Abstract
Notch receptor signaling is active during cardiac development and silenced in myocytes after birth. Conversely, outward K+ Kv currents progressively appear in postnatal myocytes leading to shortening of the action potential (AP) and acquisition of the mature electrical phenotype. In the present study, we tested the possibility that Notch signaling modulates the electrical behavior of cardiomyocytes by interfering with Kv currents. For this purpose, the effects of Notch receptor activity on electrophysiological properties of myocytes were evaluated using transgenic mice with inducible expression of the Notch1 intracellular domain (NICD), the functional fragment of the activated Notch receptor, and in neonatal myocytes after inhibition of the Notch transduction pathway. By patch clamp, NICD-overexpressing cells presented prolonged AP duration and reduced upstroke amplitude, properties that were coupled with reduced rapidly activating Kv and fast Na+ currents, compared with cells obtained from wild-type mice. In cultured neonatal myocytes, inhibition of the proteolitic release of NICD with a γ-secretase antagonist increased transcript levels of the Kv channel-interacting proteins 2 (KChIP2) and enhanced the density of Kv currents. Collectively, these results indicate that Notch signaling represents an important regulator of the electrophysiological behavior of developing and adult myocytes by repressing, at least in part, repolarizing Kv currents.
NEW & NOTEWORTHY We investigated the effects of Notch receptor signaling on the electrical properties of cardiomyocytes. Our results indicate that the Notch transduction pathway interferes with outward K+ Kv currents, critical determinants of the electrical repolarization of myocytes.
Keywords: Notch receptor, electrophysiology, myocytes, Kv currents
INTRODUCTION
Outward K+ Kv-mediated currents are important determinant of the repolarization phase of the action potential (AP) of cardiomyocytes (57). Alterations in the expression of Kv channels and regulatory proteins during development, physiological, and pathological circumstances affect the profile of the AP with consequences on myocardial excitation-contraction coupling and electrical stability of the heart (68).
The Kv channel-interacting protein 2 (KChIP2), which is part of the Kv channel multiprotein complex assembly, has emerged as a key modulator of Kv currents and AP duration (17, 46, 74). KChIP2 levels are attenuated in the heart during early phases of development (35, 79) and in response to diseased conditions (46, 59, 62), scenarios that are associated with weaker repolarizing Kv currents and prolonged duration of the AP (4, 11, 19, 34, 52, 54, 69, 76, 78, 80). However, molecular cues governing the expression of Kv, KChIP2, and other ion channel subunits dictating AP properties in myocytes in homeostatic and pathological conditions remain largely unknown.
Notch receptor signaling is active during embryonic development and critical in cardiogenesis by regulating trabeculation, myocyte proliferation, and valve formation (8, 12, 20, 30, 60, 65). Notch signaling is progressively silenced in the postnatal heart, but it is partly restored in the adult myocardium after ischemic injury (22, 44), hypertrophy (10), or heart failure (40), a factor that appears to preserve the structural and functional integrity of the organ by promoting prosurvival and proliferating signaling (10, 21, 22, 44, 67). Activation of the Notch transduction system involves cell-to-cell contact and binding of the extracellular domain of the Notch receptor to delta-like and/or Jagged families of membrane-bound ligands. After receptor-ligand interaction, the multisubunit protease complex γ-secretase cleaves the intracellular domain of the Notch receptor (NICD) (6, 14, 42), which, in turn, translocates to the nucleus. NICD then interacts with transcription regulators of the CSL family, promoting the activation of genes comprising the hairy and enhancer of split (HES) and hairy-related transcription factor (HRT) families (31, 33, 55) involved in cell fate determination. But whether the Notch receptor signaling axis ultimately interferes with the electrophysiological properties of myocytes in the developing and adult heart remains to be clarified.
In the present study, we examined the possibility that NICD modulates the density of ionic currents in cardiomyocytes. We found that overexpression of NICD in adult cells alters the repolarization phase of the AP by decreasing Kv currents, whereas suppression of NICD signaling in neonatal cells promotes the appearance of Kv currents and enhances KChIP2 gene expression. Thus, Notch signaling represents an important regulator of the electrophysiological properties of neonatal and adult cardiomyocytes.
METHODS
Transgenic animals.
C57Bl/6 neonatal mice at postnatal day 2 (n = 8 litters) or postnatal days 5–7 (n = 4) and adult transgenic mice of either sex (n = 54) and C57Bl/6 adult mice (n = 8) were used in this study. Experiments were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and approved by the local animal care committees (Institutional Animal Care and Use Committee at New York Medical College and at Brigham and Women's Hospital, Harvard Medical School). To activate Notch1 signaling in cardiomyocytes, a Cre-conditional NICD expression mouse model was used (49). This was achieved by crossing homozygous mice for tamoxifen-inducible Cre recombinase under the cardiac-specific α-myosin heavy chain promoter (αMHC-MerCreMer, MCM+/+ mice; stock no. 5657, The Jackson Laboratory) (71) with hemizygous ZEG-NICD mice, in which the transgenic construct uses a loxP-flanked STOP sequence between a cytomegalovirus enhancer chicken β-actin promoter and the coding sequence for intracellular portion of the Notch1 protein (NICD), with an internal ribosomal entry site (IRES) linked the enhanced green fluorescence protein (GFP) gene (NICD-GFP+/− mice, stock no. 6850, The Jackson Laboratory) (49). The progeny consisted of double transgenic (αMHC-MerCreMer and NICD-GFP, MCM-NICD-GFP) and single transgenic [αMHC-MerCreMer, MCM-wild type (WT)] mice born with a normal Mendelian ratio of ~1:1.
To account for the potential off-target effects of tamoxifen treatment and expression of Cre and GFP in myocytes (41), mice with tamoxifen-inducible Cre recombinase (MCM+/+ mice) were crossed with transgenic mice with inducible cell membrane-localized green fluorescence in Cre recombinase-expressing cells (stock no. 7676, The Jackson Laboratory) (53). The progeny consisted of double transgenic animals (αMHC-MerCreMer and GFP, MCM-GFP mice).
Cre recombinase was induced by administration of tamoxifen (Sigma-Aldrich) for 4 consecutive days (0.1 g·kg body wt−1·day−1 ip) (29). Age- and sex-matched MCM-NICD-GFP and MCM-WT mice were treated with tamoxifen, and cardiomyocytes were isolated 19–58 days later.
Histological analysis.
Mouse hearts were fixed in phosphate-buffered formalin (10%, Sigma) and embedded in paraffin (9). Thin sections of the left ventricle (LV) were indirectly immunolabeled for the detection of GFP (Anti-GFP Antibody, catalog no. ab5450, Abcam) and KChIP2 (KCNIP2 Polyclonal Antibody, catalog no. PA5-41075, ThermoFisher Scientific). Nuclei were counterstained with DAPI (Sigma-Aldrich). Secondary antibodies included donkey anti-goat [Alexa Fluor 488 AffiniPure Donkey Anti-Goat IgG (H+L), catalog no. 705-545-147, Jackson ImmunoReasearch Laboratories] and goat anti-rabbit [Alexa Fluor 647 AffiniPure Goat Anti-Rabbit IgG (H+L), catalog no. 111-605-003, Jackson ImmunoReasearch Laboratories]. Images were acquired using a Zeiss LSM 710 confocal microscope with a ×63 objective. For comparison of KChIP2 expression levels in the myocardium of MCM-WT and MCM-NICD-GFP mice, digital images obtained from immunofluorescent-stained sections were analyzed using ImageJ software. Mean fluorescence intensity in the channel of KChIP2 labeling was measured in areas corresponding to cardiomyocytes, as determined by cell shape and GFP expression.
Isolation and culture of neonatal mouse cardiomyocytes.
Neonatal mouse cardiomyocytes were prepared from hearts of 2-day-old pups. Briefly, mice were anesthetized by hypothermia and euthanized by decapitation with sharp surgical scissors. Hearts were explanted, and ventricles were dissected, minced, incubated in trypsin dissolved in HBSS medium (1 mg/ml, Sigma-Aldrich) at 4°C for ~12 h, and then serially digested with collagenase type II (1 mg/ml, 285 U/mg, Worthington Biochemical) at 37°C. Alternatively, minced myocardial ventricles were digested in a solution containing the following (in mM): 116 NaCl, 4.7 KCl, 0.8 MgSO4, 20 HEPES, 5.6 glucose, 20 taurine, 0.005 creatine, 0.005 Na pyruvate, and 0.8 NaH2PO4 (pH 7.4), in which 0.4 mg/ml of collagenase type II (285 U/mg, Worthington Biochemical) and 0.6 mg/ml of pancreatin from the porcine pancreas (Sigma-Aldrich) were added. Collagenase type IV (Worthington Biochemical) was used for one isolation. After dissociation, cells were pooled and preplated in two consecutive steps of 30–45 min to allow fibroblasts to adhere. The myocyte-enriched population remaining in suspension was plated in culture dishes or coverglasses precoated with 0.1% gelatin (Sigma-Aldrich) for 1 h. Myocytes were cultured in DMEM (Life Technologies) containing 10% FBS (GIBCO) and the antibiotics penicillin-streptomycin (100 U/ml penicillin and 0.1 mg/ml streptomycin, Sigma-Aldrich). After 24 h, the medium was changed and cells were cultured in the presence of 20 µM γ-secretase inhibitor (DAPT; Sigma-Aldrich) or vehicle only (DMSO; Sigma-Aldrich). The treatment was repeated every 48 h.
Isolation of adult cardiomyocytes.
With the animal under deep anesthesia (isoflurane), thoracotomy was performed, the heart was excised, and LV myocytes were enzymatically dissociated as previously reported (52, 69, 70, 72). Briefly, the heart was connected to a plastic cannula for retrograde perfusion through the aorta in a Langendorff system (Radnoti) at 37°C. The perfusate consisted of a Ca2+-free solution gassed with 85% O2-15% N2. After 5 min, 0.1 mM CaCl2, 274 U/ml collagenase (type 2; Worthington Biochemical), and 0.57 U/ml protease (type XIV, Sigma-Aldrich) were added to solution that contained the following (in mM): 126 NaCl, 4.4 KCl, 5 MgCl2, 20 HEPES, 22 glucose, 20 taurine, 5 creatine, 5 Na pyruvate, and 5 NaH2PO4 (pH 7.4). At completion of digestion, the atria and right ventricle were dissected and the LV was cut in small pieces; these fragments were shaken in resuspension solution and filtered using a 200-µm nylon mesh (Spectrum Laboratories). For electrophysiological experiments, only rod-shaped myocytes exhibiting cross striations and showing no spontaneous contractions or contractures were selected; cells were used within 8 h after enzymatic digestion.
Whole cell patch-clamp experiments.
Isolated adult LV myocytes or neonatal myocytes plated on a coverglass were placed in a bath on the stage of an Olympus IX51, Olympus IX71, or Zeiss Axiovert 200 microscopes for patch-clamp measurements (52, 66, 69, 70, 73). Data were acquired by means of the whole cell patch-clamp technique in voltage- and current-clamp modes using Multiclamp 700A or 700B amplifiers (Molecular Devices). Electrical signals were digitized using 250-kHz 16-bit resolution analog-to-digital converters (Digidata 1322, 1440A, or 1550B, Molecular Devices) and recorded using pCLAMP 9.0 or 10 software (Molecular Devices) with low-pass filtering at 2 kHz. Membrane capacitance (Cm) was measured in voltage-clamp mode using a 5-mV voltage step and pCLAMP software algorithm; this parameter was used to normalize transmembrane currents (69, 70). Pipettes were pulled by means of a vertical (PB-7, Narishige) or horizontal (P-97, Sutter Instruments) glass microelectrode puller; when filled with intracellular solution, pipettes had a resistance of 1–3 MΩ.
For AP measurements, cells were stimulated with current pulses 1–1.5 times threshold (52, 66, 69, 70, 72, 73). Myocytes were bathed with Tyrode solution containing the following (in mM): 140 NaCl, 5.4 KCl, 1 MgCl2, 5 HEPES, 5.5 glucose, and 1 CaCl2 (pH 7.4 adjusted with NaOH). The composition of the pipette solution was as follows (in mM): 10 NaCl, 113 KCl, 0.5 MgCl2, 5 K2-ATP, 5.5 glucose, 5 EGTA, and 10 HEPES (pH 7.2 with KOH). AP measurements were conducted at 37°C. Data were analyzed using Clampfit (pCLAMP) software.
The fast Na+ current (INa) was measured in adult myocytes with a previously reported protocol (69). Cells were bathed with modified Tyrode solution of the following composition (in mM): 5 NaCl, 1 MgCl2, 1 CaCl2, 0.1, CdCl2, 20 HEPES, 11 glucose, and 132.5 CsCl (pH 7.4 with CsOH). The composition of the pipette solution was as follows (in mM): 5 NaCl, 135 CsF, 10 EGTA, 5 Mg-ATP, and 5 HEPES (pH 7.2 with CsOH). Current-voltage (I–V) relations were determined by applying depolarizing steps 200 ms in duration from a holding potential (Vh) of −120 mV in 5-mV increments. The interpulse interval was 3 s. INa amplitude was measured as the current difference between the inward peak current and the current at the end of the 200-ms step. INa was normalized by Cm.
At each membrance potential (Vm) tested, Na+ conductance (g) was calculated as follows: g = INa/(Vm − ENa) (1), where INa is the amplitude of the Na+ current at Vm and ENa is the reversal potential, which was defined by the intercept of the I–V relation to the zero-current axis.
Conductance-voltage relations were plotted and fitted with the following Boltzmann function: g = gmax/{1 + exp[(V1/2G − Vm)/kG]} (2), where gmax is the maximal conductance, V1/2G is the potential at which conductance is halfway between 0 and gmax, and kG is the slope of the conductance curve.
Values of normalized Na+ conductance (G = g/gmax) were plotted to obtain activation curves; half-maximal activation potential (V1/2G) and the slope of the activation curve (kG) were obtained by fitting the data with the following Boltzmann equation: G = {1 + exp[(V1/2G − Vm)/kG]}−1 (3).
A two-pulse protocol was used to assess the voltage dependence of steady-state inactivation of INa (69). Prepulses were introduced to depolarize the cell to different membrane voltages starting from −120 mV for 300 ms in 5-mV increments. Each prepulse was followed by a single 30-ms test pulse, which depolarized the cell to −40 mV. Values of normalized Na+ conductance (G = g/gmax) were plotted with Vm relative to the preconditioning steps to compute the steady-state inactivation curves, which were fitted with a Boltzmann equation, as indicated above (3).
INa reactivation was studied using a two-pulse protocol with a variable interpulse duration (69) ranging from 1 to 50 ms in increments of 1 ms. With each pulse, INa was activated by depolarizing cells from a Vh of −120 to −40 mV for 30 ms. The double pulse protocol was repeated every 3 s. For each interpulse duration (t), INa reactivation was calculated by dividing the amplitude of the current measured during the second pulse [I(t)] by the amplitude of the current measured during the first pulse (Imax). Values of INa reactivation were plotted to obtain reactivation curves.
In adult cardiomyocytes, voltage-gated outward K+ Kv currents were assessed with a previously reported protocol (48, 69) to dissect K+ currents with rapid activation and fast (Ito), intermediate (IK,slow1), and slow (IK,slow2) kinetics of inactivation, together with a sustained (noninactivating) component (Iss) (48, 57). Cells were bathed with modified Tyrode solution containing the following (in mM): 140 NaCl, 4 KCl, 1 MgCl2, 10 HEPES, 10 glucose, 1.2 CaCl2, and 0.3 mM CdCl2 to block L-type Ca2+ current (pH 7.4 adjusted with NaOH) (48, 69). The composition of the pipette solution was as follows (in mM): 5 NaCl, 20, KCl, 120 K-aspartate, 1 MgCl2, 5 Mg-ATP, 10 EGTA, and 10 HEPES (pH 7.2 with KOH) (48, 69). After Cm and series resistance compensation (≥70%), Kv currents were activated using depolarizing voltage steps from a Vh of −80 to +60 mV for 25 s in duration. By this protocol, loss of voltage control was minimized due to the proximity to the reversal potential of Na+. Interpulse interval was 60 s. Ito, IK,slow1, IK,slow2, and Iss amplitudes and time constants were obtained by fitting the current decay during the depolarizing step with a triexponential function using Clampfit 10 software (48, 69). For cells lacking Ito, a biexponential function was used for fitting. Currents (expressed as pA/pF) were normalized by Cm. A representative multiexponential fitting curve for total outward currents and monoexponential fitting curve for each Kv current component are reported.
To further dissect the two components of Ito with fast (Ito,f) and slow (Ito,s) inactivation kinetics (7, 83), the decay phases of outward currents evoked by 4.5-s depolarization steps from a Vh of −80 to +60 mV were analyzed with a triexponential function to evaluate amplitude of Ito,f, Ito,s, and IK,slow based on their distinct time constants of decay that differ by an order of magnitude (7, 23, 25, 83). For cells lacking Ito,f, Ito,s, or both components, biexponential or monoexponential functions were used. The interpulse interval was 5.5 s. Currents (expressed as pA/pF) were normalized by Cm. The representative multiexponential fitting curves for total outward currents and monoexponential fitting curve for each Kv current component are reported. In addition, the Kv channel inhibitor 4-aminopyridine was used at a high concentration (5 mM) (48, 83) to evaluate the nature of Kv currents affected by Notch signaling. For the same cell, the 4-aminopyridine-sensitive current was assessed as the difference current obtained by subtracting traces recorded in 4-aminopyridine from those measured in Tyrode solution for the same cell.
The inward rectifier K+ current (IK1) was evaluated at 37°C using a depolarizing ramp (18, 66) from −110 to +80 mV for 10 s in duration using solutions comparable to those used for AP measurements.
Rapidly activating outward K+ Kv currents were assessed in neonatal myocytes with Tyrode solution containing 0.3 mM CdCl2 to block L-type Ca2+ current. The composition of the pipette solution was as follows (in mM): 10 NaCl, 113 KCl, 0.5 MgCl2, 5 K2-ATP, 5.5 glucose, 5 EGTA, and 10 HEPES (79). I–V relations were determined applying depolarizing steps 500 ms in duration from a Vh of −70 mV in 10-mV increments. Rapidly activating K+ current amplitude was measured as the difference between the peak outward current at the beginning of the step and Iss, the current at the end of the 500-ms step (79), using Clampfit 10 software.
Quantitative RT-PCR.
Total cellular RNA was prepared from cultured neonatal mouse ventricular myocytes or myocardial tissue using RNeasy Plus Micro kit (Qiagen) or Quick-RNA MiniPrep kits (Zymo Research) following the manufacturer's instructions. The kit contains gDNA Eliminator Mini Spin Columns to avoid genomic DNA contaminations. cDNA was obtained from total RNA using the MultiScribe reverse transcriptase kit (Applied Biosystems) or Moloney murine leukemia virus reverse transcriptase (Promega). Real-time RT-PCR was performed with the primers shown in Table 1. To avoid the influence of genomic contamination, forward and reverse primers for each gene were located in different exons. The StepOnePlus Real-Time PCR system (Applied Biosystems) was used for quantitative RT-PCR. In each case, cDNA was combined with Power SYBR Green Master Mix (Applied Biosystems) or Perfecta SYBR green FastMix Rox qPCR master mix (Quanta BioSciences) in a 20-μl reaction. Cycling conditions were as follows: 95°C for 10 min followed by 40 cycles of amplification (95°C denaturation for 10–15 s, 60°C annealing and extension for 1 min). The melting curve was then obtained. Threshold cycle (Ct) values were normalized with respect to β2-microglobulin or hypoxanthine guanine phosphoribosyl transferase.
Table 1.
Primers for PCR analysis
| Gene | Primers |
|---|---|
| GFP | |
| Forward | 5′-GCACGACTTCTTCAAGTCCGCCATGCC-3′ |
| Reverse | 5′-GCGGATCTTGAAGTTCACCTTGATGCC-3′ |
| Mouse Kcna4 | |
| Forward | 5′-AAAGGGGAAACAAATCACCG-3′ |
| Reverse | 5′-GCAGGAAATGAAGAGCATCC-3′ |
| Mouse Kcna5 | |
| Forward | 5′-ATGAGGCCCATCACTGTAGG-3′ |
| Reverse | 5′-AAAATTGGAGACGATGACGG-3′ |
| Mouse Kcnd2 | |
| Forward | 5′-TCAGCAAGCAAGTTCACCAG-3′ |
| Reverse | 5′-TTCCCTGCTATGGTTTTTGG-3′ |
| Mouse Kcnd3 | |
| Forward | 5′-TCTGCCAGCAAGTTCACAAG-3′ |
| Reverse | 5′-TTCCCTGCGATTGTCTTAGG-3′ |
| Mouse Kcnip2 | |
| Forward | 5′-TGAGGGTCTGGAACAACTCC-3′ |
| Reverse | 5′-GGGACATTCGTTCTTGAAGC-3′ |
| Mouse Knip2a | |
| Forward | 5′-CTCCAGGGCCCAGTAAAAA-3′ |
| Reverse | 5′-TGGGGCAGCTAATGTTTCAC-3′ |
| Mouse Knip2b | |
| Forward | 5′-TCAGTGAAAACAGCGTGGAG-3′ |
| Reverse | 5′-TGCGTGTGAACTTGGTTTGT-3′ |
| Mouse Knip2c | |
| Forward | 5′-AGCTTACGGACAGCGTGGA-3′ |
| Reverse | 5′-ACTCTCTGCGTGTGAACTTGG-3′ |
| Mouse Notch1 | |
| Forward | 5′-AAGTGGGACCTGCCTGAAT-3′ |
| Reverse | 5′-CTGAGGCAAGGATTGGAGTC-3′ |
| Mouse Hes1 | |
| Forward | 5′-GGAAATGACTGTGAAGCACCT-3′ |
| Reverse | 5′-GTCACCTCGTTCATGCACTC-3′ |
| Mouse Hey1 | |
| Forward | 5′-CACCTGAAAATGCTGCACAC-3′ |
| Reverse | 5′-ATGCTCAGATAACGGGCAAC-3′ |
| Mouse Hprt | |
| Forward | 5′-AGCCCCAAAATGGTTAAGGT-3′ |
| Reverse | 5′-CAAGGGCATATCCAACAACA-3′ |
| Mouse β2m | |
| Forward | 5′-CTCGGTGACCCTGGTCTTTCTG-3′ |
| Reverse | 5′-ATGTGAGGCGGGTGGAACTG-3′ |
GFP, green fluorescent protein; Hprt, hypoxanthine guanine phosphoribosyl transferase; β2m, β2-microglobulin.
Data analysis.
Data are presented as means ± SE or means ± SE and scatterplots. Statistical analysis was performed using SigmaPlot 11.0. Data were initially tested for normality (Shapiro-Wilk) and equal variance for assignment to parametric or nonparametric analysis. Parametric tests included Student’s t-test or one-way repeated ANOVA followed by the Bonferroni test for two groups or multiple comparisons, respectively. Nonparametric tests included the Mann-Whitney rank sum test or Kruskal-Wallis one-way ANOVA on ranks followed by Dunn’s method for two groups or multiple comparisons, respectively (69, 73). P < 0.05 was considered significant.
RESULTS
Notch1 alters the AP in adult myocytes.
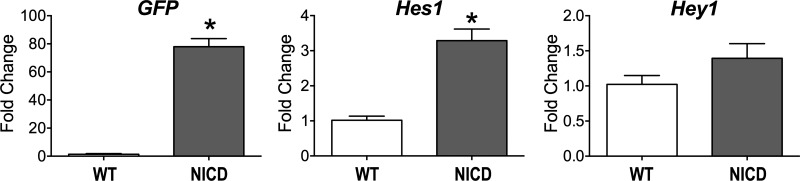
To establish the effects of Notch1 signaling on the electrical properties of cardiomyocytes, mice with inducible myocyte-specific expression of NICD were used. Specifically, the MerCreMer system allowed tamoxifen-inducible synthesis of Cre recombinase in cardiomyocytes, promoting the expression of NICD and GFP (MCM-NICD-GFP). By RT-PCR, transcripts for GFP were apparent in isolated cell preparations obtained from MCM-NICD-GFP mice, together with significant increase in levels of the Notch downstream target Hes1, with respect to cells from WT mice (Fig. 1), which is consistent with NICD overexpression.
Fig. 1.
Activation of Notch signaling in MCM-NICD-GFP myocytes. Transcript levels for green fluorescence protein (GFP) and downstream targets of Notch1–Notch4 signaling Hes1 and Hey1 in isolated myocyte preparations from C57Bl/6 [wild type (WT); n = 4] and MCM-NICD-GFP mice [Notch1 intracellular domain (NICD); n = 4] are shown. *P < 0.001 vs. WT.
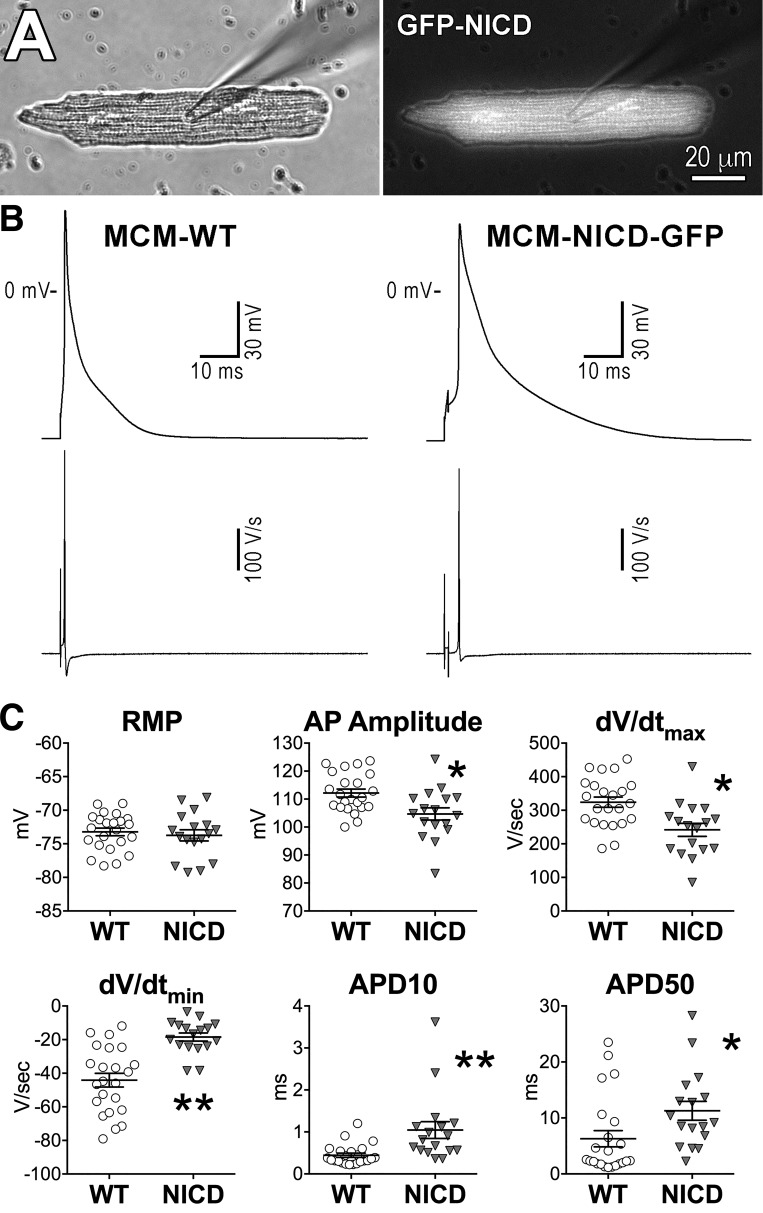
For electrophysiological experiments, LVs were enzymatically digested and GFP-positive myocytes from MCM-NICD-GFP animals were compared with GFP-negative cells obtained from single transgenic mice with tamoxifen-inducible MerCreMer transgene (MCM-WT). By patch-clamp analysis, MCM-NICD-GFP myocytes presented substantial (P < 0.001) prolongation of the early repolarization phase of the AP and reduction of the maximal velocity of repolarization (dV/dtmin) with respect to MCM-WT cells (Fig. 2). Conversely, resting Vm was similar in the two groups, but upstroke amplitude and velocity were attenuated in MCM-NICD-GFP cells. Thus, overexpression of NICD in myocytes is coupled with altered electrical properties.
Fig. 2.
The Notch1 intracellular domain (NICD) prolongs the duration of the action potential (AP) of adult myocytes. A: images for a left ventricular (LV) cardiomyocyte from a tamoxifen-treated MCM-NICD-GFP mouse obtained in bright-field (left) and fluorescent light (right). B: APs recorded in a green fluorescent protein (GFP)-negative myocytes isolated from a wild-type (WT) mouse (MCM-WT) and a GFP-positive cell obtained from a transgenic mouse with NICD expression (MCM-NICD-GFP). The derivative of the transmembrane potential signal for the two cells is shown at the bottom. C: AP properties of GFP-negative myocytes from MCM-WT mice (WT, n = 23; from 9 animals) and GFP-positive myocytes from MCM-NICD-GFP mice (NICD, n = 17; from 8 animals) are shown as means ± SE and scatterplots. RMP, resting membrane potential; APD, AP duration. *P < 0.05 vs. WT; **P < 0.001 vs. WT.
Notch1 attenuates fast INa in adult myocytes.
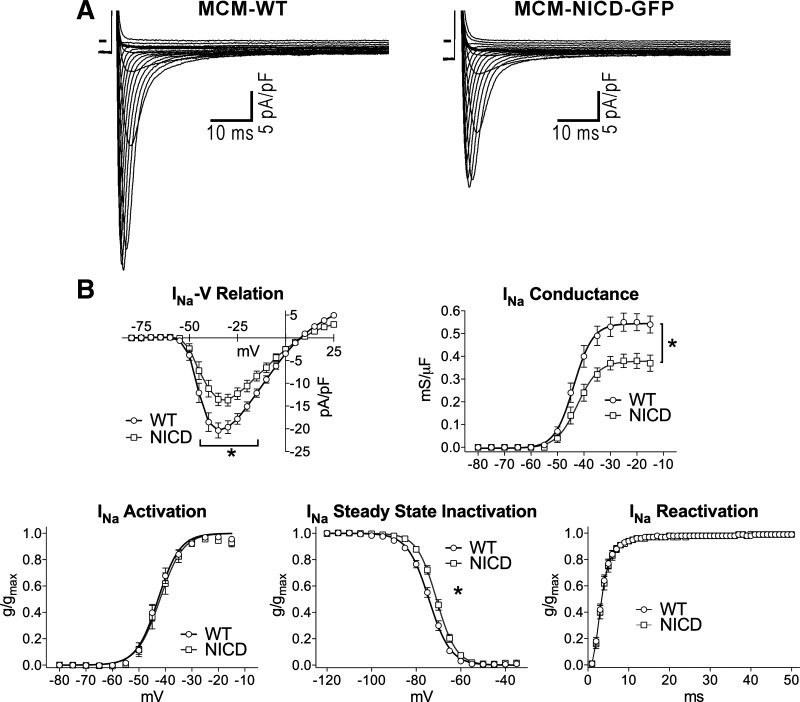
To define the basis for the attenuated depolarization of the AP in myocytes overexpressing NICD, fast INa, which is responsible for the AP upstroke, was evaluated in the two groups of cells. With respect MCM-WT myocytes, peak INa density and channel conductance were reduced, respectively, by 33% and 32% in MCM-NICD-GFP cells (Fig. 3). INa activation and reactivation properties were comparable in the two groups, but steady-state inactivation was shifted toward more positive potentials in cells overexpressing NICD. Thus, reduced INa density may account, at least in part, for the slower depolarization rate and attenuated AP amplitude in cells with active Notch signaling.
Fig. 3.
The Notch1 intracellular domain (NICD) reduces fast Na+ current (INa) in adult myocytes. A: whole cell voltage-gated INa recorded in voltage clamp in a green fluorescent protein (GFP)-negative myocyte isolated from a wild-type (WT) mouse (MCM-WT) and a GFP-positive cell obtained from a transgenic mouse with NICD expression (MCM-NICD-GFP). The voltage-clamp protocol consisted of a family of depolarizing steps in 5-mV increments. B: current-voltage (I–V) relations, voltage dependency of conductance, steady-state activation, steady inactivation, and time dependency of reactivation for INa in GFP-negative myocytes from MCM-WT mice (WT, n = 14–16; from 3 animals) and GFP-positive myocytes from MCM-NICD-GFP mice (NICD, n = 11–14; from 4 animals) are shown as means ± SE. gmax, maximal conductance. *P < 0.05 vs. WT. Fitting parameters are shown in Table 2.
Table 2.
Fitting parameters for INa properties
| gmax, mS/µF | V1/2G, mV | kG, mV | R2 | P | ||
|---|---|---|---|---|---|---|
| INa conductance | ||||||
| WT | 0.54 ± 0.02 | −43.9 ± 0.7 | 3.61 ± 0.6 | 0.81 | <0.0001 | |
| NICD | 0.38 ± 0.01 | −42.4 ± 0.8 | 3.98 ± 0.7 | 0.79 | ||
| INa activation | ||||||
| WT | −42.8 ± 0.3 | 4.1 ± 0.3 | 0.94 | 0.559 | ||
| NICD | −42.0 ± 0.4 | 4.3 ± 0.4 | 0.89 | |||
| INa steady-state inactivation | ||||||
| WT | −74.3 ± 0.2 | 4.8 ± 0.2 | 0.98 | 0.0419 | ||
| NICD | −70.9 ± 0.2 | 4.4 ± 0.2 | 0.98 | |||
Values are means ± SE. INa, fast Na+ current; gmax, maximal conductance; V1/2G, potential at which conductance is halfway between 0 and gmax; kG, slope of the conductance curve; WT, wild type; NICD, Notch1 intracellular domain.
Notch1 reduces Kv currents in adult myocytes.
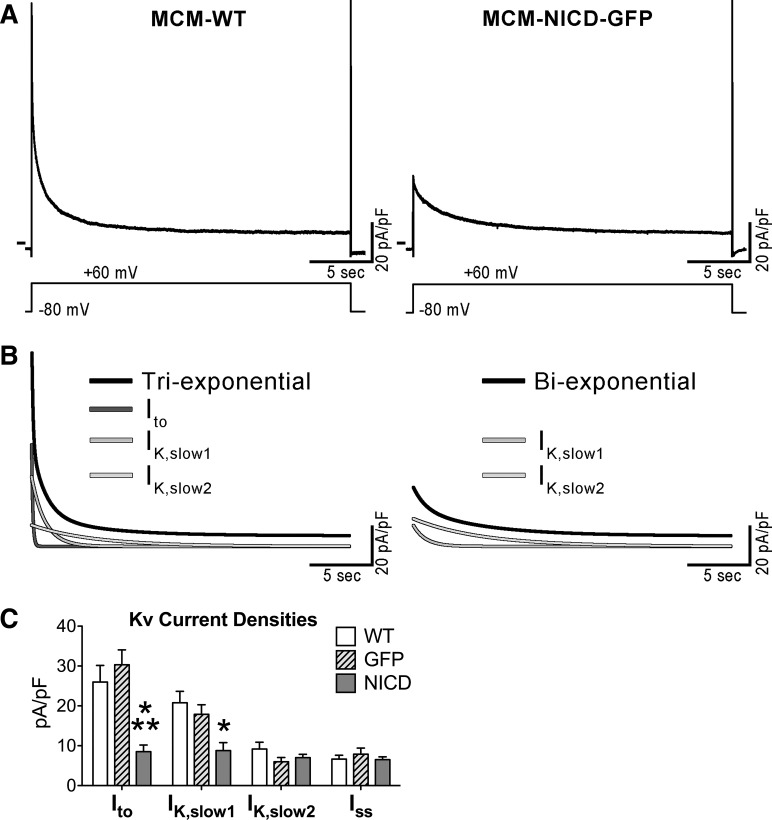
Voltage-gated outward K+ Kv currents are the primary determinants of AP repolarization (57, 69), and, therefore, voltage-clamp experiments were performed to test the influence of Notch signaling on Kv current densities. Specifically, the amplitudes of Ito, IK,slow1, IK,slow2, and Iss were studied by fitting the decay phase of the total outward K+ current elicited by a 25 s-depolarizing step (17, 48, 69). The analysis was performed in 1) GFP-negative myocytes from MCM-WT mice, 2) GFP-NICD-positive myocytes from MCM-NICD-GFP mice, and 3) GFP-positive myocytes from animals with tamoxifen-inducible MerCreMer transgene-driving myocyte-restricted expression of GFP (MCM-GFP). The latter group of cells was introduced to evaluate the potential effects of the Cre recombinase system on K+ currents. Quantitatively, Ito and IK,slow1 components of Kv currents were significantly reduced in NICD-expressing myocytes, whereas IK,slow2 and Iss were comparable in the three groups of cells (Fig. 4).
Fig. 4.
The Notch1 intracellular domain (NICD) reduces Kv current components of adult myocytes. A: whole cell voltage-gated K+ currents recorded in voltage clamp in a green fluorescent protein (GFP)-negative myocyte isolated from a wild-type (WT) mouse (MCM-WT) and a GFP-positive cell obtained from a transgenic mouse with NICD expression (MCM-NICD-GFP). The voltage-clamp protocol consisting of a 25 s-depolarizing step is shown at the bottom. B: fitted curves of current traces shown in A with multiexponential function (black curves) and monoexponential fitted components (shades of gray curves). For the MCM-NICD-GFP myocyte, a biexponential function was used because this cell lacked transiet outward K+ current (Ito). C: quantitative data for the amplitude of Kv current components in GFP-negative myocytes from MCM-WT mice (WT, n = 17; from 3 animals), GFP-positive myocytes from a MCM-GFP mouse (GFP, n = 11), and GFP-positive myocytes from MCM-NICD-GFP mice (NICD, n = 18; from 4 animals) are shown as means ± SE. Ito, K+ currents with rapid activation; IK,slow1 and IK,slow2, intermediate and slow kinetics of inactivation; Iss, sustained (noninactivating) component. *P < 0.05 vs. WT; **P < 0.05 vs. GFP.
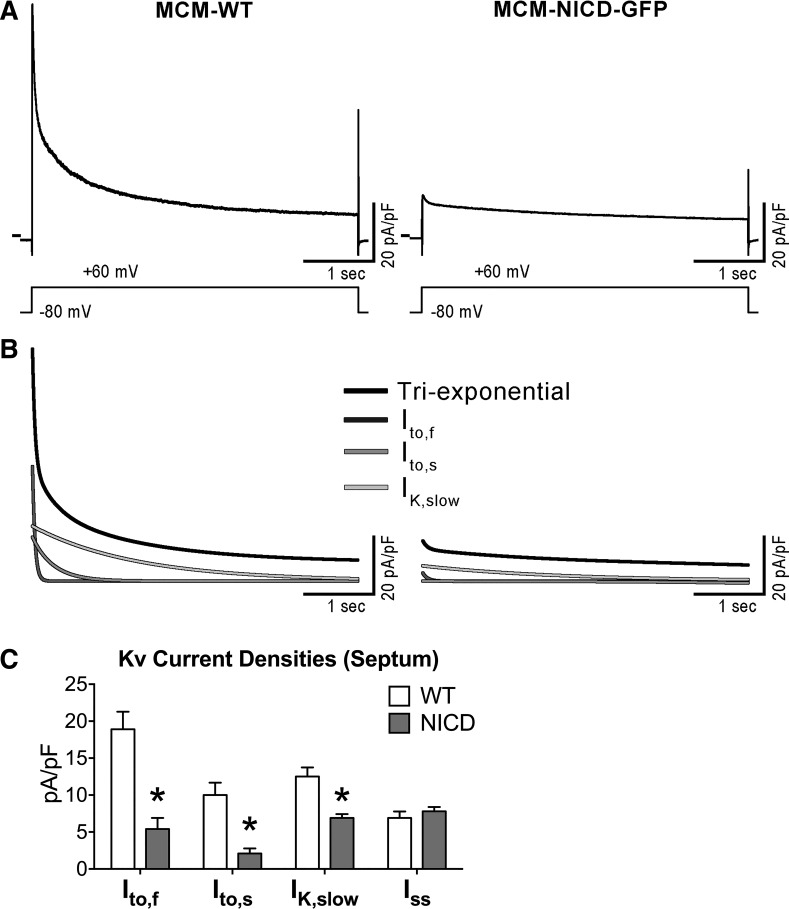
The transient outward current (Ito) can be further separated in components with fast (Ito,f) and slow (Ito,s) inactivation kinetics (17, 23, 25, 48, 83). Thus, we implemented additional tests to determine which of these currents were affected by the activation of Notch signaling. For this purpose, we studied cells obtained from the intraventricular septum, the anatomic region of the heart where myocytes present both Ito,f and Ito,s (17, 83). Specifically, the decay phases of outward currents evoked by a 4.5 s-depolarization steps were analyzed with a triexponential function to dissect Ito,f, Ito,s, and IK,slow based on their distinct time constants of decay, which differ by an order of magnitude (23, 25, 83). Both Ito,f and Ito,s were substantially reduced in NICD-expressing myocytes compared with WT myocytes, contributing in a similar manner to the overall reduction of Ito. Additionally, NICD-expressing cells presented diminished IK,slow, whereas Iss was not affected (Fig. 5).
Fig. 5.
The Notch1 intracellular domain (NICD) affects fast and slow components of the transient outward current (Ito). A: whole cell voltage-gated K+ currents recorded in voltage clamp in a green fluorescent protein (GFP)-negative myocyte isolated from the intraventricular septum of a wild-type (WT) mouse (MCM-WT) and a GFP-positive cell from the intraventricular septum of a transgenic mouse with NICD expression (MCM-NICD-GFP). The voltage-clamp protocol consisting of a 4.5 s-depolarizing step is shown at the bottom. B: fitted curves of current traces shown in A with triexponential function (black curves) and relative monoexponential fitted components (shades of gray curves). C: quantitative data for the amplitude of Kv current components in GFP-negative myocytes from the ventricular septum of MCM-WT mice (WT, n = 18; from 3 animals) and GFP-positive myocytes from the ventricular septum of MCM-NICD-GFP mice (NICD, n = 17; from 5 animals) are shown as means ± SE. *P < 0.01 vs. WT.
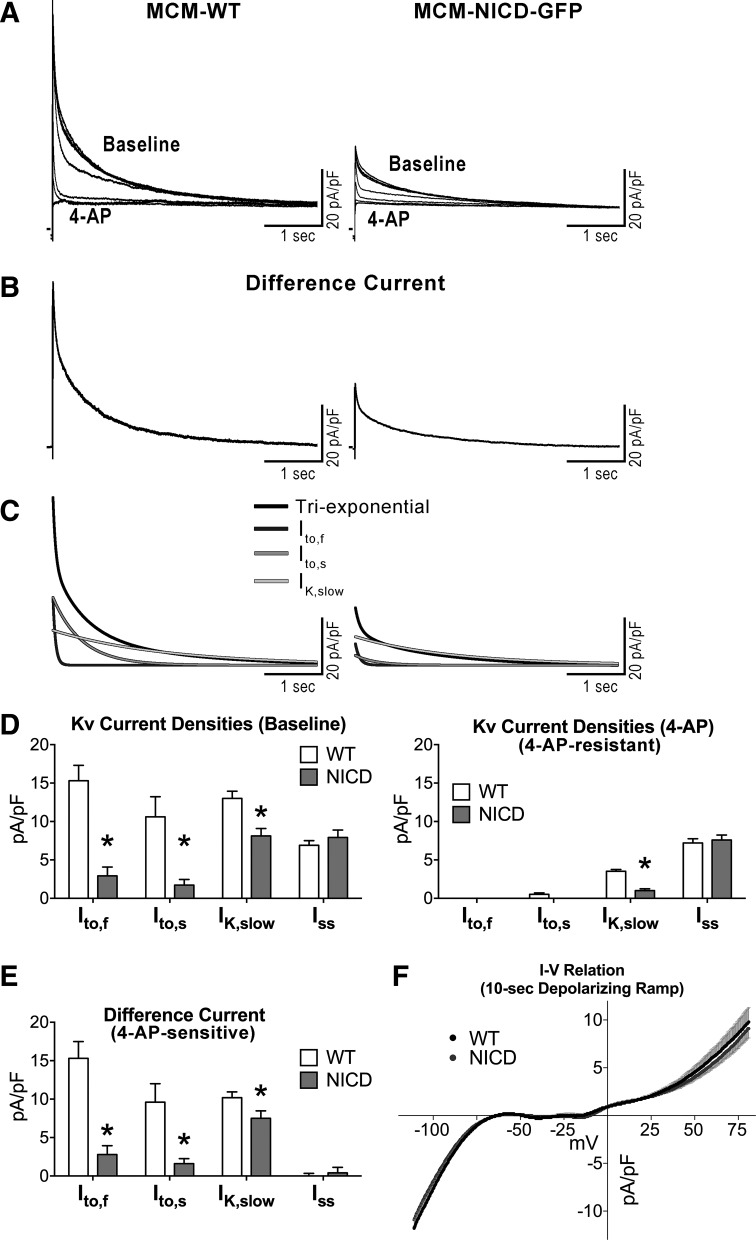
To better define the nature of K+ currents altered by Notch signaling activation, myocytes from WT and NICD-overexpressing mice were exposed to 4-aminopyridine, a blocker of Kv1 and Kv4 channel subunits (48, 83). K+ currents were elicited using 4.5-s depolarization steps before and after application of 5 mM 4-aminopyridine; the amplitude of the 4-aminopyridine-sensitive current was evaluated for the two groups of cells by subtracting currents in the presence of the drug from currents obtained in baseline condition (Fig. 6, A–C). For both cell types, exposure to 4-aminopyridine eliminated Ito,f and Ito,s and reduced IK,slow, whereas no major changes were observed for Iss (Fig. 6D). In the presence of the drug, the component of IK,slow not affected by 4-aminopyridine remained larger in WT myocytes with respect to NICD myocytes. Importantly, quantification of the difference current before and after exposure to the blocker indicated that the 4-aminopyridine-sensitive components of Ito,f, Ito,s, and IK,slow were reduced in myocytes with active NICD (Fig. 6E). Thus, Notch signaling activation reduces Kv currents that are sensitive to 4-aminopyridine (i.e., Ito,f, Ito,s, and part of IK,slow) or resistant to this blocker (i.e., a component of IK,slow).
Fig. 6.
The Notch1 intracellular domain (NICD) affects 4-aminopyridine (4-AP)-sensitive currents. A: whole cell voltage-gated K+ currents recorded in voltage clamp in a green fluorescent protein (GFP)-negative myocyte isolated from a wild-type (WT) mouse (MCM-WT) and a GFP-positive cell obtained from a transgenic mouse with NICD expression (MCM-NICD-GFP) before (Baseline) and after progressive exposure to 5 mM 4-AP. B: 4-AP-sensitive (Difference) current obtained by subtracting current traces before and after exposure to 4-AP shown in A. C: fitted curves of difference current traces shown in B with triexponential function (black curves) and relative monoexponential fitted components (shades of gray curves). D: quantitative data for the amplitude of Kv current components before (left) and after (right) exposure to 5 mM 4-AP for GFP-negative myocytes from MCM-WT mice (WT, n = 14; from 3 animals) and GFP-positive myocytes from MCM-NICD-GFP mice (NICD, n = 9; from 2 animals) are shown as means ± SE. *P < 0.05 vs. WT. E: quantitative data for the amplitude of 4-AP-sensitive (Difference) currents for myocytes reported in D. F: current-voltage (I–V) relations obtained by 10 s-depolarizing ramps from −110 to +80 mV. Data for MCM-WT mice (WT, n = 16; from 5 animals) and GFP-positive myocytes from MCM-NICD-GFP mice (NICD, n = 20; from 5 animals) are shown as means ± SE. Inward current at negative potential reflects inward rectifier K+ current.
To test whether activation of Notch signaling had any consequences on IK1, which controls resting Vm in ventricular myocytes, slow depolarizing ramps were used (18, 66). No differences were observed between WT and NICD myocytes (Fig. 6F) in the range of potential where IK1 is operative. Thus, Notch signaling reduces Kv current components with fast and intermediate inactivating kinetics, contributing, at least in part, to the protracted repolarization phase of the AP.
Notch interferes with Kv currents and KChIP2 expression in neonatal myocytes.
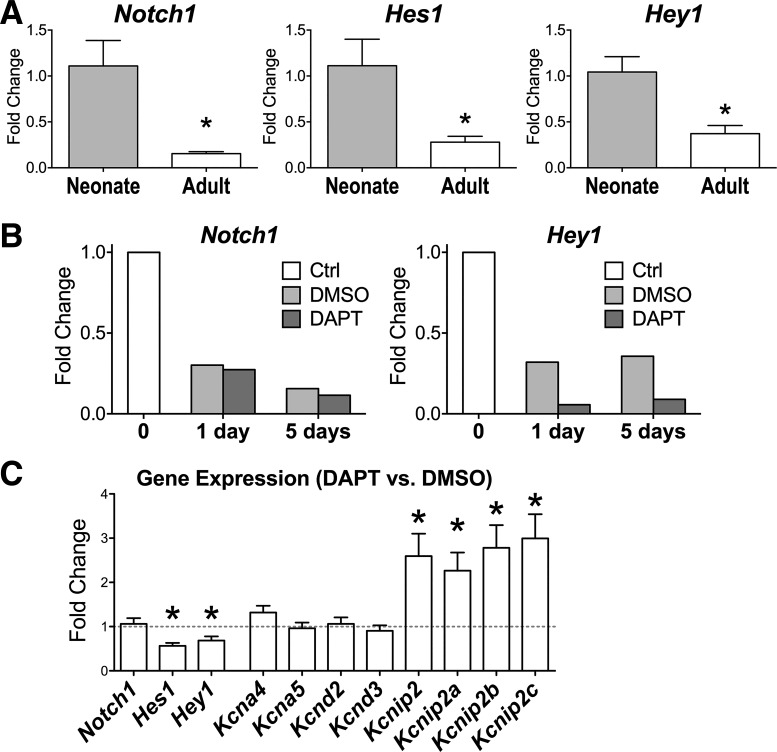
The expression of Notch receptors is progressively reduced in postnatal hearts (22), whereas outward K+ currents gradually appear in maturing cardiomyocytes, resulting in a progressive shortening of the AP duration (19, 34, 78). To test the possibility of a causative link between Notch activation and electrophysiological properties of developing myocytes, Notch signaling was perturbed using an antagonist of γ-secretase, the enzyme responsible for the cleavage of the Notch receptor and subsequent translocation of active NICD to the nucleus (14, 16). The γ-secretase inhibitor DAPT was used based on the ability of this compound to interfere with Notch signaling in various cell types under in vitro conditions (1, 38, 45, 77, 84). As previously reported (22), transcripts for the Notch1 receptor and downstream targets of Notch signaling were highly expressed in neonatal hearts compared with adult hearts (Fig. 7A). Thus, neonatal cells obtained from C57Bl/6 WT mice at postnatal day 2 were cultured and treated with DAPT or vehicle alone (DMSO). Transcripts of the Notch1 receptor and Hey1 gradually declined when neonatal myocytes were cultured for 1 or 5 days in the presence of DAPT or DMSO, but the γ-secretase inhibitor further decreased expression of Hey1, indicating that this compound effectively interferes with Notch signaling (Fig. 7B). Thus, we used this approach to modulate Notch signaling in cardiomyocytes and test the effects of this intervention on the expression of Kv channel subunit and Kv currents. Quantitatively, compared with vehicle-treated cells, levels of Kcna4, Kcna5, Kcnd2, and Kcnd3, which encode the K+ channel subunits Kv1.4, Kv1.5, Kv4.2, and Kv4.3, respectively, were not affected by DAPT treatment. Conversely, levels of Kcnip2 and relative isoforms Kcnip2a, Kcnip2b, and Kcnip2c, which encode KChIP2, modulators of Ito (35, 59, 79), were increased in the group of cells with perturbed Notch signaling (Fig. 7C).
Fig. 7.
Inhibition of Notch1 intracellular domain (NICD) formation in neonatal myocytes alters their gene expression profile. A: transcript levels, by RT-PCR, for the Notch1 receptor and downstream target genes Hes1 and Hey1 in the myocardium of C57Bl/6 mice at 5–7 days (Neonate, n = 4) and 3.8 mo (Adult, n = 4) of age. *P < 0.05 vs. neonate. B: transcript levels for the Notch1 receptor and its downstream target gene Hey1 relative to a preparation of isolated neonatal cardiomyocytes before [control (Ctrl)] and after culture with a γ-secretase inhibitor (DAPT) or vehicle (DMSO) for 1 or 5 days. C: average changes for the expression of transcripts in cultured neonatal myocytes treated with the γ-secretase inhibitor (DAPT) relative to vehicle-treated cells (dashed line) obtained from the same isolations (n = 11 cell cultures for each condition). Cells were cultured for 1–6 days after the initial treatment. Data are shown as means ± SE. *P < 0.05 DAPT- vs. vehicle-treated cells.
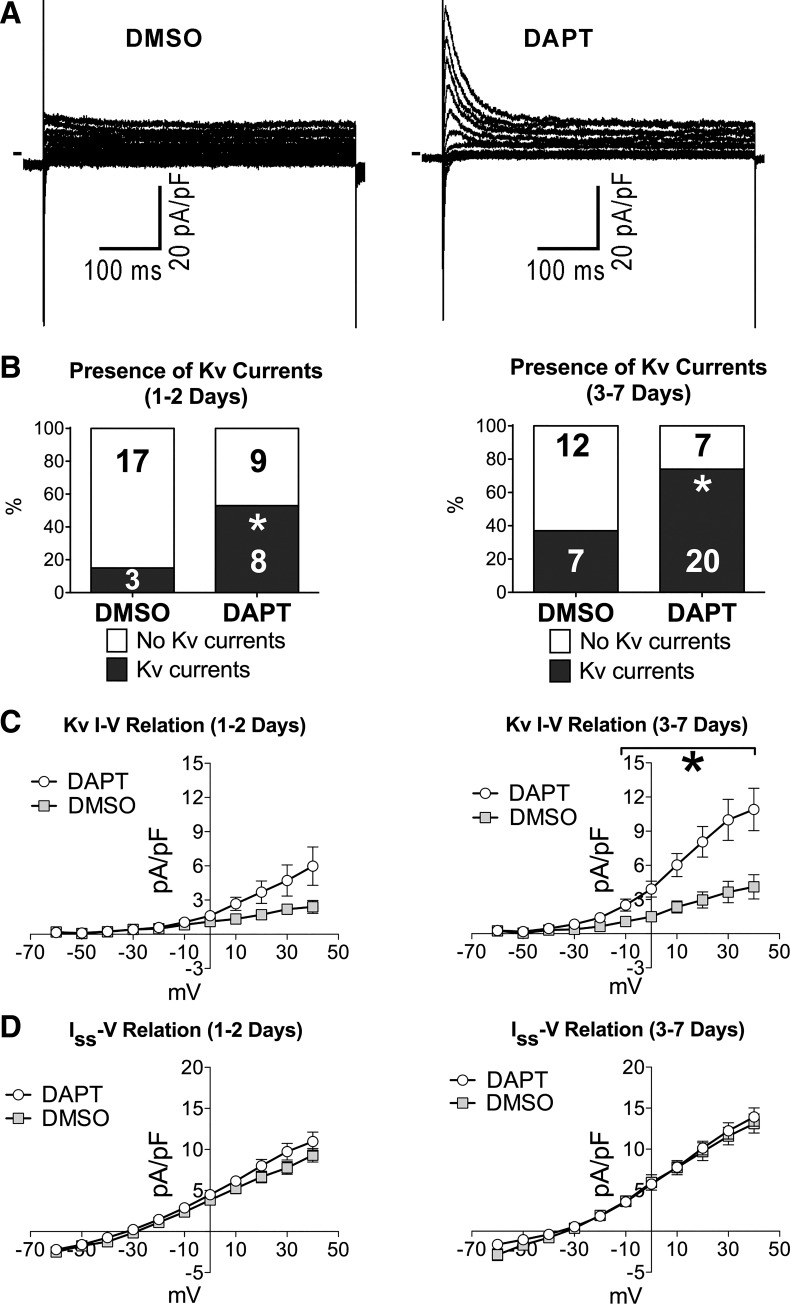
The effects of Notch signaling inhibition on Kv currents were established at 1, 2, and 3−7 days after treatment with DAPT. By patch clamp, the fraction of neonatal myocytes presenting fast activating and rapid inactivating K+ currents was progressively elevated with culture time and was twofold greater in myocytes treated with DAPT compared with vehicle-treated myocytes (Fig. 8, A and B). Additionally, overall Kv current density was severalfold larger in cells exposed to the γ-secretase inhibitor relative to control, but the amplitude of the sustained component (Iss) was comparable (Fig. 8C). These results point to a positive correlation between activated KChIP2 transcription and enhanced Ito densities in myocytes with reduced Notch signaling.
Fig. 8.
Inhibition of Notch1 intracellular domain (NICD) formation in neonatal myocytes promotes Kv currents. A: whole cell voltage-gated K+ currents recorded in voltage clamp in neonatal myocytes in cultures treated with vehicle alone (DMSO) or a γ-secretase inhibitor (DAPT). Currents were elicited by a family of depolarizing steps from a holding potential (Vh) of −70 mV. B: fraction of myocytes presenting outward K+ Kv components in the groups of cells treated with vehicle (DMSO, n = 20 at 1–2 days; n = 19 at 3–7 days) or γ-secretase inhibitor (DAPT, n = 17 at 1–2 days; n = 27 at 3–7 days). The numbers of cells expressing or negative for Kv currents are shown in the graphs. *P < 0.05 vs. DMSO. C: quantitative data for the rapidly activating outward Kv currents in cells reported in B. *P < 0.05 vs. DMSO. D: quantitative data for the sustained current (Iss) measured during the last 50 ms of depolarizing steps in cells shown in B and C.
Notch interferes with KChIP2 expression in adult myocytes.
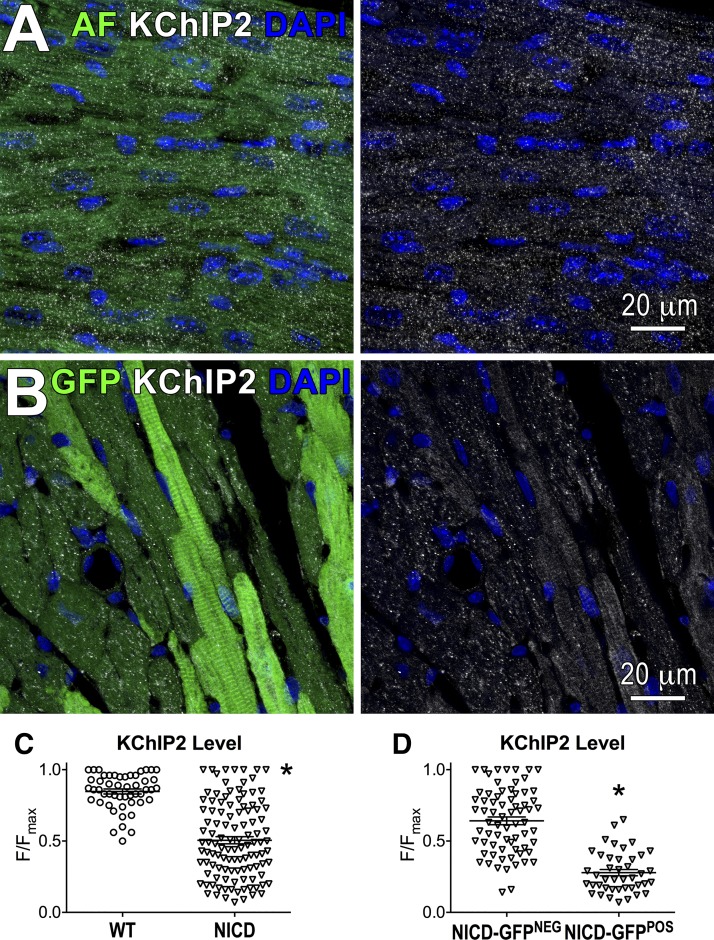
Based on in vitro findings, expression of KChIP2 was evaluated in the heart of transgenic mice with active NICD to substantiate the possibility of a causative link between Notch1 activation and modulation of KChIP2 expression. By immunohistochemistry, KChIP2 was homogeneously distributed in the myocardium of MCM-WT mice, whereas levels of this protein were variably localized in the ventricular tissue of MCM-NICD-GFP mice. Specifically, levels of KChIP2 were reduced in GFP-positive myocytes with respect to GFP-negative myocytes (Fig. 9). Thus, Notch signaling activation represses KChIP2 expression.
Fig. 9.
Notch signaling activation reduces Kv channel-interacting protein 2 (KChIP2) expression in cardiomyocytes. A: KChIP2 (white) expression in the left ventricular (LV) myocardium of a MCM-WT mouse. Nuclei are stained by DAPI. Autofluorescence (AF) in the green channel was collected to visualize the structure of myocardial tissue. The green channel is omitted at right. B: green fluorescent protein (GFP; green) and KChIP2 (white) expression in the LV myocardium of a MCM-NICD-GFP mouse. Nuclei are stained by DAPI. The green channel is omitted at right. C: quantification of KChIP2 levels in myocytes in LV tissue of MCM-WT mice [wild type (WT), n = 2 mice] and MCM-NICD-GFP mice [Notch1 intracellular domain (NICD), n = 3 mice]. For each acquired image, the intensity of the fluorescent signal (F) from individual myocytes was normalized with respect to the signal of the myocyte presenting the highest fluorescent intensity (Fmax). Normalized data are presented as F/Fmax. *P < 0.001 vs. WT. D: quantification of relative KChIP2 levels in NICD-GFP-negative and NICD-GFP-positive myocytes in the myocardium of the MCM-NICD-GFP mice (NICD) shown in C. *P < 0.001 vs. NICD-GFP negative.
DISCUSSION
The results of the present study indicate that Notch signaling controls the electrophysiological properties of cardiomyocytes by negatively regulating Kv and INa densities. Also, our findings support the possibility that the modulatory action of NICD on the electrical behavior of neonatal and adult cells involves, at least in part, repression of KChIP2 gene expression.
The ability of cleaved NICD to modify the cellular electrophysiology of mouse ventricular myocytes has been previously reported (63, 64), but the ionic basis for the protracted electrical recovery found in cells with Notch activation was not clarified. A close resemblance of the AP properties of Notch-positive cells and myocytes of the cardiac conduction system was noted, together with the identification of a conduction-like phenotype of the transcriptional profile of newborn myocytes with ectopic Notch expression (64). More recently, additional studies have provided novel information on the mechanisms of action of Notch signaling on the electrophysiological aspects of ventricular and atrial myocytes, which include the regulation of Kv currents (40, 61). Our results complement these findings and strengthen the notion that Notch signaling has the ability to modulate the electrical behavior of cardiomyocytes during development and adult life.
Protracted electrical repolarization is a typical feature of neonatal cells (78, 81), which is also observed in myocytes of the postinfarcted, hypertrophied, failing, and aged heart (2–4, 27, 28, 32, 37, 58, 69, 73, 76). Thus, common gene regulatory networks active in the fetal and diseased myocardium may account for these phenotypic similarities. The finding that Notch levels decline postnatally and are partly restored after ischemic insults (22, 44), hypertrophy (10), or heart failure (40) favors the possibility that this receptor contributes to the electrophysiological adaptations of cardiomyocytes during development and with intervening pathologies. Thus, additional studies are necessary to define a causative relationship between Notch expression and electrical remodeling in myocytes under diseased conditions. Moreover, abundance of Notch ligands may represent an important variable in the electrophysiological alterations occurring in the damaged heart and this aspect also requires further clarification.
Our data indicate that Notch signaling maintains neonatal myocytes in an immature electrophysiological state and has the capability to remodel the AP of adult cells. Prolongation of the AP under conditions of increased workload of the myocardium represents an adaptive mechanism of inotropic support (5, 39, 68, 69, 80), whereas long AP in myocytes during the perinatal period seems the necessary condition to sustain sarcolemmal Ca2+ entry for cell contraction, in view of the immature t-tubular/sarcoplasmic reticulum systems of developing cells (43). Thus, by modulating the electrical properties of myocytes, Notch signaling may have important consequences on myocardial mechanical function.
Outward Kv currents in cardiomyocytes comprise components that differ in biophysical and pharmacological properties: Ito,f (encoded by subunits Kv4.2 and Kv4.3), Ito,s (encoded by Kv1.4), IK,slow (encoded by Kv1.5 and Kv2.1), and Iss (based on the two-pore domain K+ channel TASK1) (26, 48, 50, 51, 74, 82). The analysis of the decay of K+ currents and the use of 4-aminopyridine in WT and NICD myocytes allowed us to identify that Ito,f, Ito,s, and IK,slow are critically reduced by Notch1 signaling. We found that KChIP2 levels declined in NICD-positive myocytes, constituting, at least in part, the molecular basis for the decrease of Ito,f. In fact, KChIP2 was reported to coimmunoprecipate (24, 47), stabilize Kv4.2 and Kv4.3 proteins, and generate Ito,f in myocytes (17, 46, 74). However, KChIP2 does not modulate Ito,s (17, 74), and, although it reduces Kv1.5-based current in a heterologous expression system (47), there is no indication that KChIP2 interferes with the 4-aminopyridine-resistant component of IK,slow, which was found to be declined in NICD myocytes. Thus, the molecular basis for the observed reduction of Kv currents in cardiomyocytes in the presence of Notch activation remains to be defined. Interestingly, silencing of KChIP2 reduces the expression of the Na+ channel protein Nav1.5 (15, 56), a factor that may have contributed to the decline in INa density, attenuation of AP amplitude, and slower rate of depolarization in NICD-expressing cells.
Four Notch proteins (Notch1–Notch4) have been identified in mammals (42), raising the possibility that effects observed with γ-secretase inhibition in neonatal myocytes may have resulted from perturbation of multiple receptors. Therefore, caution has to be exercised when interpreting the consequences of the pharmacological modulation of Notch signaling in neonatal cells and overexpression of the Notch1 intracellular domain in adult transgenic myocytes. Specifically, whether the reduction of Kv currents is mediated exclusively by Notch1 activation or is achieved by the redundant action of Notch2–Notch4 signaling remains to be established. In this regard, a mutation of Notch3, which has been linked to a hereditary form of cerebral small vessel disease (CADSIL syndrome) (36), led to upregulation of Kv1 channel current density in smooth muscle cells from rodent cerebral arteries (13). This condition closely mimics the effects of γ-secretase inhibition in neonatal myocytes and supports the possibility of redundancy in the role of Notch1–Notch4 signaling.
Study limitations.
Kv current and INa were initially evaluated in NICD-expressing myocytes based on consistent differences in the early repolarization phase of the AP and reduced upstroke amplitude and velocity with respect to WT cells. Although our results indicate that conductance of Kv and Na+ channels is critically modulated by Notch signaling, we cannot exclude with certainty that other ionic currents are also affected. In this regard, KChIP2 expression alters L-type Ca2+ current by binding to the NH2-terminal inhibitory module of Cav1.2 (75) and influencing Cav1.2 protein expression (56), a cascade of events that may have occurred in NICD-expressing myocytes.
Conclusions.
Our results support the notion that Notch signaling represents an important regulator of the physiological properties of neonatal and adult cardiomyocytes. Studies aimed at clarifying the sequence of events associated with Notch receptor activation and defining the role of this signaling axis on the electrical remodeling of myocytes in pathological conditions are warranted.
GRANTS
This work was supported by National Institute of Health Grants R01-HL-091021, R01- HL-114346, P01-AG-043353, P01-HL-092868, R01-AG-037495, R01-HL-111183, R01-AG-037490, R01-HL-105532, and R37-HL-081737 and intramural resources at New York Medical College (NYMC) including funds from the NYMC Translation Science Institute.
DISCLOSURES
P. Anversa is a member of Autologous, LLP, and P. Anversa and A. Leri are members of AAL Scientifics Corporation. All other authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
G.B., C.A.E., S.S., A.S., K.K., A.A.-V., J.G.E., M.N., K.Q., L.M.E., and M.R. performed experiments; G.B., S.S., A.S., K.K., and M.R. analyzed data; G.B., S.S., A.S., and M.R. interpreted results of experiments; G.B., S.S., L.M.E., and M.R. prepared figures; G.B., S.S., and M.R. drafted manuscript; G.B., C.A.E., S.S., A.S., K.K., A.A.-V., J.G.E., M.N., K.Q., D.S., P.G., A.L., P.A., L.M.E., J.T.J., T.H.H., and M.R. edited and revised manuscript; G.B., C.A.E., S.S., A.S., K.K., A.A.-V., J.G.E., M.N., K.Q., D.S., P.G., A.L., P.A., L.M.E., J.T.J., T.H.H., and M.R. approved final version of manuscript; M.R. conceived and designed research.
REFERENCES
- 1.Al Haj Zen A, Oikawa A, Bazan-Peregrino M, Meloni M, Emanueli C, Madeddu P. Inhibition of delta-like-4-mediated signaling impairs reparative angiogenesis after ischemia. Circ Res 107: 283–293, 2010. doi: 10.1161/CIRCRESAHA.110.221663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armoundas AA, Wu R, Juang G, Marbán E, Tomaselli GF. Electrical and structural remodeling of the failing ventricle. Pharmacol Ther 92: 213–230, 2001. doi: 10.1016/S0163-7258(01)00171-1. [DOI] [PubMed] [Google Scholar]
- 3.Aronson RS. Characteristics of action potentials of hypertrophied myocardium from rats with renal hypertension. Circ Res 47: 443–454, 1980. doi: 10.1161/01.RES.47.3.443. [DOI] [PubMed] [Google Scholar]
- 4.Beuckelmann DJ, Näbauer M, Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res 73: 379–385, 1993. doi: 10.1161/01.RES.73.2.379. [DOI] [PubMed] [Google Scholar]
- 5.Bouchard RA, Clark RB, Giles WR. Effects of action potential duration on excitation-contraction coupling in rat ventricular myocytes. Action potential voltage-clamp measurements. Circ Res 76: 790–801, 1995. doi: 10.1161/01.RES.76.5.790. [DOI] [PubMed] [Google Scholar]
- 6.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7: 678–689, 2006. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 7.Brunet S, Aimond F, Li H, Guo W, Eldstrom J, Fedida D, Yamada KA, Nerbonne JM. Heterogeneous expression of repolarizing, voltage-gated K+ currents in adult mouse ventricles. J Physiol 559: 103–120, 2004. doi: 10.1113/jphysiol.2004.063347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chau MD, Tuft R, Fogarty K, Bao ZZ. Notch signaling plays a key role in cardiac cell differentiation. Mech Dev 123: 626–640, 2006. doi: 10.1016/j.mod.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cimini M, Cannatá A, Pasquinelli G, Rota M, Goichberg P. Phenotypically heterogeneous podoplanin-expressing cell populations are associated with the lymphatic vessel growth and fibrogenic responses in the acutely and chronically infarcted myocardium. PLoS One 12: e0173927, 2017. doi: 10.1371/journal.pone.0173927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Croquelois A, Domenighetti AA, Nemir M, Lepore M, Rosenblatt-Velin N, Radtke F, Pedrazzini T. Control of the adaptive response of the heart to stress via the Notch1 receptor pathway. J Exp Med 205: 3173–3185, 2008. doi: 10.1084/jem.20081427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crumb WJ Jr, Pigott JD, Clarkson CW. Comparison of Ito in young and adult human atrial myocytes: evidence for developmental changes. Am J Physiol Heart Circ Physiol 268: H1335–H1342, 1995. [DOI] [PubMed] [Google Scholar]
- 12.D’Amato G, Luxán G, del Monte-Nieto G, Martínez-Poveda B, Torroja C, Walter W, Bochter MS, Benedito R, Cole S, Martinez F, Hadjantonakis AK, Uemura A, Jiménez-Borreguero LJ, de la Pompa JL. Sequential Notch activation regulates ventricular chamber development. Nat Cell Biol 18: 7–20, 2016. doi: 10.1038/ncb3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dabertrand F, Krøigaard C, Bonev AD, Cognat E, Dalsgaard T, Domenga-Denier V, Hill-Eubanks DC, Brayden JE, Joutel A, Nelson MT. Potassium channelopathy-like defect underlies early-stage cerebrovascular dysfunction in a genetic model of small vessel disease. Proc Natl Acad Sci USA 112: E796–E805, 2015. doi: 10.1073/pnas.1420765112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature 398: 518–522, 1999. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 15.Deschênes I, Armoundas AA, Jones SP, Tomaselli GF. Post-transcriptional gene silencing of KChIP2 and Navbeta1 in neonatal rat cardiac myocytes reveals a functional association between Na and Ito currents. J Mol Cell Cardiol 45: 336–346, 2008. doi: 10.1016/j.yjmcc.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dovey HF, John V, Anderson JP, Chen LZ, de Saint Andrieu P, Fang LY, Freedman SB, Folmer B, Goldbach E, Holsztynska EJ, Hu KL, Johnson-Wood KL, Kennedy SL, Kholodenko D, Knops JE, Latimer LH, Lee M, Liao Z, Lieberburg IM, Motter RN, Mutter LC, Nietz J, Quinn KP, Sacchi KL, Seubert PA, Shopp GM, Thorsett ED, Tung JS, Wu J, Yang S, Yin CT, Schenk DB, May PC, Altstiel LD, Bender MH, Boggs LN, Britton TC, Clemens JC, Czilli DL, Dieckman-McGinty DK, Droste JJ, Fuson KS, Gitter BD, Hyslop PA, Johnstone EM, Li WY, Little SP, Mabry TE, Miller FD, Audia JE. Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J Neurochem 76: 173–181, 2001. doi: 10.1046/j.1471-4159.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- 17.Foeger NC, Wang W, Mellor RL, Nerbonne JM. Stabilization of Kv4 protein by the accessory K+ channel interacting protein 2 (KChIP2) subunit is required for the generation of native myocardial fast transient outward K+ currents. J Physiol 591: 4149–4166, 2013. doi: 10.1113/jphysiol.2013.255836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glynn P, Musa H, Wu X, Unudurthi SD, Little S, Qian L, Wright PJ, Radwanski PB, Gyorke S, Mohler PJ, Hund TJ. Voltage-gated sodium channel phosphorylation at Ser571 regulates late current, arrhythmia, and cardiac function in vivo. Circulation 132: 567–577, 2015. doi: 10.1161/CIRCULATIONAHA.114.015218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grandy SA, Trépanier-Boulay V, Fiset C. Postnatal development has a marked effect on ventricular repolarization in mice. Am J Physiol Heart Circ Physiol 293: H2168–H2177, 2007. doi: 10.1152/ajpheart.00521.2007. [DOI] [PubMed] [Google Scholar]
- 20.Grego-Bessa J, Luna-Zurita L, del Monte G, Bolós V, Melgar P, Arandilla A, Garratt AN, Zang H, Mukouyama YS, Chen H, Shou W, Ballestar E, Esteller M, Rojas A, Pérez-Pomares JM, de la Pompa JL. Notch signaling is essential for ventricular chamber development. Dev Cell 12: 415–429, 2007. doi: 10.1016/j.devcel.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gude N, Joyo E, Toko H, Quijada P, Villanueva M, Hariharan N, Sacchi V, Truffa S, Joyo A, Voelkers M, Alvarez R, Sussman MA. Notch activation enhances lineage commitment and protective signaling in cardiac progenitor cells. Basic Res Cardiol 110: 29, 2015. doi: 10.1007/s00395-015-0488-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gude NA, Emmanuel G, Wu W, Cottage CT, Fischer K, Quijada P, Muraski JA, Alvarez R, Rubio M, Schaefer E, Sussman MA. Activation of Notch-mediated protective signaling in the myocardium. Circ Res 102: 1025–1035, 2008. doi: 10.1161/CIRCRESAHA.107.164749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo W, Jung WE, Marionneau C, Aimond F, Xu H, Yamada KA, Schwarz TL, Demolombe S, Nerbonne JM. Targeted deletion of Kv4.2 eliminates Ito,f and results in electrical and molecular remodeling, with no evidence of ventricular hypertrophy or myocardial dysfunction. Circ Res 97: 1342–1350, 2005. doi: 10.1161/01.RES.0000196559.63223.aa. [DOI] [PubMed] [Google Scholar]
- 24.Guo W, Li H, Aimond F, Johns DC, Rhodes KJ, Trimmer JS, Nerbonne JM. Role of heteromultimers in the generation of myocardial transient outward K+ currents. Circ Res 90: 586–593, 2002. doi: 10.1161/01.RES.0000012664.05949.E0. [DOI] [PubMed] [Google Scholar]
- 25.Guo W, Li H, London B, Nerbonne JM. Functional consequences of elimination of ito,f and ito,s: early afterdepolarizations, atrioventricular block, and ventricular arrhythmias in mice lacking Kv1.4 and expressing a dominant-negative Kv4 alpha subunit. Circ Res 87: 73–79, 2000. doi: 10.1161/01.RES.87.1.73. [DOI] [PubMed] [Google Scholar]
- 26.Guo W, Xu H, London B, Nerbonne JM. Molecular basis of transient outward K+ current diversity in mouse ventricular myocytes. J Physiol 521: 587–599, 1999. doi: 10.1111/j.1469-7793.1999.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gwathmey JK, Copelas L, MacKinnon R, Schoen FJ, Feldman MD, Grossman W, Morgan JP. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ Res 61: 70–76, 1987. doi: 10.1161/01.RES.61.1.70. [DOI] [PubMed] [Google Scholar]
- 28.Gwathmey JK, Slawsky MT, Hajjar RJ, Briggs GM, Morgan JP. Role of intracellular calcium handling in force-interval relationships of human ventricular myocardium. J Clin Invest 85: 1599–1613, 1990. doi: 10.1172/JCI114611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol 244: 305–318, 2002. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 30.High FA, Epstein JA. The multifaceted role of Notch in cardiac development and disease. Nat Rev Genet 9: 49–61, 2008. doi: 10.1038/nrg2279. [DOI] [PubMed] [Google Scholar]
- 31.Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol 194: 237–255, 2003. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- 32.Janse MJ. Electrophysiological changes in heart failure and their relationship to arrhythmogenesis. Cardiovasc Res 61: 208–217, 2004. doi: 10.1016/j.cardiores.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 33.Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A. Signalling downstream of activated mammalian Notch. Nature 377: 355–358, 1995. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- 34.Jeck CD, Boyden PA. Age-related appearance of outward currents may contribute to developmental differences in ventricular repolarization. Circ Res 71: 1390–1403, 1992. doi: 10.1161/01.RES.71.6.1390. [DOI] [PubMed] [Google Scholar]
- 35.Jin H, Hadri L, Palomeque J, Morel C, Karakikes I, Kaprielian R, Hajjar R, Lebeche D. KChIP2 attenuates cardiac hypertrophy through regulation of Ito and intracellular calcium signaling. J Mol Cell Cardiol 48: 1169–1179, 2010. doi: 10.1016/j.yjmcc.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, Alamowitch S, Domenga V, Cécillion M, Marechal E, Maciazek J, Vayssiere C, Cruaud C, Cabanis EA, Ruchoux MM, Weissenbach J, Bach JF, Bousser MG, Tournier-Lasserve E. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature 383: 707–710, 1996. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- 37.Kääb S, Nuss HB, Chiamvimonvat N, O’Rourke B, Pak PH, Kass DA, Marban E, Tomaselli GF. Ionic mechanism of action potential prolongation in ventricular myocytes from dogs with pacing-induced heart failure. Circ Res 78: 262–273, 1996. doi: 10.1161/01.RES.78.2.262. [DOI] [PubMed] [Google Scholar]
- 38.Kanungo J, Zheng YL, Amin ND, Pant HC. The Notch signaling inhibitor DAPT down-regulates cdk5 activity and modulates the distribution of neuronal cytoskeletal proteins. J Neurochem 106: 2236–2248, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaprielian R, Wickenden AD, Kassiri Z, Parker TG, Liu PP, Backx PH. Relationship between K+ channel down-regulation and [Ca2+]i in rat ventricular myocytes following myocardial infarction. J Physiol 517: 229–245, 1999. doi: 10.1111/j.1469-7793.1999.0229z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khandekar A, Springer S, Wang W, Hicks S, Weinheimer C, Diaz-Trelles R, Nerbonne JM, Rentschler S. Notch-mediated epigenetic regulation of voltage-gated potassium currents. Circ Res 119: 1324–1338, 2016. doi: 10.1161/CIRCRESAHA.116.309877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koitabashi N, Bedja D, Zaiman AL, Pinto YM, Zhang M, Gabrielson KL, Takimoto E, Kass DA. Avoidance of transient cardiomyopathy in cardiomyocyte-targeted tamoxifen-induced MerCreMer gene deletion models. Circ Res 105: 12–15, 2009. doi: 10.1161/CIRCRESAHA.109.198416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137: 216–233, 2009. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korhonen T, Hänninen SL, Tavi P. Model of excitation-contraction coupling of rat neonatal ventricular myocytes. Biophys J 96: 1189–1209, 2009. doi: 10.1016/j.bpj.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kratsios P, Catela C, Salimova E, Huth M, Berno V, Rosenthal N, Mourkioti F. Distinct roles for cell-autonomous Notch signaling in cardiomyocytes of the embryonic and adult heart. Circ Res 106: 559–572, 2010. doi: 10.1161/CIRCRESAHA.109.203034. [DOI] [PubMed] [Google Scholar]
- 45.Kumar V, Palermo R, Talora C, Campese AF, Checquolo S, Bellavia D, Tottone L, Testa G, Miele E, Indraccolo S, Amadori A, Ferretti E, Gulino A, Vacca A, Screpanti I. Notch and NF-kB signaling pathways regulate miR-223/FBXW7 axis in T-cell acute lymphoblastic leukemia. Leukemia 28: 2324–2335, 2014. doi: 10.1038/leu.2014.133. [DOI] [PubMed] [Google Scholar]
- 46.Kuo HC, Cheng CF, Clark RB, Lin JJ, Lin JL, Hoshijima M, Nguyêñ-Trân VT, Gu Y, Ikeda Y, Chu PH, Ross J Jr, Giles WR, Chien KR. A defect in the Kv channel-interacting protein 2 (KChIP2) gene leads to a complete loss of Ito and confers susceptibility to ventricular tachycardia. Cell 107: 801–813, 2001. doi: 10.1016/S0092-8674(01)00588-8. [DOI] [PubMed] [Google Scholar]
- 47.Li H, Guo W, Mellor RL, Nerbonne JM. KChIP2 modulates the cell surface expression of Kv 1.5-encoded K+ channels. J Mol Cell Cardiol 39: 121–132, 2005. doi: 10.1016/j.yjmcc.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 48.Liu J, Kim KH, London B, Morales MJ, Backx PH. Dissection of the voltage-activated potassium outward currents in adult mouse ventricular myocytes: Ito,f, Ito,s, IK,slow1, IK,slow2, and Iss. Basic Res Cardiol 106: 189–204, 2011. doi: 10.1007/s00395-010-0134-z. [DOI] [PubMed] [Google Scholar]
- 49.Liu J, Lobe CG. Cre-conditional expression of constitutively active Notch1 in transgenic mice. Genesis 45: 259–265, 2007. doi: 10.1002/dvg.20282. [DOI] [PubMed] [Google Scholar]
- 50.London B, Guo W, Pan Xh, Lee JS, Shusterman V, Rocco CJ, Logothetis DA, Nerbonne JM, Hill JA. Targeted replacement of KV1.5 in the mouse leads to loss of the 4-aminopyridine-sensitive component of IK,slow and resistance to drug-induced qt prolongation. Circ Res 88: 940–946, 2001. doi: 10.1161/hh0901.090929. [DOI] [PubMed] [Google Scholar]
- 51.Marionneau C, Aimond F, Brunet S, Niwa N, Finck B, Kelly DP, Nerbonne JM. PPARalpha-mediated remodeling of repolarizing voltage-gated K+ (Kv) channels in a mouse model of metabolic cardiomyopathy. J Mol Cell Cardiol 44: 1002–1015, 2008. doi: 10.1016/j.yjmcc.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meo M, Meste O, Signore S, Sorrentino A, Cannata A, Zhou Y, Matsuda A, Luciani M, Kannappan R, Goichberg P, Leri A, Anversa P, Rota M. Reduction in Kv current enhances the temporal dispersion of the action potential in diabetic myocytes: insights from a novel repolarization algorithm. J Am Heart Assoc 5: e003078, 2016. doi: 10.1161/JAHA.115.003078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis 45: 593–605, 2007. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 54.Näbauer M, Kääb S. Potassium channel down-regulation in heart failure. Cardiovasc Res 37: 324–334, 1998. doi: 10.1016/S0008-6363(97)00274-5. [DOI] [PubMed] [Google Scholar]
- 55.Nakagawa O, McFadden DG, Nakagawa M, Yanagisawa H, Hu T, Srivastava D, Olson EN. Members of the HRT family of basic helix-loop-helix proteins act as transcriptional repressors downstream of Notch signaling. Proc Natl Acad Sci USA 97: 13655–13660, 2000. doi: 10.1073/pnas.250485597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nassal DM, Wan X, Liu H, Deschênes I. Myocardial KChIP2 expression in guinea pig resolves an expanded electrophysiologic role. PLoS One 11: e0146561, 2016. doi: 10.1371/journal.pone.0146561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev 85: 1205–1253, 2005. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- 58.Nordin C, Siri F, Aronson RS. Electrophysiologic characteristics of single myocytes isolated from hypertrophied guinea-pig hearts. J Mol Cell Cardiol 21: 729–739, 1989. doi: 10.1016/0022-2828(89)90614-7. [DOI] [PubMed] [Google Scholar]
- 59.Ozgen N, Lau DH, Shlapakova IN, Sherman W, Feinmark SJ, Danilo P Jr, Rosen MR. Determinants of CREB degradation and KChIP2 gene transcription in cardiac memory. Heart Rhythm 7: 964–970, 2010. doi: 10.1016/j.hrthm.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pedrazzini T. Control of cardiogenesis by the notch pathway. Trends Cardiovasc Med 17: 83–90, 2007. doi: 10.1016/j.tcm.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Qiao Y, Lipovsky C, Hicks S, Bhatnagar S, Li G, Khandekar A, Guzy R, Woo KV, Nichols CG, Efimov IR, Rentschler S. Transient Notch activation induces long-term gene expression changes leading to sick sinus syndrome in mice. Circ Res 121: 549–563, 2017. doi: 10.1161/CIRCRESAHA.116.310396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Radicke S, Cotella D, Graf EM, Banse U, Jost N, Varró A, Tseng GN, Ravens U, Wettwer E. Functional modulation of the transient outward current Ito by KCNE beta-subunits and regional distribution in human non-failing and failing hearts. Cardiovasc Res 71: 695–703, 2006. doi: 10.1016/j.cardiores.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 63.Rayatzadeh H, Cabral-Da Silva MC, Ferreira-Martins J, Ide-Iwata N, Steadman E, Gurusamy N, Zheng H, Arranto C, Sanada F, Ogorek B, Fiorini C, Anastasia L, Goichberg P, Kajstura J, Anversa P, Hosoda T, Urbanek K, Leri A, Rota M. Notch1 overexpression reprograms adult myocytes to an immature functional phenotype. Circulation 122: A14700, 2010. [Google Scholar]
- 64.Rentschler S, Yen AH, Lu J, Petrenko NB, Lu MM, Manderfield LJ, Patel VV, Fishman GI, Epstein JA. Myocardial Notch signaling reprograms cardiomyocytes to a conduction-like phenotype. Circulation 126: 1058–1066, 2012. doi: 10.1161/CIRCULATIONAHA.112.103390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rones MS, McLaughlin KA, Raffin M, Mercola M. Serrate and Notch specify cell fates in the heart field by suppressing cardiomyogenesis. Development 127: 3865–3876, 2000. [DOI] [PubMed] [Google Scholar]
- 66.Rota M, Vassalle M. Patch-clamp analysis in canine cardiac Purkinje cells of a novel sodium component in the pacemaker range. J Physiol 548: 147–165, 2003. doi: 10.1113/jphysiol.2003.039263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Russell JL, Goetsch SC, Gaiano NR, Hill JA, Olson EN, Schneider JW. A dynamic notch injury response activates epicardium and contributes to fibrosis repair. Circ Res 108: 51–59, 2011. doi: 10.1161/CIRCRESAHA.110.233262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sah R, Ramirez RJ, Oudit GY, Gidrewicz D, Trivieri MG, Zobel C, Backx PH. Regulation of cardiac excitation-contraction coupling by action potential repolarization: role of the transient outward potassium current (Ito). J Physiol 546: 5–18, 2003. doi: 10.1113/jphysiol.2002.026468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Signore S, Sorrentino A, Borghetti G, Cannata A, Meo M, Zhou Y, Kannappan R, Pasqualini F, O’Malley H, Sundman M, Tsigkas N, Zhang E, Arranto C, Mangiaracina C, Isobe K, Sena BF, Kim J, Goichberg P, Nahrendorf M, Isom LL, Leri A, Anversa P, Rota M. Late Na+ current and protracted electrical recovery are critical determinants of the aging myopathy. Nat Commun 6: 8803, 2015. doi: 10.1038/ncomms9803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Signore S, Sorrentino A, Ferreira-Martins J, Kannappan R, Shafaie M, Del Ben F, Isobe K, Arranto C, Wybieralska E, Webster A, Sanada F, Ogórek B, Zheng H, Liu X, Del Monte F, D’Alessandro DA, Wunimenghe O, Michler RE, Hosoda T, Goichberg P, Leri A, Kajstura J, Anversa P, Rota M. Inositol 1, 4, 5-trisphosphate receptors and human left ventricular myocytes. Circulation 128: 1286–1297, 2013. doi: 10.1161/CIRCULATIONAHA.113.002764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, Penninger JM, Molkentin JD. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res 89: 20–25, 2001. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 72.Sorrentino A, Borghetti G, Zhou Y, Cannata A, Meo M, Signore S, Anversa P, Leri A, Goichberg P, Qanud K, Jacobson JT, Hintze TH, Rota M. Hyperglycemia induces defective Ca2+ homeostasis in cardiomyocytes. Am J Physiol Heart Circ Physiol 312: H150–H161, 2017. doi: 10.1152/ajpheart.00737.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sorrentino A, Signore S, Qanud K, Borghetti G, Meo M, Cannata A, Zhou Y, Wybieralska E, Luciani M, Kannappan R, Zhang E, Matsuda A, Webster A, Cimini M, Kertowidjojo E, D’Alessandro DA, Wunimenghe O, Michler RE, Royer C, Goichberg P, Leri A, Barrett EG, Anversa P, Hintze TH, Rota M. Myocyte repolarization modulates myocardial function in aging dogs. Am J Physiol Heart Circ Physiol 310: H873–H890, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thomsen MB, Sosunov EA, Anyukhovsky EP, Ozgen N, Boyden PA, Rosen MR. Deleting the accessory subunit KChIP2 results in loss of Ito,f and increased IK,slow that maintains normal action potential configuration. Heart Rhythm 6: 370–377, 2009. doi: 10.1016/j.hrthm.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thomsen MB, Wang C, Ozgen N, Wang HG, Rosen MR, Pitt GS. Accessory subunit KChIP2 modulates the cardiac L-type calcium current. Circ Res 104: 1382–1389, 2009. doi: 10.1161/CIRCRESAHA.109.196972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tomaselli GF, Marbán E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res 42: 270–283, 1999. doi: 10.1016/S0008-6363(99)00017-6. [DOI] [PubMed] [Google Scholar]
- 77.Trombly DJ, Woodruff TK, Mayo KE. Suppression of Notch signaling in the neonatal mouse ovary decreases primordial follicle formation. Endocrinology 150: 1014–1024, 2009. doi: 10.1210/en.2008-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang L, Duff HJ. Developmental changes in transient outward current in mouse ventricle. Circ Res 81: 120–127, 1997. doi: 10.1161/01.RES.81.1.120. [DOI] [PubMed] [Google Scholar]
- 79.Wang Y, Xu H, Kumar R, Tipparaju SM, Wagner MB, Joyner RW. Differences in transient outward current properties between neonatal and adult human atrial myocytes. J Mol Cell Cardiol 35: 1083–1092, 2003. doi: 10.1016/S0022-2828(03)00200-1. [DOI] [PubMed] [Google Scholar]
- 80.Wickenden AD, Kaprielian R, Kassiri Z, Tsoporis JN, Tsushima R, Fishman GI, Backx PH. The role of action potential prolongation and altered intracellular calcium handling in the pathogenesis of heart failure. Cardiovasc Res 37: 312–323, 1998. doi: 10.1016/S0008-6363(97)00256-3. [DOI] [PubMed] [Google Scholar]
- 81.Wickenden AD, Kaprielian R, Parker TG, Jones OT, Backx PH. Effects of development and thyroid hormone on K+ currents and K+ channel gene expression in rat ventricle. J Physiol 504: 271–286, 1997. doi: 10.1111/j.1469-7793.1997.271be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu H, Barry DM, Li H, Brunet S, Guo W, Nerbonne JM. Attenuation of the slow component of delayed rectification, action potential prolongation, and triggered activity in mice expressing a dominant-negative Kv2 alpha subunit. Circ Res 85: 623–633, 1999. doi: 10.1161/01.RES.85.7.623. [DOI] [PubMed] [Google Scholar]
- 83.Xu H, Guo W, Nerbonne JM. Four kinetically distinct depolarization-activated K+ currents in adult mouse ventricular myocytes. J Gen Physiol 113: 661–678, 1999. doi: 10.1085/jgp.113.5.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang CP, Yang JL, Zhang J, Li L, Huang L, Ji SY, Hu ZY, Gao F, Liu YX. Notch signaling is involved in ovarian follicle development by regulating granulosa cell proliferation. Endocrinology 152: 2437–2447, 2011. doi: 10.1210/en.2010-1182. [DOI] [PubMed] [Google Scholar]