Abstract
The carotid body chemoreceptors are activated during hypoglycemia and contribute to glucoregulation during prolonged exercise in dogs. Low-dose intravenous infusions of dopamine have been shown to blunt the activation of the carotid body chemoreceptors during hypoxia. Therefore, we tested the hypotheses that dopamine would blunt glucoregulatory responses and attenuate plasma glucose during prolonged aerobic exercise in healthy participants. Twelve healthy participants completed two randomized exercise sessions at 65% peak oxygen consumption for up to 120 min. Saline was infused during one exercise session, and dopamine (2 μg·kg−1·min−1) was infused during the other session. Arterial plasma glucose, growth hormone, glucagon, cortisol, norepinephrine, and epinephrine were measured every 10 min. Exercise duration during dopamine infusion was 107 ± 6 and 119 ± 0.8 min during saline infusion. Glucose area under the curve during exercise was lower during dopamine (9,821 ± 686 vs. 11,194 ± 395 arbitrary units; P = 0.016). The ratio of circulating growth hormone to glucose and the ratio of glucagon to glucose were greater during dopamine (P = 0.045 and 0.037, respectively). These results indicate that the infusion of dopamine during aerobic exercise impairs glucoregulation. This suggests that the carotid body chemoreceptors contribute to glucoregulation during prolonged exercise in healthy exercise-trained humans.
Keywords: epinephrine, norepinephrine, glucagon, cortisol, growth hormone
INTRODUCTION
The carotid body chemoreceptors are the primary oxygen sensors in the body (24). These receptors are also activated by a number of additional stimuli, including carbon dioxide, pH, hyperinsulinemia, heat, and hypoglycemia (40). Additionally, the carotid body chemoreceptors contribute to the glucoregulatory response to a hyperinsulinemic/hypoglycemic clamp in healthy humans (51). However, the use of insulin to induce hypoglycemia makes it difficult to discern if the carotid body chemoreceptors are being activated by low plasma glucose, elevated insulin, or both (20). In this context, prolonged high levels of exercise, which result in reduced levels of plasma glucose, may be a useful model to study the contribution of the carotid body chemoreceptors to the glucoregulatory response to hypoglycemia. For example, Koyama et al. found lower plasma glucose and blunted glucoregulatory hormone responses during 180 min of aerobic exercise in carotid body-resected dogs compared with control dogs (22). However, it is not known if the carotid body chemoreceptors contribute to the glucoregulatory response to exercise-induced hypoglycemia in humans.
Exogenous dopamine infusion inhibits carotid sinus nerve discharge (4, 5, 10, 18, 25, 29, 30) and reduces the ventilatory response to hypoxia (1, 2, 6, 13, 16, 35, 43, 46, 48–50, 52), a response mediated by the carotid body chemoreceptors. Hence, blunting the activation of the carotid body chemoreceptors during exercise-induced hypoglycemia will help us understand the role of carotid body chemoreceptors in glucoregulation. The purpose of this study was to determine the role of the carotid body chemoreceptors in glucoregulation during prolonged aerobic exercise by intravenously infusing low-dose dopamine. We hypothesized that the infusion of low-dose dopamine would blunt the glucoregulatory response during prolonged aerobic exercise. We also hypothesized that, when carotid body chemoreceptor activation was blunted with dopamine, we would observe lower plasma glucose values compared with saline infusion.
METHODS
The Mayo Clinic Institutional Review Board approved the study, which was performed in accordance with the standards set by the latest revision of the Declaration of Helsinki. All participants provided written informed consent before participation.
Participants
Twelve healthy participants (2 women, 33 ± 2 yr) free of any neurological, metabolic, cardiovascular, or respiratory disease participated in the study. All participants were nonobese (body mass index: 22 ± 1 kg/m2) and were not taking any medications, and women were confirmed to not be pregnant via a urine pregnancy test. All participants self-reported to be exercise trained and participate in competitive cycling events that last >150 min. Participants first performed a graded exercise test to determine peak oxygen uptake (V̇o2peak) followed by two randomized study visits. All testing was performed at least 7 days apart. For 3 days before each study visit, a registered dietician provided participants with weight-maintenance meals and snacks with a macronutrient content of 50% carbohydrates, 30% fat, and 20% protein with a caloric intake matched to self-reported physical activity. Participants stayed overnight in the Clinical Research Trial Unit (CRTU) of the Mayo Clinic Center for Translational Science Activities (CTSA) the night before each study visit, with the last meal before the study at ~1830 the night before the study. Participants were asked to perform 45–60 min of moderate-intensity aerobic exercise on the third and second day before each study visit. At 1730 the night before each study visit, participants performed 30 min of aerobic exercise at 65% of V̇o2peak in the CRTU. Intestinal temperature was obtained from seven participants who ingested a telemetry pill at ~2200 the night before each study visit (HQ, Palmetto, FL).
Graded Exercise Test Day
Each participant performed a graded exercise test on an electronically braked cycle ergometer (Excalibur Sport, Lode, Groningen, The Netherlands) to assess V̇o2peak within 30 days before the first study visit. Heart rate was monitored using a 12-lead ECG. Breath-by-breath ventilation and expired gases were measured and averaged every 30 s using a metabolic cart (Ultima CardiO2; MCG Diagnostics, St. Paul, MN). Arterial oxygen saturation was measured using a forehead pulse oximeter (Nellcor N-560; Covidien, Minneapolis, MN) and interfaced with the metabolic cart. Following 5 min of resting measurements, the exercise test was initiated at 100 W for women and 140 W for men, increased 30 or 40 W, respectively, every 3 min, and continued until volitional fatigue. V̇o2peak was defined as the highest attained V̇o2 averaged over a 30-s period.
Study Days
Instrumentation and monitoring.
At ~0700 the morning of the study days, a radial artery catheter was placed using local anesthesia (2% lidocaine) under aseptic techniques for blood pressure monitoring (FloTrac; Edwards Lifesciences) and blood sampling. A forearm intravenous catheter was placed under local anesthesia and using aseptic techniques. Dopamine or an equivolume of normal saline was infused intravenously at a rate of 2 μg·kg−1·min−1 throughout the study visit. Exogenously infused dopamine does not cross the blood-brain barrier (3, 31, 36). Heart rate was monitored using a 3-lead ECG (Cardiocap/5; Datex-Ohmeda, Louisville, CO), and blood oxygen saturation was monitored with a forehead pulse oximeter and interfaced with the metabolic cart. Breath-by-breath ventilation, V̇o2, carbon dioxide production (V̇co2), and the pressure of end-tidal carbon dioxide () were averaged every 30 s using the metabolic cart.
Blood sampling.
Arterial blood was immediately placed on ice and centrifuged at 4°C, and plasma was stored at −80°C until analysis. Plasma was analyzed for glucose, growth hormone, cortisol, glucagon, epinephrine, norepinephrine, and lactate using standard techniques employed in the CRTU of the Mayo Clinic CTSA as previously described (51).
Experimental protocol.
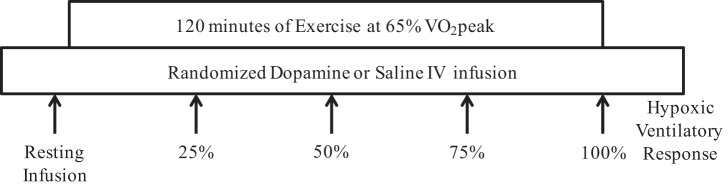
Figure 1 outlines the timeline during the study visits. Baseline blood samples were collected and hemodynamic and ventilatory measurements were performed for 5 min starting at ~0800 with participants in the semirecumbent position. After baseline measurements, the infusion of dopamine or saline commenced. Study visits were randomized, and participants and study staff were blinded to the infusion condition. Fifteen minutes after the dopamine or saline infusion was initiated, another set of resting measurements was made for 5 min. Next, participants were transitioned to the cycle ergometer, and the exercise protocol commenced. Participants exercised at a power output that elicited 65% V̇o2peak for up to 120 min or until volitional fatigue. This exercise stimulus produces consistent plasma glucose responses in exercise-trained participants across multiple study visits (11). Every 10 min blood samples were collected, and ratings of perceived exertion [RPE; 6–20 point scale (7)] were obtained during exercise. Hemodynamic and ventilatory measurements were made for 4 min (starting at minute 8) every 10 min. Participants breathed through the mouth piece only during the period of ventilatory measurements and were allowed to remove the mouth piece when ventilatory data were not collected. Participants drank water ad libitum. If a participant was unable to maintain a pedaling rate of 40 revolutions/min, reported a rating of perceived exertion >17, or wanted to discontinue exercise, the session was terminated. If a participant was unable to complete 120 min of exercise, hemodynamic and ventilatory measurements were made for 2 min, and blood samples were collected immediately before exercise termination.
Fig. 1.
Study visit timeline. After instrumentation, the continuous infusion of either 2 μg·kg−1·min−1 of intravenous dopamine or volume-matched saline commenced. Resting infusion measurements were performed 15 min after the initiation of infusion. After resting infusion measurements, participants cycled on an electronically braked cycle ergometer at 65% peak oxygen uptake (V̇o2peak) for 120 min or until volitional fatigue. Blood draws and respiratory, hemodynamic, and perceptual measurements were performed every 10 min during exercise. After the end of exercise (15 min), a hypoxic ventilatory response assessment was performed during exercise at 30 W. The infusion of dopamine or saline was discontinued after the hypoxic ventilatory response test.
Postexercise hypoxic ventilatory response.
Fifteen minutes following exercise, a hypoxic ventilatory response test was performed while participants exercised on the cycle ergometer at 30 W to assess carotid body chemosensitivity. The hypoxic ventilatory response consisted of 3 min breathing room air, 3 min breathing 15% oxygen (balance nitrogen), and 3 min breathing 10% oxygen (balance nitrogen). During the hypoxic ventilatory response test, small amounts of 5% carbon dioxide were added to the inspirate to avoid a large drop in . The hypoxic ventilatory response was terminated early if arterial oxygen saturation fell below 75% or if participants experienced dizziness or nausea. The arterial oxygen saturation and ventilatory values over the last 60 s of each stage were plotted against each other, and the slope of the linear regression line was used as an index of carotid body chemosensitivity (32). Additionally, because the carotid body chemoreceptors increase heart rate and blood pressure when activated, the heart rate and mean arterial pressure values over the last 60 s of each stage were plotted against arterial oxygen saturation. The slopes of the linear regression lines between heart rate and arterial oxygen saturation and between mean arterial pressure and arterial oxygen saturation were used as indexes of carotid body chemosensitivity. Participants who were experiencing postexercise hypotension or felt ill following exercise (n = 4) did not perform the hypoxic ventilatory response test.
Data analyses.
Heart rate and blood pressure values were collected at 1,000 Hz and analyzed using data acquisition software (ADinstruments, Colorado Springs, CO). Resting hemodynamic and ventilatory measurements were averaged over the 5-min data collection period. During exercise, hemodynamic and ventilatory measurements were averaged over the 4-min data collection period. As a measure of the volume of the responses, the area under the curve for each blood variable was calculated during exercise from the values obtained at 25, 50, 75, and 100% of total exercise duration. To determine if there was a disproportionate hormonal response to plasma glucose between saline and dopamine, we calculated the ratio of the glucoregulatory hormones to plasma glucose values.
Statistical analyses.
One participant was not able to complete 120 min of exercise during saline, and four were unable to complete the entire dopamine session. Therefore, the change from baseline data were placed in time bins that were based on the percentage of total exercise time completed (i.e., 25, 50, 75, and 100% of time completed). These data were analyzed using repeated-measures ANOVA, and baseline data were analyzed using paired t-tests. If a significant interaction or main effect was observed, the Holm-Sidak procedure for multiple comparisons was used to determine where differences existed. Paired t-tests were used to determine if differences existed between conditions for the hypoxic ventilatory responses and area under the curve for each blood variable. Data are presented as means ± SE, and P values are reported.
RESULTS
The mean peak V̇o2 during the graded exercise test was 54 ± 2 ml·kg−1·min−1. Oxygen consumption was not different between conditions during exercise (saline: 64.7 ± 1.0% V̇o2peak vs. dopamine: 64.1 ± 0.9% V̇o2peak; P = 0.534). Of the 12 participants, 11 completed the 120 min of exercise during saline infusion, and 8 completed 120 min of exercise during dopamine infusion. Exercise duration during dopamine infusion was 107 ± 6 and 119 ± 0.8 min during saline infusion. Baseline metabolic, ventilatory, and hemodynamic data are presented in Table 1. Changes from baseline data during exercise for metabolic, ventilatory, and hemodynamic variables and RPE data are presented in Table 2.
Table 1.
Baseline metabolic, ventilatory, and hemodynamic variables during intravenous infusion of saline and dopamine
| n | Saline | Dopamine | P Value | |
|---|---|---|---|---|
| Glucose, mg/dl | 12 | 101 ± 3 | 103 ± 2 | 0.740 |
| Growth hormone, ng/ml | 12 | 2.9 ± 0.9 | 5.2 ± 1.3 | 0.056 |
| Glucagon, pg/ml | 12 | 77 ± 7 | 83 ± 7 | 0.413 |
| Cortisol, µg/ml | 12 | 14.8 ± 2.1 | 12.4 ± 1.2 | 0.314 |
| Insulin, µU/ml | 10 | 6.6 ± 1.0 | 7.1 ± 0.8 | 0.715 |
| Norepinephrine, pg/ml | 11 | 297 ± 50 | 324 ± 68 | 0.582 |
| Epinephrine, pg/ml | 12 | 116 ± 28 | 121 ± 26 | 0.669 |
| Free glycerol, mg/dl | 10 | 8.3 ± 0.8 | 8.7 ± 0.8 | 0.664 |
| Lactate, mmol/l | 12 | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.054 |
| Respiratory exchange ratio | 11 | 0.83 ± 0.03 | 0.78 ± 0.03 | 0.129 |
| Ventilation, l/min | 11 | 9.6 ± 1.3 | 9.1 ± 1.0 | 0.334 |
| V̇e/V̇co2 | 11 | 36.9 ± 1.8 | 35.7 ± 1.2 | 0.430 |
| Oxygen consumption, ml·kg−1·min−1 | 11 | 4.6 ± 0.5 | 4.3 ± 0.4 | 0.419 |
| , mmHg | 11 | 39 ± 1 | 40 ± 1 | 0.248 |
| Intestinal temperature, °C | 7 | 36.9 ± 0.1 | 37.0 ± 0.1 | 0.386 |
| Heart rate, beats/min | 12 | 63 ± 3 | 65 ± 3 | 0.398 |
| Mean arterial pressure, mmHg | 12 | 92 ± 3 | 90 ± 3 | 0.325 |
Values are means ± SE; n, no. of experiments. V̇e/V̇co2, ventilatory equivalent for carbon dioxide production; , partial pressure of end-tidal carbon dioxide.
Table 2.
Change from baseline data for metabolic, ventilatory, and hemodynamic variables and perceptual responses during prolonged exercise with saline and dopamine intravenous infusions
| n | 25% |
50% |
75% |
100% |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Saline | Dopamine | Saline | Dopamine | Saline | Dopamine | Saline | Dopamine | ||
| Insulin, µU/ml | 10 | −3.6 ± 0.9 | −4.1 ± 0.8 | −4.5 ± 1.0 | −5.0 ± 0.7 | −5.3 ± 1.0 | −5.9 ± 0.8 | −6.0 ± 1.0 | −6.4 ± 0.8 |
| Free glycerol, mg/dl | 10 | 16.0 ± 1.9 | 14.8 ± 2.0 | 25.9 ± 2.8 | 26.0 ± 3.4 | 34.5 ± 2.8 | 34.5 ± 3.4 | 43.3 ± 2.4 | 42.7 ± 3.8 |
| Lactate, mmol/l | 12 | 0.8 ± 0.2 | 0.8 ± 0.1 | 0.9 ± 0.2 | 0.8 ± 0.1 | 1.0 ± 0.4 | 1.0 ± 0.2 | 1.4 ± 0.5 | 1.5 ± 0.2 |
| Respiratory exchange ratio† | 11 | 0.04 ± 0.03 | 0.13 ± 0.04 | 0.02 ± 0.03 | 0.11 ± 0.04 | 0.02 ± 0.03 | 0.10 ± 0.04 | 0.02 ± 0.03 | 0.10 ± 0.04 |
| Ventilation, l/min | 11 | 49 ± 2 | 52 ± 4 | 51 ± 3 | 56 ± 5 | 54 ± 3 | 60 ± 7 | 58 ± 4 | 65 ± 8 |
| V̇e/V̇co2 | 11 | −9.1 ± 1.1 | −9.1 ± 0.9 | −7.9 ± 1.2 | −6.5 ± 0.9 | −6.2 ± 1.1 | −4.5 ± 1.8 | −4.5 ± 1.8 | −1.8 ± 3.0 |
| Oxygen consumption, %peak | 11 | 63.7 ± 1.1 | 63.6 ± 1.5 | 64.7 ± 1.1 | 64.2 ± 1.8 | 64.8 ± 1.1 | 64.4 ± 1.6 | 65.5 ± 1.1 | 64.2 ± 1.8 |
| , mmHg | 11 | 3 ± 1 | 4 ± 1 | 1 ± 1 | 1 ± 1 | 0 ± 1 | −1 ± 1 | −1 ± 1 | −3 ± 1* |
| Intestinal temperature, °C | 7 | 1.2 ± 0.2 | 1.0 ± 0.1 | 1.5 ± 0.2 | 1.4 ± 0.2 | 1.6 ± 0.2 | 1.4 ± 0.2 | 1.6 ± 0.2 | 1.4 ± 0.2 |
| Heart rate, beats/min | 12 | 76 ± 4 | 78 ± 3 | 81 ± 4 | 83 ± 3 | 84 ± 4 | 85 ± 3 | 89 ± 4 | 89 ± 3 |
| Mean arterial pressure, mmHg | 12 | 0 ± 2 | 5 ± 3 | −3 ± 2 | 2 ± 4 | −1 ± 2 | 1 ± 4 | −1 ± 3 | 3 ± 4 |
| RPE | 12 | 11 ± 1 | 11 ± 1 | 13 ± 1 | 13 ± 1 | 14 ± 1 | 14 ± 1 | 15 ± 1 | 16 ± 1 |
Values are means ± SE; n, no. of experiments. Data are for 25, 50, 75, and 100% of total exercise time. RPE, rating of perceived exertion. All values are change from baseline except oxygen consumption and RPE.
P < 0.05 vs. saline.
Condition main effect, P < 0.05.
Hypoxic Ventilatory Response
Eight participants completed the hypoxic ventilatory response assessments following exercise during both saline and dopamine infusion conditions. Arterial oxygen saturation was not different between conditions during normoxia (saline: 97 ± 1% vs. dopamine: 97 ± 1%; P = 0.835) or during each level of hypoxia (16% O2: saline: 91 ± 1% vs. dopamine: 90 ± 1%; P = 0.755) (10% O2: saline: 84 ± 1% vs. dopamine: 84 ± 1%; P = 0.835). The hypoxic ventilatory response was statistically indistinguishable between conditions (saline: −1.25 ± 0.28 vs. dopamine: −0.93 ± 0.18 l·min−1·%SaO2−1; P = 0.165). However, five of eight participants had an attenuated hypoxic ventilatory response during dopamine infusion. There were no differences between conditions in the heart rate response (saline: −1.46 ± 0.13 vs. dopamine: −1.22 ± 0.23 beats·min−1·%SaO2−1; P = 0.222) or mean arterial pressure response (saline: −0.29 ± 0.11 vs. dopamine: −0.17 ± 0.13 mmHg/%SaO2; P = 0.231) to hypoxia. Three participants had lower heart rate responses to hypoxia, and four participants had lower mean arterial pressure responses to hypoxia during dopamine infusion. Seven of the eight participants had at least one marker of carotid body chemosensitivity that was attenuated during dopamine infusion.
Glucose
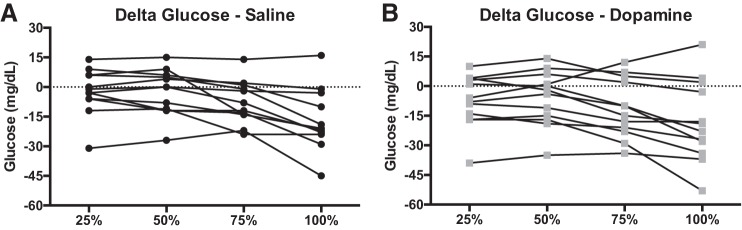
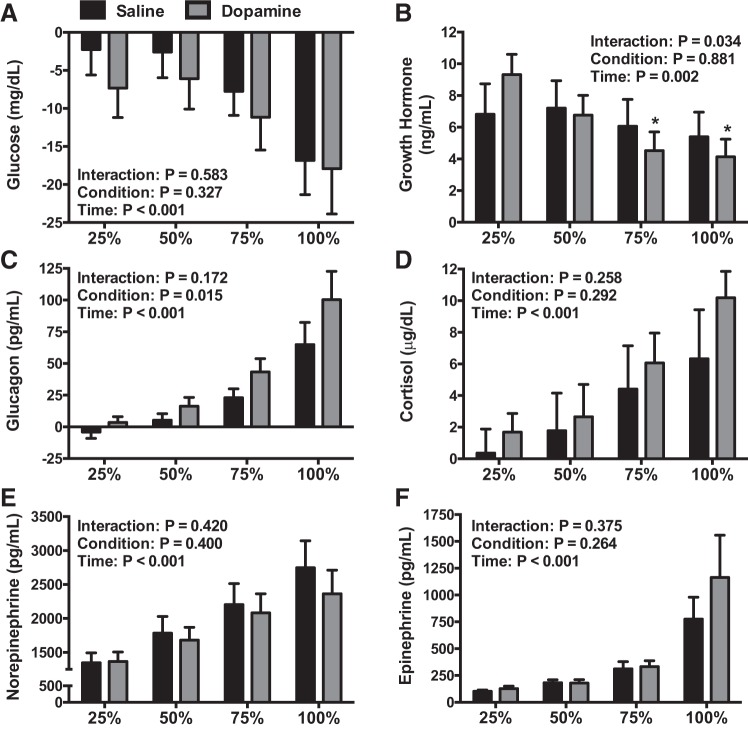
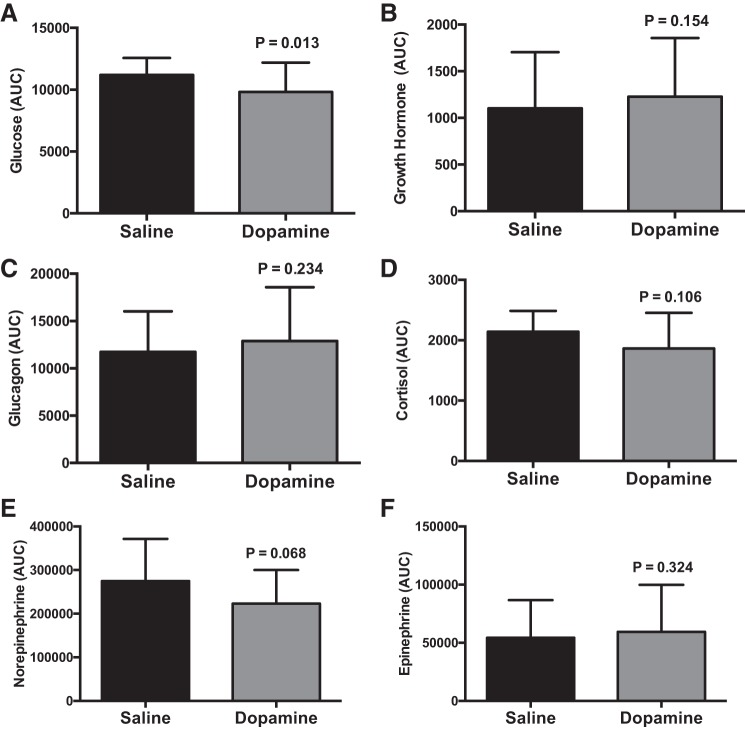
Data are presented in Figs. 2, 3, and 4. Glucose progressively fell throughout exercise (P < 0.001), but there were no differences between saline and dopamine during exercise (P = 0.327) (Fig. 3A). The glucose area under the curve during dopamine, which represents the cumulative glucose response during exercise, was 13 ± 5% lower than saline infusion (P = 0.016) (Fig. 4A). Participants who had a lower hypoxic ventilatory response during dopamine (n = 5) had a 16 ± 12% lower glucose area under the curve during dopamine vs. saline. Participants who had a greater hypoxic ventilatory response during dopamine (n = 3) had a 1 ± 3% lower glucose area under the curve during dopamine vs. saline.
Fig. 2.
Individual (n = 12) plasma glucose responses as a change from resting infusion during saline (A) and dopamine (B) infusion. Eight of 12 participants had lower plasma glucose values at exercise termination during dopamine infusion.
Fig. 3.
The change from resting infusion for plasma glucose (n = 12, A), growth hormone (n = 12, B), glucagon (n = 12, C), cortisol (n = 12, D), norepinephrine (n = 11, E), and epinephrine (n = 12, F) during each study visit. Data were analyzed using repeated-measures ANOVA followed by the Holm-Sidak procedure for multiple comparisons if a significant interaction or main effect was found. *Different from saline (P < 0.050). Values are means ± SE.
Fig. 4.
Area under the exercise curve values for blood glucose (n = 12, A), growth hormone (n = 12, B), glucagon (n = 12, C), cortisol (n = 12, D), norepinephrine (n = 11, E), and epinephrine (n = 12, F) during each study visit. Data were analyzed using paired t-tests. Values are means ± SE.
Glucoregulatory Hormones
Data are shown in Figs. 3 and 4. Growth hormone values were lower during dopamine at 75% (P < 0.001) and 100% (P < 0.001) compared with 25% of exercise time (Fig. 3B). The area under curve for growth hormone was statistically indistinguishable between conditions (P = 0.154) (Fig. 4B). During exercise, glucagon was greater during dopamine infusion (P = 0.015) (Fig. 3C); however, the area under the curve was not different between dopamine and saline (P = 0.234) (Fig. 4C). Circulating cortisol (P < 0.001), norepinephrine (P < 0.001), and epinephrine (P < 0.001) concentrations increased during exercise but were not different between infusion conditions (P = 0.292, 0.400, and 0.264, respectively) (Fig. 3, D–F). Area under the curve values for cortisol (P = 0.106), norepinephrine (P = 0.068), and epinephrine (P = 0.324) were statistically indistinguishable between conditions (Fig. 4, D–F).
Glucoregulatory Hormone: Plasma Glucose Ratios
We calculated the ratio of glucoregulatory hormones to plasma glucose to determine if there was a disproportionate hormonal response to glucose values between conditions. These ratios are presented in Fig. 5. The growth hormone-to-glucose ratio was greater during dopamine (P = 0.045), but there were no changes during exercise (P = 0.202) (Fig. 5A). The ratio of glucagon to plasma glucose was greater during dopamine infusion (P = 0.037) and increased during exercise in both conditions (P < 0.001) (Fig. 5B). The cortisol-to-glucose ratio (P = 0.931) (Fig. 5C), norepinephrine-to-glucose ratio (P = 0.907) (Fig. 5D), and epinephrine-to-glucose ratio (P = 0.242) (Fig. 5E) was not different between conditions, but each of these ratios increased during exercise (P < 0.001 for all).
Fig. 5.
Ratio of growth hormone (n = 12, A), glucagon (n = 12, B), cortisol (n = 12, C), norepinephrine (n = 11, D), and epinephrine (n = 12, E) to blood glucose during each study visit. Data were analyzed using repeated-measures ANOVA. There were no statistically significant differences between saline and dopamine infusions. Values are means ± SE.
DISCUSSION
We found that the administration of dopamine decreases the cumulative plasma glucose response to prolonged aerobic exercise, despite the increase in certain glucoregulatory hormones. These results indicate that the carotid body chemoreceptors or other organs with dopamine receptors (e.g., pancreas or pituitary gland) contribute to the complex regulation of plasma glucose during prolonged aerobic exercise in exercise-trained participants.
After exercise, we found that at least one measure of carotid body chemosensitivity was attenuated in seven of eight participants during dopamine infusion, that is, some participants had attenuated ventilatory responses to hypoxia during dopamine infusion without a corresponding decrease in hemodynamic responses and vice versa. Although measuring the ventilatory responses to acute hypoxia is the most common method to describe carotid body chemosensitivity, assessing the hemodynamic responses have also recently been proposed as markers of carotid body chemosensitivity (26, 34, 35, 39). In this context, it has been postulated that the carotid body chemoreceptors are comprised of type I glomus cells that initiate either ventilatory or hemodynamic responses upon activation (38). Therefore, we speculate that the exogenous infusion of dopamine could have resulted in a nonuniform distribution throughout the perfused carotid bodies and lead to a heterogeneous inhibitory response. While we were unable to perform hypoxic ventilatory response assessments in all participants during both infusion conditions, the collective ventilatory, heart rate, and blood pressure responses to hypoxia suggest that carotid body chemosensitivity was attenuated during dopamine infusion in a majority of our participants.
We observed a 13% reduction in the cumulative plasma glucose response (represented by the glucose area under the curve) during dopamine infusion vs. saline. This indicates that dopamine either attenuated the rate of glucose appearance or increased the rate of glucose disposal during exercise. Similarly, breathing hyperoxia during aerobic exercise reduces the rate of glucose appearance and disappearance as well as circulating catecholamines versus hypoxia breathing in healthy participants (12). Furthermore, breathing hyperoxia during aerobic exercise reduces circulating catecholamines compared with room air breathing in healthy participants (42). The effect of breathing hyperoxia on glucose regulation appears to be greater than the infusion of dopamine. However, this difference might be because of changes in intracellular metabolism observed during hyperoxia breathing (42).
Circulating glucose is 6% lower during aerobic exercise in carotid body-denervated dogs versus predenervation (22). The discrepancy between our data and the animal data is currently unclear. Chemosensitivity in carotid body-denervated animals improves over time (15, 17), whereas our participants were acutely exposed to low-dose dopamine. The carotid body-denervated dogs were >16 days from denervation, and it is possible that they relied on heightened chemosensitivity by other chemoreceptor regions to improve glucose regulation. Therefore, the relatively abrupt administration of dopamine in humans appears to have a greater impact on aerobic exercise glucoregulation vs. carotid body denervation in animals.
Carotid body denervation removes all carotid body afferent signals to the brain (22, 23), whereas exogenous dopamine suppresses carotid body activation of the carotid sinus nerve. Therefore, the physical ability of the carotid body chemoreceptors to transmit afferent nerve signals was still intact during dopamine infusion. It is likely that the reduction in blood glucose during exercise with dopamine was sensed by the carotid body chemoreceptors, but dopamine was unable to fully blunt afferent signals. For instance, carotid body resection in humans results in little to no ventilatory response to hypoxia (28, 34), but increases in ventilation during hypoxia are still observed during dopamine infusion (1, 2, 6, 13, 16, 26, 35, 43, 46, 48, 49, 52). Therefore, our data might underestimate the contribution of the carotid body chemoreceptors to glucoregulation during prolonged aerobic exercise in humans, or the exogenous infusion of dopamine might have influenced other chemosensitive regions or altered metabolism and/or glucose delivery. For instance, blood flow to skeletal working muscles increases and a reduction in sympathetic constraint are observed when the carotid body chemoreceptors are inhibited in healthy participants performing handgrip exercise (14, 44). If these blood flow and sympathetic responses are consistent with prolonged cycle ergometer exercise during dopamine infusion, glucose delivery and glucose disposal might have been enhanced during our study. We observed a trend for lower norepinephrine area under the curve response during dopamine infusion, which suggests that sympathetic restraint to blood flow directed toward working skeletal muscle might have been attenuated.
We observed a small increase in the respiratory exchange ratio during dopamine infusion, which indicates an increase in glucose metabolism. The increase in glucose metabolism could account for a rise in the rate of glucose disposal and the attenuated cumulative glucose response during dopamine infusion. However, the increase in the respiratory exchange ratio was small and likely does not reflect a shift in cellular metabolism that was sufficient enough to elicit the changes we observed in plasma glucose during dopamine infusion. This is supported by our lactate and free glycerol concentrations during exercise, which were both not different between conditions.
In contrast to the previous study conducted on denervated dogs that found an attenuated hormonal response during exercise (22), we found that growth hormone and glucagon were disproportionately elevated during dopamine (Fig. 5, A and B). This suggests that dopamine attenuated the rate of glucose appearance or influenced hormonal feedback mechanisms to allow growth hormone and glucagon to be augmented. The mechanisms that underpin these possible responses during exercise are currently not clear. Previous evidence indicates that an exogenous dopamine infusion of 4 μg·kg−1·min−1 can increase circulating growth hormone during resting conditions (47). Our resting baseline data extend the previous findings to include that infusing 2 μg·kg−1·min−1 of dopamine increases circulating growth hormone. This is most likely because of inhibition of somatostatin secretion by the hypothalamus (47). It is currently not clear how the elevated release of growth hormone during dopamine infusion does not also raise plasma glucose values at rest (Table 1). We speculate that the initial increase in growth hormone during dopamine could have influenced subsequent growth hormone release during the later portions of exercise (Fig. 3B), since circulating growth hormone inhibits growth hormone secretion (37, 45).
Dopamine receptors are located on the pancreas (41, 53) and, when activated, inhibit the release of insulin (41), which might allow for an increase in glucagon secretion (21). However, insulin was not different between saline and dopamine conditions; therefore, this mechanism of glucagon release is unlikely. How the exogenous infusion of dopamine increases circulating glucagon without a corresponding increase in plasma glucose during exercise warrants further investigation.
Peak oxygen consumption and exercise time during graded exercise tests are increased in heart failure patients when the carotid body chemoreceptors are inhibited using hyperoxia (8, 9, 33). If V̇o2peak was elevated during dopamine infusion in our study, the relative exercise intensity would have been lower, which could have influenced glucose metabolism and exercise duration. Although we did not assess V̇o2peak during dopamine infusion, Janssen et al. found that peak oxygen uptake and power output were not influenced by low-dose dopamine infusion in healthy participants (19). Therefore, it is unlikely that V̇o2peak increased during dopamine infusion. Hence, it is also doubtful that our participants exercised at a lower relative exercise intensity during dopamine.
Experimental Considerations
There are several experimental considerations that are pertinent to the interpretation of our results. First, we achieved end-exercise plasma glucose values <70 mg/dl in only three participants (all during the dopamine condition). A longer exercise duration and/or greater exercise intensity could have exacerbated the fall in plasma glucose in both conditions. Second, although plasma glucose was attenuated during prolonged exercise with dopamine infusion, the intersubject variability was large, and 8 of the 12 participants we tested had a greater decrease in plasma glucose at the end of exercise during dopamine infusion. Similarly, not all of the participants had an attenuated hypoxic ventilatory response during low-intensity exercise with dopamine. Therefore, it is possible that the dose of dopamine we used was inadequate to completely blunt carotid body activation during exercise. We previously demonstrated a large intersubject variability in the hypoxic ventilatory response to a range of dopamine doses (26). However, most participants had the greatest attenuation of the hypoxic ventilatory response when we infused dopamine at 2 μg·kg−1·min−1 (26). Identifying the dose of dopamine that maximally blunts carotid body chemoreceptor activation within each participant might have led to a more robust and consistent reduction in end-exercise plasma glucose values. Third, plasma glucose values at baseline were normal, despite a controlled dietary intake and exercise regimen in the 3 days preceding each study visit. A further restriction in carbohydrate intake and an increase in exercise intensity and/or duration in the days before the exercise session could have led to more rapid decrease in plasma glucose during exercise. Fourth, the carotid body chemoreceptors are also stimulated by increases in lactate, epinephrine, angiotensin II, and potassium, and decreases in pH (24), all of which occur during intense aerobic exercise. Therefore, we cannot determine if dopamine exclusively blunted the response to low circulating glucose or if dopamine also attenuated the response to the increase in other circulating activators of the carotid body chemoreceptors during exercise. Finally, we did not include a study visit that examined the influence of 120 min of low-dose dopamine infusion on glucoregulation and other carotid body chemoreceptor stimuli during a nonexercise condition. This approach might have provided additional information regarding some of the confounding effects of low-dose dopamine that we observed, such as the increase in growth hormone, since dopamine has been shown to inhibit hypothalamic somatostatin secretion (47).
Conclusions
Although the carotid body chemoreceptors are not the primary glucosensors or the primary initiator of the glucoregulatory hormone response, our results do indicate that plasma glucose and secretion of certain glucoregulatory hormones are attenuated when low-dose dopamine has been exogenously administered during prolonged aerobic exercise in trained participants. Therefore, it appears as though the carotid body chemoreceptors, or other possible glucoregulatory organs with dopamine receptors, contribute to glucoregulation during prolonged aerobic exercise in trained individuals.
Perspectives and Significance
The control of blood glucose relies on complex feedback mechanisms that control the secretion of glucoregulatory hormones. The finding that the carotid body chemoreceptors contribute to glucoregulation during prolonged exercise is important because exercise-induced glucose reductions do not rely on the infusion of insulin. Therefore, exercise represents a natural glucose challenge that is analogous to hypoglycemia unawareness because participants typically do not perceive changes in blood glucose during exercise.
GRANTS
Support for this study was provided by National Institutes of Health Grants DK-090541 (M. J. Joyner), NS-32352 (M. J. Joyner), and 1UL1-RR-024150 (Mayo Clinic Center for Translational Science Activities, M. J. Joyner, American Heart Association Midwest Affiliate Grant 13POST-14380027 (B. D. Johnson), and Mobility Grant Abroad “José Castillejo” for Young PhD CAS14/00239 (A. B. Peinado).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.D.J., T.B.C., and M.J.J. conceived and designed research; B.D.J., A.B.P., S.M.R., T.B.C., and M.J.J. performed experiments; B.D.J. analyzed data; B.D.J., A.B.P., S.M.R., T.B.C., and M.J.J. interpreted results of experiments; B.D.J. prepared figures; B.D.J. drafted manuscript; B.D.J., A.B.P., S.M.R., T.B.C., and M.J.J. edited and revised manuscript; B.D.J., A.B.P., S.M.R., T.B.C., and M.J.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the participants for their time and effort. We also thank Pamela Engrav, Luke J. Matzek, Shelly K. Roberts, Jennifer L. Taylor, and Sarah C. Wolhart for assistance with recruitment and data collection.
REFERENCES
- 1.Bainbridge CW, Heistad DD. Effect of haloperidol on ventilatory responses to dopamine in man. J Pharmacol Exp Ther 213: 13–17, 1980. [PubMed] [Google Scholar]
- 2.Bascom DA, Clement ID, Dorrington KL, Robbins PA. Effects of dopamine and domperidone on ventilation during isocapnic hypoxia in humans. Respir Physiol 85: 319–328, 1991. doi: 10.1016/0034-5687(91)90071-P. [DOI] [PubMed] [Google Scholar]
- 3.Bertler A, Falck B, Owman C, Rosengrenn E. The localization of monoaminergic blood-brain barrier mechanisms. Pharmacol Rev 18: 369–385, 1966. [PubMed] [Google Scholar]
- 4.Bisgard GE, Forster HV, Klein JP, Manohar M, Bullard VA. Depression of ventilation by dopamine in goats–effects of carotid body excision. Respir Physiol 40: 379–392, 1980. doi: 10.1016/0034-5687(80)90036-5. [DOI] [PubMed] [Google Scholar]
- 5.Black AM, Comroe JH Jr, Jacobs L. Species difference in carotid body response of cat and dog to dopamine and serotonin. Am J Physiol 223: 1097–1102, 1972. [DOI] [PubMed] [Google Scholar]
- 6.Boetger CL, Ward DS. Effect of dopamine on transient ventilatory response to exercise. J Appl Physiol (1985) 61: 2102–2107, 1986. [DOI] [PubMed] [Google Scholar]
- 7.Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics, 1998. [Google Scholar]
- 8.Chua TP, Harrington D, Ponikowski P, Webb-Peploe K, Poole-Wilson PA, Coats AJS. Effects of dihydrocodeine on chemosensitivity and exercise tolerance in patients with chronic heart failure. J Am Coll Cardiol 29: 147–152, 1997. doi: 10.1016/S0735-1097(96)00446-9. [DOI] [PubMed] [Google Scholar]
- 9.Chua TP, Ponikowski PP, Harrington D, Chambers J, Coats AJ. Contribution of peripheral chemoreceptors to ventilation and the effects of their suppression on exercise tolerance in chronic heart failure. Heart 76: 483–489, 1996. doi: 10.1136/hrt.76.6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciarka A, Vincent J-L, van de Borne P. The effects of dopamine on the respiratory system: friend or foe? Pulm Pharmacol Ther 20: 607–615, 2007. doi: 10.1016/j.pupt.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Coggan AR, Coyle EF. Reversal of fatigue during prolonged exercise by carbohydrate infusion or ingestion. J Appl Physiol (1985) 63: 2388–2395, 1987. [DOI] [PubMed] [Google Scholar]
- 12.Cooper DM, Wasserman DH, Vranic M, Wasserman K. Glucose turnover in response to exercise during high- and low- breathing in man. Am J Physiol Endocrinol Metab 251: E209–E214, 1986. [DOI] [PubMed] [Google Scholar]
- 13.Dahan A, Ward D, van den Elsen M, Temp J, Berkenbosch A. Influence of reduced carotid body drive during sustained hypoxia on hypoxic depression of ventilation in humans. J Appl Physiol (1985) 81: 565–572, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Edgell H, Stickland MK. Activation of the carotid chemoreflex secondary to muscle metaboreflex stimulation in men. Am J Physiol Regul Integr Comp Physiol 306: R693–R700, 2014. doi: 10.1152/ajpregu.00472.2013. [DOI] [PubMed] [Google Scholar]
- 15.Forster HV. Plasticity in the control of breathing following sensory denervation. J Appl Physiol (1985) 94: 784–794, 2003. doi: 10.1152/japplphysiol.00602.2002. [DOI] [PubMed] [Google Scholar]
- 16.Henson LC, Ward DS, Whipp BJ. Effect of dopamine on ventilatory response to incremental exercise in man. Respir Physiol 89: 209–224, 1992. doi: 10.1016/0034-5687(92)90051-W. [DOI] [PubMed] [Google Scholar]
- 17.Hodges MR, Forster HV. Respiratory neuroplasticity following carotid body denervation: central and peripheral adaptations. Neural Regen Res 7: 1073–1079, 2012. doi: 10.3969/j.issn.1673-5374.2012.14.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ide T, Shirahata M, Chou CL, Fitzgerald RS. Effects of a continuous infusion of dopamine on the ventilatory and carotid body responses to hypoxia in cats. Clin Exp Pharmacol Physiol 22: 658–664, 1995. doi: 10.1111/j.1440-1681.1995.tb02084.x. [DOI] [PubMed] [Google Scholar]
- 19.Janssen C, Beloka S, Kayembe P, Deboeck G, Adamopoulos D, Naeije R, van de Borne P. Decreased ventilatory response to exercise by dopamine-induced inhibition of peripheral chemosensitivity. Respir Physiol Neurobiol 168: 250–253, 2009. doi: 10.1016/j.resp.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Joyner MJ, Limberg JK. Hitting the wall: glycogen, glucose and the carotid bodies. J Physiol 592: 4413–4414, 2014. doi: 10.1113/jphysiol.2014.281790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaneko K, Shirotani T, Araki E, Matsumoto K, Taguchi T, Motoshima H, Yoshizato K, Kishikawa H, Shichiri M. Insulin inhibits glucagon secretion by the activation of PI3-kinase in In-R1-G9 cells. Diabetes Res Clin Pract 44: 83–92, 1999. doi: 10.1016/S0168-8227(99)00021-2. [DOI] [PubMed] [Google Scholar]
- 22.Koyama Y, Coker RH, Denny JC, Lacy DB, Jabbour K, Williams PE, Wasserman DH. Role of carotid bodies in control of the neuroendocrine response to exercise. Am J Physiol Endocrinol Metab 281: E742–E748, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Koyama Y, Coker RH, Stone EE, Lacy DB, Jabbour K, Williams PE, Wasserman DH. Evidence that carotid bodies play an important role in glucoregulation in vivo. Diabetes 49: 1434–1442, 2000. doi: 10.2337/diabetes.49.9.1434. [DOI] [PubMed] [Google Scholar]
- 24.Kumar P, Prabhakar NR. Peripheral chemoreceptors: function and plasticity of the carotid body. Compr Physiol 2: 141–219, 2012. doi: 10.1002/cphy.c100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lahiri S, Nishino T. Inhibitory and excitatory effects of dopamine on carotid chemoreceptors. Neurosci Lett 20: 313–318, 1980. doi: 10.1016/0304-3940(80)90166-4. [DOI] [PubMed] [Google Scholar]
- 26.Limberg JK, Johnson BD, Holbein WW, Ranadive SM, Mozer MT, Joyner MJ. Interindividual variability in the dose-specific effect of dopamine on carotid chemoreceptor sensitivity to hypoxia. J Appl Physiol (1985) 120: 138–147, 2016. doi: 10.1152/japplphysiol.00723.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Limberg JK, Taylor JL, Mozer MT, Dube S, Basu A, Basu R, Rizza RA, Curry TB, Joyner MJ, Wehrwein EA. Effect of bilateral carotid body resection on cardiac baroreflex control of blood pressure during hypoglycemia. Hypertension 65: 1365–1371, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Llados F, Zapata P. Effects of dopamine analogues and antagonists on carotid body chemosensors in situ. J Physiol 274: 487–499, 1978. doi: 10.1113/jphysiol.1978.sp012162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Löllgen H, Drexler H. Use of inotropes in the critical care setting. Crit Care Med Suppl 18: S56–S60, 1990. doi: 10.1097/00003246-199001002-00011. [DOI] [PubMed] [Google Scholar]
- 31.Lorenzi M, Karam JH, McIlroy MB, Forsham PH. Increased growth hormone response to dopamine infusion in insulin-dependent diabetic subjects: indication of possible blood-brain barrier abnormality. J Clin Invest 65: 146–153, 1980. doi: 10.1172/JCI109644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lourenço RV. Clinical methods for the study of regulation of breathing. Chest Suppl 70: 109–112, 1976. doi: 10.1378/chest.70.1_Supplement.109-a. [DOI] [PubMed] [Google Scholar]
- 33.Moore DP, Weston AR, Hughes JM, Oakley CM, Cleland JG. Effects of increased inspired oxygen concentrations on exercise performance in chronic heart failure. Lancet 339: 850–853, 1992. doi: 10.1016/0140-6736(92)90288-E. [DOI] [PubMed] [Google Scholar]
- 34.Niewinski P, Janczak D, Rucinski A, Tubek S, Engelman ZJ, Jazwiec P, Banasiak W, Sobotka PA, Hart EC, Paton JF, Ponikowski P. Dissociation between blood pressure and heart rate response to hypoxia after bilateral carotid body removal in men with systolic heart failure. Exp Physiol 99: 552–561, 2014. doi: 10.1113/expphysiol.2013.075580. [DOI] [PubMed] [Google Scholar]
- 35.Niewinski P, Tubek S, Banasiak W, Paton JFR, Ponikowski P. Consequences of peripheral chemoreflex inhibition with low-dose dopamine in humans. J Physiol 592: 1295–1308, 2014. doi: 10.1113/jphysiol.2013.266858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oldendorf WH. Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. Am J Physiol 221: 1629–1639, 1971. [DOI] [PubMed] [Google Scholar]
- 37.Patel YC. Growth hormone stimulates hypothalamic somatostatin. Life Sci 24: 1589–1593, 1979. doi: 10.1016/0024-3205(79)90020-1. [DOI] [PubMed] [Google Scholar]
- 38.Paton JF, Ratcliffe L, Hering D, Wolf J, Sobotka PA, Narkiewicz K. Revelations about carotid body function through its pathological role in resistant hypertension. Curr Hypertens Rep 15: 273–280, 2013. doi: 10.1007/s11906-013-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfoh JR, Tymko MM, Abrosimova M, Boulet LM, Foster GE, Bain AR, Ainslie PN, Steinback CD, Bruce CD, Day TA. Comparing and characterizing transient and steady-state tests of the peripheral chemoreflex in humans. Exp Physiol 101: 432–447, 2016. doi: 10.1113/EP085498. [DOI] [PubMed] [Google Scholar]
- 40.Prabhakhar NR, Joyner MJ. Tasting arterial blood: what do the carotid chemoreceptors sense? Front Physiol 5: 524, 2015. doi: 10.3389/fphys.2014.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubí B, Ljubicic S, Pournourmohammadi S, Carobbio S, Armanet M, Bartley C, Maechler P. Dopamine D2-like receptors are expressed in pancreatic beta cells and mediate inhibition of insulin secretion. J Biol Chem 280: 36824–36832, 2005. doi: 10.1074/jbc.M505560200. [DOI] [PubMed] [Google Scholar]
- 42.Stellingwerff T, Leblanc PJ, Hollidge MG, Heigenhauser GJ, Spriet LL. Hyperoxia decreases muscle glycogenolysis, lactate production, and lactate efflux during steady-state exercise. Am J Physiol Endocrinol Metab 290: E1180–E1190, 2006. doi: 10.1152/ajpendo.00499.2005. [DOI] [PubMed] [Google Scholar]
- 43.Stickland MK, Fuhr DP, Haykowsky MJ, Jones KE, Paterson DI, Ezekowitz JA, McMurtry MS. Carotid chemoreceptor modulation of blood flow during exercise in healthy humans. J Physiol 589: 6219–6230, 2011. doi: 10.1113/jphysiol.2011.218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stickland MK, Morgan BJ, Dempsey JA. Carotid chemoreceptor modulation of sympathetic vasoconstrictor outflow during exercise in healthy humans. J Physiol 586: 1743–1754, 2008. doi: 10.1113/jphysiol.2007.147421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tannenbaum GS. Evidence for autoregulation of growth hormone secretion via the central nervous system. Endocrinology 107: 2117–2120, 1980. doi: 10.1210/endo-107-6-2117. [DOI] [PubMed] [Google Scholar]
- 46.van de Borne P, Oren R, Somers VK. Dopamine depresses minute ventilation in patients with heart failure. Circulation 98: 126–131, 1998. doi: 10.1161/01.CIR.98.2.126. [DOI] [PubMed] [Google Scholar]
- 47.Vance ML, Kaiser DL, Frohman LA, Rivier J, Vale WW, Thorner MO. Role of dopamine in the regulation of growth hormone secretion: dopamine and bromocriptine augment growth hormone (GH)-releasing hormone-stimulated GH secretion in normal man. J Clin Endocrinol Metab 64: 1136–1141, 1987. doi: 10.1210/jcem-64-6-1136. [DOI] [PubMed] [Google Scholar]
- 48.Ward DS. Stimulation of hypoxic ventilatory drive by droperidol. Anesth Analg 63: 106–110, 1984. doi: 10.1213/00000539-198402000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Ward DS, Bellville JW. Reduction of hypoxic ventilatory drive by dopamine. Anesth Analg 61: 333–337, 1982. doi: 10.1213/00000539-198204000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Ward DS, Voter WA, Karan S. The role of the carotid bodies in the counter-regulatory response to hypoglycemia. Adv Exp Med Biol 648: 273–280, 2009. doi: 10.1007/978-90-481-2259-2_31. [DOI] [PubMed] [Google Scholar]
- 51.Wehrwein EA, Joyner MJ, Hart ECJ, Wallin BG, Karlsson T, Charkoudian N. Blood pressure regulation in humans: calculation of an “error signal” in control of sympathetic nerve activity. Hypertension 55: 264–269, 2010. doi: 10.1161/HYPERTENSIONAHA.109.141739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Welsh MJ, Heistad DD, Abboud FM. Depression of ventilation by dopamine in man. Evidence for an effect on the chemoreceptor reflex. J Clin Invest 61: 708–713, 1978. doi: 10.1172/JCI108983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, Zheng R, Meng X, Wang L, Liu L, Gao Y. Pancreatic Endocrine Effects of Dopamine Receptors in Human Islet Cells. Pancreas 44: 925–929, 2015. doi: 10.1097/MPA.0000000000000357. [DOI] [PubMed] [Google Scholar]