Abstract
Background
Ventricular arrhythmias are common in patients with left ventricular assist devices (LVADs) but are often hemodynamically tolerated. Optimal implantable cardioverter defibrillator (ICD) tachy‐programming strategies in patients with LVAD have not been determined. We sought to determine if an ultra‐conservative ICD programming strategy in patients with LVAD affects ICD shocks.
Methods and Results
Adult patients with an existing ICD undergoing continuous flow LVAD implantation were randomized to standard ICD programming by their treating physician or an ultra‐conservative ICD programming strategy utilizing maximal allowable intervals to detection in the ventricular fibrillation and ventricular tachycardia zones with use of ATP. Patients with cardiac resynchronization therapy (CRT) devices were also randomized to CRT ON or OFF. Patients were followed a minimum of 6 months. The primary outcome was time to first ICD shock. Among the 83 patients studied, we found no statistically significant difference in time to first ICD shock or total ICD shocks between groups. In the ultra‐conservative group 16% of patients experienced at least one shock compared with 21% in the control group (P=0.66). There was no difference in mortality, arrhythmic hospitalization, or hospitalization for heart failure. In the 41 patients with CRT ICDs fewer shocks were observed with CRT‐ON but this was not statistically significant: 10% of patients with CRT‐ON (n=21) versus 38% with CRT‐OFF (n=20) received shocks (P=0.08).
Conclusions
An ultra‐conservative programming strategy did not reduce ICD shocks. Programming restrictions on ventricular tachycardia and ventricular fibrillation zone therapy should be reconsidered for the LVAD population. The role of CRT in patients with LVAD warrants further investigation.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT01977703.
Keywords: cardiac resynchronization therapy, implantable cardioverter defibrillator, left ventricular assist device
Subject Categories: Arrhythmias, Catheter Ablation and Implantable Cardioverter-Defibrillator, Ventricular Fibrillation
Clinical Perspective
What Is New?
This study is the first to prospectively examine the effects of implantable cardioverter‐defibrillator (ICD) programming on the timing and rate of ICD shocks in patients with implanted left ventricular assist devices in a randomized fashion.
The study additionally demonstrates that ICD shocks are common in this group, though in no case did ventricular arrhythmia result in symptoms.
Patients with cardiac resynchronization therapy (CRT) devices were also randomized to CRT ON or OFF, and we observed fewer ICD shocks in patients with CRT ON.
What Are the Clinical Implications?
Currently available maximally conservative programming schema do not appear to be sufficient to meaningfully decrease ICD shocks in the left ventricular assist devices population.
The effect of CRT on ventricular arrhythmias and ICD shocks requires further prospective evaluation.
Pivotal randomized controlled trials have established the role of the implantable cardioverter‐defibrillator (ICD) in the treatment of patients with systolic heart failure.1, 2, 3, 4, 5 Patients with class D advanced heart failure are more likely to die of progressive pump failure than sudden cardiac death,6 and as such ICD therapy has not been recommended in this population.7 However, patients in this population supported with continuous flow left ventricular assist devices (LVAD) are not subject to the same risk of progressive pump failure. For this reason the use of ICD therapy has been generally recommended in patients with heart failure supported by an LVAD.8 Additionally, ventricular arrhythmias (VAs) are common in this population, though their clinical implications are uncertain. The data addressing this question are limited to small, retrospective trials and meta‐analyses.9, 10, 11, 12, 13, 14 Small clinical studies have demonstrated that VAs may result in reduced cardiac performance in patients with LVAD, likely due to reduced right ventricular function.15 However, our own clinical experience, as well as published clinical examples, demonstrate that malignant VAs often result in minimal symptoms in patients with LVAD.16, 17, 18 For this reason, we feel that the traditional ICD programming strategies used in patients without LVAD and the manufacturer restrictions on duration for detection of VAs, are likely inappropriate for the LVAD population. Additionally, while cardiac resynchronization therapy (CRT) has an established role in the treatment of patients with congestive heart failure and intraventricular conduction delay, this effect is thought to be mediated through augmentation of left ventricular function. Once an LVAD is placed, offloading the left ventricle, the role of CRT becomes unclear. We sought to address these 2 areas of uncertainty in the management of cardiac rhythm devices in the LVAD population though a prospective randomized trial examining the effects of an ultra‐conservative (UC) ICD programming strategy as well as CRT pacing.
Methods
Study Design
Patients with an existing ICD undergoing de novo continuous flow LVAD implantation at Vanderbilt University Medical Center were randomized to either a UC ICD programming strategy or programming at the discretion of their treating physician during their index hospitalization. In addition, those patients with a CRT capable ICD were randomized to either have CRT turned OFF or remain ON. Patients with complete atrioventricular block and no escape rhythm (pacemaker dependent) were excluded from CRT randomization. Enrollment and ICD programming occurred after the patient had left the cardiac intensive care unit following LVAD implant but before hospital discharge to minimize the impact of peri‐procedural arrhythmias on outcomes. All patients were followed for a minimum of 6 months after enrollment. Patients were required to have an existing transvenous ICD and had to be a minimum of 18 years of age at the time of enrollment. The primary end point within each randomization was time to first ICD shock. Secondary end points included inappropriate shocks (defined as ICD shock for a rhythm other than ventricular tachycardia or ventricular fibrillation as determined by review of stored electrograms), hospitalization for arrhythmia, implantable cardioverter‐defibrillator hospitalization for congestive heart failure, and death. All patients provided written informed consent. This study was approved by the institutional review board of Vanderbilt University. Participants received no compensation for participation in the study. The trial was designed as a single center pilot study, and as such a strict sample size calculation was not performed. We planned to enroll 80 patients over the course of 2 years based on the clinical volume at our center. Assuming a 25% incidence of ICD shocks, our trial would have 70% power to detect an absolute risk reduction of 15% in ICD shocks with UC programming. The power to detect an effect of CRT therapy was lower than this given the smaller sample size. The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure upon individual requests addressed to the corresponding author.
ICD Programming
Patients randomized to an UC programming strategy underwent changes to tachycardia therapies on their devices with the intention of allowing maximal time to detection of ventricular arrhythmia, as well as treating only rapid tachycardia. Programming parameters are shown in Table 1. No changes were made to tachycardia therapies in patients randomized to the control group. In patients with CRT capable ICDs randomized to CRT‐OFF, CRT (left ventricular pacing) was inactivated provided a reliable baseline rhythm >35 beats per minute (bpm) was present. In the CRT OFF group devices were reprogrammed to DDI 40 or VVI 40 for dual and single chamber devices respectively, minimizing ventricular pacing.
Table 1.
ICD Programming Schema
| Manufacturer | VT Zone Detection | VT Zone Therapy | VF Zone Detection | VF Zone Therapy |
|---|---|---|---|---|
| Medtronic Inc |
Rate: 180 bpm 100 intervals (33 s) to detection |
ATP×5, 25 J×2 |
Rate: 222 bpm 120/160 intervals to detection (32.4 s) |
25 J, 35 J×5 |
| Boston Scientific Inc |
Rate: 180 bpm 30 s to detection |
ATP×8, 21 J, 41 J×6 |
Rate: 220 bpm 15 s to detection |
29 J, 41 J×7 |
| St. Jude Medical |
Rate: 180 bpm 100 intervals (33 s) to detection |
ATP×3, 36 J, 40 J×2 |
Rate: 240 bpm 100 intervals to detection (25 s) |
36 J, 40 J×5 |
ICD programming schema for the three device manufacturers involved in the trial. A number followed by “J” indicates a shock therapy with the number referring to the shock energy in joules (J). ATP indicates anti‐tachycardia pacing; bpm, beats per minute; ICD, implantable cardioverter‐defibrillator; VF, ventricular fibrillation; VT, ventricular tachycardia.
Follow‐Up
Follow‐up data were obtained by in‐office or remote monitoring ICD checks, review of the electronic medical record, phone calls with patients and/or their treating physicians, or direct in‐office visits with the LVAD and advanced heart failure service. Initial follow‐up occurred at 1‐month and then at 6‐month intervals thereafter. Follow‐up continued for all subjects until all patients had been followed for a minimum of 6 months. At each follow‐up interval ICD shocks, hospitalizations for either heart failure or arrhythmia, and mortality were assessed. All ICD shocks were reviewed by the study investigators and adjudicated as either appropriate or inappropriate. Shocks for either sustained ventricular tachycardia (VT) or ventricular fibrillation (VF), as determined by available clinical information, were deemed appropriate. All shock events were reviewed for tachycardia cycle length, zone of therapy, delivery of ATP, acceleration of tachycardia by ATP, and report of patient symptoms.
Statistical Analysis
Baseline characteristics are expressed as median (interquartile range) for continuous variables and frequency (percentage) for categorical variables, respectively. Univariate comparisons were performed using the Pearson chi‐square test for categorical variables or the Wilcoxon Rank‐sum test for continuous variables. Statistical significance was taken as a two‐tailed P≤0.05. Outcomes were assessed using an intention to treat analysis. Time to first ICD shock as well as mortality was assessed using the Kaplan–Meier method for the UC versus control analysis. Statistical analysis was performed using R version 3.4.2 (September 28, 2017).
Results
Eighty‐three patients, recruited from November 2013 to April 2016, were included in the final analysis (44 UC and 39 control). One patient withdrew consent on the day of enrollment and 5 patients were removed within a month of enrollment due to conflicts with other investigations (Figure 1). Median duration of follow‐up was 11 months (4 to 18 interquartile range). The majority of patients were white males. A similar proportion of patients had ischemic and non‐ischemic cardiomyopathy. Twenty of the 83 patients (24%) had an LVAD implanted as destination therapy. Baseline characteristics were similar between the 2 groups as presented in Table 2. Programming in the control group is summarized in Table 3. Of the patients in the control group (n=39), all patients had a VF tachy‐therapy zone active with a median detection rate of 214 bpm [200 to 228] and a median of 16 intervals to detection. Twenty‐six patients (68%) had a VT therapy zone active with median rate of 176 bpm [167 to 181] and 19 intervals to detection. All VT programming zones had ATP therapy active before shocks. Nine patients (24%) had a second VT zone active with median rate of 187 bpm [182 to 188] and 24 intervals to detection. ATP therapy was active in all fast VT (FVT)/VT‐2 zones.
Figure 1.
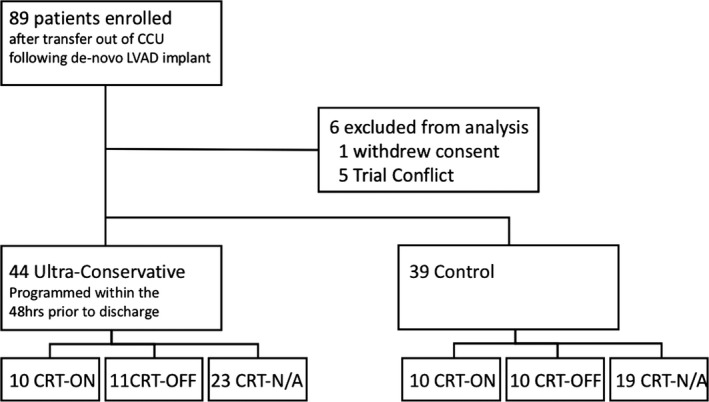
Randomization strategy and enrollment. CCU indicates cardiac critical care unit; CRT, cardiac resynchronization therapy; LVAD, left ventricular assist device.
Table 2.
Baseline Characteristics
| Ultra‐Conservative (N=44) | Control (N=39) | Combined (N=83) | P Value | |
|---|---|---|---|---|
| Age, y | 55 (47 to 62) | 57 (48 to 63) | 56 (48 to 63) | 0.51 |
| Male | 34 (77%) | 31 (82%) | 65 (79%) | 0.63 |
| Ethnicity | 0.68 | |||
| White | 33 (75%) | 30 (79%) | 63 (77%) | |
| Black | 8 (18%) | 7 (18%) | 15 (18%) | |
| Other | 3 (7%) | 1 (3%) | 4 (5%) | |
| Heart failure etiology | 0.35 | |||
| Ischemic | 18 (41%) | 19 (51%) | 37 (46%) | |
| Non‐ischemic | 26 (59%) | 18 (49%) | 44 (54%) | |
| Diabetic | 17 (39%) | 17 (41%) | 34 (40%) | 0.53 |
| CRT pacing | 0.84 | |||
| CRT‐ON | 10 (23%) | 10 (27%) | 20 (25%) | |
| CRT‐OFF | 11 (25%) | 10 (27%) | 21 (26%) | |
| No CRT | 23 (52%) | 19 (46%) | 40 (49%) | |
| Comorbidities | 0.52 | |||
| Prior VT/VF therapy | 4 (9%) | 3 (8%) | 7 (8%) | |
| Arrhythmic syncope | 3 (7%) | 2 (5%) | 5 (6%) | |
| RBBB, LBBB, or IVCD | 11 (25%) | 13 (33%) | 24 (29%) | |
| Atrial fibrillation, flutter, or SVT | 9 (20%) | 3 (8%) | 12 (14%) | |
| Destination LVAD therapy | 11 (25%) | 9 (23%) | 20 (24%) | 0.67 |
All values presented as number of patients followed by percent of group, other than age, which is presented as the median and interquartile range. Wilcoxon rank test used for continuous variables and Pearson test used for ordinal variables. CRT indicates cardiac resynchronization therapy; IVCD, intraventricular conduction delay; LBBB, left bundle branch block; LVAD, left ventricular assist device; RBBB, right bundle branch block; SVT, supraventricular tachycardia; VF, ventricular fibrillation; VT, ventricular tachycardia.
Table 3.
Control Group Programming
| VF zone active | 38 patients (100%) |
| Detection rate | 214 bpm (200 to 228) |
| Intervals to detection | 16 (12 to 24) |
| Shocks active | 38 (100%) |
| ATP active | 31 (82%) |
| VT zone active | 26 patients (68%) |
| Detection rate | 176 (167 to 181) |
| Intervals to detection | 19 (16 to 27) |
| Shocks active | 24 of 26 (92%) |
| ATP active | 26 of 26 (100%) |
| FVT/VT‐2 zone active | 9 patients (24%) |
| Detection rate | 187 (182 to 188) |
| Intervals to detection | 24 (18 to 30) |
| Shocks active | 9 of 9 (100%) |
| ATP active | 9 of 9 (100%) |
Values are presented as the number of patients with each zone active followed by the percentage of the control group (n=38 patients), or the median followed by the interquartile range. ATP indicates anti‐tachycardia pacing; bpm, beats per minute; FVT, fast ventricular tachycardia; VF, ventricular fibrillation; VT, ventricular tachycardia.
UC Programming
No statistically significant difference was seen in the total number of ICD shocks between patients randomized to a UC programming strategy versus control, with 16% of patients in the UC group and 21% in the control group experiencing at least one shock (P=0.66). Additionally, there was no difference in the time to first ICD shock in the Kaplan–Meier analysis (Table 4 and Figure 2). Inappropriate shocks remained common with 6% of patients receiving at least one inappropriate shock. Even in the UC group, subjects received inappropriate shocks for rapid atrial fibrillation despite maximally extended detection when ventricular rate was sustained above the FVT or VF detection limit. Details of shock events are presented in Table 5. The majority of shocks were delivered within the VF therapy zone in both the UC and control group. ATP was frequently delivered (10 of 23 shock events), acceleration was observed once. No differences were observed in the rates of mortality, arrhythmic hospitalization, or hospitalization for congestive heart failure between groups. Five patients (2 UC, 3 control) died within 30 days of LVAD implantation, all during their initial hospitalization. None of these deaths were due to arrhythmia.
Table 4.
Outcomes
| Ultra‐Conservative (N=44) | Control (N=39) | Combined (N=83) | P Value | |
|---|---|---|---|---|
| Patients experiencing ICD shock | 7 (16%) | 8 (21%) | 15 (18%) | 0.66 |
| Patients experiencing inappropriate shocks | 4 (9%) | 1 (3%) | 5 (6%) | 0.35 |
| Patients hospitalized for arrhythmia or CHF | 11 (26%) | 8 (22%) | 19 (24%) | 0.16 |
| Mortality | 8 (18%) | 8 (21%) | 16 (19%) | 0.79 |
| CRT‐ON (n=20) | CRT‐OFF (n=21) | Combined (n=41) | P Value | |
|---|---|---|---|---|
| Patients experiencing ICD shocks | 2 (10%) | 8 (38%) | 10 (24%) | 0.08 |
| Patients experiencing inappropriate shocks | 0 (0%) | 2 (10%) | 2 (5%) | 0.16 |
| Patients hospitalized for arrhythmia | 0 (0%) | 2 (9.5%) | 2 (4.8%) | 0.16 |
| Patients hospitalized for CHF | 5 (25%) | 6 (28%) | 11 (27%) | 0.71 |
All values presented as number of patients followed by percent of group, P‐value calculated using Pearson test. CHF indicates congestive heart failure; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter‐defibrillator.
Figure 2.
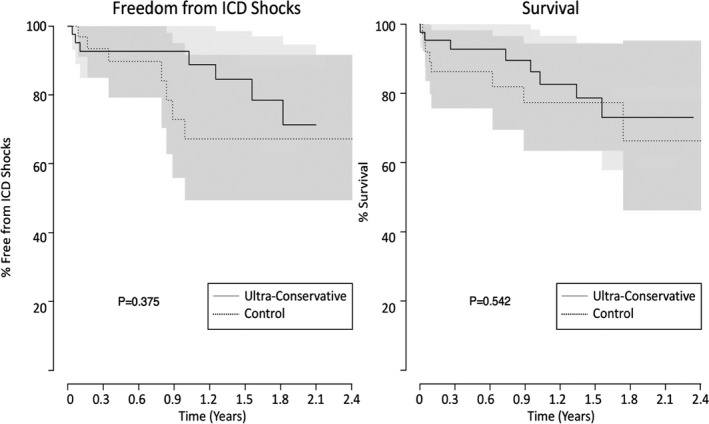
Kaplan–Meier analysis of time to first implantable cardioverter‐defibrillator (ICD) shock as well as survival in patients randomized to an ultra‐conservative ICD programming strategy vs programming at the discretion of their treating physician.
Table 5.
Shock Details
| Therapy zone | Ultra‐Conservative | Control |
|---|---|---|
| Appropriate shocks | ||
| VT/VT‐1 | 0 | 1 |
| Median TCL, ms | NA | 380 |
| FVT/VT‐2 | 2 | 1 |
| Median TCL, ms | 310 | 300 |
| VF | 4 | 9 |
| Median TCL, ms | 235 | 265 |
| ATP delivered (% of events) | 5 (83%) | 2 (18%) |
| Acceleration w/ATP | 1 | 0 |
| Symptomatic | 0 | 0 |
| Inappropriate shocks | ||
| VT/VT‐1 | 0 | 0 |
| Median TCL, ms | NA | NA |
| FVT/VT‐2 | 3 | 0 |
| Median TCL, ms | 310 | NA |
| VF | 2 | 1 |
| Median TCL, ms | 285 | 280 |
| ATP delivered (% of events) | 2 (40%) | 1 (100%) |
| Acceleration w/ATP | 0 | 0 |
| Symptomatic | 0 | 0 |
Values are presented as the number of patients experiencing a shock within each therapy zone. Acceleration refers to acceleration of a tachycardia with ATP, ie, a decrease in average tachycardia cycle length of 10 ms. ATP indicates anti‐tachycardia pacing; TCL, tachycardia cycle length; VF, ventricular fibrillation; VT, ventricular tachycardia.
Cardiac Resynchronization Therapy
No baseline differences were observed in patients with CRT‐ON versus OFF. In patients with CRT‐ON the median percent BiV pacing was 99% [94 to 99%]. We found a nonsignificant trend toward reduction in ICD shocks with CRT‐ON compared with CRT‐OFF, with 10% of patients with CRT‐ON versus 38% with CRT‐OFF receiving at least one shock (P=0.08). No differences were observed in the time to first ICD shock, rates of inappropriate shocks, arrhythmic hospitalization, or hospitalization for congestive heart failure between groups. While inappropriate shocks were rare in the CRT cohort, we did observe an instance of inappropriate shocks for regular SVT in the CRT OFF group when inactivating CRT pacing altered intrinsic QRS morphology leading to a lack of waveform recognition.
Discussion
The role of ICD therapy in patients with LVAD is unclear. Further, optimal programming of these devices is entirely uninvestigated. This trial is the first to prospectively investigate any ICD programming strategy in the LVAD population. We assessed the effect of a UC ICD programming strategy in patients with an existing transvenous ICD undergoing de novo LVAD implantation. While we did not demonstrate a reduction in total ICD shocks delivered, no adverse outcome was seen such as an increase in arrhythmic or heart failure‐related hospitalizations or mortality. Notably, no patient in our trial who received a shock experienced symptoms related to their arrhythmia. As such, more conservative approaches may be safe in the LVAD population and different programming restrictions should be considered for these patients. When designing the UC programming parameters, we were significantly limited by the Food and Drug Administration and manufacturer restrictions. For example, the maximal time to detection in the VT zone that we could program for any manufacturer was 33 s (or 100 intervals at 180 bpm). While this may be appropriate in a patient without LVAD support, in this population it would be preferable to extend this time frame much farther, potentially into the range of minutes to hours. The variation amongst manufacturers in programming limits at the extremes of VF and VT zone therapy largely stems from intellectual property restrictions and established Food and Drug Administration approval of the firmware in ICD generators, which cannot be altered. Opening ICD programming limits to practitioners managing patients with LVAD has the potential to improve quality of life and reduce healthcare utilization in this resource‐intensive population by avoiding ICD shocks, limiting emergency room visits for stable VAs, and potentially extending device longevity.
In the LVAD population with ICDs in place there may be a role for a VA monitoring‐only strategy using remote alerts. With this approach, care providers could be prompted to call patients in sustained VA and evaluate symptoms before determining treatment. Thus, a monitoring‐only strategy would allow VA to be treated similarly to atrial fibrillation, with cardioversions occurring electively in the outpatient setting or observation for spontaneous conversion, which is known to occur even 24 hours after the onset of sustained VT. While ICDs have not been proven to reduce mortality in the LVAD population, there remains a reluctance to turn off all ICD therapies, particularly in patients awaiting heart transplantation. If a robust monitoring‐only strategy was available, this may be a more viable option. As has recently been proposed,19, 20 randomized prospective evaluation of the role of ICD therapy in this population is desperately needed. While this trial does not directly address the question of whether ICD therapy is necessary in the LVAD population, we feel that this work, along with the clinical experience of ourselves and others, supports the safety of such a prospective evaluation.
The benefit of CRT once the left ventricle is hemodynamically supported is also uncertain. Based on our observations, active CRT pacing appears to be protective from VA and ICD shocks. Conversely, turning off the left ventricular lead or disabling CRT may significantly improve CRT‐D longevity and spare the LVAD patient from additional unnecessary ICD generator changes, which themselves carry significant risk in this vulnerable population. As such, which of these strategies is the “right” approach is an important question. Data aimed at addressing this are extremely limited. A single retrospective study suggested that the presence of a CRT‐D as compared with an ICD did not affect mortality, hospitalization rates, or ICD shocks.21 However, a single‐center prospective evaluation of patients with CRT inactivated in a nonrandomized fashion following LVAD implantation did demonstrate decreased incidence of ICD shocks in patients with CRT active.22 An antiarrhythmic effect of CRT, at least in CRT responders, has been demonstrated in patients without LVAD.23 While our investigation did not demonstrate any difference in hospitalizations for congestive heart failure or arrhythmia, fewer ICD shocks were seen in patients with CRT‐ON, possibly in part because of the antiarrhythmic effect of CRT. Notably, when we turned CRT‐OFF the pacing mode was changed to minimize ventricular pacing, as such our study does not address the effect of RV only pacing in this population. Given the importance of RV function in the LVAD population, this is another area that requires further investigation.
Study Limitations
Despite the high volume of LVAD implants at our center, the main limitation of this pilot study is its small sample size, which limits our statistical power to detect small, clinically meaningful differences in outcomes. A multicenter investigation will be necessary to overcome this limitation. Additionally, the majority of shocks in our control group were delivered in the VF zone, this suggests that patients in the control group were already programmed conservatively. This may have limited our ability to observe a clinical difference with the UC programming strategy.
Conclusions
Due to the small sample size in our trial we were not able to demonstrate that a UC ICD programming strategy affected time to ICD shock or reduced the number of total ICD shocks in patients with an LVAD. As the rate of ICD shocks remained high in both groups, it is likely that a strategy well beyond the programming limits of current ICDs, potentially monitor‐only programming, would be necessary to reduce total shocks. Fewer patients in the CRT‐ON arm had shocks compared with CRT‐OFF. While the role of CRT in patients with LVAD remains poorly defined, our study raises the possibility that an antiarrhythmic effect of CRT may exist in the LVAD population.
Overall, as the presence of an LVAD converts even the most malignant VA to one unlikely to result in sudden cardiac death, we feel a traditional ICD programming strategy in this population is far from ideal. Additionally, the role of CRT in the LVAD population is unknown. This pilot study suggests that there is no difference between a UC ICD programming strategy and standard programming in the LVAD population and that CRT may be beneficial for reducing ICD shocks. Based on the results of this pilot study, a larger multi‐center prospective investigation into the optimal tachycardia therapy ICD programming strategy as well as the effect of CRT for LVAD patients is justified. Finally, in the absence of randomized data evaluating the use of ICD therapy in the LVAD population, an incremental step in this direction would be prospective evaluation of a monitoring only strategy.
Sources of Funding
This work was supported equally by Thoratec Inc. (Pleasanton, CA), Heartware International Inc (Framingham, MA), and Boston Scientific Inc. (Marlborough, MA). The sponsors were involved in the approval of the investigators' study design. The sponsors had no role in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Disclosures
Ellis reports research funding from Medtronic, Atricure, Abbott, Boston Scientific, St. Jude Medical, Thoratec, Heartware; Advisory board, consulting: Medtronic, Sentre Heart, Spectranetics, Biosense Webster, Boston Scientific, and Atricure. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2018;7:e007748 DOI: 10.1161/JAHA.117.007748.)29475875
References
- 1. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp‐Channing N, Davidson‐Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH; Sudden Cardiac Death in Heart Failure Trial (SCD‐HeFT) Investigators . Amiodarone or an implantable cardioverter–defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 2. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 3. Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996;335:1933–1940. [DOI] [PubMed] [Google Scholar]
- 4. The AVID Investigators . A comparison of antiarrhythmic‐drug therapy with implantable defibrillators in patients resuscitated from near‐fatal ventricular arrhythmias. N Engl J Med. 1997;337:1576–1584. [DOI] [PubMed] [Google Scholar]
- 5. Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. N Engl J Med. 1999;341:1882–1890. [DOI] [PubMed] [Google Scholar]
- 6. Group M‐HS . Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in‐congestive heart failure (MERIT‐HF). Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 7. Epstein AE, DiMarco JP, Ellenbogen KA, Estes NAM, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device‐based therapy of cardiac rhythm abnormalities. Circulation. 2013;127:e283–e352. [DOI] [PubMed] [Google Scholar]
- 8. Feldman D, Pamboukian SV, Teuteberg JJ, Birks E, Lietz K, Moore SA, Morgan JA, Arabia F, Bauman ME, Buchholz HW, Deng M, Dickstein ML, El‐Banayosy A, Elliot T, Goldstein DJ, Grady KL, Jones K, Hryniewicz K, John R, Kaan A, Kusne S, Loebe M, Massicotte MP, Moazami N, Mohacsi P, Mooney M, Nelson T, Pagani F, Perry W, Potapov EV, Eduardo Rame J, Russell SD, Sorensen EN, Sun B, Strueber M, Mangi AA, Petty MG, Rogers J; International Society for Heart and Lung Transplantation . The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant. 2013;32:157–187. [DOI] [PubMed] [Google Scholar]
- 9. Enriquez AD, Calenda B, Miller MA, Anyanwu AC, Pinney SP. The role of implantable cardioverter‐defibrillators in patients with continuous flow left ventricular assist devices. Circ Arrhythm Electrophysiol. 2013;6:668–674. [DOI] [PubMed] [Google Scholar]
- 10. Vakil K, Kazmirczak F, Sathnur N, Adabag S, Cantillon DJ, Kiehl EL, Koene R, Cogswell R, Anand I, Roukoz H. Implantable cardioverter‐defibrillator use in patients with left ventricular assist devices: a systematic review and meta‐analysis. JACC Heart Fail. 2016;4:772–779. [DOI] [PubMed] [Google Scholar]
- 11. Cantillon DJ, Tarakji KG, Kumbhani DJ, Smedira NG, Starling RC, Wilkoff BL. Improved survival among ventricular assist device recipients with a concomitant implantable cardioverter‐defibrillator. Heart Rhythm. 2010;7:466–471. [DOI] [PubMed] [Google Scholar]
- 12. Garan AR, Yuzefpolskaya M, Colombo PC, Morrow JP, Te‐Frey R, Dano D, Takayama H, Naka Y, Garan H, Jorde UP, Uriel N. Ventricular arrhythmias and implantable cardioverter‐defibrillator therapy in patients with continuous‐flow left ventricular assist devices: need for primary prevention? J Am Coll Cardiol. 2013;61:2542–2550. [DOI] [PubMed] [Google Scholar]
- 13. Agrawal S, Garg L, Nanda S, Sharma A, Bhatia N, Manda Y, Singh A, Fegley M, Shirani J. The role of implantable cardioverter‐defibrillators in patients with continuous flow left ventricular assist devices—a meta‐analysis. Int J Cardiol. 2016;222:379–384. [DOI] [PubMed] [Google Scholar]
- 14. Ambardekar AV, Allen LA, Lindenfeld J, Lowery CM, Cannon AP, Cleveland JC, Brieke A, Sauer WH. Implantable cardioverter‐defibrillator shocks in patients with a left ventricular assist device. J Heart Lung Transplant. 2010;29:771–776. [DOI] [PubMed] [Google Scholar]
- 15. Cantillon DJ, Saliba WI, Wazni OM, Kanj M, Starling RC, Tang WHW, Wilkoff BL. Low cardiac output associated with ventricular tachyarrhythmias in continuous‐flow LVAD recipients with a concomitant ICD (LoCo VT Study). J Heart Lung Transplant. 2014;33:318–320. [DOI] [PubMed] [Google Scholar]
- 16. Healy C, Viles‐Gonzalez JF, Sacher F, Coffey JO, d'Avila A. Management of ventricular arrhythmias in patients with mechanical ventricular support devices. Curr Cardiol Rep. 2015;17:59. [DOI] [PubMed] [Google Scholar]
- 17. Fitzgibbon J, Kman NE, Gorgas D. Asymptomatic sustained polymorphic ventricular tachycardia in a patient with a left ventricular assist device: case report and what the emergency physician should know. J Emerg Med. 2016;50:e135–e141. [DOI] [PubMed] [Google Scholar]
- 18. Oz MC, Rose EA, Slater J, Kuiper JJ, Catanese KA, Levin HR. Malignant ventricular arrhythmias are well tolerated in patients receiving long‐term left ventricular assist devices. J Am Coll Cardiol. 1994;24:1688–1691. [DOI] [PubMed] [Google Scholar]
- 19. Kociol RD. Time for MADIT‐VAD? JACC Heart Fail. 2016;4:780–782. [DOI] [PubMed] [Google Scholar]
- 20. Kutyifa V. Is there a need for an implantable cardioverter defibrillator in patients with left ventricular assist devices? Time for MADIT‐VAD! Expert Rev Med Devices. 2016;14:1–2. [DOI] [PubMed] [Google Scholar]
- 21. Gopinathannair R, Birks EJ, Trivedi JR, McCants KC, Sutton BS, Deam AG, Slaughter MS, Hottigoudar RU. Impact of cardiac resynchronization therapy on clinical outcomes in patients with continuous‐flow left ventricular assist devices. J Card Fail. 2015;21:226–232. [DOI] [PubMed] [Google Scholar]
- 22. Schleifer JW, Mookadam F, Kransdorf EP, Nanda U, Adams JC, Cha S, Pajaro OE, Steidley DE, Scott RL, Carvajal T, Saadiq RA, Srivathsan K. Effect of continued cardiac resynchronization therapy on ventricular arrhythmias after left ventricular assist device implantation. Am J Cardiol. 2016;118:556–559. [DOI] [PubMed] [Google Scholar]
- 23. Barsheshet A, Wang PJ, Moss AJ, Solomon SD, Al‐Ahmad A, McNitt S, Foster E, Huang DT, Klein HU, Zareba W, Eldar M, Goldenberg I. Reverse remodeling and the risk of ventricular tachyarrhythmias in the MADIT‐CRT (Multicenter Automatic Defibrillator Implantation Trial‐Cardiac Resynchronization Therapy). J Am Coll Cardiol. 2011;57:2416–2423. [DOI] [PubMed] [Google Scholar]


