Abstract
Moderate acute intermittent hypoxia (mAIH) elicits a form of respiratory motor plasticity known as phrenic long-term facilitation (pLTF). Preconditioning with modest protocols of chronic intermittent hypoxia enhances pLTF, demonstrating pLTF metaplasticity. Since “low-dose” protocols of repetitive acute intermittent hypoxia (rAIH) show promise as a therapeutic modality to restore respiratory (and nonrespiratory) motor function in clinical disorders with compromised breathing, we tested 1) whether preconditioning with a mild rAIH protocol enhances pLTF and hypoglossal (XII) LTF and 2) whether the enhancement is regulated by glycolytic flux. In anesthetized, paralyzed, and ventilated adult male Lewis rats, mAIH (three 5-min episodes of 10% O2) elicited pLTF (pLTF at 60 min post-mAIH: 49 ± 5% baseline). rAIH preconditioning (ten 5-min episodes of 11% O2/day with 5-min normoxic intervals, 3 times per week, for 4 wk) significantly enhanced pLTF (100 ± 16% baseline). XII LTF was unaffected by rAIH. When glycolytic flux was inhibited by 2-deoxy-d-glucose (2-DG) administered via drinking water (~80 mg·kg−1·day−1), pLTF returned to normal levels (58 ± 8% baseline); 2-DG had no effect on pLTF in normoxia-pretreated rats (59 ± 7% baseline). In ventral cervical (C4/5) spinal homogenates, rAIH increased inducible nitric oxide synthase mRNA vs. normoxic controls, an effect blocked by 2-DG. However, there were no detectable effects of rAIH or 2-DG on several molecules associated with phrenic motor plasticity, including serotonin 2A, serotonin 7, brain-derived neurotrophic factor, tropomyosin receptor kinase B, or VEGF mRNA. We conclude that modest, but prolonged, rAIH elicits pLTF metaplasticity and that a drug known to inhibit glycolytic flux (2-DG) blocks pLTF enhancement.
Keywords: glycolytic activity, intermittent hypoxia, long-term facilitation, phrenic nerve, respiratory metaplasticity
INTRODUCTION
Moderate acute intermittent hypoxia (mAIH) triggers a progressive, posthypoxia increase in the amplitude of integrated phrenic and hypoglossal nerve activity known as long-term facilitation (LTF). Intermittent hypoxia (IH) preconditioning amplifies LTF, demonstrating metaplasticity in LTF (17). Specifically, 1 wk of IH (12 h/night, 5-min hypoxic episodes, 5-min intervals) nearly doubles phrenic (pLTF) and hypoglossal (XII) LTF in anesthetized Sprague-Dawley rats (36), and a similar effect is observed in ventilatory LTF (45). In Brown Norway rats [a strain with minimal LTF (4)], preconditioning with daily acute IH (ten 5-min episodes per day, 5-min intervals, for 7 days) enhances XII LTF, with similar, but only marginally statistically significant, trends in pLTF (P = 0.054) (64).
“Low-dose” IH protocols, referred to here as repetitive acute IH (rAIH), have considerable therapeutic potential to restore breathing capacity in clinical disorders that compromise breathing and nonrespiratory movements (8, 25, 43, 44). Thus it is important to understand the cumulative benefits of rAIH with prolonged exposures. Preconditioning with a modest rAIH protocol (10 episodes/day, 3 days/wk, for 10 wk) increases expression of proteins critical for pLTF within the phrenic motor nucleus (58). The first goal of this study was to test the hypothesis that a similar rAIH protocol [ten 5-min hypoxic episodes per day, 5-min normoxic intervals, 3 times/wk (3×W AIH), for 4 wk] enhances pLTF and XII LTF in normal Lewis rats (i.e., metaplasticity).
IH preconditioning may enhance pLTF via cell signaling and/or gene regulation, linking cellular energy metabolism with neuronal plasticity. For example, late-phase hippocampal LTP is regulated by the energy sensor AMP-activated protein kinase (AMPK) (55); AMPK inhibition with the glycolytic inhibitor 2-deoxy-d-glucose (2-DG) blocks LTP. Similarly, in a rat model of temporal lobe epilepsy, twice-daily hippocampal stimulation elicits kindling, an effect that is blocked by 2-DG (21). 2-DG increases NAD levels by slowing glycolytic flux, enabling the repressor element-1-silencing transcription factor-COOH-terminal binding protein complex to methylate histone 3, reducing brain-derived neurotrophic factor (BDNF) and tropomyosin receptor kinase B (TrkB) mRNA expression, thereby undermining neuroplasticity. Conversely, increased glucose availability enhances learning and memory in rodents, supporting the concept that multiple forms of plasticity are sensitive to glucose metabolism/glycolytic flux (3, 23, 33, 47). On the basis of these reports, our second goal was to test the hypothesis that glycolytic flux is necessary for rAIH-induced phrenic and XII metaplasticity and that glycolytic flux suppression with 2-DG restores normal LTF.
Although prior studies elucidate aspects of cellular mechanisms giving rise to pLTF and XII LTF, mechanisms of IH preconditioning-induced metaplasticity in pLTF and XII LTF are largely unknown (17). pLTF metaplasticity may arise from amplification of the same elements that give rise to mAIH-induced pLTF in normal rats. Basic pLTF mechanisms, including spinal serotonin (5-HT) 2 receptor activation (6, 20, 32, 39), downstream signaling via ERK MAP kinases, TrkB, and protein kinase Cθ (9, 12, 29), new BDNF protein synthesis (5), reactive oxygen species formation (38, 41), and neuronal nitric oxide (NO) synthase (nNOS) activity (40), have been reviewed in recent years (7, 13, 15, 42, 48). In support of the idea that these molecules play an important role in rAIH-induced pLTF metaplasticity, rAIH increases BDNF protein expression in ventral cervical segments containing the phrenic motor nucleus (64) and within phrenic motor neurons per se (37, 58). Similarly, 3×W AIH for 10 wk upregulates 5-HT terminal density, 5-HT2A receptors, BDNF, TrkB, and phosphorylated ERK MAP kinases in the phrenic motor nucleus (58).
On the other hand, rAIH also upregulates proteins associated with distinct cellular mechanisms of phrenic motor plasticity, including 5-HT7 receptors (46), phosphorylated Akt (24, 27), vascular endothelial growth factor (VEGF), and erythropoietin (10). Thus the third goal of this study was to test the impact of 3 × W AIH on mRNA expression of select molecules involved in phrenic motor plasticity within ventral cervical spinal homogenates, including 5-HT2A and 5-HT7 receptors, BDNF, TrkB, VEGF, and inducible NO synthase (iNOS). We predicted that any changes would be reversed by 2-DG if they were critical for LTF metaplasticity.
MATERIALS AND METHODS
Experiments were performed on 3- to 4-mo-old male Lewis rats (colony 202A, Harlan). All procedures were carried out in accordance with the National Institutes of Health guidelines for care and use of laboratory animals and were approved by the Animal Care and Use Committee at the University of Wisconsin-Madison. Appropriate measures were taken to minimize the use of animals and possible pain and suffering.
rAIH preconditioning.
Rats were randomly assigned to receive either rAIH or continuous normoxia (control). AIH consisted of ten 5-min episodes of hypoxia (11% inspired O2) separated by 5-min intervals of normoxia (Fig. 1). rAIH consisted of 3 days (Monday, Wednesday, and Friday) of AIH per week for 4 consecutive wk (3×W AIH). On exposure days, rats were placed in individual, custom-designed cylindrical exposure chambers with a flat insert to support the rats and enable urine to pass below the rat’s level in the chamber. Each chamber was connected to computer-controlled mass flow controllers (Aarlborg) to regulate gas flow through the chambers (~4 l/min), with targeted inspired O2 levels (Fig. 1). This system allowed automated cycling between hypoxia and room air. Normoxia-exposed (Nx) control rats were placed in the same chambers but received continuous room air (~4 l/min). Exposures commenced at ~9 AM each day. During their first week of exposure, rats were allowed 30 min to acclimate to the chamber before initiation of IH. During subsequent exposures, rats were observed in behavioral sleep once they were placed in the chamber. The rats were returned to their home cages at the end of AIH exposures.
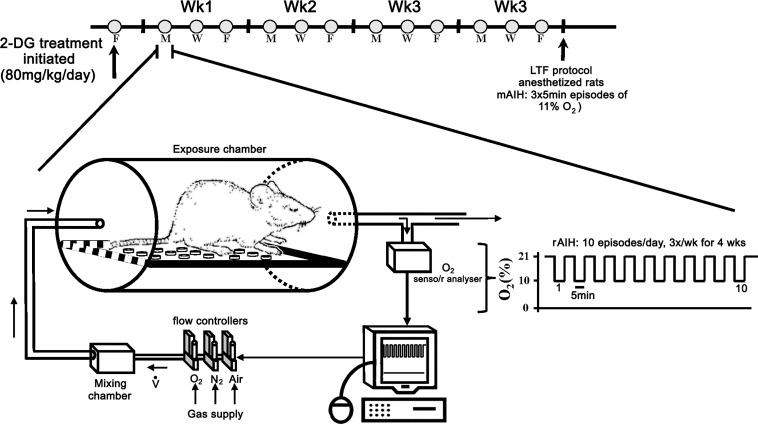
Fig. 1.
Timeline of the repetitive acute intermittent hypoxia (rAIH) and 2-deoxy-d-glucose (2-DG) treatment paradigm. An automated gas flow system enabled rAIH exposure (ten 5-min exposures to 10% O2 with 5-min intervals of room air) in unanesthetized rats on 3 days [Monday (M), Wednesday (W), and Friday (F)] for 4 consecutive wk. O2 and CO2 were measured in excurrent gas of the chambers. 2-DG treatment (~80 mg·kg−1·day−1) was initiated 3 days before the start of rAIH; 2-DG was dissolved in drinking water. Rats were weighed on the days they were placed in exposure chambers (i.e., M, W, and F). The amount of 2-DG administered was calculated by daily water intake. Moderate acute intermittent hypoxia (mAIH, three 5-min hypoxic episodes)-induced phrenic and hypoglossal (XII) long-term facilitation (LTF) were measured, and tissues were collected from urethane-anesthetized rats on the day after their last AIH exposure.
2-DG.
Additional groups of rats were treated with 2-DG (~80 mg·kg−1·day−1; Sigma) throughout the rAIH or normoxic (sham) exposure protocol. This 2-DG dose was chosen on the basis of ED50 values that were reported to reduce seizure activity in both mice and rats (61). 2-DG was administered via drinking water beginning 3 days before the first AIH exposure (Fig. 1). On each day (Monday, Wednesday, and Friday) of AIH exposure, water bottles were filled with a known volume (300 ml); on subsequent exposure days, the remaining water volume was measured to determine daily water consumption. After daily water intake was calculated, the bottles were refilled (300 ml). On the basis of average daily water consumption and the rat’s body weight, an appropriate amount of 2-DG was added to the water to maintain a dose of 80 mg·kg−1·day−1. Control (non-2-DG-treated) rats underwent the same protocol for determination of daily water consumption, but 2-DG was omitted from the drinking water. Average daily water intake was not statistically different between groups (Fig. 2). Rats were housed in pairs, and water/2-DG intake was assumed to be equal for each rat. On the day after their final AIH exposure, rats were anesthetized and surgically prepared for phrenic and XII nerve recordings to assess LTF. A separate group of rats were treated with 2-DG and exposed to normoxia for 4 wk.
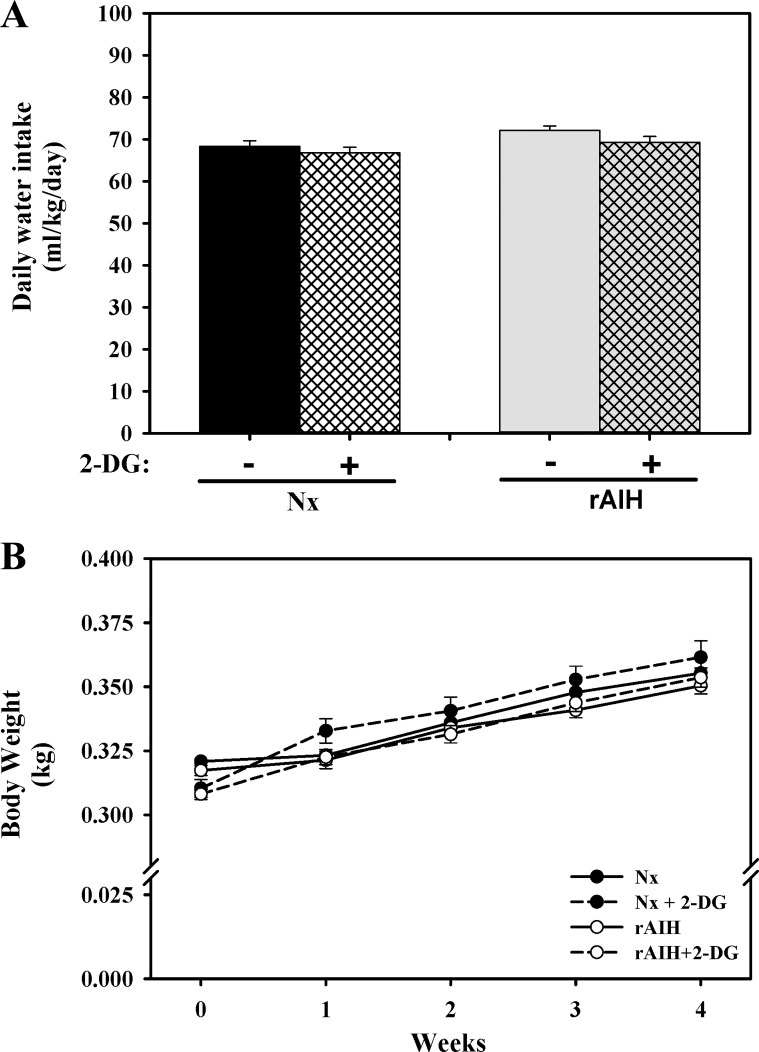
Fig. 2.
Daily water intake (A) averaged throughout the 4-wk exposure period, as well as weekly body weights (B), of normoxia-exposed (Nx, n = 15), Nx + 2-DG (n = 15), rAIH (n = 15), and rAIH + 2-DG (n = 15) rats. There was no significant difference in water consumption or body weight between treatment groups. Values are means ± SE.
Surgical preparation.
On the day after (<24 h) the last AIH exposure, rats were prepared for phrenic and XII nerve recordings. Rats were anesthetized with isoflurane, tracheotomized through a ventral midline neck incision, and mechanically ventilated (tidal volume ~2.5 ml, 70 breaths/min) using a rodent ventilator (model 683, Harvard Apparatus, South Natick, MA). Isoflurane anesthesia (3.5% in 50% O2-balance N2) was maintained for the duration of surgical procedures (~30 min); rats were then slowly (over ~15 min) weaned from isoflurane during urethane infusion (1.8 mg/kg) via a tail vein catheter (24-gauge; Surflo, Elkton, MD). The adequacy of anesthetic depth was confirmed periodically by assessment of the foot-withdrawal reflex response to toe pinch. Surgery was performed on a custom-made stainless steel surgical table connected to an adjustable water bath (Isotemp 1006S, Fisher Scientific, Pittsburgh, PA) for temperature control. Rectal temperature was monitored continuously with a temperature sensor (Fisher Scientific) and maintained constant by adjustment of water bath temperature. Inspired O2 levels were monitored throughout experiments with a fuel-cell O2 sensor (model TED 60T, Teledyne Analytical Instruments, City of Industry, CA) inserted into the tubing upstream from the isoflurane vaporizer. A 1:1 ratio of lactated Ringer solution-hetastarch was infused (1.5–2 ml/h; Cole-Palmer, Vernon Hills, IL) via the intravenous catheter to regulate blood pressure (6% hetastarch; Hospira, Lake Forest, IL) and arterial base excess levels (lactated Ringer solution; Baxter, Deerfield, IL). Sodium bicarbonate (8.4%; Hospira) was also added to the infusion solution (1:20 ratio). Once isoflurane anesthesia had been established rats received an initial 1-ml intravenous injection of lactated Ringer solution over 5 min to minimize any initial base excess disturbances. After a ventral midline neck incision, both vagi were transected. A polyethylene (PE-50) catheter (0.58 mm ID, 0.965 mm OD; Intramedic, Becton Dickinson, Sparks, MD) was inserted into the right femoral artery and connected to a pressure transducer (model P23, Gould) to monitor mean arterial pressure. The femoral artery catheter also enabled periodic blood sampling during the protocol for analysis of partial pressure of O2 (Po2) and CO2 (Pco2) and pH with a blood gas analyzer (model ABL 800, Radiometer, Copenhagen, Denmark); base excess was calculated by the analyzer. The rat was then placed in a prone position to enable dorsal isolation of the phrenic and XII nerves.
Phrenic and XII nerve recordings.
After the left phrenic and XII nerves were dissected and exposed via a dorsal approach, they were cut distally, desheathed, submerged in mineral oil, and placed on bipolar silver recording electrodes. Nerve activity was amplified (gain 10,000; A-M Systems, Everett, WA), band-pass-filtered (100 Hz–10 kHz), rectified, and integrated (CWE 821 filter, Paynter, Ardmore, PA; time constant 50 ms). The signal was digitized and recorded using the WinDaq data acquisition system (DATAQ Instruments, Akron, OH). Data were analyzed using custom software based on a LabVIEW platform (National Instruments, Austin, TX). Once electrical activity was detected, the rats were again assessed for adequate depth of anesthesia by examination for transient increases in blood pressure and/or respiratory neural output in response to toe pinch. Supplemental urethane was provided at this time if necessary. Rats were then paralyzed with pancuronium bromide (1.2 ml iv, 1 mg/ml). End-tidal CO2 was monitored using a flow-through capnograph (model 1265, Novametrix, Wallingford, CT), and rats were allowed ~30 min for stabilization of the nerve signal. Stable phrenic and XII nerve activity was established while the rat was ventilated with a hyperoxic mixture [fraction of inspired O2 () ~0.50, arterial Po2 () >250 mmHg], and sufficient inspired CO2 was delivered to maintain end-tidal Pco2 above the apneic threshold (typically 40-45 mmHg). After nerve recordings stabilized, the apneic threshold for phrenic nerve activity was determined by progressive lowering of the inspired CO2 during monitoring of end-tidal CO2 until fictive breathing ceased. End-tidal Pco2 was then progressively increased in 1-mmHg increments approximately every minute until nerve activity resumed (i.e., recruitment threshold). End-tidal Pco2 was raised ~2 mmHg above the CO2 recruitment threshold to establish baseline nerve activity.
mAIH protocol.
After the CO2 recruitment threshold was established and a further 20 min of stable nerve recordings were obtained, a baseline blood sample was taken to assess , arterial Pco2 (), pH, and base excess before mAIH (or sham) exposure. mAIH consisted of three 5-min episodes of ~11% O2 (adjusted to target a of ~40 mmHg) separated by 5 min of baseline conditions ( ~0.50). Blood samples were collected in 60-µl heparinized capillary tubes (Radiometer, Copenhagen, Denmark) before AIH (baseline), during the first hypoxic episode, and at 15, 30, and 60 min post-AIH to ensure that blood gases remained constant. If during the first episode of hypoxia was not within ±5 mmHg of the target (40 mmHg) or if changed >2 mmHg from baseline values, or fraction of inspired CO2 () was adjusted, and another blood sample was taken during the second hypoxic episode to ensure that variables met our criteria. If O2 or CO2 levels could not be regulated within their target range on the second blood measurement, the rat was excluded from the study. Nerve activity was monitored for 60 min after the last hypoxic episode while arterial blood gases were maintained at baseline conditions. Blood gases were assessed 15, 30, and 60 min post-AIH to ensure that was within 1 mmHg of baseline values; was adjusted, if necessary, to meet the 1-mmHg criterion.
Phrenic and XII nerve burst amplitude and frequency were assessed for 60 s just before arterial blood samples were taken. In all treatment groups, corresponding time controls (TCs), in which the anesthetized rats were not exposed to mAIH, were included. At the end of experiments, rats were given a lethal urethane injection, the cervical (C4/5) spinal cord was removed, and tissues were immediately stored at −80°C for later analysis.
Quantitative RT-PCR and mRNA expression.
Cervical (C4/5) spinal segments were placed on a freezing microtome, and successive 50-μm sections of dorsal horn were removed until the ventral aspect of the central canal was visible. Total RNA was extracted from remaining ventral spinal tissue using TRIzol (1 ml; Invitrogen, Carlsbad, CA). From each sample, 1 μg of RNA was obtained for reverse transcriptase analysis using oligo(dT) primers and Moloney murine leukemia virus reverse transcriptase (Invitrogen). Quantitative RT-PCR was performed in duplicate via a Step-One Real Time PCR system (Applied Biosystems, Framingham, MA). mRNA for BDNF, 5-HT2A, and 5-HT7 receptors, iNOS, and VEGF were analyzed using the following primer sequences: 5′-CTGACACTTTTGAGCACGTGATC-3′ (forward) and 5′-AGGCTCCAAAGGCACTTGACT-3′ (reverse) for BDNF, 5′-TGCCTGTGTCCATGTTAACCA-3′ (forward) and 5′-AAGAGCACATCCAGGTAAATCCA-3′ (reverse) for 5-HT2A receptors, 5′-CTAACCCCCTCCCACCAATAA-3′ (forward) and 5′-CACCCAGACTACTACATAGCAACATTT-3′ (reverse) for 5-HT7 receptors, 5′-TGAGGAGCAGGTTGAGGATTACT-3′ (forward) and 5′-TTGCCCTTTTTTGCTCCATAG-3′ (reverse) for iNOS, 5′-TTG AGA CCC TGG TGG ACA TCT-3′ (forward) and 5′-CAC ACA GGA CGG CTT GAA GA-3′ (reverse) for VEGF, and 5′-GGCTGTGGTACCCGATCAGT-3′ (forward) and 5′-CACTCACCCTTCGCCACCTA-3′ (reverse) for TrkB.
Primer specificity was confirmed by dissociation (melting) curve analysis. Data were analyzed using the comparative cycle threshold (CT) method. CT values were set in the linear range of gene amplification. The gene of interest was normalized as a difference from the amplification cycle at threshold for 18S mRNA. Nontemplate controls (lacking cDNA) were generated for each gene to control for baseline reagent contamination. The 18S primer sequence was 5′-AACGAGACTCTCGGCATGCTAA-3′ (forward) and 5′-CCGGACATCTAAGGGCATCA-3′ (reverse).
Statistical analysis.
Blood gases and integrated phrenic nerve burst amplitude and frequency (bursts/min) were averaged in 1-min bins at each recorded data point (baseline, 1st hypoxia, and 15, 30, and 60 min post-mAIH). Burst amplitude was normalized as percent change from baseline. Burst frequency data were normalized as a change (bursts/min) from baseline.
A two-way repeated-measures ANOVA was used to compare blood gases and changes in nerve activity at each time between rAIH-exposed and unexposed rats (and between TCs). Statistical analysis of spinal mRNA expression for each gene of interest was performed between groups using a two-way ANOVA on ΔCT values.
Daily water intake was averaged across 4-wk treatment periods, and comparisons between groups were made via one-way ANOVA. Two-way repeated-measures ANOVA was used to analyze changes in body weight throughout the treatment period (Fig. 2) and to compare phrenic and XII nerve activity with and without mAIH exposure (Fig. 3) or rAIH rats with and without 2-DG treatment (60 min post-AIH; Fig. 4). Student-Newman-Keuls post hoc test was used for individual comparisons. All data were assessed for normality using the Shapiro-Wilk test. Differences were considered significant if P < 0.05. Values are means ± SE.
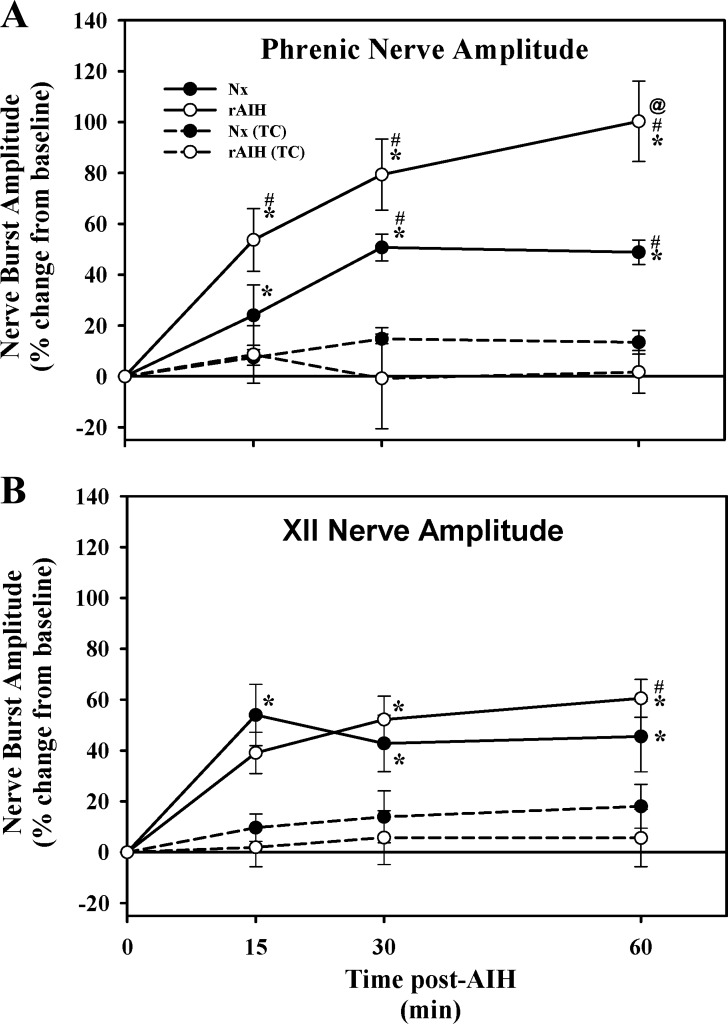
Fig. 3.
Phrenic (A) and XII (B) nerve burst amplitude at 15, 30, and 60 min post-mAIH in anesthetized Nx (n = 8) and rAIH (n = 9) rats. Results from time-control (TC) experiments in anesthetized Nx (n = 5) and rAIH (n = 15) rats that did not receive mAIH are also shown. Compared with TC groups, phrenic nerve burst amplitude was significantly elevated in Nx and rAIH rats at every time post-mAIH, demonstrating pLTF. Phrenic burst amplitude was significantly greater in rAIH than Nx rats (i.e., rats not pretreated with rAIH) at 60 min post-AIH, demonstrating pLTF metaplasticity. XII LTF was expressed in both treatment groups relative to baseline values, but not when compared at corresponding times in TC rats; one exception was significantly greater expression of XII LTF in rAIH rats at 60 min post-mAIH than in TC rats. There was no difference in XII nerve burst amplitude between rAIH and Nx rats at any time (i.e., rAIH did not enhance pLTF). Values are means ± SE. *Significantly different from baseline; @significantly different from Nx at corresponding time; #significantly different from corresponding TC within treatment group (P < 0.05).
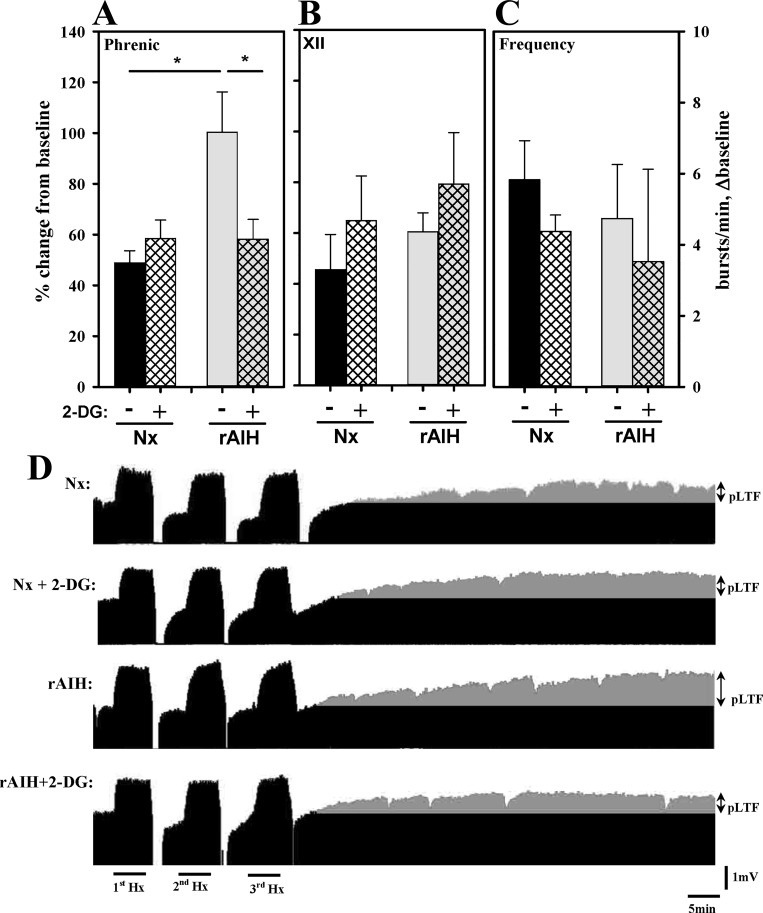
Fig. 4.
Phrenic (A) and XII (B) nerve burst amplitude and nerve burst frequency (C) 60 min post-mAIH in anesthetized Nx or rAIH rats with (+) or without (−) 2-DG treatment. D: representative phrenic neurograms (D) for each group. rAIH-induced pLTF enhancement was prevented by 2-DG, whereas pLTF of Nx rats was not affected by 2-DG. Values are means ± SE; n = 8 Nx and rAIH + 2-DG and n = 9 Nx + 2-DG and rAIH. *P < 0.05.
RESULTS
Daily water intake and growth during rAIH exposure and 2-DG treatment.
Average daily water intake (ml·kg−1·day−1) across the 4-wk exposure period was similar in Nx (68 ± 1), Nx + 2-DG (67 ± 1), rAIH (72 ± 1), and rAIH + 2-DG (69 ± 1) groups (Fig. 2A). Body weight significantly increased weekly in all groups but did not differ between groups at any time (Fig. 2B).
rAIH enhances pLTF but not XII LTF.
Integrated phrenic nerve burst amplitude following mAIH is shown in Fig. 3. mAIH caused a progressive increase in phrenic nerve burst amplitude in Nx control rats, reaching statistical significance vs. corresponding values in TC rats at 30 and 60 min post-mAIH, demonstrating pLTF (Fig. 3A). Rats preconditioned with rAIH also exhibited a progressive increase in phrenic nerve burst amplitude post-AIH, reaching statistical significance vs. TC rats as early as 15 min post-mAIH. However, mAIH-induced pLTF was significantly greater in rats preconditioned with rAIH than in rats preconditioned with normoxia (100.3 ± 15.8 vs. 48.8 ± 4.8% baseline, P < 0.05); this difference was statistically significant at 60 min post-mAIH (Fig. 2A). Thus, rAIH preconditioning enhances mAIH-induced pLTF, demonstrating pLTF metaplasticity.
mAIH significantly increased XII nerve burst amplitude above baseline conditions in both Nx and rAIH rats (i.e., XII LTF; Fig. 3), thereby demonstrating XII LTF. However, this XII LTF was not robust, since only rAIH rats exhibited a significant increase in XII amplitude vs. TC rats at a corresponding time point; this difference was significant only at 60 min postexposure (60.5 ± 11.2 vs. 5.6 ± 11.2% baseline, P < 0.05; Fig. 3B). There were no significant differences between Nx and rAIH rats (Figs. 3 and 4), demonstrating that rAIH did not elicit XII LTF metaplasticity in Lewis rats.
2-DG pretreatment has no effect on phrenic LTF but blocks rAIH enhancement.
In Nx control rats, 2-DG had no effect on pLTF (Fig. 4A). Specifically, the magnitude of pLTF post-mAIH (60 min) in Nx control rats was not significantly different from that in rats without 2-DG (58.5 ± 7.3 and 48.8 ± 4.8% baseline, respectively; Fig. 4A). In contrast, in rAIH rats, phrenic nerve amplitude at 60 min post-mAIH was significantly reduced compared with rAIH rats without 2-DG (58.1 ± 7.9 vs. 100.3 ± 15.8% baseline) and was similar in magnitude to Nx control rats (Fig. 4, A and D). Collectively, these data demonstrate that 2-DG blocked pLTF enhancement following rAIH preconditioning but had no effect on pLTF in normal rats. 2-DG had no effect on either XII nerve burst amplitude or frequency (Fig. 4, B and C). Representative phrenic nerve recordings for all treatment groups are shown in Fig. 4D.
Baseline nerve activity and hypoxic phrenic response after rAIH + 2-DG.
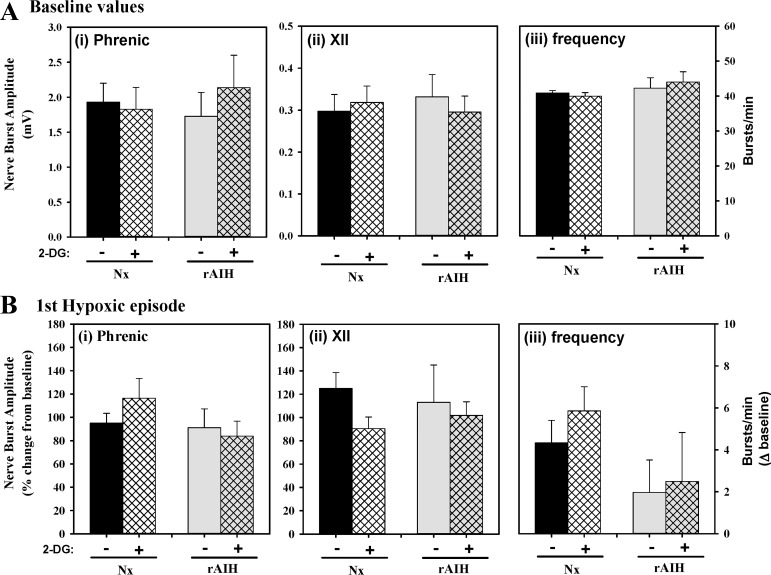
Neither rAIH nor 2-DG had significant effects on baseline phrenic or XII nerve burst amplitude or frequency (Fig. 5A). Similarly, there were no rAIH or 2-DG effects on phrenic or XII nerve burst amplitude (expressed as %change from baseline) or frequency (expressed as change from baseline, in bursts/min) within hypoxic episodes of the mAIH protocol (Fig. 5B).
Fig. 5.
Phrenic and XII nerve burst amplitude at baseline (A) and 1st hypoxic episode (B) in anesthetized Nx or rAIH rats with (+) or without (−) 2-DG treatment. There were no differences in phrenic nerve burst amplitude (i), XII nerve burst amplitude (ii), or frequency (iii) between treatment groups at baseline or in response to acute hypoxia. Values are means ± SE; n = 8 Nx and rAIH + 2-DG and n = 9 Nx + 2-DG and rAIH.
Ventral cervical spinal mRNA.
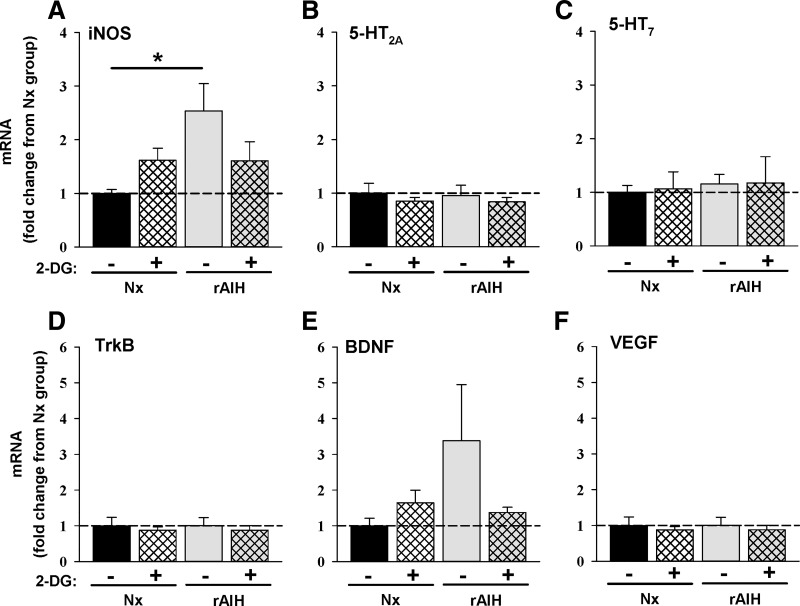
In ventral cervical spinal homogenates, we evaluated mRNA expression of select molecules associated with phrenic motor facilitation following rAIH exposure and 2-DG pretreatment (Fig. 6). To our surprise, only iNOS mRNA was significantly affected by rAIH exposure (~2.5-fold increase vs. Nx); this increase was prevented by 2-DG (Fig. 6A). There were no significant changes in 5-HT2A, 5-HT7, or TrkB receptor mRNA or in BDNF or VEGF mRNA in response to rAIH or 2-DG (Fig. 6, B–F). There was a nonsignificant trend for increased BDNF mRNA following rAIH that appeared to be reversed by 2-DG (Fig. 6E). These measurements are complicated by substantial dilution of phrenic motor neuron mRNA by mRNA from other cells in the ventral spinal homogenates.
Fig. 6.
Cervical spinal (C4–C5) mRNA expression for inducible nitric oxide synthase (NOS, A), 5-HT2A (B), 5-HT7 (C), and tropomyosin receptor kinase B (TrkB, D) receptors, as well as brain-derived neurotrophic factor (BDNF, E) and VEGF (F), in Nx and rAIH rats with (+) or without (−) 2-DG treatment. Data are expressed as fold change from Nx rats that did not (−) receive 2-DG. Values are means ± SE; n = 6 rats in each group. *P < 0.05.
Other physiological variables during neurophysiology experiments.
In anesthetized rats, body temperature and blood gas variables were not significantly different among treatment groups during baseline conditions (Table 1). was the same at all time points in all groups, indicating that isocapnic conditions were successfully maintained throughout the experiments. As expected, (mmHg) was reduced equally during the first hypoxic episode in all treatment groups [45 ± 2 (Nx), 45 ± 1 (Nx + 2-DG), 46 ± 0.9 (rAIH), and 44 ± 2 (rAIH + 2-DG)]. Although there were no differences in baseline (mmHg) among groups [289 ± 16 (Nx), 304 ± 9 (Nx + 2-DG), 317 ± 5 (rAIH), and 286 ± 11 (rAIH + 2-DG)], was significantly decreased vs. baseline in Nx + 2-DG and rAIH rats; these values were not different from Nx or rAIH + 2-DG rats. Since levels were >250 mmHg before and after mAIH in all rats, small differences in were not regarded as physiologically significant. As is typical in this experimental preparation, mean arterial pressure was reduced during the first hypoxic episode in all groups and decreased relative to baseline 60 min post-mAIH; however, there were no significant differences among groups at any time post-mAIH.
Table 1.
Average body temperature, blood gas parameters, and MAP for Nx or rAIH rats with (+) or without (−) 2-DG at baseline, during the first hypoxic episode, and 60 min after the last mAIH episode
| Treatment |
Blood Gas Parameters |
|||||
|---|---|---|---|---|---|---|
| Nx/rAIH | ± 2-DG | Tb, °C | , mmHg | , mmHg | pH | MAP, mmHg |
| Baseline | ||||||
| Nx | − | 37.7 ± 0.1 | 289.1 ± 16.4 | 51.4 ± 0.9 | 7.339 ± 0.006 | 67.2 ± 2.0 |
| Nx | + | 37.7 ± 0.1 | 303.8 ± 8.6 | 51.0 ± 0.9 | 7.335 ± 0.005 | 66.8 ± 3.6 |
| rAIH | − | 37.9 ± 0.2 | 316.9 ± 4.9 | 51.1 ± 1.2 | 7.316 ± 0.006 | 72.3 ± 2.4 |
| rAIH | + | 37.8 ± 0.1 | 286.3 ± 10.9 | 53.3 ± 1.6 | 7.323 ± 0.008 | 75.7 ± 5.2 |
| Hypoxia (1st episode) | ||||||
| Nx | − | 37.6 ± 0.1 | 44.9 ± 2.2* | 50.6 ± 0.9 | 7.339 ± 0.006 | 39.8 ± 1.6* |
| Nx | + | 37.6 ± 0.1 | 44.8 ± 1.3* | 50.3 ± 1.0 | 7.335 ± 0.006 | 43.7 ± 3.1* |
| rAIH | − | 37.8 ± 0.1 | 46.1 ± 0.9* | 49.9 ± 1.0 | 7.317 ± 0.009 | 50.4 ± 5.1* |
| rAIH | + | 37.7 ± 0.1 | 44.3 ± 1.8* | 51.9 ± 1.3 | 7.329 ± 0.008 | 44.6 ± 3.6* |
| 60 min | ||||||
| Nx | − | 37.9 ± 0.2 | 275.9 ± 6.9 | 50.8 ± 0.8 | 7.341 ± 0.006 | 54.2 ± 2.7* |
| Nx | + | 37.9 ± 0.1 | 271.1 ± 8.2* | 50.7 ± 1.0 | 7.335 ± 0.007 | 54.0 ± 3.0* |
| rAIH | − | 37.8 ± 0.2 | 279.4 ± 7.3* | 51.6 ± 1.1 | 7.309 ± 0.005 | 52.5 ± 1.8* |
| rAIH | + | 38.0 ± 0.1 | 266.0 ± 9.9 | 53.3 ± 1.6 | 7.307 ± 0.011 | 54.3 ± 2.8* |
Values are means ± SE; n = 8 Nx and rAIH + 2-DG and n = 9 Nx + 2-DG and rAIH. Nx, normoxia; rAIH, repetitive acute intermittent hypoxia; 2-DG, 2-deoxy-d-glucose; Tb, body temperature; , arterial Po2; , arterial Pco2; MAP, mean arterial pressure.
Significantly different from baseline (P < 0.05).
DISCUSSION
The essential findings of this study are as follows: 1) preconditioning with low-dose, rAIH enhances moderate AIH-induced pLTF (but not XII LTF) long-term facilitation in Lewis rats, and 2) an inhibitor of glycolytic flux (2-DG) blocks this form of respiratory metaplasticity. Since 2-DG had no detectable effect on moderate AIH-induced pLTF in control rats, the impact of glycolytic inhibition is unique to the mechanisms enhancing pLTF, and not normal pLTF mechanisms (7, 13, 17).
Although earlier reports demonstrate that rAIH preconditioning upregulates key proteins necessary for pLTF in phrenic motor neurons (37, 58, 64), mRNA expression for BDNF, VEGF, and TrkB, 5-HT2A, and 5-HT7 receptors was unchanged in ventral cervical spinal homogenates. However, iNOS mRNA increased, and since NO is sufficient to elicit phrenic LTF (40), this finding may have functional significance. Specifically, constitutive NO production from the induced iNOS could layer an independent form of phrenic motor facilitation on the normal mAIH-induced pLTF. On the other hand, NO appears to act by enhancing spinal 5-HT release, activating the normal Q pathway to pLTF following moderate AIH (39). Since enhanced pLTF here appears to arise from a distinct mechanism (likely the S pathway to phrenic motor facilitation), the role of iNOS remains unclear. A number of caveats, particularly the expected dilution of phrenic motor neuron mRNA in ventral spinal homogenates and possible differences in mRNA vs. protein expression, limit our ability to make strong inferences concerning the potential roles of mRNAs studied in spinal homogenates in rAIH-enhanced pLTF.
Collectively, our findings 1) demonstrate that low-dose rAIH preconditioning enhances mAIH-induced pLTF and 2) establish a link between metabolic processes (e.g., glycolytic flux) and enhanced, but not normal, pLTF. Although mechanisms linking glycolysis and pLTF metaplasticity are not yet clear, possible mechanisms are discussed.
rAIH preconditioning enhances pLTF.
This is the first study demonstrating that 3×W AIH for 4 wk elicits pLTF metaplasticity. However, enhanced pLTF is consistent with previous studies utilizing more extreme protocols of IH preconditioning (17). For example, chronic IH (CIH), consisting of 5-min hypoxic episodes with 5-min normoxic intervals for 12 h/night, is associated with enhanced pLTF (36, 54) and ventilatory LTF (45, 46). Similarly, ventilatory LTF during the day in humans with obstructive sleep apnea is enhanced (22, 34, 44). pLTF after rAIH has only been reported in one study using daily AIH (10 episodes) for 7 days in Brown Norway rats (64). In that study, daily AIH had only marginally significant effects on pLTF, although there was a near doubling of its value (P = 0.054). Conversely, XII LTF was revealed by daily AIH, a form of plasticity not ordinarily observed in Brown Norway rats (64); the discrepancy in the effects of IH preconditioning on XII LTF between Brown Norway rats and the Lewis rats used in the current study could be explained by strain differences in sensitivity to preconditioning. In striking contrast to mild-to-moderate IH preconditioning protocols, even a single day of intense IH preconditioning (2-min episodes, 2-min intervals, over 8 h) abolishes moderate AIH-induced pLTF due to spinal neuroinflammation through a p38 MAP kinase-dependent mechanism (30). Thus the impact of IH preconditioning on mAIH-induced pLTF is highly dose-dependent.
Glycolytic flux and pLTF.
2-DG competitively inhibits the production of glucose 6-phosphate, slowing the overall rate of glycolytic flux (63). Since glycolytic flux inhibition with 2-DG had no impact on moderate AIH-induced pLTF (or XII LTF) in normoxic rats, the basic mechanism of moderate AIH-induced pLTF is not sensitive to reduced glycolytic flux. With moderate hypoxia, pLTF normally arises from a cellular cascade known as the Q pathway (7), which requires spinal 5-HT release, 5-HT2 receptor activation on or near phrenic motor neurons (6, 20, 31, 39), ERK MAP kinase activation (29), new BDNF synthesis (5, 29), TrkB activation within phrenic motor neurons (9), and downstream signaling via protein kinase Cθ (1, 12). mAIH-induced pLTF also requires reactive oxygen species formation via NADPH oxidase (38, 41) and nNOS activity (40). We conclude that 2-DG, and glycolytic flux inhibition, does not impact these individual steps. However, severe AIH elicits pLTF via a distinct, adenosine-dependent mechanism that could be sensitive to glycolytic inhibition (13, 14, 52). This possibility was not tested here.
Glycolytic flux and pLTF metaplasticity.
Several observations argue against the possibility that rAIH preconditioning simply amplifies normal mAIH-induced pLTF mechanisms. For example, with modest CIH preconditioning (5-min episodes, 5-min intervals, 12 h/day, over 7 consecutive days), pLTF is enhanced by a mechanism that requires both 5-HT2 and 5-HT7 receptor activation (17); in striking contrast, 5-HT7 receptors constrain mAIH-induced pLTF in normal rats (28). Thus it appears that unique mechanisms contribute to pLTF following rAIH preconditioning.
Although rAIH upregulates proteins necessary for mAIH-induced pLTF within phrenic motor neurons (58), we found little evidence for increased mRNA expression of these same molecules, including BDNF, TrkB, and 5-HT2A receptor mRNA (Fig. 6). These observations do not eliminate the possibility of rAIH-induced Q pathway upregulation but are consistent with the hypothesis that alternative mechanisms enhance pLTF.
The differential response to 2-DG in Nx vs. rAIH rats is consistent with recruitment of new mechanisms following rAIH preconditioning that enhance pLTF. Two distinct but competing mechanisms can account for AIH-induced pLTF under different conditions in normal rats. With mAIH (as used here), the 5-HT2 receptor-initiated Q pathway dominates pLTF; concurrent 5-HT7 receptor activation actually constrains pLTF via a protein kinase A-dependent mechanism (16, 28). In contrast, when spinal 5-HT7 receptors are activated without hypoxia, robust phrenic motor facilitation results from the S pathway (17, 27), a distinct mechanism that requires adenylate cyclase/cAMP, EPAC (exchange protein directly activated by cAMP), Akt, and mTOR (mechanistic target of rapamycin) signaling, with new TrkB (not BDNF) synthesis. Thus the positive contribution of 5-HT7 receptors to mAIH-induced pLTF following IH preconditioning reflects a unique situation: 5-HT7 receptor activation no longer inhibits, but actually contributes to, enhanced pLTF (17, 36, 46). If pLTF metaplasticity following rAIH preconditioning results from combined Q and S pathway contributions to pLTF, it leaves novel targets for unique 2-DG interference with rAIH enhancement (S pathway) vs. the normal Q pathway to pLTF. Shifting 5-HT7 receptors from a constraint toward a contributor to pLTF would require diminished cross-talk inhibition between these competing mechanisms (13, 17), although mechanisms that could account for changing the Q-S pathway interaction are unknown.
Other molecules/mechanisms that elicit phrenic motor facilitation and are likely impacted by rAIH include VEGF (10), erythropoietin (11), and NO (40). VEGF and erythropoietin synthesis are regulated by O2-sensitive transcription factors, such as hypoxia-inducible factor 1 (19, 60). Although 3×W AIH preconditioning increases hypoxia-inducible factor 1, VEGF, and erythropoietin within motor neurons (10, 58, 59), these mechanisms are unlikely to account for pLTF metaplasticity, since rAIH had no effect on ventral spinal VEGF mRNA levels (Fig. 6) and VEGF-induced phrenic motor facilitation was unaffected by rAIH (10). This tentative conclusion requires additional testing.
Our observation that rAIH elicited a 2-DG-dependent increase in cervical spinal iNOS mRNA (Fig. 6) raises other possible explanations for pLTF metaplasticity. nNOS, but not iNOS, is necessary for moderate AIH-induced pLTF in normal rats (40). On the other hand, low spinal doses of the NO donor sodium nitroprusside induce phrenic motor facilitation (40). Thus, after rAIH, iNOS may supplement nNOS-derived NO, triggering greater phrenic motor facilitation. Since 2-DG reversed the increase in iNOS expression, it would be expected to undermine rAIH-enhanced pLTF. Of considerable interest, iNOS gene expression is sensitive to glycolytic flux. Won et al. reported that LPS-induced increases in glial iNOS gene expression are enhanced by exogenous glucose (65); in their study, 2-DG prevented glucose-induced changes in iNOS gene expression. The impact of 2-DG on iNOS mRNA expression correlates with changes in the glycolytic flux-sensitive transcription factor CAAT/enhancing-binding protein (C/EBP). C/EBP is involved in numerous forms of synaptic plasticity and learning and memory, suggesting the potential for involvement of C/EBP in pLTF metaplasticity (2). The possible involvement of these molecules in enhanced pLTF following IH preconditioning warrants further investigation.
Perspectives and Significance
Glucose levels and glycolytic flux are important regulators of neural plasticity (3, 21, 23, 33, 47, 55). Our observations extend these findings to a novel form of metaplasticity in respiratory motor control, namely, enhanced pLTF following rAIH preconditioning. The link between pLTF metaplasticity and glycolytic flux is demonstrated by the observation that 2-DG blocks rAIH-induced pLTF metaplasticity but has no effect on normal pLTF. The significance of understanding metaplasticity in respiratory (and nonrespiratory) motor control is considerable, given the emergence of rAIH as a promising new therapeutic modality to restore lost respiratory and nonrespiratory function in severe clinical disorders that compromise movement, such as spinal injury and amyotrophic lateral sclerosis (8, 25, 51).
Since rAIH is emerging as a therapeutic modality to restore lost breathing capacity or walking ability following spinal injury (8, 25, 49) or during amyotrophic lateral sclerosis (8, 53), it is essential to demonstrate that it has persistent efficacy without eliciting significant pathology. rAIH-enhanced pLTF demonstrates that the functional benefits of low-dose IH likely accumulate with prolonged exposures and are not subject to inhibition within the time frame investigated here. Indeed, rAIH protocols similar to those used here improve breathing capacity in rats with chronic incomplete cervical spinal injury (50) and walking ability in humans with chronic, incomplete spinal cord injury (49).
The therapeutic value of rAIH will be limited to doses where plasticity is initiated/enhanced without meaningful pathology (8, 51). CIH intended to mimic aspects of obstructive sleep apnea is not suitable, since it undermines pLTF (30) and elicits systemic hypertension (18), hippocampal cell loss (26), metabolic syndrome (35, 62), and learning deficits (56, 57). In contrast, rAIH protocols, including daily AIH for 7 days and 3×W AIH for 10 wk elicit minimal (if any) hypertension (64), hippocampal cell loss, or reactive gliosis (37, 58, 59). Here, we confirm that 3×W AIH for 4 wk does not increase baseline blood pressure (Table 1).
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-080209 and HL-69064 (G. S. Mitchell). P. M. MacFarlane was supported by the Francis Families Foundation. S. Vinit was supported by the Craig H. Neilsen Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.M.M. conceived and designed research; P.M.M. and S.V. performed experiments; P.M.M. and S.V. analyzed data; P.M.M., S.V., and G.S.M. interpreted results of experiments; P.M.M. prepared figures; P.M.M. and G.S.M. drafted manuscript; P.M.M., S.V., and G.S.M. edited and revised manuscript; P.M.M., S.V., and G.S.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. A. Roopra for input into the design of these experiments.
REFERENCES
- 1.Agosto-Marlin IM, Mitchell GS. Spinal BDNF induced phrenic motor facilitation requires PKCθ activity. J Neurophysiol 118: 2755–2762, 2017. doi: 10.1152/jn.00945.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol Rev 89: 121–145, 2009. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azari NP. Effects of glucose on memory processes in young adults. Psychopharmacology (Berl) 105: 521–524, 1991. doi: 10.1007/BF02244373. [DOI] [PubMed] [Google Scholar]
- 4.Baker-Herman TL, Bavis RW, Dahlberg JM, Mitchell AZ, Wilkerson JE, Golder FJ, Macfarlane PM, Watters JJ, Behan M, Mitchell GS. Differential expression of respiratory long-term facilitation among inbred rat strains. Respir Physiol Neurobiol 170: 260–267, 2010. doi: 10.1016/j.resp.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci 7: 48–55, 2004. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- 6.Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci 22: 6239–6246, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dale-Nagle EA, Hoffman MS, MacFarlane PM, Mitchell GS. Multiple pathways to long-lasting phrenic motor facilitation. Adv Exp Med Biol 669: 225–230, 2010. doi: 10.1007/978-1-4419-5692-7_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dale EA, Ben Mabrouk F, Mitchell GS. Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function. Physiology (Bethesda) 29: 39–48, 2014. doi: 10.1152/physiol.00012.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dale EA, Fields DP, Devinney MJ, Mitchell GS. Phrenic motor neuron TrkB expression is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation. Exp Neurol 287: 130–136, 2017. doi: 10.1016/j.expneurol.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dale EA, Mitchell GS. Spinal vascular endothelial growth factor (VEGF) and erythropoietin (EPO) induced phrenic motor facilitation after repetitive acute intermittent hypoxia. Respir Physiol Neurobiol 185: 481–488, 2013. doi: 10.1016/j.resp.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dale EA, Satriotomo I, Mitchell GS. Cervical spinal erythropoietin induces phrenic motor facilitation via extracellular signal-regulated protein kinase and Akt signaling. J Neurosci 32: 5973–5983, 2012. doi: 10.1523/JNEUROSCI.3873-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devinney MJ, Fields DP, Huxtable AG, Peterson TJ, Dale EA, Mitchell GS. Phrenic long-term facilitation requires PKCθ activity within phrenic motor neurons. J Neurosci 35: 8107–8117, 2015. doi: 10.1523/JNEUROSCI.5086-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devinney MJ, Huxtable AG, Nichols NL, Mitchell GS. Hypoxia-induced phrenic long-term facilitation: emergent properties. Ann NY Acad Sci 1279: 143–153, 2013. doi: 10.1111/nyas.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devinney MJ, Nichols NL, Mitchell GS. Sustained hypoxia elicits competing spinal mechanisms of phrenic motor facilitation. J Neurosci 36: 7877–7885, 2016. doi: 10.1523/JNEUROSCI.4122-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26: 239–266, 2003. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fields DP, Mitchell GS. Divergent cAMP signaling differentially regulates serotonin-induced spinal motor plasticity. Neuropharmacology 113, Pt A: 82–88, 2017. doi: 10.1016/j.neuropharm.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fields DP, Mitchell GS. Spinal metaplasticity in respiratory motor control. Front Neural Circuits 9: 2, 2015. doi: 10.3389/fncir.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fletcher EC, Lesske J, Behm R, Miller CC 3rd, Stauss H, Unger T. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol (1985) 72: 1978–1984, 1992. doi: 10.1152/jappl.1992.72.5.1978. [DOI] [PubMed] [Google Scholar]
- 19.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16: 4604–4613, 1996. doi: 10.1128/MCB.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol (1985) 90: 2001–2006, 2001. doi: 10.1152/jappl.2001.90.5.2001. [DOI] [PubMed] [Google Scholar]
- 21.Garriga-Canut M, Schoenike B, Qazi R, Bergendahl K, Daley TJ, Pfender RM, Morrison JF, Ockuly J, Stafstrom C, Sutula T, Roopra A. 2-Deoxy-d-glucose reduces epilepsy progression by NRSF-CtBP-dependent metabolic regulation of chromatin structure. Nat Neurosci 9: 1382–1387, 2006. doi: 10.1038/nn1791. [DOI] [PubMed] [Google Scholar]
- 22.Gerst DG 3rd, Yokhana SS, Carney LM, Lee DS, Badr MS, Qureshi T, Anthouard MN, Mateika JH. The hypoxic ventilatory response and ventilatory long-term facilitation are altered by time of day and repeated daily exposure to intermittent hypoxia. J Appl Physiol (1985) 110: 15–28, 2011. doi: 10.1152/japplphysiol.00524.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gold PE. Glucose modulation of memory storage processing. Behav Neural Biol 45: 342–349, 1986. doi: 10.1016/S0163-1047(86)80022-X. [DOI] [PubMed] [Google Scholar]
- 24.Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J Neurosci 28: 2033–2042, 2008. doi: 10.1523/JNEUROSCI.3570-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez-Rothi EJ, Lee KZ, Dale EA, Reier PJ, Mitchell GS, Fuller DD. Intermittent hypoxia and neurorehabilitation. J Appl Physiol (1985) 119: 1455–1465, 2015. doi: 10.1152/japplphysiol.00524.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gozal E, Row BW, Schurr A, Gozal D. Developmental differences in cortical and hippocampal vulnerability to intermittent hypoxia in the rat. Neurosci Lett 305: 197–201, 2001. doi: 10.1016/S0304-3940(01)01853-5. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman MS, Mitchell GS. Spinal 5-HT7 receptor activation induces long-lasting phrenic motor facilitation. J Physiol 589: 1397–1407, 2011. doi: 10.1113/jphysiol.2010.201657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffman MS, Mitchell GS. Spinal 5-HT7 receptors and protein kinase A constrain intermittent hypoxia-induced phrenic long-term facilitation. Neuroscience 250: 632–643, 2013. doi: 10.1016/j.neuroscience.2013.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffman MS, Nichols NL, Macfarlane PM, Mitchell GS. Phrenic long-term facilitation after acute intermittent hypoxia requires spinal ERK activation but not TrkB synthesis. J Appl Physiol (1985) 113: 1184–1193, 2012. doi: 10.1152/japplphysiol.00098.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huxtable AG, Smith SM, Peterson TJ, Watters JJ, Mitchell GS. Intermittent hypoxia-induced spinal inflammation impairs respiratory motor plasticity by a spinal p38 MAP kinase-dependent mechanism. J Neurosci 35: 6871–6880, 2015. doi: 10.1523/JNEUROSCI.4539-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinkead R, Mitchell GS. Time-dependent hypoxic ventilatory responses in rats: effects of ketanserin and 5-carboxamidotryptamine. Am J Physiol Regul Integr Comp Physiol 277: R658–R666, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Kinkead R, Zhan WZ, Prakash YS, Bach KB, Sieck GC, Mitchell GS. Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonin-dependent long-term facilitation of respiratory motor output in rats. J Neurosci 18: 8436–8443, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kopf SR, Baratti CM. Memory modulation by post-training glucose or insulin remains evident at long retention intervals. Neurobiol Learn Mem 65: 189–191, 1996. doi: 10.1006/nlme.1996.0020. [DOI] [PubMed] [Google Scholar]
- 34.Lee DS, Badr MS, Mateika JH. Progressive augmentation and ventilatory long-term facilitation are enhanced in sleep apnoea patients and are mitigated by antioxidant administration. J Physiol 587: 5451–5467, 2009. doi: 10.1113/jphysiol.2009.178053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Thorne LN, Punjabi NM, Sun CK, Schwartz AR, Smith PL, Marino RL, Rodriguez A, Hubbard WC, O’Donnell CP, Polotsky VY. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res 97: 698–706, 2005. doi: 10.1161/01.RES.0000183879.60089.a9. [DOI] [PubMed] [Google Scholar]
- 36.Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB Jr, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci 21: 5381–5388, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lovett-Barr MR, Satriotomo I, Muir GD, Wilkerson JE, Hoffman MS, Vinit S, Mitchell GS. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J Neurosci 32: 3591–3600, 2012. doi: 10.1523/JNEUROSCI.2908-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacFarlane PM, Satriotomo I, Windelborn JA, Mitchell GS. NADPH oxidase activity is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation. J Physiol 587: 1931–1942, 2009. doi: 10.1113/jphysiol.2008.165597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacFarlane PM, Vinit S, Mitchell GS. Serotonin 2A and 2B receptor-induced phrenic motor facilitation: differential requirement for spinal NADPH oxidase activity. Neuroscience 178: 45–55, 2011. doi: 10.1016/j.neuroscience.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacFarlane PM, Vinit S, Mitchell GS. Spinal nNOS regulates phrenic motor facilitation by a 5-HT2B receptor- and NADPH oxidase-dependent mechanism. Neuroscience 269: 67–78, 2014. doi: 10.1016/j.neuroscience.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacFarlane PM, Wilkerson JE, Lovett-Barr MR, Mitchell GS. Reactive oxygen species and respiratory plasticity following intermittent hypoxia. Respir Physiol Neurobiol 164: 263–271, 2008. doi: 10.1016/j.resp.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol 92: 27–37, 2007. doi: 10.1113/expphysiol.2006.033720. [DOI] [PubMed] [Google Scholar]
- 43.Mateika JH, Komnenov D. Intermittent hypoxia initiated plasticity in humans: a multipronged therapeutic approach to treat sleep apnea and overlapping co-morbidities. Exp Neurol 287: 113–129, 2017. doi: 10.1016/j.expneurol.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 44.Mateika JH, Syed Z. Intermittent hypoxia, respiratory plasticity and sleep apnea in humans: present knowledge and future investigations. Respir Physiol Neurobiol 188: 289–300, 2013. doi: 10.1016/j.resp.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGuire M, Zhang Y, White DP, Ling L. Chronic intermittent hypoxia enhances ventilatory long-term facilitation in awake rats. J Appl Physiol (1985) 95: 1499–1508, 2003. doi: 10.1152/japplphysiol.00044.2003. [DOI] [PubMed] [Google Scholar]
- 46.McGuire M, Zhang Y, White DP, Ling L. Serotonin receptor subtypes required for ventilatory long-term facilitation and its enhancement after chronic intermittent hypoxia in awake rats. Am J Physiol Regul Integr Comp Physiol 286: R334–R341, 2004. doi: 10.1152/ajpregu.00463.2003. [DOI] [PubMed] [Google Scholar]
- 47.McNay EC, McCarty RC, Gold PE. Fluctuations in brain glucose concentration during behavioral testing: dissociations between brain areas and between brain and blood. Neurobiol Learn Mem 75: 325–337, 2001. doi: 10.1006/nlme.2000.3976. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB Jr. Intermittent hypoxia and respiratory plasticity. J Appl Physiol (1985) 90: 2466–2475, 2001. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- 49.Navarrete-Opazo A, Alcayaga J, Sepulveda O, Rojas E, Astudillo C. Repetitive intermittent hypoxia and locomotor training enhances walking function in incomplete spinal cord injury subjects: a randomized, triple-blind, placebo-controlled clinical trial. J Neurotrauma 34: 1803–1812, 2017. doi: 10.1089/neu.2016.4478. [DOI] [PubMed] [Google Scholar]
- 50.Navarrete-Opazo A, Dougherty BJ, Mitchell GS. Enhanced recovery of breathing capacity from combined adenosine 2A receptor inhibition and daily acute intermittent hypoxia after chronic cervical spinal injury. Exp Neurol 287: 93–101, 2017. doi: 10.1016/j.expneurol.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Navarrete-Opazo A, Mitchell GS. Recruitment and plasticity in diaphragm, intercostal, and abdominal muscles in unanesthetized rats. J Appl Physiol (1985) 117: 180–188, 2014. doi: 10.1152/japplphysiol.00130.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nichols NL, Dale EA, Mitchell GS. Severe acute intermittent hypoxia elicits phrenic long-term facilitation by a novel adenosine-dependent mechanism. J Appl Physiol (1985) 112: 1678–1688, 2012. doi: 10.1152/japplphysiol.00060.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nichols NL, Van Dyke J, Nashold L, Satriotomo I, Suzuki M, Mitchell GS. Ventilatory control in ALS. Respir Physiol Neurobiol 189: 429–437, 2013. doi: 10.1016/j.resp.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peng YJ, Prabhakar NR. Reactive oxygen species in the plasticity of respiratory behavior elicited by chronic intermittent hypoxia. J Appl Physiol (1985) 94: 2342–2349, 2003. doi: 10.1152/japplphysiol.00613.2002. [DOI] [PubMed] [Google Scholar]
- 55.Potter WB, O’Riordan KJ, Barnett D, Osting SM, Wagoner M, Burger C, Roopra A. Metabolic regulation of neuronal plasticity by the energy sensor AMPK. PLoS One 5: e8996, 2010. doi: 10.1371/journal.pone.0008996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Row BW, Kheirandish L, Neville JJ, Gozal D. Impaired spatial learning and hyperactivity in developing rats exposed to intermittent hypoxia. Pediatr Res 52: 449–453, 2002. doi: 10.1203/00006450-200209000-00024. [DOI] [PubMed] [Google Scholar]
- 57.Row BW, Liu R, Xu W, Kheirandish L, Gozal D. Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am J Respir Crit Care Med 167: 1548–1553, 2003. doi: 10.1164/rccm.200209-1050OC. [DOI] [PubMed] [Google Scholar]
- 58.Satriotomo I, Dale EA, Dahlberg JM, Mitchell GS. Repetitive acute intermittent hypoxia increases expression of proteins associated with plasticity in the phrenic motor nucleus. Exp Neurol 237: 103–115, 2012. doi: 10.1016/j.expneurol.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Satriotomo I, Nichols NL, Dale EA, Emery AT, Dahlberg JM, Mitchell GS. Repetitive acute intermittent hypoxia increases growth/neurotrophic factor expression in non-respiratory motor neurons. Neuroscience 322: 479–488, 2016. doi: 10.1016/j.neuroscience.2016.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Semenza GL, Nejfelt MK, Chi SM, Antonarakis SE. Hypoxia-inducible nuclear factors bind to an enhancer element located 3′ to the human erythropoietin gene. Proc Natl Acad Sci USA 88: 5680–5684, 1991. doi: 10.1073/pnas.88.13.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stafstrom CE, Ockuly JC, Murphree L, Valley MT, Roopra A, Sutula TP. Anticonvulsant and antiepileptic actions of 2-deoxy-d-glucose in epilepsy models. Ann Neurol 65: 435–447, 2009. doi: 10.1002/ana.21603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tasali E, Ip MS. Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation. Proc Am Thorac Soc 5: 207–217, 2008. doi: 10.1513/pats.200708-139MG. [DOI] [PubMed] [Google Scholar]
- 63.Wick AN, Drury DR, Nakada HI, Wolfe JB. Localization of the primary metabolic block produced by 2-deoxyglucose. J Biol Chem 224: 963–969, 1957. [PubMed] [Google Scholar]
- 64.Wilkerson JE, Mitchell GS. Daily intermittent hypoxia augments spinal BDNF levels, ERK phosphorylation and respiratory long-term facilitation. Exp Neurol 217: 116–123, 2009. doi: 10.1016/j.expneurol.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Won JS, Im YB, Key L, Singh I, Singh AK. The involvement of glucose metabolism in the regulation of inducible nitric oxide synthase gene expression in glial cells: possible role of glucose-6-phosphate dehydrogenase and CCAAT/enhancing binding protein. J Neurosci 23: 7470–7478, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]