Abstract
Background
Although diastolic blood pressure (DBP) is independently associated with an increased risk of adverse cardiovascular outcomes in the general population, it is unclear if a similar relationship exists in patients with heart failure with preserved ejection fraction.
Methods and Results
This analysis included 1703 (mean age, 72±10 years; 50% men; 78% white) patients with heart failure with preserved ejection fraction enrolled in the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) Trial from the Americas who were treated for hypertension. Multivariable Cox regression was used to examine the risk of hospitalization for heart failure, death, and cardiovascular death associated with DBP. The relationship between hospitalization for heart failure and DBP was linear, with an increased risk observed with decreasing DBP values (≥90 mm Hg: referent; 80–89 mm Hg: hazard ratio [HR], 1.44; 95% confidence interval [CI], 0.85–2.44; 70–79 mm Hg: HR, 1.18; 95% CI, 0.69–2.01; 60–69 mm Hg: HR, 1.54; 95% CI, 0.90–2.63; <60 mm Hg: HR, 2.12; 95% CI, 1.20–3.74; P=0.0055 for trend). The associations of DBP with death (≥90 mm Hg: HR, 1.86; 95% CI, 1.12–3.06; 80–89 mm Hg: HR, 1.23; 95% CI, 0.89–1.70; 70–79 mm Hg: referent; 60–69 mm Hg: HR, 1.20; 95% CI, 0.90–1.59; <60 mm Hg: HR, 1.68; 95% CI, 1.21–2.33) and cardiovascular death (≥90 mm Hg: HR, 2.02; 95% CI, 1.10–3.71; 80–89 mm Hg: HR, 1.17; 95% CI, 0.77–1.79; 70–79 mm Hg: referent; 60–69 mm Hg: HR, 1.16; 95% CI, 0.80–1.70; <60 mm Hg: HR, 1.85; 95% CI, 1.21–2.82) were nonlinear, with a greater risk of each outcome observed with DBP values ≥90 and <60 mm Hg.
Conclusions
DBP values ≥90 and <60 mm Hg are associated with a significant risk of adverse outcomes in patients with heart failure with preserved ejection fraction who are treated for hypertension. Further research is needed to determine optimal DBP targets to reduce the risk of adverse events in patients with heart failure with preserved ejection fraction.
Keywords: blood pressure, heart failure, preserved left ventricular function
Subject Categories: Heart Failure, High Blood Pressure
Clinical Perspective
What Is New?
Although recent reports have implicated diastolic blood pressure (DBP) as an important parameter for predicting future cardiovascular disease events, it was unknown if a similar association exists in patients with heart failure with preserved ejection fraction who are treated for hypertension.
What Are the Clinical Implications?
In patients with heart failure with preserved ejection fraction, DBP values ≥90 and <60 mm Hg were associated with an increased risk of adverse events.
The risk of hospitalization for heart failure was greatest for patients with DBP values <60 mm Hg, whereas the risk for death and cardiovascular death demonstrated a U‐shaped association, with a greater risk observed with DBP values ≥90 and <60 mm Hg.
Our data suggest that careful attention is needed to DBP in patients with heart failure with preserved ejection fraction who are treated for hypertension.
Heart failure with preserved ejection fraction (HFpEF) accounts for >50% of all heart failure cases, and this condition is increasing in frequency, along with associated morbidity, mortality, and healthcare costs.1 HFpEF represents a complex clinical syndrome with multiple comorbidities, such as hypertension, diabetes mellitus, renal disease, metabolic syndrome, and atrial fibrillation.2 Systemic hypertension is the most prevalent modifiable risk factor,1 and its presence confers an increased risk of developing clinically apparent heart failure.3, 4
Although hypertension treatment represents an important aspect of heart failure management,5 a relative paucity of data exist on the management of this common comorbid condition in patients with HFpEF. The 2017 American College of Cardiology/American Heart Association focused update of the 2013 guidelines for the management of heart failure recommended a target systolic blood pressure (SBP) of <130 mm Hg in patients with HFpEF.5, 6 However, goals for diastolic blood pressure (DBP) in this population were ignored. Previous reports from prospective cohort studies representative of the general population have demonstrated that lower DBP values are associated with an increased risk for adverse cardiovascular outcomes,7, 8 and a similar relationship possibly exists in HFpEF. To address this gap in knowledge, we examined the association between levels of DBP and adverse outcomes in patients with HFpEF from the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) Trial.
Methods
Study Design and Patients
The present study used data from the TOPCAT Trial obtained from the National Heart, Lung, and Blood Institute, and the data and study materials have been made available to other researchers.9 The TOPCAT Trial was a multicenter, international randomized, double‐blind, placebo‐controlled study to examine the efficacy of spironolactone in patients with HFpEF. The design, inclusion criteria, and baseline characteristics of the trial have been published previously.10, 11 Briefly, 3445 patients with symptomatic HFpEF from 270 sites in 6 countries were enrolled between August 2006 and January 2012. The primary goal of the trial was to determine if spironolactone was associated with a reduction in the composite outcome of cardiovascular mortality, aborted cardiac arrest, or heart failure hospitalization in patients with HFpEF (eg, documented ejection fraction ≥45%). In this analysis, we examined the relationship between DBP levels and the risk of hospitalization for heart failure, death, and cardiovascular death. The analysis was limited to patients with HFpEF who were receiving at least 1 antihypertensive medication at the time of enrollment. This was done because most patients with HFpEF were treated for hypertension, and we aimed to examine the association between DBP and outcomes in patients who were treated for hypertension. In addition, because of differences in the baseline characteristics and event rates observed between patients recruited in Russia and Georgia versus the Americas,12 we limited our analysis to TOPCAT Trial patients who were enrolled from the Americas. This current analysis was approved by the institutional review board at Emory University School of Medicine (Atlanta, GA), and subjects gave written informed consent before participation in the TOPCAT Trial.
Baseline Characteristics
Patients who participated in the TOPCAT Trial underwent a detailed baseline evaluation.11 Age, sex, race, and smoking were obtained by self‐reported history. Smoking was defined as the current use of cigarettes. Medical history for the following diagnoses was obtained by self‐report and medical record review: coronary heart disease, stroke, New York Heart Association class, and prior heart failure hospitalization. Diabetes mellitus was ascertained by self‐reported history, medical record review, and the use of diabetes mellitus medications (eg, insulin and oral hypoglycemic agents). SBP, DBP, and body mass index were obtained by trained staff, and laboratory data included serum creatinine. Medication data included aspirin, statins, and antihypertensive medications (β blockers, angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers, calcium channel blockers, diuretics, long‐acting nitrates, and other antihypertensive medications).
Outcomes
Outcomes in the TOPCAT Trial were adjudicated by a clinical end point committee, and the details of this process and definitions for each outcome examined have been described.10, 13 The outcomes examined in this analysis included hospitalization for heart failure, death, and cardiovascular death. Briefly, hospitalization for heart failure was defined as the unexpected presentation to an immediate care facility requiring overnight stay with symptoms and physical examination findings consistent with heart failure, and treatment with intravenous vasodilators or inotropes, mechanical fluid removal, or hemodynamic support. Cardiovascular death was defined as death attributable to one of the following: myocardial infarction, worsening heart failure, sudden death, stroke, pulmonary embolism, death occurring during a cardiovascular‐related procedure, or other cardiovascular death. Death included the composite of cardiovascular and noncardiovascular death.
Statistical Analysis
Participants were stratified into 5 categories on the basis of DBP (<60, 60–69, 70–79, 80–89, and ≥90 mm Hg). The following categories were used because these have been previously demonstrated to detect clinically apparent differences in the risk of adverse events in the general population.8 Baseline characteristics were compared across the aforementioned DBP categories. Categorical variables were reported as frequency and percentage, whereas continuous variables were recorded as mean±SD. Statistical significance for categorical variables was tested using the χ2 method; and for continuous variables, the analysis of variance procedure was used.
Because of the potential for nonlinear associations between DBP and outcomes,7 we tested for linearity between the associations of DBP with each outcome. This was done graphically using a restricted cubic spline model with incorporated knots at the 5th, 50th, and 95th percentiles,14 and also using a likelihood ratio test for linearity. The relationship between DBP and hospitalization for HF was found to be linear (likelihood ratio test for nonlinearity, P=0.26), and the referent group for this outcome was DBP ≥90 mm Hg. A nonlinear relationship was observed for death (likelihood ratio test for nonlinearity, P=0.0016) and cardiovascular death (likelihood ratio test for nonlinearity, P=0.0066), and the optimal referent group for these outcomes was DBP between 70 and 79 mm Hg on the basis of the restricted cubic spline analysis.
Follow‐up time was defined as the time from randomization until one of the following: outcome of interest, death, unavailable for follow‐up, or end of follow‐up. Kaplan‐Meier estimates were used to examine the unadjusted cumulative incidence estimates of heart failure hospitalization, and differences were compared using the log‐rank procedure. Cox regression was used to examine the risk of each outcome associated with each DBP category. Multivariable models were constructed with the following clinically relevant variables: model 1 adjusted for age, sex, and race; model 2 adjusted for model 1 covariates plus smoking, SBP, serum creatinine, diabetes mellitus, body mass index, aspirin, statin, randomization group, New York Heart Association class, coronary heart disease, and stroke. A test of trend was computed per category increase for hospitalization for heart failure.
The proportional hazards assumption was not violated in our analyses. Statistical significance, including interaction terms, was defined as P<0.05. SAS Version 9.4 (SAS Institute Inc, Cary, NC) was used for all analyses.
Results
This analysis included 1703 (mean age, 72±10 years; 50% men; 78% white) participants from the TOPCAT Trial who were enrolled in the Americas. There were 116 (7%), 380 (22%), 485 (29%), 500 (29%), and 222 (13%) participants who had DBP values of ≥90, 80 to 89, 70 to 79, 60 to 69, and ≤60 mm Hg, respectively. Baseline characteristics across DBP values are shown in Table 1.
Table 1.
Baseline Characteristics (N=1703)
| Characteristic | Diastolic Blood Pressure (mm Hg) | P Valuea | ||||
|---|---|---|---|---|---|---|
| ≥90 (n=116) | 80–89 (n=380) | 70–79 (n=485) | 60–69 (n=500) | <60 (n=222) | ||
| Age, y | 67±10 | 70±10 | 72±10 | 72±8.9 | 74±10 | <0.001 |
| Male sex | 40 (34) | 189 (50) | 240 (49) | 260 (52) | 122 (55) | 0.0066 |
| White race | 73 (63) | 287 (76) | 375 (77) | 412 (82) | 180 (81) | <0.001 |
| Current smoker | 12 (10) | 34 (9) | 31 (6) | 28 (6) | 10 (5) | 0.084 |
| Diabetes mellitus | 51 (44) | 144 (38) | 226 (47) | 226 (45) | 115 (52) | 0.014 |
| Coronary heart disease | 28 (24) | 115 (30) | 168 (35) | 213 (43) | 104 (47) | <0.001 |
| Stroke | 12 (10) | 33 (9) | 39 (8) | 44 (9) | 26 (12) | 0.58 |
| Systolic blood pressure, mm Hg | 143±10 | 134±12 | 129±14 | 121±15 | 117±17 | <0.001 |
| Body mass index, kg/m2 | 36±8.3 | 34±7.4 | 34±8.2 | 34±8.3 | 32±8.9 | 0.0012 |
| Serum creatinine, mg/dL | 1.07±0.34 | 1.11±0.32 | 1.13±0.33 | 1.19±0.35 | 1.25±0.35 | <0.001 |
| New York Heart Association class III–IV | 35 (30) | 117 (31) | 170 (35) | 185 (37) | 95 (43) | 0.029 |
| Prior heart failure hospitalization | 78 (67) | 222 (58) | 287 (59) | 280 (56) | 135 (61) | 0.24 |
| Aspirin use | 61 (53) | 200 (53) | 283 (58) | 305 (61) | 149 (67) | 0.0044 |
| Statin use | 60 (52) | 213 (56) | 323 (67) | 346 (69) | 170 (77) | <0.001 |
| Spironolactone use | 60 (52) | 190 (50) | 255 (53) | 245 (49) | 111 (50) | 0.84 |
| β‐Blocker use | 78 (67) | 282 (74) | 397 (82) | 418 (84) | 179 (81) | <0.001 |
| ACEI/ARB use | 105 (91) | 315 (83) | 377 (78) | 385 (77) | 174 (78) | 0.0061 |
| Calcium channel blocker use | 52 (45) | 152 (40) | 184 (38) | 192 (38) | 89 (40) | 0.70 |
| Diuretic use | 107 (92) | 336 (88) | 425 (88) | 457 (91) | 203 (91) | 0.20 |
| Long‐acting nitrate use | 16 (14) | 50 (13) | 92 (19) | 91 (18) | 45 (20) | 0.086 |
| Other antihypertensive medication use | 24 (21) | 56 (15) | 80 (16) | 78 (16) | 46 (21) | 0.25 |
Data are given as mean±SD or number (percentage). ACEI/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker.
Statistical significance for continuous data was tested using the analysis of variance, and categorical data were tested using the χ2 test.
During a median follow‐up of 2.9 years (25th–75th percentile, 1.9–4.1 years), a total of 386 hospitalizations for heart failure, 372 deaths, and 218 cardiovascular deaths occurred. The cumulative incidence estimates for hospitalization for heart failure are shown in Figure 1. As shown, a higher number of hospitalizations for heart failure was observed as DBP decreased. The relationship between hospitalization for heart failure and DBP was linear, with an increased risk observed with decreasing DBP values (P=0.0055 for trend), and the risk of hospitalization for heart failure was greatest for DBP values <60 mm Hg (Table 2). The risk of hospitalization for heart failure across DBP values is depicted graphically in Figure 2.
Figure 1.
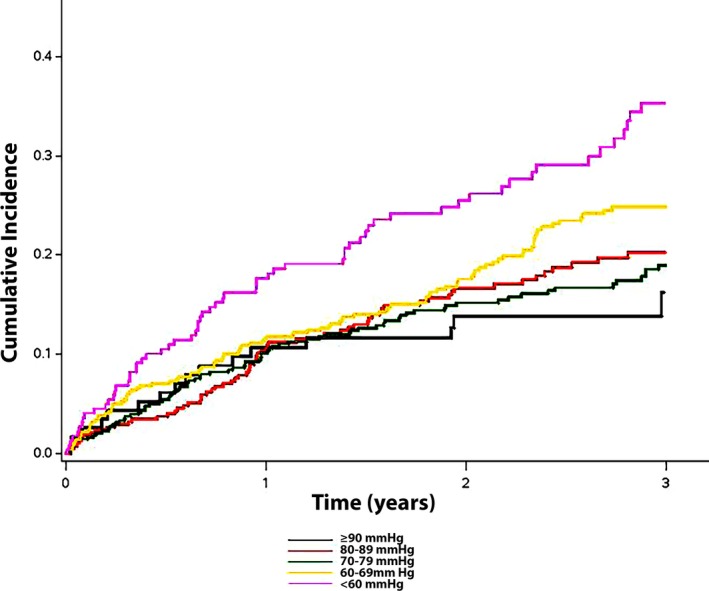
Unadjusted cumulative incidence of hospitalization for heart failure. The cumulative incidence curves for hospitalization for heart failure (log‐rank P<0.001) are shown.
Table 2.
Risk of Hospitalization for Heart Failure, Death, and Cardiovascular Death With DBP (N=1703)
| Outcome | Events/No. at Risk | Model 1a | Model 2b | ||
|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| Hospitalization for heart failure | |||||
| ≥90 mm Hg | 17/116 | Reference | ··· | Reference | ··· |
| 80–89 mm Hg | 80/380 | 1.38 (0.82–2.34) | 0.23 | 1.44 (0.85–2.44) | 0.18 |
| 70–79 mm Hg | 95/485 | 1.24 (0.74–2.09) | 0.42 | 1.18 (0.69–2.01) | 0.54 |
| 60–69 mm Hg | 121/500 | 1.62 (0.97–2.71) | 0.067 | 1.54 (0.90–2.63) | 0.12 |
| <60 mm Hg | 73/222 | 2.43 (1.42–4.16) |
0.0011 (P trend<0.001) |
2.12 (1.20–3.74) |
0.0096 (P trend=0.0055) |
| Death | |||||
| ≥90 mm Hg | 21/116 | 1.55 (0.95–2.51) | 0.077 | 1.86 (1.12–3.06) | 0.016 |
| 80–89 mm Hg | 70/380 | 1.16 (0.84–1.59) | 0.37 | 1.23 (0.89–1.70) | 0.22 |
| 70–79 mm Hg | 85/485 | Reference | ··· | Reference | ··· |
| 60–69 mm Hg | 119/500 | 1.37 (1.04–1.81) | 0.027 | 1.20 (0.90–1.59) | 0.22 |
| <60 mm Hg | 77/222 | 2.09 (1.54–2.86) | <0.001 | 1.68 (1.21–2.33) | 0.0020 |
| Cardiovascular death | |||||
| ≥90 mm Hg | 15/116 | 1.80 (1.00–3.24) | 0.049 | 2.02 (1.10–3.71) | 0.024 |
| 80–89 mm Hg | 42/380 | 1.17 (0.78–1.77) | 0.45 | 1.17 (0.77–1.79) | 0.46 |
| 70–79 mm Hg | 50/485 | Reference | ··· | Reference | ··· |
| 60–69 mm Hg | 65/500 | 1.29 (0.89–1.86) | 0.18 | 1.16 (0.80–1.70) | 0.44 |
| <60 mm Hg | 46/222 | 2.18 (1.46–3.26) | <0.001 | 1.85 (1.21–2.82) | 0.0046 |
CI indicates confidence interval; DBP, diastolic blood pressure; and HR, hazard ratio.
Adjusted for age, sex, and race.
Adjusted for model 1 covariates plus smoking, systolic blood pressure, serum creatinine, diabetes mellitus, body mass index, aspirin, statin, randomization group, New York Heart Association class, coronary heart disease, and stroke.
Figure 2.
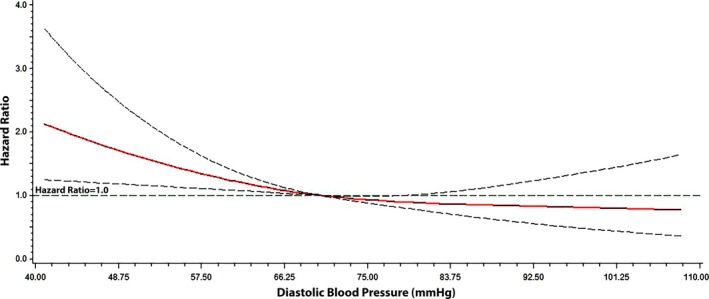
Risk of hospitalization for heart failure across diastolic blood pressure. Each hazard ratio was computed with the median diastolic blood pressure value of 70 mm Hg as the reference and was adjusted for age, sex, race, smoking, systolic blood pressure, serum creatinine, diabetes mellitus, body mass index, aspirin, statin, randomization group, New York Heart Association class, coronary heart disease, and stroke. Dotted lines represent the 95% confidence interval.
The associations of DBP with death and cardiovascular death were nonlinear, with a greater risk of each outcome observed with DBP values ≥90 and <60 mm Hg (Table 2). The U‐shaped association between DBP and death is depicted in Figure 3, with a lower risk of death among patients with DBP values between 70 and 79 mm Hg. A similar U‐shaped relationship was observed for cardiovascular death (data not shown).
Figure 3.
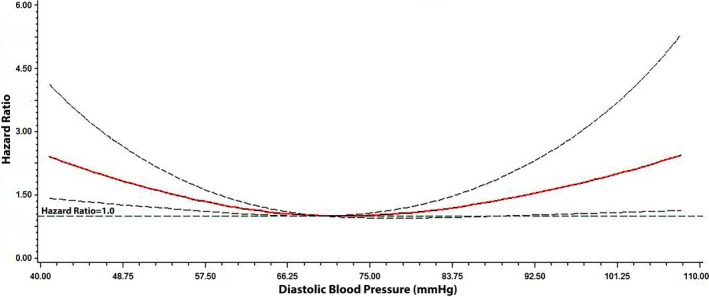
Risk of death across diastolic blood pressure. Each hazard ratio was computed with the median diastolic blood pressure value of 70 mm Hg as the reference and was adjusted for age, sex, race, smoking, systolic blood pressure, serum creatinine, diabetes mellitus, body mass index, aspirin, statin, randomization group, New York Heart Association class, coronary heart disease, and stroke. Dotted lines represent the 95% confidence interval.
Discussion
In this analysis from the TOPCAT Trial, DBP values ≥90 and <60 mm Hg were associated with an increased risk of adverse outcomes in patients with HFpEF who were treated for hypertension. Specially, the risk of hospitalization for heart failure was greatest for patients with DBP values <60 mm Hg, whereas the risk for death and cardiovascular death demonstrated a U‐shaped association, with a greater risk observed with DBP values ≥90 and <60 mm Hg. Overall, our data highlight the importance of DBP in patients with HFpEF who are treated for hypertension and suggest that careful attention is needed regarding this hemodynamic parameter among patients with HFpEF.
The importance of DBP and its association with adverse cardiovascular events have been well described. In the INVEST (International Verapamil SR‐Trandolapril) trial of >22 000 patients with coronary artery disease who were treated for hypertension, low DBP (eg, <70 mm Hg) was associated with a 2‐fold increase in the risk of the primary outcome (death, nonfatal myocardial infarction, or nonfatal stroke).15 In addition, data from the community‐based ARIC (Atherosclerosis Risk In Communities) study demonstrated that DBP values <70 mm Hg were associated with increased risks of coronary heart disease events and death, and the risk was greatest for values <60 mm Hg.8 The report from the ARIC study was able to demonstrate that the relationship between low DBP and adverse events possibly is related to subclinical myocardial injury, because DBP <70 mm Hg was associated with elevated high‐sensitivity cardiac troponin‐T levels (≥14 ng/L).8 Data from the MESA (Multi‐Ethnic Study of Atherosclerosis) also demonstrated that DBP values <60 mm Hg were associated with coronary heart disease development and death.16
The finding that DBP <60 mm Hg is associated with an increased risk of death and cardiovascular death in our report fits well with previous data, and it is likely explained by underlying subclinical myocardial injury in patients with HFpEF. In addition, we demonstrated that low DBP (<60 mm Hg) increases the risk for hospitalization for heart failure in patients who have HFpEF. The association between low DBP and hospitalization for heart failure possibly is related to the precipitation of cardiovascular symptoms. Data from SPRINT have demonstrated a higher risk of hypotension, bradycardia, and syncope in patients with aggressive SBP goals (eg, <120 mm Hg),6 and the same possibility is true in our cohort, because patients in our analysis with low DBP (eg, <60 mm Hg) had concomitantly low SBP (mean SBP, 117 mm Hg). Therefore, low DBP may result in decreased coronary perfusion that precipitates acute decompensated HFpEF, especially with simultaneously low SBP. However, the association between DBP and hospitalization for heart failure is speculative, and further research is needed to understand this finding. Nonetheless, we have identified a subgroup of patients with HFpEF in whom decompensation is likely, and DBP values <60 mm Hg should alert clinicians to the need for frequent cardiovascular assessment and optimization of heart failure therapies.
The findings of this analysis also demonstrate that DBP values ≥90 mm Hg are associated with an increased risk of death and cardiovascular death in patients with HFpEF. The initiation of antihypertensive treatment with DBP ≥90 mm Hg to a goal <90 mm Hg reduces the risk of cerebrovascular events and overall mortality, and this is recommended in the general population.17 Therefore, the finding of adverse outcomes in patients with HFpEF with DBP ≥90 mm Hg likely reflects that diastolic hypertension also is an important marker of cardiovascular risk in HFpEF, similar to the general population. In addition, this finding highlights the importance of hypertension as a modifiable risk factor to reduce future cardiovascular disease events in patients who have HFpEF.
Although recent trials have examined the benefit of certain antihypertensive agents in HFpEF, none have examined the benefit of strict blood pressure control. The 2017 American College of Cardiology/American Heart Association focused update on the management of heart failure guidelines recommended a target SBP <130 mm Hg in patients with established HFpEF.5 This recommendation was based on the findings from SPRINT.6 DBP goals and their influence on adverse events in HFpEF were ignored, and this is related to the relative paucity of data that exist on DBP in patients with HFpEF. Although our data suggest a benefit of DBP targets <90 mm Hg, an increased risk for adverse events, including hospitalization for heart failure, was observed with values <60 mm Hg. Therefore, the findings in this analysis suggest that careful attention is needed on titration of DBP in patients with HFpEF who are treated for hypertension. However, we acknowledge that the findings of our analysis are speculative, because the primary purpose of the TOPCAT Trial was not to determine DBP goals in patients with HFpEF, and further research is needed before changes in clinical practice are made.
Our analysis has several limitations that merit attention. Several baseline characteristics were self‐reported and subjected our analysis to recall bias. Blood pressure measurements were obtained at a single time period in patients who were treated with antihypertensive medications. Accordingly, it is possible that our findings vary with repeated measurements or with changes in blood pressure medications during the study period. We also acknowledge that the main aim of the TOPCAT Trial was not to investigate the role of DBP in patients with HFpEF, and our hypothesis about blood pressure control in this patient group is speculative. Furthermore, we acknowledge that further studies, including clinical trials, are needed to appropriately investigate the role of DBP in patients who have HFpEF. Finally, we acknowledge the possibility of residual confounding in our multivariable models.
In conclusion, we have demonstrated that DBP values ≥90 and <60 mm Hg are associated with an increased risk of adverse outcomes in patients with HFpEF who are treated for hypertension. Further investigation is needed to confirm our findings and to identify optimal DBP goals in this high‐risk population.
Sources of Funding
Sandesara is supported by the Abraham J. & Phyllis Katz Foundation (Atlanta, GA). O'Neal is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number F32‐HL134290. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
None.
Acknowledgments
This manuscript was prepared using TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) Trial research materials obtained from the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the TOPCAT Trial or the National Heart, Lung, and Blood Institute.
(J Am Heart Assoc. 2018;7:e007475 DOI: 10.1161/JAHA.117.007475.)29475874
References
- 1. Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype‐specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation. 2016;134:73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sharma K, Kass DA. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circ Res. 2014;115:79–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–1562. [PubMed] [Google Scholar]
- 4. Ho JE, Enserro D, Brouwers FP, Kizer JR, Shah SJ, Psaty BM, Bartz TM, Santhanakrishnan R, Lee DS, Chan C, Liu K, Blaha MJ, Hillege HL, van der Harst P, van Gilst WH, Kop WJ, Gansevoort RT, Vasan RS, Gardin JM, Levy D, Gottdiener JS, de Boer RA, Larson MG. Predicting heart failure with preserved and reduced ejection fraction: the international collaboration on heart failure subtypes. Circ Heart Fail. 2016;9:e003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. [DOI] [PubMed] [Google Scholar]
- 6. SPRINT Research Group , Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vidal‐Petiot E, Ford I, Greenlaw N, Ferrari R, Fox KM, Tardif JC, Tendera M, Tavazzi L, Bhatt DL, Steg PG; CLARIFY Investigators . Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet. 2016;388:2142–2152. [DOI] [PubMed] [Google Scholar]
- 8. McEvoy JW, Chen Y, Rawlings A, Hoogeveen RC, Ballantyne CM, Blumenthal RS, Coresh J, Selvin E. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: implications for blood pressure control. J Am Coll Cardiol. 2016;68:1713–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT). https://biolincc.nhlbi.nih.gov/studies/topcat. Accessed October 26, 2016.
- 10. Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, McKinlay S, O'Meara E, Shaburishvili T, Pitt B, Pfeffer MA. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J. 2011;162(966–972):e10. [DOI] [PubMed] [Google Scholar]
- 11. Shah SJ, Heitner JF, Sweitzer NK, Anand IS, Kim HY, Harty B, Boineau R, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Lewis EF, Markov V, O'Meara E, Kobulia B, Shaburishvili T, Solomon SD, Pitt B, Pfeffer MA, Li R. Baseline characteristics of patients in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail. 2013;6:184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O'Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131:34–42. [DOI] [PubMed] [Google Scholar]
- 13. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM; TOPCAT Investigators . Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. [DOI] [PubMed] [Google Scholar]
- 14. Desquilbet L, Mariotti F. Dose‐response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29:1037–1057. [DOI] [PubMed] [Google Scholar]
- 15. Messerli FH, Mancia G, Conti CR, Hewkin AC, Kupfer S, Champion A, Kolloch R, Benetos A, Pepine CJ. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med. 2006;144:884–893. [DOI] [PubMed] [Google Scholar]
- 16. Rahman F, Al Rifai M, Blaha MJ, Nasir K, Budoff MJ, Psaty BM, Post WS, Blumenthal RS, McEvoy JW. Relation of diastolic blood pressure and coronary artery calcium to coronary events and outcomes (from the Multi‐Ethnic Study of Atherosclerosis). Am J Cardiol. 2017;120:1797–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. James PA, Oparil S, Carter BL, Cushman WC, Dennison‐Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E. 2014 Evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]


