Abstract
Previously, we showed that receptor for activated C kinase 1 (Rack1) regulates growth of colon cells in vitro, partly by suppressing Src kinase activity at key cell cycle checkpoints, in apoptotic and cell survival pathways and at cell-cell adhesions. Here, we generated mouse models of Rack1 deficiency to assess Rack1’s function in intestinal epithelia in vivo. Intestinal Rack1 deficiency resulted in proliferation of crypt cells, diminished differentiation of crypt cells into enterocyte, goblet, and enteroendocrine cell lineages, and expansion of Paneth cell populations. Following radiation injury, the morphology of Rack1-deleted small bowel was strikingly abnormal with development of large polypoid structures that contained many partly formed villi, numerous back-to-back elongated and regenerating crypts, and high-grade dysplasia in surface epithelia. These abnormalities were not observed in Rack1-expressing areas of intestine or in control mice. Following irradiation, apoptosis of enterocytes was strikingly reduced in Rack1-deleted epithelia. These novel findings reveal key functions for Rack1 in regulating growth of intestinal epithelia: suppressing crypt cell proliferation and regeneration, promoting differentiation and apoptosis, and repressing development of neoplasia.
NEW & NOTEWORTHY Our findings reveal novel functions for receptor for activated C kinase 1 (Rack1) in regulating growth of intestinal epithelia: suppressing crypt cell proliferation and regeneration, promoting differentiation and apoptosis, and repressing development of neoplasia.
Keywords: receptor for activated C kinase 1, apoptosis, cell proliferation, gene knockout, intestinal epithelium
INTRODUCTION
Receptor for activated C kinase 1 (Rack1) is an evolutionarily conserved protein that is expressed in many cell types and tissues. In vitro studies show that Rack1 is involved in diverse processes including protein translation, cell growth, cell cycle progression, cytokinesis, apoptosis, cell survival, cell adhesion, migration, and stress responses [reviewed by Adams et al. (1) and Li and Xie (24)].
Although in yeast the Rack1 ortholog is dispensable for growth in enriched media (13, 38), Rack1 deletion is lethal to Caenorhabditis elegans (39) and Drosophila melanogaster (20). Rack1 is required for notochord cell polarization, oriented cell division, and convergent extension during gastrulation and for neurulation in zebrafish (43). These data suggest that Rack1 plays an important role in the development of eukaryotes. Little is known about the in vivo function of Rack1 in higher animals.
We discovered a novel function of Rack1 in regulating growth of human colon cells in vitro (31–33). We showed (by overexpressing Rack1, depleting Src or Rack1, and utilizing cell-permeable peptides that perturb Rack1’s interaction with Src) that Rack1 regulates growth of colon cells partly by suppressing Src activity at gap 1 phase (G1) and mitotic checkpoints and consequently delaying cell cycle progression. Activated Src rescues Rack1-inhibited growth of HT-29 cells (31). Conversely, inhibiting Src activity abolishes growth promoted by Rack1 depletion in normal colon cells (31). We identified two potential mechanisms whereby Rack1 regulates mitotic exit: suppression of Src phosphorylation of Src-associated in mitosis 68-kDa protein (Sam68) and maintenance of the cyclin-dependent kinase 1 (Cdk1)-cyclin B complex in an active state (31). Our results revealed novel mechanisms of cell cycle control in G1 and mitosis of colon cells.
We also discovered a novel proapoptotic function of Rack1 in colon cells in vitro: suppressing Src activity in the intrinsic apoptotic and in the protein kinase B (Akt) cell survival pathways (30). We showed that Rack1 is required for staurosporin-induced mitochondrial cell death, the activation and translocation of Bcl2-associated X protein (Bax) and Bcl2-interacting mediator of cell death (Bim) to mitochondria, the oligomerization of Bax, and caspase activation.
We identified another novel in vitro function of Rack1 in maintaining junctional homeostasis of intestinal epithelia by regulating Src- and hepatocyte growth factor-induced endocytosis of E-cadherin (41). We found that Rack1 promotes cell-cell adhesion and reduces invasive properties of colon cancer cells by regulating E-cadherin tyrosine phosphorylation and endocytosis and by diverting E-cadherin from a degradative to a recycling pathway. Collectively, our in vitro studies demonstrate that Rack1 regulates intestinal cell growth partly by suppressing Src activity at G1 and mitotic checkpoints, inducing apoptosis, and promoting epithelial cell-cell adhesions.
However, how Rack1 functions in vivo in intestinal epithelia of higher animals is an unanswered and important question with broad and profound implications to the regulation of cell growth and death during health and disease. For example, if Rack1 regulates growth by dual mechanisms (that of inhibiting proliferation and that of inducing apoptosis), exploitation of these dual functions could lead to new and more powerful and selective strategies for treating human colon cancer.
We hypothesized that Rack1 regulates growth of intestinal epithelial cells in vivo, as it does in vitro. Utilizing mouse models of Rack1 deficiency that we generated, we show, for the first time, that Rack1 regulates growth of intestinal epithelia in vivo as crypt cells proliferate, regenerate, differentiate, and undergo apoptosis.
MATERIALS AND METHODS
Mice.
Mice were bred and maintained at the Stanford Veterinary Service Center. The animal protocol and procedures for the studies were approved by the Stanford institutional animal care and use committee, known as the Administrative Panel on Laboratory Animal Care.
Construction of the floxed-Rack1 targeting vector.
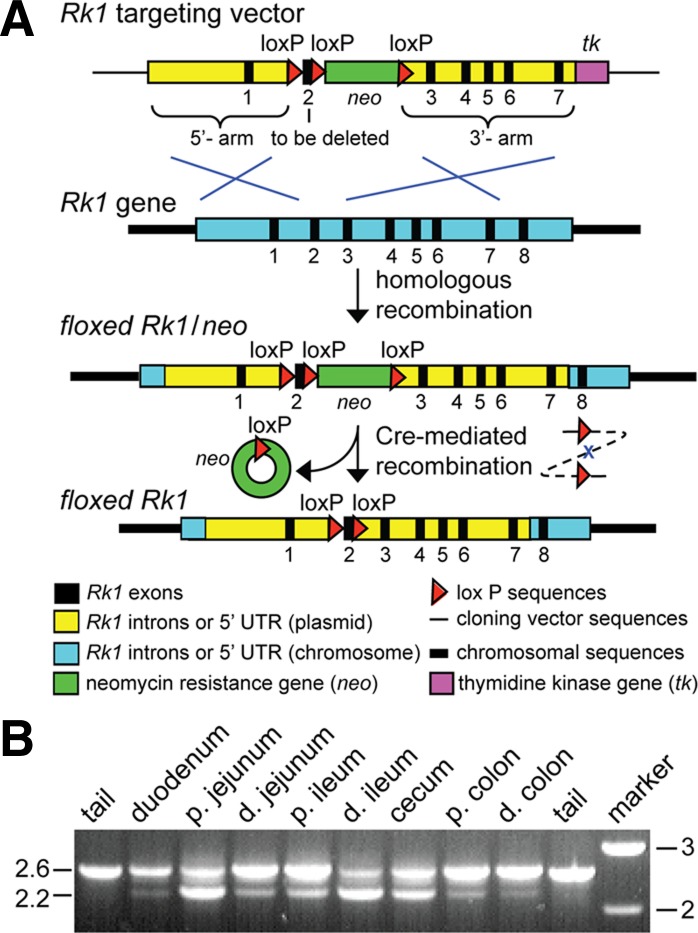
To generate the floxed-Rack1 targeting vector (17, 34; Fig. 1A, 1st panel), the following steps were taken: 1) the 3′-arm (Rack1 sequence extending from intron 2 to 7; 4.29 kb) was amplified by polymerase chain reaction (PCR) from 129/Sv mouse genomic DNA (Jackson Laboratory) using primers Gnb2l1-3f4 (5′-AAT CAT TGA TCA GTC TCC CCT TGG TAA TTG GTC CTT TTG-3′) and Gnb2l1-3r3 (5′-TTT TTT CCG CGG CTG TTA CTG CTT ACA TGC CAG GAT AG-3′), digested with BclI and SacII, and inserted at the BamHI-SacII site of the cloning vector PL452, which contained a neomycin resistance gene (neo) flanked by loxP sequences (26); 2) the 5′-arm (Rack1 5′-untranslated region (UTR), exon 1 and most of intron 1 followed by a loxP sequence; 2.15 kb) was amplified using primers Gnb2l1-5f3 (5′-AA AGT CGA CCA AAC ACA CAT GGC AGG AGA GAA-3′) and Gnb2l1-5loxP-r-ApaLI (5′-AAA AAA GTG CAC ATA ACT TCG TAT AGC ATA CAT TAT ACG AAG TTA TCA TTT ACA GTC TTA AAG TCA CTG GGG-3′); 3) a Rack1 fragment containing exon 2 (0.27 kb) was amplified using primers Gnb2l1-5loxP-f-ApaLI (5′-TAA ATG GTG CAC AGT AAG TAG GCT TAT AC-3′) and Gnb2l1-5r4 (5′-ACG AAT TCT ACC TCA GTT CTG CCC ACT TTC C-3′); 4) the 5′-arm (digested by SalI and ApaLI) and the exon 2 fragment (digested by ApaLI and EcoRI) were ligated and inserted into the PL452 vector (containing the 3′-arm) at the SalI and EcoRI site (upstream of the loxP-flanked neo gene); and 5) a DNA fragment (2.00 kb) carrying the thymidine kinase (tk) gene from herpes simplex virus for negative selection (17, 34) was excised from plasmid PL253 (26) with BamHI (blunted) and SfoI and inserted into the SalI (blunted) site downstream of the 3′-arm to generate the targeting vector. The loxP sites were selected to avoid disruption or deletion of the small nucleolar RNAs present in the Rack1 introns.
Fig. 1.
Generation of a mouse line with receptor for activated C kinase 1 (Rack1) deleted in intestinal epithelia. A: generation of floxed-Rack1 mice. First panel: Rack1 (Rk1) targeting vector. PL452 vector containing mouse genomic Rack1 fragments (amplified from mouse 129/Sv genomic DNA) with a loxP sequence inserted into intron 1 and a neo gene (flanked by loxP) inserted into intron 2. 5′-Arm (2.15 kb): Rack1 5′-untranslated region (UTR), exon 1, most of intron 1, and a loxP sequence added to the 3′-end. 3′-Arm (4.29 kb): Rack1 sequence extending from intron 2 to 7. Second panel: the chromosomal Rack1 gene. Third panel: the floxed-Rack1 allele containing the neo gene. Following electroporation of the targeting vector into embryonic stem (ES) cells, ES clones were selected by neo+ (positive selection) and tk− (negative selection), and homologous recombinants were identified by PCR and Southern blot analyses. Fourth panel: the floxed-Rack1 allele. To generate a chromosomal copy of floxed-Rack1 in the ES cell line, neo was deleted by transient expression of Cre recombinase. B: Rack1 deletion in the intestine of a mouse carrying vil-Cre and floxed-Rack1 (vil-Cre:Rack1+/fl). Intestinal DNA from a vil-Cre:Rack1+/fl mouse was examined for Rack1 deletion by PCR analyses. Tail DNA analysis was included as a control. The 2.6-kb fragment corresponds to the floxed-Rack1 allele (exon 2 present), and the 2.2-kb fragment corresponds to the mutant Rack1 allele (exon 2 deleted). p., proximal; d., distal.
Generation of floxed-Rack1 mice.
The floxed-Rack1 targeting vector (Fig. 1A, 1st panel) was linearized and introduced by electroporation into R1 embryonic stem (ES) cells derived from 129/SvJ and 129/Sv mice (23) by the Stanford Transgenic Facility. ES clones that were neo+ and tk- were selected (Fig. 1A, 3rd panel; 19). Of 130 clones obtained, 13 true homologous recombinants were identified by PCR and Southern blot analyses. Two clones were sequenced, and both contained the correct floxed-Rack1 sequence. The two clones (G6 and E1) were expanded and transiently transfected with a Cre recombinase (Cre)-expressing plasmid pOG231-Cre (23) to delete the neo gene (Fig. 1A, 4th panel). One ES clone (E1F3) out of 554 clones screened by PCR analysis (from 4 transfections) carried the desired floxed-Rack1 sequence. Sequencing of the entire targeting region of the E1F3 clone confirmed that the correct recombination had occurred and no mutations other than the genetically engineered loxP sequences had been introduced. The clone was then expanded and microinjected into C57BL/6 host blastocysts, which were transferred into pseudopregnant female recipients (23) to generate the floxed-Rack1 mice.
Chimeric mice resulting from ES cells injected into host embryos are mosaics of the heterozygous mutant cells and the host embryo cells, with the ES clone contributing brown (agouti) hair and the host contributing black. Ten chimeric mice were obtained: 7 were male (6 were 95% agouti and 1 was 40%). Male chimeric mice were backcrossed to C57BL6/J mice (Jackson Laboratory), and brown offspring were genotyped for presence of the floxed-Rack1 allele (founder mice).
Generation of mice with Rack1 deleted in intestinal epithelia.
To target Rack1 deletion to intestinal epithelia, floxed-Rack1 mice were bred to those expressing Cre under the control of the villin promoter (vil-Cre; Jackson Laboratory; 28) to generate vil-Cre:Rack1fl/fl mice. Offspring were examined by PCR analyses of tail DNA for the presence of vil-Cre and floxed-Rack1. PCR analysis of intestinal DNA from a vil-Cre:Rack1+/fl mouse was performed to confirm the presence of the mutant Rack1 allele (exon 2 deleted) in the intestine. The primers used for the PCR analysis are 5′-GCT GAA CAA ATC GGC TGT AGC CAA ACA C-3′ and 5′-CCT CGA GGG ACC TAA TAA CTT CGT ATA GCA TAC ATT-3′.
To assess Cre activity in vil-Cre:Rack1fl/fl mice, vil-Cre:Rack1+/fl mice were crossed with Rosa26-tdTomato mice (Jackson Laboratory; 27) to generate vil-Cre:tdTomato:Rack1fl/fl mice.
Generation of mice with Rack1 deleted ubiquitously.
Floxed-Rack1 mice were bred to those expressing Cre under the control of the human cytomegalovirus minimal promoter (CMV-Cre; Jackson Laboratory; 37) in an attempt to generate CMV-Cre:Rack1fl/fl mice or to those expressing a tamoxifen-dependent Cre under control of the CAG promoter (CAG-Cre-ERTM mice; Jackson Laboratory; 18) to generate CAG-Cre-ERTM:Rack1fl/fl mice. For mice carrying CAG-Cre-ERTM, Cre activation was induced upon tamoxifen treatment (dose, 0.2 g/kg via oral gavage every day for 5 days; 2).
Whole body X-ray radiation.
To assess the effect of Rack1 deletion on regeneration of crypt cells, mice were irradiated using a Polaris SC-500 Series II X-ray generator with a dose of 8 Gy at a dose rate of 1.1 Gy/min (225 kV, 13.3 mA, with a 0.5-mm-thick copper filter). Irradiated mice were euthanized 72 or 96 h after irradiation. Necropsies were performed by board-certified veterinarians in the Stanford Veterinary Service Center.
Tissue harvesting and immunofluorescent and histopathologic analyses.
Mice were euthanized in a standard chamber with carbon dioxide administered through a controlled gas cylinder. Intestines were removed en bloc, flushed intraluminally once with cold PBS unless otherwise indicated, coiled into Swiss rolls, and fixed in phosphate-buffered formalin (10%) for 24 h. Fixed tissues were embedded in paraffin, cut into 4-μm sections, and stained for hematoxylin and eosin by the Stanford Comparative Medicine Animal Histology Service Center. Antigen retrieval was performed by soaking deparaffinized and rehydrated sections in subboiling 10 mM Tris base-1 mM EDTA buffer (pH 9) for 10 min. Immunofluorescent (IF) and immunohistochemical staining was performed using standard techniques. Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) analyses were performed per the manufacturer’s instructions. Light and fluorescent images were captured using a Nikon Eclipse E600 microscope.
For frozen sections, intestinal segments were fixed in 4% paraformaldehyde for 1 h at room temperature, soaked in PBS containing 30% sucrose at 4°C overnight, frozen in optimal cutting temperature compound (OCT), and cut into 10-μm sections. After antigen retrieval using subboiling Tris-EDTA buffer (pH 9) for 5 min, tissue sections were subjected to IF staining.
Reagents.
Primary antibodies were as follows: mouse monoclonal antibody (mAb) Rack1 (B-3) and rabbit mucin-2 (H-300; Santa Cruz Biotechnology), mouse mAb PCNA (BD Transduction Laboratories), mouse mAb bromodeoxyuridine (BrdU, BU-33; Sigma-Aldrich), rat mAb cluster of differentiation 44 (CD44; BD Transduction Laboratories), rabbit mAb proliferation marker protein Ki-67 (Ki67, SP6; GeneTex), rabbit transcription factor SOX-9 (Sox9, AB5535; Millipore), cleaved caspase-3 (Asp175; Cell Signaling), rabbit mAb Rack1 (EPR7388) and synaptophysin (EP1098Y; Abcam), rabbit lysozyme (180039; Invitrogen), and rabbit sucrase isomaltase (a gift from Gary Gray and Eric Sibley). Secondary antibodies were as follows: Alexa Fluor 488 goat anti-mouse IgG, Alexa Fluor 488 goat anti-rabbit IgG, Alexa Fluor 568 goat anti-rabbit IgG, Alexa Fluor 594 goat anti-rat IgG, and Alexa Fluor 594 goat anti-mouse IgG (Invitrogen Molecular Probes). Other reagents were as follows: Hoechst 33342 and Click-iT Plus TUNEL Assay for in situ Apoptosis Detection Alexa Fluor 594 dye (Invitrogen Molecular Probes); Vectastain ABC HRP kit, hematoxylin, and VectorShield mounting medium (Vector Laboratories); and tamoxifen base (Sigma).
RESULTS
Rack1 deficiency promotes proliferation of intestinal crypt cells.
Previously, we showed that Rack1 regulates growth of colon cells in vitro, partly by inhibiting Src activity at key cell cycle checkpoints, in apoptotic and cell survival pathways, and at cell-cell adhesions (30, 31, 33, 41). We hypothesized that Rack1 also regulates growth of intestinal epithelia in vivo. To test this, we utilized a Cre-loxP recombination system to generate mouse models in which Rack1 is deleted in intestinal epithelia. To do so, we generated Rack1 transgenic mice with two loxP sequences inserted in the genome flanking exon 2 of the Rack1 gene (floxed-Rack1; Fig. 1A). Necropsies of mice homozygous or heterozygous for floxed-Rack1 (Rack1fl/fl or Rack1+/fl mice, respectively) showed that their phenotype was not different from that of wild-type mice (data not shown). Histopathology of the small intestine (Figs. 3A, 7A, and 8A) and colon (Figs. 2B and 3B) was not different from that of wild-type mice.
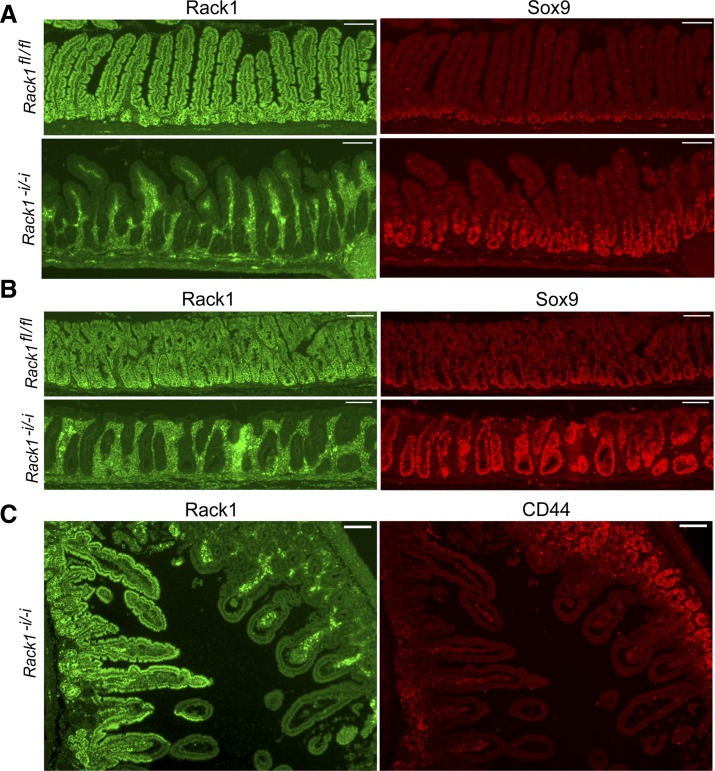
Fig. 3.
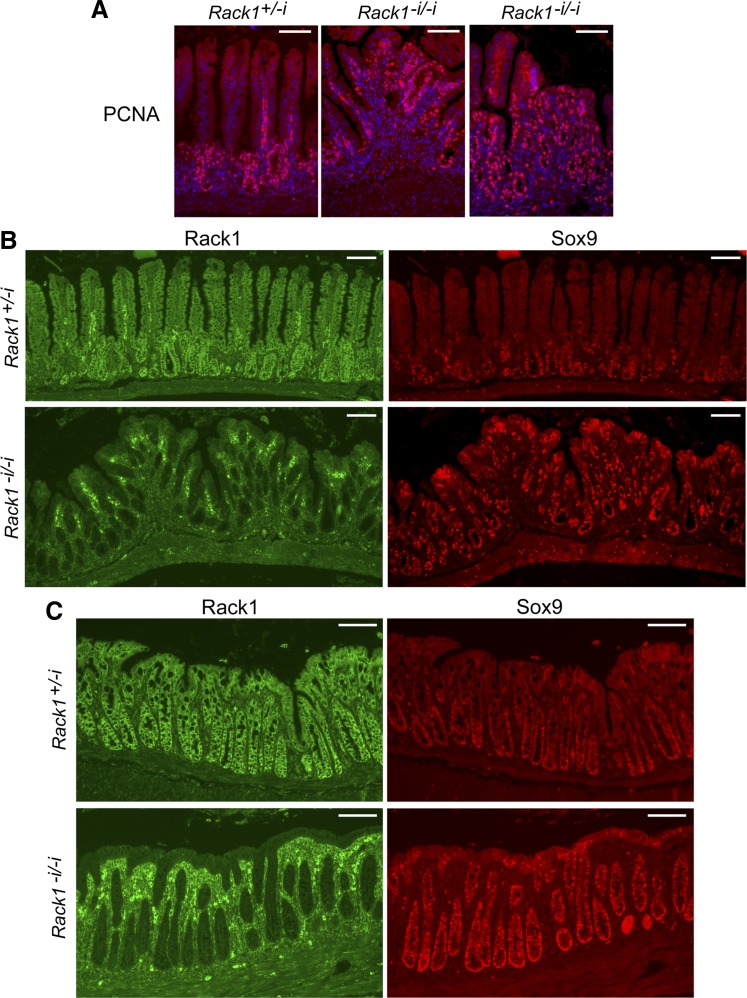
Rack1 deficiency induces proliferation of a population of intestinal crypt cells enriched for stem and progenitor cells. A and B: immunofluorescent costaining for Rack1 (green, panels at left) and Sox9 (red, panels at right) in the small intestine (A) or colon (B) of a Rack1−i/−i mouse (panels at bottom) and a Rack1fl/fl littermate (panels at top). C: costaining for Rack1 (panel at left) and CD44 (panel at right) in the small intestine of a Rack1−i/−i mouse. Scale bar: 100 μm. Data are representative of up to 50 fields examined for each of 3–8 mice.
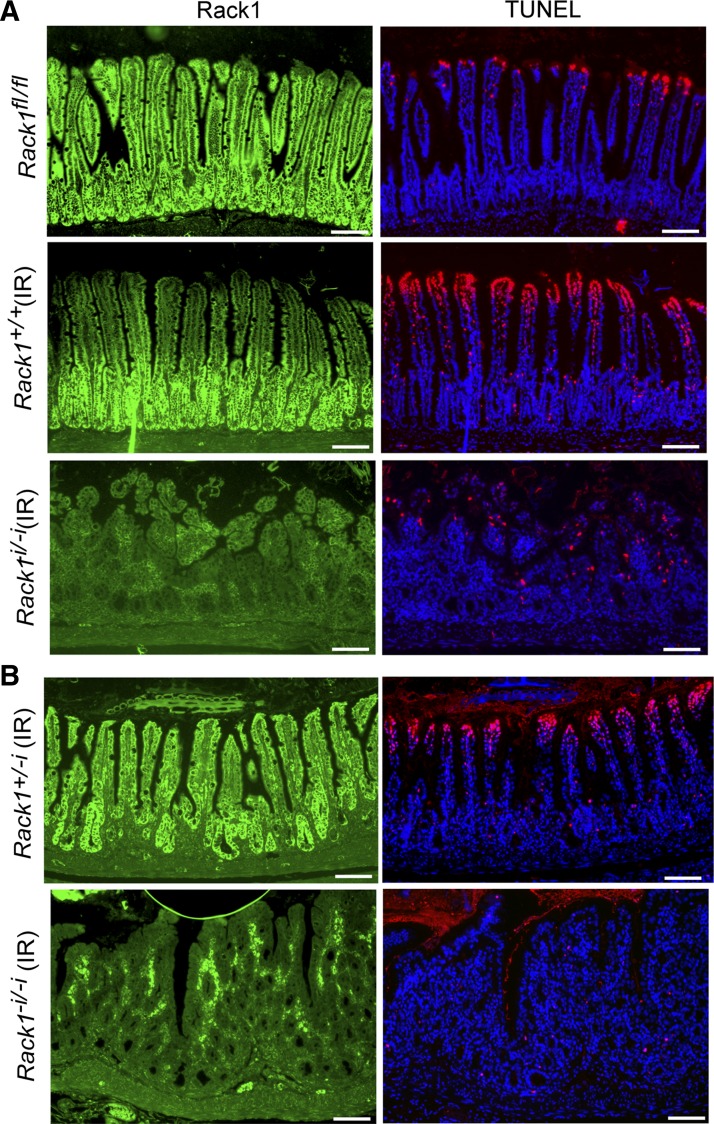
Fig. 7.
Rack1 deficiency reduces apoptotic activity as measured by DNA fragmentation following radiation injury. Mice received whole body X-ray radiation, as indicated (IR), and were euthanized 72 h later. Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assays for DNA fragmentation were performed as described in materials and methods. Fluorescent staining for Rack1 (green, panels at left) and DNA fragments (red, panels at right) from a litter containing a nonirradiated Rack1fl/fl control (A, panels at top), an irradiated Rack1+/+ control (A, panels at middle), and an irradiated Rack1−i/−i mouse (A, panels at bottom) or from another litter containing an irradiated Rack1+/−i control (B, panels at top) and an irradiated Rack1−i/−i mouse (B, panels at bottom). Scale bar: 100 μm. Data are representative of up to 50 fields examined from 4 mice.
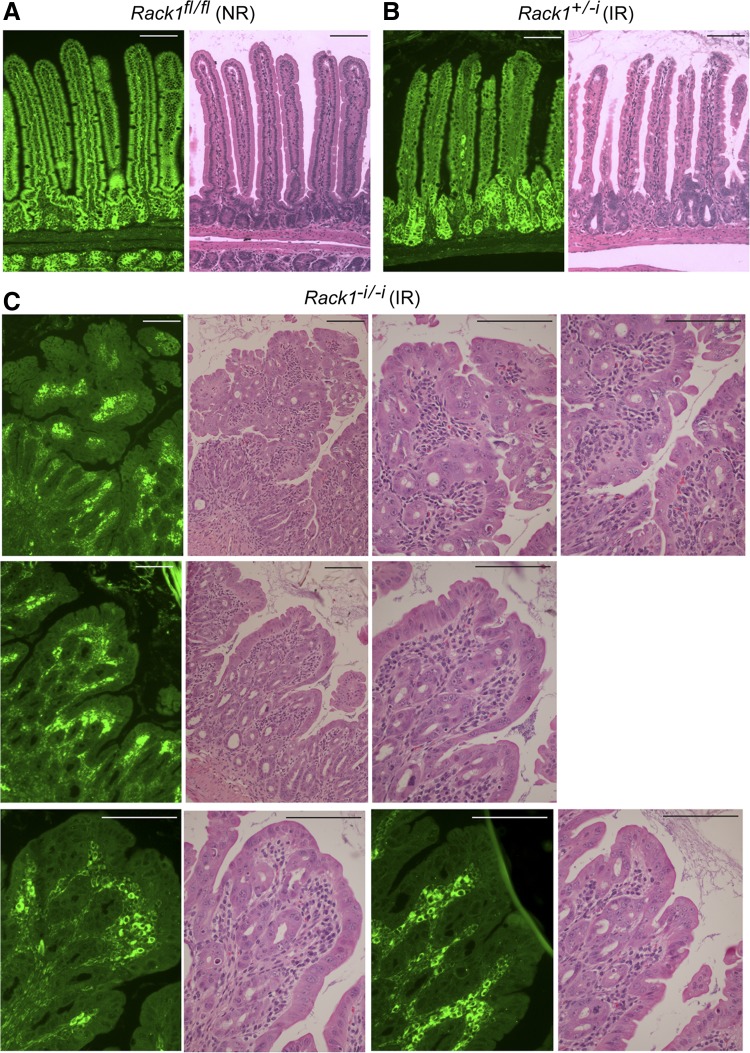
Fig. 8.
Rack1-deleted epithelia of irradiated (IR) Rack1−i/−i mice contain areas of high-grade dysplasia. Mice received whole body X-ray radiation, as indicated. Immunofluorescent staining for Rack1 (green, panels at left) or hematoxylin and eosin analysis of small intestinal sections from a nonirradiated (NR) Rack1fl/fl mouse (A) or an irradiated (IR) Rack1−i/−i mouse (C) and its irradiated Rack1+/−i littermate control (B). Scale bar: 100 μm. Data are representative of up to 50 fields examined from 5 mice.
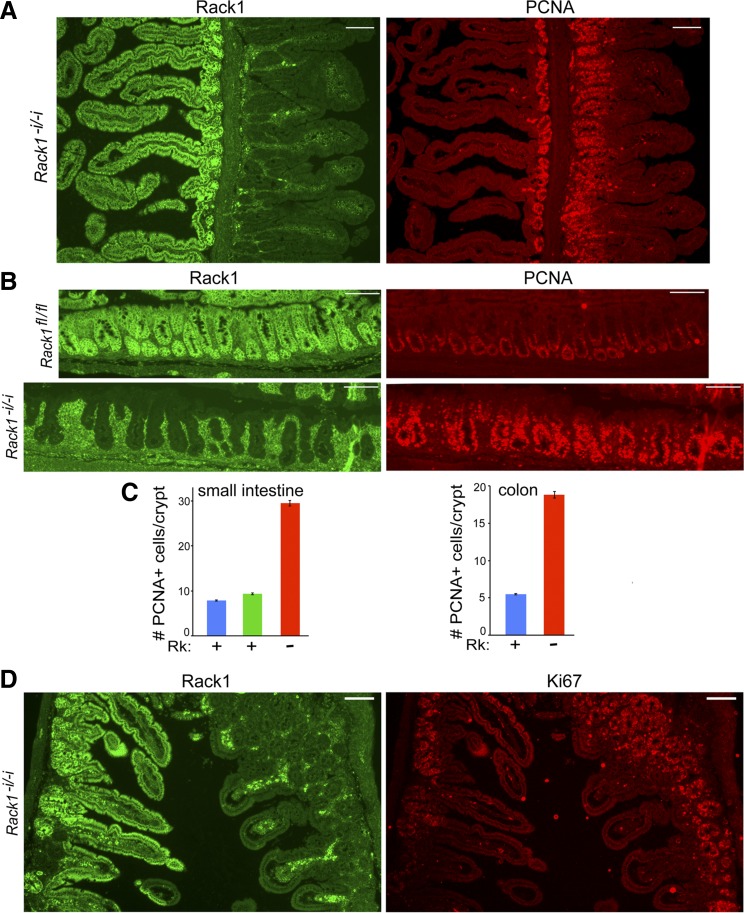
Fig. 2.
Rack1 deficiency results in proliferation of intestinal crypt cells. Rack1−i/−i or Rack1fl/fl littermate control mice were euthanized, and intestines were formalin fixed, paraffin embedded, and sectioned, as described in materials and methods. A and D: immunofluorescent costaining for Rack1 (green, panels at left) and PCNA (A) or proliferation marker protein Ki-67 (Ki67; D) (red, panels at right) in the small intestine of Rack1−i/−i mice. Enhanced staining of Rack1 in lamina propria near Rack1-deleted epithelia was observed (panels at left). B: costaining for Rack1 (panels at left) and PCNA (panels at right) in the colon of a Rack1−i/−i mouse (panels at bottom) and a Rack1fl/fl littermate control (panels at top). Data are representative of up to 50 fields examined for each of 8 mice. Images were captured using a Nikon Eclipse E600 microscope at ×100 magnification. Scale bar: 100 μm. C: quantitative analysis of the number of PCNA-positive cells per crypt in Rack1-expressing (Rk+) and Rack1-depleted (Rk−) crypts in the small intestine (graph at left) and colon (graph at right). Data shown represent means ± SE; n, number of crypts counted. Small intestine: Rk− crypts (red bar, n = 77) and Rk+ crypts (green bar, n = 70) from a Rack1−i/−i mouse and Rk+ crypts from a Rack1fl/fl littermate control (blue bar, n = 103). Colon: Rk− crypts from a Rack1−i/−i mouse (red bar, n = 56) and Rk+ crypts from a Rack1fl/fl littermate control (blue bar, n = 85).
Floxed-Rack1 mice were crossed with those expressing Cre under the control of the villin promoter to generate mice with deletion of Rack1 in intestinal epithelia (vil-Cre:Rack1fl/fl or Rack1−i/−i) or crossed with CAG-Cre-ER mice to generate mice with deletion of Rack1 upon tamoxifen treatment (CAG-Cre-ERTM:Rack1fl/fl or inducible Rack1−/− mice). For the vil-Cre:Rack1fl/fl or vil-Cre:Rack1+/fl mice, PCR analyses of genomic DNA showed that the mutant Rack1 allele (exon 2 deleted) was detected only in the intestine and not in the tail (Fig. 1B) or other organs (data not shown), confirming that the Rack1 deletion is Cre dependent.
Rack1−i/−i mice were viable and fertile and had no overt phenotype other than that their body weights were lower than those of control littermates (data not shown). IF analyses of intestine from Rack1−i/−i mice revealed a mosaic pattern of Rack1 deletion in epithelia of the small intestine (Figs. 2, A and D, 3C, and 4, A–D, panels at left, as indicated) and colon (Fig. 4E, panel at left).
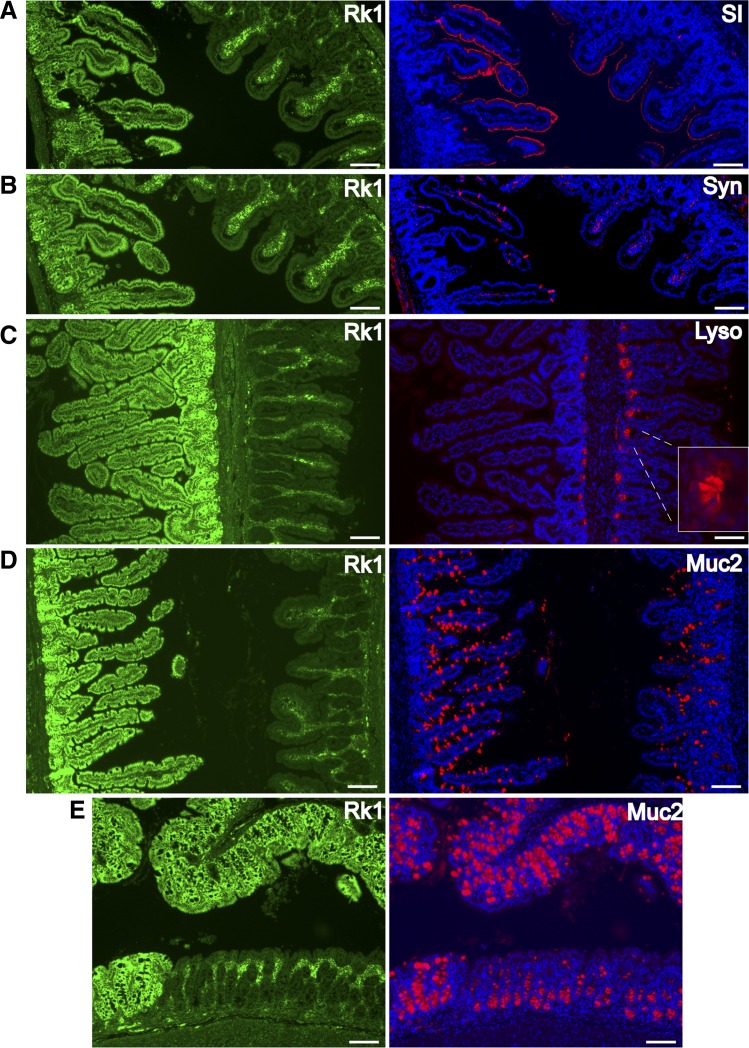
Fig. 4.
Rack1 deficiency decreases differentiation of crypt cells into enterocyte, goblet, and enteroendocrine cell lineages and promotes differentiation into Paneth cells. Immunofluorescent costaining for Rack1 (Rk1; green, panels at left) and the differentiation markers (red, panels at right) sucrase isomaltase (SI; A), synaptophysin (Syn; B), lysozyme (Lyso; C), or mucin-2 (Muc2; D and E) in the small intestine (A–D) or colon (E) of Rack1−i/−i mice. DNA is labeled by Hoechst 33342 fluorescent staining (blue). Scale bar: 100 μm. Data are representative of up to 50 fields examined from 3 or more mice.
On the basis of our in vitro results, we hypothesized that Rack1 deficiency would promote crypt cell proliferation. To test this, IF analyses were performed using PCNA and Ki67 as markers for cell proliferation (14, 21, 22). We observed that Rack1-depleted crypts in the small intestines or colons of Rack1−i/−i mice contained many more proliferating cells than did Rack1-expressing crypts, as detected by PCNA (Fig. 2, A and B, panels at right, and Fig. 2C quantitation) or Ki67 (Fig. 2D, panel at right) staining. Quantitative analysis revealed that Rack1-depleted crypts in the small intestine or colon of Rack1−i/−i mice contained over 3 times more PCNA-positive cells than did Rack1-expressing crypts in the same mice or in littermate controls (Fig. 2C).
Because of the striking increase in proliferation of crypt cells, we then stained for Sox9 and CD44, which mark populations of crypt cells that encompass stem and progenitor cells (6, 11, 12, 15, 16, 44). We observed that Rack1-depleted crypts in the small intestine or colon of Rack1−i/−i mice contained many more Sox9-positive cells than did Rack1-expressing crypts of control mice (Fig. 3, A and B, compare panels at bottom right with panels at top right). Similarly, Rack1-depleted crypts in the small intestine of Rack1−i/−i mice contained many more CD44-positive cells than did Rack1-expressing crypts from the same mouse (Fig. 3C, panel at right). Collectively, our results from Ki67, PCNA, CD44, and Sox9 staining indicate that Rack1 deficiency promotes crypt cell proliferation.
Morphologic changes in Rack1-depleted epithelia included striking elongation of crypts in both the small and large intestine and the development of broader villi in the small intestine (Figs. 2–4).
Rack1 deficiency decreases differentiation of crypt cells into enterocyte, goblet, and enteroendocrine cell lineages and promotes differentiation into Paneth cells.
Previously, we showed that Src activity decreases as crypt cells differentiate and migrate along the crypt-villus axis of chicken duodenum (7). We hypothesized that Rack1, perhaps via its inhibitory influence on Src activity, induces differentiation of crypt cells into enterocytes, goblet, and enteroendocrine cell lineages. To test this, we assessed the effect of Rack1 loss on cell differentiation using markers specific for each cell lineage (5). We observed that Rack1 deficiency reduces differentiation into the following: enterocytes, as identified by sucrase isomaltase expression (SI; Fig. 4A), enteroendocrine cells, as identified by synaptophysin expression (Syn; Fig. 4B), and goblet cells, as identified by mucin-2 expression (Muc2; Fig. 4, D and E). Quantitative analyses revealed that the number of Syn-positive cells per villus was 0.12 ± 0.04 (mean ± SE) in Rack1-deleted epithelia of a Rack1−i/−i mouse (n = 84 villi counted), 6.03 ± 0.19 in Rack1-positive epithelia from the same Rack1−i/−i mouse (n = 112), and 7.15 ± 0.16 in Rack1-positive epithelia of a littermate control (not shown; n = 102). The number of Muc2-positive cells per colonic crypt was 6.25 ± 0.18 in Rack1-deleted epithelia of a Rack1−i/−i mouse (n = 73 crypts counted) and 36.2 ± 0.87 in Rack1-positive epithelia of a littermate control (not shown; n = 53).
On the basis of our finding that Rack1 loss induced a striking expansion of CD44- and Sox9-positive crypt cells (Fig. 3), which encompass stem and progenitor cells, we hypothesized that Rack1 loss would induce differentiation into Paneth cells, which support the stem cell niche. We observed a striking increase in Paneth cells, as identified by lysozyme expression, in Rack1-depleted crypts (Fig. 4C). Together, these results suggest that Rack1 promotes differentiation of crypt cells into enterocyte, goblet, and enteroendocrine cell lineages and inhibits differentiation into Paneth cells.
Rack1 loss promotes morphologic abnormalities and crypt cell regeneration following radiation injury.
On the basis of our results showing that Rack1 regulates crypt cell proliferation (Figs. 2 and 3), we hypothesized that Rack1 also regulates regeneration of intestinal epithelia following radiation injury. To test this, we irradiated mice and assessed for Rack1’s influence on postradiation regeneration. Following whole body X-ray radiation, we observed that the morphology of Rack1-depleted small bowel was strikingly abnormal with development of large polypoid structures that contained many partly formed villi and numerous back-to-back elongated crypts (Fig. 5A, panels at center and right, and Figs. 5B, 6A, and 7, A and B, panels at bottom) that were not found in Rack1-expressing epithelia or in control mice (panels at top). The crypts contained actively proliferating cells as measured by PCNA (Fig. 5A, panels at center and right) or Sox9 (Fig. 5B, panel at bottom right) staining. The morphology of Rack1-depleted epithelia of the colon was also abnormal, and like that of the small intestine, the crypts were elongated (Fig. 5C, panels at bottom) and there was a striking increase in Sox9-positive cells within the crypts (Fig. 5C, panel at bottom right). Collectively, these results indicate that Rack1 regulates crypt cell regeneration following radiation injury.
Fig. 5.
Rack1 deficiency results in morphologic abnormalities and proliferation of crypts following radiation injury. Rack1−i/−i or Rack1+/−i littermate control mice received whole body X-ray radiation (8 Gy), as described in materials and methods, and were euthanized 72 h later. A: immunofluorescent staining for PCNA (red) in the small intestine of a Rack1−i/−i mouse (panels in center and at right) and a Rack1+/−i littermate control (panel at left). DNA is labeled by Hoechst 33342 fluorescent staining (blue). B and C: immunofluorescent costaining for Rack1 (green, panels at left) and Sox9 (red, panels at right) in the small intestine (B) and colon (C) of a Rack1−i/−i mouse (panels at bottom) and a Rack1+l-i littermate control (panels at top). Scale bar: 100 μm. Data are representative of up to 50 fields examined from 3 or more mice.
Fig. 6.
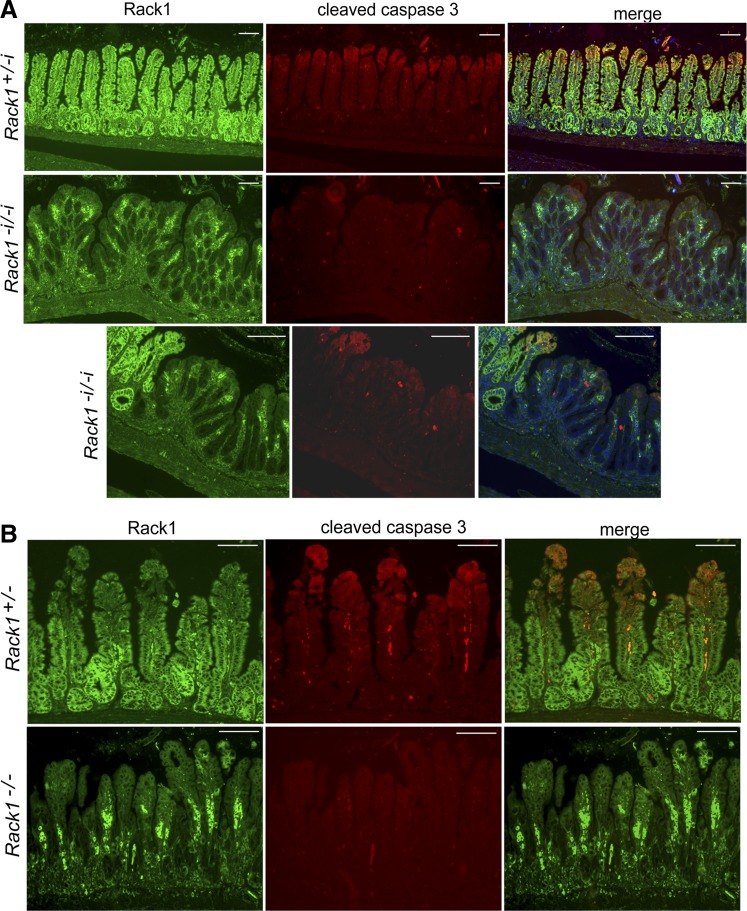
Rack1 deficiency reduces apoptotic activity as measured by cleavage of caspase-3 following radiation injury. A: Rack1+/−i and Rack1−i/−i mice received whole body X-ray radiation and were euthanized 72 h later. B: CAG-Cre-ER:Rack1+/fl and CAG-Cre-ER:Rack1fl/fl mice treated with tamoxifen (0.2 g/kg) for 5 days (Rack1+/− and Rack1−/− mice, respectively) were irradiated and euthanized 96 h later. Immunofluorescent costaining for Rack1 (green, panels at left) and cleaved caspase-3 (red, panels in center) was performed in small intestinal sections. Merged images are shown in the panels at right. Scale bar: 100 μm. Data are representative of up to 50 fields examined from 3 or more mice (A) or 2 mice (B).
Rack1 loss reduces apoptosis of intestinal epithelial cells.
Small intestinal epithelia renew every 3–5 days with differentiated cells at the villus tip removed by apoptosis. This rapid and well-characterized renewal process makes intestinal epithelium an excellent system to study how tissue homeostasis is achieved in vivo through the balance of cell birth and death. On the basis of our in vitro results showing that Rack1 induces apoptosis of intestinal cells partly by suppressing Src activity in the intrinsic and Akt pathways (30), we hypothesized that Rack1-depleted intestinal epithelia would be more resistant to apoptosis than would epithelia of control mice. To test this, spontaneous and radiation-induced apoptotic activity was compared in Rack1−i/−i and control mice using IF analyses to detect active, cleaved caspase-3. We observed only subtle differences in spontaneous apoptosis between Rack1−i/−i mice and control mice (data not shown). However, following whole body X-ray radiation, there was a striking decrease in apoptosis activity, as measured by the absence of active/cleaved caspase-3 in epithelia of Rack1−i/−i mice (Fig. 6A, rows 2 and 3, panels in center) but not in control mice (Fig. 6A, 1st row, panel in center). Moreover, clusters of caspase-3-negative cells piled up at villus tips of Rack1−i/−i mice, presumably because they could not undergo apoptosis. Similarly, in irradiated Rack1−/− mice that were inducibly depleted of Rack1 following tamoxifen treatment, apoptosis was partially arrested in epithelial cells (Fig. 6B, 2nd row, panel in center).
When DNA fragmentation was analyzed by TUNEL analysis in a complementary approach for assessing apoptosis, we again observed a striking decrease in apoptotic activity in Rack1-deleted epithelia (Fig. 7A, compare panel at bottom right with panels at middle right and top right, and Fig. 7B, compare panel at bottom right with panel at top right). We did detect some apoptosis in the crypts of control mice (e.g., Figure 7A, panel at middle right), although it was not nearly as striking as that observed in villus tips. Together, our results from two different mouse models of Rack1 deficiency, and utilizing two different assays for apoptosis, demonstrate that Rack1 promotes apoptosis of intestinal epithelial cells in vivo, as it does in vitro.
Rack1 deficiency promotes development of intestinal neoplasia.
On the basis of our results showing that Rack1 loss results in marked proliferation of crypt cells (Figs. 2, 3, and 5) and a decrease in apoptotic activity (Figs. 6 and 7), we hypothesized that this might lead to development of intestinal neoplasia. We observed that Rack1-depleted surface epithelia of irradiated Rack1−i/−i mice contained areas of high-grade dysplasia (Fig. 8C) that were not present in irradiated control mice (Fig. 8B) or in nonradiated Rack1fl/fl mice (Fig. 8A), as read blindly and independently by three pathologists. Dysplastic features included nuclear enlargement, nuclear crowding, loss of nuclear polarity, vesicular nuclei, and prominent nucleoli. Other dysplastic features included marked proliferation and crowding of the crypts with areas of cribriform growth. In addition, there was increased mitotic activity, mucin depletion, and reduced numbers of apoptotic bodies (not shown). Rack1-depleted surface epithelia of nonradiated Rack1−i/−i mice contained rare areas of low-grade dysplasia (data now shown).
Together, our findings suggest that Rack1 suppresses development of intestinal neoplasia and that Rack1 deficiency promotes development of dysplasia in regenerating epithelia because of multiple consequences of Rack1 loss: proliferation of crypt cells, inhibition of differentiation, and partial arrest of apoptosis.
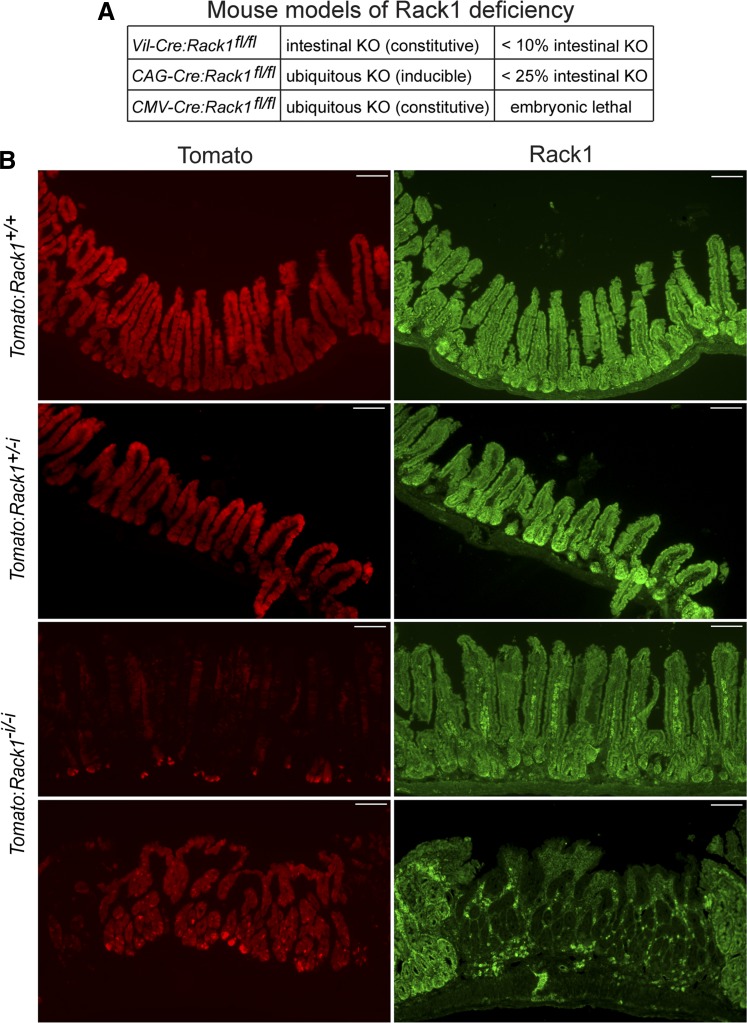
Other than slightly low body weight, we did not detect an overt phenotype in the constitutive Rack1−i/−i mice, presumably because Rack1 was deleted in only a small percentage (we estimate <10%) of the total surface area of their intestinal epithelia (Fig. 9A). The induced Rack1−/− mice also lost weight and had a very mosaic pattern of Rack1 loss in their intestines and other organs. We estimate that Rack1 was deleted in <25% of the total surface area of their intestinal epithelia (Fig. 9A).
Fig. 9.
A: estimates of the percentage of the total surface area of the intestinal epithelia with deletion of Rack1 in various mouse models of Rack1 deficiency. Resected small intestines and colons from Rack1−i/−i mice (vil-Cre:Rack1fl/fl) and induced Rack1−/− mice (CAG-Cre:Rack1fl/fl) were prepared and fixed as coiled Swiss rolls, as described in materials and methods. Sections containing coiled, full-length small intestine and colon were subjected to immunofluorescent staining for Rack1 and scanned by fluorescent microscopy. The percentage of the total surface area of the intestinal epithelia with deletion of Rack1 was estimated for each mouse individually and then collectively for Rack1−i/−i mice, or for induced Rack1−/− mice. The data shown represent the overall estimate based on analysis of 45 Rack1−i/−i mice or 13 Rack1−/− mice. Embryonic lethality was observed in a mouse model of ubiquitous Rack1 deletion (CMV-Cre:Rack1fl/fl). KO, knockout. B: tdTomato reporter expression is low in vil-Cre:tdTomato:Rack1fl/fl mice. A vil-Cre:tdTomato:Rack1fl/fl (Tomato:Rack1−i/−i) mouse and two littermate controls, vil-Cre:tdTomato:Rack1+/fl (Tomato:Rack1+/−i) and vil-Cre:tdTomato:Rack1+/+ (Tomato:Rack1+/+), were euthanized, and resected intestines were fixed and frozen, as described in materials and methods. Tissue sections were subjected to immunofluorescent staining for Rack1 (green, panels at right). The fluorescent tdTomato reporter (red) is shown in the panels at left. Images were captured at ×100 magnification. Scale bar: 100 μm.
To determine whether the highly mosaic pattern of Rack1 deletion in the Rack1−i/−i mice was due to the vil-Cre promoter, we introduced the Rosa26-tdTomato reporter gene (as an indicator of Cre activity) into the vil-Cre:Rack1fl/fl mice to generate vil-Cre:tdTomato:Rack1fl/fl (Tomato:Rack1−i/−i) mice. We observed that tdTomato was strongly expressed throughout the intestinal epithelia of control mice: vil-Cre:tdTomato:Rack1+/+ (Tomato:Rack1+/+) mice and vil-Cre:tdTomato:Rack1+/fl (Tomato:Rack1+/−i) mice (Fig. 9B, rows 1 and 2, respectively, panels at left). Thus the vil-Cre promoter was functioning as expected in the control mice by driving expression of active Cre. However, in Tomato:Rack1−i/−i mice, expression of tdTomato was strikingly suppressed in most of the intestinal epithelia (Fig. 9B, 3rd row, panel at left). Rack1 was expressed in those areas (Fig. 9B, 3rd row, panel at right), presumably because Cre activity was low. It was only in rare areas that tdTomato was highly expressed (Fig. 9B, 4th row, panel at left), and in those areas Rack1 was deleted and the morphology was strikingly abnormal with development of numerous elongated crypts and blunted abnormal villi (Fig. 9B, 4th row, panel at right). This observation suggests that in Rack1−i/−i mice there is a strong selection pressure against Cre activity and thereby Rack1 deletion. Although further studies are needed to define the mechanisms involved, our results suggest that strong safeguards are in place to ensure Rack1 expression and thereby function in intestinal epithelia.
Consistent with these findings, we observed that Rack1−i/−i mice, while viable and fertile, were born at a frequency that is lower than expected on the basis of Mendelian predictions (Table 1), suggesting that there is a selection bias against Rack1 deletion in intestinal epithelia during development. Moreover, we observed embryonic lethality in a mouse model of ubiquitous Rack1 deletion (CMV-Cre:Rack1fl/fl) that we generated (Fig. 9A, and data not shown), and as published by others (42).
Table 1.
Breeding for vil-Cre:Rack1fl/fl (Rack1−i/−i) mice: comparison of actual and predicted birth rates
| Parental Genotype |
|||||
|---|---|---|---|---|---|
| Female | Male | No. of Pups Genotyped | No. of Rack1-i/−i Pups | % of Pups That Were Rack1-i/−i | % of Pups Expected to Be Rack1-i/−i* |
| Rack1fl/fl | vil-Cre:Rack1+/fl | 421 | 46 | 10.9 | 25.0 |
| Rack1+/fl | vil-Cre:Rack1+/fl | 410 | 25 | 6.1 | 12.5 |
Percentage of pups expected to be Rack1-i/−i on the basis of Mendelian predictions.
DISCUSSION
In this study we identify a novel function of Rack1 in vertebrates: regulating the growth of intestinal epithelial cells. Rack1 works by suppressing proliferation (Figs. 2 and 3) and regeneration (Fig. 5) of crypt cells, promoting differentiation of crypt cells into enterocyte, goblet, and enteroendocrine cell lineages while inhibiting differentiation into Paneth cells (Fig. 4), inducing apoptosis (Figs. 6 and 7), and suppressing development of intestinal neoplasia (Fig. 8). Thus Rack1 targets both cell proliferation and cell death pathways to regulate growth.
Recent studies from Dr. Biffo’s laboratory (42) and ours (Fig. 9A and data not shown) reveal that ubiquitous deletion of Rack1 in the germ line is embryonically lethal, indicating a crucial role for Rack1 during vertebrate development. Our finding that Rack1−i/−i mice are born at a frequency that is lower than expected on the basis of Mendelian predictions (Table 1) and that they demonstrate a highly mosaic pattern of Rack1 expression (Figs. 2–4) suggests a selection bias for Rack1 expression.
Our in vivo results demonstrating that Rack1 suppresses crypt cell proliferation (Figs. 2 and 3) confirm our in vitro results demonstrating that Rack1 suppresses growth of normal colon cells and colon carcinoma cells (31). Similarly, our in vivo results showing that Rack1 promotes apoptosis (Figs. 6 and 7) confirm our in vitro results showing that Rack1 promotes apoptosis in HT-29 colon carcinoma cells (30). Our in vitro analyses also show that Rack1 is required for staurosporin-induced mitochondrial cell death, the activation and translocation of Bax and Bim to mitochondria, the oligomerization of Bax, and caspase activation (30). Thus our in vivo findings validate our in vitro findings. Both identify novel and key functions for Rack1 in regulating intestinal epithelial cell growth and cell death.
In Rack1-depleted crypts we observed a striking increase in proliferating cells (as measured by Ki67 and PCNA) compared with Rack1-expressing crypts (Fig. 2, A–D). Consistent with these results, we observed an increase in BrdU incorporation in Rack1-depleted crypt cells (data not shown). Interestingly, Rack1-depleted crypts also showed a striking increase in both Sox9- and CD44-expressing cells (Fig. 3 and Fig. 5, B and C) and Paneth cells (Fig. 4C). Sox9 and CD44 are known to mark populations of crypt cells that encompass stem and progenitor cells (6, 11, 12, 15, 16, 44). Moreover, Sox9 is expressed in Paneth cells and is required for their differentiation (4, 36). This raises an intriguing question as to whether Rack1 regulates growth of intestinal stem cells and their differentiation into Paneth cells and whether loss of that regulation results in expansion of the stem/progenitor cell populations and the niche cells needed to support them. Experiments are underway to test this possibility.
Crypt homeostasis is a balance between intestinal stem cell self-renewal and cell loss caused by differentiation, apoptosis, and environmental damage [reviewed by Andersson-Rolf et al. (3) and Smith et al. (40)]. Homeostasis is governed by a complex signaling network encompassing Wnt, Notch, EGF, and bone morphogenetic protein (BMP) signals. Wnt is a major driver of stem, crypt, and Paneth cell proliferation. Notch activation leads to expansion of the stem cell compartment, resulting in increased stem cell proliferation and inhibition of secretory cell differentiation (9). In Rack1-deleted epithelia, the striking increase in proliferating crypt cells (Figs. 2 and 3) together with the striking decrease in differentiation into goblet and enteroendocrine secretory lineages (Fig. 4, B, D, and E) suggests high levels of Notch signaling. The increase in crypt cell proliferation together with expansion of the Paneth cell population (Fig. 4C) suggests high levels of Wnt signaling. Collectively, this raises the intriguing possibility that under normal physiologic conditions, Rack1 negatively regulates Notch and Wnt signaling in crypt stem and progenitor cells and thereby suppresses their proliferation.
In support of this hypothesis, Rack1 has been implicated in the negative regulation of Wnt signaling via multiple mechanisms (8, 10, 25). In zebrafish, Rack1 promotes Wnt/planar cell polarity signaling by interacting with van Gogh-like 2 (Vangl2) while attenuating canonical Wnt activity (25). In gastric cancer cells, Rack1 negatively regulates Wnt signaling by stabilizing the β-catenin destruction complex and acts as a tumor suppressor in gastric cancer cells (10). Recently, Rack1 was shown to promote Dishevelled protein degradation via autophagy thereby antagonizing Wnt signaling (8). Collectively, these studies indicate that Rack1 can regulate Wnt signaling via various mechanisms.
Future studies are needed to determine whether Rack1 modulates Wnt and Notch signaling in intestinal crypt cells and, if so, by what mechanism and to determine whether Rack1 loss releases this inhibition, resulting in increased Wnt and Notch signaling and consequently increased crypt cell proliferation (including Paneth cells) and diminished differentiation into other secretory lineages.
The observation that tdTomato was strongly expressed throughout the intestinal epithelia of vil-Cre:tdTomato:Rack1+/+ and vil-Cre:tdTomato:Rack1+/fl control mice (Fig. 9B, rows 1 and 2, panels at left) indicates that the vil-Cre promoter was functioning as expected in these mice by driving expression of active Cre recombinase. The observation that TdTomato was not expressed in most of the epithelia of the Tomato:Rack1−i/−i mice (Fig. 9B, 3rd row, panel at left) suggests a selection pressure against Cre activity and thereby Rack1 deletion. What mediates this selection pressure is unknown. There appears to be a compensatory mechanism that is present only in homozygous Rack1−i/−i mice and not in heterozygous or wild-type littermate control mice that downregulates the vil-Cre promoter (and thereby expression of Cre and possibly villin), destabilizes the Cre protein, and/or inhibits Cre enzymatic activity. Although further studies are needed to define these mechanisms, our results suggest that strong safeguards are in place to ensure Rack1 expression and thereby function in intestinal epithelia. They imply a critical role for Rack1 in maintaining intestinal homeostasis. Historically, variability in Cre activity leading to inconsistent mosaicism in target tissues is well described [reviewed by Magnuson and Osipovich (29)], including when Cre is expressed under the control of the villin promoter (e.g., 35), but the mechanisms underlying this are poorly understood.
In summary, our results reveal a novel mechanism by which intestinal epithelial cells regulate their growth. They show that Rack1 exerts powerful and pervasive control over growth as crypt cells proliferate and regenerate, and as progeny differentiate and undergo apoptosis. Exploitation of this knowledge could lead to new cancer therapies that mimic Rack1 function.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants 43743 (to C. A. Cartwright) and 56339.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.-F.C. and C.A.C. conceived and designed research; Z.-F.C. performed experiments; Z.-F.C., R.K.P., and C.A.C. analyzed data; Z.-F.C. and C.A.C. interpreted results of experiments; Z.-F.C. and C.A.C. prepared figures; Z.-F.C. and C.A.C. drafted manuscript; Z.-F.C. and C.A.C. edited and revised manuscript; Z.-F.C., R.K.P., and C.A.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Stanford Transgenic Facility for assistance with generating the floxed-Rack1 mice, Pauline Chu for tissue sectioning and hematoxylin and eosin staining, pathologists Donna Bouley and Gerald Berry for review of hematoxylin and eosin slides, Drs. Neal Copeland and Nancy Jenkins (National Cancer Institute) for providing plasmids PL452 and PL253, and Gary Gray and Eric Sibley for the gift of sucrase isomaltase antibody.
REFERENCES
- 1.Adams DR, Ron D, Kiely PA. RACK1, a multifaceted scaffolding protein: structure and function. Cell Commun Signal 9: 22, 2011. doi: 10.1186/1478-811X-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anastassiadis K, Glaser S, Kranz A, Berhardt K, Stewart AF. A practical summary of site-specific recombination, conditional mutagenesis, and tamoxifen induction of CreERT2. Methods Enzymol 477: 109–123, 2010. doi: 10.1016/S0076-6879(10)77007-5. [DOI] [PubMed] [Google Scholar]
- 3.Andersson-Rolf A, Zilbauer M, Koo BK, Clevers H. Stem cells in repair of gastrointestinal epithelia. Physiology (Bethesda) 32: 278–289, 2017. doi: 10.1152/physiol.00005.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bastide P, Darido C, Pannequin J, Kist R, Robine S, Marty-Double C, Bibeau F, Scherer G, Joubert D, Hollande F, Blache P, Jay P.. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol 178: 635–648, 2007. doi: 10.1083/jcb.200704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjerknes M, Cheng H. Intestinal epithelial stem cells and progenitors. Methods Enzymol 419: 337–383, 2006. doi: 10.1016/S0076-6879(06)19014-X. [DOI] [PubMed] [Google Scholar]
- 6.Blache P, van de Wetering M, Duluc I, Domon C, Berta P, Freund JN, Clevers H, Jay P. SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. J Cell Biol 166: 37–47, 2004. doi: 10.1083/jcb.200311021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cartwright CA, Mamajiwalla S, Skolnick SA, Eckhart W, Burgess DR. Intestinal crypt cells contain higher levels of cytoskeletal-associated pp60c-Src protein tyrosine kinase activity than do differentiated enterocytes. Oncogene 8: 1033–1039, 1993. [PubMed] [Google Scholar]
- 8.Cheng M, Xue H, Cao W, Li W, Chen H, Liu B, Ma B, Yan X, Chen YG. Receptor for activated C kinase 1 (RACK1) promotes dishevelled protein degradation via autophagy and antagonizes Wnt signaling. J Biol Chem 291: 12871–12879, 2016. doi: 10.1074/jbc.M115.708818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demitrack ES, Samuelson LC. Notch regulation of gastrointestinal stem cells. J Physiol 594: 4791–4803, 2016. doi: 10.1113/JP271667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng YZ, Yao F, Li JJ, Mao ZF, Hu PT, Long LY, Li G, Ji XD, Shi S, Guan DX, Feng YY, Cui L, Li DS, Liu Y, Du X, Guo MZ, Xu LY, Li EM, Wang HY, Xie D. RACK1 suppresses gastric tumorigenesis by stabilizing the β-catenin destruction complex. Gastroenterology 142: 812–823, 2012. doi: 10.1053/j.gastro.2011.12.046. [DOI] [PubMed] [Google Scholar]
- 11.Feng Y, Sentani K, Wiese A, Sands E, Green M, Bommer GT, Cho KR, Fearon ER. Sox9 induction, ectopic Paneth cells, and mitotic spindle axis defects in mouse colon adenomatous epithelium arising from conditional biallelic Apc inactivation. Am J Pathol 183: 493–503, 2013. doi: 10.1016/j.ajpath.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Formeister EJ, Sionas AL, Lorance DK, Barkley CL, Lee GH, Magness ST. Distinct SOX9 levels differentially mark stem/progenitor populations and enteroendocrine cells of the small intestine epithelium. Am J Physiol Gastrointest Liver Physiol 296: G1108–G1118, 2009. doi: 10.1152/ajpgi.00004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerbasi VR, Weaver CM, Hill S, Friedman DB, Link AJ. Yeast Asc1p and mammalian RACK1 are functionally orthologous core 40S ribosomal proteins that repress gene expression. Mol Cell Biol 24: 8276–8287, 2004. doi: 10.1128/MCB.24.18.8276-8287.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer 31: 13–20, 1983. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- 15.Gracz AD, Fuller MK, Wang F, Li L, Stelzner M, Dunn JC, Martin MG, Magness ST. Brief report: CD24 and CD44 mark human intestinal epithelial cell populations with characteristics of active and facultative stem cells. Stem Cells 31: 2024–2030, 2013. doi: 10.1002/stem.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gracz AD, Ramalingam S, Magness ST. Sox9 expression marks a subset of CD24-expressing small intestine epithelial stem cells that form organoids in vitro. Am J Physiol Gastrointest Liver Physiol 298: G590–G600, 2010. doi: 10.1152/ajpgi.00470.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall B, Limaye A, Kulkarni AB. Overview: generation of gene knockout mice. Curr Protoc Cell Biol 19: 19.12, 2009. 10.1002/0471143030.cb1912s44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol 244: 305–318, 2002. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 19.Horie K, Nishiguchi S, Maeda S, Shimada K. Structures of replacement vectors for efficient gene targeting. J Biochem 115: 477–485, 1994. doi: 10.1093/oxfordjournals.jbchem.a124362. [DOI] [PubMed] [Google Scholar]
- 20.Kadrmas JL, Smith MA, Pronovost SM, Beckerle MC. Characterization of RACK1 function in Drosophila development. Dev Dyn 236: 2207–2215, 2007. doi: 10.1002/dvdy.21217. [DOI] [PubMed] [Google Scholar]
- 21.Kee N, Sivalingam S, Boonstra R, Wojtowicz JM. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods 115: 97–105, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Kubben FJ, Peeters-Haesevoets A, Engels LG, Baeten CG, Schutte B, Arends JW, Stockbrügger RW, Blijham GH. Proliferating cell nuclear antigen (PCNA): a new marker to study human colonic cell proliferation. Gut 35: 530–535, 1994. doi: 10.1136/gut.35.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LePage DF, Conlon RA. Animal models for disease: knockout, knock-in, and conditional mutant mice. Methods Mol Med 129: 41–67, 2006. doi: 10.1385/1-59745-213-0:41. [DOI] [PubMed] [Google Scholar]
- 24.Li JJ, Xie D. RACK1, a versatile hub in cancer. Oncogene 34: 1890–1898, 2015. doi: 10.1038/onc.2014.127. [DOI] [PubMed] [Google Scholar]
- 25.Li S, Esterberg R, Lachance V, Ren D, Radde-Gallwitz K, Chi F, Parent JL, Fritz A, Chen P. Rack1 is required for Vangl2 membrane localization and planar cell polarity signaling while attenuating canonical Wnt activity. Proc Natl Acad Sci USA 108: 2264–2269, 2011. doi: 10.1073/pnas.1013170108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res 13: 476–484, 2003. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140, 2010. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madison BB, Dunbar L, Qiao XT, Braunstein K, Braunstein E, Gumucio DL. cis Elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem 277: 33275–33283, 2002. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 29.Magnuson MA, Osipovich AB. Pancreas-specific Cre driver lines and considerations for their prudent use. Cell Metab 18: 9–20, 2013. doi: 10.1016/j.cmet.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mamidipudi V, Cartwright CA. A novel pro-apoptotic function of RACK1: suppression of Src activity in the intrinsic and Akt pathways. Oncogene 28: 4421–4433, 2009. doi: 10.1038/onc.2009.293. [DOI] [PubMed] [Google Scholar]
- 31.Mamidipudi V, Dhillon NK, Parman T, Miller LD, Lee KC, Cartwright CA. RACK1 inhibits colonic cell growth by regulating Src activity at cell cycle checkpoints. Oncogene 26: 2914–2924, 2007. doi: 10.1038/sj.onc.1210091. [DOI] [PubMed] [Google Scholar]
- 32.Mamidipudi V, Miller LD, Mochly-Rosen D, Cartwright CA. Peptide modulators of Src activity in G1 regulate entry into S phase and proliferation of NIH 3T3 cells. Biochem Biophys Res Commun 352: 423–430, 2007. doi: 10.1016/j.bbrc.2006.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mamidipudi V, Zhang J, Lee KC, Cartwright CA. RACK1 regulates G1/S progression by suppressing Src kinase activity. Mol Cell Biol 24: 6788–6798, 2004. doi: 10.1128/MCB.24.15.6788-6798.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mansour SL, Thomas KR, Capecchi MR. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature 336: 348–352, 1988. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- 35.McConnell BB, Kim SS, Bialkowska AB, Yu K, Sitaraman SV, Yang VW. Kruppel-like factor 5 protects against dextran sulfate sodium-induced colonic injury in mice by promoting epithelial repair. Gastroenterology 140: 540–549, 2011. doi: 10.1053/j.gastro.2010.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mori-Akiyama Y, van den Born M, van Es JH, Hamilton SR, Adams HP, Zhang J, Clevers H, de Crombrugghe B. SOX9 is required for the differentiation of Paneth cells in the intestinal epithelium. Gastroenterology 133: 539–546, 2007. doi: 10.1053/j.gastro.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 37.Schwenk F, Baron U, Rajewsky K.. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res 23: 5080–5081, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shor B, Calaycay J, Rushbrook J, McLeod M.. Cpc2/RACK1 is a ribosome-associated protein that promotes efficient translation in Schizosaccharomyces pombe. J Biol Chem 278: 49119–49128, 2003. doi: 10.1074/jbc.M303968200. [DOI] [PubMed] [Google Scholar]
- 39.Skop AR, Liu H, Yates J 3rd, Meyer BJ, Heald R. Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science 305: 61–66, 2004. doi: 10.1126/science.1097931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith RJ, Rao-Bhatia A, Kim TH. Signaling and epigenetic mechanisms of intestinal stem cells and progenitors: insight into crypt homeostasis, plasticity, and niches. Wiley Interdiscip Rev Dev Biol 6: e281, 2017. doi: 10.1002/wdev.281. [DOI] [PubMed] [Google Scholar]
- 41.Swaminathan G, Cartwright CA. Rack1 promotes epithelial cell-cell adhesion by regulating E-cadherin endocytosis. Oncogene 31: 376–389, 2012. doi: 10.1038/onc.2011.242. [DOI] [PubMed] [Google Scholar]
- 42.Volta V, Beugnet A, Gallo S, Magri L, Brina D, Pesce E, Calamita P, Sanvito F, Biffo S. RACK1 depletion in a mouse model causes lethality, pigmentation deficits and reduction in protein synthesis efficiency. Cell Mol Life Sci 70: 1439–1450, 2013. doi: 10.1007/s00018-012-1215-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wehner P, Shnitsar I, Urlaub H, Borchers A.. RACK1 is a novel interaction partner of PTK7 that is required for neural tube closure. Development 138: 1321–1327, 2011. doi: 10.1242/dev.056291. [DOI] [PubMed] [Google Scholar]
- 44.Zeilstra J, Joosten SP, Vermeulen L, Koster J, Medema JP, Versteeg R, Spaargaren M, Pals ST. CD44 expression in intestinal epithelium and colorectal cancer is independent of p53 status. PLoS One 8: e72849, 2013. doi: 10.1371/journal.pone.0072849. [DOI] [PMC free article] [PubMed] [Google Scholar]