Abstract
Background
The associations between high‐sensitivity troponin I (hsTnI) levels and coronary artery disease (CAD) severity and progression remain unclear. We investigated whether there is an association between hsTnI and angiographic severity and progression of CAD and whether the predictive value of hsTnI level for incident cardiovascular outcomes is independent of CAD severity.
Methods and Results
In 3087 patients (aged 63±12 years, 64% men) undergoing cardiac catheterization without evidence of acute myocardial infarction, the severity of CAD was calculated by the number of major coronary arteries with ≥50% stenosis and the Gensini score. CAD progression was assessed in a subset of 717 patients who had undergone ≥2 coronary angiograms >3 months before enrollment. Patients were followed up for incident all‐cause mortality and incident cardiovascular events. Of the total population, 11% had normal angiograms, 23% had nonobstructive CAD, 20% had 1‐vessel CAD, 20% had 2‐vessel CAD, and 26% had 3‐vessel CAD. After adjusting for age, sex, race, body mass index, smoking, hypertension, diabetes mellitus history, and renal function, hsTnI levels were independently associated with the severity of CAD measured by the Gensini score (log 2 ß=0.31; 95% confidence interval, 0.18–0.44; P<0.001) and with CAD progression (log 2 ß=0.36; 95% confidence interval, 0.14–0.58; P=0.001). hsTnI level was also a significant predictor of incident death, cardiovascular death, myocardial infarction, revascularization, and cardiac hospitalizations, independent of the aforementioned covariates and CAD severity.
Conclusions
Higher hsTnI levels are associated with the underlying burden of coronary atherosclerosis, more rapid progression of CAD, and higher risk of all‐cause mortality and incident cardiovascular events. Whether more aggressive treatment aimed at reducing hsTnI levels can modulate disease progression requires further investigation.
Keywords: atherosclerosis, coronary angiography, coronary artery disease, troponin
Subject Categories: Mortality/Survival, Angiography
Clinical Perspective
What Is New?
High levels of high‐sensitivity cardiac troponin I are associated with more severe coronary artery disease and its accelerated angiographic progression.
In patients with coronary artery disease, high‐sensitivity cardiac troponin I levels can be used to risk stratify and predict future cardiovascular outcomes.
What Are the Clinical Implications?
High‐sensitivity cardiac troponin I is potentially a surrogate biomarker to monitor therapeutic responses in patients with coronary artery disease.
Whether interventions aimed at reducing its levels are associated with improved outcomes requires further investigations.
Although coronary artery disease (CAD) may be present early in life, its progression over time is highly unpredictable. Coronary atherosclerosis can progress at variable rates, ranging from a gradual increase in luminal narrowing to an abrupt progression to total luminal occlusion, the latter often as a result of disruption of a vulnerable nonstenotic plaque attributable to rupture or erosion and subsequent thrombosis.1 Thus, CAD progression may be silent and gradual or sudden and catastrophic, leading to acute coronary syndrome or death.2 However, it appears that rapid CAD progression, whether “silent” or associated with clinical manifestations, is an independent predictor of adverse events.3
Availability of the new generation of high‐sensitivity assays enables detection of low concentrations of circulating cardiac troponins. In contrast to the conventional troponin assays, the high‐sensitivity troponin I (hsTnI) assay detects circulating troponin I in most patients with stable CAD in the absence of myocardial necrosis. High levels of circulating hsTnI or high‐sensitivity troponin T have been associated with prevalent obstructive coronary atherosclerosis and with adverse incident cardiovascular events in patients with stable CAD or the general and elderly population.4, 5, 6, 7, 8, 9 hsTnI levels have been shown to be associated with CAD severity quantified by angiograms and atherosclerotic plaque burden using computed tomographic angiography in relatively small studies.9, 10, 11, 12 However, the relationship between hsTnI levels and CAD progression has not been studied to date. Moreover, whether the predictive value of hsTnI for cardiovascular outcomes is independent of CAD severity remains unknown.
In this study, we investigated the association between plasma hsTnI levels and the following: (1) the angiographic severity of CAD, (2) the progression of CAD, and (3) whether the predictive value of hsTnI levels for incident cardiovascular events is independent of CAD severity.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Design and Population
We studied 3087 adults ≥18 years from the Emory Cardiovascular Biobank, a prospective cohort of patients undergoing left‐sided heart catheterization for suspected or confirmed CAD at 3 Emory healthcare sites in Atlanta, GA, between 2003 and 2015. Subjects with congenital heart disease, heart transplantation, severe anemia, and cancer were excluded. Participants were interviewed to collect demographic characteristics, medical history, medication use, and behavioral habits, as previously described.13 Risk factor prevalence was determined by physician diagnosis and/or treatment for hypertension, hyperlipidemia, and diabetes mellitus. Blood pressure, heart rate, weight, and height were measured. Chronic kidney disease was defined as estimated glomerular filtration rate <60 mL/min per 1.73 m2 (estimated glomerular filtration rate calculated using the Chronic Kidney Disease Epidemiology Collaboration equation) or urine albumin/creatinine ratio >30 mg/g. Medical records and International Classification of Diseases, Ninth Revision (ICD‐9) diagnostic codes were reviewed to confirm self‐reported medical history. The study complies with the Declaration of Helsinki and was approved by the institutional review board at Emory University (Atlanta, GA). All subjects provided written informed consent at enrollment.
Identification of CAD and Severity Scoring
All coronary angiograms were scored for luminal narrowing using a modified American Heart Association/American College of Cardiology classification.14 Patients were designated as having no CAD, nonobstructive CAD (visible plaque resulting in <50% luminal stenosis), or significant CAD (at least 1 major epicardial vessel with ≥50% stenosis). The severity of their CAD was calculated on the basis of angiographic disease of their native vessels before revascularization. Those with a history of coronary artery bypass grafting or percutaneous coronary intervention were all labeled as having significant CAD. Quantitative angiographic scoring was performed using the Gensini score, which quantifies CAD severity by a nonlinear point system for degree of luminal narrowing, along with a multiplier for specific coronary tree locations. For example, 1 point is equivalent to a 25% lesion in the right coronary artery. The score has prognostic significance.15 Severity of CAD was also quantified by number of major epicardial coronary arteries with ≥50% stenosis.
CAD Progression
A subset of 717 patients who had undergone ≥2 coronary angiograms at least 3 months before enrollment were identified, and the 2 angiograms furthest apart in time were quantified using the Gensini score. The net change in angiographic score was divided by number of years between the angiograms to calculate the Gensini progression rate/year. For example, development of a new 75% stenosis in the right coronary artery in an angiogram performed after 1 year will be associated with a Gensini change of 4 points.
Sample Collection and Measurement of hsTnI Level
Fasting arterial blood samples were collected at cardiac catheterization and stored at −80°C. High‐sensitivity troponin I (hsTnI) was measured using the Abbott ARCHITECT analyzer (Abbott Laboratories, North Chicago, IL), which has a limit of detection of 1.2 pg/mL and an interassay coefficient of variation of <10% at 4.7 pg/mL. The upper reference limit (99th centile) ranges between 24 and 30 pg/mL in healthy populations, with a sex‐specific upper reference range of 36 pg/mL for men and 15 pg/mL for women.16, 17, 18, 19, 20 Samples were centrifuged twice before analysis and according to manufacturer's instructions. Serum high‐sensitivity C‐reactive protein (hs‐CRP) levels were determined in 2127 patients using a particle‐enhanced immunoturbidimetry assay (FirstMark, a division of GenWay Biotech) that has a lower limit of detection of 0.03 mg/L.21
Follow‐Up and Outcomes
We conducted follow‐up, as previously described, to identify prespecified incident adverse cardiovascular outcomes, including death, cardiovascular death, myocardial infarction (MI), coronary revascularizations, hospitalizations attributable to cardiac causes, and combined major adverse cardiovascular events, which include all‐cause mortality, MI, and coronary revascularization.13 Follow‐up was conducted by telephone, electronic medical record review, social security death index, and state records, and adjudication was conducted by personnel blinded to the hsTnI data.
Statistical Analysis
Subject characteristics were reported as descriptive statistics, with means, medians, SDs, and ranges. Differences between groups were assessed using the t test for continuous variables and χ2 for categorical variables, where appropriate. Two‐tailed P≤0.05 was considered statistically significant. For nonnormally distributed variables, such as hsTnI and CRP levels, comparisons between 2 groups were performed with the Mann‐Whitney U test. Differences between ≥3 groups were assessed using Kruskal‐Wallis tests in unadjusted analyses. For multivariable analyses, hsTnI levels were examined as both categorical variables stratified by quartiles (ranges: quartile 1, 0–2.7; quartile 2, 2.8–4.4; quartile 3, 4.5–7.8; and quartile 4, 7.9–35) and continuous variables. Characteristics incorporated in multivariable analyses included age, sex, race, body mass index, smoking history, hypertension, diabetes mellitus, estimated glomerular filtration rate, statin use, antiplatelet therapy, angiotensin pathway antagonist use, β‐blocker therapy, and hs‐CRP levels, when available. Spearman correlation coefficients were used to assess the relationship between hsTnI levels and angiographic CAD severity. A linear regression model was used for independent predictors of CAD severity and progression quantified by the Gensini rate. Regression coefficients are presented as point estimates with 95% confidence intervals (CIs). The Kaplan‐Meier curves as well as the Cox proportional‐hazards regression model were used to examine the association between hsTnI and all‐cause death, cardiovascular death, MI, revascularization, hospitalizations, and major adverse cardiovascular events. Analysis was conducted using available data (9% with missing data) under the assumption of missing completely at random. Analyses were performed using IBM SPSS Statistics Version 22 (IBM, Armonk, NY) and R 3.2.2 (R Core Team, Vienna, Austria). Last, we examined the incremental value of adding hsTnI levels to a clinical model for predicting death. The C‐statistic, continuous net reclassification improvement, and integrated discrimination improvement were calculated to evaluate the improvement in predictive ability of the models with and without hsTnI using the R packages survC1 and survIDINRI.
Results
Baseline characteristics of the 3087 patients, aged 63±12 years, by CAD severity are included in Table 1.
Table 1.
Baseline Characteristics
| Characteristics | Normal Angiogram (n=345) | Nonobstructive CAD (n=720) | 1‐Vessel CAD (n=611) | 2‐Vessel CAD (n=615) | 3‐Vessel CAD (n=796) | P Valuea |
|---|---|---|---|---|---|---|
| Age, mean (SD), y | 56.9 (11.5) | 61.1 (12.1) | 64.3 (11.5) | 65.5 (10.2) | 66.4 (10.3) | <0.001 |
| Male sex, n (%) | 152 (44.1) | 379 (52.6) | 401 (65.6) | 435 (70.7) | 622 (78.1) | <0.001 |
| Black race, n (%) | 79 (22.9) | 172 (23.9) | 94 (15.4) | 88 (14.3) | 103 (12.9) | <0.001 |
| Body mass index, mean (SD), kg/m2 | 30.7 (7.4) | 30.1 (6.9) | 29.6 (5.6) | 29.3 (6) | 29.3 (5.7) | 0.181 |
| Estimated GFR, mean (SD), mL/min per 1.73 m2 | 82.2 (19.9) | 78.3 (20.8) | 73.6 (21.5) | 71.9 (21.8) | 70.7 (21) | <0.001 |
| Smoking, n (%) | 195 (56.5) | 453 (62.9) | 441 (72.2) | 424 (68.9) | 553 (69.5) | <0.001 |
| Diabetes mellitus, n (%) | 62 (18) | 196 (27.6) | 215 (35.7) | 199 (32.4) | 333 (42.2) | <0.001 |
| Hypertension, n (%) | 222 (64.5) | 487 (67.9) | 488 (80) | 496 (80.7) | 639 (80.6) | <0.001 |
| Hyperlipidemia, n (%) | 178 (51.7) | 426 (59.4) | 473 (77.5) | 467 (76.4) | 658 (82.9) | <0.001 |
| History of stroke, n (%) | 11 (3.2) | 56 (7.8) | 58 (9.6) | 60 (9.8) | 90 (11.5) | 0.001 |
| History of heart failure, n (%) | 40 (11.6) | 157 (21.8) | 165 (27) | 143 (23.3) | 228 (28.6) | <0.001 |
| Ejection fraction, mean (SD), % | 58 (9.2) | 56.2 (10.5) | 55 (11) | 55 (10) | 52 (12) | <0.001 |
| History of myocardial infarction, n (%) | 5 (1.5) | 44 (6.3) | 136 (22.7) | 162 (27) | 293 (37.7) | <0.001 |
| History of PCI, n (%) | 6 (1.7) | 60 (8.3) | 336 (55) | 407 (66.2) | 545 (68.5) | <0.001 |
| History of CABG, n (%) | 2 (0.6) | 26 (3.6) | 77 (12.6) | 163 (26.5) | 481 (60.4) | <0.001 |
| ACE/ARB use, n (%) | 131 (38) | 332 (46.1) | 360 (58.9) | 390 (63.4) | 528 (66.3) | <0.001 |
| Aspirin use, n (%) | 167 (48.4) | 425 (59) | 494 (80.9) | 535 (87) | 671 (84.3) | <0.001 |
| Clopidogrel use, n (%) | 9 (2.6) | 75 (10.4) | 325 (53.2) | 415 (67.5) | 485 (60.9) | <0.001 |
| Statin use, n (%) | 134 (38.8) | 383 (53.2) | 464 (75.9) | 522 (84.9) | 671 (84.3) | <0.001 |
| β‐Blocker use, n (%) | 148 (42.9) | 362 (50.3) | 409 (66.9) | 459 (74.6) | 594 (74.6) | <0.001 |
| hs‐CRP, median (IQR), mg/dL | 2.55 (1–5.3) | 3.2 (1.3–6.33) | 2.4 (1–6.2) | 2.4 (1–5.4) | 2.3 (1–6) | 0.006 |
| hsTnI, median (IQR), pg/mL | 2.9 (1.8–4.3) | 3.6 (2.2–6) | 4.3 (2.7–7.7) | 5.1 (3–8.6) | 5.7 (3.4–9.8) | <0.001 |
ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; CAD, coronary artery disease; GFR, glomerular filtration rate; hs‐CRP, high‐sensitivity C‐reactive protein; hsTnI, high‐sensitivity troponin I; IQR, interquartile range; and PCI, percutaneous coronary intervention.
P value compares patients with CAD.
Relationship Between hsTnI Levels and Angiographic CAD Severity
hsTnI levels were detected in almost all patients (99.9%) and were lowest in subjects without significant native CAD (median, 3.3 pg/mL; interquartile range [IQR], 2.2–6 pg/mL) compared with those with 1‐vessel CAD (median, 4.3 pg/mL; IQR, 2.7–7.7 pg/mL), 2‐vessel CAD (median, 5.1 pg/mL; IQR, 3–8.6 pg/mL), or 3‐vessel CAD (median, 5.7 pg/mL; IQR, 3.4–9.8 pg/mL) (P<0.0001) (Figure 1A). Similarly, hsTnI levels were associated with severity of CAD, measured by the Gensini score (ρ=0.26, P<0.00001) (Figure 1B). Thus, prevalence of 3‐vessel CAD was greater in those with hsTnI in the highest versus lowest quartile (34.6% versus 13.2%; P<0.001). Bivariate correlations between continuous variables and CAD severity are demonstrated in Table 2. In multivariable analyses, independent predictors of worse CAD severity, measured by Gensini score, included age, sex, race, body mass index, diabetes mellitus, hypertension, hyperlipidemia, history of MI, antiplatelets, statin use, β‐blocker use, and hsTnI levels (Table 3). For each doubling in hsTnI levels, there was a 35% increase (95% CI, 1.19–1.52; P<0.0001) in the risk of higher CAD severity. In contrast, hs‐CRP was not independently associated with angiographic CAD severity (P=0.56). In subgroups analysis, high‐density lipoprotein was negatively associated with more severe CAD (β=−0.27; 95% CI −0.37 to −0.17; P<0.0001), whereas low‐density lipoprotein was not associated with CAD severity (P=0.54). hsTnI remained significantly associated with CAD severity, despite adjusting for low‐ and high‐density lipoproteins (P<0.0001).
Figure 1.
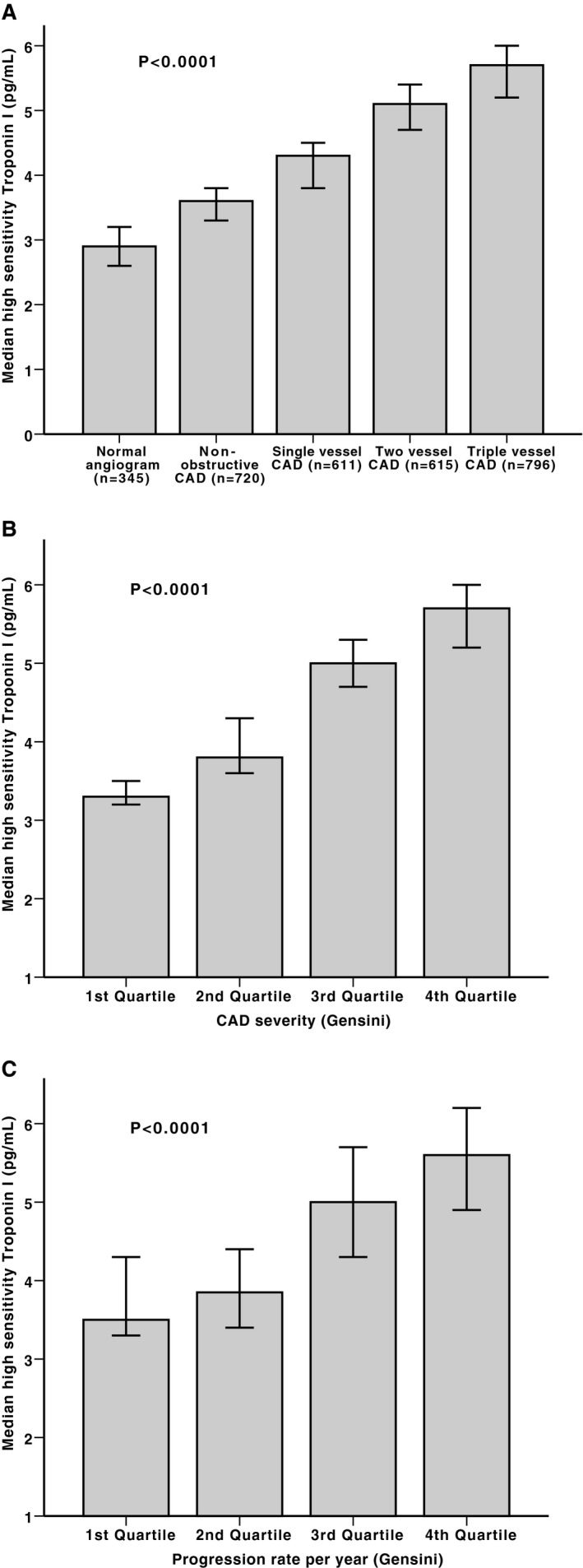
Boxplots demonstrating the correlation between high‐sensitivity troponin I levels and prevalent coronary artery disease (CAD) severity measured as number of obstructed vessels (A), prevalent CAD severity measured by Gensini score quartiles (B), and CAD progression scores measured as quartiles of Gensini progression rate (C). P values for trend.
Table 2.
Bivariate Correlations Between Continuous Variables and CAD Severity (Gensini Score) and Progression Rate (Gensini Progression Rate per Year)
| Variable | CAD Severity | CAD Progression Rate | ||
|---|---|---|---|---|
| ρ | P Value | ρ | P Value | |
| Age | 0.23 | <0.0001 | 0.06 | 0.104 |
| Body mass index | −0.04 | 0.061 | −0.09 | 0.018 |
| High‐density lipoprotein | −0.18 | <0.0001 | −0.05 | 0.201 |
| Low‐density lipoprotein | −0.16 | <0.0001 | −0.03 | 0.47 |
| Estimated GFR | −0.17 | <0.0001 | −0.05 | 0.197 |
| hs‐CRP level | −0.06 | 0.003 | −0.04 | 0.276 |
| hsTnI level | 0.26 | <0.0001 | 0.20 | <0.0001 |
CAD indicates coronary artery disease; GFR, glomerular filtration rate; hs‐CRP, high‐sensitivity C‐reactive protein; and hsTnI, high‐sensitivity troponin I.
Table 3.
Linear Regression Model for the Predictors of CAD Severity (Log 2 Gensini Score)
| Variable | β | Lower CI | Upper CI | OR (95% CI) | P Value |
|---|---|---|---|---|---|
| Myocardial infarction history | 2.34 | 2.04 | 2.65 | 10.4 (7.67–14.11) | <0.0001 |
| Male sex | 1.36 | 1.1 | 1.63 | 3.91 (3.01–5.08) | <0.0001 |
| Antiplatelet therapy | 1.09 | 0.76 | 1.42 | 2.98 (2.14–4.16) | <0.0001 |
| Statin use | 0.95 | 0.64 | 1.27 | 2.59 (1.89–3.55) | <0.0001 |
| Hyperlipidemia | 0.87 | 0.59 | 1.16 | 2.39 (1.8–3.18) | <0.0001 |
| Diabetes mellitus | 0.59 | 0.33 | 0.86 | 1.81 (1.38–2.36) | <0.0001 |
| β‐Blocker | 0.58 | 0.3 | 0.86 | 1.79 (1.35–2.36) | <0.0001 |
| Age (10‐y increase) | 0.40 | 0.03 | 0.05 | 1.49 (1.03–1.05) | <0.0001 |
| hsTnI (log 2) | 0.30 | 0.17 | 0.42 | 1.35 (1.19–1.52) | <0.0001 |
| Hypertension | 0.29 | −0.02 | 0.59 | 1.33 (0.98–1.81) | 0.063 |
| Smoking history | 0.17 | −0.08 | 0.43 | 1.19 (0.92–1.54) | 0.185 |
| Angiotensin pathway antagonist | 0.05 | −0.22 | 0.32 | 1.05 (0.81–1.38) | 0.7 |
| GFR (10‐U increase) | −0.05 | −0.01 | 0.001 | 0.95 (0.99–1) | 0.121 |
| Heart failure history | −0.11 | −0.4 | 0.19 | 0.9 (0.67–1.21) | 0.47 |
| BMI (5‐U increase) | −0.12 | −0.04 | −0.003 | 0.89 (0.96–0.99) | 0.02 |
| Black race | −0.68 | −1.01 | −0.35 | 0.51 (0.36–0.71) | <0.0001 |
BMI indicates body mass index; CAD, coronary artery disease; CI, confidence interval; GFR, glomerular filtration rate; hsTnI, high‐sensitivity troponin I; and OR, odds ratio.
Relationship Between hsTnI Levels and Angiographic Progression of CAD
Of the 3087 patients enrolled, 717 had coronary angiography at least 3 months before enrollment. Patients who had prior angiograms were more likely to be older and male, have a higher prevalence of diabetes mellitus and hyperlipidemia, have worse renal function, and were more likely to have a history of coronary intervention (Table 4). hsTnI levels were not different between those with and without previous angiograms. The median length of time between angiograms was 3.8 years (IQR, 1.7–6.6 years). Bivariate correlations between CAD progression rate and continuous variables are shown in Table 2. There was a significant correlation between the hsTnI levels and the progression of CAD measured as Gensini rate/year (ρ=0.2, P<0.0001) (Figure 1C). Progression rate was 3.6‐fold higher in those with hsTnI in the highest versus lowest quartile (1.5 versus 5.4; P<0.001). However, hs‐CRP levels did not correlate with the Gensini rate of progression (P=0.28).
Table 4.
Adjusted Cox Regression Model for the Association Between hsTnI Levels and Incident Events
| Variable | Unadjusted HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value |
|---|---|---|---|---|
| Death (n=431) | ||||
| hsTnI (100% increase) | 1.68 (1.56–1.82) | <0.0001 | 1.53 (1.39–1.69) | <0.0001 |
| Quartile 2 vs 1 | 1.57 (1.12–2.2) | 0.009 | 1.21 (0.84–1.75) | 0.304 |
| Quartile 3 vs 1 | 2.87 (2.11–3.91) | <0.0001 | 1.99 (1.42–2.81) | <0.0001 |
| Quartile 4 vs 1 | 5.02 (3.75–6.73) | <0.0001 | 3.35 (2.39–4.71) | <0.0001 |
| Cardiovascular death (n=239) | ||||
| hsTnI (100% increase) | 1.84 (1.66–2.05) | <0.0001 | 1.71 (1.5–1.95) | <0.0001 |
| Quartile 2 vs 1 | 1.57 (0.96–2.56) | 0.073 | 1.14 (0.67–1.95) | 0.628 |
| Quartile 3 vs 1 | 3.27 (2.11–5.07) | <0.0001 | 2.33 (1.44–3.77) | 0.001 |
| Quartile 4 vs 1 | 6.65 (4.4–10.06) | <0.0001 | 4.30 (2.68–6.9) | <0.0001 |
| MI (n=99) | ||||
| hsTnI (100% increase) | 1.6 (1.36–1.88) | <0.0001 | 1.37 (1.12–1.68) | 0.002 |
| Quartile 2 vs 1 | 2 (0.99–4.04) | 0.053 | 1.47 (0.69–3.13) | 0.313 |
| Quartile 3 vs 1 | 3.15 (1.63–6.1) | 0.001 | 2.01 (0.98–4.11) | 0.057 |
| Quartile 4 vs 1 | 4.76 (2.52–9.01) | <0.0001 | 2.5 (1.22–5.14) | 0.012 |
| Revascularization (n=680) | ||||
| hsTnI (100% increase) | 1.26 (1.18–1.34) | <0.0001 | 1.1 (1.02–1.19) | 0.018 |
| Quartile 2 vs 1 | 1.38 (1.11–1.72) | 0.003 | 1.18 (0.93–1.49) | 0.170 |
| Quartile 3 vs 1 | 1.46 (1.18–1.81) | 0.001 | 1.11 (0.88–1.42) | 0.383 |
| Quartile 4 vs 1 | 2.02 (1.64–2.48) | <0.0001 | 1.34 (1.05–1.71) | 0.019 |
| Combined all‐cause mortality, MI, or coronary revascularization (n=875) | ||||
| hsTnI (100% increase) | 1.36 (1.29–1.44) | <0.0001 | 1.19 (1.11–1.28) | <0.0001 |
| Quartile 2 vs 1 | 1.37 (1.12–1.67) | 0.002 | 1.14 (0.92–1.41) | 0.224 |
| Quartile 3 vs 1 | 1.7 (1.4–2.06) | <0.0001 | 1.25 (1.01–1.55) | 0.042 |
| Quartile 4 vs 1 | 2.5 (2.08–3.01) | <0.0001 | 1.64 (1.32–2.03) | <0.0001 |
| Cardiac hospitalizations (n=1022) | ||||
| hsTnI (100% increase) | 1.35 (1.28–1.42) | <0.0001 | 1.24 (1.17–1.32) | <0.0001 |
| Quartile 2 vs 1 | 1.28 (1.06–1.54) | 0.009 | 1.13 (0.93–1.38) | 0.214 |
| Quartile 3 vs 1 | 1.67 (1.39–1.99) | <0.0001 | 1.35 (1.11–1.64) | 0.003 |
| Quartile 4 vs 1 | 2.5 (2.11–2.96) | <0.0001 | 1.90 (1.56–2.32) | <0.0001 |
Adjusted Cox regression model for age, sex, race, body mass index, smoking, diabetes mellitus, hypertension, hyperlipidemia, heart failure, estimated glomerular filtration rate, statin use, antiplatelet therapy, angiotensin pathway antagonist use, β‐blocker therapy, and coronary artery disease severity (Gensini score). Quartiles ranges are as follows: quartile 1, 0 to 2.7 pg/mL; quartile 2, 2.8 to 4.4 pg/mL; quartile 3, 4.5 to 7.8 pg/mL; and quartile 4, 7.9 to 35 pg/mL. CI indicates confidence interval; HR, hazard ratio; hsTnI, high‐sensitivity troponin I; and MI, myocardial infarction.
Furthermore, after adjustment for clinical variables (age, sex, race, body mass index, smoking, diabetes mellitus, hypertension, hyperlipidemia, heart failure, estimated glomerular filtration rate, statin use, antiplatelet therapy, angiotensin pathway antagonist use, β‐blocker therapy, and CAD severity), the hsTnI level was independently associated with the progression rate (ß=0.36; 95% CI, 0.14–0.58; P=0.001). For each doubling in hsTnI levels, there was a 43% increase (95% CI, 1.15–1.78) in the risk of progression rate. Similar results were observed when CAD progression was defined as >4 Gensini points/year as a cutoff (correlates with new 75% stenosis in the right coronary artery); thus, for each 100% higher level of hsTnI, there was a 40% increase in the likelihood of progression (95% CI, 1.2–1.7; P=0.0001). Other predictors of CAD progression included male sex (hazard ratio [HR], 1.7; 95% CI, 1.1–2.6; P=0.012), baseline Gensini score (HR, 1.10; 95% CI, 1.05–1.16; P=0.0002), and β‐blocker use (HR, 1.7; 95% CI, 1.1–2.7; P=0.015). Subjects with CAD progression (defined as Gensini progression rate >4) had an increased risk of mortality (HR, 1.64; 95% CI, 1.14–2.33; P=0.008), cardiovascular death/MI (HR, 1.92; 95% CI, 1.26–2.93; P=0.002), and major adverse cardiovascular events (HR, 2.01; 95% CI, 1.58–2.55; P<0.001).
Relationship Between hsTnI Levels and Incident Cardiovascular Events
After a mean follow‐up of 5.2±3.1 years, there were 431 deaths (15.5%) from all causes, 239 deaths (8.6%) from cardiovascular causes, 99 incident MIs (3.6%), 680 coronary revascularization procedures (24.3%), and 1022 hospitalizations for cardiac causes (36.6%). In unadjusted analyses, increase in hsTnI level was associated with increased risk of all‐cause death, cardiovascular death, MI, revascularization procedures, cardiac hospitalizations, and major adverse cardiovascular event rate (Table 5). Kaplan‐Meier survival curves for association between hsTnI quartiles and clinical outcomes are shown in Figure 2.
Table 5.
Baseline Characteristics for Patients With and Without Previous Angiograms
| Characteristics | Patients Without Previous Angiogram (n=2370) | Patients With Previous Angiogram (n=717) | P Valuea |
|---|---|---|---|
| Age, mean (SD), y | 63.1 (11.8) | 64.7 (10.4) | <0.0001 |
| Male sex, n (%) | 1480 (62.4) | 509 (71) | <0.0001 |
| Black race, n (%) | 447 (18.9) | 89 (12.4) | <0.0001 |
| Body mass index, mean (SD), kg/m2 | 29.7 (6.2) | 29.9 (6.4) | 0.711 |
| Estimated GFR, mean (SD), mL/min per 1.73 m2 | 75.1 (21.7) | 72.9 (20.5) | 0.004 |
| Smoking, n (%) | 1587 (67) | 479 (66.8) | 0.938 |
| Diabetes mellitus, n (%) | 727 (31) | 278 (38.8) | <0.0001 |
| Hypertension, n (%) | 1786 (75.6) | 546 (76.2) | 0.77 |
| Hyperlipidemia, n (%) | 1639 (69.5) | 563 (78.5) | <0.0001 |
| History of stroke, n (%) | 206 (8.8) | 69 (9.7) | 0.469 |
| History of heart failure, n (%) | 555 (23.4) | 178 (24.8) | 0.438 |
| Ejection fraction, mean (SD), % | 55 (11) | 54 (11) | <0.0001 |
| History of myocardial infarction, n (%) | 367 (15.9) | 273 (38.1) | <0.0001 |
| History of PCI, n (%) | 824 (34.8) | 530 (73.9) | <0.0001 |
| History of CABG, n (%) | 469 (19.8) | 280 (39.1) | <0.0001 |
| ACE/ARB use, n (%) | 1249 (52.7) | 492 (68.6) | <0.0001 |
| Aspirin use, n (%) | 1704 (71.9) | 588 (82) | <0.0001 |
| Clopidogrel use, n (%) | 889 (37.5) | 420 (58.6) | <0.0001 |
| Statin use, n (%) | 1594 (67.3) | 580 (80.9) | <0.0001 |
| β‐Blocker use, n (%) | 1452 (61.3) | 520 (72.5) | <0.0001 |
| hs‐CRP, median (IQR), mg/dL | 2.6 (1.1–6.4) | 2.4 (1–5.7) | 0.185 |
| hsTnI, median (IQR), pg/mL | 4.4 (2.6–7.9) | 4.4 (2.8–7.5) | 0.493 |
ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; GFR, glomerular filtration rate; hs‐CRP, high‐sensitivity C‐reactive protein; hsTnI, high‐sensitivity troponin I; IQR, interquartile range; and PCI, percutaneous coronary intervention.
P value compares patients with and without previous angiogram.
Figure 2.
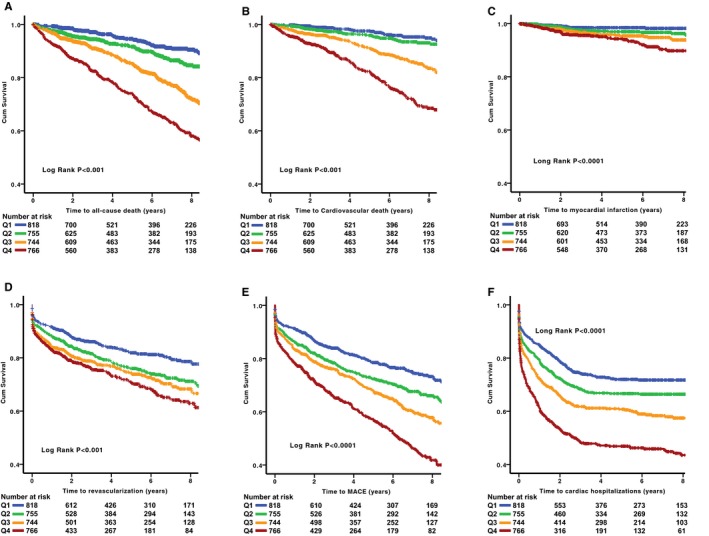
Kaplan‐Meier curves for association between levels of high‐sensitivity troponin I (hsTnI) by quartiles for the primary end point of all‐cause death (A), cardiovascular death (B), incident myocardial infarction (C), revascularization (D) major adverse cardiovascular events (MACEs; E) and cardiac hospitalizations (F). Colored lines represent quartiles (Qs) of hsTnI levels (blue, lowest Q [0–2.7 pg/mL]; green, second Q [2.8–4.4 pg/mL]; orange, third Q [4.5–7.8 pg/mL]; red, highest Q [7.9–35 pg/mL]). Cum indicates cumulative.
After adjusting for demographic and clinical characteristics, as previously detailed, hsTnI levels, both as a continuous variable and in quartiles, remained an independent predictor of all the aforementioned clinical outcomes (Table 5). These results remained significant even after adjusting for high‐density lipoproteins, low‐density lipoproteins, and hs‐CRP levels in a subset of 2127 patients. We also derived an optimal cutoff using Youden's index (sensitivity+specificity−1) for the outcome of cardiovascular death (5.15 pg/mL [57th percentile]), above which the risk of cardiovascular death is as follows: unadjusted HR, 3.9; 95% CI, 3.0 to 5.0 (P<0.0001); adjusted HR, 2.8; 95% CI, 2.1 to 3.8 (P<0.0001) (Table 6). We found no significant heterogeneity in the HR for mortality on the basis of age, sex, race, and presence of individual risk factors like smoking, diabetes mellitus, hypertension, or hyperlipidemia, heart failure, statin use, or conservative versus invasive management. However, we found a significant trend for hsTnI to have better predictive value in those with versus without chronic kidney disease (interaction P=0.03) (Figure 3).
Table 6.
Adjusted Cox Regression Model for the Association Between hsTnI Levels (Using Cutoff of 5.15 pg/mL) and Incident Events
| Variable | Unadjusted | P Value | Adjusted | P Value |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Death (n=431) | 2.93 (2.44–3.52) | <0.001 | 2.14 (1.73–2.65) | <0.001 |
| Cardiovascular death (n=239) | 3.86 (2.97–5) | <0.001 | 2.85 (2.11–3.86) | <0.001 |
| MI (n=99) | 2.73 (1.86–4.02) | <0.001 | 2.00 (1.27–3.10) | 0.002 |
| Revascularization (n=680) | 1.5 (1.3–1.73) | <0.001 | 1.16 (0.98–1.37) | 0.076 |
| Combined all‐cause mortality, MI, or coronary revascularization (n=875) | 1.89 (1.68–2.13) | <0.001 | 1.40 (1.21–1.62) | <0.001 |
| Cardiac hospitalizations (n=1022) | 1.9 (1.69–2.14) | <0.001 | 1.57 (1.37–1.80) | <0.001 |
Adjusted Cox regression model for age, sex, race, body mass index, smoking, diabetes mellitus, hypertension, hyperlipidemia, heart failure, estimated glomerular filtration rate, statin use, antiplatelet therapy, angiotensin pathway antagonist use, β‐blocker therapy, and coronary artery disease severity (Gensini score). CI indicates confidence interval; HR, hazard ratio; hsTnI, high‐sensitivity troponin I; and MI, myocardial infarction.
Figure 3.
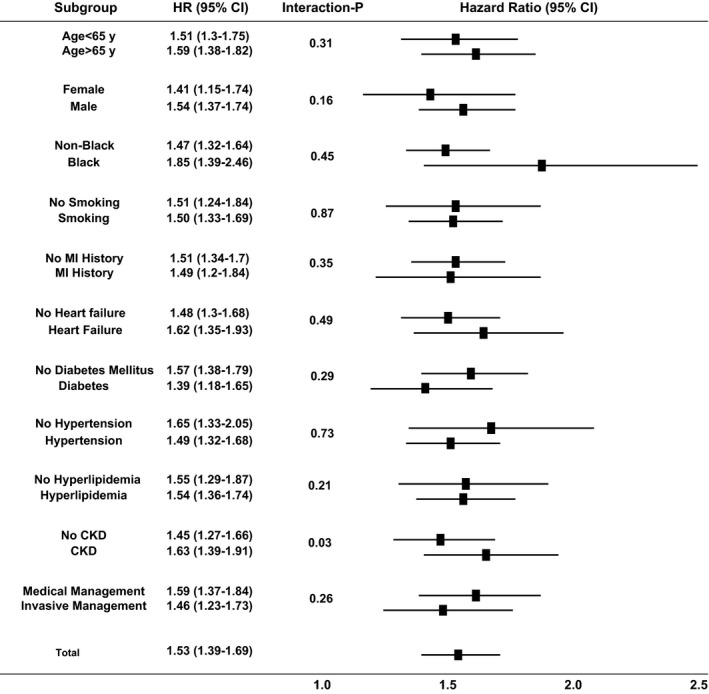
Forest plot interaction of high‐sensitivity troponin I with clinical covariates for the outcomes of death. CI indicates confidence interval; CKD, chronic kidney disease; HR, hazard ratio; and MI, myocardial infarction.
CAD severity, measured by Gensini score, was an independent predictor of cardiovascular death/MI and additive to hsTnI. To assess the incremental prognostic value of hsTnI level in relation to established risk predictors and CAD severity, patients were stratified into groups according to combined strata of CAD severity and hsTnI quartiles (Figure 4). Compared with the group within the lowest hsTnI quartile and normal angiogram (reference group), patients within the highest hsTnI quartile and 3‐vessel CAD had an HR of 11.1 (95% CI, 3.4–36.7) for cardiovascular death/MI (P<0.0001).
Figure 4.
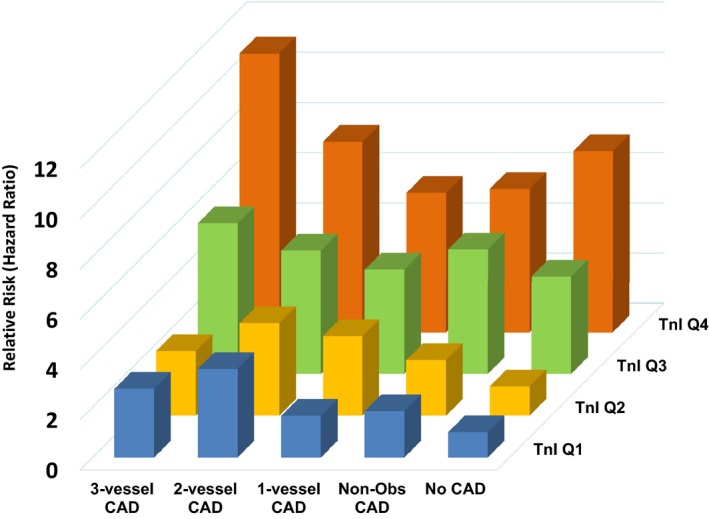
Relative risk (hazard ratio) of cardiovascular death/myocardial infarction in combined strata of coronary artery disease (CAD) severity and high‐sensitivity troponin I quartiles. Non‐Obs indicates nonobstructive; and Tnl, troponin I.
Risk Prediction Performance
We tested the incremental value of adding hsTnI level to a model with significant traditional risk factors and clinical characteristics (including age, sex, race, body mass index, smoking history, hypertension, diabetes mellitus, hyperlipidemia, estimated glomerular filtration rate, history of MI, history of heart failure, and CAD severity) in predicting incident cardiovascular death/MI events. Addition of hsTnI significantly improved the C‐statistic (from 0.683 to 0.710; ∆=0.027 [95% CI, 0.008–0.044]), the continuous net reclassification improvement (0.250 [95% CI, 0.113–0.420]; P=0.02), and the integrated discrimination improvement (0.046 [95% CI, 0.020–0.087]; P=0.02).
Discussion
In this large cohort of patients with suspected or confirmed CAD, we demonstrate that elevated circulating levels of hsTnI are associated with the severity and progression of angiographic CAD and its adverse outcomes. hsTnI levels are higher in those with greater severity of CAD, confirmed by the number of narrowed coronary arteries or angiographic atherosclerotic burden, independent of cardiovascular risk factors. Furthermore, for the first time, we found a robust association between hsTnI and CAD progression. More important, our data showed that hsTnI is a strong predictor of incident mortality and morbidity, independent of and additive to CAD severity. Thus, compared with those in the lowest quartile, patients in the highest quartile of hsTnI levels had an adjusted >4‐fold increased risk of cardiovascular death during follow‐up. hsTnI significantly improved discrimination of future cardiovascular outcomes over a standard clinical model, as evidenced by improvement in the C‐statistic, integrated discrimination improvement, and net reclassification improvement.
Although hsTnI has been extensively studied in populations with and without cardiovascular disease, its relationship to CAD severity has only been explored in few studies with limited numbers of patients. hsTnI was found to be associated with angiographic and computed tomographic CAD severity in few smaller studies.9, 10, 11, 12, 22 We confirmed the association between hsTnI levels and comprehensively analyzed angiographic native CAD severity in a much larger population.
Elevated circulating levels of hsTnI do not necessarily implicate myocardial cell death.23, 24, 25, 26, 27 Increased metabolic demand of the heart can lead to cleavage and release of cardiac hsTnI. In addition, microinjury caused by dislodgement of thrombi in small coronary vessels could be a potential cause for elevated levels of hsTnI and may explain the observation that more severe CAD was associated with higher hsTnI level.12, 28
Another important finding is the association between the elevated hsTnI level and accelerated coronary atherosclerosis. Higher hsTnI levels were associated with progression in terms of both frequency and severity. Those in the highest quartile of hsTnI levels had >3‐fold higher rate of CAD progression. As previously reported, CAD progression was not predicted by hs‐CRP level,29, 30 a finding that may be explained by the fact that the gradual progression of CAD may not be associated with inflammation to the same extent as an abrupt progression of a vulnerable and inflamed plaque. Although previous studies showed the prognostic utility of hsTnI in CAD populations from large trials, including HOPE (Heart Outcomes Prevention Evaluation) and PEACE (Prevention of Events With Angiotensin Converting Enzyme Inhibition), none of them adjusted for the underlying severity of CAD.31, 32 In this cohort of >3000 patients who were carefully phenotyped for CAD severity, we demonstrate strong predictive value of hsTnI levels for all cardiovascular outcomes independent of and additive to CAD severity. Thus, in comparison to those without CAD with hsTnI levels in the lowest quartile, those with 3‐vessel CAD and hsTnI levels in the highest quartile had >11‐fold higher mortality.
Using receiver‐operating curve analysis, we found 5.2 pg/mL to be the optimal cutoff in a population enriched for CAD. Interestingly, this corresponds to the same cutoff identified by a recent study of patients without established CAD.33 hsTnI levels are modifiable by interventions such as statin use, and these changes are predictive of outcomes, as shown in previous studies.4, 33 Therefore, hsTnI could serve as a potential surrogate biomarker to monitor therapeutic responses in patients with CAD. This can be addressed in an adequately powered randomized clinical trial.
Our study has important strengths, including a large cohort size with detailed phenotyping of CAD burden, long‐term follow‐up with a large number of incident cardiovascular events, and exploration of the interaction of hsTnI levels with CAD severity. Although coronary angiography remains the gold standard for documenting the severity of CAD, the selection of patients for repeated angiography and its timing may have introduced bias because only subjects with symptoms were undergoing repeated angiography. This may have selected a cohort with more comorbidities and advanced CAD. As a consequence, we may have overestimated the true overall incidence of CAD progression. Nevertheless, 31% of those who had repeated angiograms had no significant CAD progression and served as a control group and those with retrospective progression had worse clinical outcomes during follow‐up. Because this was a retrospective and observational study, we are unable to establish the association between hsTnI and prospective CAD progression. Given potential selection bias and the paucity of research on CAD progression, this study generates questions that should be answered with large prospective studies.
In conclusion, an elevated hsTnI level is closely associated with more severe CAD, with its accelerated progression, and with incident adverse cardiovascular events, independent of other clinical risk factors and hs‐CRP levels. This study provides additional support for the potential role of hsTnI as a marker of the presence, progression, and outcomes in CAD. Whether more aggressive treatment aimed at reducing hsTnI levels can positively alter disease course requires further investigation.
Sources of Funding
This work was supported partially by Abbott. Quyyumi is supported by National Institutes of Health (NIH) grants 5P01HL101398‐02, 1P20HL113451‐01, 1R56HL126558‐01, 1RF1AG051633‐01, R01 NS064162‐01, R01 HL89650‐01, HL095479‐01, 1U10HL110302‐01, 1DP3DK094346‐01, and 2P01HL086773‐06A1. Samman Tahhan is supported by the Abraham J. & Phyllis Katz Foundation grant (Atlanta, GA) and NIH/National Institute on Aging grant AG051633.
Disclosures
None.
(J Am Heart Assoc. 2018;7:e007914 DOI: 10.1161/JAHA.117.007914.)29467150
References
- 1. Ambrose JA, Tannenbaum MA, Alexopoulos D, Hjemdahl‐Monsen CE, Leavy J, Weiss M, Borrico S, Gorlin R, Fuster V. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol. 1988;12:56–62. [DOI] [PubMed] [Google Scholar]
- 2. Pencina MJ, D'Agostino RB Sr, Larson MG, Massaro JM, Vasan RS. Predicting the 30‐year risk of cardiovascular disease: the Framingham Heart Study. Circulation. 2009;119:3078–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Waters D, Craven TE, Lesperance J. Prognostic significance of progression of coronary atherosclerosis. Circulation. 1993;87:1067–1075. [DOI] [PubMed] [Google Scholar]
- 4. Eggers KM, Venge P, Lindahl B, Lind L. Cardiac troponin I levels measured with a high‐sensitive assay increase over time and are strong predictors of mortality in an elderly population. J Am Coll Cardiol. 2013;61:1906–1913. [DOI] [PubMed] [Google Scholar]
- 5. Everett BM, Brooks MM, Vlachos HE, Chaitman BR, Frye RL, Bhatt DL; BARI 2D Study Group . Troponin and cardiac events in stable ischemic heart disease and diabetes. N Engl J Med. 2015;373:610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neumann JT, Havulinna AS, Zeller T, Appelbaum S, Kunnas T, Nikkari S, Jousilahti P, Blankenberg S, Sydow K, Salomaa V. Comparison of three troponins as predictors of future cardiovascular events: prospective results from the FINRISK and BiomaCaRE studies. PLoS One. 2014;9:e90063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sinning C, Keller T, Zeller T, Ojeda F, Schluter M, Schnabel R, Lubos E, Bickel C, Lackner KJ, Diemert P, Munzel T, Blankenberg S, Wild PS; Gutenberg Health Study . Association of high‐sensitivity assayed troponin I with cardiovascular phenotypes in the general population: the population‐based Gutenberg health study. Clin Res Cardiol. 2014;103:211–222. [DOI] [PubMed] [Google Scholar]
- 8. White HD, Tonkin A, Simes J, Stewart R, Mann K, Thompson P, Colquhoun D, West M, Nestel P, Sullivan D, Keech AC, Hunt D, Blankenberg S; LIPID Study Investigators . Association of contemporary sensitive troponin I levels at baseline and change at 1 year with long‐term coronary events following myocardial infarction or unstable angina: results from the LIPID Study (long‐term intervention with pravastatin in ischaemic disease). J Am Coll Cardiol. 2014;63:345–354. [DOI] [PubMed] [Google Scholar]
- 9. Ndrepepa G, Braun S, Schulz S, Mehilli J, Schomig A, Kastrati A. High‐sensitivity troponin T level and angiographic severity of coronary artery disease. Am J Cardiol. 2011;108:639–643. [DOI] [PubMed] [Google Scholar]
- 10. Laufer EM, Mingels AM, Winkens MH, Joosen IA, Schellings MW, Leiner T, Wildberger JE, Narula J, Van Dieijen‐Visser MP, Hofstra L. The extent of coronary atherosclerosis is associated with increasing circulating levels of high sensitive cardiac troponin T. Arterioscler Thromb Vasc Biol. 2010;30:1269–1275. [DOI] [PubMed] [Google Scholar]
- 11. Schulz O, Reinicke M, Berghoefer GH, Bensch R, Kraemer J, Schimke I, Jaffe AS. High‐sensitive cardiac troponin I (hs‐cTnI) values in patients with stable cardiovascular disease: an initial foray. Clin Chim Acta. 2010;411:812–817. [DOI] [PubMed] [Google Scholar]
- 12. Korosoglou G, Lehrke S, Mueller D, Hosch W, Kauczor HU, Humpert PM, Giannitsis E, Katus HA. Determinants of troponin release in patients with stable coronary artery disease: insights from CT angiography characteristics of atherosclerotic plaque. Heart. 2011;97:823–831. [DOI] [PubMed] [Google Scholar]
- 13. Eapen DJ, Manocha P, Patel RS, Hammadah M, Veledar E, Wassel C, Nanjundappa RA, Sikora S, Malayter D, Wilson PW, Sperling L, Quyyumi AA, Epstein SE. Aggregate risk score based on markers of inflammation, cell stress, and coagulation is an independent predictor of adverse cardiovascular outcomes. J Am Coll Cardiol. 2013;62:329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Neeland IJ, Patel RS, Eshtehardi P, Dhawan S, McDaniel MC, Rab ST, Vaccarino V, Zafari AM, Samady H, Quyyumi AA. Coronary angiographic scoring systems: an evaluation of their equivalence and validity. Am Heart J. 2012;164:547–552.e541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gensini GG. Coronary Arteriography. Mt. Kisco, NY: Futura; 1975. [Google Scholar]
- 16. Apple FS, Ler R, Murakami MM. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin Chem. 2012;58:1574–1581. [DOI] [PubMed] [Google Scholar]
- 17. Keller T, Zeller T, Ojeda F, Tzikas S, Lillpopp L, Sinning C, Wild P, Genth‐Zotz S, Warnholtz A, Giannitsis E, Mockel M, Bickel C, Peetz D, Lackner K, Baldus S, Munzel T, Blankenberg S. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA. 2011;306:2684–2693. [DOI] [PubMed] [Google Scholar]
- 18. Zeller T, Tunstall‐Pedoe H, Saarela O, Ojeda F, Schnabel RB, Tuovinen T, Woodward M, Struthers A, Hughes M, Kee F, Salomaa V, Kuulasmaa K, Blankenberg S; MORGAM Investigators . High population prevalence of cardiac troponin I measured by a high‐sensitivity assay and cardiovascular risk estimation: the MORGAM Biomarker Project Scottish Cohort. Eur Heart J. 2014;35:271–281. [DOI] [PubMed] [Google Scholar]
- 19. Aw TC, Phua SK, Tan SP. Measurement of cardiac troponin I in serum with a new high‐sensitivity assay in a large multi‐ethnic Asian cohort and the impact of gender. Clin Chim Acta. 2013;422:26–28. [DOI] [PubMed] [Google Scholar]
- 20. Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Alkhoder A, Obideen M, Abdelhadi N, Fang S, Ibeanu I, Pimple P, Mohamed Kelli H, Shah AJ, Pearce B, Sun Y, Garcia EV, Kutner M, Long Q, Ward L, Bremner JD, Esteves F, Raggi P, Sheps D, Vaccarino V, Quyyumi AA. Association between high‐sensitivity cardiac troponin levels and myocardial ischemia during mental stress and conventional stress. JACC Cardiovasc Imaging. 2017. Available at: https://www.sciencedirect.com/science/article/pii/S1936878X17301572?via%3Dihub. Accessed January 29, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jeppesen J, Hansen TW, Olsen MH, Rasmussen S, Ibsen H, Torp‐Pedersen C, Hildebrandt PR, Madsbad S. C‐reactive protein, insulin resistance and risk of cardiovascular disease: a population‐based study. Eur J Cardiovasc Prev Rehabil. 2008;15:594–598. [DOI] [PubMed] [Google Scholar]
- 22. Seifarth H, Schlett CL, Lehman SJ, Bamberg F, Donnelly P, Januzzi JL, Koenig W, Truong QA, Hoffmann U. Correlation of concentrations of high‐sensitivity troponin T and high‐sensitivity C‐reactive protein with plaque progression as measured by CT coronary angiography. J Cardiovasc Comput Tomogr. 2014;8:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hogas S, Bilha SC, Branisteanu D, Hogas M, Gaipov A, Kanbay M, Covic A. Potential novel biomarkers of cardiovascular dysfunction and disease: cardiotrophin‐1, adipokines and galectin‐3. Arch Med Sci. 2017;13:897–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feng YJ, Chen C, Fallon JT, Lai T, Chen L, Knibbs DR, Waters DD, Wu AH. Comparison of cardiac troponin I, creatine kinase‐MB, and myoglobin for detection of acute ischemic myocardial injury in a swine model. Am J Clin Pathol. 1998;110:70–77. [DOI] [PubMed] [Google Scholar]
- 25. Suleiman MS, Lucchetti V, Caputo M, Angelini GD. Short periods of regional ischaemia and reperfusion provoke release of troponin I from the human hearts. Clin Chim Acta. 1999;284:25–30. [DOI] [PubMed] [Google Scholar]
- 26. Sabatine MS, Morrow DA, de Lemos JA, Jarolim P, Braunwald E. Detection of acute changes in circulating troponin in the setting of transient stress test‐induced myocardial ischaemia using an ultrasensitive assay: results from TIMI 35. Eur Heart J. 2009;30:162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. White HD. Pathobiology of troponin elevations: do elevations occur with myocardial ischemia as well as necrosis? J Am Coll Cardiol. 2011;57:2406–2408. [DOI] [PubMed] [Google Scholar]
- 28. Rittersma SZ, van der Wal AC, Koch KT, Piek JJ, Henriques JP, Mulder KJ, Ploegmakers JP, Meesterman M, de Winter RJ. Plaque instability frequently occurs days or weeks before occlusive coronary thrombosis: a pathological thrombectomy study in primary percutaneous coronary intervention. Circulation. 2005;111:1160–1165. [DOI] [PubMed] [Google Scholar]
- 29. Wilson PW, Nam BH, Pencina M, D'Agostino RB Sr, Benjamin EJ, O'Donnell CJ. C‐reactive protein and risk of cardiovascular disease in men and women from the Framingham Heart Study. Arch Intern Med. 2005;165:2473–2478. [DOI] [PubMed] [Google Scholar]
- 30. Khera A, de Lemos JA, Peshock RM, Lo HS, Stanek HG, Murphy SA, Wians FH Jr, Grundy SM, McGuire DK. Relationship between C‐reactive protein and subclinical atherosclerosis: the Dallas Heart Study. Circulation. 2006;113:38–43. [DOI] [PubMed] [Google Scholar]
- 31. Kavsak PA, Xu L, Yusuf S, McQueen MJ. High‐sensitivity cardiac troponin I measurement for risk stratification in a stable high‐risk population. Clin Chem. 2011;57:1146–1153. [DOI] [PubMed] [Google Scholar]
- 32. Omland T, Pfeffer MA, Solomon SD, de Lemos JA, Rosjo H, Saltyte Benth J, Maggioni A, Domanski MJ, Rouleau JL, Sabatine MS, Braunwald E; PEACE Investigators . Prognostic value of cardiac troponin I measured with a highly sensitive assay in patients with stable coronary artery disease. J Am Coll Cardiol. 2013;61:1240–1249. [DOI] [PubMed] [Google Scholar]
- 33. Ford I, Shah AS, Zhang R, McAllister DA, Strachan FE, Caslake M, Newby DE , Packard CJ, Mills NL. High‐sensitivity cardiac troponin, statin therapy, and risk of coronary heart disease. J Am Coll Cardiol. 2016;68:2719–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]


