Abstract
Background
Collagen biomarkers may correlate with incident heart failure (HF) and its subtypes. We hypothesized that circulating procollagen type III N‐terminal propeptide (PIIINP) and collagen type I carboxy‐terminal telopeptide (ICTP) predict incident HF.
Methods and Results
We used a stratified sampling design in a multiethnic sample of 3187 subjects, initially aged 45 to 84 years and free of cardiovascular disease. We assayed baseline serum PIIINP and ICTP concentrations using radioimmunoassay. Incident HF was adjudicated, distinguishing reduced ejection fraction (HFrEF; EF <45%) from preserved EF (HFpEF; EF ≥45%). The incidence density for HFpEF and HFrEF was computed using Poisson regression per SD for each of PIIINP and ICTP, adjusting in model 1 for age, race, sex, and renal function or in model 2 for these variables plus blood pressure and medication. Mean (SD) ICTP was 3.38±1.77 μg/L, and mean (SD) PIIINP was 5.48±2.04 μg/L. Among the HF cases, 96 were HFrEF and 107 were HFpEF. Neither ICTP nor PIIINP significantly predicted incident HFrEF. The incidence density for HFpEF per 100 people observed for 13 years was 1.65 for low PIIINP (lower 6 octiles) versus 3.00 for higher PIIINP (P=0.002) in model 1 and correspondingly 1.45 versus 2.59 (P=0.003) in model 2. For low ICTP (lower 7 octiles) versus higher ICTP (octile 8), incidence densities were 1.79 versus 3.64 (P=0.002) in model 1 and 1.58 versus 3.12 (P=0.002) in model 2.
Conclusions
High levels of circulating ICTP and PIIINP as collagen biomarkers appear to be associated with incident HFpEF, but not HFrEF.
Keywords: diastolic dysfunction, fibrosis, collagen type I carboxy‐terminal telopeptide, procollagen type III N‐terminal propeptide, systolic dysfunction
Subject Categories: Heart Failure, Epidemiology, Biomarkers, Fibrosis, Race and Ethnicity
Clinical Perspective
What is New?
The value of circulating procollagen type III N‐terminal propeptide and collagen type I carboxy‐terminal telopeptide for risk stratification for heart failure (HF) with preserved ejection fraction (EF) versus HF with reduced EF at long‐term in subjects free of overt cardiovascular disease is relatively unknown.
Elevated collagen biomarkers, especially procollagen type III N‐terminal propeptide, are associated with new onset of HF with preserved EF but not with HF with reduced EF in subjects free of overt cardiovascular disease.
What Are the Clinical Implications?
These findings may be clinically useful in future diagnostic prevention strategies for early risk stratification for HF with preserved EF beyond age, sex, ethnicity, cardiovascular risk factors, and markers of inflammation.
Alterations of the structure and composition of the cardiomyocyte and noncardiomyocyte compartments of the myocardium appear to play a central role in the pathogenesis of heart failure (HF).1, 2, 3 Among these alterations, changes in the quantity and quality of the extracellular matrix, involving the collagen network and associated myocardial remodeling, ultimately lead to deterioration of left ventricular (LV) function and facilitate the development of HF.4, 5, 6, 7, 8, 9 Such changes differ, however, according to the different pathophysiological mechanisms leading to the 2 major subtypes of HF, HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF). HFrEF is a consequence of cardiomyocyte loss from ischemic or other insults, which is accompanied by replacement fibrosis, whereas HFpEF is associated with cardiomyocyte preservation but increased diffuse interstitial fibrosis.9
Circulating procollagen type III N‐terminal propeptide (PIIINP) and collagen type I carboxy‐terminal telopeptide (ICTP), the 2 major collagen types in the heart, reflect collagen synthesis and degradation (PIIINP) and degradation (ICTP); they have been proposed as potentially useful to improve diagnosis, prognosis, and therapy in cardiac diseases associated with HF.10, 11, 12 Most data about the predictive value of collagen biomarkers, however, have been obtained in post–myocardial infarction and/or prevalent HF settings.11, 13, 14, 15, 16
There is limited information available in individuals free of overt cardiovascular disease (CVD) about the association of such collagen biomarkers with the development of HF and its subtypes. Available data come from a single longitudinal study of older adults of mostly European descent, the CHS (Cardiovascular Health Study). In an initial report involving a subset of the CHS cohort, both PIIINP and ICTP, but not procollagen type I carboxy‐terminal propeptide, were significantly associated with HF.17 The small sample size precluded separate evaluation of HF subtypes. This was followed by larger‐scale measurements of serum PIIINP in the CHS cohort study, which validated the independent relationship of this marker with incident HF.18 There was no difference, however, in HFpEF and HFrEF. It remains unclear, however, whether these associations observed late in life apply earlier in the life course or across different race‐ethnic groups. We sought to evaluate the relationship of circulating ICTP and PIIINP with HF subtypes in a multiethnic cohort of middle‐aged to older adults free overt CVD at baseline (2000–2002).
Methods
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing or expanding on the results after application to and approval by the MESA (Multi‐Ethnic Study of Atherosclerosis) Publications and Presentations Committee (described at http://www.mesa-nhlbi.org).
Study Sample
MESA was initiated to investigate the prevalence, correlates, and progression of subclinical CVD in people initially free of overt clinical CVD, radiation‐ or chemotherapy‐treated cancer, other serious major illness, or cognitive impairment in the judgment of the screening interviewer before baseline.19 Between 2000 and 2002, 6814 men and women of white, black, Hispanic, or Chinese race/ethnicity, aged 45 to 84 years, were enrolled. The institutional review boards at all participating centers approved the study, and all participants signed informed consent.
Collagen biomarkers were assessed in a MESA subsample; our data analysis accounted for design features of that substudy. The subsample studied was selected with the original purpose of studying collagen markers and CVD in relation to measurements of a blood pressure (BP) waveform. In the present stratified design, we excluded 1000 randomly selected MESA participants whose blood sample inventory had been depleted in earlier studies, as well as those who were missing the baseline continuous BP waveform measurement (the latter based on the original purpose of the design). We analyzed examination 1 blood samples in all participants with adjudicated CVD before 2011 plus a 56% random sample of all remaining participants, after the previously described exclusions. Sample size was 3151 participants for ICTP and 3187 participants for PIIINP, after exclusions for missing covariates.
Laboratory Measurements
Collagen biomarkers
Blood was drawn after a 12‐hour fast, and EDTA plasma was stored at −70°C. EDTA plasma PIIINP (μg/L) and ICTP (μg/L) were selected as measures reflecting type III and type I collagen, respectively. Assays were performed in the Molecular Epidemiology and Biomarker Research Laboratory (University of Minnesota; under direction of Myron Gross, PhD) using competitive radioimmunoassay kits (UNIQ no. 06099 [ICTP] and UniQ no. 06098 [PIIINP]; Orion Diagnostica, Espoo, Finland). In the PIIINP assay, a known amount of labeled PIIINP and an unknown amount of unlabeled PIIINP in the sample compete for the limited number of high‐affinity binding sites of the antibody. After separating the free antigen, the amount of labeled PIIINP in the sample tube is inversely proportional to the amount of PIIINP in the sample. The description of the ICTP assay is parallel to that for PIIINP, using an ICTP‐specific antibody. The concentrations in unknown samples are obtained from a calibration curve. Coefficients of variation for internal quality control samples during the main runs were 9.3% for PIIINP high control, 16.5% for PIIINP low control, 6.3% for ICTP high control, and 8.8% for ICTP low control.
Lipids and inflammatory markers
Lipids were measured at the Collaborative Studies Clinical Laboratory (Fairview‐University Medical Center, Minneapolis, MN) within 2 weeks of sample collection, using Centers for Disease Control and Prevention/National Heart, Lung, and Blood Institute standards, as previously reported.20 High‐sensitivity C‐reactive protein was measured using the BNII nephelometer (Dade Behring Inc, Deerfield, IL). Interleukin 6 was measured by ultrasensitive ELISA (Quantikine HS Human IL‐6 Immunoassay; R&D Systems, Minneapolis, MN). D‐dimer was measured using an immunoturbidimetric assay on an Sta‐R analyzer (Liatest D‐DI; Diagnostica Stago, Parsippany, NJ). GlycA (a biomarker that reflects integrated concentrations and glycosylation states of several acute‐phase proteins) was assayed using nuclear magnetic resonance on the basis of the LipoProfile test spectra. The methods for inflammatory markers were previously described.20, 21 Glomerular filtration rate was estimated on the basis of serum creatinine calibrated to standards provided by the National Institute of Standards and Technology, using a formula from the Chronic Kidney Disease Epidemiology Collaboration.22 Serum creatinine is measured by rate reflectance spectrophotometry using thin film adaptation of the creatine amidinohydrolase method on the Vitros analyzer (14650; Johnson & Johnson Clinical Diagnostics, Inc, Rochester, NY) at the Collaborative Studies Clinical Laboratory at Fairview‐University Medical Center. The reference range in adult women is 0.4 to 1.1 mg/dL, and the reference range in adult men is 0.5 to 1.2 mg/dL. The laboratory analytical coefficient of variation is 2.2%.
HF Events
As previously described, participants were contacted at 9‐ to 12‐month intervals for 13 years to identify deaths and hospitalizations. HF events were adjudicated by physicians on the basis of medical records.20, 21, 23 We used EF reported in the hospital record to define HFrEF if EF <45% versus HFpEF if EF ≥45%. Sixteen HF events were omitted because there was no EF in their reviewed medical records.
Statistical Analysis
All analyses were weighted inversely to the sampling weight (1 or 0.56, in effect treating each participant who did not have a CVD event before 2011 as representing 1/0.56=1.79 participants). Mean and SD or counts and percentages were calculated for description. We first examined whether any association of PIIINP and ICTP with HFpEF and HFrEF looked linear across quartiles of the predictors. For PIIINP, we found that associations with HFpEF appeared to be of a threshold nature; this observation was consistent in examination of octiles of both PIIINP and ICTP. Therefore, we present the analysis of octiles for prediction of HFpEF. For HFrEF, in which there was no apparent threshold, we computed the relative incidence density (ID) using Poisson regression over a median follow‐up of 13 years per SD for each of PIIINP and ICTP using 2 levels of adjustment: (1) age, race, sex, and estimated glomerular filtration (eGFR); and (2) these variables plus systolic and diastolic BP levels and use of antihypertensive medications. For HFpEF, in which there was an apparent threshold between the sixth and seventh octiles of PIIINP and between the seventh and eighth octiles of ICTP, we performed Poisson regression with the same 2 sets of covariates. We compared IDs of incident HFpEF between the lower 6 octiles of PIIINP (the low PIIINP group) with the upper 2 octiles (the high PIIINP group). We similarly grouped the lower 7 octiles versus the highest octile of ICTP and compared these groups in Poisson regression. To further examine the model fit, we did sensitivity analysis by forming intervals (with equal intervals of 1 μg/L for each of PIIINP and ICTP) and also examined regression with a continuous (linear) term for PIIINP and for ICTP.
The logic of covariate selection was that demographic variables were considered to be confounding for both PIIINP and ICTP. eGFR was included as a confounder because ICTP is a small molecule that is filtered by the kidney, whereas addition of eGFR to the model did not alter the PIIINP findings compared with a model with only demographic covariates. The BP variables were added because they were the only variables among possible mediators that were differential between HFpEF and HFrEF. Additional exploratory models were run, including possibly mediating variables (height, heart rate, body mass index, former and current smoking, diabetes mellitus, total cholesterol, high‐density lipoprotein cholesterol, triglycerides, cholesterol‐lowering medication, high‐sensitivity C‐reactive protein, interleukin 6, D‐dimer, and GlycA). We considered P<0.05 to be noteworthy in general screening of findings. Analyses were performed using PC‐SAS, version 9.4 (SAS Institute, Cary, NC).
Results
There were race/ethnic differences in the occurrence of either form of HF, compared with no HF (χ2, 6 df, P=0.0005) and for sex (χ2, 2 df, P<0.0001). As shown in Table 1, comparing relative incidences of HFpEF (n=107) versus HFrEF (n=96), Chinese participants developed mainly HFpEF and nearly no HFrEF (P=0.001), whereas black participants developed nearly 2 times as much HFrEF as HFpEF (P=0.04). Occurrence of HFpEF and HFrEF was not significantly different between Hispanics and whites (P=0.05 for Hispanics). Occurrence of HFpEF and HFrEF did not differ significantly within male participants (P=0.07), whereas female participants were at higher risk for HFpEF (P=0.001).
Table 1.
Subject Characteristics With HFpEF and With HFrEF
| Characteristics | HFpEF (n=107) | HFrEF (n=96) | P Value for Difference (HFpEF vs HFrEF) |
|---|---|---|---|
| Race | |||
| White (37.0%) | 2.3 | 1.8 | 0.18 |
| Chinese (12.4%) | 1.9 | 0.2 | 0.001 |
| Black (27.4%) | 2.0 | 3.3 | 0.04 |
| Hispanic (23.2%) | 2.5 | 2.0 | 0.05 |
| Sex | |||
| Male (49.0%) | 2.1 | 3.0 | 0.07 |
| Female (51.0%) | 2.3 | 1.2 | 0.001 |
Data are given as percentage incidence. HFpEF indicates heart failure with preserved ejection fraction; and HFrEF, heart failure with reduced ejection fraction.
Table 2 summarizes the CVD risk factors, inflammatory markers, and collagen biomarkers of the groups who developed HFpEF or HFrEF, versus the subjects who did not develop HF. The subjects who developed HFpEF and HFrEF were older at baseline and shared other clinical and inflammatory risk characteristics compared with the subjects who did not develop HF. Reduced eGFR was 12.2% in the HFrEF group compared with 4.8% in those without HF (P=0.005), but it did not differ significantly from the 9.6% low eGFR among those with HFpEF. However, blood lipid levels did not relate significantly to HF. The primary traditional risk variable that differed between HF types was systolic BP, which was higher in the HFpEF group. Like BP and several other variables, baseline PIIINP was significantly higher in subjects with HFpEF than in subjects without HF.
Table 2.
Cardiovascular Risk Factors, Inflammatory Markers, and Collagen Biomarkers in Subjects With No HF, With HFpEF, and With HFrEF (N=3187)a
| Variable | No HF (n=2984) | HFpEF (n=107) | HFrEF (n=96) | P Value for Difference | ||
|---|---|---|---|---|---|---|
| No HF vs HFpEF | No HF vs HFrEF | HFpEF vs HFrEF | ||||
| ICTP, μg/mL | 3.38±0.02 | 3.68±0.15 | 3.68±0.16 | 0.05 | 0.06 | 0.99 |
| PIIINP, μg/mL | 5.47±0.03 | 5.89±0.18 | 5.55±0.19 | 0.03 | 0.68 | 0.20 |
| Age, y | 62.2±0.19 | 69.3±1.21 | 67.2±1.27 | <0.0001 | <0.0001 | 0.23 |
| Age, race/ethnicity, and sex adjusted | ||||||
| SBP, mm Hg | 126.5±0.35 | 137.4±2.32 | 129.3±2.42 | <0.0001 | 0.27 | 0.01 |
| DBP, mm Hg | 71.9±0.17 | 74.3±1.13 | 72.6±1.18 | 0.04 | 0.58 | 0.29 |
| Hypertension, % | 45.1 | 67.4 | 53.0 | <0.0001 | 0.17 | 0.07 |
| BP Rx, % | 33.4 | 52.6 | 37.8 | 0.0004 | 0.43 | 0.05 |
| HR, bpm | 62.9±0.17 | 65.9±1.12 | 63.9±1.17 | 0.008 | 0.40 | 0.21 |
| Height, cm | 166.3±0.11 | 167.1±0.75 | 167.4±0.78 | 0.31 | 0.18 | 0.79 |
| BMI, kg/m2 | 28.1±0.09 | 30.6±0.59 | 29.4±0.62 | <0.0001 | 0.04 | 0.17 |
| LDL‐C, mg/dL | 117.5±0.58 | 110.8±3.81 | 118.1±3.94 | 0.08 | 0.88 | 0.18 |
| HDL‐C, mg/dL | 51.1±0.24 | 48.7±1.61 | 49.3±1.68 | 0.14 | 0.28 | 0.80 |
| Triglycerides, mg/dL | 129.7±1.41 | 144.3±9.29 | 136.8±9.7 | 0.12 | 0.47 | 0.57 |
| Chol Rx, % | 17.2 | 15.6 | 16.6 | 0.72 | 0.89 | 0.88 |
| eGFR, mL/min per 1.73 m2 | 89.2±0.25 | 87.1±1.67 | 86.3±1.74 | 0.22 | 0.10 | 0.72 |
| eGFR <60 mL/min per 1.73 m2, % | 4.8 | 9.6 | 12.2 | 0.06 | 0.005 | 0.47 |
| Diabetes mellitus, % | 11.6 | 25.6 | 22.1 | 0.0003 | 0.008 | 0.53 |
| Current smoking, % | 11.7 | 15 | 18.3 | 0.39 | 0.10 | 0.56 |
| hs‐CRP, mg/L | 3.61±0.1 | 4.72±0.64 | 4.78±0.67 | 0.09 | 0.09 | 0.95 |
| IL‐6, pg/mL | 1.53±0.02 | 2.01±0.14 | 1.92±0.14 | 0.0007 | 0.009 | 0.64 |
| D‐dimer, μg/mL | 0.36±0.01 | 0.56±0.09 | 0.48±0.09 | 0.02 | 0.20 | 0.50 |
| GlycA, μmol/L | 379.8±1.1 | 402.4±7.0 | 397.2±7.3 | 0.002 | 0.02 | 0.61 |
Data are given as mean±SD unless otherwise indicated. Serum ICTP and PIIINP levels are given. BMI indicates body mass index; bpm, beats/min; BP Rx, blood pressure–lowering therapy; Chol Rx, cholesterol‐lowering therapy; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate21; HDL‐C, high‐density lipoprotein cholesterol; HF, heart failure; HFpEF, HF with preserved ejection fraction; HFrEF, HF with reduced ejection fraction; HR, heart rate; hs‐CRP, high‐sensitivity C‐reactive protein; ICTP, collagen type I carboxy‐terminal telopeptide; IL‐6, interleukin 6; LDL‐C, low‐density lipoprotein cholesterol; PIIINP, procollagen type III N‐terminal propeptide; and SBP, systolic blood pressure.
N value varies slightly for some variables: LDL‐C, n=3145; IL‐6, n=3117; D‐dimer, n=3182; GlycA, n=3183.
The Figure shows the relationships of ICTP and PIIINP with HFpEF and with HFrEF, with adjustment for confounders (age, race, sex, and eGFR) in model 1 and adding BP‐related variables in model 2 (model 1+systolic BP, diastolic BP, and BP‐lowering medication). Neither ICTP nor PIIINP significantly predicted incident HFrEF in either model. Assuming for HFpEF that the visual thresholds in the Figure are correct, the ID associated with low PIIINP (lower 6 octiles; cut point, 6.18 μg/mL) versus high PIIINP is 1.65 versus 3.00 per 100 people observed for 13 years (P=0.002) in model 1; in model 2, the corresponding IDs are 1.45 versus 2.59 (P=0.003). The apparent threshold for ICTP was more extreme (low ICTP in the lower 7 octiles; cut point, 4.55 μg/mL), with IDs of 1.79 versus 3.64 (P=0.002) in model 1 and 1.58 versus 3.12 (P=0.002) in model 2. Further adjustment for many other possibly mediating variables did not change the character of the threshold relationships, although the P values comparing above versus below the stated thresholds both had P=0.05 (data not shown).
Figure 1.
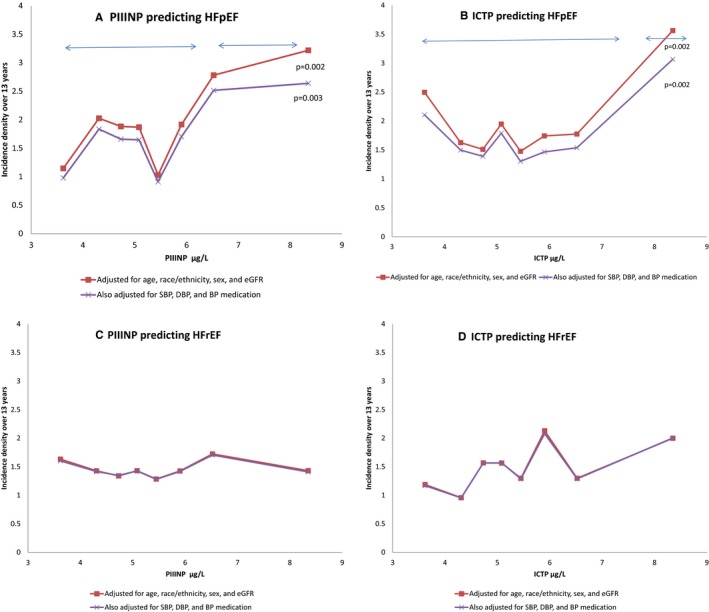
Prediction of incident heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF) from octiles of collagen type I carboxy‐terminal telopeptide (ICTP) and procollagen type III N‐terminal propeptide (PIIINP). Each panel is based on Poisson regression, with 2 levels of adjustment. A and B, PIIINP and ICTP predicting HFpEF. The apparent thresholds above the sixth octile (6.18 μg/mL) for PIIINP and above the seventh octile (4.55 μg/mL) for ICTP are marked, and P values for tests for difference in the incidence densities between low and high predictor values are shown. C and D, PIIINP and ICTP predicting HFrEF. No associations were found. The 2 models are nearly indistinguishable (in C and D). BP indicates blood pressure; DBP, diastolic BP; eGFR, estimated glomerular filtration rate; and SBP, systolic BP.
Sensitivity analyses over equal 1‐μg/L intervals supported the previously described interpretations, especially for PIIINP. Across a range of low PIIINP of ≈3 to 6 μg/L, the variability in HFpEF risk appeared to fluctuate randomly. However, when the highest 25% of participants were split into smaller categories centered at 7, 8, 9, and 12 μg/L, IDs increased to >3 and 5.6 HFpEF events per 100 people observed for 13 years (the highest category based on only 56 people, ≈1.8% of the sample). This agreement with the octile analysis was further supported by linear regression, in which the relative ID per SD of PIIINP was 1.31 (P=0.03) after restricting the covariates by backwards regression to age, race/ethnicity, sex, heart rate, systolic BP, use of antihypertensive medication, body mass index, current smoking, diabetes mellitus, and GlycA. Parallel sensitivity analyses for ICTP did not find this consistency of increasing risk in the highest equal interval categories of ICTP, but did agree with the octile analysis that HFpEF risk was somewhat higher among those with the highest ICTP values, compared with lower values. No association was seen with HFrEF in sensitivity analyses.
Discussion
Although many variables correlated with incident HF, our study found few variables that distinguished HFpEF from HFrEF. These included little HFrEF in Chinese individuals and women, primarily HFrEF in blacks, and higher levels of systolic BP in people who developed HFpEF than in those who developed HFrEF. Both PIIINP and ICTP appeared to distinguish HFpEF from those without HF, both in threshold functions (ie, only when their circulating concentrations were high and consistency across different analysis strategies was greater for PIIINP than for ICTP). However, neither PIIINP nor ICTP predicted HFrEF.
There is limited information about the association of collagen biomarkers and HF in apparently healthy subjects. The FHS (Framingham Heart Study) measured plasma PIIINP concentration at baseline in 967 subjects (mean age, 56 years; and 60% women) free of prior myocardial infarction and HF.24 They found no association of plasma PIIINP with LV structure and function assessed by echocardiography. In the CHS, incident HF was studied in relation to PIIINP in 2568 subjects (mean age, 78 years) during 14 years of follow‐up.18 The hazard ratio of PIIINP for HF was 1.13 per 1.78 μg/L (95% confidence interval, 1.06–1.21; P<001), with adjustment for age, race, sex, and clinic; in a second model adjusted for the demographics and CVD risk factors, the hazard ratio was 1.08 (95% confidence interval, 1.01–1.16; P=0.03). In a smaller subgroup of the CHS (880 participants; mean age, 77 years), serum levels of carboxyl‐terminal peptide of procollagen type I, ICTP, and PIIINP were measured in 4 groups: HFrEF (n=146), HFpEF (n=175), controls with CVD risk factors but not HF (n=280), and healthy controls free of CVD (n=279).17 In healthy controls with CVD risk factors, ICTP was associated with incident HF, whereas in participants with HFpEF, ICTP was associated with hospitalization for HF. No collagen biomarker was associated with hospitalization for HF in participants with HFrEF, and carboxyl‐terminal peptide of procollagen type I was not associated with outcome in the cohort or its subgroups. Zhang et al recruited 782 subjects at random (mean age, 50.5 years) from FLEMENGHO (Flemish Study on Environment, Genes, and Health Outcomes).25 They assessed diastolic LV function from the early and late diastolic peak velocities of the transmitral blood flow and of the mitral annulus: 182 participants (23.3%) had subclinical diastolic LV dysfunction, on the basis of age‐specific echocardiographic criteria. They measured serum carboxyl‐terminal peptide of procollagen type I, ICTP, and PIIINP. By sequencing urinary peptides, they identified 70 urinary collagen fragments, urinary collagen I and collagen III fragments. Carboxyl‐terminal peptide of procollagen type I, ICTP, and PIIINP were associated with diastolic LV dysfunction. The serum collagen biomarkers increased in relation to urinary collagen fragments.
Our data suggest that renal function, CVD risk factors, inflammation, and fibrosis are key players for which a model of independent effects is not a good description (ie, there is joint action in relation to the development of HFpEF). Collagen turnover occurs for normal maintenance of the different organs and tissues and is primarily regulated by fibroblasts during normal physiological functions. Under pathologic conditions, morphologically distinct cells, myofibroblasts, appear. These cells are defined by their dual functions: fibroblast like, in terms of extracellular matrix synthesis and smooth muscle; myocyte like, in terms of migration. Myofibroblast‐mediated collagen turnover is regulated by autocrine and paracrine factors generated within the myocardium and by endocrine hormones derived from the circulation.26 In the case of disrupted tissue, elastin is replaced by collagen, repairing tissue with adequate, but less functional, tissue. Both forms of collagen activity in pathologic features result in procollagen fragments that circulate. Thus, the blood is a sink for both normal collagen activity and collagen repair activity. The large amount of collagen turnover activity in normal physiological functions dampens the signal from circulating collagen biomarkers related to repair activity. Furthermore, the influence of repair activity in the circulating collagen biomarkers is competitive, depending on the differential amounts of collagen needed for repair activity in the different organs.
We previously reported that neither ICTP nor PIIINP tracked over 10 years (each had close to zero correlation with itself after 10 years).23 We speculate that this lack of correlation relates to the concept of flare‐up, with increased repair activity when needed. In a repair episode, less functional tissue is formed, which may be a disadvantage for long‐term health, long after the collagen flare‐up has subsided. For all these reasons, circulating collagen biomarkers may only weakly mark repair activity, especially for repair activity restricted to a single organ. Nevertheless, both ICTP and PIIINP were strongly correlated with both death and chronic inflammatory‐related severe hospitalization and death, as we have described in MESA.23 In this context, we examined HF with particular focus on HFpEF versus HFrEF. PIIINP and ICTP were related to incident HFpEF (threshold association appearing in quartile 4 of PIIINP and in octile 8 of ICTP), but neither related to incident HFrEF.
Interpretation of collagen biomarker studies is complicated by the nature of the circulating collagen biomarker molecules, because these markers are imperfect representations of fibrotic repair processes. The amount in the blood depends on the source. There may be substantial fibrosis in small tissue beds that does not influence the level of ICTP or PIIINP in the blood. Nevertheless, the scenario that we believe is operating in this case is reasonable and consistent with the current theory about HFpEF.26
Current knowledge on the pathogenesis of diastolic HF predominantly rests on case‐control studies involving symptomatic patients with preserved EF and relying on invasive diagnostic procedures, including endomyocardial biopsy. Diastolic tissue indexes derived from echocardiographic measurements have been associated with the degree of collagen expression and cross‐linking studied in endomyocardial biopsy specimens obtained in subjects with HFpEF.27 A novel paradigm for HFpEF has been described in which comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation.26
The strength of our study is the large, well‐characterized, community‐based multiethnic population sample and the availability of a diverse range of adjustment variables to minimize confounding in multivariable models. Although this study is limited to 2 collagen biomarkers, other CVD risk factors and inflammatory markers were taken into consideration for adjustment. It is likely that use of multiple biomarkers will be valuable for prediction of incident HF of the outcome of prevalent HF.
In conclusion, among asymptomatic subjects free of overt CVD, PIIINP and ICTP have potential to predict HFpEF. A caution is that the evidence presented is stronger for high PIIINP than for ICTP. Neither marker was useful in the prediction of HFrEF. Our study potentially adds to the understanding of the cause of HFpEF. Further studies are warranted to explore the value of the collagen biomarkers within a broader scope of biomarkers of HF.
Sources of Funding
MESA (Multi‐Ethnic Study of Atherosclerosis) was supported by contracts N01‐HC‐95159, N01‐HC‐95160, N01‐HC‐95161, N01‐HC‐95162, N01‐HC‐95163, N01‐HC‐95164, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168, and N01‐HC‐95169 from the National Heart, Lung, and Blood Institute and by grants UL1‐TR‐000040 and UL1‐TR‐001079 from the National Center for Research Resources (the last 2 are Clinical and Translational Science Institute acknowledgements from Columbia and Johns Hopkins University). Collagen biomarkers were funded by R01 HL098382 from the National Heart, Lung, and Blood Institute (Jacobs and Duprez).
Disclosures
None.
(J Am Heart Assoc. 2018;7:e007885 DOI: 10.1161/JAHA.117.007885.)29475876
References
- 1. López B, González A, Díez J. Circulating biomarkers of collagen metabolism in cardiac diseases. Circulation. 2010;121:1645–1654. [DOI] [PubMed] [Google Scholar]
- 2. Díez J, Laviades C, Mayor G, Gil MJ, Monreal I. Increased serum concentrations of procollagen peptides in essential hypertension: relation to cardiac alterations. Circulation. 1995;91:1450–1456. [DOI] [PubMed] [Google Scholar]
- 3. Poulsen SH, Andersen NH, Heickendorff L, Mogensen CE. Relation between plasma amino‐terminal propeptide of procollagen type III and left ventricular longitudinal strain in essential hypertension. Heart. 2005;91:624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weber KT. Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol. 1989;13:1637–1652. [DOI] [PubMed] [Google Scholar]
- 5. Valiente‐Alandi I, Schafer AE, Blaxall BC. Extracellular matrix‐mediated cellular communication in the heart. J Mol Cell Cardiol. 2016;91:228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takawale A, Sakamuri SS, Kassiri Z. Extracellular matrix communication and turnover in cardiac physiology and pathology. Compr Physiol. 2015;5:687–719. [DOI] [PubMed] [Google Scholar]
- 7. López B, González A, Ravassa S, Beaumont J, Moreno MU, San José G, Querejeta R, Díez J. Circulating biomarkers of myocardial fibrosis: the need for a reappraisal. J Am Coll Cardiol. 2015;65:2449–2456. [DOI] [PubMed] [Google Scholar]
- 8. Janicki JS, Brower GL, Gardner JD, Chancey AL, Stewart JA Jr. The dynamic interaction between matrix metalloproteinase activity and adverse myocardial remodeling. Heart Fail Rev. 2004;9:33–42. [DOI] [PubMed] [Google Scholar]
- 9. Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57:891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zannad F, Rossignol P, Iraqi W. Extracellular matrix fibrotic markers in heart failure. Heart Fail Rev. 2010;15:319–329. [DOI] [PubMed] [Google Scholar]
- 11. Zannad F, Alla F, Dousset B, Perez A, Pitt B; RALES Investigators . Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES). Circulation. 2000;102:2700–2706. [DOI] [PubMed] [Google Scholar]
- 12. Zile MR, Baicu CF. Biomarkers of diastolic dysfunction and myocardial fibrosis: application to heart failure with a preserved ejection fraction. J Cardiovasc Transl Res. 2013;6:501–515. [DOI] [PubMed] [Google Scholar]
- 13. Iraqi W, Rossignol P, Angioi M, Fay R, Nuée J, Ketelslegers JM, Vincent J, Pitt B, Zannad F. Extracellular cardiac matrix biomarkers in patients with acute myocardial infarction complicated by left ventricular dysfunction and heart failure: insights from the Eplerenone Post‐Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) study. Circulation. 2009;119:2471–2479. [DOI] [PubMed] [Google Scholar]
- 14. Klappacher G, Franzen P, Haab D, Mehrabi M, Binder M, Plesch K, Pacher R, Grimm M, Pribill I, Eichler HG, Glogar HD. Measuring extracellular matrix turnover in the serum of patients with idiopathic or ischemic dilated cardiomyopathy and impact on diagnosis and prognosis. Am J Cardiol. 1995;75:913–918. [DOI] [PubMed] [Google Scholar]
- 15. Martos R, Baugh J, Ledwidge M, O'Loughlin C, Murphy NF, Conlon C, Patle A, Donnelly SC, McDonald K. Diagnosis of heart failure with preserved ejection fraction: improved accuracy with the use of markers of collagen turnover. Eur J Heart Fail. 2009;11:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kitahara T, Takeishi Y, Arimoto T, Niizeki T, Koyama Y, Sasaki T, Suzuki S, Nozaki N, Hirono O, Nitobe J, Watanabe T, Kubota I. Serum carboxy‐terminal telopeptide of type I collagen (ICTP) predicts cardiac events in chronic heart failure patients with preserved left ventricular systolic function. Circ J. 2007;71:929–935. [DOI] [PubMed] [Google Scholar]
- 17. Barasch E, Gottdiener JS, Aurigemma G, Kitzman DW, Han J, Kop WJ, Tracy RP. Association between elevated fibrosis markers and heart failure in the elderly: the Cardiovascular Health Study. Circ Heart Fail. 2009;2:303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Agarwal I, Glazer NL, Barasch E, Biggs ML, Djousse L, Fitzpatrick AL, Gottdiener JS, Ix JH, Kizer JR, Rimm EB, Sicovick DS, Tracy RP, Mukamal KJ. Fibrosis‐related biomarkers and incident cardiovascular disease in older adults: the cardiovascular health study. Circ Arrhyth Electrophysiol. 2014;7:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi‐Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 20. Duprez DA, Otvos J, Tracy RP, Feingold KR, Greenland P, Gross MD, Lima JA, Mackey RH, Neaton JD, Sanchez OA, Jacobs DR. High‐density lipoprotein subclasses and noncardiovascular, noncancer chronic inflammatory‐related events versus cardiovascular events: the Multi‐Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2015;4:e002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duprez DA, Otvos J, Sanchez OA, Mackey RH, Tracy R, Jacobs DR Jr. Comparison of the predictive value of GlycA and other biomarkers for total death, incident cardiovascular events, noncardiovascular and noncancer inflammatory‐related events. Clin Chem. 2016;62:1020–1031. [DOI] [PubMed] [Google Scholar]
- 22. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD‐EPI Investigators . Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duprez DA, Gross MD, Sanchez OA, Kizer JR, Ix JH, Lima J, Tracy RP, Jacobs DR Jr. Collagen turnover markers in relation to future cardiovascular and noncardiovascular disease: the multi‐ethnic study of atherosclerosis. Clin Chem. 2017;63:1237–1247. [DOI] [PubMed] [Google Scholar]
- 24. Wang TJ, Larson MG, Benjamin EJ, Siwik DA, Safa R, Guo CY, Corey D, Sundstrom J, Sawyer DB, Colucci WS, Vasan RS. Clinical and echocardiographic correlates of plasma procollagen type III amino‐terminal peptide levels in the community. Am Heart J. 2007;154:291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang ZY, Ravassa S, Yang WY, Petit T, Pejchinovski M, Zürbig P, López B, Wei FF, Pontillo C, Thijs L, Jacobs L, González A, Koeck T, Delles C, Voigt JU, Verhamme P, Kuznetsova T, Díez J, Mischak H, Staessen JA. Diastolic left ventricular function in relation to urinary and serum collagen biomarkers in a general population. PLoS One. 2016;11:e0167582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 27. Kasner M, Westermann D, Lopez B, Gaub R, Escher F, Kühl U, Schultheiss HP, Tschöpe C. Diastolic tissue Doppler indexes correlate with the degree of collagen expression and cross‐linking in heart failure and normal ejection fraction. J Am Coll Cardiol. 2011;57:977–985. [DOI] [PubMed] [Google Scholar]


