Abstract
Background
Uninephrectomy (UNX) is performed for various reasons, including kidney cancer or donation. Kidneys being the main site of l‐arginine production in the body, we tested whether UNX mediated kidney mass reduction impacts l‐arginine metabolism and thereby nitric oxide production and blood pressure regulation in mice.
Methods and Results
In a first series of experiments, we observed a significant increase in arterial blood pressure 8 days post‐UNX in female and not in male mice. Further experimental series were performed in female mice, and the blood pressure increase was confirmed by telemetry. l‐citrulline, that is used in the kidney to produce l‐arginine, was elevated post‐UNX as was also asymmetric dimethylarginine, an inhibitor of nitric oxide synthase that competes with l‐arginine and is a marker for renal failure. Interestingly, the UNX‐induced blood pressure increase was prevented by supplementation of the diet with 5% of the l‐arginine precursor, l‐citrulline. Because l‐arginine is metabolized in the kidney and other peripheral tissues by arginase‐2, we tested whether the lack of this metabolic pathway also compensates for decreased l‐arginine production in the kidney and/or for local nitric oxide synthase inhibition and consecutive blood pressure increase. Indeed, upon uninephrectomy, arginase‐2 knockout mice (Arg‐2−/−) neither displayed an increase in asymmetric dimethylarginine and l‐citrulline plasma levels nor a significant increase in blood pressure.
Conclusions
UNX leads to a small increase in blood pressure that is prevented by l‐citrulline supplementation or arginase deficiency, 2 measures that appear to compensate for the impact of kidney mass reduction on l‐arginine metabolism.
Keywords: arginine, arginine metabolism, blood pressure, kidney, l‐citrulline, sex differences, telemetry
Subject Categories: Nephrology and Kidney, High Blood Pressure, Hypertension, Physiology
Clinical Perspective
What Is New?
Uninephrectomy (UNX) causes in female mice an increase in plasma asymmetric dimethyl arginine, l‐citrulline, and blood pressure.
This UNX‐induced blood pressure increase is attenuated by l‐citrulline supplementation and in arginase‐2 deficient (Arg‐2−/−) mice.
What Are the Clinical Implications?
Experiments performed in mice suggest that UNX‐mediated kidney mass reduction interferes with l‐arginine metabolism and thereby with blood pressure regulation, in particular in females.
Asymmetric dimethyl arginine is suggested to mediate UNX‐induced blood pressure increase by competing with l‐arginine for nitric oxide synthesis.
Dietary supplementation with the l‐arginine precursor, l‐citrulline, or deletion of l‐arginine metabolizing enzyme arginase‐2 prevents blood pressure dysregulation in this murine model of live kidney donation.
Future studies in animal models and humans are required to clarify the apparent sex difference regarding effects of UNX on l‐arginine metabolism and blood pressure control.
Removal of 1 kidney (uninephrectomy; UNX) causes a hypertrophy of the remnant kidney without an increase in the number of nephrons given that in humans nephrogenesis ceases at birth.1 Until very recently, not much was known about what triggers this compensatory enlargement of the remaining kidney post‐UNX and regulates its extent. Recently, the laboratory of R.C. Harris2 has shown that the observed increase in blood flow through the remnant kidney and the consecutively increased amino acid delivery activates a class III phosphatidylinositol‐3 kinase that induces mechanistic target of rapamycin complex 1 (mTORC1)/p70 ribosomal protein kinase 1 (S6K1) signaling and thereby the compensatory increase in kidney size. However, the compensatory growth of the remnant kidney amounts to ≈20%3, 4 such that not only the filtration and transport capacities, but also the metabolic functions are presumably not fully compensated.
l‐arginine is 1 of the 20 naturally occurring amino acids and is categorized as a conditionally essential amino acid in humans. It plays an important role, acting as a precursor for a variety of physiologically important substances. For l‐arginine homeostasis, the proximal tubule of the kidney not only fulfills the typical epithelial transport function consisting in amino acid reabsorption from the primary urine, but also plays a central role in its metabolism. In particular, the majority of l‐arginine is produced in the kidney from circulating l‐citrulline. Indeed, when l‐arginine is absorbed from food, it is, to a large extent, metabolized to l‐citrulline in the intestine.5 This metabolite of arginine then escapes further modifications when passing through the liver, unlike l‐arginine that is efficiently metabolized there by arginase‐1 into urea and l‐ornithine that, in turn, may be further metabolized for producing polyamines or l‐proline. Circulating l‐citrulline, in contrast, is, to a large extent, metabolized back to l‐arginine, mostly in the kidney.6 The other major metabolic pathway using l‐arginine as substrate is the production of nitric oxide (NO) by the various NO synthases. Whereas for the function of NO synthase the availability of l‐arginine has been shown to be limiting, methylated forms of l‐arginine, in contrast, inhibit NO synthesis.7, 8 Methylation of protein arginines, namely monomethylation to NG monomethyl‐l‐arginine and dimethylation to the symmetric or asymmetric NGNGdimethyl‐l‐arginines (symmetric dimethylarginine or asymmetric dimethylarginine [ADMA]) are post‐translational modifications that play an important role, for instance, in proteins involved in transcriptional regulation, RNA metabolism, and signal transduction. Breakdown of these methylated proteins releases the free methylated arginines of which both ADMA and NG monomethyl‐l‐arginine can inhibit all isoforms of NO synthase.9 These competitive inhibitors are found to be elevated and of particular consequence in chronic kidney disease.10, 11 Furthermore, population studies have shown that symmetric dimethylarginine levels correlate with cardiovascular mortality.12 Therefore, a therapy used sometimes in patients suffering from chronic kidney disease or cardiovascular disease is to increase the bioavailability of l‐arginine. This may be done by administering l‐citrulline, because this l‐arginine precursor escapes modifications in the intestine or the liver and can be processed in the kidneys to produce l‐arginine. This increased l‐arginine may then be used for NO production, in particular by endothelial NO synthase (NOS3), and thereby it is expected to contribute to the alleviation of high blood pressure.13, 14, 15
Another enzyme that plays a role in l‐arginine metabolism, and thus its availability, is arginase, which catalyzes the breakdown of l‐arginine to urea and l‐ornithine. Arginase is present in 2 isoforms. Arginase‐1 is cytosolic and predominantly expressed in the liver. Its dysfunction leads to argininemia, which is characterized by hyperammonemia.16 Arginase‐2, on the other hand, is apparently mostly mitochondrial and expressed in non‐hepatic tissues, particularly in the kidney.17 The physiological role of this enzyme continues to be poorly understood, although to better understand its role, arginase‐2 knockout (Arg‐2−/−) mice were generated more than a decade ago.18 These mice were viable and no major differences were detected compared with wild‐type mice. Nonetheless, plasma l‐arginine levels were elevated, which indicates a role of arginase‐2 in systemic l‐arginine homeostasis.
Various studies in patients and with animal models have indicated that UNX as performed in kidney donors may lead to changes in different parameters, including, next to glomerular filtration rate, also blood pressure.19, 20, 21 UNX in adult mice mimics the situation of living kidney donation of humans, a condition that becomes increasingly frequent because of the lack of sufficient deceased donors and increased demand.
In this study, we tested whether the decrease in renal mass consecutive to UNX influenced renal l‐arginine production and transport and thereby also the control of blood pressure for which l‐arginine‐derived NO plays an important role. Because a first series of experiments had revealed, only in female mice, a clear effect of UNX on blood pressure, subsequent experiments were performed only with females. Two strategies were then used to partially compensate the effects of a presumably decreased renal l‐arginine production post‐UNX, supplementation with l‐citrulline, and the use of Arg‐2−/− mice.
Materials and Methods
The authors declare that all supporting data are available within the article and its online supplementary file.
Animals
Experiments were performed in C57/BL6 mice purchased from Charles River Laboratories (Munich, Germany) and Arg‐2−/− mice,18 which have been back‐crossed into the same background.22 All procedures for mice handling and experimental interventions were performed in accord with Swiss Animal Welfare laws and approved by the Cantonal Veterinary Office, Zürich. Before the start of experimentation, animals were allowed to acclimatize for a 2‐week period. During the experiments, animals had access to food and water ad libitum. Between 7 and 20 animals were included in each experimental group (see Results). The normal diet contained 18% protein, whereas for the l‐citrulline supplementation experiments, the normal diet was supplemented with 5% (w/w) of l‐citrulline. For the l‐citrulline supplementation study, animals were placed on the l‐citrulline diet for 8 days and then on the normal diet for 8 days presurgery. Post‐UNX, animals continued on the normal diet and were again switched to the l‐citrulline diet for 8 days. Blood pressure was measured on the l‐citrulline diet on days −8 and 8 and on the normal diet on days −1 and 8.
Uninephrectomy
Mice (10–12 weeks) were randomly assigned to undergo UNX or sham surgery. For the surgery, mice were briefly anesthetized with 3% isoflurane and then maintained with 1% to 2% isoflurane with oxygen as the carrier. Briefly, the region undergoing surgery was shaved and an incision was made on the left side. The adrenal was carefully separated from the upper pole of the kidney before ligating the renal pedicle. The left kidney was then removed and the peritoneum and the skin sutured. For sham surgeries, the same process was followed and the kidneys were exposed but not ablated.
Metabolic Cage Measurements
Mice were placed individually in metabolic cages (Techniplast, Buguggiate, Italy) with food and drinking water available ad libitum first for 8 hours on 3 consecutive days for adaptation. Then, after 1 day in a normal cage, they were placed again in the metabolic cages for a 24‐hour collection period of feces and urine (under mineral oil), during which food and water consumption and body weight were measured. At the end of the 24‐hour period, after removing access to food for 30 minutes, blood was collected from the tail vein of mice and plasma was prepared (5000 RCF, 5 minutes, 4°C) and frozen for further analysis.
Blood Pressure Measurement With Tail Cuff
Systolic blood pressure was measured using a noninvasive tail‐cuff method (BP2000; Visitech Systems, Apex, NC) in restrained mice as described previously.23 Mice were adapted for 5 days before the start of the experiment. This adaptation has been shown to reduce stress‐related side effects.23 In each case, 10 preliminary measurements were conducted, followed by 15 actual measurements. Averages of systolic blood pressure (SBP) measurements having SDs less than 10 mm Hg were considered for further analysis. Measurements were taken 2 to 3 hours after the beginning of the light phase.
Blood Pressure Measurement by Telemetry
Radiotelemetry is considered to be the gold standard for measuring blood pressure in rodents, initially in rats and then also in mice.24 Manufacturer instructions were followed, and the transmitter was implanted as previously described.25 Briefly, mice were anesthetized with the aid of isoflurane and implanted with a PA‐C‐10 transmitter (Data Sciences International [DSI], St. Paul, MN). The catheter was implanted in the carotid artery taking care to place the pressure‐sensitive tip in the aortic arch. Mice were given analgesic for 3 days postsurgery along with pre‐emptive analgesic. One week after telemetry implantation, a test measurement was conducted to ensure that animals had recovered and that the circadian clock was back to normal. UNX was performed 8 days after these measurements.
Measurements were recorded with Dataquest ART (version 3.1) and RespiRate (DSI). Recordings were made for a 3‐day period every week. Recordings were continuously taken for 30 seconds every 5 minutes during this time. Systolic, diastolic, and mean blood pressure, heart beat, and activity were analyzed separately for the light versus dark phases.
Plasma Amino Acid Measurements
Amino acids were measured after extraction with methanol without derivatization (Neobase Kit; Perkin Elmer, Turku, Finland) on an XEVO‐TQD tandem mass spectrometer (Waters, Milford, MA). Briefly, 4 μL of plasma or urine were spotted onto prepunched plain filter paper (Ahlstom 226) in 96‐well plain uncoated microtiter plates. Amino acids were extracted with 100 μL of methanolic extraction solution containing stable isotope‐labeled internal standards by shaking for 45 minutes at 45°C. Twenty microliters of extract were then injected with a flow rate of 10 μL/min directly into the tandem mass spectrometer and measured afterward in multiple reaction monitoring mode. In addition to the amino acids, which are incorporated in the Neobase kit, ADMA was added to the multiple reaction monitoring experiment with the following settings: parent ion, 203.1 Da; daughter ion, 46.1 Da; dwell time, 0.05 seconds; cone voltage, 26 V; and collision energy, 37 eV.
Immunofluorescence Staining and Confocal Microscopy
Rabbit antibody against arginase‐2 (sc‐20151) was from Santa Cruz Technology Inc (Nunningen, Switzerland); Alexa Fluor 488–conjugated goat antirabbit IgG (H+L) secondary antibody (A‐11008) was from Invitrogen/Thermo Fisher Scientific (Waltham, MA).
Kidneys from wild‐type and Arg‐2−/− mice were isolated and fixed with 3.7% PFA and embedded in paraffin. After deparaffinization in xylene (3 times, 5 minutes for each), hydration in ethanol (twice in 100% ethanol, twice in 95% ethanol, and once in 80%, 70%, 50% ethanol for 5 minutes, sequentially), heat‐induced epitope retrieval in Tris‐EDTA buffer (10 mmol of Tris Base, 1 mmol/L of EDTA, and 0.05% Tween‐20; pH 9.0) was performed in a pressure cooker for 3 minutes to unmask antigens present in renal tissue. Tissue sections (5 μm) were then blocked with 10% BSA in PBS for 1 hour and followed by an incubation with primary antibodies (arginase‐2 1:100, B0AT3 1:200) overnight at 4°C and subsequently exposed to fluorescence‐labeled secondary antibodies (1:400) at room temperature for 2 hours, respectively. Immunofluorescence signals were visualized under Leica's DIM6000 confocal microscope (Leica Microsystems, Wetzlar, Germany).
Statistical Analysis
GraphPad Prism software (version 6; GraphPad Software Inc, San Diego, CA) was used to perform statistical analysis. Differences between groups were determined by Student t test for 2 groups (paired in case the same animal was being followed over time) or 1‐way repeated‐measures ANOVA for ≥3 groups with a Dunnett post‐hoc test versus a control group. Values are presented as means±SEM and were considered different when P<0.05.
Results
UNX Leads to Increased Blood Pressure in Female Mice
In a first series of experiments, wild‐type C57BL6 mice were subjected to left UNX or sham surgery as described in Materials and Methods section, and their blood pressure was measured by the tail‐cuff technique pre‐ and postsurgery. Both female (Figure 1A) and male (Figure 1B) UNX mice showed an increase in weight of the remnant right kidney. UNX provoked an increase in SBP that was significant only in female (Figure 1C) but not in male mice (Figure 1D). Therefore, and also because ADMA was increased only in females (Figure S1 and below), further experiments were performed with female mice. SBP in female mice presurgery and 8, 10, and 30 days postsurgery was 122.2±2.1, 131.6±2.5, 134.9±2.5, and 135.6±2.6 mm Hg, respectively (Figure 1E). Importantly, sham‐operated animals displayed no significant change in mean SBP during this period (123±0.5 mm Hg).
Figure 1.
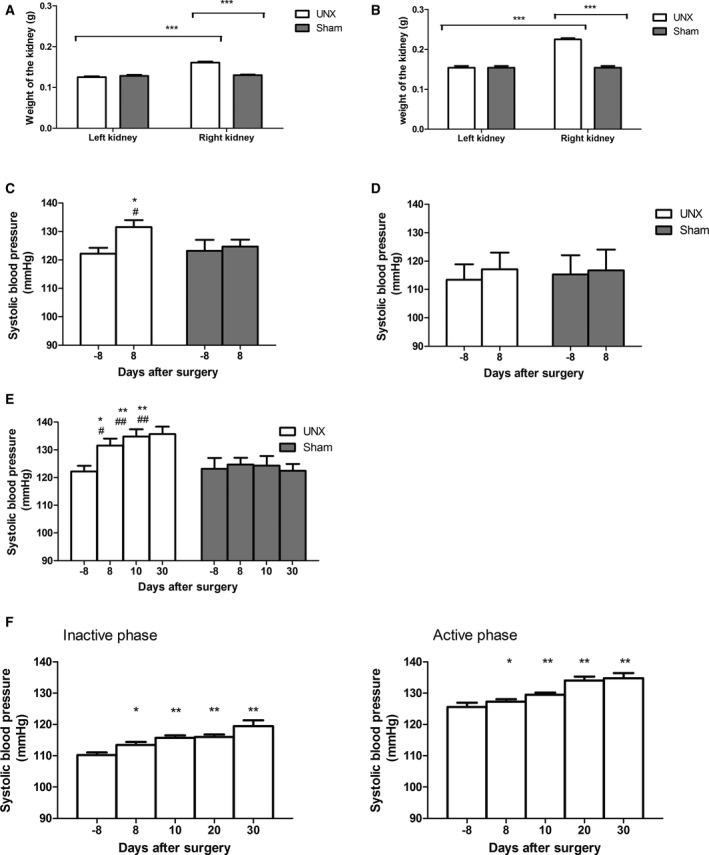
Effect of uninephrectomy (UNX) on remnant kidney weight and systolic blood pressure. A and B, UNX caused an increase in weight of the remnant right kidney in both male and female mice (white bars) compared with pre‐UNX value (left kidney) and to the right kidney of sham‐operated mice (gray bars; n=10). ***P<0.001. C and D, Measurements performed by the tail‐cuff technique at an age of 10 to 12 weeks showed (C) in female mice submitted to UNX (white bars; n=10–20) an increase in systolic blood pressure and no change in sham‐operated mice (gray bars; n=10–20). *P<0.05 compared with pre‐UNX; # P<0.05 compared with sham. D, Male mice submitted to UNX (white bars) as sham‐operated ones (gray bars; each n=10) showed no blood pressure change. E, Female mice submitted to UNX (white bars) continued to show an increase in systolic blood pressure up to 30 days post‐UNX when compared with sham‐operated animals (gray bars) or their values pre‐UNX. *P<0.05, **P<0.01 compared with pre‐UNX; # P<0.05, ## P<0.01 compared with sham. F, Telemetric blood pressure measurements performed in 10‐ to 12‐week‐old female mice submitted to UNX 2 weeks after device implantation confirmed the post‐UNX increase in blood pressure that was observed during both the inactive light and active dark phases (n=7–10). *P<0.05, **P<0.01, comparing before and after surgery.
To verify the results obtained by the tail‐cuff technique, blood pressure measurements were performed in female mice using telemetry. Similarly to the observation made with the tail‐cuff technique, SBP was found to be increased post‐UNX in female mice, actually both during the inactive and the active phase (light/dark cycle). Representative traces from 1 mouse (8 days pre‐UNX and 10 days post‐UNX) are shown in Figure S2. Specifically, mean SBP was increased within 30 days postsurgery from 110.2±0.9 to 119.4±1.8 mm Hg and from 125.6±1.3 to 134.8±1.6 mm Hg during the inactive and active phases, respectively (Figure 1F). Similarly, diastolic pressure was increased from 81.5±0.8 to 91.5±0.9 mm Hg and from 94.6±0.8 to 103.2±1.8 mm Hg during the inactive and active phases, respectively.
Impact of UNX on Plasma Amino Acid Levels
Plasma levels of amino acids before and at various points after surgery were measured from wild‐type mice subjected to UNX and from control‐operated mice (sham) to identify potential changes attributed to UNX and not to a general effect of the surgery, anesthesia, analgesia, or environmental factors, etc. Whereas surgery appeared to impact onto the level of several of the measured amino acids and metabolites, UNX per se did not modify the level of any of the measured proteinogenic amino acids (Figure S3). In contrast, the level of ADMA and that of the l‐arginine precursor, l‐citrulline, were both significantly altered by UNX (Figure 2A and 2B). However, the level of arginine itself and of the other tested amino acids involved in its metabolism, such as l‐ornithine and the methylated l‐arginines NG monomethyl‐l‐arginine and symmetric dimethylarginine, was not differentially affected by UNX versus sham operation (Figure S3).
Figure 2.
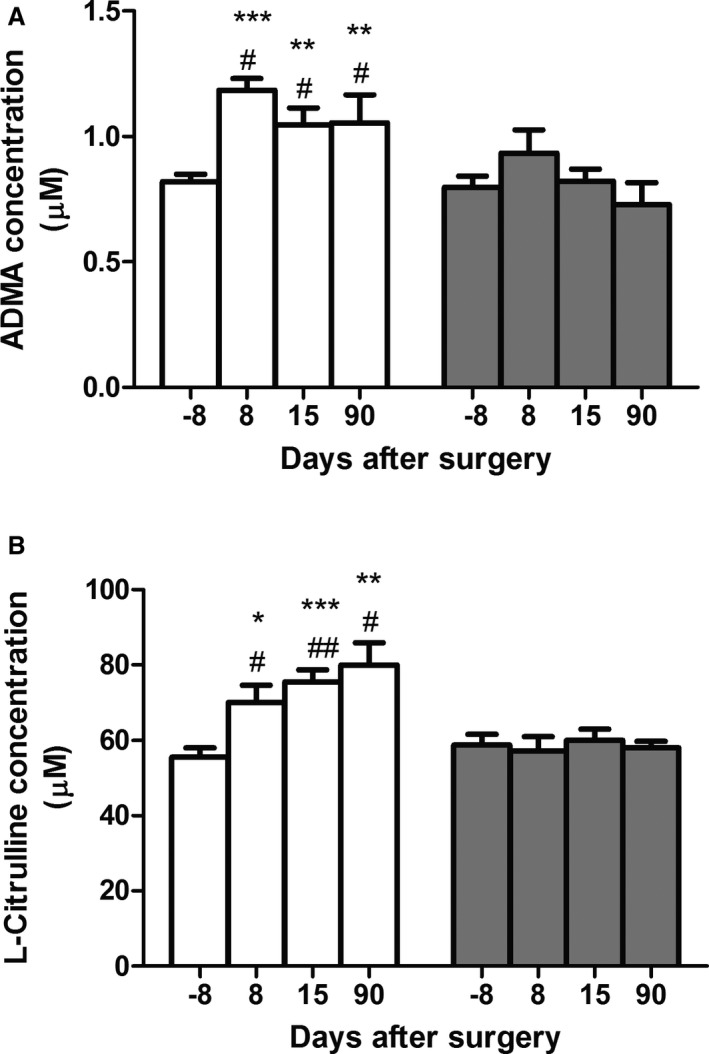
Effect of uninephrectomy (UNX) on plasma asymmetric dimethylarginine (ADMA) and l‐citrulline concentrations. UNX animals (white bars) showed (A) an increase in plasma ADMA and (B) l‐citrulline levels postsurgery. *P<0.05, **P<0.01, ***P<0.001 comparing UNX before and after surgery; # P<0.05, ## P<0.01 comparing with sham (gray bars). n=7 to 28 animals per group.
Citrulline Supplementation Largely Prevents Blood Pressure Increase Post‐UNX
l‐citrulline has been used for therapeutic purposes to increase the availability of l‐arginine because it is not metabolized in the liver and can be used by the kidney to produce l‐arginine.26 We tested the effect of this l‐arginine precursor on blood pressure by first placing kidney‐intact mice on a diet supplemented with 5% l‐citrulline (w/w) and switched then to a normal diet. Measurements of SBP performed by the tail‐cuff method revealed that the l‐citrulline‐supplemented diet and the switch back to control diet did not impact on blood pressure of kidney‐intact mice (Figure 3). Half of these animals were then subjected to UNX and the other half to sham surgery. Tail‐cuff blood pressure was again recorded when mice were maintained first on control‐ and then on l‐citrulline‐supplemented diet. As expected, under normal diet, UNX mice showed an increase in SBP compared with sham‐operated mice. When the diet was switched again to l‐citrulline supplementation, SBP decreased nonsignificantly in UNX mice, however reaching the same level as in sham‐operated mice. From this experiment, we conclude that l‐citrulline supplementation prevents UNX‐induced blood pressure increase.
Figure 3.
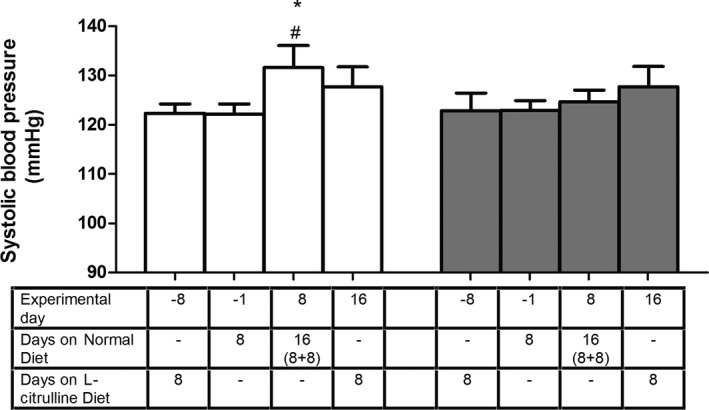
Effect of 5% l‐citrulline supplemented diet (CD) on uninephrectomy (UNX)‐induced systolic blood pressure changes. On a normal diet post‐UNX (white bars), animals showed an elevated systolic blood pressure as in previous cases (measured by the tail‐cuff method), but when they were shifted to an l‐citrulline supplemented diet, this elevation in blood pressure was no longer observed, indicating that l‐citrulline supplementation prevents the blood pressure increase observed post‐UNX. *P<0.05 comparing UNX before and after surgery; # P<0.05 comparing to corresponding sham (gray bars). n=9 animals per group.
Arg−/− Mice Do Not Show an Increase in Blood Pressure Post‐UNX
We hypothesized that in the absence of arginase‐2, more l‐arginine might be available for NO synthesis and thus the impact of UNX on blood pressure would be reduced. Using arginase‐2 antibodies, we first confirmed that arginase‐2 is indeed expressed in the kidney, and show that this is mostly in the straight part of the proximal tubule cells as confirmed by the expression of the luminal B0AT3 amino acid transporter in the same cells.27, 28 As expected, arginase‐2 expression is absent in Arg‐2−/− mice (Figure 4).
Figure 4.
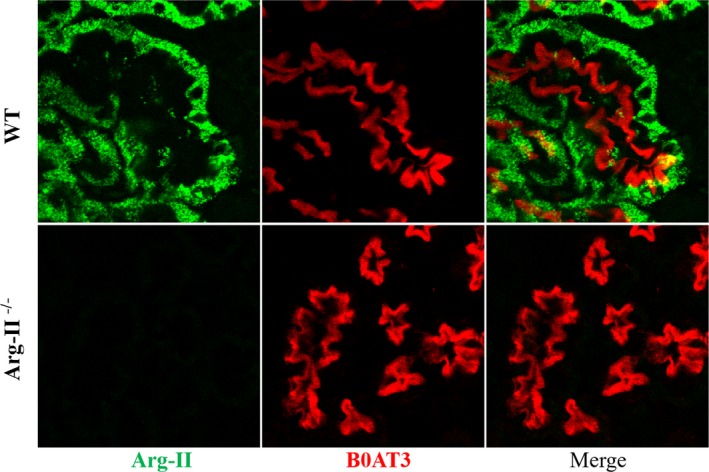
Immunofluorescence imaging of arginase‐2 and B0AT3. Confocal immunofluorescence staining on consecutive kidney sections of wildtype and Arg‐2−/− mice show Arg‐2 (green) staining in cells of wild‐type (WT) mice positive for the luminal proximal tubule amino acid transporter B0AT3 (Slc6a18) highly expressed in the S3 straight segments of the proximal tubule. Arg‐II indicates arginase‐2.
We then tested the impact of UNX on blood pressure in Arg‐2−/− mice by telemetry. Unlike wild‐type mice, they did not show a significant increase in SBP after UNX surgery in either the inactive or active phase (Figure 5A and 5B). During the inactive phase, SBP of unoperated Arg‐2−/− mice appeared to be more elevated than that of wild‐type mice (compare with Figure 1F), in accord with a previous report.14 Importantly, however, this value (119.9±1.7 presurgery) remained unchanged upon UNX (119.7±1.4 mm Hg 20 days postsurgery; Figure 5A). During the active phase, their SBP nonsignificantly increased from 126.5±3.0 presurgery to 132.3±1.5 mm Hg 20 days postsurgery (Figure 5B). Diastolic pressure was unchanged from 112.3±1.5 to 112.4±1.6 mm Hg and nonsignificantly increased from 114.7±1.8 to 119.3±1.8 mm Hg during the inactive and active phases, respectively. Also, plasma levels of ADMA and l‐citrulline, which were increased in wild‐type animals, remained unchanged in Arg‐2−/− mice post‐UNX surgery (Figure 5C and 5D), suggesting that both the alterations of arginine metabolism and of blood pressure were prevented by the lack of arginase‐2.
Figure 5.
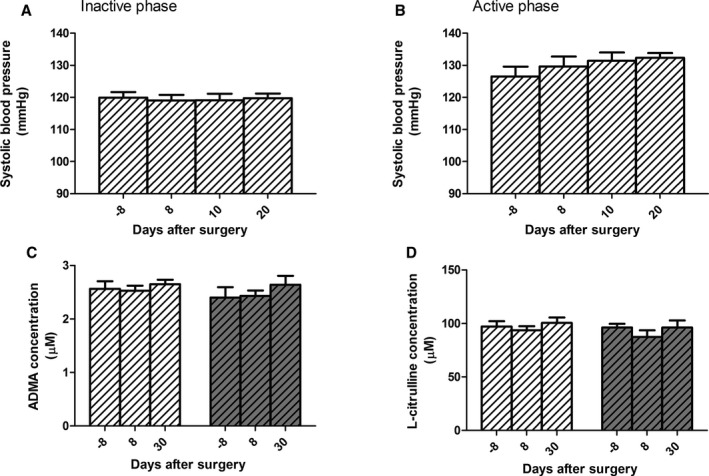
No uninephrectomy (UNX)‐induced changes in blood pressure and plasma asymmetric dimethylarginine (ADMA) and l‐citrulline levels in arginase‐2 knockout (Arg‐2−/−) mice. Arg‐2−/− mice displayed no significant increase in blood pressure post‐UNX (dashed bars with white background) both in the inactive (A) and in active (B) phases as measured by telemetry. They showed also no changes in plasma levels of ADMA (C) and l‐citrulline (D) compared with sham‐operated mice (dashed bars with gray background). n=9 animals per group.
Discussion
It has been known for decades that renal mass reduction by UNX induces a compensatory hypertrophy of the remnant kidney. However, only in recent years have actual mechanisms involved in regulation of this compensatory growth been reported (Chen et al,2 references therein and Introduction). Also, UNX is increasingly often performed in humans for kidney donation because of the demand linked to the continuously high incidence of end‐stage renal disease, lack of sufficient deceased donor kidneys, and comparatively better outcome of transplantation with kidneys from live donors.29, 30 In view of the fact that l‐arginine production from l‐citrulline takes place primarily in the proximal tubule of the kidney,31 we hypothesized that a decrease in renal mass would have an adverse effect on l‐arginine homeostasis and, consequently, on blood pressure regulation by decreasing substrate availability for NO synthesis.
Interestingly, we observed, in our initial experiments, that UNX induced a significant increase in blood pressure in female mice, but not in male mice. This sex difference is per se an interesting observation that we, however, did not follow, focusing for the remainder of the study on female mice. Actually, it is interesting to note that published studies with precise blood pressure measurements pre‐ and post‐UNX were as yet performed, to our knowledge, in male mice (see, eg, Crowley et al32, 33). We observed that compensatory kidney growth was possibly more effective in males than females (Figure 1A and 1B, difference not significant), but whether this might impact onto the differential blood pressure response observed in this study is not clear.34, 35 Actually, we are not aware of previous studies indicating that kidney mass reduction leads to a sex‐specific blood pressure increase. Concerning human kidney donors, addressing the question of whether UNX impacts on blood pressure control has been debated in particular because of the lack of appropriate control groups. A recent study on the Swiss Organ Living‐Donor Health Registry comparing the occurrence of hypertension with appropriately corrected estimates from the Framingham hypertension risk score suggested that kidney donation increases the risk of hypertension by 3.6% after 1 year (Thiel et al36 and references therein), This study did, however, not mention a possible sex difference in the occurrence of hypertension. Also, the question of sex differences in renal transplant outcomes has been debated and addressed recently in a very large retrospective study on the Scientific Registry of Transplant Recipients database. Interestingly, this study revealed an increased number of graft failure in (young) female versus male recipients. However, there is as yet no explanation for this sex difference, and the question of kidney growth and blood pressure was not addressed in this study.37
The endothelial dysfunction observed in chronic renal failure is often considered to be resulting from a decrease in l‐arginine bioavailability for NO synthesis that is related to an increase in competing l‐arginine analogues.38, 39 Indeed, increased ADMA levels might explain the arginine paradox, namely the observation that administering acutely l‐arginine (or l‐citrulline) to patients with cardiovascular and renal diseases increases their NO production and thereby improves their endothelial function, although plasma levels of l‐arginine are similar to those of healthy individuals and much higher than the Km of NO synthases.40, 41 However, ADMA levels tend to be significantly higher in these patients compared with healthy individuals, and this modified l‐arginine functions as a competitive inhibitor of l‐arginine for NO synthesis.7, 42 Because our experiments showed an unchanged l‐arginine concentration in plasma, we investigated whether it may be a decrease of its relative availability for NO synthesis attributed to the competition by ADMA that interferes with blood pressure control after kidney mass reduction. To potentially increase the local intracellular availability of l‐arginine for NO synthesis, we tested the effect of dietary l‐citrulline, the precursor of kidney‐produced l‐arginine that is not catabolized upon passage through the liver.43 Despite the fact that l‐citrulline was actually already increased, to some extent, post‐UNX (≈ +30%), this additional dietary l‐citrulline prevented, to a large extent, the UNX‐induced increase in blood pressure, supporting this hypothesis.
Yet another approach to testing the role of l‐arginine availability on the observed UNX‐induced blood pressure increase was to test the impact of UNX in Arg‐2−/− mice that lack an important intracellular pathway consuming l‐arginine. Arg‐2−/− mice indeed did not develop high blood pressure post‐UNX, again supporting the hypothesis that the local availability of l‐arginine is a limiting factor for NO synthesis and thus blood pressure control. Taken together, the l‐citrulline diet and Arg‐2−/− experiments support our hypothesis that renal mass reduction leads to an elevated blood pressure that is mediated by the defective bioavailability of l‐arginine for NO synthesis. Based on the plasma amino acid and dimethylated arginine values measured in UNX mice, this defective bioavailability of l‐arginine does not appear to be attributed primarily to a decrease in l‐arginine concentration related to the kidney mass reduction. It is rather the increased level of ADMA that is suggested to play a central role in mediating the observed blood pressure increase. This ADMA increase may be attributed, to a large extent, to a decreased availability of its metabolizing enzyme, dimethylarginine dimethylaminohydrolase, whose overall amount is presumably altered by renal mass reduction. Thus, in UNX female mice, it is presumably the increase in ADMA that mediates the observed increase in blood pressure by interfering with endothelial NO synthesis.
Conclusions and Perspectives
Taken together, this study links metabolic effects of moderate kidney mass reduction, in particular as regards l‐arginine production from l‐citrulline and metabolism by arginase‐2 as well as ADMA metabolism, to blood pressure control. The present experiments performed in female mice suggest possible interventions such as dietary supplementation with the l‐arginine precursor, l‐citrulline, and the inhibition of arginase‐2 function as means of preventing blood pressure dysregulation in cases of kidney mass reduction such as upon live kidney donation. Future experiments will need to clarify the apparent sex difference regarding effects of nephrectomy on l‐arginine metabolism and blood pressure control both in animal models and humans.
Sources of Funding
The research leading to these results was supported the Swiss National Centre for Competence in Research NCCR Kidney.CH to Verrey and the European Community's Seventh Framework Programme (FP7/2007‐2013) under grant agreement No. 246539 to Pillai.
Disclosures
None.
Supporting information
Figure S1. Plasma ADMA and l‐citrulline levels change upon UNX, compared with sham‐operated animals, only in female mice. The surgery performed at day 0 induced an increase in the plasma concentration of ADMA and l‐citrulline in both UNX‐ and sham‐operated male mice. An increase was observed for plasma ADMA and l‐citrulline levels only in female UNX mice. White and gray bars indicate UNX‐ and sham‐operated animals, respectively. ***P<0.001, comparing before and after surgery; # P<0.05, ## P<0.01 comparing UNX‐ and sham‐operated animals. n=9 to 18 animals per group.
Figure S2. Representative traces from 1 mouse (8 days before and 10 days after UNX). Green, brown, and red traces indicate diastolic, mean, and systolic blood pressure, respectively.
Figure S3. No changes in plasma arginine, ornithine, SDMA, and NMMA levels between UNX‐ and sham‐operated mice. Surgery performed at day 0 induced changes in plasma concentration of several amino acids that were, however, not different in UNX‐ and sham‐operated animals. White and gray bars indicate UNX‐ and sham‐operated animals, respectively. *P<0.05, **P<0.01, ***P<0.001, comparing before and after surgery. n=9 to 18 animals per group.
Acknowledgments
We are grateful to the Zurich Integrative Rodent Physiology (ZIRP) for the support provided during animal experiments.
(J Am Heart Assoc. 2018;7:e008025 DOI: 10.1161/JAHA.117.008025.)29478971
References
- 1. Kramp RA, MacDowell M, Gottschalk CW, Oliver JR. A study by microdissection and micropuncture of the structure and the function of the kidneys and the nephrons of rats with chronic renal damage. Kidney Int. 1974;5:147–176. [DOI] [PubMed] [Google Scholar]
- 2. Chen JK, Nagai K, Chen J, Plieth D, Hino M, Xu J, Sha F, Ikizler TA, Quarles CC, Threadgill DW, Neilson EG, Harris RC. Phosphatidylinositol 3‐kinase signaling determines kidney size. J Clin Invest. 2015;125:2429–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Williams CR, Wynne BM, Walker M, Hoover RS, Gooch JL. Compensatory renal hypertrophy following uninephrectomy is calcineurin‐independent. J Cell Mol Med. 2014;18:2361–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen KW, Wu MW, Chen Z, Tai BC, Goh YS, Lata R, Vathsala A, Tiong HY. Compensatory hypertrophy after living donor nephrectomy. Transplant Proc. 2016;48:716–719. [DOI] [PubMed] [Google Scholar]
- 5. Levillain O, Rabier D, Duclos B, Gaudreau P, Vinay P. l‐arginine metabolism in dog kidney and isolated nephron segments. Metabolism. 2008;57:9–23. [DOI] [PubMed] [Google Scholar]
- 6. Cynober L, Moinard C, De Bandt JP. The 2009 ESPEN Sir David Cuthbertson. Citrulline: a new major signaling molecule or just another player in the pharmaconutrition game? Clin Nutr. 2010;29:545–551. [DOI] [PubMed] [Google Scholar]
- 7. Baylis C. Arginine, arginine analogs and nitric oxide production in chronic kidney disease. Nat Clin Pract Nephrol. 2006;2:209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol. 2007;34:906–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leiper J, Vallance P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc Res. 1999;43:542–548. [DOI] [PubMed] [Google Scholar]
- 10. Sharma M, Zhou Z, Miura H, Papapetropoulos A, McCarthy ET, Sharma R, Savin VJ, Lianos EA. ADMA injures the glomerular filtration barrier: role of nitric oxide and superoxide. Am J Physiol Renal Physiol. 2009;296:F1386–F1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. MacAllister R, Rambausek M, Vallance P, Williams D, Hoffmann KH, Ritz E. Concentration of dimethyl‐l‐arginine in the plasma of patients with end‐stage renal failure. Nephrol Dial Transplant. 1996;11:2449–2452. [DOI] [PubMed] [Google Scholar]
- 12. Atzler D, Wallaschofski H, Schwedhelm E, Nauck M, Volker U, Kroemer HK, Volzke H, Boger RH, Friedrich N, Dorr M. Incidence of all‐cause and cardiovascular mortality predicted by symmetric dimethylarginine in the population‐based study of health in pomerania. Circulation. 2013;128:A14456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vliet BN, Chafe LL, Montani JP. Characteristics of 24 h telemetered blood pressure in eNOS‐knockout and C57Bl/6J control mice. J Physiol. 2003;549:313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. [DOI] [PubMed] [Google Scholar]
- 15. Romero MJ, Platt DH, Caldwell RB, Caldwell RW. Therapeutic use of citrulline in cardiovascular disease. Cardiovasc Drug Rev. 2006;24:275–290. [DOI] [PubMed] [Google Scholar]
- 16. Snyderman SE, Sansaricq C, Chen WJ, Norton PM, Phansalkar SV. Argininemia. J Pediatr. 1977;90:563–568. [DOI] [PubMed] [Google Scholar]
- 17. Magri E, Baldoni G, Grazi E. On the biosynthesis of creatine. Intramitochondrial localization of transamidinase from rat kidney. FEBS Lett. 1975;55:91–93. [DOI] [PubMed] [Google Scholar]
- 18. Shi O, Morris SM, Zoghbi H, Porter CW, O'Brien WE. Generation of a mouse model for arginase II deficiency by targeted disruption of the arginase II gene. Mol Cell Biol. 2001;21:811–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boudville N, Prasad GV, Knoll G, Muirhead N, Thiessen‐Philbrook H, Yang RC, Rosas‐Arellano MP, Housawi A, Garg AX; Donor Nephrectomy Outcomes Research (DONOR) Network . Meta‐analysis: risk for hypertension in living kidney donors. Ann Intern Med. 2006;145:185–196. [DOI] [PubMed] [Google Scholar]
- 20. Rodríguez‐Gómez I, Wangensteen R, Pérez‐Abud R, Quesada A, Raimundo G, Osuna A, O'Valle F, de Dios Luna J, Vargas F. Long‐term consequences of uninephrectomy in male and female rats. Hypertension. 2012;60:1458–1463. [DOI] [PubMed] [Google Scholar]
- 21. Moody WE, Ferro CJ, Edwards NC, Chue CD, Lin EL, Taylor RJ, Cockwell P, Steeds RP, Townend JN; CRIB‐Donor Study Investigators . Cardiovascular effects of unilateral nephrectomy in living kidney donors. Hypertension. 2016;67:368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ming XF, Rajapakse AG, Yepuri G, Xiong Y, Carvas JM, Ruffieux J, Scerri I, Wu Z, Popp K, Li J, Sartori C, Scherrer U, Kwak BR, Montani JP, Yang Z. Arginase II promotes macrophage inflammatory responses through mitochondrial reactive oxygen species, contributing to insulin resistance and atherogenesis. J Am Heart Assoc. 2012;1:e000992 DOI: 10.1161/JAHA.112.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krege JH, Hodgin JB, Hagaman JR, Smithies O. A noninvasive computerized tail‐cuff system for measuring blood pressure in mice. Hypertension. 1995;25:1111–1115. [DOI] [PubMed] [Google Scholar]
- 24. Carlson SH, Wyss JM. Long‐term telemetric recording of arterial pressure and heart rate in mice fed basal and high NaCl diets. Hypertension. 2000;35:e1–e5. [DOI] [PubMed] [Google Scholar]
- 25. Huetteman DA, Bogie H. Direct blood pressure monitoring in laboratory rodents via implantable radio telemetry. Methods Mol Biol. 2009;573:57–73. [DOI] [PubMed] [Google Scholar]
- 26. Persson P, Fasching A, Teerlink T, Hansell P, Palm F. l‐citrulline, but not l‐arginine, prevents diabetes mellitus‐induced glomerular hyperfiltration and proteinuria in rat. Hypertension. 2014;64:323–329. [DOI] [PubMed] [Google Scholar]
- 27. Nikolaeva S, Ansermet C, Centeno G, Pradervand S, Bize V, Mordasini D, Henry H, Koesters R, Maillard M, Bonny O, Tokonami N, Firsov D. Nephron‐specific deletion of circadian clock gene Bmal1 alters the plasma and renal metabolome and impairs drug disposition. J Am Soc Nephrol. 2016;27:2997–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Romeo E, Dave MH, Bacic D, Ristic Z, Camargo SM, Loffing J, Wagner CA, Verrey F. Luminal kidney and intestine SLC6 amino acid transporters of B0AT‐cluster and their tissue distribution in Mus musculus . Am J Physiol Renal Physiol. 2006;290:F376–F383. [DOI] [PubMed] [Google Scholar]
- 29. Mueller TF, Luyckx VA. The natural history of residual renal function in transplant donors. J Am Soc Nephrol. 2012;23:1462–1466. [DOI] [PubMed] [Google Scholar]
- 30. Summers DM, Watson CJ, Pettigrew GJ, Johnson RJ, Collett D, Neuberger JM, Bradley JA. Kidney donation after circulatory death (DCD): state of the art. Kidney Int. 2015;88:241–249. [DOI] [PubMed] [Google Scholar]
- 31. Levillain O, Marescau B, de Deyn PP. Guanidino compound metabolism in rats subjected to 20% to 90% nephrectomy. Kidney Int. 1995;47:464–472. [DOI] [PubMed] [Google Scholar]
- 32. Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, Spurney RF, Kim HS, Smithies O, Le TH, Coffman TM. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin‐angiotensin system. J Clin Invest. 2005;115:1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA. 2006;103:17985–17990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Azurmendi PJ, Oddo EM, Toledo JE, Martin RS, Ibarra FR, Arrizurieta EE. Sexual hormones modulate compensatory renal growth and function. Medicina (B Aires). 2013;73:513–519. [PubMed] [Google Scholar]
- 35. Schlondorff D, Trizna W, De Rosis E, Korth‐Schutz S. Effect of testosterone on compensatory renal hypertrophy in the rat. Endocrinology. 1977;101:1670–1675. [DOI] [PubMed] [Google Scholar]
- 36. Thiel GT, Nolte C, Tsinalis D. Prospective Swiss cohort study of living‐kidney donors: study protocol. BMJ Open. 2011;1:e000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lepeytre F, Dahhou M, Zhang X, Boucquemont J, Sapir‐Pichhadze R, Cardinal H, Foster BJ. Association of sex with risk of kidney graft failure differs by age. J Am Soc Nephrol. 2017;28:3014–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schwartz IF, Ingbir M, Chernichovski T, Reshef R, Chernin G, Litvak A, Weinstein T, Levo Y, Schwartz D. Arginine uptake is attenuated, through post‐translational regulation of cationic amino acid transporter‐1, in hyperlipidemic rats. Atherosclerosis. 2007;194:357–363. [DOI] [PubMed] [Google Scholar]
- 39. Brunini TM, Mendes‐Ribeiro AC, Ellory JC, Mann GE. Platelet nitric oxide synthesis in uremia and malnutrition: a role for l‐arginine supplementation in vascular protection? Cardiovasc Res. 2007;73:359–367. [DOI] [PubMed] [Google Scholar]
- 40. Bode‐Boger SM, Scalera F, Ignarro LJ. The l‐arginine paradox: importance of the l‐arginine/asymmetrical dimethylarginine ratio. Pharmacol Ther. 2007;114:295–306. [DOI] [PubMed] [Google Scholar]
- 41. Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y. CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch. 2004;447:532–542. [DOI] [PubMed] [Google Scholar]
- 42. Mihout F, Shweke N, Bige N, Jouanneau C, Dussaule JC, Ronco P, Chatziantoniou C, Boffa JJ. Asymmetric dimethylarginine (ADMA) induces chronic kidney disease through a mechanism involving collagen and TGF‐beta1 synthesis. J Pathol. 2011;223:37–45. [DOI] [PubMed] [Google Scholar]
- 43. van de Poll MC, Soeters PB, Deutz NE, Fearon KC, Dejong CH. Renal metabolism of amino acids: its role in interorgan amino acid exchange. Am J Clin Nutr. 2004;79:185–197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Plasma ADMA and l‐citrulline levels change upon UNX, compared with sham‐operated animals, only in female mice. The surgery performed at day 0 induced an increase in the plasma concentration of ADMA and l‐citrulline in both UNX‐ and sham‐operated male mice. An increase was observed for plasma ADMA and l‐citrulline levels only in female UNX mice. White and gray bars indicate UNX‐ and sham‐operated animals, respectively. ***P<0.001, comparing before and after surgery; # P<0.05, ## P<0.01 comparing UNX‐ and sham‐operated animals. n=9 to 18 animals per group.
Figure S2. Representative traces from 1 mouse (8 days before and 10 days after UNX). Green, brown, and red traces indicate diastolic, mean, and systolic blood pressure, respectively.
Figure S3. No changes in plasma arginine, ornithine, SDMA, and NMMA levels between UNX‐ and sham‐operated mice. Surgery performed at day 0 induced changes in plasma concentration of several amino acids that were, however, not different in UNX‐ and sham‐operated animals. White and gray bars indicate UNX‐ and sham‐operated animals, respectively. *P<0.05, **P<0.01, ***P<0.001, comparing before and after surgery. n=9 to 18 animals per group.


