Summary
The epidemiology of lumbar degenerative spondylolisthesis (DS) remains controversial. We performed a systematic review with the aim of gaining a better understanding of the prevalence of DS in the general population. The results showed that the prevalence of DS is very gender- and age-specific. Few women and men develop DS before they are 50 years old. After 50 years of age, both women and men begin to develop DS, with women having a faster rate of development than men. For elderly Chinese (≥ 65 years, mean age: 72.5 years), large population-based studies MsOS (Hong Kong, females: n = 2000) and MrOS (Hong Kong, males: n = 2000) showed DS prevalence was 25.0% in women and 19.1% in men. The female:male (F:M) prevalence ratio was 1.3:1. The published data for MsOS (USA) and MrOS (USA) studies seem to show that elderly Caucasian Americans have a higher DS prevalence, being approximately 60–70% higher than elderly Chinese; however, the F:M prevalence ratio was similar to the elderly Chinese population. Patient data showed that female patients more often received surgical treatment than male and preliminary data showed the ratio of female to male patients receiving surgical treatment did not differ between Northeast Asians (Chinese, Japanese, and Korean), Europeans, and American Caucasians, being around 2:1 in the elderly population. The existing data also suggest that menopause may be a contributing factor for the accelerated development of DS in postmenopausal women.
The translational potential of this article: A better understanding of epidemiology of lumbar degenerative spondylolisthesis can support patient consultation and treatment planning.
Keywords: Caucasian, Chinese, degenerative spondylolisthesis, men, prevalence, women
Introduction and basic concepts
Degenerative spondylolisthesis (DS) is a disorder that causes the slip of one vertebral body over the one below due to degenerative changes. It differs from spondylolytic spondylolisthesis by the absence of a pars interarticularis defect (spondylolysis), i.e., in DS, the whole upper vertebra (vertebral body and posterior part of the vertebra including neural arch and processes) slips relative to the lower vertebra. Both DS and spondylolytic spondylolisthesis are commonly seen as incidental findings in asymptomatic patients. A good understanding of the natural history of these conditions is important to counsel patients and determine a course of action. The plain radiographic features include the essential finding of spondylolisthesis on a lateral view of forward (or backward) displacement of L4 on L5 or, less commonly, L5 on S1 or L3 on L4 in the presence of an intact neural arch. The “listhesis” is a rotary deformity and not a simple forward (or backward) displacement [1]. Radiograph can also show small compensating curves in the upper lumbar and lower thoracic spine [1]. The major local reasons of DS that probably lead to the development of degenerative vertebral slippage are: (1) arthritis of the facet joints with loss of their normal structural support; (2) malfunction of the ligamentous stabilizing component, probably due to hyperlaxity; and (3) ineffectual muscular stabilization [2], [3], [4], [5], [6], [7]. Disc degeneration leads to segmental instability in the sagittal plane and may also result in DS [8]. Pregnancy and sports activities are also associated with DS [9], [10], [11], [12], [13], [14].
Separation of the pars interarticularis can occur when spondylolysis is present (Figure 1). Spondylolysis can be congenital or caused by a stress fracture of the bone and is especially common in adolescents who overtrain in sports activities [2], [12], [13], [14]. The pars interarticularis is vulnerable to fracture during spinal hyperextension, especially when combined with rotation or when experiencing a force during landing. This stress fracture most commonly occurs where the concave lumbar spine transitions to the convex sacrum (L5–S1). A significant number of individuals with spondylolysis will develop spondylolisthesis, accounting for 50–81% of this particular population. It is believed that both repetitive trauma and an inherent genetic weakness can make an individual more susceptible to spondylolysis [15], [16].
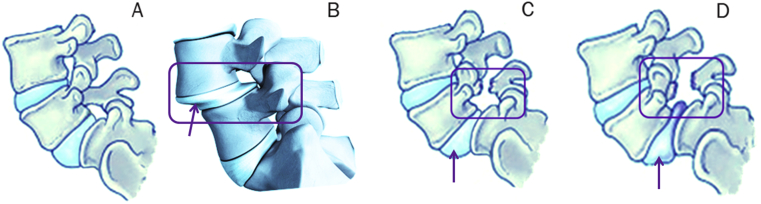
Figure 1.
(A) Normal anatomy L5/S1; (B) degenerative spondylolisthesis of L4/L5; and (C,D) different extents of spondylolytic spondylolisthesis of L5/S1.
After degenerative changes unlocked the intervertebral joint, the vertebral body slipping occurs along a direction that roughly depends on two factors: (1) the symmetry of facet joint lesions, and (2) the distribution of weight-bearing forces. When facet joint subluxation is symmetric, slipping is mainly sagittal, but with asymmetric subluxation, a rotatory displacement also occurs. Defects of the pars interarticularis seen on lateral or bilateral oblique views help to distinguish between DS and isthmic spondylolisthesis. Additional findings include disc space narrowing, endplate sclerosis, peridiscal osteophytes, facet sclerosis, and hypertrophy. In the last stage, osteophytes and advanced disc space narrowing lead to restabilization of the intervertebral level with decrease or disappearance of the range of movement [17], [18].
The natural course of spondylolysis and spondylolisthesis has been studied [19], [20], [21]. A prospective study was initiated in 1955 with a radiographic and clinical study of 500 first-grade children. A total of 22 individuals of 6 years of age were found to have a lytic defect of the pars interarticularis, giving a rate of 4.4%. Thirty adult individuals consisting of 10 females and 20 males (F:M ratio 1:2) were found to have pars lesions with a prevalence rate of 6%. Of the 30 individuals, 22 had bilateral L5 pars defects and 8 individuals had unilateral defects. All bilateral pars defects were at L5. Over the course of the study, spondylolisthesis developed in 18 of the 22 individuals with bilateral L5 lesions (spondylolisthesis prevalence = 18/500 = 3.6%). The average slip was 11% for all individuals with initial spondylolisthesis. The average slip in the 1999 studies for this group was 18%. There appeared to be a marked slowing of slip progression with each decade, while no individual had reached a 40% slip. It was suggested that most patients live active, pain-free, fully functional lives. There is no correlation between changes in clinical symptoms and progression of spondylolisthesis. DS, although progressive, rarely exceeds Grade II. Low-grade isthmic spondylolisthesis rarely progresses, and it has a benign clinical course in majority of patients [20]. Spur formation, sclerosis, and ossification of ligaments limit the progression of degenerative spondylolisthesis. Fredrickson et al [21] suggested that a child with spondylolysis or spondylolisthesis can be permitted to enjoy a normal childhood and adolescence without restriction of activities and without fear of progressive olisthesis or disabling pain.
Progressive degeneration of the intervertebral disc, thickening of the ligamentum flavum, and translation of the vertebra all contribute to the compromise of the canal and central spinal stenosis. The process may also cause foraminal narrowing due to the impingement of the superior articular process in the neuroforamina. Patients presenting with DS may have any combination of low back pain, neurogenic claudication, and radiculopathy [22]. Hamstring spasm is the most frequently associated neurologic abnormality. Lumbar radiculopathy and bowel or bladder symptoms are rare, but may occur in individuals with severe isthmic spondylolisthesis. In the case of DS with nerve root compression, the anatomic type of vertebral slippage has an influence on the pattern of the neurologic symptoms. In the case of symmetric spondylolisthesis with only mild or no rotatory component, nerve root involvement that is typically bilateral and pluriradicular is related to the compression of the thecal sac in the central spinal canal and to a bilateral lateral recess stenosis. Conversely, in the case of asymmetric spondylolisthesis with a marked rotatory displacement, nerve root involvement is frequently unilateral, involving one or two nerve roots on the side of maximal facet joint subluxation because of the compression of the nerve roots in the ipsilateral lateral recess and foramen. In a meta-analysis of surgically treated DS, radiculopathy and neurogenic claudication were found preoperatively in 32% and 3% of the patients, respectively [23]. Harris and Weinstein [24] studied the long-term outcome in patients with Meyerding Grades III or IV spondylolisthesis (≥ 51% slip) and found that 36% of the patients treated nonsurgically were asymptomatic, 55% had occasional back pain, and 45% had neurologic symptoms; none of the patients was incontinent.
Only 10% to 15% of patients seeking treatment will eventually have surgery [25]. Also, a multilevel slip, or a greater degree of slip (> 25%), does not increase the prevalence and/or severity of symptoms [26], [27]. In symptomatic patients with DS whose debilitating condition is nonresponsive to conservative management, surgical intervention is performed. For the clinical management of spondylolysis and spondylolisthesis, readers are advised to refer to references [28], [29], [30].
Classifications
The Wiltse-Newman classification (Table 1) is the most widely used classification of spondylolisthesis [31]. Of the five types, Types I and II apply commonly to the child and adolescent. Type I, the dysplastic type, defines spondylolisthesis secondary to congenital abnormalities of the lumbosacral articulation, including maloriented or hypoplastic facets and sacral deficiency. The pars is poorly developed, which allows for elongation or eventual separation and forward slippage of L5 on the sacrum with repetitive loading over time. Type I is less common, comprising 14% to 21% of congenital cases [32], [33]. Type II, the isthmic type, defines spondylolisthesis that results from defects of the pars interarticularis. This group is subdivided into three subtypes. Type IIA, the most common subtype, is caused by fatigue failure of the pars from repetitive loading, resulting in a complete radiolucent defect. Type IIB is caused by an elongated pars secondary to repeated microfractures that heal. This type can be difficult to distinguish radiographically from the dysplastic type. Type IIC refers to a pars fracture that results from an acute injury. Wiltse hypothesized that isthmic defects are the result of chronic loading of a pars interarticularis that is genetically predisposed to fatigue failure [34]. Marchetti and Bartolozzi [35] proposed an alternative classification system with two broad categories—developmental and acquired. The developmental category defines spondylolisthesis resulting from an inherited dysplasia of the pars, lumbar facets, discs, and vertebral endplates, combining the dysplastic and isthmic categories of Wiltse-Newman. Acquired spondylolysis and spondylolisthesis define failure of the pars secondary to repetitive spinal loading related to specific activities.
Table 1.
Classification systems for spondylolisthesis.
| Wiltse-Newman | Marchetti-Bartolozzi |
|---|---|
| I. Dysplastic | Developmental |
| II. Isthmic | High dysplastic |
| IIA. Disruption of pars as a result of stress fracture | With lysis |
| IIB. Elongation of pars without disruption related to repeated, healed microfractures | With elongation |
| IIC. Acute fracture through pars | Low dysplastic |
| III. Degenerative | With lysis |
| IV. Traumatic | With elongation |
| V. Pathologic | Acquired |
| Traumatic | |
| Acute fracture | |
| Stress fracture | |
| Postsurgery | |
| Direct surgery | |
| Indirect surgery | |
| Pathologic | |
| Local pathology | |
| Systemic pathology | |
| Degenerative | |
| Primary | |
| Secondary |
In dysplastic spondylolisthesis (Wiltse-Newman Type I), the L5 vertebra with intact posterior elements slips forward on the sacrum. The resulting lumbar stenosis may cause L5 nerve radiculopathy as well as bowel and bladder dysfunction from compression of sacral nerve roots. Children and adolescents with dysplastic spondylolisthesis are more likely to develop neurologic injury and carry greater risk of progressive deformity than patients with isthmic spondylolisthesis (Wiltse-Newman type II). McPhee et al [36] reported a higher frequency of progression in the dysplastic type (32%) than in the isthmic type (4%). Furthermore, patients with dysplastic spondylolisthesis are more likely to require surgical treatment [32], [37]. Children who are diagnosed before their adolescent growth spurt, girls, and those presenting with > 50% slip are most likely to progress [38]. Spondylolisthesis associated with congenital dysplasia of the lumbosacral facets and sacrum allows anterior translation of the L5 vertebral body with intact posterior elements that can compress the L5 and sacral nerve roots.
Radiodiagnostics
Radiograph is the first line of investigation for suspected spondylolisthesis (Figure 2). The best approach for making a diagnosis of spondylolisthesis remains controversial, and it also remains unknown whether the selection of radiodiagnostic techniques will influence clinical management. Standing posteroanterior (PA) and lateral radiographs of the thoracolumbar spine, with supine oblique views of the lumbosacral spine, are usually used to assess potential spondylolysis or spondylolisthesis. The standard PA radiographic view allows evaluation of coexisting scoliosis that may be secondary to paraspinal spasm, whether idiopathic or olisthetic (i.e., the result of asymmetric forward vertebral translation at the level of the spondylolisthesis). The standing lateral view is useful for identifying spondylolytic defects and documenting the degree of spondylolisthesis. The abnormal translation may increase in standing position compared to recumbent position. Vertebral sagittal slip is higher in standing position than in recumbent supine position [39]. In the coronal plane, a slight disruption of the alignment of the spinous processes and of the lateral border of the vertebral bodies with or without a lateral slip (laterolisthesis) should be checked carefully. Supine oblique and spot lateral radiographic views of the lumbosacral junction improve the likelihood of diagnosing stress reactions and spondylolytic defects. Harvey et al [40] suggested that the coned lateral of the lumbosacral junction and the anteroposterior (AP) view with 30 degrees cranial angulation are particularly important. Plain radiography of the pars is sometimes difficult since the pars lies oblique to all three orthogonal planes. A trapezoidal shape of the fifth lumbar vertebra is probably a result of the slipping, not a cause [21]. Spina bifida occulta occurs more frequently in patients with a pars interarticularis defect than in patients without a defect.
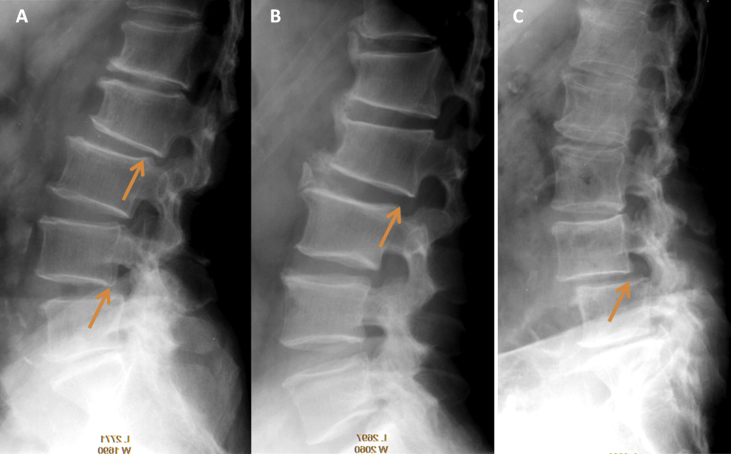
Figure 2.
(A) Multiple-level spondylolisthesis of L2 (Grade I posterolithesis) and L4 (Grade I anterolisthesis); (B) spondylolisthesis of L2 (Grade I posterolithesis) with formation of osteophytes probably as a mechanism to compensate for stabilization; and (C) L4 spondylolisthesis (Grade I anterolisthesis).
Flexion-extension positioning is a technique used by many surgeons to assess the degree of lumbar instability in spondylolisthesis [41]. However, it has been speculated that tightness or spasm in the paraspinal muscles secondary to pain when standing may have a splinting effect, thus reducing the apparent instability on flexion-extension radiographs. Pain leads to decreased intervertebral motion in symptomatic patients with spondylolisthesis [42]. While static radiographs seem to show the greatest slip in standing position, in cases of hypermobile spondylolisthesis the lateral decubitus position may reveal an even higher abnormal translation because the stiffness of the splinting muscles is reduced [43]. Adequate functional radiographs depend on the patient's cooperation and the examiner's proper control and can lead to different results from test to test [44]. According to Danielson et al [45], [46], a slight variation in patient positioning or in gantry tilting may result in a 10% to 15% variation in the range of vertebral displacement. Patient positioning and direction of the X-ray beam have to be accurate and reproducible to allow optimal measurement. However, the way to perform functional radiographs and the method to measure displacements are still not standardized. Anderson et al [47] reported that initial radiographs and nuclear planar bone scans failed to demonstrate 19% of the pars lesion in one of their studies.
Factors requiring additional radiological investigations include significant and progressive neurologic claudication or radiculopathies and clinical suspicion of other conditions such as metastatic disease. An absolute indication is the presence of bladder or bowel complaints [48]. Additional studies that may be selected include technetium bone scanning, particularly when a metastatic tumor is suspected. Single-photon emission computed tomography (SPECT) of the lumbosacral spine is the most effective method for detecting spondylolysis when plain radiographs are normal and the patient's history and physical examination are suggestive of the diagnosis. Increased radionuclide uptake in an intact pars, lamina or pedicle is consistent with a stress reaction. A relative decrease in tracer uptake on serial SPECT scans has been correlated with improvement of clinical symptoms and signs in treated, symptomatic patients [49]. As spondylolisthesis develops and progresses, the SPECT scan again becomes positive. SPECT scanning in spondylolysis is not a positive or negative examination, but varies with the time and stability of the spondylolytic spine.
Stress reactions that have not progressed to complete defects will be radiographically occult. If the radiographic series is nondiagnostic, limited thin section computed tomography (CT) technique will demonstrate both stress reactions and established defects, though establishing a diagnosis of spondylolysis as the cause of the pain is more difficult. Thin-section CT, performed with a reverse gantry angle, is the best modality for defining the bony anatomy of spondylolysis [50]. Magnetic resonance imaging (MRI) is indicated when neurologic symptoms and signs are present in conjunction with spondylolysis and spondylolisthesis. Nerve root compression, lumbar disc abnormalities, spinal cord anomalies, and neoplasm of the spinal cord or vertebral spinal column are other causes of low back pain that are best assessed with MRI [51]. MRI may demonstrate intraosseous edema of the affected areas in these patients.
A few methods have been proposed to grade spondylolisthesis. The first is the method of Meyerding (Figure 3) [52]. The AP diameter of the superior surface of the lower vertebral body is divided into quarters and Grade I–IV is assigned to slips of one, two, three, or four quarters of the superior vertebra, respectively. The second method, first described by Taillard (Figure 3) [53], expresses the degree of slip as a percentage of the AP diameter of the top of the lower vertebra. The second method is favoured by most authors as it is more accurately reproducible [48]. A simpler classification system divides spondylolisthesis into cases with translation of ≤ 50% (stable) and those with translation of > 50% (unstable). Patients with higher grades of spondylolisthesis and higher slip angles, a measure of lumbosacral kyphosis, have a higher risk of progression. Measurement of the slip and its apparent progression should however be viewed with caution. Studies have shown that there can be interobserver and intraobserver error of up to 15% [45], [46]. This variation can increase if there is an element of rotation. The position of the radiograph at different times will also affect the measurement results.
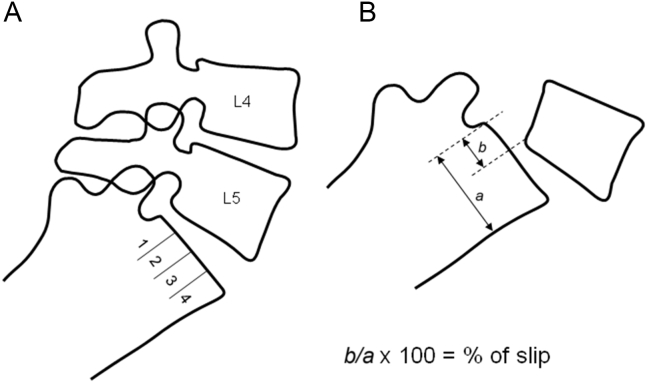
Figure 3.
Scheme of spondylolisthesis grading methods: (A) Meyerding, and (B) Taillard.
For more discussion on radiological investigations of spondylolisthesis, see references [50], [51], [54], [55], [56].
Epidemiology I: results of population based studies
The published epidemiological data on DS varies greatly one from another. The prevalence, F:M prevalence ratio, and risk factors remain controversial. For example, in an elderly Chinese population (≥ 65 years) of 4000 individuals (half females and half males, mean age: 72.5 years), we found the overall prevalence of spondylolisthesis was 25.0% for females and 19.1% for males, with an F:M ratio of 1.3:1 [27]. In the Copenhagen Osteoarthritis Study (1533 males, mean age of 62 years, range 23–93 years; 2618 females, mean age: 65 years, range 22–9 years), Jacobsen et al [57] reported the prevalence of spondylolisthesis was 2.7% for males and 8.4% for females, with an F:M ratio of 6.4:1. Farfan studied 460 lumbar spine autopsies (mean age: 64 years) and found the prevalence of DS was 4.1% [1]. In a professional taxi driver cohort in Taipei (mean age: 44.5 ± 8.7 years, predominately males), Chen et al [58] reported the prevalence of spondylolisthesis was 3.2%. Kalichman et al [59] studied 188 adult community-based population (mean: 52.7 ± 10.8 years) with CT, and found the DS prevalence was 7.7% (males) vs. 21.3% (females) with an F:M ratio = 3:1. These controversies are complicated by the fact that imaging techniques and radiographic landmarks used for the measurements vary between reported studies and radiographic magnification is not always taken into account. For this review, we searched systemically the published literature, with the aim: (1) to have a clearer understanding of age-specific and gender-specific prevalence of DS; (2) to determine whether there is a prevalence difference of DS between Northeast Asians (Chinese, Japanese, and Korean) and European/American Caucasians; and (3) to determine the potential association between menopause and DS development.
PubMed (https://www.ncbi.nlm.nih.gov/pubmed) was used to search the literature. To broadly include data, only the word ‘spondylolisthesis’ was used for the search. The search was carried out on April 24, 2016, and 5255 items were found. This was again updated on September 18, 2016, with 120 new items found. These 5375 items in total were evaluated by the authors according their relevance to the current study. Relevant articles containing original data were reviewed. The emphasis of this study is degenerative spondylolisthesis, therefore papers solely dealing with congenital spondylolisthesis were not selected. Spondylolysis without listhesis, iatrogenic spondylolisthesis [60], traumatic spondylolisthesis, and spondylolisthesis among athletes are not the focus of this literature survey.
Reported data shows that fetal incidence of spondylolisthesis (i.e., with a vertebral slip) is close to zero [21], [61], [62], [63]. The incidence of dysplastic or isthmic spondylolisthesis in the general population is 4% to 8% [19], [59], [61], [62], [63], [64], [65], [66], [67]. Beutler et al [19] reported that the prevalence rate of lytic defects of the pars interarticularis was 6%, and the spondylolisthesis prevalence was 3.6%. The male prevalence is likely to be twice that for females [19]. In a study involving 2000 individuals from the Japanese general population with multidetector computed tomography scan, Sakai et al [66] reported lumbar spondylolysis was found in 5.9% of participants and the F:M ratio was 1:2. They also noted that spondylolisthesis was found in 74.5% of participants with bilateral spondylolysis, and in 7.7% of those with unilateral spondylolysis. One study reported that CT showed higher spondylolysis rate (11%) than radiograph [59]. However, this was not confirmed in other CT-based studies [66], [67]. Some ethnic groups may have higher prevalence rate and that may be related to genetic factors [68], [69], [70], [71]. A higher prevalence in the Inuit and black American female populations have been reported [64], [68], [72].
Our literature review shows DS is strongly age-specific and gender-specific, and it is relatively rare before 50 years of age (Figure 4). In the Copenhagen Osteoarthritis Study, DS increases with increasing age in both sexes, while very few individuals (about 4% of all DS cases) had spondylolisthesis at L4 to L5 level before the age of 50 years (Figure 4A). Kalichman et al [59] also reported that no cases of DS were observed in men less than 40 years or in women less than 50 years of age (Figure 4B). Similar results were seen in Chen et al's study [58]. The degree of DS slip is usually Grade I [26], [27]. The DS level most commonly involved was L4–L5, followed by L5–S1 and L3–L4 (approximately 12% each) [26], [27], [73], [74]. It has been demonstrated that the progression of slipping is slow and not correlated to age at diagnosis and initial degree of spondylolisthesis. Disc height reduction at the spondylolytic level occurs at an earlier age and is more severe than in a normal group. Symptoms were correlated to radiographic pathology. Risk factors for low-back symptoms were greater than 25% slipping, spondylolysis at the L4 level, and early disc degeneration [75], [76], [77].
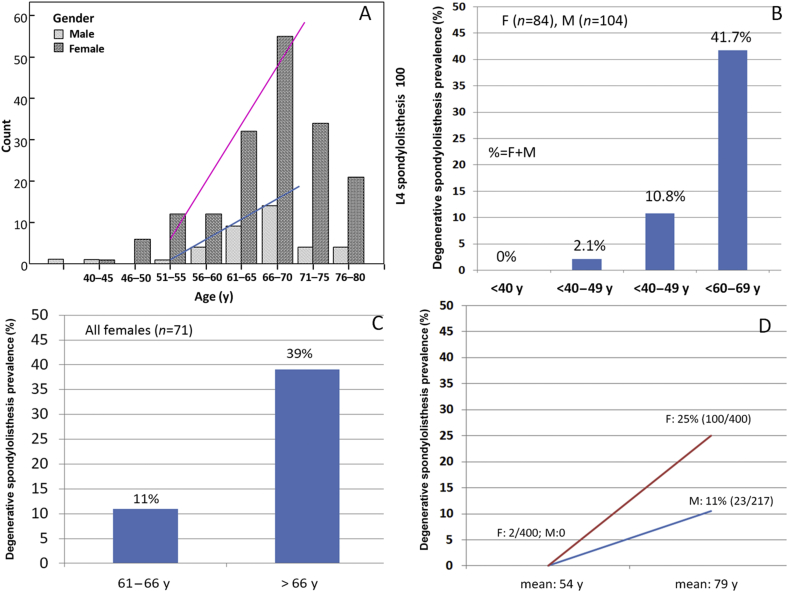
Figure 4.
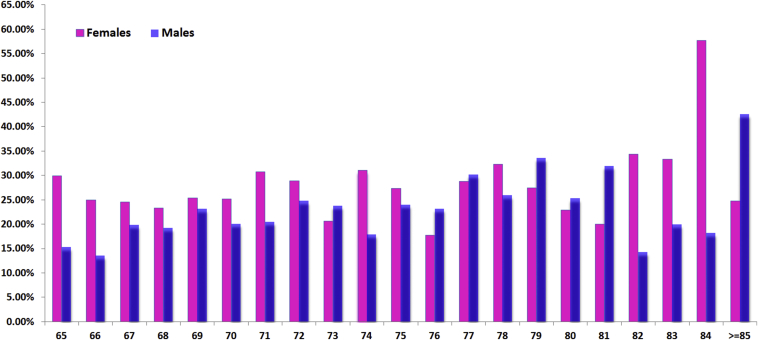
The prevalence of DS is very gender-specific and age-specific. Both women and men have a low incidence of DS before 50 years of age. After 50 years, both women and men start to develop DS, with women beginning to develop DS at a faster rate than men (A–D). (A) Raw data from reference [57]; (B) raw data from reference [59]; (C) raw data from reference [78]; and (D) raw data from reference [26].
With the population-based epidemiology of ours [27], 2000 elderly Chinese men and 2000 elderly Chinese women were recruited during the period August 2001 to March 2003 [27]. The prevalence of lumbar spondylolisthesis was shown to be 25.0% in women and 19.1% in men, and the F:M ratio was 1.3:1 (Table 2, Figure 5). Our study did not differentiate between DS and spondylolytic spondylolisthesis as only lateral radiographs were obtained; therefore our data probably overestimated prevalence by 4% for this reason. It is also probable that our data underestimated the prevalence by around 8% because standing radiographs were not obtained [37], [41], [53]. Hensinger [37] reported that the average slip of 9% on the supine radiographs increased to 10% on the erect views and this was not significant; while in a low back pain patient cohort, Iguchi et al [41] reported that 15% of spondylolisthetic lesions can only be seen by a standing lateral radiograph. Our study could not be compared with Chen et al's results as their participants were younger [58]. In a small-cohort (male, n = 306; female, n = 486) population-based study reported by Chaiwanichsiri et al [79], the prevalence was 14.4% (females age: 60.88 ± 7.9 years) vs. 8.8% (males 61.39 ± 7.7 years), with an F:M ratio of 1.63:1. In our cohort aged 65–69 years, the prevalence was 21.1% (females) vs. 14.7% (males), with an F:M ratio of 1.45:1 [27]. These data are probably comparable since our patients were older than Chaiwanichsiri et al's.
Table 2.
A comparison of degenerative spondylolisthesis prevalence and 4-year progression in elderly Chinese and elderly Caucasian Americans [27], [73], [80], [81].
| Age (y), mean (range) | Prevalence (%) | Progression (%) | De novo (%) | |
|---|---|---|---|---|
| MsOS (Hong Kong) Year 0a | 72.6 (65–98) | 25 | ||
| MsOS (USA) Year 0b | 71.5 (65–89) | 43.1 | ||
| MrOS (Hong Kong) Year 0c | 72.4 (65–92) | 19.1 | ||
| MrOS (USA) Year 0d | 31 | |||
| MsOS (Hong Kong) Year 4e | 75.7 (68–102) | 41.2 | 16.5 | 12.7 |
| MrOS (Hong Kong) Year 4f | 75.5 (68–95) | 31.5 | 13.0 | 12.4 |
| MrOS (USA) Year 4g | 43h | 12 | 12 |
n = 1994 participants.
n = 788 participants.
n = 1996 participants.
n = 295 participants.
n = 1546 participants.
n = 1519 participants.
n = 190 participants.
Estimated from baseline data plus de novo number. The F:M ratio of MsOS (Hong Kong) and MrOS (Hong Kong) is 1.3:1 while the F:M ratio of MsOS (USA) and MrOS (USA) is 1.38:1.
Figure 5.
The elderly Chinese DS prevalence based on elderly Chinese MsOS (Hong Kong) and MrOS (Hong Kong) studies. Baseline data and Year-4 follow-up data have been combined. x-Axis: age in years; y-axis: prevalence (in %). Source data from references [27] and [73].
The design of our MsOS (Hong Kong) and MrOS (Hong Kong) studies is similar to the MsOS (USA) and MrOS (USA) studies [27], [73], [80], [81]. In our study, spondylolisthesis was defined as a forward slip (anterolisthesis) or backward slip (retrolisthesis) of one vertebral body by at least 5% in relation to the next most caudal vertebral body. This would be roughly translated to 3 mm. For women in the MsOS (USA) study (n = 788 subjects analyzed), Vogt et al [79] used greater than 3 mm as the threshold of spondylolisthesis and measured at the lower lumbar level (L3–S1). The prevalence of DS was 43.1% (anterolisthesis = 28.9 %, retrolisthesis = 14.2%), higher than our results of 25% (Table 2). Compared with the MrOS (USA) study (n = 295 subjects analyzed), our male cohort also had a lower anterolisthesis prevalence (19.1% vs. 31%) [27], [81]. These results suggest that elderly Caucasian Americans have a higher DS prevalence, approximately 60–70% higher than elderly Chinese. However, the F:M ratio was similar to the Chinese population, being 1.38:1 (Table 2). The 4-year de novo DS is also similar between Chinese men and Caucasian American men, being around 12%. That Caucasians have higher DS prevalence has been indicated in smaller cohort studies by Kalichman et al [59] (Figure 4B) and Marty-Poumarat et al [78] (Figure 4C). On the other hand, the Japanese population may also have a lower DS prevalence than the Caucasian population. In a cross-sectional study of the elderly population from a single Japanese village with 205 elderly men (mean age, 70.7 years) and 323 elderly women (mean age, 70.5 years), Horikawa et al [82] reported spondylolisthesis prevalence of 4.9% for males, and 11.5% for females. In a cohort of 3259 Japanese patients with low back and/or leg pain (mean age approximately 65 years), Iguchi et al [41] reported a DS prevalence of about 8.7% (F:M ratio = 1.3:1). Additionally, Aono et al [83] followed up for 12.1 years (8–14 years) in 142 female subjects without spondylolisthesis at baseline radiographs (mean baseline age: 54.7 years, range: 40–77yrs), and the incidence of newly developed DS was 12.7%. The same group [84] reported when the female participants were 68.5 ± 9.2 years old, the DS prevalence was 24.8% (50 participants out of 289). These data suggest that elderly Japanese females and elderly Chinese females in Hong Kong have similar DS prevalence and progression rates. Of note, a few studies showed body height was not associated with the incidence of DS [27], [57], [73].
Based on the observations above (Table 2, Figure 4), we can conclude that elderly Chinese, probably all Northeast Asians, have a substantially lower prevalence of DS than European and American Caucasians. The precise difference is difficult to confirm due to the fact that the DS prevalence is very age-specific and gender-specific, and few age-matched studies are available for comparison. The results of Table 2 suggest that the F:M ratio (1.38:1) in elderly Caucasian Americans is similar to Chinese, in contrast to the much larger F:M ratios reported by some groups [57], [64]. Iguchi et al's [41] cohort of DS with low back and/or leg pain also had an F:M ratio of 1.3:1 (115 women and 86 men). Of note, Meana et al's study on Canadian women reported that in the age group 65 years and older, Chinese males and females had a lower rate of chronic pain than other ethnic groups [85]. Deyo et al [86] also estimated that back pain prevalence and hospital/clinics visit rates from U.S. national surveys in 2002 showed that American Indians and Alaska natives had the highest prevalence of back pain, while Asian Americans had the lowest prevalence (Asian Americans vs. non-Hispanic whites = 19.0% vs. 27.2%, or 1:1.43).
Epidemiology II: ratio of female and male patients who received surgical treatment
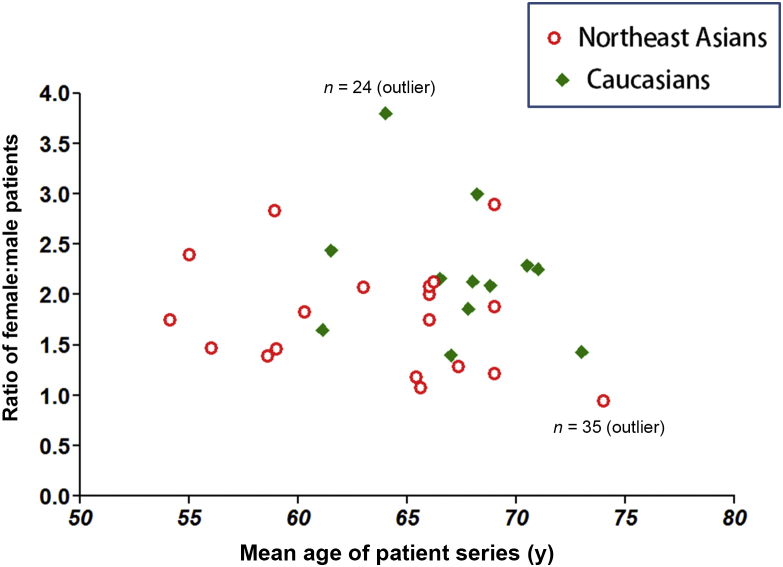
Our previous study showed elderly Chinese, Japanese, and Korean women have very similar age-specific osteoporotic vertebral fracture prevalence, but elderly Caucasian women have slightly higher age-specific osteoporotic vertebral fracture prevalence than Northeast Asians (the F:M ratio for elderly American females and elderly Chinese females is around 1.3:1—see Table 6 of reference [87]). Surgical patient series on European Caucasian, American (the majority being whites) and Northeast Asians (Chinese, Japanese, and Korean) were retrieved for the current study. The criterion for being included in the current analysis was gender specific age information was provided in the publications; or the age distribution was rather narrow, so that female patients and male patients were close to be age-matched and F:M ratio can be computed. We only included the series with no less than 24 patients. The result is shown in Figure 6.
Figure 6.
Surgical patient series age-specific female:male ratio for European/American Caucasian and Northeast Asian groups. Data were extracted from references [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114].
In Figure 6 there were 19 patient series for Asians and 12 patient series for Caucasians. Excluding two outliers, the mean age was 63.0 ± 5.10 years for Asians and 67.6 ± 2.58 years for Caucasians, respectively (p = 0.01); and the mean F:M ratio was 1.81 ± 0.53 for Asians and 2.0 ± 0.34 for Caucasians, respectively (p = 0.22). It is unknown whether Asians were likely to be symptomatic earlier, or they had easier access to early treatment. During the literature search, two surgical patient series publications on the Mexican population which otherwise met our inclusion criteria were also retrieved. The F:M ratio was 1.3 and 1.15, and mean ages were 58.9 years and 60.3 years, respectively [115], [116]. Therefore, these limited data did not show F:M ratio difference between Latin American, Asian, and Caucasian patients.
That higher F:M ratio for DS surgery shown in Figure 6, being approximately 2:1, is more than the population based DS prevalence F:M ratio of 1.3:1. This can be explained by the fact that women have a lower threshold for back pain and women are more likely to seek pain treatment than men [117]. There is a higher prevalence of pain in females for headache, migraine, temporomandibular pain, burning mouth pain, neck pain, shoulder pain, back pain, knee pain, abdominal pain, and fibromyalgia [117]. Women have been shown to have a lower threshold of perception for pain and in reacting to it [118], [119]. It has been observed that females are more likely to report symptoms, even when physician verified abnormalities are approximately equal to those of males [120], [121]. There are more female patients than male in general in low back pain clinics [122], [123].
There are a number of limitations of this patient series review. The majority of published papers did not separately report the age information for females and males. The age of females and males in some publications might be actually matched without providing the necessary information. However, in these cases, we could not use these papers for Figure 6. In addition, we found that the majority of patient series reported in the Chinese language did not separate isthmic spondylolisthesis and degenerative spondylolisthesis. Some of the patient series were limited in patient number and the F:M ratio might be coincidental. In some series, the F:M ratio might be due to the type of treatment selected. We analyzed publications in English, French, German, Chinese, Japanese, Spanish, and Hungarian. Publications in some other languages such as Polish were not analyzed unless a detailed English abstract was available. More studies are required to further confirm the pattern seen in Figure 6.
The role of menopause on degenerative spondylolisthesis
The association between menopause and DS was proposed with the consideration of the higher incidence of DS in postmenopausal women than in age-match men. Before the age of 50 years, DS is rare; and the prevalence of congenital spondylolisthesis is actually more common in men as discussed above [19], [66]. Low levels of female sex hormones in postmenopausal women can be associated with (1) accelerated degeneration of disc degeneration and disc space narrowing [124], [125], [126], [127], [128]; (2) higher prevalence of osteoarthritis, including that of facet joints [129], [130]; and (3) general laxity of the paraspinal ligaments. It has been shown that hormone replacement treatment (HRT) preserves muscle strength in postmenopausal women [131], [132]. Taaffe et al [133] showed that HRT preserves or improves skeletal muscle quality in early postmenopausal women, and has a positive effect on muscle performance. In experiments on the elastic properties of the capsular ligament of the hip, periodontal tissues and the uterus, oestrogen was proved to have a considerable influence on collagen and elastin synthesis [134], [135], [136], [137]. Fischer and Swain [138] found that oestrogen reduced the collagen content and increased the elastin content of the arterial wall in rats. Shikata et al [134] in an experimental study on hip dislocation stated that oestrogen deficit after oophorectomy induced a loss of elasticity of the capsular ligament.
In 1994 Imada et al [139] performed a case-control study on the influence of oophorectomy on the development of DS. The mean period between oophorectomy and review was 6.3 ± 2.8 years. They found bilateral oophorectomy with no hormonal replacement therapy was a risk factor for DS with an odds ratio of 7.5 (95% confidence interval, 1.6–46). The incidence (n = 20) of DS in 69 oophorectomised patients (mean age at oophorectomy: 47.4 ± 5.6 years) was about three times higher than in 69 nonoophorectomised matched control subjects (n = 6, 29.0% vs. 8.7%, p < 0.005). Their results suggest that the abrupt decrease in oestradiol level caused by oophorectomy may be a predisposing factor in degenerative spondylolisthesis at L4/5. The loss of elasticity in the paraspinal ligamentous system produced by the hormonal changes caused by oophorectomy may contribute to degeneration and to the development of the vertebral slip at L4/S. Of note, bilateral oophorectomy has acute and dramatic impact on the physiology of musculoskeletal system [140], [141].
Marty-Poumarat et al [78] evaluated the influence of HRT on degenerative scoliosis and lateral rotatory olisthesis (LRO). A cross-sectional study was conducted in 146 postmenopausal women, 75 women had received HRT for more than 1 year (HRT>1, age: 65.0 ± 5.4 years) and 71 women had never received HRT or received HRT for less than 1 year (HRT<1, age: 66.7 ± 5.9 years, p = 0.07). All the women had been in menopause for more than 5 years. There was no difference in body mass index in the two groups (24.2 ± 3.4 vs. 25.0 ± 2.9 kg/m2, p = 0.11), and the mean time since menopause was not significantly different in the two groups (14.8 ± 6.3 vs. 16.8 ± 7.2 years, p = 0.09). The HRT duration was 8.7 ± 6.1 years for HRT > 1 group. The prevalence of LRO was significantly lower in HRT > 1 group than in HRT < 1 group (8% vs. 30%). LRO increased with age only in HRT < 1 group (11% when aged ≤ 66 years vs. 39% when aged > 66 years, p = 0.013), whereas the prevalence of LRO remained stable in HRT > 1 group.
If we assume the prevalence of DS is low (estimated to be 5%, note our method did not exclude congenital DS in the MsOS (Hong Kong) cohort) before 50 years, then Figure 4 shows there was substantial increase of DS during menopause (around 50 years) and 65 years for females (while the extent is also substantial for males). On the other hand, the new incidence DS rate is less high after 65 years (Figure 5). We believe menopause may have triggered the accelerated DS development after the immediate postmenopausal phase in women. A further population-based study involving both females and males and covering the age span of 50 to 65 years may confirm this hypothesis.
Our MsOS (Hong Kong) and MrOs (Hong Kong) studies demonstrated the factors for DS occurrence include to control the body weight and have moderate exercises to increase protective muscle strength, and also control blood pressure, particularly for women [27], [73]. Actually, we think one of the possible reasons why men have lower vertebral slip rate is that men tend to have stronger protective muscles and ligaments. HRT initiated at an early postmenopausal phase may be protective for intervertebral disc degeneration [142], osteoarthritis of facet joints, and maintain muscle and ligament tone in lumbar regions, as well as attenuate atherosclerosis [143]. A regimen of HRT may be considered in cases of women with anatomical high risk of developing DS or DS progression, such as high lumbar lordosis, vertebral end-plate inclination, severe disc degeneration and loss of height, and facet joint sagittal orientation [3], [7], particularly cases with clinical symptoms. Optimal selection of dose regimen, combination of oestrogen with progestins versus oestrogen alone, the administration route, and duration of treatment such as the choice of repetitive or periodic administration simulating the menstrual cycle may lead to better benefits [144], [145]. Women presenting to their medical providers during the menopausal transition provide a unique opportunity for risk assessment, counselling and the institution of various preventive measures [145].
In conclusion, our review demonstrated that the prevalence of DS is very gender-specific and age-specific. Few women and men have DS before 50 years old and after 50 years both women and men begin to develop DS with faster development rate in women than men. Elderly Caucasian Americans have a higher DS prevalence, being approximately 60% to 70% higher, than elderly Chinese. The female:male prevalence ratio is estimated to be around 1.3:1. Further studies on DS epidemiology should report gender-specific and age-specific information to allow better interstudy comparison and data synthesis. Evidence suggests that hormone replacement therapy may alleviate the development of DS in postmenopausal women.
Conflicts of interest
We declare that there are no conflicts of interest for this paper.
References
- 1.Farfan H.F. The pathological anatomy of degenerative spondylolisthesis. A cadaver study. Spine (Phila Pa 1976) 1980;5(5):412–418. doi: 10.1097/00007632-198009000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Newman P.H. The etiology of spondylolisthesis. J Bone Joint Surg [Br] 1963;45:39–59. [Google Scholar]
- 3.Devine J.G., Schenk-Kisser J.M., Skelly A.C. Risk factors for degenerative spondylolisthesis: a systematic review. Evid Based Spine Care J. 2012;3(2):25–34. doi: 10.1055/s-0031-1298615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hosoe H., Ohmori K. Degenerative lumbosacral spondylolisthesis: possible factors which predispose the fifth lumbar vertebra to slip. J Bone Joint Surg Br. 2008;90(3):356–359. doi: 10.1302/0301-620X.90B3.19606. [DOI] [PubMed] [Google Scholar]
- 5.Berlemann U., Jeszenszky D.J., Bühler D.W., Harms J. The role of lumbar lordosis, vertebral end-plate inclination, disc height, and facet orientation in degenerative spondylolisthesis. J Spinal Disord. 1999;12(1):68–73. [PubMed] [Google Scholar]
- 6.Schwab F.J., Farcy J.P., Roye D.P., Jr. The sagittal pelvic tilt index as a criterion in the evaluation of spondylolisthesis. Preliminary observations. Spine (Phila Pa 1976) 1997;22(14):1661–1667. doi: 10.1097/00007632-199707150-00026. [DOI] [PubMed] [Google Scholar]
- 7.Cinotti G., Postacchini F., Fassari F., Urso S. Predisposing factors in degenerative spondylolisthesis. A radiographic and CT study. Int Orthop. 1997;21(5):337–342. doi: 10.1007/s002640050180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sengupta D.K., Herkowitz H.N. Degenerative spondylolisthesis: review of current trends and controversies. Spine (Phila Pa 1976) 2005;30(Suppl):S71–S81. doi: 10.1097/01.brs.0000155579.88537.8e. [DOI] [PubMed] [Google Scholar]
- 9.Sanderson P.L., Fraser R.D. The influence of pregnancy on the development of degenerative spondylolisthesis. J Bone Joint Surg [Br] 1996;78:951–954. doi: 10.1302/0301-620x78b6.1291. [DOI] [PubMed] [Google Scholar]
- 10.Tallarico R.A., Madom I.A., Palumbo M.A. Spondylolysis and spondylolisthesis in the athlete. Sports Med Arthrosc. 2008;16:32–38. doi: 10.1097/JSA.0b013e318163be50. [DOI] [PubMed] [Google Scholar]
- 11.Toueg C.W., Mac-Thiong J.M., Grimard G., Parent S., Poitras B., Labelle H. Prevalence of spondylolisthesis in a population of gymnasts. Stud Health Technol Inform. 2010;158:132–137. [PubMed] [Google Scholar]
- 12.Jackson D.W., Wiltse L.L., Cirincione R.J. Spondylolysis in the female gymnast. Clinical Orthopaedics. 1976;117:68–73. [PubMed] [Google Scholar]
- 13.Rossi F. Spondylolysis, spondylolisthesis and sports. J Sports Med Phys Fitness. 1988;18:317–340. [PubMed] [Google Scholar]
- 14.Congeni J., McCulloch J., Swanson K. Lumbar spondylolysis: A study of natural progression in athletes. Am J Sports Med. 1997;25:248–253. doi: 10.1177/036354659702500220. [DOI] [PubMed] [Google Scholar]
- 15.Syrmou E., Tsitsopoulos P.P., Marinopoulos D., Tsonidis C., Anagnostopoulos I., Tsitsopoulos P.D. Spondylolysis: a review and reappraisal. Hippokratia. 2010;14:17–21. [PMC free article] [PubMed] [Google Scholar]
- 16.Virta L., Ronnemaa T., Osterman K., Aalto T., Laakso M. Prevalence of isthmic lumbar spondylolisthesis in middle-aged subjects from eastern and western Finland. J Clin Epidemiol. 1992;45:917–922. doi: 10.1016/0895-4356(92)90075-x. [DOI] [PubMed] [Google Scholar]
- 17.Kirkaldy-Willis W.H., Farfan H.F. Instability of the lumbar spine. Clin Orthop Relat Res. 1982;165:110–123. [PubMed] [Google Scholar]
- 18.Matsunaga S., Sakou T., Morizono Y., Masuda A., Demirtas A.M. Natural history of degenerative spondylolisthesis. Pathogenesis and natural course of the slippage. Spine (Phila Pa 1976) 1990;15(11):1204–1210. doi: 10.1097/00007632-199011010-00021. [DOI] [PubMed] [Google Scholar]
- 19.Beutler W.J., Fredrickson B.E., Murtland A., Sweeney C.A., Grant W.D., Baker D. The natural history of spondylolysis and spondylolisthesis: 45-year follow up evaluation. Spine (Phila Pa 1976) 2003;28:1027–1035. doi: 10.1097/01.BRS.0000061992.98108.A0. [DOI] [PubMed] [Google Scholar]
- 20.Frennered A.K., Danielson B.I., Nachemson A.L. Natural history of symptomatic isthmic low-grade spondylolisthesis in children and adolescents: A seven-year follow-up study. J Pediatr Orthop. 1991;11:209–213. doi: 10.1097/01241398-199103000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Fredrickson B.E., Baker D., McHolick W.J., Yuan H.A., Lubicky J.P. The natural history of spondylolysis and spondylolisthesis. J Bone Joint Surg [Am] 1984;66:699–707. [PubMed] [Google Scholar]
- 22.Denard P.J., Holton K.F., Miller J., Fink H.A., Kado D.M., Marshall L.M. Osteoporotic Fractures in Men (MrOS) Study Group. Back pain, neurogenic symptoms, and physical function in relation to spondylolisthesis among elderly men. Spine J. 2010;10(10):865–873. doi: 10.1016/j.spinee.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mardjetko S., Connolly P., Shott S. Degenerative lumbar spondylolisthesis: A meta-analysis of literature 1970–1993. Spine (Phila Pa 1976) 1994;19(20 Suppl):2256S–2265S. [comments: Spine (Phila Pa 1976). 1995;20(17):1957–8] [PubMed] [Google Scholar]
- 24.Harris I.E., Weinstein S.L. Long-term follow-up of patients with grade-III and IV spondylolisthesis: Treatment with and without posterior fusion. J Bone Joint Surg Am. 1987;69:960–969. [PubMed] [Google Scholar]
- 25.Postacchini F., Cinotti G., Perugia D. Degenerative lumbar spondylolisthesis II. Surgical treatment. Ital J Orthop Traumatol. 1991;17:467–477. [PubMed] [Google Scholar]
- 26.Kauppila L.I., Eustace S., Kiel D.P., Felson D.T., Wright A.M. Degenerative displacement of lumbar vertebrae. A 25-year follow-up study in Framingham. Spine (Phila Pa 1976) 1998;23(17):1868–1873. doi: 10.1097/00007632-199809010-00014. discussion 1873–4. [DOI] [PubMed] [Google Scholar]
- 27.He L.C., Wang Y.X., Gong J.S., Griffith J.F., Zeng X.J., Kwok A.W. Prevalence and risk factors of lumbar spondylolisthesis in elderly Chinese men and women. Eur Radiol. 2014;24(2):441–448. doi: 10.1007/s00330-013-3041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.North American Spine Society . North American Spine Society; Burr Ridge, USA: 2008. Clinical guidelines for multidisciplinary spine care. Diagnosis and treatment of degenerative lumbar spondylolisthesis. [Google Scholar]
- 29.Watters W.C., 3rd, Bono C.M., Gilbert T.J., Kreiner D.S., Mazanec D.J., Shaffer W.O. North American Spine Society. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spondylolisthesis. Spine J. 2009;9(7):609–614. doi: 10.1016/j.spinee.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 30.Sys J., Michielsen J., Bracke P., Martens M., Verstreken J. Nonoperative treatment of active spondylolysis in elite athletes with normal x-ray findings: Literature review and results of conservative treatment. Eur Spine J. 2001;10:498–504. doi: 10.1007/s005860100326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiltse L.L., Newman P.H., Macnab I. Classification of spondylolisis and spondylolisthesis. Clin Orthop Relat Res. 1976;117:23–29. [PubMed] [Google Scholar]
- 32.Boxall D., Bradford D.S., Winter R.B., Moe J.H. Management of severe spondylolisthesis in children and adolescents. J Bone Joint Surg [Am] 1979;61:479–495. [PubMed] [Google Scholar]
- 33.Newman P.H. A clinical syndrome associated with severe lumbo-sacral subluxation. J Bone Joint Surg [Br] 1965;47:472–481. [PubMed] [Google Scholar]
- 34.Wiltse L.L., Widell E.H., Jr., Jackson D.W. Fatigue fracture: The basic lesion in isthmic spondylolisthesis. J Bone Joint Surg [Am] 1975;57:17–22. [PubMed] [Google Scholar]
- 35.Marchetti P.G., Bartolozzi P. Lippincott-Raven; Philadelphia, PA: 1997. Classification of spondylolisthesis as a guideline for treatment, in Textbook of Spinal Surgery, ed 2; pp. 1211–1254. [Google Scholar]
- 36.McPhee I.B., O'Brien J.P., McCall I.W., Park W.M. Progression of lumbosacral spondylolisthesis. Australas Radiol. 1981;25:91–95. doi: 10.1111/j.1440-1673.1981.tb02225.x. [DOI] [PubMed] [Google Scholar]
- 37.Hensinger R.N. Spondyloysis, spondylolisthesis in children, adolescents. J Bone Joint Surg [Am] 1989;71:1098–1107. [PubMed] [Google Scholar]
- 38.Boxall D., Bradford D.S., Winter R.B., Moe J.H. Management of severe spondylolisthesis in children and adolescents. J Bone Joint Surg [Am] 1979;61:479–495. [PubMed] [Google Scholar]
- 39.Luk K.D., Chow D.H., Holmes A. Vertical instability in spondylolisthesis: a traction radiographic assessment technique and the principle of management. Spine (Phila Pa 1976) 2003;28(8):819–827. doi: 10.1097/00007632-200304150-00016. [DOI] [PubMed] [Google Scholar]
- 40.Harvey C.J., Richenberg J.L., Saifuddin A., Wolman R.L. Pictorial review: The radiological investigation of lumbar spondylolysis. Clin Radiol. 1998;53:723–728. doi: 10.1016/s0009-9260(98)80313-9. [DOI] [PubMed] [Google Scholar]
- 41.Iguchi T., Wakami T., Kurihara A., Kasahara K., Yoshiya S., Nishida K. Lumbar multilevel degenerative spondylolisthesis: radiological evaluation and factors related to anterolisthesis and retrolisthesis. J Spinal Disord Tech. 2002;15:93–99. doi: 10.1097/00024720-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Pearcy M., Portek I., Shepherd J. The effect of low-back pain on lumbar spinal movements measured by three-dimensional X-ray analysis. Spine (Phila Pa 1976) 1985;10(2):150–153. doi: 10.1097/00007632-198503000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Wood K.B., Popp C.A., Transfeldt E.E., Geissele A.E. Radiographic evaluation of instability in spondylolisthesis. Spine (Phila Pa 1976) 1994;19(15):1697–1703. doi: 10.1097/00007632-199408000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Quinnell R.C., Stockdale H.R. Flexion and extension radiography of the lumbar spine: a comparison with lumbar discography. Clin Radiol. 1983;34(4):405–411. doi: 10.1016/s0009-9260(83)80224-4. [DOI] [PubMed] [Google Scholar]
- 45.Danielson B., Frennerd K., Irstam L. Roentgenologic assessment of spondylolisthesis. I: a study of measurement variations. Acta Radiol. 1988;29:345–351. [PubMed] [Google Scholar]
- 46.Danielson B., Frennerd K., Selvik G., Irstram L. Roentgenologic assessment of spondylolisthesis. II: an evaluation of progression. Acta Radiol. 1989;30:65–68. [PubMed] [Google Scholar]
- 47.Anderson K., Sarwark J.F., Conway J.J., Logue E.S., Schafer M.F. Quantitative assessment with SPECT imaging of stress injuries of the pars interarticularis and response to bracing. J Pediatr Orthop. 2000;20(1):28–33. [PubMed] [Google Scholar]
- 48.Butt S., Saifuddin A. The imaging of lumbar spondylolisthesis. Clin Radiol. 2005;60:533–546. doi: 10.1016/j.crad.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 49.Lusins J.O., Elting J.J., Cicoria A.D., Goldsmith S.J. SPECT evaluation of lumbar spondylolysis and spondylolisthesis. Spine (Phila Pa 1976) 1994;19:608–612. doi: 10.1097/00007632-199403000-00018. [DOI] [PubMed] [Google Scholar]
- 50.Grogan J.P., Hemminghytt S., Williams A.L., Carrera G.F., Haughton V.M. Spondylolysis studied with computed tomography. Radiology. 1982;145:737–742. doi: 10.1148/radiology.145.3.7146406. [DOI] [PubMed] [Google Scholar]
- 51.Nizard R.S., Wybier M., Laredo J.D. Radiologic assessment of lumbar intervertebral instability and degenerative spondylolisthesis. Radiol Clin North Am. 2001;39(1):55–71. doi: 10.1016/s0033-8389(05)70263-3. [DOI] [PubMed] [Google Scholar]
- 52.Meyerding H.W. Spondyloptosis. Surg Gynaecol Obstet. 1932;54:371–377. [Google Scholar]
- 53.Taillard W. Le spondylolisthesis chez l'enfant et l'adolescent. Acta Orthop Scand. 1954;24:115–144. [PubMed] [Google Scholar]
- 54.Lowe R.W., Hayes T.D., Kaye J., Bagg R.J., Luekens C.A. Standing roentgenograms in spondylolisthesis. Clin Orthop Relat Res. 1976;10:80–84. [PubMed] [Google Scholar]
- 55.Câmara J.R., Keen J.R., Asgarzadie F. Functional radiography in examination of spondylolisthesis. AJR Am J Roentgenol. 2015;204(4):W461–W469. doi: 10.2214/AJR.14.13139. [DOI] [PubMed] [Google Scholar]
- 56.Cabraja M., Mohamed E., Koeppen D., Kroppenstedt S. The analysis of segmental mobility with different lumbar radiographs in symptomatic patients with a spondylolisthesis. Eur Spine J. 2012;21(2):256–261. doi: 10.1007/s00586-011-1870-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacobsen S., Sonne-Holm S., Rovsing H., Monrad H., Gebuhr P. Degenerative lumbar spondylolisthesis: an epidemiological perspective: the Copenhagen Osteoarthritis Study. Spine (Phila Pa 1976) 2007;32(1):120–125. doi: 10.1097/01.brs.0000250979.12398.96. [DOI] [PubMed] [Google Scholar]
- 58.Chen J.C., Chan W.P., Katz J.N., Chang W.P., Christiani D.C. Occupational and personal factors associated with acquired lumbar spondylolisthesis of urban taxi drivers. Occup Environ Med. 2004;61(12):992–998. doi: 10.1136/oem.2003.011775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kalichman L., Kim D.H., Li L., Guermazi A., Berkin V., Hunter D.J. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine (Phila Pa 1976) 2009;34:199–205. doi: 10.1097/BRS.0b013e31818edcfd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guha D., Heary R.F., Shamji M.F. Iatrogenic spondylolisthesis following laminectomy for degenerative lumbar stenosis: systematic review and current concepts. Neurosurg Focus. 2015;39(4):E9. doi: 10.3171/2015.7.FOCUS15259. [DOI] [PubMed] [Google Scholar]
- 61.Hensinger R.N., Lang J.R., MacEwen G.D. Surgical management of spondylolisthesis in children and adolescents. Spine (Phila Pa 1976) 1976;1:207–216. [Google Scholar]
- 62.Wiltse L.L. Spondylolisthesis in children. Clin Orthop. 1961;21:156–163. [PubMed] [Google Scholar]
- 63.Wiltse L.L. The etiology of spondylolisthesis. J Bone Joint Surg [Am] 1962;44:539–559. [PubMed] [Google Scholar]
- 64.Hu S.S., Tribus C.B., Diab M., Ghanayem A.J. Spondylolisthesis and spondylolysis. Instr Course Lect. 2008;57:431–445. [PubMed] [Google Scholar]
- 65.Osterman K., Schlenzka D., Poussa M., Seitsalo S., Virta L. Isthmic spondylolisthesis in symptomatic and asymptomatic subjects, epidemiology, and natural history with special reference to disk abnormality and mode of treatment. Clin Orthop. 1993;297:65–70. [PubMed] [Google Scholar]
- 66.Sakai T., Sairyo K., Takao S., Nishitani H., Yasui N. Incidence of lumbar spondylolysis in the general population in Japan based on multidetector computed tomography scans from two thousand subjects. Spine (Phila Pa 1976) 2009;34(21):2346–2350. doi: 10.1097/BRS.0b013e3181b4abbe. [DOI] [PubMed] [Google Scholar]
- 67.Belfi L.M., Ortiz A.O., Katz D.S. Computed tomography evaluation of spondylolysis and spondylolisthesis in asymptomatic patients. Spine (Phila Pa 1976) 2006;31(24):E907–E910. doi: 10.1097/01.brs.0000245947.31473.0a. [DOI] [PubMed] [Google Scholar]
- 68.Tower S.S., Pratt W.B. Spondylolysis and associated spondylolisthesis in Eskimo and Athabascan populations. Clin Orthop Relat Res. 1990;250:171–175. [PubMed] [Google Scholar]
- 69.Albanese M., Pizzutillo P.D. Family study of spondylolysis and spondylolisthesis. J Pediatr Orthop. 1982;2:496–499. doi: 10.1097/01241398-198212000-00006. [DOI] [PubMed] [Google Scholar]
- 70.Cai T., Yang L., Cai W., Guo S., Yu P., Li J. Dysplastic spondylolysis is caused by mutations in the diastrophic dysplasia sulfate transporter gene. Proc Natl Acad Sci U S A. 2015;112(26):8064–8069. doi: 10.1073/pnas.1502454112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stewart T. The incidence of neural-arch defects in Alaskan natives, considered from the standpoint of etiology. J Bone Joint Surg [Am] 1953;35:937–950. [PubMed] [Google Scholar]
- 72.Vogt M.T., Rubin D.A., Palermo L., Christianson L., Kang J.D., Nevitt M.C. Lumbar spine listhesis in older African American women. Spine J. 2003;3(4):255–261. doi: 10.1016/s1529-9430(03)00024-x. [DOI] [PubMed] [Google Scholar]
- 73.Wáng Y.X., Deng M., Griffith J.F., Kwok A.W., Leung J.C., Ahuja A.T. Lumbar Spondylolisthesis Progression and De Novo Spondylolisthesis in Elderly Chinese Men and Women: A Year-4 Follow-up Study. Spine (Phila Pa 1976) 2016;41(13):1096–1103. doi: 10.1097/BRS.0000000000001507. [DOI] [PubMed] [Google Scholar]
- 74.Williams R., Cheung J.P., Goss B., Rajasekaran S., Kawaguchi Y., Acharya S. An International Multicenter Study Assessing the Role of Ethnicity on Variation of Lumbar Facet Joint Orientation and the Occurrence of Degenerative Spondylolisthesis in Asia Pacific: A Study from the AOSpine Asia Pacific Research Collaboration Consortium. Global Spine J. 2016;6(1):35–45. doi: 10.1055/s-0035-1555655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rosenberg N.J. Degenerative spondylolisthesis. Predisposing factors. J Bone Joint Surg Am. 1975;57:467–474. [PubMed] [Google Scholar]
- 76.Saraste H. Long-term clinical and radiological follow-up of spondylolysis and spondylolisthesis. J Pediatr Orthop. 1987;7:631–638. [PubMed] [Google Scholar]
- 77.Fennered A.K., Danielson B.I., Nachemson A.L. Natural history of symptomatic isthmic low-grade spondylolisthesis in children and adolescents: A seven year follow-up study. J Pediatr Orthop. 1991;11:209–213. doi: 10.1097/01241398-199103000-00014. [DOI] [PubMed] [Google Scholar]
- 78.Marty-Poumarat C., Ostertag A., Baudoin C., Marpeau M., de Vernejoul M.C., Cohen-Solal M. Does hormone replacement therapy prevent lateral rotatory spondylolisthesis in postmenopausal women? Eur Spine J. 2012;21(6):1127–1134. doi: 10.1007/s00586-011-2048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chaiwanichsiri D., Jiamworakul A., Jitapunkul S. Lumbar disc degeneration in Thai elderly: a population-based study. J Med Assoc Thai. 2007;90:2477–2481. [PubMed] [Google Scholar]
- 80.Vogt M.T., Rubin D., Valentin R.S., Palermo L., Donaldson W.F., 3rd, Nevitt M. Lumbar olisthesis and lower back symptoms in elderly white women. The study of osteoporotic fractures. Spine (Phila Pa 1976) 1998;23:2640–2647. doi: 10.1097/00007632-199812010-00020. [DOI] [PubMed] [Google Scholar]
- 81.Denard P.J., Holton K.F., Miller J., Fink H.A., Kado D.M., Yoo J.U. Osteoporotic Fractures in Men (MrOS) Study Group. Lumbar spondylolisthesis among elderly men: prevalence, correlates and progression. Spine (Phila Pa 1976) 2010;35:1072–1078. doi: 10.1097/BRS.0b013e3181bd9e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Horikawa K., Kasai Y., Yamakawa T., Sudo A., Uchida A. Prevalence of osteoarthritis, osteoporotic vertebral fractures, and spondylolisthesis among the elderly in a Japanese village. J Orthop Surg (Hong Kong) 2006;14(1):9–12. doi: 10.1177/230949900601400103. [DOI] [PubMed] [Google Scholar]
- 83.Aono K., Kobayashi T., Jimbo S., Atsuta Y., Matsuno T. Radiographic analysis of newly developed degenerative spondylolisthesis in a mean twelve-year prospective study. Spine (Phila Pa 1976) 2010;35(8):887–891. doi: 10.1097/BRS.0b013e3181cdd1aa. [DOI] [PubMed] [Google Scholar]
- 84.Kobayashi T., Chiba H., Jimbo S., Senoo I., Shimizu M., Atsuta Y. Clinical, physical, and radiographic analyses of lumbar degenerative kyphosis and spondylolisthesis among community-based cohort. Eur Spine J. 2016;25(8):2384–2389. doi: 10.1007/s00586-016-4615-0. [DOI] [PubMed] [Google Scholar]
- 85.Meana M., Cho R., DesMeules M. Chronic Pain: The Extra Burden on Canadian Women. BMC Womens Health. 2004;4(Suppl 1):S17. doi: 10.1186/1472-6874-4-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deyo R.A., Mirza S.K., Martin B.I. Back pain prevalence and visit rates: Estimates from U.S. national surveys, 2002. Spine (Phila Pa 1976) 2006;31:2724–2727. doi: 10.1097/01.brs.0000244618.06877.cd. [DOI] [PubMed] [Google Scholar]
- 87.Kwok A.W., Gong J.S., Wang Y.X., Leung J.C., Kwok T., Griffith J.F. Prevalence and risk factors of radiographic vertebral fractures in elderly Chinese men and women: results of Mr. OS (Hong Kong) and Ms. OS (Hong Kong) studies. Osteoporos Int. 2013;24(3):877–885. doi: 10.1007/s00198-012-2040-8. [DOI] [PubMed] [Google Scholar]
- 88.Ren H., Geng W., Ma J., Xu H., Li Z., Pang L. [Correlation analysis of changes of spine-pelvic sagittal parameters before and after operation and effective in patients with lumbar spondylolisthesis] [Article in Chinese] Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2015;29(10):1269–1274. [PubMed] [Google Scholar]
- 89.Wang Y.P., Fei Q., Qiu G.X., Zhao H., Zhang J.G., Tian Y. Outcome of posterolateral fusion versus circumferential fusion with cage for lumbar stenosis and low degree lumbar spondylolisthesis. Chin Med Sci J. 2006;21(1):41–47. [PubMed] [Google Scholar]
- 90.Eliades P., Rahal J.P., Herrick D.B., Corliss B.M., Riesenburger R., Hwang S. Unilateral Pedicle Screw Fixation is Associated with Reduced Cost and Similar Outcomes in Selected Patients Undergoing Minimally Invasive Transforaminal Lumbar Interbody Fusion for L4-5 Degenerative Spondylolisthesis. Cureus. 2015;7(2):e249. doi: 10.7759/cureus.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee S.H., Lee J.H., Hong S.W., Chung S.E., Yoo S.H., Lee H.Y. Spinopelvic alignment after interspinous soft stabilization with a tension band system in grade 1 degenerative lumbar spondylolisthesis. Spine (Phila Pa 1976) 2010;35(15):E691–E701. doi: 10.1097/BRS.0b013e3181d2607e. [DOI] [PubMed] [Google Scholar]
- 92.Blumenthal C., Curran J., Benzel E.C., Potter R., Magge S.N., Harrington J.F., Jr. Radiographic predictors of delayed instability following decompression without fusion for degenerative grade I lumbar spondylolisthesis. J Neurosurg Spine. 2013;18(4):340–346. doi: 10.3171/2013.1.SPINE12537. [DOI] [PubMed] [Google Scholar]
- 93.Enyo Y., Yoshimura N., Yamada H., Hashizume H., Yoshida M. Radiographic natural course of lumbar degenerative spondylolisthesis and its risk factors related to the progression and onset in a 15-year community-based cohort study: the Miyama study. J Orthop Sci. 2015;20(6):978–984. doi: 10.1007/s00776-015-0759-8. [DOI] [PubMed] [Google Scholar]
- 94.Ahmadian A., Verma S., Mundis G.M., Jr., Oskouian R.J., Jr., Smith D.A., Uribe J.S. Minimally invasive lateral retroperitoneal transpsoas interbody fusion for L4-5 spondylolisthesis: clinical outcomes. J Neurosurg Spine. 2013;19(3):314–320. doi: 10.3171/2013.6.SPINE1340. [DOI] [PubMed] [Google Scholar]
- 95.Toyone T., Tanaka T., Kato D., Kaneyama R., Otsuka M. Anatomic changes in lateral spondylolisthesis associated with adult lumbar scoliosis. Spine (Phila Pa 1976) 2005 Nov 15;30(22):E671–E675. doi: 10.1097/01.brs.0000186581.44715.df. [DOI] [PubMed] [Google Scholar]
- 96.Wu C.H., Kao Y.H., Yang S.C., Fu T.S., Lai P.L., Chen W.J. Supplementary pedicle screw fixation in spinal fusion for degenerative spondylolisthesis in patients aged 65 and over: outcome after a minimum of 2 years follow-up in 82 patients. Acta Orthop. 2008;79(1):67–73. doi: 10.1080/17453670710014789. [DOI] [PubMed] [Google Scholar]
- 97.Schaeren S., Broger I., Jeanneret B. Minimum four-year follow-up of spinal stenosis with degenerative spondylolisthesis treated with decompression and dynamic stabilization. Spine (Phila Pa 1976) 2008;33(18):E636–E642. doi: 10.1097/BRS.0b013e31817d2435. [DOI] [PubMed] [Google Scholar]
- 98.Klessinger S. Radiofrequency neurotomy for treatment of low back pain in patients with minor degenerative spondylolisthesis. Pain Physician. 2012;15(1):E71–E78. [PubMed] [Google Scholar]
- 99.Fernández-Fairen M., Sala P., Ramírez H., Gil J. A prospective randomized study of unilateral versus bilateral instrumented posterolateral lumbar fusion in degenerative spondylolisthesis. Spine (Phila Pa 1976) 2007;32(4):395–401. doi: 10.1097/01.brs.0000255023.56466.44. [DOI] [PubMed] [Google Scholar]
- 100.Kanamori M., Yasuda T., Hori T., Suzuki K., Kawaguchi Y. Minimum 10-Year Follow-up Study of Anterior Lumbar Interbody Fusion for Degenerative Spondylolisthesis: Progressive Pattern of the Adjacent Disc Degeneration. Asian Spine J. 2012;6(2):105–114. doi: 10.4184/asj.2012.6.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Matsudaira K., Yamazaki T., Seichi A., Takeshita K., Hoshi K., Kishimoto J. Spinal stenosis in grade I degenerative lumbar spondylolisthesis: a comparative study of outcomes following laminoplasty and laminectomy with instrumented spinal fusion. J Orthop Sci. 2005;10(3):270–276. doi: 10.1007/s00776-005-0887-7. [DOI] [PubMed] [Google Scholar]
- 102.Lee S.H., Lee J.H., Hong S.W., Shim C.S., Chung S.E., Yoo S.H. Factors affecting clinical outcomes in treating patients with grade 1 degenerative spondylolisthesis using interspinous soft stabilization with a tension band system: a minimum 5-year follow-up. Spine (Phila Pa 1976) 2012;37(7):563–572. doi: 10.1097/BRS.0b013e31821c0b97. [DOI] [PubMed] [Google Scholar]
- 103.Archavlis E., Carvi y Nievas M. Comparison of minimally invasive fusion and instrumentation versus open surgery for severe stenotic spondylolisthesis with high-grade facet joint osteoarthritis. Eur Spine J. 2013;22(8):1731–1740. doi: 10.1007/s00586-013-2732-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Takahashi T., Hanakita J., Minami M., Kitahama Y., Kuraishi K., Watanabe M. Clinical outcomes and adverse events following transforaminal interbody fusion for lumbar degenerative spondylolisthesis in elderly patients. Neurol Med Chir (Tokyo) 2011;51(12):829–835. doi: 10.2176/nmc.51.829. [DOI] [PubMed] [Google Scholar]
- 105.Fay L.Y., Wu J.C., Tsai T.Y., Wu C.L., Huang W.C., Cheng H. Dynamic stabilization for degenerative spondylolisthesis: evaluation of radiographic and clinical outcomes. Clin Neurol Neurosurg. 2013;115(5):535–541. doi: 10.1016/j.clineuro.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 106.Aoki Y., Yamagata M., Ikeda Y., Nakajima F., Ohtori S., Nakagawa K. A prospective randomized controlled study comparing transforaminal lumbar interbody fusion techniques for degenerative spondylolisthesis: unilateral pedicle screw and 1 cage versus bilateral pedicle screws and 2 cages. J Neurosurg Spine. 2012;17(2):153–159. doi: 10.3171/2012.5.SPINE111044. [DOI] [PubMed] [Google Scholar]
- 107.Nakanishi K., Tanaka N., Fujimoto Y., Okuda T., Kamei N., Nakamae T. Medium-term clinical results of microsurgical lumbar flavectomy that preserves facet joints in cases of lumbar degenerative spondylolisthesis: comparison of bilateral laminotomy with bilateral decompression by a unilateral approach. J Spinal Disord Tech. 2013;26(7):351–358. doi: 10.1097/BSD.0b013e318247f1fd. [DOI] [PubMed] [Google Scholar]
- 108.Kotani Y., Abumi K., Ito M., Sudo H., Abe Y., Minami A. Mid-term clinical results of minimally invasive decompression and posterolateral fusion with percutaneous pedicle screws versus conventional approach for degenerative spondylolisthesis with spinal stenosis. Eur Spine J. 2012;21(6):1171–1177. doi: 10.1007/s00586-011-2114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sato J., Ohtori S., Orita S., Yamauchi K., Eguchi Y., Ochiai N. Radiographic evaluation of indirect decompression of mini-open anterior retroperitoneal lumbar interbody fusion: oblique lateral interbody fusion for degenerated lumbar spondylolisthesis. Eur Spine J. 2015 doi: 10.1007/s00586-015-4170-0. [DOI] [PubMed] [Google Scholar]
- 110.Kleinstueck F.S., Fekete T.F., Mannion A.F., Grob D., Porchet F., Mutter U. To fuse or not to fuse in lumbar degenerative spondylolisthesis: do baseline symptoms help provide the answer? Eur Spine J. 2012;21(2):268–275. doi: 10.1007/s00586-011-1896-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Love T.W., Fagan A.B., Fraser R.D. Degenerative spondylolisthesis. Developmental or acquired? J Bone Joint Surg [Br] 1999;81(4):670–674. doi: 10.1302/0301-620x.81b4.9682. [DOI] [PubMed] [Google Scholar]
- 112.Tsuji T., Watanabe K., Hosogane N., Fujita N., Ishii K., Chiba K. Risk factors of radiological adjacent disc degeneration with lumbar interbody fusion for degenerative spondylolisthesis. J Orthop Sci. 2016;21(2):133–137. doi: 10.1016/j.jos.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 113.Ghogawala Z., Benzel E.C., Amin-Hanjani S., Barker F.G., 2nd, Harrington J.F., Magge S.N. Prospective outcomes evaluation after decompression with or without instrumented fusion for lumbar stenosis and degenerative Grade I spondylolisthesis. J Neurosurg Spine. 2004;1(3):267–272. doi: 10.3171/spi.2004.1.3.0267. [DOI] [PubMed] [Google Scholar]
- 114.Epstein N.E., Epstein J.A., Carras R., Lavine L.S. Degenerative spondylolisthesis with an intact neural arch: a review of 60 cases with an analysis of clinical findings and the development of surgical management. Neurosurgery. 1983;13(5):555–561. doi: 10.1227/00006123-198311000-00012. [DOI] [PubMed] [Google Scholar]
- 115.Vázquez-Aguilar A., Torres-Gómez A., Atlitec-Castillo P.T., De León-Martínez J.E. [Degenerative espondylolisthesis. Body mass index influence on the post-surgical evolution]. [in Spanish] Acta Ortop Mex. 2016;30(1):13–16. [PubMed] [Google Scholar]
- 116.Juárez-Jiménez H.G., Zarate-Kalfópulos B., Alpizar-Aguirre A., Sánchez-Bringas M.G., Rosales-Olivarez L.M., Reyes-Sánchez A.A. [Utility of ligamentoplasty for the prevention of adjacent segment disease above 360 degree arthrodesis in degenerative lumbar spondylosis. Preliminary report]. [in Spanish] Acta Ortop Mex. 2013;27(5):324–330. [PubMed] [Google Scholar]
- 117.Manchikanti L., Singh V., Datta S., Cohen S.P., Hirsch J.A. American Society of Interventional Pain Physicians. Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Physician. 2009;12:E35–E70. [PubMed] [Google Scholar]
- 118.Wolfe F., Ross K., Anderson J., Russell I.J. Aspects of fibromyalgia in the general population: sex, pain threshold, and fibromyalgia symptoms. J Rheumatol. 1995;22:151–156. [PubMed] [Google Scholar]
- 119.Rollman G.B., Lautenbacher S. Sex differences in musculoskeletal pain. Clin J Pain. 2001;17:20–24. doi: 10.1097/00002508-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 120.Cunningham L.S., Kelsey J.L. Epidemiology of musculoskeletal impairments and associated disability. Am J Public Health. 1984;74:574–579. doi: 10.2105/ajph.74.6.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wáng Y.X., Wáng J.Q., Káplár Z. Increased low back pain prevalence in females than in males after menopause age: evidences based on synthetic literature review. Quant Imaging Med Surg. 2016;6(2):199–206. doi: 10.21037/qims.2016.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kent P.M., Keating J.L. The epidemiology of low back pain in primary care. Chiropr Osteopat. 2005;13:13. doi: 10.1186/1746-1340-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Walker B.F., Muller R., Grant W.D. Low back pain in Australian adults health provider utilization and care seeking. J Manipulative Physiol Ther. 2004;27(5):327–335. doi: 10.1016/j.jmpt.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 124.Wang Y.X., Griffith J.F., Zeng X.J., Deng M., Kwok A.W., Leung J.C. Prevalence and sex difference of lumbar disc space narrowing in elderly chinese men and women: osteoporotic fractures in men (Hong Kong) and osteoporotic fractures in women (Hong Kong) studies. Arthritis Rheum. 2013;65(4):1004–1010. doi: 10.1002/art.37857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang Y.X., Griffith J.F., Ma H.T., Kwok A.W., Leung J.C., Yeung D.K. Relationship between gender, bone mineral density, and disc degeneration in the lumbar spine: a study in elderly subjects using an eight-level MRI-based disc degeneration grading system. Osteoporos Int. 2011;22(1):91–96. doi: 10.1007/s00198-010-1200-y. [DOI] [PubMed] [Google Scholar]
- 126.Akeda K., Yamada T., Inoue N., Nishimura A., Sudo A. Risk factors for lumbar intervertebral disc height narrowing: a population-based longitudinal study in the elderly. BMC Musculoskelet Disord. 2015;16:344. doi: 10.1186/s12891-015-0798-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang Y.X., Griffith J.F. Effect of menopause on lumbar disk degeneration: potential etiology. Radiology. 2010;257(2):318–320. doi: 10.1148/radiol.10100775. [DOI] [PubMed] [Google Scholar]
- 128.Wang Y.X. Post-menopausal Chinese women have accelerated lumbar disc degeneration compared with Chinese men. J Orthop Translat. 2015;3:205–211. doi: 10.1016/j.jot.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Srikanth V.K., Fryer J.L., Zhai G., Winzenberg T.M., Hosmer D., Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13:769–781. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 130.Dodge H.J., Mikkelsen W.M., Duff I.F. Age-sex specific prevalence of radiographic abnormalities of the joints of the hands, wrists and cervical spine of adult residents of the Tecumseh, Michigan, Community Health Study area, 1962–1965. J Chronic Dis. 1970;23:151–159. [PubMed] [Google Scholar]
- 131.Phillips S.K., Rook K.M., Bruce N.C., Woledge R.C. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci. 1993;84:95–98. doi: 10.1042/cs0840095. [DOI] [PubMed] [Google Scholar]
- 132.Skelton D.A., Phillips S.K., Bruce S.A., Naylor C.H., Woledge R.C. Hormone replacement therapy increases isometric muscle strength of adductor pollicis in post-menopausal women. Clin Sci. 1999;96:357–364. [PubMed] [Google Scholar]
- 133.Taaffe D.R., Sipila S., Cheng S., Puolakka J., Toivanen J., Suominen H. The effect of hormone replacement therapy and/or exercise on skeletal muscle attenuation in postmenopausal women: a yearlong intervention. Clin Physiol Funct Imaging. 2005;25:297–304. doi: 10.1111/j.1475-097X.2005.00628.x. [DOI] [PubMed] [Google Scholar]
- 134.Shikata J., Sanada H., Tamamuro T., Takeda T. Experimental studies of the elastic fiber of the capsular ligament. Connect lissue Res. 1979;7:21–27. doi: 10.3109/03008207909152349. [DOI] [PubMed] [Google Scholar]
- 135.Yamamuro T., Hama H., Takeda T., Shikata J., Sanada H. Biomechnical and hormonal factors in the etiology of congenital dislocation of the hip joint. Int Orthop. 1977;1:231–236. [Google Scholar]
- 136.Dyer R.F., Sodek J., Heersche J.N. The effect of 17β-estradiol on collagen and non-collagenous protein synthesis in the uterus and some penodontaltissues. Endocrinology. 1980;107:1014–1024. doi: 10.1210/endo-107-4-1014. [DOI] [PubMed] [Google Scholar]
- 137.Sanada H., Shikata J., Hamamoto H., Ueba Y., Yamamuro T., Takeda T. Changes in collagen cross-linking and lysyl oxidase by estrogen. Biochim Biophys Acta. 1978;541(3):408–413. doi: 10.1016/0304-4165(78)90199-x. [DOI] [PubMed] [Google Scholar]
- 138.Fischer G.M., Swain M.L. Effect of sex hormones on blood pressure and vascular connective tissue in castrated and noncastrated male rats. Am J Physiol. 1977;232:H617–H621. doi: 10.1152/ajpheart.1977.232.6.H617. [DOI] [PubMed] [Google Scholar]
- 139.Imada K., Matsui H., Tsuji H. Oophorectomy predisposes to degenerative spondylolisthesis. J Bone Joint Surg [Br] 1995;77:126–130. [PubMed] [Google Scholar]
- 140.Wáng Y.X., Griffith J.F., Deng M., Yeung D.K., Yuan J. Rapid increase in marrow fat content and decrease in marrow perfusion in lumbar vertebra following bilateral oophorectomy: an MR imaging-based prospective longitudinal study. Korean J Radiol. 2015;16:154–159. doi: 10.3348/kjr.2015.16.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Deng M., Wang Y.X., Griffith J.F., Lu G., Ahuja A.T., Poon W.S. Characteristics of rat lumbar vertebral body bone mineral density and differential segmental responses to sex hormone deficiency: a clinical multidetector computed tomography study. Biomed Environ Sci. 2012;25(6):607–613. doi: 10.3967/0895-3988.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 142.Baron Y.M., Brincat M.P., Galea R., Calleja N. Intervertebral disc height in treated and untreated overweight post-menopausal women. Hum Reprod. 2005;20:3566–3570. doi: 10.1093/humrep/dei251. [DOI] [PubMed] [Google Scholar]
- 143.Tostes R.C., Nigro D., Fortes Z.B., Carvalho M.H. Effects of estrogen on the vascular system. Braz J Med Biol Res. 2003;36:1143–1158. doi: 10.1590/s0100-879x2003000900002. [DOI] [PubMed] [Google Scholar]
- 144.Wang Y.X. Menopause as a potential cause for higher prevalence of low back pain in women than in age-matched men. J Orthop Translat. 2015;8:1–4. doi: 10.1016/j.jot.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lobo R.A., Davis S.R., De Villiers T.J., Gompel A., Henderson V.W., Hodis H.N. Prevention of diseases after menopause. Climacteric. 2014;17(5):540–556. doi: 10.3109/13697137.2014.933411. [DOI] [PubMed] [Google Scholar]