Abstract
Recent studies suggest that circadian rhythms regulate intestinal barrier integrity, but it is not clear whether there are daily variations in barrier integrity. This study investigated daily variations in intestinal barrier integrity, including whether there are differences in alcohol-induced intestinal barrier dysfunction after an alcohol binge at different times of day and whether this is associated with concurrent liver injury. C57BL6/J male mice were fed a standard chow diet, an alcohol-containing liquid diet, or an alcohol control diet for 4 wk. During week 5 (i.e., on days 43–45), mice received three once-daily gavages of alcohol (6 g/kg) or the control (phosphate-buffered saline) at the same time each day. Immediately after the binge on the second day, intestinal permeability was assessed. Four hours after the third and final binge, mice were euthanized and tissue samples collected. The results demonstrated diet-specific and outcome-specific effects of time, alcohol, and/or time by alcohol interaction. Specifically, the alcohol binge robustly influenced markers of intestinal barrier integrity, and liver markers were robustly influenced by time of day. Only intestinal permeability (i.e., sucralose) demonstrated a significant effect of time and also showed a binge by time interaction, suggesting that the time of the alcohol binge influences colonic permeability.
NEW & NOTEWORTHY This study investigated daily variations in intestinal barrier integrity, including whether there are differences in alcohol-induced intestinal barrier dysfunction after an alcohol binge at different times of day and whether this is associated with concurrent liver injury. We conclude that 1) alcohol binge significantly impacted markers of intestinal permeability, 2) time of day significantly affected liver outcomes, and 3) the time of day influenced colonic permeability.
Keywords: alcohol binge, circadian rhythms, diurnal oscillations, intestinal permeability, intestine, liver
INTRODUCTION
The intestinal epithelium is a selectively permeable barrier that permits the absorption of nutrients and water while it inhibits the passage of proinflammatory luminal contents from the intestine into the lamina propria and the systemic circulation. Dysfunction of this barrier is associated with a wide variety of diseases, including alcoholic liver disease (8, 9, 15, 18). Our group has shown that circadian rhythm disruption is also a factor that causes intestinal barrier dysfunction (34–36). Circadian rhythms regulate a wide variety of behaviors, biological functions, and gene expression in mammals (30, 49), including the expression of proteins that regulate intestinal barrier integrity (29a, 34). Emerging evidence suggests that there are diurnal fluctuations in intestinal barrier integrity; however, there is no consensus identifying features of intestinal diurnal variation (29a, 37). In addition, it is not known how diet (e.g., high-fat diet, high-sugar diet, or alcohol) influences diurnal variations in barrier integrity or whether diurnal fluctuations in barrier integrity confer time-of-day-specific vulnerability to a secondary insult such as alcohol. Evaluating this potentially clinically impactful phenomenon was the goal of this study.
We examined markers of intestinal barrier integrity and liver pathology at six different times of the day, using a chow diet and the Nanji diet (i.e., alcohol-containing and control diet) to determine whether there are daily fluctuations in intestinal barrier integrity and whether/how these daily variations are impacted by an alcohol binge. Alcohol consumption disrupts the intestinal barrier, and the endotoxemia that results from barrier dysfunction is one important mechanism that promotes liver damage (16, 18). However, it is not clear whether susceptibility to alcohol-induced intestinal hyperpermeability exhibits daily variation. Binge drinking has become increasingly prevalent in the US, with nearly 25% of the population engaging in this activity in any given 30-day period (33). Thus, we used a binge-drinking animal model 1) as a relevant example of alcohol consumption and 2) as a precise method to determine how the timing of alcohol consumption impacts intestinal barrier integrity and liver pathology.
MATERIALS AND METHODS
Ethics Statement
All mice were housed and handled in accordance with federal animal welfare guidelines and in compliance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and the Guide for the Use and Care of Laboratory Animals. All experiments were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at Rush University Medical Center (study protocol no. 13-067) before being conducted.
Mice and Housing
Studies utilized male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME). Young adult (6–8 wk old) mice were housed individually in cages housed within IACUC-approved, ventilated, light-tight cabinets. Mice were acclimated to the facility for 1 wk before the study was initiated. Food intake and body weight were measured throughout the experiment. Mice were maintained on a constant 12-h:12-h light-dark cycle for the duration of the experiment. By convention, the time of light onset is referred to as Zeitgeber time (ZT) 0, whereas the lights-off time is referred to as ZT12 on a normal 12-h:12-h light-dark cycle. Mice were randomly assigned to each treatment group. All procedures performed during dark conditions (i.e., ZT12 to ZT0) were performed under red light conditions.
Treatment Protocols
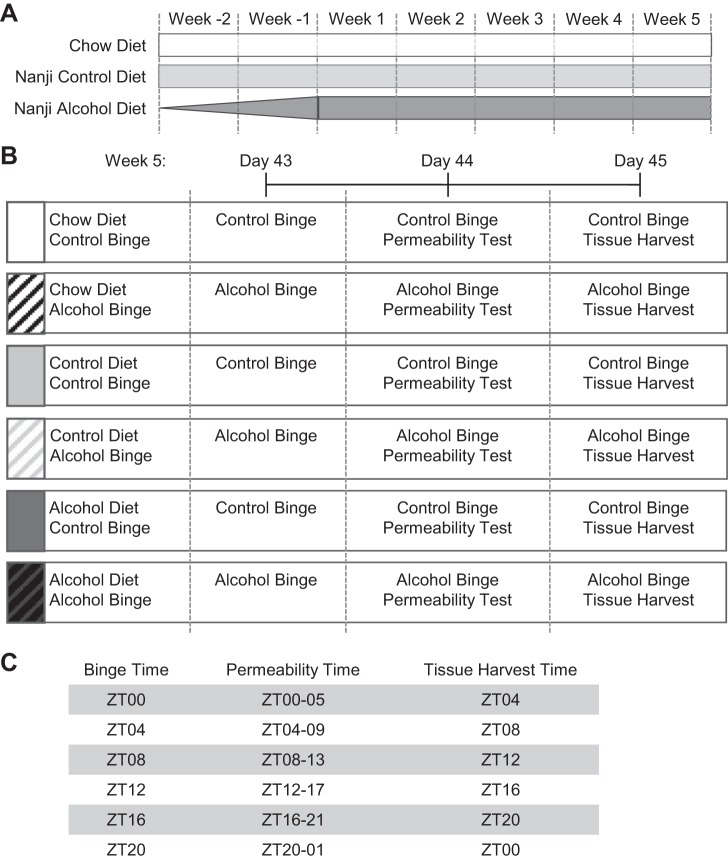
Mice consumed one of three diets: a standard rodent chow diet (Teklad Envigo no. 2018; Teklad, Madison, WI), an alcohol-containing liquid diet, or the alcohol-free control liquid diet. The alcohol diet was the Nanji diet (28, 40), which is a modification of the Lieber DeCarli diet, in which the fat calories come from fish oil to promote liver pathology. Alcohol-fed mice had a 2-wk gradual introduction of alcohol into the diet, followed by 4 wk on the full alcohol concentration (4.5% vol/vol, 29% of total daily calories from alcohol; Fig. 1A). Control mice were fed an isocaloric diet in which the calories from alcohol were replaced by dextrose (i.e., a high-fat, high-carbohydrate diet). We have used this experimental diet previously and found that alcohol-fed and control-fed mice consume statistically indistinguishable amounts of each diet (34). The components of the Nanji diet include mineral mix, vitamin mix, choline bitartrate, d-l-methionine, lactalbumin, xanthan gum, dextrose (all obtained from Dyets, Bethlehem, PA), fish oil (from Menhaden, Sigma, St. Louis, MO), ethanol (Sigma), and Hershey’s chocolate syrup to improve palatability. The caloric composition of the diet was 36% protein, 29% carbohydrate/alcohol, and 35% fat. The Nanji diet was prepared fresh daily and was provided to the mice in individual, specialized, graduated sipper tubes (Bio-Serv, Frenchtown, NJ) to allow for convenient monitoring of daily food intake, which took place between 12 PM and 2 PM. Daily food intake was not monitored in chow-fed mice, nor was new food presented on a daily basis; therefore, chow-fed mice were analyzed separately from Nanji diet-fed mice since these procedural differences may have influenced outcomes. All diets and water were available ad libitum.
Fig. 1.
Experimental design. A: mice were fed 1 of 3 diets: a chow diet, a Nanji control diet (i.e., a high-fat, high-sugar diet), or a Nanji alcohol-containing diet. The alcohol content in the alcohol-containing diet was gradually increased over the first 2 wk (week −2, week −1), followed by 4 wk on the full alcohol concentration (4.5% vol/vol, 29% of total daily calorie intake from alcohol). B and C: during week 5, mice were given 3 once/daily gavages of alcohol (6 g/kg) or the vehicle control (i.e., PBS) at the same time each day. On day 43, mice were given a gavage and immediately returned to the home cage; on day 44, mice were given a gavage, followed immediately by a 5-h test for intestinal permeability; and on day 45, mice were given a gavage, and 4 h later, liver and blood were collected. ZT, zeitgeber time.
During week 5 (i.e., study days 43–45), mice were given a once-daily gavage (i.e., binge) of alcohol (6 g·kg−1·day−1) or the vehicle control (i.e., PBS) (Fig. 1B). The alcohol treatment paradigm in this study is similar to chronic alcohol (i.e., 2–12 wk) plus three alcohol binge (4–6 g/kg body weight) models (the NIAAA or Gao-binge model) that have been used previously (1, 2, 19, 23, 24). The binge (i.e., vehicle or alcohol) was administered at the same time each day. To determine whether an alcohol binge at different times of the day influenced the response to the alcohol binge, each group of mice received the binge at a different time: ZT0, ZT4, ZT8, ZT12, ZT16, or ZT20 (Fig. 1C). This experimental design yielded a total of 36 experimental groups (3 diets × 2 binge types × 6 ZTs).
In vivo Intestinal Permeability Testing
Mice were fasted overnight before the intestinal permeability test, which was performed on day 44 (after the second binge; Fig. 1B). A 200-µl sugar solution [a combination of nonabsorbable, poorly digestible sugars, including lactulose (3.2 mg), sucrose (0.45 mg), sucralose (0.45 mg), and mannitol (0.9mg)] was administered to mice via oral gavage, followed immediately by a 2-ml, 0.9% saline injection subcutaneously on the hind quarter to promote urine production. Following administration of the sugar solution and saline, mice were placed into a metabolic chamber for 5 h, and urine was collected. The 5-h time frame was selected to account for transit time of the sugar through the intestine, including the colon. Intestinal permeability was assessed by measuring excreted urinary sugar content using gas chromatography (3, 7, 11, 13, 25, 26, 41). Lactulose and mannitol are not digested in the small intestine but are digested by bacteria in the colon; thus, these sugar probes represent small intestine permeability (3, 7, 11, 13, 26, 41). Sucralose is not metabolized by bacteria in the colon, making it an estimate of whole intestine permeability (25).
Tissue Collection and Analysis
Four hours after the third and final gavage (study day 45), mice were euthanized by decapitation (Fig. 1, B and C). Liver and blood were collected for analyses. Liver was fixed in 10% formalin for 24 h, paraffin embedded, and stained with hematoxylin and eosin. Blind assessments of liver samples were conducted by a hepatologist (C. Aloman). Histological analysis, including steatosis and inflammation, were scored according to the following criteria: steatosis, scored as percent hepatocyte involvement corresponding to the fraction of lipid-containing hepatocytes (0 = <5%, 1 = 5–33%, 2 = 34–66%, and 3 = >67%); inflammation, scored based on the number of inflammatory foci per ×200 field (0 = no foci, 1 = 1 focus, 2 = 2–4 foci, and 3 = >4 foci). These markers (steatosis, inflammation) were selected because they are well-established markers of alcoholic steatohepatitis.
Myeloperoxidase.
Neutrophils are the predominant inflammatory cell in alcohol-induced liver injury, and myeloperoxidase (MPO) is released by activated polymorphonuclear neutrophils. Liver MPO levels were assessed using an ELISA according to the manufacturer’s instructions (Hycult Biotech, Plymouth Meeting, PA).
Blood was allowed to clot at room temperature before centrifugation for serum collection. Serum was stored at −80°C until use.
Serum alcohol.
Serum alcohol levels were measured by head space chromatography (5, 34). Briefly, serum was precipitated with perchloric acid-thiourea containing 1 mM 2-propanol, which was included as the internal standard. Samples were heated to 60°C, and the vapor phase was quantified for alcohol concentrations, using a Perkin-Elmer gas chromatograph.
Endotoxemia.
Lipopolysaccharide (LPS), a component in the outer membrane of gram-negative bacteria, was used as a marker of barrier dysfunction and endotoxemia. Serum collected at the time of euthanasia was used to measure systemic LPS levels using the Pyrogen Recombinant Factor C kit according to the manufacturer’s instructions (Lonza, Walkersville, MD). LPS-binding protein (LBP) is a type 1 acute-phase protein that binds to LPS to facilitate an immune response in conjunction with cell-surface pattern recognition receptors and was used as an indicator of LPS exposure. Serum collected at the time of euthanasia was used to measure systemic LBP levels using an LBP ELISA kit (HK205; Hycult Biotech) according to the manufacturer’s instructions.
Serum alanine aminotransferase/aspartate aminotransferase.
Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured by ANTECH Diagnostics (Lake Success, NY).
Statistical Analyses
All data are shown as means ± SE. Statistical evaluations of data were performed using SPSS (version 19), R (version 3.2.3), or GraphPad Prism (version 5). Binge time and tissue harvest time are not linearly independent (i.e., the tissue harvest is always 4 h after the binge time); therefore, an estimation of all terms in the model is not using analysis of variance (ANOVA). To incorporate both time factors into the analysis, a stepwise analysis was conducted. First, a true score analysis was performed to determine the linear relationship between tissue harvest time and the outcome of interest. The residuals resulting from this initial analysis were then used as the dependent variable in the ANOVA analysis. Two-way or three-way analysis of variance (ANOVA) was used to assess the impact of time and/or treatment (i.e., diet and binge) on experimental outcomes. Histological analyses were assessed with nonparametric statistics.
RESULTS
The primary goals of our study were to determine whether 1) there are time-of-day differences in intestinal barrier integrity and/or liver pathology and 2) how fluctuations in barrier integrity and liver pathology are affected by dietary alcohol consumption and/or alcohol binge. We utilized two different feeding protocols, with the first being a standard chow diet and the other the Nanji diet consisting of a control diet and an alcohol-containing diet.
Chow-fed mice
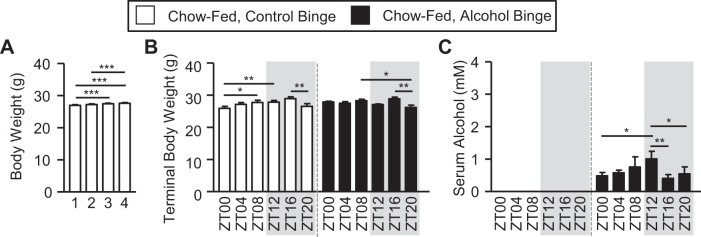
First, we examined chow-fed mice. Over the course of 4 wk, there was a small but significant increase in body weight (1-way ANOVA, P < 0.00; Fig. 2A). Analysis of terminal body weight (i.e., assessed just before tissue collection) revealed no significant effects of binge or time (i.e., ZT), nor an interaction (Fig. 2B and Table 1). Although time was not a significant main effect, significant post hoc differences were observed between mice given the binge at ZT16 weighing more than those at ZT20 in both groups (i.e., control and alcohol binge) (Fig. 2B). Serum alcohol content was significantly increased by the alcohol binge with a main effect of binge (Fig. 2C and Table 1). Although time was not a significant main factor, post hoc analysis revealed that serum alcohol levels at ZT12 were the highest in those mice receiving the alcohol binge (Fig. 2C).
Fig. 2.
Chow-fed mice exhibit treatment-induced effects on body weight and serum alcohol levels. Mice were fed a standard chow diet for 4 wk, and during the 5th wk (i.e., days 43–45) mice were administered a once/daily binge of alcohol (6 g/kg) or the vehicle control for alcohol (i.e., PBS) at a specific time indicated by the zeitgeber time (ZT), and mice were euthanized 4 h after the 3rd and final once/daily binge. A: mice were weighed weekly, and body weight significantly increased over the 4 wk of the study (1-way ANOVA, P < 0.00). Post hoc Tukey’s test revealed significant between-week differences. Numbers indicate study week. B: analysis of body weight before tissue collection (i.e., following the 3rd and final binge) revealed a significant main effect of time (2-way ANOVA, P < 0.00) but no effect of binge or an interaction. Post hoc Tukey’s test revealed significant time-of-day differences. C: serum alcohol levels assessed 4 h after the 3rd and final binge and revealed a significant main effect of binge (P < 0.00) but no effect of time nor a binge-by-time interaction (2-way ANOVA). Post hoc Tukey’s test revealed significant time-of-day differences in serum alcohol levels. ZT indicates binge time. Between n = 5 and 10 mice were included in each treatment group at each time point. Post hoc Tukey’s test: *P < 0.05; **P < 0.01; ***P < 0.001.
Table 1.
Chow-fed mice statistical outcomes
| Binge | Time | Binge × Time | |
|---|---|---|---|
| Terminal body weight | P = 0.38 | P = 0.59 | P = 0.23 |
| Serum alcohol | P < 0.00* | P = 0.31 | P = 0.29 |
| Markers of intestinal permeability | |||
| Lactulose | P < 0.00* | P = 0.53 | P = 0.25 |
| Mannitol | P < 0.00* | P = 0.53 | P = 0.17 |
| Sucralose | P < 0.00* | P < 0.00* | P < 0.00* |
| LPS | P = 0.32 | P = 0.02* | P = 0.60 |
| LBP | P = 0.10 | P = 0.91 | P = 0.09 |
| Markers of liver pathology | |||
| Steatosis | P < 0.00*† | P = 0.08† | NA† |
| Inflammation | P = 0.32† | P = 0.10† | NA† |
| MPO | P = 0.62 | P = 0.50 | P = 0.80 |
| ALT | P = 0.03* | P = 0.89 | P = 0.76 |
| AST | P < 0.00* | P = 0.23 | P = 0.93 |
LPS, lipopolysaccharide; LBP; lipopolysaccharide-binding protein; MPO, myeloperoxidase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; NA, not applicable. Chow-fed mice exhibit treatment-induced effects on body weight, serum alcohol concentrations, markers of intestinal permeability, and markers of liver pathology. Between 5 and 10 mice were included at each treatment group at each time point. Data were analyzed using a 2-way ANOVA, except where indicated.
Data were analyzed via a nonparametric Kruskal-Wallis test;
P < 0.05.
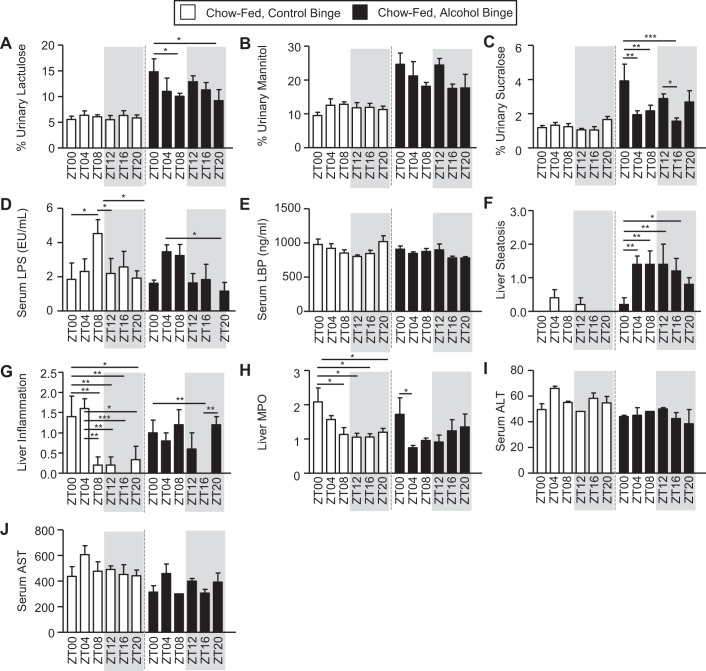
We next examined intestinal barrier integrity. A solution of nonabsorbable, poorly digestible sugars containing mannitol, lactulose, sucralose, and sucrose was administered to the mice via gavage, and urine was collected for 5 h. A high level of sugar in the urine is an indication of impaired intestinal barrier integrity (i.e., intestinal hyperpermeability). Lactulose (i.e., primarily a marker of small intestine permeability) exhibited a significant main effect of binge (Fig. 3A and Table 1), and although there was not a main effect of time, post hoc analysis revealed that mice exhibited greater alcohol binge-induced intestinal barrier dysfunction when the alcohol binge was administered at ZT0 compared with the other times (Fig. 3A). Likewise, mannitol (i.e., primarily a marker of proximal small intestine permeability) exhibited a significant main effect of binge (Fig. 3B and Table 1). Sucralose (i.e., a marker of whole intestine and colonic permeability) exhibited a significant effect of binge and was the only urinary sugar to show a main effect of time and a binge-by-time interaction. Post hoc analysis again revealed that alcohol binge-induced intestinal barrier dysfunction was greatest when the binge was administered at ZT0 (Fig. 3C and Table 1). Lipopolysaccharide (LPS) is a component found in the outer membrane of gram-negative bacteria and is considered a valid marker of microbiota translocation, and therefore, it is indicated intestinal barrier dysfunction. Analysis of LPS levels revealed a significant main effect of time, with the highest levels observed at ZT8 in control binged mice and at ZT4 in alcohol-binged mice (Fig. 3D and Table 1). LPS binding protein (LBP) is an acute-phase protein produced by the liver that binds to LPS, and we used this marker to assess intestinal barrier integrity. Despite the changes observed in LPS, LBP levels were not affected by binge or time, nor was there an interaction (Fig. 3E and Table 1).
Fig. 3.
Binge- and time-dependent effects on markers of intestinal barrier integrity and liver inflammation in chow-fed mice. Mice were fed a chow diet for 4 wk, and during the 5th wk (i.e., days 43–45), mice were administered a once/daily binge of alcohol (6 g/kg) or the vehicle control for alcohol (i.e., PBS) at a specific time indicated by the zeitgeber time (ZT). After the first binge, mice were immediately returned to the home cage; then they were given a test for intestinal permeability immediately following the 2nd binge, and then they were euthanized 4 h after the 3rd and final once/daily binge. Intestinal barrier integrity and liver pathology were assessed. A–C: intestinal barrier integrity was evaluated following the bolus administration of a sugar solution, and urinary sugar was measured as an index of barrier integrity; higher urinary sugar equates to greater barrier dysfunction. A: urinary lactulose exhibited a significant effect of binge (P < 0.00) but no effect of diet nor an interaction. Post hoc Tukey’s test revealed significant time-of-day effects. B: urinary mannitol exhibited a significant effect of binge (P < 0.00) but no effect of diet or an interaction. Post hoc Tukey’s test revealed no significant effects. C: urinary sucralose revealed a significant effect of binge (P < 0.00) and time (P = 0.04) but no interaction. Post hoc Tukey’s test revealed significant time-of-day effects. D and E: intestinal barrier integrity was also evaluated using serum markers, including lipopolysaccharide (LPS) and LPS-binding protein (LBP). D: serum LPS exhibited a significant effect of time (P = 0.01) but no effect of binge or an interaction. Post hoc Tukey’s test revealed significant time-of-day effects. E: serum LBP levels were not affected by binge or time, nor was there an interaction. F–J: next, we assessed liver pathology using a combination of histological and serum analysis approaches. F: liver steatosis exhibited a significant effect of binge (Kruskal-Wallis, P < 0.00) but no effect of time. Post hoc Tukey’s test revealed significant time-of-day effects. G: liver inflammation exhibited a significant effect of time (Kruskal-Wallis, P < 0.00) but no effect of binge or an interaction. Post hoc Tukey’s test revealed significant time of day effects. H: liver myeloperoxidase (MPO) exhibited a significant main effect of time (P = 0.01) but no effect of binge or an interaction. Post hoc Tukey’s test revealed significant time-of-day effects. I: serum alanine aminotransferase (ALT) exhibited a significant effect of binge (P = 0.03) but not time, nor was there an interaction. Post hoc Tukey’s test revealed no significant effects. J: serum aspartate aminotransferase (AST) exhibited a significant effect of binge (P < 0.00) but no effect of time or an interaction. Post hoc Tukey’s test revealed no significant differences. Between n = 5 and 10 mice were included in each treatment group at each time point. Two-way ANOVA, followed by post hoc Tukey’s test (*P < 0.05; **P < 0.01; ***P < 0.001), was used for analysis unless noted. ZT, zeitgeber time (binge time).
We also examined markers of liver pathology, including steatosis, inflammation (MPO and histology), ALT, and AST. Liver steatosis, or fat accumulation, was examined as one pathological marker of alcohol consumption. Histological scoring of steatosis histology revealed a significant main effect of binge; post hoc analysis revealed that liver steatosis was lowest at ZT0 in mice that were given an alcohol binge (Fig. 3F and Table 1). In contrast, liver inflammation, defined as immune cell infiltration in the liver, showed no main effects of binge or time (Fig. 3G and Table 1). Despite seeing no significant main effects, post hoc analysis of control binged mice showed that ZT0 and ZT4 had the highest levels of liver inflammation, whereas alcohol-binged mice had no detectable inflammation at ZT16 (Fig. 3G). Other indices of liver pathology were examined, including liver myleloperoxidiase (MPO), serum alanine aminotransferase (ALT), and serum aspartate aminotransferase (AST). Liver MPO exhibited no significant main effects of binge or time, nor was there an interaction (Fig. 3H and Table 1). Post hoc analysis revealed that ZT0 had the highest MPO levels in both control and alcohol-binged mice (Fig. 3H). Analysis of serum ALT revealed a significant effect of binge but no effect of time or an interaction (Fig. 3I and Table 1). Serum AST exhibited a significant effect of binge but no effect of time, nor was there an interaction (Fig. 3J and Table 1).
Nanji Diet-Fed Mice
We next examined the impact of time and an alcohol binge on mice consuming the Nanji diet (i.e., control and alcohol). Food consumption was averaged for each week (i.e., weeks 1–4). The analysis revealed a significant effect of diet (P < 0.00) and week (i.e., time, P < 0.00) as well as a diet-by-week interaction (P < 0.00) (Fig. 4A). Analysis of body weight, assessed weekly (i.e., weeks 1–4), revealed a significant effect of week (i.e., time) on body weight (P < 0.00) but no effect of diet (P = 0.72), nor was there an interaction (P = 0.08) (Fig. 4B); thus, although alcohol-fed mice consumed slightly less food, their mean body weight was statistically indistinguishable from control-fed mice.
Fig. 4.
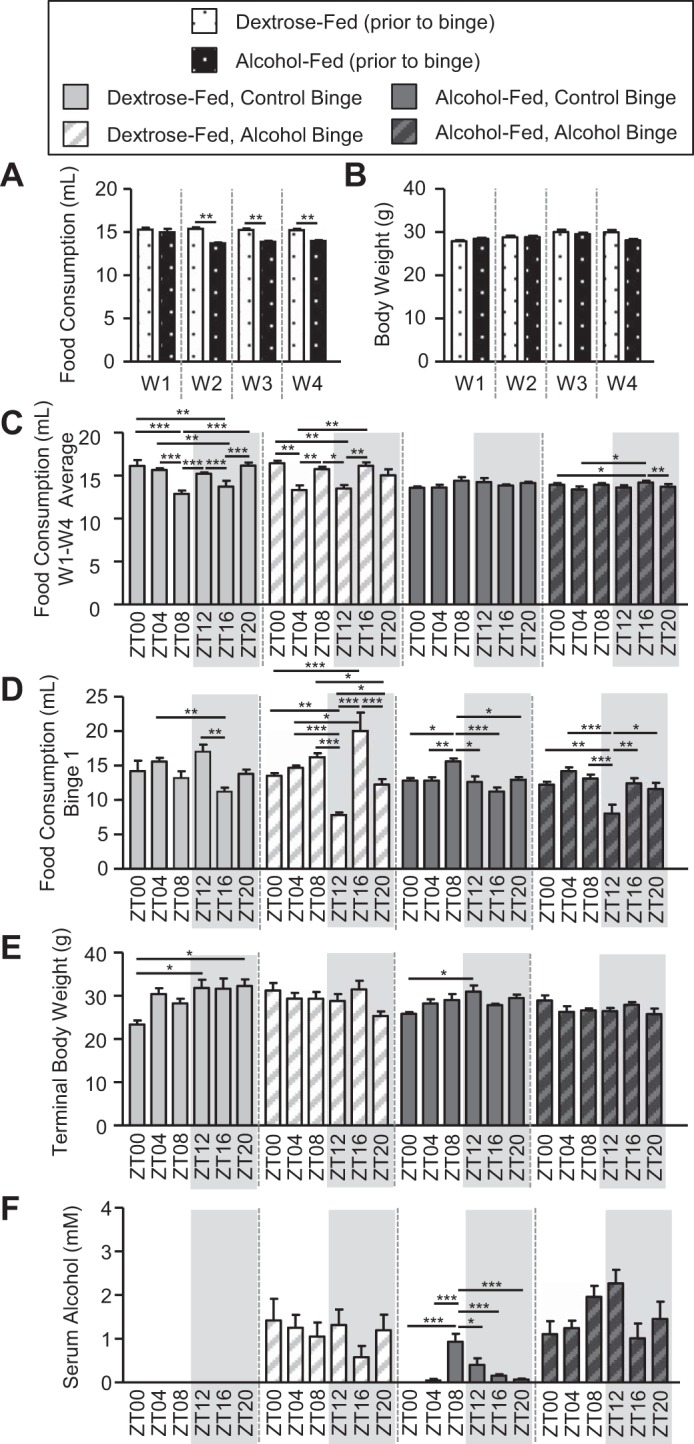
Nanji diet-fed mice exhibit treatment-induced effects on food consumption, body weight, and serum alcohol levels. Mice were fed a Nanji diet for 4 wk, and during the 5th wk (i.e., days 43–45) mice were administered a once/daily binge of alcohol (6 g/kg) or the vehicle control for alcohol (i.e., PBS) at a specific time indicated by the zeitgeber time (ZT), and mice were euthanized 4 h after the 3rd and final once/daily binge. A: food consumption was monitored daily, and weekly averages were calculated. There was a significant effect of diet (P < 0.00), time (P < 0.00), and diet × time interaction (P < 0.00) (2-way ANOVA). Post hoc Tukey’s test revealed significant differences between control-fed and alcohol-fed mice. B: mice were weighed weekly, and body weight was significantly influenced by time (P < 0.00, 2-way ANOVA). Post hoc Tukey revealed no significant differences. C and D: next, we assessed whether the time of food presentation and/or binge influenced food consumption. C: food consumption was monitored daily, and then food consumption over the entire 4-wk study was averaged for each ZT individually. There was a significant effect of diet (P < 0.00), a diet × time interaction (P < 0.00), a binge × time interaction (P < 0.00), and a diet × binge × time interaction (P < 0.00). Post hoc Tukey’s test revealed significant time-of-day effects. D: food consumption was monitored during the 24-h encompassing the first binge. There was a significant effect of diet (P < 0.00), binge (P = 0.04), and time (P < 0.00) as well as a binge × time interaction (P < 0.00) and a diet × binge × time interaction (P < 0.00). Post hoc analysis revealed significant time-of-day effects. E: terminal body weight measured before euthanasia revealed a significant effect of diet (P = 0.01) and a binge × time interaction (P < 0.00). Post hoc testing revealed significant time-of-day effects. F: serum alcohol exhibited a significant main effect of diet (P < 0.00), binge (P < 0.00), time (P = 0.01), and diet × time interaction (P = 0.02). Post hoc Tukey’s test revealed significant time-of-day effects. Between n = 5 and 10 mice were included in each treatment group at each time point. A 3-way ANOVA followed by post hoc Tukey’s test was used unless otherwise noted. *P < 0.05; **P < 0.01; ***P < 0.001. W, week.
The next important question to consider was how the timing of the daily food presentation impacted food consumption. Although food was prepared fresh daily and provided to the mice between 12 and 2 PM, the food was actually provided to the mice at different ZT because different groups of mice were housed under different lights on-lights off cycles, which allowed us to euthanize mice at six different times. To address the potential impact of feeding at different times, food consumption was averaged for each group. This analysis revealed a significant effect of binge as well as a diet-by-time interaction, a binge-by-time interaction, and a diet-by-binge-by-time interaction (Fig. 4C and Table 2). Post hoc analysis showed many between-group differences in control-fed mice, and with fewer differences observed among alcohol-fed mice, the lack of consistency between groups makes it difficult to interpret the results. The time of the binge may have also affected food consumption since the binge was administered at different times of day. After the first binge, significant main effects of diet, binge, time, a binge-by-time interaction, and a diet-by-binge-by-time interaction were observed (Fig. 4D and Table 2). Post hoc analysis revealed many between-group differences. Specifically, mice that received the alcohol binge at ZT12 consumed less food than those mice that received the binge at the other ZTs (Fig. 4D). The mean terminal body weight following the third and final binge (i.e., the weight immediately before euthanasia) showed a significant main effect of diet and a binge-by-time interaction (Fig. 4E and Table 2). Post hoc analysis revealed that mice given the binge at ZT0 weighed significantly less than the other ZTs; however, this was observed only in the control binged groups (i.e., binged with PBS; Fig. 4E). Therefore, although time impacted food consumption, time did not significantly influence terminal body weight. Moreover, the reduced food intake following the alcohol binge had only a minor effect, since the alcohol binge affected markers of intestinal permeability and liver pathology more than those of chronic alcohol consumption alone (see results below). Analysis of serum alcohol levels revealed significant main effects of diet, binge, and time as well as a diet-by-time interaction (Fig. 4F and Table 2). Post hoc analysis revealed that serum alcohol levels were highest at ZT8 in alcohol-fed mice with a control binge (Fig. 4F).
Table 2.
Nanji diet-fed mice statistical outcomes
| Diet | Binge | Time | Diet × Binge | Diet × Time | Binge × Time | Diet × Binge × Time | |
|---|---|---|---|---|---|---|---|
| Food consumption/body weight/serum alcohol | |||||||
| Food consumption (weekly average) | P < 0.00* | P = 0.84 | P = 0.05 | P = 0.48 | P < 0.00* | P < 0.00* | P < 0.00* |
| Food consumption (binge 1) | P < 0.00* | P = 0.04* | P < 0.00* | P = 0.08 | P = 0.09 | P < 0.00* | P < 0.00* |
| Terminal body weight | P = 0.01* | P = 0.12 | P = 0.76 | P = 0.35 | P = 0.59 | P < 0.00* | P = 0.51 |
| Serum alcohol | P < 0.00* | P < 0.00* | P = 0.01* | P = 0.60 | P = 0.02* | P = 0.22 | P = 0.87 |
| Markers of intestinal permeability | |||||||
| Lactulose | P = 0.38 | P < 0.00* | P = 0.86 | P = 0.63 | P = 0.17 | P = 0.79 | P = 0.07 |
| Mannitol | P = 0.50 | P < 0.00* | P = 0.72 | P = 0.88 | P = 0.15 | P = 0.21 | P = 0.14 |
| Sucralose | P = 0.16 | P < 0.00* | P = 0.13 | P = 0.75 | P = 0.25 | P = 0.01* | P = 0.33 |
| LPS | P = 0.03* | P = 0.01* | P < 0.00* | P = 0.31 | P < 0.00* | P < 0.00* | P = 0.02* |
| LBP | P = 0.35 | P = 0.95 | P = 0.20 | P = 0.08 | P = 0.11 | P = 0.51 | P = 0.58 |
| Markers of liver pathology | |||||||
| Steatosis | P = 0.80† | P = 0.01*† | P < 0.00*† | NA† | NA† | NA† | NA† |
| Inflammation | P = 0.87† | P = 0.43† | P < 0.00*† | NA† | NA† | NA† | NA† |
| MPO | P = 0.74 | P < 0.00* | P = 0.54 | P = 0.19 | P = 0.96 | P = 0.12 | P = 0.58 |
| ALT | P = 0.52 | P = 0.28 | P = 0.01* | P = 0.82 | P = 0.60 | P = 0.02* | P = 0.95 |
| AST | P = 0.16 | P = 0.01* | P = 0.02* | P = 0.61 | P = 0.22 | P = 0.21 | P = 0.78 |
LPS, lipopolysaccharide; LBP, lipopolysaccharide-binding protein; MPO, myeloperoxidase; ALT, alanine aminotransferase; AST, aspartate aminotransferase. Nanji diet-fed mice exhibit treatment induced effects on body weight, serum alcohol concentrations, markers of intestinal permeability, and markers of liver pathology. Between 5 and 10 mice were included at each treatment group at each time point. Data were analyzed using a 2-way ANOVA, except where indicated.
Data were analyzed via a nonparametric Kruskal-Wallis;
P < 0.05.
We next analyzed markers of intestinal barrier integrity. Analysis of urinary sugars is used to assess intestinal barrier integrity, and higher urinary sugar content equates to intestinal barrier dysfunction (the so-called “leaky gut”). These data revealed that both lactulose and mannitol exhibited a significant main effect of binge but were statistically unaffected by diet and time, nor were there any interactions (Fig. 5, A and B, and Table 2). Sucralose also exhibited a significant main effect of binge and in addition demonstrated a significant binge-by-time interaction, indicating that the effects of the binge on urinary sucralose were influenced by time (Fig. 5C and Table 2). Post hoc analysis revealed a significant difference between ZT4 and ZT16 only in the control-fed, alcohol-binged mice (Fig. 5C). Serum LPS levels exhibited significant main effects of diet, binge, and time as well as a significant diet-by-time interaction, binge-by-time interaction, and a diet-by-binge-by-time interaction (Fig. 5D and Table 2). Post hoc analysis revealed that serum LPS levels were highest at ZT8 in the alcohol-fed mice receiving the control binge (Fig. 5D). No effects of diet, binge, binge time, or any interactions were observed for serum LBP (Fig. 5E and Table 2).
Fig. 5.
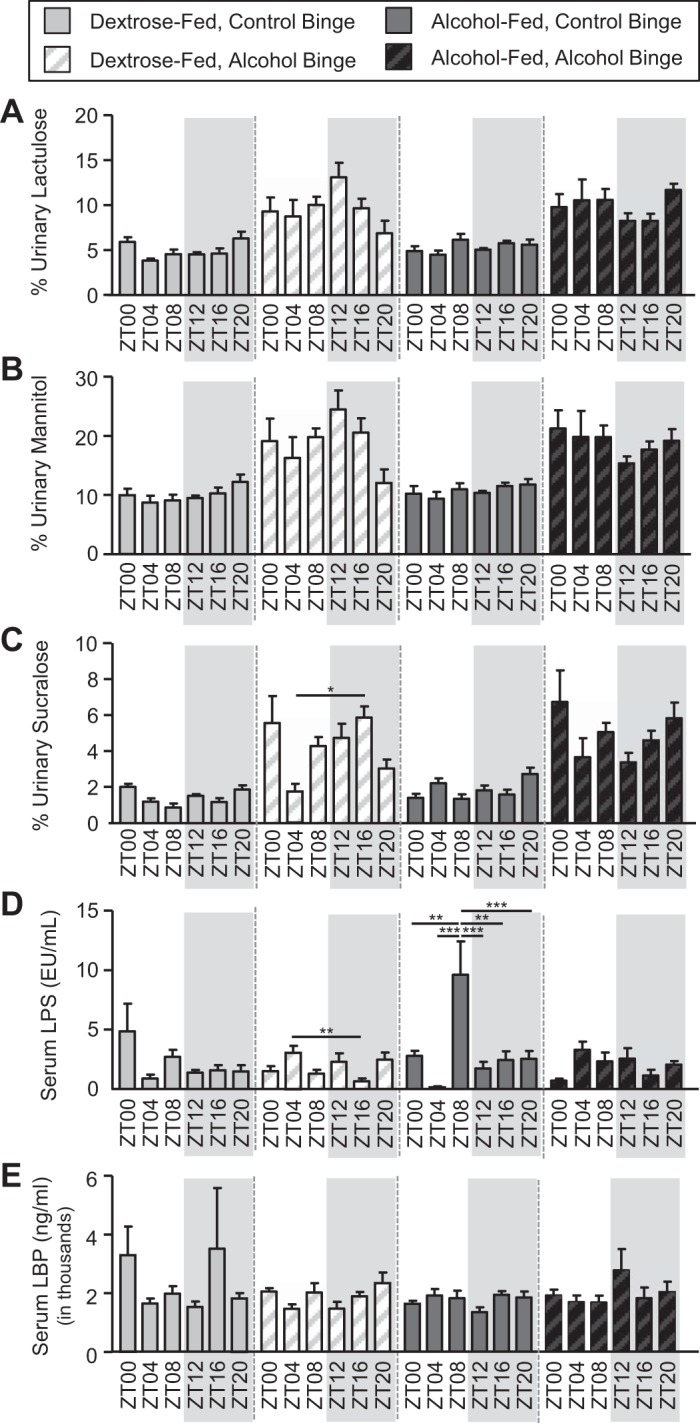
Diet-, binge-, and time-dependent effects on markers of intestinal barrier integrity in Nanji diet-fed mice. Mice were fed a Nanji diet for 4 wk, and during the 5th wk (i.e., days 43–45) mice were administered a once/daily binge of alcohol (6 g/kg) or the vehicle control for alcohol (i.e., PBS) at a specific time indicated by the zeitgeber time (ZT); mice were given a test for intestinal permeability immediately following the 2nd binge; and mice were euthanized 4 h after the 3rd and final once/daily binge. Intestinal barrier integrity and liver pathology were assessed. A–C: intestinal barrier integrity was evaluated following the bolus administration of a sugar solution, and urinary sugar was measured as an index of barrier integrity; higher urinary sugar equates to greater barrier dysfunction. A: urinary lactulose exhibited a significant effect of binge (binge: P < 0.00). Post hoc Tukey’s test revealed significant time-of-day effects. B: urinary mannitol exhibited a significant effect of binge (binge: P < 0.00). Post hoc Tukey’s test revealed no significant effects. C: urinary sucralose revealed a significant effect of binge (P < 0.00) as well as a binge × time interaction (P = 0.01). Post hoc Tukey’s test revealed significant time-of-day effects. D and E: intestinal barrier integrity was also evaluated using serum markers, including lipopolysaccharide (LPS) and lipopolysaccharide-binding protein (LBP). D: serum LPS exhibited a significant effect of diet (P = 0.03), binge (P = 0.01), and time (P < 0.00) as well as a diet × time interaction (P < 0.00), a binge × time interaction (P < 0.00), and a diet × binge × time interaction (P = 0.02). Post hoc analysis revealed significant time-of-day effects. E: serum LBP levels were unaffected by binge or time. Between n = 5 and 10 were mice were included in each treatment group at each time point. Three-way ANOVA followed by post hoc Tukey’s test (*P < 0.05; **P < 0.01; ***P < 0.001) was used for all analyses.
We next examined markers of liver pathology. Histological analysis revealed a significant effect of binge and time on liver steatosis, with the post hoc analysis revealing many between-group differences, which was limited primarily to mice receiving the alcohol binge, suggesting that the binge induces a large fluctuation in liver steatosis (Fig. 6A and Table 2). Histological analysis of liver inflammation revealed a significant main effect of time (Fig. 6B and Table 2). Post hoc analysis revealed many between-group differences, with the highest liver inflammation appearing at ZT0/ZT4 in control-fed mice and ZT8 in alcohol-fed mice (Fig. 6B). Analysis of liver MPO revealed a significant main effect of binge but no post hoc significance (Fig. 6C and Table 2). Analysis of serum ALT revealed a significant effect of time as well as a significant binge-by-time interaction (Fig. 6D and Table 2). Post hoc analysis revealed that ALT levels were highest at ZT0 in alcohol-fed mice given a control binge (Fig. 6D). Serum AST demonstrated a significant effect of binge and time but no post hoc significance (Fig. 6E and Table 2).
Fig. 6.
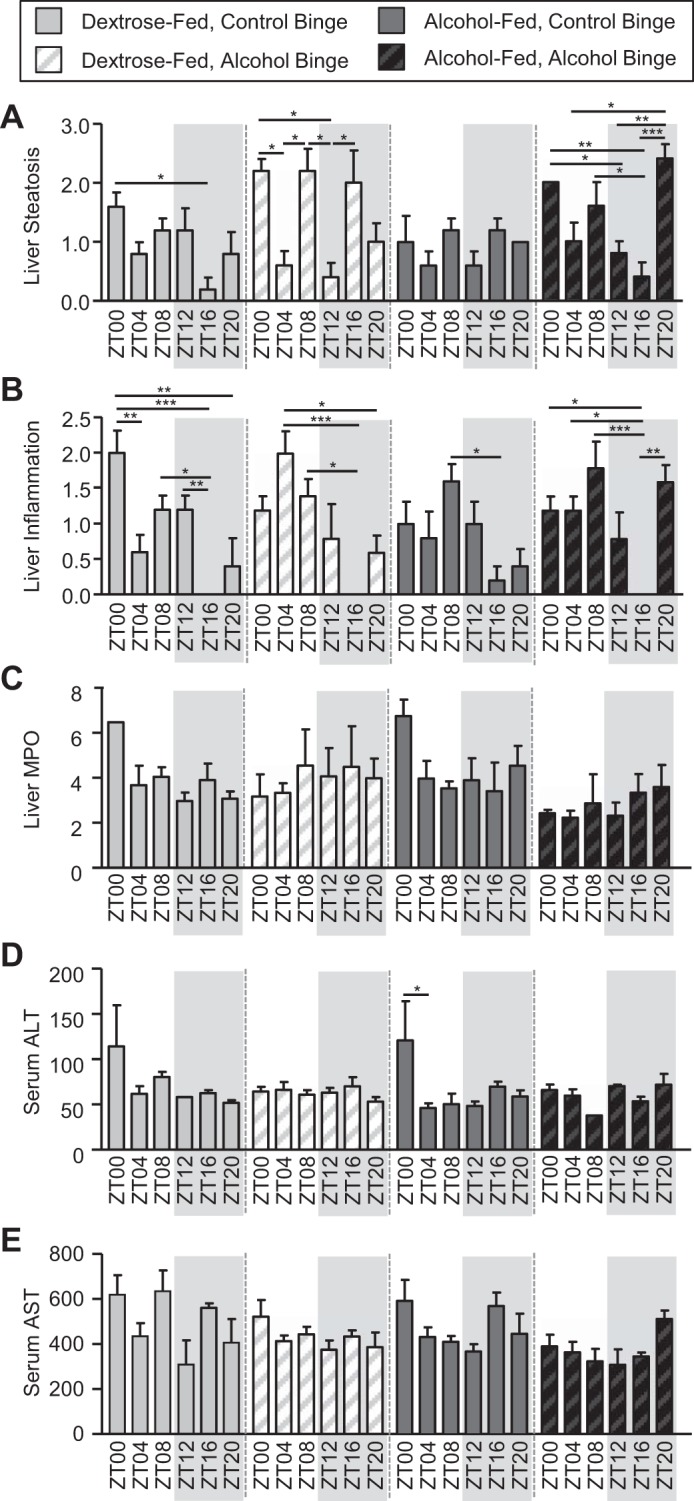
Diet-, binge-, and time-dependent effects on markers of liver pathology in Nanji diet-fed mice. Mice were fed a Nanji diet for 4 wk, and during the 5th wk (i.e., days 43–45) mice were administered a once/daily binge of alcohol (6 g/kg) or the vehicle control for alcohol (i.e., PBS) at a specific time indicated by the zeitgeber time (ZT), and mice were euthanized 4 h after the 3rd and final once/daily binge. Liver pathology was assessed using a combination of histological and serum analyses. A: liver steatosis exhibited a significant effect of binge (P = 0.01) and time (P < 0.00) (nonparametric Kruskal-Wallis). Post hoc Tukey’s test showed significant time-of-day effects. B: liver inflammation exhibited a significant effect of time (P < 0.00) (nonparametric Kruskal-Wallis). Post hoc analysis revealed significant time-of-day effects. C: liver myeloperoxidase (MPO) exhibited a significant effect of binge (P < 0.00). Post hoc analysis revealed no significant effects. D: serum alanine aminotransferase (ALT) exhibited a significant effect of time (P = 0.01) as well as a binge × time interaction (P = 0.02). Post hoc Tukey’s test revealed only a single time-of-day difference. E: serum aspartate aminotransferase (AST) exhibited a significant effect of binge (P = 0.00) and time (P = 0.01). Post hoc Tukey’s test revealed no significant differences. Two-way ANOVA followed by post hoc Tukey’s test (*P < 0.05; **P < 0.01; ***P < 0.001) were used for analysis unless noted.
DISCUSSION
In this study, we examined the impact of alcohol binge at different times of the day on markers of intestinal barrier integrity and markers of liver pathology.
As would be expected, chow-fed mice demonstrated a significant main effect of alcohol binge on markers of intestinal barrier integrity, including lactulose, mannitol, and sucralose. Alcohol binge caused intestinal barrier dysfunction, but only sucralose demonstrated a significant main effect of time, with LPS also showing a significant effect of time. In addition, sucralose demonstrated a significant binge-by-time interaction. These data suggest that the magnitude of alcohol-induced colonic permeability and microbiota translocation may be impacted by the time that the alcohol binge was administered. This finding makes sense since high urinary sucralose, in the absence of increased lactulose and mannitol, likely indicates barrier dysfunction in the colon, which harbors the highest concentration of LPS-producing gram-negative bacteria and other intestinal microbiota. In agreement with intestinal outcomes, markers of liver pathology showed a significant main effect of the alcohol binge-including steatosis, ALT, and AST. No marker showed a significant effect of time or a significant binge-by-binge time interaction.
Nanji diet-fed mice demonstrated a different profile from chow-fed mice. Although 4 wk of dietary alcohol consumption was sufficient to impact serum alcohol levels, it was not sufficient to induce intestinal barrier dysfunction (i.e., intestinal leakiness indicated by higher urinary sugar content), nor did it increase markers of liver pathology. However, there was a significant main effect of 4 wk of alcohol consumption on serum LPS levels, which was likely be a consequence of alcohol-induced changes in the intestinal microbiota (i.e., increase in gram-negative bacteria or bacterial overgrowth). In contrast to dietary alcohol consumption, the effects of the alcohol binge on serum alcohol levels and markers of intestinal barrier integrity were profound. The alcohol binge also significantly affected serum LPS levels, which were opposite of what might be expected and may suggest that the alcohol binge reduced the levels of LPS-producing gram-negative bacteria. Alcohol binge also impacted markers of liver pathology, including an increase in steatosis and a decrease in both MPO and AST. Time was a significant factor impacting LPS and nearly all the markers of liver pathology, including steatosis, inflammation, ALT, and AST, with only MPO not demonstrating a significant effect of time. Critically, there was a significant binge-by-binge time interaction for sucralose and LPS, suggesting that time significantly impacted the effects of the alcohol binge.
Diurnal Oscillations in Colonic Permeability
There was a significant main effect of the alcohol binge on intestinal permeability observed in both chow-fed and Nanji diet-fed mice. However, time was a significant factor only for sucralose (a marker of whole intestine permeability) in chow-fed mice, whereas lactulose and mannitol (markers of small intestine permeability) did not. Likewise, only sucralose demonstrated a significant binge-by-time interaction. Specifically, urinary sucralose was highest when the alcohol binge was administered at ZT0 (i.e., at the start of the rest period). Two recent studies describe differences in diurnal measurements of intestinal permeability in mice (29a, 37); one shows greatest permeability at ZT16 (i.e., during the dark period) (29a), whereas the other shows the greatest permeability around ZT8 (i.e., during the light period) (37). These studies assessed intestinal permeability using methods that differed from those used in the current study, and they examined only two time points over 24 h, whereas our study examined six different ZTs. There are several factors that may contribute to diurnal oscillations in barrier integrity. One factor that may contribute to daily variations is the circadian regulation of tight-junction protein expression, as reported in the kidney (45) and colon (29a), or diurnal changes in the intestinal microbiota (i.e., bacterial community structure and/or function) that can significantly influence barrier integrity (14, 42–44, 46–48). Interestingly, although our data revealed a significant main effect of time and a binge-by-time interaction for urinary sucralose in chow-fed mice, this was not observed in mice fed the Nanji diet. The Nanji diet contains 35% of daily calories from fat with the fat source derived from fish oil; diet robustly influences the intestinal microbiota, and diets high in fat alter diurnal fluctuations in bacterial community structure and function (14, 43, 44, 46–48). Fluctuations in the colon microbiome dramatically influence gene expression in both the colon and liver (38), and these likely include genes that regulate intestinal permeability. Indeed, abnormal intestinal microbiota composition (i.e., dysbiosis) is associated with intestinal hyperpermeability (10, 17, 27). Thus, in the current study, diet-induced changes in the microbiome could explain why Nanji diet-fed mice did not demonstrate a significant effect of time on intestinal permeability whereas chow-fed mice did exhibit an effect of time.
Daily variations in colonic permeability are supported by our data demonstrating diurnal fluctuations in serum LPS levels, with LPS levels exhibiting a tendency to peak around ZT8 (i.e., during the rest period). It is intriguing that the increase in LPS often occurred several hours after the observed peak in urinary sucralose to suggest that the increase in LPS was due primarily to disruption of the intestinal barrier in the colon. Of course, this pattern was not observed in all groups; therefore, the contribution of intestinal dysbiosis cannot be excluded. Indeed, diurnal oscillations in intestinal barrier integrity are driven by alterations in the circadian expression (or cellular localization) of tight-junction proteins (29a, 34, 37). Likewise, diurnal fluctuations in bacterial populations have been reported (21, 22, 38, 39). Further evaluations will be necessary; therefore, studies are currently underway to analyze stool and intestinal mucosa-associated microbiota to determine the contribution the microbiome may play in diurnal fluctuations in barrier integrity or liver pathology.
Immune Cell Trafficking in the Liver
There was a significant effect of time on a number of liver outcomes, including steatosis, liver inflammation, ALT, and AST, in mice fed the Nanji diet. Trafficking of leukocytes, including mononuclear phagocytes (i.e., monocytes and dendritic cells) in the blood and their localization to specific tissues, exhibits circadian rhythms (4, 6, 29). For example, polymorphonuclear leukocyte concentration in the blood peaks during the light period (around ZT5) in mice (32), and another study reports maximum leukocyte numbers in murine muscle tissue during the light period (6, 12, 31). These results are similar to the peak liver inflammation observed in the current study, with inflammatory cells peaking between ZT4 and ZT8. As noted above, this could also be a consequence of circadian variation in colonic permeability but may also be due to circadian rhythms of the microbiota that influence hepatic gene expression (38). One intriguing aspect was the observation that liver inflammation was nearly absent in all groups when the tissue was collected at ZT20, independent of whether the mice had a control or an alcohol binge at ZT16. These findings may reflect normal diurnal fluctuations in immune cell trafficking. If the presence of immune cells in the liver is contributing to liver pathology, then the absence of immune cells in the liver should be a time when the liver is protected from the negative effects of alcohol. However, the absence of the immune cells at ZT20 does not seem to be a time when the liver is protected from the pathological effects of alcohol. Future studies will be necessary to evaluate the inflammatory potential of liver immune cells at different times of the day. Finally, we did observe immune cells in the liver of chow-fed control binged mice, which likely reflects the stress induced by the gavage procedure.
Conclusion
Our data revealed that the alcohol binge significantly influenced food consumption; therefore, it is possible that the reduced food intake immediately following the binge may have influenced the outcomes in this study. However, since 4 wk of alcohol consumption (i.e., the Nanji diet) did not significantly impact our markers of intestinal barrier dysfunction, it is unlikely that the reduced food consumption following the alcohol binge on days 43–45 markedly impacted the outcomes presented here. However, altered food consumption should be taken into account when interpreting the data presented in this study. With that caveat in place, we conclude that 1) alcohol binge significantly impacted markers of intestinal permeability, 2) time of day significantly affected liver outcomes, and 3) time of day influenced colonic permeability with a time of day-dependent effect of the alcohol binge under certain dietary conditions.
GRANTS
This study was supported by National Institutes of Health Grant NIAAA-AA020216.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.M.V., C.B.F., and A.K. conceived and designed research; R.M.V., C.B.F., M.S., L.Z., S.R., C.A., N.Z.P., and T.M.D. performed experiments; R.M.V., M.S., C.A., T.M.D., and L.F.F. analyzed data; R.M.V., C.B.F., L.F.F., and A.K. interpreted results of experiments; R.M.V. prepared figures; R.M.V. drafted manuscript; R.M.V., C.B.F., C.A., T.M.D., and A.K. edited and revised manuscript; R.M.V., C.B.F., M.S., L.Z., S.R., C.A., N.Z.P., T.M.D., L.F.F., and A.K. approved final version of manuscript.
REFERENCES
- 1.Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat Protoc 8: 627–637, 2013. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertola A, Park O, Gao B. Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury in mice: a critical role for E-selectin. Hepatology 58: 1814–1823, 2013. doi: 10.1002/hep.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjarnason I, Maxton D, Reynolds AP, Catt S, Peters TJ, Menzies IS. Comparison of four markers of intestinal permeability in control subjects and patients with coeliac disease. Scand J Gastroenterol 29: 630–639, 1994. doi: 10.3109/00365529409092484. [DOI] [PubMed] [Google Scholar]
- 4.Curtis AM, Bellet MM, Sassone-Corsi P, O’Neill LA. Circadian clock proteins and immunity. Immunity 40: 178–186, 2014. doi: 10.1016/j.immuni.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Donohue TM Jr, Curry-McCoy TV, Todero SL, White RL, Kharbanda KK, Nanji AA, Osna NA. L-Buthionine (S,R) sulfoximine depletes hepatic glutathione but protects against ethanol-induced liver injury. Alcohol Clin Exp Res 31: 1053–1060, 2007. doi: 10.1111/j.1530-0277.2007.00393.x. [DOI] [PubMed] [Google Scholar]
- 6.Druzd D, de Juan A, Scheiermann C. Circadian rhythms in leukocyte trafficking. Semin Immunopathol 36: 149–162, 2014. doi: 10.1007/s00281-013-0414-4. [DOI] [PubMed] [Google Scholar]
- 7.Dumas F, Aussel C, Pernet P, Martin C, Giboudeau J. Gas chromatography applied to the lactulose-mannitol intestinal permeability test. J Chromatogr B Biomed Appl 654: 276–281, 1994. doi: 10.1016/0378-4347(94)00041-7. [DOI] [PubMed] [Google Scholar]
- 8.Farhadi A, Banan A, Fields J, Keshavarzian A. Intestinal barrier: an interface between health and disease. J Gastroenterol Hepatol 18: 479–497, 2003. doi: 10.1046/j.1440-1746.2003.03032.x. [DOI] [PubMed] [Google Scholar]
- 9.Farhadi A, Gundlapalli S, Shaikh M, Frantzides C, Harrell L, Kwasny MM, Keshavarzian A. Susceptibility to gut leakiness: a possible mechanism for endotoxaemia in non-alcoholic steatohepatitis. Liver Int 28: 1026–1033, 2008. doi: 10.1111/j.1478-3231.2008.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, Estes JD, Dodiya HB, Keshavarzian A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One 6: e28032, 2011. doi: 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodges S, Ashmore SP, Patel HR, Tanner MS. Cellobiose: mannitol differential permeability in small bowel disease. Arch Dis Child 64: 853–855, 1989. doi: 10.1136/adc.64.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.House SD, Ruch S, Koscienski WF III, Rocholl CW, Moldow RL. Effects of the circadian rhythm of corticosteroids on leukocyte-endothelium interactions in the AM and PM. Life Sci 60: 2023–2034, 1997. doi: 10.1016/S0024-3205(97)00167-7. [DOI] [PubMed] [Google Scholar]
- 13.Johnston SD, Smye M, Watson RG, McMillan SA, Trimble ER, Love AH. Lactulose-mannitol intestinal permeability test: a useful screening test for adult coeliac disease. Ann Clin Biochem 37: 512–519, 2000. doi: 10.1177/000456320003700413. [DOI] [PubMed] [Google Scholar]
- 14.Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci 9: 392, 2015. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol 50: 538–547, 2009. doi: 10.1016/j.jhep.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keshavarzian A, Fields JZ, Vaeth J, Holmes EW. The differing effects of acute and chronic alcohol on gastric and intestinal permeability. Am J Gastroenterol 89: 2205–2211, 1994. [PubMed] [Google Scholar]
- 17.Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, Mutlu E, Shannon KM. Colonic bacterial composition in Parkinson’s disease. Mov Disord 30: 1351–1360, 2015. doi: 10.1002/mds.26307. [DOI] [PubMed] [Google Scholar]
- 18.Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol 94: 200–207, 1999. doi: 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- 19.Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, Gao B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology 52: 1291–1300, 2010. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leone V, Gibbons SM, Martinez K, Hutchison AL, Huang EY, Cham CM, Pierre JF, Heneghan AF, Nadimpalli A, Hubert N, Zale E, Wang Y, Huang Y, Theriault B, Dinner AR, Musch MW, Kudsk KA, Prendergast BJ, Gilbert JA, Chang EB. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 17: 681–689, 2015. doi: 10.1016/j.chom.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang X, Bushman FD, FitzGerald GA. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc Natl Acad Sci USA 112: 10479–10484, 2015. doi: 10.1073/pnas.1501305112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathews S, Feng D, Maricic I, Ju C, Kumar V, Gao B. Invariant natural killer T cells contribute to chronic-plus-binge ethanol-mediated liver injury by promoting hepatic neutrophil infiltration. Cell Mol Immunol 13: 206–216, 2016. doi: 10.1038/cmi.2015.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathews S, Xu M, Wang H, Bertola A, Gao B. Animals models of gastrointestinal and liver diseases. Animal models of alcohol-induced liver disease: pathophysiology, translational relevance, and challenges. Am J Physiol Gastrointest Liver Physiol 306: G819–G823, 2014. doi: 10.1152/ajpgi.00041.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meddings JB, Sutherland LR, Byles NI, Wallace JL. Sucrose: a novel permeability marker for gastroduodenal disease. Gastroenterology 104: 1619–1626, 1993. doi: 10.1016/0016-5085(93)90637-R. [DOI] [PubMed] [Google Scholar]
- 26.Müller M, Walker-Smith J, Shmerling DH, Curtius HC, Prader A. Lactulose: a gas-liquid chromatography method of determination and evaluation of its use to assess intestinal mucosal damage. Clin Chim Acta 24: 45–49, 1969. doi: 10.1016/0009-8981(69)90139-9. [DOI] [PubMed] [Google Scholar]
- 27.Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, Kwasny M, Lau CK, Keshavarzian A. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol 302: G966–G978, 2012. doi: 10.1152/ajpgi.00380.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nanji AA, Zhao S, Sadrzadeh SM, Dannenberg AJ, Tahan SR, Waxman DJ. Markedly enhanced cytochrome P450 2E1 induction and lipid peroxidation is associated with severe liver injury in fish oil-ethanol-fed rats. Alcohol Clin Exp Res 18: 1280–1285, 1994. doi: 10.1111/j.1530-0277.1994.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science 341: 1483–1488, 2013. doi: 10.1126/science.1240636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29a.Oh-oka K, Kono H, Ishimaru K, Miyake K, Kubota T, Ogawa H, Okumura K, Shibata S, Nakao A. Expressions of tight junction proteins Occludin and Claudin-1 are under the circadian control in the mouse large intestine: implications in intestinal permeability and susceptibility to colitis. PLoS One 9: e98016, 2014. doi: 10.1371/journal.pone.0098016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109: 307–320, 2002. doi: 10.1016/S0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 31.Scheiermann C, Kunisaki Y, Lucas D, Chow A, Jang JE, Zhang D, Hashimoto D, Merad M, Frenette PS. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity 37: 290–301, 2012. doi: 10.1016/j.immuni.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schloss MJ, Horckmans M, Nitz K, Duchene J, Drechsler M, Bidzhekov K, Scheiermann C, Weber C, Soehnlein O, Steffens S. The time-of-day of myocardial infarction onset affects healing through oscillations in cardiac neutrophil recruitment. EMBO Mol Med 8: 937–948, 2016. doi: 10.15252/emmm.201506083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Substance Abuse and Mental Health Services Administration (SAMHSA) 2014 National Survey on Drug Use and Health (NSDUH). Table 2.46B-Alcohol use, binge alcohol use, and heavy alcohol use in the past month among persons aged 18 or older, by demographic characteristics: Percentages, 2013 and 2014 (Online) https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs2014/NSDUH-DetTabs2014.pdf [2016].
- 34.Summa KC, Voigt RM, Forsyth CB, Shaikh M, Cavanaugh K, Tang Y, Vitaterna MH, Song S, Turek FW, Keshavarzian A. Disruption of the circadian clock in mice increases intestinal permeability and promotes alcohol-induced hepatic pathology and inflammation. PLoS One 8: e67102, 2013. doi: 10.1371/journal.pone.0067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swanson G, Forsyth CB, Tang Y, Shaikh M, Zhang L, Turek FW, Keshavarzian A. Role of intestinal circadian genes in alcohol-induced gut leakiness. Alcohol Clin Exp Res 35: 1305–1314, 2011. doi: 10.1111/j.1530-0277.2011.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swanson GR, Gorenz A, Shaikh M, Desai V, Kaminsky T, Van Den Berg J, Murphy T, Raeisi S, Fogg L, Vitaterna MH, Forsyth C, Turek F, Burgess HJ, Keshavarzian A. Night workers with circadian misalignment are susceptible to alcohol-induced intestinal hyperpermeability with social drinking. Am J Physiol Gastrointest Liver Physiol 311: G192–G201, 2016. doi: 10.1152/ajpgi.00087.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanabe K, Kitagawa E, Wada M, Haraguchi A, Orihara K, Tahara Y, Nakao A, Shibata S. Antigen exposure in the late light period induces severe symptoms of food allergy in an OVA-allergic mouse model. Sci Rep 5: 14424, 2015. doi: 10.1038/srep14424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thaiss CA, Levy M, Korem T, Dohnalová L, Shapiro H, Jaitin DA, David E, Winter DR, Gury-BenAri M, Tatirovsky E, Tuganbaev T, Federici S, Zmora N, Zeevi D, Dori-Bachash M, Pevsner-Fischer M, Kartvelishvily E, Brandis A, Harmelin A, Shibolet O, Halpern Z, Honda K, Amit I, Segal E, Elinav E. Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell 167: 1495–1510.e12, 2016. doi: 10.1016/j.cell.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, Abramson L, Katz MN, Korem T, Zmora N, Kuperman Y, Biton I, Gilad S, Harmelin A, Shapiro H, Halpern Z, Segal E, Elinav E. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 159: 514–529, 2014. doi: 10.1016/j.cell.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 40.Tipoe GL, Liong EC, Casey CA, Donohue TM Jr, Eagon PK, So H, Leung TM, Fogt F, Nanji AA. A voluntary oral ethanol-feeding rat model associated with necroinflammatory liver injury. Alcohol Clin Exp Res 32: 669–682, 2008. doi: 10.1111/j.1530-0277.2008.00623.x. [DOI] [PubMed] [Google Scholar]
- 41.Ukabam SO, Cooper BT. Small intestinal permeability to mannitol, lactulose, and polyethylene glycol 400 in celiac disease. Dig Dis Sci 29: 809–816, 1984. doi: 10.1007/BF01318423. [DOI] [PubMed] [Google Scholar]
- 42.Voigt RM, Forsyth CB, Green SJ, Mutlu E, Engen P, Vitaterna MH, Turek FW, Keshavarzian A. Circadian disorganization alters intestinal microbiota. PLoS One 9: e97500, 2014. doi: 10.1371/journal.pone.0097500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Liu Y, Kirpich I, Ma Z, Wang C, Zhang M, Suttles J, McClain C, Feng W. Lactobacillus rhamnosus GG reduces hepatic TNFα production and inflammation in chronic alcohol-induced liver injury. J Nutr Biochem 24: 1609–1615, 2013. doi: 10.1016/j.jnutbio.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Liu Y, Sidhu A, Ma Z, McClain C, Feng W. Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. Am J Physiol Gastrointest Liver Physiol 303: G32–G41, 2012. doi: 10.1152/ajpgi.00024.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamato M, Ito T, Iwatani H, Yamato M, Imai E, Rakugi H. E-cadherin and claudin-4 expression has circadian rhythm in adult rat kidney. J Nephrol 23: 102–110, 2010. [PubMed] [Google Scholar]
- 46.Yu LC, Wang JT, Wei SC, Ni YH. Host-microbial interactions and regulation of intestinal epithelial barrier function: From physiology to pathology. World J Gastrointest Pathophysiol 3: 27–43, 2012. doi: 10.4291/wjgp.v3.i1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zakostelska Z, Kverka M, Klimesova K, Rossmann P, Mrazek J, Kopecny J, Hornova M, Srutkova D, Hudcovic T, Ridl J, Tlaskalova-Hogenova H. Lysate of probiotic Lactobacillus casei DN-114 001 ameliorates colitis by strengthening the gut barrier function and changing the gut microenvironment. PLoS One 6: e27961, 2011. doi: 10.1371/journal.pone.0027961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zareie M, Johnson-Henry K, Jury J, Yang PC, Ngan BY, McKay DM, Soderholm JD, Perdue MH, Sherman PM. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut 55: 1553–1560, 2006. doi: 10.1136/gut.2005.080739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci USA 111: 16219–16224, 2014. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]