. 2008;1:184–191. [DOI] [PubMed] [Google Scholar]
- 35. Guttmann OP, Pavlou M, O'Mahony C, Monserrat L, Anastasakis A, Rapezzi C, Biagini E, Gimeno JR, Limongelli G, Garcia‐Pavia P, McKenna WJ, Omar RZ, Elliott PM; Hypertrophic Cardiomyopathy Outcomes Investigators . Predictors of atrial fibrillation in hypertrophic cardiomyopathy. Heart. 2017;103:672–678. [DOI] [PubMed] [Google Scholar]
- 36. Maron BJ, Maron MS. The 25‐year genetic era in hypertrophic cardiomyopathy: revisited. Circ Cardiovasc Genet. 2014;7:401–404. [DOI] [PubMed] [Google Scholar]
- 37. Ciampi Q, Olivotto I, Gardini C, Mori F, Peteiro J, Monserrat L, Fernandez X, Cortigiani L, Rigo F, Lopes LR, Cruz I, Cotrim C, Losi M, Betocchi S, Beleslin B, Tesic M, Dikic AD, Lazzeroni E, Lazzeroni D, Sicari R, Picano E. Prognostic role of stress echocardiography in hypertrophic cardiomyopathy: the International Stress Echo Registry. Int J Cardiol. 2016;219:331–338. [DOI] [PubMed] [Google Scholar]
- 38. Peteiro J, Fernandez X, Bouzas‐Mosquera A, Monserrat L, Mendez C, Rodriguez‐Garcia E, Soler R, Couto D, Castro‐Beiras A. Exercise echocardiography and cardiac magnetic resonance imaging to predict outcome in patients with hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2015;16:423–432. [DOI] [PubMed] [Google Scholar]
- 39. Maron BJ, Rowin EJ, Casey SA, Link MS, Lesser JR, Chan RH, Garberich RF, Udelson JE, Maron MS. Hypertrophic cardiomyopathy in adulthood associated with low cardiovascular mortality with contemporary management strategies. J Am Coll Cardiol. 2015;65:1915–1928. [DOI] [PubMed] [Google Scholar]


