Abstract
Peroxisome proliferator-activated receptor α (PPARα) is a nuclear hormone receptor that promotes fatty acid β-oxidation (FAO) and oxidative phosphorylation (OXPHOS). We and others have recently shown that PPARα and its target genes are downregulated, and FAO and OXPHOS are impaired in autosomal dominant polycystic kidney disease (ADPKD). However, whether PPARα and FAO/OXPHOS are causally linked to ADPKD progression is not entirely clear. We report that expression of PPARα and FAO/OXPHOS genes is downregulated, and in vivo β-oxidation rate of 3H-labeled triolein is reduced in Pkd1RC/RC mice, a slowly progressing orthologous model of ADPKD that closely mimics the human ADPKD phenotype. To evaluate the effects of upregulating PPARα, we conducted a 5-mo, randomized, preclinical trial by treating Pkd1RC/RC mice with fenofibrate, a clinically available PPARα agonist. Fenofibrate treatment resulted in increased expression of PPARα and FAO/OXPHOS genes, upregulation of peroxisomal and mitochondrial biogenesis markers, and higher β-oxidation rates in Pkd1RC/RC kidneys. MRI-assessed total kidney volume and total cyst volume, kidney-weight-to-body-weight ratio, cyst index, and serum creatinine levels were significantly reduced in fenofibrate-treated compared with untreated littermate Pkd1RC/RC mice. Moreover, fenofibrate treatment was associated with reduced kidney cyst proliferation and infiltration by inflammatory cells, including M2-like macrophages. Finally, fenofibrate treatment also reduced bile duct cyst number, cyst proliferation, and liver inflammation and fibrosis. In conclusion, our studies suggest that promoting PPARα activity to enhance mitochondrial metabolism may be a useful therapeutic strategy for ADPKD.
Keywords: fatty acid oxidation, fenofibrate, peroxisome proliferator-activated receptor α, polycystic kidney disease
INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) is among the most common human monogenic disorders and the leading genetic cause of kidney failure in the United States (12, 25). ADPKD is primarily caused by mutations in the PKD1 or PKD2 genes. The hallmark of this disorder is the presence of numerous fluid-filled, renal tubule-derived cysts that grow over time causing massive bilateral kidney enlargement. Approximately 50% of ADPKD patients develop end-stage renal disease (ESRD). In addition to kidney cysts, nearly 80% of the patients also develop liver disease primarily involving bile duct-derived cysts (2). Despite the recent advances, the pathogenesis of this disorder is incompletely understood, and an FDA-approved therapy is still lacking.
Kidneys require large amounts of energy to execute their daily function. Fatty acid β-oxidation (FAO) and oxidative phosphorylation (OXPHOS) are the major pathways for ATP production in the normal kidney (14, 33). However, unlike the normal kidney, renal tubules in cystic kidneys are rapidly proliferating. Thus, a more pressing need for cystic kidneys is to generate new organelles, DNA/RNA, and cell membranes for dividing cells. To meet these needs, cyst epithelial cells appear to rewire their metabolism by activating alternative pathways of glycolysis and glutaminolysis (11, 27). As an additional component of this rewiring, we and others have recently shown that FAO/OXPHOS is reduced in ADPKD models (9, 23). Collectively, the metabolic reprogramming could provide abundant carbon for the production of building blocks (nucleotides, proteins, lipids) that are needed for the formation of new cells. Targeting metabolic derangements has emerged as a new therapeutic approach for ADPKD (6). Indeed, inhibiting glycolysis has been shown to slow cyst proliferation and growth. However, whether enhancing FAO/OXPHOS could be an alternative or perhaps even a complementary approach to slow cyst growth is currently unknown.
Peroxisome proliferator-activated receptor alpha (PPARα) is a nuclear hormone receptor that is activated by fatty acids (and its derivatives) and regulates the expression of numerous metabolism-related genes (35). Accordingly, activation of PPARα promotes peroxisomal and mitochondrial FAO. PPARα is expressed primarily in the organs that metabolize fatty acids, such as the liver, adipose tissue, kidney, and heart. PPARα is the main target of a class of widely used drugs called fibrates, which includes fenofibrate, clofibrate, and others (21, 30). These drugs function as agonists of PPARα and are commonly prescribed for the treatment of cholesterol and triglyceride disorders.
We have recently demonstrated that PPARα is downregulated in aggressive mouse models of ADPKD and primary human ADPKD cells, suggesting that decreased PPARα function may underlie the impaired FAO and OXPHOS observed in ADPKD (9). Moreover, deleting PPARα exacerbates cyst growth in an ADPKD mouse model. Thus, the goal of this study was to test whether upregulating PPARα would enhance FAO/OXPHOS and attenuate cyst growth. We have previously shown that fenofibrate treatment slows cyst growth in the Pkd2-KO mouse model of ADPKD (9). However, this is an aggressive and short-lived ADPKD model, and fenofibrate treatment was performed only for 10 days. On the basis of observations from this short-term study, we designed a randomized, 6-mo preclinical trial in a long-term, slowly progressing, clinically relevant orthologous model of ADPKD. Here, we report that fenofibrate upregulates PPARα, improves FAO/OXPHOS, and slows kidney and liver cyst growth, suggesting that normalizing PPARα activity may be a novel therapeutic strategy for ADPKD.
MATERIALS AND METHODS
Mice.
Pkd1RC/RC mice were used for these studies. At 50 days of age Pkd1RC/RC mice were randomized to receive a standard chow diet or standard chow diet supplemented with fenofibrate (Sigma) at a dose of 400 mg·day−1·kg body wt−1 based on an average food intake of 160 mg·day−1·kg body wt−1. Equal males and females were used in both groups. At 180 days, all mice underwent MRI and were subsequently euthanized at 200 days of age for histological and molecular analysis. All experiments involving animals were conducted under the auspices of the University of Texas Southwestern Animal Care and Use Committee.
MRI imaging and analysis.
MRI scans were performed using a 7-T small animal MRI scanner [Agilent (Varian), Palo Alto, CA] equipped with a 40-mm Millipede RF coil (ExtendMR LLC, Milpitas, CA). Under anesthesia by inhalation of 1–3% isoflurane mixed in with medical-grade oxygen via nose cone, the animals were placed supine with the respiratory sensor, head first with the kidneys centered with respect to the center of a RF coil. MRI acquisitions were gated using the respiratory triggering. The bore temperature was kept at 28 ± 1°C. Two-dimensional scout images on three orthogonal planes (axial, coronal, and sagittal) were acquired to ensure the positioning. For kidney volume measurements, the high-resolution T2-weighted fast spin-echo (FSE) images were acquired on the coronal sections by applying a 6-ms sinc presaturation pulse to remove the fat signal. Some of the major imaging parameters were TR/TE = 2,500/60 ms, FOV = 32 × 32 mm, matrix size = 256 × 256 (affording 125-µm in-plane resolution), slice thickness = 1 mm, slice number = 9–15 (dependent on the kidney size), no gap, and number of average = 6. Volumetric analysis was performed using ImageJ software.
Tissue harvesting and analysis.
Mice were anesthetized under approved protocols, blood was obtained by cardiac puncture, and the right kidney was flash frozen for molecular analysis. The left kidney was perfused with cold PBS and 4% (wt/vol) paraformaldehyde and then harvested. Kidneys were fixed with 4% paraformaldehyde for 2 h and then embedded in paraffin for sectioning. Sagittal sections of kidneys were stained with hematoxylin and eosin (H&E) or picosirius red for additional analysis using ImageJ software.
Renal function tests.
Serum creatinine was measured using capillary electrophoresis by the UT Southwestern O’Brien Kidney Core Center.
3H-triolein uptake and β-oxidation.
Endogenous triolein clearance rates and β-oxidation rates in kidneys were performed as previously described (18). Briefly, 150-day-old wild-type or Pkd1RC/RC mice were injected with 3H-triolein (2 μCi per mouse in 100 μl of 5% Intralipid) after an 8-h fast. Twenty minutes later, mice were euthanized, blood samples were taken, and kidneys were harvested, and weighed and frozen at −80°C until processing. Lipids were extracted, and radioactivity content was quantified.
RNA isolation and quantitative RT-PCR.
Total RNA was isolated from mouse kidneys and livers using miRNeasy mini kits (Qiagen). First-strand cDNA was synthesized from mRNA using the iScript cDNA synthesis kit (Bio-Rad), and quantitiative PCR (Q-PCR) was performed using the iQ SYBR Green Supermix (Bio-Rad). The samples were loaded in triplicate on a CFX Connect real-time PCR detection system. 18S was used to normalize expression of mRNA. Data were analyzed using the Bio-Rad CFX software. The sequences of the PCR primers are shown in Table 1.
Table 1.
List of qPCR primers
| Gene | Primer Sequence |
|---|---|
| Ppara | |
| Forward | 5′-CCTCAAAGTCTGAGCGGTCT-3′ |
| Reverse | 5′-CTAACCTTGGGCCACACCT-3′ |
| Ppargc1a | |
| Forward | 5′-GTGAACATTCAAAGCAGCAGAG-3′ |
| Reverse | 5′-TTCTTCGTACAGCCATCAAAAA-3′ |
| Pparg | |
| Forward | 5′-AGCTCCAAGAATACCAAAGTGC-3′ |
| Reverse | 5′-GATGCTTTATCCCAGACTC-3′ |
| Acox1 | |
| Forward | 5′-CCGCCACCTTCAATCCAGAG-3′ |
| Reverse | 5′-CAAGTTCTCGATTTCTCGACGG-3′ |
| Cpt1a | |
| Forward | 5′-CACCAGTGATGATGCCATTCT-3′ |
| Reverse | 5′-CTCCGCCTGAGCCATGAAG-3′ |
| Cpt1b | |
| Forward | 5′-GCACACCAGGCAGTAGCTTT-3′ |
| Reverse | 5′-CAGGAGTTGATTCCAGACAGGTA-3′ |
| Slc27a2 | |
| Forward | 5′-CGAGACGAGACGCTCACCTA-3′ |
| Reverse | 5′-ACGAATGTTGTAGTTGAGGCAC-3′ |
| Cd36 | |
| Forward | 5′-ATGGGCTGTGATCGGAACTG-3′ |
| Reverse | 5′-TTTGCCACGTCATCTGGGTTT-3′ |
| Etfb | |
| Forward | 5′-CTGTCAAGAGGTCATCGACT-3′ |
| Reverse | 5′-CACAGAAGGGGTTCATGGAGT-3′ |
| Etfdh | |
| Forward | 5′-GTGCGACTAACCCTGTC-3′ |
| Reverse | 5′-GGATGAACAGTGTAGTGAGTGG-3′ |
| Pdk4 | |
| Forward | 5′-AGGGAGGTCGAGCTGTTCTC-3′ |
| Reverse | 5′-GGAGTGTTCACTAAGCGGTCA-3′ |
| F4/80 | |
| Forward | 5′-TGACTCACCTTGTGGTCCTAA-3′ |
| Reverse | 5′-CTTCCCAGAATCCAGTCTTTCC-3′ |
| Cd68 | |
| Forward | 5′-TGTCTGATCTTGCTAGGACCG-3′ |
| Reverse | 5′-TTCGGCGTCCATTTTCTTTGG-3′ |
| Mip2 | |
| Forward | 5′-GCTGGCCACCAACCACCAG-3′ |
| Reverse | 5′-AGCGAGGCACATCAGGTA-3′ |
Immunofluorescence staining.
The following antibodies and dilutions were used on paraffin-embedded sections for immunofluorescence staining: anti-Ppara (Abcam ab8934; 1:200), anti-F4/80 (Abcam; ab6640, 1:50), anti-Pmp70 (EMD Millipore ABT12; 1:200), anti-phosphohistone H3 (1:400; Sigma-Aldrich H0412), and anti-mannose receptor (Abcam ab64693; 1:400).
Proliferation quantification.
Liver cyst and kidney cyst proliferation was performed using the automated ImageJ’s Find Maxima feature. Noise cut-offs of 30 and 200 were used to count DAPI-positive and phosphohistone-H3-positive cells, respectively.
Liver cyst quantification.
Ten random ×20 fields per liver were scored with a 0 (cyst present) or 1 (cyst absent), and the percentage of positive field was calculated. The person performing the quantification was blinded to the two groups.
Western blot analysis.
Total protein was extracted from kidneys and livers. Ten micrograms of protein loaded on a 4–15% SDS-polyacrylamide gel, and the proteins were transferred to a nitrocellulose membrane. The membrane was blocked with 5% BSA and probed overnight at 4°C with anti-Ppara (1:1,000), anti-Pmp70 (1:1,000), or Anti-Pgc-1a (Abcam ab54481, 1:1,000) antibodies. Goat-anti-rabbit HRP-conjugated IgG was used as a secondary antibody, and the blot was developed using the SuperSignal West Dura Extended Duration substrate from Pierce. The protein bands were quantified using Quantity One imaging software from Bio-Rad.
Statistical analysis.
Data are shown as the means ± SE. Statistical analysis was performed using unpaired, two-tailed, Studentʼs t-test. P < 0.05 was considered significant. Primary outcomes (kidney volume, cyst volume, cyst index, and serum creatinine) are statistically powered to achieve an α error <5% and power of >80%.
RESULTS
PPARα and FAO/OXPHOS are downregulated in Pkd1RC/RC mice.
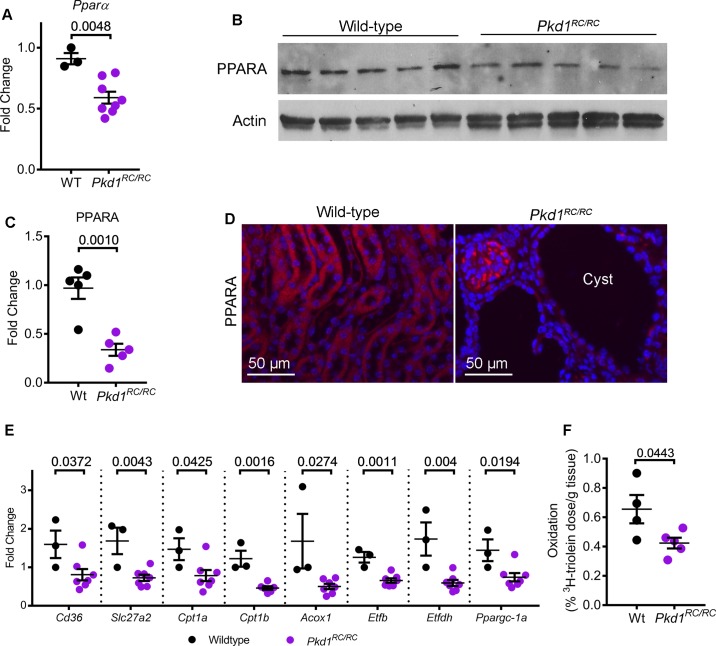
We began by examining PPARα expression in the Pkd1R3277C/R3277C (Pkd1RC/RC) mice that harbor germline hypomorphic Pkd1 mutations (10). These mice develop slowly progressing kidney cysts that closely mimic the human ADPKD phenotype. First, we performed quantitative real-time PCR (Q-PCR) to measure Ppara mRNA expression levels in 200-day-old control and Pkd1RC/RC mice (n = 5). Ppara mRNA expression was decreased by 35.1% in kidneys from Pkd1RC/RC mice compared with noncystic control mice (Fig. 1A). Similarly, Western blot analysis showed that PPARα protein levels were also reduced 65.6% in Pkd1RC/RC mice compared with control mice (Fig. 1, B and C). Moreover, immunofluorescence staining demonstrated that PPARα levels were reduced specifically in kidney cyst epithelial cells of Pkd1RC/RC mice (Fig. 1D).
Fig. 1.
Peroxisome proliferator-activated receptor α (PPARα) is downregulated in Pkd1RC/RC mice. A: quantitative PCR (Q-PCR) analysis showed that Ppara expression was reduced by 35.1% in the kidneys of 200-day-old Pkd1RC/RC mice (n = 8) compared to age-matched wild-type control mice (n = 3). B and C: Western blot analysis and quantification demonstrated that PPARα protein expression was reduced 65.6% in Pkd1RC/RC mice (n = 5) compared to wild-type controls (n = 5). Actin was used as the loading control. D: immunofluorescence staining revealed that PPARα (red) expression was specifically reduced in kidney cyst epithelial cells Pkd1RC/RC mice. E: expression of fatty acid oxidation/oxidative phosphorylation (FAO/OXPHOS) genes regulated by PPARα was measured using Q-PCR. Compared to control mice kidneys (n = 3), Pkd1RC/RC mice kidneys (n = 6 or 7) demonstrated significant downregulation of FAO/OXPHOS gene expression. F: to determine whether reduced FAO/OXPHOS gene expression resulted in reduced mitochondrial metabolism, wild-type (n = 4) and Pkd1RC/RC (n = 5) were injected with 3H-labeled triolein tracer to measure in vivo kidney FAO. Oxidation of 3H-labeled triolein was reduced in Pkd1RC/RC mice kidneys compared to wild-type kidneys. Error bars indicate means ± SE.
To determine whether reduced PPARα is associated with a decrease in FAO/OXPHOS, we measured expression of FAO/OXPHOS-related PPARα target genes in 200-day-old control (n = 3) and Pkd1RC/RC mice (n = 7) (Fig. 1E). Q-PCR analysis showed that the expression of Cd36, which encodes the membrane protein that imports fatty acids into the cells, and Slc27a2, which encodes the enzyme that activates fatty acids to their CoA derivatives was decreased by 49% and 57% in the kidneys of Pkd1RC/RC mice compared with age-matched wild-type control mice. Cpt1a and Cpt1b, which encode carnitine palmitoyltransferase I enzyme, which catalyzes the essential step of transporting fatty acids into the mitochondria, and Acox1, which encodes the first enzyme of the FAO pathway, were downregulated by 47%, 62%, and 70%, respectively, in Pkd1RC/RC mice kidneys compared with wild-type kidneys. Etfb and Etfdh, which collectively encode the OXPHOS enzyme electron transfer flavoprotein and Ppargc1a, which is a transcriptional coactivator and a master regulator of mitochondrial biogenesis, were also decreased by 48%, 66%, and 49%, respectively. Finally, to determine whether downregulation of PPARα targets and markers of mitochondrial biogenesis translates into reduced mitochondrial metabolism, we injected Pkd1RC/RC mice (n = 5) and wild-type mice (n = 4) with 3H-triolein tracer to measure in vivo kidney FAO. This assay measures radioactive hydrogen content in aqueous (oxidized 3H-triolein) and organic fractions (unoxidized 3H-triolein) of the kidney in relation to the total systemically injected 3H-triolein. The β-oxidation rate of 3H-triolein was reduced by 35% in the kidneys of Pkd1RC/RC mice compared with wild-type mice kidneys (Fig. 1F). Thus, Ppara mRNA and PPARα protein are downregulated in kidney cysts of the Pkd1RC/RC mouse model, and this is associated with a functional decrease in FAO/OXPHOS.
Fenofibrate augments PPARα and enhances FAO/OXPHOS in Pkd1RC/RC mice.
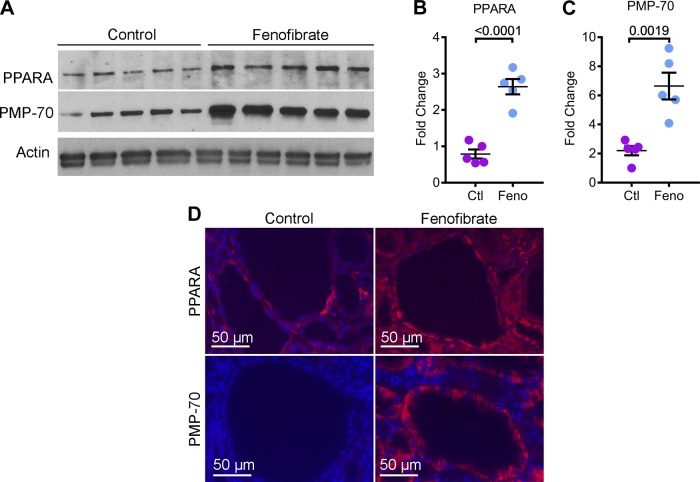
We next studied the effects of upregulating PPARα in Pkd1RC/RC mice. Fifty-day-old littermate Pkd1RC/RC mice were randomly assigned to receive either a standard chow diet (control group, n = 8) or a standard chow diet supplemented with fenofibrate (fenofibrate group, n = 8). An equal number of males and females were included in each group. These mice were euthanized after 5 mo of treatment for phenotypic analysis. We first determined whether fenofibrate treatment regulated PPARα expression in kidneys of Pkd1RC/RC mice. Western blot analysis using total kidney lysate showed a 3.34-fold increase in PPARα expression in the fenofibrate group compared with the control group (Fig. 2, A and B). Moreover, immunofluorescence staining revealed that PPARα expression was specifically upregulated in cyst epithelial cells (Fig. 2D). This result also indicated that systemic administration was sufficient to deliver fenofibrate to kidney cysts. To determine whether PPARα upregulation was associated with increased PPARα function, we first assessed whether fenofibrate treatment affected the abundance of peroxisomes. Kidney sections from mice supplemented with fenofibrate or controls were stained with an antibody against PMP70, an integral peroxisomal protein. PMP70 expression was markedly increased in cyst epithelial cells of fenofibrate-treated compared with control mice (Fig. 2D). Furthermore, Western blot analysis revealed a three-fold increase in PMP70 in fenofibrate-treated compared with control kidney (Fig. 2, A and C).
Fig. 2.
Fenofibrate treatment augments PPARα expression. 50-day-old Pkd1RC/RC mice were randomized to receive either standard chow diet (Ctl) or a diet supplemented with fenofibrate (Feno) for 5 mo. At 200 days of age, these mice were euthanized for analysis. A–C: Western blot analysis of total kidney lysate (A) and subsequent quantification (B and C) demonstrated increased expression of PPARα and PMP-70, a marker of peroxisomes, in fenofibrate-treated Pkd1RC/RC mice (n = 5) compared to control-treated Pkd1RC/RC mice (n = 5). D: immunofluorescence staining revealed that fenofibrate-treated Pkd1RC/RC mice showed increased expression of PPARα (red) and PMP-70(red) in kidney cyst epithelial cells compared to control-treated Pkd1RC/RC mice. Error bars indicate means ± SE.
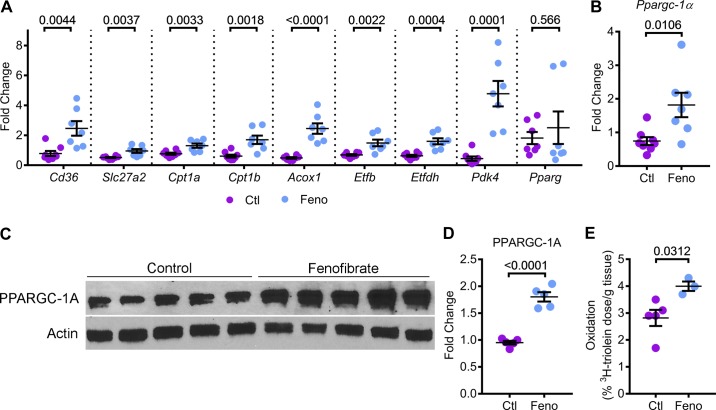
Next, we determined whether PPARα upregulation enhanced mitochondrial metabolism in Pkd1RC/RC mice. Q-PCR analysis showed that the expression of Cd36 and Slc27a2 were upregulated by 213% and 85.2%, respectively, in kidneys of fenofibrate-treated mice compared with control-treated mice. Cpt1a, Cpt1b, and Acox1 were also upregulated by 70%, 170%, and 400%, respectively, in fenofibrate-treated kidneys compared with control kidneys. Similarly, fenofibrate treatment increased the expression of Etfb and Etfdh by 115% and 150%, respectively (Fig. 3A). The expression of Pdk4 (pyruvate dehydrogenase kinase 4), which encodes the enzyme that inhibits pyruvate dehydrogenase, thereby, decreasing glucose utilization and increasing FAO, was upregulated more than four-fold in fenofibrate-treated kidneys compared with control kidneys. Interestingly, the expression of Pparg (peroxisome proliferator-activated receptor γ), which regulates glucose metabolism did not change between the two groups, suggesting that fenofibrate specifically upregulates PPARα, but not the closely related family member PPARγ. To determine whether fenofibrate treatment promoted mitochondrial biogenesis, we measured Ppargc1a mRNA and PPARGC1α protein levels. Q-PCR and Western blot analysis revealed that Ppargc1a mRNA and PPARGC1α protein were upregulated by 140% and 90%, respectively, in the kidneys of fenofibrate-treated mice compared with control-treated mice (Fig. 3, B–D). Finally, to determine whether upregulation of PPARα targets and markers of mitochondrial biogenesis translates into increased mitochondrial metabolism, we measured in vivo kidney FAO in fenofibrate-treated and control-treated Pkd1RC/RC mice. The β-oxidation rate of 3H-triolein was increased by 41.8% in kidneys of Pkd1RC/RC mice supplemented with fenofibrate compared with mice on the control diet (Fig. 3E). Taken together, these results indicated that fenofibrate treatment activates PPARα and promotes mitochondrial biogenesis, thereby enhancing FAO and OXPHOS in Pkd1RC/RC mice.
Fig. 3.
Fenofibrate enhances FAO and OXPHOS in Pkd1RC/RC mice. PPARα regulates several aspects of mitochondrial metabolism, including OXPHOS and FAO. Therefore, we tested whether fenofibrate treatment and PPARα upregulation affected kidney mitochondrial metabolism. A: Q-PCR analysis of FAO and OXPHOS genes that are directly regulated by PPARα was performed (n = 7 or 8). Expression of metabolism-related genes Cd36, Slc27a2, Cpt1a, Cpt1b, Acox1, Etfb, Etfdh, and Pdk4 was significantly increased in kidneys of fenofibrate-treated Pkd1RC/RC mice compared with control-treated Pkd1RC/RC mice. Fenofibrate treatment did not affect the expression of Pparg, which encodes PPARγ, a member of the PPAR subfamily. B–D: Q-PCR (n = 7 or 8) and Western blot analysis (n = 5) showed that the expression of Ppargc-1a mRNA and PPARGC1-α protein levels were increased in the kidneys of fenofibrate-treated Pkd1RC/RC mice compared to control-treated Pkd1RC/RC mice, suggesting that fenofibrate treatment was associated with enhanced mitochondrial biogenesis. E: to measure whether improved PPARα metabolic gene network was associated with improved mitochondrial function, control (n = 5) and fenofibrate-treated (n = 3) Pkd1RC/RC mice were injected with 3H-labeled triolein tracer to measure in vivo kidney FAO. Oxidation of 3H-labeled triolein was increased by 41.8% in fenofibrate-treated compared to control-treated Pkd1RC/RC mice. Ctl, control; feno, fenofibrate. Error bars represent means ± SE.
Fenofibrate attenuates cyst growth in Pkd1RC/RC mice.
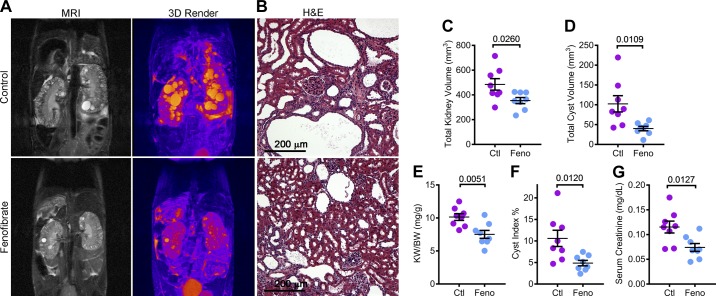
To determine whether PPARα upregulation and improved mitochondrial metabolism impacted the cystic phenotype of Pkd1RC/RC mice, we measured total kidney and total cyst volume by performing kidney MRIs (Fig. 4A). Total kidney volume was reduced by 26.9%, and total cyst volume was reduced by 60.9% in Pkd1RC/RC mice treated with fenofibrate compared with mice on a standard diet (Fig. 4, C and D). Next, we euthanized these mice for histological and molecular analysis. Consistent with the MRI analysis, gross examination showed that the kidney-weight-to-body-weight ratio and histological cyst index were also reduced in fenofibrate-treated mice by 25.8% and 54.1%, respectively. (Fig. 4, E and F). Moreover, the serum creatinine level was reduced by 35.7% in the Pkd1RC/RC fenofibrate group compared with the control group, indicating improved renal function (Fig. 4G). Together, these findings suggest that fenofibrate treatment attenuates cyst growth in a slowly progressing mouse model of ADPKD.
Fig. 4.
Fenofibrate slows kidney cyst growth in Pkd1RC/RC mice. Kidney MRIs and histological analysis were performed to determine whether fenofibrate treatment affected PKD progression in Pkd1RC/RC mice. A and B: representative T2-weighted MRI images, three-dimensional reconstruction of the MRI and hematoxylin and eosin (H&E)-stained sections of the same control and fenofibrate-treated mice are shown. C and D: quantification showed that MRI-assessed total kidney volume and total cyst volume was reduced in fenofibrate-treated compared to control-treated Pkd1RC/RC mice. E and F: consistent with the MRI analysis, kidney-weight-to-body-weight ratio and histological cyst index were reduced in fenofibrate-treated compared to control-treated Pkd1RC/RC mice. G: biochemical analysis showed that serum creatinine levels were reduced in fenofibrate-treated compared to control-treated Pkd1RC/RC mice. Fenofibrate (feno) group, n = 8 and control (Ctl) group, n = 8. Error bars represent means ± SE.
Fenofibrate treatment inhibits cyst proliferation and interstitial inflammation.
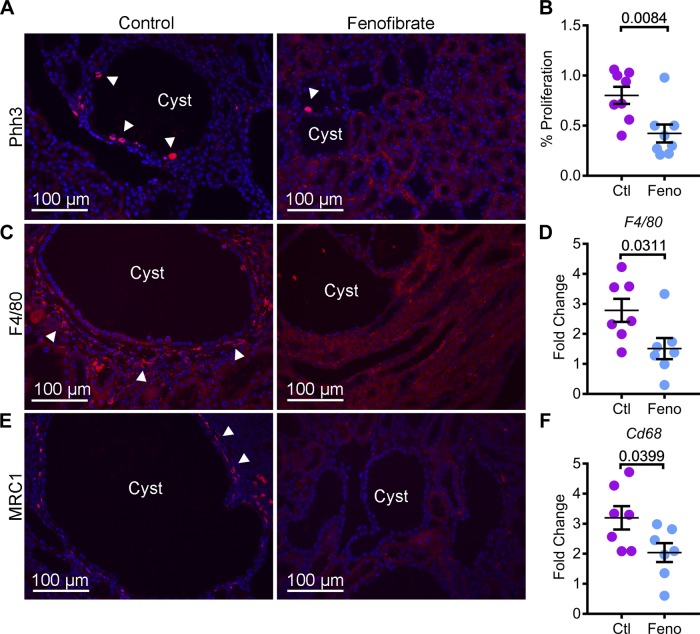
Excessive renal tubule proliferation and dysregulated inflammatory response underlie ADPKD pathogenesis (8). PPARα inhibits both proliferation and inflammation in other organs. Hence, we next determined whether administration of fenofibrate was associated with a change in these processes in cystic kidneys. Kidney sections from Pkd1RC/RC mice from control and fenofibrate groups were stained with an antibody against phospho-histone H3, a marker of cells undergoing mitosis (Fig. 5A). Quantification revealed that phospho-histone H3-positive cells were decreased by 47.3% in fenofibrate-treated kidneys compared with control kidneys (Fig. 5B). To assess inflammation, we stained kidney sections from both groups with an antibody against EGF-like module-containing mucin-like hormone receptor-like 1 (F4/80), a pan-macrophage marker (Fig. 5C). Immunofluorescence analysis demonstrated a marked reduction in macrophages surrounding cyst epithelial cells. Moreover, Q-PCR analysis of F4/80 and Cd68 (cluster of differentiation), another global macrophage marker, showed that their expression was reduced by 45.6% and 36.1%, respectively, in the fenofibrate group compared with control group. (Fig. 5, D and F). Macrophages surrounding cyst epithelia are a heterogeneous population, which includes M1-like and M2-like macrophages. M2-like macrophages are thought to promote cyst growth (31). Thus, we evaluated whether the reduction in total macrophages was associated with a decrease in the abundance of M2-like macrophages. Kidney sections were stained with an antibody against mannose receptor 1 (MRC1), a M2-macrophage marker. Immunofluorescence analysis revealed reduced MRC1-positive cells in kidneys of fenofibrate-treated Pkd1RC/RC mice compared with control Pkd1RC/RC mice (Fig. 5E). Thus, these results indicate that PPARα upregulation attenuates cyst growth in Pkd1RC/RC mice by inhibiting cyst proliferation and reducing the number of M2-like macrophages surrounding cyst epithelial cells.
Fig. 5.
Fenofibrate treatment inhibits cyst proliferation and interstitial inflammation. A and B: kidney sections from control and fenofibrate-treated Pkd1RC/RC mice were stained with phospho-histone H3 (Phh3), a marker of proliferation. Immunofluorescence analysis and quantification of Phh3-positive cells (red, arrowheads) showed that proliferation was reduced in the kidneys of fenofibrate-treated (n = 8) compared to control-treated mice (n = 8). To assess inflammation, kidney sections from control and fenofibrate-treated Pkd1RC/RC mice were stained using an antibody against F4/80, a pan-macrophage marker, and MRC1, a marker of M2-like macrophages. C: immunofluorescence analysis revealed that the abundance of F4/80-positive inflammatory cells (red, arrowheads) was reduced in fenofibrate-treated Pkd1RC/RC mice compared to control-treated Pkd1RC/RC mice. D: Q-PCR analysis also demonstrated decreased F4/80 expression in fenofibrate-treated Pkd1RC/RC mice (n = 7) compared with control-treated Pkd1RC/RC mice (n = 7). E: expression of MRC1 (red, arrowheads) was reduced in fenofibrate-treated Pkd1RC/RC mice. F: Q-PCR analysis demonstrated that the expression of Cd68, another marker of macrophages, was downregulated in fenofibrate-treated (n = 8) compared with control-treated (n = 7) Pkd1RC/RC mice. Ctl, control; feno, fenofibrate. Error bars indicate means ± SE.
Fenofibrate slows cystic liver disease.
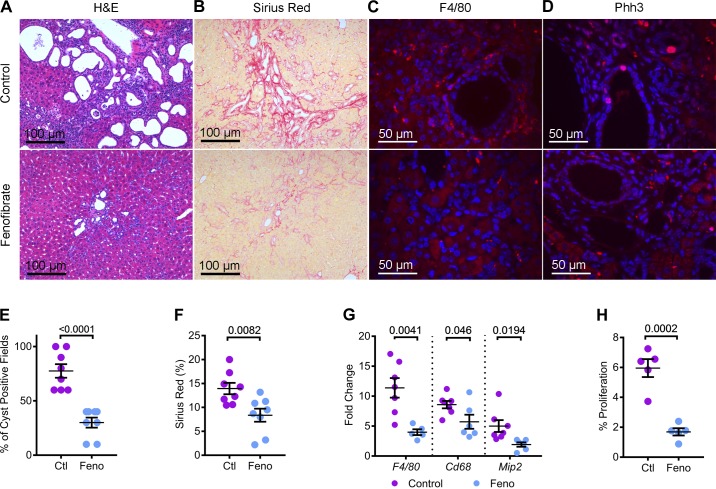
In addition to kidney cysts, the Pkd1RC/RC mouse also develops liver disease, which includes bile duct cyst and liver inflammation and fibrosis (10). Therefore, we examined whether fenofibrate treatment affected the liver phenotype. H&E staining of liver sections revealed fewer and smaller bile duct cysts in fenofibrate-treated compared with the control-treated Pkd1RC/RC mice (Fig. 6A). Quantification showed that 77.5% of microscopic fields (×20) contained at least one bile duct cyst in control Pkd1RC/RC mice (Fig. 6E). In contrast, bile duct cysts were observed only in 30% of microscopic fields of livers from fenofibrate-treated Pkd1RC/RC mice. To evaluate liver fibrosis, we performed picrosirius red staining of liver sections from both groups of mice. Picrosirius red-positive area was reduced by 40% in fenofibrate-treated compared with control-treated mice (Fig. 6, B and F). Immunofluorescence staining using an anti-F4/80 antibody revealed marked inflammatory infiltration in livers of Pkd1RC/RC mice on the control diet (Fig. 6C). Fenofibrate treatment significantly reduced this inflammatory infiltration. Moreover, Q-PCR analysis demonstrated that the expression of markers of inflammation F4/80 (down by 62.1%), Cd68 (down by 33.3%), and Mip2 (down by 61.6%) was also significantly reduced in fenofibrate-treated compared with control Pkd1RC/RC mice (Fig. 6G). Finally, we examined whether fenofibrate treatment affected liver cyst proliferation. Liver sections were stained with an antibody against Phospho-histone H3 (Fig. 6D). Quantification revealed that proliferation of liver cysts was reduced by 71.6% in the Pkd1RC/RC mice on the standard chow diet supplemented with fenofibrate compared with those on the control diet (Fig. 6H). Taken together, our results indicate that fenofibrate also abrogates liver disease in Pkd1RC/RC mice.
Fig. 6.
Fenofibrate attenuates liver cysts, fibrosis, and inflammation in Pkd1RC/RC mice. A and B: H&E and picrosirius red-stained liver sections showed the marked regression in cyst burden and fibrosis in fenofibrate-treated Pkd1RC/RC mice. C and D: immunofluorescence staining using antibodies against F4/80 and Phh3 revealed reduced inflammation and proliferation in the livers of fenofibrate-treated Pkd1RC/RC mice, respectively, compared to control-treated Pkd1RC/RC mice. E: quantification of cyst-positive fields in H&E-stained liver sections showed fewer cysts in fenofibrate-treated (n = 8) Pkd1RC/RC mice compared with control-treated (n = 8) Pkd1RC/RC mice. F: similarly, quantification of picrosirius red-positive area was reduced in fenofibrate-treated Pkd1RC/RC mice, indicating reduced liver fibrosis (n = 8). G: consistent with immunofluorescence staining, Q-PCR analysis demonstrated reduced expression of macrophage markers (F4/80, Cd68, and Mip2) in fenofibrate-treated Pkd1RC/RC mice compared with control-treated Pkd1RC/RC mice (n = 6 or 7). H: quantification revealed that the number of Phh3-positive cyst epithelial cells were reduced by 71.6% in the Pkd1RC/RC mice on a chow diet supplemented with fenofibrate compared with the control diet (n = 5). Ctl, control; feno, fenofibrate. Error bars represent means ± SE.
DISCUSSION
The major conclusion of our work is that PPARα is a new drug target for ADPKD. First, we demonstrated that expression of PPARα and FAO/OXPHOS genes is downregulated, and in vivo, the β-oxidation rate of 3H-labeled triolein is reduced in Pkd1RC/RC mice. Reduced PPARα expression is also observed in human ADPKD kidney samples and multiple other ADPKD rodent models (29). Moreover, FAO and OXPHOS, the two metabolic processes regulated by PPARα, are impaired in other ADPKD mouse models and PKD1 mutant cell lines (9, 23). These observations suggest that inhibition of PPARα function may be a common feature of ADPKD. The mechanisms that lead to PPARα downregulation in ADPKD are not well understood. Our previous work has shown that one potential mechanism may involve direct repression of PPARα by pathogenic microRNAs such as miR-17, miR-19, and miR-21 that are upregulated in ADPKD (5, 9, 19, 36). As a second line of evidence implicating PPARα in ADPKD pathogenesis, we showed that treatment with PPARα agonist fenofibrate upregulated PPARα expression, enhanced kidney FAO, slowed cyst proliferation, and attenuated polycystic kidney disease progression in Pkd1RC/RC mice. We have previously observed similar beneficial effects of fenofibrate in the Pkd2-KO model of ADPKD (9).
Metabolic reprogramming of cyst epithelial cells involving the activation of glycolysis and glutaminolysis is thought to underlie ADPKD progression. Several recent studies have shown that an important additional layer of this metabolic reprogramming is the inhibition of FAO and OXPHOS in cyst epithelial cells (9, 23). In fact, reduced OXPHOS may be a primary event in this metabolic rewiring considering that the polycystin 1 and polycystin 2 complex is thought to directly regulate mitochondrial function (24). Several monogenetic human disorders further support the idea that reduced FAO/OXPHOS can independently drive kidney cystogenesis. For example, mutations in CPT2, an enzyme that transports FA into mitochondria, or mutations of either ETFA, ETFB, or ETFDH that collectively encode the OXPHOS enzyme electron transfer flavoprotein, are associated with PKD (16, 22, 34). Activating glycolysis and glutaminolysis and inhibiting FAO/OXPHOS would together shuttle carbon away from mitochondrial ATP generation to other pathways that produce the building blocks critically needed for rapidly dividing cells. Inhibition of glycolysis, which would lead to the depletion of carbon available for dividing cells, has been shown to slow cyst proliferation. As an alternative approach, our work suggests that improving FAO/OXPHOS is also an effective therapeutic strategy to target the dysregulated proproliferative, metabolic pathways in ADPKD. Enhancing FAO/OXPHOS could potentially shuttle carbon to mitochondria, thereby limiting the amount of carbon available for anabolic purposes (making cell components) and, thereby, slowing proliferation (3, 26, 28).
In addition to regulating FAO/OXPHOS, PPARα is also known to inhibit inflammatory pathways. Infiltration of immune cells, in particular, M2-like macrophages is observed in kidneys from mouse models and human ADPKD patients. Importantly, depleting macrophages in PKD mouse models has been shown to retard cyst growth (15). Consistent with the observations in other organs, we found that fenofibrate treatment was associated with a reduced number F4/80-positive cells, including M2-like macrophages in kidneys of Pkd1RC/RC mice. Whether these findings are due to effects of fenofibrate on cyst epithelial cells or a direct effect on macrophages remains to be explored.
ADPKD is a systemic disorder with nearly 80% of patients developing bile duct cysts and liver inflammation and fibrosis. As an added benefit, we observed that fenofibrate treatment and PPARα upregulation was associated with a marked attenuation of liver disease in Pkd1RC/RC mice. Similar beneficial effects of fenofibrate treatment have been seen in other types of inflammatory and fibrotic liver diseases. For example, fenofibrate appears to be effective in patients with primary biliary cirrhosis, a rare condition in which bile ducts are damaged, causing cirrhosis (7). Similarly, fenofibrate has shown some efficacy in patients with nonalcoholic fatty liver disease, which is characterized by steatosis, liver inflammation, and eventually liver fibrosis (17). The favorable liver effects of fenofibrate are especially noteworthy since liver toxicity is the main potential limitation of tolvaptan, the only currently available treatment for ADPKD (32).
PPARγ, another member of the PPAR subfamily of the nuclear hormone receptor, has also been implicated in ADPKD pathogenesis (4, 20, 37). PPARγ is a ligand-activated transcription factor that regulates glucose metabolism and promotes adipocyte differentiation and insulin sensitivity. PPARγ agonists (for example, pioglitazone and rosiglitazone) are clinically used for the treatment of Type 2 diabetes. Similar to PPARα, PPARγ expression is downregulated, and treatment with its agonists attenuates PKD progression in rodents. Pioglitazone is currently being evaluated in a human ADPKD clinical trial. Interestingly, we found that fenofibrate did not upregulate PPARγ expression, suggesting that the cyst-reducing effects of fenofibrate are mediated due to upregulation of PPARα. Therefore, it is possible that a combined pioglitazone and fenofibrate treatment may have synergistic effects.
Fenofibrate is FDA-approved, cheap, and has been clinically used for decades. Moreover, fenofibrate was found to reduce albuminuria in a large randomized clinical trial of patients with Type 2 diabetes, suggesting that it may have beneficial effects on the kidney (13). Hence, testing whether this agent demonstrates therapeutic efficacy in a human ADPKD clinical trial is extremely appealing. However, paradoxically, fenofibrate use has been linked to elevations in serum blood urea nitrogen, creatinine, and cystatin C levels in some individuals with chronic kidney disease (CKD). Moreover, fenofibrate accumulates in the serum of individuals with CKD, which could lead to toxicity (1). Therefore, designing a clinical trial and monitoring therapeutic efficacy of this drug in ADPKD patients with low GFR may prove challenging. Nevertheless, our work suggests that other approaches aimed at stabilizing PPARα or promoting FAO and OXPHOS expression may have therapeutic value in ADPKD.
In summary, we have shown that PPARα downregulation is associated with ADPKD progression. Activation of PPARα results in enhanced FAO/OXPHOS and attenuation of polycystic kidney and liver disease in mice. Our preclinical studies indicate that PPARα is a novel drug target for ADPKD.
GRANTS
We thank the University of Texas (UT) Southwestern O’Brien Kidney Research Core Center (P30DK-079328), the Touchstone Diabetes Center at UT Southwestern, and the Mayo Translation PKD center (DK-090728) for providing critical reagents and services. The work from the authors’ laboratory is supported by National Institutes of Health Grants R01-DK-102572 (to V. Patel).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.L. and V.P. conceived and designed research; R.L., M.Y., A.F., E.B.Q.-S., and W.L.H. performed experiments; R.L., M.Y., A.F., E.B.Q.-S., W.L.H., and V.P. analyzed data; R.L., M.Y., and V.P. interpreted results of experiments; R.L. and M.Y. prepared figures; R.L. and V.P. drafted manuscript; R.L., M.Y., A.F., E.B.Q.-S., W.L.H., and V.P. approved final version of manuscript; V.P. edited and revised manuscript.
REFERENCES
- 1.Attridge RL, Frei CR, Ryan L, Koeller J, Linn WD. Fenofibrate-associated nephrotoxicity: a review of current evidence. Am J Health Syst Pharm 70: 1219–1225, 2013. doi: 10.2146/ajhp120131. [DOI] [PubMed] [Google Scholar]
- 2.Bae KT, Zhu F, Chapman AB, Torres VE, Grantham JJ, Guay-Woodford LM, Baumgarten DA, King BF Jr, Wetzel LH, Kenney PJ, Brummer ME, Bennett WM, Klahr S, Meyers CM, Zhang X, Thompson PA, Miller JP; Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) . Magnetic resonance imaging evaluation of hepatic cysts in early autosomal-dominant polycystic kidney disease: the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort. Clin J Am Soc Nephrol 1: 64–69, 2006. doi: 10.2215/CJN.00080605. [DOI] [PubMed] [Google Scholar]
- 3.Beuster G, Zarse K, Kaleta C, Thierbach R, Kiehntopf M, Steinberg P, Schuster S, Ristow M. Inhibition of alanine aminotransferase in silico and in vivo promotes mitochondrial metabolism to impair malignant growth. J Biol Chem 286: 22323–22330, 2011. doi: 10.1074/jbc.M110.205229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blazer-Yost BL, Haydon J, Eggleston-Gulyas T, Chen JH, Wang X, Gattone V, Torres VE. Pioglitazone attenuates cystic burden in the PCK rodent model of polycystic kidney disease. PPAR Res 2010: 274376, 2010. doi: 10.1155/2010/274376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chau BN, Xin C, Hartner J, Ren S, Castano AP, Linn G, Li J, Tran PT, Kaimal V, Huang X, Chang AN, Li S, Kalra A, Grafals M, Portilla D, MacKenna DA, Orkin SH, Duffield JS. MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci Transl Med 4: 121ra18, 2012. doi: 10.1126/scitranslmed.3003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiaravalli M, Rowe I, Mannella V, Quilici G, Canu T, Bianchi V, Gurgone A, Antunes S, D’Adamo P, Esposito A, Musco G, Boletta A. 2-Deoxy-d-glucose ameliorates PKD progression. J Am Soc Nephrol 27: 1958–1969, 2016. doi: 10.1681/ASN.2015030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuperus FJ, Halilbasic E, Trauner M. Fibrate treatment for primary biliary cirrhosis. Curr Opin Gastroenterol 30: 279–286, 2014. doi: 10.1097/MOG.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 8.Grantham JJ. 1992 Homer Smith Award. Fluid secretion, cellular proliferation, and the pathogenesis of renal epithelial cysts. J Am Soc Nephrol 3: 1841–1857, 1993. [DOI] [PubMed] [Google Scholar]
- 9.Hajarnis S, Lakhia R, Yheskel M, Williams D, Sorourian M, Liu X, Aboudehen K, Zhang S, Kersjes K, Galasso R, Li J, Kaimal V, Lockton S, Davis S, Flaten A, Johnson JA, Holland WL, Kusminski CM, Scherer PE, Harris PC, Trudel M, Wallace DP, Igarashi P, Lee EC, Androsavich JR, Patel V. microRNA-17 family promotes polycystic kidney disease progression through modulation of mitochondrial metabolism. Nat Commun 8: 14395, 2017. doi: 10.1038/ncomms14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hopp K, Ward CJ, Hommerding CJ, Nasr SH, Tuan HF, Gainullin VG, Rossetti S, Torres VE, Harris PC. Functional polycystin-1 dosage governs autosomal dominant polycystic kidney disease severity. J Clin Invest 122: 4257–4273, 2012. doi: 10.1172/JCI64313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell 134: 703–707, 2008. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 12.Igarashi P, Somlo S. Polycystic kidney disease. J Am Soc Nephrol 18: 1371–1373, 2007. doi: 10.1681/ASN.2007030299. [DOI] [PubMed] [Google Scholar]
- 13.Jun M, Zhu B, Tonelli M, Jardine MJ, Patel A, Neal B, Liyanage T, Keech A, Cass A, Perkovic V. Effects of fibrates in kidney disease: a systematic review and meta-analysis. J Am Coll Cardiol 60: 2061–2071, 2012. doi: 10.1016/j.jacc.2012.07.049. [DOI] [PubMed] [Google Scholar]
- 14.Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, Park AS, Tao J, Sharma K, Pullman J, Bottinger EP, Goldberg IJ, Susztak K. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med 21: 37–46, 2015. doi: 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karihaloo A, Koraishy F, Huen SC, Lee Y, Merrick D, Caplan MJ, Somlo S, Cantley LG. Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol 22: 1809–1814, 2011. doi: 10.1681/ASN.2011010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kjaergaard S, Graem N, Larsen T, Skovby F. Recurrent fetal polycystic kidneys associated with glutaric aciduria type II. APMIS 106: 1188–1193, 1998. doi: 10.1111/j.1699-0463.1998.tb00276.x. [DOI] [PubMed] [Google Scholar]
- 17.Kostapanos MS, Kei A, Elisaf MS. Current role of fenofibrate in the prevention and management of non-alcoholic fatty liver disease. World J Hepatol 5: 470–478, 2013. doi: 10.4254/wjh.v5.i9.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kusminski CM, Holland WL, Sun K, Park J, Spurgin SB, Lin Y, Askew GR, Simcox JA, McClain DA, Li C, Scherer PE. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med 18: 1539–1549, 2012. doi: 10.1038/nm.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lakhia R, Hajarnis S, Williams D, Aboudehen K, Yheskel M, Xing C, Hatley ME, Torres VE, Wallace DP, Patel V. MicroRNA-21 aggravates cyst growth in a model of polycystic kidney disease. J Am Soc Nephrol 27: 2319–2330, 2016. doi: 10.1681/ASN.2015060634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu C, Zhang Y, Yuan L, Fu L, Mei C. Rosiglitazone inhibits insulin-like growth factor-1-induced polycystic kidney disease cell growth and p70S6 kinase activation. Mol Med Rep 8: 861–864, 2013. doi: 10.3892/mmr.2013.1588. [DOI] [PubMed] [Google Scholar]
- 21.McKeage K, Keating GM. Fenofibrate: a review of its use in dyslipidaemia. Drugs 71: 1917–1946, 2011. doi: 10.2165/11208090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Meir K, Fellig Y, Meiner V, Korman SH, Shaag A, Nadjari M, Soffer D, Ariel I. Severe infantile carnitine palmitoyltransferase II deficiency in 19-week fetal sibs. Pediatr Dev Pathol 12: 481–486, 2009. doi: 10.2350/08-10-0548.1. [DOI] [PubMed] [Google Scholar]
- 23.Menezes LF, Lin CC, Zhou F, Germino GG. Fatty acid oxidation is impaired in an orthologous mouse model of autosomal dominant polycystic kidney disease. EBioMedicine 5: 183–192, 2016. doi: 10.1016/j.ebiom.2016.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Padovano V, Kuo IY, Stavola LK, Aerni HR, Flaherty BJ, Chapin HC, Ma M, Somlo S, Boletta A, Ehrlich BE, Rinehart J, Caplan MJ. The polycystins are modulated by cellular oxygen-sensing pathways and regulate mitochondrial function. Mol Biol Cell 28: 261–269, 2017. doi: 10.1091/mbc.E16-08-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel V, Chowdhury R, Igarashi P. Advances in the pathogenesis and treatment of polycystic kidney disease. Curr Opin Nephrol Hypertens 18: 99–106, 2009. doi: 10.1097/MNH.0b013e3283262ab0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poteet E, Choudhury GR, Winters A, Li W, Ryou MG, Liu R, Tang L, Ghorpade A, Wen Y, Yuan F, Keir ST, Yan H, Bigner DD, Simpkins JW, Yang SH. Reversing the Warburg effect as a treatment for glioblastoma. J Biol Chem 288: 9153–9164, 2013. doi: 10.1074/jbc.M112.440354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowe I, Chiaravalli M, Mannella V, Ulisse V, Quilici G, Pema M, Song XW, Xu H, Mari S, Qian F, Pei Y, Musco G, Boletta A. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat Med 19: 488–493, 2013. doi: 10.1038/nm.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schulz TJ, Thierbach R, Voigt A, Drewes G, Mietzner B, Steinberg P, Pfeiffer AF, Ristow M. Induction of oxidative metabolism by mitochondrial frataxin inhibits cancer growth: Otto Warburg revisited. J Biol Chem 281: 977–981, 2006. doi: 10.1074/jbc.M511064200. [DOI] [PubMed] [Google Scholar]
- 29.Song X, Di Giovanni V, He N, Wang K, Ingram A, Rosenblum ND, Pei Y. Systems biology of autosomal dominant polycystic kidney disease (ADPKD): computational identification of gene expression pathways and integrated regulatory networks. Hum Mol Genet 18: 2328–2343, 2009. doi: 10.1093/hmg/ddp165. [DOI] [PubMed] [Google Scholar]
- 30.Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 98: 2088–2093, 1998. doi: 10.1161/01.CIR.98.19.2088. [DOI] [PubMed] [Google Scholar]
- 31.Swenson-Fields KI, Vivian CJ, Salah SM, Peda JD, Davis BM, van Rooijen N, Wallace DP, Fields TA. Macrophages promote polycystic kidney disease progression. Kidney Int 83: 855–864, 2013. doi: 10.1038/ki.2012.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS; TEMPO 3:4 Trial Investigators . Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tran MT, Zsengeller ZK, Berg AH, Khankin EV, Bhasin MK, Kim W, Clish CB, Stillman IE, Karumanchi SA, Rhee EP, Parikh SM. PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature 531: 528–532, 2016. doi: 10.1038/nature17184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitfield J, Hurst D, Bennett MJ, Sherwood WG, Hogg R, Gonsoulin W. Fetal polycystic kidney disease associated with glutaric aciduria type II: an inborn error of energy metabolism. Am J Perinatol 13: 131–134, 1996. doi: 10.1055/s-2007-994309. [DOI] [PubMed] [Google Scholar]
- 35.Wu J, Chen L, Zhang D, Huo M, Zhang X, Pu D, Guan Y. Peroxisome proliferator-activated receptors and renal diseases. Front Biosci (Landmark Ed) 14: 995–1009, 2009. doi: 10.2741/3291. [DOI] [PubMed] [Google Scholar]
- 36.Yheskel M, Patel V. Therapeutic microRNAs in polycystic kidney disease. Curr Opin Nephrol Hypertens 26: 282–289, 2017. doi: 10.1097/MNH.0000000000000333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshihara D, Kurahashi H, Morita M, Kugita M, Hiki Y, Aukema HM, Yamaguchi T, Calvet JP, Wallace DP, Nagao S. PPAR-γ agonist ameliorates kidney and liver disease in an orthologous rat model of human autosomal recessive polycystic kidney disease. Am J Physiol Renal Physiol 300: F465–F474, 2011. doi: 10.1152/ajprenal.00460.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]