Abstract
This study tested the hypothesis that sacral neuromodulation, i.e., electrical stimulation of afferent axons in sacral spinal root, can block pudendal afferent inhibition of the micturition reflex. In α-chloralose-anesthetized cats, pudendal nerve stimulation (PNS) at 3–5 Hz was used to inhibit bladder reflex activity while the sacral S1 or S2 dorsal root was stimulated at 15–30 Hz to mimic sacral neuromodulation and to block the bladder inhibition induced by PNS. The intensity threshold (T) for PNS or S1/S2 dorsal root stimulation (DRS) to induce muscle twitch of anal sphincter or toe was determined. PNS at 1.5–2T intensity inhibited the micturition reflex by significantly (P < 0.01) increasing bladder capacity to 150–170% of control capacity. S1 DRS alone at 1–1.5T intensity did not inhibit bladder activity but completely blocked PNS inhibition and restored bladder capacity to control level. At higher intensity (1.5–2T), S1 DRS alone inhibited the micturition reflex and significantly increased bladder capacity to 135.8 ± 6.6% of control capacity. However, the same higher intensity S1 DRS applied simultaneously with PNS, suppressed PNS inhibition and significantly (P < 0.01) reduced bladder capacity to 126.8 ± 9.7% of control capacity. S2 DRS at both low (1T) and high (1.5–2T) intensity failed to significantly reduce PNS inhibition. PNS and S1 DRS did not change the amplitude and duration of micturition reflex contractions, but S2 DRS at 1.5–2T intensity doubled the duration of the contractions and increased bladder capacity. These results are important for understanding the mechanisms underlying sacral neuromodulation of nonobstructive urinary retention in Fowler’s syndrome.
Keywords: sacral, pudendal, neuromodulation, bladder, cat
INTRODUCTION
Sacral neuromodulation is a Food and Drug Administration-approved therapy that can treat both overactive bladder (OAB) and nonobstructive urinary retention (NOUR) (9, 11, 24). It requires surgical implantation of a programmable stimulator subcutaneously, which delivers electrical stimulation via an electrode lead inserted through the sacral foramen to continuously stimulate the sacral S3 spinal nerve (12). Currently, the mechanisms underlying sacral neuromodulation therapy are still not fully understood; and it is especially difficult to understand how the same sacral neuromodulation therapy can treat two opposite bladder conditions, i.e., bladder overactivity (OAB) and bladder underactivity. Our previous studies in cats showed that sacral neuromodulation inhibits both normal and overactive bladder activity (26, 27) and that multiple inhibitory neurotransmitter receptors (GABA, opioid, and β-adrenergic) are involved in sacral neuromodulation of bladder overactivity (1, 10). However, currently there is no animal model for studying the neurotransmitters involved in sacral neuromodulation of bladder underactivity or NOUR.
In 1988, Dr. Clare Fowler described a syndrome in women with NOUR in which the external urethral sphincter (EUS) EMG exhibits abnormal periodic complex activity (7). Later, it was discovered that sacral neuromodulation treats NOUR particularly well in women with Fowler’s syndrome (6, 20). While the pathophysiological mechanisms underlying Fowler’s syndrome are still uncertain, it is known that activation of the EUS (4) or external anal sphincter (15) in animals elicits afferent activity in the pudendal nerve that inhibits the micturition reflex and that electrical stimulation of afferent axons in the pudendal nerve produces similar inhibition (5, 21, 22). These observations led to the hypothesis that in Fowler’s syndrome the abnormal periodic EUS activity generates EUS afferent firing that inhibits micturition causing urinary retention, while sacral neuromodulation activates afferent nerves in the sacral spinal root that suppress the inhibition and normalize bladder function (12).
In women with Fowler’s syndrome, it was also found that abnormal EMG activity of the external anal sphincter (EAS) commonly coexists with abnormal EUS EMG activity (17, 25). Therefore, it is logical to further hypothesize that abnormal afferent firing originating from the EUS as well as the EAS and possibly from the pelvic floor muscles may inhibit the micturition reflex in the central nervous system (CNS) and cause bladder underactivity or NOUR, while sacral neuromodulation can suppress this inhibition. Testing this hypothesis in animal models may provide insights into the mechanisms underlying the beneficial effects of sacral neuromodulation in women with Fowler’s syndrome and provide a better understanding of sacral neuromodulation therapy in general.
In this study using anesthetized cats, pudendal nerve stimulation (PNS) was used to activate the afferent nerves innervating the EUS/EAS and pelvic floor muscles. PNS inhibits the micturition reflex and increases bladder capacity, mimicking in part the bladder underactivity caused by abnormal EUS/EAS afferent activity in Fowler’s syndrome. Sacral dorsal roots (S1 or S2) were stimulated to mimic sacral neuromodulation and to determine if the stimulation can suppress/block the pudendal afferent-induced inhibition. This study established an animal model for sacral neuromodulation of pudendal afferent inhibition of the micturition reflex and provided basic science evidence to support the hypothesis that sacral neuromodulation treats NOUR in women with Fowler’s syndrome by blocking the abnormal EUS/EAS afferent-induced inhibition of micturition.
MATERIALS AND METHODS
The protocol and animal use in this study were approved by the Animal Care and Use Committee at the University of Pittsburgh.
Surgical procedures.
A total of seven cats (2 male and 5 female, 2.8–4.1 kg; Liberty Research, Waverly, NY) were used in this study. The animals were anesthetized with isoflurane (2–5% in oxygen) during surgery and then switched to α-chloralose anesthesia (initial 65 mg/kg iv and supplemented as needed) during data collection. The left cephalic vein was catheterized for administration of anesthetics and fluid. A tracheotomy was performed, and a tube was inserted to keep the airway patent. A catheter was inserted into right carotid artery to monitor systemic blood pressure. Heart rate and blood oxygen were monitored by a pulse oximeter (9847V; NONIN Medical, Plymouth, MN) attached to the tongue. Through an abdominal incision, the ureters were isolated, tied, and cut for external drainage. A double lumen catheter was inserted into the bladder via a small cut in the proximal urethra and secured by a ligature around the urethra. One lumen was connected to a pump to slowly (1–4 ml/min) infuse saline into the bladder. The other lumen was attached to a pressure transducer to measure bladder pressure. The right pudendal nerve was dissected via a 3- to 4-cm incision in the sciatic notch lateral to the tail, and the intact nerve was mounted on a tripolar cuff electrode (NC223pt; MicroProbe, Gaithersburg, MD). During the experiment, the cuff electrode was connected to an electrical stimulator (S88; Grass Medical Instruments, Quincy, MA) via a constant voltage stimulus isolator (SIU5; Grass Medical Instruments). The surgical incisions including the skin and muscle layers were closed by sutures.
The spinal cord and cauda equina were exposed between the L5 and S3 vertebrae via a dorsal laminectomy. The spinal dura was cut, and the S1-S2 sacral dorsal roots on the right side were separated for electrical stimulation. A bipolar stainless steel hook electrode was used during the experiment to stimulate individual S1/S2 dorsal roots by delivering electrical pulses that were generated by an electrical stimulator (S88; Grass Medical Instruments). The animal was mounted in a modified Narishige “Eccles” spinal cord frame in which the hip was supported by metal pins, and the spinous process at the rostral end of the laminectomy was secured with a clamp. The skin, cut mid-sagittally from L4 to S3, was tied along each margin to form a pool that was filled with warmed (35–37°C) mineral oil. The temperature of the animal was maintained at 36–38°C using a heating pad during the experiments.
Experimental protocol.
At the beginning of each experiment, uniphasic rectangular pulses (5-Hz frequency, 0.2-ms pulse width) were used to stimulate the pudendal nerve and the S1 or S2 dorsal roots to determine the intensity threshold (T) for inducing observable twitching of the anal sphincter or toe. We assume that T represents the intensity threshold for large diameter group I afferents because sphincter reflexes can be elicited by electrical stimulation of afferents in the sacral spinal roots at intensity thresholds below those of large diameter motor axons innervating the sphincter muscles (8). Then, multiple cystometrograms (CMGs) were performed by slowly infusing the bladder with saline to determine the bladder capacity defined as the bladder volume threshold to induce a micturition reflex contraction of large amplitude (>30 cmH2O) and long duration (>20 s). Once the control bladder capacity stabilized during repeated saline CMGs, preliminary tests were conducted by applying PNS (3 or 5 Hz, 0.2 ms) or S1 dorsal root stimulation (DRS; 15 or 30 Hz, 0.2 ms) during several CMGs to determine the minimal PNS intensity (1.5–2T) that increased bladder capacity >1 ml or the maximal S1 DRS intensity (1–1.5T) that did not increase bladder capacity (<1 ml). After the preliminary tests, two to three control CMGs without stimulation were performed, followed by additional five CMGs in the following order: 1) CMG during S1 DRS, 2) CMG during PNS, 3) CMG during combined PNS and S1 DRS, 4) CMG during PNS, and 5) control CMG to examine any poststimulation effect. Then, the above sequence of CMG tests was performed again after the intensity of S1 DRS was increased to 1.5–2T that induced bladder inhibition and increased bladder capacity during the CMGs. The bladder was emptied after each CMG. A 2- to 3-min waiting period was inserted between CMGs, which in previous studies (10, 13, 27) allowed the bladder reflex to fully recover.
After S1 DRS was tested, the same experimental protocol was also used to test S2 DRS (30 Hz, 0.2 ms) at 1T intensity that did not change bladder capacity during the CMGs. Then, the same experimental protocol was tested again with S2 DRS at 1.5–2T intensity that inhibited reflex bladder activity and increased bladder capacity during the CMGs.
Data analysis.
The control bladder capacity for each animal was determined by averaging the measurements during two to three control CMGs before testing different combinations of PNS and S1/S2 DRS. Then, the control bladder capacity immediately before testing the stimulation and the bladder capacity during the testing of different stimulations were measured and normalized to the averaged control capacity in the same animal. The maximal amplitude, duration, and area under curve of the micturition reflex contractions were also measured in each CMG and normalized to the averaged measurements of the control CMGs. The data from different animals are presented as means ± SE. Statistical significance (P < 0.05) was determined by repeated-measures one-way ANOVA followed by Dunnett’s multiple comparison.
RESULTS
S1 DRS completely blocks pudendal inhibition of the micturition reflex.
S1 DRS (15 or 30 Hz) at a low intensity (1–1.5T) did not change bladder capacity during CMGs, while PNS at 1.5–2T intensity inhibited the micturition reflex and significantly (P < 0.01) increased bladder capacity to 150.8 ± 8.7% of control capacity (12.8 ± 3.4 ml) (Fig. 1, n = 7 cats). When the 1–1.5T S1 DRS was combined with PNS, it completely blocked the PNS inhibition and restored bladder capacity to the control level (Fig. 1). After S1 DRS was terminated, PNS could again inhibit the micturition reflex and significantly (P < 0.01) increased bladder capacity to 147.7 ± 9.2% of control capacity (Fig. 1). Neither PNS or S1 DRS nor the combined stimulation elicited a poststimulation effect (see the last CMG trace in Fig. 1A). The amplitude, duration, and area under curve of the micturition reflex contractions were not significantly changed by either PNS, S1 DRS, or the combined stimulation (Fig. 2).
Fig. 1.
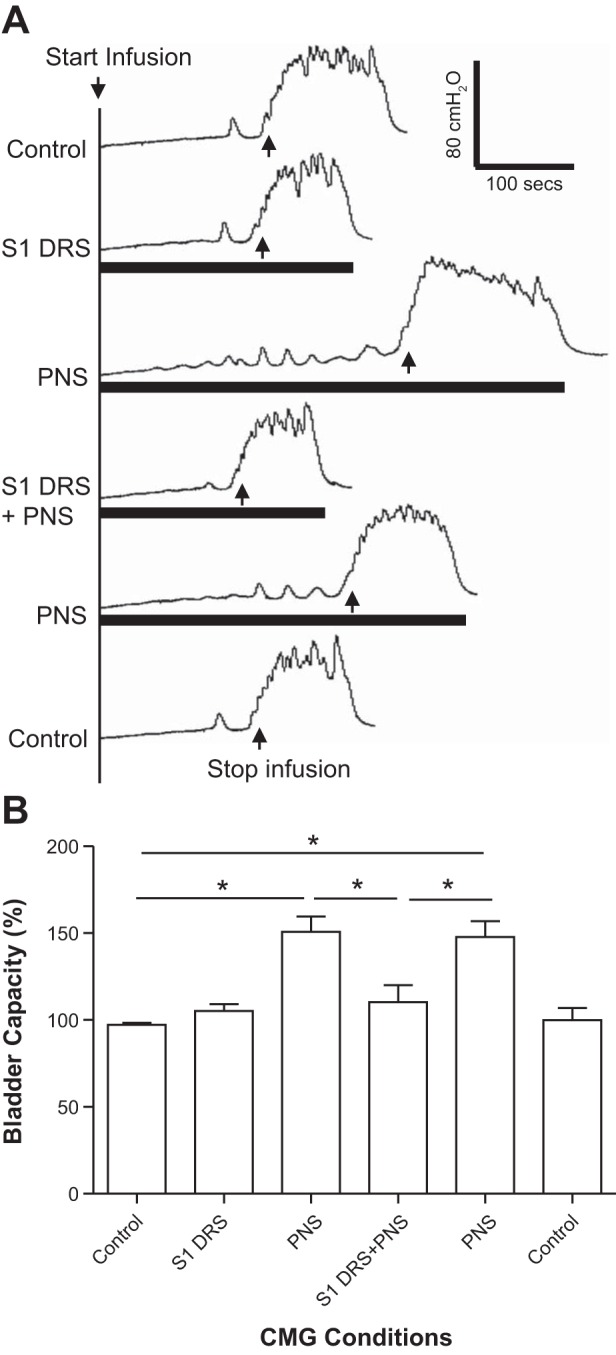
Electrical stimulation (1–1.5T) of the S1 dorsal root did not increase bladder capacity but removed the increase in bladder capacity induced by pudendal nerve stimulation (PNS). A: repeated cystometrogram (CMG) traces with/without PNS and/or S1 dorsal root stimulation (DRS). The black bars under the CMG traces indicate the duration of PNS and/or S1 DRS. PNS: 5 Hz, 0.2 ms, 1.5T = 0.36 V. S1 DRS: 30 Hz, 0.2 ms, 1.5T = 0.3 V. T: threshold intensity for inducing anal twitch; infusion rate: 2 ml/min. B: summarized bladder capacities measured during different CMGs (n = 7 cats). *Significant difference (P < 0.01, one-way ANOVA). PNS: 3 or 5 Hz, 0.2 ms, 1.5–2T, T = 0.2–0.6 V. S1 DRS: 15 or 30 Hz, 0.2 ms, 1–1.5T, T = 0.16–0.6 V.
Fig. 2.
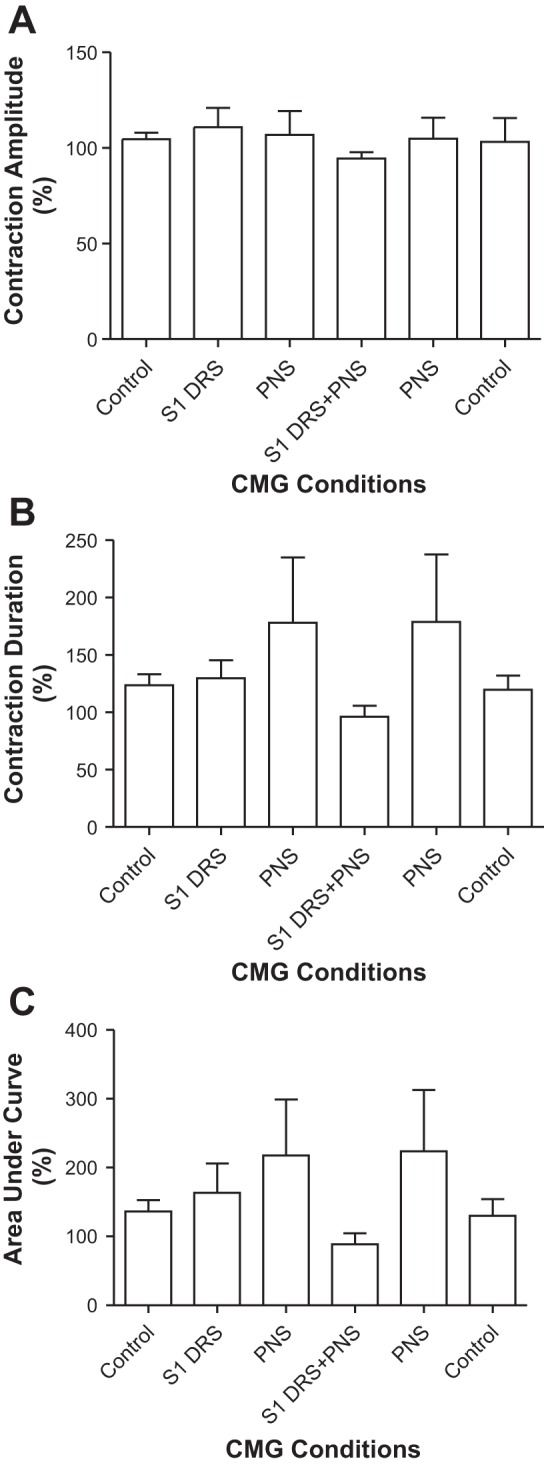
Effects of pudendal nerve stimulation (PNS) and/or S1 dorsal root stimulation (DRS) on the amplitude (A), duration (B), and area under curve (C) of micturition reflex contraction (n = 7 cats). PNS: 3 or 5 Hz, 0.2 ms, 1.5–2T, T = 0.2–0.6 V. S1 DRS: 15 or 30 Hz, 0.2 ms, 1–1.5T, T = 0.16–0.6 V.
S1 DRS at a higher intensity (1.5–2T) significantly (P < 0.05) increased bladder capacity to 135.8 ± 6.6% of control capacity (10.3 ± 2.8 ml; Fig. 3, n = 6 cats). PNS at 1.5–2T intensity also significantly (P < 0.01) increased bladder capacity to 171.6 ± 10.4% of control capacity (Fig. 3). When the same S1 DRS was combined with PNS, it suppressed the PNS inhibition and significantly (P < 0.01) reduced bladder capacity to 126.8 ± 9.7% of control capacity that was not significantly different from the control (Fig. 3). After the S1 DRS was terminated, PNS again significantly (P < 0.01) increased bladder capacity to 174.7 ± 7.1% of control capacity (Fig. 3). Neither PNS or S1 DRS nor the combined stimulation elicited a poststimulation effect (see the last CMG trace in Fig. 3A). The amplitude, duration, and area under curve of the micturition reflex contractions were not significantly changed by either PNS, S1 DRS, or the combined stimulation (Fig. 4).
Fig. 3.
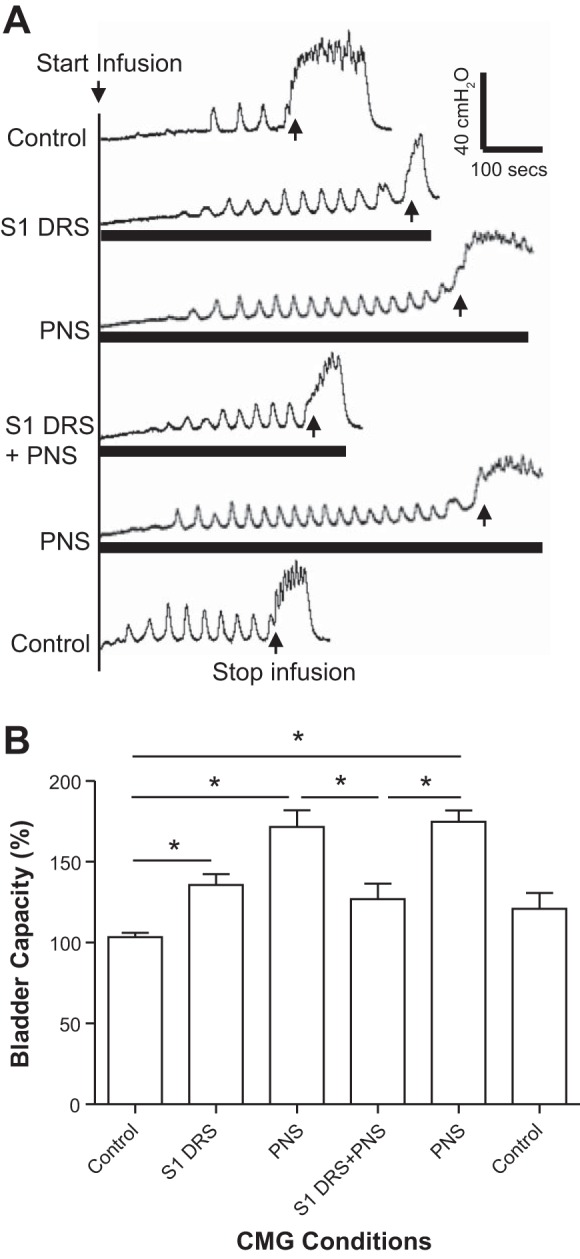
Electrical stimulation (1.5–2T) of the S1 dorsal root increased bladder capacity, but also removed the increase in bladder capacity induced by pudendal nerve stimulation (PNS). A: repeated cystometrogram (CMG) traces with/without PNS and/or S1 dorsal root stimulation (DRS). The black bars under the CMG traces indicate the durations of PNS and/or S1 DRS. PNS: 5 Hz, 0.2 ms, 1.5T = 0.9 V. S1 DRS: 15 Hz, 0.2 ms, 2T = 0.4 V. T: threshold intensity for inducing anal twitch; infusion rate: 3 ml/min. B: summarized bladder capacities measured during different CMGs (n = 6 cats). *Significant difference (P < 0.05, one-way ANOVA). PNS: 3–5 Hz, 0.2 ms, 1.5–2T, T = 0.2–0.6 V. S1 DRS: 15 or 30 Hz, 0.2 ms, 1.5–2T, T = 0.16–0.36 V.
Fig. 4.
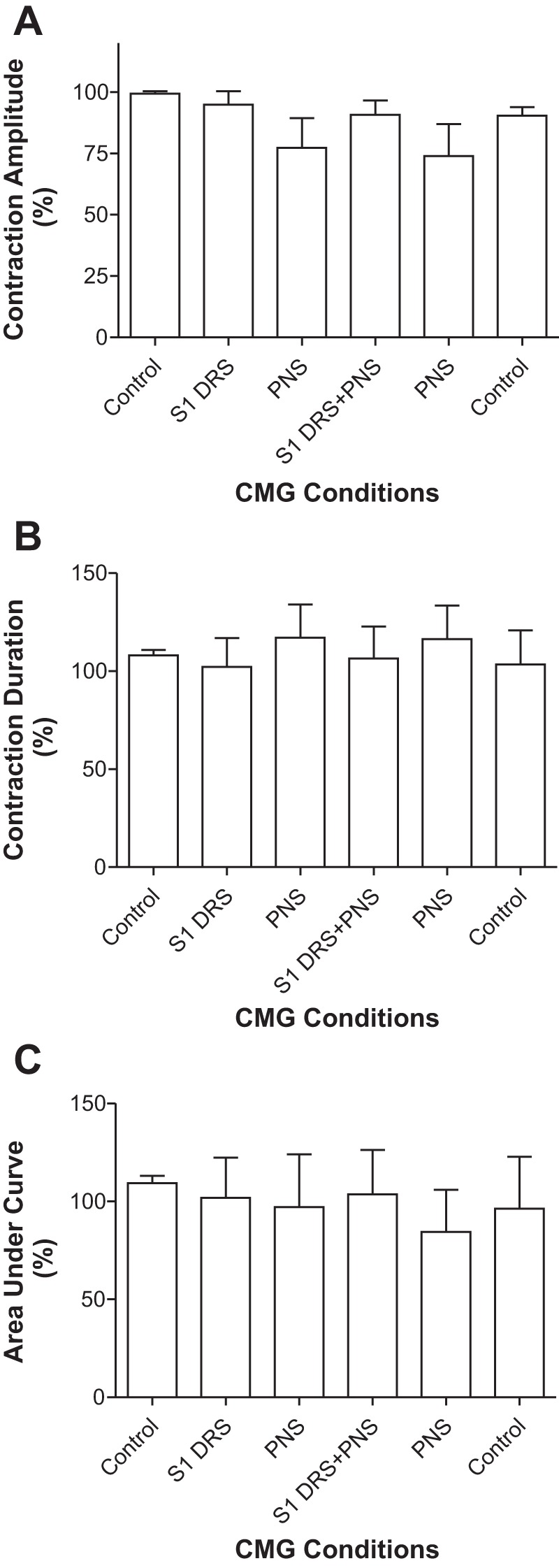
Effects of pudendal nerve stimulation (PNS) and/or S1 dorsal root stimulation (DRS) on the amplitude (A), duration (B), and area under curve (C) of micturition reflex contractions (n = 6 cats). PNS: 3–5 Hz, 0.2 ms, 1.5–2T, T = 0.2–0.6 V. S1 DRS: 15 or 30 Hz, 0.2 ms, 1.5–2T, T = 0.16–0.36 V.
S2 DRS failed to block pudendal inhibition of the micturition reflex.
S2 DRS at a low intensity (1T) did not change bladder capacity during CMGs, while PNS at 1.5–2T intensity significantly (P < 0.01) increased bladder capacity to 155.7 ± 21.0% of control capacity (11.3 ± 3.4 ml; Fig. 5, n = 5 cats). However, when the same intensities of S2 DRS and PNS were applied simultaneously, the bladder capacity was not significantly (P > 0.05) different from that during PNS alone (Fig. 5), i.e., S2 DRS failed to significantly reduce PNS inhibition. After the S2 DRS was terminated, PNS was repeated and significantly (P < 0.01) increased bladder capacity to 155.2 ± 12.1% of control capacity (Fig. 5). Neither PNS or S2 DRS nor the combined stimulation elicited a poststimulation effect (see the last CMG trace in Fig. 5A). The amplitude, duration, and area under curve of the micturition reflex contractions were not significantly changed by either PNS, S2 DRS, or the combined stimulation (Fig. 6).
Fig. 5.
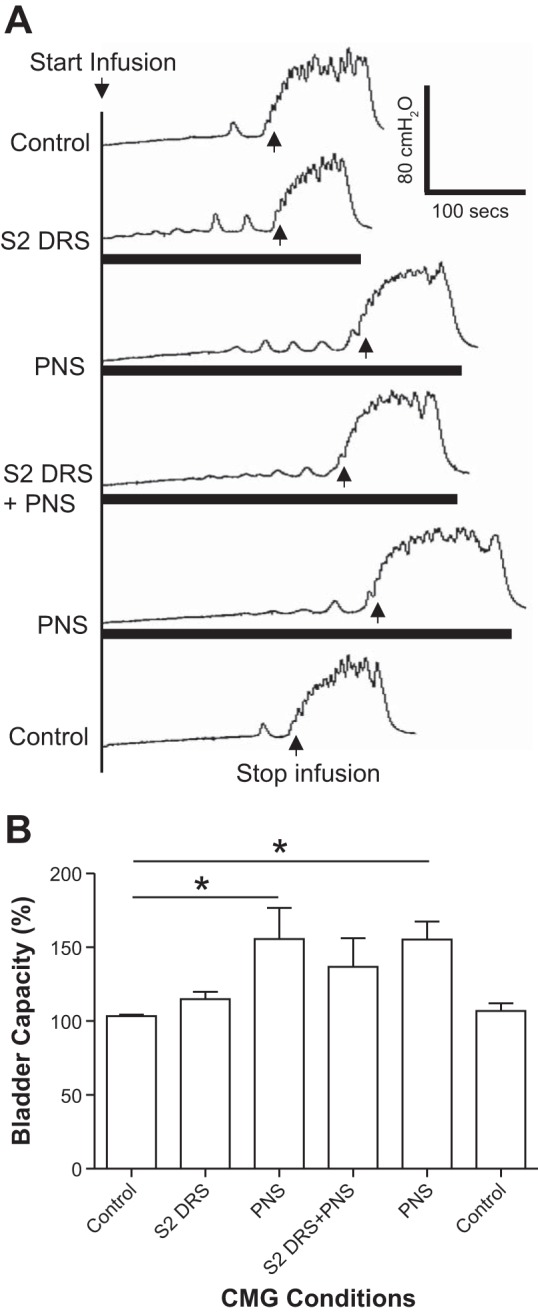
Electrical stimulation (1T) of S2 dorsal root did not increase bladder capacity but slightly reduced the increase in bladder capacity induced by pudendal nerve stimulation (PNS). A: repeated cystometrogram (CMG) traces with/without PNS and/or S2 dorsal root stimulation (DRS). The black bars under the CMG traces indicate the durations of PNS and/or S2 DRS. PNS: 5 Hz, 0.2 ms, 1.5T = 0.36 V. S2 DRS: 30 Hz, 0.2 ms, 1T = 0.3 V. T: threshold intensity for inducing anal twitch; infusion rate: 2 ml/min. B: summarized bladder capacities measured during different CMGs (n = 5 cats). *Significant difference (P < 0.01, one-way ANOVA). PNS: 3–5 Hz, 0.2 ms, 1.5–2T, T = 0.2–0.56 V. S2 DRS: 30 Hz, 0.2 ms, 1T, T = 0.14–0.3 V.
Fig. 6.
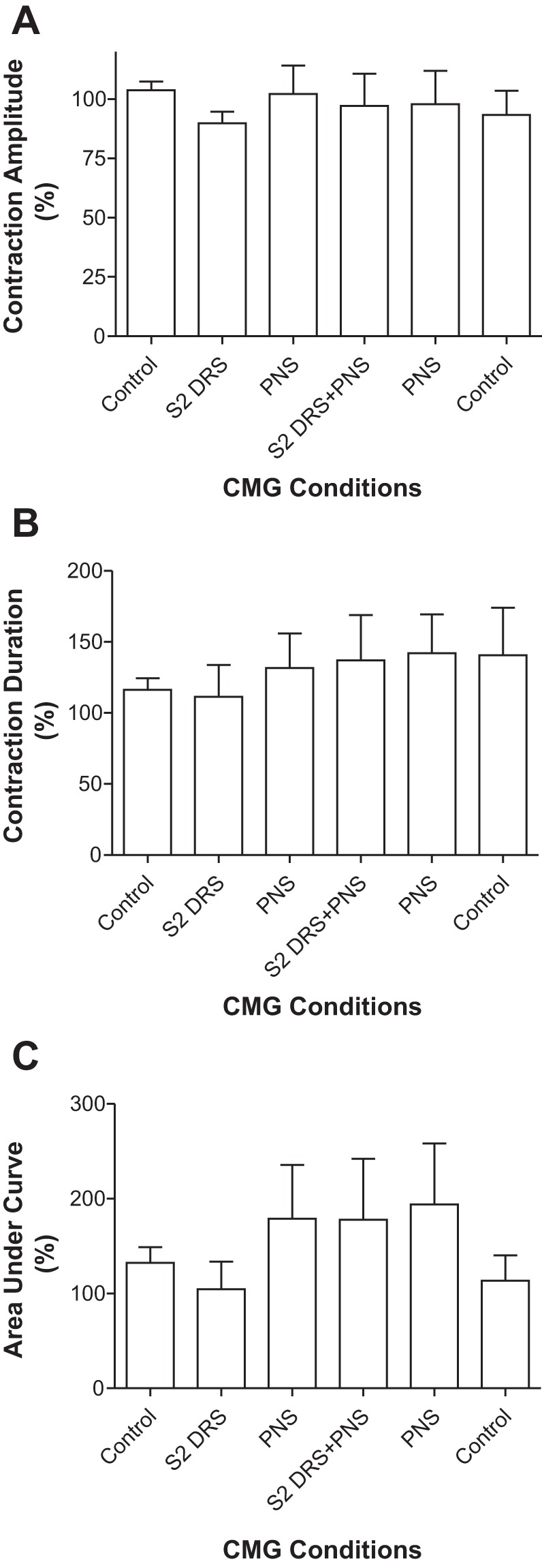
Effects of pudendal nerve stimulation (PNS) and/or S2 dorsal root stimulation (DRS) on the amplitude (A), duration (B), and area under curve (C) of micturition reflex contractions (n = 5 cats). PNS: 3–5 Hz, 0.2 ms, 1.5–2T, T = 0.2–0.56 V. S2 DRS: 30 Hz, 0.2 ms, 1T, T = 0.14–0.3 V.
S2 DRS at a higher intensity (1.5–2T) significantly (P < 0.01) increased bladder capacity to 134.4 ± 7.6% of control capacity, while PNS alone at 1.5–2T intensity also significantly (P < 0.01) increased bladder capacity to 163.3 ± 12.9% of control capacity (Fig. 7). When the same intensities of S2 DRS and PNS were applied simultaneously, the bladder capacity significantly (P < 0.01) increased to 157.9 ± 12.5% of control capacity, which was not different from that during PNS alone (Fig. 7). After the S2 DRS was terminated, PNS significantly (P < 0.01) increased bladder capacity to 156.5 ± 11.2% of control capacity (Fig. 7). A small poststimulation effect after combined PNS and S2 DRS did occur in four animals (see the last CMG trace in Fig. 7A), but this effect was not significant in the average from 5 animals (Fig. 7B). The amplitude and area under curve of the micturition reflex contractions were not significantly changed by either 1T S2 DRS, PNS, or the combined stimulation (Fig. 8). However, S2 DRS at a higher intensity (1.5–2T) significantly (P < 0.01) increased the duration of the micturition reflex contraction to 214.5 ± 55.6% of the control duration (115.6 ± 12.2 s) (Figs. 7A and 8B).
Fig. 7.
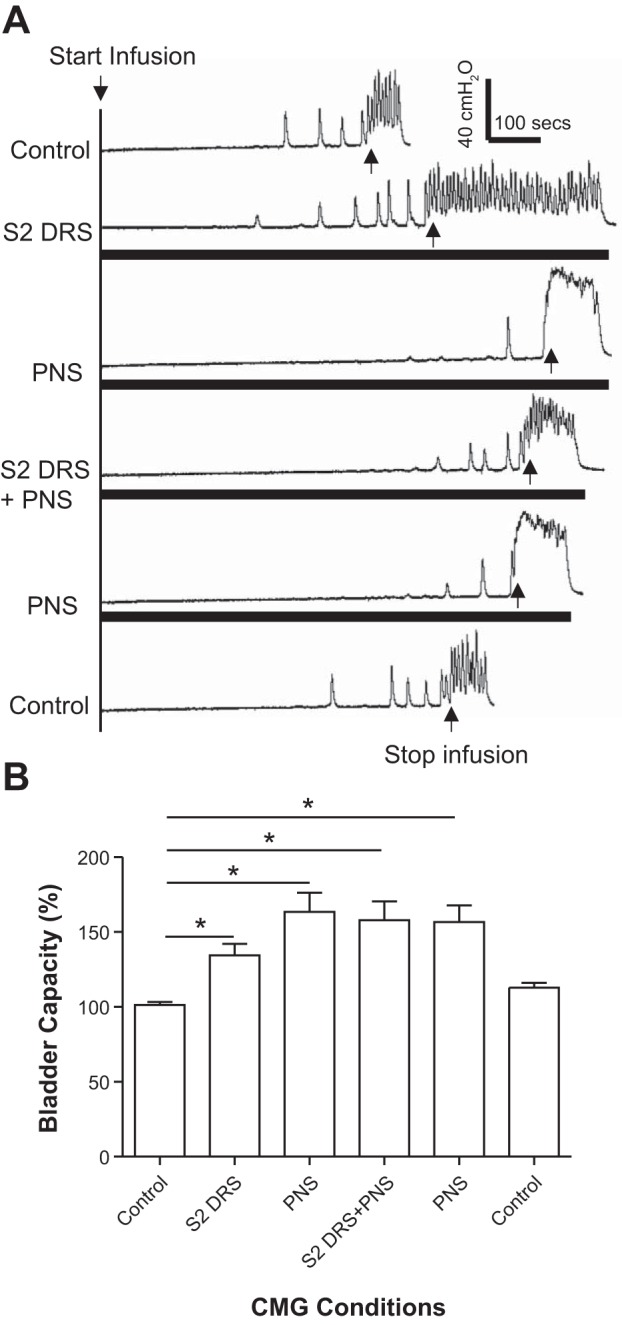
Electrical stimulation (1.5–2T) of S2 dorsal root increased bladder capacity but did not reduce the increase in bladder capacity induced by pudendal nerve stimulation (PNS). A: repeated cystometrogram (CMG) traces with/without PNS and/or S2 dorsal root stimulation (DRS). The black bars under the CMG traces indicate the durations of PNS and/or S1 DRS. PNS: 5 Hz, 0.2 ms, 2T = 0.48 V. S2 DRS: 30 Hz, 0.2 ms, 2T = 0.28 V. T: threshold intensity for inducing anal twitch; infusion rate: 1 ml/min. B: summarized bladder capacities measured during different CMGs (n = 5 cats). *Significant difference (P < 0.01, one-way ANOVA). PNS: 3–5 Hz, 0.2 ms, 1.5–2T, T = 0.2–0.56 V. S2 DRS: 30 Hz, 0.2 ms, 1.5–2T, T = 0.14–0.3 V.
Fig. 8.
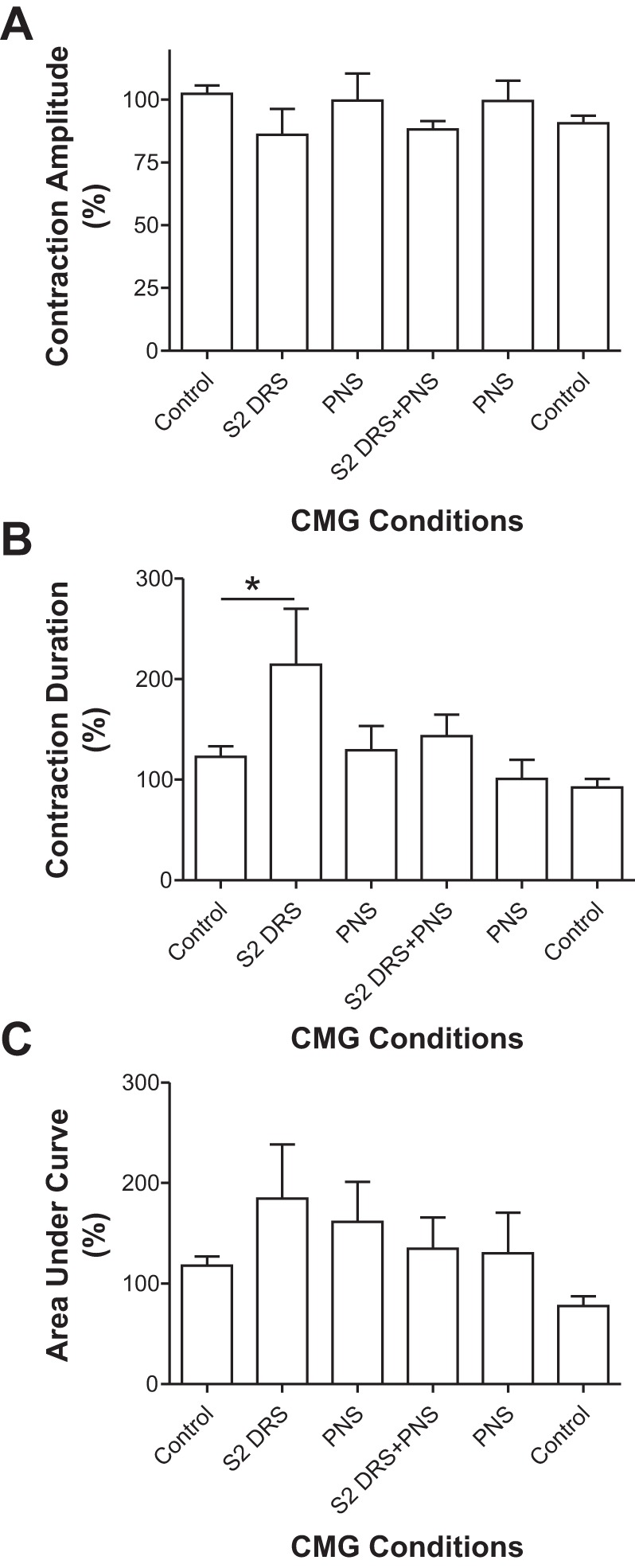
Effects of pudendal nerve stimulation (PNS) and/or S2 dorsal root stimulation (DRS) on the amplitude (A), duration (B), and area under curve (C) of micturition reflex contractions (n = 5 cats). PNS: 3–5 Hz, 0.2 ms, 1.5–2T, T = 0.2–0.56 V. S2 DRS: 30 Hz, 0.2 ms, 1.5–2T, T = 0.14–0.3 V.
DISCUSSION
This study in cats revealed that sacral neuromodulation elicited by S1 but not S2 DRS can partially suppress or completely block the increase in bladder capacity induced by PNS (Figs. 1, 3, 5, and 7). However, neither S1 DRS or PNS nor combined stimulation altered the amplitude or duration of the micturition reflex contractions (Fig. 2 and Fig. 4). On the other hand, S2 DRS at a higher intensity prolonged the duration of the micturition reflex contraction (Fig. 8). Because the PNS inhibitory effect on bladder capacity is mediated by suppression of bladder reflex pathways in the CNS (13, 14), it is reasonable to conclude that the suppression of the PNS inhibitory effect by S1 DRS must also occur in the CNS and be mediated by a modulation of synaptic transmission in the PNS-bladder inhibitory pathway. Figure 9 shows a hypothetical neural circuit in the sacral spinal cord that could mediate PNS inhibition of the micturition reflex and a circuit that could mediate the suppression of PNS inhibition by S1 DRS. Similar inhibitory interactions could also exist in the brain.
Fig. 9.
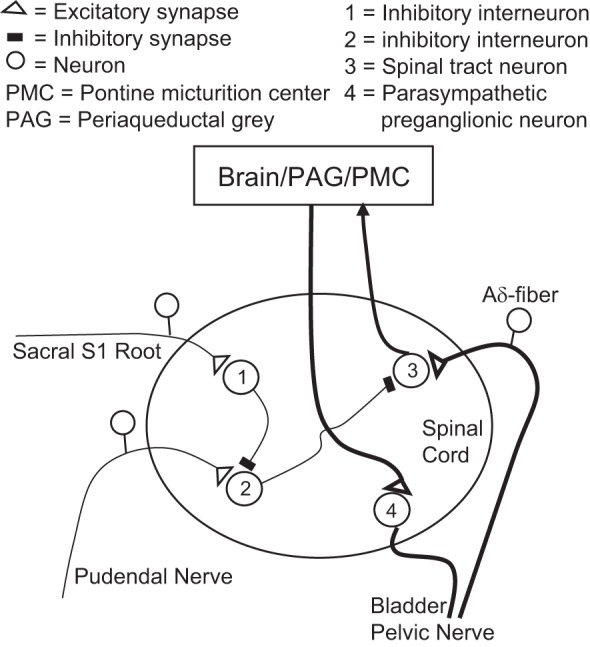
Hypothetical spinal mechanisms that underlie: first, the inhibition of the micturition reflex pathway by afferent axons in the pudendal nerve, and second, the suppression of the pudendal afferent inhibitory pathway by electrical stimulation of the sacral S1 dorsal root. Pudendal afferents activate an inhibitory interneuron (2) that suppresses activity of a spinal tract neuron (3) on the ascending limb of the micturition reflex. S1 dorsal root stimulation activates an inhibitory interneuron (1) that suppresses activity of the interneuron (2) in the pudendal afferent inhibitory pathway.
The results of our study in combination with the results of earlier animal experiments provide considerable support for the hypothesis (12, 17) that sacral neuromodulation reverses the bladder sensory and voiding dysfunctions in women with Fowler’s syndrome or NOUR by blocking CNS inhibitory mechanisms induced by abnormal EUS/EAS afferent nerve activity passing through the pudendal nerve to the sacral spinal cord. First, inhibition of reflex bladder activity can be induced by electrical stimulation of EAS in the nonhuman primates (15) or by activation of EAS and EUS contractions by electrical stimulation of motor axons in the S1 ventral root in anesthetized cats (4). The latter inhibition is eliminated by transecting the pudendal nerve or paralyzing striated muscle by administration of a neuromuscular blocking agent. Thus afferent nerves responding to sphincter muscle contractions must be able to inhibit bladder activity generated by central neural mechanisms. Furthermore, the inhibition elicited by EAS stimulation must occur in part within the lumbosacral spinal cord because it persists after transection of the thoracic spinal cord (15).
In the present experiments, the intact pudendal nerve was stimulated by intensities (1.5–2T) sufficient to activate efferent motor axons, group I (Aα) afferent axons innervating the EUS and EAS and also group II (Aβ) afferent axons innervating the skin of the perineum and genital organs (18). Thus, the increased bladder capacity induced by PNS could be due to direct activation of multiple afferent pathways or indirect activation of sphincter afferent pathways in response to the sphincter contractions. Nevertheless, the inhibition elicited by PNS was completely eliminated by simultaneous activation of the S1 dorsal root indicating that multiple somato-bladder inhibitory mechanisms that might be activated by PNS share the common characteristic of reversal by S1 DRS.
It is noteworthy that in the cat either S1 or S2 DRS increases bladder capacity when applied alone during saline or acetic acid CMGs (26, 27), yet only S1 DRS applied during PNS reverses the PNS inhibition and decreases bladder capacity. These competing effects of S1 DRS that can occur under different experimental conditions resemble the seemingly paradoxical clinical effects of sacral neuromodulation to improve, on the one hand, storage function of the bladder in OAB patients but also improve voiding function in patients with Fowler’s syndrome. However, based on the stimulus intensities which elicit these opposite effects in cats, it appears that the reversal of PNS inhibition is mediated by low intensities (1–1.5T) of S1 DRS that do not elicit inhibition of the bladder, indicating that the reversal of PNS inhibition is triggered by activation of lower-threshold, larger-diameter group I afferent axons than higher-threshold (1.5–2T) group II afferent axons (18) that elicit the increase in bladder capacity. Higher intensity (1.5–2T) S1 DRS, which alone produces an inhibition of bladder activity, still counteracts the inhibitory effect of pudendal afferent stimulation because combined S1 DRS at the higher intensity and PNS always produces a bladder capacity similar to the capacity during the inhibition elicited by S1 DRS alone (Figs. 1 and 3). Thus S1 DRS even at higher intensities must eliminate PNS inhibition suggesting that the PNS and sacral neuromodulation-induced inhibitions probably occur by different mechanisms. Furthermore, the elimination of PNS inhibition by S1 DRS at an intensity that alone has no effect on bladder capacity indicates that this effect of S1 DRS is not due to excitation of the bladder reflex pathway or suppression of a tonic inhibitory mechanism but rather is due to selectively targeting the PNS induced inhibitory mechanism (Fig. 9).
Clinical results showed that sacral neuromodulation, which enables NOUR patients to better sense their bladder, only slightly (not significantly) enhances bladder contractions during voiding (3). In cats, S1 DRS, which suppressed the PNS inhibition (Figs. 1 and 3), also did not significantly enhance the micturition reflex contractions (Figs. 2 and 4). However, significant enhancement of bladder contractions did occur during S2 DRS at 1.5–2T intensity (Fig. 8B). This enhancement is probably due to activation of afferent axons that originate in the S2 dorsal root ganglia and then pass through the pelvic and pudendal nerves to innervate the pelvic viscera, external genital organs, and perineum (16, 23). Stimulation of many of these afferents is known to enhance reflex bladder contractions (5, 21, 22). However, the enhancement is unlikely due to activation of Aδ bladder afferent axons because these axons have a high threshold and would require >4T stimulus intensities (18).
When sacral neuromodulation is used in patients to reverse the symptoms of Fowler’s syndrome, the stimulation is usually applied to S3 spinal root (12). This root is equivalent to the S1/S2 roots in cats due to spinal segmental differences between cats and humans. Humans have only five lumbar segments, and cats have seven lumbar segments. Our previous studies in cats showed that S1/S2 DRS is effective in inhibiting both normal and overactive bladder activity, but low-intensity stimulation of sacral ventral roots has no effect (26, 27). Previous clinical studies also showed that the therapeutic effects of sacral neuromodulation are due to stimulation of afferent nerve fibers instead of efferent nerve fibers in the spinal roots (8, 19). Therefore, this study established an appropriate animal model that can be used to determine the sites of action and possible neurotransmitter mechanisms underlying the reversal by sacral neuromodulation of urinary retention and bladder sensory dysfunction in Fowler’s syndrome.
The effects of PNS and S1 DRS on reflex bladder activity could occur in both ascending and descending pathways of the supraspinal micturition reflex (Fig. 9). The increase in bladder capacity by PNS indicates that pudendal afferent input suppresses bladder sensory input to the brain either at synapses in the spinal cord or in the periaqueductal gray/pontine micturition center (PMC) in the brainstem (Fig. 9), thereby delaying the activation of the bulbospinal efferent pathway originating in the PMC. The delay in triggering of a micturition reflex contraction without changing the amplitude of the micturition reflex contraction indicates a selective action on bladder sensory mechanisms. Therefore, S1 DRS is likely to act by blocking PNS inhibition on the ascending sensory limb of the micturition reflex pathway (Fig. 9). Brain imaging studies in patients with Fowler’s syndrome suggest a similar sensory dysfunction and a reversal of that dysfunction by sacral neuromodulation (2). However, a previous study in decerebrate cats (14) also revealed that PNS inhibits the bladder contractions induced by electrical stimulation of the PMC and activation of the descending micturition reflex pathway. This inhibition was mediated by reflex activation of the lumbar sympathetic inhibitory pathway to the bladder. Although different experimental conditions (i.e., intact neuraxis vs. decerebration) might account for the unmasking of this second mechanism of PNS inhibition, further studies are needed to test the effect of S1 DRS on PNS inhibition of bladder contractions induced by stimulation of the PMC.
In the present experiments, PNS and S1 DRS were both applied to the nerves on the same side. Our previous studies in cats showed that 5-Hz PNS is inhibitory to the bladder but 20- to 30-Hz PNS is excitatory to the bladder (21, 22). It is possible that S1 DRS at 15–30 Hz might simply summate with the 5-Hz pudendal afferent firing to generate a 20- to 35-Hz input to the spinal cord, thereby eliminating the pudendal inhibitory effect. However, this mechanism seems unlikely because 1) the intensity of S1 DRS was below the threshold for eliciting inhibition and therefore was not likely to activate pudendal afferent axons projecting through the dorsal roots; 2) pudendal afferent nerves project into the spinal cord predominately via S2 dorsal roots (23), and therefore the majority of pudendal afferent nerves traveling in the S2 dorsal root would not be influenced by S1 DRS; and 3) although both S1 DRS and S2 DRS could activate pudendal afferent axons, only S1 DRS is effective in blocking pudendal inhibition (Figs. 1, 3, 5, and 7). Therefore, it is highly likely that S1 dorsal root afferent input interacts with pudendal afferent input in the spinal cord or brain (Fig. 9) to achieve the effects observed in this study.
When used clinically to treat NOUR or OAB symptoms, sacral neuromodulation is applied continuously, indicating that the beneficial effects are mediated by transient inhibitory mechanisms. However, it has been noted that the effects of neuromodulation in patients with Fowler’s syndrome can be delayed in onset at the beginning of treatment and can persist for 48 h after terminating treatment. Our study, which established an acute model of neurogenic underactive bladder in chloralose-anesthetized cats, could demonstrate only a transient reversal of pudendal inhibition by sacral neuromodulation. It will be important in future experiments to determine if the reversal is more prolonged in the absence of anesthesia.
Perspectives and Significance
In summary, this study provides basic science evidence supporting the hypothesis that sacral neuromodulation treats NOUR in women with Fowler’s syndrome by blocking the inhibition of micturition induced by abnormal EUS/EAS afferent activity (12). The results are important for understanding why sacral neuromodulation can treat both OAB and NOUR. This study, which established the first animal model for demonstrating the effects of sacral neuromodulation on neurogenic bladder underactivity, provides an opportunity to explore the sites of action and possible neurotransmitter mechanisms underlying sacral neuromodulation therapy for Fowler’s syndrome.
GRANTS
This study is supported by the National Institutes of Diabetes, Digestive and Kidney Diseases Grants DK-094905, DK-102427, and DK-111382.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X.L., J.U., M.Y., S.L., K.T., J.B., B.S., J.W., J.R.R., W.C.d.G., and C.T. conceived and designed research; X.L., J.U., M.Y., S.L., K.T., J.B., B.S., J.W., J.R.R., W.C.d.G., and C.T. performed experiments; X.L., J.U., M.Y., S.L., K.T., J.B., B.S., J.W., J.R.R., W.C.d.G., and C.T. analyzed data; X.L., J.U., M.Y., S.L., K.T., J.B., B.S., J.W., J.R.R., W.C.d.G., and C.T. interpreted results of experiments; X.L., J.U., M.Y., S.L., K.T., J.B., B.S., J.W., J.R.R., W.C.d.G., and C.T. prepared figures; X.L., J.U., M.Y., S.L., K.T., J.B., B.S., J.W., J.R.R., W.C.d.G., and C.T. drafted manuscript; X.L., J.U., M.Y., S.L., K.T., J.B., B.S., J.W., J.R.R., W.C.d.G., and C.T. edited and revised manuscript; X.L., J.U., M.Y., S.L., K.T., J.B., B.S., J.W., J.R.R., W.C.d.G., and C.T. approved final version of manuscript.
REFERENCES
- 1.Bandari J, Bansal U, Zhang Z, Shen B, Wang J, Lamm V, Chang V, Roppolo JR, de Groat WC, Tai C. Neurotransmitter mechanisms underlying sacral neuromodulation of bladder overactivity in cats. Neuromodulation 20: 81–87, 2017. doi: 10.1111/ner.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dasgupta R, Critchley HD, Dolan RJ, Fowler CJ. Changes in brain activity following sacral neuromodulation for urinary retention. J Urol 174: 2268–2272, 2005. doi: 10.1097/01.ju.0000181806.59363.d1. [DOI] [PubMed] [Google Scholar]
- 3.DasGupta R, Fowler CJ. Urodynamic study of women in urinary retention treated with sacral neuromodulation. J Urol 171: 1161–1164, 2004. doi: 10.1097/01.ju.0000113201.26176.8f. [DOI] [PubMed] [Google Scholar]
- 4.de Groat WC, Fraser MO, Yoshiyama M, Smerin S, Tai C, Chancellor MB, Yoshimura N, Roppolo JR. Neural control of the urethra. Scand J Urol Nephrol Suppl 207: 35–43, 2001. [DOI] [PubMed] [Google Scholar]
- 5.de Groat WC, Ryall RW. Reflexes to sacral parasympathetic neurones concerned with micturition in the cat. J Physiol 200: 87–108, 1969. doi: 10.1113/jphysiol.1969.sp008683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Ridder D, Ost D, Bruyninckx F. The presence of Fowler’s syndrome predicts successful long-term outcome of sacral nerve stimulation in women with urinary retention. Eur Urol 51: 229–233, 2007. doi: 10.1016/j.eururo.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 7.Fowler CJ, Christmas TJ, Chapple CR, Parkhouse HF, Kirby RS, Jacobs HS. Abnormal electromyographic activity of the urethral sphincter, voiding dysfunction, and polycystic ovaries: a new syndrome? BMJ 297: 1436–1438, 1988. doi: 10.1136/bmj.297.6661.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fowler CJ, Swinn MJ, Goodwin RJ, Oliver S, Craggs M. Studies of the latency of pelvic floor contraction during peripheral nerve evaluation show that the muscle response is reflexly mediated. J Urol 163: 881–883, 2000. doi: 10.1016/S0022-5347(05)67826-3. [DOI] [PubMed] [Google Scholar]
- 9.Goodwin RJ, Swinn MJ, Fowler CJ. The neurophysiology of urinary retention in young women and its treatment by neuromodulation. World J Urol 16: 305–307, 1998. doi: 10.1007/s003450050072. [DOI] [PubMed] [Google Scholar]
- 10.Jiang X, Fuller TW, Bandari J, Bansal U, Zhang Z, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Contribution of GABAA, glycine, and opioid receptors to sacral neuromodulation of bladder overactivity in cats. J Pharmacol Exp Ther 359: 436–441, 2016. doi: 10.1124/jpet.116.235846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jonas U, Fowler CJ, Chancellor MB, Elhilali MM, Fall M, Gajewski JB, Grünewald V, Hassouna MM, Hombergh U, Janknegt R, van Kerrebroeck PE, Lycklama á Nijholt AA, Siegel SW, Schmidt RA. Efficacy of sacral nerve stimulation for urinary retention: results 18 months after implantation. J Urol 165: 15–19, 2001. doi: 10.1097/00005392-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Kessler TM, Fowler CJ. Sacral neuromodulation for urinary retention. Nat Clin Pract Urol 5: 657–666, 2008. doi: 10.1038/ncpuro1251. [DOI] [PubMed] [Google Scholar]
- 13.Larson JA, Ogagan PD, Chen G, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Involvement of metabotropic glutamate receptor 5 in pudendal inhibition of nociceptive bladder activity in cats. J Physiol 589: 5833–5843, 2011. doi: 10.1113/jphysiol.2011.215657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyon TD, Ferroni MC, Kadow BT, Slater RC, Zhang Z, Chang V, Lamm V, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Pudendal but not tibial nerve stimulation inhibits bladder contractions induced by stimulation of pontine micturition center in cats. Am J Physiol Regul Integr Comp Physiol 310: R366–R374, 2016. doi: 10.1152/ajpregu.00490.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGuire E, Morrissey S, Zhang S, Horwinski E. Control of reflex detrusor activity in normal and spinal injured non-human primates. J Urol 129: 197–199, 1983. doi: 10.1016/S0022-5347(17)51982-5. [DOI] [PubMed] [Google Scholar]
- 16.Morgan C, Nadelhaft I, de Groat WC. The distribution of visceral primary afferents from the pelvic nerve to Lissauer’s tract and the spinal gray matter and its relationship to the sacral parasympathetic nucleus. J Comp Neurol 201: 415–440, 1981. doi: 10.1002/cne.902010308. [DOI] [PubMed] [Google Scholar]
- 17.Osman NI, Chapple CR. Fowler’s syndrome–a cause of unexplained urinary retention in young women? Nat Rev Urol 11: 87–98, 2014. doi: 10.1038/nrurol.2013.277. [DOI] [PubMed] [Google Scholar]
- 18.Sato A, Sato Y, Schmidt RF. Reflex bladder activity induced by electrical stimulation of hind limb somatic afferents in the cat. J Auton Nerv Syst 1: 229–241, 1980. doi: 10.1016/0165-1838(80)90019-3. [DOI] [PubMed] [Google Scholar]
- 19.Schurch B, Reilly I, Reitz A, Curt A. Electrophysiological recordings during the peripheral nerve evaluation (PNE) test in complete spinal cord injury patients. World J Urol 20: 319–322, 2003. doi: 10.1007/s00345-002-0299-7. [DOI] [PubMed] [Google Scholar]
- 20.Swinn MJ, Kitchen ND, Goodwin RJ, Fowler CJ. Sacral neuromodulation for women with Fowler’s syndrome. Eur Urol 38: 439–443, 2000. doi: 10.1159/000020321. [DOI] [PubMed] [Google Scholar]
- 21.Tai C, Chen M, Shen B, Wang J, Liu H, Roppolo JR, de Groat WC. Plasticity of urinary bladder reflexes evoked by stimulation of pudendal afferent nerves after chronic spinal cord injury in cats. Exp Neurol 228: 109–117, 2011. doi: 10.1016/j.expneurol.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tai C, Smerin SE, de Groat WC, Roppolo JR. Pudendal-to-bladder reflex in chronic spinal-cord-injured cats. Exp Neurol 197: 225–234, 2006. doi: 10.1016/j.expneurol.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Thor KB, Morgan C, Nadelhaft I, Houston M, de Groat WC. Organization of afferent and efferent pathways in the pudendal nerve of the female cat. J Comp Neurol 288: 263–279, 1989. doi: 10.1002/cne.902880206. [DOI] [PubMed] [Google Scholar]
- 24.van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, Lycklama á Nijholt AA, Siegel S, Jonas U, Fowler CJ, Fall M, Gajewski JB, Hassouna MM, Cappellano F, Elhilali MM, Milam DF, Das AK, Dijkema HE, van den Hombergh U. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol 178: 2029–2034, 2007. doi: 10.1016/j.juro.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 25.Webb RJ, Fawcett PR, Neal DE. Electromyographic abnormalities in the urethral and anal sphincters of women with idiopathic retention of urine. Br J Urol 70: 22–25, 1992. doi: 10.1111/j.1464-410X.1992.tb15657.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Bandari J, Bansal U, Shen B, Wang J, Lamm V, Roppolo JR, de Groat WC, Tai C. Sacral neuromodulation of nociceptive bladder overactivity in cats. Neurourol Urodyn 36: 1270–1277, 2017. doi: 10.1002/nau.23105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang F, Zhao S, Shen B, Wang J, Nelson DE, Roppolo JR, de Groat WC, Tai C. Neural pathways involved in sacral neuromodulation of reflex bladder activity in cats. Am J Physiol Renal Physiol 304: F710–F717, 2013. doi: 10.1152/ajprenal.00334.2012. [DOI] [PubMed] [Google Scholar]


