Abstract
Enteric glia play an important neuroprotective role in the enteric nervous system (ENS) by producing neuroprotective compounds such as the antioxidant reduced glutathione (GSH). The specific cellular pathways that regulate glial production of GSH and how these pathways are altered during, or contribute to, neuroinflammation in situ and in vivo are not fully understood. We investigated this issue using immunohistochemistry to localize GSH synthesis enzymes within the myenteric plexus and tested how the inhibition of GSH synthesis with the selective inhibitor l-buthionine sulfoximine impacts neuronal survival and inflammation. Both enteric glia and neurons express the cellular machinery necessary for GSH synthesis. Furthermore, glial GSH synthesis is necessary for neuronal survival in isolated preparations of myenteric plexus. In vivo depletion of GSH does not induce colitis but alters myenteric plexus neuronal phenotype and survival. Importantly, global depletion of glutathione is protective against some macroscopic and microscopic measures of colonic inflammation. Together, our data highlight the heterogeneous roles of GSH in the myenteric plexus of the ENS and during gastrointestinal inflammation.
NEW & NOTEWORTHY Our results show that both enteric glia and neurons express the cellular machinery necessary for glutathione (GSH) synthesis and that glial GSH synthesis is necessary for neuronal survival in isolated enteric nervous system (ENS) preparations. In vivo depletion of GSH with the selective inhibitor l-buthionine sulfoximine is not sufficient to induce inflammation but does alter neuronal neurochemical composition and survival. Together, our data highlight novel heterogeneous roles for GSH in the ENS and during gastrointestinal inflammation.
Keywords: glutathione, enteric glia, inflammatory bowel disease
INTRODUCTION
The enteric nervous system (ENS) is a network of neurons and glial cells that controls the reflexes and behaviors of the gastrointestinal (GI) tract. Inflammation, among many other insults, can disrupt the control of GI reflexes by altering the function and/or survival of enteric neurons, and these changes contribute to common functional GI motility disorders (22, 32, 33, 40). Enteric glial cells have been shown to play active roles in maintaining ENS function and regulating enteric neuron death (20, 23). The activation of glia contributes to neuron death via pathways that involve connexin-43 hemichannels and oxidants such as nitric oxide (7). Enteric glial networks are disrupted or altered in Crohn’s disease patients (6, 9), and reactive gliosis in enteric glia is a common feature of animal models of inflammation (46). Glia also serve an important neuroprotective role in the ENS, and the targeted ablation of glial cells results in enteric neurodegeneration and gut inflammation (8, 10). The specific cellular mechanisms that regulate the production and secretion of glial neuroprotective factors, and how these are altered in (patho)physiology, are poorly understood.
Enteric glia synthesize and release a number of neuroprotective compounds such as the prostaglandin derivative 15-deoxy-prostaglandin-J2 (2), the neurotrophic factor glial-derived neurotrophic factor (GDNF; 3), the endogenous antioxidant reduced glutathione (1), and the glutathione derivative S-nitrosoglutathione (48). Reduced glutathione (GSH) is the primary endogenous antioxidant in the body and promotes neuronal survival in the central and peripheral nervous systems (12, 15, 29, 30, 37). In the central nervous system, astroglia are the primary cell type responsible for the production of GSH and contain large quantities of GSH in vivo and in vitro (43). Furthermore, astrocytes have the ability to secrete GSH, and glial-derived GSH can be taken up by neurons or elicit its antioxidant functions in the extracellular space (14, 24, 52). Given the similarities between astrocytes and enteric glia, we hypothesized that enteric glia play a similar role in the production and regulation of GSH in the ENS.
We tested our hypothesis by studying the cellular localization of glutathione synthesis enzymes within the myenteric plexus, one division of the ENS, and the impact of GSH depletion on myenteric neuron health and GI inflammation in situ and in vivo. Our data show that both enteric neurons and glia express the cellular machinery necessary for the production of GSH. GSH production is necessary for the survival of myenteric neurons in situ, but surprisingly, in vivo depletion of GSH was protective against certain measures of GI inflammation. Together, our results provide novel insight into the cellular regulation of a key antioxidant in the intestine. GSH plays complex roles in the context of 2,4-dinitrobenzene sulfonic acid-induced colitis (DNBS colitis), and its cytoprotective effects may contribute to disease pathogenesis.
MATERIALS AND METHODS
Animals.
All work was approved by the Michigan State University Institutional Animal Care and Use Committee. All experiments used male C57BL/6 mice (Charles River, Hollister, CA) aged 6–10 wk and maintained on a 12-h:12-h light-dark cycle with ad libitum access to food and water.
In vivo colitis.
Colitis was induced in male C57BL/6 mice as previously described (7, 22). An enema of DNBS (5.5 mg/mouse in 0.1 ml 50:50 ethanol-saline) was administered via a gavage needle inserted 3 cm into the colon. Experimental controls were given an enema of saline only. Animals were killed 48 h after DNBS/saline treatment, and macroscopic damage to the colon was assessed with a well-established scoring system (50). Tissue was then fixed overnight in Zamboni’s fixative for use in immunohistochemical assays. Animal body weight and appearance were recorded daily following the induction of colitis.
In vivo glutathione depletion.
Colonic glutathione was depleted as described by Watanabe et al. (53). Briefly, mice were given ad libitum access to drinking water containing 20 mM l-buthionine-sulfoximine (BSO) or normal drinking water (H2O) for a period of 14 days. Mice were observed daily, and changes in appearance, body weight, and fluid consumption were recorded.
In situ model of neuroinflammation.
Enteric neuron death was driven in live longitudinal muscle myenteric plexus (LMMP) preparations in situ by the P2X purinoceptor 7 (P2X7) receptor agonist 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate triethylammonium salt (BzATP, 300 μM; 7, 22). Live LMMPs were prepared from segments of mouse distal colon tissue in Sylgard-coated 35-mm2 dishes containing DMEM F-12 media as described previously (7). LMMPs were incubated with BzATP (300 μM, to activate P2X7 receptor) for 2 h in 95% air-5% CO2 at 37°C. LMMPs were then rinsed three times with fresh Krebs buffer and incubated for an additional 2 h in Krebs buffer. LMMPs were fixed overnight at 4°C in Zamboni’s fixative for immunohistochemical (IHC) analysis. To deplete GSH in situ, this protocol was modified, and preparations were incubated in BSO (100 μM, to deplete GSH) for 2 h, followed by a 2-h incubation in Krebs buffer. γ-Glutamyl-cysteine (γ-GC), A740003, and 10Panx were applied for a half hour before BSO treatment and cotreated with BSO in experiments using these drugs.
Whole mount IHC.
Whole mount LMMP preparations were prepared from colonic tissue fixed overnight in Zamboni’s fixative at 4°C. Briefly, preparations were permeabilized by three 10-min washes in 0.1% Triton X-100 in phosphate-buffered saline (PBS). Following a 45-min block in 4% normal goat or donkey serum, LMMP preparations were incubated in primary antibody (see Table 1) either overnight at room temperature (RT) or for 48 h at 4°C. Finally, preparations were incubated in secondary antibodies (see Table 2) for 2 h at RT before mounting and imaging.
Table 1.
Primary antibodies used
| Antibody | Dilution | Source | Catalog No. |
|---|---|---|---|
| Chicken anti-GFAP | 1:1,000 | Abcam, Cambridge, MA | ab4674 |
| Biotinylated mouse anti-human HuC/D | 1:200 | Invitrogen, Carlsbad, CA | A21272 |
| Rabbit anti-GCLC | 1:200 | Abcam | ab190685 |
| Rabbit anti-GS | 1:200 | Abcam | ab133592 |
| Rabbit anti-MPO | 1:200 | Abcam | ab9353 |
| Rat anti-MHC II | 1:200 | Novus Biologicals, Littleton, CO | NBP2-21789 |
| Goat anti-calretinin | 1:1,000 | Swant, Marly, Switzerland | CG1 |
| Sheep anti-nNOS | 1:500 | Millipore, Darmstadt, Germany | ab1529 |
| Rabbit anti-synaptophysin | 1:2,000 | Abcam | ab32127 |
GFAP, glial fibrillary acidic protein; GCLC, glutamate-cysteine ligase catalytic subunit; GS, glutathione synthetase; MPO, myeloperoxidase; MHC II, major histocompatibility complex II; nNOS, neuronal nitric oxide synthase.
Table 2.
Secondary antibodies used
| Antibody | Source | Catalog No. |
|---|---|---|
| Streptavidin DyLight Fluor 405 | Jackson ImmunoResearch, West Grove, PA | 016-470-084 |
| Goat anti-chicken Alexa Fluor 488 | Invitrogen, Carlsbad, CA | A-11039 |
| Donkey anti-goat Alexa Fluor 488 | Jackson ImmunoResearch | 705-545-003 |
| Donkey anti-sheep Alexa Fluor 488 | Jackson ImmunoResearch | 713-545-003 |
| Goat anti-rabbit Alexa Fluor 568 | Invitrogen | A-11036 |
| Streptavidin Alexa Fluor 594 | Jackson ImmunoResearch | 016-580-084 |
All secondary antibodies were used at 1:400 dilution.
Images were acquired using the ×40 objective (0.75 numerical aperture, Plan Fluor; Nikon, Melville, NY) of an upright epifluorescence microscope (Nikon Eclipse Ni) with a Retiga 2000R camera (QImaging, Surrey, BC, Canada) controlled by QCapture Pro 7.0 (QImaging) software or by confocal imaging through the Plan-Apochromat ×60 oil immersion objective (1.42 numerical aperture) of an inverted Olympus Fluoview FV1000 microscope (Olympus, Center Valley, PA).
Dual labeling with primary antibodies raised in the same host.
Dual labeling with primary antibodies raised in the same host was achieved using Tyramide Signal Amplification Kit no. 14 (T20924) containing horseradish peroxidase-conjugated goat anti-rabbit IgG and Alexa Fluor 568 tyramide from Life Technologies (Carlsbad, CA), using a modification of the protocol described by Gulbransen et al. (21) and verified in control experiments where the second primary antibody was omitted. The first primary antibody (rabbit anti-synaptophysin) was used at a 1:2,000 dilution and detected via tyramide signal amplification reaction while the second primary antibody [rabbit anti-glutamate-cysteine ligase catalytic subunit (GCLC)] was detected via normal IHC staining protocols. Preparations were incubated in the first primary antibody overnight at RT after endogenous peroxidases were quenched by a 60-min preincubation in 3% H2O2 diluted in PBS. Preparations were next incubated with horseradish peroxidase-goat anti-rabbit IgG for 2 h at RT followed by a 10-min incubation in Alexa Fluor 568 tyramide and an overnight incubation with unlabeled goat anti-rabbit Fab fragments (1:50) at RT. Finally, preparations were labeled with the second primary and secondary antibody pair as described above (whole mount IHC).
Quantification of neuronal thiol oxidation.
Cellular oxidative stress was quantified using a neuronal thiol oxidation ratiometric analysis as previously described (7, 39). Reduced [sulfhydryl (SH)] and oxidized [disulfide (SS)] thiols were labeled with 1 μM Alexa Fluor 680 C2 maleimide and 1 μM Alexa Fluor 546 C5 maleimide, respectively (Life Technologies). Oxidized thiols were reduced to free thiols for labeling using 5 mM Tris·(2-carboxyethyl)phosphine hydrochloride (Sigma) in PBS. Images were obtained by epifluorescence microscopy as described above, and the ratio of 546-maleimide to 680-maleimide (SS/SH) was calculated with ImageJ software (https://imagej.nih.gov/ij/).
Calcein AM cell viability assay.
Cell viability in myenteric plexus in situ preparations was determined using calcein AM (41), a reliable marker of live, membrane-intact cells, as previously described (7). Live LMMP preparations were incubated with 4 μM calcein AM for 30 min at 37°C and then immediately imaged using a Neo scientific complementary metal-oxide semiconductor (sCMOS) digital camera (Andor, South Windsor, CT) through the ×40 water immersion objective (LUMPlan N, 0.8 numerical aperture) of an upright Olympus BX51W1 fixed-stage microscope controlled by Andor IQ3 software.
Chemicals/drugs.
BzATP, N-ethylmaleimide, Tris·(2-carboxyethyl)phosphine hydrochloride (TCEP), l-buthione-sulfoximine (BSO), γ-glutamyl-cysteine (γ-GC), and A740003 were purchased from Sigma-Aldrich (St. Louis, MO). The pannexin-1 mimetic peptide 10Panx was purchased from Anaspec (Fremont, CA), and calcein AM was purchased from Thermo Fisher Scientific (Waltham, MA).
Solutions and media.
Live tissue was collected in Dulbecco’s modified Eagle’s medium nutrient mixture F-12 (DMEM/F-12; Life Technologies) supplemented with 3 μM nicardipine and 1 μM scopolamine. Normal goat/donkey serum contained 4% normal goat/donkey serum, 0.4% Triton X-100, and 1% bovine serum albumin in PBS. Krebs buffer contained (in mM): 121 NaCl, 5.9 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 10 HEPES, 21.2 NaHCO3, 1 pyruvic acid, and 8 glucose (pH adjusted to 7.4 with NaOH) with 3 mM nicardipine and 1 mM scopolamine.
Data and statistical analysis.
Average ganglionic neuronal density was determined by calculating the number of HuC/D-immunoreactive neurons per ganglionic area using the cell counter tool in ImageJ, in at least 10 ganglia per animal. Cell viability in the calcein AM viability assay was calculated as the number of fluorescent (viable) cells per ganglionic area. Average ganglionic fluorescence was quantified using the measure tool in ImageJ. Thiol oxidation ratios were calculated using the region of interest (ROI) tool in ImageJ. Fluorescence of 546-maleimide (oxidized thiols) and 680-maleimide (reduced thiols) was measured in 15–20 neurons per ganglia in a minimum of 10 ganglia per animal. SS-to-SH (546-maleimide-to-680-maleimide) ratios were calculated per neuron and averaged, first per ganglia and then per mouse.
Subpopulations of excitatory (calretinin-positive) and inhibitory [neuronal nitric oxide synthase (nNOS)-positive] neurons were calculated by expressing the number of calretinin- or nNOS-positive neurons as a percent of HuC/D (total) neurons in a minimum of 10 ganglia per mouse. Density of infiltrating neutrophils was calculated using the cell counter tool in ImageJ to quantify the number of myeloperoxidase (MPO)-positive neutrophils in a 76,800-μm2 field of view surrounding a single myenteric ganglion. Similarly, activation of major histocompatibility complex class II (MHC II) muscularis macrophages was quantified by measuring average fluorescence of a 76,800-μm2 field of view surrounding a single myenteric ganglion. Both measurements were performed in a minimum of 10 ganglia per mouse.
Colonic macroscopic damage was quantified using the well-characterized scoring system described by Storr et al. (50). The presence of fecal blood, diarrhea, and/or hemorrhage received one point per feature present. Adhesion of the colon to other organs and peritoneal space was scored 0, 1, or 2 on the basis of the severity of adhesions. Colon length and thickness were also scored with greater points for a shortened and/or thickened colon.
Data were analyzed by Student’s t-test, one-way or two-way ANOVA (with Dunnett’s or Tukey’s post hoc test) using GraphPad Prism 7 (GraphPad Software, San Francisco, CA). Values are presented as means ± SE.
RESULTS
Glutathione synthesis enzymes are expressed in myenteric ganglia.
Enteric glia are thought to serve important neuroprotective roles in the enteric nervous system because the ablation of glia leads to widespread neuron death (8, 9). How glia protect neurons in the gut is not clear. Reduced glutathione (GSH) is a neuroprotective antioxidant that is secreted by enteric glia in culture (1), and mice lacking the regulatory glutathione peroxidase enzymes develop spontaneous colitis (16). However, whether enteric glia express the enzymes necessary for glutathione synthesis and contribute to the protection against colitis in vivo is not known.
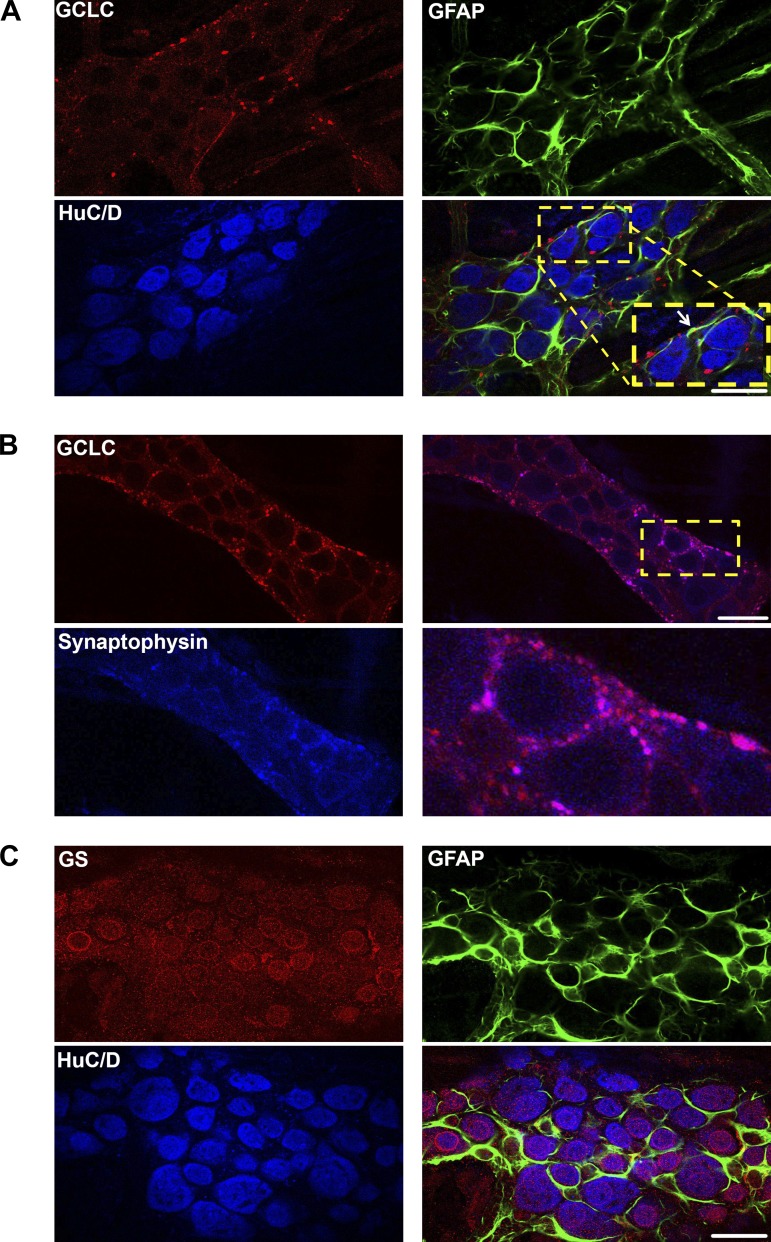
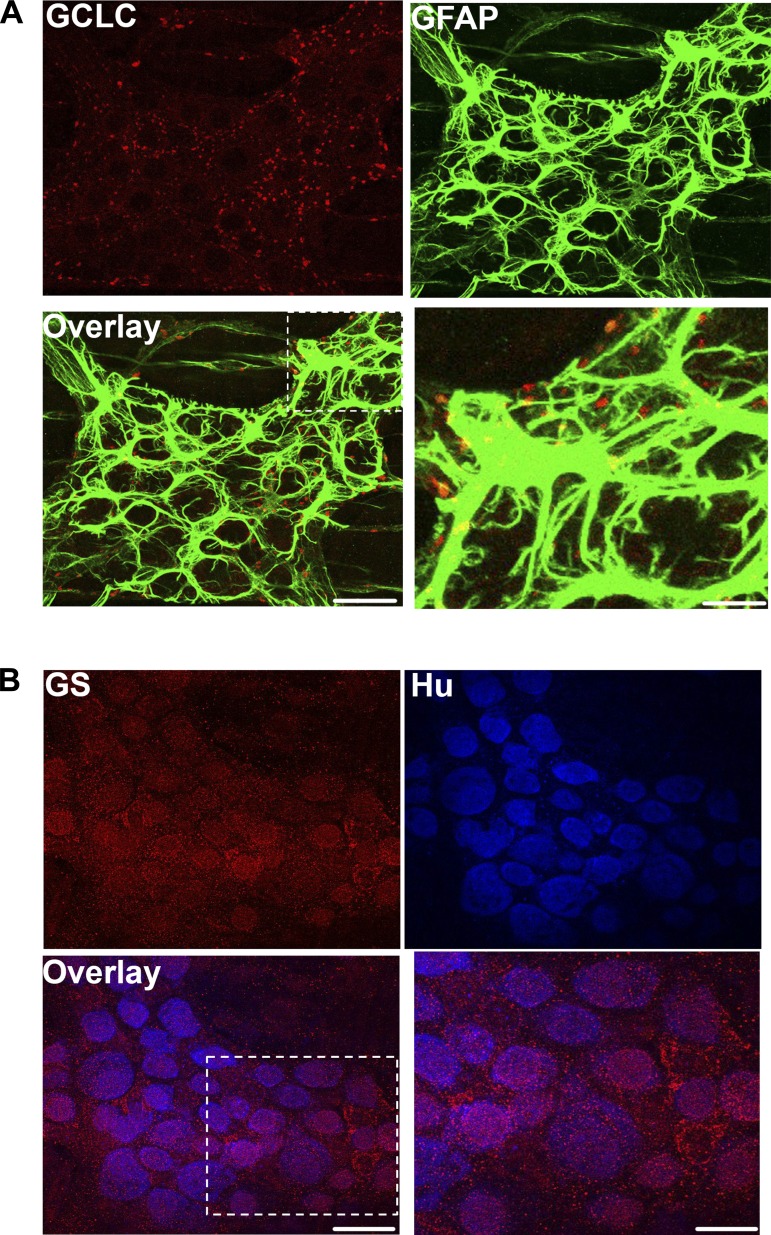
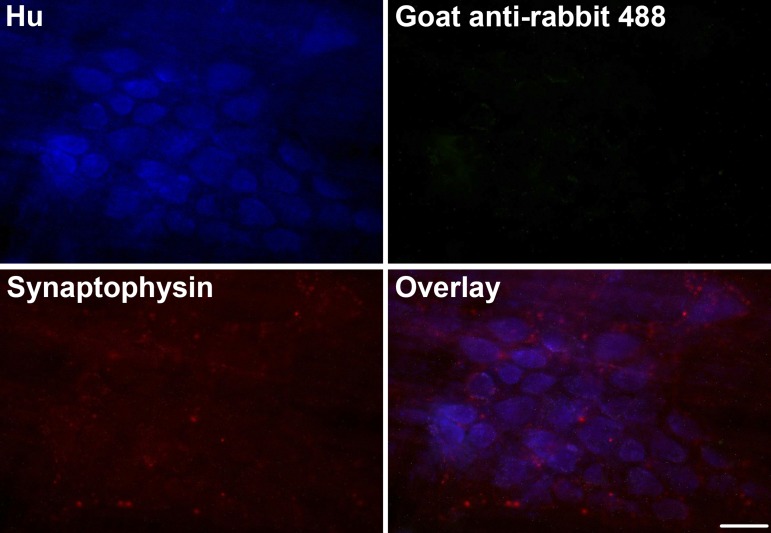
We tested whether glia express the machinery required for glutathione synthesis in vivo by labeling for two key enzymes: glutamate-cysteine ligase and glutathione synthetase. Glutathione is synthesized in a two-step pathway (36). In the first and rate-limiting step, glutamate-cysteine ligase (GCL) catalyzes the formation of γ-glutamylcysteine (γ-GC) from glutamate and cysteine. γ-GC then combines with glycine in a step catalyzed by glutathione synthetase (GS) to form reduced GSH (γ-glutamyl-cysteinyl-glycine). Our data show that the catalytic subunit of GCL (GCLC) is expressed in mouse myenteric ganglia and colocalizes with the glial cell marker, glial fibrillary acidic protein [GFAP; Fig. 1A (white arrow) and Fig. 2A]. Furthermore, GCLC is also expressed in neuronal varicosities, and its labeling colocalizes well with the presynaptic vesicle marker synaptophysin (Fig. 1B). We performed control experiments to rule out the possibility that colocalization was caused by cross-reactivity between the multiple antibodies used in this experiment and found that our labeling was specific for each antigen (Fig. 3). The second synthesis enzyme, GS, is also expressed by enteric glia, but interestingly, the majority of GS labeling was localized to HuC/D-immunoreactive myenteric neurons (Figs. 1C and 2B). This pattern of expression is consistent with the known cellular distribution in the central nervous system, where neurons express GSH synthesis enzymes and can synthesize GSH when supplied with the dipeptide precursors, such as CysGly, from astrocytes (13, 14). These results show that enteric glia express the enzymes necessary for GSH synthesis and, furthermore, suggest a novel pathway where enteric neurons are capable of producing GSH.
Fig. 1.
Expression of the glutathione synthesis enzymes, glutamate-cysteine ligase and glutathione synthetase, in the enteric nervous system. Representative mouse myenteric ganglia showing immunoreactivity for the catalytic subunit of glutamate-cysteine ligase (GCLC, red; A and B) and glutathione synthetase (GS, red; C). Enteric neurons are labeled with the neuronal marker HuC/D (blue), enteric glia are labeled with the glial marker glial fibrillary acidic protein (GFAP, green), and presynaptic vesicles are labeled with the vesicular marker synaptophysin (blue). A white arrow in the overlay image of A highlights an area of colocalization between glutathione synthesis enzymes and GFAP. (Scale bars = 20 μm.)
Fig. 2.
Localization of the glutathione synthesis enzymes glutamate-cysteine ligase and glutathione synthetase in three-dimensional images of myenteric ganglia. Representative images showing immunohistochemistry for the catalytic subunit of glutamate-cysteine ligase (GCLC, red; A) and glutathione synthetase (GS, red; B) in full-thickness three-dimensional z-stack images of mouse myenteric ganglia. Enteric neurons are labeled with the neuronal marker HuC/D (blue) and enteric glia with the glial marker glial fibrillary acidic protein (GFAP, green). Areas of colocalization in the overlay panel are pseudocolored yellow in A and purple in B. The outlined region in the overlay images is shown at increased magnification in the panel at bottom right of both A and B. (Scale bar = 30 μm for labeled single stain and overlay images, 10 μm in zoomed image in A, and 15 μm in zoomed image in B.)
Fig. 3.
Experimental controls for same-host dual labeling using tyramide signal amplification (TSA). Representative images of mouse myenteric ganglia where presynaptic vesicles are labeled via the TSA reaction with rabbit anti-synaptophysin (1:2,000) and Alexa Fluor 568 tyramide (red). Enteric neurons are labeled with HuC/D (Hu, blue) and subsequently incubated with goat anti-rabbit Alexa Fluor 488 via conventional immunohistochemistry. No fluorescence was detectable at the 488-nm wavelength in the absence of a second rabbit primary antibody [rabbit anti-glutamate-cysteine ligase catalytic subunit (GCLC)]. (Scale bar = 30 μm.)
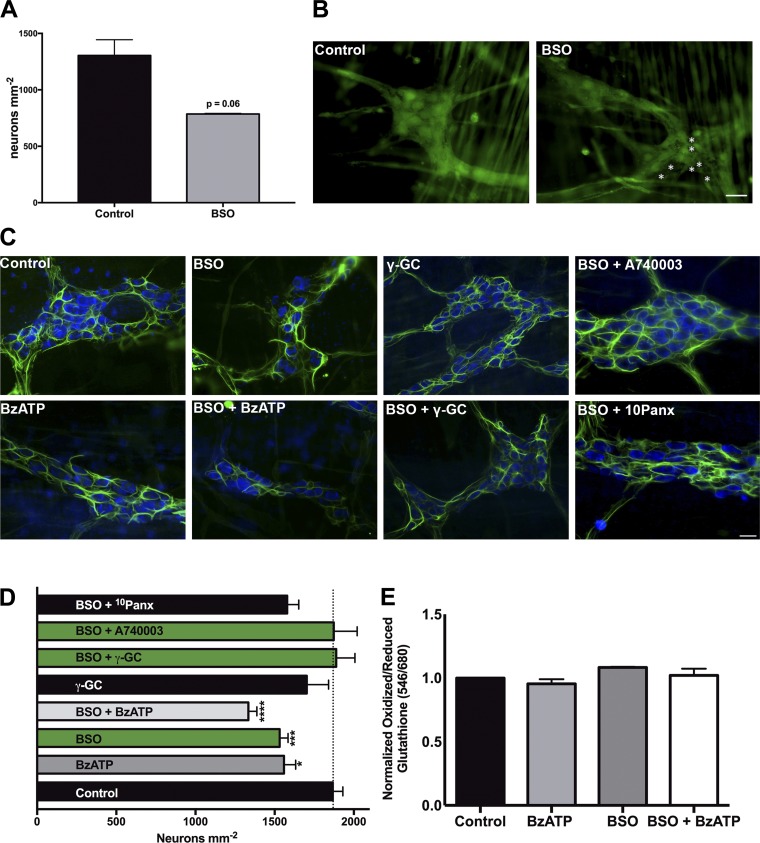
In situ inhibition of GSH synthesis with l-buthionine sulfoximine decreases myenteric neuronal density.
We next tested the role of glial glutathione production in the maintenance of enteric neuron survival by selectively blocking GCL activity with the specific inhibitor, l-buthionine sulfoximine (BSO, 100 μM; 18). GCL is the rate-limiting enzyme in GSH synthesis, and the inhibition of GCL with BSO is sufficient to deplete GSH in vitro and in vivo (17, 30, 53). We first measured neuronal survival using the calcein AM cell viability assay, which fluorescently labels live, membrane-intact cells. Depletion of GSH with BSO decreased ganglionic density of myenteric neurons by ~50% (Fig. 4, A and B). Similarly, the inhibition of GCL in whole mount preparations decreased HuC/D-immunoreactive myenteric neuron packing density by 14.1 ± 4% (1,867 ± 64 neurons/mm2 in control vs. 1,532 ± 52 neurons/mm2 in BSO-treated tissue; Fig. 4, C and D) within 4 h. Glutathione depletion drove neuron death to an extent similar to that of the activation of P2X7 receptors (P2X7R) on enteric neurons with BzATP, a known mechanism of enteric neuron death during inflammation (1,532 ± 52 neurons/mm2 with BSO vs. 1,559 ± 74 neurons/mm2 with BzATP; 7, 22). Importantly, the effects of BSO and BzATP were not additive (1,334 ± 54 neurons/mm2), suggesting either that BSO and BzATP drive neuron death through the same pathways or that they act through different neurotoxic mechanisms in the same subpopulation of neurons.
Fig. 4.
In situ inhibition of glutathione (GSH) synthesis contributes to neuron death in a P2X purinoceptor 7 receptor (P2X7R)-dependent manner. Quantification (A) and representative images (B) of live enteric neurons in myenteric ganglia following in situ depletion of GSH with l-buthionine-sulfoximine (BSO, 100 μM) or buffer control treatment. Live cells are labeled with the fluorescent marker calcein AM (green), and dead neurons are denoted by white asterisks. Representative images (C) and quantification (D) of mean neuronal packing density in mouse myenteric ganglia following inhibition of GSH synthesis with the glutamate-cysteine ligase catalytic subunit (GCLC) inhibitor BSO (100 μM). Whole mount preparations of myenteric plexus were incubated with BSO in the presence, or absence, of the GCLC product γ-glutamyl-cysteine (γ-GC, 100 μM), the P2X7R agonist 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate triethylammonium salt (BzATP, 300 μM), the P2X7R antagonist A740003 (10 μM) or the pannexin mimetic peptide 10Panx (100 μM). Inhibition of BSO decreases neuronal density to the same extent as P2X7R activation, while simultaneous treatment with γ-GC, or inhibition of P2X7R, protects against BSO-mediated cell death. (*P ≤ 0.05, ***P ≤ 0.0005, ****P ≤ 0.0001, 1-way ANOVA with Dunnett’s multiple-comparison test vs. control, n = 3–19 myenteric preparations per group from 15 animals.) Enteric neurons are labeled with HuC/D (blue), and enteric glia are labeled with glial fibrillary acidic protein (GFAP, green) in all panels. E: neuronal thiol oxidation measurements (ratio of fluorescently labeled oxidized glutathione to reduced glutathione) from in situ myenteric preparations treated with BSO (100 μM) in the presence or absence of BzATP (300 μm) (n = 3–6 ganglia per group). (Scale bar = 30 μm in all images.)
To differentiate between these two possibilities and better elucidate the mechanism of BSO-mediated cell death, we treated LMMP preparations with the P2X7R antagonist A740003 or the pannexin-1 mimetic peptide 10Panx (in the presence of BSO) since BzATP-driven neuron death requires activation of neuronal P2X7Rs and pannexin-1 channels (22). Inhibition of 10Panx was not protective against BSO-mediated neuron death (1,532 ± 52 neurons/mm2 in BSO-treated tissue vs. 1,570 ± 73 neurons/mm2 with BSO + 10Panx; Fig. 4, C and D). However, blocking neuronal P2X7Rs with A740003 maintained myenteric neuronal density at control levels during BSO treatment (1,867 ± 64 neurons/mm2 in control vs. 1,873 ± 148 neurons/mm2 with BSO + A740003), supporting the hypothesis that BSO and BzATP drive neuron death through the same pathways. Finally, to address the possible off-target neurotoxic effects during BSO treatment, we treated tissue with γ-glutamyl-cysteine (γ-GC), the product of GCL, to “rescue” the depletion of GSH by BSO. Treatment with γ-GC was protective against BSO-mediated neuron death (1,889 ± 118 neurons/mm2 with γ-GC + BSO vs. 1,532 ± 52 neurons/mm2 with BSO only; Fig. 4, C and D), indicating that BSO-mediated neuron death is primarily through direct inhibition of GSH synthesis.
Treatment with BSO in vitro depletes cellular GSH, increases free radicals, and drives cellular oxidative stress (17, 26), and we hypothesized that increased oxidative stress following BSO inhibition of GCL is the most likely mechanistic explanation for neuron death in our experiments. We measured neuronal oxidative stress in myenteric preparations after in situ treatment with BSO and BzATP using a thiol oxidation ratiometric analysis that quantifies the ratio of oxidized thiols to reduced thiols. Surprisingly, in situ oxidative stress was not significantly increased despite the cell death associated with BSO and BzATP treatment (Fig. 4E). These results demonstrate that in situ inhibition of glial GSH synthesis with BSO is neurotoxic to myenteric neurons but does not alter neuronal thiol state.
In vivo inhibition of glutathione synthesis does not cause overt inflammation but is protective against macroscopic measures of inflammation.
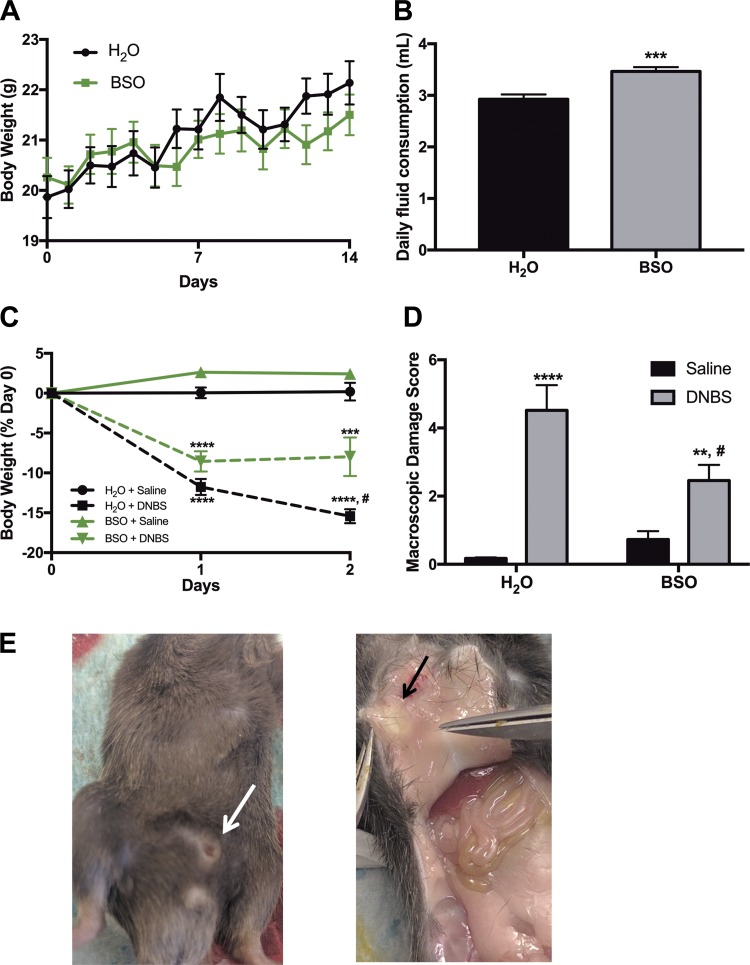
To better understand the role of glial GSH during GI pathology, we treated mice ad libitum with BSO in drinking water (20 mM) for 14 days before induction of in vivo inflammation with DNBS colitis. This treatment paradigm decreases colonic GSH content by 84% in adult mice (53), and liver GSH by 86% in pregnant rats (42), without any toxic effects. In our experiments, BSO treatment produced no significant adverse effects as evaluated by daily body weight measurements and observations of behavior and appearance (Fig. 5A). Half of BSO-treated mice developed preputial gland abscesses around day 7 of treatment (white and black arrows; Fig. 5E). However, all abscesses self-ruptured by day 10, and mice were alert and in healthy condition before DNBS treatment on day 14. To determine whether there was an aversion to water containing BSO, we measured daily fluid consumption in both treatment groups. Mice drinking water containing BSO consumed similar volumes of fluid daily compared with mice drinking normal H2O (Fig. 5B). In fact, mice drinking BSO consumed slightly more fluid (2.9 ml H2O vs. 3.5 ml BSO in H2O·mouse−1·day−1).
Fig. 5.
Inhibition of glutathione synthesis alone is not sufficient to cause overt inflammation but does alter susceptibility to inflammatory insults. A: daily body weight of mice consuming either normal drinking water (H2O, black) or water containing the glutamate-cysteine ligase catalytic subunit (GCLC) inhibitor l-buthionine sulfoximine (BSO at 20 mM, green) for 2 wk. B: average daily ad libitum fluid consumption for mice drinking normal water (H2O) or water containing 20 mM BSO (***P < 0.001, Student’s t-test). Daily percent weight loss (C) and macroscopic damage score (D) of BSO-treated mice (green lines and gray bars) or their normal drinking water controls (H2O, black lines and bars) after 2,4-dinitrobenzene sulfonic acid-induced colitis (DNBS colitis; dashed lines in C, gray bars in D) or saline control treatment (solid lines in C, black bars in D). (**P ≤ 0.01, ***P ≤ 0.005, ****P ≤ 0.0001 compared with H2O-Saline, #P ≤ 0.05 compared with H2O-DNBS via 2-way ANOVA with Tukey’s post hoc test, n = 6–9 animals per group.) E: external (white arrow) and internal (black arrow) view of preputial gland abscess in BSO-treated mice on day of death.
We next induced inflammation with an enema of 2,4-dinitrobenzene sulfonic acid (DNBS), while control mice received a saline enema. BSO consumption without exposure to an inflammatory insult (Fig. 5C, BSO-Saline, green solid line) did not induce colonic inflammation as determined by weight loss (body weight 2.6 ± 0.4 and 2.4 ± 0.5% of control at days 1 and 2, respectively; Fig. 5C). Mice that consumed normal drinking water before inflammation (H2O-DNBS, black dashed line) lost significant body weight 1 and 2 days post-DNBS compared with H2O-Saline controls (11.8 ± 1% at day 1 and 15.4 ± 0.9% at day 2; Fig. 5C). Similarly, mice drinking BSO that received a DNBS enema (BSO-DNBS, green dashed line) also lost significant body weight compared with H2O-Saline controls (8.5 ± 1.3% at day 1 and 7.9 ± 2.4% at day 2, Fig. 5C). Importantly, BSO consumption before and during inflammation was protective against inflammatory damage, as BSO-DNBS mice lost significantly less weight than H2O-DNBS mice 2 days post-DNBS (7.9 ± 2.4 vs. 15.4 ± 0.9%; Fig. 5C).
At death, mouse colons were collected, and macroscopic damage score was assessed as an additional measure of inflammatory damage (50). BSO consumption in drinking water was not sufficient to increase macroscopic markers of inflammation (Fig. 5D; BSO-Saline). Macroscopic damage score was increased in DNBS-treated mice, regardless of H2O or BSO consumption before DNBS treatment (gray bars, Fig. 5D). In agreement with the trend seen in body weight, BSO treatment before DNBS was protective against increased colonic macroscopic damage (4.5 ± 0.7 and 2.5 ± 0.5 in H2O-DNBS and BSO-DNBS, respectively; Fig. 5D). Collectively, these results show that whole body glutathione depletion does not induce overt colonic inflammation. Rather, BSO treatment is protective against some key features of colitis in mice.
Glutathione depletion decreases neutrophil infiltration during DNBS colitis but does not alter reactivity of MHC II-expressing macrophages or neuronal thiol oxidation levels.
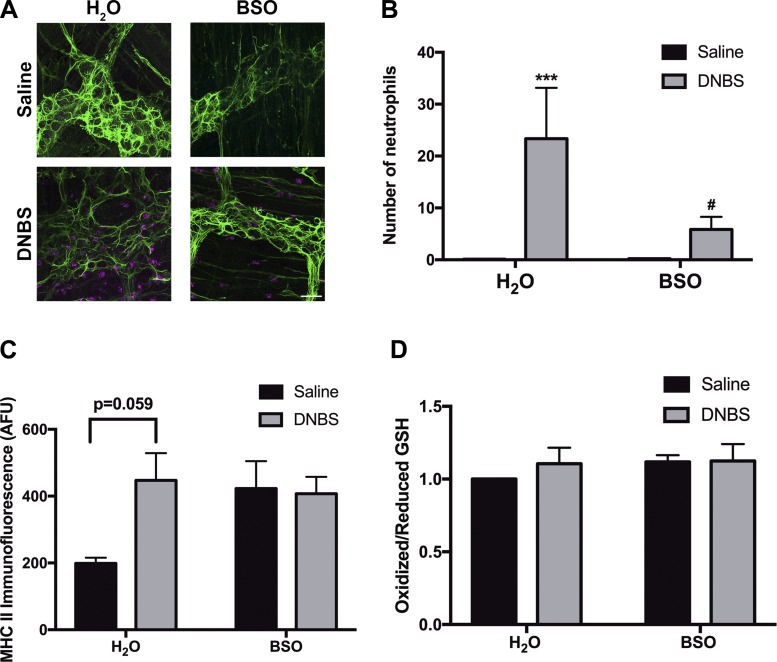
We next investigated how in vivo glutathione depletion affects microscopic markers of inflammation. Increased immune cell infiltration is a key feature of many animal models of colitis, including DNBS (32). In agreement with prior reports, we observed increased infiltration of myeloperoxidase (MPO)-positive neutrophils into a 76,800-μm2 field-of-view area in the myenteric plexus in water drinking-inflamed animals (23.3 ± 10 neutrophils in H2O-DNBS vs. 0.1 ± 0.04 neutrophils in H2O-Saline, Fig. 6, A and B). The frequency of neutrophils within the myenteric plexus of healthy animals drinking water supplemented with BSO was comparable to that of healthy animals drinking water alone (0.2 ± 0.05 neutrophils in BSO-Saline, Fig. 6, A and B). However, mice drinking BSO during the course of colitis exhibited significantly attenuated myenteric plexus neutrophil infiltration compared with inflamed animals that did not receive BSO (5.8 ± 2.5 neutrophils in BSO-DNBS vs. 23.3 ± 10 neutrophils in H2O-DNBS; Fig. 6, A and B).
Fig. 6.
Effects of glutathione depletion on neutrophil infiltration into the myenteric plexus, major histocompatibility complex class II (MHC II)-positive muscularis macrophage infiltration, and neuronal thiol oxidation levels during 2,4-dinitrobenzene sulfonic acid-induced colitis (DNBS colitis) in mice. Representative images (A) and quantification (B) of the infiltration of myeloperoxidase-positive neutrophils (magenta) in myenteric ganglia of mice drinking normal water (H2O) or water with 20 mM l-buthionine sulfoximine (BSO) before induction of colitis with DNBS (gray bars) or saline control (black bars). Enteric glia are labeled with glial fibrillary acidic protein (GFAP, green). (***P ≤ 0.005 compared with H2O-Saline, #P ≤ 0.05 compared with H2O-DNBS via 2-way ANOVA with Tukey’s post hoc test, n = 3–6 animals per group.) C: fluorescence of MHC II-immunoreactive muscularis macrophages in mouse myenteric plexus after DNBS colitis (gray bars) or saline control animals (black bars), drinking either normal H2O or water containing 20 mM BSO (2-way ANOVA with Dunnett’s multiple-comparison test, n = 3 animals per group; AFU, average fluorescence units). D: neuronal ganglionic oxidation measurements [ratio of fluorescently labeled oxidized glutathione (GSH) to reduced glutathione] (n = 3–6 ganglia per group).
We next measured changes in major histocompatibility complex class II (MHC II) immunoreactivity to quantify the reactivity of antigen-presenting cells in a 76,800-μm2 area surrounding individual myenteric ganglia. This population of cells predominantly consists of muscularis macrophages that reside at the level of the myenteric plexus in the colonic wall (38, 54). Colonic inflammation increased the immunoreactivity of MHC II-expressing muscularis macrophages in the myenteric plexus (Fig. 6C). MHC II expression was also induced by glutathione depletion as both inflamed (DNBS) and control (Saline) animals that consumed BSO showed a nonsignificant increase in ganglionic MHC II expression (Fig. 6C). In agreement with our in situ data (Fig. 6C), neuronal thiol oxidation state was not significantly altered following induction of colonic inflammation (H2O-DNBS) or depletion of cellular glutathione in normal and inflamed animals (BSO-Saline and BSO-DNBS; Fig. 6D). Together, these results show that BSO treatment attenuates the infiltration of neutrophils during DNBS colitis but does not significantly affect the infiltration or reactive state of MHC II-positive cells or neuronal thiol oxidation levels.
In vivo inhibition of glutathione synthesis decreases neuronal density and alters myenteric plexus neurochemical coding.
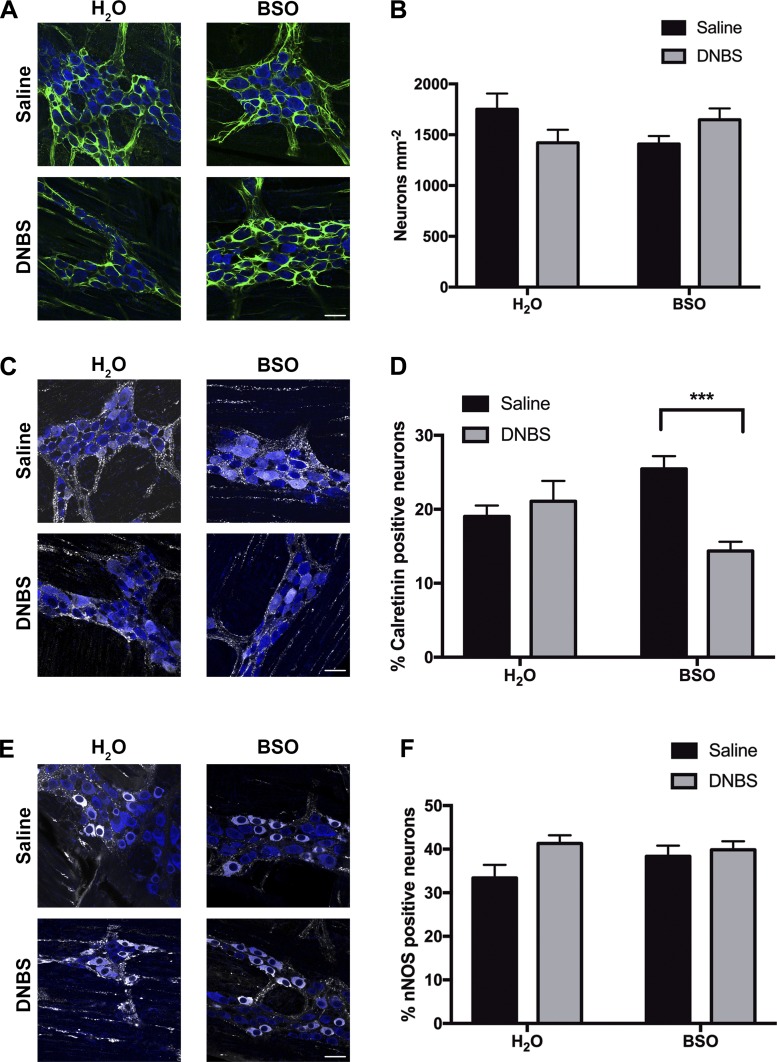
We next assessed how in vivo depletion of glutathione affects myenteric neuron density and neurochemical coding. In agreement with our prior published findings (7, 22), DNBS colitis decreased neuronal density by 19 ± 7% in animals drinking normal water (1,751 ± 155 neurons/mm2 in H2O-Saline vs. 1,421 ± 127 neurons/mm2 in H2O-DNBS animals, P = 0.17 via 2-way ANOVA; Fig. 7, A and B). In vivo glutathione depletion with BSO decreased HuC/D-immunoreactive neuron density to a similar extent as in vivo inflammation (1,409 ± 78 neurons/mm2 in BSO-Saline animals). Consistent with our in situ results (Fig. 4D), glutathione depletion during DNBS administration does not potentiate neuron loss during colitis (1,647 ± 111 neurons/mm2 in BSO-DNBS mice; Fig. 7, A and B).
Fig. 7.
Inhibition of glutathione synthesis in vivo decreases enteric neuron survival and alters myenteric plexus neurochemical coding. Representative images (A) and mean packing density (B) of HuC/D-immunoreactive myenteric neurons (blue) from mice consuming normal drinking water (H2O) or water with 20 mM l-buthionine sulfoximine (BSO) before 2,4-dinitrobenzene sulfonic acid (DNBS, gray bars) or saline (black bars) enema administration. Enteric glia are labeled with glial fibrillary acidic protein (GFAP, green). C and E: representative images showing excitatory (calretinin, gray; C) and inhibitory [neuronal nitric oxide synthase (nNOS), gray; E] neuronal subpopulations in myenteric ganglia. D and F: percentage of HuC/D-positive neurons that are immunoreactive for calretinin (D) and nNOS (F) in mice drinking water (H2O) or water with 20 mM BSO before DNBS (gray bars) or saline (black bars) enema administration. Myenteric neurons are labeled with the pan-neuronal marker HuC/D (blue), excitatory neurons are labeled with calretinin (gray), and inhibitory neurons are labeled with nNOS (gray). (***P ≤ 0.005, 2-way ANOVA with Tukey’s post hoc test, n = 4–9 animals per group, scale bars = 20 μm.)
Glutathione depletion is known to reduce levels of inhibitory neurochemicals in gastrointestinal tissue (28). However, how glutathione depletion specifically alters neurochemical coding within myenteric ganglia is not known. We used immunohistochemical markers for specific classes of myenteric neurons to investigate how neurochemical coding is altered in myenteric ganglia after glutathione depletion. Neither glutathione depletion nor inflammation altered the percentage of calretinin-positive excitatory neurons in healthy animals (19 ± 1.5% in H2O-Saline, 21 ± 2.8% in H2O-DNBS, and 25.5 ± 1.7% in BSO-Saline; Fig. 7, C and D). In contrast, glutathione depletion caused the percentage of calretinin-positive neurons to decrease by ~10% during inflammation (25.5 ± 1.7% in BSO-Saline vs. 14.4 ± 1.2% in BSO-DNBS; Fig. 7, C and D). Conversely, glutathione depletion did not significantly affect the percentage of nNOS-positive inhibitory neurons in healthy or inflamed animals (33 ± 3% in H2O-Saline vs. 38 ± 2.5% in BSO-Saline vs. 39 ± 2% in BSO-DNBS; Fig. 7, E and F). Together, these results show that glutathione is necessary to maintain both the phenotype and survival of neurons in the myenteric plexus of the colon.
Inflammation alters the expression of the glutathione synthesis enzymes, glutamate-cysteine ligase and glutathione synthetase, in the ENS.
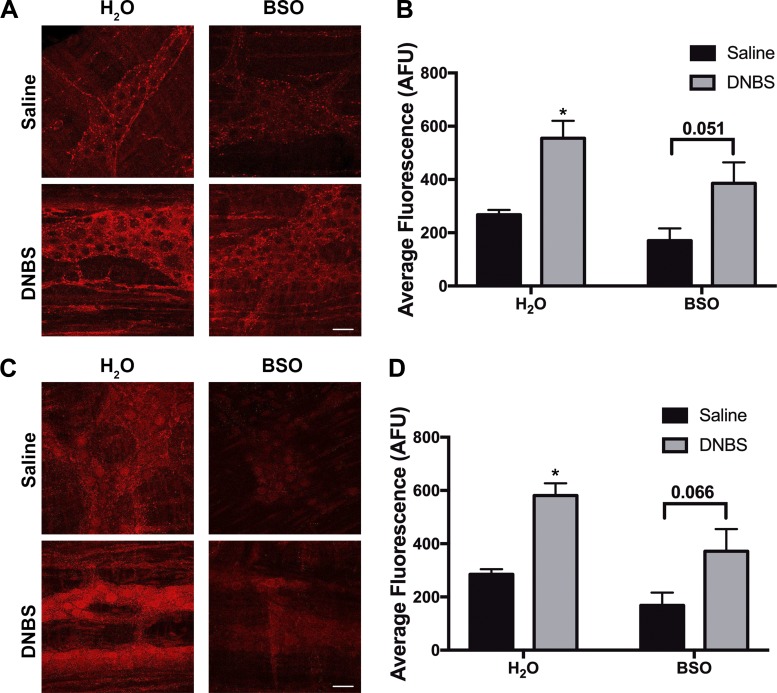
Finally, we investigated how in vivo inflammation affects the localization and expression of glutathione synthesis enzymes. DNBS colitis did not alter the localization of either GCLC or GS. GCLC expression remained localized to enteric glia and presynaptic neuronal vesicles (Fig. 8A), and GS expression remained localized to glial cells and myenteric neuron cell bodies (Fig. 8C). However, both in vivo inflammation and BSO treatment altered the expression levels of GCLC and GS. In vivo inhibition of glutathione synthesis with the GCLC inhibitor BSO (20 mM) decreased ganglionic GCLC expression by 36% (mean fluorescence: 267 ± 18 in H2O-Saline vs. 170 ± 46 in BSO-Saline; Fig. 8B). This result supports well-established data showing that cellular GSH concentrations regulate GCLC activity and expression via a negative feedback loop (19, 44). BSO-treated healthy animals also show a ~40% decrease in expression of the second glutathione synthesis enzyme GS (mean fluorescence: 284 ± 19 in H2O-Saline animals vs. 168 ± 48 in BSO-Saline animals; Fig. 8D).
Fig. 8.
Inflammation alters the expression of the glutathione synthesis enzymes, glutamate-cysteine ligase and glutathione synthetase, in the enteric nervous system. Representative images (A and C) and corresponding quantification (B and D) of immunoreactivity for the catalytic subunit of glutamate-cysteine ligase (GCLC, red; A and B), the rate-limiting enzyme in glutathione synthesis, or glutathione synthetase (GS, red; C and D) in mouse myenteric ganglia from healthy (saline) or inflamed [2,4-dinitrobenzene sulfonic acid (DNBS)] mice drinking either normal water (H2O) or water supplemented with l-buthionine sulfoximine (BSO, 20 mM). (*P ≤ 0.05 compared with H2O-Saline via 2-way ANOVA with Tukey’s post hoc analysis, n = 7–9 animals per group; AFU, average fluorescence units.)
In vivo inflammation doubled the ganglionic expression of GCLC in both normal and BSO-treated animals (mean fluorescence: 267 ± 18 vs. 555 ± 65 in H2O Saline vs. DNBS, 170 ± 46 vs. 386 ± 79 in BSO Saline vs. DNBS; Fig. 8, A and B). Similarly, GS expression increased ~200% following inflammation in both normal animals and animals with depleted glutathione (mean fluorescence: 285 ± 19 vs. 581 ± 46 in H2O Saline vs. DNBS animals, 169 ± 48 vs. 372 ± 83 in BSO Saline vs. DNBS animals; Fig. 8D). These results demonstrate a dynamic relationship between in vivo inflammation and regulation of glutathione synthesis within the ENS.
DISCUSSION
Our study investigates the role of the endogenous antioxidant reduced glutathione (GSH) in the ENS and probes how enteric glial GSH contributes to neuroprotection during in situ and in vivo neuroinflammation. Our findings show that glutathione is important in supporting neuronal survival and phenotype in the gut. However, the systemic inhibition of glutathione synthesis in vivo is neuroprotective during colitis, possibly by limiting the infiltration of immune cells.
Glutathione and its derivatives are well known for their neuroprotective effects in the ENS. Cultured enteric glia produce and secrete reduced glutathione, which is neuroprotective to enteric neurons in the myenteric plexus (1). In the mucosa, the glial-derived S-nitrosoglutathione helps maintain mucosal barrier function and promotes tight junction formation (48). However, no work to date has directly shown that enteric glia express the enzymes necessary for glutathione synthesis. Our data here show that the enteric glia express the molecular machinery necessary for glutathione synthesis in vivo. These findings provide important support for previous work that measured the release of glutathione and its derivatives from cultured enteric glia (1, 48).
The expression pattern of the glutathione synthesis enzymes in the ENS is similar to that in the central nervous system, where astroglia are primarily responsible for the production and secretion of neuroprotective GSH (43, 47). An unexplored hypothesis in the ENS was the ability of enteric neurons to also produce GSH. In the brain, neurons express the GSH synthesis enzymes, albeit at much lower levels, and are capable of producing limited quantities of GSH (13). In addition, neurons rely heavily on astrocytes to provide key precursor molecules necessary for GSH synthesis (14, 37). Our findings show a similar relationship in the ENS where enteric neurons express GCLC and GS in neuronal varicosities and cell bodies, respectively. This suggests that enteric neurons have the ability to produce GSH, and we hypothesize a novel neuroglia relationship similar to that in the central nervous system in regard to GSH synthesis and release.
Our data show that glutathione is necessary to support neuronal survival because the inhibition of glial GSH synthesis with the selective GCL inhibitor BSO decreased myenteric neuron density, and decreased the number of calcein AM-positive cells, in acutely isolated tissues. One possibility is that BSO could produce direct neurotoxic effects on enteric neurons independent of its inhibition of glutathione synthesis. However, this is unlikely because other reports of in vitro BSO treatment show decreased GSH content in concurrence with cell death (26, 30). Furthermore, treatment with GSH (30) or its precursors was sufficient to protect against BSO-mediated cell death (15; Fig. 4, C and D), suggesting that the cytotoxic effects of BSO are directly related to its inhibition of GSH synthesis.
Glutathione depletion drove neuron death to the same extent as direct activation of the neuronal P2X7 receptor, but the effects were not additive, and BSO treatment did not potentiate BzATP-driven neuron death in situ. P2X7R-mediated neuron death requires activation of P2X7R and opening of neuronal pannexin-1 channels (22). Blocking pannexin-1 with 10Panx did not protect against BSO-mediated neuron death, suggesting that glutathione depletion does not affect pannexin-1 channel opening and subsequent ATP release. Conversely, inhibition of P2X7R with the specific antagonist A740003 was sufficient to protect against neuron death during glutathione depletion with BSO (Fig. 4, C and D). This demonstrates that the mechanism of BSO-mediated cell death requires activation of neuronal P2X7Rs by large amounts of extracellular ATP. Enteric glial cells are an important source of ATP during GI inflammation and contribute to neuron death via connexin-43 (Cx43)-dependent ATP release (7). As Cx43 hemichannels are gated by oxidative stress (46a), one hypothesis is that glutathione depletion causes neuron death by altering the glial cellular environment to a prooxidative state, which in turn increases Cx43 channel opening and ATP release, leading to increased P2X7R activation and neuron death.
Glutathione depletion is reported to increase cellular oxidative stress and cellular susceptibility to oxidative damage (17). However, neuronal thiol oxidation (a measure of cellular oxidative stress) was not increased in our live whole mount preparations following treatment with BSO, suggesting that in situ inhibition of GSH synthesis does not affect cellular thiol state. A number of factors may explain this finding. First, the thiol oxidation ratiometric analysis quantifies the oxidation states of all cellular thiols inclusive of, but not limited to, cellular glutathione. GSH depletion, while decreasing total thiol levels, may not alter the state of other proteinaceous thiols. In our treatment paradigm, live whole mounts are incubated with BSO for 2 h followed by a 2-h incubation in buffer only. BSO has been shown to dissociate from 1 to 3% of bound GCL enzyme per hour when a BSO treatment solution is replaced with normal buffer (19). Thus it is also possible that dissociation of BSO during the second 2-h incubation is sufficient to return cellular thiol levels to normal but not to reverse the cytotoxicity and cell death induced by the 2-h incubation with BSO.
GSH is synthesized in a two-step process catalyzed by the rate-limiting GCL and GS. Regulation of GCL and GS activity and expression are important mechanisms in the regulation of cellular glutathione content (35). In fact, GSH synthesis is controlled by a negative feedback loop where GSH acts as a nonallosteric inhibitor on the rate-limiting GCL enzyme (19, 44). Similarly, GCL transcription and protein expression are induced by increased oxidative stress (51, 55). We observed this in our studies, where increased oxidative stress during colitis increased expression of both GCL and GS. Increased expression of both enzymes is reported to significantly increase cellular GSH capacity and is thus an expected result in response to GI inflammation (25, 34).
A primary goal of our study was to investigate the effects of in vivo inhibition of GSH synthesis and how GSH depletion affects macroscopic and microscopic damage during inflammation. To this end, we administered 20 mM BSO in vivo via drinking water for 14 days before the induction of inflammation. This treatment paradigm has been previously well characterized as a suitable animal model for tissue GSH depletion without cytotoxicity (53). In support, we observed few adverse effects in healthy BSO-treated animals throughout the treatment period or at the time of death. A proportion of animals did develop preputial gland abscesses, a common condition seen in nonbreeder male mice (5). If severe, this ailment requires surgical rupturing and antibiotic treatment. However, our mice developed mild abscesses that self-ruptured and did not significantly affect animal health and welfare. Specifically, depletion of GSH did not decrease body weight, increase macroscopic colon damage, or increase the infiltration of neutrophils in otherwise healthy animals.
Reduced glutathione is the primary endogenous antioxidant in the body and is an important cellular tool in protection against oxidative stress (4). Typically, antioxidant depletion increases cellular oxidative stress and increases cell susceptibility to exogenous toxicants including oxidative stressors (35). However, our results show that decreased glutathione is protective during in vivo inflammation. Specifically, GSH depletion before inflammation decreases colonic macroscopic damage score and neutrophil infiltration, partially protects against colitis-associated weight loss, and prevents additional loss of myenteric neurons. One explanation of our findings is that GSH supports glial cells in a pathogenic state and that decreasing glial GSH is protective against glial activation and the subsequent neuron death. GSH serves a similar unexpected function in central nervous system gliomas. In this disease, increased glioma GSH synthesis is associated with the glutamate excitotoxicity responsible for many glioma symptoms (11). In support, inhibition of the cysteine/glutamate system xc− transporter on glioma cells with sulfasalazine, a system xc− inhibitor currently used to treat Crohn’s disease patients (31), decreases cellular GSH synthesis and protects against symptoms (45). Thus decreased glial synthesis of the normally protective GSH may have therapeutic benefits in some pathogenic conditions.
The infiltration of immune cells is an important aspect of colitis pathology (32). An alternate hypothesis for our findings is that the protective effects of GSH depletion are mediated via altered immune cell behavior. In some bacterial infections, decreased neutrophil GSH content is associated with reduced neutrophil cytokine production and decreased migration (27). This aligns with our own finding showing decreased neutrophil infiltration into the myenteric plexus of animals with depleted GSH before inflammation.
In our study, depletion of GSH, by inhibiting GSH synthesis with BSO, produced differential results in our in situ vs. in vivo experiments. Production of GSH is necessary for in situ neuronal survival (Fig. 4, C and D). Conversely, in vitro whole body GSH did not statistically decrease myenteric neuronal density but was protective against key features of DNBS colitis. A number of factors underlie the heterogeneity of in situ vs. in vivo findings. First, the in situ LMMP tissue preparation features less cellular complexity than our whole animal in vivo subject. Thus it is possible that the protective effects of BSO require intermediate cell types that are not present in our LMMP preparations (e.g., immune cells). Second, the time course of treatment varied between our two models. The in situ treatment was given acutely while in vivo depletion of GSH requires a more chronic administration of BSO. These differences in treatment time may also explain the heterogeneous findings between our in situ and in vivo experiments.
In conclusion, our findings show evidence for both myenteric glial and neuronal production of GSH in the ENS. Furthermore, we demonstrate that in situ GSH synthesis is necessary for neuroprotection and present a novel hypothesis where in vivo GSH depletion is protective against inflammation. These observations present a potential therapeutic target for improved GI pathology during inflammation.
GRANTS
This project was supported by the National Institutes of Health Grants R01-DK-103723 (to B. D. Gulbransen) and F31-107232 (to I. A. M. Brown) and the Crohn’s & Colitis Foundation Senior Research Grant Award 327058 (to B. D. Gulbransen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.A.M.B. and B.D.G. conceived and designed research; I.A.M.B. performed experiments; I.A.M.B. analyzed data; I.A.M.B. and B.D.G. interpreted results of experiments; I.A.M.B. prepared figures; I.A.M.B. drafted manuscript; I.A.M.B. and B.D.G. edited and revised manuscript; I.A.M.B. and B.D.G. approved final version of manuscript.
REFERENCES
- 1.Abdo H, Derkinderen P, Gomes P, Chevalier J, Aubert P, Masson D, Galmiche JP, Vanden Berghe P, Neunlist M, Lardeux B. Enteric glial cells protect neurons from oxidative stress in part via reduced glutathione. FASEB J 24: 1082–1094, 2010. doi: 10.1096/fj.09-139519. [DOI] [PubMed] [Google Scholar]
- 2.Abdo H, Mahé MM, Derkinderen P, Bach-Ngohou K, Neunlist M, Lardeux B. The omega-6 fatty acid derivative 15-deoxy-Δ12,14-prostaglandin J2 is involved in neuroprotection by enteric glial cells against oxidative stress. J Physiol 590: 2739–2750, 2012. doi: 10.1113/jphysiol.2011.222935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anitha M, Gondha C, Sutliff R, Parsadanian A, Mwangi S, Sitaraman SV, Srinivasan S. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest 116: 344–356, 2006. doi: 10.1172/JCI26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aquilano K, Baldelli S, Ciriolo MR. Glutathione: new roles in redox signaling for an old antioxidant. Front Pharmacol 5: 196, 2014. doi: 10.3389/fphar.2014.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertrand HG, Thomas AA, Ellen YC, Dorward RS, Flecknell PA. A surgical approach in the treatment of preputial gland abscesses in mice. BMC Vet Res 12: 16, 2016. doi: 10.1186/s12917-016-0636-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Boyen GB, Schulte N, Pflüger C, Spaniol U, Hartmann C, Steinkamp M. Distribution of enteric glia and GDNF during gut inflammation. BMC Gastroenterol 11: 3, 2011. doi: 10.1186/1471-230X-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown IA, McClain JL, Watson RE, Patel BA, Gulbransen BD. Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide. Cell Mol Gastroenterol Hepatol 2: 77–91, 2016. doi: 10.1016/j.jcmgh.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell 93: 189–201, 1998. doi: 10.1016/S0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- 9.Cornet A, Savidge TC, Cabarrocas J, Deng WL, Colombel JF, Lassmann H, Desreumaux P, Liblau RS. Enterocolitis induced by autoimmune targeting of enteric glial cells: a possible mechanism in Crohn’s disease? Proc Natl Acad Sci USA 98: 13306–13311, 2001. doi: 10.1073/pnas.231474098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Giorgio R, Giancola F, Boschetti E, Abdo H, Lardeux B, Neunlist M. Enteric glia and neuroprotection: basic and clinical aspects. Am J Physiol Gastrointest Liver Physiol 303: G887–G893, 2012. doi: 10.1152/ajpgi.00096.2012. [DOI] [PubMed] [Google Scholar]
- 11.de Groot J, Sontheimer H. Glutamate and the biology of gliomas. Glia 59: 1181–1189, 2011. doi: 10.1002/glia.21113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickinson DA, Forman HJ. Cellular glutathione and thiols metabolism. Biochem Pharmacol 64: 1019–1026, 2002. doi: 10.1016/S0006-2952(02)01172-3. [DOI] [PubMed] [Google Scholar]
- 13.Dringen R, Gutterer JM, Hirrlinger J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem 267: 4912–4916, 2000. doi: 10.1046/j.1432-1327.2000.01597.x. [DOI] [PubMed] [Google Scholar]
- 14.Dringen R, Pfeiffer B, Hamprecht B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci 19: 562–569, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol 62: 649–671, 2000. doi: 10.1016/S0301-0082(99)00060-X. [DOI] [PubMed] [Google Scholar]
- 16.Esworthy RS, Aranda R, Martín MG, Doroshow JH, Binder SW, Chu FF. Mice with combined disruption of Gpx1 and Gpx2 genes have colitis. Am J Physiol Gastrointest Liver Physiol 281: G848–G855, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Gegg ME, Clark JB, Heales SJ. Co-culture of neurones with glutathione deficient astrocytes leads to increased neuronal susceptibility to nitric oxide and increased glutamate-cysteine ligase activity. Brain Res 1036: 1–6, 2005. doi: 10.1016/j.brainres.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 18.Griffith OW. Mechanism of action, metabolism, and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. J Biol Chem 257: 13704–13712, 1982. [PubMed] [Google Scholar]
- 19.Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med 27: 922–935, 1999. doi: 10.1016/S0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- 20.Grubišić V, Gulbransen BD. Enteric glia: the most alimentary of all glia. J Physiol 595: 557–570, 2017. doi: 10.1113/JP271021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gulbransen B, Silver W, Finger TE. Solitary chemoreceptor cell survival is independent of intact trigeminal innervation. J Comp Neurol 508: 62–71, 2008. doi: 10.1002/cne.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, Muruve DA, McKay DM, Beck PL, Mawe GM, Thompson RJ, Sharkey KA. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med 18: 600–604, 2012. doi: 10.1038/nm.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gulbransen BD, Sharkey KA. Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol 9: 625–632, 2012. doi: 10.1038/nrgastro.2012.138. [DOI] [PubMed] [Google Scholar]
- 24.Hirrlinger J, Schulz JB, Dringen R. Glutathione release from cultured brain cells: multidrug resistance protein 1 mediates the release of GSH from rat astroglial cells. J Neurosci Res 69: 318–326, 2002. doi: 10.1002/jnr.10308. [DOI] [PubMed] [Google Scholar]
- 25.Huang ZZ, Li H, Cai J, Kuhlenkamp J, Kaplowitz N, Lu SC. Changes in glutathione homeostasis during liver regeneration in the rat. Hepatology 27: 147–153, 1998. doi: 10.1002/hep.510270123. [DOI] [PubMed] [Google Scholar]
- 26.Ibi M, Sawada H, Kume T, Katsuki H, Kaneko S, Shimohama S, Akaike A. Depletion of intracellular glutathione increases susceptibility to nitric oxide in mesencephalic dopaminergic neurons. J Neurochem 73: 1696–1703, 1999. doi: 10.1046/j.1471-4159.1999.731696.x. [DOI] [PubMed] [Google Scholar]
- 27.Kewcharoenwong C, Rinchai D, Nithichanon A, Bancroft GJ, Ato M, Lertmemongkolchai G. Glibenclamide impairs responses of neutrophils against Burkholderia pseudomallei by reduction of intracellular glutathione. Sci Rep 6: 34794, 2016. doi: 10.1038/srep34794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koch TR, Fink JG, Ruan E, Petro A, Opara EC. Chronic glutathione depletion alters expression of enteric inhibitory neurochemicals in the mouse. Neurosci Lett 235: 77–80, 1997. doi: 10.1016/S0304-3940(97)00726-X. [DOI] [PubMed] [Google Scholar]
- 29.Lee M, Cho S, Roh K, Chae J, Park JH, Park J, Lee MA, Kim J, Auh CK, Yeom CH, Lee S. Glutathione alleviated peripheral neuropathy in oxaliplatin-treated mice by removing aluminum from dorsal root ganglia. Am J Transl Res 9: 926–939, 2017. [PMC free article] [PubMed] [Google Scholar]
- 30.Lee M, Cho T, Jantaratnotai N, Wang YT, McGeer E, McGeer PL. Depletion of GSH in glial cells induces neurotoxicity: relevance to aging and degenerative neurological diseases. FASEB J 24: 2533–2545, 2010. doi: 10.1096/fj.09-149997. [DOI] [PubMed] [Google Scholar]
- 31.Lim WC, Wang Y, MacDonald JK, Hanauer S. Aminosalicylates for induction of remission or response in Crohn’s disease. Cochrane Database Syst Rev 7: CD008870, 2016. doi: 10.1002/14651858.CD008870.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linden DR, Couvrette JM, Ciolino A, McQuoid C, Blaszyk H, Sharkey KA, Mawe GM. Indiscriminate loss of myenteric neurones in the TNBS-inflamed guinea-pig distal colon. Neurogastroenterol Motil 17: 751–760, 2005. doi: 10.1111/j.1365-2982.2005.00703.x. [DOI] [PubMed] [Google Scholar]
- 33.Linden DR. Enhanced excitability of guinea pig ileum myenteric AH neurons during and following recovery from chemical colitis. Neurosci Lett 545: 91–95, 2013. doi: 10.1016/j.neulet.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu SC, Huang ZZ, Yang H, Tsukamoto H. Effect of thioacetamide on the hepatic expression of gamma-glutamylcysteine synthetase subunits in the rat. Toxicol Appl Pharmacol 159: 161–168, 1999. doi: 10.1006/taap.1999.8729. [DOI] [PubMed] [Google Scholar]
- 35.Lu SC. Regulation of glutathione synthesis. Mol Aspects Med 30: 42–59, 2009. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu SC. Glutathione synthesis. Biochim Biophys Acta 1830: 3143–3153, 2013. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin HL, Teismann P. Glutathione: a review on its role and significance in Parkinson’s disease. FASEB J 23: 3263–3272, 2009. doi: 10.1096/fj.08-125443. [DOI] [PubMed] [Google Scholar]
- 38.Muller PA, Koscsó B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li XM, Mucida D, Stanley ER, Dahan S, Margolis KG, Gershon MD, Merad M, Bogunovic M. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158: 300–313, 2014. [Erratum in Cell 158(5): 1210, 2014. doi: 10.1016/j.cell.2014.08.002.]. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mullett SJ, Di Maio R, Greenamyre JT, Hinkle DA. DJ-1 expression modulates astrocyte-mediated protection against neuronal oxidative stress. J Mol Neurosci 49: 507–511, 2013. doi: 10.1007/s12031-012-9904-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neunlist M, Aubert P, Toquet C, Oreshkova T, Barouk J, Lehur PA, Schemann M, Galmiche JP. Changes in chemical coding of myenteric neurones in ulcerative colitis. Gut 52: 84–90, 2003. doi: 10.1136/gut.52.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palma PF, Baggio GL, Spada C, Silva RD, Ferreira SI, Treitinger A. Evaluation of annexin V and Calcein-AM as markers of mononuclear cell apoptosis during human immunodeficiency virus infection. Braz J Infect Dis 12: 108–114, 2008. doi: 10.1590/S1413-86702008000200003. [DOI] [PubMed] [Google Scholar]
- 42.Reyes E, Ott S, Robinson B, Contreras R. The effect of in utero administration of buthionine sulfoximine on rat development. Pharmacol Biochem Behav 50: 491–497, 1995. doi: 10.1016/0091-3057(94)00320-3. [DOI] [PubMed] [Google Scholar]
- 43.Rice ME, Russo-Menna I. Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience 82: 1213–1223, 1998. doi: 10.1016/S0306-4522(97)00347-3. [DOI] [PubMed] [Google Scholar]
- 44.Richman PG, Meister A. Regulation of gamma-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J Biol Chem 250: 1422–1426, 1975. [PubMed] [Google Scholar]
- 45.Robert SM, Buckingham SC, Campbell SL, Robel S, Holt KT, Ogunrinu-Babarinde T, Warren PP, White DM, Reid MA, Eschbacher JM, Berens ME, Lahti AC, Nabors LB, Sontheimer H. SLC7A11 expression is associated with seizures and predicts poor survival in patients with malignant glioma. Sci Transl Med 7: 289ra86, 2015. doi: 10.1126/scitranslmed.aaa8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenbaum C, Schick MA, Wollborn J, Heider A, Scholz CJ, Cecil A, Niesler B, Hirrlinger J, Walles H, Metzger M. Activation of myenteric glia during acute inflammation in vitro and in vivo. PLoS One 11: e0151335, 2016. doi: 10.1371/journal.pone.0151335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46a.Sáez JC, Retamal MA, Basilio D, Bukauskas FF, Bennett MV. Connexin-based gap junction hemichannels: gating mechanisms. Biochim Biophys Acta 1711: 215–224, 2005. doi: 10.1016/j.bbamem.2005.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sagara J, Makino N, Bannai S. Glutathione efflux from cultured astrocytes. J Neurochem 66: 1876–1881, 1996. doi: 10.1046/j.1471-4159.1996.66051876.x. [DOI] [PubMed] [Google Scholar]
- 48.Savidge TC, Newman P, Pothoulakis C, Ruhl A, Neunlist M, Bourreille A, Hurst R, Sofroniew MV. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology 132: 1344–1358, 2007. doi: 10.1053/j.gastro.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 50.Storr MA, Keenan CM, Zhang H, Patel KD, Makriyannis A, Sharkey KA. Activation of the cannabinoid 2 receptor (CB2) protects against experimental colitis. Inflamm Bowel Dis 15: 1678–1685, 2009. doi: 10.1002/ibd.20960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tian L, Shi MM, Forman HJ. Increased transcription of the regulatory subunit of gamma-glutamylcysteine synthetase in rat lung epithelial L2 cells exposed to oxidative stress or glutathione depletion. Arch Biochem Biophys 342: 126–133, 1997. doi: 10.1006/abbi.1997.9997. [DOI] [PubMed] [Google Scholar]
- 52.Wang XF, Cynader MS. Astrocytes provide cysteine to neurons by releasing glutathione. J Neurochem 74: 1434–1442, 2000. doi: 10.1046/j.1471-4159.2000.0741434.x. [DOI] [PubMed] [Google Scholar]
- 53.Watanabe T, Sagisaka H, Arakawa S, Shibaya Y, Watanabe M, Igarashi I, Tanaka K, Totsuka S, Takasaki W, Manabe S. A novel model of continuous depletion of glutathione in mice treated with l-buthionine (S,R)-sulfoximine. J Toxicol Sci 28: 455–469, 2003. doi: 10.2131/jts.28.455. [DOI] [PubMed] [Google Scholar]
- 54.Wehner S, Engel DR. Resident macrophages in the healthy and inflamed intestinal muscularis externa. Pflügers Arch 469: 541–552, 2017. doi: 10.1007/s00424-017-1948-4. [DOI] [PubMed] [Google Scholar]
- 55.Yamane Y, Furuichi M, Song R, Van NT, Mulcahy RT, Ishikawa T, Kuo MT. Expression of multidrug resistance protein/GS-X pump and gamma-glutamylcysteine synthetase genes is regulated by oxidative stress. J Biol Chem 273: 31075–31085, 1998. doi: 10.1074/jbc.273.47.31075. [DOI] [PubMed] [Google Scholar]