Abstract
Stem cells mechanosense the stiffness of their microenvironment, which impacts differentiation. Although tissue hydration anti-correlates with stiffness, extracellular matrix (ECM) stiffness is clearly transduced into gene expression via adhesion and cytoskeleton proteins that tune fates. Cytoskeletal reorganization of ECM can create heterogeneity and influence fates, with fibrosis being one extreme.
Introduction to Mechanosensing
Brain tissue is very soft and bone is rigid, but do cells sense and respond to such physical differences? Understanding most physiological functions has invariably relied on physics as well as chemistry, but a test-tube vision of well-stirred biology ignores the mechanical properties of most tissues as well as any collective effects on cells. New opportunities to address such issues are provided by the sensitivity of stem cells in differentiation and the many types of stem cells that have been identified or generated de novo.
Embryonic stem cells and the related induced pluripotent stem cells (iPS cells) give rise to hundreds of specialized cell types in the human body. Beyond functionally committed cells such as neurons, muscle cells, or bone cells, pluripotent stem cells also generate adult stem cells that are multi-potent. Adult stem cells reside in specific tissues to provide for more restricted regeneration throughout life. To drive stem cells to an appropriate fate, coordination of inductive cues is necessary, and various soluble factors in “cocktails” are certainly potent in this respect. However, physical factors—specifically the softness or stiffness of the microenvironment—can also contribute to differentiation (23).
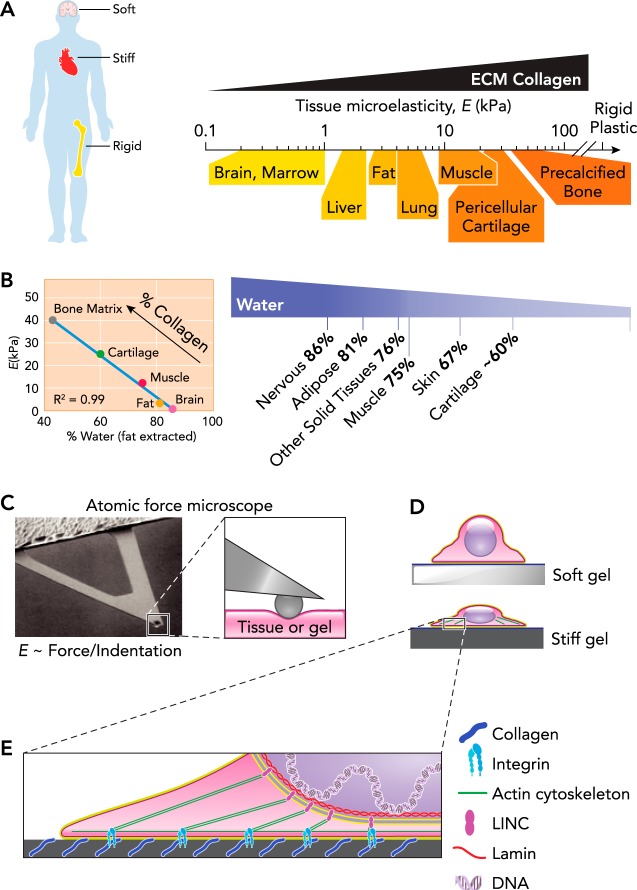
The ease of deforming tissue or a cell is described by its mechanical properties, and to first order excludes changes in volume since we are, of course, mostly incompressible water. Biological tissues deform when a mechanical stress (force per unit area) is applied, and the mechanical properties of solid and semi-solid tissues are often simplified to an elastic modulus (mechanical stress per strain) that varies widely across tissues (20). Brain tissue requires very little stress to extend or shear it and has a low elastic modulus (E < 1 kPa), making the tissue “soft,” whereas “rigid” calcified bone has an elastic modulus orders of magnitude higher (E > 1 GPa); all other solid tissues fall between these two extremes (31, 67, 97) (FIGURE 1A). Water content also decreases with tissue stiffness (FIGURE 1B) as various (non-fat) constituents increase in weight fraction, particularly ECM proteins such as collagens that are the most abundant proteins in the body; tissue softness and tissue water content are thus “colligative” properties. Most reductionist studies with stem-cell cultures nonetheless use rigid and hydrophobic tissue culture plastic, even though cultures of committed cells on soft hydrogels has been known since Pelham and Wang (71) to dramatically limit cell spreading and adhesive signaling relative to stiff substrates. Control over both adhesive ligands (i.e., surface biochemistry) and gel mechanics (FIGURE 1C) was essential to proving this point, and much earlier work might be interpreted as implying such matrix mechanosensitivity (5, 90). However, none of these early studies related mechanical properties of tissues to culture substrates, likely because the needed instruments are rare in physiology and cell biology laboratories. Micro-scale tools such as atomic force microscopes (AFM) have indeed been essential for the mechanical characterization not only of tissues and stem-cell niches on cellular and subcellular scales but also the gels used to mimic them (49). AFM remains a workhorse for measuring substrate mechanics on the cellular scale, and a variety of techniques are now available to also measure cellular forces and displacements (76).
FIGURE 1.
Universal scale of micro-stiffness for tissues
A: stem cells derive the tissues across that body that vary in stiffness of wide scales, from fluid like in the marrow at <1 kPa to rigid bone in the GPa range. The stiffness measured as microelasticity correlates with expression of collagen across the range of tissues but is generally much softer than the rigid plastic typically used in cell culture (21). B: hydration level of several human tissues after extraction of fat from a 46-yr-old male (26). Cartilage hydration state is age dependent and approximated for a 46-yr-old male (1). Bone matrix hydration is determined as a percentage of water and organic bone matrix (55, 56). The hydration state of tissues is inversely proportional to the tissue microelasticity (E) and collagen content. C: AFM is used to probe tissue or gel stiffness on the scale of the cell. The microelasticity is determined by measuring the restoring force relative to the indentation distance and depends on the probe tip (88). D: various cell types spread out when placed on a substrate with collagen coating of a stiff underlying gel. The spread-out cells contain a robust cytoskeleton with abundant actin stress fibers. E: collagen of the ECM provides adhesion sites for transmembrane integrins of the cell that form the basis of focal adhesions. Focal adhesions anchor the actin cytoskeleton at the membrane, whereas the LINC complex anchors the cytoskeleton at the nuclear membrane. LINC complexes also interact with the nuclear stiffness, determining lamins just inside the nuclear membrane, which provides a direct link to chromatin and DNA.
Adhesive ligands are of course crucial for cells to molecularly engage their surroundings, and such ligands would appear abundantly displayed on extracellular matrix (ECM) molecules. Synthetic gels can likewise be modified to display sufficient ligand, such that adhesion is not limiting, although this must always be verified when working with synthetic gels. Using polyacrylamide gels (PA) that were first covalently modified with constant collagen (PA is inert and thus widely used for protein separations), Pelham and Wang varied substrate stiffness through cross-linking and then demonstrated that 3T3 fibroblasts spread less on softer gels (71). Stiff substrates, including rigid plastic and glass, promote spreading of most cells, whereas soft substrates limit spreading and produce more rounded cell morphologies (FIGURE 1D). Although PA hydrogels are widely used to control substrate stiffness, other synthetic polymers, such as polydimethylsiloxane (PDMS) elastomers, in addition to modified biopolymers, such as alginate and hyaluronic acid (HA), have also been used (35). In general, gel stiffness is controlled by adjusting the concentration of polymer and/or cross-linker, and the gels are functionalized with collagen, fibronectin, or other ECM protein to provide the biochemistry needed for cell adhesion.
In tissues, ECM proteins also confer structure and mechanics. Most cells interact with ECM typically by adhering, but ECM also acts as a depot for semi-soluble growth factors, some of which depend on ECM mechanical properties for release (39). Fibrillar collagens (collagen-1, 2, 3, 5, 6 . . .) are the primary shear-resistant proteins of ECM, and the wide range of stiffnesses reported for tissues indeed correlate well with the amounts of collagen, over 4–5 logs in collagen level (84). Tissues that sustain high mechanical stress (muscle, bone) have the most collagen and are the stiffest, whereas tissues that are relatively protected from mechanical stress (brain, marrow) have low collagen and are soft. Collagenase addition to such tissues will soften them in tens of minutes (84), which is of course consistent with methods for isolating primary cells. Purified collagen self-assembles into a fibrous gel with a concentration-dependent elastic modulus, for which collagen fiber elastic modulus, bending modulus, lattice spacing, and cross-linking can all modulate gel stiffness (79). Fibers also tend to align with the direction of strain and generally stiffen under tension (64). Non-fibrillar collagens, proteoglycans, and other matrix components also modulate the mechanical properties of ECM, either on their own or through their interactions with collagen fibrous network.
For cells to sense their mechanical microenvironment, they must apply a force on that environment, just as we need to push and tug on a material surface to probe its mechanics. Such cell forces are generated at least in part by the myosin II mini-filaments that pull on the actin cytoskeleton and adhesions to drive cell and ECM contraction (FIGURE 1E). Stiff substrates provide a mechanical resistance or load that favors acto-myosin assembly and cell spreading. Inhibition of myosin II (or F-actin) pharmacologically indeed leads to cell rounding regardless of the stiffness of the substrate, and so cells lose all mechanosensitivity to matrix. Cell spreading while contracting on a stiff substrate requires stable adhesions to the ECM. Integrins coalesce into focal adhesion complexes while binding specific ECM ligands (83), and focal adhesions grow with tension to license cells to spread while pulling on the matrix (8).
Although stiffness-dependent adhesion and spreading occur within short time scales of 1–2 h (71), more profound and lasting changes in phenotype, such as lineage specification of stem cells, requires specific transcriptional programs to be activated and/or repressed in a process of mechanotransduction from the ECM to the nucleus. Among the mechanosensitive signaling pathways that transmit ECM stiffness signals to transcriptional machinery are the integrin-based focal adhesions with focal adhesion kinase (FAK) that initiates multiple mechanosensitive pathways (32). These include ERK, JNK, Wnt-β-catenin, and Hippo pathways (45). Many of these pathways regulate transcription factors that translocate into the nucleus in response to microenvironment mechanics. Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) are examples: they are usually in the cytosol of cells cultured on soft matrices, but when cell contractility increases on a stiff matrix, YAP/TAZ translocate into the nucleus, seemingly independent of the canonical Hippo signaling pathway (22). YAP/TAZ influences differentiation of stem cells by such pathways or others, and illustrate the broad range of mechanisms by which differentiation of stem cells can be manipulated by their mechanical environment.
Mechanosensitive signaling pathways with soluble cytoplasmic factors undoubtedly contribute (45), but mechanotransduction is also likely through direct physical linkage from ECM all the way to DNA. Filamentous actin that engages integrins together with microtubules and intermediate filaments connect to the nucleus through nesprins that span the outer nuclear membrane. Nesprins can in turn engage SUN proteins that span the inner nuclear membrane and bind to the underlying lamina proteins (17). A- and B-type lamins form the primary structural meshwork of the nuclear envelope and determine the mechanical properties of the nucleus (70). A-type lamin levels vary widely between tissues, but the ratio of A-type to B-type lamins scales systematically with tissue collagen levels and tissue stiffness over several orders of magnitude, so that nuclear stiffness increases with ECM stiffness (84). Importantly, the nuclear lamins directly regulate transcription factors with broad roles in differentiation such as serum response factor (SRF) and retinoic acid receptors (RAR). The lamins also interact with and control integral membrane proteins, such as lamin-B receptor (LBR), that bind and regulate chromatin factors to complete the linkage from ECM to DNA (68). These linkages have been shown to influence the differentiation of adult stem cells (84).
Matrix Stiffness Directed Differentiation
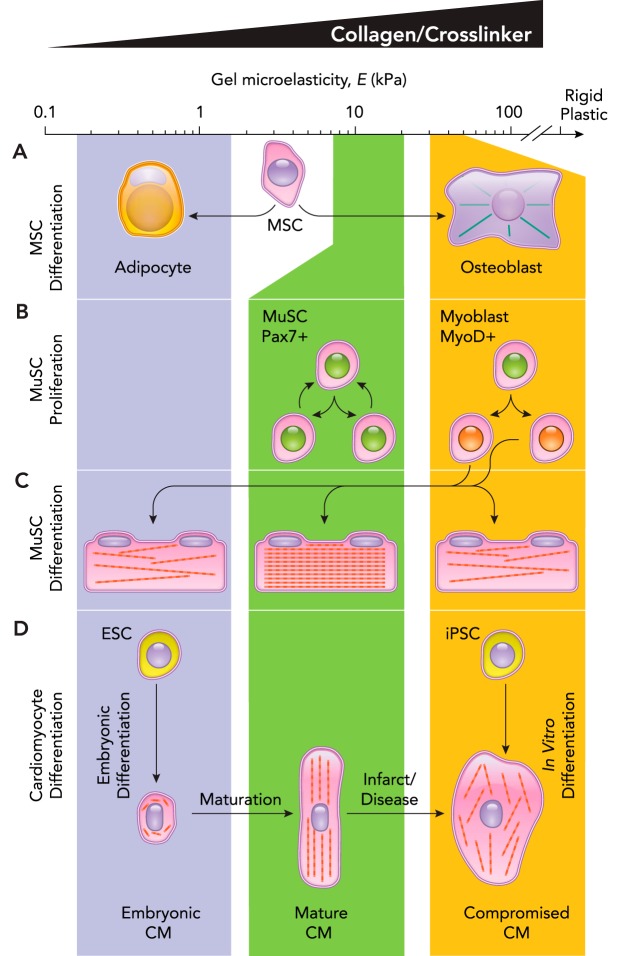
Human bone marrow-derived mesenchymal stem cells (MSCs) likely differentiate in situ into bone, cartilage, and fat (86); unlike mouse marrow, human adult marrow in long bones is filled with fat. Mouse marrow MSCs clearly express nestin in situ, which suggests some neuronal origins or character (65). Human MSCs were the first stem cells used to demonstrate the in vitro influence of matrix stiffness on stem cell differentiation (23). To test mechanosensitive lineage specification of MSCs, PA gel formulations to mimic the tissue stiffness of brain, muscle, and osteoid (bone surface) were used. Importantly, the gels were coated with equal concentrations of collagen so that all of the MSCs cultured atop the gel interacted with the same ligand. Based on protein and transcript expression, MSCs differentiated over several days or more toward neural, muscle, and bone cells on the tissue-matched substrates (FIGURE 2A). This mechanotransduction was lost when cells were treated with blebbistatin, which inhibits myosin II and thereby blocks the ability of MSCs to pull on their substrate and “feel” matrix stiffness. Importantly, phenotypic changes in cell morphology occur in hours: neuron-like dendritic branching on soft gels, myoblast-like elongation on intermediate stiffness gels, and osteoblastic spreading on stiff gels. Although it is likely that such human marrow-derived MSCs do not become neurons or muscle cells in vivo (13), multiple methods of controlling substrate stiffness have demonstrated clear effects on MSC differentiation. For example, arrays of micropillars can be made that allow cells to adhere to the tops with short and unbendable pillars or else with long and flexible pillars (28), and MSCs on the short pillars responded to the high stiffness by undergoing osteogenesis, whereas MSCs on the longer pillars responded to the soft substrate and underwent adipogenesis. Soft gels that are made very thin on top of rigid glass likewise favor osteogenesis, with high lamin-A and low LBR, whereas thick versions of the same soft gels coordinately repress lamin-A and upregulate LBR, which is interesting for its role in lipid biopsynthesis (8a). The use of 2D gels may be appropriate to mimic the process of MSCs adhering to the bone surface and differentiating into osteoblasts, but adipogenesis likely involves tissue building more in 3D. Embedding cells within 3D hydrogels presents some technical challenges, but alginate modified for cell adhesion showed that soft gels favored MSC adipogenesis in 3D, whereas stiffer gels favored osteogenesis (43).
FIGURE 2.
Stiffness-based differentiation of stem cells
A: MSCs plated on collagen functionalized gels of the indicated stiffness differentiate over the course of a week into cells from tissues corresponding to the stiffness of the gel. Soft gels direct adipogenic differentiation, whereas the more stiff gels induce osteoblast differentiation. B: substrate stiffness drives the type of proliferation among MuSC, where substrates with tissue-like stiffness often maintain their stem-like properties (defined by expression of Pax7), stiff substrates deplete the stem cell pool by inducing differentiation of both daughter cells (defined by expression of MyoD). C: muscle progenitors eventually differentiate and fuse into multinucleated muscle fibers filled with contractile sarcomeres. The formation of sarcomeres is most prevalent on tissue-like stiffness, whereas stiffness above or below that level leads to impaired sarcomerogenesis. D: immature cardiomyocytes are derived along a series of steps from embryonic stem cells in the soft embryo. As the heart matures, it becomes more stiff, and the cardiomyocytes become more aligned and contractile. However, in a stiffer fibrotic heart, contraction of cardiomyocytes is impaired. Alternatively, iPSCs can be induced to form cardiomyocytes on plastic; however, they are not able to produce the contractile force of a healthy mature cardiomyocyte.
Differentiation to multiple lineages is characteristic of stem cells, of course, but an ability to self-renew without differentiation is also a defining property of stem cells that has shown to be downstream of matrix mechanosensing. Skeletal muscle maintains a resident quiescent population of muscle stem cells (MuSC) that becomes activated and undergoes 1) symmetric division into muscle progenitors, 2) symmetric division that maintains stemness, or 3) asymmetric division to produce one daughter cell of each cell type. Culture systems that constrain mouse MuSCs on asymmetric fibronectin-coated substrates leads to these asymmetric divisions (96). Symmetric divisions that maintain stemness are important to preserve the stem-cell population for future injuries. Using a gel stiffness to mimic skeletal muscle stiffness, MuSC underwent significant symmetric stem-cell division that was not observed on stiffer substrates (96), as well as being able to maintain the quiescent state on softer gels (62) (FIGURE 2B). MuSCs grown on gels with the stiffness of healthy muscle were even able to repopulate the MuSC niche when injected back into a mouse muscle as opposed to cells grown on stiffer substrates (33). To repair muscle, MuSCs eventually differentiate into contractile myotubes and myofibers. In vitro, this is typically done on plastic and results in myotubes that are stuck in an immature state and do not progress to form highly aligned and packed sarcomeres that are observed in mature muscle. However, when C2C12 cells, a MuSC-like cell line, were differentiated on PA gels with an intermediate stiffness, as in healthy muscle, sarcomeric striation was maximized compared with softer gels and stiffer gels (16) (FIGURE 2C). Quiescent MuSCs are present in a complex 3D environment sandwiched between a mature muscle fiber and the basement membrane. Recent efforts have been made to engineer synthetic muscle fibers as a substrate for MuSCs, again showing that, when the stiffness of these synthetic fibers is matched to that of real fibers, the MuSCs maintained a quiescent stem cell pool and an enhanced ability to engraft in vivo (74).
Cardiac muscle, on the other hand, has a more limited adult stem-cell progenitor pool. Along with its obviously critical function, this makes deriving cardiomyocytes from iPSCs a major regenerative medicine target. Many protocols seek to use iPSCs to generate functional cardiomyocytes relying on transcriptional induction on rigid plastic, with limited success or else unknown contributions from ECM made by the cells (78). However, the ECM stiffness that a cardiomyocyte experiences in an embryo and through development is much softer than the rigid plastic used in such studies (59). It is interesting that, transplanting human PSC-derived cardiomyocytes into a neonatal rat heart with a soft microenvironment facilitates nearly full maturation of the cardiomyocytes, which is not observed when the cardiomyocytes are transplanted into a stiffer adult rat heart (14). Embryonic cardiomyocytes also tune their beating to the stiffness of their substrate. On very soft gels, they disassemble the contractile machinery of the sarcomere that allows them to contract and produce mechanical work on stiff gels (59) (FIGURE 2D). However, when the gel substrate becomes too stiff, mimicking more fibrotic post-infarct scars, sarcomeres are disrupted, and beating is inhibited. In addition to sarcomeric organization and contractility being downstream of matrix stiffness, the transcriptional mechanosensitivity of heart has also been shown across many disease states to have collagen I expression—a gauge for ECM stiffness—coupled to lamin A/C expression in the nucleus (15). Although the precise mechanisms remain to be elucidated, on stiff matrices, YAP/TAZ shuttles into the nucleus (22, 34), whereas NKX-2.5, a repressor of smooth muscle actin (ACTA2), shuttles out of the nucleus (19). These systems highlight how stem cells and progenitors rely on mechanical cues from the extracellular matrix to modulate the transcriptional program used to drive differentiation and maturation of cells.
Osmotic Regulation of Matrix Mechanosensing
Soft matrix suppresses myosin II activity and cytoskeletal assembly, but hypotonic media causes similar cell rounding with disruption of the actin cytoskeleton and changes in ion channel activity (24, 61). Changes in osmotic pressure well below or above isotonic pressure cause proportional volume changes of cells and nuclei, with hypotonic conditions causing chromatin decondensation and hypertonic conditions resulting in chromatin condensation (46). Physiological differences in osmotic pressure are not evident for tissues that otherwise vary in stiffness by orders of magnitude (FIGURE 1A), but regulation of MSC differentiation on soft and stiff gels by osmotic pressure (36) is nonetheless understandable, based at least on the long-established effects of osmotic pressure on the cytoskeleton (61). Indeed, given the higher water content in soft tissues and the lower water content in stiff tissues (FIGURE 1B), the pleiotropic effects of osmotic pressure changes and hydration motivate further stem-cell differentiation experiments that combine osmotic-hydration changes with drugs that target specific molecules of the cytoskeleton. It will be interesting to clarify, for example, whether osteogenesis on soft matrix that can be increased by ~50% using hypertonic media (36) (which dehydrates cells and drives actomyosin assembly) is suppressed or not by simultaneous inhibition of myosin II.
Stress or Strain Relaxation and Matrix Heterogeneity
Many stem cells express ECM components in a mechanosensitive manner. Using void-forming alginate hydrogels, murine MSCs express the most collagen with an (osteoid-like) alginate stiffness of 20–60 kPa (44). Cells also degrade ECM using matrix metalloproteinases (MMPs). Primary mouse fibroblasts transduced with the four reprogramming factors (“Yamanaka” factors) and cultured in 3D hydrogels of different initial stiffness, MMP-degradability, and adhesive ligand illustrate the importance of matrix malleability (9). The reprogramming efficiency to iPSCs proved highest with highly degradable gels of initial stiffness of 0.6 kPa; such a stiffness is similar to that of embryos, which are also likely to yield and flow under stress similar to brain tissue (59).
Cell forces can make ECM flow as occurs macroscopically with soft brain tissue under strain, but ECM in some tissues can also fully recover its shape and exhibit elasticity, as is a requirement of cardiac tissue (59). Not all tissues flow under cell-generated stresses or strains (or else our tissues would have no shape when isolated), but the ability to confer gels with relaxation properties has recently been accomplished with relaxation time scales that are tunable from 1 to 40 min, which affects differentiation (12). MSCs in 9-kPa gels undergo more adipogenesis with slow-relaxing gels compared with fast-relaxing gels, whereas MSCs in 17-kPa gels exhibit maximal osteogenesis with fast-relaxing gels (98). Importantly, MSCs pull on the fast-relaxing gel and surround themselves with more ECM and more ligand. As a general rule in polymer physics, a high concentration of polymer will have a higher stiffness. Synthetic fiber matrices with fine control of individual fiber stiffness, size, and cross-linking have also been made, although sparse meshworks limit the presentation of ligand for cell adhesion (2). MSCs spread more on soft-fiber meshes compared with stiff fiber meshes, but once again the soft meshes condense and likely stiffen beneath the cell (FIGURE 3A). Both of these examples demonstrate the importance of taking care to distinguish the initial bulk mechanics from the final heterogeneous mechanics (and ligand) of a microenvironment.
FIGURE 3.
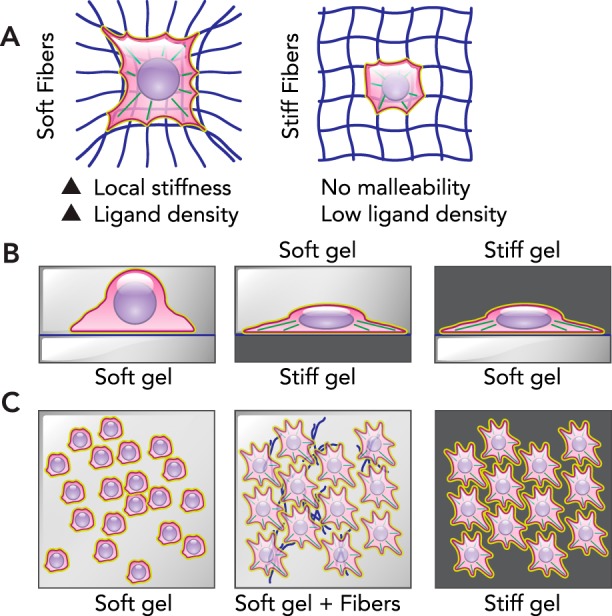
ECM malleability and heterogeneity
A: MSCs placed in soft fibrous networks are able to pull the fibers and pack them near the cell, creating heterogeneity with high stiffness and ligand presentation local to the cell. However, in fibrous networks with stiff fibers, the cell is unable to deform the network and change the local stiffness. B: simple sandwich gels demonstrate that stiff regions dominate matrix mechanosensing. When a soft gel is placed on top of cells on either soft or stiff lower substrates, the cells maintain their rounded or spread morphology, respectively. However, when rounded cells are on a soft substrate or overlaid with a stiff gel, they actively spread to adopt the morphology expected from a stiff substrate. C: gels can also be made with heterogenous underlying collagen fibers that also introduce heterogenous stiffness within the 2D gel. Reminiscent of a fibrotic environment, these gels are termed “scar in a dish.” Although MSCs on a soft or stiff substrate adopt a rounded or spread morphology, respectively, the mechanical heterogeneity creates a near homogenous population of MSCs with a spread morphology. This spread morphology includes robust αSMA, which is a hallmark of fibrotic cells.
Some tissues (such as liver) might have relatively isotropic mechanics at the level of the cell, but many musculoskeletal tissues (such as tendon) experience forces more in one direction and exhibit anisotropic ECM with aligned collagen fibers. Understandably, the orientation of ECM stiffness can also impact stem-cell differentiation. Collagen fiber nano-films on mica exhibit very long-range fiber orientation, and MSCs extend along such fibers in a more myogenic-like morphology; however, when the fibers are cross-linked, the MSCs show no orientation preference and undergo osteogenesis on the stiffer films (47). Skeletal muscle progenitors (MuSCs) also exhibit enhanced differentiation and maturation on stiff fiber substrates compared with the typical isotropic gels or culture plastic, even generating functional neuromuscular junctions in cocultures with motoneurons (38). Mechanical anisotropy of ECM can affect morphology, maturation, and/or differentiation, similar to experimentally controlled cell shapes, by patterning ECM ligand on a substrate; with cardiomyocytes, for example, rod-shape patterns produced more forceful contractions relative to circular patterns (7). Printing a rod-shaped pattern of ligand on gels with physiological stiffness understandably maximized the maturation of iPSC-derived cardiomyocytes, which exhibited greater mechanical work and calcium handling as well as more physiological intracellular structure (77).
Mechanical anisotropy in 3D has also been investigated with gel overlays or “sandwich” gels that present distinct mechanical properties above and below the cell. Some niches in tissues are similarly stratified: MSCs undergo osteogenesis on top of bone with soft marrow above, and MuSCs typically have semi-stiff muscle below and semi-stiff basement membrane above. Bio-compatible gels for overlays include HA that can be synthetically modified to control elasticity on either side of sandwiched cells (FIGURE 3B). Bonding between the gels should be minimized to allow cells to freely change morphology at the gel-gel interface. MSC morphology indeed responds to the stiffer gel, regardless of being above or below the cells (75); the stiffest medium thus dominates cell responses. Hepatic stellate cells (HSCs) are also mechanosensitive, maintaining quiescence on soft substrates and activating toward a myofibroblast-like state on stiff substrates, but HSCs on a soft gel plus are also activated by a rigid overlay (69).
Cells are only able to mechanically sense approximately one cell length away on gels such as PA gels, but within a purely fibrous network, cells, including MSCs, are able to increase their sensing radius 10-fold (87). By mixing soluble collagen I into a soft PA hydrogel, collagen-I fibers bundle and segregate into a heterogeneous distribution of branched fibers that mimic fibrotic scar tissue (19). The heterogeneously soft- and stiff-gel regions are then uniformly functionalized with collagen for adhesion, and cultures of MSCs respond as if cultured on a uniformly stiff hydrogel rather than a soft hydrogel in terms of multiple mechanosensitive markers, including α-SMA (FIGURE 3C), which is an important cellular maker of contractility as well as scarring. α-SMA is often observed in fibrotic tissues and even precedes the overexpression of collagen in chemically induced models of liver fibrosis (69). The stiffest components of a heterogenous ECM thus dominate mechanosensitive differentiation.
Fibrosis, the Pathologic Matrix, and Differentiation
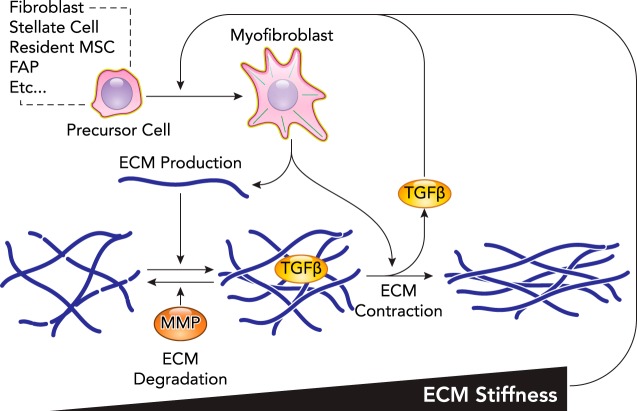
Fibrosis is the pathologic accumulation of ECM within a tissue and is generally associated with aberrant wound-healing mechanisms that result in scarring. Dysregulated repair can initiate feed-forward systems that result in progressive fibrotic tissue accumulation that undermines stem cell-based repair (37). Given the association of fibrosis with a wide array of tissue injuries, it contributes to nearly half of all deaths in the developed world (95). Fibrosis reflects an imbalance between synthesis and degradation of ECM, both of which can be influenced by mechanical stress (FIGURE 4). Since ECM is the major determinant of tissue mechanical properties, the disrupted ECM architecture in fibrosis has mechanical consequences. For example, healthy lung tissue with an elasticity of 2 kPa stiffens to >15 kPa in idiopathic pulmonary fibrosis (6). In vivo elastography has become a reliable way with ultrasound to assess such trends in situ; for example, healthy liver has an elasticity of <5 kPa, and cirrhotic liver has an elasticity of >12 kPa (18). Similar increases in tissue stiffness and collagen content are also observed in fibrotic striated muscle (81) and kidney (51). Many studies show altered mechanosensing in fibrotic ECM is responsible for both the impaired ability of healthy cells to regenerate functioning tissue as well as promoting fibroblast activation—a type of differentiation—to myofibroblasts that secrete even more ECM (10).
FIGURE 4.
Schematic of stem-cell mechanosensing in fibrosis
Fibrosis is the pathologic accumulation of ECM that is driven by myofibroblast in many tissues. A host of resident stem cells are known to differentiate into myofibroblast, which in many cases is known to be mechanosensitive. Myofibroblasts secrete matrix components that incorporate into the matrix to increase stiffness. Myofibroblasts are also highly contractile and stress the matrix, which leads to even higher levels of stiffness and also limits the ability of MMPs to degrade the matrix. Mechanical stress on the matrix has also been demonstrated to release active TGFB from the matrix, a critical soluble factor in the differentiation of myofibroblasts and other fibrotic programs. These processes demonstrate the critical nature of ECM mechanics in the positive feedback loop that results in progressive fibrosis.
Mechanical properties likely stimulate the activation of myofibroblasts to produce ECM in skin (40), heart (29), lung (42), liver (69), and kidney (57). Mechanosensing of matrix seems critical to the positive feedback in fibro-proliferative disorders (92). Unfortunately, myofibroblasts do not easily revert to their previous phenotype upon matrix softening (3); reprogramming is feasible under some conditions (30, 60), but soft and malleable matrix remains best for reprogramming any cell to an iPSC, as cited above. The increased contractility of myofibroblasts and other resident cells in the fibrotic tissue can mechanically impact nearby cells. In the liver, activation of the HSCs around sinusoidal endothelial cells restricts blood flow in the damaged region (82). Given the known role of hypoxia signaling in collagen expression, this further propagates fibrosis (58). Macrophages from the circulation or already resident in tissue are also recognized as mediators of fibrosis in many tissues by secretion of pro-inflammatory signals and wound-healing signals. Macrophages are mechanosensitive, but whether stiff matrices cause them to differentiate into pro-inflammatory (73) or anti-inflammatory (27) phenotypes remains unclear.
Although many cell types with differentiation potential are mechanosensitive in culture, the specific cell type responsible for mechanosensing the fibrotic environment in vivo appears tissue-dependent. The resident fibroblast population in idiopathic pulmonary fibrosis senses stiffness and becomes activated into a myofibroblast phenotype in response to stiffness (63), whereas in liver the HSCs are primarily responsible for the fibrotic response and require a stiff environment for activation (69). In skeletal muscle, fibro-adipogenic progenitors fulfill a similar role (48), although their matrix mechanosensing has not been investigated. Tissue-resident MSCs are a major contributor to myofibroblast populations (52) and likely overlap with the populations of hepatic stellate cells in liver (50) or fibro-adipogenic progenitors in muscle (85). In mice, when Gli1+ resident MSCs are genetically ablated, fibrosis is ameliorated in a range of tissues, and cardiac function is preserved (52).
The process of epithelial-mesenchymal transition (EMT) involves the transdifferentiation of functional epithelial cells into mesenchymal cells, which can be important during development and wound healing, but may also go awry in fibrosis (41, 53). Epithelial cells from lung (11) and kidney (54) have been shown to undergo EMT and progress into fibrogenic myofibroblasts, but the role of EMT in the liver has been called into question (91). EMT critically involves not only the recruitment of additional matrix-modifying cells, but also the loss of cells fulfilling the tissue’s primary function. TGF-β signaling has been described as the primary driver of EMT; however, multiple reports have shown that both TGF-β signaling and matrix rigidity are necessary to elicit EMT (66, 89). In addition to EMT, lineage-tracing experiments have also demonstrated the possibility of endothelial-to-mesenchymal transitions (EndoMT) in fibrosis of the heart, lung, and kidney (72); however, the role of mechanosensing in (EndoMT) is less well known. Importantly, mechanosensing can also disrupt proper functioning of resident cells even without undergoing transdifferentiation processes.
In addition to the mechanosensitive differentiation of many cell types, ECM itself is mechanosensitive, with fibrils under load being protected from MMP-based degradation (25). This “use it or lose it” mechanism applied in a fibrotic context makes existing scar tissues more difficult to remodel. The mechanical signal produced by myofibroblast contraction is propagated further through alignment of collagen fibrils between contractile elements that enable long-range force transmission (4, 87). In vitro experiments show collagen is compacted and well aligned between contractile groups of cells (80), creating an ECM architecture reminiscent of bridging fibrosis in the liver. This propagation of strain through the fibrotic ECM can also impact signaling by soluble factors. Myofibroblast contraction on stiff matrices directly activates latent TGF-β, which further cranks the wheel of positive feedback in fibrosis (94). This brings into focus the interplay between soluble, cellular, and mechanical signals in fibrotic disease (93). Although soluble factors have long been a focus of scientific inquiry in fibrosis, the impact of mechanosensing in fibrosis underscores the broader importance of matrix mechanosensing in cell and tissue differentiation.
Concluding Prospectus
Various types of stem and progenitor cells have a demonstrated ability to sense the stiffness of ECM. The morphology, cytoskeleton, and migration of these cells are all responsive within hours to ECM mechanics, whereas effects on proliferation and/or differentiation follow over days. Stem cells can also reorganize and sometimes synthesize their ECM, which creates a local niche and adds mechanical heterogeneity. This is important because the stiffest ECM dominates the cell response. In fibrosis, this can feed forward with stiffer ECM leading to increased fibrogenic states of cells and further stiffening. Various engineered gels systems have clearly demonstrated matrix mechanosensitivity, but a myriad of factors in vivo continue to make it challenging to isolate the precise role of matrix mechanosensing in tissue differentiation. Although MSCs have been useful for documenting many aspects of mechanosensitive differentiation, further investigation of how iPSCs and other stem and progenitor cells respond to ECM mechanics—in combination with potent soluble factors—will likely prove critical to unlocking the therapeutic potential of such cells.
Acknowledgments
The authors were supported by the U.S. National Institutes of Health National Cancer Institute under PSOC Award No. U54 CA-193417, by the National Heart Lung and Blood Institute under Award Nos. R01 HL-124106 and R21 HL-128187, by the National Institute of Arthritis and Musculoskeletal and Skin under Award No. K99 AR-067867 (L.S.), and also by the U.S. National Science Foundation Materials Science and Engineering Center (MRSEC) and Science & Technology Center (CMMI-1548571) grants to Penn. Additional support was provided by the U.S.-Israel Binational Science Foundation (BSF) and the Human Frontiers Sciences Program (HFSP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other granting agencies.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: L.R.S., S.C., and D.E.D. interpreted results of experiments; L.R.S. and D.E.D. prepared figures; L.R.S. and D.E.D. drafted manuscript; L.R.S., S.C., and D.E.D. edited and revised manuscript; L.R.S., S.C., and D.E.D. approved final version of manuscript.
References
- 1.Amadò R, Werner G, Neukom H. Water content of human articular cartilage and its determination by gas chromatography. Biochem Med 16: 169–172, 1976. doi: 10.1016/0006-2944(76)90020-X. [DOI] [PubMed] [Google Scholar]
- 2.Baker BM, Trappmann B, Wang WY, Sakar MS, Kim IL, Shenoy VB, Burdick JA, Chen CS. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat Mater 14: 1262–1268, 2015. doi: 10.1038/nmat4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balestrini JL, Chaudhry S, Sarrazy V, Koehler A, Hinz B. The mechanical memory of lung myofibroblasts. Integr Biol 4: 410–421, 2012. doi: 10.1039/c2ib00149g. [DOI] [PubMed] [Google Scholar]
- 4.Bates JH, Davis GS, Majumdar A, Butnor KJ, Suki B. Linking parenchymal disease progression to changes in lung mechanical function by percolation. Am J Respir Crit Care Med 176: 617–623, 2007. doi: 10.1164/rccm.200611-1739OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben-Ze’ev A, Farmer SR, Penman S. Protein synthesis requires cell-surface contact while nuclear events respond to cell shape in anchorage-dependent fibroblasts. Cell 21: 365–372, 1980. doi: 10.1016/0092-8674(80)90473-0. [DOI] [PubMed] [Google Scholar]
- 6.Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, Weiss K, Horowitz JC, Fiore VF, Barker TH, Moore BB, Martinez FJ, Niklason LE, White ES. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med 186: 866–876, 2012. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bray MA, Sheehy SP, Parker KK. Sarcomere alignment is regulated by myocyte shape. Cell Motil Cytoskeleton 65: 641–651, 2008. doi: 10.1002/cm.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burridge K, Guilluy C. Focal adhesions, stress fibers and mechanical tension. Exp Cell Res 343: 14–20, 2016. doi: 10.1016/j.yexcr.2015.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Buxboim A, Irianto J, Swift J, Athirasala A, Shin JW, Rehfeldt F, Discher DE. Coordinated increase of nuclear tension and lamin-A with matrix stiffness outcompetes lamin-B receptor that favors soft tissue phenotypes. Mol Biol Cell 28: 3333–3348, 2017. doi: 10.1091/mbc.E17-06-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caiazzo M, Okawa Y, Ranga A, Piersigilli A, Tabata Y, Lutolf MP. Defined three-dimensional microenvironments boost induction of pluripotency. Nat Mater 15: 344–352, 2016. doi: 10.1038/nmat4536. [DOI] [PubMed] [Google Scholar]
- 10.Carver W, Goldsmith EC. Regulation of tissue fibrosis by the biomechanical environment. BioMed Res Int 2013: 101979, 2013. doi: 10.1155/2013/101979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman HA. Epithelial-mesenchymal interactions in pulmonary fibrosis. Annu Rev Physiol 73: 413–435, 2011. doi: 10.1146/annurev-physiol-012110-142225. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee HP, Lippens E, Duda GN, Mooney DJ. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater 15: 326–334, 2016. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Q, Shou P, Zheng C, Jiang M, Cao G, Yang Q, Cao J, Xie N, Velletri T, Zhang X, Xu C, Zhang L, Yang H, Hou J, Wang Y, Shi Y. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ 23: 1128–1139, 2016. doi: 10.1038/cdd.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho GS, Lee DI, Tampakakis E, Murphy S, Andersen P, Uosaki H, Chelko S, Chakir K, Hong I, Seo K, Chen HV, Chen X, Basso C, Houser SR, Tomaselli GF, O’Rourke B, Judge DP, Kass DA, Kwon C. Neonatal transplantation confers maturation of PSC-derived cardiomyocytes conducive to modeling cardiomyopathy. Cell Reports 18: 571–582, 2017. doi: 10.1016/j.celrep.2016.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho S, Irianto J, Discher DE. Mechanosensing by the nucleus: from pathways to scaling relationships. J Cell Biol 216: 305–315, 2017. doi: 10.1083/jcb.201610042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper ST, Maxwell AL, Kizana E, Ghoddusi M, Hardeman EC, Alexander IE, Allen DG, North KN. C2C12 co-culture on a fibroblast substratum enables sustained survival of contractile, highly differentiated myotubes with peripheral nuclei and adult fast myosin expression. Cell Motil Cytoskeleton 58: 200–211, 2004. doi: 10.1002/cm.20010. [DOI] [PubMed] [Google Scholar]
- 17.Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D. Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol 172: 41–53, 2006. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Degos F, Perez P, Roche B, Mahmoudi A, Asselineau J, Voitot H, Bedossa P; FIBROSTIC study group . Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol 53: 1013–1021, 2010. doi: 10.1016/j.jhep.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 19.Dingal PC, Bradshaw AM, Cho S, Raab M, Buxboim A, Swift J, Discher DE. Fractal heterogeneity in minimal matrix models of scars modulates stiff-niche stem-cell responses via nuclear exit of a mechanorepressor. Nat Mater 14: 951–960, 2015. doi: 10.1038/nmat4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science 310: 1139–1143, 2005. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 21.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science 324: 1673–1677, 2009. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature 474: 179–183, 2011. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 23.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 126: 677–689, 2006. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 24.Fernández P, Pullarkat PA. The role of the cytoskeleton in volume regulation and beading transitions in PC12 neurites. Biophys J 99: 3571–3579, 2010. doi: 10.1016/j.bpj.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flynn BP, Bhole AP, Saeidi N, Liles M, Dimarzio CA, Ruberti JW. Mechanical strain stabilizes reconstituted collagen fibrils against enzymatic degradation by mammalian collagenase matrix metalloproteinase 8 (MMP-8). PLoS One 5: e12337, 2010. doi: 10.1371/journal.pone.0012337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forbes RM, Cooper AR, Mitchell HH. The composition of the adult human body as determined by chemical analysis. J Biol Chem 203: 359–366, 1953. [PubMed] [Google Scholar]
- 27.Friedemann M, Kalbitzer L, Franz S, Moeller S, Schnabelrauch M, Simon JC, Pompe T, Franke K. Instructing Human Macrophage Polarization by Stiffness and Glycosaminoglycan Functionalization in 3D Collagen Networks. Adv Healthc Mater 6: 1600967, 2017. doi: 10.1002/adhm.201600967. [DOI] [PubMed] [Google Scholar]
- 28.Fu J, Wang YK, Yang MT, Desai RA, Yu X, Liu Z, Chen CS. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Methods 7: 733–736, 2010. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galie PA, Westfall MV, Stegemann JP. Reduced serum content and increased matrix stiffness promote the cardiac myofibroblast transition in 3D collagen matrices. Cardiovasc Pathol 20: 325–333, 2011. doi: 10.1016/j.carpath.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaça MD, Zhou X, Issa R, Kiriella K, Iredale JP, Benyon RC. Basement membrane-like matrix inhibits proliferation and collagen synthesis by activated rat hepatic stellate cells: evidence for matrix-dependent deactivation of stellate cells. Matrix Biol 22: 229–239, 2003. doi: 10.1016/S0945-053X(03)00017-9. [DOI] [PubMed] [Google Scholar]
- 31.Gefen A, Margulies SS. Are in vivo and in situ brain tissues mechanically similar? J Biomech 37: 1339–1352, 2004. doi: 10.1016/j.jbiomech.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 32.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol 10: 21–33, 2009. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 33.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329: 1078–1081, 2010. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graham DM, Burridge K. Mechanotransduction and nuclear function. Curr Opin Cell Biol 40: 98–105, 2016. doi: 10.1016/j.ceb.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gribova V, Crouzier T, Picart C. A material’s point of view on recent developments of polymeric biomaterials: control of mechanical and biochemical properties. J Mater Chem 21: 14354–14366, 2011. doi: 10.1039/c1jm11372k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo M, Pegoraro AF, Mao A, Zhou EH, Arany PR, Han Y, Burnette DT, Jensen MH, Kasza KE, Moore JR, Mackintosh FC, Fredberg JJ, Mooney DJ, Lippincott-Schwartz J, Weitz DA. Cell volume change through water efflux impacts cell stiffness and stem cell fate. Proc Natl Acad Sci U S A 114: E8618–E8627, 2017. doi: 10.1073/pnas.1705179114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 453: 314–321, 2008. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 38.Happe CL, Tenerelli KP, Gromova AK, Kolb F, Engler AJ. Mechanically patterned neuromuscular junctions-in-a-dish have improved functional maturation. Mol Biol Cell 28: 1950–1958, 2017. doi: 10.1091/mbc.E17-01-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hinz B. The extracellular matrix and transforming growth factor-β1: Tale of a strained relationship. Matrix Biol 47: 54–65, 2015. doi: 10.1016/j.matbio.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 40.Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol 159: 1009–1020, 2001. doi: 10.1016/S0002-9440(10)61776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol 170: 1807–1816, 2007. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, Ding Q, Thannickal VJ, Zhou Y. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol 47: 340–348, 2012. doi: 10.1165/rcmb.2012-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater 9: 518–526, 2010. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huebsch N, Lippens E, Lee K, Mehta M, Koshy ST, Darnell MC, Desai RM, Madl CM, Xu M, Zhao X, Chaudhuri O, Verbeke C, Kim WS, Alim K, Mammoto A, Ingber DE, Duda GN, Mooney DJ. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat Mater 14: 1269–1277, 2015. doi: 10.1038/nmat4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 15: 802–812, 2014. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Irianto J, Swift J, Martins RP, McPhail GD, Knight MM, Discher DE, Lee DA. Osmotic challenge drives rapid and reversible chromatin condensation in chondrocytes. Biophys J 104: 759–769, 2013. doi: 10.1016/j.bpj.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ivanovska IL, Swift J, Spinler K, Dingal D, Cho S, Discher DE. Cross-linked matrix rigidity and soluble retinoids synergize in nuclear lamina regulation of stem cell differentiation. Mol Biol Cell 28: 2010–2022, 2017. doi: 10.1091/mbc.E17-01-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12: 153–163, 2010. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jorba I, Uriarte JJ, Campillo N, Farré R, Navajas D. Probing micromechanical properties of the extracellular matrix of soft tissues by atomic force microscopy. J Cell Physiol 232: 19–26, 2017. doi: 10.1002/jcp.25420. [DOI] [PubMed] [Google Scholar]
- 50.Kordes C, Sawitza I, Götze S, Herebian D, Häussinger D. Stellate cells are mesenchymal stem cells. Eur J Med Res 19, Suppl 1: S6, 2014. doi: 10.1186/2047-783X-19-S1-S6. [DOI] [Google Scholar]
- 51.Korsmo MJ, Ebrahimi B, Eirin A, Woollard JR, Krier JD, Crane JA, Warner L, Glaser K, Grimm R, Ehman RL, Lerman LO. Magnetic resonance elastography noninvasively detects in vivo renal medullary fibrosis secondary to swine renal artery stenosis. Invest Radiol 48: 61–68, 2013. doi: 10.1097/RLI.0b013e31827a4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, Humphreys BD. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16: 51–66, 2015. doi: 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 15: 178–196, 2014. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R. Origin and function of myofibroblasts in kidney fibrosis. Nat Med 19: 1047–1053, 2013. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lees S. Considerations regarding the structure of the mammalian mineralized osteoid from viewpoint of the generalized packing model. Connect Tissue Res 16: 281–303, 1987. doi: 10.3109/03008208709005616. [DOI] [PubMed] [Google Scholar]
- 56.Lees S, Escoubes M. Vapor pressure isotherms, composition and density of hyperdense bones of horse, whale and porpoise. Connect Tissue Res 16: 305–322, 1987. doi: 10.3109/03008208709005617. [DOI] [PubMed] [Google Scholar]
- 57.Leight JL, Wozniak MA, Chen S, Lynch ML, Chen CS. Matrix rigidity regulates a switch between TGF-β1-induced apoptosis and epithelial-mesenchymal transition. Mol Biol Cell 23: 781–791, 2012. doi: 10.1091/mbc.E11-06-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lokmic Z, Musyoka J, Hewitson TD, Darby IA. Hypoxia and hypoxia signaling in tissue repair and fibrosis. Int Rev Cell Mol Biol 296: 139–185, 2012. doi: 10.1016/B978-0-12-394307-1.00003-5. [DOI] [PubMed] [Google Scholar]
- 59.Majkut S, Idema T, Swift J, Krieger C, Liu A, Discher DE. Heart-specific stiffening in early embryos parallels matrix and myosin expression to optimize beating. Curr Biol 23: 2434–2439, 2013. doi: 10.1016/j.cub.2013.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marinković A, Liu F, Tschumperlin DJ. Matrices of physiologic stiffness potently inactivate idiopathic pulmonary fibrosis fibroblasts. Am J Respir Cell Mol Biol 48: 422–430, 2013. doi: 10.1165/rcmb.2012-0335OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mills JW, Schwiebert EM, Stanton BA. Evidence for the role of actin filaments in regulating cell swelling. J Exp Zool 268: 111–120, 1994. doi: 10.1002/jez.1402680207. [DOI] [PubMed] [Google Scholar]
- 62.Monge C, DiStasio N, Rossi T, Sébastien M, Sakai H, Kalman B, Boudou T, Tajbakhsh S, Marty I, Bigot A, Mouly V, Picart C. Quiescence of human muscle stem cells is favored by culture on natural biopolymeric films. Stem Cell Res Ther 8: 104, 2017. doi: 10.1186/s13287-017-0556-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moore MW, Herzog EL. Regulation and relevance of myofibroblast responses in idiopathic pulmonary fibrosis. Curr Pathobiol Rep 1: 199–208, 2013. doi: 10.1007/s40139-013-0017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Motte S, Kaufman LJ. Strain stiffening in collagen I networks. Biopolymers 99: 35–46, 2013. doi: 10.1002/bip.22133. [DOI] [PubMed] [Google Scholar]
- 65.Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466: 829–834, 2010. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O’Connor JW, Gomez EW. Biomechanics of TGFβ-induced epithelial-mesenchymal transition: implications for fibrosis and cancer. Clin Transl Med 3: 23, 2014. doi: 10.1186/2001-1326-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oftadeh R, Perez-Viloria M, Villa-Camacho JC, Vaziri A, Nazarian A. Biomechanics and mechanobiology of trabecular bone: a review. J Biomech Eng 137: 0108021–01080215, 2015. doi: 10.1115/1.4029176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olins AL, Rhodes G, Welch DB, Zwerger M, Olins DE. Lamin B receptor: multi-tasking at the nuclear envelope. Nucleus 1: 53–70, 2010. doi: 10.4161/nucl.1.1.10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olsen AL, Bloomer SA, Chan EP, Gaça MD, Georges PC, Sackey B, Uemura M, Janmey PA, Wells RG. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol 301: G110–G118, 2011. doi: 10.1152/ajpgi.00412.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pajerowski JD, Dahl KN, Zhong FL, Sammak PJ, Discher DE. Physical plasticity of the nucleus in stem cell differentiation. Proc Natl Acad Sci USA 104: 15619–15624, 2007. doi: 10.1073/pnas.0702576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pelham RJ Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA 94: 13661–13665, 1997. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol 179: 1074–1080, 2011. doi: 10.1016/j.ajpath.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Previtera ML, Sengupta A. Substrate stiffness regulates proinflammatory mediator production through TLR4 activity in macrophages. PLoS One 10: e0145813, 2015. doi: 10.1371/journal.pone.0145813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Quarta M, Brett JO, DiMarco R, De Morree A, Boutet SC, Chacon R, Gibbons MC, Garcia VA, Su J, Shrager JB, Heilshorn S, Rando TA. An artificial niche preserves the quiescence of muscle stem cells and enhances their therapeutic efficacy. Nat Biotechnol 34: 752–759, 2016. doi: 10.1038/nbt.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rehfeldt F, Brown AE, Raab M, Cai S, Zajac AL, Zemel A, Discher DE. Hyaluronic acid matrices show matrix stiffness in 2D and 3D dictates cytoskeletal order and myosin-II phosphorylation within stem cells. Integr Biol 4: 422–430, 2012. doi: 10.1039/c2ib00150k. [DOI] [PubMed] [Google Scholar]
- 76.Rehfeldt F, Schmidt CF. Physical probing of cells. J Phys D Appl Phys 50: 463001, 2017. doi: 10.1088/1361-6463/aa8aa6. [DOI] [Google Scholar]
- 77.Ribeiro AJ, Ang YS, Fu JD, Rivas RN, Mohamed TM, Higgs GC, Srivastava D, Pruitt BL. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc Natl Acad Sci USA 112: 12705–12710, 2015. doi: 10.1073/pnas.1508073112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robertson C, Tran DD, George SC. Concise review: maturation phases of human pluripotent stem cell-derived cardiomyocytes. Stem Cells 31: 829–837, 2013. doi: 10.1002/stem.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sharma A, Licup AJ, Jansen KA, Rens R, Sheinman M, Koenderink GH, MacKintosh FC. Strain-controlled criticality governs the nonlinear mechanics of fibre networks. Nat Phys 12: 584–587, 2016. doi: 10.1038/nphys3628. [DOI] [Google Scholar]
- 80.Shi Q, Ghosh RP, Engelke H, Rycroft CH, Cassereau L, Sethian JA, Weaver VM, Liphardt JT. Rapid disorganization of mechanically interacting systems of mammary acini. Proc Natl Acad Sci USA 111: 658–663, 2014. doi: 10.1073/pnas.1311312110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smith LR, Lee KS, Ward SR, Chambers HG, Lieber RL. Hamstring contractures in children with spastic cerebral palsy result from a stiffer ECM and increased in vivo sarcomere length. J Physiol 589: 2625–2639, 2011. doi: 10.1113/jphysiol.2010.203364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Soon RK Jr, Yee HF Jr. Stellate cell contraction: role, regulation, and potential therapeutic target. Clin Liver Dis 12: 791–803, 2008. doi: 10.1016/j.cld.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun Z, Guo SS, Fässler R. Integrin-mediated mechanotransduction. J Cell Biol 215: 445–456, 2016. doi: 10.1083/jcb.201609037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, Rehfeldt F, Speicher DW, Discher DE. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341: 1240104, 2013. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Uezumi A, Ikemoto-Uezumi M, Tsuchida K. Roles of nonmyogenic mesenchymal progenitors in pathogenesis and regeneration of skeletal muscle. Front Physiol 5: 68, 2014. doi: 10.3389/fphys.2014.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep 35: e00191, 2015. doi: 10.1042/BSR20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang H, Abhilash AS, Chen CS, Wells RG, Shenoy VB. Long-range force transmission in fibrous matrices enabled by tension-driven alignment of fibers. Biophys J 107: 2592–2603, 2014. doi: 10.1016/j.bpj.2014.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y-L, Discher DE. Cell Mechanics, Volume 83 (Methods in Cell Biology). Cambridge, MA: Academic Press, 2007. [Google Scholar]
- 89.Wei SC, Fattet L, Tsai JH, Guo Y, Pai VH, Majeski HE, Chen AC, Sah RL, Taylor SS, Engler AJ, Yang J. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat Cell Biol 17: 678–688, 2015. doi: 10.1038/ncb3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weiss P, Garber B. Shape and movement of mesenchyme cells as functions of the physical structure of the medium: contributions to a quantitative morphology. Proc Natl Acad Sci USA 38: 264–280, 1952. doi: 10.1073/pnas.38.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wells RG. The epithelial-to-mesenchymal transition in liver fibrosis: here today, gone tomorrow? Hepatology 51: 737–740, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wells RG. Tissue mechanics and fibrosis. Biochim Biophys Acta 1832: 884–890, 2013. doi: 10.1016/j.bbadis.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wells RG, Discher DE. Matrix elasticity, cytoskeletal tension, and TGF-beta: the insoluble and soluble meet. Sci Signal 1: pe13, 2008. doi: 10.1126/stke.110pe13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol 179: 1311–1323, 2007. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest 117: 524–529, 2007. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yennek S, Burute M, Théry M, Tajbakhsh S. Cell adhesion geometry regulates non-random DNA segregation and asymmetric cell fates in mouse skeletal muscle stem cells. Cell Reports 7: 961–970, 2014. doi: 10.1016/j.celrep.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 97.Yoshikawa Y, Yasuike T, Yagi A, Yamada T. Transverse elasticity of myofibrils of rabbit skeletal muscle studied by atomic force microscopy. Biochem Biophys Res Commun 256: 13–19, 1999. doi: 10.1006/bbrc.1999.0279. [DOI] [PubMed] [Google Scholar]
- 98.Zemel A, Rehfeldt F, Brown AE, Discher DE, Safran SA. Optimal matrix rigidity for stress fiber polarization in stem cells. Nat Phys 6: 468–473, 2010. doi: 10.1038/nphys1613. [DOI] [PMC free article] [PubMed] [Google Scholar]