Abstract
Background
Virtually no reports on the effects of exercise in patients with a small abdominal aortic aneurysm (AAA) exist.
Methods and Results
We conducted a retrospective cohort study on 1515 patients with a small AAA before surgery at 2 high‐volume hospitals in Tokyo, Japan, from April 2004 to September 2015. A carefully modified cardiac rehabilitation program without excessive blood pressure elevation during exercise was prescribed to 50 patients with an AAA. Using propensity score matching, mortality and clinical outcomes, including AAA expansion rate, were compared between 2 groups: rehabilitation group and nonrehabilitation group. The background characteristics of the rehabilitation group (n=49) and the nonrehabilitation group (n=163) were almost identical. The risk for AAA repair was much lower in the rehabilitation group after matching (before matching: hazard ratio, 0.43; 95% confidence interval, 0.25–0.72; P=0.001; and after matching: hazard ratio, 0.19; 95% confidence interval, 0.07–0.50; P<0.001). AAA expansion rate was slower in the rehabilitation group (before matching: rehabilitation versus nonrehabilitation group, 2.3±3.7 versus 3.8±3.4 mm/y [P=0.008]; after matching: rehabilitation versus nonrehabilitation group, 2.1±3.0 versus 4.5±4.0 mm/y [P<0.001]). Elevation of blood pressure during exercise was positively correlated with AAA expansion rate after the rehabilitation program (r=0.569, P<0.001).
Conclusions
Cardiac rehabilitation protects against the expansion of small AAAs and mitigates the risk associated with AAA repair, possibly because of the decreased elevation of blood pressure during exercise.
Clinical Trial Registration
URL: upload.umin.ac.jp. Unique identifier: UMIN000028237.
Keywords: abdominal aortic aneurysm, cardiac rehabilitation, exercise training, systolic blood pressure
Subject Categories: Exercise, Aneurysm, Vascular Disease, Mortality/Survival, Secondary Prevention
Clinical Perspective
What Is New?
In patients with a small abdominal aortic aneurysm (AAA), the long‐term effectiveness of a carefully modified cardiac rehabilitation program without excessive blood pressure elevation during exercise was investigated.
Our supervised safely performed cardiac rehabilitation program clearly suppressed the expansion of patients' AAAs.
This may have been because of the stabilization of patients' systolic blood pressure during exercise.
What Are the Clinical Implications?
Prescription of cardiac rehabilitation to patients with preoperative AAAs tends to be avoided, for fear that a patient's blood pressure elevation during exercise may cause expansion or rupture of his or her AAA.
Judging by our present findings, patients with an AAA should participate in a supervised carefully modified cardiac rehabilitation program without excessive blood pressure elevation during exercise, so that AAA expansion can be prevented.
Introduction
The prevalence of abdominal aortic aneurysm (AAA) is 4.0% to 7.6% in whites, according to 4 large population‐based AAA screening randomized controlled trials.1 Atherosclerosis is well known to contribute to the occurrence of AAA, and the major risk factors for AAA2 are identical to those for coronary artery disease (CAD).3 Concordantly, there is a high prevalence of AAA in patients with CAD.4, 5 It is recommended that patients with CAD participate in a cardiac rehabilitation program, which results in ameliorated risk factor profiles and reduced morbidity and mortality.6
How about exercise for patients with AAA? The blood pressure (BP) of patients with AAA should be strictly controlled, to prevent AAA expansion and rupture. As such, some physicians are hesitant to prescribe a rehabilitation program to AAA patients, for fear that BP elevation during exercise may cause AAA expansion or rupture, despite AAA not being regarded as a contraindication for exercise in the guidelines for rehabilitation.5, 7 There are almost no data that indicate that a carefully modified exercise program has a detrimental effect on the prognoses of patients with AAA. Rather, some reports have demonstrated the safety of exercise in patients with AAA and its preventive effect on AAA expansion.8, 9 In particular, Myers et al demonstrated the safety and efficacy of exercise training in patients with small AAAs: no increases in AAA size were observed during the 3‐year follow‐up period.10 An experimental study using an AAA mice model demonstrated the protective effect of exercise on the occurrence of AAA, through the preservation of endothelial integrity, reduction in inflammation and oxidative stress, and inhibition of the osteogenic pathway.11 However, the long‐term effectiveness of cardiac rehabilitation in patients with AAA has not yet been investigated. We hypothesized that a carefully modified relatively mild exercise program, during which high BP is not induced during exercise, would be favorable for patients with AAA. Therefore, in patients with small AAAs, we examined whether such a program had a beneficial effect on their AAA expansion rates, as well as on their risk for AAA repair.
Methods
We declare that all supporting data are available within the article. This study was approved by the ethical committees of The University of Tokyo Hospital (Tokyo, Japan) and the Sakakibara Heart Institute Hospital (Tokyo, Japan). Informed consent was obtained from all patients, according to the protocol approved by both ethical committees.
Study Population
Patients ≥45 years with small AAAs, defined as a maximal diameter of >30 and <55 mm, were retrospectively taken into consideration. A total of 1515 patients were managed at The University of Tokyo Hospital or Sakakibara Heart Institute from April 1, 2004 to September 30, 2015. Exclusion criteria were as follows: patients who underwent AAA repair within 150 days of diagnosis; patients with a short follow‐up of <150 days; patients who had no follow‐up computed tomographic scan; and those diagnosed as having Marfan syndrome, IgG4‐related disease, saccular aneurysm, infectious aneurysm, traumatic aneurysm, inflammatory aneurysm, and congenital aneurysm. After these exclusions, 213 patients with small AAAs remained, and of those, 50 were recruited into the cardiac rehabilitation program for any reason other than AAA (Figure 1).
Figure 1.
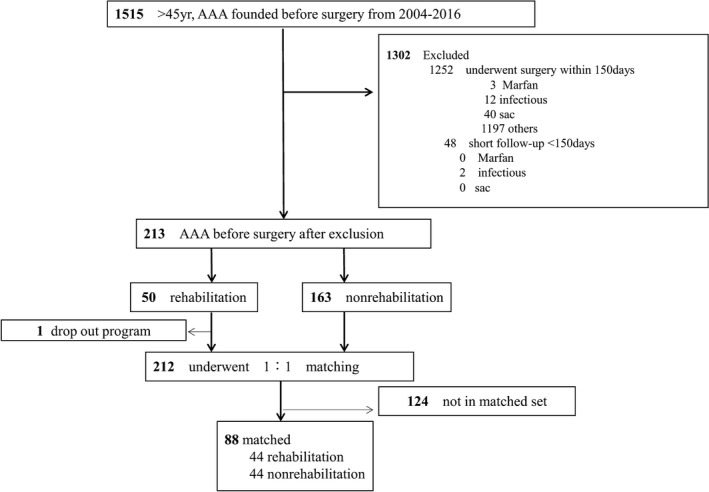
Flow chart of abdominal aortic aneurysm (AAA) patient inclusion and reasons for exclusion. Patients with small AAAs, defined as a maximal diameter of >30 mm and <55 mm, were retrospectively taken into consideration. Of a total 1515 patients, those who underwent AAA repair within 150 days of diagnosis, those with a short follow‐up of <150 days, those who had no follow‐up computed tomographic scan, and those diagnosed as having Marfan syndrome, IgG4‐related disease, saccular aneurysm, infectious aneurysm, traumatic aneurysm, inflammatory aneurysm, or congenital aneurysm were excluded. After these exclusions, 213 patients with small AAAs remained, and of those patients, 49 participated in the cardiac rehabilitation program. Finally, 88 patients remained after propensity score matching.
All patients were Asian. All medical decisions on treatment, including cardiac rehabilitation, were made by the attending physicians without any intervention by the researchers. An initial biomechanical analysis of AAA was performed for the purposes of the research, using a simple aneurysm model, according to a method previously reported.12 Computed tomographic scan data were imported using DICOM viewer and DICOM server software (SonicDICOM; JIUN Co, Japan) and evaluated using a modeling workstation (Osirix 9.0‐DICOM viewer; Pixmeo SARL, Switzerland) to calculate aspect ratios (vertical/horizontal diameter) for each patient's AAA.
Patients who lived far from the hospitals or who had no difficulty exercising because of their young age did not participate in the program. Those who had busy schedules also did not participate in the program, although patients who were thought to be vulnerable to the recurrence of cardiovascular events were strongly recommended by medical staff to participate in the program. Basically, the more severe the patient's condition, the more support he or she received, including the comprehensive rehabilitation program. Because the rehabilitation program is supported by health insurance in Japan, the nonparticipation rates attributable to participants' low incomes that have been reported in other countries13 were not seen in this study. After starting the program, 1 patient dropped out. Finally, after propensity score matching, 88 patients remained (Figure 1).
Outcomes
The end points of this study were defined as death by any cause, any major adverse cardiovascular events (MACEs; cardiac death, acute coronary syndrome, cerebral infarction, hospitalization for heart failure, and AAA rupture), and AAA repair; information on all of these end points was obtained from the patients' medical records. Because patients with a short follow‐up period (within 150 days of their diagnosis of AAA) were excluded from this study, the clinical outcomes of patients in the nonrehabilitation group were obtained from 150 days after their diagnosis of AAA, and the clinical outcomes of patients in the rehabilitation group were obtained from the beginning of the cardiac rehabilitation program. In our institutes, patients underwent AAA repair in accordance with the Guidelines for Diagnosis and Treatment of Aortic Aneurysm and Aortic Dissection, for 1998, 2004, and 2011.14, 15, 16 According to these guidelines, for AAAs with a maximum minor‐axis diameter of >50 mm, AAAs with an expansion rate of >5 mm/6 months, or patients with symptoms such as abdominal pain, lumbago, or back pain, an AAA repair is considered.
Cardiac rehabilitation
In our institutes, the comprehensive cardiac rehabilitation program is routinely performed during courses of cardiovascular treatment, in accordance with the Guidelines for Rehabilitation in Patients With Cardiovascular Disease (JCS 2012).7 According to these guidelines, indications of cardiac rehabilitation are AAA, aortic dissection, angina pectoris, myocardial infarction, open heart surgery, acute heart failure, and peripheral arterial occlusive diseases complicated by intermittent claudication. In addition, patients with chronic heart failure, defined by the left ventricular ejection fraction of ≤40%, peak oxygen uptake (peak VO2) of ≤80% of the reference value, or brain natriuretic peptide of ≥80 pg/mL, are also considered appropriate candidates for exercise programs. Patients with cardiovascular diseases are recruited into a 150‐day rehabilitation program, a supervised exercise program in hospital at an anaerobic threshold (AT) level of 1 to 3 times a week. Vital signs are checked, followed by stretches for 10 minutes as a gradual warm‐up before exercise (Figure 2). The duration of continuous exercise is ≈30 minutes, without interval, and vital signs are checked by medical staff repeatedly, every 10 minutes. In‐hospital training consists of treadmill walking and use of a bicycle ergometer. The strength exercises, anaerobic exercises, and a blood flow moderation exercise known as Kaatsu were not incorporated into this program. After the exercise, there is a gradual cooling‐down period, the duration of which is ≈10 minutes. During the exercise program, patients are supervised by rehabilitation staff composed of cardiologists, cardiac care nurses, expert physical therapists, clinical psychologists, and registered dietitians.
Figure 2.
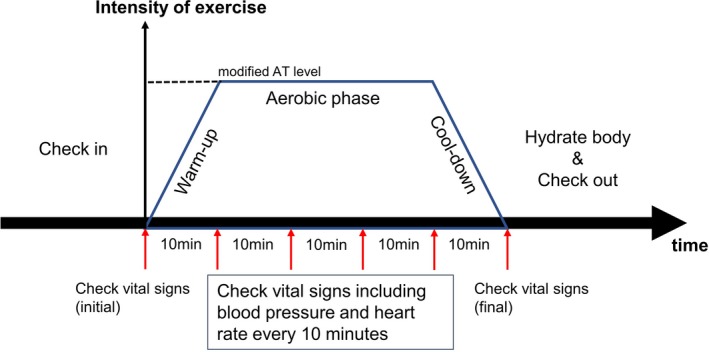
Explanation of the exercise procedure. Vital signs, including blood pressure and heart rate, were checked, followed by stretches for 10 minutes as a gradual warm‐up before exercise. The duration of continuous exercise (an ergometry with a modified anaerobic threshold [AT] level) was ≈30 minutes without interval, and vital signs were checked by medical staff repeatedly, every 10 minutes. Exercise was discontinued when blood pressure during exercise was >150/100 mm Hg. The duration of the gradual cooling‐down period after exercise was ≈10 minutes. Patients were recommended to consume water, according to their medical status, before checkout.
The exercise intensity level of regular AT is expressed as 60% to 80% of a patient's maximum heart rate (% HR) or 0.4 to 0.6 of the exercise factor from Karvonen formula.7 For the patients with AAA in this study, considering the hazardous effect of BP elevation during exercise, the load corresponded to 0.2 of the exercise factor for the HR reserve using the Karvonen formula, and this was designated as exercise with a “modified AT level” (Figure 3). The exercise program with this modified AT level was prescribed to the patients, but it not prescribed when a patient's BP at rest was >130/90 mm Hg; it was discontinued when a patient's BP during exercise was >150/100 mm Hg.
Figure 3.
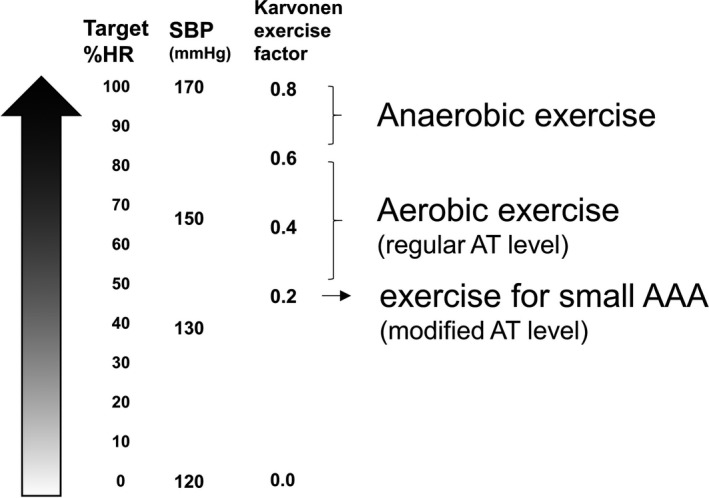
Degree of exercise stress. The exercise intensity level was set using percentage maximum heart rate (% HR) or the exercise factor from the Karvonen formula. For convenience, the approximate systolic blood pressure (SBP) corresponding to each exercise intensity in our institutes is described. The exercise stress with a modified anaerobic threshold (AT) level was much lower than that of other cardiac rehabilitation programs. Exercise with a modified AT level corresponds to an SBP of <150 mm Hg. AAA indicates abdominal aortic aneurysm.
A cardiopulmonary exercise test (CPX) was performed 1 week and 3 months after the introduction of the cardiac rehabilitation program, to assess exercise capacity. The CPX was performed using the Aero Monitor AS 300S (Minato Medical, Japan). Routine blood tests were performed with patients in both groups at the time of admission to the study and again 1 year later. AAA evaluations using computed tomographic scans were performed with patients in both groups every 6 months.
Statistical Analysis
To minimize bias caused by patients' baseline characteristics, propensity score matching was performed to identify patients with similar backgrounds, using IBM SPSS statistical software, version 22 (SPSS, Inc, Chicago, IL). One‐to‐one nearest neighbor matching was used on a common support, with caliper 0.1.17 Covariables, including age, sex, body mass index, hypertension, dyslipidemia, diabetes mellitus, current smoking, former smoking, CAD, cerebrovascular disease, β and αβ blockers, angiotensin‐converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, statins, and initial AAA diameter, were adjusted using propensity score matching. Before and after matched pairs had been formed, patients' characteristics were compared using the χ2 test for noncontinuous variables or an unpaired t test if the continuous data distributed normally and the Wilcoxon signed‐rank test if not. Kaplan‐Meier and Cox hazard analyses were used to analyze patients' mortality and clinical outcomes before matching. The log‐rank test and the Cox proportional hazard models, stratified by matched pairs, were used to compare the data set after matching.18 To accurately compare the clinical outcomes of the rehabilitation group and the nonrehabilitation group, after matching, the beginning of the observation period for a nonrehabilitation patient was consistently set with a paired rehabilitation patient. To check the proportional hazards assumption for all cohort and matched pairs, model assessment based on cumulative sums of martingale residuals and follow‐up times was performed using the model of Lin et al19 and the log‐log plot of a Kaplan‐Meier curve.20 Pearson's r was used as a correlation statistic to measure the degree of the relationship between linearly related variables in the parametric test. Spearman's rank correlation was used to measure the degree of the association between 2 variables, in the case of the nonparametric test. To evaluate the predictive value for accelerated AAA expansion >5 mm/y, a receiver operating characteristic curve analysis was performed. We used a paired t test to analyze the repeated CPX measurements. A logistic regression analysis was used to examine the risk factors for accelerated AAA expansion (>5 mm/y) in the rehabilitation group.
Results
The baseline characteristics before propensity score matching are shown in Table 1. Male sex, former smoking, family history of CAD, and cerebrovascular disease were more prevalent in the rehabilitation group compared with the nonrehabilitation group at the beginning of the study (Table 1). Current smoking and chronic obstructive pulmonary disease were more prevalent in the nonrehabilitation group. β and αβ Blockers, angiotensin II receptor blockers, and statins were used more frequently in the rehabilitation group. The baseline findings of the echocardiography and blood test are also shown (Table 1). In the rehabilitation group, the ejection fraction was lower and the left ventricular posterior wall was thicker, compared with the nonrehabilitation group. In addition, the rehabilitation group's serum levels of total cholesterol and high‐density lipoprotein cholesterol were lower. The rehabilitation group's follow‐up was statistically shorter (4.7±3.0 versus 6.3±3.3 years; P<0.01). After matching, there were significant differences between groups but only in the existence of a family history of CAD and high‐density lipoprotein cholesterol levels. Although aspect ratios (vertical/horizontal diameter) were calculated to evaluate the geometry of each patient's AAA, the ratios were not statistically different between the rehabilitation group and the nonrehabilitation group (Table 2).
Table 1.
Patient Background Before and After Propensity Score Matching
| Characteristics | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
| Rehabilitation (n=49) | No Rehabilitation (n=163) | P Value | Rehabilitation (n=44) | No Rehabilitation (n=44) | P Value | |
| Age, y | 72±8 | 74±8 | 0.15 | 72±8 | 72±8 | 0.94 |
| Male sex, n (%) | 45 (92) | 126 (77) | 0.04 | 40 (91) | 38 (86) | 0.50 |
| BMI, kg/m2 | 24±3 | 24±4 | 0.81 | 24±3 | 23±3 | 0.29 |
| Hypertension, n (%) | 36 (73) | 121 (74) | 0.33 | 33 (75) | 33 (75) | 1.00 |
| Dyslipidemia, n (%) | 30 (61) | 108 (66) | 0.13 | 28 (64) | 31 (70) | 0.49 |
| Diabetes mellitus, n (%) | 8 (16) | 52 (32) | 0.10 | 8 (18) | 13 (30) | 0.22 |
| Current smoking, n (%) | 7 (14) | 55 (34) | 0.01 | 7 (16) | 9 (20) | 0.58 |
| Former smoking, n (%) | 35 (71) | 60 (37) | <0.01 | 30 (68) | 32 (73) | 0.64 |
| Former smoking, n (%) | 4 (8) | 5 (3) | 0.22 | 4 (9) | 1 (2) | 0.17 |
| Family history of CAD, n (%) | 13 (27) | 11 (7) | <0.01 | 12 (27) | 4 (9) | 0.04 |
| Family history of AAA, n (%) | 2 (4) | 7 (4) | 0.33 | 2 (5) | 3 (7) | 0.61 |
| CAD, n (%) | 33 (67) | 98 (60) | 0.50 | 28 (64) | 20 (45) | 0.50 |
| CVD, n (%) | 2 (4) | 0 (0) | 0.02 | 2 (5) | 0 (0) | 0.15 |
| COPD, n (%) | 2 (4) | 48 (29) | <0.01 | 2 (5) | 7 (16) | 0.08 |
| β or αβ Blocker, n (%) | 19 (39) | 39 (24) | 0.02 | 17 (39) | 9 (20) | 0.06 |
| ACE inhibitor, n (%) | 9 (18) | 21 (13) | 0.31 | 7 (16) | 4 (9) | 0.35 |
| ARB, n (%) | 25 (51) | 57 (35) | <0.01 | 24 (55) | 16 (36) | 0.11 |
| Calcium channel blocker, n (%) | 30 (61) | 86 (53) | 0.29 | 26 (59) | 21 (48) | 0.24 |
| Statin, n (%) | 25 (51) | 54 (33) | <0.01 | 20 (45) | 12 (27) | 0.08 |
| EF, % | 56±13 | 61±14 | 0.03 | 57±11 | 63±11 | 0.05 |
| Dd, mm | 49±9 | 48±7 | 0.42 | 49±9 | 50±6 | 0.83 |
| Ds, mm | 35±10 | 32±9 | 0.08 | 33±8 | 33±8 | 0.73 |
| IVSth, mm | 11±2 | 10±1 | 0.06 | 11±2 | 11±2 | 0.37 |
| PWth, mm | 11±2 | 10±1 | <0.01 | 11±2 | 10±1 | 0.05 |
| Creatinine, mg/dLa | 1.1±0.4 | 1.0±0.4 | 0.30 | 1.1±0.4 | 1.0±0.4 | 0.20 |
| hsCRP, mg/dL | 1.7±1.6 | 0.8±2.6 | 0.05 | 1.8±1.7 | 1.0±2.1 | 0.09 |
| HbA1c (NGSP), % | 5.9±0.7 | 5.7±1.1 | 0.33 | 5.9±0.7 | 5.8±0.5 | 0.24 |
| T‐chol, mg/dL | 170±27 | 189±35 | <0.01 | 171±28 | 176±34 | 0.54 |
| LDL‐C, mg/dL | 104±23 | 113±29 | 0.30 | 105±23 | 110±30 | 0.60 |
| HDL‐C, mg/dL | 42±11 | 50±14 | <0.01 | 42±11 | 50±14 | 0.02 |
| Triglycerides, mg/dL | 131±70 | 144±91 | 0.48 | 135±71 | 116±42 | 0.19 |
| Peak VO2, mL/kg per min | 14±4 | 18±6 | ||||
| AT, mL/kg per min | 9±1 | 12±3 | ||||
| ΔVE/ΔVCO2 slope | 36±10 | 34±10 | ||||
| ΔVO2/ΔWR, mL/min per watt | 8±2 | 9±3 | ||||
| Rehabilitation period, d | 57±60 | 59±62 | ||||
| Follow‐up, y | 4.7±3.0 | 6.3±3.3 | <0.01 | 4.8±3.0 | 5.1±3.3 | 0.62 |
Data are given as mean±SD unless otherwise indicated. AAA indicates abdominal aortic aneurysm; ACE, angiotensin‐converting enzyme; AT, anaerobic threshold; ARB, angiotensin II receptor blocker; BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disorder; Dd, diastolic diameter; Ds, systolic diameter; EF, ejection fraction; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; hsCRP, high‐sensitivity C‐reactive protein; IVSth, interventricular septal thickness; LDL‐C, low‐density lipoprotein cholesterol; NGSP, National Glycohemoglobin Standardization Program; peak VO2, peak oxygen uptake; PWth, posterior wall thickness; T‐chol, total cholesterol; VCO2, CO2 output; VE, ventilation; and WR, work rate.
The patients undergoing hemodialysis were excluded.
Table 2.
Comparison of AAA Diameter, AAA Expansion Rate, and hsCRP
| Variable | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
| Rehabilitation | No Rehabilitation | P Value | Rehabilitation | No Rehabilitation | P Value | |
| AAA diameter, mm | ||||||
| Initial | 38±8 | 39±9 | 0.45 | 39±7 | 41±8 | 0.23 |
| After 1 y | 42±9 | 44±9 | 0.12 | 42±8 | 45±2 | 0.04 |
| Latest | 45±10 | 50±9 | <0.01 | 45±10 | 49±7 | 0.04 |
| Expansion rate, mm/y | 2.3±3.7 | 3.8±3.4 | <0.01 | 2.1±3.0 | 4.5±4.0 | <0.01 |
| Follow‐up of AAA diameter, y | 3.1±3.2 | 3.7±2.6 | 0.26 | 2.8±2.3 | 2.5±2.2 | 0.48 |
| hsCRP, mg/dL | ||||||
| Initial | 1.7±1.7 | 0.8±2.6 | 0.06 | 1.8±1.7 | 1.0±2.1 | 0.09 |
| After 1 y | 0.8±1.8 | 1.0±2.2 | 0.54 | 0.6±1.4 | 1.4±2.8 | 0.11 |
| ΔhsCRP, mg/dL per y | −0.9±2.1 | 0.2±3.3 | <0.01 | −1.2±1.7 | 0.4±3.1 | <0.01 |
| Aspect ratio (initial)a | 2.6±0.7 | 2.5±0.6 | 0.20 | 2.6±0.7 | 2.4±0.5 | 0.59 |
Data are given as mean±SD. The AAA diameters at initial and 1 year after registration were compared between the rehabilitation group and the nonrehabilitation group, before and after matching. The latest AAA diameter was the size of AAA at the latest follow‐up computed tomographic (CT) scan. Expansion rate was calculated as the change of AAA diameter per year (mm/y). Follow‐up of AAA diameter was the period from initial CT scan to the latest follow‐up CT scan. AAA indicates abdominal aortic aneurysm; and hsCRP, high‐sensitivity C‐reactive protein.
The aspect ratio (vertical/horizontal diameter) of AAA was calculated using simple aneurysm model, according to the method previously reported.12
Before matching, the risk of AAA repair was much lower in the rehabilitation group (hazard ratio, 0.43; 95% confidence interval, 0.25–0.72; P=0.001 according to the Cox regression and P=0.003 according to the log‐rank test), but mortality was similar between the 2 groups (P=0.331) (Figure 4). The reasons for death in the rehabilitation group were bleeding (n=3), pneumonia (n=1), and heart failure (n=1). The reasons for death in the nonrehabilitation group were CAD (n=7), AAA rupture (n=4), cancer (n=4), heart failure (n=2), and infection (n=1). The 5‐year survival rates free from AAA repair were 86% in the rehabilitation group and 38% in the nonrehabilitation group (P<0.001). After matching, there was also a reduced risk of AAA repair in the rehabilitation group (hazard ratio, 0.19; 95% confidence interval, 0.07–0.50; P<0.001 according to the Cox regression and P<0.001 according to the stratified log‐rank test), and mortality remained similar between the 2 groups (P=0.655). After matching, there remained no difference in the risk for MACEs, according to the stratified log‐rank test (P=0.257). By testing assumption for the Cox regression model before and after matching, the calculated P value for each cohort was 0.84, 0.90 and nonsignificant, indicating that the deviation from the assumption is small. Also, the proportional hazards assumption was confirmed by the log‐log plot of a Kaplan‐Meier curve for both groups.
Figure 4.
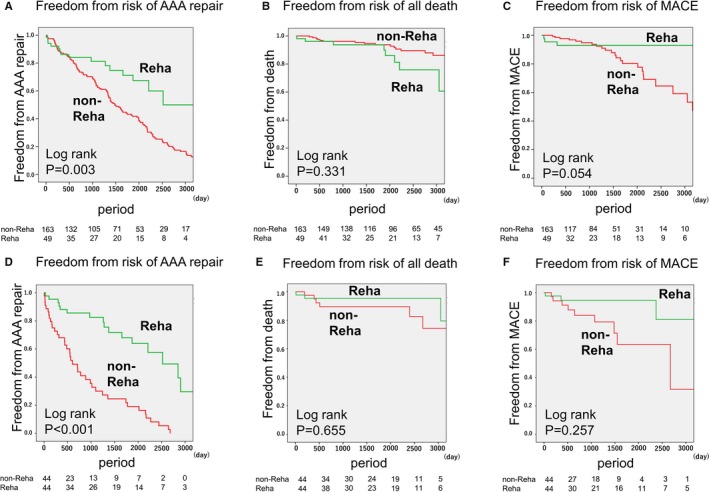
Risks of abdominal aortic aneurysm (AAA) repair, death by any cause, and major adverse cardiovascular events (MACEs) in patients with small AAAs. The risks of AAA repair, death by any cause, and MACEs in patients with small AAAs were analyzed using the Kaplan‐Meier method. A through C, Comparison between the rehabilitation group (n=49) and the nonrehabilitation group (n=163) before matching. D through F, Comparison between the rehabilitation group (n=44) and the nonrehabilitation group (n=44) after matching. Reha indicates rehabilitation.
Next, we evaluated AAA expansion rates. AAA maximum diameters were evaluated using computed tomographic scans and regularly monitored until AAA repair. Two patients in the nonrehabilitation group received AAA repair within a year. Although initial AAA size was similar between the rehabilitation group and the nonrehabilitation group after matching (rehabilitation versus nonrehabilitation group, 39±7 versus 41±8 mm; P=0.23), after a year, AAA size in the rehabilitation group was smaller than that of the nonrehabilitation group (rehabilitation versus nonrehabilitation group, 42±8 versus 45±2 mm; P=0.04). The AAA expansion rate was smaller in the rehabilitation group (before matching: rehabilitation versus nonrehabilitation group, 2.3±3.7 versus 3.8±3.4 mm/y [P=0.008]; after matching: rehabilitation versus nonrehabilitation group, 2.1±3.0 versus 4.5±4.0 mm/y [P<0.001]) (Table 2). Concordantly, there were fewer patients in the rehabilitation group receiving AAA repair than in the nonrehabilitation group.
Moreover, after matching, high‐sensitivity C‐reactive protein (hsCRP) values a year after admission to this study improved more in the rehabilitation group compared with the nonrehabilitation group (rehabilitation versus nonrehabilitation group, −1.2±1.7 versus 0.4±3.1 mg/dL; P=0.006). However, the correlations between AAA expansion rate and CRP values, including the initial CRP values, follow‐up CRP values after 1 year, and ΔCRP (follow‐up CRP values after 1 year–the initial CRP values), were not statistically significant (r=−0.14, P=0.08; r=−0.12, P=0.12; and r=0.07, P=0.58, respectively). According to the receiver operating characteristic curve analysis for the accelerated AAA expansion rate (>5 mm/y), any hsCRP value could not reflect accelerated AAA expansion (Figure 5A).
Figure 5.
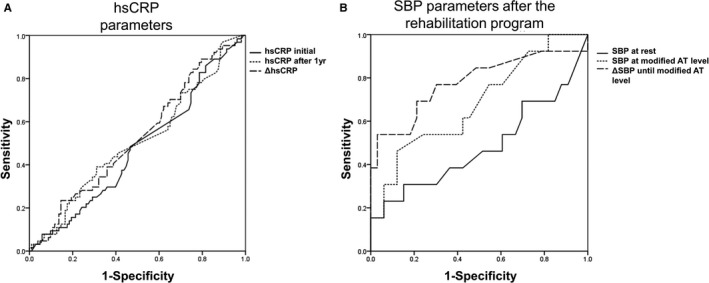
Receiver operating characteristic (ROC) curves for the accelerated abdominal aortic aneurysm (AAA) expansion rate. A, Areas under ROC curves for the accelerated AAA expansion rate (>5 mm/y) of the initial high‐sensitivity C‐reactive protein (hsCRP) values, hsCRP values after 1 year, and ΔCRP (hsCRP values after 1 year–initial hsCRP values) (n=213) were 0.475, 0.506, and 0.525, respectively (P=0.581, P=0.416, and P=0.594, respectively). B, After the rehabilitation program (n=49), the areas under the ROC curves for accelerated AAA expansion rate (>5 mm/y) of systolic blood pressure (SBP) at rest, SBP at exercise with a modified anaerobic threshold (AT) level, and ΔSBP during exercise (SBP at exercise with modified AT level–SBP at rest) were 0.483, 0.683, and 0.780, respectively (P=0.855, P=0.055, and P=0.003, respectively).
To analyze the reason for the preventive effect of the cardiac rehabilitation program on AAA expansion, we investigated the elevation of BP during exercise in the rehabilitation group. None of the antihypertensive drugs of the patients in the rehabilitation group were changed. The systolic BP (SBP) at rest was not statistically significantly different before and after the rehabilitation program (123±22 versus 115±16 mm Hg; P=0.06). ΔSBP during exercise (SBP at exercise with modified AT level–SBP at rest) significantly decreased after the rehabilitation program (before versus after program: 9±14 versus 4±13 mm Hg; P=0.035). Before the rehabilitation program, ΔSBP during exercise was not correlated with AAA expansion rate (Figure 6A through 6C). However, after the rehabilitation program, there was a positive correlation between AAA expansion rate and SBP at exercise with modified AT level (Pearson r=0.315, P=0.031), and ΔSBP during exercise was positively correlated with the AAA expansion rate (r=0.569, P<0.001) (Figure 6D through 6F). Despite this, there was no significant relationship between AAA expansion rate and diastolic BP at exercise with modified AT level before or after the rehabilitation program. Neither HR at rest nor diastolic BP at rest was related to AAA expansion rate. With regard to the receiver operating characteristic curve analysis for the accelerated AAA expansion rate, the area under curve of ΔSBP during exercise after the rehabilitation program was 0.780 (P=0.003) (Figure 5B). ΔSBP during exercise after the rehabilitation program was the most powerful marker for reflecting AAA expansion rate. Reflecting high sensitivity and specificity, the cutoff point of ΔSBP during exercise after the rehabilitation program was 6.50 mm Hg (sensitivity, 0.692; and specificity, 0.794). ΔSBP during exercise of >6.50 mm Hg after the rehabilitation program was the risk factor for AAA repair (hazard ratio, 1.079; P=0.020). In the analysis on the risk factors for accelerated AAA expansion (>5 mm/y) in the rehabilitation group (Figure 7), ΔSBP during exercise of >6.50 mm Hg after the rehabilitation program was a potent risk factor for AAA expansion (hazard ratio, 5.20; P=0.021). After adjusting for other factors (ie, age, male sex, initial AAA diameter of >40 mm, hypertension, dyslipidemia, diabetes mellitus, current smoking, former smoking, family history of CAD, existence of CAD, ejection fraction <40%, β and αβ blockers, angiotensin‐converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, and statins), ΔSBP during exercise of >6.50 mm Hg remained an independent risk factor for accelerated AAA expansion (hazard ratio, 19.7; P=0.040).
Figure 6.
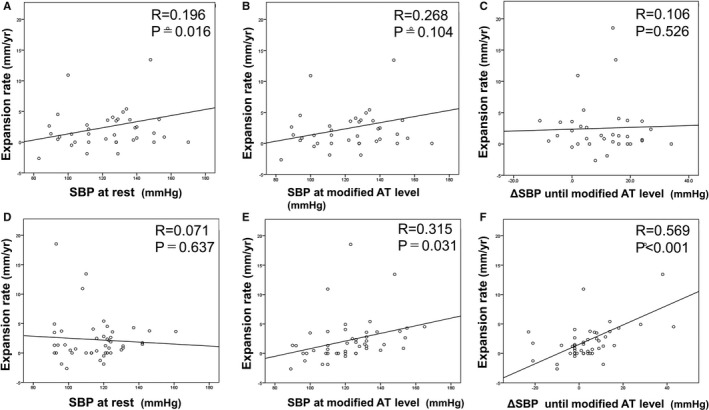
Correlation between the abdominal aortic aneurysm (AAA) expansion rate and systolic blood pressure (SBP) parameters in the rehabilitation group (n=49). Before the rehabilitation program, the AAA expansion rate had no notable correlation with SBP at rest (A), no correlation with SBP at exercise with a modified anaerobic threshold (AT) level (B), and no ΔSBP during exercise (SBP at exercise with modified AT level–SBP at rest) (C). After the rehabilitation program, the AAA expansion rate was not correlated with SBP at rest (D) but was significantly correlated with SBP at exercise with a modified AT level (E) and ΔSBP during exercise (F).
Figure 7.
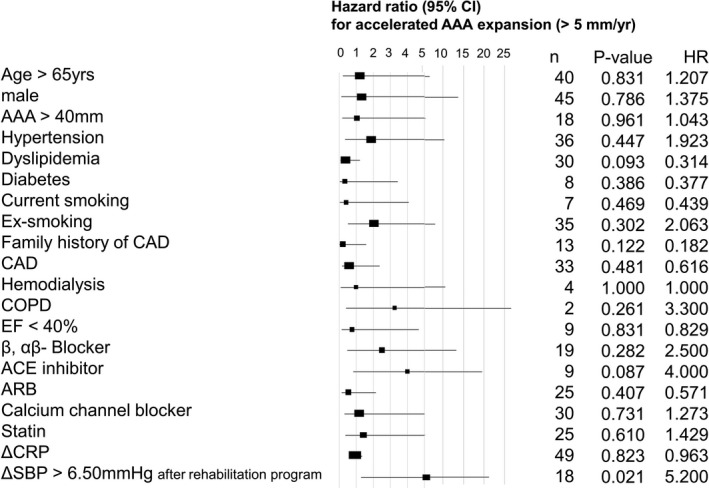
Risk factors for accelerated abdominal aortic aneurysm (AAA) expansion (>5 mm/y) in the rehabilitation group (n=49). With the logistic univariable regression analysis, change in systolic blood pressure (ΔSBP) during exercise >6.5 mm Hg after the rehabilitation program was correlated with accelerated AAA expansion (>5 mm/y). ACE indicates angiotensin‐converting enzyme; ARB, angiotensin II receptor blocker; CAD, coronary artery disease; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; EF, ejection fraction; and HR, hazard ratio.
In this study, 19 of 49 patients in the rehabilitation group underwent the CPX 1 week and 3 months after starting the rehabilitation program (Table 3). Indicators of cardiopulmonary ability, including HR, BP, AT, peak VO2, ΔVE/ΔCO2 output slope, and ΔVO2/ΔWR, were examined. Peak VO2 was not significantly elevated in the rehabilitation group. In addition, the AT, which was determined not by the Karvonen method but by the CPX, did not statistically significantly improve after the rehabilitation program. SBP at exercise with regular AT level, ΔSBP during exercise from at rest to exercise with regular AT level, and SBP at peak exercise did not statistically significantly decrease after the rehabilitation program.
Table 3.
CPX Results Before and 3 Months After the Rehabilitation Program in the Rehabilitation Group (n=19)
| Variable | Time After Rehabilitation | P Value | |
|---|---|---|---|
| 1 wk | 3 mo | ||
| At rest | |||
| HR, bpm | 71±16 | 71±16 | 0.327 |
| SBP, mm Hg | 125±15 | 126±13 | 0.545 |
| DBP, mm Hg | 74±10 | 73±7 | 0.684 |
| At AT | |||
| HR, bpm | 88±17 | 87±17 | 1.000 |
| SBP, mm Hg | 146±25 | 146±20 | 0.651 |
| DBP, mm Hg | 77±11 | 75±8 | 0.306 |
| From rest to AT | |||
| ΔHR, bpm | 16±6 | 17±7 | 1.000 |
| ΔSBP, mm Hg | 21±20 | 21±19 | 0.552 |
| ΔDBP, mm Hg | 3±9 | 3±7 | 0.541 |
| At peak | |||
| HR, bpm | 110±26 | 110±25 | 1.000 |
| SBP, mm Hg | 171±40 | 169±34 | 0.790 |
| DBP, mm Hg | 83±13 | 80±12 | 0.238 |
| Peak VO2, mL/kg per min | 15.0±3.6 | 16.5±4.2 | 0.211 |
| AT, mL/kg per min | 10.0±1.6 | 10.4±1.8 | 0.431 |
| ΔVE/ΔVCO2 slope | 32.9±5.8 | 32.2±4.0 | 0.630 |
| ΔVO2/ΔWR, mL/min per watt | 9.0±2.2 | 9.1±1.8 | 0.922 |
Data are given as mean±SD. AT indicates anaerobic threshold; bpm, beats/min; CPX, cardiopulmonary exercise test; DBP, diastolic blood pressure; HR, heart rate; and SBP, systolic blood pressure; VCO2, CO2 output; VE, ventilation; VO2, oxygen uptake; and WR, work rate.
Discussion
For patients with atherosclerotic disease (eg, myocardial infarction and cerebral infarction), participation in an exercise program is recommended to prevent MACEs and death.7 Because AAA tends to coexist with atherosclerotic diseases, and MACE is the main cause of death in patients with AAA,3 exercise should be effective at least for the prevention of MACEs and death in these patients with AAA. However, the participation of patients with AAA in such programs is often avoided, for fear that exercise may cause AAA expansion or rupture because of BP elevation during exercise, although we have neither evidence nor case reports to support this. Also, AAA is not regarded as a contraindication for exercise in the guidelines for rehabilitation.7 In our institutes, on the basis of empirical evidence, we have prescribed a carefully modified exercise program to patients with AAA that takes into account BP elevation. This study has confirmed the safety of a carefully modified relatively mild exercise program for patients with AAAs of an average size of ≈40 mm and demonstrated its effect on AAA expansion rates and on the timing of AAA repair.
In this study, AAA rupture did not occur in the rehabilitation group, whereas 4 patients in the nonrehabilitation group died of AAA rupture, which indicates that patients with AAA can safely participate in a carefully modified exercise program. In addition, we clearly demonstrated that the exercise program, despite being of mild intensity, can suppress AAA expansion and delay the timing of AAA repair. The 5‐year survival free from AAA repair rate was much higher in the rehabilitation group than in the nonrehabilitation group. To date, few studies have reported the safety and effectiveness of exercise in patients with AAA. In a recent report on an exercise program in a mouse model of Marfan syndrome, mild aerobic exercise blocks elastin fiber fragmentation and aortic dilatation.21 Two pilot studies in 20098 and 201022 reported on the safety and tolerance of exercise in a few patients with AAA. From the 12‐week supervised program of moderate‐intensity endurance exercise for patients (n=11) with small AAAs, no increases in AAA size nor any other adverse clinical events were observed at 12 weeks,23 but there were no follow‐up data on patients' clinical outcomes after the exercise program. Subsequently, a combination of rehabilitation in a Department of Veterans Affairs Palo Alto Health Care System (VAPAHCS) facility and home training for up to 3 years (n=72), in which BP during exercise was not monitored and exercise intensity was higher than ours, had no influence on AAA expansion rate.10 Precisely regulating the BPs of patients with AAA during home‐based exercise is difficult, and home‐based exercise is sometimes less effective than supervised exercise.24 For patients with AAA, BP during exercise should be monitored carefully, under supervision, and exercise programs should be carefully modified to avoid excessive BP elevation during exercise. In our exercise program with modified AT levels, the average SBP during exercise reached ≈130 mm Hg. Although the AT and peak VO2 evaluated by the CPX did not improve, participation in this carefully modified exercise program could have a beneficial effect on patients' BP and clinical outcomes, including AAA expansion.
How did our study's mild exercise program have a beneficial effect on clinical outcomes (ie, what was the mechanism)? The exercise‐induced effects of lowering BP25, 26 and stabilizing BP elevation during exercise27 have been reported previously. The BP‐lowering effect can be explained by the reduction in total peripheral resistance28 or improvement in autonomic function29 after exercise training. Furthermore, the lowered plasma norepinephrine levels after exercise training may play a role in lowering BP. Exercise training could also reduce insulin resistance, followed by lowering BP.30 In our study, after the rehabilitation program, patients' SBP at rest decreased from 123 to 115 mm Hg, which is a finding in agreement with a previous report that low‐intensity training (corresponding to 53% of maximal oxygen consumption) can effectively lower BP in old people with essential hypertension.26 Indeed, hypertension is a known risk factor for AAA occurrence3 and rupture,31 whereas there is no correlation between hypertension and AAA expansion.32 Although the correlation between BP elevation during exercise and AAA expansion has not been debated until now, judging by our data that ΔSBP during exercise after a rehabilitation program was an independent risk for the acceleration of AAA expansion (Figure 7), our carefully modified mild exercise program had a protective effect on AAA expansion through its suppressive effect on BP elevation during exercise.
Consistent with the results of previous studies,22, 23 hsCRP levels significantly improved in the rehabilitation group compared with the nonrehabilitation group; however, individual AAA expansion rate by itself was not correlated with individual ΔhsCRP value. Because initial hsCRP values were measured before the beginning of the rehabilitation program, the timing of which could have corresponded to the phase immediately after the onset of any cardiovascular events, in this study ΔhsCRP values may not necessarily reflect only the changes in local inflammatory status related to AAA.
Further studies are required to clarify the precise mechanism of why even a mild exercise program can suppress AAA expansion. On the basis of our findings, stabilized BP elevation during exercise was mainly related to the beneficial effect of our rehabilitation program. At present, there is virtually no established strategy for AAA expansion. A carefully modified mild exercise program, as described herein, should be recommended to patients with small AAAs to prevent AAA expansion. Indeed, on the basis of the present findings, we have started a prospective study to examine the effect of a carefully modified cardiac rehabilitation in patients with small AAAs.
Strengths
This is the first report on long‐term prognosis after a supervised carefully modified exercise program in patients with small AAAs. To date, there have been some reports on the safety of exercise for patients with AAAs. We compared clinical outcomes, including AAA expansion rate, in 49 patients with small AAAs (a mean diameter of 38 mm) after a supervised mild exercise program (the rehabilitation group), the intensity of which was set at a modified AT level to avoid excessive BP elevation during exercise, and 163 patients with small AAAs (a mean diameter of 39 mm) without an exercise program (the nonrehabilitation group). As a result, we demonstrated the safety and effectiveness of a supervised carefully modified exercise program for patients with small AAAs.
Limitations
Because of the inherent nature of a hospital‐based retrospective cohort study, this study has some limitations. Because our institutes are referral hospitals located in metropolitan Tokyo, 1252 of 1515 patients with AAA underwent AAA repair within 150 days of the initial referral. After the exclusions, only 213 patients with AAA qualified for inclusion in this study. Of these patients, 50 had been recruited into the rehabilitation program for a cardiovascular disease other than AAA, whose exercise intensity was carefully modified because of their coexistence of AAA. In addition, 5 of 49 patients in the rehabilitation group were excluded from the matching analysis. The asymmetry of AAA could have a great influence on the distribution of tangent wall tensions. Evaluation of the aspect ratios for AAA, which are closely related to peak wall stress,12 was performed as the biomechanical AAA analysis of this study. However, direct evaluation of AAA peak wall stress using finite element simulations33 could not be performed in this study. Despite this, because a saccular aneurysm was excluded from this study in advance (Figure 1), the biomechanical effects of AAA on its expansion rate may be comparably smaller.12 It may be that because the timing of the hsCRP level measurement before the beginning of the rehabilitation program could correspond to the short‐term phase of other cardiovascular diseases, the mediation of the anti‐inflammation effect of exercise on the suppression of the AAA expansion rate may not have been precisely evaluated. Furthermore, the hazard ratio for accelerated AAA could be calculated only in 49 patients, and the CPX was performed with only 19 of the 49 patients in the rehabilitation group, and with no patients in the nonrehabilitation group.
Conclusion
A carefully modified cardiac rehabilitation program that was safely performed with patients with small AAAs protected against AAA expansion and postponed the timing of AAA repair, through stabilization of the elevation of BP during exercise. This is the first report to demonstrate the beneficial effect of a mild exercise program on AAA expansion.
Sources of Funding
This study was supported by Grants‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology in Japan.
Disclosures
None.
(J Am Heart Assoc. 2018;7:e007959 DOI: 10.1161/JAHA.117.007959.)29487112
Contributor Information
Atsuko Nakayama, Email: st7089-fki@umin.ac.jp.
Hiroyuki Morita, Email: hmrt-tky@umin.net.
References
- 1. Guirguis‐Blake JM, Beil TL, Senger CA, Whitlock EP. Ultrasonography screening for abdominal aortic aneurysms: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160:321–329. [DOI] [PubMed] [Google Scholar]
- 2. Kent KC. Clinical practice: abdominal aortic aneurysms. N Engl J Med. 2014;371:2101–2108. [DOI] [PubMed] [Google Scholar]
- 3. Forsdahl SH, Singh K, Solberg S, Jacobsen BK. Risk factors for abdominal aortic aneurysms: a 7‐year prospective study: the Tromsø Study, 1994–2001. Circulation. 2009;119:2202–2208. [DOI] [PubMed] [Google Scholar]
- 4. Durieux R, Van Damme H, Labropoulos N, Yazici A, Legrand V, Albert A, Defraigne JO, Sakalihasan N. High prevalence of abdominal aortic aneurysm in patients with three‐vessel coronary artery disease. Eur J Vasc Endovasc Surg. 2014;47:273–278. [DOI] [PubMed] [Google Scholar]
- 5. Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B, Fletcher GF, Gordon NF, Pate RR, Rodriguez BL, Yancey AK, Wenger NK; American Heart Association Council on Clinical Cardiology Subcommittee on Exercise, Rehabilitation, and Prevention; American Heart Association Council on Nutrition, Physical Activity, and Metabolism Subcommittee on Physical Activity . Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation. 2003;107:3109–3116. [DOI] [PubMed] [Google Scholar]
- 6. Dorn J, Naughton J, Imamura D, Trevisan M. Results of a multicenter randomized clinical trial of exercise and long‐term survival in myocardial infarction patients: the National Exercise and Heart Disease Project (NEHDP). Circulation. 1999;100:1764–1769. [DOI] [PubMed] [Google Scholar]
- 7. JCS Joint Working Group . Guidelines for rehabilitation in patients with cardiovascular disease (JCS 2012). Circ J. 2014;78:2022–2093. [DOI] [PubMed] [Google Scholar]
- 8. Kothmann E, Batterham AM, Owen SJ, Turley AJ, Cheesman M, Parry A, Danjoux G. Effect of short‐term exercise training on aerobic fitness in patients with abdominal aortic aneurysms: a pilot study. Br J Anaesth. 2009;103:505–510. [DOI] [PubMed] [Google Scholar]
- 9. Barakat HM, Shahin Y, Barnes R, Gohil R, Souroullas P, Khan J, McCollum PT, Chetter IC. Supervised exercise program improves aerobic fitness in patients awaiting abdominal aortic aneurysm repair. Ann Vasc Surg. 2014;28:74–79. [DOI] [PubMed] [Google Scholar]
- 10. Myers J, McElrath M, Jaffe A, Smith K, Fonda H, Vu A, Hill B, Dalman R. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc. 2014;46:2–9. [DOI] [PubMed] [Google Scholar]
- 11. Matsumoto Y, Adams V, Jacob S, Mangner N, Schuler G, Linke A. Regular exercise training prevents aortic valve disease in low‐density lipoprotein‐receptor‐deficient mice. Circulation. 2010;121:759–767. [DOI] [PubMed] [Google Scholar]
- 12. Akai T, Hoshina K, Yamamoto S, Takeuchi H, Nemoto Y, Ohshima M, Shigematsu K, Miyata T, Yamauchi H, Ono M, Watanabe T. Biomechanical analysis of an aortic aneurysm model and its clinical application to thoracic aortic aneurysms for defining “saccular” aneurysms. J Am Heart Assoc. 2015;4:e001547 DOI: 10.1161/JAHA.114.001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cooper AF, Jackson G, Weinman J, Horne R. Factors associated with cardiac rehabilitation attendance: a systematic review of the literature. Clin Rehabil. 2002;16:541–552. [DOI] [PubMed] [Google Scholar]
- 14. JCS Joint Working Group . Guidelines for diagnosis and treatment of aortic aneurysm and aortic dissection (JCS 1988). Circ J. 2000;64:1249–1283. [Google Scholar]
- 15. JCS Joint Working Group . Guidelines for diagnosis and treatment of aortic aneurysm and aortic dissection (JCS 2006). J Cardiol. 2007;50:547–577. [PubMed] [Google Scholar]
- 16. JCS Joint Working Group . Guidelines for diagnosis and treatment of aortic aneurysm and aortic dissection (JCS 2011). Circ J. 2013;77:789–828. [DOI] [PubMed] [Google Scholar]
- 17. Bangalore S, Guo Y, Samadashvili Z, Blecker S, Xu J, Hannan EL. Everolimus‐eluting stents or bypass surgery for multivessel coronary disease. N Engl J Med. 2015;372:1213–1222. [DOI] [PubMed] [Google Scholar]
- 18. Velazquez EJ, Samad Z, Al‐Khalidi HR, Sangli C, Grayburn PA, Massaro JM, Stevens SR, Feldman TE, Krucoff MW. The MitraClip and survival in patients with mitral regurgitation at high risk for surgery: a propensity‐matched comparison. Am Heart J. 2015;170:1050–1059. [DOI] [PubMed] [Google Scholar]
- 19. Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale‐based residuals. Biometrika. 1993;80:557–572. [Google Scholar]
- 20. Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York, NY: John Wiley & Sons; 1980:Chapter 2. [Google Scholar]
- 21. Gibson C, Nielsen C, Alex R, Cooper K, Farney M, Gaufin D, Cui JZ, van Breemen C, Broderick TL, Vallejo‐Elias J, Esfandiarei M. Mild aerobic exercise blocks elastin fiber fragmentation and aortic dilatation in a mouse model of Marfan syndrome associated aortic aneurysm. J Appl Physiol. 2017;123:147–160. [DOI] [PubMed] [Google Scholar]
- 22. Myers JN, White JJ, Narasimhan B, Dalman RL. Effects of exercise training in patients with abdominal aortic aneurysm: preliminary results from a randomized trial. J Cardiopulm Rehabil Prev. 2010;30:374–383. [DOI] [PubMed] [Google Scholar]
- 23. Tew GA, Moss J, Crank H, Mitchell PA, Nawaz S. Endurance exercise training in patients with small abdominal aortic aneurysm: a randomized controlled pilot study. Arch Phys Med Rehabil. 2012;93:2148–2153. [DOI] [PubMed] [Google Scholar]
- 24. Cowie A, Thow MK, Granat MH, Mitchell SL. A comparison of home and hospital‐based exercise training in heart failure: immediate and long‐term effects upon physical activity level. Eur J Cardiovasc Prev Rehabil. 2011;18:158–166. [DOI] [PubMed] [Google Scholar]
- 25. Taylor RS, Brown A, Ebrahim S, Jolliffe J, Noorani H, Rees K, Skidmore B, Stone JA, Thompson DR. Oldridge exercise‐based rehabilitation for patients with coronary heart disease: systematic review and meta‐analysis of randomized controlled trials. Am J Med. 2004;116:682–692. [DOI] [PubMed] [Google Scholar]
- 26. Hagberg JM, Montain SJ, Martin WH III, Ehsani AA. Effect of exercise training in 60‐ to 69‐year‐old persons with essential hypertension. Am J Cardiol. 1989;64:348–353. [DOI] [PubMed] [Google Scholar]
- 27. Cornelissen VA, Buys R, Smart NA. Endurance exercise beneficially affects ambulatory blood pressure: a systematic review and meta‐analysis. J Hypertens. 2013;31:639–648. [DOI] [PubMed] [Google Scholar]
- 28. Hambrecht R, Gielen S, Linke A, Fiehn E, Yu J, Walther C, Schoene N, Schuler G. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: a randomized trial. JAMA. 2000;283:3095–3101. [DOI] [PubMed] [Google Scholar]
- 29. Iellamo F, Legramante JM, Massaro M, Raimondi G, Galante A. Effects of a residential exercise training on baroreflex sensitivity and heart rate variability in patients with coronary artery disease: a randomized, controlled study. Circulation. 2000;102:2588–2592. [DOI] [PubMed] [Google Scholar]
- 30. Iellamo F, Caminiti G, Sposato B, Vitale C, Massaro M, Rosano G, Volterrani M. Effect of high‐intensity interval training versus moderate continuous training on 24‐h blood pressure profile and insulin resistance in patients with chronic heart failure. Intern Emerg Med. 2014;9:547–552. [DOI] [PubMed] [Google Scholar]
- 31. Gokani VJ, Sidloff D, Bath MF, Bown MJ, Sayers RD, Choke E. A retrospective study: factors associated with the risk of abdominal aortic aneurysm rupture. Vascul Pharmacol. 2015;65–66:13–16. [DOI] [PubMed] [Google Scholar]
- 32. Takagi H, Umemoto T; ALICE (All‐Literature Investigation of Cardiovascular Evidence) Group . Association of hypertension with abdominal aortic aneurysm expansion. Ann Vasc Surg. 2017;39:74–89. [DOI] [PubMed] [Google Scholar]
- 33. Gasser TC, Auer M, Labruto F, Swedenborg J, Roy J. Biomechanical rupture risk assessment of abdominal aortic aneurysms: model complexity versus predictability of finite element simulations. Eur J Vasc Endovasc Surg. 2010;40:176–185. [DOI] [PubMed] [Google Scholar]


