Abstract
Background
The optimal duration of β‐blocker therapy in patients with acute myocardial infarction (AMI) is unknown. We aimed to evaluate the late effect of β‐blockers in patients with AMI.
Methods and Results
We enrolled all consecutive patients who presented with AMI at Seoul National University Bundang Hospital, between June 3, 2003 and February 24, 2015. The primary end point was 5‐year all‐cause mortality, depending on the use of β‐blockers at discharge, 1 year after AMI, and 3 years after AMI. Of 2592 patients, the prescription rates of β‐blockers were 72%, 69%, 63%, and 60% at discharge and 1, 3, and 5 years after AMI, respectively. The patients who were receiving β‐blocker therapy had more favorable clinical characteristics, such as younger age (62 versus 65 years; P<0.001). They received reperfusion therapy more often (92% versus 80%; P<0.001) than those without β‐blocker prescription. In the univariate analysis, the patients with β‐blocker prescription had lower 5‐year mortality at all time points. In the Cox model after adjustment for significant covariates, β‐blocker prescription at discharge was associated with a 29% reduced mortality risk (hazard ratio, 0.71; 95% confidence interval, 0.55–0.90; P=0.006); however, β‐blocker prescriptions at 1 and 3 years after AMI were not associated with reduced mortality.
Conclusions
The beneficial effect of β‐blocker therapy after AMI may be limited until 1 year after AMI. Whether late β‐blocker therapy beyond 1 year after AMI offers clinical benefits should be confirmed in further clinical trials.
Keywords: acute myocardial infarction, β‐blocker, mortality, secondary prevention
Subject Categories: Myocardial Infarction, Mortality/Survival, Secondary Prevention, Hypertension
Clinical Perspective
What Is New?
Although β‐blockers appear to be beneficial in patients with acute myocardial infarction, the beneficial effect may be limited beyond 1 year after acute myocardial infarction.
What Are the Clinical Implications?
Whether late β‐blocker therapy beyond 1 year after acute myocardial infarction offers clinical benefit and whether β‐blockers should be switched to other classes of antihypertensive agents in patients after acute myocardial infarction with hypertension should be confirmed in further clinical trials.
Patients with acute myocardial infarction (AMI) are a high‐risk group with increased mortality even after successful revascularization; thus, the current practice guidelines emphasize the importance of intensive risk factor modification, including hypertension in patients with AMI.1
β‐Blockers have improved survival and are one of the cornerstones in the treatment of ischemic heart disease; they exert an antianginal effect by reducing the myocardial workload and oxygen demand.2 Besides, they also have antiarrhythmic and antiremodeling effects.3 The effects of β‐blockers have been extensively investigated in patients with AMI,4, 5, 6, 7 and the current practice guidelines also recommend the use of β‐blockers in all patients after AMI unless contraindicated.1, 8
Although the effects of β‐blockers on short‐term outcomes are well established, data on their long‐term effects are scarce, especially beyond 1 year after AMI. In addition, the β‐blocker prescription may change during follow‐up, so recent studies that examined long‐term survival effect according to β‐blocker prescription at discharge might have grave limitations.9, 10 Therefore, whether β‐blocker use beyond 1 year after AMI truly reduces mortality in the current era of AMI and post‐AMI care remains inconclusive, particularly in patients with preserved left ventricular (LV) systolic function. In low‐risk patients (preserved ejection fraction, young age, absence of arrhythmias, or residual ischemia), prolonged use of β‐blockers is unlikely to confer any mortality benefit.11 These findings continuously raise a question to clinicians on whether to discontinue β‐blockers in low‐risk patients or to switch β‐blockers to other classes of antihypertensive agents with stronger evidence for secondary prevention of cardiovascular adverse outcomes.
In this study, we aimed to examine the late effect of β‐blockers in patients with AMI. For this purpose, the β‐blocker status and clinical characteristics of each patient were reevaluated at discharge, 1 year after AMI, and 3 years after AMI.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Population
Seoul National University Bundang Hospital is a paperless hospital, and all patients’ information on medication and laboratory findings is electronically recorded and archived. Seoul National University Bundang Hospital was the first outside the United States that reached stage 7 of the Healthcare Information and Management System Society. Stage 7 implies an environment where paper charts are no longer used, thereby enabling true sharing, information exchange, and immediate delivery of patient data to improve process performance, quality of care, and patient safety.
We selected all consecutive patients who presented with AMI between June 3, 2003 and February 24, 2015 by using a searching machine called “clinical data warehouse,” which is designed to search and retrieve patient information from electronic medical records. In our hospital, we monitor all patients admitted with an initial diagnosis of AMI12 and adjudicate the discharge diagnosis in the framework for continuous quality improvement. Therefore, in the present study, we included the following patients: (1) patients who were admitted to the coronary care unit via the emergency department and (2) patients who had AMI as a discharge diagnosis. Patients were treated with revascularization with thrombolysis, stent implantation, or coronary artery bypass graft surgery; or they received medical treatment only without revascularization.
Variables, including demographic and baseline characteristics, medical history, clinical presentation, laboratory tests, hospital course, and clinical outcomes during admission and at discharge, were collected from each patient. Almost all patients underwent echocardiography during hospitalization for AMI.
The study protocol was approved by the institutional review board, and written informed consent was waived (institutional review board no. B‐1411/276‐115). The study complied with the principles of the Declaration of Helsinki.
β‐Blockers
We collected data on the dose and type of β‐blocker for each patient before admission, at discharge, and 1 and 3 years after AMI.10, 13, 14 β‐Blocker prescription was defined as a binary variable (yes or no), regardless of the β‐blocker type or dose. We also collected data on β‐blocker type and dose.
Study End Point
The primary outcome was 5‐year all‐cause mortality. We were especially interested in the late effect of β‐blockers beyond 1 year, so we performed landmark analyses at 1 and 3 years after AMI. Therefore, we have 3 study populations at 3 different time points (ie, at discharge and 1 and 3 years after AMI). Population 1 included patients with AMI, who were discharged alive. Population 2 included patients who visited our institution at 1 year after discharge, excluding those who were referred out or died. Population 3 included patients who visited our institution at 3 years after discharge, excluding those who died or were referred out between 1 and 3 years after discharge.
The prescription of β‐blockers in each patient was reevaluated, and 5‐year all‐cause mortality was reassessed from these landmarks. The vital statuses of all the patients were collected from the National Insurance data or National Death Records.
Statistical Analyses
Data are presented as numbers and frequencies for categorical variables and as mean±SD for continuous variables. For comparisons among the groups, the χ2 test (or Fisher's exact test when any expected count was <5 for a 2×2 table) was used for categorical variables, and the unpaired Student t test or 1‐way analysis of variance was used for continuous variables.
Five‐year mortality was analyzed using the Kaplan‐Meier method. Survival times were censored at the date of death, last follow‐up, or last vital status collection. A multivariable Cox proportional‐hazards regression model was used to determine the effect of β‐blocker as an independent predictor of all‐cause death. Covariates for the adjustment were selected using the stepwise Akaike information criterion method. The following variables were included in the multivariable model as confounding factors: age; sex; diabetes mellitus; hypertension; body mass index; dyslipidemia; initial presentation (ST‐segment–elevation myocardial infarction [STEMI] versus non‐STEMI); multivessel disease; the use of aspirin, P2Y12 inhibitors, renin‐angiotensin system (RAS) inhibitors, diuretics, calcium channel blockers, and statins; LV ejection fraction; serum creatinine level; and mode of treatment (versus medical therapy only). The proportional hazard assumption of each variable was tested. Because calcium channel blocker use in population 2 violated proportionality, it was removed from the model.
The propensity score was estimated using multivariable logistic regression analysis with the variables listed in the second adjustment model. A propensity score–matched cohort was created using the nearest neighbor method without replacement, in a 1:1 ratio. “MatchIt” package of the R programming was used for the matching.
A 2‐sided probability value of <0.05 was considered indicative of a statistically significant difference. Statistical analyses were performed using R programming, version 3.1.0 (The R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org). All the analyses were performed by a professional statistician (S.‐H.K.).
Results
Patients
From 2003 to 2015, 2753 patients with AMI were admitted. Of the patients, 161 died during hospitalization, so that only the data of 2592 patients were available for the current analysis (Figure 1).
Figure 1.
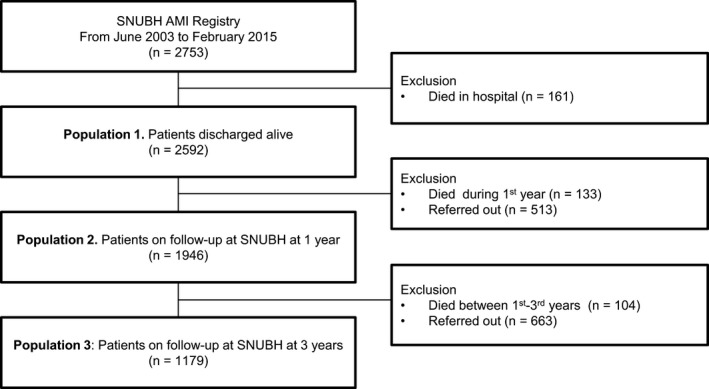
Flow chart of the study population. AMI indicates acute myocardial infarction; and SNUBH, Seoul National University Bundang Hospital.
For the baseline characteristics of population 1, the mean age was 62.7 years. Of the patients, 76.4% were men, 51% had hypertension, 28% had diabetes mellitus, 51% had STEMI, and 49% had non‐STEMI. For the revascularization method, 4.1% underwent thrombolysis: 86.1%, percutaneous coronary intervention; 6.8%, coronary artery bypass graft surgery; and 8.17%, medical therapy only without any revascularization procedure.
The prescription rates of β‐blockers were 72%, 69%, 63%, and 60% at discharge and 1, 3, and 5 years after AMI, respectively (Figure 2A). At discharge, the most commonly prescribed agents were carvedilol (70%), followed by bisoprolol (28%) and nebivolol (1%; Figure 2B). β‐Blocker prescription status had significantly changed during follow‐up (Figure 2C). No significant difference in systolic blood pressure was found between the groups at discharge and during follow‐up in the 3 populations (Figure 2D). Table 1 shows the baseline characteristics of the patients according to β‐blocker use. The patients who were receiving β‐blocker had more favorable clinical characteristics, such as younger age (62 versus 65 years; P<0.001), lower NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) level (2062±6844 versus 3882±10 693 pg/mL; P<0.001), and more RAS inhibitor use (83.6% versus 68.5%; P<0.001). They received reperfusion therapy more often (94% versus 85%; P<0.001) than those without β‐blocker prescription. For their medications, they received RAS blockers (84% versus 69%; P<0.001) and statins (92% versus 78%; P<0.001) more frequently.
Figure 2.
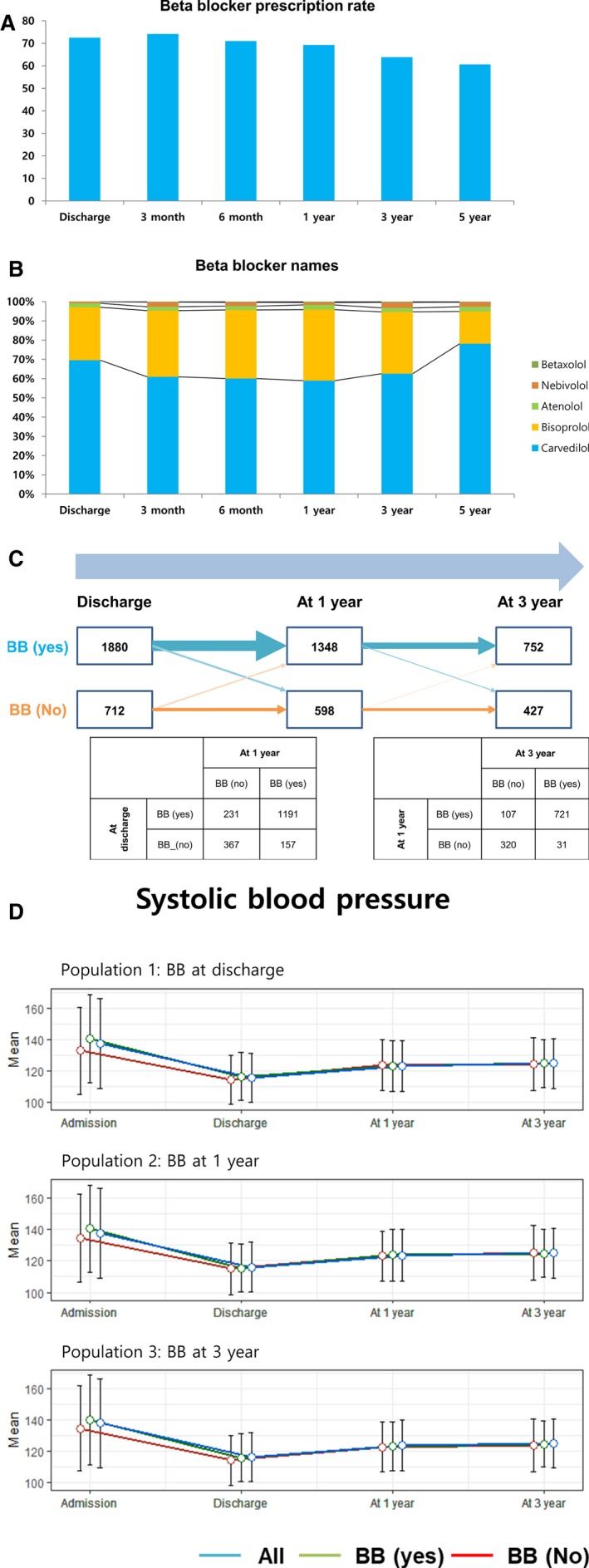
β‐Blockers (BBs) and blood pressure of the study patients. A, Prescription rates of BBs were 72%, 69%, 63%, and 60% at discharge and 1, 3, and 5 years after acute myocardial infarction, respectively. B, At discharge, the most commonly prescribed agents were carvedilol (70%), followed by bisoprolol (28%) and nebivolol (1%). C, BB prescription status changed during follow‐up and, thus, it was significantly different in the 3 populations analyzed in this study. Of the patients, 20% and 11% changed their BB statuses from discharge to 1 year and from 1 to 3 years, respectively. D, The difference in systolic blood pressure between the patients with and those without BBs at discharge and during follow‐up was not significant in the 3 populations.
Table 1.
Baseline Characteristics of the Study Population
| Characteristics | Population 1 | Population 2 | Population 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All (N=2592) | β‐Blocker Yes (N=1880) | β‐Blocker No (N=712) | P Value | β‐Blocker Yes (N=1348) | β‐Blocker No (N=598) | P Value | β‐Blocker Yes (N=752) | β‐Blocker No (N=427) | P Value | |
| Age, y | 62.6±13.5 | 61.7±13.3 | 64.9±13.7 | <0.001 | 60.6±13.0 | 63.9±13.4 | <0.001 | 59.3±12.8 | 62.7±13.1 | <0.001 |
| Male sex, % | 76.5 | 77.4 | 74.0 | 0.079 | 79.2 | 74.1 | 0.016 | 80.3 | 78.5 | 0.491 |
| Diabetes mellitus, % | 28.0 | 27.0 | 30.7 | 0.070 | 24.9 | 33.2 | <0.001 | 24.5 | 28.1 | 0.193 |
| Hypertension, % | 51.6 | 51.1 | 51.9 | 0.888 | 50.2 | 51.5 | 0.625 | 49.7 | 46.8 | 0.371 |
| Dyslipidemia, % | 23.1 | 23.3 | 22.7 | 0.798 | 23.5 | 27.3 | 0.077 | 26.4 | 25.8 | 0.875 |
| Previous MI, % | ||||||||||
| >30 d | 4.6 | 4.1 | 6.1 | 0.0 | 3.0 | 6.1 | 0.0 | 3.5 | 5.2 | 0.3 |
| 7–30 d | 1.7 | 1.7 | 1.7 | 95 | 1.9 | 1.2 | 03 | 2.3 | 1.9 | 25 |
| Heart failure, % | 0.7 | 0.4 | 1.3 | 0.027 | 0.4 | 0.7 | 0.507 | 0.4 | 0.9 | 0.262 |
| Stroke, % | 6.2 | 5.4 | 8.1 | 0.013 | 4.9 | 7.5 | 0.027 | 5.7 | 5.6 | 1.000 |
| Previous CVD, % | 12.1 | 11.0 | 15.0 | 0.007 | 9.7 | 14.6 | 0.002 | 11.2 | 12.7 | 0.498 |
| Smoking, % | ||||||||||
| Current | 42.7 | 43.4 | 40.8 | 0.112 | 46.2 | 39.7 | 0.028 | 46.7 | 43.3 | 0.149 |
| Former | 25.2 | 24.0 | 28.0 | 24.1 | 27.9 | 24.4 | 29.6 | |||
| Never | 32.2 | 32.6 | 31.2 | 29.7 | 32.3 | 28.8 | 27.1 | |||
| BMI, kg/m2 | 24.3±3.5 | 24.4±3.5 | 23.7±3.6 | <0.001 | 24.6±3.4 | 23.9±3.4 | <0.001 | 24.8±3.4 | 24.0±3.2 | <0.001 |
| Heart rate, bpm | 73.5±14.3 | 72.8±13.5 | 75.5±16.2 | <0.001 | 66.6±11.3 | 70.2±13.1 | <0.001 | 68.1±12.2 | 70.2±13.2 | 0.038 |
| STEMI, % | 51.16 | 51.2 | 50.7 | 0.857 | 55.2 | 48.0 | 0.004 | 59.8 | 51.8 | 0.009 |
| Multivessel disease, % | 68.4 | 67.3 | 71.2 | 0.074 | 33.6 | 29.8 | 0.130 | 70.7 | 71.8 | 0.752 |
| Systolic BP, mm Hg | 123.5±16.3 | 116.6±15.4 | 114.4±15.8 | 0.002 | 123.7±16.4 | 123.0±15.8 | 0.428 | 124.6±14.6 | 123.9±16.8 | 0.520 |
| Diastolic BP, mm Hg | 73.4±9.8 | 66.6±10.4 | 64.7±10.0 | <0.001 | 73.9±9.7 | 72.4±9.8 | 0.006 | 75.2±8.9 | 73.4±9.2 | 0.003 |
| Medication, % | ||||||||||
| Aspirin | 99.2 | 99.7 | 98.0 | <0.001 | 97.4 | 75.6 | <0.001 | 92.2 | 71.2 | <0.001 |
| P2Y12 | 95.8 | 97.7 | 90.4 | <0.001 | 77.5 | 54.3 | <0.001 | 51.2 | 37.5 | <0.001 |
| Warfarin | 4.0 | 3.3 | 5.9 | 0.004 | 5.4 | 3.8 | 0.173 | 4.1 | 3.7 | 0.872 |
| RAS inhibitor | 79.5 | 83.6 | 68.5 | <0.001 | 83.6 | 61.9 | <0.001 | 82.2 | 59.0 | <0.001 |
| Diuretics | 26.6 | 21.9 | 39.0 | <0.001 | 27.9 | 28.1 | 0.971 | 27.1 | 23.4 | 0.184 |
| CCB | 10.9 | 10.9 | 10.8 | 1.000 | 32.1 | 25.3 | 0.003 | 31.1 | 23.7 | 0.008 |
| Statin | 88.0 | 91.9 | 77.7 | <0.001 | 96.4 | 71.4 | <0.001 | 96.4 | 71.7 | <0.001 |
| Echocardiography | ||||||||||
| EF, % | 53.2±10.8 | 53.7±10.2 | 51.7±12.2 | <0.001 | 54.2±9.9 | 52.8±11.1 | 0.008 | 53.9±9.5 | 53.9±10.0 | 0.962 |
| EF <40%, % | 12.4 | 10.0 | 18.7 | <0.001 | 8.8 | 14.9 | <0.001 | 9.3 | 10.1 | 0.746 |
| LVEDD, mm | 48.8±5.7 | 48.5±5.3 | 49.5±6.6 | <0.001 | 48.8±5.1 | 49.3±6.0 | 0.103 | 49.1±5.2 | 49.2±5.4 | 0.813 |
| LVESD, mm | 33.3±6.6 | 33.0±6.3 | 34.1±7.5 | 0.001 | 33.0±6.2 | 33.4±6.9 | 0.222 | 33.2±6.1 | 32.8±6.1 | 0.329 |
| Laboratory findings | ||||||||||
| Creatinine, mg/dL | 1.15±1.05 | 1.1±1.0 | 1.2±1.1 | 0.005 | 1.1±0.8 | 1.2±1.3 | 0.003 | 1.1±0.9 | 1.1±0.7 | 0.991 |
| GFR, mL/min per 1.72 m2 | 77.9±34.2 | 79.6±33.3 | 71.9±36.5 | <0.001 | 80.8±33.9 | 72.9±32.5 | <0.001 | 72.5±28.5 | 69.0±23.8 | 0.077 |
| Troponin I | 75.5±116.4 | 75.6±112.0 | 75.2±127.4 | 0.947 | 78.4±112.8 | 69.0±113.4 | 0.092 | 83.5±110.3 | 71.9±117.2 | 0.094 |
| NT‐proBNP, pg/L | 2588±8184 | 2062±6844 | 3882±10 693 | <0.001 | 1690±6571 | 3260±10 625 | 0.003 | 1334±10 451 | 2282±10 451 | 0.098 |
| CK‐MB, mg/dL | 66.9±123.7 | 65.1±120.9 | 71.6±130.7 | 0.254 | 71.7±129.4 | 69.8±129.7 | 0.757 | 79.6±136.7 | 69.6±133.9 | 0.222 |
| CRP, mg/dL | 1.2±3.2 | 1.0±2.8 | 1.8±3.9 | <0.001 | 0.9±2.5 | 1.3±2.9 | 0.012 | 0.9±2.2 | 1.1±2.9 | 0.132 |
| Hs‐CRP, mg/dL | 4.9±6.4 | 4.2±6.0 | 6.2±7.1 | <0.001 | 3.8±5.8 | 5.1±6.2 | 0.005 | 3.6±5.8 | 4.7±6.2 | 0.038 |
| Cholesterol, mg/dL | 173.8±42.8 | 175.9±42.9 | 168.3±42.3 | <0.001 | 179.7±42.8 | 169.2±40.1 | <0.001 | 183.0±42.2 | 175.3±39.7 | 0.003 |
| HDL, mg/dL | 43.7±10.9 | 43.5±10.6 | 44.1±11.5 | 0.218 | 43.5±10.7 | 43.4±11.0 | 0.825 | 43.8±10.8 | 44.0±11.5 | 0.712 |
| LDL, mg/dL | 107.4±36.8 | 110.0±37.8 | 99.8±32.6 | <0.001 | 113.1±37.9 | 99.7±33.1 | <0.001 | 111.5±38.0 | 103.0±32.0 | <0.001 |
| Triglyceride, mg/dL | 140.1±120.5 | 146.7±129.3 | 121.8±89.6 | <0.001 | 144.3±125.1 | 127.7±102.4 | 0.003 | 143.8±124.2 | 123.8±93.3 | 0.002 |
| Treatment | ||||||||||
| None | 8.1 | 5.6 | 14.5 | <0.001 | 4.1 | 10.5 | <0.001 | 2.7 | 9.6 | <0.001 |
| Thrombolysis | 4.1 | 4.1 | 3.9 | 4.9 | 3.5 | 5.9 | 4.2 | |||
| PCI | 81.1 | 85.7 | 68.8 | 85.3 | 76.8 | 86.3 | 78.7 | |||
| CABG | 6.8 | 4.5 | 12.8 | 5.7 | 9.2 | 5.2 | 7.5 | |||
Data are given as mean±SD unless otherwise indicated. BMI indicates body mass index; BP, blood pressure; bpm, beats/min; CABG, coronary artery bypass graft; CCB, calcium channel blocker; CK‐MB, creatine kinase‐muscle/brain; CRP, C‐reactive protein; CVD, cardiovascular disease; EF, ejection fraction; GFR, glomerular filtration rate; HDL, high‐density lipoprotein; Hs‐CRP, high‐sensitivity CRP; LDL, low‐density lipoprotein; LVEDD, left ventricular end‐diastolic diameter; LVESD, left ventricular end‐systolic diameter; MI, myocardial infarction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PCI, percutaneous coronary intervention; RAS, renin‐angiotensin system; and STEMI, ST‐segment–elevation myocardial infarction.
Population 1 Outcomes: β‐Blocker at Hospital Discharge
Overall, 320 patients died during a median follow‐up of 1364 days (interquartile range, 784–2190 days). The mortality rate for each time point is presented in Table 2. The patients who were receiving β‐blockers at discharge had lower 5‐year all‐cause and cardiovascular mortality than those without β‐blocker prescription (Figure 3A and 3D). In the Cox proportional hazard regression analysis after adjustment for significant covariates, β‐blocker use was associated with a 29% reduced risk of 5‐year all‐cause mortality (hazard ratio [HR], 0.71; 95% confidence interval [CI], 0.56–0.91; P=0.008) and 36% reduced risk for cardiovascular mortality (HR, 0.64; 95% CI, 0.42–0.97; P=0.035) (Table 3). Body mass index, the use of RAS inhibitors, statins, percutaneous coronary intervention, and coronary artery bypass graft surgery were associated with reduced mortality, whereas advanced age, diabetes mellitus, previous cardiovascular disease, low LV dysfunction, and creatinine level were associated with increased mortality.
Table 2.
Mortality Rates of the Study Population
| Population | Mortality | ||
|---|---|---|---|
| 1 y | 2 y | 5 y | |
| 1 | 133 (5.13) | 188 (7.25) | 227 (10.69) |
| 2 | 56 (2.88) | 104 (5.34) | 159 (8.17) |
| 3 | 24 (2.04) | 40 (3.39) | 79 (6.70) |
Data are given as number (percentage).
Figure 3.
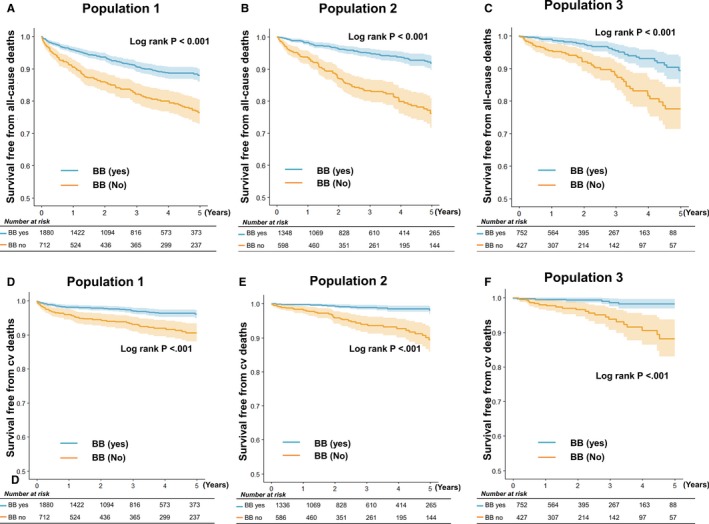
Five‐year all‐cause and cardiovascular (cv) mortality. In the Kaplan‐Meier survival analysis, the patients with a β‐blocker (BB) prescription in population 1 (at discharge) (A), population 2 (1 year after discharge) (B), and population 3 (3 years after discharge) (C) had lower all‐cause mortality rates than their counterparts without BB use. Similarly, patients with a BB prescription had lower cv mortality in population 1 (D), population 2 (E), and population 3 (F).
Table 3.
Cox Proportional Hazard Model for All‐Cause Deaths
| Variable | At Discharge | At 1 y | At 3 y | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Multivariate | Multivariate | |||||||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| β‐Blocker | 0.48 | 0.38–0.60 | <0.001 | 0.71 | 0.56–0.91 | 0.008 | 0.74 | 0.50–1.11 | 0.150 | 0.79 | 0.45–1.40 | 0.418 |
| Age | 1.10 | 1.08–1.11 | <0.001 | 1.08 | 1.06–1.09 | <0.001 | 1.07 | 1.05–1.09 | <0.001 | 1.07 | 1.04–1.09 | <0.001 |
| Male sex | 0.51 | 0.40–0.65 | <0.001 | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· |
| Diabetes mellitus | 1.71 | 1.34–2.18 | <0.001 | 1.36 | 1.05–1.78 | 0.022 | ··· | ··· | ··· | ··· | ··· | ··· |
| Hypertension | 1.90 | 1.49–2.45 | <0.001 | ··· | ··· | ··· | 1.46 | 1.00–2.13 | 0.051 | 1.92 | 1.09–3.41 | 0.025 |
| Body mass index | 0.79 | 0.76–0.82 | <0.001 | 0.90 | 0.86–0.94 | <0.001 | 0.91 | 0.87–0.96 | <0.001 | 0.88 | 0.82–0.95 | 0.002 |
| Dyslipidemia | 0.59 | 0.43–0.82 | 0.001 | 0.78 | 0.56–1.07 | 0.126 | ··· | ··· | ··· | ··· | ··· | ··· |
| Diagnosis STEMI | 0.67 | 0.53–0.85 | 0.001 | ··· | ··· | ··· | 0.78 | 0.56–1.09 | 0.144 | ··· | ··· | ··· |
| Previous CVD | 2.67 | 2.04–3.49 | <0.001 | 1.88 | 1.43–2.49 | <0.001 | 1.82 | 1.25–2.63 | 0.002 | 2.74 | 1.69–4.46 | <0.001 |
| Aspirin | 0.20 | 0.08–0.49 | <0.001 | 0.31 | 0.12–0.81 | 0.016 | 0.41 | 0.22–0.76 | 0.005 | 0.52 | 0.25–1.12 | 0.095 |
| P2Y12 | 0.65 | 0.40–1.04 | 0.073 | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· |
| RAS inhibitor | 0.58 | 0.44–0.75 | <0.001 | 0.75 | 0.57–0.99 | 0.04 | 0.51 | 0.32–0.80 | 0.003 | 0.40 | 0.21–0.73 | 0.003 |
| Diuretics | 2.59 | 2.05–3.28 | <0.001 | ··· | ··· | ··· | 2.28 | 1.53–3.41 | <0.001 | 2.24 | 1.27–3.97 | 0.006 |
| CCB | 1.62 | 1.16–2.25 | 0.004 | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· |
| Statin | 0.31 | 0.24–0.41 | <0.001 | 0.63 | 0.48–0.84 | 0.001 | 0.48 | 0.29–0.79 | 0.004 | 0.49 | 0.26–0.94 | 0.032 |
| EF <40% | 3.09 | 2.37–4.03 | <0.001 | 1.70 | 1.28–2.27 | <0.001 | ··· | ··· | ··· | ··· | ··· | ··· |
| Creatinine | 1.01 | 1.00–1.02 | 0.011 | 1.22 | 1.14–1.29 | <0.001 | 1.23 | 1.12–1.34 | <0.001 | 1.31 | 1.09–1.59 | 0.004 |
| Treatment | ||||||||||||
| Thrombolysis | 0.22 | 0.10–0.48 | <0.001 | 0.54 | 0.24–1.21 | 0.135 | ··· | ··· | ··· | ··· | ··· | ··· |
| PCI | 0.35 | 0.25–0.48 | <0.001 | 0.53 | 0.37–0.76 | <0.001 | ··· | ··· | ··· | ··· | ··· | ··· |
| CABG | 0.69 | 0.43–1.10 | 0.115 | 0.46 | 0.28–0.76 | 0.002 | ··· | ··· | ··· | ··· | ··· | ··· |
CABG indicates coronary artery bypass graft; CCB, calcium channel blocker; CI, confidence interval; CVD, cardiovascular disease; EF, ejection fraction; HR, hazard ratio; PCI, percutaneous coronary intervention; RAS, renin‐angiotensin system; and STEMI, ST‐segment–elevation myocardial infarction.
Population 2 and 3 Outcomes: β‐Blocker at 1 and 3 Years
At 1 year, the data of 1946 patients were available. Of the patients, 69% received β‐blockers at 1 year, and again, they had more favorable clinical characteristics that slightly differed from those at discharge (Table 1). In the Kaplan‐Meier survival analysis, the patients with β‐blockers at 1 year had lower 5‐year all‐cause and cardiovascular deaths (Figure 3B and 3E). However, in the Cox model, β‐blocker prescription was not associated with survival after adjustment for significant covariates; RAS inhibitors and statins were beneficial, and hypertension was hazardous.
At 3 years, the data of 1179 patients were available. Similarly, patients with β‐blocker prescription (63.8%) had better clinical characteristics than those without β‐blocker prescription (Table 1). However, the difference between the groups was markedly smaller than that at discharge or 1 year. Once again, patients with β‐blocker prescription at 3 years had a lower 5‐year all‐cause mortality in the Kaplan‐Meier survival analysis (Figure 3C and 3F). Nevertheless, in the Cox model after adjustment, β‐blocker use was not associated with reduced all‐cause and cardiovascular mortality, whereas hypertension, RAS inhibitors, and statins were a significant predictor of 5‐year mortality.
Subgroup Analysis
The effect of β‐blockers may be dependent on comorbidities, such as LV dysfunction and hypertension; thus, we performed an exploratory subgroup analysis. We found no significant interaction between β‐blockers and various subgroups at discharge. In population 2, among the patients <65 years and those who were receiving RAS inhibitors, the β‐blocker prescription seemed to be beneficiary. In population 3, β‐blockers showed a protective effect only in the patients <65 years (Figure 4).
Figure 4.
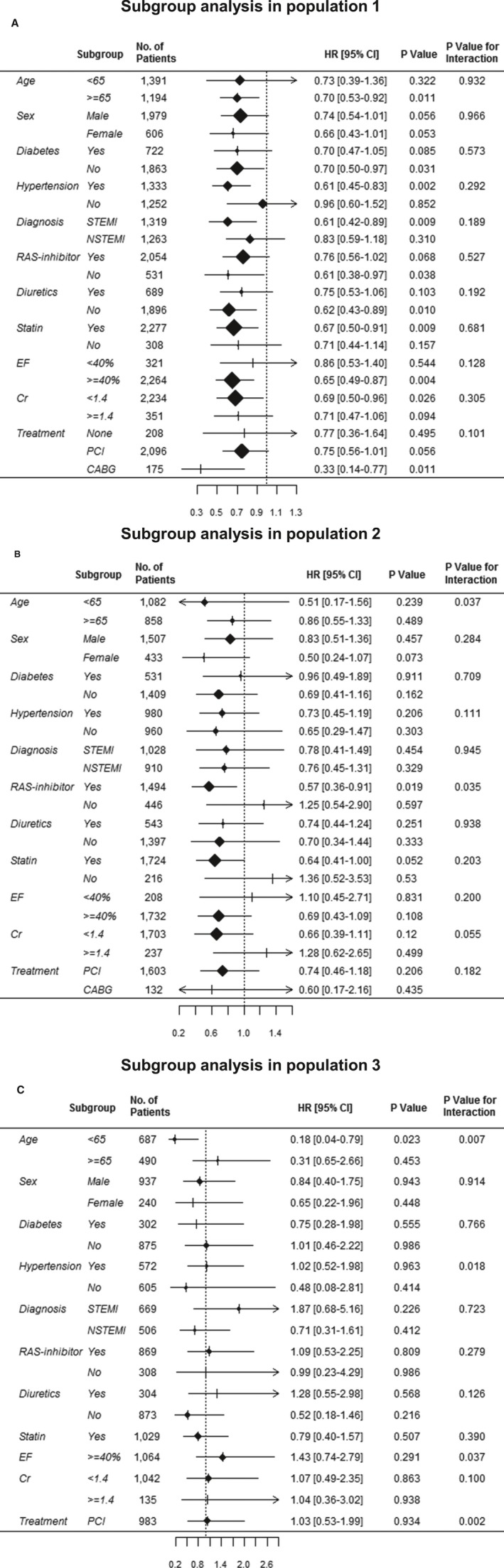
Exploratory subgroup analyses. A, In population 1, no significant interaction was observed between β‐blocker and mortality across all subgroups. B, In population 2, among the patients <65 years and those who were receiving renin‐angiotensin system (RAS) inhibitors, β‐blocker therapy seemed to demonstrate a protective effect. C, In population 3, β‐blocker therapy showed a protective effect only in the patients <65 years. CABG indicates coronary artery bypass graft; CI, confidence interval; Cr, creatinine; EF, ejection fraction; HR, hazard ratio; NSTEMI, non‐STEMI; PCI, percutaneous coronary intervention; and STEMI, ST‐segment–elevation myocardial infarction.
Propensity Score–Matched Cohort
A total of 1258, 824, and 556 patients were matched on the basis of propensity score at discharge and 1 and 3 years after AMI, respectively. The baseline characteristics of the cohort after matching were well balanced (Table 4, Figure S1). The C‐statistics showed area under the curve values of 0.85, 0.89, and 0.89 for populations 1, 2 and 3, respectively. In the matched population, β‐blocker at discharge was associated with a 30% reduced risk (HR, 0.70; 95% CI, 0.52–0.94; P=0.019); however, β‐blocker use at 1 year (HR, 0.85; 95% CI, 0.53–1.36; P=0.489) and at 3 years (HR, 0.89; 95% CI, 0.44–1.78; P=0.733) after AMI was not associated with improved survival (Figure 5).
Table 4.
Propensity Score–Matched Cohort
| Variable | Discharge | At 1 y | At 3 y | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β‐Blocker Yes (N=629) | β‐Blocker No (N=629) | P Value | SD | β‐Blocker Yes (N=412) | β‐Blocker No (N=412) | P Value | SD | β‐Blocker Yes (N=278) | β‐Blocker No (N=278) | P Value | SD | |
| Age, y | 64.52±13.06 | 64.78±14.78 | 0.732 | 0.019 | 62.90±13.02 | 62.75±13.24 | 0.867 | 0.012 | 60.66±13.00 | 61.24±13.05 | 0.598 | 0.045 |
| Male sex | 75.4 | 74.4 | 0.745 | 0.022 | 76.7 | 76.9 | >0.999 | 0.006 | 79.1 | 80.9 | 0.671 | 0.045 |
| Diabetes mellitus | 30.5 | 30.7 | >0.999 | 0.003 | 33.3 | 33.0 | >0.999 | 0.005 | 25.9 | 28.8 | 0.505 | 0.065 |
| Hypertension | 50.9 | 52.8 | 0.535 | 0.038 | 53.9 | 50.2 | 0.329 | 0.073 | 49.6 | 47.5 | 0.671 | 0.043 |
| Body mass index, kg/m2 | 23.97±3.64 | 23.79±3.62 | 0.401 | 0.047 | 24.14±3.44 | 24.16±3.36 | 0.928 | 0.006 | 24.49±3.28 | 24.24±3.03 | 0.359 | 0.078 |
| Dyslipidemia | 23.2 | 23.5 | 0.947 | 0.008 | 33.0 | 30.1 | 0.410 | 0.063 | 27.7 | 28.8 | 0.851 | 0.024 |
| Diagnosis STEMI | 51.2 | 53.7 | 0.397 | 0.051 | 49.8 | 47.8 | 0.626 | 0.039 | 56.8 | 54.3 | 0.609 | 0.051 |
| Previous CVD | 17.2 | 14.5 | 0.216 | 0.074 | 15.3 | 14.6 | 0.845 | 0.020 | 12.2 | 13.7 | 0.705 | 0.043 |
| Aspirin | 99.4 | 98.9 | 0.545 | 0.051 | 95.6 | 95.9 | >0.999 | 0.012 | 88.8 | 87.4 | 0.694 | 0.044 |
| P2Y12 | 74.3 | 72.8 | 0.358 | 0.058 | 72.6 | 68.7 | 0.251 | 0.085 | 50.4 | 46.8 | 0.445 | 0.072 |
| RAS inhibitor | 73.1 | 70.6 | 0.347 | 0.057 | 79.6 | 79.6 | >0.999 | 0.000 | 77.3 | 76.6 | 0.920 | 0.017 |
| Diuretics | 35.0 | 38.5 | 0.219 | 0.073 | 33.0 | 36.4 | 0.341 | 0.071 | 28.1 | 28.1 | >0.999 | 0.000 |
| CCB | 10.5 | 10.2 | 0.926 | 0.010 | 34.0 | 31.8 | 0.553 | 0.047 | 28.4 | 28.4 | >0.999 | 0.000 |
| Statin | 82.7 | 80.0 | 0.247 | 0.069 | 92.5 | 91.3 | 0.610 | 0.044 | 91.4 | 91.7 | >0.999 | 0.013 |
| EF <40% | 16.7 | 17.8 | 0.654 | 0.029 | 12.1 | 13.3 | 0.676 | 0.036 | 9.7 | 8.6 | 0.769 | 0.037 |
| Admission creatinine, mg/dL | 1.21±1.14 | 1.25±1.16 | 0.507 | 0.037 | 1.20±1.12 | 1.23±1.32 | 0.711 | 0.026 | 1.17±1.04 | 1.10±0.53 | 0.346 | 0.080 |
| Treatment | 0.532 | 0.641 | 0.259 | |||||||||
| Thrombolysis | 3.2 | 3.7 | 0.026 | 3.6 | 3.6 | 0.000 | 6.8 | 4.3 | 0.109 | |||
| PCI | 74.7 | 71.5 | 0.072 | 78.6 | 75.2 | 0.081 | 81.3 | 79.1 | 0.054 | |||
| CABG | 10.5 | 12.9 | 0.074 | 9.7 | 10.9 | 0.040 | 6.8 | 8.6 | 0.067 | |||
Data are given as mean±SD or percentage. CABG indicates coronary artery bypass graft; CCB, calcium channel blocker; CVD, cardiovascular disease; EF, ejection fraction; PCI, percutaneous coronary intervention; RAS, renin‐angiotensin system; SD, standardized difference; and STEMI, ST‐segment–elevation myocardial infarction.
Figure 5.
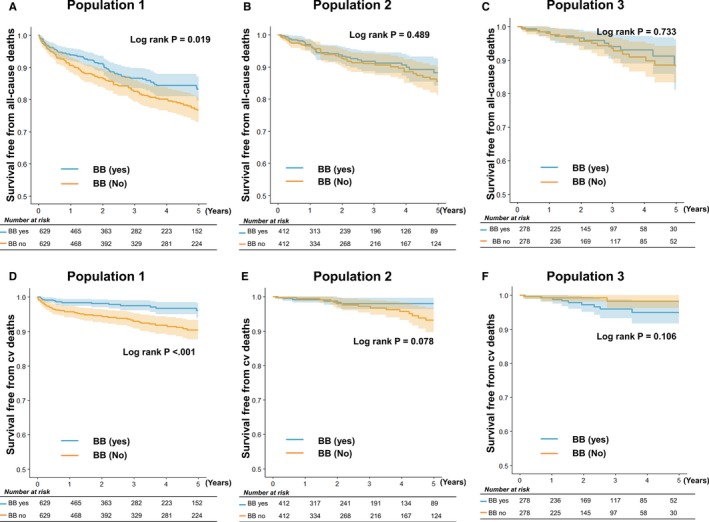
Five‐year all‐cause and cardiovascular (cv) mortality in propensity score–matched cohort. A, In the Kaplan‐Meier survival analysis, the patients with a β‐blocker (BB) prescription in population 1 had lower mortality rate than their counterparts without BB use. However, the 5‐year mortality did not differ between patients with or without BB prescription in population 2 (B) and population 3 (C). For 5‐year cv mortality, the patients with a BB prescription in population 1 had lower mortality rate than their counterparts without BB use (D); however, there was no difference between patients with or without BB prescription in populations 2 (E) and 3 (F).
One‐Year Later Outcomes
Because of the lag time between prescription and 5‐year mortality, we also analyzed 1‐year outcome to minimize the confounding, and the 1‐year outcomes were similar to those of 5‐year outcomes (Figure 6).
Figure 6.
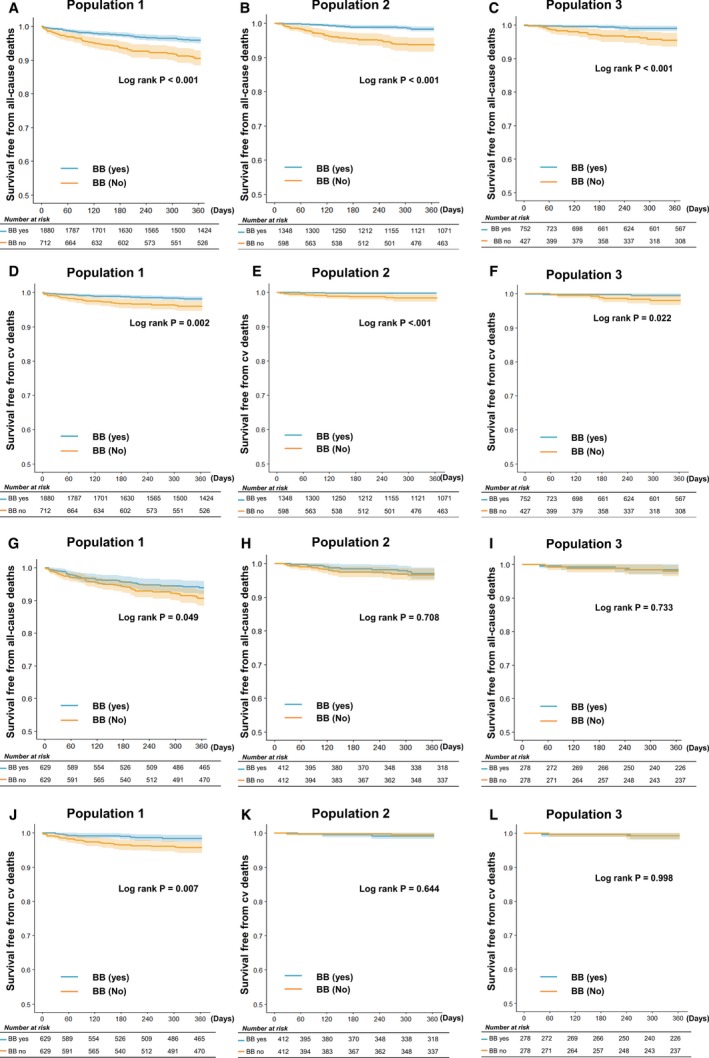
One‐year mortality. A through C, 1‐year all‐cause mortality. D through F, 1‐year cardiovascular (cv) mortality. G through I, 1‐year all‐cause mortality in propensity‐matched cohort. J through L, 1‐year cv mortality in propensity‐matched cohort. BB indicates β‐blocker.
Discussion
In patients with AMI, early β‐blocker prescription improves short‐ and long‐term survival; however, data that evaluate the late effect of β‐blockers are scarce. Consequently, no consensus has been reached among cardiologists on the appropriate duration of β‐blocker therapy in patients after AMI who have normal LV ejection fraction and do not have angina symptoms, arrhythmia, or hypertension.15 The present study was designed to evaluate the late effect of β‐blockers in patients with AMI and demonstrated that the use of β‐blockers at discharge was associated with a 29% reduced risk of mortality. However, β‐blocker prescription at 1 and 3 years after AMI was no longer associated with improved survival.
Effect of β‐Blockers in Patients With AMI
β‐Blockers are one of the cornerstones for treatment of coronary artery disease; they reduce the myocardial oxygen demand, increase oxygen supply, and lower blood pressure.16 Besides the antianginal effect, β‐blockers reduce infarct size, malignant ventricular arrhythmia, and sudden cardiac death, especially during the early period of AMI.3, 5, 17
Nonetheless, the optimal duration of β‐blockers in patients with AMI is unknown. For the short‐term effect, 15‐day treatment with metoprolol after AMI did not reduce mortality,17 whereas 90‐day administration of metoprolol showed 36% mortality reduction.18 For the long‐term effect, timolol reduced mortality by 39% during a mean follow‐up of 17 months, and the mortality curves continued to diverge up to 30 months.19 In an extensive follow‐up of the same study, the survival curves continue to diverge until 6 years but only in high‐risk patients.20 In the BHAT (β‐Blocker Heart Attack Trial) trial, propranolol showed a mortality reduction of 25%; nevertheless, the survival curves became parallel after 1 year.21 In a long‐term follow‐up study of the BHAT trial, propranolol showed a beneficial effect only among patients with recurrent ischemic events and congestive heart failure during the first 1 year after AMI,22 indicating that the beneficial effect of propranolol beyond 1 year after AMI was confined to high‐risk patients. However, these initial β‐blocker studies were performed in the era of AMI treatment without reperfusion therapy.
In the present era of effective reperfusion with percutaneous coronary intervention and coronary artery bypass graft surgery, the effect of β‐blockers may be different. Early intravenous administration of metoprolol therapy in (predominantly) patients with STEMI decreased the risks of reinfarction and ventricular fibrillation, but not mortality, for up to 4 weeks of treatment in the COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction) trial.23 For the long‐term outcome, β‐blocker therapy was associated with improved survival in a subgroup of patients with reduced LV ejection fraction,11 but not in those without heart failure and LV systolic dysfunction.10, 24
In this study, the patients who were receiving β‐blockers at discharge had a better 5‐year survival, and the survival curves continued to diverge during the entire follow‐up duration. Although the patients with β‐blocker prescription at discharge had more favorable baseline characteristics, β‐blocker prescription was associated with a 29% reduced risk of mortality after adjustment for significant covariates, confirming the beneficial effect of β‐blocker prescription at discharge after AMI. This benefit seemed unrelated to the blood pressure–lowering effect because no difference in blood pressure was observed until 3 years after AMI. In the prespecified landmark analyses at 1 and 3 years, we reevaluated all the patients on their baseline characteristics and β‐blocker prescription because these important factors may change during follow‐up. Many studies do not provide data on whether β‐blockers were continued until the end of follow‐up.25 More important, β‐blocker use at 1 and 3 years after AMI was no longer associated with reduced mortality in the Cox model, which suggests that the beneficial effect is attributed to the differences in baseline characteristics, not to β‐blocker effect per se. Similarly, Puymirat et al showed in a French prospective registry that discontinuation of β‐blocker treatment in the year after the AMI was not related to a higher risk of mortality up to 5 years.13
New‐Generation β‐Blockers
Despite the extensive use of β‐blockers in patients after AMI, data on their benefits are derived from old studies5, 19 or extrapolated from studies on chronic angina and heart failure.8, 26 Therefore, data on the long‐term effect of newer generations of β‐blockers, such as bisoprolol, nebivolol, and carvedilol, are limited, which may have different properties and distinct effects from those of the first‐generation β‐blockers (ie, propranolol or timolol). In patients with heart failure and reduced ejection fraction, only long‐acting metoprolol, bisoprolol, and carvedilol have proven survival benefits, which indicates that the protective effect of β‐blockers is not a “class effect,” as seen in angiotensin‐converting enzyme inhibitors.7, 27, 28 In this study, 70% and 27% received third‐generation carvedilol and second‐generation bisoprolol, respectively, and our study results did not demonstrate different efficacies in patients with AMI, as reported in previous studies.13, 29
Switch of β‐Blockers to RAS Inhibitors
After 1 year, hypertension became an important risk factor of long‐term mortality in this study, and the use of RAS inhibitors at 1 or 3 years showed a significant survival benefit. β‐Blockers are not recommended as the first‐line therapy for management of hypertension because of their inferior efficacy to that of RAS inhibitors.30, 31 RAS inhibitors, such as angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers, exert pleiotropic effects and reduce oxidative stress, cardiovascular fibrosis, and vascular and cardiac muscle hypertrophies,32 independently of their blood pressure–lowering property. Whether β‐blockers should be switched to other classes of antihypertensive agents in patients with hypertension 1 year after AMI or later should be confirmed in further clinical trials.
Limitations
The current data are not results of a randomized controlled study but are from a single‐center registry. Consequently, the risk factors showed a nonuniform distribution between the groups. Patients who did not receive β‐blocker at discharge were sicker and at higher risk, which could potentially affect the results. Although we adjusted for significant risk factors, unmeasured covariates, such as instrumental variables, may have influenced the study outcomes. Although some statistical techniques can be used to eliminate unmeasured covariates,33 only well‐designed randomized controlled clinical trials are likely to be free of measured and unmeasured confounders. Furthermore, because of attrition of sample size in populations 2 and 3, we cannot exclude the possibility of type 1 error for the neutral association between β‐blockers and mortality.
The main strength is that we could collect the information of all the patients who were undergoing regular follow‐up, with a relatively small follow‐up loss, which is rare at a tertiary hospital. Our hospital is a paperless hospital, and its electronic medical record system complies with stage 7 of the Healthcare Information and Management System Society; thus, we could reevaluate all patients’ information at discharge and 1 and 3 years after discharge. This unique design enabled us to determine the optimal duration of β‐blocker therapy, in comparison with studies that evaluated long‐term effects according to the β‐blocker prescription statuses at discharge and 1 and 3 years after AMI. Nevertheless, randomized trials that evaluate the effect of β‐blockers beyond 1 year in comparison with that of placebo are necessary before inclusion of the use of β‐blockers in practice guidelines.
Conclusions
The beneficial effect of β‐blocker therapy after AMI may be limited to until 1 year after AMI. Whether late β‐blocker therapy beyond 1 year after AMI offers clinical benefit and whether β‐blockers should be switched to other classes of antihypertensive agents in patients after AMI with hypertension should be confirmed in further clinical trials.
Sources of Funding
This research was supported by a grant (2011‐E63009‐00) from the Research of Korea Centers for Disease Control and Prevention; by the Industrial Strategic Technology Development Program (10052980; for the development of microbiorobotic systems for surgical treatment of chronic total occlusion, funded by the Ministry of Trade, Industry, and Energy [Korea]); by a grant from the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI17C1799); and by a grant from the SNUBH Research Fund (14‐2017‐012).
Disclosures
None.
Supporting information
Figure S1. Distribution of propensity score.
(J Am Heart Assoc. 2018;7:e007567 DOI: 10.1161/JAHA.117.007567.)29502101
References
- 1. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ. 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–e228. [DOI] [PubMed] [Google Scholar]
- 2. Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985;27:335–371. [DOI] [PubMed] [Google Scholar]
- 3. Olsson G, Rehnqvist N. Evaluation of antiarrhythmic effect of metoprolol treatment after acute myocardial infarction: relationship between treatment responses and survival during a 3‐year follow‐up. Eur Heart J. 1986;7:312–319. [DOI] [PubMed] [Google Scholar]
- 4. The β‐Blocker Heart Attack Trial (BHAT) research group . A randomized trial of propranolol in patients with acute myocardial infarction, I: mortality results. JAMA. 1982;247:1707–1714. [DOI] [PubMed] [Google Scholar]
- 5. First International Study of Infarct Survival Collaborative Group . Randomised trial of intravenous atenolol among 16 027 cases of suspected acute myocardial infarction: ISIS‐1. Lancet. 1986;2:57–66. [PubMed] [Google Scholar]
- 6. Freemantle N, Cleland J, Young P, Mason J, Harrison J. Beta blockade after myocardial infarction: systematic review and meta regression analysis. BMJ. 1999;318:1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH; US Carvedilol Heart Failure Study Group. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med. 1996;334:1349–1355. [DOI] [PubMed] [Google Scholar]
- 8. O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis‐Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman EM, Stevenson WG, Yancy CW. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–e140. [DOI] [PubMed] [Google Scholar]
- 9. Yang JH, Hahn JY, Song YB, Choi SH, Choi JH, Lee SH, Kim JH, Ahn YK, Jeong MH, Choi DJ, Park JS, Kim YJ, Park HS, Han KR, Rha SW, Gwon HC. Association of beta‐blocker therapy at discharge with clinical outcomes in patients with ST‐segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. JACC Cardiovasc Interv. 2014;7:592–601. [DOI] [PubMed] [Google Scholar]
- 10. Dondo TB, Hall M, West RM, Jernberg T, Lindahl B, Bueno H, Danchin N, Deanfield JE, Hemingway H, Fox KAA, Timmis AD, Gale CP. Beta‐blockers and mortality after acute myocardial infarction in patients without heart failure or ventricular dysfunction. J Am Coll Cardiol. 2017;69:2710–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ozasa N, Kimura T, Morimoto T, Hou H, Tamura T, Shizuta S, Nakagawa Y, Furukawa Y, Hayashi Y, Nakao K, Matsuzaki M, Nobuyoshi M, Mitsudo K; j‐Cypher Registry Investigators . Lack of effect of oral beta‐blocker therapy at discharge on long‐term clinical outcomes of ST‐segment elevation acute myocardial infarction after primary percutaneous coronary intervention. Am J Cardiol. 2010;106:1225–1233. [DOI] [PubMed] [Google Scholar]
- 12. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction , Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez‐Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035.22923432 [Google Scholar]
- 13. Puymirat E, Riant E, Aissoui N, Soria A, Ducrocq G, Coste P, Cottin Y, Aupetit JF, Bonnefoy E, Blanchard D, Cattan S, Steg G, Schiele F, Ferrieres J, Juilliere Y, Simon T, Danchin N. Beta blockers and mortality after myocardial infarction in patients without heart failure: multicentre prospective cohort study. BMJ. 2016;354:i4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–719. [DOI] [PubMed] [Google Scholar]
- 15. Kezerashvili A, Marzo K, De Leon J. Beta blocker use after acute myocardial infarction in the patient with normal systolic function: when is it “ok” to discontinue? Curr Cardiol Rev. 2012;8:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kjekshus JK. Importance of heart rate in determining beta‐blocker efficacy in acute and long‐term acute myocardial infarction intervention trials. Am J Cardiol. 1986;57:43F–49F. [DOI] [PubMed] [Google Scholar]
- 17. The MIAMI Trial Research Group . Metoprolol in acute myocardial infarction (MIAMI): a randomised placebo‐controlled international trial. Eur Heart J. 1985;6:199–226. [PubMed] [Google Scholar]
- 18. Hjalmarson A, Elmfeldt D, Herlitz J, Holmberg S, Malek I, Nyberg G, Ryden L, Swedberg K, Vedin A, Waagstein F, Waldenstrom A, Waldenstrom J, Wedel H, Wilhelmsen L, Wilhelmsson C. Effect on mortality of metoprolol in acute myocardial infarction: a double‐blind randomised trial. Lancet. 1981;2:823–827. [DOI] [PubMed] [Google Scholar]
- 19. Norwegian Multicenter Study Group . Timolol‐induced reduction in mortality and reinfarction in patients surviving acute myocardial infarction. N Engl J Med. 1981;304:801–807. [DOI] [PubMed] [Google Scholar]
- 20. Pedersen TR. Six‐year follow‐up of the Norwegian multicenter study on timolol after acute myocardial infarction. N Engl J Med. 1985;313:1055–1058. [DOI] [PubMed] [Google Scholar]
- 21. Beta‐Blocker Heart Attack Study Group . The beta‐blocker heart attack trial. JAMA. 1981;246:2073–2074. [PubMed] [Google Scholar]
- 22. Viscoli CM, Horwitz RI, Singer BH. Beta‐blockers after myocardial infarction: influence of first‐year clinical course on long‐term effectiveness. Ann Intern Med. 1993;118:99–105. [DOI] [PubMed] [Google Scholar]
- 23. Chen ZM, Pan HC, Chen YP, Peto R, Collins R, Jiang LX, Xie JX, Liu LS; COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group; Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo‐controlled trial. Lancet. 2005;366:1622–1632. [DOI] [PubMed] [Google Scholar]
- 24. Huang BT, Huang FY, Zuo ZL, Liao YB, Heng Y, Wang PJ, Gui YY, Xia TL, Xin ZM, Liu W, Zhang C, Chen SJ, Pu XB, Chen M, Huang DJ. Meta‐analysis of relation between oral beta‐blocker therapy and outcomes in patients with acute myocardial infarction who underwent percutaneous coronary intervention. Am J Cardiol. 2015;115:1529–1538. [DOI] [PubMed] [Google Scholar]
- 25. Cucherat M, Boissel JP, Leizorovicz A; The APSI Investigators. Acebutolol et Prevention Secondaire de de l'infarctus. Persistent reduction of mortality for five years after one year of acebutolol treatment initiated during acute myocardial infarction. Am J Cardiol. 1997;79:587–589. [DOI] [PubMed] [Google Scholar]
- 26. Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left‐ventricular dysfunction: the capricorn randomised trial. Lancet. 2001;357:1385–1390. [DOI] [PubMed] [Google Scholar]
- 27. Hjalmarson A, Goldstein S, Fagerberg B, Wedel H, Waagstein F, Kjekshus J, Wikstrand J, El Allaf D, Vitovec J, Aldershvile J, Halinen M, Dietz R, Neuhaus KL, Janosi A, Thorgeirsson G, Dunselman PH, Gullestad L, Kuch J, Herlitz J, Rickenbacher P, Ball S, Gottlieb S, Deedwania P; MERIT‐HF Study Group. Effects of controlled‐release metoprolol on total mortality, hospitalizations, and well‐being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT‐HF). JAMA. 2000;283:1295–1302. [DOI] [PubMed] [Google Scholar]
- 28. CIBIS‐II Investigators and Committees . The Cardiac Insufficiency Bisoprolol Study II (CIBIS‐II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 29. Seo GW, Kim DK, Kim KH, Seol SH, Jin HY, Yang TH, Ahn Y, Jeong MH, Song PS, Kim DI; Other Korea Acute Myocardial Infarction Registry Investigators . Impact of carvedilol versus beta1‐selective beta blockers (bisoprolol, metoprolol, and nebivolol) in patients with acute myocardial infarction undergoing percutaneous coronary intervention. Am J Cardiol. 2015;116:1502–1508. [DOI] [PubMed] [Google Scholar]
- 30. Lindholm LH, Ibsen H, Dahlof B, Devereux RB, Beevers G, de Faire U, Fyhrquist F, Julius S, Kjeldsen SE, Kristiansson K, Lederballe‐Pedersen O, Nieminen MS, Omvik P, Oparil S, Wedel H, Aurup P, Edelman J, Snapinn S; LIFE Study Group . Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:1004–1010. [DOI] [PubMed] [Google Scholar]
- 31. Bradley HA, Wiysonge CS, Volmink JA, Mayosi BM, Opie LH. How strong is the evidence for use of beta‐blockers as first‐line therapy for hypertension? Systematic review and meta‐analysis. J Hypertens. 2006;24:2131–2141. [DOI] [PubMed] [Google Scholar]
- 32. Verdecchia P, Reboldi G, Angeli F, Gattobigio R, Bentivoglio M, Thijs L, Staessen JA, Porcellati C. Angiotensin‐converting enzyme inhibitors and calcium channel blockers for coronary heart disease and stroke prevention. Hypertension. 2005;46:386–392. [DOI] [PubMed] [Google Scholar]
- 33. Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA. 2007;297:278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Distribution of propensity score.


