Abstract
Background
To determine whether daily physical activity (PA), as measured by implanted devices (through accelerometer sensor), was related to the risk of developing atrial arrhythmias during long‐term follow‐up in a population of heart failure (HF) patients with an implantable cardioverter defibrillator (ICD).
Methods and Results
The study population was divided into 2 equally sized groups (PA cutoff point: 3.5 h/d) according to their mean daily PA recorded by the device during the 30‐ to 60‐day period post‐ICD implantation. Propensity score matching was used to compare 2 equally sized cohorts with similar characteristics between lower and higher activity patients. The primary end point was time free from the first atrial high‐rate episode (AHRE) of duration ≥6 minutes. Secondary end points were: first AHRE ≥6 hours, first AHRE ≥48 hours, and a combined end point of death or HF hospitalization. Data from 770 patients (65±15 years; 66% men; left ventricular ejection fraction 35±12%) remotely monitored for a median of 25 months were analyzed. A PA ≥3.5 h/d was associated with a 38% relative reduction in the risk of AHRE ≥6 minutes (72‐month cumulative survival: 75.0% versus 68.1%; log rank P=0.025), and with a reduction in the risk of AHRE ≥6 hours, AHRE ≥48 hours, and the combined end point of death or HF hospitalization (all P<0.05).
Conclusions
In HF patients with ICD, a low level of daily PA was associated with a higher risk of atrial arrhythmias, regardless of the patients' baseline characteristics. In addition, a lower daily PA predicted death or HF hospitalization.
Keywords: atrial fibrillation, heart failure, implanted cardioverter defibrillator, physical exercise
Subject Categories: Atrial Fibrillation, Heart Failure, Exercise, Lifestyle
Clinical Perspective
What Is New?
In heart failure patients with implantable devices, a low level of baseline daily physical activity as measured by the devices (through the accelerometer sensor) was associated with a higher risk of atrial arrhythmias.
A low level of baseline physical activity was also associated with a higher risk of death or heart failure hospitalization.
What Are the Clinical Implications?
The data already available to physicians through physical activity measurement could prove effective in foreseeing atrial arrhythmic episodes and improving outcomes.
Introduction
A low level of daily physical activity (PA) is an independent predictor of all‐cause mortality, cardiovascular events, and atrial fibrillation (AF) in the general population.1, 2 Several observations suggest that daily PA also predicts outcome in various chronic diseases, including heart failure (HF).3, 4, 5, 6, 7, 8
Information on PA is recorded and stored automatically by many implantable cardioverter‐defibrillators (ICDs) through accelerometer sensors incorporated into the device. These data provide an easily accessible quantitative measure that may reflect the individual's functional status.7, 9 Previous studies evaluating patient activity by means of ICDs, whether alone7, 8 or integrated with other diagnostics,10, 11, 12 in HF patients have suggested an inverse relationship with survival, after adjustment for other clinical factors. The relationship between daily PA and the long‐term risk of developing AF in HF patients is unknown.
Remote monitoring (RM) enables the level of daily PA of patients with implantable devices to be monitored over time. In addition, it allows the continuous, rapid, and accurate detection of AF episodes (including subclinical atrial high‐rate episodes [AHREs]), over long periods.13
The aim of the present study was to determine whether daily PA, as measured by implanted devices, is related to the risk of developing atrial arrhythmias during long‐term follow‐up in a population of HF patients. A secondary aim was to assess the relationship between daily PA and clinical outcomes, such as death and HF.
Methods
The authors declare that all supporting data are available within the article. IMPLANTED (The Italian Multicentre Observational Registry on Patients With Implantable Devices Remotely Monitored) is a multicenter, retrospective registry endorsed by the Italian Association of Arrhythmology and Cardiac Pacing (AIAC), enrolling patients from 7 Italian high‐volume arrhythmia centers. All consecutive patients aged ≥18 years who underwent pacemaker or ICD implantation in accordance with current guidelines14 from January 1, 2009, to September 30, 2016, and followed up by means of RM in addition to conventional in‐office examination, were enrolled in the registry.
The study was approved by Ethics Committees of all participating institutions, and was registered on www.clinicaltrials.gov under identifier NCT03061747. All patients gave their written informed consent at the time of enrollment.
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Population
For the purpose of this study, patients who met the following inclusion criteria were included in the analysis: (1) implantation of an ICD with an atrial lead, and equipped with a software platform for daily recording and uploading of patient activity; (2) availability of complete data on daily PA collected by the device in the period from 30 to 60 days after implantation (activity window, see below); (3) availability of at least 1 RM transmission (manual or automatic) performed not earlier than 30 days after the end of the activity window. In order to test the real effect of PA, patients were excluded from the analysis if they already had at least 1 AHRE (≥180 bpm atrial, documented by the ICD via its atrial lead and stored digitally) of ≥6 minutes during the first 60 days after implantation, and if they died or underwent another hospitalization during the 30‐day activity window.
Remote Monitoring
All patients enrolled in the study received an ICD equipped with RM capabilities; 38.7% of patients were enrolled in the Medtronic CareLink Network (Medtronic Inc, Minneapolis, MN), 27.1% in the Boston Scientific Latitude Patient Management System (Boston Scientific, St Paul, MN), 17.7% in the Biotronik Home Monitoring system (Biotronik Gmbh, Berlin, Germany), and 16.5% in the St Jude Medical Merlin.net system (St Jude Medical, Sylmar, CA). These 4 RM systems operate in a similar manner and have previously been described in detail.4, 12, 13 Briefly, the remote system consists of a base station that is placed in the patient's home and is capable of full device interrogation and transmission. These interrogations may be patient initiated or (for some models) performed automatically by wireless telemetry at scheduled intervals. Data are then transferred by telephone line and are accessible for routine clinical care through a secure website administered by the manufacturer. In the case of devices with wireless communication capabilities, the ICD allows unscheduled alert‐based transmissions without patient intervention. Specifically, in the case of programmable alert conditions, the system can transmit data and notify the physician via phone or e‐mail. Each transmission contains the complete device diagnostic information, including counts of arrhythmia episodes and therapies, rhythm information, and technical parameters.
All patients included in the registry were enrolled in the remote follow‐up program and performed a first successful transmission within 3 months after implantation. The routine remote transmissions were scheduled at intervals of 1 to 3 months, according to physician decision.
Device Measurement of Daily Physical Activity
The PA is measured by the device's accelerometer sensor, which is designed to capture normal daily activities, including walking at a slow pace. The software platform for recording daily PA operates in a similar manner for all manufacturers. The accelerometer detects both the frequency and amplitude of the patient's motion and translates this into a proportional electrical signal. The number of minutes during which the patient is active per day is counted. A minute is considered to be “active” if a threshold that incorporates both the number and magnitude of deflections in the accelerometer signal is reached. For the purpose of this study, the daily time in which the patient was active was expressed in hours per day.
All the devices implanted during the study period were capable of storing up to 1 year of daily PA data. At each RM transmission, all available activity data were uploaded.
The baseline daily PA was defined as the mean daily activity (in h/d) recorded in the period from 30 to 60 days after device implantation (this period was dubbed the “activity window”) (Figure 1). This window was selected a priori to take into account clinical recovery after the device implantation procedure.7, 8
Figure 1.
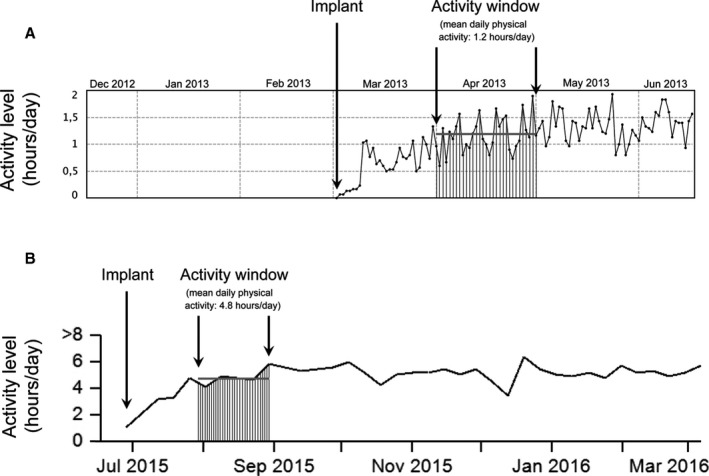
Two examples of calculation of baseline daily physical activity (PA). Baseline daily PA was calculated as the mean daily activity (in h/d) recorded in the period from 30 to 60 days after device implantation (activity window). A, Patient enrolled in the Boston Scientific Latitude Patient Management System (Boston Scientific, St Paul, MN). The mean daily PA recorded during the activity window was 1.2 h/d (lower‐activity patient). B, Patients enrolled in the Medtronic CareLink Network (Medtronic Inc, Minneapolis, MN). The mean daily PA recorded during the activity window was 4.8 h/d (higher‐activity patient).
Data Collection
Clinical history, cardiovascular risk factors, comorbidity, cardiovascular medications, and ICD indications were collected for all patients. Transthoracic echocardiography was performed in all patients prior to ICD implantation.
A complete review of all remote transmissions was performed for all patients, in order to calculate and record the mean daily PA during the activity window, and to detect all arrhythmic episodes from the end of the activity window to the last available transmission. For the purpose of this study, the time between the end of the activity window and the date of the last available transmission was considered as the observation period.
After implantation, in addition to undergoing RM, all patients were followed up at their referring out‐of‐hospital clinics on a 6‐ or 12‐month basis, or earlier in case of unscheduled visits.
Information about clinical outcomes such as hospitalizations, deaths, and causes of death were collected during hospital visits or by phone calls for patients who missed programmed visits.
Deaths were classified according to a modified Hinkle‐Thaler classification,15 and categorized into 3 predefined groups: non–sudden cardiac death (further categorized into HF death, coronary death, and cardiac death but unable to classify further), noncardiac death, and sudden death.
Data regarding ICD implantation, baseline characteristics of patients, and clinical outcomes were collected prospectively at the enrolling centers and retrospectively analyzed for the purpose of this study.
End Points
The primary end point was the time free from first AHRE of ≥6 minutes. This cutoff was based on previous evidence, which defined 6‐minute episodes as significantly associated with an increased risk of strokes and thromboembolic events.16, 17 Secondary end points were (1) time free from first AHRE ≥6 hours; (2) time free from first AHRE ≥48 hours; (3) time free from a predefined combined end point of death and HF hospitalization; (4) time free from all‐cause death; and (5) time free from HF hospitalization.
Only hospitalizations requiring at least 1 overnight stay, and which were adjudicated as related to HF by a blinded Endpoint Advisory Committee contributed to the end point.
Statistical Analysis
Descriptive statistics are reported as mean±standard deviation for normally distributed continuous variables and compared by means of Student t test and analysis of variance. Continuous variables with skewed distribution are reported as medians with 25th to 75th percentiles. Categorical data are expressed as percentages, reported in contingency tables and compared by means of χ2 test or Fisher exact test, as appropriate. Relative risks are reported with their 95% confidence intervals.
The effect of individual variables on the risk of AHRE ≥6 minutes was investigated by using univariate Cox proportional hazards models applied to the whole study population. Variables that showed an effect on the risk of AHRE ≥6 minutes with a significance level <0.2 on univariate analyses were entered into multivariable Cox proportional hazards models. Cox model findings are presented as hazard ratios (HRs), tests of significance, and 95% confidence intervals (CIs). In this model, daily PA was considered as a continuous variable. Interactions between the covariates were tested for significance in the model.
Subsequently, in order to assess the relationship between daily PA and study end points, all patients were stratified into 2 equal‐sized groups on the basis of their mean daily PA value recorded during the 30‐ to 60‐day window post‐ICD implantation, with a cut point of 3.5 h/d (median). A propensity score for the likelihood of lower daily PA was obtained by means of multiple logistic regression. The variables included in the score were age, left ventricular ejection fraction (LVEF), New York Heart Association (NYHA) class, CHA2DS2‐VASC2 score, ischemic cardiomyopathy, other cardiac structural diseases, secondary prevention, history of AF, diabetes, chronic renal disease, obstructive sleep apnea, previous stroke or transient ischemic attack, coronary artery disease, previous coronary arterial bypass graft, medical therapy with angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, furosemide, antiplatelets, ivabradine, mineralocorticoid receptor antagonists, amiodarone, and other antiarrhythmic drugs. Matching was then performed on log‐transformed propensity score in a 1:1 fashion with a caliper of 0.1,to take into account the differences in baseline characteristics between patients with low and high daily PA. Kaplan‐Meier analyses and a log rank P test were used to compare the end points between the 2 patient groups. P values of <0.05 were considered statistically significant. The data were analyzed by means of the statistical software package Statistica version 6.1 (StatSoft Inc, Tulsa, OK).
Results
General Population
Of the 1107 patients enrolled in the IMPLANTED registry, 770 were eligible for analysis (Figure 2).
Figure 2.
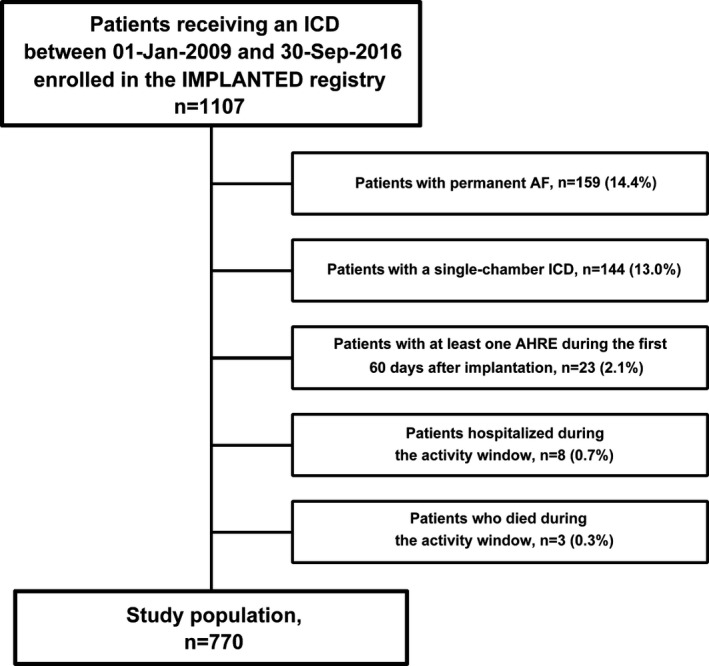
Study flow with derivation of the study population. AF indicates atrial fibrillation; AHRE, atrial high‐rate episode; ICD, implantable cardioverter defibrillator; IMPLANTED, Italian Multicentre Observational Registry on Patients With Implantable Devices Remotely Monitored.
Table 1 shows the baseline characteristics of the general population stratified into 2 groups according to daily PA, and the propensity score‐matched groups.
Table 1.
Baseline Characteristics of Patients Subdivided According to h/d Physical Activity Measured by the Device
| Characteristics | General Population | Propensity Score Matched | ||||
|---|---|---|---|---|---|---|
| Low Activity (<3.5 h/d) (n=387) | High Activity (≥3.5 h/d) (n=383) | P Value | Low Activity (<3.5 h/d) (n=223) | High Activity (≥3.5 h/d) (n=223) | P Value | |
| Baseline characteristics | ||||||
| Male, n (%) | 251 (64.9) | 260 (67.9) | 0.374 | 133 (59.6) | 141 (63.2) | 0.436 |
| Age, y, mean+SD | 70.1±12.5 | 60.8±14.0 | <0.001 | 66.4±13.1 | 64.4±13 | 0.105 |
| LVEF in %, mean±SD | 34.4±11.0 | 36.2±13.2 | 0.034 | 35.4±11.8 | 35.1±12.3 | 0.839 |
| Mean (±SD) NYHA class | 2.2±0.8 | 2.0±0.7 | <0.001 | 2.0±0.7 | 2.0±0.7 | 0.838 |
| NYHA class, n (%) | <0.001 | 0.998 | ||||
| I | 56 (14.5) | 72 (18.8) | 39 (17.5) | 38 (17.0) | ||
| II | 208 (53.7) | 243 (63.4) | 136 (61.0) | 138 (61.9) | ||
| III | 114 (29.5) | 66 (17.2) | 47 (21.1) | 46 (20.6) | ||
| IV | 9 (2.3) | 2 (0.5) | 1 (0.4) | 1 (0.4) | ||
| CHA2DS2‐VASc score, mean±SD | 4±1.5 | 3.1±1.6 | <0.001 | 3.6±1.6 | 3.4±1.6 | 0.192 |
| CHA2DS2‐VASc score, n (%) | <0.001 | 0.346 | ||||
| 0 (low risk) | 6 (1.6) | 19 (5.0) | 4 (1.8) | 8 (3.6) | ||
| 1 (intermediate risk) | 22 (5.7) | 57 (14.9) | 19 (8.5) | 24 (10.8) | ||
| 2 to 9 (high risk) | 359 (92.8) | 307 (80.2) | 200 (89.7) | 191 (85.7) | ||
| Cardiac resynchronization therapy, n (%) | 233 (60.2) | 168 (43.9) | <0.001 | 104 (46.6) | 104 (46.6) | 1.000 |
| Daily physical activity in h/d, mean±SD | 2.1±0.9 | 6.3±3.7 | <0.001 | 2.1±0.9 | 5.5±2.2 | <0.001 |
| Etiology, n (%) | 0.005 | 0.232 | ||||
| Ischemic cardiomyopathy | 193 (49.9) | 154 (40.2) | 103 (46.2) | 87 (39.0) | ||
| Nonischemic cardiomyopathy | 146 (37.7) | 154 (40.2) | 84 (37.7) | 101 (45.3) | ||
| Other | 48 (12.4) | 75 (19.6) | 36 (16.1) | 35 (15.7) | ||
| Indication for ICD, n (%) | 0.009 | 0.842 | ||||
| Primary prevention | 350 (90.5) | 365 (95.3) | 209 (93.7) | 210 (94.2) | ||
| Secondary prevention | 37 (9.6) | 18 (4.7) | 14 (6.3) | 13 (5.8) | ||
| Associated disorders | ||||||
| Arterial hypertension, n (%) | 294 (76.0) | 276 (72.1) | 0.216 | 160 (71.7) | 163 (73.1) | 0.751 |
| Diabetes mellitus, n (%) | 145 (37.5) | 118 (30.8) | 0.051 | 77 (34.5) | 71 (31.8) | 0.546 |
| Dyslipidemia, n (%) | 243 (62.8) | 233 (60.8) | 0.577 | 137 (61.4) | 134 (60.1) | 0.771 |
| Obesity, n (%) | 108 (27.9) | 119 (31.1) | 0.336 | 72 (32.3) | 63 (28.3) | 0.353 |
| Chronic renal disease, n (%) | 134 (34.6) | 71 (18.5) | <0.001 | 53 (23.8) | 49 (22.0) | 0.652 |
| COPD, n (%) | 97 (25.1) | 105 (27.4) | 0.459 | 59 (26.5) | 60 (26.9) | 0.915 |
| Obstructive sleep apnea, n (%) | 46 (11.9) | 28 (7.3) | 0.031 | 22 (9.9) | 16 (7.2) | 0.309 |
| Previous stroke/TIA, n (%) | 35 (9.0) | 18 (4.7) | 0.017 | 18 (8.1) | 11 (4.9) | 0.179 |
| Paroxysmal/persistent AF, n (%) | 59 (15.2) | 54 (14.1) | 0.653 | 30 (13.5) | 33 (14.8) | 0.683 |
| CAD, n (%) | 181 (46.8) | 140 (36.6) | 0.004 | 96 (43.0) | 82 (36.8) | 0.176 |
| Previous PCI n (%) | 106 (27.4) | 120 (31.3) | 0.230 | 57 (25.6) | 64 (28.7) | 0.456 |
| Previous CABG n (%) | 85 (22.0) | 47 (12.3) | <0.001 | 37 (16.6) | 26 (11.7) | 0.135 |
| Cardiovascular medications | ||||||
| Beta‐blockers, n (%) | 364 (94.1) | 355 (92.7) | 0.446 | 210 (94.2) | 206 (92.4) | 0.450 |
| ACE‐Is/ARBs, n (%) | 309 (79.8) | 322 (84.1) | 0.127 | 197 (88.3) | 198 (88.8) | 0.882 |
| Furosemide, n (%) | 325 (84.0) | 267 (69.7) | <0.001 | 182 (81.6) | 183 (82.1) | 0.902 |
| Statins, n (%) | 219 (56.6) | 215 (56.1) | 0.899 | 132 (59.2) | 125 (56.1) | 0.502 |
| Antiplatelet drugs, n (%) | 258 (66.7) | 272 (71.0) | 0.192 | 159 (71.3) | 158 (70.9) | 0.917 |
| Ivabradine, n (%) | 32 (8.3) | 55 (14.4) | 0.008 | 22 (9.9) | 21 (9.4) | 0.873 |
| MRAs, n (%) | 239 (61.8) | 212 (55.4) | 0.071 | 129 (57.8) | 147 (65.9) | 0.079 |
| Amiodarone, n (%) | 127 (32.8) | 83 (21.7) | <0.001 | 64 (28.7) | 49 (22.0) | 0.102 |
| Oral anticoagulants, n (%) | 83 (21.4) | 69 (18.0) | 0.232 | 47 (21.1) | 43 (19.3) | 0.637 |
| Digoxin, n (%) | 27 (7.0) | 24 (6.3) | 0.692 | 15 (6.7) | 13 (5.8) | 0.696 |
| Other AADs, n (%) | 3 (0.8) | 7 (1.8) | 0.197 | 2 (0.9) | 5 (2.2) | 0.253 |
AAD indicates antiarrhythmic drug; ACE‐I, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; CABG, coronary arterial bypass graft; COPD, chronic obstructive pulmonary disease; ICD, implantable cardioverter‐defibrillator; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; TIA, transient ischemic attack.
Data on daily PA recorded in the 30‐ to 60‐day window post‐ICD implantation was available for all enrolled patients and averaged 3.8±3.4 h/d. Lower‐activity patients were on average 10 years older, presented a worse NYHA class, had a higher CHA2DS2‐VASC2 score, and presented a lower LVEF on enrollment. They were more likely to receive cardiac resynchronization therapy combined with ICD, and to have an ischemic cardiomyopathy. They also had more concomitant diseases such as diabetes mellitus, chronic renal disease, and previous stroke or TIA. Lower‐activity patients were more often treated with furosemide, mineralocorticoid receptor antagonists, amiodarone, and other antiarrhythmic drugs (Table 1).
During a median follow‐up of 25 months (first to third quartile, 12–50 months), the numbers of patients who experienced at least 1 AHRE of duration ≥6 minutes, ≥6 hours and ≥48 hours were 88 (11.4%), 83 (10.8%) and 61 (7.9%), respectively. The number of deaths and HF hospitalizations recorded was 35 (2.7%) and 111 (14.4%), respectively.
In the study population, univariate analysis showed that an higher daily PA was significantly associated with lower risk of at least 1 AHRE ≥6 minutes during follow‐up. Patient factors associated with greater risk of AHRE ≥6 minutes on univariate analysis were higher age, higher NYHA class, higher CHA2DS2‐VASc score, cardiac resynchronization therapy, chronic renal failure, history of paroxysmal/persistent AF, and the use of amiodarone. Conversely, a higher LVEF was associated with lower risk of AHRE ≥6 minutes (Table 2). After multivariable analysis, the predictive factors that were still associated with greater risk of AHRE ≥6 minutes were higher age and a history of paroxysmal/persistent AF. A higher LVEF and a higher daily PA remained associated with lower risk of AHRE ≥6 minutes. Interactions among the covariates were not significant.
Table 2.
Predictors of AHRE Lasting ≥6 Minutes in the Overall Study Population (n=770): Univariate and Multivariable Cox Proportional Hazards Analysis
| Variable | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Age (per 1‐y increase) | 1.035 (1.02–1.06) | <0.001 | 1.047 (1.02–1.08) | 0.003 |
| Left ventricular ejection fraction (per 1% increase) | 0.977 (0.96–0.99) | 0.031 | 0.904 (0.84–0.98) | 0.011 |
| NYHA class (per 1 increase) | 1.620 (1.18–2.22) | 0.003 | 1.158 (0.78–1.71) | 0.460 |
| CHA2DS2‐VASc score (per 1 increase) | 1.216 (1.06–1.41) | 0.007 | 0.962 (0.77–1.19) | 0.724 |
| Cardiac resynchronization therapy | 2.010 (1.26–3.21) | 0.004 | 1.540 (0.91–2.61) | 0.108 |
| Daily physical activity (per 1 h/d increase) | 0.997 (0.99–0.99) | <0.001 | 0.998 (0.99–0.99) | 0.007 |
| Chronic renal failure | 1.668 (1.05–2.66) | 0.032 | 0.833 (0.48–1.43) | 0.507 |
| Paroxysmal/persistent AF | 5.312 (3.33–8.48) | <0.001 | 4.039 (2.45–6.65) | <0.001 |
| Amiodarone | 2.490 (1.58–3.92) | <0.001 | 1.828 (0.87–3.84) | 0.111 |
AF indicates atrial fibrillation; AHRE, atrial high‐rate episode; CI, confidence interval; NYHA, New York Heart Association.
According to quintiles of daily PA, patients of the whole study population were divided into 5 groups: <2.0 h/d (n=154); from 2.0 to 2.9 h/d (n=154); from 3.0 to 4.0 h/d (n=154); from 4.1 to 5.9 h/d (n=154); and >5.9 h/d (n=154). Figure 3 shows a forest plot displaying HRs for primary end point of the 5 groups after multivariable analysis.
Figure 3.
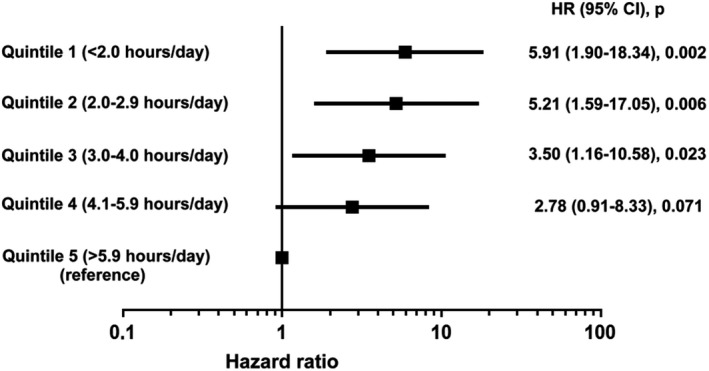
Forest plot showing the risk of AHREs lasting ≥6 minutes in the whole study population (n=770) by quintiles of daily physical activity after multivariable analysis. AHRE indicates atrial high‐rate episode; CI, confidence interval; HR, hazard ratio.
Propensity Score–Matched Groups
After propensity score matching for the likelihood of having a lower daily PA, we selected 223 lower‐activity and 223 higher‐activity patients with similar baseline characteristics on 35 variables.
A baseline daily PA ≥3.5 h/d was significantly associated with a 38% relative reduction of risk of the primary end point (first AHRE ≥6 minutes) (relative risk [RR]=0.62; 95% CI, 0.41–0.95; P=0.025). There were also significant associations with first AHRE ≥6 hours (RR=0.58; 95% CI, 0.34–0.99; P=0.040), first AHRE ≥48 hours (RR=0.48; 95% CI, 0.24–0.96; P=0.033), the combined end point of death or HF hospitalization (RR=0.66; 95% CI, 0.44–0.98; P=0.039), and first HF hospitalization (RR=0.61; 95% CI, 0.37–0.99; P=0.041).
Figure 4 shows event‐free survival at 72 months with patients stratified into 2 equal‐sized groups according to baseline daily PA (cut point 3.5 h/d). Survival from the primary end point was 75.0% for higher‐activity patients and 68.1% for lower‐activity patients (log rank P=0.025) (Figure 4A). Survival from first AHRE ≥6 hours was 81.5% for higher‐activity patients and 75.9% for lower‐activity patients (log rank P=0.048) (Figure 4B). Survival from first AHRE ≥48 hours was 87.3% for higher‐activity patients and 81.7% for lower‐activity patients (log rank P=0.042) (Figure 4C). Survival from the combined end point of death and HF hospitalization was 78.1% for higher‐activity patients and 67.2% for lower‐activity patients (log rank P=0.038) (Figure 4D). Survival from all‐cause death was 94.1% for higher‐activity patients and 84.7% for lower‐activity patients (log rank P=0.143) (Figure 4E). Survival from first HF hospitalization was 87.0% for higher‐activity patients and 72.4% for lower‐activity patients (log rank P=0.048) (Figure 4F).
Figure 4.
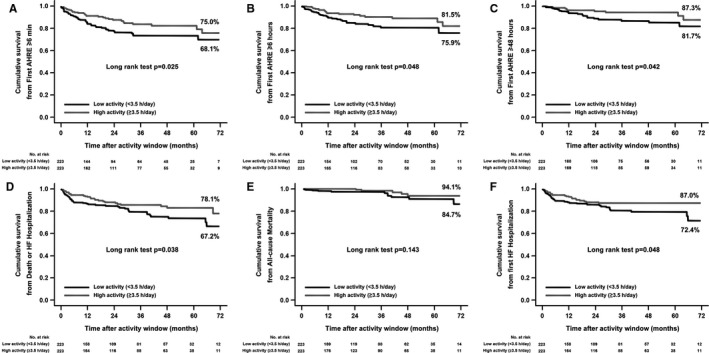
Kaplan‐Meier estimates of the cumulative time free from first atrial high‐rate episodes (AHREs) detected by the device, and from clinical events in the low and high physical activity propensity score–matched groups (n=446). A, First AHRE lasting ≥6 minutes. B, First AHRE lasting ≥6 hours. C, First AHRE lasting ≥48 hours. D, All‐cause death or heart failure (HF) hospitalization. E, All‐cause death. F, HF hospitalization. Events are counted after closure of the activity window used to determine baseline physical activity level.
Discussion
The main finding of the present study is that the daily PA measured by the accelerometer sensor of implanted ICDs over a 30‐day window starting 30 days after implantation predicted the risk of AHREs detected by the device in patients with HF. The association between daily PA and risk of AHREs was independent of the baseline characteristics of patients.
Several studies have examined the association between PA and risk of AF, and have demonstrated that in the general population a regular PA seems to have a protective role in development of AF.18, 19 A recent analysis enrolling a large, multiethnic cohort of individuals undergoing a graded exercise treadmill test, demonstrated an inverse association between cardiorespiratory fitness status and risk of incident AF.2 In addition, epidemiological studies have shown that there is a close correlation between a low level of daily PA, obesity, and increased risk of AF.2, 18, 20 The relationship between PA and AF in patients with HF and ICD has not been previously investigated.
Patients with defibrillators are at high risk of atrial arrhythmias, particularly AF, which predispose them to embolic events and worsening congestive HF, with a negative prognostic impact.21, 22, 23, 24, 25 Atrial arrhythmias can also trigger inappropriate shocks.25
Modern cardiac implantable devices with implanted atrial lead, through analytic software allow the continuous detection and characterization of individual AHREs over long periods. Studies have indicated that the detection of such episodes correlates well with electrocardiographic documentation of AF. Subclinical atrial tachyarrhythmias detected by implantable devices, even if of short duration, are associated with a significantly increased risk of ischemic stroke or systemic embolism16, 17, 26 and are independent predictors of cardiovascular mortality.27, 28 For these reasons, the prevention and/or reduction of atrial arrhythmias is an important goal in the management of patients with HF and ICD.
ICDs and CRT devices are routinely equipped with activity sensors in order to adjust heart rates during patient activity. Thus, data on daily PA assessed by these sensors can easily be obtained on ICD/CRT interrogation (remote or in‐office), and it correlates strongly with data obtained from validated external accelerometers.28 This information is available irrespective of the activation of the rate response function.
The results of our study suggest that the level of daily PA measured by implanted devices predicts the risk of episodes of atrial arrhythmias detected by the device. Specifically, we found a significant association both with short and subclinical episodes and with those of longer duration (>6 and >48 hours) requiring clinical intervention. The results of the study do not provide a definite explanation for these findings. It is possible that daily PA is a clinical status marker, and that a lower level of daily PA identifies the most compromised patients, who have a higher risk of developing atrial arrhythmias. It is also possible that a lower level of PA has a negative influence on cardiovascular risk factors, thereby increasing the risk of atrial arrhythmias. Because AF has multiple cardiovascular and noncardiovascular risk factors, the reduction in the risk of AF in patients with higher level of daily PA may be partly mediated through the improvement of preexisting risk factors. Previous studies showed that higher physical fitness is associated with a lower level of inflammatory markers (eg, C‐reactive protein).29 Thus, it is likely that the reduction in the risk of AF at a higher level of daily PA is partly mediated through reducing the incidence of inflammation. Overweight and obesity are also important risk factors for AF2, 18, 20 and may induce AF by increasing left atrial size and volume.30 It is therefore likely that the inverse association between PA and AF is also mediated through the regulation of body mass index.
The results of several studies on ICD patients have shown that a reduction in daily PA level detected by device identifies patients at a higher risk of HF hospitalizations within the subsequent month.10, 11, 12 In addition, a baseline low daily PA measured by implantable devices after implantation is a strong predictor of negative long‐term outcomes, being associated with higher all‐cause mortality and higher risk of HF hospitalization.7, 8 The results of the present study confirmed this evidence, as a baseline daily PA <3.5 h/d was significantly associated with a higher risk of death or HF hospitalization and of HF hospitalization alone, even after correction for disease severity.
Study Limitations
Although data were collected prospectively at the enrolling centers for an observational registry, the analysis performed was retrospective and is therefore subject to all of the limitations of a retrospective analysis. Moreover, while propensity score matching is one of the best techniques in order to reduce the intrinsic biases of nonrandomized studies, our results should be interpreted with caution, as confounding factors cannot be entirely excluded. Moreover, further prospective, large‐population analyses are needed to confirm them. In addition, the definition of our activity window from 30 to 60 days postimplantation is (although based on common sense) arbitrary, and different periods could have provided slightly different results. Moreover, extending our results to a broader HF population with dissimilar baseline characteristics and to non‐ICD recipients is not warranted.
In contrast with previous similar studies,7, 8 the reduced risk for all‐cause mortality associated with a lower level of daily PA did not reach statistical significance (Figure 4E). It is likely that statistical significance has not been reached due to the small sample size, the low number of events, and the relative short duration of follow‐up.
Finally, while modern implantable devices offer a reliable and sensitive mean of detecting both subclinical arrhythmias and PA, different manufacturers may be slightly different in function and related software, thus yielding slightly different measurements.
Conclusions
In HF patients with implantable devices, a low level of baseline daily PA was associated with a higher risk of atrial arrhythmias, regardless of the baseline characteristics of the patients. In addition, a lower daily PA predicted death or HF hospitalization, as well as HF hospitalization alone.
The data already available to physicians through PA measurement could prove effective in foreseeing arrhythmic episodes, thus helping in reducing mortality and HF hospitalizations in patients with implantable devices. Further research is needed in order to confirm the present findings and to understand the possible mechanisms underlying our observations.
Appendix
Members of the Italian Association of Arrhythmology and Cardiac Pacing (AIAC) National Directive Board 2016‐2018 who contributed to this study: Gian Luca Botto, Emanuele Bertaglia, Massimo Zoni Berisso, Vincenzo Nissardi, Luca Santini, Ezio Soldati, Giuseppe Stabile, Maurizio Landolina, and Luigi Padeletti.
Disclosures
None.
(J Am Heart Assoc. 2018;7:e008146 DOI: 10.1161/JAHA.117.008146.)29478022
Contributor Information
Pietro Palmisano, Email: dr.palmisano@libero.it.
the Italian Association of Arrhythmology and Cardiac Pacing (AIAC):
Gian Luca Botto, Emanuele Bertaglia, Massimo Zoni Berisso, Vincenzo Nissardi, Luca Santini, Ezio Soldati, Giuseppe Stabile, Maurizio Landolina, and Luigi Padeletti
References
- 1. Paffenbarger RS Jr, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical‐activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328:538–545. [DOI] [PubMed] [Google Scholar]
- 2. Qureshi WT, Alirhayim Z, Blaha MJ, Juraschek SP, Keteyian SJ, Brawner CA, Al‐Mallah MH. Cardiorespiratory fitness and risk of incident atrial fibrillation: results from the Henry Ford Exercise Testing (FIT) Project. Circulation. 2015;131:1827–1834. [DOI] [PubMed] [Google Scholar]
- 3. Doukky R, Mangla A, Ibrahim Z, Poulin MF, Avery E, Collado FM, Kaplan J, Richardson D, Powell LH. Impact of physical inactivity on mortality in patients with heart failure. Am J Cardiol. 2016;117:1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walsh JT, Charlesworth A, Andrews R, Hawkins M, Cowley AJ. Relation of daily activity levels in patients with chronic heart failure to long‐term prognosis. Am J Cardiol. 1997;79:1364–1369. [DOI] [PubMed] [Google Scholar]
- 5. Flynn KE, Piña IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, Fine LJ, Howlett JG, Keteyian SJ, Kitzman DW, Kraus WE, Miller NH, Schulman KA, Spertus JA, O'Connor CM, Weinfurt KP; HF‐ACTION Investigators . Effects of exercise training on health status in patients with chronic heart failure: HF‐ACTION randomized controlled trial. JAMA. 2009;301:1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jehn M, Schmidt‐Trucksäss A, Schuster T, Weis M, Hanssen H, Halle M, Koehler F. Daily walking performance as an independent predictor of advanced heart failure: prediction of exercise capacity in chronic heart failure. Am Heart J. 2009;157:292–298. [DOI] [PubMed] [Google Scholar]
- 7. Conraads VM, Spruit MA, Braunschweig F, Cowie MR, Tavazzi L, Borggrefe M, Hill MR, Jacobs S, Gerritse B, van Veldhuisen DJ. Physical activity measured with implanted devices predicts patient outcome in chronic heart failure. Circ Heart Fail. 2014;7:279–287. [DOI] [PubMed] [Google Scholar]
- 8. Kramer DB, Mitchell SL, Monteiro J, Jones PW, Normand SL, Hayes DL, Reynolds MR. Patient activity and survival following implantable cardioverter‐defibrillator implantation: the ALTITUDE activity study. J Am Heart Assoc. 2015;4:e001775 DOI: 10.1161/JAHA.115.001775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kadhiresan VA, Pastore J, Auricchio A, Sack S, Doelger A, Girouard S, Spinelli JC; PATH‐CHF Study Group . Pacing therapies in congestive heart failure: a novel method–the activity log index–for monitoring physical activity of patients with heart failure. Am J Cardiol. 2002;89:1435–1437. [DOI] [PubMed] [Google Scholar]
- 10. Whellan DJ, Ousdigian KT, Al‐Khatib SM, Pu W, Sarkar S, Porter CB, Pavri BB, O'Connor CM; PARTNERS Study Investigators . Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients With Heart Failure) study. J Am Coll Cardiol. 2010;55:1803–1810. [DOI] [PubMed] [Google Scholar]
- 11. Boehmer JP, Hariharan R, Devecchi FG, Smith AL, Molon G, Capucci A, An Q, Averina V, Stolen CM, Thakur PH, Thompson JA, Wariar R, Zhang Y, Singh JP. A multisensor algorithm predicts heart failure events in patients with implanted devices: results from the MultiSENSE study. JACC Heart Fail. 2017;5:216–225. [DOI] [PubMed] [Google Scholar]
- 12. Burri H, da Costa A, Quesada A, Ricci RP, Favale S, Clementy N, Boscolo G, Villalobos FS, Mangoni di S Stefano L, Sharma V, Boriani G; MORE‐CARE Investigators . Risk stratification of cardiovascular and heart failure hospitalizations using integrated device diagnostics in patients with a cardiac resynchronization therapy defibrillator. Europace. 2017. . Available at: https://academic.oup.com/europace/advance-article-abstract/doi/10.1093/europace/eux206/3904548?redirectedFrom=fulltext. Accessed February 17, 2018. [DOI] [PubMed] [Google Scholar]
- 13. Varma N, Epstein AE, Irimpen A, Schweikert R, Love C; TRUST Investigators . Efficacy and safety of automatic remote monitoring for implantable cardioverter‐defibrillator follow‐up: the Lumos‐T Safely Reduces Routine Office Device Follow‐up (TRUST) trial. Circulation. 2010;122:325–332. [DOI] [PubMed] [Google Scholar]
- 14. Priori SG, Blomström‐Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez‐Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Van Veldhuisen DJ. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 15. Hinkle LE Jr, Thaler HT. Clinical classification of cardiac deaths. Circulation. 1982;65:457–464. [DOI] [PubMed] [Google Scholar]
- 16. Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, Lau CP, Fain E, Yang S, Bailleul C, Morillo CA, Carlson M, Themeles E, Kaufman ES, Hohnloser SH; ASSERT Investigators . Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. [DOI] [PubMed] [Google Scholar]
- 17. Witt CT, Kronborg MB, Nohr EA, Mortensen PT, Gerdes C, Nielsen JC. Early detection of atrial high rate episodes predicts atrial fibrillation and thromboembolic events in patients with cardiac resynchronization therapy. Heart Rhythm. 2015;12:2368–2375. [DOI] [PubMed] [Google Scholar]
- 18. Calvo N, Ramos P, Montserrat S, Guasch E, Coll‐Vinent B, Domenech M, Bisbal F, Hevia S, Vidorreta S, Borras R, Falces C, Embid C, Montserrat JM, Berruezo A, Coca A, Sitges M, Brugada J, Mont L. Emerging risk factors and the dose‐response relationship between physical activity and lone atrial fibrillation: a prospective case‐control study. Europace. 2016;18:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bapat A, Zhang Y, Post WS, Guallar E, Soliman EZ, Heckbert SR, Lima J, Bertoni AG, Alonso A, Nazarian S. Relation of physical activity and incident atrial fibrillation (from the Multi‐Ethnic Study of Atherosclerosis). Am J Cardiol. 2015;116:883–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huxley RR, Misialek JR, Agarwal SK, Loehr LR, Soliman EZ, Chen LY, Alonso A. Physical activity, obesity, weight change, and risk of atrial fibrillation: the Atherosclerosis Risk in Communities study. Circ Arrhythm Electrophysiol. 2014;7:620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Santini M, Gasparini M, Landolina M, Lunati M, Proclemer A, Padeletti L, Catanzariti D, Molon G, Botto GL, La Rocca L, Grammatico A, Boriani G; cardiological centers participating in ClinicalService Project . Device‐detected atrial tachyarrhythmias predict adverse outcome in real‐world patients with implantable biventricular defibrillators. J Am Coll Cardiol. 2011;57:167–172. [DOI] [PubMed] [Google Scholar]
- 22. Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. [DOI] [PubMed] [Google Scholar]
- 23. Dries DL, Exner DV, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 1998;32:695–703. [DOI] [PubMed] [Google Scholar]
- 24. Middlekauff HR, Stevenson WG, Stevenson LW. Prognostic significance of atrial fibrillation in advanced heart failure: a study of 390 patients. Circulation. 1991;84:40–48. [DOI] [PubMed] [Google Scholar]
- 25. vanBoven N , Theuns D, Bogaard K, Ruiter J, Kimman G, Berman L, VAN DER Ploeg T, Kardys I, Umans V. Atrial fibrillation in cardiac resynchronization therapy with a defibrillator: a risk factor for mortality, appropriate and inappropriate shocks. J Cardiovasc Electrophysiol. 2013;24:1116–1122. [DOI] [PubMed] [Google Scholar]
- 26. Glotzer TV, Hellkamp AS, Zimmerman J, Sweeney MO, Yee R, Marinchak R, Cook J, Paraschos A, Love J, Radoslovich G, Lee KL, Lamas GA; MOST Investigators . Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation. 2003;107:1614–1619. [DOI] [PubMed] [Google Scholar]
- 27. Gonzalez M, Keating RJ, Markowitz SM, Liu CF, Thomas G, Ip JE, Lerman BB, Cheung JW. Newly detected atrial high rate episodes predict long‐term mortality outcomes in patients with permanent pacemakers. Heart Rhythm. 2014;11:2214–2221. [DOI] [PubMed] [Google Scholar]
- 28. Pressler A, Danner M, Esefeld K, Haller B, Scherr J, Schömig A, Halle M, Kolb C. Validity of cardiac implantable electronic devices in assessing daily physical activity. Int J Cardiol. 2013;168:1127–1130. [DOI] [PubMed] [Google Scholar]
- 29. Aronson D, Sheikh‐Ahmad M, Avizohar O, Kerner A, Sella R, Bartha P, Markiewicz W, Levy Y, Brook GJ. C‐Reactive protein is inversely related to physical fitness in middle‐aged subjects. Atherosclerosis. 2004;176:173–179. [DOI] [PubMed] [Google Scholar]
- 30. Wang TJ, Parise H, Levy D, D'Agostino RB Sr, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new‐onset atrial fibrillation. JAMA. 2004;292:2471–2477. [DOI] [PubMed] [Google Scholar]


