Abstract
Chemogenetic technologies enable selective pharmacological control of specific cell populations. An increasing number of approaches have been developed that modulate different signaling pathways. Selective pharmacological control over G protein-coupled receptor signaling, ion channel conductances, protein association, protein stability, and small molecule targeting allows modulation of cellular processes in distinct cell types. Here, we review these chemogenetic technologies and instances of their applications in complex tissues in vivo and ex vivo.
I. INTRODUCTION
Electrochemical gradients facilitate basic cellular functions such as nutrient transport, energy production, intracellular signaling, and long-range electrical transmission. Ion channels control cellular electrical activity and are thus prime targets for influencing cellular functions. Ion channels can be perturbed directly with small molecule or peptide agonists or antagonists for ligand-gated ion channels (LGICs) or indirectly via G protein-coupled receptor (GPCR) signaling (87, 140). Pharmacological control of LGICs and GPCRs has long been used to acutely alter cell function (37, 78, 86, 101). However, the precision of these manipulations has been limited by insufficient selectivity; most LGICs and GPCRs are expressed across multiple cell populations. To address this issue, engineered LGICs and GPCRs have been developed as exogenously applied tools to obtain remote control over cellular electrical activity (62, 180, 199). Chemogenetic tools (180) are comprised of a transgenic actuator for a cellular pathway that is targeted to specific cell populations and can be rapidly switched on or off by delivery of a chemical ligand.
Chemogenetics generalizes chemical control of cellular pathways by engineering a limited set of tunable, modular, and selective receptor/ligand systems that can be applied to virtually any cell population. Optimal chemogenetic tools possess two core properties: 1) the transgenic actuator is normally nonperturbative to cells, i.e., it has low constitutive activity and low responsiveness to endogenous ligands; and 2) the exogenously applied ligand is nonperturbative to cells that lack the actuator transgene. An increasing number of chemogenetic tools have been developed to perturb cellular electrical activity in a variety of ways. Here, we will primarily review work focusing on cellular control with LGIC and GPCR tools, along with some additional chemogenetic strategies that are useful for controlling other aspects of cell function. Because chemogenetic technologies are used quite extensively, the objective of this review is to highlight the various ways in which these tools are used but not to exhaustively cover all chemogenetic studies.
II. GPCR-BASED TOOLS
GPCRs have extensive functional consequences in cells by influencing signal transduction pathways, gene expression, ion channels, and synapses (181). Intensive investigation over several decades has revealed a number of structural and functional characteristics that can be leveraged to tune the properties of GPCR-based tools (157). Furthermore, many GPCR ligands can have high potency, which is a useful foundation for optimizing receptor selectivity and also for dosing in animal experiments.
A. GPCR Signaling Pathways
1. Gα signaling
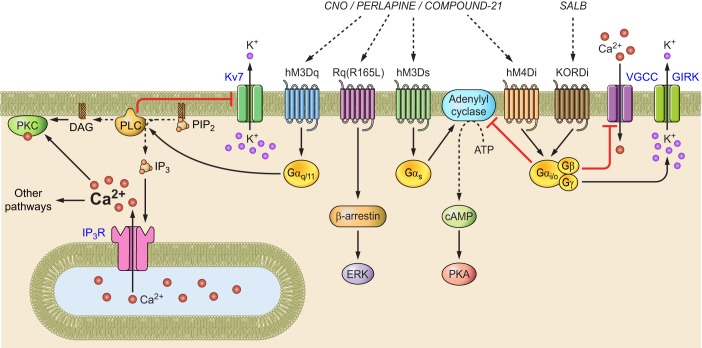
GPCRs interact with a G protein complex comprised of a Gα subunit bound to guanosine diphosphate (GDP), along with tightly associated Gβ and Gγ subunits (FIGURE 1) (74). Upon agonist binding, GPCRs catalyze exchange of Gα-GDP for guanosine triphosphate (GTP). Gα-GTP dissociates from the Gβγ complex, and both Gα-GTP and Gβγ engage separate downstream signaling processes. There are multiple G protein classes, and GPCRs are often distinguished by which type of Gα subunit they interact with. GPCRs that couple to a Gαs (Gs) protein increase the activity of adenylyl cyclase, which produces cAMP, thereby increasing protein kinase A (PKA) activity along with effects on gene expression. GPCRs that couple to Gi proteins inhibit adenylyl cyclase and reduce PKA activity. GPCRs that couple to Gq proteins activate phospholipase C, which cleaves phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol 1,4,5-trisphosphate (IP3) and diacyglycerol (DAG). IP3 binds receptors on the endoplasmic reticulum, leading to Ca2+ release from internal stores. DAG and Ca2+ activate protein kinase C (PKC), which engages multiple additional intracellular signaling processes.
FIGURE 1.
DREADDs and their downstream intracellular signal transduction pathways.
2. Gβγ signaling
The Gβγ complex also initiates downstream signaling, notably activating G protein-regulated inward rectifier potassium (GIRK) channels (122) and inhibiting N-type and P/Q-type voltage-gated Ca2+ channels (VGCCs) (43, 52). Interestingly, these effects seem to be observed selectively with activation of Gi protein-coupled GPCRs despite the fact that different individual Gβγ subunits do not show this selectivity (53). Thus Gi protein-coupled GPCRs activate GIRKs and inhibit VGCCs, both of which result in reduced cellular excitability. The mechanism by which the Gα subtype influences the cellular effects of the Gβγ subunits remains unclear (102). Nevertheless, different GPCRs have well-characterized, distinct functional consequences that provide a sound basis for chemogenetic tool development.
B. RASSLs
GPCRs have been extensively analyzed by mutagenesis to establish the influence of specific amino acid residues on ligand activation. Structure-function studies identified mutated GPCRs with greatly diminished ability to bind an endogenous ligand while preserving the capacity to be activated by synthetic ligands (39, 171, 182). These Receptors Activated Solely by Synthetic Ligands (RASSLs) were used as early tools for cell type-specific perturbations (38, 39). RASSLs are expressed in a desired cell type and are nonperturbative because they show low sensitivity to endogenous agonists. Once expressed in a desired cell type, RASSLs can be engaged by an exogenous synthetic ligand to selectively perturb cellular function. The major limitation of RASSLs is that the synthetic ligands retain the ability to engage the endogenous receptors.
One striking application of RASSLs is an experiment that examined taste sensory systems to test a hypothesis that sweet taste receptor-expressing neurons were a “labeled line” for the hedonic properties of sweet tastes irrespective of the identity of the receptor. After expressing a RASSL derived from the κ-opioid receptor (KOR) (152) in sweet receptor-expressing cells in taste buds, mice avidly consumed the normally nonpreferred synthetic ligand spiradoline at nanomolar concentrations (211). Although spiradoline can activate endogenous KORs, the delivery was limited to the tongue, and control experiments showed no significant effect of the ligand on consumption. Nevertheless, in many other circumstances, the selectivity profile of this system would be unsuitable, for example, in experiments involving systemic ligand administration. For that, a synthetic ligand with no endogenous activity is required.
C. DREADDs
1. Design
Development of GPCR tools with low sensitivity to endogenous ligands and high potency for otherwise inert exogenous ligands required more demanding design principles. This was first achieved by engineering a high-potency GPCR interaction with clozapine-N-oxide (CNO), which is an inactive metabolite of the antipsychotic drug clozapine. Clozapine is an agonist for endogenous muscarinic acetylcholine receptors, and CNO has very low potency at these receptors. With the use of random mutagenesis, a modified muscarinic acetylcholine receptor 3 (hM3) was used to create a library of mutant receptors that were assayed in a yeast selection system in which growth is dependent on G protein signaling (5). Yeast clones that grew on selective media in the presence of CNO were subjected to additional rounds of random mutagenesis and screening at progressively lower CNO concentrations until a receptor with two mutations was arrived at that exhibited high CNO sensitivity. Two additional criteria were taken into consideration during the clone selection process: minimal sensitivity to acetylcholine and low constitutive activity (5). Application of these mutations to hM3 created a Designer Receptor Exclusively Activated by a Designer Drug (DREADD), which was named hM3D (5). Because hM3D couples to Gq-type G proteins, it is often referred to as hM3Dq. CNO treatment of cells expressing hM3Dq increased IP3 production with an EC50 of 17 nM, which was 480-fold selective over CNO activation of unmutated hM3 (5). Conversely, acetylcholine potency was reduced by >50,000-fold for hM3D. Although constitutive activity of hM3D was modestly increased by twofold over hM3 (5), heterologous expression of hM3D in cortical neuron cultures did not significantly affect membrane potential (5). The same modification was also applied to human M1, M2, M4, and M5 receptors to generate additional CNO-sensitive DREADDs (5).
2. hM3Dq
hM3Dq was one of the first GPCR-based DREADDs, and it proved well suited to activate neuronal firing. Administration of CNO depolarized neurons and resulted in action potential firing in a PLC-dependent manner (2). Multiple mechanisms for hM3Dq-mediated neuron depolarization can be predicted from studies on signaling from endogenous Gq-coupled GPCRs. Muscarinic activation reduces the M-current (22), which is a PIP2-sensitive K+ conductance mediated by Kcnq channels (FIGURE 1) (210). At neuronal resting membrane potentials, K+ channels reduce neuronal excitability (87). CNO treatment of cells expressing hM3D converts PIP2 to IP3, and reduced PIP2 decreases M-current in Kcnq-expressing cells, consequently increasing neuronal excitability (210). However, the ionic mechanisms of hM3Dq signaling are likely more complicated than this. hM3Dq activation also releases Ca2+ from internal stores (5). Release of internal Ca2+ can activate the Na+/Ca2+ exchanger (18), which is electrogenic and can lead to a depolarizing inward current when internal Ca2+ is elevated (108, 141), complementing depolarization from M-current suppression. Countervailing receptors can also be engaged, including activation of calcium-sensitive potassium channels, which suppress neuronal activity and thus work against the desired neuron activation (15). Clearly, the effectiveness of hM3Dq for neuron activation reflects the expression of relevant accessory channels (such as Ca2+-sensitive K+ channels) in a cell type of interest. For this reason, verification of cell activation by electrophysiology is important when using hM3Dq (2, 105).
For such verification purposes, the immediate early gene Fos is often used as an in vivo marker for cellular activation after activating hM3Dq as an alternative to electrophysiology. One caveat of this validation approach for hM3Dq perturbations in animals is that Fos expression is also sensitive to elevated cellular Ca2+, which can be derived from internal stores in addition to external sources via VGCCs (69). Consistent with this, Fos expression has been shown, in some cases, to not correlate with independently measured neuronal activity (146). Thus the expression of Fos in response to hM3Dq activation may be related to pathways that are independent of electrical activity.
3. hM3Ds
To create a Gs-coupled DREADD, the modularity of hM3Dq signaling was exploited by maintaining the mutations that confer CNO sensitivity while altering a region that confers Gα-protein selectivity. By substituting intracellular loop sequences from the Gs-coupled β-adrenergic receptor into hM3Dq, hM3Ds was generated, which increases cAMP production but not IP3 or intracellular Ca2+ in the presence of CNO (61, 81). These experiments showed that because GPCRs have separate regions for ligand binding and G protein coupling, additional chemogenetic tools can be generated with distinct signaling properties while maintaining CNO responsiveness.
4. hM4Di
A Gi-coupled DREADD was generated by applying the hM3Dq mutations at homologous residues in hM4, which is an endogenous Gi-coupled receptor. hM4Di activation with CNO reduced forskolin-induced cAMP production (5). Expression of hM4Di in neurons rendered cells sensitive to CNO-induced hyperpolarization and reduction of action firing due to activation of GIRK channels (5). Further analysis of hM4Di demonstrated that it is an effective inhibitor of synaptic release (131, 173). Stachniak et al. (173) showed that the synaptic silencing function of hM4Di was likely more relevant to inhibiting neuronal output than was the suppression of electrical activity. This was demonstrated by tagging hM4Di with a portion of neurexin receptor 1a (hM4Dnrxn) to produce a receptor that was predominantly trafficked to the axonal compartment. hM4Dnrxn showed minimal effects on somatic conductance with CNO application, but synaptic silencing remained intact as did in vivo behavioral efficacy (173). Thus hM4Di and hM4Dnrxn are powerful tools for selectively blocking synaptic release from axon projections.
5. DREADD[Rq(R165L)]-β-arrestin signaling
In addition to G protein activation, GPCRs also influence cell signaling by recruitment of β-arrestin. Following agonist activation of GPCR signaling, the GPCR is phosphorylated, which recruits β-arrestin, and this promotes association with other signaling enzymes, such as extracellular signal-regulated kinase (ERK) (FIGURE 1) (191). Although β-arrestin signaling is activated by all DREADDs, a set of hM3Dq mutations that prevent G protein coupling permitted creation of a DREADD selective for β-arrestin signaling, called DREADD[Rq(R165L)] (138). This DREADD stimulated insulin release in response to CNO from a cultured pancreatic β-cell line (138).
6. Caveats and new ligands
Additional extensions of the DREADD approach include improvements in the chemogenetic agonist. Of note here, CNO administration at commonly used doses and in the absence of any DREADD expression was reported to affect amphetamine-induced dopamine release, locomotion, and acoustic startle reflex (129), possibly due to retro-conversion to clozapine (31). Nevertheless, the doses used in this study (1–5 mg/kg) are greater than the recommended in vivo dose of 0.3 mg/kg (158). The potency of behavioral effects in chemogenetic experiments is also affected by the excess receptor expression such that signaling from just a fraction of ligand-bound receptors is sufficient for a behavioral response (158). Another concern is a report that, in nonhuman primate brains, CNO is a substrate for the P-glycoprotein efflux pump, resulting in low partition of plasma CNO into the brain (136, 151). However, different studies in nonhuman primates report contradictory results regarding the levels of retro-converted clozapine in the cerebrospinal fluid of CNO-treated rhesus macaques (136, 151). More recently, Gomez, et al. (75a) used autoradiography and positron emission tomography with [11C]CNO to show that CNO does not get into the brain in rats. This study provided multiple lines of evidence that CNO is not the active agent for in vivo chemogenetic experiments and that, instead, it is the small amount of retro-converted clozapine that is responsible for muscarinic DREADD activation. Although only 2% of CNO is converted to clozapine, the considerably higher potency of clozapine for hM3Dq and hM4Di relative CNO is suggested to be what permits this chemogenetic system to function in the brain (75a). Due to concern about the metabolic back reaction of CNO to clozapine, a new potent and metabolically stable small molecule agonist, called compound 21, has been identified (34). Compound 21 is a promising tool to replace CNO with DREADDs derived from human muscarinic receptors. The drug perlapine also activates hM3Dq-derived DREADDs but has sedative side effects (34).
7. KORDi
The molecular evolution process for producing DREADDs is applicable to other GPCRs. This was demonstrated for kappa opioid receptor (KOR), which is a Gi-coupled receptor that is potently activated by the hallucinogen salvinorin A (SalvA). Directed molecular evolution identified mutations in KOR that rendered it sensitive to salvinorin B (SalvB), which is a metabolite of SalvA that is inactive at KOR (193). KORD activation by SalvB inhibited neuron electrical activity and synaptic release in the presence of SalvB (193). With hM3Dq and KORDi, chemogenetic neuron activation and silencing could be demonstrated in the same neuron, although neuron inhibition was only observed for ~1 h due to the short in vivo half-life of SalvB (193). The development of orthogonal DREADD systems allows for multiple, distinct chemogenetic perturbations in the same organism.
8. Summary
Directed evolution of the hM3 receptor to install sensitivity to the normally inactive drug metabolite CNO led to the identification of two mutations that reduce acetylcholine responsiveness and dramatically enhanced CNO sensitivity. The sites mutated in hM3Dq are conserved across other muscarinic receptors, conferring CNO sensitivity on functionally distinct muscarinic receptors. Moreover, existing structure-function analysis of GPCRs can be applied to hM3D receptors to produce receptors with distinct properties such as Gs- and β-arrestin-selective signaling. Thus the discovery of mutations that produce new pharmacological selectivity has been exploited in conjunction with other structure-function information to produce a range of powerful chemogenetic tools for control of different aspects of cellular signaling. Moreover, the platform for discovering DREADD mutations can be applied to multiple GPCRs as evidenced by the KORDi DREADD. This expanding toolbox enables selective pharmacological control over distinct signaling pathways, offering new capabilities for sophisticated analysis of cellular and circuit functions.
D. Neuronal Applications
1. DREADD delivery
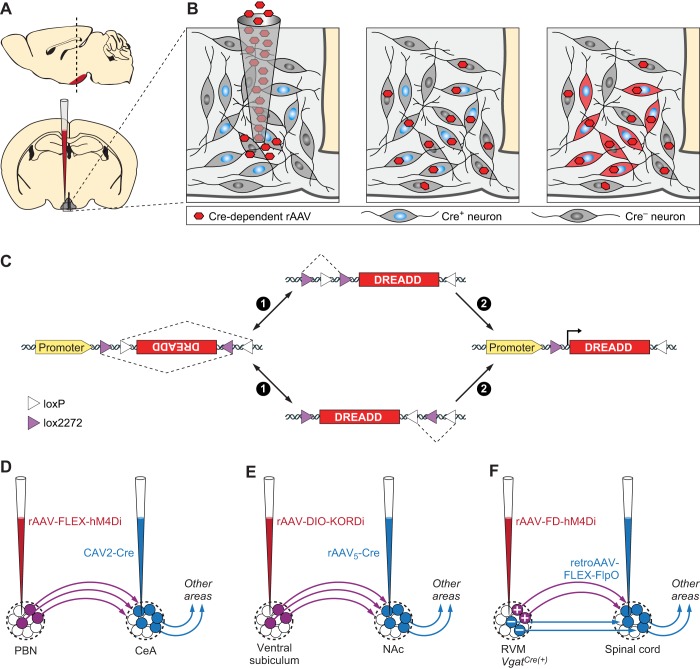
Two of the most commonly employed strategies for genetic targeting of DREADDs involve locally delivered viral injections and expression from genetically engineered animal models. The relatively short DREADD coding sequences allow efficient packaging into viral vectors. Recombinant AAV vectors carrying DREADD transgenes under multiple promoters and fluorescent tags have been developed and are available from plasmid repositories and multiple viral vector core facilities. Stereotactic delivery of DREADD-expressing viral vectors permits anatomically restricted expression. In addition to regionally limited distribution, further specificity for distinct cell types can be achieved using recombinases, such as Cre and Flp, expressed under cell type-specific promoters in transgenic or knock-in mouse lines (FIGURE 2, A–C). Isolating and manipulating neuron subpopulations with specific projection input/output patterns have been achieved using viral vectors that can be transmitted either anterogradely or retrogradely, often with AAV, HSV, or CAV, driving Cre-recombinase expression (FIGURE 2, D–F) (27, 67, 132, 174, 187, 214).
FIGURE 2.
Cell type-specific or projection-specific transgene expression. A and B: Cre-dependent expression of DREADDs in neurons. A: schematic injection of Cre-dependent virus into brain via stereotactic delivery. B: selective DREADD expression FLEX switch. C: schematic of FLEX-switch used in Cre-dependent viruses and two step Cre-recombination events involving loxP and lox2272 heterotypic recombination sites for permanent inversion of DREADD transgenes. D and E: two-way intersectional viral strategies to express DREADDs in projection-specific neuronal subpopulations. D: axonally targeted CAV2-Cre injected into CeA for retrograde labeling and somatically targeted rAAV-FLEX-hM3Dq injected into PBN for expression only in CeA projecting PBN neurons. E: axonally targeted rAAV5-Cre injected into NAc for retrograde labeling and somatically targeted rAAV-FLEX-KORDi injected into central subiculum for expression only in NAc projecting subiculum neurons. F: a 3-way intersectional strategy to express hM4Di in a molecularly defined neuron population with a defined axon projection. First, axon-targeted injection of a retroAAV expressing Flp recombinase in a Cre-recombinase-dependent manner (retroAAV-FLEX-FlpO) was made into spinal cord of Vgat-cre mice. Then somatic injection of Flp recombinase-inducible rAAV virus expressing hM4Di (rAAV-FD-hM4Di) targeted to RVM. In this configuration only the spinal cord projecting GABAergic neurons of RVM will be expressing hM4Di.
Despite the advantages of speed and flexibility with virally mediated DREADD expression, stereotactic viral delivery strategy leads to variation in transduction efficiency across animal subjects, which requires post hoc evaluation of viral transduction (6). In addition, for loss of function studies, it is frequently important for most of the targeted cells to be silenced, which may be difficult to achieve with viral delivery. Transgenic DREADD-expressing mouse lines have been developed to overcome these issues. An early example was based on a tet-off system, in which removal of doxycycline permitted hM3D expression from a CamKII promoter (2). This line had expression reportedly restricted to excitatory neurons. For targeting DREADD expression to other cell types, two Cre-dependent reporter lines have been developed by knocking DREADD genes into ROSA26 locus under a CAG promoter and Lox-Stop-Lox cassette (84, 212). With the increasing number of Cre-driver choices available, these DREADD lines open a panoply of research applications.
2. Appetite circuits
Energy balance is regulated by a complex interplay of molecularly defined cell types and circuits influencing appetite, satiety, and energy expenditure (179). Thus DREADDs have been used extensively for cell type-specific manipulation of appetite-regulating neuroendocrine circuits (178). An early application used acute chemogenetic activation of AGRP neurons with a virally transduced hM3Dq actuator to stimulate food search and consumption behaviors (105). Interestingly, activation of the Gs-coupled DREADD hM3Ds (61) in AGRP neurons had a slower but much more prolonged hyperphagic effect than activating Gq-coupled GPCR pathway due to selective upregulation of AGRP release (137). Using hM3Dq activation of AGRP neurons in various knockout mouse backgrounds, researchers have shown that either NPY or GABA (but not AGRP) signaling is sufficient for acutely activating food intake (106). Of note, chemogenetic inhibition of AGRP neurons by hM4Di rapidly reduces food intake in fasted animals (105) as well as overeating induced by ethanol (25), but it is less effective at inhibiting palatable food intake, due to the necessity of other circuits (47).
DREADDs have been used to deconstruct the contributions of distinct cellular nodes in appetite circuits. Based on circuit connectivity, early studies suggested that AGRP neurons regulated appetite by antagonizing intermingled POMC neurons (40). However, hM4Di-dependent inhibition of ARCPOMC neurons did not rapidly increase feeding but instead elevated food consumption after 24-h of POMC neuron inhibition (6), suggesting that POMC neuron inhibition is not the main mode of action for AGRP neuron-mediated acute activation of appetite. In addition, hM3Dq-dependent activation of ARCPOMC neurons did not acutely reduce food intake, although chronic activation of these neurons using multiple dose of daily CNO injection significantly reduced food intake (209), a result consistent with an earlier optogenetic activation study (3). Instead, another group of neurons in the arcuate nucleus defined by overlapping vGlut2 expression have been shown to be responsible for rapid suppression of feeding (63). Thus acute cell type-specific manipulation of neuron activity was critical for determining the relative role of these cells types in appetite and on various timescales.
Several hypothalamic and extrahypothalamic areas receive AGRP axonal input (177). AGRP neuron projections to the PVH were shown to be inhibitory, and hM4Di-dependent or KORDi direct inhibition of PVH neurons (marked by Sim1 expression) resulted in elevated food intake and increased motivation to work for food (6, 193). DREADD-dependent inhibition of a subpopulation of PVH neurons expressing MC4R was sufficient to elicit feeding and increased motivation for food in sated mice (71). Conversely, chemogenetic activation of PVH neurons marked by NOS1, MC4R, or AVP expression suppressed feeding in food-deprived mice (6, 71, 144, 183).
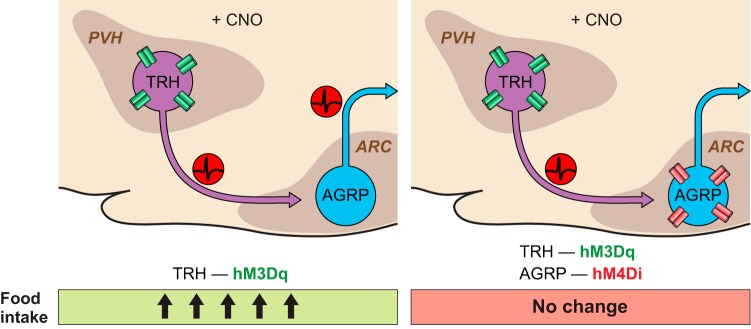
DREADDs have also been used for neural circuit epistasis analysis to examine the functional dependency of two connected pairs of neurons. After establishing that a direct excitatory connection exists between TRH neurons in the PVH and AGRP neurons, the behavioral significance of this connection was investigated by activating or inhibiting PVHTRH neurons with hM3Dq or HM4Di, which increased or decreased feeding, respectively (107). The dependency of PVHTRH neuron elevation of food intake on the activity of AGRP neurons was tested by simultaneous activation of TRH neurons and AGRP inhibition, using dual viral targeting with hM3Dq and hM4Di, respectively (FIGURE 3). Together, these manipulations blunted the food intake-stimulating effect of PVHTRH neuron activation, supporting the conclusion that the appetite stimulating properties of PVHTRH neurons were due to their synaptic connection to AGRP neurons (107). Such manipulations are also facilitated by the development of new DREADDs, and KORDi and hM3Dq have been coexpressed in AGRP neurons to selectively activate or inhibit feeding in the same mice (193).
FIGURE 3.
Schematic of neural circuit epistasis for the PVHTRH→ARCAGRP feeding circuit. TRH activation using hM3Dq drives food intake which is abolished by concomitant hM4Di silencing of downstream AGRP neurons.
Because Gi-coupled DREADDs like hM4Di and KORDi suppress synaptic release, these DREADDs can also be used to functionally map the role of synaptic connections to specified brain regions (173, 193). Appetite regulating projections of PVHSIM1 neurons have been traced to a number of hindbrain locations. Expression of hM4Dnrxn, an axon-targeted Gi-coupled DREADD, in PVH neurons was used to show through a series of intracranial CNO injections that a site in the vicinity of the periaqueductal grey and dorsal raphe led to increased food intake when these axon terminals were silenced (173). Dose responses with CNO and injections into nearby sites were used to localize this response. Importantly, these experiments indicated that hM4Di does not block action potential transmission along axons, making this a useful tool to inhibit synaptic output to a targeted region without blocking transmission through fibers of passage (173).
Visceral signals of satiety and also nausea are integrated through a hindbrain pathway. Hormonal and visceral information enters the nucleus of the solitary tract (NTS) and area postrema (AP). DREADD-mediated activation of two distinct NTS neuronal subpopulations marked by CCK and DBH expression suppress appetite and increase Fos activity in PBN (parabrachial nucleus) neurons (155). Optogenetic mapping confirmed that both NTSCCK and NTSDBH neurons directly project to and excite a subpopulation of PBN neurons marked by calcitonin gene-related peptide (CGRP) expression (PBNCGRP) (155). CNO-mediated activation of hM3Dq-expressing PBNCGRP neurons suppressed food intake and also proved to be aversive (27). Conversely, NTSCCK neurons also send long-range projections to the PVH, and hM3Dq activation suppressed appetite as well, but unlike those that activate PBNCGRP, stimulating NTSCCK→PVH axons elicited positive valence (45). In addition to DBH and CCK, at least two other NTS neuronal subpopulations, expressing POMC and GLP1, are appetite suppressing. Unlike ARCPOMC neurons, hM3Dq-dependent activation of NTSPOMC neurons rapidly and robustly suppresses food intake (209). Chemogenetic activation of NTSGLP1 fibers with hM3Dq, targeted using Phox2b-Cre mice, with intracranial CNO injections to the VTA selectively suppressed high-fat feeding, which suggests a role for conveying caloric reward via this projection (197). Thus DREADDs have been instrumental in teasing apart the distinct role of intermingled hindbrain populations that have been found to have subtly different effects on control of satiety.
The hindbrain anorectic circuit defined by PBNCGRP has been traced to the central nucleus of the amygdala (CeA), a region associated with aversive emotional states. Chemogenetic inhibition of PBNCGRP neurons that were labeled retrogradely from CeA with a Cre-expressing canine adenovirus (CAV) was sufficient to suppress the appetite-lowering effects of satiety and nausea-inducing agents (26, 27) (FIGURE 2D). Fibers from PBNCGRP neurons directly activated PKC-δ+ neurons in CeA, a connection suggested to be critical for generating an aversive state (83). Activating PKC-δ+ neurons suppressed feeding, and conversely, chemogenetic inhibition of these neurons with hM4Di blunts the anorectic response to nausea- and satiety-inducing agents and increases food intake (24).
Chemogenetic approaches have been used to dissect other hunger-related circuits as well. hM3Dq-dependent activation of lateral hypothalamic VGAT neurons stimulated food-seeking and consumption irrespective of caloric content (96, 139). Conversely, hM3Dq activation of lateral hypothalamus-projecting septal VGAT neurons suppressed feeding (185). Chemogenetic activation of pyramidal neurons in medial prefrontal cortex (mPFC) enhanced operant performance for food reward without effecting unconditioned hedonic or homeostatic food consumption (198).
3. Glucose homeostasis circuits
Neuronal circuits for appetite regulation are intermingled with neurons that regulate blood glucose levels. Hypoglycemia induces a counterregulatory response (CRR) to restore blood glucose to normal levels. A subpopulation of PBN neurons defined by leptin receptor expression was critical for this reflex. hM4Di-mediated inhibition of PBNLepRb blunts the CRR induced by 2-deoxyglucose (2-DG)-mediated glucoprivation, whereas PBNLepRb chemogenetic activation with hM3Dq mimicked CRR leading to hyperglycemia (64). Many PBNLepRb neurons coexpress CCK, are activated by glucoprivation, and project axons to the ventromedial hypothalamic nucleus (VMH). With the use of chemogenetic epistasis experiments, PBNCCK→VMH was shown to be necessary for CRR by simultaneous activation of PBNCCK neurons and silencing of VMHSF1 neurons (72).
Manipulations of other neuron populations also impact glucose homeostasis. Activation of hypocretin/orexin neurons with hM3Dq acutely increased circulating glucose (90). Glucose-sensitive neurons in the lateral hypothalamus marked by MCH expression are another component of the central glucose regulating system. Chronic MCH overexpression led to insulin resistance; however, acute hM3Dq-mediated chemogenetic activation of these neurons did not, suggesting that chronic activity would be required (84). In contrast, systemic insulin resistance can be acutely triggered by hM3Dq-mediated activation of AGRP neurons (176).
4. Learning and memory
Innovative chemogenetic methods have facilitated investigation of previously intractable problems in the field of learning and memory. Several aspects of memory formation, storage, and retrieval have been studied using chemogenetic manipulations.
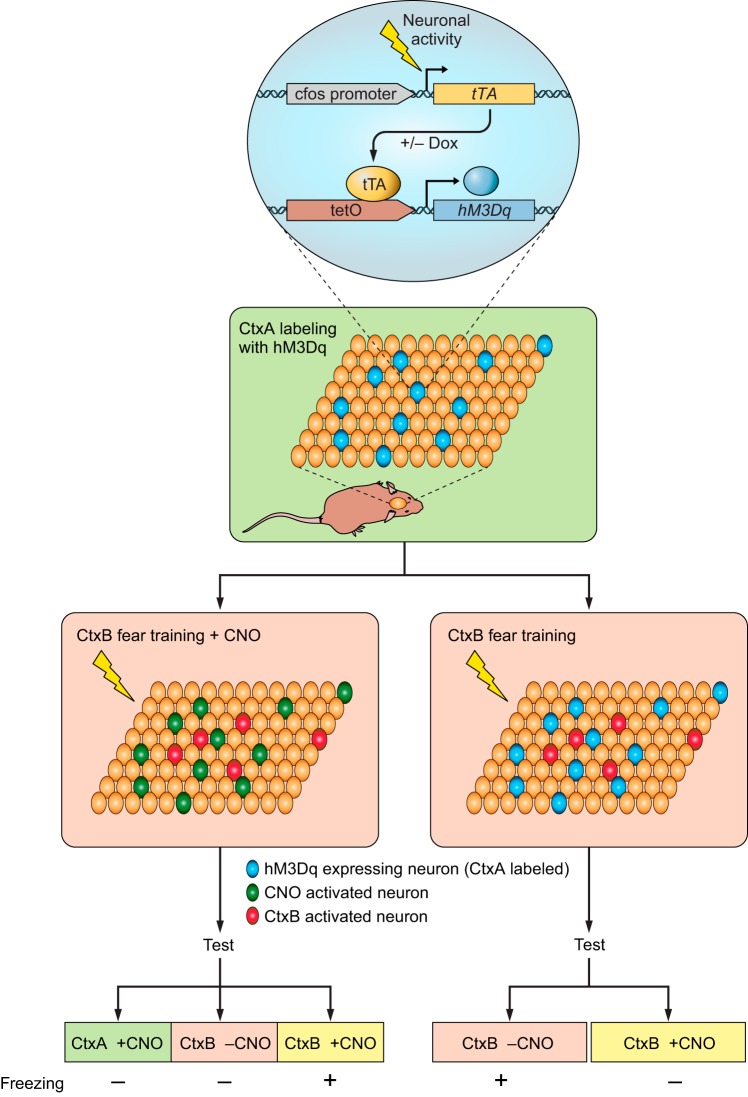
Distributed ensembles of neuronal activity during learning are thought to be necessary for forming association between environmental contexts and emotional states. Within certain brain regions, this activation pattern is suggested to be specific for distinct context-dependent sensory inputs. Chemogenetics has been used to activate context-specific ensembles. To achieve this, Garner et al. (73) used transgenic mice expressing hM3Dq under the activity-dependent promoter Fos, which allowed selective hM3Dq expression in subset of neurons that were active during a given sensory context (FIGURE 4). This allowed subsequent access to these neurons (which were mainly located in hippocampus, basolateral amygdala, and cortex) and the ability to reactivate them using CNO to investigate how their activation impacted memory formation and retrieval. They used a contextual fear learning task in which a freezing response is stimulated in mice after repeated exposure to an unconditional stimulus (e.g., foot shock) paired with a contextual conditional stimulus. Neurons were marked by hM3Dq expression in context A and then reactivated using CNO during contextual fear learning in context B, such that the resulting conditioned fear memory response could not be activated by presenting context B or CNO alone but instead required joint context B and hM3Dq activation of the context A-associated ensemble. This result suggests that artificially activated neurons associated with context A formed a hybrid representation with context B that was uniquely associated with representation of the fear memory. If hM3Dq-expressing neurons associated with context A were activated not during context B fear conditioning but during its retrieval, then CNO-induced activation of the competing ensemble interfered with the learned spatial code of context B and thus degraded its recognition. These experiments provided insights into how distinct contextual representations are distributed and retrieved during learning as well as about memorization process of different context.
FIGURE 4.
Summary diagram depicting the experimental strategies to test the impact of synthetically activated memory engrams during fear learning and recall.
A similar activity-dependent DREADD expression strategy was also used during Pavlovian fear conditioning (206). Animals form an association between a neutral conditioned stimulus (CS) and a noxious unconditioned stimulus (US), but fear responses to the CS eventually extinguish if presented in the absence of the US. DREADDs were used to investigate whether the original association can be reinstated by activating the ensemble associated with the fear response (freezing). Mice were trained by Pavlovian fear conditioning while the memory engram was tagged by hM3Dq expression under the control of Fos promoter, which labeled dorsal hippocampal CA1 area, subiculum, the cerebral cortex, and the basolateral amygdala (BLA). After mice underwent extinction training, they no longer responded to the CS; however, when these animals were given CNO, they displayed significant freezing responses. These results suggested that artificial reactivation of original neuronal ensemble was sufficient to recapitulate the fear response and that the original memory engram persisted, even after extinction.
Chemogenetic silencing has also been used in a number of learning and memory studies to replace lesions or common chemical inhibitors of neuron activity such as muscimol or tetrodotoxin. For example, the retrosplenial cortex (RSC) is a structure that receives input from multiple cortical and thalamic sensory areas as well as postrhinal, medial entorhinal cortices. The RSC is involved in stimulus-stimulus association such that preconditioning with two cues paired in the absence of reward followed by conditioning with the pairing of one cue with a food reward renders the other preconditioned cue effective at inducing conditioned responding for food. Examining the role of stimulus-stimulus association in the RSC with reversible inactivation has been challenging due to the large size of this structure, making it difficult to inhibit the entire region with traditional pharmacological inhibitors of neuron activity. Reversible and anatomically encompassing RSC perturbations were achieved after making multiple AAV injections of hM4Di to cover the entire rostrocaudal axis of the RSC in rats (154). Animals with RSC activity inhibited by CNO injection during preconditioning were unable to form associations between neutral sensory stimuli. This study also points to a considerable advantage of DREADDs over existing pharmacological manipulations where perturbations on anatomically large or even disconnected regions can be achieved by stereotactic viral delivery. Chemogenetic silencing has also been used to investigate fear memory consolidation in the CA1 region of the hippocampus. Using AAV vectors under control of Ca2+/calmodulin kinase II (CaMKII) promoter in dorsal or ventral hippocampal CA1 neurons, hM4Di was expressed selectively in CA1 glutamatergic neurons (213). Injection of CNO to inhibit CA1 neurons immediately after contextual fear conditioning disrupted fear memory in the ventral but not dorsal hippocampus. However, if CNO was given 6 h after training, memory was not affected (213). This suggests that the window for memory consolidation lies somewhere between 0 and 6 h after learning and requires the ventral hippocampus, representing an additional advantage of using AAV vectors to deliver DREADDs to enable anatomical, cell type-specific, and temporal control of neuron activity.
The time period over which consolidated memories require the hippocampus for retrieval has also been examined in fear conditioning. With stereotactic AAV delivery, hM4Di was expressed in the entire length of mouse hippocampus (194). After fear conditioning, the hippocampal dependence of memory recall was tested at various time points after training by reversible hippocampal inhibition with CNO. Disrupting hippocampal activity produced a temporally graded amnesia in which recall of recent memories (2 days) was impaired but distant memories (>7 wk) apparently no longer required intact hippocampal activity.
Learning to avoid predators has also been studied in the context of hypothalamic fear encoding. The VMH plays a role in defensive responses to predators. Silva et al. (166) generated a transgenic mouse in which hM4Di is expressed under the control of Nr5a1 promoter to restrict its expression to VMH. They silenced VMH activity during training sessions in which mice were exposed to a predatory rat to test whether this region is involved in acquisition of fear memory. Mice in which VMH activity was suppressed during predatory fear conditioning showed significantly decreased fear response when they were reexposed to the predator-associated context. Furthermore, mice conditioned with predatory fear showed impaired fear responses if VMH was silenced during reexposure, suggesting that VMH is involved in both acquisition and recall of predator fear.
5. Sleep
DREADD chemogenetic technology has also been deployed to decipher sleep regulation circuits. Acute activation of parabrachial nucleus neurons in mice with hM3Dq and CNO caused rapid induction of wakefulness and alert statelike electroencephalogram (EEG) activity that can be sustained up to 4 days by repeated injections (150). The PBN sends projections to a variety of brain regions, and to determine which downstream target region is responsible, a projection target-specific labeling strategy was used. Each of the potential downstream projection targets was injected with a Cre recombinase expressing AAV serotype 6, which can be taken up by PBN axons and retrogradely transported. By making a second AAV injection into PBN carrying a Cre-dependent hM3Dq, they specifically expressed this DREADD in a subset of PBN neurons that send axons to a specific target. The wakefulness response was recapitulated by activating PBN neurons that send projections to basal forebrain or hypothalamus but not thalamus.
The role of lateral hypothalamic hypocretin/orexin neurons has also been examined in sleep with chemogenetics. Expression of hM3Dq and hM4Di in Orexin-cre mice was used to measure the impact of chemogenetic activation and inhibition on sleep. hM3Dq-mediated activation of hypocretin/orexin neurons significantly increased time spent in wakefulness and reduced the non-rapid-eye-movement (NREM) and rapid-eye-movement (REM) sleep times, while hM4Di-dependent inhibition had an opposite effect on wakefulness and NREM sleep (161). In a related study, DREADD-dependent manipulations were performed in basal forebrain (BF) cholinergic neurons in Chat-ires-cre mice (32). Activating BFCHAT neurons increased wakefulness, whereas inhibition had an opposite effect. This group also took advantage of DREADD activation of BFCHAT neurons and performed a Fos analysis to mark activated downstream regions. Finally, Chen et al. (33) used DREADD technology and viral lesions to probe the role of various brain stem nuclei in sleep regulation and atonia. Acute chemogenetic activation of presuperior olivary medulla (pSOM) selectively suppressed REM but not NREM sleep, while activation of rostral ventromedial medulla (RVM) increased wakefulness and decreased sleep.
6. Pain
DREADDs have started to find widespread use in itch and pain research. One of the major goals in pain research is to control nociceptive afferent excitability. To silence pain transmitting C-fibers, a Trpv-1-Cre mouse was crossed with Cre-conditional hM4Di mouse line (159). A single dose of CNO rapidly decreased their excitability and significantly increased pain threshold, thereby producing analgesia lasting up to 3 h (159). Notably, these researchers have also observed CNO-independent effects of hM4Di expression on sensory neurons, including significant alterations in Na+ current, increased NaV1.7 expression, and decreased voltage-gated calcium channel conductance (159). Furthermore, in hM4Di-expressing neurons, endogenous Gi-coupled signaling was also disrupted. Such reports on off-target effects of DREADD expression have been rare, and it is not known whether other neuronal cell types that express DREADDs also have altered functional properties. Nevertheless, these results highlight the need for careful consideration of the consequences of overexpressing any protein, including DREADD-based GPCRs in cells.
Afferent nociceptors were targeted by others as well. Instead of intersectional transgenic mouse breeding, viral injections into sciatic nerve were used to target sensory neurons (94). The analgesic effects were compared for chemogenetic (hM4Di) silencing and optogenetic (step function optogenetic inhibitor SwiChR) inhibition of unmyelinated DRG neurons that carry pain signal and observed similar increases in mechanical and thermal pain threshold.
DREADDs have also been used in pain and itch signaling circuits in the dorsal horn of the spinal cord. Mechanical hypersensitivity was increased when neurons marked by transient expression of vGlut3 in the dorsal horn were activated by hM3Dq (145). A role of NPY-expressing dorsal horn neurons in light touch mechanical itch was investigated by crossing Npy-Cre transgenic mice with a conditional hM4Di-DREADD expressing mouse line for acute silencing of NPY neurons (21). After CNO injection, light mechanical stimulation produced robust scratching responses, suggesting that acute NPY neuronal inhibition abolished a cellular circuit node that normally suppressed itch. It should be noted that the intersectional approach to access NPY-expressing neurons described here would drive DREADD expression in other brain regions. Therefore, systemic delivery of CNO likely inhibited non-spinal NPY-expressing populations as well.
Pain threshold is modulated, in part, by descending inputs to the spinal cord that affect pain transmission. An important part of this circuit is thought to be from RVM and local enkephalinergic neurons in the dorsal horn. Francois et al. (67) explored this pathway in detail using DREADDs. They intrathecally delivered AAV expressing Cre-dependent hM4Di to inhibit spinal enkephalinergic neurons in Penk-cre mice, which induced robust mechanical hypersensitivity in the presence of CNO. Viral tracing showed that spinal encephalin neurons receive direct inhibitory input from GABA-releasing RVM neurons. Spinal cord projecting RVMVGAT neurons were targeted with hM4Di using an intersectional approach. RetroAAV-FLEX-FlpO, an axonally transported viral vector expressing FLP-recombinase protein in a Cre-dependent manner, was injected into the dorsal horn of Vgat-Cre mice. In this way, they retrogradely targeted FLP to all VGAT expressing neurons that project to the dorsal horn (FIGURE 2F). By injecting a second AAV into RVM that expresses hM4Di in a FLP-dependent fashion, they were able to selectively target hM4Di to spinal cord projecting RVMVGAT neurons. Inhibition of these RVM neurons reduced mechanical sensitivity.
Pain signaling in higher brain centers has also been explored using DREADDs; for example, hM3Dq-mediated activation of periaqueductal gray and dorsal raphe (PAG/DR) tyrosine hydroxylase positive neurons in mice has an anti-nociceptive effect (112). Fos activation mapping for putative downstream mediators of acute pancreatitis-induced visceral pain identified the PVT, PAG, and PFC (97). Selective expression of DREADDs in these regions was used to show that inhibition of PVT neurons or activation of downstream glutamatergic neurons in mPFC (but not PAG neurons) attenuates visceral nociception. Finally, Wakaizumi et al. (196) investigated exercise-induced hypoalgesia and showed that selective suppression of nucleus accumbens projecting VTA dopaminergic neurons offsets antinociceptive effects of low-intensity exercise.
7. Primate applications
The noninvasive and reversible properties of DREADDs have made these tools attractive for primate research as well. In a chemogenetic dissociation study, hM4Di-expressing viral vectors were delivered to rhinal cortex (Rh) in one hemisphere and orbitofrontal (OFC) cortex in the other hemisphere in rhesus monkeys (56). Silencing these two regions contralaterally disrupts intrahemispheric communication between the two regions, effectively dissociating the two structures. When CNO was injected, these animals had significantly reduced sensitivity to changes in reward size.
Related work examined reward value processing by chemogenetically silencing rostromedial caudate (rmCD), using virally expressed hM4Di in monkeys (136). Inactivating rmCD bilaterally resulted in significant loss of sensitivity to reward value. In most studies verification of DREADD expression is performed through post mortem histological analysis. However, Nagai et al. (136) also developed a method to visualize DREADD expression in vivo. They used a positron emission tomography (PET)-sensitive DREADD ligand, 11C-labeled clozapine. Injecting monkeys with this ligand provided information about the size and location of hM4Di expression, and by using a blocking protocol they were able to observe receptor occupancy by CNO. This approach has significant advantages over post mortem analysis, especially in primate research, which requires investment of substantial time and effort for each subject.
DREADDs have also been used in conjunction with magnetic resonance imaging (MRI) in primates. In rhesus monkeys, chemogenetic silencing of amygdala was combined with functional connectivity MRI, a configuration that allowed investigation of the brain-wide functional connectivity in vivo (79). Silencing the amygdala affected not only amygdalo-cortical connectivity, but also corticocortical coupling across multiple functional systems was disrupted, although a high CNO concentration (10 mg/kg) was used. In these studies, DREADD technology has provided significant improvement over classical approaches that have limitations imposed by necessity to use implant to manipulate neuronal activity or by compensatory changes that can occur after lesions.
A related technology has been developed by Michaelides et al. (134) termed DREADD-assisted metabolic mapping (DREAMM). This approach is based on making remote-controlled cell type specific perturbations with DREADDs and observing the brain-wide functional consequences in awake-behaving animals under PET scan. In this study authors described PET measurement of whole-brain activity by monitoring brain glucose metabolism with injected [18F]fluorodeoxyglucose (FDG) as they hM4Di-inhibit distinct nucleus accumbens shell subpopulations. Although this work was performed in rats, a similar approach could be used in other rodents and primates. Furthermore, different PET probes could be used to monitor changes in different cellular processes of downstream targets such as neurotransmitter dynamics or enzymatic activities. Thus DREAMM has the potential to map global long-range functional connectivity networks by combining advantages provided by cell type specificity of DREADD manipulations and noninvasiveness of PET imaging.
Nevetheless, the use of CNO-based DREADD strategies in non-human primates must consider the limited access of CNO to the brain (75a, 136, 151). In addition, retro-conversion of CNO has been measured to give significant concentrations of clozapine in cerebrospinal fluid (CSF) in one study (151), but CSF clozapine was not detected in a different report (136) is the active agent in the brain. Thus alternative DREADD ligands should be examined for use in non-human primates.
E. Non-neuronal Applications
1. Glial cells
Glial cells are the predominant cell type in the nervous system, and they can be marked by glial fibrillary associated protein (GFAP). Specific manipulation of these cell populations is important for understanding their role in vivo. Transgenic mice expressing hM3D under a GFAP promoter fragment were used to assess physiological impact of glia specific activation. CNO-mediated activation of Gq-coupled signaling in glia throughout the body altered autonomic nervous system functions by increasing heart rate, blood pressure, saliva formation, and decreasing body temperature (1). Glial targeting can be achieved in wild-type animals by using viral vectors expressing DREADDs under a GFAP promoter. Using this approach in rats, hM3Dq activation in glial cells in the nucleus accumbens core induced glutamate release, which was sufficient to inhibit cue-induced reinstatement of cocaine (163) and ethanol seeking (23), suggesting a potential in vivo role for glial signaling to influence behavior. CNO-mediated activation of hypothalamic astrocytes has been shown to suppress feeding without effecting emotional states (184, 203). The influence of in vivo astrocytic Gq signaling on blood flow was investigated using hM3Dq and was found to be insufficient to alter cortical hemodynamics (20). Glial cells of the enteric nervous system were also activated by hM3Dq, resulting in neurogenic contractions as well as intestinal and colonic motility, showing that gut reflexes can also be regulated by glial cells (133).
A non-neuronal contribution to pain pathways outside the brain have been examined by DREADDs. Grace et al. (77) investigated the role of spinal cord microglia in morphine-induced neuropathic pain in rats. A common agent for pain management, morphine, induces inflammasome in microglial cells. To test causal involvement of microglia, they intrathecally delivered hM4Di under the direction p38 microglial promoter. Activation of microglial hM4Di inhibited proinflammatory signals and reversed morphine-induced sensitization, confirming a causal role for these cells.
2. Other cell types
GPCR signaling is important for nearly all cell types. Thus DREADDs can be applied to assess the general function of these cell signaling pathways independently of endogenous GPCRs. For example, the role of Gq-coupled signaling was probed in hepatocytes using hM3Dq, which was shown to stimulate glycogen breakdown and gluconeogenesis (113). Activation of Gs and Gq pathway activation in pancreatic β-cells using transgenic mice showed a protective role for activating Gq pathway against diabetes (81, 95).
hM4Di has been used to interfere with intracellular signaling and metastasis in breast cancer cells (202). Researchers have also used hM4Di in T-lymphocytes to engineer chemotaxis toward a CNO source (143). DREADDs have been used in kidney research as well. Gq signaling has been studied in podocytes, specialized cells that wrap glomerular capillaries and are thought to influence glomerular filtration. Due to technical limitations it has been difficult to study the role of Ca2+ signaling in these cells in vivo; therefore, most evidence was based on cultured studies. A mouse line with podocyte specific hM3Dq expression enabled CNO-dependent activation of Gq signaling in vivo. This elicited Ca2+ transients in podocytes, but surprisingly this manipulation did not have any impact on glomerular perfusion or filtration, suggesting that more prolonged Ca2+ level alterations might be required to alter these parameters (103).
In summary, a rapidly expanding body of literature, some of which is mentioned here, suggests that DREADDs have already started to transform neuroscience and hold significant promise for non-neuroscience applications as well.
III. LIGAND-GATED ION CHANNEL CHEMOGENETIC TOOLS
LGICs permit direct pharmacological control over ion conductance. The functional properties of ion channels are primarily dictated by their ion selectivity. Inward flux of cations or outward flux of anions depolarizes cells, and correspondingly inward flux of anions or outward flux of cations leads to cellular hyperpolarization (98). Some channels also pass Ca2+ and influence other signal transduction pathways independently of the effect on cellular membrane potential. Additional important characteristics can be tuned with specific mutations, such as conductance magnitude, localization, and desensitization properties (87). Several LGIC families have been developed as chemogenetic tools, including Cys-loop receptors, ATP-sensitive channels, and transient receptor potential (TRP) channels.
A. Cys-Loop Receptors
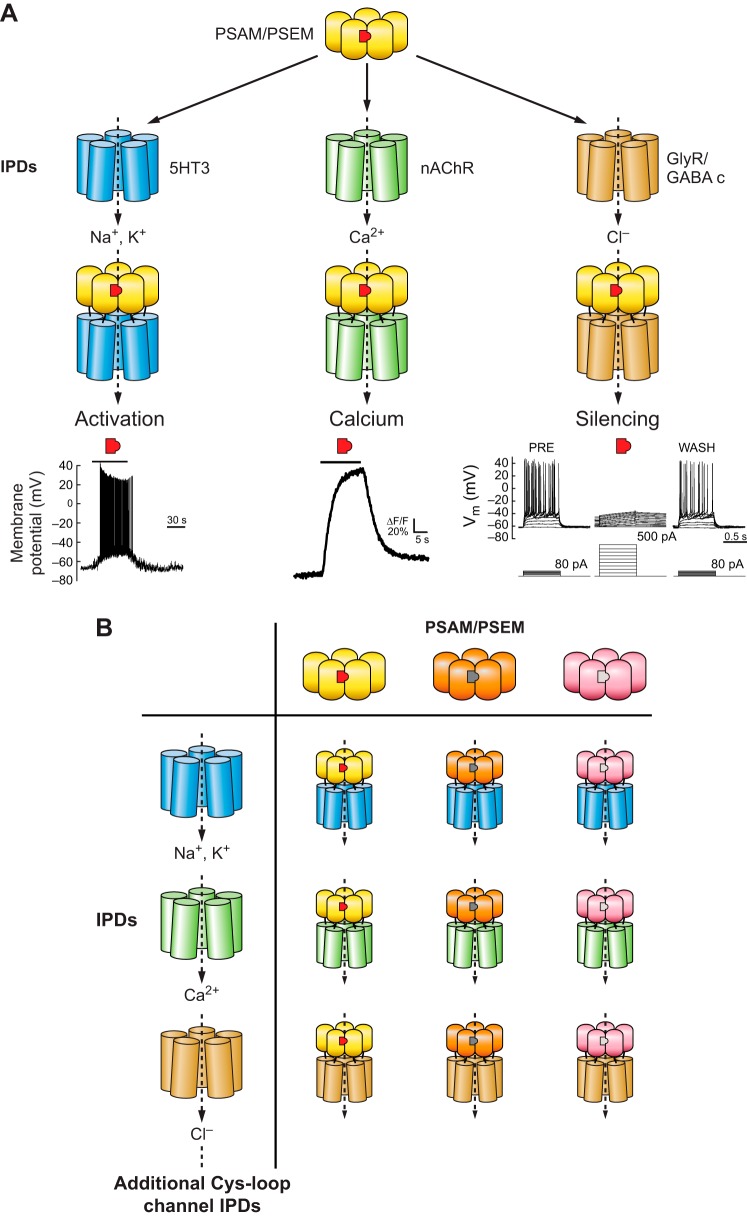
The Cys-loop receptors are a family of pentameric ion channels named for conserved vicinal cysteine residues in a loop that is important for channel gating (168, 188). Cys-loop receptors include nicotinic receptors (nAChR), serotonin receptor 3 (5HT3), GABA receptors, glycine receptors (GlyR), and glutamate-gated chloride channels (GluCl), many of which are heteromers that are comprised of multiple subunits (FIGURE 5). Several Cys-loop ion channels form as pentameric homomers, which facilitates use of these ion channels as tools for manipulating cellular function because only a single ion channel subunit needs to be expressed. These include α7 nAChR, α9 nAChR, 5HT3, GABA-C, and GlyR. Agonist binding at Cys-loop receptors occurs at the interface between protomer subunits. Binding at the principal subunit face is associated with interaction with the agonist pharmacophore, a cationic moiety that is stabilized by interactions with a backbone carbonyl and cation-π interactions with aromatic tyrosines and tryptophans (111). The complementary face of the ligand binding domain (LBD) is contributed by the adjacent protomer, which largely affects the specificity of ligand-receptor interactions. Ligand binding leads to a contraction of the LBD, and these structural changes are transmitted to the transmembrane domain to permit ion permeation. Ion selectivity is determined in part by cytoplasmic residues that can be altered to switch cation channels into anion-selective channels and vice versa (147, 169).
FIGURE 5.
Chemogenetic LGIC tools for activating and inhibiting neurons.
1. GABA-C receptor
One of the first chemogenetic ion channel tools involved heterologous expression of the GABA-C (also called GABA rho) receptor in hippocampal neurons. This LGIC is not normally expressed in hippocampal neurons and rendered them selectively sensitive to the GABA-C agonist cis-4-aminocrotonic acid (CACA) (36). Therefore, this system revealed successful neuronal expression of a heterologous LGIC and used a selective ligand for that channel to control neuron excitability. Nevertheless, this approach was limited by the remaining sensitivity to the endogenous ligand GABA, which was perturbative to neuron activity.
2. GluCl/ivermectin
An improved approach applied GluCl subunits from Caenorhabditis elegans (41) to potently control neuron excitability (170). These channels are sensitive to the natural product ivermectin (IVM), which is also used clinically as an antiparasitic (41). GluCl is a heteromeric channel comprised of two subunits (GluClα and GluClβ); however, more recent results indicate that the GluClα subunit can form homomeric channels (68). A mutation in the glutamate binding site reduced glutamate efficacy by sixfold, although glutamate potency and IVM sensitivity were not substantially changed (114, 170). One limitation of IVM is that it also activates or modulates GABA receptors, glycine receptors, α7 nAChRs, and P2X4-R albeit with lower potency than for GluCl (208), and this is associated with toxicity in the central nervous system (192). Therefore, low doses of IVM are essential for use as a selective chemogenetic tool. To aid in more selective usage of IVM, a modified version of GluCl has been developed that is activated with ~10 nM IVM, which is a >10-fold improvement in potency (68). Although use of this channel has not yet been reported in vivo, the original modified GluCl system has been used in several studies.
In mice, expression of GluCl in the striatum, an area associated with motor function, was found to be well tolerated and to not show any obvious changes in locomotor activity. Upon administration of IVM to mice intraperitoneally, the mice displayed elevated rotational behavior in conjunction with amphetamine injection, consistent with loss-of-function in the striatum (110). In another study, this system was used to suppress the function of the ventrolateral portion of ventromedial hypothalamic nucleus (VMHvl) to suppress attack in a resident intruder aggression assay (119). In each of these experiments in mice, the pharmacokinetics timescale of the chemogenetic loss-of-function perturbation was slow to develop and was especially prolonged, with effects reported 1 day following injection and lasting for more than a week (110, 119). This corresponds to the known pharmacokinetic profile of IVM, which is largely distributed into fatty depots forming a reservoir of the drug that leaches back into circulation over multiday timescales (11). Thus GluCl provides an effective chemogenetic framework, but it has some limitations such as the need for heteromeric receptors, and the toxicity and slow pharmacokinetics of IVM.
3. Glycine receptor/ivermectin
An alternative IVM-sensitive chemogenetic system was developed based on an engineered glycine receptor (GlyR) that has one LBD mutation to eliminate glycine sensitivity and a second IPD mutation to increase IVM potency (127, 128). This channel is activated by 19 nM IVM (126). The engineered receptors are homomeric channels that are selective for chloride and can reduce neuron electrical activity. Two Cre-dependent mouse lines based on this channel have been reported, where IVM administration is reported to reduce neuron activity in vivo and ex vivo (89, 142).
Another version of this GlyR-based chemogenetic system exploits IVM sensitization of glycine responsiveness. In this channel, IVM sensitivity is enhanced with an IPD mutation, and glycine sensitivity is reduced. However, in the presence of IVM, glycine sensitivity is potentiated, which can suppress neuron activity (92). The appeal of this system is that it does not lead to a constant chloride shunt of the cell, which in some cases can increase cellular excitability if the chloride gradient is collapsed.
An IVM-sensitive GlyR chemogenetic system has been converted to cation selectivity by applying three mutations to the intracellular domain of the channel (91). This system can be applied to depolarize neurons to fire action potentials. Thus IVM/GlyR chemogenetic tools can be used as neuron silencing and activation tools, but they require coping with the complex pharmacology of IVM.
4. PSAM/PSEM engineered ion channels and ligands
a) Design.
To utilize the functional diversity of the Cys-loop ion channel family, a set of chemogenetic tools was created based on chimeric LGICs derived from α7 nicotinic acetylcholine receptor (nAChR) and other Cys-loop family members. The α7 nicotinic acetylcholine receptor (nAChR) was chosen because it has been intensively investigated as a drug target for schizophrenia therapies and thus had a number of published small molecule structure activity relationships (49). In addition, mutagenesis studies of α7 nAChR had previously identified residues important for binding acetylcholine and there are also crystal structures of the homologous acetylcholine binding protein bound to agonists such as nicotine, carbamoylcholine, and epibatidine (29, 115). α7 nAChR forms homopentameric channels, simplifying its use as a tool by requiring expression of only a single subunit. Finally, the extracellular ligand binding domain (LBD) of α7 nAChR is transferrable to the transmembrane ion pore domains (IPDs) of other members of the Cys-loop LGIC family (55, 80, 130). This property allows the pharmacology of the α7 nAChR to be maintained while accessing the ion conductance properties of other LGICs, such as the 5HT3 receptor, GlyR, and the GABA-C receptor.
To leverage these characteristics, mutagenesis of the α7 nAChR LBD was performed to identify interactions with small molecules that did not activate unmutated α7 nAChRs. For this, a chimeric channel consisting of the α7 nAChR LBD and 5HT3 IPD (α7–5HT3) was selectively mutated to all other possible amino acids at four sites on the complementary face of the ligand binding pocket formed at the interface of two protomer subunits. These mutant channels were screened in cell-based assays using fluorescent membrane potential dyes against a library of small molecules synthesized to have characteristics previously indicated to have poor potency against the unmodified α7 nAChR (19). This resulted in identification of three mutations (W77F, Q79G, L141F) that each conferred selective agonist activity to structurally distinct small molecules with low to no activity against α7–5HT3 (130). Moreover, additional molecules could be synthesized that were also selective between each mutant channel as well as the unmutated channel. Each of these selectivity-conferring mutations could be combined with an additional mutation that reduced ACh potency (Y115F, Q139G, L141S). These mutated LBDs were shown to be transferrable to four IPDs and were termed pharmacologically selective actuator modules (PSAMs, pronounced as SAMs) (130). The cognate synthetic ligands are called pharmacologically selective effector molecules (PSEMs, pronounced as SEMs). Together, PSAM/PSEMs offer flexible tools for selective LGIC pharmacology (FIGURE 6).
FIGURE 6.
Combination of pharmacological and functional Cys-loop modules to generate engineered chimeric ion channels for controlling ion flux. A: for a given PSAM (yellow)/PSEM (red) pair, combination with different IPDs allows multiple functional outcomes to be achieved ranging from neuronal activation, controlling calcium flux, or neuronal silencing. B: multiple orthogonal PSAM/PSEM pairs have been developed, which enables combinatorial generation of diverse chemogenetic ion channel tools.
PSAMs can be applied to construct a variety of chimeric ion channels with distinct functional properties. PSAM-5HT3 provides prolonged depolarizing currents in the presence of the corresponding PSEM89S, consistent with the functional properties of α7–5HT3. The addition of three mutations to the IPD of this chimeric channel increased conductance over 100-fold (PSAM-5HT3 HC) (130). PSAMQ79G,L141S-nAChR V13’T allows for primarily Ca2+ conductance with the V13’T ion pore mutation greatly curtailing the desensitization normally associated with α7 nAChR channels. Pharmacologically selective inhibitory channels were generated by PSAM-GlyR and PSAM-GABA C channels. Each of these shows large conductances as well as long steady-state window currents to maintain silencing as long as the agonist is present (130). Cells expressing PSAM-GlyR channels dramatically reduce input resistance by 70%, making it difficult to fire action potentials. Correspondingly, rheobase (the current required to fire an action potential) increases by at least eightfold (130). This makes PSAM-GlyR channels extremely powerful tools for silencing neuronal activity.
b) ex vivo applications: neural circuit analysis.
These engineered chimeric LGICs and their corresponding PSEMs have been used in a number of ex vivo and in vivo applications. For ex vivo analysis of circuit functions, PSAM-GlyR has been selected because it strongly suppresses neuron activity. PSAM-GlyR has been used in analysis of interneuron populations in acute hippocampal microcircuits (12, 125, 156) and retinal (195) ex vivo tissue preparations. These studies reported complete suppression of electrical activity from interneuron populations expressing PSAM-GlyRL141F in the presence of cognate PSEMs. Effective neuronal silencing facilitates analysis of circuit computations in the presence and absence of signaling through a particular circuit node.
c) learning and memory.
The powerful neuronal silencing functions of PSAM-GlyR in analysis of cortical and hippocampal circuits have been used to investigate the role of molecularly defined interneuron populations in different learning processes. Building on ex vivo studies using PSAML141F-GlyR silencing (125), somatostatin (SOM) interneurons were silenced in vivo by intraperitoneal administration of PSEM89S (124). Consistent with the established fast pharmacokinetics of this molecule (130), in vivo silencing was rapid and recovered after 20–30 min postinjection (124). Silencing SOM interneurons but not parvalbumin (PV) interneurons blocked contextual fear conditioning, consistent with the inability to distinguish contextual cues appropriate for fear responding in the absence of firing from this population (124).
In another study (13), the role of long-range inhibitory projections from the lateral entorhinal cortex (LEC) to hippocampus in contextual fear conditioning was examined for by axonal silencing with PSAML141F-GlyR. The potent PSEM308 was delivered by intracranial injection to the distal axon projections of LEC to the hippocampus. Silencing these axons in a contextual fear conditioning assay did not prevent freezing in the conditioned context, but it led to inappropriate generalization such that the mice showed freezing in a distinct context, which is not typically observed. These studies demonstrated that the output of local axon projections could be silenced by local PSEM delivery.
Cortical and hippocampal CA3 PV interneuron activity during a foreign object recognition experiment, spatial learning, and contextual fear memory was examined using bidirectional control of PV neuron activity with PSAML141F,Y115F-5HT3 HC and PSAML141F-GlyR (50, 51, 100). Activation of PV neurons increased PV immunoreactivity and PV synapse number on CA3 pyramidal cells. Conversely, inhibition using the same ligand in different mice led to a corresponding reduction in PV immunoreactivity and synapse number. PV neuron inhibition reduced PV expression levels, promoted memory acquisition, and promoted FOR, while PV neuron activation showed opposite outcomes (51). These experiments demonstrate bidirectional control over PV, an activity-dependent gene, along with activity-dependent synaptic plasticity, and associated consequences for the FOR behavioral task. A related set of experiments used PSAML141F-GlyR to investigate SOM and VIP interneuron influences on cortical integration (135). The use of LGICs to make these perturbations is consistent with the conclusion that the cellular and behavioral consequences of these manipulations are activity dependent, which is less certain for perturbations with GPCRs that can directly affect gene expression independently of effects on activity.
Chemogenetic silencing of a hindbrain premotor nucleus established its role in learning motor tasks like reaching or rotorod balancing (58). This study compared the effectiveness of reversible neuronal silencing with PSAML141F-GlyR of acute neuronal ablation. The behavioral impact on rotorod balance duration or reach success rate was very similar between permanent neuron ablation and reversible silencing (FIGURE 7A). Moreover, learning was tested over multiple days, and the effectiveness of silencing was maintained with repetitive application of the ligand, indicating the durability of the PSAML141F-GlyR response. Importantly, however, once the ligand was no longer administered, mice quickly learned the rotorod task (58). Thus these inhibitory channels show similar neuron suppression efficacy as neuron ablation but allow for reversible, daily control of cell function.
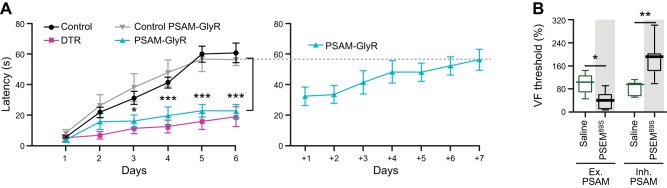
FIGURE 7.
Efficacy, durability, and bidirectionality of PSAM/PSEM neuron perturbations. A: daily, reversible silencing of premotor neurons with PSAM-GlyR during rotorod motor learning compared with permanent neuron ablation (left). Subsequent training without PSEM injection led to efficient learning of the task. [From Esposito et al. (58), with permission from Nature Publishing Group.] B: bidirectional activation (Ex. PSAM) or silencing (Inh. PSAM) of D2R neurons in the striatum alters Von Frey (VF) threshold in spared nerve injury (SNI) pain model. [From Ren et al. (153), with permission from Nature Publishing Group.]
d) amyotrophic lateral sclerosis.
Bidirectional control of neuron electrical activity also had dramatic effects on motorneuron survival in an amyotrophic lateral sclerosis (ALS) mouse model. Using PSAML141F,Y115F-5HT3 HC and the chloride conducting PSAML141F-GlyR, once daily activation or silencing increased or decreased, respectively, survival of motorneurons expressing a mutated superoxide dismutase (SODmut) (162). This was associated with an increase in muscle innervation in mice with once daily motorneuron activation, relative to GFP-expressing control motorneurons on the opposite side of the spinal cord. This is a striking demonstration of the “use it or lose it” aphorism applied to cell viability in a model for this debilitating human disease. In addition, the increased survival of sensitive SODmut expressing motor neurons in the presence of PSAML141F,Y115F-5HT3 HC and the ligand PSEM308 indicates that this channel is well tolerated, even in sensitive cells.
e) pain.
Pain responses can also be modulated bidrectionally with opposite neuron activity perturbations (153). Using spared nerve injury mouse model, expression of either the cation conducting PSAML141F,Y115F-5HT3 HC or the chloride conducting PSAML141F,Y115F-GlyR in D2R neurons (FIGURE 7B) showed that D2R neuron activation increases pain while D2R neuron inhibition decreases pain sensitivity.
Activation of a different population in the hindbrain called POMC neurons also reduced thermal pain withdrawal responses, presumably due to β-endorphin release (30). The magnitude of the analgesic effect was similar to morphine in the same assay; however, the chemogenetic effect was reversed by 45 min (30), consistent with the pharmacokinetics of PSEM89S (130).
f) blood pressure and appetite.
PSAM/PSEM systems have also been used for bidirectional control of blood pressure by targeting activators and silencers to leptin receptor-expressing neurons in the dorsomedial hypothalamic nucleus (DMHLepr). Activation of DMHLepr neurons increased blood pressure, while neuron inhibition reduced blood pressure (167).
Neuronal silencing of AGRP neurons in the hypothalamus was shown to suppress eating and also to promote learning about cues associated with AGRP neuron inhibition (16). Notably, the effectiveness of preference learning was proportional to the number of AGRP neurons that were virally transduced with the inhibitory ion channel (16). This highlights the importance of post hoc analysis of viral transductions to provide additional evidence that a behavioral response is due to the degree of neuronal silencing.
g) summary.
Experiments using PSAM/PSEM chemogenetic silencing and activation tools have demonstrated bidirectional control over neuron activity. The influence of these LGICs on cells is limited to direct modulation of electrical activity, which is not the case in systems that rely on GPCRs. Both chemogenetic silencing and activation are sustained because the channels have a substantial window current in the presence of the corresponding ligand that maintains channel signaling (130). Neuron perturbations can be applied daily without loss of effectiveness. The channels and the ligands are well tolerated, even in neurons sensitized by SODmut. Fast-acting pharmacokinetics of the reported ligands is useful for studies involving perturbations that influence learning and memory in a short behavioral session. Additional ligands with longer-acting pharmacokinetics can presumably be generated as well. Therefore, engineered LGICs are powerful and straightforward chemogenetic tools for cell type-selectively controlling cellular functions in animals.
Engineered LGICs, such as PSAMs, and GPCR tools such as DREADDs are among the most widely used chemogenetic tools for activating or inhibiting cells in vivo. Although these tools are used in similar applications, they have notable functional, mechanistic, and temporal differences. DREADDs utilize endogenous cell signaling pathways, which are common to many but not all cell types, to indirectly modulate cell activity. PSAM-based LGICs and other engineered LGICs can activate or silence cells directly. Inhibitory DREADDs modestly affect cellular activity, but they are often very effective synaptic silencers (173), while inhibitory PSAM LGICs are very powerful inhibitors of cellular electrical activity due to an electrical shunt that renders cells insensitive even to injection of hundreds of picoamperes of current (130). Inhibitory PSAM LGICs also can be used to block axonal transmission (13), but this is likely due to shunting the axon conductances, while hM4Di inhibits synaptic release but not axonal action potential propagation (173). Conversely, neuron activation is also direct with PSAM-5HT3 channels, and the depolarization magnitude can be controlled by the dose of the cognate PSEM ligand. However, application of these LGIC tools to some neuronal cell types may lead to depolarization block in response to constant depolarizing current. DREADD activators utilize a second messenger pathway to modestly depolarize neurons, and to our knowledge depolarization block has not been reported with this system. Finally, existing DREADD ligands persist in vivo ~8 h for CNO to ~1 h for SalvB. Published PSEM ligands have short half-lives of 0.5–1 h in mice. As a practical matter, PSAM and DREADD-based neuron perturbation methods have been largely interchangeable, but characterization of these chemogenetic systems in any cell type under investigation is advised to firmly establish the effect on neuron activation or inhibition.
B. TRP and P2X2
Additional approaches for chemogenetic control over electrical activity used TRP channels. TRPV1 is a channel that is important for heat sensation (28). Expression of TRPV1 in neurons has been shown to be suitable for neuron activation in some cell types using the ligand capsaicin or thermal energy (207). Because TRPV1 is present in mammals, it is used primarily on a knockout background, which requires additional mouse breeding (4, 48, 82). TRPV1 is permeable to calcium (28) and can promote synaptic vesicle release (165), but the degree of channel activation must be restrained to avoid calcium toxicity and cell death (82). A ferritin-modified TRPV1 has also been shown to be susceptible to electromagnetic gating, which avoids the endogenous activity of capsaicin. TRPM8 (207) and the ATP-sensitive channel P2X2 (207) have also be shown to allow heterologous control of neuron activity. In invertebrates, the ATP-sensitive P2X2 channel can be used as a selective chemogenetic/optogenetic system with caged ATP, but their application as chemogenetic tools in mammals requires use of a knockout background (118).
IV. CELL TYPE-SPECIFIC PHARMACOLOGY
Cell type-specific pharmacology is the selective perturbation of targeted cell populations via chemical control. DREADDs and PSAM/PSEM systems are prominent examples of cell type-specific pharmacology applied to GPCRs and LGICs, respectively. Other instances that involve genetically targeting sensitivity to an otherwise inert small molecule ligand include dimerizer technologies, “pharmacological kinase alleles,” control of protein stability, and small molecule unmasking methods (FIGURE 8).
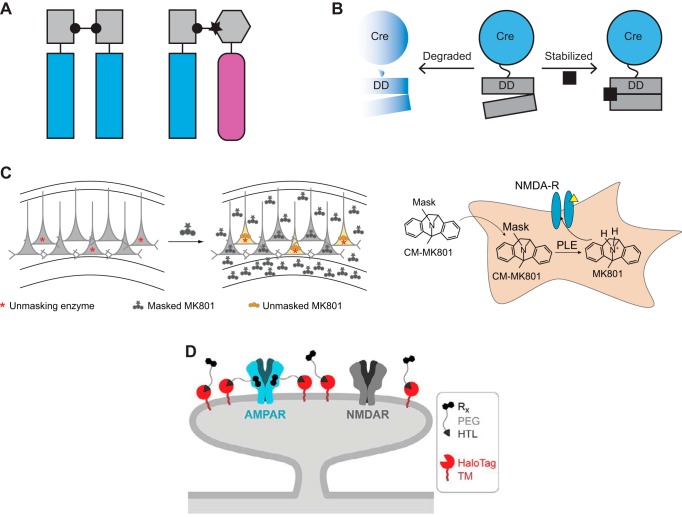
FIGURE 8.
Additional cell type-specific pharmacology methods. A: homodimerization (left) and heterodimerization (right). B: Cre recombinase fused to a destabilization domain (DD) can be stabilized by addition of the small molecule trimethoprim. C: targeting of the small molecule MK801 to specific cell populations by expressing porcine liver esterase (PLE) that can unmask CM-MK801. D: DART method for cell type-specific pharmacology in vivo. [From Shields et al. (164), with permission from American Association for the Advancement of Science.]
A. Dimerizer Technologies
Chemically induced dimerization is an extensively used chemogenetic technology for small molecule control of protein association. Dimerization domains are proteins for which there exists a high-potency dimeric ligand, such that by fusing the dimerization domains to a protein of interest, the dimeric ligand can be used to control the interaction between two protein subunits. Although these approaches have been used extensively in cell culture, applications are more limited in vivo. Chemically induced homodimerization was first achieved by fusing FKBP to proteins using a dimeric version of the FKBP ligand, FK506, which was correspondingly called FK1012 (172). Heterodimerization was accomplished with a number of FKBP-based systems (14, 117) but has been widely performed by fusing FKBP to one protein and FRB to another protein and using rapamycin to join them (88). Further modification of rapamycin, and FRB, allowed for selective heterodimerization without perturbation of the endogenous mTor/FRAP protein targets of rapamycin (116). Simpler synthetic molecules, such AP20187, and mutations of FKBP were identified that enabled homodimerization without binding to endogenous FKBP (204). Chemogenetic control over synaptic vesicle fusion was achieved by fusing the protein of a mutated FKBP to the synaptic vesicle protein synaptobrevin. In the presence of the dimeric FKBP ligand AP20187, synaptic vesicle fusion was suppressed both ex vivo and in vivo (99). FKBP-based dimerizer technologies have also been used in mice to kill senescent cells in mice by chemically induced dimerization of apoptosis (7) and also to provide selective activation of chimeric antigen receptor T cells (200).
Additional heterodimerization systems have been developed based on glucocorticoid receptor and dihydrofolate reductase (DHFR) fusions that can be associated by a dexamethasone-methotrexate heterodimerizer (120). A more selective heterodimerizer system uses E. coli DHFR (ecDHFR) and FKBP12 fusions with the heterodimerizer from trimethoprim-SLF, which does not bind endogenous protein targets (44).
B. Destabilization Domains
Traditionally small molecule control of protein abundance has been mediated by small molecule-dependent transcriptional systems such as the doxycycline controlled tetracycline transactivator (76). Alternative approaches have been developed based on small molecule control over protein stability. A serendipitous observation revealed that GSK3β-FRB fusion led to rapid degradation of the fusion protein that could be reversed with a rapamycin variant that recruited FKBP12 (175). Based on this work showing that recruitment of FKBP can stabilize a rapidly degraded protein, chemogenetic control over protein stability has been extensively investigated (9, 75, 85, 109).
Mutated FKBP variants can act as degradation domains (DD) that lead to rapid degradation of associated fusion proteins. These fusion proteins can be stabilized in the presence of a high-affinity ligand (Shield-1), allowing for rapid control over protein levels in cells (8, 54). Additional DDs have been developed based on a mutated ecDHFR, which can be stabilized by the antibiotic trimethoprim (TMP) (93). Fusion of ecDHFR to Cre-recombinase (DD-Cre) rendered Cre recombinase dependent on TMP (160). Prior work had developed small molecule control over Cre activity by fusion to the estrogen receptor which was anchored in the cytoplasm until it was bound by the ligand tamoxifen (46). However, tamoxifen is an endocrine disruptor, which can be problematic for certain experiments. For DD-Cre, treatment with the antibiotic trimethoprim stabilizes the Cre protein and allows it to perform recombination, enabling small molecule temporal control over Cre. Trimethoprim crosses the blood-brain barrier and is thus suitable for use in the central nervous system. Destabilization domains from FKBP12, FRB, and ecDHFR permit independent small molecule control over their respective fusion proteins, thus enabling rapid posttranscriptional control over protein function.
C. Cell Type-Selective Inhibition of Engineered Kinases
Selective chemogenetic control over kinase signaling was first described using the bump-hole approach of producing a small molecule kinase antagonist with an atomic “bump” that reduced affinity for endogenous kinases but could bind to a kinase with single amino acid mutation to create a corresponding hole (17). These “chemical alleles” for different kinases have been used extensively in cell biological studies (70). This strategy has been implemented in mice by knock-in to the TrkB locus of an altered TrkB allele that allows selective small molecule inhibition with a kinase antagonist modified to selectively inhibit the mutated TrkB receptor. This mouse model has been used to selectively inhibit TrkB kinase involvement in neurotrophin signaling (35) and epilepsy (121). Targeting pharmacologically selective kinase alleles to specific neuron populations affords cell-type-specific modulation of these signaling pathways with a high degree of temporal control, although replacement of the endogenous allele using a knock-in genetic strategy is usually required.
D. Targeting Small Molecules to Cell Types Using Enzyme/prodrug Pairs
Although most chemogenetic systems ideally involve engineering a protein that binds an inert small molecule to influence cellular function, another strategy has been to devise methods to target small molecules to specific cell types where they can modulate endogenous proteins.
1. β-Galactosidase
Expression of Escherichia coli β-galactosidase in cells has been used as a suicide gene therapy to deliver galactose-derivatized daunomycin (Daun02) as a possible anti-cancer therapy (60). A similar approach was adapted for neurobiology applications by generating transgenic mice with E. coli β-galactosidase under control of the immediate early gene Fos promoter. Neurons activated by cocaine could be selectively killed by transcranial targeting of Daun02, which blocked subsequent cocaine-induced locomotion (104). This approach has also been applied to examine the necessity of Fos-activated neurons in cortical regions following heroin (59) or alcohol (148) administration.
2. Nitroreductase
The bacterial nitroreductase enzyme NfsB has also been used for chemically induced cell killing in zebrafish. NfsB converts the drug metronidazole to a toxin, resulting in cell killing in 12–24 h. Reduction of metronidazole results in a cell-impermeant molecule and thus minimizes bystander-effect cell killing (42, 149).
3. Esterase targeting of fluorophores and MK801
To target other molecules to specific cell types, the enzyme porcine liver esterase (PLE) was used in conjunction with a cyclopropylmethyl carboxyl (CM) ester masking group. CM esters were found to be stable in neurons and in HEK cells but could be cleaved with transgenically expressed PLE. CM masking of fluorescein showed that it could be targeted to neurons and cultured cells (189). In addition, the small molecule monastrol was target to specific HEK cells in culture by unmasking the corresponding CM ester with PLE (189). A CM ester was also used to mask the NMDA receptor antagonist MK801 (205). Neuron recordings in brain slices showed that PLE was well tolerated in transfected cortical neurons and that expression of the enzyme rendered them sensitive to NMDA-R blockade in the presence in CM-MK801. Importantly, adjacent, untransfected neurons did not show a reduction in NMDA-R currents, demonstrating the selectivity of CM-MK801 for PLE expressing cells in heterogeneous brain tissue. This approach was extended to test the role of dopamine neuron NMDA receptors in cocaine-induced synaptic plasticity in brain slices (205). Currently, this method has not been used in vivo. The major challenge is the reduced solubility of masked molecules such that high co-solvent concentrations become needed to achieve suitable concentrations for unmasking by PLE. Moreover, CM esters are not expected to survive systemic administration due to the action of peripheral esterases; thus intracranial delivery would be required for this masking strategy. Further development of cell type-specific pharmacology by exploring other masking groups and unmasking enzymes is important to add cell type specificity to traditional pharmacology.
4. Chemogenetic ligand tethering
A wide range of ion channels engineered to react with a light-sensitive chemical agonist or antagonist have been developed as optical chemogenetic tools. These permit optical control of engineered ion channels that have been introduced to cells. Ion channels are either engineered with a cysteine residue (10) or use a naturally occurring nucleophile in the channel (65) near a ligand binding site. This can form a covalent attachment with a maleimide-derivatized linker containing an azobenzene photoswitch that is tethered to an agonist or antagonist. This chemogenetic approach has been applied to Drosophila Shaker-type K+ channels (10), as well as Kv1.3, Kv3.1, Kv7.2, and SK2 K+ channels (66), ligand-gated ion channels such as α3β4 and α4β2 acetylcholine receptors (190), and kainate receptor (186), which has been used in vivo in zebrafish locomotor circuits (186, 201).
An alternative approach, called DART (drugs acutely restricted by tethering), allows chemogenetic control of endogenous ion channels and GPCRs (FIGURE 8D) (164). The method exogenously targets a membrane-bound HaloTag, which is an engineered bacterial enzyme designed to covalently react with a chloroalkane substrate called HaloTag ligand (HTL) (123). By generating bifunctional molecules with HTL at one end and antagonists for AMPA receptors or muscarinic acetylcholine receptors on the other end of a tether, high local concentration of the antagonist is attained, permitting cell type specific inhibition of the receptors. This method was demonstrated in vivo by cell type-specific antagonism of glutamate receptors unilaterally in the striatum, which led to an increased bias in turning direction (123).
V. CONCLUSION
Engineering cells to express receptors that are controlled by selective chemical probes offers the capability to streamline pharmacological analyses. A diversity of tools and methods has been described that includes selective agonist methods to activate specific pathways, such as with DREADDs and PSAM/PSEM LGICs. Also, selective antagonist methods are available both via genetic engineering of the target, as with kinases, or chemogenetically targeting small molecule to specific cell types. The options to activate or inhibit protein function through chemical control of protein association, or chemical reversal or potentiation of protein degradation provide an extensive toolbox for selective pharmacological control over a diverse range of cell signaling pathways. The impact on basic research is already apparent, and hundreds of chemogenetic experiments have been used to dissect cell signaling pathways and cellular roles in physiology and behavior over the past 30 years (see TABLE 1). Perhaps one of the most exciting prospects for the future involves applying chemogenetics in conjunction with gene or cell clinical therapies in humans. As in research studies, chemogenetic therapeutic approaches could achieve pharmacological selectivity that would be otherwise unattainable without the capability to engineer both the drug and the receptor to optimize selectivity and efficacy while minimizing potential side effects (57). Existing chemogenetic technologies offer a solid foundation on which to build towards this aspiration.
Table 1.
Applications of chemogenetic tools
| Actuator Type | Actuator | Tissue and Cell Type | Application | Phenotype | Reference Nos. |
|---|---|---|---|---|---|
| RASSL | RASSL κ-opioid receptor | TongueT1R2 | Artificial sensory perception | Taste preference for synthetic ligand, spiradoline | 211 |
| DREADDs | DREADD[Rq(R165L)] (β-arrestin) | β-Cell line | Cell culture | Insulin release | 138 |
| KORDi | (VTA/SN)VGAT | Salvinorin-B, ip | Increased locomotion | 193 | |
| hM3Dq, hM3Ds, hM4Di | ARCAGRP | CNO, ip | Bidirectional modulation of food intake | 105, 137 | |
| hM4Dnrxn | PVHSIM1→vlPAG | CNO, intracranial, dose response | Increased food intake | 173 | |
| hM4Di | CeA→PBNCGRP | Retrogradely labeled PBN neurons by CAV-Cre | Reduced nausea susceptibility | 26, 27 | |
| hM4Di | PBNLepRb | CNO, ip | Decreased counterregulatory response to hypoglycemia | 64 | |
| hM3Dq | Fos-expressing active neurons | Context-specific ensemble activation | Formation of hybrid memories | 73 | |
| hM4Di | Retrosplenial cortex | Rat, CNO | Impaired learning | 154 | |
| hM3Dq | PBN→basal forebrain | Retrograde labeling by AAV6-Cre, CNO, ip | Wakefulness | 150 | |
| hM4Di | RVMVGAT→spinal cord | Retrograde labeling by AAV-Flex-Flpo, CNO, ip | Reduced mechanical touch sensitivity | 67 | |
| hM4Di | OFC | CNO in primates | Reduced sensitivity to changes in reward size | 56 | |
| hM4Di | Amygdala | CNO and fMRI in primates | Impaired amygdala-cortical functional connectivity | 79 | |
| hM4Di | Caudate nucleus | PET pharmacodynamics in primates | Reduced sensitivity to reward value | 136 | |
| No DREADD | Rat, locomotor effects of CNO | Off target effects of CNO | 129, 151 | ||
| Primate CNO→clozapine | |||||
| hM3Dq | Glial cells, accumbens | CNO, ip | Reduced cue induced reinstatement of cocaine | 163 | |
| hM3Dq | Glomerular podocytes | CNO, ip | Increased Ca2+ transients | 103 | |
| LGICs | GluCl/IVM | VMHvl | IVM, ip | Reduced aggression | 119 |
| GlyR/IVM | Hippocampus | IVM, ip | Impaired spatial working memory | 142 | |
| PSAML141F-GlyR | Hippocampus | PSEM89S, ip | Impaired fear conditioning | 124 | |
| PSAML141F-GlyR | MdV hindbrain premotor neurons | Comparison of reversible silencing and neuron ablation | Impaired locomotor function | 58 | |
| PSAML141F-GlyR | LECGad2→CA1 | Axonal inhibition by intracranial PSEM308 into hippocampus | Inappropriate generalization of freezing context | 13 | |
| PSAML141F-GlyR | Cortical and CA3 PV neurons | PSEM308 | Bidirectional control of foreign object recognition learning | 50, 51, 100 | |
| PSAML141F, Y115F–5HT3 HC | |||||
| PSAML141F, Y115F–5HT3 HC | Motor neurons of SODmut mice | PSEM308, ip | Increased motor neuron survival in ALS model | 162 | |
| PSAML141F, Y115F–GlyR | D1 neurons in NAc | PSEM89S, ip | Bidirectional control of pain responsiveness | 153 | |
| PSAML141F, Y115F–5HT3 HC | |||||
| PSAML141F, Y115F–5HT3 HC | NTSPOMC neurons | PSEM89S, ip | Analgesia, morphine comparison | 30 | |
| PSAML141F-GlyR | DMHLepr neurons | PSEM308, ip | Bidirectional control of heart rate and blood pressure | 167 | |
| PSAML141F, Y115F–5HT3 HC | |||||
| TRPV1 | DAT neurons | Capsaicin, ip; dose response | Elevated locomotion | 82 | |
| Cell type-specific pharmacology | FKBP | Synaptobrevin-chimeric protein | Intracranial AP20187 | Inhibition of vesicle fusion, reduced coordination | 99 |
| Inhibition of Purkinje neuron | |||||
| ecDHFR (DD) | Neuronal synaptobrevin | Inducible Cre recombinase trimethoprim, ip | Impaired spatial memory | 160 | |
| PLE | Primary neuronal cultures | CM ester of MK801 | Cell type-selective NMDA receptor blockade | 205 | |
| DART | Mouse striatum | YM90K-DART | Cell type-selective AMPA receptor blockade | 123 |
ip, Intraperitoneal.
GRANTS
D. Atasoy is supported by a European Molecular Biology Organization (EMBO) Installation Grant. S. M. Sternson is funded by the Howard Hughes Medical Institute.
DISCLOSURES
S. M. Sternson has patents issued and pending on chemogenetic technologies involving PSAM/PSEM ion channels and ligands. S. M. Sternson is a founder of Redpin Therapeutics that is developing clinical chemogenetic applications.
ACKNOWLEDGMENTS
Address for reprint requests and other correspondence: S. M. Sternson, Janelia Research Campus, HHMI, 19700 Helix Dr., Ashburn, VA 20147 (e-mail: sternsons@janelia.hhmi.org) or D. Atasoy, Dept. of Physiology, School of Medicine, Istanbul Medipol University, Istanbul, Turkey (e-mail: datasoy@medipol.edu.tr).
REFERENCES
- 1.Agulhon C, Boyt KM, Xie AX, Friocourt F, Roth BL, McCarthy KD. Modulation of the autonomic nervous system and behaviour by acute glial cell Gq protein-coupled receptor activation in vivo. J Physiol 591: 5599–5609, 2013. doi: 10.1113/jphysiol.2013.261289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO, Roth BL. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63: 27–39, 2009. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci 14: 351–355, 2011. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arenkiel BR, Klein ME, Davison IG, Katz LC, Ehlers MD. Genetic control of neuronal activity in mice conditionally expressing TRPV1. Nat Methods 5: 299–302, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA 104: 5163–5168, 2007. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature 488: 172–177, 2012. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530: 184–189, 2016. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banaszynski LA, Chen LC, Maynard-Smith LA, Ooi AG, Wandless TJ. A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell 126: 995–1004, 2006. doi: 10.1016/j.cell.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banaszynski LA, Sellmyer MA, Contag CH, Wandless TJ, Thorne SH. Chemical control of protein stability and function in living mice. Nat Med 14: 1123–1127, 2008. doi: 10.1038/nm.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banghart M, Borges K, Isacoff E, Trauner D, Kramer RH. Light-activated ion channels for remote control of neuronal firing. Nat Neurosci 7: 1381–1386, 2004. doi: 10.1038/nn1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baraka OZ, Mahmoud BM, Marschke CK, Geary TG, Homeida MM, Williams JF. Ivermectin distribution in the plasma and tissues of patients infected with Onchocerca volvulus. Eur J Clin Pharmacol 50: 407–410, 1996. doi: 10.1007/s002280050131. [DOI] [PubMed] [Google Scholar]
- 12.Basu J, Srinivas KV, Cheung SK, Taniguchi H, Huang ZJ, Siegelbaum SA. A cortico-hippocampal learning rule shapes inhibitory microcircuit activity to enhance hippocampal information flow. Neuron 79: 1208–1221, 2013. doi: 10.1016/j.neuron.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basu J, Zaremba JD, Cheung SK, Hitti FL, Zemelman BV, Losonczy A, Siegelbaum SA. Gating of hippocampal activity, plasticity, and memory by entorhinal cortex long-range inhibition. Science 351: aaa5694, 2016. doi: 10.1126/science.aaa5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belshaw PJ, Ho SN, Crabtree GR, Schreiber SL. Controlling protein association and subcellular localization with a synthetic ligand that induces heterodimerization of proteins. Proc Natl Acad Sci USA 93: 4604–4607, 1996. doi: 10.1073/pnas.93.10.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berridge MJ. Neuronal calcium signaling. Neuron 21: 13–26, 1998. doi: 10.1016/S0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 16.Betley JN, Xu S, Cao ZFH, Gong R, Magnus CJ, Yu Y, Sternson SM. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521: 180–185, 2015. doi: 10.1038/nature14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bishop AC, Ubersax JA, Petsch DT, Matheos DP, Gray NS, Blethrow J, Shimizu E, Tsien JZ, Schultz PG, Rose MD, Wood JL, Morgan DO, Shokat KM. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature 407: 395–401, 2000. doi: 10.1038/35030148. [DOI] [PubMed] [Google Scholar]
- 18.Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev 79: 763–854, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Bodnar AL, Cortes-Burgos LA, Cook KK, Dinh DM, Groppi VE, Hajos M, Higdon NR, Hoffmann WE, Hurst RS, Myers JK, Rogers BN, Wall TM, Wolfe ML, Wong E. Discovery and structure-activity relationship of quinuclidine benzamides as agonists of alpha7 nicotinic acetylcholine receptors. J Med Chem 48: 905–908, 2005. doi: 10.1021/jm049363q. [DOI] [PubMed] [Google Scholar]
- 20.Bonder DE, McCarthy KD. Astrocytic Gq-GPCR-linked IP3R-dependent Ca2+ signaling does not mediate neurovascular coupling in mouse visual cortex in vivo. J Neurosci 34: 13139–13150, 2014. doi: 10.1523/JNEUROSCI.2591-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bourane S, Duan B, Koch SC, Dalet A, Britz O, Garcia-Campmany L, Kim E, Cheng L, Ghosh A, Ma Q, Goulding M. Gate control of mechanical itch by a subpopulation of spinal cord interneurons. Science 350: 550–554, 2015. doi: 10.1126/science.aac8653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature 283: 673–676, 1980. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- 23.Bull C, Freitas KC, Zou S, Poland RS, Syed WA, Urban DJ, Minter SC, Shelton KL, Hauser KF, Negus SS, Knapp PE, Bowers MS. Rat nucleus accumbens core astrocytes modulate reward and the motivation to self-administer ethanol after abstinence. Neuropsychopharmacology 39: 2835–2845, 2014. doi: 10.1038/npp.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai H, Haubensak W, Anthony TE, Anderson DJ. Central amygdala PKC-δ(+) neurons mediate the influence of multiple anorexigenic signals. Nat Neurosci 17: 1240–1248, 2014. doi: 10.1038/nn.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cains S, Blomeley C, Kollo M, Rácz R, Burdakov D. Agrp neuron activity is required for alcohol-induced overeating. Nat Commun 8: 14014, 2017. doi: 10.1038/ncomms14014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campos CA, Bowen AJ, Schwartz MW, Palmiter RD. Parabrachial CGRP Neurons Control Meal Termination. Cell Metab 23: 811–820, 2016. doi: 10.1016/j.cmet.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature 503: 111–114, 2013. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 29.Celie PH, van Rossum-Fikkert SE, van Dijk WJ, Brejc K, Smit AB, Sixma TK. Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AChBP crystal structures. Neuron 41: 907–914, 2004. doi: 10.1016/S0896-6273(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 30.Cerritelli S, Hirschberg S, Hill R, Balthasar N, Pickering AE. Activation of Brainstem Pro-opiomelanocortin Neurons Produces Opioidergic Analgesia, Bradycardia and Bradypnoea. PLoS One 11: e0153187, 2016. doi: 10.1371/journal.pone.0153187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang WH, Lin SK, Lane HY, Wei FC, Hu WH, Lam YWF, Jann MW. Reversible metabolism of clozapine and clozapine N-oxide in schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry 22: 723–739, 1998. doi: 10.1016/S0278-5846(98)00035-9. [DOI] [PubMed] [Google Scholar]
- 32.Chen L, Yin D, Wang TX, Guo W, Dong H, Xu Q, Luo YJ, Cherasse Y, Lazarus M, Qiu ZL, Lu J, Qu WM, Huang ZL. Basal Forebrain Cholinergic Neurons Primarily Contribute to Inhibition of Electroencephalogram Delta Activity, Rather Than Inducing Behavioral Wakefulness in Mice. Neuropsychopharmacology 41: 2133–2146, 2016. doi: 10.1038/npp.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen MC, Vetrivelan R, Guo CN, Chang C, Fuller PM, Lu J. Ventral medullary control of rapid eye movement sleep and atonia. Exp Neurol 290: 53–62, 2017. doi: 10.1016/j.expneurol.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen X, Choo H, Huang XP, Yang X, Stone O, Roth BL, Jin J. The first structure-activity relationship studies for designer receptors exclusively activated by designer drugs. ACS Chem Neurosci 6: 476–484, 2015. doi: 10.1021/cn500325v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X, Ye H, Kuruvilla R, Ramanan N, Scangos KW, Zhang C, Johnson NM, England PM, Shokat KM, Ginty DD. A chemical-genetic approach to studying neurotrophin signaling. Neuron 46: 13–21, 2005. doi: 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Cheng Q, Kulli JC, Yang J. Suppression of neuronal hyperexcitability and associated delayed neuronal death by adenoviral expression of GABA(C) receptors. J Neurosci 21: 3419–3428, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology 115: 427–429, 1984. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- 38.Conklin BR, Hsiao EC, Claeysen S, Dumuis A, Srinivasan S, Forsayeth JR, Guettier JM, Chang WC, Pei Y, McCarthy KD, Nissenson RA, Wess J, Bockaert J, Roth BL. Engineering GPCR signaling pathways with RASSLs. Nat Methods 5: 673–678, 2008. doi: 10.1038/nmeth.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coward P, Wada HG, Falk MS, Chan SD, Meng F, Akil H, Conklin BR. Controlling signaling with a specifically designed Gi-coupled receptor. Proc Natl Acad Sci USA 95: 352–357, 1998. doi: 10.1073/pnas.95.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411: 480–484, 2001. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 41.Cully DF, Vassilatis DK, Liu KK, Paress PS, Van der Ploeg LH, Schaeffer JM, Arena JP. Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature 371: 707–711, 1994. doi: 10.1038/371707a0. [DOI] [PubMed] [Google Scholar]
- 42.Curado S, Stainier DY, Anderson RM. Nitroreductase-mediated cell/tissue ablation in zebrafish: a spatially and temporally controlled ablation method with applications in developmental and regeneration studies. Nat Protoc 3: 948–954, 2008. doi: 10.1038/nprot.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Currie KP. G protein modulation of CaV2 voltage-gated calcium channels. Channels (Austin) 4: 497–509, 2010. doi: 10.4161/chan.4.6.12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Czlapinski JL, Schelle MW, Miller LW, Laughlin ST, Kohler JJ, Cornish VW, Bertozzi CR. Conditional glycosylation in eukaryotic cells using a biocompatible chemical inducer of dimerization. J Am Chem Soc 130: 13186–13187, 2008. doi: 10.1021/ja8037728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D’Agostino G, Lyons DJ, Cristiano C, Burke LK, Madara JC, Campbell JN, Garcia AP, Land BB, Lowell BB, Dileone RJ, Heisler LK. Appetite controlled by a cholecystokinin nucleus of the solitary tract to hypothalamus neurocircuit. eLife 5: e12225, 2016. doi: 10.7554/eLife.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol 8: 1323–1326, 1998. doi: 10.1016/S0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- 47.Denis RG, Joly-Amado A, Webber E, Langlet F, Schaeffer M, Padilla SL, Cansell C, Dehouck B, Castel J, Delbès AS, Martinez S, Lacombe A, Rouch C, Kassis N, Fehrentz JA, Martinez J, Verdié P, Hnasko TS, Palmiter RD, Krashes MJ, Güler AD, Magnan C, Luquet S. Palatability Can Drive Feeding Independent of AgRP Neurons. Cell Metab 22: 646–657, 2015. doi: 10.1016/j.cmet.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dietrich MO, Zimmer MR, Bober J, Horvath TL. Hypothalamic Agrp neurons drive stereotypic behaviors beyond feeding. Cell 160: 1222–1232, 2015. doi: 10.1016/j.cell.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dineley KT, Pandya AA, Yakel JL. Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol Sci 36: 96–108, 2015. doi: 10.1016/j.tips.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donato F, Chowdhury A, Lahr M, Caroni P. Early- and late-born parvalbumin basket cell subpopulations exhibiting distinct regulation and roles in learning. Neuron 85: 770–786, 2015. doi: 10.1016/j.neuron.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 51.Donato F, Rompani SB, Caroni P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature 504: 272–276, 2013. doi: 10.1038/nature12866. [DOI] [PubMed] [Google Scholar]
- 52.Dunlap K, Fischbach GD. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones. J Physiol 317: 519–535, 1981. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dupré DJ, Robitaille M, Rebois RV, Hébert TE. The role of Gbetagamma subunits in the organization, assembly, and function of GPCR signaling complexes. Annu Rev Pharmacol Toxicol 49: 31–56, 2009. doi: 10.1146/annurev-pharmtox-061008-103038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Egeler EL, Urner LM, Rakhit R, Liu CW, Wandless TJ. Ligand-switchable substrates for a ubiquitin-proteasome system. J Biol Chem 286: 31328–31336, 2011. doi: 10.1074/jbc.M111.264101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eiselé JL, Bertrand S, Galzi JL, Devillers-Thiéry A, Changeux JP, Bertrand D. Chimaeric nicotinic-serotonergic receptor combines distinct ligand binding and channel specificities. Nature 366: 479–483, 1993. doi: 10.1038/366479a0. [DOI] [PubMed] [Google Scholar]
- 56.Eldridge MA, Lerchner W, Saunders RC, Kaneko H, Krausz KW, Gonzalez FJ, Ji B, Higuchi M, Minamimoto T, Richmond BJ. Chemogenetic disconnection of monkey orbitofrontal and rhinal cortex reversibly disrupts reward value. Nat Neurosci 19: 37–39, 2016. doi: 10.1038/nn.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.English JG, Roth BL. Chemogenetics-A Transformational and Translational Platform. JAMA Neurol 72: 1361–1366, 2015. doi: 10.1001/jamaneurol.2015.1921. [DOI] [PubMed] [Google Scholar]
- 58.Esposito MS, Capelli P, Arber S. Brainstem nucleus MdV mediates skilled forelimb motor tasks. Nature 508: 351–356, 2014. doi: 10.1038/nature13023. [DOI] [PubMed] [Google Scholar]
- 59.Fanous S, Goldart EM, Theberge FR, Bossert JM, Shaham Y, Hope BT. Role of orbitofrontal cortex neuronal ensembles in the expression of incubation of heroin craving. J Neurosci 32: 11600–11609, 2012. doi: 10.1523/JNEUROSCI.1914-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farquhar D, Pan BF, Sakurai M, Ghosh A, Mullen CA, Nelson JA. Suicide gene therapy using E. coli beta-galactosidase. Cancer Chemother Pharmacol 50: 65–70, 2002. doi: 10.1007/s00280-002-0438-2. [DOI] [PubMed] [Google Scholar]
- 61.Farrell MS, Pei Y, Wan Y, Yadav PN, Daigle TL, Urban DJ, Lee HM, Sciaky N, Simmons A, Nonneman RJ, Huang XP, Hufeisen SJ, Guettier JM, Moy SS, Wess J, Caron MG, Calakos N, Roth BL. A Gαs DREADD mouse for selective modulation of cAMP production in striatopallidal neurons. Neuropsychopharmacology 38: 854–862, 2013. doi: 10.1038/npp.2012.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci 34: 389–412, 2011. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fenselau H, Campbell JN, Verstegen AM, Madara JC, Xu J, Shah BP, Resch JM, Yang Z, Mandelblat-Cerf Y, Livneh Y, Lowell BB. A rapidly acting glutamatergic ARC→PVH satiety circuit postsynaptically regulated by α-MSH. Nat Neurosci 20: 42–51, 2017. doi: 10.1038/nn.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flak JN, Patterson CM, Garfield AS, D’Agostino G, Goforth PB, Sutton AK, Malec PA, Wong JT, Germani M, Jones JC, Rajala M, Satin L, Rhodes CJ, Olson DP, Kennedy RT, Heisler LK, Myers MG Jr. Leptin-inhibited PBN neurons enhance responses to hypoglycemia in negative energy balance. Nat Neurosci 17: 1744–1750, 2014. doi: 10.1038/nn.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fortin DL, Banghart MR, Dunn TW, Borges K, Wagenaar DA, Gaudry Q, Karakossian MH, Otis TS, Kristan WB, Trauner D, Kramer RH. Photochemical control of endogenous ion channels and cellular excitability. Nat Methods 5: 331–338, 2008. doi: 10.1038/nmeth.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fortin DL, Dunn TW, Fedorchak A, Allen D, Montpetit R, Banghart MR, Trauner D, Adelman JP, Kramer RH. Optogenetic photochemical control of designer K+ channels in mammalian neurons. J Neurophysiol 106: 488–496, 2011. doi: 10.1152/jn.00251.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.François A, Low SA, Sypek EI, Christensen AJ, Sotoudeh C, Beier KT, Ramakrishnan C, Ritola KD, Sharif-Naeini R, Deisseroth K, Delp SL, Malenka RC, Luo L, Hantman AW, Scherrer G. A Brainstem-Spinal Cord Inhibitory Circuit for Mechanical Pain Modulation by GABA and Enkephalins. Neuron 93: 822–839.e6, 2017. doi: 10.1016/j.neuron.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frazier SJ, Cohen BN, Lester HA. An engineered glutamate-gated chloride (GluCl) channel for sensitive, consistent neuronal silencing by ivermectin. J Biol Chem 288: 21029–21042, 2013. doi: 10.1074/jbc.M112.423921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fujii T, Kawashima K. Calcium signaling and c-Fos gene expression via M3 muscarinic acetylcholine receptors in human T- and B-cells. Jpn J Pharmacol 84: 124–132, 2000. doi: 10.1254/jjp.84.124. [DOI] [PubMed] [Google Scholar]
- 70.Gallion SL, Qian D. Chemical genetic approaches to kinase drug discovery. Curr Opin Drug Discov Devel 8: 638–645, 2005. [PubMed] [Google Scholar]
- 71.Garfield AS, Li C, Madara JC, Shah BP, Webber E, Steger JS, Campbell JN, Gavrilova O, Lee CE, Olson DP, Elmquist JK, Tannous BA, Krashes MJ, Lowell BB. A neural basis for melanocortin-4 receptor-regulated appetite. Nat Neurosci 18: 863–871, 2015. doi: 10.1038/nn.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garfield AS, Shah BP, Madara JC, Burke LK, Patterson CM, Flak J, Neve RL, Evans ML, Lowell BB, Myers MG Jr, Heisler LK. A parabrachial-hypothalamic cholecystokinin neurocircuit controls counterregulatory responses to hypoglycemia. Cell Metab 20: 1030–1037, 2014. doi: 10.1016/j.cmet.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garner AR, Rowland DC, Hwang SY, Baumgaertel K, Roth BL, Kentros C, Mayford M. Generation of a synthetic memory trace. Science 335: 1513–1516, 2012. doi: 10.1126/science.1214985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gilman AG. G proteins: transducers of receptor-generated signals. Annu Rev Biochem 56: 615–649, 1987. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 75.Glass M, Busche A, Wagner K, Messerle M, Borst EM. Conditional and reversible disruption of essential herpesvirus proteins. Nat Methods 6: 577–579, 2009. doi: 10.1038/nmeth.1346. [DOI] [PubMed] [Google Scholar]
- 75a.Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, Pomper MG, Bonci A, Michaelides M. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357: 503–507, 2017. doi: 10.1126/science.aan2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gossen M, Freundlieb S, Bender G, Müller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science 268: 1766–1769, 1995. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 77.Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, Fabisiak TJ, Anderson ND, Cheng K, Greene LI, Berkelhammer D, Zhang Y, Ellis AL, Yin HH, Campeau S, Rice KC, Roth BL, Maier SF, Watkins LR. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc Natl Acad Sci USA 113: E3441–E3450, 2016. doi: 10.1073/pnas.1602070113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grandison L, Guidotti A. Stimulation of food intake by muscimol and beta endorphin. Neuropharmacology 16: 533–536, 1977. doi: 10.1016/0028-3908(77)90019-3. [DOI] [PubMed] [Google Scholar]
- 79.Grayson DS, Bliss-Moreau E, Machado CJ, Bennett J, Shen K, Grant KA, Fair DA, Amaral DG. The Rhesus Monkey Connectome Predicts Disrupted Functional Networks Resulting from Pharmacogenetic Inactivation of the Amygdala. Neuron 91: 453–466, 2016. doi: 10.1016/j.neuron.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grutter T, de Carvalho LP, Dufresne V, Taly A, Edelstein SJ, Changeux JP. Molecular tuning of fast gating in pentameric ligand-gated ion channels. Proc Natl Acad Sci USA 102: 18207–18212, 2005. doi: 10.1073/pnas.0509024102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guettier JM, Gautam D, Scarselli M, Ruiz de Azua I, Li JH, Rosemond E, Ma X, Gonzalez FJ, Armbruster BN, Lu H, Roth BL, Wess J. A chemical-genetic approach to study G protein regulation of beta cell function in vivo. Proc Natl Acad Sci USA 106: 19197–19202, 2009. doi: 10.1073/pnas.0906593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Güler AD, Rainwater A, Parker JG, Jones GL, Argilli E, Arenkiel BR, Ehlers MD, Bonci A, Zweifel LS, Palmiter RD. Transient activation of specific neurons in mice by selective expression of the capsaicin receptor. Nat Commun 3: 746, 2012. doi: 10.1038/ncomms1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Han S, Soleiman MT, Soden ME, Zweifel LS, Palmiter RD. Elucidating an Affective Pain Circuit that Creates a Threat Memory. Cell 162: 363–374, 2015. doi: 10.1016/j.cell.2015.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hausen AC, Ruud J, Jiang H, Hess S, Varbanov H, Kloppenburg P, Brüning JC. Insulin-Dependent Activation of MCH Neurons Impairs Locomotor Activity and Insulin Sensitivity in Obesity. Cell Reports 17: 2512–2521, 2016. doi: 10.1016/j.celrep.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 85.Herm-Götz A, Agop-Nersesian C, Münter S, Grimley JS, Wandless TJ, Frischknecht F, Meissner M. Rapid control of protein level in the apicomplexan Toxoplasma gondii. Nat Methods 4: 1003–1005, 2007. doi: 10.1038/nmeth1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. J Neurophysiol 53: 266–291, 1985. [DOI] [PubMed] [Google Scholar]
- 87.Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer, 2001. [Google Scholar]
- 88.Ho SN, Biggar SR, Spencer DM, Schreiber SL, Crabtree GR. Dimeric ligands define a role for transcriptional activation domains in reinitiation. Nature 382: 822–826, 1996. doi: 10.1038/382822a0. [DOI] [PubMed] [Google Scholar]
- 89.Hu L, Lan W, Guo H, Chai G-D, Huang K, Zhang L, Huang Y, Chen X-F, Zhang L, Song N-N, Chen L, Lang B, Wang Y, Wang Q-X, Zhang J-B, McCaig C, Xu L, Ding Y-Q. A mouse line for inducible and reversible silencing of specific neurons. Mol Brain 7: 68, 2014. doi: 10.1186/s13041-014-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Inutsuka A, Inui A, Tabuchi S, Tsunematsu T, Lazarus M, Yamanaka A. Concurrent and robust regulation of feeding behaviors and metabolism by orexin neurons. Neuropharmacology 85: 451–460, 2014. doi: 10.1016/j.neuropharm.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 91.Islam R, Keramidas A, Xu L, Durisic N, Sah P, Lynch JW. Ivermectin-Activated, Cation-Permeable Glycine Receptors for the Chemogenetic Control of Neuronal Excitation. ACS Chem Neurosci 7: 1647–1657, 2016. doi: 10.1021/acschemneuro.6b00168. [DOI] [PubMed] [Google Scholar]
- 92.Islam R, Zhang Y, Xu L, Sah P, Lynch JW. A Chemogenetic Receptor That Enhances the Magnitude and Frequency of Glycinergic Inhibitory Postsynaptic Currents Without Inducing a Tonic Chloride Flux. ACS Chem Neurosci 8: 460–467, 2017. doi: 10.1021/acschemneuro.6b00382. [DOI] [PubMed] [Google Scholar]
- 93.Iwamoto M, Björklund T, Lundberg C, Kirik D, Wandless TJ. A general chemical method to regulate protein stability in the mammalian central nervous system. Chem Biol 17: 981–988, 2010. doi: 10.1016/j.chembiol.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iyer SM, Vesuna S, Ramakrishnan C, Huynh K, Young S, Berndt A, Lee SY, Gorini CJ, Deisseroth K, Delp SL. Optogenetic and chemogenetic strategies for sustained inhibition of pain. Sci Rep 6: 30570, 2016. doi: 10.1038/srep30570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jain S, Ruiz de Azua I, Lu H, White MF, Guettier JM, Wess J. Chronic activation of a designer G(q)-coupled receptor improves β cell function. J Clin Invest 123: 1750–1762, 2013. doi: 10.1172/JCI66432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jennings JH, Ung RL, Resendez SL, Stamatakis AM, Taylor JG, Huang J, Veleta K, Kantak PA, Aita M, Shilling-Scrivo K, Ramakrishnan C, Deisseroth K, Otte S, Stuber GD. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell 160: 516–527, 2015. doi: 10.1016/j.cell.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jurik A, Auffenberg E, Klein S, Deussing JM, Schmid RM, Wotjak CT, Thoeringer CK. Roles of prefrontal cortex and paraventricular thalamus in affective and mechanical components of visceral nociception. Pain 156: 2479–2491, 2015. doi: 10.1097/j.pain.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 98.Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. New York: McGraw-Hill, 2000, p. 1414. [Google Scholar]
- 99.Karpova AY, Tervo DG, Gray NW, Svoboda K. Rapid and reversible chemical inactivation of synaptic transmission in genetically targeted neurons. Neuron 48: 727–735, 2005. doi: 10.1016/j.neuron.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 100.Karunakaran S, Chowdhury A, Donato F, Quairiaux C, Michel CM, Caroni P. PV plasticity sustained through D1/5 dopamine signaling required for long-term memory consolidation. Nat Neurosci 19: 454–464, 2016. doi: 10.1038/nn.4231. [DOI] [PubMed] [Google Scholar]
- 101.Kelly J, Rothstein J, Grossman SP. GABA and hypothalamic feeding systems. I. Topographic analysis of the effects of microinjections of muscimol. Physiol Behav 23: 1123–1134, 1979. doi: 10.1016/0031-9384(79)90306-8. [DOI] [PubMed] [Google Scholar]
- 102.Khan SM, Sleno R, Gora S, Zylbergold P, Laverdure JP, Labbé JC, Miller GJ, Hébert TE. The expanding roles of Gβγ subunits in G protein-coupled receptor signaling and drug action. Pharmacol Rev 65: 545–577, 2013. doi: 10.1124/pr.111.005603. [DOI] [PubMed] [Google Scholar]
- 103.Koehler S, Brähler S, Kuczkowski A, Binz J, Hackl MJ, Hagmann H, Höhne M, Vogt MC, Wunderlich CM, Wunderlich FT, Schweda F, Schermer B, Benzing T, Brinkkoetter PT. Single and Transient Ca(2+) Peaks in Podocytes do not induce Changes in Glomerular Filtration and Perfusion. Sci Rep 6: 35400, 2016. doi: 10.1038/srep35400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Koya E, Golden SA, Harvey BK, Guez-Barber DH, Berkow A, Simmons DE, Bossert JM, Nair SG, Uejima JL, Marin MT, Mitchell TB, Farquhar D, Ghosh SC, Mattson BJ, Hope BT. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nat Neurosci 12: 1069–1073, 2009. doi: 10.1038/nn.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest 121: 1424–1428, 2011. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Krashes MJ, Shah BP, Koda S, Lowell BB. Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell Metab 18: 588–595, 2013. doi: 10.1016/j.cmet.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida N, Liberles SD, Lowell BB. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 507: 238–242, 2014. doi: 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee K, Boden PR. Characterization of the inward current induced by metabotropic glutamate receptor stimulation in rat ventromedial hypothalamic neurones. J Physiol 504: 649–663, 1997. doi: 10.1111/j.1469-7793.1997.649bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Leong HS, Lizardo MM, Ablack A, McPherson VA, Wandless TJ, Chambers AF, Lewis JD. Imaging the impact of chemically inducible proteins on cellular dynamics in vivo. PLoS One 7: e30177, 2012. doi: 10.1371/journal.pone.0030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lerchner W, Xiao C, Nashmi R, Slimko EM, van Trigt L, Lester HA, Anderson DJ. Reversible silencing of neuronal excitability in behaving mice by a genetically targeted, ivermectin-gated Cl− channel. Neuron 54: 35–49, 2007. doi: 10.1016/j.neuron.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 111.Lester HA, Dibas MI, Dahan DS, Leite JF, Dougherty DA. Cys-loop receptors: new twists and turns. Trends Neurosci 27: 329–336, 2004. doi: 10.1016/j.tins.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 112.Li C, Sugam JA, Lowery-Gionta EG, McElligott ZA, McCall NM, Lopez AJ, McKlveen JM, Pleil KE, Kash TL. Mu Opioid Receptor Modulation of Dopamine Neurons in the Periaqueductal Gray/Dorsal Raphe: A Role in Regulation of Pain. Neuropsychopharmacology 41: 2122–2132, 2016. doi: 10.1038/npp.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li JH, Jain S, McMillin SM, Cui Y, Gautam D, Sakamoto W, Lu H, Jou W, McGuinness OP, Gavrilova O, Wess J. A novel experimental strategy to assess the metabolic effects of selective activation of a G(q)-coupled receptor in hepatocytes in vivo. Endocrinology 154: 3539–3551, 2013. doi: 10.1210/en.2012-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li P, Slimko EM, Lester HA. Selective elimination of glutamate activation and introduction of fluorescent proteins into a Caenorhabditis elegans chloride channel. FEBS Lett 528: 77–82, 2002. doi: 10.1016/S0014-5793(02)03245-3. [DOI] [PubMed] [Google Scholar]
- 115.Li SX, Huang S, Bren N, Noridomi K, Dellisanti CD, Sine SM, Chen L. Ligand-binding domain of an α7-nicotinic receptor chimera and its complex with agonist. Nat Neurosci 14: 1253–1259, 2011. doi: 10.1038/nn.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liberles SD, Diver ST, Austin DJ, Schreiber SL. Inducible gene expression and protein translocation using nontoxic ligands identified by a mammalian three-hybrid screen. Proc Natl Acad Sci USA 94: 7825–7830, 1997. doi: 10.1073/pnas.94.15.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Licitra EJ, Liu JO. A three-hybrid system for detecting small ligand-protein receptor interactions. Proc Natl Acad Sci USA 93: 12817–12821, 1996. doi: 10.1073/pnas.93.23.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lima SQ, Miesenböck G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell 121: 141–152, 2005. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 119.Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ. Functional identification of an aggression locus in the mouse hypothalamus. Nature 470: 221–226, 2011. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lin H, Abida WM, Sauer RT, Cornish VW. Dexamethasone−Methotrexate: An Efficient Chemical Inducer of Protein Dimerization In Vivo. J Am Chem Soc 122: 4247–4248, 2000. doi: 10.1021/ja9941532. [DOI] [Google Scholar]
- 121.Liu G, Gu B, He X-P, Joshi RB, Wackerle HD, Rodriguiz RM, Wetsel WC, McNamara JO. Transient inhibition of TrkB kinase after status epilepticus prevents development of temporal lobe epilepsy. Neuron 79: 31–38, 2013. doi: 10.1016/j.neuron.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Logothetis DE, Kim DH, Northup JK, Neer EJ, Clapham DE. Specificity of action of guanine nucleotide-binding regulatory protein subunits on the cardiac muscarinic K+ channel. Proc Natl Acad Sci USA 85: 5814–5818, 1988. doi: 10.1073/pnas.85.16.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Los GV, Encell LP, McDougall MG, Hartzell DD, Karassina N, Zimprich C, Wood MG, Learish R, Ohana RF, Urh M, Simpson D, Mendez J, Zimmerman K, Otto P, Vidugiris G, Zhu J, Darzins A, Klaubert DH, Bulleit RF, Wood KV. HaloTag: a novel protein labeling technology for cell imaging and protein analysis. ACS Chem Biol 3: 373–382, 2008. doi: 10.1021/cb800025k. [DOI] [PubMed] [Google Scholar]
- 124.Lovett-Barron M, Kaifosh P, Kheirbek MA, Danielson N, Zaremba JD, Reardon TR, Turi GF, Hen R, Zemelman BV, Losonczy A. Dendritic inhibition in the hippocampus supports fear learning. Science 343: 857–863, 2014. doi: 10.1126/science.1247485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lovett-Barron M, Turi GF, Kaifosh P, Lee PH, Bolze F, Sun XH, Nicoud JF, Zemelman BV, Sternson SM, Losonczy A. Regulation of neuronal input transformations by tunable dendritic inhibition. Nat Neurosci 15: 423–430, 2012. doi: 10.1038/nn.3024. [DOI] [PubMed] [Google Scholar]
- 126.Lynagh T, Lynch JW. A glycine residue essential for high ivermectin sensitivity in Cys-loop ion channel receptors. Int J Parasitol 40: 1477–1481, 2010. doi: 10.1016/j.ijpara.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 127.Lynagh T, Lynch JW. An improved ivermectin-activated chloride channel receptor for inhibiting electrical activity in defined neuronal populations. J Biol Chem 285: 14890–14897, 2010. doi: 10.1074/jbc.M110.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lynagh T, Webb TI, Dixon CL, Cromer BA, Lynch JW. Molecular determinants of ivermectin sensitivity at the glycine receptor chloride channel. J Biol Chem 286: 43913–43924, 2011. doi: 10.1074/jbc.M111.262634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.MacLaren DA, Browne RW, Shaw JK, Krishnan Radhakrishnan S, Khare P, España RA, Clark SD. Clozapine N-Oxide Administration Produces Behavioral Effects in Long-Evans Rats: Implications for Designing DREADD Experiments. eNeuro 3: 0219-16, 2016. doi: 10.1523/ENEURO.0219-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Magnus CJ, Lee PH, Atasoy D, Su HH, Looger LL, Sternson SM. Chemical and genetic engineering of selective ion channel-ligand interactions. Science 333: 1292–1296, 2011. doi: 10.1126/science.1206606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, Wilson SP, Deisseroth K, Woodward JJ, Aston-Jones G. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat Neurosci 17: 577–585, 2014. doi: 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Marchant NJ, Whitaker LR, Bossert JM, Harvey BK, Hope BT, Kaganovsky K, Adhikary S, Prisinzano TE, Vardy E, Roth BL, Shaham Y. Behavioral and Physiological Effects of a Novel Kappa-Opioid Receptor-Based DREADD in Rats. Neuropsychopharmacology 41: 402–409, 2016. doi: 10.1038/npp.2015.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McClain JL, Fried DE, Gulbransen BD. Agonist-evoked Ca2+ signaling in enteric glia drives neural programs that regulate intestinal motility in mice. Cell Mol Gastroenterol Hepatol 1: 631–645, 2015. doi: 10.1016/j.jcmgh.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Michaelides M, Anderson SA, Ananth M, Smirnov D, Thanos PK, Neumaier JF, Wang GJ, Volkow ND, Hurd YL. Whole-brain circuit dissection in free-moving animals reveals cell-specific mesocorticolimbic networks. J Clin Invest 123: 5342–5350, 2013. doi: 10.1172/JCI72117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Muñoz W, Tremblay R, Levenstein D, Rudy B. Layer-specific modulation of neocortical dendritic inhibition during active wakefulness. Science 355: 954–959, 2017. doi: 10.1126/science.aag2599. [DOI] [PubMed] [Google Scholar]
- 136.Nagai Y, Kikuchi E, Lerchner W, Inoue KI, Ji B, Eldridge MA, Kaneko H, Kimura Y, Oh-Nishi A, Hori Y, Kato Y, Hirabayashi T, Fujimoto A, Kumata K, Zhang MR, Aoki I, Suhara T, Higuchi M, Takada M, Richmond BJ, Minamimoto T. PET imaging-guided chemogenetic silencing reveals a critical role of primate rostromedial caudate in reward evaluation. Nat Commun 7: 13605, 2016. doi: 10.1038/ncomms13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nakajima K, Cui Z, Li C, Meister J, Cui Y, Fu O, Smith AS, Jain S, Lowell BB, Krashes MJ, Wess J. Gs-coupled GPCR signalling in AgRP neurons triggers sustained increase in food intake. Nat Commun 7: 10268, 2016. doi: 10.1038/ncomms10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nakajima K, Wess J. Design and functional characterization of a novel, arrestin-biased designer G protein-coupled receptor. Mol Pharmacol 82: 575–582, 2012. doi: 10.1124/mol.112.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Navarro M, Olney JJ, Burnham NW, Mazzone CM, Lowery-Gionta EG, Pleil KE, Kash TL, Thiele TE. Lateral Hypothalamus GABAergic Neurons Modulate Consummatory Behaviors Regardless of the Caloric Content or Biological Relevance of the Consumed Stimuli. Neuropsychopharmacology 41: 1505–1512, 2016. doi: 10.1038/npp.2015.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nicoll RA. The coupling of neurotransmitter receptors to ion channels in the brain. Science 241: 545–551, 1988. doi: 10.1126/science.2456612. [DOI] [PubMed] [Google Scholar]
- 141.Niggli E, Lederer WJ. Activation of Na-Ca exchange current by photolysis of “caged calcium”. Biophys J 65: 882–891, 1993. doi: 10.1016/S0006-3495(93)81105-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Obenhaus HA, Rozov A, Bertocchi I, Tang W, Kirsch J, Betz H, Sprengel R. Causal Interrogation of Neuronal Networks and Behavior through Virally Transduced Ivermectin Receptors. Front Mol Neurosci 9: 75, 2016. doi: 10.3389/fnmol.2016.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Park JS, Rhau B, Hermann A, McNally KA, Zhou C, Gong D, Weiner OD, Conklin BR, Onuffer J, Lim WA. Synthetic control of mammalian-cell motility by engineering chemotaxis to an orthogonal bioinert chemical signal. Proc Natl Acad Sci USA 111: 5896–5901, 2014. doi: 10.1073/pnas.1402087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pei H, Sutton AK, Burnett KH, Fuller PM, Olson DP. AVP neurons in the paraventricular nucleus of the hypothalamus regulate feeding. Mol Metab 3: 209–215, 2014. doi: 10.1016/j.molmet.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Peirs C, Williams SP, Zhao X, Walsh CE, Gedeon JY, Cagle NE, Goldring AC, Hioki H, Liu Z, Marell PS, Seal RP. Dorsal Horn Circuits for Persistent Mechanical Pain. Neuron 87: 797–812, 2015. doi: 10.1016/j.neuron.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Peter M, Bathellier B, Fontinha B, Pliota P, Haubensak W, Rumpel S. Transgenic mouse models enabling photolabeling of individual neurons in vivo. PLoS One 8: e62132, 2013. doi: 10.1371/journal.pone.0062132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Peters JA, Hales TG, Lambert JJ. Molecular determinants of single-channel conductance and ion selectivity in the Cys-loop family: insights from the 5-HT3 receptor. Trends Pharmacol Sci 26: 587–594, 2005. doi: 10.1016/j.tips.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 148.Pfarr S, Meinhardt MW, Klee ML, Hansson AC, Vengeliene V, Schönig K, Bartsch D, Hope BT, Spanagel R, Sommer WH. Losing Control: Excessive Alcohol Seeking after Selective Inactivation of Cue-Responsive Neurons in the Infralimbic Cortex. J Neurosci 35: 10750–10761, 2015. doi: 10.1523/JNEUROSCI.0684-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pisharath H, Rhee JM, Swanson MA, Leach SD, Parsons MJ. Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase. Mech Dev 124: 218–229, 2007. doi: 10.1016/j.mod.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Qiu MH, Chen MC, Fuller PM, Lu J. Stimulation of the Pontine Parabrachial Nucleus Promotes Wakefulness via Extra-thalamic Forebrain Circuit Nodes. Curr Biol 26: 2301–2312, 2016. doi: 10.1016/j.cub.2016.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Raper J, Morrison RD, Daniels JS, Howell L, Bachevalier J, Wichmann T, Galvan A. Metabolism and Distribution of Clozapine-N-oxide: Implications for Nonhuman Primate Chemogenetics. ACS Chem Neurosci 8: 1570–1576, 2017. doi: 10.1021/acschemneuro.7b00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Redfern CH, Coward P, Degtyarev MY, Lee EK, Kwa AT, Hennighausen L, Bujard H, Fishman GI, Conklin BR. Conditional expression and signaling of a specifically designed Gi-coupled receptor in transgenic mice. Nat Biotechnol 17: 165–169, 1999. doi: 10.1038/6165. [DOI] [PubMed] [Google Scholar]
- 153.Ren W, Centeno MV, Berger S, Wu Y, Na X, Liu X, Kondapalli J, Apkarian AV, Martina M, Surmeier DJ. The indirect pathway of the nucleus accumbens shell amplifies neuropathic pain. Nat Neurosci 19: 220–222, 2016. doi: 10.1038/nn.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Robinson S, Todd TP, Pasternak AR, Luikart BW, Skelton PD, Urban DJ, Bucci DJ. Chemogenetic silencing of neurons in retrosplenial cortex disrupts sensory preconditioning. J Neurosci 34: 10982–10988, 2014. doi: 10.1523/JNEUROSCI.1349-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Roman CW, Derkach VA, Palmiter RD. Genetically and functionally defined NTS to PBN brain circuits mediating anorexia. Nat Commun 7: 11905, 2016. doi: 10.1038/ncomms11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rosen ZB, Cheung S, Siegelbaum SA. Midbrain dopamine neurons bidirectionally regulate CA3-CA1 synaptic drive. Nat Neurosci 18: 1763–1771, 2015. doi: 10.1038/nn.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rosenbaum DM, Rasmussen SGF, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature 459: 356–363, 2009. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Roth BL. DREADDs for Neuroscientists. Neuron 89: 683–694, 2016. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Saloman JL, Scheff NN, Snyder LM, Ross SE, Davis BM, Gold MS. Gi-DREADD Expression in Peripheral Nerves Produces Ligand-Dependent Analgesia, as well as Ligand-Independent Functional Changes in Sensory Neurons. J Neurosci 36: 10769–10781, 2016. doi: 10.1523/JNEUROSCI.3480-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sando R III, Baumgaertel K, Pieraut S, Torabi-Rander N, Wandless TJ, Mayford M, Maximov A. Inducible control of gene expression with destabilized Cre. Nat Methods 10: 1085–1088, 2013. doi: 10.1038/nmeth.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sasaki K, Suzuki M, Mieda M, Tsujino N, Roth B, Sakurai T. Pharmacogenetic modulation of orexin neurons alters sleep/wakefulness states in mice. PLoS One 6: e20360, 2011. doi: 10.1371/journal.pone.0020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Saxena S, Roselli F, Singh K, Leptien K, Julien JP, Gros-Louis F, Caroni P. Neuroprotection through excitability and mTOR required in ALS motoneurons to delay disease and extend survival. Neuron 80: 80–96, 2013. doi: 10.1016/j.neuron.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 163.Scofield MD, Boger HA, Smith RJ, Li H, Haydon PG, Kalivas PW. Gq-DREADD Selectively Initiates Glial Glutamate Release and Inhibits Cue-induced Cocaine Seeking. Biol Psychiatry 78: 441–451, 2015. doi: 10.1016/j.biopsych.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shields BC, Kahuno E, Kim C, Apostolides PF, Brown J, Lindo S, Mensh BD, Dudman JT, Lavis LD, Tadross MR. Deconstructing behavioral neuropharmacology with cellular specificity. Science 356: eaaj2161, 2017. doi: 10.1126/science.aaj2161. [DOI] [PubMed] [Google Scholar]
- 165.Sikand P, Premkumar LS. Potentiation of glutamatergic synaptic transmission by protein kinase C-mediated sensitization of TRPV1 at the first sensory synapse. J Physiol 581: 631–647, 2007. doi: 10.1113/jphysiol.2006.118620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Silva BA, Mattucci C, Krzywkowski P, Cuozzo R, Carbonari L, Gross CT. The ventromedial hypothalamus mediates predator fear memory. Eur J Neurosci 43: 1431–1439, 2016. doi: 10.1111/ejn.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Simonds SE, Pryor JT, Ravussin E, Greenway FL, Dileone R, Allen AM, Bassi J, Elmquist JK, Keogh JM, Henning E, Myers MG Jr, Licinio J, Brown RD, Enriori PJ, O’Rahilly S, Sternson SM, Grove KL, Spanswick DC, Farooqi IS, Cowley MA. Leptin mediates the increase in blood pressure associated with obesity. Cell 159: 1404–1416, 2014. doi: 10.1016/j.cell.2014.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sine SM, Engel AG. Recent advances in Cys-loop receptor structure and function. Nature 440: 448–455, 2006. doi: 10.1038/nature04708. [DOI] [PubMed] [Google Scholar]
- 169.Sine SM, Wang HL, Hansen S, Taylor P. On the origin of ion selectivity in the Cys-loop receptor family. J Mol Neurosci 40: 70–76, 2010. doi: 10.1007/s12031-009-9260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Slimko EM, McKinney S, Anderson DJ, Davidson N, Lester HA. Selective electrical silencing of mammalian neurons in vitro by the use of invertebrate ligand-gated chloride channels. J Neurosci 22: 7373–7379, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Small KM, Brown KM, Forbes SL, Liggett SB. Modification of the beta 2-adrenergic receptor to engineer a receptor-effector complex for gene therapy. J Biol Chem 276: 31596–31601, 2001. doi: 10.1074/jbc.M102734200. [DOI] [PubMed] [Google Scholar]
- 172.Spencer DM, Wandless TJ, Schreiber SL, Crabtree GR. Controlling signal transduction with synthetic ligands. Science 262: 1019–1024, 1993. doi: 10.1126/science.7694365. [DOI] [PubMed] [Google Scholar]
- 173.Stachniak TJ, Ghosh A, Sternson SM. Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus→midbrain pathway for feeding behavior. Neuron 82: 797–808, 2014. doi: 10.1016/j.neuron.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Stamatakis AM, Jennings JH, Ung RL, Blair GA, Weinberg RJ, Neve RL, Boyce F, Mattis J, Ramakrishnan C, Deisseroth K, Stuber GD. A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron 80: 1039–1053, 2013. doi: 10.1016/j.neuron.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Stankunas K, Bayle JH, Gestwicki JE, Lin YM, Wandless TJ, Crabtree GR. Conditional protein alleles using knockin mice and a chemical inducer of dimerization. Mol Cell 12: 1615–1624, 2003. doi: 10.1016/S1097-2765(03)00491-X. [DOI] [PubMed] [Google Scholar]
- 176.Steculorum SM, Ruud J, Karakasilioti I, Backes H, Engström Ruud L, Timper K, Hess ME, Tsaousidou E, Mauer J, Vogt MC, Paeger L, Bremser S, Klein AC, Morgan DA, Frommolt P, Brinkkötter PT, Hammerschmidt P, Benzing T, Rahmouni K, Wunderlich FT, Kloppenburg P, Brüning JC. AgRP Neurons Control Systemic Insulin Sensitivity via Myostatin Expression in Brown Adipose Tissue. Cell 165: 125–138, 2016. doi: 10.1016/j.cell.2016.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sternson SM, Atasoy D. Agouti-related protein neuron circuits that regulate appetite. Neuroendocrinology 100: 95–102, 2014. doi: 10.1159/000369072. [DOI] [PubMed] [Google Scholar]
- 178.Sternson SM, Atasoy D, Betley JN, Henry FE, Xu S. An Emerging Technology Framework for the Neurobiology of Appetite. Cell Metab 23: 234–253, 2016. doi: 10.1016/j.cmet.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 179.Sternson SM, Eiselt AK. Three Pillars for the Neural Control of Appetite. Annu Rev Physiol 79: 401–423, 2017. doi: 10.1146/annurev-physiol-021115-104948. [DOI] [PubMed] [Google Scholar]
- 180.Sternson SM, Roth BL. Chemogenetic tools to interrogate brain functions. Annu Rev Neurosci 37: 387–407, 2014. doi: 10.1146/annurev-neuro-071013-014048. [DOI] [PubMed] [Google Scholar]
- 181.Strader CD, Fong TM, Tota MR, Underwood D, Dixon RA. Structure and function of G protein-coupled receptors. Annu Rev Biochem 63: 101–132, 1994. doi: 10.1146/annurev.bi.63.070194.000533. [DOI] [PubMed] [Google Scholar]
- 182.Strader CD, Gaffney T, Sugg EE, Candelore MR, Keys R, Patchett AA, Dixon RA. Allele-specific activation of genetically engineered receptors. J Biol Chem 266: 5–8, 1991. [PubMed] [Google Scholar]
- 183.Sutton AK, Pei H, Burnett KH, Myers MG Jr, Rhodes CJ, Olson DP. Control of food intake and energy expenditure by Nos1 neurons of the paraventricular hypothalamus. J Neurosci 34: 15306–15318, 2014. doi: 10.1523/JNEUROSCI.0226-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sweeney P, Qi Y, Xu Z, Yang Y. Activation of hypothalamic astrocytes suppresses feeding without altering emotional states. Glia 64: 2263–2273, 2016. doi: 10.1002/glia.23073. [DOI] [PubMed] [Google Scholar]
- 185.Sweeney P, Yang Y. An Inhibitory Septum to Lateral Hypothalamus Circuit That Suppresses Feeding. J Neurosci 36: 11185–11195, 2016. doi: 10.1523/JNEUROSCI.2042-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Szobota S, Gorostiza P, Del Bene F, Wyart C, Fortin DL, Kolstad KD, Tulyathan O, Volgraf M, Numano R, Aaron HL, Scott EK, Kramer RH, Flannery J, Baier H, Trauner D, Isacoff EY. Remote control of neuronal activity with a light-gated glutamate receptor. Neuron 54: 535–545, 2007. doi: 10.1016/j.neuron.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 187.Tervo DG, Hwang BY, Viswanathan S, Gaj T, Lavzin M, Ritola KD, Lindo S, Michael S, Kuleshova E, Ojala D, Huang CC, Gerfen CR, Schiller J, Dudman JT, Hantman AW, Looger LL, Schaffer DV, Karpova AY. A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons. Neuron 92: 372–382, 2016. doi: 10.1016/j.neuron.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Thompson AJ, Lester HA, Lummis SC. The structural basis of function in Cys-loop receptors. Q Rev Biophys 43: 449–499, 2010. doi: 10.1017/S0033583510000168. [DOI] [PubMed] [Google Scholar]
- 189.Tian L, Yang Y, Wysocki LM, Arnold AC, Hu A, Ravichandran B, Sternson SM, Looger LL, Lavis LD. Selective esterase-ester pair for targeting small molecules with cellular specificity. Proc Natl Acad Sci USA 109: 4756–4761, 2012. doi: 10.1073/pnas.1111943109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tochitsky I, Banghart MR, Mourot A, Yao JZ, Gaub B, Kramer RH, Trauner D. Optochemical control of genetically engineered neuronal nicotinic acetylcholine receptors. Nat Chem 4: 105–111, 2012. doi: 10.1038/nchem.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tohgo A, Pierce KL, Choy EW, Lefkowitz RJ, Luttrell LM. beta-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J Biol Chem 277: 9429–9436, 2002. doi: 10.1074/jbc.M106457200. [DOI] [PubMed] [Google Scholar]
- 192.Trailovic SM, Nedeljkovic JT. Central and peripheral neurotoxic effects of ivermectin in rats. J Vet Med Sci 73: 591–599, 2011. doi: 10.1292/jvms.10-0424. [DOI] [PubMed] [Google Scholar]
- 193.Vardy E, Robinson JE, Li C, Olsen RHJ, DiBerto JF, Giguere PM, Sassano FM, Huang XP, Zhu H, Urban DJ, White KL, Rittiner JE, Crowley NA, Pleil KE, Mazzone CM, Mosier PD, Song J, Kash TL, Malanga CJ, Krashes MJ, Roth BL. A New DREADD Facilitates the Multiplexed Chemogenetic Interrogation of Behavior. Neuron 86: 936–946, 2015. doi: 10.1016/j.neuron.2015.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Varela C, Weiss S, Meyer R, Halassa M, Biedenkapp J, Wilson MA, Goosens KA, Bendor D. Tracking the Time-Dependent Role of the Hippocampus in Memory Recall Using DREADDs. PLoS One 11: e0154374, 2016. doi: 10.1371/journal.pone.0154374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Vlasits AL, Bos R, Morrie RD, Fortuny C, Flannery JG, Feller MB, Rivlin-Etzion M. Visual stimulation switches the polarity of excitatory input to starburst amacrine cells. Neuron 83: 1172–1184, 2014. doi: 10.1016/j.neuron.2014.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wakaizumi K, Kondo T, Hamada Y, Narita M, Kawabe R, Narita H, Watanabe M, Kato S, Senba E, Kobayashi K, Kuzumaki N, Yamanaka A, Morisaki H, Narita M. Involvement of mesolimbic dopaminergic network in neuropathic pain relief by treadmill exercise: a study for specific neural control with Gi-DREADD in mice. Mol Pain 12: 1–11, 2016. doi: 10.1177/1744806916681567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wang XF, Liu JJ, Xia J, Liu J, Mirabella V, Pang ZP. Endogenous Glucagon-like Peptide-1 Suppresses High-Fat Food Intake by Reducing Synaptic Drive onto Mesolimbic Dopamine Neurons. Cell Reports 12: 726–733, 2015. doi: 10.1016/j.celrep.2015.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Warthen DM, Lambeth PS, Ottolini M, Shi Y, Barker BS, Gaykema RP, Newmyer BA, Joy-Gaba J, Ohmura Y, Perez-Reyes E, Güler AD, Patel MK, Scott MM. Activation of Pyramidal Neurons in Mouse Medial Prefrontal Cortex Enhances Food-Seeking Behavior While Reducing Impulsivity in the Absence of an Effect on Food Intake. Front Behav Neurosci 10: 63, 2016. doi: 10.3389/fnbeh.2016.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.White B, Osterwalder T, Keshishian H. Molecular genetic approaches to the targeted suppression of neuronal activity. Curr Biol 11: R1041–R1053, 2001. doi: 10.1016/S0960-9822(01)00621-2. [DOI] [PubMed] [Google Scholar]
- 200.Wu C-Y, Roybal KT, Puchner EM, Onuffer J, Lim WA. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science 350: aab4077, 2015. doi: 10.1126/science.aab4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wyart C, Del Bene F, Warp E, Scott EK, Trauner D, Baier H, Isacoff EY. Optogenetic dissection of a behavioural module in the vertebrate spinal cord. Nature 461: 407–410, 2009. doi: 10.1038/nature08323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yagi H, Tan W, Dillenburg-Pilla P, Armando S, Amornphimoltham P, Simaan M, Weigert R, Molinolo AA, Bouvier M, Gutkind JS. A synthetic biology approach reveals a CXCR4-G13-Rho signaling axis driving transendothelial migration of metastatic breast cancer cells. Sci Signal 4: ra60, 2011. doi: 10.1126/scisignal.2002221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yang L, Qi Y, Yang Y. Astrocytes control food intake by inhibiting AGRP neuron activity via adenosine A1 receptors. Cell Reports 11: 798–807, 2015. doi: 10.1016/j.celrep.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 204.Yang W, Rozamus LW, Narula S, Rollins CT, Yuan R, Andrade LJ, Ram MK, Phillips TB, van Schravendijk MR, Dalgarno D, Clackson T, Holt DA. Investigating protein-ligand interactions with a mutant FKBP possessing a designed specificity pocket. J Med Chem 43: 1135–1142, 2000. doi: 10.1021/jm9904396. [DOI] [PubMed] [Google Scholar]
- 205.Yang Y, Lee P, Sternson SM. Cell type-specific pharmacology of NMDA receptors using masked MK801. eLife 4: e10206, 2015. doi: 10.7554/eLife.10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yoshii T, Hosokawa H, Matsuo N. Pharmacogenetic reactivation of the original engram evokes an extinguished fear memory. Neuropharmacology 113, Pt A: 1–9, 2017. doi: 10.1016/j.neuropharm.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 207.Zemelman BV, Nesnas N, Lee GA, Miesenbock G. Photochemical gating of heterologous ion channels: remote control over genetically designated populations of neurons. Proc Natl Acad Sci USA 100: 1352–1357, 2003. doi: 10.1073/pnas.242738899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zemkova H, Tvrdonova V, Bhattacharya A, Jindrichova M. Allosteric modulation of ligand gated ion channels by ivermectin. Physiol Res 63, Suppl 1: S215–S224, 2014. [DOI] [PubMed] [Google Scholar]
- 209.Zhan C, Zhou J, Feng Q, Zhang JE, Lin S, Bao J, Wu P, Luo M. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J Neurosci 33: 3624–3632, 2013. doi: 10.1523/JNEUROSCI.2742-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhang H, Craciun LC, Mirshahi T, Rohács T, Lopes CMB, Jin T, Logothetis DE. PIP2 activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron 37: 963–975, 2003. doi: 10.1016/S0896-6273(03)00125-9. [DOI] [PubMed] [Google Scholar]
- 211.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell 115: 255–266, 2003. doi: 10.1016/S0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 212.Zhu H, Aryal DK, Olsen RH, Urban DJ, Swearingen A, Forbes S, Roth BL, Hochgeschwender U. Cre-dependent DREADD (Designer Receptors Exclusively Activated by Designer Drugs) mice. Genesis 54: 439–446, 2016. doi: 10.1002/dvg.22949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhu H, Pleil KE, Urban DJ, Moy SS, Kash TL, Roth BL. Chemogenetic inactivation of ventral hippocampal glutamatergic neurons disrupts consolidation of contextual fear memory. Neuropsychopharmacology 39: 1880–1892, 2014. doi: 10.1038/npp.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zingg B, Chou XL, Zhang ZG, Mesik L, Liang F, Tao HW, Zhang LI. AAV-Mediated Anterograde Transsynaptic Tagging: Mapping Corticocollicular Input-Defined Neural Pathways for Defense Behaviors. Neuron 93: 33–47, 2017. doi: 10.1016/j.neuron.2016.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]