Summary
Background
Percutaneous kyphoplasty (PKP), a minimally invasive treatment, has been widely used for osteoporotic vertebral compression fractures (OVCFs).
Objective
To retrospectively analyse the therapeutic effects of PKP using a series of key techniques in a multicentre study.
Methods
From May 2000 to December 2016, PKP was performed using a series of key techniques (puncture, reduction, and perfusion techniques) for the treatment of 4532 OVCF patients. The pain visual analog scale (VAS) and the Oswestry Disability Index (ODI) questionnaire prior to the operation, at postoperative Day 2, and at the last follow-up were analysed by paired t-test analysis. The leakage of bone cement was evaluated by postoperative radiography and/or computed tomography. Four-year survival was calculated at the last follow-up.
Results
The average follow-up was 63 months (1–116 months). The VAS score decreased from 8.9 (preoperative) to 2.3 (2 days postoperative) to 1.9 (last follow-up). The ODI score of the patients decreased from 86.7 (preoperative) to 31.6 (2 days postoperative) to 25.3 (last follow-up). Both VAS score and ODI score improved significantly. The bone cement leakage rate was 3.5%, with no clinical symptoms. The 4-year survival rate was 77.5%.
Conclusion
This study suggests that PKP with key techniques would be an effective technique to treat OVCF with less risk and better therapeutic effect. Such diagnostic methods and surgical techniques lead to the development and progress of treatment for OVCF.
The translational potential of this article: PKP with key techniques would be an effective technique to treat and lead to the development and progress of treatment for OVCF.
Keywords: kyphoplasty, multicentre, OVCF, percutaneous, therapeutic effect
Introduction
According to estimated data from the World Health Organization, the population of the elderly over 60 years old around the world was 900 million in 2015 and may reach 2 billion by 2050 [1], [2], [3], [4]. Osteoporosis and osteoporotic fractures have become global concerns along with this increasing population, affecting the quality of life and incurring a high economic burden to the patients, as well as society. Currently, more than 8.9 million patients suffer from osteoporotic fractures annually all over the world, 40% of which are osteoporotic vertebral compression fractures (OVCFs) [4], [5]. Most patients with OVCF (>75%) had only minor injuries (such as coughing and sneezing) or no definite trauma. A small number (<25%) of cases are caused by trauma, such as falls. Thereafter, patients with fractures often experience only thoracic/lumbar back pain, with no obvious swelling or deformity. The majority of patients can reluctantly stand and may be able to slightly walk. Therefore, patients often consider it to be a lumbar sprain; thus, they do not pay sufficient attention to it, and do not go to the hospital for treatment. Even if patients are still experiencing chest/back pain after resting in bed, they usually do not go to the department of orthopaedics for treatment. Thus, the misdiagnosis rate is high. The results of a retrospective study in the UK showed that the misdiagnosis rate of OVCF was as high as 82% in all patients who underwent chest radiography for chest pain [6]. Thus, the actual incidence rate of OVCF is much higher than the current rate from hospital statistics.
For elderly patients with OVCF, bed rest and other conservative treatments often lead to further loss of bone mass, worse osteoporosis, development of hypostatic pneumonia, bedsores, urinary tract infection, and other complications. Additionally, they can reduce the patients' quality of life, and even endanger their lives. The mortality rate of OVCF is as high as 49.4% within 4 years [7], [8], [9]. Percutaneous kyphoplasty (PKP) has been widely accepted and adopted because of its satisfactory clinical efficacy and ability to quickly relieve the patient's thoracic/lumbar back pain and improve the patient's quality of life. This study was conducted from May 2000 to December 2016 in three hospitals, including The First Affiliated Hospital of Soochow University, The First Affiliated Hospital of Sun Yat-sen University and The First Affiliated Hospital of Nanjing Medical University. The clinical efficacy of PKP in the treatment of patients with OVCF was analysed retrospectively, and the therapeutic effect of PKP was also evaluated to provide clinical references.
Materials and methods
Patients
The clinical study was approved by the Ethics Committee of The First Affiliated Hospital of Soochow University, The First Hospital of Sun Yat-sen University and The First Affiliated Hospital of Nanjing Medical University, and written informed consents were obtained from all participants. A total of 4532 patients with OVCF treated by PKP in three hospitals between May 2000 and December 2016 were included in the study, including 1036 men and 3496 women, with an average age of 69.1 years (range, 50–99 years). All patients were examined using radiography, computed tomography (CT), and magnetic resonance imaging (MRI) prior to operation. Painful vertebrae in each patient were identified preoperatively by chief complaint (local back pain), physical examination (percussion pain at local vertebral spinal process), and MRI (low signal intensity on T1-weighted imaging, high or equal signal intensity on T2-weighted imaging, and high signal intensity on fat suppression sequence imaging) [10]. The bone mineral density of the lumbar spine was measured with dual-energy X-ray absorptiometry. The T-score was −2.6 to −4.1 (average, −3.4). All patients underwent a radiographic examination of the spine after operation and during follow-up.
Operation procedures using key techniques
The procedure was performed under general anaesthesia. The C-arm X-ray was used to capture standard anteroposterior and lateral images for the operative vertebral bodies, and safety puncture techniques for the vertebral bodies (baseline positioning, puncture point positioning, puncture direction adjustment, and step-by-step insertion) were also used. The bilateral/unilateral pedicle and vertebral pedicle puncture approach were used to reach the front of the posterior margin of the vertebral bodies (about 3 mm); guide wires, expansion cannula, and working cannula were then sequentially inserted. Inflatable balloons were placed into the vertebral body through a working cannula. They were then inflated under fluoroscopic guidance. A series of bone cement perfusion techniques (time-window determination of injection, incremental temperature cement delivery, real-time perfusion pressure detection, and secondary cement preparation based perfusion and blocking) was used; the prepared polymethylmethacrylate cement was slowly inserted into the vertebral body through a cannula, accompanied by perspective monitoring, until the bone cement filled the cavity. This process was stopped when it was close to exceeding the range of the vertebral body, and the cannula was removed. The patients were allowed to walk 12-hours postoperatively.
Curative efficacy evaluation
The pain visual analog scale (VAS) was applied to evaluate the patients' subjective symptoms (0–10; 0 as painless and 10 as the most painful) preoperatively, 2-days postoperatively, and at the last follow-up. The Oswestry Disability Index (ODI) questionnaire was used to assess the patients' quality of life. All patients underwent postoperative radiography and/or CT examination of the vertebral body to assess the leakage of bone cement, and survival was also observed.
Statistical methods
The SPSS 13.0 (IBM Corporation, 233 South Warker Drive, Chicago IL, USA) was used to analyse the data, and statistical values are expressed as the mean ± standard deviation. A paired t-test analysis was performed, and p < 0.05 indicates that the difference was statistically significant.
Results
All patients were followed up for 1–116 months, with an average of 63 months. Postoperative radiography and/or CT examination showed that bone cement leakage occurred in 159 cases (leakage rate = 3.5%), with no clinical symptoms. OVCF nonunion occurred in 467 patients (10.3%), of whom 58 (12.4%) had bone cement leakage. The 4-year survival rate was 77.5% (3512/4532). The detailed evaluation results preoperatively, 2 days after surgery, and at the last follow-up are shown in Table 1. The differences in VAS score and ODI score between postoperative Day 2 and the last follow-up were statistically significant (p < 0.05).
Table 1.
Efficacy outcomes.
| Preoperative | Postoperative Day 2 | Last follow-up | |
|---|---|---|---|
| VAS score | 8.9 ± 0.8 | 2.3 ± 0.6* | 1.9 ± 0.9* |
| ODI score | 86.7 ± 3.6 | 31.6 ± 4.0* | 25.3 ± 1.2* |
*Indicates a significant difference in each evaluation index before the operation and at the postoperative follow-up (p < 0.05).
ODI = Oswestry Disability Index; VAS = visual analog scale.
Illustrative case
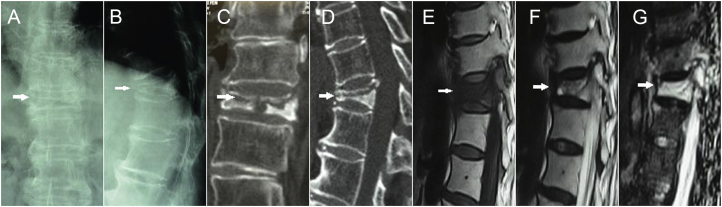
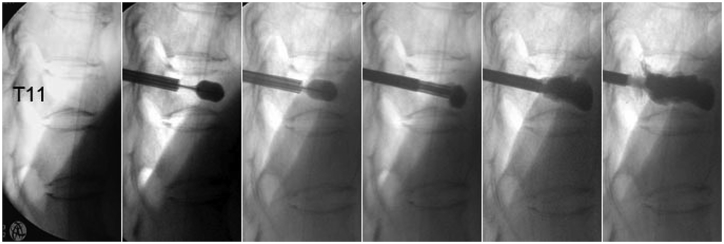
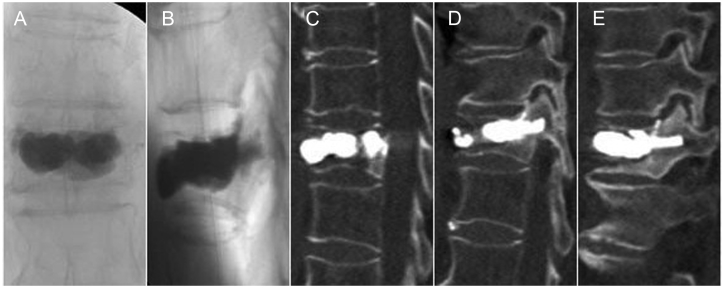
A 68-year-old woman had back pain for 4 months. Radiography showed T11 OVCF nonunion, based on the “painful vertebrae” theory (Figure 1). The key techniques of PKP were performed for this patient (Figure 2). Postoperative radiography and CT showed a sufficient anchoring between the bone cement and the trabecular bones, and no bone cement leakage was detected (Figure 3).
Figure 1.
Preoperative images. (A, B) Anteroposterior-lateral radiograph and (C,D) computed tomography scans showed T11 (arrows) intravertebral cleft and marginal sclerosis. (E) T1-weighted sagittal magnetic resonance imaging showed low signal. (F) T2- and (G) STIR-weighted sagittal magnetic resonance imaging showed showing high signal. STIR: short time inversion recovery.
Figure 2.
Operative images. The “secondary cement preparation-based perfusion and blocking” and “cement-bone sufficient anchoring” techniques were used during surgery.
Figure 3.
Postoperative images. (A, B) Radiograph and (C, D and E) computed tomography scans showed a sufficient anchoring between the bone cement and trabecular bones, and no bone cement leakage was detected.
Discussion
PKP shows great superiority in the treatment of OVCF. A meta-analysis by Eck et al. [11] showed that PKP was able to reduce the VAS score from 8.06 preoperatively to 3.46 postoperatively, with an improvement in pain rate of up to 57%; therefore, this method is effective in relieving pain and improving the quality of life of patients with OVCF. In this study, PKP was used in the treatment of 4532 patients with OVCF, and the VAS score decreased from 8.9 (preoperative) to 2.3 (2 days postoperative) to 1.9 (last follow-up). The pain improvement rates were 74.2% and 78.7% at postoperative Day 2 and the last follow-up, respectively, which are better than those found in Eck et al.'s [11] meta-analysis. To evaluate the types of spinal fractures, vertebral collapse degree, and vertebral marrow oedema signals, radiography, CT, and MRI scans were suggested before operation. We established the “painful vertebrae” therapeutic strategy [10] to solve the surgical target of the vertebral body during clinical diagnosis, which includes: (1) low signal intensity on T1-weighted MRI, high or equal signal intensity on T2-weighted imaging, and fat suppression sequence imaging on the vertebral body with high signal intensity; (2) for patients not able to undergo MRI examination, a radionuclide bone scan can be used to show the radionuclide concentration of the vertebral body; and (3) physical examination of the corresponding segment with pressure pain and percussion pain. The “painful vertebrae” therapeutic strategy was especially useful to locate the vertebral level needed to be operated in multi-level OVCFs. Not every vertebral fracture is a source of pain or necessitates surgery. Being able to determine which vertebral fractures are associated with mobility might be key to the success or failure of treatment. Therefore, we proposed this principle of the OVCF treatment: PKP is only suitable for “painful vertebrae.”
In addition, we found the phenomenon of the nonunion of OVCFs and established the diagnosis criteria [12]: (1) history of pain for several months at the fracture site; (2) low T1- and high T2-signal on magnetic resonance images; (3) widening of the fracture line on routine radiographs; and (4) movement of the endplate and changes of anterior vertebral heights on hyperextension radiographs. Such cases were often accompanied by obvious anterior and side wall bone defects; bone sclerosis was always observed around the clefts; and bone cement leakage or dislodge usually occurred, which is known as the “forbidden area”. We used the “secondary cement preparation-based perfusion and blocking” and “cement-bone sufficient anchoring” techniques for the treatment of 467 patients with OVCF with nonunion, and satisfactory clinical effects were observed. The postoperative radiograph showed sufficient anchoring between the bone cement and trabecular bones, and we observed a bone cement leakage rate of 12.4%, compared with the reported 79% [13], with no case of cement dislodge. Patients who did not undergo any treatment can now accept this safe, effective, minimally invasive treatment to avoid the collapse of the vertebral body, which can cause paralysis or even death.
It is very important to select the appropriate puncture point and angle before the minimally invasive puncturing during PKP. If improper selection occurs, it is easy to damage important adjacent vessels and nerves, leading to paralysis and even death. In addition, the minimally invasive treatment of OVCF, especially multi-level OVCFs, may lead to excessive reduction and new fractures, which can increase the risk of bone cement leakage if the initial height of the fractured vertebral body is not properly estimated. Therefore, it is important to estimate the initial height of the fractured vertebral body. Bone cement leakage is the most common complication of PKP; a meta-analysis by Lee et al. [14] reported that the PKP bone cement leakage rate was 14%, and, of all clinical complications seen in patients who undergo PKP, 73% are related to bone cement leakage. If the bone cement infiltrates and results in spinal cord compression, it will cause paralysis. If the bone cement infiltrates into the vascular system, it can cause pulmonary embolism and even death. Ninety percent of bone leakage is related to the modulation and perfusion of bone cement. In the event of blood vessel, nerve root, or spinal cord injury during OVCF treatment, especially the treatment of OVCF nonunion, bone cement leakage easily leads to paralysis and death, as well as other complications. A series of key techniques were established, based on “puncture, reduction, and perfusion”, such as the core strategy of PKP, including the “baseline positioning,” “time-window determination of injection,” “incremental temperature cement delivery” techniques, etc. [10], [12], [15]. In this study, the minimally invasive OVCF techniques were successfully used in PKP, and the bone cement leakage rate was 3.5%, which was lower than the 7–14% reported in the literature [10], [14]; there were also no cases of catastrophic complications. In addition, new bone cement materials, such as strontium-containing bioactive bone cement, are expected to achieve the biological reconstruction of bone fractures and effectively reduce bone cement leakage [16].
Edidin et al. [9] followed a group of more than 850,000 patients with OVCF for 4 years. The survival rate with conservative treatment was 50%, with vertebroplasty therapy it was 57.3%, and with PKP treatment it was 62.8%. This minimally invasive surgery can effectively improve the survival rate of patients with OVCF. Our team used the key techniques of PKP, clearly recognised the indications for surgery, and guided the patients to accept rehabilitation and treatment of osteoporosis. The results showed that the ODI score of the patients decreased from 86.7 (preoperative) to 31.6 (2-days postoperative) to 25.3 (last follow-up), and the functional improvement rates were 63.6% and 70.8% at 2-days postoperatively and the last follow-up, respectively. The total 4-year survival rate was 77.5%.
Conclusion
We have shown that PKP with key techniques is an effective technique to treat OVCF with less risk and better therapeutic effect.
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
Funding/Acknowledgements
This study was supported by National High Technology Research and Development Program of China (2015AA020316), National Natural Science Foundation of China (81472132, 81572183 and 81672220), and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jot.2017.04.003.
Contributor Information
Huilin Yang, Email: hlyang@suda.edu.cn.
Jun Zou, Email: jzou@suda.edu.cn.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Czerwinski E., Badurski J.E., Marcinowska-Suchowierska E., Osieleniec J. Current understanding of osteoporosis according to the position of the World Health Organization (WHO) and International Osteoporosis Foundation. Ortop Traumatol Rehabil. 2007;9:337–356. [PubMed] [Google Scholar]
- 2.Watts N.B., Lewiecki E.M., Miller P.D., Baim S. National Osteoporosis Foundation 2008 clinician's guide to prevention and treatment of osteoporosis and the World Health Organization Fracture Risk Assessment Tool (FRAX): what they mean to the bone densitometrist and bone technologist. J Clin Densitom. 2008;11:473–477. doi: 10.1016/j.jocd.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Shuler F.D., Conjeski J., Kendall D., Salava J. Understanding the burden of osteoporosis and use of the World Health Organization FRAX. Orthopedics. 2012;35:798–805. doi: 10.3928/01477447-20120822-12. [DOI] [PubMed] [Google Scholar]
- 4.Shuler F.D., Scott K., Wilson-Byrne T., Morgan L., Olajide O.B. Improving rural bone health and minimizing fracture risk in West Virginia: validation of the World Health Organization FRAX assessment tool as a phone survey for osteoporosis detection. W V Med J. 2016;112:84–88. [PubMed] [Google Scholar]
- 5.Si L., Winzenberg T.M., Jiang Q., Chen M., Palmer A.J. Projection of osteoporosis-related fractures and costs in China: 2010–2050. Osteoporos Int. 2015;26:1929–1937. doi: 10.1007/s00198-015-3093-2. [DOI] [PubMed] [Google Scholar]
- 6.Gehlbach S.H., Bigelow C., Heimisdottir M., May S., Walker M., Kirkwood J.R. Recognition of vertebral fracture in a clinical setting. Osteoporos Int. 2000;11:577–582. doi: 10.1007/s001980070078. [DOI] [PubMed] [Google Scholar]
- 7.Dittmer D.K., Teasell R. Complications of immobilization and bed rest. Part 1: musculoskeletal and cardiovascular complications. Can Fam Physician. 1993;39:1428–1432. 35–7. [PMC free article] [PubMed] [Google Scholar]
- 8.Teasell R., Dittmer D.K. Complications of immobilization and bed rest. Part 2: other complications. Can Fam Physician. 1993;39:1440–1442. 5–6. [PMC free article] [PubMed] [Google Scholar]
- 9.Edidin A.A., Ong K.L., Lau E., Kurtz S.M. Morbidity and mortality after vertebral fractures: comparison of vertebral augmentation and nonoperative management in the Medicare population. Spine. 2015;40:1228–1241. doi: 10.1097/BRS.0000000000000992. [DOI] [PubMed] [Google Scholar]
- 10.Yang H.L., Wang G.L., Niu G.Q., Liu J.Y., Hiltner E., Meng B. Using MRI to determine painful vertebrae to be treated by kyphoplasty in multiple-level vertebral compression fractures: a prospective study. J Int Med Res. 2008;36:1056–1063. doi: 10.1177/147323000803600524. [DOI] [PubMed] [Google Scholar]
- 11.Eck J.C., Nachtigall D., Humphreys S.C., Hodges S.D. Comparison of vertebroplasty and balloon kyphoplasty for treatment of vertebral compression fractures: a meta-analysis of the literature. Spine J. 2008;8:488–497. doi: 10.1016/j.spinee.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Yang H., Wang G., Liu J., Ebraheim N.A., Niu G., Hiltner L. Balloon kyphoplasty in the treatment of osteoporotic vertebral compression fracture nonunion. Orthopedics. 2010;33:24. doi: 10.3928/01477447-20091124-28. [DOI] [PubMed] [Google Scholar]
- 13.Peh W.C., Gelbart M.S., Gilula L.A., Peck D.D. Percutaneous vertebroplasty: treatment of painful vertebral compression fractures with intraosseous vacuum phenomena. AJR Am J Roentgenol. 2003;180:1411–1417. doi: 10.2214/ajr.180.5.1801411. [DOI] [PubMed] [Google Scholar]
- 14.Lee M.J., Dumonski M., Cahill P., Stanley T., Park D., Singh K. Percutaneous treatment of vertebral compression fractures: a meta-analysis of complications. Spine. 2009;34:1228–1232. doi: 10.1097/BRS.0b013e3181a3c742. [DOI] [PubMed] [Google Scholar]
- 15.Yang H., Liu H., Wang S., Wu K., Meng B., Liu T. Review of percutaneous kyphoplasty in China. Spine. 2016;41:B52–B58. doi: 10.1097/BRS.0000000000001804. [DOI] [PubMed] [Google Scholar]
- 16.Lu W.W., Cheung K.M., Li Y.W., Luk K.D., Holmes A.D., Zhu Q.A. Bioactive bone cement as a principal fixture for spinal burst fracture: an in vitro biomechanical and morphologic study. Spine. 2001;26:2684–2690. doi: 10.1097/00007632-200112150-00010. discussion 2690–1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.