Abstract
Negative and positive muscle sympathetic nerve activity (MSNA) responders have been observed during mental stress. We hypothesized that similar MSNA response patterns could be identified during the first minute of static handgrip and contribute to the interindividual variability throughout exercise. Supine measurements of multiunit MSNA (microneurography) and continuous blood pressure (Finometer) were recorded in 29 young healthy men during the first (HG1) and second (HG2) minute of static handgrip (30% maximal voluntary contraction) and subsequent postexercise circulatory occlusion (PECO). Responders were identified on the basis of differences from the typical error of baseline total MSNA: 7 negative, 12 positive, and 10 nonresponse patterns. Positive responders demonstrated larger total MSNA responses during HG1 (P < 0.01) and HG2 (P < 0.0001); however, the increases in blood pressure throughout handgrip exercise were similar between all groups, as were the changes in heart rate, stroke volume, cardiac output, total vascular conductance, and respiration (all P > 0.05). Comparing negative and positive responders, total MSNA responses were similar during PECO (P = 0.17) but opposite from HG2 to PECO (∆40 ± 46 vs. ∆-21 ± 62%, P = 0.04). Negative responders also had a shorter time-to-peak diastolic blood pressure during HG1 (20 ± 20 vs. 44 ± 14 s, P < 0.001). Total MSNA responses during HG1 were associated with responses to PECO (r = 0.39, P < 0.05), the change from HG2 to PECO (r = −0.49, P < 0.01), and diastolic blood pressure time to peak (r = 0.50, P < 0.01). Overall, MSNA response patterns during the first minute of static handgrip contribute to interindividual variability and appear to be influenced by differences in central command, muscle metaboreflex activation, and rate of loading of the arterial baroreflex.
Keywords: blood pressure, muscle metaboreflex, muscle sympathetic nerve activity, static handgrip, sympathetic nervous system
INTRODUCTION
Examining the neural and hemodynamic responses to stress can offer unique physiological information not obtained under resting conditions (1, 13, 22, 41, 44) and assist in identifying subgroups at heightened clinical risk (6, 13, 44). Static handgrip exercise represents a classic sympathoexcitatory stress used to evoke increases in peripheral sympathetic activity and blood pressure (25, 27). With respect to the former, static handgrip exercise completed at low intensity [e.g., 30% maximal voluntary contraction (MVC)] increases muscle sympathetic nerve activity (MSNA) during the second or third minute of contraction, coincident with stimulation of chemosensitive group III/IV afferents and activation of the potent muscle metaboreflex (27, 31, 34, 45, 46). The small, statistically insignificant, increases or decreases in MSNA observed during the first minute of static handgrip exercise (27, 34, 45, 46) have been taken as evidence that feedforward control by higher brain centers (i.e., central command) and feedback from mechanosensitive skeletal muscle afferents exert minimal effects on efferent sympathetic outflow to skeletal muscle vasculature (15, 31, 45). More recent work studying the neural response to the onset (20–30 s) of 30% MVC static handgrip exercise, which may rely to a greater extent on input from central command (15, 31, 45), has reported both reductions (18) and increases (24) in MSNA raising the possibility of sympathetic responder types. Unfortunately, each of these studies examined only mean group data, which may aggregate two (or more) distinct populations of responders.
Prior work investigating MSNA responses to central stress challenges (e.g., mental arithmetic, Stroop test, arousal) has demonstrated similar large interindividual variability (4, 9–12, 17), including the presence of reproducible negative and positive responders (10, 17). Whether specific sympathetic responder types can be identified during exercise, a stress involving both central and peripheral afferent regulation, is unclear. Exercise studies examining the neural contribution of feedforward central command have reported contrasting results on sympathetic outflow (3, 27, 45). MSNA is decreased in anticipation of cycling exercise (3) and during a 2-min voluntary (but not electrically stimulated) static bicep contraction at 20% MVC (27), yet increased during near-maximal static handgrip exercise when motor output is reduced with neuromuscular blockade (45). A recent examination of interindividual MSNA responses during 2 min of static handgrip exercise at 35% MVC failed to identify negative responders (12). However, characterization of responder type was based on the mean change over the entire stress, a potential limitation given the potent sympathoexcitation elicited by activation of the muscle metaboreflex later in the contraction (27, 31, 34, 45, 46). Whether sympathetic responder types are present during the first minute of static handgrip exercise when the net effect of central command is greatest has not been studied.
Therefore, the purpose of the present investigation was to examine the interindividual MSNA responses to static handgrip exercise. On the basis of prior evidence for central responders types (4, 9–12), we hypothesized that 1) during the first minute of static handgrip exercise, we could identify groups of participants who exhibit either a mean decrease (negative responders), no change (nonresponders), or an increase (positive responders) in MSNA from baseline; and 2) that these group differences would persist throughout the second minute of static handgrip exercise, providing a mechanism for between-subject variability in MSNA responses. Additionally, we sought to investigate whether interindividual MSNA responses to static handgrip exercise were linked to differences in MSNA responses during anticipation and cessation of exercise (central command), postexercise circulatory occlusion (isolation of the muscle metaboreflex), or differences in diastolic blood pressure time to peak or rate of rise (arterial baroreflex loading).
METHODS
Participants.
Data were obtained from 29 healthy men (age: 24 ± 5 yr; BMI 24 ± 3 kg/m2; hand grip MVC: 47 ± 11 kg). All participants were in sinus rhythm; in addition, they were nonsmokers, with no history of cardiovascular disease, and they had not been prescribed any pharmacological medications. Participants completed an introductory familiarization visit and were instructed and reminded to abstain from vigorous physical activity for 48 h, as well as to abstain from caffeine and alcohol for 24 h before the study visit. All procedures were approved by the University of Guelph Research Ethics Board, and written informed consent was obtained from all participants.
Measurements.
Continuous heart rate was collected using single-lead electrocardiography. Respiratory excursions were monitored using a piezoelectric transducer placed around the mid-to-upper abdomen (model no. 1132 Pneumotrace II; UFI, Morro Bay, CA). The respiratory trace was also used to estimate within-participant peak-to-peak displacement (mV) since MSNA can be modulated by lung volume (40). To assess changes in blood pressure, beat-to-beat alterations were measured using a cuff placed on the right middle finger (Finometer MIDI; Finapress Medical Systems, Enschede, The Netherlands). From the continuous blood pressure waveform, the ModelFlow method (2) was used to calculate stroke volume, permitting determination of cardiac output and total vascular conductance. Discrete brachial blood pressure was monitored from the left upper arm every minute during the baseline using an automated oscillometric device (model BPM-200, BpTRU, Coquitlam, BC). Discrete blood pressure measurements were used to validate the accuracy of continuous blood pressure values.
Multiunit postganglionic efferent MSNA was measured from the right fibular nerve by microneurography, as described previously (28, 29, 33). A 2-MΩ tungsten microelectrode was inserted percutaneously into a motor fascicle and adjusted until spontaneous pulse-synchronous bursts of integrated sympathetic activity could be observed clearly from the background noise (3:1 signal-to-noise ratio). Confirmation of muscle sympathetic activity was made by observing reflexive increases in response to an end-expiratory apnea and the absence of responsiveness to unexpected clapping (startle response) or light stroking of the skin. The MSNA signal was amplified (75,000×), band-pass filtered (0.7–2.0 kHz), rectified, and integrated using a 0.1-s time constant to obtain the mean voltage multiunit neurogram (Nerve Traffic Analyzer, model 662C-4; Absolute Design and Manufacturing Services, Salon, IA). The neural signal was monitored both audibly and visually to ensure no change in site throughout the study protocol.
All continuous data were acquired at a frequency of 1,000 Hz, except the raw MSNA neurogram (10,000 Hz), using LabChart (version 8; PowerLab, ADInstruments, New South Wales, Australia).
Experimental protocol.
All testing was conducted in a light- and temperature-controlled laboratory. Following collection of anthropometric data, participants were positioned supine on a comfortable bed and asked to complete two MVC in their left hand (one participant was left-handed) (model 78010, Hand Dynamometer, Lafayette Instrument, Lafayette, LA). Each contraction was separated by 30–60 s of rest, and the highest force was designated as MVC.
After instrumentation and 10 min of quiet rest, participants underwent data collection of all continuous measurements during a 3-min baseline period, a 45-s transition period, 2 min of static handgrip contraction at 30% of MVC, and 3 min of postexercise circulatory occlusion (PECO). During the first 15 s of the transition period, the oscillometric blood pressure cuff was replaced with an aneroid sphygmomanometer and cuff to administer PECO. In anticipation of exercise, participants were given 30, 10, and 3-2-1-s verbal reminders. The same experimenter provided the verbal cues, monitored each participant throughout the entire duration of the static handgrip exercise and provided real-time feedback to ensure the desired contraction intensity was consistently achieved and maintained. To initiate PECO, the aneroid sphygmomanometer was inflated to 220 mmHg, which in all cases was greater than systolic blood pressure. PECO was used to trap local metabolites produced from exercise, permitting continued activation of the muscle metaboreflex, while removing the confounding cardiovascular influences of the muscle mechanoreflex and central command (27, 32, 34, 45, 46).
Data analysis.
Resting hemodynamic, respiratory, and MSNA values were averaged over the 3-min baseline and used to calculate change (Δ) during each minute of static handgrip exercise and last minute of PECO. Offline analysis of the integrated MSNA neurogram was performed using a custom LabView program (28, 29, 33) and expressed as burst frequency (bursts/min), burst incidence (bursts/100 heartbeats), and total MSNA (burst frequency × mean burst integral) (48). To account for between-subject differences in electrode placement, the average baseline measurement of total MSNA was set at 100, and the responses were expressed as a percent change. Baseline spontaneous arterial sympathetic baroreflex sensitivity was calculated as the slope of the weighted linear regression line between the likelihood of a MSNA burst (incidence) within 2-mmHg bins of diastolic blood pressure (23). To be accepted, the regression line was required to possess an r value ≥ 0.5. Two participants did not meet this criterion and were excluded from the analysis.
In contrast to previous studies, which classified MSNA responders on the basis of directionality of responses or self-selected cut-off values (4, 12), we divided participants on the basis of whether their total MSNA response during the first minute of static handgrip exercise differed from the baseline typical error of measurement. The typical error of measurement describes the within-participant variation between measurements (i.e., reliability) (19). Total MSNA was selected as the primary variable, as it represents a more comprehensive assessment of peripheral sympathetic activity than burst frequency or burst amplitude alone (43). The mean typical error of measurement was calculated on the basis of minute-to-minute changes in total MSNA during the 3-min baseline period. This time period was selected to align with the epoch durations assessed during static handgrip exercise. Participant responses that were greater than the typical error were classified as positive responders, those within the typical error as nonresponders, and those with decreases below the typical error as negative responders. Using the typical error of measurement (instead of the change from zero) reduces the risk of falsely classifying a participant based on fluctuations between measurements caused by biological or technological (methodological) variations.
After the identification of both negative and positive responders, we sought to compare directly within these groups potential neural mechanisms responsible for the divergent response patterns. To investigate the role of central command, we calculated the change in total MSNA from baseline to the 30-s period immediately before initiating static handgrip exercise (i.e., anticipation of exercise). Our laboratory has shown previously that 30-s epochs can provide valid and reliable measures of MSNA (33). We also examined the change in total MSNA from the second minute of static handgrip (HG2) to PECO (i.e., removal of central command and muscle mechanoreflex activation) (21). To examine muscle metaboreflex activation, total MSNA responses were assessed during the final minute of PECO. Finally, recent work has reported that negative MSNA responders to mental stress exhibit a faster time to peak and greater rate of rise in diastolic blood pressure (12), the input stimulus for arterial baroreflex control of MSNA (42). Diastolic blood pressure time to peak (seconds) was defined as the time required to reach peak diastolic pressure (single cardiac cycle). Diastolic blood pressure rate of rise was calculated as the peak change (from baseline) in diastolic pressure divided by the time to peak (mmHg/s). The dynamics of diastolic blood pressure responses were examined over the first minute, and the peak response over the entire static handgrip contraction.
Statistical analysis.
Data are presented as means ± SD unless specified otherwise. Hemodynamic and MSNA responses during static handgrip exercise in the entire cohort were analyzed using one-way ANOVAs. Following the division of participants into responder groups, baseline characteristics were examined using one-way ANOVAs. Between-group changes in MSNA and hemodynamic responses during static handgrip exercise [minute 1 (HG1) and 2 (HG2)] were made using two-way mixed-model repeated-measures ANOVAs. Bonferroni post hoc procedures with correction for multiple comparisons were used. Secondary analyses examining total MSNA and hemodynamic responses during exercise anticipation and cessation, PECO, and diastolic blood pressure time to peak and rate of rise between negative and positive responders were compared using unpaired t-tests. Pearson correlation coefficients were used to assess associations between study parameters. All data were analyzed using GraphPad Prism (GraphPad Software, La Jolla, CA) and P < 0.05 was considered statistically significant.
RESULTS
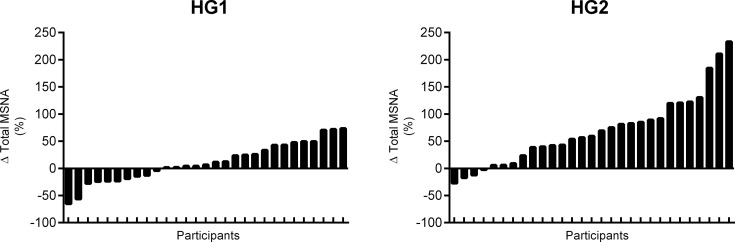
All participants completed the full study protocol. Technical difficulties precluded the analysis of the respiratory signal in four participants. As expected (Table 1), heart rate, blood pressure, and cardiac output increased during HG1 and HG2 (all P < 0.01). Stroke volume, total vascular conductance, and respiration rate were unchanged during static handgrip exercise (All P > 0.05), but respiration depth increased during HG2 (P < 0.01). MSNA burst frequency, burst incidence, and total MSNA were unchanged from baseline during HG1 (all P > 0.05), while MSNA burst frequency and total MSNA were increased during HG2 (both P < 0.0001). Considerable interindividual variability in total MSNA responses was present during static handgrip exercise (Fig. 1).
Table 1.
Mean response in hemodynamic, respiratory, and muscle sympathetic variables during the first and second minute of static handgrip exercise
| Variable | Baseline | HG1 | HG2 |
|---|---|---|---|
| Heart rate, beats/min | 60 ± 9 | 72 ± 10* | 76 ± 10* |
| Systolic BP, mmHg | 109 ± 7 | 118 ± 12* | 126 ± 14*† |
| Diastolic BP, mmHg | 64 ± 6 | 71 ± 7* | 78 ± 9*† |
| Mean arterial pressure, mmHg | 79 ± 6 | 87 ± 8* | 94 ± 10*† |
| Cardiac output, l/min | 6.0 ± 1.0 | 7.2 ± 1.4* | 7.4 ± 1.3* |
| Stroke volume, ml | 100 ± 14 | 100 ± 16 | 98 ± 16 |
| Total vascular conductance, ml·min−1·mmHg−1 | 75 ± 12 | 83 ± 15 | 79 ± 13 |
| Respiration rate, breaths/min | 15 ± 4 | 17 ± 5 | 17 ± 6 |
| Respiration depth, % of baseline | 100 | 137 ± 65 | 165 ± 89* |
| MSNA burst frequency, bursts/min | 18 ± 7 | 19 ± 10 | 25 ± 8*† |
| MSNA burst incidence, bursts/100 heartbeats | 30 ± 12 | 27 ± 14 | 33 ± 11 |
| Total MSNA, % of baseline | 100 | 111 ± 36 | 170 ± 65*† |
Values are presented as means ± SD. BP, blood pressure; HG1, first minute of static handgrip exercise; HG2, second minute of static handgrip exercise; MSNA, muscle sympathetic nerve activity. Respiratory data analysis completed on n = 25.
P < 0.01 vs. baseline.
P < 0.01 vs. HG1.
Fig. 1.
Histogram plot of the total multi-unit muscle sympathetic nerve activity (MSNA) responses during the first (HG1) and second (HG2) minute of static handgrip exercise.
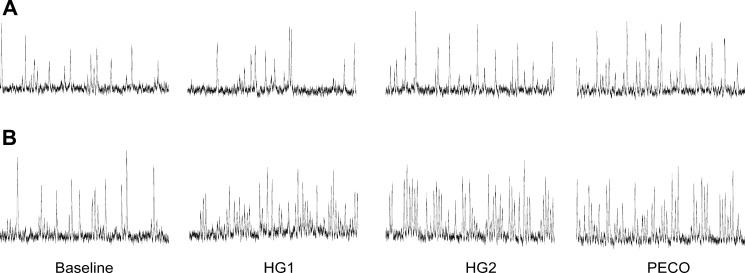
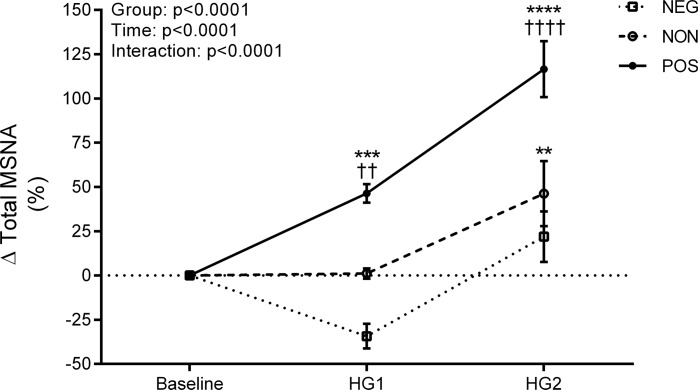
The baseline minute-to-minute typical error of measurement for total MSNA was ± 19%. As a result, 7 participants were identified as negative responders, 10 as nonresponders, and 12 as positive responders. Representative microneurographic recordings from one negative and one positive MSNA responder are shown in Fig. 2. All baseline participant characteristics, including sympathetic baroreflex sensitivity, were similar between responder groups (All P > 0.05; Table 2). Mean group responses in heart rate, blood pressure, stroke volume, cardiac output, total vascular conductance, and respiration during the first and second minute of static handgrip exercise were similar between each of the responder groups (All P > 0.05; Table 3). Total MSNA responses (Fig. 3) were larger in positive responders during HG1 (P < 0.01) and HG2 (P < 0.0001) compared with both negative responders and nonresponders. No differences were detected between negative responders and nonresponders (both P > 0.05). The change in total MSNA between HG1 and HG2 was similar between negative responders, nonresponders, and positive responders (∆56 ± 35 vs. ∆45 ± 53 vs. ∆70 ± 48%, P > 0.05).
Fig. 2.
Representative one-minute microneurographic tracings at baseline and during the first (HG1) and second (HG2) minute of static handgrip exercise and last minute of postexercise circulatory occlusion (PECO) in a negative (A) and positive (B) responder.
Table 2.
Baseline participant characteristics
| Variable | NEG (n = 7) | NON (n = 10) | POS (n = 12) |
|---|---|---|---|
| Age, yr | 22 ± 2 | 27 ± 7 | 23 ± 3 |
| Height, cm | 180 ± 3 | 179 ± 8 | 178 ± 6 |
| Weight, kg | 77 ± 8 | 80 ± 10 | 74 ± 12 |
| BMI, kg/m2 | 24 ± 3 | 25 ± 2 | 23 ± 3 |
| Handgrip MVC, kg | 46 ± 13 | 46 ± 9 | 49 ± 11 |
| Heart rate, beats/min | 61 ± 12 | 59 ± 10 | 60 ± 7 |
| Systolic BP, mmHg | 109 ± 6 | 106 ± 8 | 111 ± 6 |
| Diastolic BP, mmHg | 64 ± 9 | 64 ± 6 | 65 ± 5 |
| Mean arterial pressure, mmHg | 79 ± 8 | 78 ± 6 | 80 ± 4 |
| Cardiac output, l/min | 6.2 ± 1.2 | 6.0 ± 0.8 | 5.8 ± 1.1 |
| Stroke volume, ml | 102 ± 9 | 103 ± 16 | 97 ± 15 |
| Total vascular conductance, ml·min−1·mmHg−1 | 78 ± 9 | 77 ± 12 | 73 ± 15 |
| Respiration rate, breaths/min | 17 ± 5 | 12 ± 4 | 16 ± 3 |
| MSNA burst frequency, bursts/min | 17 ± 7 | 17 ± 7 | 18 ± 7 |
| MSNA burst incidence, bursts/100 heartbeats | 29 ± 14 | 31 ± 12 | 31 ± 12 |
| Sympathetic baroreflex sensitivity, bursts·100 heartbeats−1·mmHg−1 | −4.6 ± 1.5 | −5.3 ± 1.8 | −4.2 ± 1.8 |
Values are expressed as means ± SD. MVC, maximal voluntary contraction; NEG, negative responders; NON, nonresponders; POS, positive responders. Data analysis completed on n = 25 for respiration (NEG, n = 7; NON, n = 6; POS, n = 12) and n = 27 for sympathetic baroreflex sensitivity (NEG, n = 7; NON, n = 9; POS, n = 11).
Table 3.
Mean change in hemodynamic, respiratory, and neural variables during the first and second minute of static handgrip exercise in negative responders, nonresponders, and positive responders
| NEG (n = 7) |
NON (n = 10) |
POS (n = 12) |
||||
|---|---|---|---|---|---|---|
| ΔHG1 | ΔHG2 | ΔHG1 | ΔHG2 | ΔHG1 | ΔHG2 | |
| Heart rate, beats/min | 17 ± 9 | 19 ± 8 | 9 ± 6 | 13 ± 10 | 12 ± 6 | 17 ± 9 |
| Systolic BP, mmHg | 10 ± 6 | 20 ± 10 | 10 ± 9 | 15 ± 9 | 8 ± 9 | 18 ± 12 |
| Diastolic BP, mmHg | 8 ± 4 | 16 ± 8 | 6 ± 4 | 11 ± 7 | 6 ± 4 | 15 ± 7 |
| Mean arterial pressure, mmHg | 9 ± 4 | 18 ± 9 | 8 ± 6 | 12 ± 7 | 7 ± 5 | 16 ± 9 |
| Cardiac output, l/min | 1.7 ± 0.9 | 1.8 ± 0.7 | 0.9 ± 0.8 | 1.2 ± 1.0 | 1.2 ± 1.1 | 1.4 ± 1.1 |
| Stroke volume, ml/l | 0 ± 7 | −2 ± 10 | −1 ± 6 | −2 ± 10 | 1 ± 8 | −3 ± 8 |
| Total vascular conductance, ml·min−1·mmHg−1 | 12 ± 10 | 5 ± 8 | 4 ± 9 | 3 ± 10 | 8 ± 11 | 2 ± 8 |
| Respiration rate, breaths/min | 3 ± 2 | 2 ± 4 | 1 ± 4 | 1 ± 5 | 1 ± 3 | 0 ± 4 |
| Respiration depth, % AU | 39 ± 68 | 72 ± 100 | 48 ± 81 | 52 ± 59 | 32 ± 61 | 67 ± 99 |
| MSNA burst frequency, bursts/min | −4 ± 3 | 4 ± 5 | −1 ± 2 | 3 ± 6 | 6 ± 4* | 12 ± 5* |
| MSNA burst incidence, bursts/100 heartbeats | −12 ± 7 | −3 ± 8 | −5 ± 5 | 0 ± 8 | 3 ± 6* | 8 ± 8* |
Values are expressed as means ± SD.
Significant interaction effect, P < 0.01 vs. negative responders and nonresponders.
Fig. 3.
Change (∆) in total muscle sympathetic nerve activity (MSNA) during the first (HG1) and second (HG2) minute of static handgrip exercise in negative responders (□, n = 7), nonresponders (○, n = 10), and positive responders (●, n = 12). Data presented as means ± SE. **P < 0.01, ***P < 0.001, ****P < 0.0001 compared with baseline. ††P < 0.01, ††††P < 0.0001 compared with negative responders and nonresponders.
Table 4 displays the results of potential neural mechanisms involved in mediating the negative and positive response patterns during static handgrip exercise. The change in total MSNA during the anticipatory period before exercise was not different between negative and positive responders (∆−25 ± 30 vs. ∆−13 ± 33%, P = 0.43), in parallel with no differences in heart rate or blood pressure responses (both P > 0.05). However, the change in total MSNA from HG2 to PECO demonstrated qualitatively opposite responses between negative and positive responders (∆40 ± 46 vs. ∆−21 ± 62%, P = 0.04). As a result, changes in total MSNA during PECO were not different between negative and positive responder groups (∆62 ± 55 vs. ∆96 ± 46%, P = 0.17). Finally, although the peak change in diastolic blood pressure was not different between negative and positive responders (∆15 ± 6 vs. ∆15 ± 4 mmHg, P = 0.94), negative responders had a shorter time to peak (∆24 ± 22 vs. ∆45 ± 15 s, P = 0.03) and a trend for a faster rate of rise (∆4.4 ± 6.8 vs. ∆0.5 ± 0.6 mmHg/s, P = 0.06).
Table 4.
Mean change in hemodynamic variables and MSNA during anticipation of exercise, the transition from the second minute of static handgrip to postexercise circulatory occlusion, postexercise circulatory occlusion, as well as the diastolic blood pressure response in negative and positive responders
| NEG (n = 7) | POS (n = 12) | P Value | |
|---|---|---|---|
| Anticipation of exercise | |||
| Heart rate, beats/min | 9 ± 7 | 7 ± 3 | 0.44 |
| Systolic BP, mmHg | 6 ± 6 | 6 ± 4 | 0.94 |
| Diastolic BP, mmHg | 3 ± 3 | 2 ± 3 | 0.70 |
| Mean arterial pressure, mmHg | 14 ± 9 | 15 ± 8 | 0.71 |
| Total MSNA, % | −25 ± 30 | −13 ± 33 | 0.43 |
| HG2 to postexercise circulatory occlusion | |||
| Heart rate, beats/min | −14 ± 8 | −14 ± 9 | 0.89 |
| Systolic BP, mmHg | −4 ± 6 | −2 ± 5 | 0.28 |
| Diastolic BP, mmHg | −5 ± 4 | −6 ± 3 | 0.82 |
| Mean arterial pressure, mmHg | −5 ± 5 | −4 ± 3 | 0.74 |
| Total MSNA, % | 40 ± 46 | −21 ± 62 | 0.04 |
| Postexercise circulatory occlusion | |||
| Heart rate, beats/min | 5 ± 6 | 4 ± 6 | 0.67 |
| Systolic BP, mmHg | 16 ± 9 | 16 ± 13 | 0.98 |
| Diastolic BP, mmHg | 11 ± 6 | 9 ± 7 | 0.61 |
| Mean arterial pressure, mmHg | 13 ± 7 | 12 ± 9 | 0.80 |
| Total MSNA, % | 62 ± 55 | 96 ± 46 | 0.17 |
| Temporal diastolic BP response | |||
| First minute of static handgrip | |||
| Peak DBP, mmHg | 16 ± 6 | 15 ± 4 | 0.94 |
| DBP time to peak, s | 24 ± 22 | 45 ± 15 | 0.03 |
| DBP rate of rise, mmHg/s | 4.4 ± 6.8 | 0.5 ± 0.6 | 0.06 |
| Two minutes of static handgrip | |||
| Peak DBP, mmHg | 24 ± 10 | 24 ± 8 | 0.97 |
| DBP time to peak, s | 109 ± 10 | 107 ± 13 | 0.68 |
| DBP rate of rise, mmHg/s | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.97 |
Values are expressed as means ± SD. DBP, diastolic blood pressure. Bolded values indicate significant difference.
Across the entire cohort, the change in total MSNA during exercise anticipation was not associated with responses during HG1 (P > 0.91). In contrast, the change in total MSNA from HG2 to PECO was associated negatively with the response during HG1 (r = −0.49, P < 0.01). Positive correlations were observed between the change in total MSNA during HG1 and the total MSNA response during PECO (r = 0.39, P = 0.04) and the diastolic blood pressure time to peak during HG1 (r = 0.50, P < 0.01).
DISCUSSION
The present study sought to investigate the existence of distinct sympathetic responder types during static handgrip exercise. In contrast to prior work, which grouped participants on the basis of the mean MSNA response over the entire contraction (12), we examined interindividual variability during the first minute of static handgrip exercise. Using this approach, we identified three MSNA response patterns, which differ from the typical error of measurement in our cohort of young healthy men. Interestingly, although positive responders had larger total MSNA responses during HG1 and HG2, all three responder groups possessed similar relative increases from the first to second minute of static handgrip exercise. Examination of potential neural mechanisms responsible for negative and positive responders identified differences in total MSNA between HG2 and PECO and diastolic blood pressure time to peak. Across the whole cohort, total MSNA responses during the first minute of static handgrip exercise were correlated positively with both the change in MSNA during PECO and diastolic blood pressure time to peak during HG1 and correlated negatively to the MSNA response between HG2 and PECO. Collectively, these results demonstrate that interindividual differences in MSNA responses to static handgrip exercise are driven mainly by distinct response patterns during the first minute of contraction, which may be influenced by differences in central command, muscle metaboreflex activation, and the rate of arterial baroreflex loading.
Investigations into the peripheral vasoconstrictor response to mental stress or arousal (i.e., central stressors) have demonstrated the existence of negative and positive MSNA responders (4, 9–12). Although such responses have not been reported previously during static exercise (27, 34, 45, 46), we hypothesized that distinct sympathetic responder types could be identified during the first minute of static handgrip exercise when the relative contribution of central command to the MSNA response is highest (i.e., before muscle metaboreflex activation). The summation of two subpopulations with opposite MSNA responses would explain earlier work demonstrating that static handgrip exercise failed to alter sympathetic outflow during the first minute of contraction (27, 34, 45, 46). Our results confirm this hypothesis, as group differences in sympathetic outflow were observed during HG1 and HG2 between negative and positive responders. Interestingly, the changes from HG1 to HG2 were similar between all responder groups, suggesting that the interindividual variability noted in Fig. 1 is the result of differences in sympathetic responses during the first minute of static handgrip exercise.
Net peripheral sympathetic outflow reflects the integration of central and peripheral afferent reflexes (8). However, the precise mechanisms responsible for the variability in total MSNA responses during static handgrip exercise are unclear. Evidence supports a sympathoinhibitory role of central command in anticipation of exercise (3) and during low-intensity static contractions (27). In contrast, we did not observe a difference in total MSNA responses during the 30-s period immediately before exercise between negative and positive responders. Acknowledging that the central regulation of sympathetic outflow may differ between states with and without volitional exertion, we investigated the total MSNA response between HG2 and PECO, which is associated with the removal of central command and muscle mechanoreflex (21). These results demonstrated a qualitatively opposite response between negative and positive responders. To our knowledge, the muscle mechanoreflex has only been demonstrated to exert sympathoexcitatory responses (31, 45), suggesting that it was not involved in the increases in total MSNA following its removal in negative responders. The total MSNA response between HG2 and PECO also correlated negatively with the MSNA response during HG1. These results suggest that central command may be capable of eliciting divergent effects on MSNA and that it is involved in mediating interindividual variability.
The identification of negative responders with reductions in total MSNA outside the baseline typical error of measurement are not consistent with the sympathoexcitatory response elicited by muscle metaboreflex activation (7, 27, 31, 46). Examination of the negative and positive responders further demonstrated no differences in total MSNA responses between HG1 and HG2 or during PECO. Nevertheless, between-subject differences in activation or sensitivity (gain) of the exercise pressor reflex could be responsible for variability in MSNA responses. In support of this, a modest positive correlation was found between the total MSNA response during the first minute of static handgrip exercise and PECO. This finding aligns with prior work demonstrating that the hemodynamic responses during the first minute of static handgrip exercise at 50% MVC are positively associated with responses during PECO (47).
The arterial baroreflex has been shown to buffer increases in MSNA during static handgrip exercise (37), and recently, negative MSNA responders to mental stress were shown to possess a higher rate of rise in diastolic blood pressure (12). In the present study, although peak diastolic blood pressure responses were similar, negative responders possessed a shorter time-to-peak diastolic blood pressure. Furthermore, time-to-peak diastolic blood pressure correlated negatively with MSNA responses during HG1. Similar to mental stress (12), a faster rise in diastolic blood pressure may result in reflexive arterial baroreflex inhibition of MSNA. It is unlikely that negative MSNA responses were mediated by loading of the cardiopulmonary baroreflex, as increases in central venous pressure during static handgrip exercise in men are associated with mean arterial pressure responses (24), which were not different between negative and positive responders. Further, prior studies have demonstrated no interactive effects on MSNA between central venous pressure and the exercise pressor reflex, as the MSNA responses to static handgrip exercise were observed to be unchanged with lower-body negative pressure (37–39).
In the present study, group differences in MSNA during static handgrip exercise were not paralleled by differences in hemodynamic responses. Similar observations of altered MSNA but equivalent blood pressure responses have been found during mental stress (4, 12) and in response to voluntary and involuntary static bicep contractions (27), and likely highlight the integrative nature of blood pressure control. Speculating on the mechanisms responsible for the observed dissociation, the similar changes in diastolic blood pressure (and total vascular conductance) between groups may suggest differences in neurovascular transduction. Sympathetic vascular responsiveness can differ between individuals and is inversely related to baseline MSNA (5). However, in the present study, each responder group exhibited similar baseline MSNA burst frequency and incidence. Alternatively, greater peripheral sympathetic outflow may be offset by concomitant β2-adrenergic vasodilation via circulating epinephrine or local nitric oxide-dependent vasodilation, both shown previously to be responsible for vasodilation during ischemic handgrip exercise (36). Finally, the most parsimonious explanation for our MSNA-blood pressure dissociation is for interindividual differences in directing central sympathetic outflow to specific vascular beds (e.g., muscle and kidneys) (30). That is, participants classified as negative MSNA responders may preferentially increase, for example, renal sympathetic outflow to a greater extent, so that net vasoconstrictor outflow is similar (as evidenced in our study by no differences in total vascular conductance). Previous research supports the notion of highly controlled, tissue-specific, sympathetic outflow in humans, demonstrating that 1) the modulation of muscle and skin vasoconstrictor outflow is differentially controlled in response to stress (14, 26) and that 2) differences in sympathetic overactivation can be organ-specific in heart failure (16).
We acknowledge several limitations. Although MSNA responses to stress have been shown to be consistent across time (17, 35), future work is needed to demonstrate the reproducibility of sympathetic responders during the first minute of static exercise. Second, our results may not be generalized to females, older, or diseased populations. For example, women have been shown to exhibit smaller MSNA and pressor responses to static handgrip exercise and PECO (20). Third, all of our studies were conducted in the supine posture, which could limit direct comparisons between studies conducted in the seated posture (12). Finally, acknowledging that MSNA responses represent a continuous variable, our study possessed a relatively modest sample size, which may have limited our ability to detect significant differences between negative and positive responder groups (e.g., during PECO). Nonetheless, the goal of our study was to provide evidence for the existence of sympathetic responder types during exercise, and future studies are needed to further probe interindividual responses and the resulting clinical implications.
Perspectives and Significance
These results demonstrate the existence of highly variable interindividual MSNA responses during the first minute of static handgrip exercise, similar to those observed with mental stress and arousal (4, 9–12, 17). Positive MSNA responders maintained significantly larger peripheral sympathetic outflow responses throughout static handgrip exercise, although blood pressure and hemodynamic responses were similar. Examination of neural mechanisms identified potential roles for central command, activation of the muscle metaboreflex, and the rate of arterial baroreflex loading. The physiological and clinical implications of peripheral sympathetic responder types warrant further investigation.
GRANTS
This research was supported by a Natural Sciences and Engineering Research Council (NSERC) of Canada Discovery Grant (P. J. Millar; no. 06019), a University of Guelph-Humber Research Fund Grant (P. J. Millar), the Canada Foundation for Innovation (P. J. Millar; no. 34379), and the Ontario Ministry of Research, Innovation, and Science (P. J. Millar; no. 34379). A. V. Incognito was supported by a Canadian Institutes of Health Research Fredrick Banting and Charles Best Canada Graduate Scholarship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.V.I. and P.J.M. conceived and designed research; A.V.I., C.J.D., J.B.L., M.J.B., and P.J.M. performed experiments; A.V.I., C.J.D., J.B.L., M.J.B., and P.J.M. analyzed data; A.V.I. and P.J.M. interpreted results of experiments; A.V.I. and P.J.M. prepared figures; A.V.I. and P.J.M. drafted manuscript; A.V.I., C.J.D., and P.J.M. edited and revised manuscript; A.V.I., C.J.D., J.B.L., M.J.B., and P.J.M. approved final version of manuscript.
REFERENCES
- 1.Barbato AL. Bedside evaluation of the autonomic system. In: Clinical Methods: The History, Physical, and Laboratory Examinations, edited by Walker HK, Hall WD, Hurst JW. Waltham, MA: Butterworth, 1990. [PubMed] [Google Scholar]
- 2.Bogert LWJ, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol 90: 437–446, 2005. doi: 10.1113/expphysiol.2005.030262. [DOI] [PubMed] [Google Scholar]
- 3.Callister R, Ng AV, Seals DR. Arm muscle sympathetic nerve activity during preparation for and initiation of leg-cycling exercise in humans. J Appl Physiol (1985) 77: 1403–1410, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Carter JR, Ray CA. Sympathetic neural responses to mental stress: responders, nonresponders and sex differences. Am J Physiol Heart Circ Physiol 296: H847–H853, 2009. doi: 10.1152/ajpheart.01234.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charkoudian N, Joyner MJ, Sokolnicki LA, Johnson CP, Eisenach JH, Dietz NM, Curry TB, Wallin BG. Vascular adrenergic responsiveness is inversely related to tonic activity of sympathetic vasoconstrictor nerves in humans. J Physiol 572: 821–827, 2006. doi: 10.1113/jphysiol.2005.104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi H-M, Stebbins CL, Lee O-T, Nho H, Lee J-H, Chun J-M, Kim K-A, Kim J-K. Augmentation of the exercise pressor reflex in prehypertension: roles of the muscle metaboreflex and mechanoreflex. Appl Physiol Nutr Metab 38: 209–215, 2013. doi: 10.1139/apnm-2012-0143. [DOI] [PubMed] [Google Scholar]
- 7.Cui J, Blaha C, Moradkhan R, Gray KS, Sinoway LI. Muscle sympathetic nerve activity responses to dynamic passive muscle stretch in humans. J Physiol 576: 625–634, 2006. doi: 10.1113/jphysiol.2006.116640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994. [DOI] [PubMed] [Google Scholar]
- 9.Donadio V, Kallio M, Karlsson T, Nordin M, Wallin BG. Inhibition of human muscle sympathetic activity by sensory stimulation. J Physiol 544: 285–292, 2002. doi: 10.1113/jphysiol.2002.019596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donadio V, Karlsson T, Elam M, Wallin BG. Interindividual differences in sympathetic and effector responses to arousal in humans. J Physiol 544: 293–302, 2002. doi: 10.1113/jphysiol.2002.020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donadio V, Liguori R, Elam M, Karlsson T, Giannoccaro MP, Pegenius G, Giambattistelli F, Wallin BG. Muscle sympathetic response to arousal predicts neurovascular reactivity during mental stress. J Physiol 590: 2885–2896, 2012. doi: 10.1113/jphysiol.2012.228981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Sayed K, Macefield VG, Hissen SL, Joyner MJ, Taylor CE. Rate of rise in diastolic blood pressure influences vascular sympathetic response to mental stress. J Physiol 594: 7465–7482, 2016. doi: 10.1113/JP272963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Everson SA, Kaplan GA, Goldberg DE, Salonen JT. Anticipatory blood pressure response to exercise predicts future high blood pressure in middle-aged men. Hypertension 27: 1059–1064, 1996. doi: 10.1161/01.HYP.27.5.1059. [DOI] [PubMed] [Google Scholar]
- 14.Fagius J, Karhuvaara S, Sundlöf G. The cold pressor test: effects on sympathetic nerve activity in human muscle and skin nerve fascicles. Acta Physiol Scand 137: 325–334, 1989. doi: 10.1111/j.1748-1716.1989.tb08760.x. [DOI] [PubMed] [Google Scholar]
- 15.Fisher JP, Young CN, Fadel PJ. Autonomic adjustments to exercise in humans. Compr Physiol 5: 475–512, 2015. doi: 10.1002/cphy.c140022. [DOI] [PubMed] [Google Scholar]
- 16.Floras JS. Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol 54: 375–385, 2009. doi: 10.1016/j.jacc.2009.03.061. [DOI] [PubMed] [Google Scholar]
- 17.Fonkoue IT, Carter JR. Sympathetic neural reactivity to mental stress in humans: test-retest reproducibility. Am J Physiol Regul Integr Comp Physiol 309: R1380–R1386, 2015. doi: 10.1152/ajpregu.00344.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greaney JL, Edwards DG, Fadel PJ, Farquhar WB. Rapid onset pressor and sympathetic responses to static handgrip in older hypertensive adults. J Hum Hypertens 29: 402–408, 2015. doi: 10.1038/jhh.2014.106. [DOI] [PubMed] [Google Scholar]
- 19.Hopkins WG. Measures of reliability in sports medicine and science. Sports Med 30: 1–15, 2000. doi: 10.2165/00007256-200030010-00001. [DOI] [PubMed] [Google Scholar]
- 20.Jarvis SS, VanGundy TB, Galbreath MM, Shibata S, Okazaki K, Reelick MF, Levine BD, Fu Q. Sex differences in the modulation of vasomotor sympathetic outflow during static handgrip exercise in healthy young humans. Am J Physiol Regul Integr Comp Physiol 301: R193–R200, 2011. doi: 10.1152/ajpregu.00562.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlsson LL, Montmerle S, Rohdin M, Linnarsson D. Central command and metaboreflex cardiovascular responses to sustained handgrip during microgravity. Respir Physiol Neurobiol 169, Suppl 1: S46–S49, 2009. doi: 10.1016/j.resp.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Khurana RK, Setty A. The value of the isometric hand-grip test–studies in various autonomic disorders. Clin Auton Res 6: 211–218, 1996. doi: 10.1007/BF02291136. [DOI] [PubMed] [Google Scholar]
- 23.Kienbaum P, Karlsson T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol 531: 861–869, 2001. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lalande S, Sawicki CP, Baker JR, Shoemaker JK. Effect of age on the hemodynamic and sympathetic responses at the onset of isometric handgrip exercise. J Appl Physiol (1985) 116: 222–227, 2014. doi: 10.1152/japplphysiol.01022.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lind AR, Taylor SH, Humphreys PW, Kennelly BM, Donald KW. The circulatiory effects of sustained voluntary muscle contraction. Clin Sci 27: 229–244, 1964. [PubMed] [Google Scholar]
- 26.Macefield VG, James C, Henderson LA. Identification of sites of sympathetic outflow at rest and during emotional arousal: concurrent recordings of sympathetic nerve activity and fMRI of the brain. Int J Psychophysiol 89: 451–459, 2013. doi: 10.1016/j.ijpsycho.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res 57: 461–469, 1985. doi: 10.1161/01.RES.57.3.461. [DOI] [PubMed] [Google Scholar]
- 28.Millar PJ, Murai H, Floras JS. Paradoxical muscle sympathetic reflex activation in human heart failure. Circulation 131: 459–468, 2015. doi: 10.1161/CIRCULATIONAHA.114.010765. [DOI] [PubMed] [Google Scholar]
- 29.Millar PJ, Murai H, Morris BL, Floras JS. Microneurographic evidence in healthy middle-aged humans for a sympathoexcitatory reflex activated by atrial pressure. Am J Physiol Heart Circ Physiol 305: H931–H938, 2013. doi: 10.1152/ajpheart.00375.2013. [DOI] [PubMed] [Google Scholar]
- 30.Morrison SF. Differential control of sympathetic outflow. Am J Physiol Regul Integr Comp Physiol 281: R683–R698, 2001. doi: 10.1152/ajpregu.2001.281.3.R683. [DOI] [PubMed] [Google Scholar]
- 31.Nobrega ACL, O’Leary D, Silva BM, Marongiu E, Piepoli MF, Crisafulli A. Neural regulation of cardiovascular response to exercise: role of central command and peripheral afferents. BioMed Res Int 2014: 478965, 2014. doi: 10.1155/2014/478965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Notarius CF, Atchison DJ, Floras JS. Impact of heart failure and exercise capacity on sympathetic response to handgrip exercise. Am J Physiol Heart Circ Physiol 280: H969–H976, 2001. doi: 10.1152/ajpheart.2001.280.3.H969. [DOI] [PubMed] [Google Scholar]
- 33.Notay K, Seed JD, Incognito AV, Doherty CJ, Nardone M, Burns MJ, Millar PJ. Validity and reliability of measuring resting muscle sympathetic nerve activity using short sampling durations in healthy humans. J Appl Physiol (1985) 121: 1065–1073, 2016. doi: 10.1152/japplphysiol.00736.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray CA, Rea RF, Clary MP, Mark AL. Muscle sympathetic nerve responses to static leg exercise. J Appl Physiol (1985) 73: 1523–1529, 1992. [DOI] [PubMed] [Google Scholar]
- 35.Ray CA, Secher NH, Mark AL. Modulation of sympathetic nerve activity during posthandgrip muscle ischemia in humans. Am J Physiol Heart Circ Physiol 266: H79–H83, 1994. [DOI] [PubMed] [Google Scholar]
- 36.Reed AS, Tschakovsky ME, Minson CT, Halliwill JR, Torp KD, Nauss LA, Joyner MJ. Skeletal muscle vasodilatation during sympathoexcitation is not neurally mediated in humans. J Physiol 525: 253–262, 2000. doi: 10.1111/j.1469-7793.2000.t01-1-00253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scherrer U, Pryor SL, Bertocci LA, Victor RG. Arterial baroreflex buffering of sympathetic activation during exercise-induced elevations in arterial pressure. J Clin Invest 86: 1855–1861, 1990. doi: 10.1172/JCI114916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scherrer U, Vissing SF, Victor RG. Effects of lower-body negative pressure on sympathetic nerve responses to static exercise in humans. Microneurographic evidence against cardiac baroreflex modulation of the exercise pressor reflex. Circulation 78: 49–59, 1988. doi: 10.1161/01.CIR.78.1.49. [DOI] [PubMed] [Google Scholar]
- 39.Seals DR. Cardiopulmonary baroreflexes do not modulate exercise-induced sympathoexcitation. J Appl Physiol (1985) 64: 2197–2203, 1988. [DOI] [PubMed] [Google Scholar]
- 40.Seals DR, Suwarno NO, Dempsey JA. Influence of lung volume on sympathetic nerve discharge in normal humans. Circ Res 67: 130–141, 1990. doi: 10.1161/01.RES.67.1.130. [DOI] [PubMed] [Google Scholar]
- 41.Soler NG. Laboratory evaluation of the autonomic system. In: Clinical Methods: The History, Physical, and Laboratory Examinations, edited by Walker HK, Hall WD, Hurst JW. Waltham, MA: Butterworth, 1990. [Google Scholar]
- 42.Sundlöf G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol 274: 621–637, 1978. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sverrisdóttir YB, Rundqvist B, Elam M. Relative burst amplitude in human muscle sympathetic nerve activity: a sensitive indicator of altered sympathetic traffic. Clin Auton Res 8: 95–100, 1998. doi: 10.1007/BF02267819. [DOI] [PubMed] [Google Scholar]
- 44.Tzemos N, Lim PO, Mackenzie IS, MacDonald TM. Exaggerated exercise blood pressure response and future cardiovascular disease. J Clin Hypertens (Greenwich) 17: 837–844, 2015. doi: 10.1111/jch.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Victor RG, Pryor SL, Secher NH, Mitchell JH. Effects of partial neuromuscular blockade on sympathetic nerve responses to static exercise in humans. Circ Res 65: 468–476, 1989. doi: 10.1161/01.RES.65.2.468. [DOI] [PubMed] [Google Scholar]
- 46.Wallin BG, Victor RG, Mark AL. Sympathetic outflow to resting muscles during static handgrip and postcontraction muscle ischemia. Am J Physiol Heart Circ Physiol 256: H105–H110, 1989. doi: 10.1152/ajpheart.1989.256.1.H105. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe K, Ichinose M, Tahara R, Nishiyasu T. Individual differences in cardiac and vascular components of the pressor response to isometric handgrip exercise in humans. Am J Physiol Heart Circ Physiol 306: H251–H260, 2014. doi: 10.1152/ajpheart.00699.2013. [DOI] [PubMed] [Google Scholar]
- 48.White DW, Shoemaker JK, Raven PB. Methods and considerations for the analysis and standardization of assessing muscle sympathetic nerve activity in humans. Auton Neurosci 193: 12–21, 2015. doi: 10.1016/j.autneu.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]