Abstract
Background
Cardiac troponin T (cTnT) is elevated after coronary artery bypass grafting surgery. The aim of this study was to determine the association between cTnT elevations between 6 and 12 hours after coronary artery bypass grafting and in‐hospital outcome.
Methods and Results
We prospectively studied 1722 patients undergoing isolated coronary artery bypass grafting. We assessed the association between conventional cTnT (749 patients) and high‐sensitivity cTnT (hs‐cTnT; 973 patients) 6 to 12 hours postoperatively with in‐hospital major adverse cardiac or cerebrovascular events (MACCE), a composite of all‐cause death, myocardial infarction, or stroke. The prespecified secondary outcome was a safety composite of MACCE, resuscitation, intensive care unit readmission or admission ≥48 hours, inotrope or vasopressor use ≥24 hours, or new‐onset renal insufficiency. Among patients with a conventional cTnT measurement, 92 experienced a MACCE (12%) and 146 experienced a safety composite event (19%). Likewise, for hs‐cTnT, 114 experienced a MACCE (12%) and 153 experienced a safety composite event (16%). Compared with cTnT ≤200 ng/L, each 200‐ng/L increment in cTnT was associated with a monotonous increase in the odds of MACCE and the safety composite outcome. Conventional and hs‐cTnT demonstrated moderate discrimination for MACCE (areas under the fitted receiver operating characteristics curve, 0.72 and 0.77 for conventional and hs‐cTnT, respectively) and the safety composite outcome (areas under the fitted receiver operating characteristics curve, 0.66 and 0.74 for conventional and hs‐cTnT, respectively) and resulted in improved prognostic performance when added to the EuroSCORE. At a cutoff of 800 ng/L, conventional and hs‐cTnT provided clinically relevant power to rule in MACCE and the safety composite outcome.
Conclusions
cTnT levels assessed between 6 and 12 hours after coronary artery bypass grafting identify patients at increased risk of MACCE or other complications.
Keywords: coronary artery bypass graft surgery, prognosis, troponin T
Subject Categories: Myocardial Infarction, Chronic Ischemic Heart Disease, Prognosis, Cardiovascular Surgery, Revascularization
Clinical Perspective
What Is New?
Early postoperative cardiac troponin T (cTnT), assessed 6 to 12 hours postoperatively in patients who had undergone coronary artery bypass grafting, demonstrated moderate discrimination for major adverse cardiac or cerebrovascular events and a safety composite outcome.
A cutoff value of 800 ng/L for conventional cTnT and high‐sensitivity cTnT provided clinically relevant power to rule in major adverse cardiac or cerebrovascular events and the safety composite outcome: patients with early conventional cTnT and hs‐cTnT levels >800 ng/L were 7 to 12 times more likely to subsequently experience a major adverse cardiac or cerebrovascular event or safety event in the hospital compared with patients with lower early cTnT levels.
What Are the Clinical Implications?
Conventional and hs‐cTnT levels after coronary artery bypass grafting are clinically useful in identifying patients at increased risk of complications.
Early cTnT elevations may allow for the emergent investigation and management of postoperative complications, which could have the potential to improve postoperative outcomes.
Coronary artery bypass grafting (CABG) surgery is an established revascularization strategy for adults with multivessel coronary artery disease. However, patients who undergo CABG may develop periprocedural complications, such as myocardial infarction (MI), cardiogenic shock, stroke, acute kidney injury, and others. Early identification of complications after CABG is important to allow quick therapeutic strategies, such as emergent reexploration of grafts, immediate coronary angiography with or without percutaneous intervention, insertion of intra‐aortic balloon pump, and additional monitoring in the intensive care unit.
Cardiac troponin is unsurprisingly elevated after CABG and may be a consequence of insufficient myocardial protection or myocardial ischemia related to inadequate reperfusion.1, 2, 3 Therefore, it remains unclear how to interpret changes of troponin values in the postoperative setting and whether troponin elevations have a prognostic value for in‐hospital complications after CABG. Existing studies on the association between troponin elevations after CABG and in‐hospital complications also have important limitations.4, 5, 6, 7 First, investigators have examined adults undergoing all cardiac surgical procedures as opposed to limiting their analysis to CABG. Because the magnitude of postoperative troponin elevation varies according to the cardiac surgical procedure,5 the clinical applicability of these studies to adults undergoing isolated CABG is limited.4, 5, 6 Second, few studies focused on troponin elevations measured earlier than 24 hours,4, 7 and the window of opportunity to meaningfully improve a patient's postoperative prognosis may have closed after the first postoperative day. Earlier postoperative assessment of troponin is, therefore, necessary to improve the clinical management of patients undergoing CABG. To our knowledge, only 3 small studies have assessed the association between troponin measurements taken at ≤12 hours postoperatively and subsequent in‐hospital complications. In the first study, troponin I (TnI) was measured immediately after CABG in 540 adults and, if elevated, was associated with a 17‐fold increase in perioperative MI or death.8 In the second study, troponin T measured between 8 and 16 hours postoperatively had moderate to high discrimination (area under the curve, ≥0.73) for perioperative MI.9 The third study was a pilot study conducted by our group.10 Among 290 adults undergoing isolated CABG, we demonstrated that a cardiac troponin T (cTnT) increase of 1000 ng/L, measured at a mean 8.1 hours postoperatively, was associated with a 7‐fold increase in in‐hospital complications, including periprocedural MI, prolonged need for vasopressors, and new‐onset renal insufficiency. We also reported that a cutoff of 800 ng/L is useful to identify patients at risk for subsequent complications. Although presurgical risk scores, such as the EuroSCORE, show some association with postsurgical outcomes, this is moderate at best. It would be desirable to have a marker assessed early in the postoperative course with a more pronounced association with postoperative course of patients that could be used for clinical decision making.
In this prospective cohort study, we wanted to determine the association between cTnT levels measured 6 to 12 hours after CABG and in‐hospital outcome, validating our previous findings, including the prespecified cutoff of 800 ng/L,10 and further investigate the clinical utility of early cTnT.
Methods
Patient Population
Using a prospectively maintained institutional registry (Intellect 1.7; Dendrite Clinical Systems, Henley‐on‐Thames, UK), we identified all consecutive patients undergoing isolated CABG at the University Hospital of Bern (Bern, Switzerland) from October 17, 2006 (when cTnT assays were introduced at our laboratory), to December 31, 2013. The study population was limited to adults with at least 1 troponin measurement between 6 and 12 hours after surgery. Patients who underwent CABG from January 1, 2008, to December 31, 2008, were analyzed as part of a previously published development cohort10 and were excluded. One of us (B.G.) had full access to all the data in the study and takes responsibility for its integrity and the data analysis. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
At our institution, isolated CABG procedures are performed almost exclusively with minimal extracorporeal circulation (MECC). Patients undergoing procedures that used standard ECC (extracorporeal circulation), those undergoing beating heart surgery, and those who required a concomitant procedure on the carotid artery were excluded to achieve a homogeneous study population. Also, patients with a recent MI occurring <7 days before their CABG procedure were excluded to avoid elevated cTnT levels before surgery. The study was approved by the local research ethics committee (Kantonale Ethikkommission Bern) in accordance with the Declaration of Helsinki. All patients provided written informed consent.
Clinical Procedures
Details of the surgical procedure and anesthesia were previously described in detail.10 Briefly, all procedures were performed via full sternotomy using MECC with moderate systemic hypothermia (32°C) with the MECC system (Jostra, Hirlingen, Germany) and a single‐shot low‐volume cardioplegia (100 mL Cardioplexol; Laboratorium Dr G. Bichsel AG, Unterseen, Switzerland). If necessary, a second dose of 50 to 100 mL was administered after 45 to 60 minutes of cross‐clamp time. Cardiac arrest characteristically occurred within 5 to 8 seconds. The left internal mammary artery, the left radialis artery, and/or a segment of the great saphenous vein were typically used as bypass conduits. The anesthetic protocol was standardized and included the use of fentanyl, midazolam, and isoflurane.
Assessments for Biomarkers
To qualify for this study, patients had to have at least 1 cTnT assessment available between 6 and 12 hours postoperatively. Routine postoperative assessments of cTnT, creatine kinase (CK), and the muscle‐brain type CK (CK‐MB; mass) were scheduled at ≈9 and 18 hours postoperatively, as calculated from the time point of completion of suture. If multiple assessments were available within the eligible time window, the value assessed closest to 9 hours was used for analysis. Patients without cTnT assessments within this window were excluded from the analysis.
Conventional cTnT was introduced at our laboratory on October 17, 2006, and replaced by a high‐sensitivity cTnT (hs‐cTnT) assay on August 1, 2010. Both conventional and hs‐cTnT assays were measured using an electrochemoluminescent enzyme immunoassay on a Modular analytics E170 platform (Roche Diagnostics, Mannheim, Germany). The 99th percentile cutoff point is 10 ng/L for the conventional cTnT assay and 14 ng/L for the hs‐cTnT assay. CK‐MB (mass) levels were measured on the same platform, and CK levels were measured using a UV test on a Roche Modular P800 (Roche Diagnostics).
Prognostic Variables
The prespecified primary prognostic variable was cTnT, assessed between 6 and 12 hours postoperatively. The secondary prognostic variables were the logistic version of the European System for Cardiac Operative Risk Evaluation (subsequently referred to as EuroSCORE) and requirement for inotropes or vasopressors within 6 hours postoperatively, as previously reported.10 For each patient, we calculated the EuroSCORE, which predicts the risk of 30‐day all‐cause mortality from preoperative factors on the basis of a logistic regression model using age, sex, comorbid conditions, patient history, and left ventricular ejection fraction.11
Clinical Outcomes
The prespecified primary outcome was in‐hospital major adverse cardiac or cerebrovascular event (MACCE), defined as a composite of death from any cause, MI, or stroke. The prespecified secondary outcome was a safety composite of MACCE, resuscitation, stay at the intensive care unit of >48 hours’ duration, readmission to the intensive care unit, need for inotropes or vasopressors for >24 hours, or new‐onset renal insufficiency, as previously reported for the analysis of the development cohort.10 The diagnosis of MI was based on signs or symptoms consistent with myocardial ischemia, ECG changes, and CK and CK‐MB (mass) levels, as specified by the Academic Research Consortium for the adjudication of events occurring after CABG.12 For periprocedural events occurring <72 hours after CABG, MI was assumed if CK‐MB (mass) or CK levels increased to >10 times the upper reference limit; or if CK‐MB (mass) or CK levels increased to >5 times the upper reference limit and new pathologic Q waves were observed in ≥2 contiguous ECG leads or recurrent signs or symptoms consistent with MI were observed. Postoperative echocardiography was performed to detect hypokinesia or akinesia only when considered clinically indicated. Events compatible with the occurrence of an MI were retrospectively adjudicated independently by a senior cardiologist (V.G.) who was unaware of observed cTnT levels while evaluating the imaging records. Stroke was defined as episode of neurological dysfunction caused by a focal cerebral infarction, with subsequent confirmation by imaging.13 New‐onset renal insufficiency was defined as a new need for dialysis or, in patients with preoperative creatinine levels <2 mg/dL (<172 μmol/L), an increase in postoperative creatinine levels >2 mg/dL and at least twice the preoperative value.14
Statistical Analysis
We conducted 2 main analyses. First, we assessed the prognostic performance of cTnT measured at 6 to 12 hours for predicting our study outcomes, with additional consideration of the EuroSCORE and the need for inotropes or vasopressors. Second, we sought to validate our previously identified cutoff of 800 ng/L for cTnT.10 All analyses were performed separately for conventional cTnT and hs‐cTnT.
For the primary prognostic variable, cTnT levels, we determined the crude association with study outcomes by first calculating odds ratios (ORs) for prespecified categories of cTnT of >200 to 400, >400 to 600, >600 to 800, >800 to 1200, and >1200 ng/L compared with the reference category of patients with cTnT of ≤200 ng/L. We performed a nonparametric test for trend among the ORs for the cTnT categories. We then used logistic regression to model the association between log cTnT values and the logit of the primary or secondary outcome, and we back‐transformed the resulting logits to probabilities and plotted them against cTnT values. The kernel density estimates of the distribution and quarters of cTnT values were superimposed on the graph to allow simultaneous presentation of the distribution of cTnT values in the studied population and their association with the risk of a future primary or secondary outcome event. We also examined univariable associations between the secondary prognostic variables and study outcomes. The need for inotropes or vasopressors within 6 hours postoperatively was included in regression models as a binary prognostic variable. To ensure that ORs for EuroSCORE were directly comparable with those calculated for binary variables, we expressed the ORs for EuroSCORE per 2 SD change15 after logarithmic transformation to correct for the skewed distribution of the data. We also included all prognostic variables (log cTnT, need for inotropes or vasopressors, and EuroSCORE) in a single multivariable model to assess their association with study outcomes. We did not include additional prognostic variables because our intention was to examine the role of cTnT as a marker of underlying risk of poor in‐hospital outcomes.
To evaluate the prognostic performance of the 3 prognostic variables in our study, we assessed area under the fitted receiver operating characteristics curve (AUC) using a maximum likelihood logistic regression model. We also assessed the corresponding integrated discrimination improvement using the EuroSCORE as a reference. The integrated discrimination improvement reflects the gain of prognostic performance when adding need for inotropes or vasopressors, log cTnT levels, or both to the reference model.16 We then used linear regression to determine the association between the logit of the specificities and log cTnT to correctly identify patients without subsequent clinical outcome using a piecewise linear function for log cTnT estimated from linear splines, with 8 knots equally distributed over the range of available log cTnT levels. Using the fitted receiver operating characteristic curve of sensitivity against 1‐specificity of cTnT levels, we then derived likelihood ratios for positive (LR+) and negative tests (LR−). The LR+ quantifies how much more likely it is to find a cTnT value at least as high as a given cutoff in patients with the outcome (sensitivity) compared with patients without the outcome (1‐specificity). A test can be considered to provide clinically relevant power to rule in the outcome if the LR+ is >5. The LR− indicates how much less likely it is to find a lower cTnT than a given cutoff (ie, a negative test result) in patients with the outcome (1‐sensitivity) compared with patients without (specificity); LR− can be considered to have a clinically relevant power to rule out the outcome if it is <0.2. Crude 2×2 tables, fitted sensitivity, specificity, LR+, LR−, and posttest probability after a positive test result were then reported for 200‐ng/L increments of cTnT from 200 to 1400 ng/L. To facilitate the clinical translation of our work, we constructed nomograms to allow clinicians to derive posttest probabilities of the primary and secondary outcomes in our study as a function of assumed pretest probabilities and cTnT values. This was done by multiplying the corresponding pretest odds of clinical outcomes with the LR+ and LR− found for different cTnT values to derive posttest odds of clinical outcomes. The posttest odds were then back‐transformed into a posttest probability.
Finally, we used logistic regression to examine the association between an early postoperative cTnT level of >800 ng/L and MACCE, the safety composite outcome, and individual components of each outcome. To assess calibration, we compared the probabilities of MACCE and safety composite below and above the prespecified cutoff of 800 ng/L cTnT in the development cohort10 with risks observed below and above the cutoff in the validation cohort, and we compared observed risks of MACCE and safety composite below and above the cutoff with predicted probabilities for cTnT and hs‐cTnT in the validation cohort. Because MI was likely to drive the association between cTnT levels and the safety composite outcome, we conducted a sensitivity analysis to determine whether the association between postoperative cTnT levels of >800 ng/L and the safety composite outcome would be maintained after exclusion of MI from the composite outcome definition. Patient and procedural characteristics were presented as numbers with percentage, mean±SD, or median with interquartile range (IQR), as appropriate. All P values and 95% confidence intervals (CIs) are 2 sided. All calculations were performed using Stata 12 (StataCorp, College Station, TX) and R (https://www.r-project.org).
Results
General Characteristics of the Study Cohort
Between October 17, 2006, and December 31, 2013, 2922 consecutive patients underwent isolated CABG surgery at our hospital and 2096 met our inclusion criteria, of whom 75 had to be excluded because cTnT was not measured in the prespecified time window suitable for our study (Figure 1). A further 299 of 2021 patients, who underwent isolated CABG between January 1, 2008, and December 31, 2008, were excluded because they were already previously examined as part of the development cohort.10 The remaining 1722 patients represent the validation cohort and were included in the current analysis.
Figure 1.
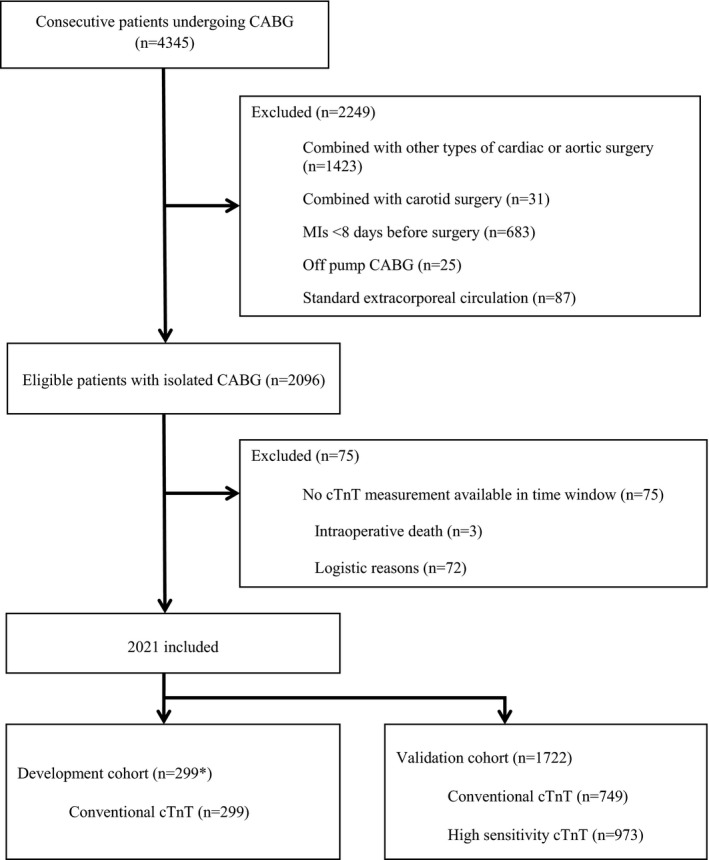
Patient flow. The asterisk indicates that 9 patients who underwent coronary artery bypass grafting (CABG) between January 1, 2008, and December 31, 2008, were originally excluded from the development cohort because they had received an ablation or a closure of a patent foramen ovale; therefore, previously published results are based on only 290 analyzed patients.10 cTnT indicates cardiac troponin T; and MI, myocardial infarction.
A total of 749 patients underwent cTnT testing with the conventional assay, and 973 patients underwent cTnT testing with the high‐sensitivity assay at a median of 8.8 hours (IQR, 8.2–9.3 hours) and 8.7 hours (IQR, 8.1–9.2 hours) postoperatively, respectively. The median conventional cTnT was 180 ng/L (IQR, 110–300 ng/L; range, 19–4300 ng/L) and the median hs‐cTnT was 320 ng/L (IQR, 220–470 ng/L; range, 54–6010 ng/L). Among adults who received a conventional cTnT test, 92 experienced a MACCE (12%) and 146 experienced a safety composite event (19%). Likewise, among adults who received an hs‐cTnT test, 114 experienced a MACCE (12%) and 153 experienced a safety composite event (16%). All MIs occurred within 72 hours after CABG.
Overall, the mean age of study participants was 66 years (Table 1). A total of 332 participants were women (19%), 563 had diabetes mellitus (33%), 452 had a history of MI (26%), and 372 had left main coronary disease (22%). A total of 335 participants (19%) were classified as having New York Heart Association stage III or IV heart failure. With respect to procedural characteristics, the mean duration of operation was 205 minutes, the mean ECC time was 70 minutes, and the mean cross‐clamp time was 44 minutes. Additional patient and procedural characteristics are presented in Table 1.
Table 1.
Patient and Procedural Characteristics Stratified by Type of cTnT
| Characteristics | All Patients | Conventional cTnT | hs‐cTnT |
|---|---|---|---|
| (N=1722) | (n=749) | (n=973) | |
| Patient characteristics | |||
| Age, y | 66.0±9.6 | 66.0±9.7 | 66.0±9.5 |
| Female sex | 332 (19) | 154 (21) | 178 (18) |
| Body mass index, kg/m2 | 27.8±4.5 | 27.6±4.4 | 27.9±4.5 |
| Diabetes mellitus | 563 (33) | 252 (34) | 311 (32) |
| Current smoker | 399 (23) | 185 (25) | 214 (22) |
| Hypertension | 1390 (81) | 595 (79) | 795 (82) |
| Positive cardiovascular family history | 554 (32) | 264 (35) | 290 (30) |
| Most recent myocardial infarction | |||
| Any history of myocardial infarction | 452 (26) | 212 (28) | 240 (25) |
| Preoperative, d | |||
| 8–21 | 106 (6) | 57 (8) | 49 (5) |
| 22–90 | 69 (4) | 39 (5) | 30 (3) |
| >90 | 277 (16) | 116 (15) | 161 (17) |
| Previous CABGa | 31 (2) | 13 (2) | 18 (2) |
| Previous stroke | 127 (7) | 43 (6) | 84 (9) |
| Extracardiac arteriopathy | 376 (22) | 138 (18) | 238 (24) |
| Chronic obstructive pulmonary disease | 193 (11) | 87 (12) | 106 (11) |
| Dialysis | 18 (1) | 5 (1) | 13 (1) |
| Last preoperative creatinine, μmol/L | 80 (69–93) | 80 (69–92) | 81 (70–94) |
| Creatinine clearance, mL/min | 85.8±31.5 | 83.4±31.8 | 87.7±31.2 |
| Sinus rhythm | 71 (4) | 18 (2) | 53 (5) |
| No. of diseased vessels | |||
| 1 | 32 (2) | 17 (2) | 15 (2) |
| 2 | 242 (14) | 129 (17) | 113 (12) |
| 3 | 1426 (83) | 593 (79) | 833 (86) |
| Left main coronary disease | 372 (22) | 148 (20) | 224 (23) |
| Ejection fraction, % | 57.4±12.1 | 56.6±12.6 | 58.1±11.6 |
| CCS III or IV | 611 (35) | 290 (39) | 321 (33) |
| NYHA III or IV | 335 (19) | 125 (17) | 210 (22) |
| Urgency | |||
| Emergency | 51 (3) | 24 (3) | 27 (3) |
| Urgent | 267 (16) | 133 (18) | 134 (14) |
| Logistic EuroSCORE | 2.6 (1.5–4.6) | 2.7 (1.5–4.8) | 2.5 (1.5–4.5) |
| Procedural characteristics | |||
| Duration of operation, min | 204.6±47.0 | 194.2±45.2 | 212.7±46.8 |
| ECC time, min | 70 (56–85) | 68 (55–82) | 71 (57–86) |
| Cross‐clamp time, min | 44 (35–54) | 43 (34–54) | 44 (35–55) |
Values are arithmetic mean±SD, number (percentage), or median (interquartile range). CABG indicates coronary artery bypass grafting; CCS, Canadian Cardiovascular Society; cTnT, cardiac troponin T; ECC, extracorporeal circulation; hs‐cTnT, high‐sensitivity cTnT; and NYHA, New York Heart Association.
Each patient was included once in our cohort; in case of a second CABG procedure during the inclusion period, only the first procedure was considered.
Prognostic Performance of cTnT
Compared with the reference category of cTnT ≤200 ng/L, we found a monotonous increase in ORs for MACCE and the safety composite outcome with increasing levels of conventional and hs‐cTnT (P<0.001 for trend, Table 2). There was a numerical decrease in the OR for conventional cTnT category of >800 to 1200 ng/L, but this was likely attributable to chance. Figure 2 presents the distribution of early cTnT levels among study participants and the associated fitted probabilities of MACCE. The estimated probabilities of MACCE for cTnT levels in increments of 200 ng/L are also presented in Table S1. For conventional cTnT, estimated probabilities of MACCE were 10.3% at 200 ng/L, 34.8% at 800 ng/L, and 49.2% at 1400 ng/L; for hs‐cTnT, the probabilities were 3.5%, 30.8%, and 55.0%, respectively.
Table 2.
Univariable ORs of MACCE and the Safety Composite by cTnT Categories
| Interval, ng/L | N | MACCE | Safety Composite | ||||
|---|---|---|---|---|---|---|---|
| Events | OR (95% CI) | P Value for Trend | Events | OR (95% CI) | P Value for Trend | ||
| Conventional cTnT | |||||||
| ≤200 | 415 | 22 | 1 (Reference) | <0.001 | 52 | 1 (Reference) | <0.001 |
| >200–400 | 208 | 32 | 3.25 (1.83–5.75) | 44 | 1.87 (1.20–2.91) | ||
| >400–600 | 72 | 16 | 5.10 (2.53–10.30) | 20 | 2.68 (1.49–4.85) | ||
| >600–800 | 20 | 6 | 7.66 (2.68–21.84) | 7 | 3.76 (1.43–9.85) | ||
| >800–1200 | 18 | 4 | 5.10 (1.55–16.80) | 9 | 6.98 (2.65–18.39) | ||
| >1200 | 16 | 12 | 53.6 (16.0–179.8) | 14 | 48.9 (10.8–221.2) | ||
| High‐sensitivity cTnT | |||||||
| ≤200 | 199 | 6 | 1 (Reference) | <0.001 | 9 | 1 (Reference) | <0.001 |
| >200–400 | 443 | 32 | 2.50 (1.03–6.09) | 52 | 2.81 (1.35–5.82) | ||
| >400–600 | 190 | 23 | 4.43 (1.76–11.1) | 28 | 3.65 (1.67–7.96) | ||
| >600–800 | 68 | 14 | 8.34 (3.06–22.7) | 19 | 8.19 (3.49–19.2) | ||
| >800–1200 | 45 | 20 | 25.7 (9.44–70.2) | 23 | 22.1 (9.08–53.6) | ||
| >1200 | 28 | 19 | 67.9 (21.8–211.4) | 22 | 77.4 (25.2–238.0) | ||
A nonparametric test for trend was performed across all troponin categories for each comparison. CI indicates confidence interval; cTnT, cardiac troponin T; MACCE, major adverse cardiac or cerebrovascular event; and OR, odds ratio.
Figure 2.
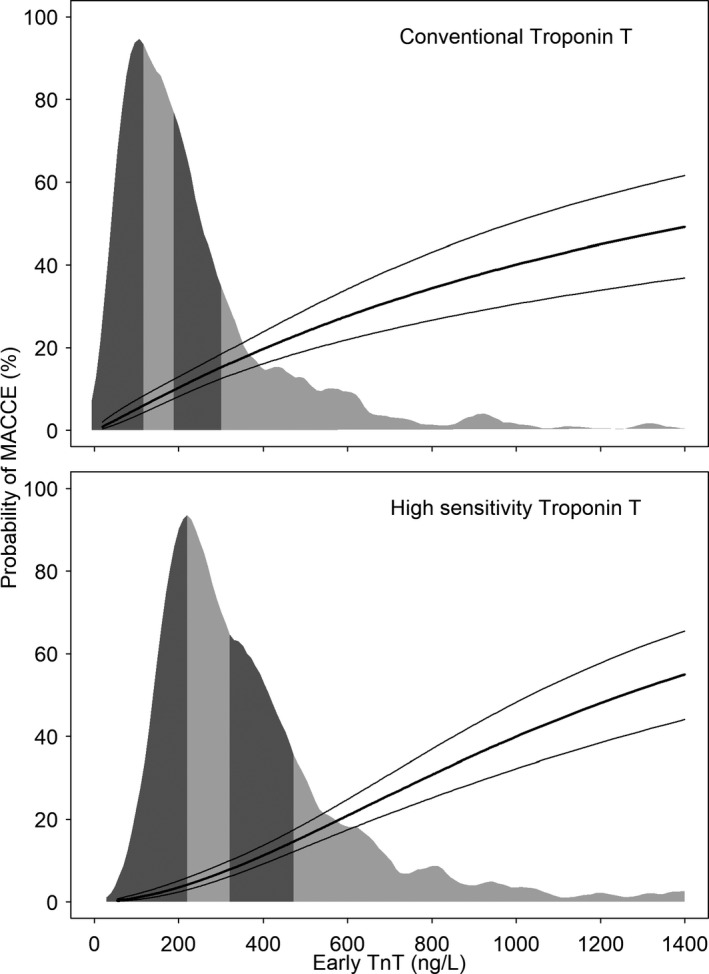
Distribution of early cardiac troponin T (cTnT) and probability of major adverse cardiac or cerebrovascular events (MACCE). Risk of in‐house MACCE; shaded region represents the distribution of conventional or high‐sensitivity troponin T measured 9 hours after coronary artery bypass grafting surgery. The corresponding figures for the safety composite outcome are shown in Figure S1.
Corresponding results for the safety composite outcome are presented in Figure S1 and Table S1. For conventional cTnT, the predicted probabilities for the safety composite outcome were 18.6% at 200 ng/L, 40.7% at 800 ng/L, and 51.2% at 1400 ng/L. For hs‐cTnT, the probabilities were 6.0%, 37.2%, and 59.3%, respectively. Finally, log cTnT remained independently associated with MACCE and the safety composite outcome in multivariable regression models that included log (logistic EuroSCORE) and need for inotropes or vasopressors within the first 6 hours after CABG (Table S2).
The AUCs for the association with MACCE were 0.72 for conventional cTnT (95% CI, 0.67–0.78) and 0.77 for hs‐cTnT (95% CI, 0.72–0.81). Likewise, the AUCs for the association with the safety composite were 0.66 for conventional cTnT (95% CI, 0.61–0.71) and 0.74 for hs‐cTnT (95% CI, 0.69–0.78). Using the EuroSCORE as reference, the addition of cTnT increased the AUC, with larger increments for MACCE (0.11 and 0.21 for conventional and hs‐cTnT, respectively) than for the safety composite (0.05 and 0.14, respectively) (Table 3). The addition of the need for inotropes or vasopressors did not result in relevant increases in the AUC.
Table 3.
Prognostic Accuracy of the Logistic EuroSCORE Alone and Combined With Prognostic Variables
| Variable | MACCE | Safety Composite | ||||
|---|---|---|---|---|---|---|
| AUC (95% CI) | IDI | P Value | AUC (95% CI) | IDI | P Value | |
| Conventional cTnT | ||||||
| EuroSCORE | 0.63 (0.57–0.69) | Reference | 0.66 (0.61–0.71) | Reference | ||
| EuroSCORE+vasopressors | 0.64 (0.59–0.70) | 0.00 | 0.25 | 0.67 (0.62–0.71) | 0.01 | 0.07 |
| EuroSCORE+cTnT | 0.74 (0.69–0.80) | 0.09 | 0.00 | 0.71 (0.66–0.76) | 0.06 | 0.00 |
| EuroSCORE+cTnT+vasopressors | 0.75 (0.70–0.80) | 0.09 | 0.00 | 0.71 (0.67–0.76) | 0.06 | 0.00 |
| High‐sensitivity cTnT | ||||||
| EuroSCORE | 0.56 (0.50–0.61) | Reference | 0.61 (0.56–0.66) | Reference | ||
| EuroSCORE+vasopressors | 0.56 (0.51–0.62) | 0.00 | 0.48 | 0.61 (0.56–0.66) | 0.00 | 0.17 |
| EuroSCORE+cTnT | 0.77 (0.72–0.82) | 0.14 | 0.00 | 0.75 (0.71–0.80) | 0.13 | 0.00 |
| EuroSCORE+cTnT+vasopressors | 0.77 (0.72–0.82) | 0.14 | 0.00 | 0.75 (0.71–0.80) | 0.13 | 0.00 |
“Vasopressors” indicate the need for vasopressors or inotropes within the first 6 hours after surgery. AUC indicates area under the receiver operating characteristic (curve); CI, confidence interval; cTnT, cardiac troponin T; IDI, integrated discrimination improvement; and MACCE, major adverse cardiac or cerebrovascular event.
Cutoff Values for cTnT
Table 4 presents 2×2 tables and corresponding fitted sensitivity, specificity, positive and negative LR, and the fitted posttest probability after a positive test result in 200 ng/L increments up to 1400 ng/L. At the prespecified cutoff of 800 ng/L, the positive LR to rule in MACCE was 7.30 for conventional cTnT (95% CI, 6.64–8.03) and 6.83 for hs‐cTnT (95% CI, 5.98–7.81). Corresponding positive LRs to rule in a safety composite event were 8.44 (95% CI, 7.86–9.06) and 7.00 (95% CI, 6.31–7.76), respectively, at this cutoff. The negative LRs at these cutoffs were consistently >0.2.
Table 4.
Cutoff Points of cTnT
| Cutoff, ng/L | No. of Operations | Sensitivity, % | Specificity, % | LR+ | LR− | Posttest Probability, % | |||
|---|---|---|---|---|---|---|---|---|---|
| TP | FN | TN | FP | ||||||
| MACCE | |||||||||
| Conventional cTnT | |||||||||
| 200 | 70 | 22 | 393 | 264 | 72 | 60 | 1.78 | 0.48 | 19 |
| 400 | 38 | 54 | 569 | 88 | 42 | 87 | 3.34 | 0.66 | 31 |
| 600 | 22 | 70 | 625 | 32 | 28 | 94 | 5.03 | 0.76 | 41 |
| 800 | 16 | 76 | 639 | 18 | 19 | 97 | 7.30 | 0.84 | 50 |
| 1000 | 12 | 80 | 648 | 9 | 13 | 99 | 9.74 | 0.88 | 57 |
| 1200 | 12 | 80 | 653 | 4 | 10 | 99 | 12.81 | 0.91 | 64 |
| 1400 | 9 | 83 | 654 | 3 | 7 | 100 | 15.52 | 0.93 | 68 |
| High‐sensitivity cTnT | |||||||||
| 200 | 108 | 6 | 193 | 666 | 95 | 22 | 1.21 | 0.22 | 14 |
| 400 | 76 | 38 | 604 | 255 | 68 | 71 | 2.33 | 0.45 | 24 |
| 600 | 53 | 61 | 771 | 88 | 43 | 89 | 4.11 | 0.63 | 36 |
| 800 | 39 | 75 | 825 | 34 | 26 | 96 | 6.83 | 0.77 | 48 |
| 1000 | 26 | 88 | 843 | 16 | 18 | 98 | 9.89 | 0.84 | 57 |
| 1200 | 19 | 95 | 850 | 9 | 13 | 99 | 12.75 | 0.88 | 63 |
| 1400 | 11 | 103 | 854 | 5 | 10 | 99 | 15.40 | 0.90 | 68 |
| Safety composite | |||||||||
| Conventional cTnT | |||||||||
| 200 | 94 | 52 | 363 | 240 | 62 | 60 | 1.57 | 0.62 | 25 |
| 400 | 50 | 96 | 527 | 76 | 35 | 88 | 3.06 | 0.73 | 39 |
| 600 | 30 | 116 | 579 | 24 | 23 | 95 | 5.03 | 0.80 | 51 |
| 800 | 23 | 123 | 592 | 11 | 15 | 98 | 8.44 | 0.87 | 64 |
| 1000 | 15 | 131 | 597 | 6 | 10 | 99 | 12.70 | 0.90 | 73 |
| 1200 | 14 | 132 | 601 | 2 | 7 | 100 | 19.27 | 0.93 | 80 |
| 1400 | 11 | 135 | 602 | 1 | 5 | 100 | 26.28 | 0.95 | 85 |
| High‐sensitivity cTnT | |||||||||
| 200 | 144 | 9 | 190 | 630 | 93 | 22 | 1.19 | 0.34 | 20 |
| 400 | 92 | 61 | 581 | 239 | 63 | 72 | 2.22 | 0.52 | 32 |
| 600 | 64 | 89 | 743 | 77 | 40 | 90 | 4.01 | 0.67 | 46 |
| 800 | 45 | 108 | 792 | 28 | 24 | 97 | 7.00 | 0.79 | 60 |
| 1000 | 29 | 124 | 807 | 13 | 16 | 98 | 10.59 | 0.85 | 69 |
| 1200 | 22 | 131 | 814 | 6 | 11 | 99 | 15.32 | 0.90 | 76 |
| 1400 | 13 | 140 | 817 | 3 | 8 | 100 | 20.55 | 0.92 | 81 |
Sensitivity, specificity, and posttest probability are fitted. Fitted posttest probability of a positive test corresponds to the positive predictive value. cTnT indicates cardiac troponin T; FN, false negative; FP, false positive; LR+, fitted positive likelihood ratio that corresponds to a troponin value above the cutoff; LR−, fitted negative likelihood ratio for a troponin value below or equal to the cutoff; MACCE, major adverse cardiac or cerebrovascular event; TN, true negative; and TP, true positive.
Nomograms of the relationship between pretest and posttest probabilities of MACCE (left panel) and safety composite (right panel) after a negative or positive test result for different cutoffs of conventional cTnT and hs‐cTnT are presented in Figure 3. In our cohort of patients undergoing isolated CABG, the average probability of MACCE in the absence of any additional information was 12%. On the basis of the bottom left nomogram in Figure 3, this pretest probability translates into a posttest probability of ≈48% after a positive test result defined by hs‐cTnT levels of >800 ng/L (dashed red lines). For a hypothetical higher‐risk cohort from a different hospital with an average probability of MACCE of 20%, an hs‐cTnT of >800 ng/L will yield a posttest probability of ≈63% (dashed blue lines). Posttest probabilities will increase with increasing cTnT cutoffs. An hs‐cTnT of >14 ng/L observed for patients in the higher‐risk cohort will yield a posttest probability of ≈80%. Nomograms for the safety composite outcome are presented on the right of Figure 3. Calibration was good for the cutoff of 800 ng/L. The probability of MACCE for cTnT values >800 ng/L was 37% (95% CI, 16%–62%) in the development cohort10 compared with a probability of 47% (95% CI, 30%–65%) for cTnT values >800 ng/L in the validation cohort. Corresponding probabilities of the safety composite were 65% (95% CI, 41%–85%) in the development cohort and 68% (95% CI, 49%–83%) in the validation cohort. Table S3 presents a comparison of observed and predicted probabilities for cTnT and hs‐cTnT in the validation cohort, again with a high concordance.
Figure 3.
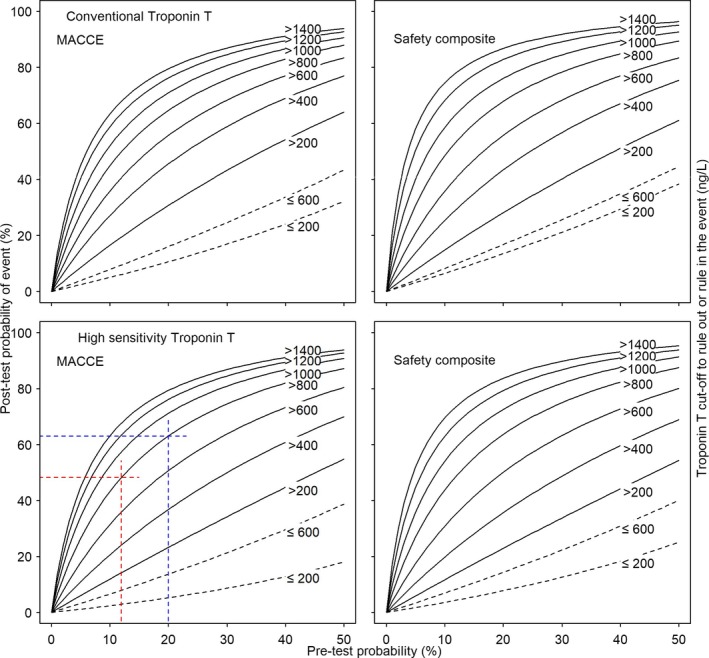
Nomograms of the relation between pretest and posttest probability of major adverse cardiac or cerebrovascular events (MACCE) or the safety composite, by troponin levels. The curves are based on pretest probabilities and fitted likelihood ratios of cardiac troponin T (cTnT) cutoffs for the risk of MACCE (left panels) and the safety composite (right panels) for conventional cTnT (top panels) and hs‐cTnT (bottom panels). Black dashed lines represent the relation between pretest and posttest probabilities, ruling out future events by cTnT levels equal to or smaller than the cutoff on the basis of negative likelihood ratios; black solid lines, the relation between pretest and posttest probabilities, ruling in future events by cTnT levels larger than the cutoff on the basis of positive likelihood ratios. Red and blue lines relate to reading examples described in the text.
Association Between cTnT >800 ng/L and Clinical Outcomes
Table 5 presents a comparison of primary and secondary composite outcomes and their individual components between patients with early cTnT levels ≤800 ng/L and patients with early cTnT levels >800 ng/L. Patients with conventional cTnT and hs‐cTnT levels >800 ng/L at 9 hours postoperatively were more likely to experience a MACCE (unadjusted OR, 7.5 [95% CI, 3.7–15.3]; and unadjusted OR, 12.6 [95% CI, 7.5–21.2], respectively) and the safety composite outcome (unadjusted OR, 10.1 [95% CI, 4.8–21.2]; and unadjusted OR, 11.8 [95% CI, 7.1–19.7], respectively) compared with patients with cTnT levels ≤800 ng/L (Table 5). With respect to individual components of MACCE, patients with conventional and hs‐cTnT levels >800 ng/L were more likely to experience an MI compared with patients with cTnT levels ≤800 ng/L (Table 5). The magnitude of the association for death and stroke was larger for conventional cTnT than hs‐cTnT (Table 5). For stroke in particular, the association with hs‐cTnT >800 ng/L was unclear, with a wide CI that was compatible with both a clinically relevant positive and negative association. In sensitivity analyses after exclusion of MI from the safety composite outcome definition, cTnT levels >800 ng/L continued to be associated with the safety composite; the association was again more pronounced for conventional cTnT (unadjusted OR, 5.7; 95% CI, 2.7–12.2) than for hs‐cTnT (unadjusted OR, 2.5; 95% CI, 1.2–5.0).
Table 5.
Adverse Events
| Events | cTnT ≤800 g/L | cTnT >800 g/L | Univariable OR (95% CI) | P Value |
|---|---|---|---|---|
| Conventional cTnT | n=715 | n=34 | ||
| MACCE, as a composite of all‐cause mortality, myocardial infarction, or stroke | 76 (11) | 16 (47) | 7.5 (3.7–15.3) | <0.001 |
| Safety composite of MACCE, acute kidney injury, resuscitation, prolongation or readmission of ICU stay, or vasopressors | 123 (17) | 23 (68) | 10.1 (4.8–21.2) | <0.001 |
| Death | 4 (1) | 2 (6) | 11.1 (2.0–62.9) | 0.027 |
| Myocardial infarction | 63 (9) | 15 (44) | 8.2 (4.0–16.9) | <0.001 |
| Stroke | 10 (1) | 3 (9) | 6.8 (1.8–26.0) | 0.018 |
| Resuscitation | 2 (0) | 2 (6) | 22.8 (3.1–167.1) | 0.011 |
| Prolonged ICU stay >48 h | 20 (3) | 8 (24) | 10.7 (4.3–26.5) | <0.001 |
| Readmission to ICU | 20 (3) | 1 (3) | 1.1 (0.1–8.1) | 1.00 |
| Vasopressors >24 h after surgery | 7 (1) | 0 (0) | 0.73 (0.04–13.1) | 1.00 |
| Acute kidney injury | 23 (3) | 4 (12) | 4.0 (1.3–12.3) | 0.030 |
| High‐sensitivity cTnT | n=900 | n=73 | ||
| MACCE, as a composite of all‐cause mortality, myocardial infarction, or stroke | 75 (8) | 39 (53) | 12.6 (7.5–21.2) | <0.001 |
| Safety composite of MACCE, acute kidney injury, resuscitation, prolongation or readmission of ICU stay, or vasopressors | 108 (12) | 45 (62) | 11.8 (7.1–19.7) | <0.001 |
| Death | 2 (0) | 1 (1) | 6.2 (0.6–69.6) | 0.21 |
| Myocardial infarction | 59 (7) | 39 (53) | 16.4 (9.6–27.8) | <0.001 |
| Stroke | 18 (2) | 1 (1) | 0.7 (0.1–5.2) | 1.00 |
| Resuscitation | 3 (0) | 3 (4) | 13.1 (2.6–66.4) | 0.006 |
| Prolonged ICU stay >48 h | 13 (1) | 2 (3) | 1.9 (0.4–8.7) | 0.31 |
| Readmission to ICU | 29 (3) | 7 (10) | 3.2 (1.3–7.5) | 0.014 |
| Vasopressors >24 h after surgery | 1 (0) | 1 (1) | 12.5 (0.8–202) | 0.14 |
| Acute kidney injury | 9 (1) | 2 (3) | 2.8 (0.6–13.2) | 0.20 |
Data are given as number (percentage) unless otherwise indicated. Vasopressors in the safety composite means need for vasopressors >24 hours after surgery. CI indicates confidence interval; cTnT, cardiac troponin T; ICU, intensive care unit; MACCE, major adverse cardiovascular or cerebrovascular event; and OR, odds ratio.
Preoperative cTnT and MACCE
Conventional cTnT and hs‐cTnT were measured preoperatively in 104 and 203 patients, respectively. The median preoperative conventional cTnT was 100 ng/L (IQR, 100–230 ng/L), and the median preoperative hs‐cTnT was 150 ng/mL (IQR, 70–400 ng/L). Preoperative conventional cTnT and hs‐cTnT were not significantly associated with MACCE (OR, 1.26 [95% CI, 0.87–1.82] for conventional cTnT; OR, 1.00 [95% CI, 0.76–1.31] for hs‐cTnT). Adjustment for preoperative cTnT increased the association of early postoperative cTnT with MACCE (Table S4).
Discussion
This study provides a detailed analysis of the prognostic importance of early postoperative cTnT measured between 6 and 12 hours after isolated CABG to predict MACCE and a safety composite outcome. The principal finding was that early postoperative cTnT demonstrated moderate discrimination for MACCE (AUC, 0.72 for conventional cTnT and 0.77 for hs‐cTnT) and the safety composite outcome (AUC, 0.66 for conventional cTnT and 0.74 for hs‐cTnT). Second, a conventional cTnT and hs‐cTnT cutoff value of 800 ng/L provided clinically relevant power (LR+ >5) to rule in MACCE and the safety composite outcome. Accordingly, patients with early conventional cTnT and hs‐cTnT levels >800 ng/L were 7 to 12 times more likely to subsequently experience a MACCE or the safety composite outcome in the hospital compared with patients with early cTnT levels ≤800 ng/L.
Strengths and Limitations
The major strength of our study is its sample size. We investigated a large and homogeneous group of consecutive patients undergoing isolated CABG, and our study is larger than previously published work on the prognostic importance of early increases in TnI or cTnT.8, 10 Another strength is that we assessed the prognostic utility of conventional troponin and high‐sensitivity troponin, which have not been previously examined. Finally, we constructed nomograms to allow clinicians to derive posttest probabilities of the primary and secondary outcomes in our study. This facilitates the clinical translation of our work. However, our study has important limitations. First, because there is no consensus about the definition of periprocedural MI,17 the rate of periprocedural MI largely varies depending on the definition used. We used a well‐established definition of periprocedural MI on the basis of CK‐MB measurements,12 which has also been used in contemporary clinical trials in adults undergoing CABG.18 Second, all MIs in our study occurred <72 hours postoperatively, but we did not have details on the exact time of diagnosis for every patient. Nevertheless, when we excluded MI from the safety composite outcome in our sensitivity analyses, there was still an association between cTnT and the safety composite. Therefore, early cTnT appears not only to be an early marker of periprocedural MI but also a useful marker for other postoperative complications.
Context
Multiple studies have examined the association between post‐CABG changes in biomarkers and subsequent midterm or long‐term adverse events. With respect to CK‐MB and TnI measurements within 24 hours of CABG, the largest study was an individual patient data meta‐analysis of 7 cohorts and including 18 908 patients. Small increments in CK‐MB of 5 to 10 times the upper limit of normal were associated with 2.9 times increase in 30‐day mortality (relative risk, 2.98; 95% CI, 1.53–5.80) compared with adults below the upper limit of normal.19 For TnI, an increase of 40 to 100 times above the upper limit of normal was the lowest threshold associated with a significantly increased risk of 30‐day mortality in 2552 patients (relative risk, 3.6; 95% CI, 1.08–12.04) compared with normal values.19 For conventional cTnT, 2 single‐center prospective cohort studies in 847 and 1350 patients suggested considerably stronger associations with clinical outcomes,1, 20 although associations were expressed using different metrics.
There has been less focus on the prognostic utility of early troponin measurements to identify in‐hospital complications after CABG. These complications occur often immediately and need emergent initiation of therapeutic measures to improve the prognosis of patients. Apart from our pilot study in 290 patients, we are only aware of an analysis of 540 patients by Eigel et al,8 which showed that an increase in TnI of 1 ng/L measured immediately after completion of the surgical intervention was associated with a 17‐fold increase in the odds of periprocedural MI or death after CABG. The same group did not perform a comprehensive analysis of the prognostic performance of troponin, and the analysis was based on patients recruited between December 1998 and January 2000. Since then, important changes have occurred in the clinical characteristics and management of patients who undergo CABG and there has been notable progress in the development of cardiac biomarkers, including the more widespread use of hs‐cTnT.
In our study, MECC was used in all operations, which is not the standard approach for CABG surgery. However, this is unlikely to affect the generalizability of our study. First, in a meta‐analysis comparing MECC and ECC,21 MECC was associated with only a small clinically irrelevant reduction of postoperative peak cardiac TnI of −180 ng/L (95% CI, −250 to −120 ng/L). Second, there is no difference in the incidence of MACCE up to 30 days between conventional ECC and off‐pump CABG,22, 23 suggesting that the event rate associated with MECC is likely comparable to conventional ECC.
Preoperative cTnT was measured in a subset of adults in our study, and there was no association between preoperative cTnT and MACCE. This is consistent with previously published observational studies reporting no association of preoperative cTnT with 30‐day major adverse events after CABG24 or with long‐term death after general cardiac surgery.25 These findings are contrasting with recently published findings in the setting of percutaneous coronary intervention, where preprocedural cTnT appears more strongly associated with 1‐ and 3‐year mortality than postprocedural cTnT.26, 27, 28, 29
Our study is a contemporary and detailed analysis of the prognostic performance of conventional and hs‐cTnT assessed 6 to 12 hours after CABG. We found that increments in early cTnT above a cutoff value of 800 ng/L provided clinically relevant power to rule in MACCE and the safety composite outcome. The association between conventional and hs‐cTnT and the safety composite outcome was robust to the exclusion of MI from the definition of the outcome. Therefore, the increase in cTnT associated with an impending periprocedural MI after CABG does not preclude the use of cTnT as a clinically relevant marker of short‐term in‐hospital prognosis beyond mere diagnosis of periprocedural MI.
Implications
Early cTnT elevations may allow for the emergent investigation and management of postoperative complications in patients undergoing CABG. For instance, adults with a high posttest probability of MACCE may be readily investigated with noninvasive imaging, such as bedside echocardiography, to assess for signs of periprocedural MI. Echocardiographic evidence of akinetic or hypokinetic myocardium may guide decision making about surgical reexploration of grafts, emergency coronary angiography, or use of an intra‐aortic balloon pump. Similarly, adults with a high posttest probability of the composite safety outcome may warrant additional monitoring in the intensive care unit or in an intermediate‐care facility to optimize treatment. Early initiation of these interventions may have the potential to improve postoperative outcomes. Given the potential for important and timely interventions in patients undergoing CABG, a randomized controlled trial may be warranted, in which patients are assigned to the use or nonuse of early cTnT to guide clinical management and to discern whether the use of early cTnT for clinical decision making is associated with a lower incidence of MACCE or other complications. Extending the use of early cTnT measurements to patients undergoing other types of cardiac surgery will also require further research on patterns of cTnT elevations postoperatively.24, 25, 30, 31
Conclusion
In conclusion, cTnT levels assessed after 6 to 12 hours after CABG are clinically useful in identifying patients at increased risk of MACCEs and other complications. A carefully designed randomized trial is required to determine whether measurement of early cTnT also may result in meaningful changes in clinical practice and improvement in clinical outcomes.
Sources of Funding
This research was completed, in part, with funding from the Canada Research Chairs Programme.
Disclosures
Peter Jüni has received research grants to the institution from Astra Zeneca, Biotronik, Biosensors International, Eli Lilly, and The Medicines Company; serves as unpaid member of the steering group of trials funded by Astra Zeneca, Biotronik, Biosensors, St Jude Medical, and The Medicines Company; and is also a Tier 1 Canada Research Chair in Clinical Epidemiology of Chronic Diseases. Hendrik Tevaearai receives personal fees from Swiss Cardio Technologies as a part‐time employee. The remaining authors have no disclosures to report.
Supporting information
Table S1. Estimated Probabilities of MACCE and the Safety Composite for Specific cTnT Levels in Increments of 200 ng/L, Stratified by Conventional Versus High Sensitivity Cardiac Troponin T
Table S2. Association of Logistic EuroSCORE, Cardiac Troponin T and Need for Vasopressors With MACCE and Safety Composite
Table S3. Comparison of Observed and Predicted Probabilities
Table S4. Association of Pre‐ and Post‐Operative Cardiac Troponin T With MACCE
Figure S1. Distribution of Early Cardiac Troponin T and Probability of Safety Composite. Risk of in‐House Safety Composite, Shaded Region Represents the Distribution of Conventional or High Sensitivity cTnT, Respectively, Measured 9 Hours After CABG Surgery.
(J Am Heart Assoc. 2018;7:e007743 DOI: 10.1161/JAHA.117.007743.)29487111
References
- 1. Mohammed AA, Agnihotri AK, van Kimmenade RR, Martinez‐Rumayor A, Green SM, Quiroz R, Januzzi JL Jr. Prospective, comprehensive assessment of cardiac troponin T testing after coronary artery bypass graft surgery. Circulation. 2009;120:843–850. [DOI] [PubMed] [Google Scholar]
- 2. White HD. Pathobiology of troponin elevations: do elevations occur with myocardial ischemia as well as necrosis? J Am Coll Cardiol. 2011;57:2406–2408. [DOI] [PubMed] [Google Scholar]
- 3. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD; Writing group on the joint ESC/ACCF/AAH/WHF task force for the universal definition of myocardial infarction, Thygesen K, Alpert JS, White HD, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, Chaitman BA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez‐Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S; ESC Committee for Practice Guidelines (CPG) . Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551–2567. [DOI] [PubMed] [Google Scholar]
- 4. Fellahi JL, Gue X, Richomme X, Monier E, Guillou L, Riou B. Short‐ and long‐term prognostic value of postoperative cardiac troponin I concentration in patients undergoing coronary artery bypass grafting. Anesthesiology. 2003;99:270–274. [DOI] [PubMed] [Google Scholar]
- 5. Januzzi JL, Lewandrowski K, MacGillivray TE, Newell JB, Kathiresan S, Servoss SJ, Lee‐Lewandrowski E. A comparison of cardiac troponin T and creatine kinase‐MB for patient evaluation after cardiac surgery. J Am Coll Cardiol. 2002;39:1518–1523. [DOI] [PubMed] [Google Scholar]
- 6. Baggish AL, MacGillivray TE, Hoffman W, Newell JB, Lewandrowski KB, Lee‐Lewandrowski E, Anwaruddin S, Siebert U, Januzzi JL. Postoperative troponin‐T predicts prolonged intensive care unit length of stay following cardiac surgery. Crit Care Med. 2004;32:1866–1871. [DOI] [PubMed] [Google Scholar]
- 7. Holmvang L, Jurlander B, Rasmussen C, Thiis JJ, Grande P, Clemmensen P. Use of biochemical markers of infarction for diagnosing perioperative myocardial infarction and early graft occlusion after coronary artery bypass surgery. Chest. 2002;121:103–111. [DOI] [PubMed] [Google Scholar]
- 8. Eigel P, van Ingen G, Wagenpfeil S. Predictive value of perioperative cardiac troponin I for adverse outcome in coronary artery bypass surgery. Eur J Cardiothorac Surg. 2001;20:544–549. [DOI] [PubMed] [Google Scholar]
- 9. Fransen EJ, Diris JH, Maessen JG, Hermens WT, van Dieijen‐Visser MP. Evaluation of “new” cardiac markers for ruling out myocardial infarction after coronary artery bypass grafting. Chest 2002;122;1316–1321. [DOI] [PubMed] [Google Scholar]
- 10. Gober V, Hohl A, Gahl B, Dick F, Eigenmann V, Carrel TP, Tevaearai HT. Early troponin T and prediction of potentially correctable in‐hospital complications after coronary artery bypass grafting surgery. PLoS One. 2013;8:e74241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roques F, Michel P, Goldstone AR, Nashef SA. The logistic EuroSCORE. Eur Heart J. 2003;24:881–882. [DOI] [PubMed] [Google Scholar]
- 12. Vranckx P, Cutlip DE, Mehran R, Kint PP, Silber S, Windecker S, Serruys PW. Myocardial infarction adjudication in contemporary all‐comer stent trials: balancing sensitivity and specificity: addendum to the historical MI definitions used in stent studies. EuroIntervention. 2010;5:871–874. [DOI] [PubMed] [Google Scholar]
- 13. Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, Hoh BL, Janis LS, Kase CS, Kleindorfer DO, Lee JM, Moseley ME, Peterson ED, Turan TN, Valderrama AL, Vinters HV; American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia, Council on Cardiovascular Radiology and Intervention, Council on Cardiovascular and Stroke Nursing, Council on Epidemiology and Prevention, Council on Peripheral Vascular Disease, Council on Nutrition, Physical Activity and Metabolism . An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. STS renal complications. http://www.sts.org/sites/default/files/documents/pdf/trainingmanuals/adult2.61/Section_P_COMPLICATIONS.pdf. Accessed January 1, 2017.
- 15. Gelman A. Scaling regression inputs by dividing by two standard deviations. Stat Med. 2008;27:2865–2873. [DOI] [PubMed] [Google Scholar]
- 16. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172; discussion 207‐212. [DOI] [PubMed] [Google Scholar]
- 17. Serruys PW, Silber S, Garg S, van Geuns RJ, Richardt G, Buszman PE, Kelbaek H, van Boven AJ, Hofma SH, Linke A, Klauss V, Wijns W, Macaya C, Garot P, DiMario C, Manoharan G, Kornowski R, Ischinger T, Bartorelli A, Ronden J, Bressers M, Gobbens P, Negoita M, van Leeuwen F, Windecker S. Comparison of zotarolimus‐eluting and everolimus‐eluting coronary stents. N Engl J Med. 2010;363:136–146. [DOI] [PubMed] [Google Scholar]
- 18. Stone GW, Sabik JF, Serruys PW, Simonton CA, Généreux P, Puskas J, Kandzari DE, Morice M‐C, Lembo N, Brown WMI, Taggart DP, Banning A, Merkely B, Horkay F, Boonstra PW, van Boven AJ, Ungi I, Bogáts G, Mansour S, Noiseux N, Sabaté M, Pomar J, Hickey M, Gershlick A, Buszman P, Bochenek A, Schampaert E, Pagé P, Dressler O, Kosmidou I, Mehran R, Pocock SJ, Kappetein AP. Everolimus‐eluting stents or bypass surgery for left main coronary artery disease. N Engl J Med. 2016;375:2223–2235. [DOI] [PubMed] [Google Scholar]
- 19. Domanski MJ, Mahaffey K, Hasselblad V, Brener SJ, Smith PK, Hillis G, Engoren M, Alexander JH, Levy JH, Chaitman BR, Broderick S, Mack MJ, Pieper KS, Farkouh ME. Association of myocardial enzyme elevation and survival following coronary artery bypass graft surgery. JAMA. 2011;305:585–591. [DOI] [PubMed] [Google Scholar]
- 20. Soraas CL, Friis C, Engebretsen KV, Sandvik L, Kjeldsen SE, Tonnessen T. Troponin T is a better predictor than creatine kinase‐MB of long‐term mortality after coronary artery bypass graft surgery. Am Heart J. 2012;164:779–785. [DOI] [PubMed] [Google Scholar]
- 21. Zangrillo A, Garozzo FA, Biondi‐Zoccai G, Pappalardo F, Monaco F, Crivellari M, Bignami E, Nuzzi M, Landoni G. Miniaturized cardiopulmonary bypass improves short‐term outcome in cardiac surgery: a meta‐analysis of randomized controlled studies. J Thorac Cardiovasc Surg. 2010;139:1162–1169. [DOI] [PubMed] [Google Scholar]
- 22. Shroyer AL, Grover FL, Hattler B, Collins JF, McDonald GO, Kozora E, Lucke JC, Baltz JH, Novitzky D; Veterans Affairs Randomized On/Off Bypass Study Group . On‐pump versus off‐pump coronary‐artery bypass surgery. N Engl J Med. 2009;361:1827–1837.19890125 [Google Scholar]
- 23. Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu S, Paolasso E, Straka Z, Piegas LS, Akar AR, Jain AR, Noiseux N, Padmanabhan C, Bahamondes JC, Novick RJ, Vaijyanath P, Reddy S, Tao L, Olavegogeascoechea PA, Airan B, Sulling TA, Whitlock RP, Ou Y, Ng J, Chrolavicius S, Yusuf S; CORONARY Investigators . Off‐pump or on‐pump coronary‐artery bypass grafting at 30 days. N Engl J Med. 2012;366:1489–1497. [DOI] [PubMed] [Google Scholar]
- 24. Petaja L, Rosjo H, Mildh L, Suojaranta‐Ylinen R, Kaukonen KM, Jokinen JJ, Salmenpera M, Hagve TA, Omland T, Pettila V. Predictive value of high‐sensitivity troponin T in addition to EuroSCORE II in cardiac surgery. Interact Cardiovasc Thorac Surg. 2016;23:133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lehrke S, Steen H, Sievers HH, Peters H, Opitz A, Muller‐Bardorff M, Wiegand UK, Katus HA, Giannitsis E. Cardiac troponin T for prediction of short‐ and long‐term morbidity and mortality after elective open heart surgery. Clin Chem. 2004;50:1560–1567. [DOI] [PubMed] [Google Scholar]
- 26. Miller WL, Garratt KN, Burritt MF, Lennon RJ, Reeder GS, Jaffe AS. Baseline troponin level: key to understanding the importance of post‐PCI troponin elevations. Eur Heart J. 2006;27:1061–1069. [DOI] [PubMed] [Google Scholar]
- 27. Prasad A, Rihal CS, Lennon RJ, Singh M, Jaffe AS, Holmes DR Jr. Significance of periprocedural myonecrosis on outcomes after percutaneous coronary intervention: an analysis of preintervention and postintervention troponin T levels in 5487 patients. Circ Cardiovasc Interv. 2008;1:10–19. [DOI] [PubMed] [Google Scholar]
- 28. Ndrepepa G, Colleran R, Braun S, Cassese S, Hieber J, Fusaro M, Kufner S, Ott I, Byrne RA, Husser O, Hengstenberg C, Laugwitz KL, Schunkert H, Kastrati A. High‐sensitivity troponin T and mortality after elective percutaneous coronary intervention. J Am Coll Cardiol. 2016;68:2259–2268. [DOI] [PubMed] [Google Scholar]
- 29. Zanchin T, Raber L, Koskinas KC, Piccolo R, Juni P, Pilgrim T, Stortecky S, Khattab AA, Wenaweser P, Bloechlinger S, Moschovitis A, Frenk A, Moro C, Meier B, Fiedler GM, Heg D, Windecker S. Preprocedural high‐sensitivity cardiac troponin T and clinical outcomes in patients with stable coronary artery disease undergoing elective percutaneous coronary intervention. Circ Cardiovasc Interv. 2016;9:e003202. [DOI] [PubMed] [Google Scholar]
- 30. Nesher N, Alghamdi AA, Singh SK, Sever JY, Christakis GT, Goldman BS, Cohen GN, Moussa F, Fremes SE. Troponin after cardiac surgery: a predictor or a phenomenon? Ann Thorac Surg. 2008;85:1348–1354. [DOI] [PubMed] [Google Scholar]
- 31. Vikenes K, Andersen KS, Melberg T, Farstad M, Nordrehaug JE. Long‐term prognostic value of cardiac troponin I and T versus creatine kinase‐MB mass after cardiac surgery in low‐risk patients with stable symptoms. Am J Cardiol. 2010;106:780–786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Estimated Probabilities of MACCE and the Safety Composite for Specific cTnT Levels in Increments of 200 ng/L, Stratified by Conventional Versus High Sensitivity Cardiac Troponin T
Table S2. Association of Logistic EuroSCORE, Cardiac Troponin T and Need for Vasopressors With MACCE and Safety Composite
Table S3. Comparison of Observed and Predicted Probabilities
Table S4. Association of Pre‐ and Post‐Operative Cardiac Troponin T With MACCE
Figure S1. Distribution of Early Cardiac Troponin T and Probability of Safety Composite. Risk of in‐House Safety Composite, Shaded Region Represents the Distribution of Conventional or High Sensitivity cTnT, Respectively, Measured 9 Hours After CABG Surgery.


