Abstract
Hypertension is a leading risk factor for vascular cognitive impairment and is strongly associated with carotid artery stenosis. In normotensive rats, chronic cerebral hypoperfusion induced by bilateral common carotid artery stenosis (BCAS) leads to cognitive impairment that is associated with impaired endothelium-dependent dilation in parenchymal arterioles (PAs). The aim of this study was to assess the effects of BCAS on PA function and structure in stroke-prone spontaneously hypertensive rats, a model of human essential hypertension. Understanding the effects of hypoperfusion on PAs in a hypertensive model could lead to the identification of therapeutic targets for cognitive decline in a model that reflects the at-risk population. We hypothesized that BCAS would impair endothelium-dependent dilation in PAs and induce artery remodeling compared with sham rats. PAs from BCAS rats had endothelial dysfunction, as assessed using pressure myography. Inhibition of nitric oxide and prostaglandin production had no effect on PA dilation in sham or BCAS rats. Surprisingly, inhibition of epoxyeicosatrienoic acid production increased dilation in PAs from BCAS rats but not from sham rats. Similar results were observed in the presence of inhibitors for all three dilatory pathways, suggesting that epoxygenase inhibition may have restored a nitric oxide/prostaglandin-independent dilatory pathway in PAs from BCAS rats. PAs from BCAS rats underwent remodeling with a reduced wall thickness. These data suggest that marked endothelial dysfunction in PAs from stroke-prone spontaneously hypertensive rats with BCAS may be associated with the development of vascular cognitive impairment.
NEW & NOTEWORTHY The present study assessed the structure and function of parenchymal arterioles in a model of chronic cerebral hypoperfusion and hypertension, both of which are risk factors for cognitive impairment. We observed that impaired dilation and artery remodeling in parenchymal arterioles and abolished cerebrovascular reserve capacity may mediate cognitive deficits.
Keywords: carotid stenosis, cognitive impairment, dilation, hypertension, parenchymal arterioles, cerebral arteries
INTRODUCTION
Chronic cerebral hypoperfusion is a major risk factor for cerebral small vessel disease, the second most common cause of vascular cognitive impairment (VCI) (68). Chronic cerebral hypoperfusion has been used to induce cognitive deficits that reflect many of the attributes of VCI (13). While the pathological neuronal changes after chronic cerebral hypoperfusion (13) have been thoroughly studied, little is known about parenchymal arterioles (PAs) that act as bottlenecks in the perfusion of the cerebral microcirculation where gas and nutrient exchange occurs (45). Interruption of blood flow through a single PA causes cognitive deficits in rats (57) highlighting the importance of studying these arterioles in a model of cerebral hypoperfusion. Moreover, patients with cerebral small vessel disease exhibit characteristic lesions in the subcortical brain regions (48, 67), a probable consequence of dysfunction of small arteries and arterioles in the parenchyma (19, 66).
Our laboratory has shown that chronic cerebral hypoperfusion, induced by bilateral common carotid artery stenosis (BCAS), impairs cognitive function and PA dilation in normotensive rats (37). Despite numerous studies emphasizing the role of midlife hypertension as a risk factor for VCI (19), the structural and functional changes in the PAs in hypertensive models with chronic cerebral hypoperfusion has been largely unexplored. In stroke-prone spontaneously hypertensive rats (SHRSP), a genetic model of essential hypertension (20), impaired endothelium-dependent dilation is evident in pial arteries and arterioles (40, 41). Cerebrovascular endothelial failure drives many of the detrimental microvascular changes in VCI patients (71). Thus, it is essential to understand the functional changes in PAs in a model of cognitive dysfunction that characterizes the comorbidities present in the VCI patient population, hypertension and chronic cerebral hypoperfusion.
Endothelium-dependent dilation is mediated by nitric oxide (NO), dilatory prostaglandins, and endothelium-derived hyperpolarizing factor (EDHF). We have shown that BCAS impairs EDHF-mediated dilation in PAs from normotensive rats through impaired epoxyeicosatrienoic acid (EET) signaling (37). Hypertension is a leading risk factor in VCI (19). VCI remains largely untreatable, and if we are to identify therapeutic targets for this condition, it is important that we understand the effects of its leading risk factors on the arteries that directly control perfusion of the parenchyma. In the present study, we hypothesized that BCAS in SHRSP, with malignant hypertension, would impair endothelium-dependent dilation and induce remodeling in PAs. In this study, we also examined the effect of BCAS on maximal reserve dilatory capacity in SHRSP since impaired maximal reserve in patients is associated with cognitive dysfunction.
METHODS
Animals and surgery.
Experimental procedures were approved by the Michigan State University Institutional Animal Care and Use Committee and was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (2011). Twenty-week-old male SHRSP, from the colony housed at Michigan State University, were randomized into two groups: one group had sham surgeries and the other group underwent BCAS surgery. Rats were maintained on a 12:12-h light-dark cycle with tap water and regular rat chow available ad libitum. To induce BCAS, rats were anesthetized with 3% isoflurane in oxygen and both common carotid arteries were exposed. A blunted 27-gauge needle was placed next to the artery, and two 6-0 silk sutures were used to firmly tie the carotid artery and needle together. After the ties were in place, the needle was carefully removed and the artery was reduced to the diameter of the needle (0.41 mm) to induce stenosis of the artery (37). The stenosis surgery had survival rate of 100%. Postsurgery, Ketoprofen (5 mg·kg−1·day−1) and Combi-pen 48 (22,000 U/day) were administered subcutaneously for 3 and 2 days, respectively. At 28–29 wk of age, rats were anesthetized with 3% isoflurane, weighed, and euthanized by decapitation after exsanguination. The organs and tissues used in this study were harvested after decapitation.
Measurement of cerebral perfusion.
Pial artery perfusion before and immediately after stenosis induction was assessed using a scanning laser-Doppler system (PeriScan PIM 3, Perimed, Stockholm, Sweden) while rats were under 3% isoflurane anesthesia with body temperature was maintained at 37°C. An incision was made in the top of the head to expose the skull, and the skull was cleaned for measurement of pial artery perfusion. Mean flow was analyzed using the LDPIwin 3.1 software (Perimed) (37).
Novel object recognition test.
Novel object recognition testing was conducted in an open box with opaque walls. Before testing, rats could explore the box for 15 min a day for 3 days. During the training phase, rats could explore two identical objects for 30 s. Ninety minutes later, during the test phase, a novel object replaced one of the familiar objects in the box. The familiar and novel objects were placed in opposite corners of the box, and the position was alternated between rats to prevent bias for a location. Exploration took place when the rat pawed at, sniffed, or whisked at a distance of 1 cm from the object (26). The time spent exploring the novel object and the total exploration time were recorded. After each trial, 70% alcohol was used to clean the objects to remove olfactory cues from the objects and box. Novel exploration quotient was expressed as a ratio of the time spent exploring the novel object to the total exploration time (43).
Morris water maze.
A circular tank (29 in. deep and 70 in. across) filled with water (30°C) was positioned in a room with external cues visible to the swimming rat. One week before surgery, rats were trained for 3 consecutive days; rats were repeatedly placed in the tank from all four possible directions (north, south, west, and east), and they learned to locate a platform 1 cm above the water level in <60 s. The test phase started 4 wk postsurgery. The test was carried out in trials of two from all four directions per session, and testing sessions were performed once a week until rats were 28 wk old. The protocol for the test was the same as training except the platform was hidden 1 in. below the surface of opaque water. Performance was measured by assessing escape latency (time required to reach the platform) (37, 69).
Acetazolamide challenge.
Rats were anesthetized and maintained at 37°C, acetazolamide (0.2 mg/g) was injected into the tail vein, and cerebral perfusion was assessed by scanning laser-Doppler as described above. Flow was measured every 5 min for 30 min (37). Cerebrovascular reserve capacity (CVR) over time was calculated using the following formula: [(flow after acetazolamide at time x – baseline pial artery perfusion)/baseline pial artery perfusion] × 100.
PA isolation and cannulation.
Eight weeks after BCAS, rats were euthanized and decapitated. The brain was removed and kept in ice-cold Ca2+-free physiological salt solution [PSS; containing (in mM) 140 NaCl, 5 KCl, 1 MgCl2·7H2O, 10 HEPES, and 10 dextrose] for the isolation of PAs. The section of brain tissue surrounding the middle cerebral artery was removed and placed in Ca2+-free PSS at 4°C with 1% BSA. The middle cerebral artery was gently separated from the surrounding tissue, and PAs branching from the middle cerebral artery were transferred to a cannulation chamber. PAs were cannulated between two glass pipettes mounted on small three-axis micromanipulators (MT XYZ, Newport, Irvine, CA), bathed in warm (37°C) PSS containing 1.8 mM Ca2+, and pressurized to 60 mmHg to allow for the generation of at least 20% spontaneous myogenic tone (37). The outer diameter of PAs was constantly tracked and recorded using MyoView 2.0 software (Danish Myo Technology, Aarhus, Denmark).
Assessment of dilatory pathways in PAs.
PAs that generated at least 20% tone were used for concentration-response experiments. Myogenic tone was calculated using the following formula: [1 − (active external diameter/passive external diameter)] × 100. A cumulative concentration response to abluminal administration of carbachol (1 nM−100 μM) was carried out. Intraluminal administration of drugs was not possible because of the small diameter and high flow resistance of the micropipettes. Each cannulated vessel was used for one concentration-response experiment. To isolate EDHF-dependent dilation, PAs were incubated with the NO synthase (NOS) inhibitor NG-nitro-l-arginine methyl ester (l-NAME; 100 μM) and the cyclooxygenase (COX) inhibitor indomethacin (Indo; 10 μM) for 30 min before the development of myogenic tone (70). EET-mediated dilation was assessed by incubating arteries with the cytochrome P-450 (CYP) epoxygenase inhibitor N-methylsulfonyl-6-(2-propargyloxyphenyl)hexanamide (MS-PPOH; 10 μM) in a similar fashion. GSK-1016790A (GSK101) was used to assess the function of transient receptor potential cation channel subfamily vanilloid member 4 (TRPV4), and sodium nitroprusside (SNP) was used to assess endothelium-independent dilation. MS-PPOH was dissolved in DMSO and GSK101 was dissolved in 100% ethanol such that the final concentration of the vehicles was <0.1%. Percent dilation was calculated using the following formula: [(external diameter at drug concentration − baseline external diameter)/(passive external diameter − baseline external diameter)] × 100.
Assessment of the structural and mechanical properties of the arterioles.
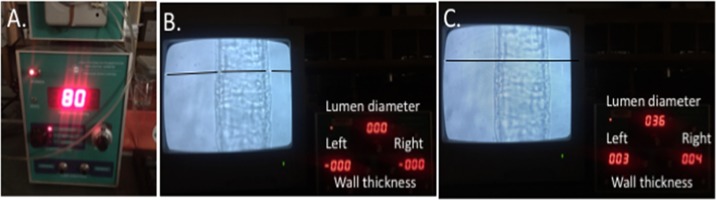
After the concentration-response curves were completed, PAs were placed in Ca2+-free PSS containing 2 mM EGTA and 10 μM SNP to assess their passive structure. A charge-coupled device camera (Hitachi Kokusai Electric) with the number of effective pixels 768 (width) × 494 (height) and a final magnification of ×1,100 was used. The camera was connected to a video dimension analyzer (Living Systems Instrumentation, Burlington, VT) that operates on the relative optical density changes of wall structures at the chosen level of a preselected scan line. The system was calibrated using a stage micrometer in accordance to the manufacturer’s protocol. Intraluminal pressure was increased from 3 to 180 mmHg in 20-mmHg increments, and lumen diameter and wall thickness were recorded after 5 min at each pressure step. Outer diameter was calculated as lumen diameter plus left and right wall thickness (Fig. 1). The wall-to-lumen ratio, passive distensibility, and circumferential wall stress were calculated as previously described (2).
Fig. 1.
Assessment of structural and mechanical properties of the arterioles. A: with the use of a PS-20 pressure servo controller with a peristaltic pump, intraluminal pressure was increased from 3 to 180 mmHg in 20-mmHg increments to assess passive structure (representative images are presented at an intraluminal pressure of 80 mmHg). B: premeasurement settings of the scan lines on the video dimension analyzer were set to identify the lumen and outer edge of the wall. All parameters measured zero while scan lines were open. C: scan lines were closed to provide the lumen diameter and left and right wall thickness. Scan lines were edited to appear darker for visual clarity.
Statistical analysis.
Novel object recognition test, myogenic tone, and resting lumen diameter data were analyzed by Student’s t-test. CVR, dilation, and passive and mechanical properties were analyzed by two-way ANOVA followed by Sidak correction for multiple comparisons. In cases of unequal variance, data were transformed to homogenize variance with the following formula: y = √y. Two-way ANOVA was then performed on the transformed data, and P < 0.05 was considered significant. Analyses were performed using GraphPad Prizm 6.0 software (La Jolla, CA).
Chemical reagents.
MS-PPOH was purchased from Cayman Chemical (Ann Arbor, MI), and acetazolamide was purchased from X-Gen (Big Flats, NY). All other chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO).
RESULTS
Eight weeks after BCAS cerebral perfusion was restored, but CVR was absent in both sham and BCAS rats.
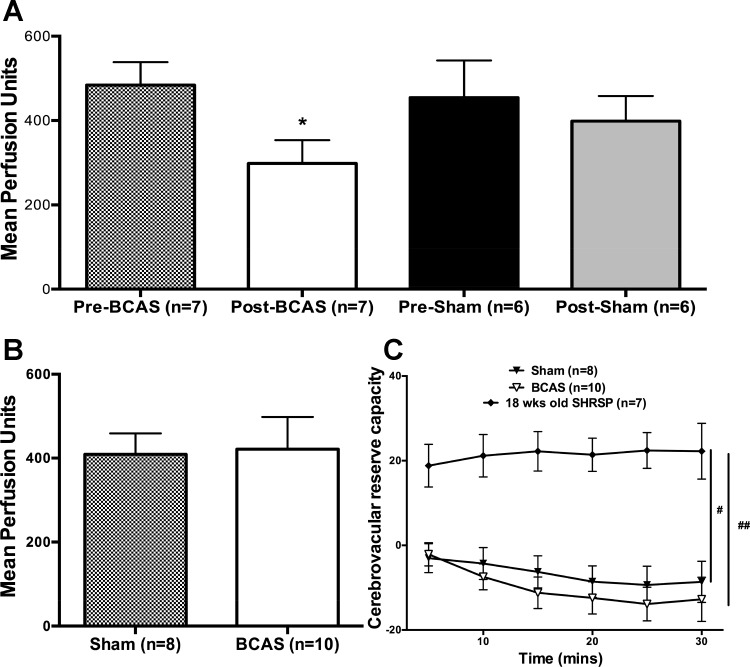
BCAS caused an immediate ≈38% reduction in cerebral blood flow, whereas the sham surgery had no effect (Fig. 2A). Blood flow in SHRSP with BCAS was restored 8 wk after stenosis such that there was no difference between sham and BCAS rats at that time point (Fig. 2B).
Fig. 2.
Cerebral perfusion was restored in bilateral common carotid artery stenosis (BCAS) rats and cerebrovascular reserve capacity was absent in both sham-operated (sham) and BCAS rats. A: BCAS surgery reduced cerebral perfusion, whereas perfusion levels were unchanged with sham surgery. B: at the end of 8 wk, there was no difference in cerebral perfusion between the groups. C: cerebrovascular reserve capacity was absent in both sham stroke-prone spontaneously hypertensive rats (SHRSP) and SHRSP with BCAS. However, 18-wk-old SHRSP had significantly higher reserve capacity compared with 28-wk-old SHRSP. *P < 0.05, different from sham rats; # and ##P < 0.05 vs. 18-wk-old SHRSP.
Acetazolamide, a carbonic anhydrase inhibitor, induces vasodilation in cerebral arteries and was used to assess CVR. Surprisingly, there was a complete absence of reserve capacity in both sham and BCAS SHRSP (Fig. 2C). To test if this absence of CVR could be attributed to chronic hypertension, we assessed CVR in younger (18 wk old) naive SHRSP. The expected increase in cerebral blood flow was observed in younger SHRSP, and CVR in 28-wk-old SHRSP and SHRSP with BCAS was significantly reduced compared with 18-wk-old SHRSP.
BCAS impaired memory function in SHRSP.
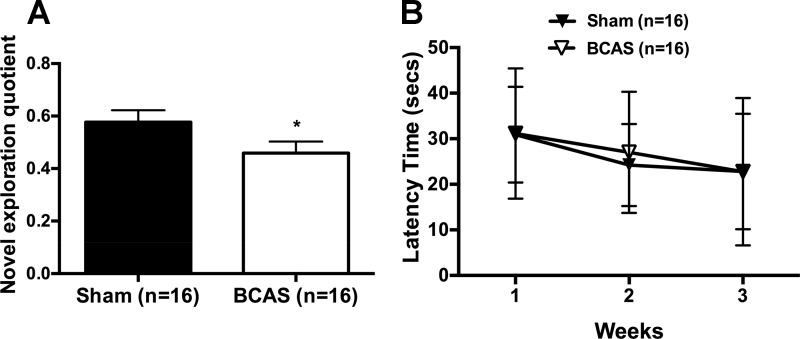
To verify that BCAS induced cognitive impairment in SHRSP, novel object recognition testing was carried out 7 wk postsurgery. The novel exploration quotient was reduced after BCAS, indicating impaired memory function (Fig. 3A). However, spatial learning abilities measured using the Morris water maze were not impaired by BCAS in SHRSP (Fig. 3B).
Fig. 3.
Bilateral common carotid artery stenosis (BCAS) impaired memory function with no change in spatial learning abilities. A: novel object recognition was reduced in stroke-prone spontaneously hypertensive rats with BCAS, signifying that they spent a smaller portion of their exploration with the novel object. B: there was no difference in the Morris water maze between groups. *P < 0.05, different from sham rats.
Altered dilatory signaling and remodeling in PAs after BCAS.
Myogenic tone generation was unchanged in PAs from BCAS rats (Fig. 4A). Dilation to the muscarinic receptor agonist carbachol was impaired in PAs from BCAS rats (Fig. 4B). To evaluate if impaired dilation was due to reduced sensitivity of smooth muscle cells to NO, a concentration-response curve to the NO donor SNP was constructed. BCAS reduced the PA sensitivity to SNP, as evidenced by an increased EC50 value (Fig. 4C). We also measured the function of TRPV4, which is a downstream target of muscarinic receptor activation (59). TRPV4-mediated dilation was not different between BCAS and sham rats (Fig. 4D).
Fig. 4.
Dilation was abolished in parenchymal arterioles (PAs) from stroke-prone spontaneously hypertensive rats with bilateral common carotid artery stenosis (BCAS). A: there was no difference in myogenic tone in PAs from the two groups at 60 mmHg. B: dilation to carbachol was abolished in PAs from BCAS rats. C: logEC50 of the nitric oxide (NO) donor sodium nitroprusside (SNP) was increased in PAs from BCAS rats. D: dilation to transient receptor potential channel vanilloid 4 agonist GSK-1016790A was not different between sham and BCAS rats. *P < 0.05, different from sham rats.
We assessed EDHF-mediated dilation, predominant in PAs (38, 58), by incubating PAs with l-NAME and Indo to inhibit NO and prostaglandin production, respectively. In the presence of l-NAME and Indo, there was no change in dilation in PAs from sham (Fig. 5A) or BCAS rats (Fig. 5B). EETs act as an EDHF in several vascular beds and are produced by CYP epoxygenase (7). Inhibition of CYP epoxygenase with MS-PPOH enhanced dilation in PAs from BCAS rats (Fig. 5D), whereas there was no significant change in dilation in PAs from sham rats (Fig. 5C). MS-PPOH in addition to l-NAME and Indo had no effect on dilation in PAs from sham rats (Fig. 5E); however, dilation in PAs from BCAS rats was enhanced similar to the increase in the presence of MS-PPOH alone (Fig. 5F).
Fig. 5.
Inhibition of cytochrome P-450 (CYP) epoxygenase restored dilation in parenchymal arterioles (PAs) from bilateral common carotid artery stenosis (BCAS) rats. A and B: inhibition of nitric oxide synthase and cyclooxygenase pathways did not change dilation in PAs from sham or BCAS rats. C: enhanced dilation was observed in PAs from sham rats in the presence of N-methylsulfonyl-6-(2-propargyloxyphenyl)hexanamide (MS-PPOH). D: dilation was restored in PAs from stroke-prone spontaneously hypertensive rats with BCAS in the presence of MS-PPOH. E: there was no difference in dilation in the presence of the MS-PPOH inhibitor NG-nitro-l-arginine methyl ester (l-NAME) and indomethacin (Indo) in PAs from sham rats. F: however, in PAs from BCAS rats, the addition of the three inhibitors enhanced dilation. *P < 0.05, different from sham rats.
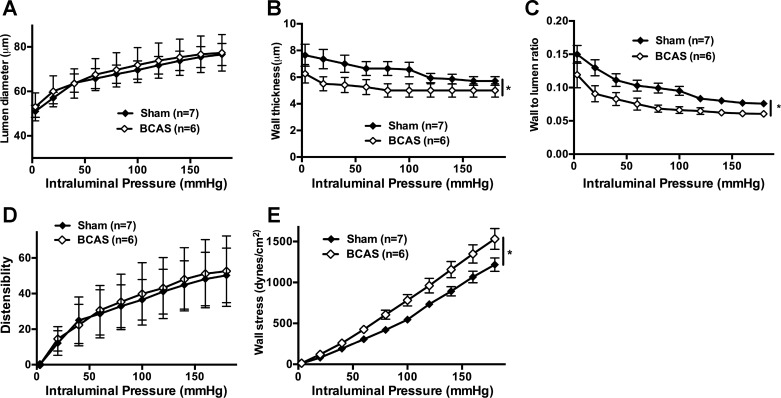
Passive artery structure was assessed under zero-flow and Ca2+-free conditions. There was no change in the lumen diameter of PAs between groups (Fig. 6A), but PAs from BCAS rats had reduced wall thickness (Fig. 6B) accompanied by a reduced wall-to-lumen ratio (Fig. 6C). The thinner walls of PAs from BCAS rats had increased wall stress (Fig. 6D), without a change in distensibility (Fig. 6E).
Fig. 6.
Hypotrophic remodeling in parenchymal arterioles (PAs) from bilateral common carotid artery stenosis (BCAS) rats. A: there was no difference in lumen diameter in PAs after BCAS. B: wall thickness was reduced in PAs after BCAS. C: the wall-to-lumen ratio was reduced in PAs after BCAS. D: there was no difference in distensibility in PAs between groups. E: there was increased wall stress in PAs after BCAS. *P < 0.05, different from sham rats.
DISCUSSION
Hypertension and carotid stenosis are risk factors for cognitive dysfunction. Thus, BCAS in SHRSP allowed us to study the effect of these comorbidities on the structure and function of PAs, which are essential for maintaining cognitive function (18, 34). We observed several salient findings regarding dilatory pathways and structure in PAs in this hypertensive model of cognitive impairment. Our study demonstrated that BCAS induced remodeling and impaired dilation in PAs with significant alterations in EDHF-mediated dilator pathways and that these changes were associated with memory deficits. By comparing these findings with our previous studies in 28-wk-old normotensive Wistar-Kyoto (WKY) rats (37), we were also able to elucidate critical differences in the responses of hypertensive and normotensive rats to carotid artery stenosis.
BCAS impaired memory function in SHRSP.
Our initial hypothesis was that the BCAS surgery would produce more marked cognitive decline in hypertensive rats than in normotensive rats. Our rationale for this was twofold; first, hypertension causes detrimental artery remodeling and endothelial dysfunction in large cerebral arteries that could exacerbate BCAS-induced vascular injury, and, second, antihypertensive drugs have the potential to prevent or delay the onset of cognitive impairment in hypertensive patients (21). However, we observed that memory impairment in SHRSP with BCAS was similar to WKY rats with BCAS (37) and that BCAS had no effect on spatial learning abilities in SHRSP. This lack of an effect of BCAS on spatial learning could be due to the fact that this capacity appears to be already impaired in sham SHRSP compared with sham WKY rats (37). Impaired learning memory in SHRSP was also reported in a passive avoidance test (31). This mildly impaired cognition may be the result of mild hypoperfusion in SHRSP; investigators have reported similar reductions in perfusion in brain regions critical for cognition in SHRSP with established hypertension (25, 28, 42).
CVR was absent in SHRSP.
To understand the vascular component of the observed cognitive deficits, we evaluated CVR with acetazolamide. Impaired CVR in patients is associated with cognitive dysfunction and is predicative of increased risk of ischemic events (8, 33, 36). Acetazolamide, a carbonic anhydrase inhibitor, causes carbonic acidosis to induce dilation (65). Reserve capacity was detected in sham WKY rats, and this was blunted in WKY rats with BCAS (37), similar to what is observed in patients with carotid artery lesions (8, 33). In SHRSP, acetazolamide did not increase blood flow in either BCAS or sham rats, suggesting that there was no cerebrovascular reserve at the time points studied. This may be a function of the reduced blood flow observed even in control/naive SHRSP (25, 28, 42); autoregulatory vasodilation occurs in response to reduced cerebral perfusion, and if the arteries in SHRSP are already maximally dilated, it may not be possible for a vasoactive stimulus such as acetazolamide to produce further dilation (50). Inward remodeling of large cerebral arteries could reduce cerebral perfusion pressure in pial arteries from SHRSP. Our finding of impaired CVR in both groups of hypertensive rats is in keeping with studies in the literature that indicate that the response to acetazolamide in impaired in patients with severe hypertension (15). In healthy volunteers, CVR declines with age (56), and age-dependent reductions in CVR were also observed in mice after unilateral internal carotid occlusion (24). To assess if aging could have contributed to the severely disrupted cerebral hemodynamics in 28-wk-old sham SHRSP, we carried out an acetazolamide challenge in 18-wk-old SHRSP. These younger SHRSP have marked hypertension, but they still had a robust CVR, suggesting that impaired reserve capacity in both groups of 28-wk-old rats is not a strain- or blood pressure-dependent effect. Taken together, these data suggest that the impaired reserve capacity in 28-wk-old SHRSP is related to increased age and the duration of hypertension. These differences in CVR between 28-wk-old sham SHRSP and WKY rats, from our previous study (37), provide additional support to the argument that hypertension accelerates normal aging of the brain (53). It should also be noted that isoflurane causes cerebral artery dilation (27); thus, it is possible that the cerebral arteries from SHRSP were maximally dilated before the administration of acetazolamide.
Dysfunctional dilatory pathways in PAs from BCAS rats.
Impaired PA function plays a critical role in the progression of cognitive impairment (3, 61). In the present study, dilation to carbachol was abolished with no change in myogenic tone in PAs from SHRSP with BCAS, similar to the changes we reported in PAs from normotensive WKY rats with BCAS (37). This prominent endothelial dysfunction in PAs from SHRSP with BCAS can be observed in patients with cerebral small vessel disease (20, 23). Cerebral small vessel disease, which can account for up to 45% of VCI cases, shows marked endothelial dysfunction in cerebral small arteries and PAs (19, 48). These pathological changes in the small arteries and PAs supplying the deep gray nuclei and white matter cause leukoariaosis (48, 60a), which is associated with a decrease in episodic memory and executive function in patients. However, the reduced sensitivity of PAs from BCAS rats to the NO donor SNP suggests that impaired dilation may not be entirely endothelium dependent and is at least in part the result of a diminished ability of smooth muscle cells to relax.
In cerebral arteries, Gq-coupled muscarinic receptor activation in endothelial cells activates TRPV4 via a PKC-dependent mechanism (11). We speculated that the signaling pathway connecting muscarinic receptor activation to TRPV4 activity could be disrupted and may have contributed to impaired dilation to carbachol. This does not appear to be the case as the dilator response to direct TRPV4 activation was similar in sham and BCAS rats (Fig. 4). It is, however, possible that hypertension itself affects this signaling pathway as TRPV4 agonist-mediated dilation was significantly impaired in SHRSP compared with sham WKY rats from our previous study (37). In small arterioles, instead of an endothelium-derived “factor” inducing smooth muscle cell hyperpolarization, direct electrotonic transfer of hyperpolarization occurs from the endothelial cell to smooth muscle cells (17). This endothelium-dependent hyperpolarization likely mediates dilation in PAs from sham SHRSP. TRPV4 activation produces Ca2+ sparklets that open Ca2+-activated K+ channels and initiate endothelium-dependent hyperpolarization (59). In hypertensive mice, attenuated Ca2+ sparklet generation leads to reduced dilation to carbachol and GSK101 in mesenteric arteries (60). This study implies that reduced dilation to carbachol and GSK101 in PAs from sham SHRSP could be due to a similar impairment in TRPV4 function (37). However, the reduced sensitivity to the NO donor in PAs from SHRSP with BCAS suggests that impaired dilation was not wholly endothelium dependent and could be due to a diminished ability of smooth muscle cells to relax.
Inhibition of NOS and COX did not alter dilation in PAs from sham rats, corroborating studies that suggest that EDHF dilation is more pronounced in arterioles compared with arteries (5). Moreover, the maximal response to SNP in PAs from BCAS rats was much higher than was the maximal response to carbachol. This result suggests that while the NO dilatory pathway is present in PAs, carbachol-mediated dilation in BCAS rats either does not have a significant NO component or that NO-dependent dilation is masked by excess production of vasoconstrictive agents. Several studies have demonstrated that EETs are an EDHF in cerebral arteries (11, 17a), and in sham WKY rats, inhibition of EET production with MS-PPOH abolished dilation (37). In distinct contrast, we observed no change in dilation in PAs from sham SHRSP in the presence of MS-PPOH alone or in combination with l-NAME and Indo. This suggests that in these arterioles, carbachol dilation is independent of NO, prostaglandins, and EETs.
While impaired dilation was observed in the presence of NOS/COX inhibitors, it was restored in the presence of the CYP epoxygenase inhibitor in PAs from BCAS rats. Dilation remained enhanced when MS-PPOH was added in combination with NOS/COX inhibitor, suggesting that improved dilation was dependent on CYP epoxygenase and independent of NOS/COX pathways in PAs from BCAS rats. The data also suggest that carotid stenosis induces CYP epoxygenase dysfunction, such that epoxygenase activity generates vasoconstrictors. Epoxidation reactions generate large amounts of ROS, such as superoxide or hydroxyl radical (16). Under normal conditions, antioxidants such as superoxide dismutase convert superoxide to hydrogen peroxide, which dilates PAs (62). However, under pathophysiological conditions with exhausted antioxidant levels, ROS impair dilation in cerebral arteries (29, 39, 44, 72). It is possible that after BCAS, ROS generated during epoxidation reactions in PAs impair dilation via ion channel dysfunction (1, 4, 14, 16, 35, 51).
Remodeling in PAs from BCAS rats.
PAs branch off the middle cerebral artery, which receives the majority of the flow from the carotid arteries. During carotid stenosis, flow through the middle cerebral arteries to PAs is likely reduced. While PAs from WKY rats with BCAS did not exhibit remodeling (37), PAs from SHRSP with BCAS had reduced wall thickness or hypotrophy. It is conceivable that after BCAS, PAs from SHRSP were exposed to reduced blood flow compared with PAs from WKY rats because of inward hypertrophic remodeling in upstrean cerebral arteries (54). Models of low blood flow in the peripheral circulation have reported hypotrophic remodeling due to loss of smooth muscle cells (6). Loss of smooth muscle cells could explain the elevated wall stress observed in PAs from BCAS rats and could initiate degenerative changes in the vessel walls that increase the incidence of lesions and hemorrhagic transformation poststroke (55, 73).
In conclusion, our study shows that the combination of malignant hypertension and carotid stenosis impaired dilation and induced remodeling in PAs from SHRSP. These changes could initiate the development of pathological features observed during cerebral small vessel disease. Additionally, impaired PA dilation could be indicative of a diminished ability of PAs to conduct dilation and enable functional hyperemia. These findings suggest that carotid stenosis could worsen impaired dilation in cerebral arterioles from hypertensive patients and warrants further investigation in restoring dilation in these arterioles.
Limitations.
Our present study has limitations that need to be discussed. First, we used scanning laser-Doppler on animals with an intact skull. The laser has a penetration depth of ~1 mm into the tissue, and the skull of a 28-wk-old SHRSP is ~0.9 mm thick; thus, the setup could only measure flow in surface pial circulation. The reduced perfusion in rats after BCAS surgery suggests that the laser-Doppler was measuring perfusion in the pial arteries and that it was not bone blood flow. This method of assessing blood flow has been published previously by another group (22). Second, cerebral flow measurements were carried out while rats were under isoflurane anesthesia, and this may have led to cerebral artery dilation (64). However, we controlled for the dilatory effect by treating all rats used in the exact same manner.
GRANTS
This work was supported by American Heart Association Grants 13GRNT1721000 (to A. M. Dorrance) and 14PRE19890001 (to N. Matin) and by National Heart, Lung, and Blood Institute Grant P01-HL-070687 (to W. F. Jackson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.M. conceived and designed research; N.M., C.F., and J.M.D.-O. performed experiments; N.M. analyzed data; N.M., W.F.J., and A.M.D. interpreted results of experiments; N.M. prepared figures; N.M. drafted manuscript; N.M., W.F.J., and A.M.D. edited and revised manuscript; N.M., C.F., W.F.J., J.M.D.-O., and A.M.D. approved final version of manuscript.
REFERENCES
- 1.Annunziato L, Pannaccione A, Cataldi M, Secondo A, Castaldo P, Di Renzo G, Taglialatela M. Modulation of ion channels by reactive oxygen and nitrogen species: a pathophysiological role in brain aging? Neurobiol Aging 23: 819–834, 2002. doi: 10.1016/S0197-4580(02)00069-6. [DOI] [PubMed] [Google Scholar]
- 2.Baumbach GL, Hajdu MA. Mechanics and composition of cerebral arterioles in renal and spontaneously hypertensive rats. Hypertension 21: 816–826, 1993. doi: 10.1161/01.HYP.21.6.816. [DOI] [PubMed] [Google Scholar]
- 3.Black S, Gao F, Bilbao J. Understanding white matter disease: imaging-pathological correlations in vascular cognitive impairment. Stroke 40, Suppl: S48–S52, 2009. doi: 10.1161/STROKEAHA.108.537704. [DOI] [PubMed] [Google Scholar]
- 4.Bondy SC, Naderi S. Contribution of hepatic cytochrome P450 systems to the generation of reactive oxygen species. Biochem Pharmacol 48: 155–159, 1994. doi: 10.1016/0006-2952(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 5.Brandes RP, Schmitz-Winnenthal FH, Félétou M, Gödecke A, Huang PL, Vanhoutte PM, Fleming I, Busse R. An endothelium-derived hyperpolarizing factor distinct from NO and prostacyclin is a major endothelium-dependent vasodilator in resistance vessels of wild-type and endothelial NO synthase knockout mice. Proc Natl Acad Sci USA 97: 9747–9752, 2000. doi: 10.1073/pnas.97.17.9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buus CL, Pourageaud F, Fazzi GE, Janssen G, Mulvany MJ, De Mey JG. Smooth muscle cell changes during flow-related remodeling of rat mesenteric resistance arteries. Circ Res 89: 180–186, 2001. doi: 10.1161/hh1401.093575. [DOI] [PubMed] [Google Scholar]
- 7.Campbell WB, Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflugers Arch 459: 881–895, 2010. doi: 10.1007/s00424-010-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chollet F, Celsis P, Clanet M, Guiraud-Chaumeil B, Rascol A, Marc-Vergnes JP. SPECT study of cerebral blood flow reactivity after acetazolamide in patients with transient ischemic attacks. Stroke 20: 458–464, 1989. doi: 10.1161/01.STR.20.4.458. [DOI] [PubMed] [Google Scholar]
- 9.Choy M, Ganesan V, Thomas DL, Thornton JS, Proctor E, King MD, van der Weerd L, Gadian DG, Lythgoe MF. The chronic vascular and haemodynamic response after permanent bilateral common carotid occlusion in newborn and adult rats. J Cereb Blood Flow Metab 26: 1066–1075, 2006. doi: 10.1038/sj.jcbfm.9600259. [DOI] [PubMed] [Google Scholar]
- 10.Earley S. Endothelium-dependent cerebral artery dilation mediated by transient receptor potential and Ca2+-activated K+ channels. J Cardiovasc Pharmacol 57: 148–153, 2011. doi: 10.1097/FJC.0b013e3181f580d9. [DOI] [PubMed] [Google Scholar]
- 11.Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res 97: 1270–1279, 2005. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- 12.Earley S, Pastuszyn A, Walker BR. Cytochrome P-450 epoxygenase products contribute to attenuated vasoconstriction after chronic hypoxia. Am J Physiol Heart Circ Physiol 285: H127–H136, 2003. doi: 10.1152/ajpheart.01052.2002. [DOI] [PubMed] [Google Scholar]
- 13.Farkas E, Luiten PG, Bari F. Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Brain Res Rev 54: 162–180, 2007. doi: 10.1016/j.brainresrev.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Fichtlscherer S, Dimmeler S, Breuer S, Busse R, Zeiher AM, Fleming I. Inhibition of cytochrome P450 2C9 improves endothelium-dependent, nitric oxide-mediated vasodilatation in patients with coronary artery disease. Circulation 109: 178–183, 2004. doi: 10.1161/01.CIR.0000105763.51286.7F. [DOI] [PubMed] [Google Scholar]
- 15.Ficzere A, Valikovics A, Fülesdi B, Juhász A, Czuriga I, Csiba L. Cerebrovascular reactivity in hypertensive patients: a transcranial Doppler study. J Clin Ultrasound 25: 383–389, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 16.Fleming I, Michaelis UR, Bredenkötter D, Fisslthaler B, Dehghani F, Brandes RP, Busse R. Endothelium-derived hyperpolarizing factor synthase (cytochrome P450 2C9) is a functionally significant source of reactive oxygen species in coronary arteries. Circ Res 88: 44–51, 2001. doi: 10.1161/01.RES.88.1.44. [DOI] [PubMed] [Google Scholar]
- 17.Garland CJ, Hiley CR, Dora KA. EDHF: spreading the influence of the endothelium. Br J Pharmacol 164: 839–852, 2011. doi: 10.1111/j.1476-5381.2010.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Gebremedhin D, Ma YH, Falck JR, Roman RJ, VanRollins M, Harder DR. Mechanism of action of cerebral epoxyeicosatrienoic acids on cerebral arterial smooth muscle. Am J Physiol Heart Circ Physiol 263: H519–H525, 1992. [DOI] [PubMed] [Google Scholar]
- 18.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol 100: 328–335, 2006. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 19.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S; American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia . Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 42: 2672–2713, 2011. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hainsworth AH, Markus HS. Do in vivo experimental models reflect human cerebral small vessel disease? A systematic review. J Cereb Blood Flow Metab 28: 1877–1891, 2008. doi: 10.1038/jcbfm.2008.91. [DOI] [PubMed] [Google Scholar]
- 21.Hanon O, Berrou JP, Negre-Pages L, Goch JH, Nádházi Z, Petrella R, Sedefdjian A, Sévenier F, Shlyakhto EV, Pathak A. Effects of hypertension therapy based on eprosartan on systolic arterial blood pressure and cognitive function: primary results of the Observational Study on Cognitive Function and Systolic Blood Pressure Reduction open-label study. J Hypertens 26: 1642–1650, 2008. doi: 10.1097/HJH.0b013e328301a280. [DOI] [PubMed] [Google Scholar]
- 22.Hardigan T, Yasir A, Abdelsaid M, Coucha M, El-Shaffey S, Li W, Johnson MH, Ergul A. Linagliptin treatment improves cerebrovascular function and remodeling and restores reduced cerebral perfusion in type 2 diabetes. Am J Physiol Regul Integr Comp Physiol 311: R466–R477, 2016. doi: 10.1152/ajpregu.00057.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hassan A, Hunt BJ, O’Sullivan M, Parmar K, Bamford JM, Briley D, Brown MM, Thomas DJ, Markus HS. Markers of endothelial dysfunction in lacunar infarction and ischaemic leukoaraiosis. Brain 126: 424–432, 2003. doi: 10.1093/brain/awg040. [DOI] [PubMed] [Google Scholar]
- 24.Hecht N, He J, Kremenetskaia I, Nieminen M, Vajkoczy P, Woitzik J. Cerebral hemodynamic reserve and vascular remodeling in C57/BL6 mice are influenced by age. Stroke 43: 3052–3062, 2012. doi: 10.1161/STROKEAHA.112.653204. [DOI] [PubMed] [Google Scholar]
- 25.Henning EC, Warach S, Spatz M. Hypertension-induced vascular remodeling contributes to reduced cerebral perfusion and the development of spontaneous stroke in aged SHRSP rats. J Cereb Blood Flow Metab 30: 827–836, 2010. doi: 10.1038/jcbfm.2009.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jablonski SA, Schreiber WB, Westbrook SR, Brennan LE, Stanton ME. Determinants of novel object and location recognition during development. Behav Brain Res 256: 140–150, 2013. doi: 10.1016/j.bbr.2013.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen NF, Todd MM, Kramer DJ, Leonard PA, Warner DS. A comparison of the vasodilating effects of halothane and isoflurane on the isolated rabbit basilar artery with and without intact endothelium. Anesthesiology 76: 624–634, 1992. doi: 10.1097/00000542-199204000-00021. [DOI] [PubMed] [Google Scholar]
- 28.Katayama Y, Katsumata T, Muramatsu H, Usuda K, Obo R, Terashi A. Effect of long-term administration of ethyl eicosapentate (EPA-E) on local cerebral blood flow and glucose utilization in stroke-prone spontaneously hypertensive rats (SHRSP). Brain Res 761: 300–305, 1997. doi: 10.1016/S0006-8993(97)00350-8. [DOI] [PubMed] [Google Scholar]
- 29.Katusic ZS, Vanhoutte PM. Superoxide anion is an endothelium-derived contracting factor. Am J Physiol Heart Circ Physiol 257: H33–H37, 1989. [DOI] [PubMed] [Google Scholar]
- 30.Kim SK, Cho KO, Kim SY. The plasticity of posterior communicating artery influences on the outcome of white matter injury induced by chronic cerebral hypoperfusion in rats. Neurol Res 31: 245–250, 2009. doi: 10.1179/174313209X382278. [DOI] [PubMed] [Google Scholar]
- 31.Kimura S, Saito H, Minami M, Togashi H, Nakamura N, Nemoto M, Parvez HS. Pathogenesis of vascular dementia in stroke-prone spontaneously hypertensive rats. Toxicology 153: 167–178, 2000. doi: 10.1016/S0300-483X(00)00312-7. [DOI] [PubMed] [Google Scholar]
- 33.Kuroda S, Houkin K, Kamiyama H, Mitsumori K, Iwasaki Y, Abe H, Yonas H, Wechsler LR, Nemoto E, Pindzola R. Long-term prognosis of medically treated patients with internal carotid or middle cerebral artery occlusion: can acetazolamide test predict it? Stroke 32: 2110–2116, 2001. doi: 10.1161/hs0901.095692. [DOI] [PubMed] [Google Scholar]
- 34.Lecrux C, Hamel E. The neurovascular unit in brain function and disease. Acta Physiol (Oxf) 203: 47–59, 2011. doi: 10.1111/j.1748-1716.2011.02256.x. [DOI] [PubMed] [Google Scholar]
- 35.Lu T, Zhang DM, Wang XL, He T, Wang RX, Chai Q, Katusic ZS, Lee HC. Regulation of coronary arterial BK channels by caveolae-mediated angiotensin II signaling in diabetes mellitus. Circ Res 106: 1164–1173, 2010. doi: 10.1161/CIRCRESAHA.109.209767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Markus H, Cullinane M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain 124: 457–467, 2001. doi: 10.1093/brain/124.3.457. [DOI] [PubMed] [Google Scholar]
- 37.Matin N, Fisher C, Jackson WF, Dorrance AM. Bilateral common carotid artery stenosis in normotensive rats impairs endothelial dependent dilation of parenchymal arterioles. Am J Physiol Heart Circ Physiol 310: H1321–H1329, 2016. 10.1152/ajpheart.00890.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matin N, Fisher C, Jackson WF, Dorrance AM. Bilateral common carotid artery stenosis in normotensive rats impairs endothelium-dependent dilation of parenchymal arterioles. Am J Physiol Heart Circ Physiol 310: H1321–H1329, 2016. doi: 10.1152/ajpheart.00890.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayhan WG. Superoxide dismutase partially restores impaired dilatation of the basilar artery during diabetes mellitus. Brain Res 760: 204–209, 1997. doi: 10.1016/S0006-8993(97)00282-5. [DOI] [PubMed] [Google Scholar]
- 40.Mayhan WG, Faraci FM, Heistad DD. Impairment of endothelium-dependent responses of cerebral arterioles in chronic hypertension. Am J Physiol Heart Circ Physiol 253: H1435–H1440, 1987. [DOI] [PubMed] [Google Scholar]
- 41.Mayhan WG, Faraci FM, Heistad DD. Responses of cerebral arterioles to adenosine 5′-diphosphate, serotonin, and the thromboxane analogue U-46619 during chronic hypertension. Hypertension 12: 556–561, 1988. doi: 10.1161/01.HYP.12.6.556. [DOI] [PubMed] [Google Scholar]
- 42.Mies G, Hermann D, Ganten U, Hossmann KA. Hemodynamics and metabolism in stroke-prone spontaneously hypertensive rats before manifestation of brain infarcts. J Cereb Blood Flow Metab 19: 1238–1246, 1999. doi: 10.1097/00004647-199911000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem 9: 49–57, 2002. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nelson CW, Wei EP, Povlishock JT, Kontos HA, Moskowitz MA. Oxygen radicals in cerebral ischemia. Am J Physiol Heart Circ Physiol 263: H1356–H1362, 1992. [DOI] [PubMed] [Google Scholar]
- 45.Nishimura N, Schaffer CB, Friedman B, Lyden PD, Kleinfeld D. Penetrating arterioles are a bottleneck in the perfusion of neocortex. Proc Natl Acad Sci USA 104: 365–370, 2007. doi: 10.1073/pnas.0609551104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 9: 689–701, 2010. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 50.Powers WJ. Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol 29: 231–240, 1991. doi: 10.1002/ana.410290302. [DOI] [PubMed] [Google Scholar]
- 51.Puntarulo S, Cederbaum AI. Production of reactive oxygen species by microsomes enriched in specific human cytochrome P450 enzymes. Free Radic Biol Med 24: 1324–1330, 1998. doi: 10.1016/S0891-5849(97)00463-2. [DOI] [PubMed] [Google Scholar]
- 53.Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev 30: 730–748, 2006. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rigsby CS, Pollock DM, Dorrance AM. Spironolactone improves structure and increases tone in the cerebral vasculature of male spontaneously hypertensive stroke-prone rats. Microvasc Res 73: 198–205, 2007. doi: 10.1016/j.mvr.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schreiber S, Bueche CZ, Garz C, Braun H. Blood brain barrier breakdown as the starting point of cerebral small vessel disease? New insights from a rat model. Exp Transl Stroke Med 5: 4, 2013. doi: 10.1186/2040-7378-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Settakis G, Molnár C, Kerényi L, Kollár J, Legemate D, Csiba L, Fülesdi B. Acetazolamide as a vasodilatory stimulus in cerebrovascular diseases and in conditions affecting the cerebral vasculature. Eur J Neurol 10: 609–620, 2003. doi: 10.1046/j.1468-1331.2003.00675.x. [DOI] [PubMed] [Google Scholar]
- 57.Shih AY, Blinder P, Tsai PS, Friedman B, Stanley G, Lyden PD, Kleinfeld D. The smallest stroke: occlusion of one penetrating vessel leads to infarction and a cognitive deficit. Nat Neurosci 16: 55–63, 2013. doi: 10.1038/nn.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, Takayanagi T, Nagao T, Egashira K, Fujishima M, Takeshita A. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol 28: 703–711, 1996. doi: 10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 59.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336: 597–601, 2012. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sonkusare SK, Dalsgaard T, Bonev AD, Hill-Eubanks DC, Kotlikoff MI, Scott JD, Santana LF, Nelson MT. AKAP150-dependent cooperative TRPV4 channel gating is central to endothelium-dependent vasodilation and is disrupted in hypertension. Sci Signal 7: ra66, 2014. doi: 10.1126/scisignal.2005052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60a.Sörös P, Whitehead S, Spence JD, Hachinski V. Antihypertensive treatment can prevent stroke and cognitive decline. Nat Rev Neurol 9: 174–178, 2013. doi: 10.1038/nrneurol.2012.255. [DOI] [PubMed] [Google Scholar]
- 61.Stanimirovic DB, Friedman A. Pathophysiology of the neurovascular unit: disease cause or consequence? J Cereb Blood Flow Metab 32: 1207–1221, 2012. doi: 10.1038/jcbfm.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sullivan MN, Gonzales AL, Pires PW, Bruhl A, Leo MD, Li W, Oulidi A, Boop FA, Feng Y, Jaggar JH, Welsh DG, Earley S. Localized TRPA1 channel Ca2+ signals stimulated by reactive oxygen species promote cerebral artery dilation. Sci Signal 8: ra2, 2015. doi: 10.1126/scisignal.2005659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Todd MM, Weeks J. Comparative effects of propofol, pentobarbital, and isoflurane on cerebral blood flow and blood volume. J Neurosurg Anesthesiol 8: 296–303, 1996. doi: 10.1097/00008506-199610000-00007. [DOI] [PubMed] [Google Scholar]
- 65.Vagal AS, Leach JL, Fernandez-Ulloa M, Zuccarello M. The acetazolamide challenge: techniques and applications in the evaluation of chronic cerebral ischemia. AJNR Am J Neuroradiol 30: 876–884, 2009. doi: 10.3174/ajnr.A1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van der Flier WM, van Straaten EC, Barkhof F, Verdelho A, Madureira S, Pantoni L, Inzitari D, Erkinjuntti T, Crisby M, Waldemar G, Schmidt R, Fazekas F, Scheltens P. Small vessel disease and general cognitive function in nondisabled elderly: the LADIS study. Stroke 36: 2116–2120, 2005. doi: 10.1161/01.STR.0000179092.59909.42. [DOI] [PubMed] [Google Scholar]
- 67.van Dijk EJ, Breteler MM, Schmidt R, Berger K, Nilsson LG, Oudkerk M, Pajak A, Sans S, de Ridder M, Dufouil C, Fuhrer R, Giampaoli S, Launer LJ, Hofman A, Consortium C; CASCADE Consortium . The association between blood pressure, hypertension, and cerebral white matter lesions: cardiovascular determinants of dementia study. Hypertension 44: 625–630, 2004. doi: 10.1161/01.HYP.0000145857.98904.20. [DOI] [PubMed] [Google Scholar]
- 68.van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study. Stroke 39: 2712–2719, 2008. doi: 10.1161/STROKEAHA.107.513176. [DOI] [PubMed] [Google Scholar]
- 69.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1: 848–858, 2006. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang R, Szabo C, Ichinose F, Ahmed A, Whiteman M, Papapetropoulos A. The role of H2S bioavailability in endothelial dysfunction. Trends Pharmacol Sci 36: 568–578, 2015. doi: 10.1016/j.tips.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 12: 483–497, 2013. doi: 10.1016/S1474-4422(13)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wei EP, Kontos HA, Christman CW, DeWitt DS, Povlishock JT. Superoxide generation and reversal of acetylcholine-induced cerebral arteriolar dilation after acute hypertension. Circ Res 57: 781–787, 1985. doi: 10.1161/01.RES.57.5.781. [DOI] [PubMed] [Google Scholar]
- 73.Yamori Y, Horie R, Handa H, Sato M, Fukase M. Pathogenetic similarity of strokes in stroke-prone spontaneously hypertensive rats and humans. Stroke 7: 46–53, 1976. doi: 10.1161/01.STR.7.1.46. [DOI] [PubMed] [Google Scholar]