Abstract
Chronic kidney disease (CKD), defined as reduced glomerular filtration rate, is increasingly becoming a major public health issue. At the histological level, renal fibrosis is the final common pathway leading to end-stage renal disease, irrespective of the initial injury. According to this view, antifibrotic agents should slow or halt the progression of CKD. However, due to multiple overlapping pathways stimulating fibrosis, it has been difficult to develop antifibrotic drugs that delay or reverse the progression of CKD. MicroRNAs (miRNAs) are small noncoding RNA molecules, 18–22 nucleotides in length, that control many developmental and cellular processes as posttranscriptional regulators of gene expression. Emerging evidence suggests that miRNAs targeted against genes involved in renal fibrosis might be potential candidates for the development of antifibrotic therapies for CKD. This review will discuss some of the miRNAs, such as Let-7, miR-21,-29, -192, -200,-324, -132, -212, -30, -126, -433, -214, and -199a, that are implicated in renal fibrosis and the potential to exploit these molecular targets for the treatment of CKD.
Keywords: chronic kidney disease, microRNA, renal fibrosis
INTRODUCTION
Chronic kidney disease (CKD), defined as reduced glomerular filtration rate, is a major public health issue (45, 84, 99). The economic burden of CKD is substantial, and the Medicaid costs for the treatment of CKD in the US now exceeds $42 billion/year (85, 118). Over the last few years, multipronged interventions have been applied to slow the progression of CKD. These include intensive glycemic control, strict control of blood pressure, correction of dyslipidemia, discontinuation of potentially nephrotoxic drugs and smoking, and blockade of the renin-angiotensin system to lower glomerular capillary pressure (47, 123, 127, 130). However, despite the use of multimodal therapeutic interventions, none of the current strategy reverses the progression to end-stage renal disease. So the need for a more efficacious approach for the treatment of CKD is as pressing as ever (3, 29, 37, 66, 101, 128, 130). At the histological level, renal fibrosis is the final common outcome of CKD, irrespective of the initial injury (57, 58, 74, 129). As discussed here, based on the strong preclinical and clinical data, the use of antifibrotic agents to slow or halt the progression of CKD is a reasonable supposition, although definitive evidence based on clinical trials is needed.
MicroRNAs (miRNAs) are small noncoding RNA molecules, 18–22 nucleotides in length, that constitute a large family of posttranscriptional regulators of gene expression controlling developmental and cellular processes in eukaryotic organisms (61). Analyses of miRNA expression profiles have identified a panel of miRNAs, including microRNA (miR)-215, miR-146a, and miR-886, along with other miRNAs (miR-192, miR-194, miR-21, miR-200a, miR-204, and let-7a–g) whose expression is enriched in the kidney relative to other organs (110). By comparing miRNA levels of normal vs. fibrotic kidneys, Gomez et al. (43) identified 21 miRNAs that were upregulated and three that were downregulated. The signature of upregulated miRNAs included several associated with cell survival in various cancers, including miR-15, -21, -200, and -451. In a comparison of results from human CKD with animal models of kidney injury with fibrosis due to unilateral ureteric obstruction (UUO) or ischemic injury, 24 miRNAs were found to be upregulated in common. These include: let-7i, miR-15b, -21, -25, -132, -199, and -214, suggesting that these miRNAs may play a role in regulating the disease process. Taken together, accumulating evidence have identified a distinct set of miRNAs involved in the process of renal fibrosis that might be a potential target for antifibrotic therapy for CKD. These genes and their targets are summarized in Fig. 1. Below we will discuss the results of recent studies relating specific miRNAs relative to molecular targets participating in renal fibrosis (Table 1). For conciseness, we use the prefix “miR” to designate all microRNAs discussed in this review. Current recommendations for nomenclature call for the use of the prefix “mir”, all lower case, to refer to precursor miRNAs and the mature microRNA to be labeled miR-#, e.g., miR-199a-5p and miR-199a-3p. Unfortunately, many of the published reports do not provide sufficient information to determine which particular strand of mature miRNA was assessed. However, based on the targeted genes reported in the studies that are discussed in our review, we have provided a list of validated mature miRNAs relevant to renal fibrosis (Table 2). In addition, the role of a specific miRNA in fibrosis can be direct, for example by downregulating collagen gene expression. In other cases, the link between a miRNA and renal fibrosis is associative and may involve changes in the expression of profibrotic cytokines or cell death (leading to reparative fibrosis). The reader is encouraged to approach the cited references from that critical perspective.
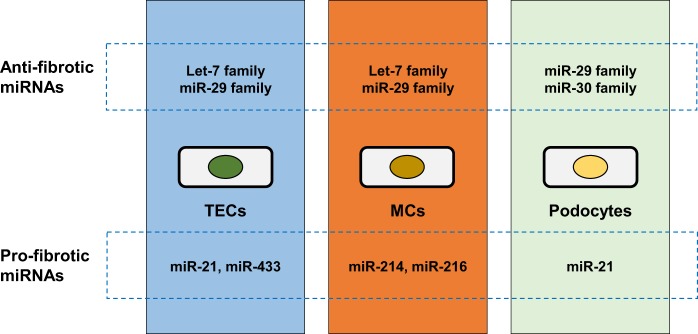
Fig. 1.
Renal pro- and antifibrotic micro (mi)RNAs for tubular epithelial cells (TECs), mesangial cells (MCs), and podocytes.
Table 1.
Summary of miRNAs involved the process of renal fibrosis and their potential targets
| MiRNAs | Potential Targets (genes, gene classes, and signaling networks) |
|---|---|
| Antifibrotic | |
| Let-7 family: Let-7a, Let-c, Let-7f, Let-7b, Let-7d, Let-7e, Let-7g, Let-7i, and miR-98 | TGF-β cell signaling pathway (COL1A2 and Col4a1), EndMT program, fibronectin, FN1, N-cadherin, THBS1, Thbs1, ZEB2, Zeb2, SMAD2, and JAG1 |
| MiR-29 family: miR-29a, -29b, and -29c | ECM genes (Col1a2, LAMC1, LAMC2, FBN1, Fbn1, Eln, and Itgb1), P53, Spry1, and Rho kinase, TGFB1, Tgfb1 |
| MiR-126 | EGFL7, VEGFA, DLK1, IRS1, Irs1, SPRED1, Spred1, HOXA11, CXCL12, PI3K/Akt pathway, AKT2 |
| MiR-30 family: miR-30a, -30b, -30c, -30d, -30e | Notch1 and p53 pathways, Ucp2, CTGF, Ctgf, NOTCH1, ZEB2, TP53, Tp53 |
| MiR-146a | IRAK1, Irak1, TLR4; TGF-β1/Smad pathway, TNF receptor-associated factor 6-nuclear factor kappa B pathway, SMAD2, Smad4, TRAF6, Traf6 |
| MiR-34c | Notch/Jag1 pathway, NOTCH1/4 |
| Profibrotic | |
| MiR-21 | Fatty acid and lipid oxidation metabolic pathways (PPARA, Mpv17l), ERK/MAPK, MMPs and TIMPs (MMP9, Mmp9, TIMP1, MMP2), PTEN/Akt pathway, PTEN, Pten, AKT2, TGF-β cell signaling pathway (SMAD7, Smad7, Smad3), Wnt-pathway (DDAH1, ADMA), TGFB1 |
| MiR-214 and -199 | PTEN; AKT1 |
| MiR-433 | TGF-β1/Smad3, AZIN1, JNK1 (MAPK8), ERK/MAPK |
| MiR-132 and -212 family: miR132, miR132*, miR-212, and miR-212* | TGF-β, STAT3/ERK pathways, MAPK1, cell proliferation (Foxo3/p300); Ep300, Mmp9 |
| MiR-324-3p | Prep, Smad7, Zeb2 |
| MiR-382 | Klk5 |
| MiR-216a | Col1a2, Tsc22, Ybx1 |
| Conflicting Findings | |
| MiR-192 family: miR-192, miR-194–2, and miR-215 | TGF-β1, miRNA-200b/c, Col1a2 and Col4a1, Zeb1, Zeb2, ZEB2, E-cadherin |
| MiR-200 family: miR-200a, −200b, -200c, -141, and -429 | TGF-β1, Zeb1, ZEB1, Zeb2, ZEB2, TGFB2, Tgfb2, Akt pathway |
Shown is a summary of the potential gene targets and signaling pathways of micro (mi)RNAs that are implicated in renal fibrosis and are discussed in this review. Validated genes (miRTarBase (23), http://mirtarbase.mbc.nctu.edu.twphp/index.php, accessed August 5, 2017) are boldfaced and italicized; human genes are in all capital letters; while the mouse/rat genes have initial capital letters.
Table 2.
Validated mature miRNAs relevant to renal fibrosis
| Family | Target | MiRNA |
|---|---|---|
| Antifibrotic | ||
| Let-7 | COL1A2 | hsa-let-7g-5p |
| FN1 | hsa-let-7g-5p | |
| JAG1 | hsa-let-7b-5p, hsa-miR-98-5p | |
| SMAD2 | hsa-let-7g-5p | |
| THBS1 | hsa-let-7a-5p, hsa-let-7b-5p, hsa-let-7c-5p, hsa-let-7d-5p, hsa-let-7e-5p, hsa-let-7f-5p, hsa-let-7g-5p, hsa-let-7i-5p, hsa-miR-98-5p | |
| Thbs1 | mmu-let-7b-5p | |
| Zeb2 | mmu-let-7b-5p | |
| ZEB2 | hsa-let-7d-5p | |
| MiR-29 | Spry1 | mmu-miR-29c-3p |
| TGFB1 | hsa-miR-29b-3p | |
| Tgfb1 | mmu-miR-29b-2-5p | |
| Col1a2 | mmu-miR-29a-3p, mmu-miR-29b-3p | |
| LAMC1 | hsa-miR-29c-3p | |
| LAMC2 | hsa-miR-29a-3p, hsa-miR-29b-3p, hsa-miR-29c-3p | |
| Fbn1 | mmu-miR-29a-3p, mmu-miR-29b-3p | |
| FBN1 | hsa-miR-29a-3p, hsa-miR-29b-3p, hsa-miR-29c-3p | |
| Eln | mmu-miR-29a-3p, mmu-miR-29b-3p, | |
| Itgb1 | rno-miR-29b-3p | |
| MiR-126 | EGFL7 | hsa-miR-126-3p |
| VEGFA | hsa-miR-126-3p | |
| IRS1 | hsa-miR-126-3p | |
| Irs1 | mmu-miR-126a-3p | |
| SPRED1 | hsa-miR-126-3p | |
| Spred1 | mmu-miR-126a-3p | |
| CXCL12 | hsa-miR-126-5p, hsa-miR-126-3p | |
| AKT2 | hsa-miR-126-3p | |
| MiR-30 | TP53 | hsa-miR-30a-5p, hsa-miR-30b-5p, hsa-miR-30c-5p, hsa-miR-30d-5p, hsa-miR-30e-5p |
| Tp53 | rno-miR-30a-5p, rno-miR-30b-5p, rno-miR-30c-5p, rno-miR-30d-5p, rno-miR-30e-5p | |
| NOTCH1 | hsa-miR-30a-5p, hsa-miR-30b-5p, hsa-miR-30c-5p, hsa-miR-30d-5p, hsa-miR-30e-5p | |
| Ucp2 | mmu-miR-30e-5p | |
| Ctgf | rno-miR-30c-5p | |
| ZEB2 | hsa-miR-30c-5p, hsa-miR-30e-5p | |
| MiR-146a | TRAF6 | hsa-miR-146a-5p |
| Traf6 | mmu-miR-146a-5p, rno-miR-146a-5p | |
| IRAK1 | hsa-miR-146a-5p | |
| Irak1 | mmu-miR-146a-5p, rno-miR-146a-5p | |
| SMAD2 | hsa-miR-146a-5p | |
| Smad4 | rno-miR-146a-5p | |
| TLR4 | hsa-miR-146a-5p, hsa-miR-146b-5p | |
| MiR-34c | NOTCH1 | hsa-miR-34c-5p |
| NOTCH4 | hsa-miR-34c-5p | |
| Profibrotic | ||
| MiR-21 | PPARA | hsa-miR-21-5p |
| MMP9 | hsa-miR-21-5p | |
| Mmp9 | mmu-miR-21a-5p | |
| MMP2 | hsa-miR-21-5p | |
| PTEN | hsa-miR-21-5p | |
| Pten | mmu-miR-21a-5p, rno-miR-21-5p | |
| AKT2 | hsa-miR-21-5p | |
| SMAD7 | hsa-miR-21-5p | |
| Smad7 | mmu-miR-21a-5p | |
| DDAH1 | hsa-miR-21-5p | |
| TGFB1 | hsa-miR-21-5p | |
| MiR-214 and -199 | PTEN | hsa-miR-214-3p |
| AKT1 | hsa-miR-199a-3p | |
| MiR-433 | AZIN1 | hsa-miR-433-3p |
| MAPK8 | hsa-miR-433-3p | |
| MiR-132 and -212 | MAPK1 | hsa-miR-132-3p |
| Foxo3 | rno-miR-132-3p, rno-miR-212-3p, mmu-miR-212-3p, mmu-miR-132-3p | |
| Ep300 | mmu-miR-132-3p | |
| Mmp9 | rno-miR-132-3p, mmu-miR-212-3p, mmu-miR-132-3p | |
| MiR-324 | Zeb2 | mmu-miR-324-3p |
| MiR-382 | Klk5 | mmu-miR-382-5p |
Source: from miRTarBase (http://mirtarbase.mbc.nctu.edu.twphp/index.php).
ANTIFIBROTIC MiRNAs IN CKD
MiRNA Let-7 family.
Lethal-7 (let-7), one of the first discovered miRNAs, has been implicated in cancer and pluripotency (41, 109). Let-7 and its family members are highly conserved across species and play a significant role in the regulation of cell reprogramming (109). In mice, 12 genes encode members of the let-7 family, which includes nine slightly different miRNAs: let-7a, let-c, let-7f (all encoded by two genes), and let-7b, let-7d, let-7e, let-7g, let-7i, and miR-98 (all encoded by one gene) (41). All let-7 family members are believed to have a similar function because they share a common seed region (nucleotides 2–8), which mediates interaction with the target mRNAs.
Recently, let-7 family members have been suggested to act as negative regulators of profibrotic processes in several disease states, including renal fibrosis (88, 91). Knockdown of let-7b in rat proximal tubular epithelial cells (pTECs) elevated transforming growth factor (TGF)-β1 receptor 1 (TGFBR1) expression and mimicked some of the profibrotic effects of TGF-β1 (113). Similarly, Park et al. (91) found that let-7 family miRNAs target extracellular matrix (ECM) gene products (Col1a2 and Col4a1) of the TGF-β signaling pathway in mouse mesangial cells (MCs) and that the let-7 family negatively modulates ECM accumulation by regulating multiple players in the pathway starting from receptor down to final gene product. Conversely, the negative regulator of let-7 biogenesis, lin28b, was upregulated by TGF-β. Lin28 is a translational enhancer and transcription factor that forms a trimeric complex with OCT4 on DNA (103). It has been widely investigated as an oncogene and was recently demonstrated to suppress the expression of let-7 miRNAs by binding to a motif in the terminal loop of pre-let-7 and recruiting ZCCHC11/TUT4 uridylyltransferase to promote terminal uridylation (10, 103, 107). Of note, in glomeruli of diabetic mice, let-7b levels were decreased, whereas lin28b, Col1a2, and Col4a1 levels were increased. These results suggest that restoring let-7 levels may play a protective role as a natural suppressor of the TGF-β pathway in renal cells during diabetic nephropathy (DN) (91). Park et al. (91) demonstrated that suppression of let-7 miRNAs by lin28 is mediated by TGF-β through the action of Smads. TGF-β-induced enrichment of Smad2/3 showed that the lin28b promoter, which contains a Smad-binding element (SBE), is responsive to TGF-β.
Endothelial-to-mesenchymal transition (EndMT) is an important source of fibroblasts in renal fibrosis. Chen et al. (19) reported that fibroblast growth factor receptor-mediated induction of let-7 miRNA acts as a negative regulator of the EndMT program by inhibiting TGF-β signaling via suppression of TGF-β, TGF-βR1, and Smad2 expression. The endogenous tetrapeptide, N-acetyl-seryl-aspartyl-lysyl-proline (AcSDKP), has antifibrotic actions in the heart and kidney due to inhibition of cellular proliferation and TGF-β signaling. Nagai et al. (86) demonstrated that the renal antifibrotic and anti-EndMT effects of AcSDKP in Type 1 diabetic CD-1 mice were associated with restoration of let-7 levels. AcSDKP is hydrolyzed almost exclusively by ACE, and administration of an ACE inhibitor plus AcSDKP ameliorated kidney fibrosis and inhibited EndMT compared with therapy with an ACE inhibitor alone. Similar findings were observed in the db/db mouse model of Type 2 DN (88).
Lipoxins are endogenously produced lipid mediators that promote resolution of inflammation and inhibit fibrosis, suggesting a possible role in modulating renal disease. Lipoxins attenuate renal fibrosis in a rat UUO model, and this is associated with increased expression of let-7c miRNA (10). Brennan et al. (10) provided evidence that lipoxins suppress TGF-β1-induced renal fibrosis through a mechanism involving upregulation of let-7c and downregulation of its target TGF-βR1. In cultured human pTECs (HK-2 cells), the lipoxin A4 attenuated TGF-β1-induced expression of fibronectin, N-cadherin, thrombospondin, and the notch ligand jagged-1. Moreover, the potential prognostic value of let-7c dysregulation in renal fibrosis is underscored by their finding that several let-7c target genes were upregulated in human CKD biopsies (10).
MiR-29 family.
MiR-29a, -29b, and -29c have a common seed sequence but are encoded by distinct genomic loci. Overall, these antifibrotic miRNAs have been reported to be downregulated in chronic kidney diseases, such as DN, focal segmental glomerulosclerosis (FSGS), membranous nephropathy, and IgA nephropathy (78, 95). Many matrix genes are targets for miR-29, including fibrillin, collagens, elastin, laminins, and integrin-β1. In fact, miR-29 has the potential to reduce the expression of ~20 genes that impact collagen formation. Thus, miR-29 normally provides tonic suppression of matrix production and turnover. Additionally, miR-29 also slows cell growth and may trigger apoptosis in certain cancer cells potentially by suppressing genes that normally silence the p53 transcription factor. Therefore, miR-29 has the potential to both suppress matrix deposition and drive cell death, which may be beneficial if the cells that undergo apoptosis are profibrogenic cells (16).
Antifibrotic effects have been reported for all of the miR-29 family members, miR-29a, miR-29b, and miR-29c. Streptozotocin-induced diabetic miR-29a transgenic mice showed improved renal function with less glomerular fibrosis and inflammation and better podocyte viability compared with diabetic wild-type (WT) mice (71). Knockdown of miR-29a promoted histone deacetylase activity that led to podocyte apoptosis, proteinuria, and subsequent renal dysfunction (71). Reduced expression of miR-29 along with the development of progressive renal fibrosis was demonstrated in obstructive nephropathy (95). In vitro studies confirmed that overexpression of miR-29b inhibited, and knockdown of miR-29 enhanced, TGF-β1-induced expression of collagens I and III by renal tubular cells. In addition, upregulation of miR-29b was implicated in the protection of SS.13BN consomic rats from renal medullary fibrosis and tubular necrosis following the development of hypertension, as compared with control Dahl salt-sensitive rats, due to the targeting of multiple collagen and ECM-related genes (75). Downregulation of miR-29c was associated with significant increases in interstitial fibrosis, collagen type II α1 (COL2A1) and tropomyosin 1α protein levels in the remnant kidney of rats subjected to 5/6 nephrectomy and in the kidneys of patients with IgA nephropathy (38).
TGF-β1 counteracts the beneficial role of miR-29 family members by downregulating their expression in pTECs, MCs, and podocytes (32). TGF-β-induced downregulation of miR-29 expression is mediated by activation of Smad3 signaling. Binding of Smad3 to an SBE in the promoter region of miR-29 suppresses its transcription in cultured pTECs and fibroblasts, as well as in vivo, in the kidney of mice following UUO (95). In contrast, Smad3 knockout (KO) mice exhibit increased expression of miR-29 along with reduced renal fibrosis in the UUO mouse model (95). Fang et al. (38) reported that cobalt chloride, which increases hypoxia-inducible factor-alpha (HIF-α) protein levels, increased miR-29c expression in human kidney epithelial HK-2 cells. Upregulation of miR-29c was significantly attenuated by knockdown of HIF-1 or HIF-2α (38). These findings suggest that HIF-1 and HIF-2α also contribute to the regulation of miR-29c expression in the kidney.
Qin et al. (95) utilized an ultrasound-microbubble-mediated gene transfer of miR-29 for the treatment of renal fibrosis in a mouse model of UUO. They demonstrated that delivery of miR-29b either before or after induction of UUO reduced renal fibrosis. However, there are some discordant findings regarding the antifibrotic effect miR-29c. Long et al. (76) reported that miR-29c increased accumulation of ECM and induced podocyte apoptosis in a db/db mouse model of Type 2 diabetes. Knockdown of miR-29c reduced albuminuria and mesangial matrix accumulation. They identified Sprouty homolog 1 (Spry1) as a direct target of miR-29c and provided evidence that downregulation of Spry1 promotes Rho kinase activation, resulting in enhanced cell apoptosis and fibronectin synthesis. Treatment with Rho kinase inhibitors attenuates the development of renal damage in different animal models (112). Although data connecting the activation of Rho/Rho kinase pathway and kidney disease in humans are still lacking, these studies have provided compelling evidence for the renoprotective effects of Rho-kinase inhibitors (39, 112, 117).
MiR-126.
MiR-126 is encoded by intron 7 of the epidermal growth factor like-7 (EGFL7) gene and has proangiogenic actions. Microvesicles derived from endothelial progenitor cells were found to protect the kidney from acute ischemic injury due to the generation of miR-126 and miR-296, which inhibited capillary rarefaction, glomerulosclerosis, and tubulointerstitial fibrosis (12). Bijkerk et al. (8) more recently reported that mice overexpressing miR-126 in the hematopoietic compartment displayed a decrease in fibrotic and injury markers after renal ischemia/reperfusion injury. The beneficial effects of miR-126 were attributed to the preservation of vascular integrity due to an increase in the number of circulating hematopoietic stem and progenitor cells and their mobilization to the kidney. Recently, Al-Kafaji et al. (2) found that decreased expression of circulating miR-126 was associated with the development of DN in Type 2 diabetic patients and suggested it may be a promising biomarker for the progression of DN.
MiR-126 regulates the responsiveness of endothelial cells to VEGF. One of the main targets of miR-126 is EGFL7, which is implicated in cell migration and blood vessel maturation and functions by interacting with the Notch receptor and Notch ligand Delta-like 4 (97). By downregulating EGFL7, miR-126 may coordinate angiogenesis and vessel maturation; however, the precise importance of this downregulation is not known (87). MiR-126 also silences Sprouty-related protein (SPRED1) (which negatively regulates VEGF signaling), DLK1, transcription factors (e.g., IRS1 and HOXA11), as well as a regulator of G protein 16 (RGS16), which is an inhibitor of G protein-coupled receptor (GPCR) signaling (40, 97). During tissue repair, delivery of miR-126 to recipient vascular cells suppresses RGS16 and enables CXCR4, a GPCR, to support an autoregulatory feedback loop that increases production of its ligand CXCL12, which in turn promotes recruitment of progenitor cells and vascular protection (131). MiR-126 may also inhibit vascular endothelial cell apoptosis by enhancing PI3K/Akt signaling by directly targeting the negative regulator PIK3R2 (phosphoinositide-3-kinase regulatory subunit 2) (18). Thus, miRNA-126 might protect the kidney via several signal pathways. Further studies are needed to discern the antifibrotic effect and its potential value for the treatment of renal disease.
MiR-30 family.
The miR-30 family consists of five evolutionarily conserved members, miR-30a through -30e (98). Members of the miR-30 family all share a common seed sequence and are encoded by six genes located on human chromosomes 1, 6, and 8 (11). The miR-30 family had been documented to be downregulated in podocytes of patients with FSGS (80, 122). Treatment of cultured human podocytes with TGF-β or puromycin aminonucleoside leads to cytoskeletal damage and apoptosis, which is ameliorated by upregulation of miR-30 expression and aggravated by knockdown of miR-30. The protective role of miR-30 was attributed to inhibition of Notch1 and p53 pathways (122).
The miR-30 family also inhibits epithelial cell ECM production and phenotype changes by downregulating mitochondrial uncoupling protein 2 (Ucp2) (51). UUO in mice was shown to induce UCP2 in tubular epithelial cells, the ablation or inhibition of which attenuated UUO-induced renal fibrosis. In cultured rat kidney epithelial cells, UCP2 expression was induced by TGF-β1, and its knockdown markedly reduced, while overexpression promoted, TGF-β1-induced epithelial-to-mesenchymal transition (EMT). Ucp2 mRNA was demonstrated to be a direct target of miR-30e, which was downregulated in tubular cells of fibrotic kidneys and TGF-β1-treated kidney epithelial cells. Moreover, tubular cell EMT was inhibited by a miR-30e mimic, whereas a miR-30e inhibitor acted like TGF-β1. In addition, connective tissue growth factor (CTGF), a key molecule in the process of renal fibrosis, is regulated by miR-30. Duisters et al. (34) demonstrated that miRNA-30 expression was inversely associated with the amount of CTGF in two animal models of heart disease, as well as in patients with left ventricular hypertrophy. In vitro, knockdown of miR-30 increased CTGF levels in cardiomyocytes and fibroblasts. Overexpression of miR-30c decreased CTGF levels and reduced the production of collagens. These results suggest that the miR-30 family is an attractive therapeutic target for fibrotic diseases including CKD since it influences the fibrotic process by regulating mitochondrial ATP and CTGF production.
PROFIBROTIC MiRNAs IN CKD
MiR-21.
MiR-21 is one of the most abundant miRNAs in human tissues and is upregulated in several animal models of kidney disease and in kidney tissue obtained from patients with CKD. In addition, the kidneys of miR-21 KO mice showed marked protection against the development of renal fibrosis (13). Microarray studies of kidneys from miR-21 WT and KO mice indicate that expression of the predicted target genes of miR-21 are not significantly different under normal conditions (14). However, after expression of miR-21 is increased by UUO or ischemia/reperfusion injury, differences in expression of the target genes of miR-21 were observed between WT and miR-21 KO mice, with miR-21 KO mice having far less renal interstitial fibrosis following injury.
Analysis of gene expression profiles in miR-21 WT and KO mice in response to injury suggested that miR-21 contributes to the development of renal fibrosis via regulation of metabolic pathways involved in fatty acid and lipid oxidation (14). Peroxisome proliferator-activated receptor (PPAR)-α is one of the most potent transcription factors that regulate fatty acid oxidation and is a validated target of miR-21. In normal kidneys, high expression levels of PPAR-α can be detected in interstitial cells, whereas after injury the expression falls secondarily to miR-21 targeting. Upregulation of PPAR-α expression in transgenic mice subjected to UUO decreased ECM accumulation and reduced renal interstitial fibrosis. Those observations confirm the importance of fatty acid oxidation in the prevention of renal fibrosis and the importance of miR-21 silencing of the PPAR-α axis.
Generation of reactive oxygen species (ROS) contributes to renal fibrosis. Mpv17-like protein is a mitochondrial inner membrane protein that regulates the production of ROS and protects against mitochondrial oxidative stress and apoptosis. Mpv17-like protein is downregulated by miR-21 during kidney injury (14). MiR-21 also upregulates extracellular signal-regulated kinases (ERK) signaling in the kidney (14). Both ERK1/2 and TGF-β/Smad signaling pathways seem to play a role in the development of kidney fibrosis in diabetic models (21).
The role of miR-21 in ECM homeostasis may be mediated through the regulation of metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs). Wang et al. (115) described the association between MMP-9/TIMP1 and miR-21 in DN using the kk-ay diabetic mouse. They confirmed computational predictions that MMP-9 is a potential target for miR-21. With the progression of DN, the expression of miR-21 increased in the kidney of kk-ay diabetic mice. They further observed that miR-21 directly downregulates MMP-9 protein expression and indirectly leads to an increase in TIMP1 mRNA expression and protein (115). Notably, treatment of kk-ay DN mice with antagomir-21 improved renal function and decreased renal TIMP1, ColIV, and FN protein levels. Additionally, global transcriptome analysis revealed miR-21 affects the expression of another target involved in ECM regulation, namely RECK (reversion-inducing-cysteine-rich protein with kazal motifs), which is a membrane-anchored glycoprotein inhibitor of MMP-9 and MMP-2 (90).
The expression of several MMPs appear to be regulated by phosphatase and tensin homolog (PTEN), a well-documented tumor-suppressor gene and negative regulator of Akt signaling that was demonstrated to be downregulated by miR-21 during TGF-β1-induced EMT (67, 116). MiR-21 knockdown in kidneys also leads to a decrease in MMP-2 (due to attenuated Akt signaling), suggesting that PTEN is also targeted by miR-21 in the kidney (14, 20). Indeed, the genes for human and mouse/rat PTEN are validated targets for miR-21 (miRTarBase 2016) (23). MMP-2 is implicated in renal interstitial fibrosis possibly via induction of EMT (33). Moreover, Chen et al. (17) demonstrated that cyclosporine A (CsA)-induced nephrotoxicity was associated with increased miR-21 and a rapid increase in phosphorylated Akt and downregulation of PTEN. Inhibition of miR-21 prevented CsA-induced Akt activation and EMT gene expression (17), as well as activation of profibrotic genes in human podocytes and tubular cells in a model of IgA nephropathy (5).
It is well established that TGF-β is a key cytokine in renal fibrosis. TGF-β signaling is regulated by miR-21. TGF-β1, in turn, induces miR-21 expression through Smad signaling (63). McClelland et al. (81) reported that the extent of fibrosis and the decline in renal function in patients with DN correlated with upregulation of miR-21, which they also observed was upregulated in various animal models of fibrotic renal disease and experimental DN. Using rat proximal tubular epithelial cells, these investigators provided evidence that TGF-β1-induced miR-21 expression augments fibrotic gene expression by TGF-β1, by enhancing TGF-β1-induced Smad3 and Akt signaling due to the downregulation of Smad7 and PTEN, respectively. Recent studies demonstrate that Smad3 stimulates the generation of miR-21. Davis et al. (27) reported that the induction of mature miR-21 and pre-miR-21 was not preceded by an increase in pri-miR-21 in response to TGF-β1 stimulation, indicating that upregulation of miR-21 occurs at the posttranscriptional level. Davis et al. (27) suggested that Smad3 associates with the Drosha/DGCR8/p68 microprocessor complex to facilitate cleavage of pri-miRNA to pre-miRNA. In a later study, TGF-β and bone morphogenetic protein (BMP) signaling was found to regulate a group of miRNAs (including miR-21) posttranscriptionally via direct binding of Smad3 to the stem regions of the target pri-miRNAs, which contain a conserved sequence similar to the SBE present in the promoters of Smad target genes (28). Recruitment of Smad3 was postulated to be required for the efficient recruitment of the microprocessor complex. Smad3 may also bind to an SBE of the promoter for the miR-21 gene to modulate its expression in response to TGF-β stimulation (133).
Recently, Liu et al. (73) found a new target for miR-21. They suggested that miR-21 interacts with the 3′-untranslated region (UTR) of the mRNA for dimethylarginine dimethylaminohydrolase 1 (DDAH1) in the Wnt pathway in human HK-2 cells. DDAH1 breaks down asymmetric dimethylarginine (ADMA), an endogenous inhibitor of nitric oxide synthase (NOS). Thus, upregulation of miR-21 may contribute to renal fibrosis by reducing DDAH1 activity/expression and increasing ADMA levels, thereby reducing production of NO.
Recent studies using anti-miR-21 oligonucleotides have demonstrated their ability to reduce renal injury and fibrosis in various animal models of acute kidney injury, as well as in Alport syndrome (14). Alport nephropathy is caused by a mutation in a capillary basement membrane protein (COL4A5) resulting in cell stress that secondarily triggers leukocyte recruitment and fibrosis. In primary cell cultures from kidneys obtained from a mouse model of Alport nephropathy, anti-miR-21 oligonucleotides protected cells from mitochondrial dysfunction due to cell stress and inhibited activation of fibroblasts by various stimuli. Weekly delivery of anti-miR-21 oligonucleotides to mice after the onset of the disease profoundly slowed disease progression and improved survival, normalized tubular function, and reduced histological end points, including interstitial fibrosis, glomerulosclerosis, tubular injury, and inflammation (42).
Anti-miR-21 oligonucleotides can be given to animals for many weeks without noticeable deleterious effects (42). Therefore, anti-miR-21 oligonucleotides represent a potential new class of therapeutics to treat fibrotic kidney diseases and have now entered phase II clinical trials (14). Although the safety of delivering modified oligonucleotides to the body has yet to be proven, phase III clinical trials are currently underway to treat a number of diseases. In addition, anti-miR-21 oligonucleotides have proven to be safe in long-term studies in primates and more recently in humans (43). On the other hand, it should be noted that ischemic preconditioning of the kidney induced upregulation of the expression of miR-21 that was shown to protect against subsequent renal ischemia/reperfusion injury by targeting proapoptotic genes (125). This study highlights the complex role of miR-21 in the regulation of fibrosis in the kidney.
MiR-214 and miR-199a.
MiR-214 is expressed at high levels in human and animal models of kidney disease (43). MiR-214 is cotranscribed with the miR-199a family on a single lncRNA strand located on the complementary strand of an intron in the dynamin-3 gene. miR-214 and miR-199a genes are upregulated by activation of the TWIST transcription factor and by hypoxia via HIF-1α. In kidney samples obtained from patients with various kidney diseases, miR-214 was detected in renal tubules, glomeruli, and infiltrating immune cells (30). Denby et al. (30) recently suggested that miR-214 functions by promoting renal fibrosis independently of TGF-β signaling. In the mouse UUO model, decreased expression of miR-214 protected against the development of fibrosis, and treatment of WT mice with anti-miR-214 before UUO resulted in similar antifibrotic effects. MiR-214 antagonism did not block Smad2/3 activation, and TGF-β blockade together with miR-214 deletion provided additional renal protection, indicating that miR-214 induces fibrotic effects independent of Smad2/3. Furthermore, the regulation of endogenous miR-214 was unaffected by inhibition of canonical TGF-β signaling (30).
The endogenous Akt signaling pathway inhibitor PTEN is also a recognized target for miR-214 (69). Wang et al. (120) recently reported that inhibition of miR-214 significantly reduced albuminuria and mesangial expansion in db/db mice, and this was associated with reduced expression of α-smooth muscle actin (SMA), SM22, and collagen IV, as well as partially restored PTEN levels. In vitro studies confirmed that PTEN is a target of miR-214 and that inhibition of miR-214 reduced fibrotic gene expression and partly restored PTEN protein levels in high glucose-stimulated human mesangial cells. Upregulation of miR-214 was specifically linked to hypertrophy in this model, as PTEN overexpression attenuated miR-214-mediated hypertrophy of the mesangial cells exposed to high glucose, whereas PTEN knockdown mimicked hypertrophy of these cells.
As mentioned, the miR-199a family is cotranscribed with miR-214. MiR-199a-5p and miR-199a-3p are produced from a single molecule, pre-miR-199p (15). Several studies in nonrenal tissues have pointed to discrete roles for these miRNAs in modulating cell migration and matrix deposition. However, the significance of the miR-199a family in kidney disease has not been established, nor has the potential differential actions of miR-199a-3p and miR-199a-5p (15). Increased renal expression of miR-199a-5p was reported in the UUO mouse model of kidney fibrosis, and miR-199a-5p was shown to be more effective than miR-199a-3p in reducing caveolin-1 levels in lung fibroblasts, a critical process in the activation of fibroblasts by TGF-β (72). Others have reported that the membrane protein Klotho is a target of miR-199a-5p in rat mesangial cells exposed to high glucose media (121). Suppression of Klotho expression by miR-199a-5p was linked to increased expression of inflammatory and profibrotic genes secondary to enhanced Toll-like receptor 4 (TLR4)/NF-κB p65/NGAL signaling. Given its positive association with inflammation and fibrosis, miR-199a-5p may also represent another therapeutic target for the treatment of renal fibrosis associated with diabetes.
MiR-433.
Early studies of miR-433 focused on its role in cancer (25). Recently, miR-433 was reported to be upregulated in renal and cardiac fibrosis (70, 106). Human miR-433, located at chromosomal region 14q32.2, belongs to the DLK1-DIO3 miRNA cluster and functions as a tumor suppressor in several human cancers. Epigenetic programming has an important role in the regulation of miR-433 (126). CpG islands surrounding the miR-433 gene are hypermethylated, and treatment with the DNA methylation inhibitor 5-aza-2'-deoxycytidine (5-aza-CdR) induces miR-433 expression.
MiR-433 was upregulated in the UUO mouse model, while knockdown of miR-433 attenuated the induction and progression of renal fibrosis in this model (70). In vitro, overexpression of miR-433 in rat tubular epithelial cells enhanced TGF-β1-induced fibrosis, while its knockdown attenuated fibrosis. Smad3 (but not Smad2) was shown to bind the miR-433 promoter to induce its expression. Thus, miR-433 is an important component of TGF-β/Smad3-induced renal fibrosis through induction of a positive feedback loop that amplifies TGF-β/Smad3 signaling. Antizyme inhibitor 1 (AZIN1), a regulator of polyamine synthesis, was identified as a target gene of miR-433 linked to TGF-β signaling in renal fibrosis (70). Overexpression of miR-433 suppressed AZIN1 expression, while AZIN1 upregulation suppressed TGF-β signaling and fibrosis. As an important component of TGF-β/Smad3-induced renal fibrosis, miR-433 may be a potential therapeutic target in renal fibrosis disease.
MiR-132 and miR-212 family.
MiR-132 and miR-212 are closely clustered in the genome and are transcribed together under the regulation by the cAMP response element binding protein (CREB), the expression of which is regulated by angiotensin II (ANG II) (35). The miR212/132 gene has an equivalent structure in all species; the two miRNAs genes are organized in tandem on chromosome 10 in the rat, 11 in mouse, and 17 in humans (96). The miR212/132 gene cluster produces four mature miRNAs: miR132, miR132*, miR212, and miR212*. MiRNA and miRNA* sequences represent the two strands of the duplex-derived from the precursor miRNA. Although they are produced in equal amounts by transcription, their accumulation differs and depends on differences in the thermodynamic stability of the 5′-end of each strand (96).
Recently, miR-132 and miR-212 were reported to be increased in the heart, aorta, and kidney of ANG II-induced hypertensive rats and decreased in human arteries obtained from patients undergoing bypass surgery, in response to ANG II type 1 receptor (AT1) blockade (35). In renal fibrosis, most of the α-SMA-positive myofibroblasts arise from perivascular cells. Bijkerk and colleagues (7) found that miR-132 increased 21-fold during pericyte-to-myofibroblast transformation in mice subjected to UUO and that miR-132 silencing decreased collagen deposition and tubular apoptosis. Further study identified a rate-limiting role for miR-132 in myofibroblast proliferation. This role of miR-132 in regulating cell proliferation was selective for the interstitial compartment. MiR-132 was found to coordinately regulate the expression of several genes involved in STAT3/ERK pathways, TGF-β signaling, and cell proliferation (Foxo3/p300) (7). In light of evidence that MMP-9 is a target of miR-132 (111), the role of MMP-9 in mediating the effect of miR-132 on renal fibrosis warrants additional consideration. Overall, the available data support miR-132 as a potential target for antifibrotic therapy in CKD (7).
MiR-324-3p.
Macconi and colleagues (79) analyzed the miRNA expression profile of glomeruli from the Munich Wistar Frömter (MWF) rat, which spontaneously develops progressive nephropathy. They found that miR-324-3p was the most upregulated miRNA. Given the remarkable antifibrotic effects of ACE inhibitors, they focused on potential targets of miR-324-3p involved in the metabolism of pro- or antifibrogenic peptides, including ANG II. Bioinformatic analysis indicated that prolyl endopeptidase (Prep) was a miR-324-3p target involved in the formation of ANG (1–7), which has renoprotective actions, and the antifibrotic peptide AcSDKP. Transient transfection of cultured tubular cells with a miR-324-3p mimic reduced Prep protein and activity. Overexpression of miR-324-3p in MWF rats reduced expression of Prep in glomeruli and tubules and the excretion of AcSDKP and increased deposition of collagen. Conversely, treatment with an ACE inhibitor downregulated glomerular and tubular miR-324-p, promoted renal Prep expression, increased plasma and urine AcSDKP levels, and attenuated renal fibrosis. These results suggest that the renoprotective effects of ACE inhibitors partially result from modulation of the miR-324-3p pathway, suggesting that it may be a potential therapeutic target for renal fibrosis (79). Recently, Xu and colleagues (124) found Smad7 is a direct target of miR-324-3p. Considering the important role of Smad7 as a negative regulator of TGF-β1/Smad3 signaling in renal fibrosis, the role of miR-324-3p in opposing Smad7 should be further investigated (119).
MiRNAs WITH CONFLICTING RESULTS
MiR-192 family.
The gene for miR-192, which is highly expressed in the normal kidney, is located on chromosome 11q13.1, 109 bp upstream of the region encoding miR-194-2 (22, 104). It is likely that miR-192 and miR-194-2 are regulated as a common transcriptional unit (104). Several studies from rodent models of kidney diseases and cell lines demonstrate a profibrotic role of miR-192 in both renal tubular and mesangial cells (24, 31, 56, 94). In this regard, Sun and collaborators (102) reported that administration of the anticancer drug Taxol interferes with cell proliferation by altering the cytoskeleton ameliorates fibrosis in the remnant kidney model by lowering miR-192 levels.
For the most part, a functional link between increased miR-192 levels and TGF-β1-driven renal fibrosis has been well documented (24, 56, 62). Like miR-21, miR-192 promotes renal fibrosis by amplifying TGF-β signaling. Glomeruli from diabetic mice show increased miR-192 expression and either TGF-β (via Smad3) or high glucose-induced miR-192 expression in TECs (24, 56). In MCs, miR-192 mediates TGF-β-induced collagen expression by downregulating zinc finger E-box binding homeobox 1/2 (Zeb1/2) expression (56). In TECs, miR-192 overexpression enhances, and its inhibition opposes, TGF-β1-induced collagen expression (24). Kato et al. (53) provided evidence that an miRNA circuit involving miR-129 mediates regulation of TGF-β1 levels in renal MCs. TGF-β1 levels were upregulated by miR-192 or miR-200b/c. Levels of miR-200b/c were also upregulated by miR-192, suggesting that miR-200b/c is a downstream target of miR-192. The activity of the TGF-β1 promoter was upregulated by TGF-β1 or miR-192, demonstrating that the miR-192/miR-200 cascade induces TGF-β1 expression. TGF-β1 increased occupancy of the activators, upstream stimulatory factors and Tfe3, and decreased that of the repressor Zeb1 on the TGF-β1 promoter E-box binding sites. Moreover, inhibitors of miR-192 decreased the expression of miR-200b/c, Col1a2, Col4a1, and TGF-β1 in mouse MCs and mouse renal cortex. Thus, miRNA-regulated circuits may amplify TGF-β1 signaling, thereby accelerating the progression of CKD (53). There is also a TGF-β-induced feedback amplification of the circuit between p53 and miR-192 in mouse MCs, via the miR-192 target Zeb2, which was implicated in the pathogenesis of DN. MiR-192-mediated reduction in Zeb2 enhanced p53 levels, which in turn drove miR-192 expression.
In contrast to these reports, Krupa et al. (62) reported that loss of miR-192 promotes fibrogenesis in DN. However, the stage of the disease may have an impact on the role of miR-192. For example, Kato and colleagues (56) observed upregulation of the renal expression of miR-192 and collagen α-2(I) in Type 1 and 2 in the early stage of mouse models of diabetes. Krupa and colleagues (62) found decreased miR-192 expression in advanced-stage human DN renal biopsy samples accompanied by tubulointerstitial fibrosis. Jenkins et al. (50) reported that TGF-β1 downregulates miR-192 and miR-194, which are cotranscribed in the shared precursor pri-miR-192/194. They identified binding sites for hepatocyte nuclear factor (HNF) and p53 within the miR-194-2/192 promoter region required for constitutive activity, which was decreased by TGF-β1 through an Alk5-dependent mechanism. TGF-β1 treatment decreased binding of HNF to the promoter, whereas knockdown of HNF-1 inhibited mature miR-192 and miR-194 expression (50). In cultured human proximal tubular cells, Krupa and colleagues (62) also reported downregulation of miR-192 expression by TGF-β1, while increased expression of this transcript repressed Zeb1 and Zeb2 expression, thereby de-repressing E-cadherin and exerting an antifibrotic effect.
Conflicting findings regarding the role of miR-194-2/192 in renal fibrosis may be attributed to intrinsic differences in the animal models used, the cell types, the disease stage studied, and/or experimental conditions used for cultured cells. One study described a biphasic induction of miR-192 by TGF-β1 in murine MCs that initially involves the Smad pathway followed by a mechanism that sustains miRNA expression by relaxing the chromatin structure of the miR-192 gene through Ets1 and histone H3 acetylation by Akt-activated p300 (54). This mechanism may contribute to DN since activation of Akt and p300 and acetylation of Ets-1 and histone H3 were found to be increased in glomeruli from diabetic db/db mice. In the kidney, HNF expression is restricted to the tubular compartment, whereas it is absent in MCs and podocytes, thus partly explaining the cell-specific regulation of miR-192 (56). Further investigation on the potential role of miR-192 and the mechanisms that regulate miR-192 expression during renal fibrosis in different species is necessary (26, 49).
MiR-215, which is located at chromosome 1q41, has the same 8-mer seed sequence as miR-192. It only differs by two nucleotides and thus is another member of the miR-192 family (52, 104). Recently, Lan et al. (64) reported that exogenous miR-215 decreased fibroblast cell proliferation, without significant apoptosis compared with controls. They concluded that miR-215 has a role in inhibiting fibroblast proliferation in the ocular surface conjunctiva. This suggests that miR-215 may have benefit in reducing renal fibrosis as well.
MiR-200 family.
The miR-200 family has been ascribed a role in maintaining epithelial cell differentiation, suggesting it might have antifibrotic actions in DN (114). This family consists of five members (miR-200a, -200b, -200c, -141, and -429), encoded by two separate genomic loci on chromosome 1 (miR-200b∼200a∼429 and miR-200c∼141) (9). Members of this family have very similar seed sequences that recognize the 3′-UTR sequence in the target transcript of the regulated genes. On the basis of their seed sequence, the family members can be classified into two functional groups. Group 1 comprises miR-200b, miR-200c, and miR-429, and group 2 comprises miR-200a and miR-141. Because of the differences in seed sequence, the two groups have different mRNA targets (93).
The mechanism for the antifibrotic effects of miR-200 may involve prevention of EMT in pTECs, which is a common pathway in renal fibrosis (93). Tang et al. (105) demonstrated that miR-200b suppresses TGF-β1-induced EMT in HK-2 cells via increased E-cadherin and reduced fibronectin mRNA and protein expression. The former was due to decreased expression of Zeb1 and Zeb2, while the latter resulted from the direct targeting of the fibronectin mRNA. Suppression of TGF-β1-induced EMT occurred independently of TGF-β1-induced phospho-Smad2/3 and phospho-p38 and p42/44 signaling. In vivo, expression of Zeb-1 and Zeb-2 increased after UUO, and administration of the miR-200b precursor blocked this effect. Despite elevated miR-200 levels following induction of UUO, delivery of miR-200b-precursor ameliorated fibrosis in these mice (89). MiR-200s are also are predicted to repress the expression of TGF-β2, which may inhibit EMT and fibrosis (6). Gregory et al. (44) also reported that members of the miR-200 family and miR-205 are downregulated during TGF-β1-mediated EMT in MDCK renal epithelial cells, and forced expression of the miR-200 family alone was sufficient to prevent EMT.
In contrast, Kato et al. (53) found that the amount of miR-200b/c increased in mouse MCs treated with TGF-β1 in vitro and in glomeruli obtained from diabetic mice. Increased miR-200b/c was linked to increased collagen expression via downregulation of Zeb1/2. Further study demonstrated that TGF-β1 levels were upregulated by miR-200b/c, creating an autoregulatory loop to amplify TGF-β1 signaling in chronic renal fibrotic diseases (53). Additionally, previous reports indicated that Smad3 directly binds to a SBE in the promoter region of miR-200b/a and functions as a transcriptional activator in a TGF-β-independent manner (1, 83). In TGF-β1-treated MCs and in glomeruli of diabetic db/db mice, miR-200b and miR-200c repressed the PI3K inhibitor FOG2, thereby enhancing PI3K/Akt signaling (92). Thus, TGF-β1-induced expression of miR-192 activates the miR-200 family (along with miR-216a and miR-217), leading to Akt activation, hypertrophy, and fibrosis in MCs.
OTHER MiRNAs AND RENAL FIBROSIS
MiR-146a was reported to inhibit inflammation by suppressing NF-κB signaling by targeting mRNAs such as tumor necrosis factor receptor-associated factor 6 (TRAF6), interleukin-1 receptor-associated kinase 1 (IRAK1), and TLR4 (68). Overexpression of miR-146a inhibited renal fibrosis, α-SMA expression, and macrophage infiltration in a UUO mouse model (82). In addition, miR-146a inhibited TGF-β1–Smad and TNF receptor-associated factor 6–NF-κB signaling pathways (82, 132). These results suggest that miR-146a attenuates renal fibrosis by inhibiting profibrotic and inflammatory signaling pathways and might be another therapeutic target for the treatment of renal fibrosis (82). On the other hand, increased miR-146a expression was observed in kidney samples obtained from patients with DN (46). Moreover, upregulation of miR-146a in the kidney of streptozotocin-induced diabetic rats was not accompanied by downregulation of inflammatory mediators, suggesting a possible defect in the miR-146a-mediated inhibitory loop in diabetes that allows for sustained activation of NF-κB (4).
Recently, Morizane et al. (83) demonstrated that overexpression of miR-34c decreased renal fibrosis and expression of α-SMA, CTGF, collagen types I and III, and fibronectin in mice with UUO. Additionally, they found that miR-34c overexpression attenuates EMT induced by TGF-β in a mouse tubular cell line. They speculated that miR-34c ameliorated renal fibrosis through suppression of the Notch/Jag1 pathway, although a direct relationship between miR-34c and Jag1 was not demonstrated (83). MiR-34c might be a novel target for the treatment of renal fibrosis as well.
In mouse MCs, TGF-β1 increased expression of Col1a2 associated with the upregulation of miR-216a (55). This effect was mediated by posttranscriptional upregulation of Tsc22 due to the alleviation of the inhibitory actions of the RNA-binding protein Ybx1 in P-bodies. Ybx1 was found to be a specific target of miR-216a. Together with the concurrent removal of the inhibitory actions of Zeb1 on the Col1a2 promoter, the interaction of Tsc22 with transcription factor E3 (Tfe3) increases Col1a2 expression in diabetes. In accordance with this model, an increase in Tsc22 was observed in the glomeruli of diabetic db/db mice (55).
UUO-induced renal interstitial fibrosis in mice has been shown to be associated with the upregulation of miR-382, which targets kallikrein 5, an enzyme involved in the degradation of several ECM proteins (60). Intravenous delivery of an anti-miR-382 prevented the decrease of kallikrein 5 protein expression and attenuated inner medullary fibrosis. Moreover, the protective effects of anti-miR-382 treatment were eliminated by knockdown of renal kallikrein 5. Previous work provided evidence that miR-382 is upregulated by TGF-β1 in human renal epithelial cells and contributes to EMT (59). This action of miR-382 was linked to downregulation of superoxide dismutase 2 (SOD2). Thus, more than one process underlies the profibrotic actions of miR-382 in the kidney.
POTENTIAL OF NOVEL ANTIFIBROTIC STRATEGIES USING MiRNAs
The growing knowledge of the role of miRNAs in the regulation of profibrotic pathways in various renal diseases has driven interest in developing agents that block pathogenic miRNAs or restore levels of renoprotective miRNAs. Nearly all aspects of TGF-β signaling that are implicated in renal fibrosis are regulated by miRNAs as presented in Fig. 2. Growing evidence illustrates that miRNAs are both downstream effectors of TGF-β-dependent renal fibrosis and upstream regulators of TGF-β-dependent signaling (32). Feedback loops between TGF-β and miRNAs have heightened the focus on the use of miRNA-based therapies for the treatment of renal disease.
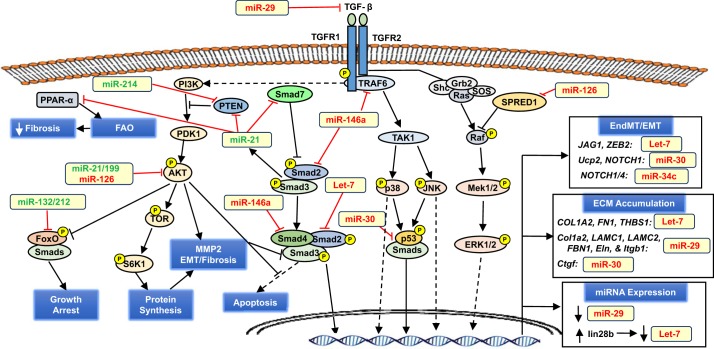
Fig. 2.
Key pro- and antifibrotic miRNAs implicated in transforming growth factor (TGF)-β signaling in the kidney. TGF-β is the central player in the initiation and progression of renal fibrosis by driving endothelial-to-mesangial transition (EndMT) and epithelial-to-mesenchymal transition (EMT), as well as extracellular matrix (ECM) remodeling and protein accumulation. Both canonical (Smad-dependent) and noncanonical (MAPKs and AKT) signaling pathways are involved and targeted by various miRNAs (boldface) that broadly favor or oppose fibrosis; however, given their broad range of gene targets, it is unlikely that a particular miRNA is strictly anti- or profibrotic. Plus, the same gene target may have conflicting actions. Although Zeb is implicated in the EMT program via in part the repression of E-cadherin, Zeb is also involved in repressing TGF-β expression. Conflicting actions are also reported for the ERK signaling cascade, which is involved in EndMT/EMT but also inhibits receptor Smads. In the text box, the human gene is shown where validated in human or in both human and rodent. Antifibrotic miRNAs are colored red; profibrotic miRNAs are colored green. See the text for additional details.
Due to their distinct features, such as short sequence and homology across species, mature miRNAs are attractive candidates as potential therapeutic agents. In vivo manipulation of the activity of specific miRNAs in the kidney has been achieved by the delivery of inhibitors to block miRNA function or mimics to restore miRNA levels. Many miRNAs are activated by stress and appear to be inactive in healthy cells, which provides a therapeutic advantage for developing safe therapies that target miRNAs. RNA oligonucleotides distribute widely throughout the body and access intracellular compartments. However, anti-miRNA oligonucleotides accumulate in the kidney and liver following systemic delivery, making them ideal for treating renal fibrotic disease. Interestingly, healthy glomeruli do not take up anti-miRNA oligonucleotides, but podocytes and other cell types in injured glomeruli do, indicating that antioligonucleotide therapy may benefit glomerular as well as tubulointerstitial diseases of the kidney (77).
Many obstacles, including safety issues and delivery methods, must be overcome before miRNA-based therapies for CKD can be translated into clinical practice. Ideally, the target miRNA should be kidney specific to avoid adverse effects in other tissues and organs. To minimize side effects, the treatment should affect only one cellular target (or targets acting in the same pathway). Recent studies have identified the targets of particular miRNAs in cells and tissues by a variety of techniques, such as precipitating RNA-induced silencing complexes (RISCs) that incorporate a specific miRNA have shown that one miRNA frequently has >100 targets (43). Commonly, many of these other targets are modestly silenced by the miRNA, although many target genes are functionally related.
Parallel with the discovery of RNA-based gene silencing was the development of anti-miRNA oligonucleotides capable of entering cells after systemic administration to suppress translation. Similar efforts have been made to generate miRNA mimics, which are double-stranded RNA molecules that separate intracellularly, with one strand loading into the RISC and functioning as a miRNA. Though miRNAs are generally stable with a long half-life, individual miRNAs can undergo rapid decay in specific cellular contexts (110). Several advances have been made to enhance RNA stability in vivo by molecular modification of the backbone (43). These modifications include replacing the phosphodiester backbone with a phosphorothioate backbone and the use of 2′-O-methyl RNA molecules and locked bicyclic nucleic acids. Also, 2′-O-methoxyethyl (MOE) and 2-oxy-methyl (OMe) modifications enhance the affinity for complementary nucleotides. Another strategy is to use three strands of RNA with two having locked nucleic acid (LNA) technology for enhanced stability. Modification of the native RNA backbone markedly reduces the type I interferon-mediated immune response cells have to extracellular RNA or DNA (77).
Although such mimics have been widely used in cultured cells, their utilization as drug candidates has been hindered by delivery and selectivity for the target organ. These limitations have been partially overcome as summarized in Fig. 3 by targeted administration or by using vectors containing kidney-specific and inducible promoters (110). Delivery of antisense or nucleic acid-based therapeutics might also benefit from nanocarrier-based delivery systems, as hydrophilic agents typically cannot cross cellular or organelle membranes. Ultrasound-microbubble-mediated gene transfer has also been used to deliver plasmids that produce various miRNAs to mammalian kidneys. High-intensity ultrasound causes microbubble oscillation, resulting in the release of the therapeutic agent(s) attached to or contained within microbubbles (36, 100).
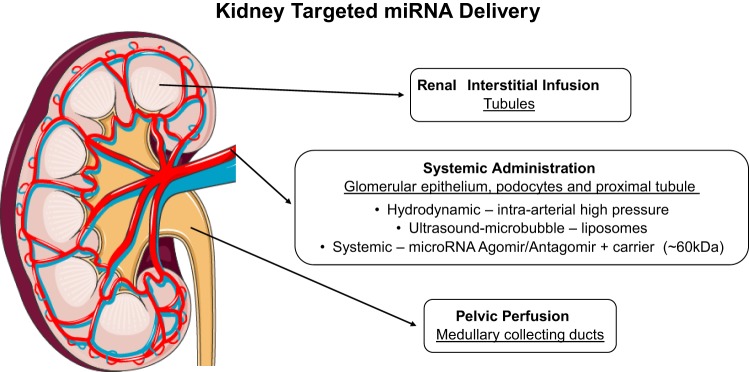
Fig. 3.
Delivery strategies for intrarenal targeting of miRNAs involving direct renal interstitial infusion, pelvic perfusion, and systemic delivery. (Kidney image adapted and reproduced with permission from the copyright holder http://servier.com).
In addition to anti-miRNAs, miRNA inhibition can be attained by expressing miRNA-target sequences able to capture pathogenic miRNAs (miRNA sponges) or by short hairpin RNA plasmids that nullify miRNA expression by RNA interference (110). Effective knockdown can also be achieved using oligonucleotides complementary to the 3′-UTR of the target mRNAs (masking approach) or to the sequence of the miRNA themselves (erasers). Many studies are now investigating the potential of miRNA targets selected on the basis of their known function in fibrosis or identification as targets genome-wide association studies of kidney disease to intervene in disease progression.
Miravirsen is an antimiR-122 for hepatitis C virus infection and the first miRNA-targeted drug to enter a phase II clinical trial (48, 65, 108). It is an LNA-modified DNA phosphorothioate antisense oligonucleotide, which acts by sequestering mature miR-122 in a stable heteroduplex. However, so far the potential for targeting miRNAs as effective antifibrotic therapy has been demonstrated only in experimental models of CKD. As most miRNAs are highly pleiotropic and act differently depending on cell type, a single miRNA may not be able to reverse the process of renal fibrosis. Rather, a complex of miRNAs or anti-miRNAs targeting different key points in the fibrotic process may prove more effective.
In conclusion, in vivo delivery of miRNA mimics or inhibitors has emerged as a promising therapeutic strategy for the treatment of CKD. Successful kidney transfection has been achieved in animal studies by intraperitoneal, intravenous, or subcutaneous injection of either mimics or inhibitors or, more frequently, by intravenous injection of plasmids expressing miRNAs or short-hairpin RNAs (110). It is likely that several agents targeting various miRNAs will enter clinical trials over the next few years and new renoprotective therapeutic agents based on this approach will enter clinical practice in the next decade.
GRANTS
This work was supported in part by National Institutes of Health grants HL-36279 (R. J. Roman) and DK-104184 (R. J. Roman), AG-050049 (F. Fan), P20GM-104357 (cores B and C, R. J. Roman; Pilot, F. Fan); American Heart Association Grant 16GRNT31200036 (F. Fan); and National Natural Science Foundation of China Fellowship Grants 81270939, 81472983, and 81571625 (W. Lv).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.L. drafted manuscript; W.L., F.F., Y.W., E.G.-F., C.W., L.Y., G.W.B., and R.J.R. approved final version of manuscript; F.F., Y.W., E.G.-F., C.W., L.Y., G.W.B., and R.J.R. edited and revised manuscript.
REFERENCES
- 1.Ahn SM, Cha JY, Kim J, Kim D, Trang HT, Kim YM, Cho YH, Park D, Hong S. Smad3 regulates E-cadherin via miRNA-200 pathway. Oncogene 31: 3051–3059, 2012. doi: 10.1038/onc.2011.484. [DOI] [PubMed] [Google Scholar]
- 2.Al-Kafaji G, Al-Mahroos G, Al-Muhtaresh HA, Skrypnyk C, Sabry MA, Ramadan AR. Decreased expression of circulating microRNA-126 in patients with type 2 diabetic nephropathy: A potential blood-based biomarker. Exp Ther Med 12: 815–822, 2016. doi: 10.3892/etm.2016.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alicic RZ, Tuttle KR. Novel therapies for diabetic kidney disease. Adv Chronic Kidney Dis 21: 121–133, 2014. doi: 10.1053/j.ackd.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Alipour MR, Khamaneh AM, Yousefzadeh N, Mohammad-nejad D, Soufi FG. Upregulation of microRNA-146a was not accompanied by downregulation of pro-inflammatory markers in diabetic kidney. Mol Biol Rep 40: 6477–6483, 2013. doi: 10.1007/s11033-013-2763-4. [DOI] [PubMed] [Google Scholar]
- 5.Bao H, Hu S, Zhang C, Shi S, Qin W, Zeng C, Zen K, Liu Z. Inhibition of miRNA-21 prevents fibrogenic activation in podocytes and tubular cells in IgA nephropathy. Biochem Biophys Res Commun 444: 455–460, 2014. doi: 10.1016/j.bbrc.2014.01.065. [DOI] [PubMed] [Google Scholar]
- 6.Benlhabib H, Guo W, Pierce BM, Mendelson CR. The miR-200 family and its targets regulate type II cell differentiation in human fetal lung. J Biol Chem 290: 22409–22422, 2015. doi: 10.1074/jbc.M114.636068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bijkerk R, de Bruin RG, van Solingen C, van Gils JM, Duijs JM, van der Veer EP, Rabelink TJ, Humphreys BD, van Zonneveld AJ. Silencing of microRNA-132 reduces renal fibrosis by selectively inhibiting myofibroblast proliferation. Kidney Int 89: 1268–1280, 2016. doi: 10.1016/j.kint.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 8.Bijkerk R, van Solingen C, de Boer HC, van der Pol P, Khairoun M, de Bruin RG, van Oeveren-Rietdijk AM, Lievers E, Schlagwein N, van Gijlswijk DJ, Roeten MK, Neshati Z, de Vries AA, Rodijk M, Pike-Overzet K, van den Berg YW, van der Veer EP, Versteeg HH, Reinders ME, Staal FJ, van Kooten C, Rabelink TJ, van Zonneveld AJ. Hematopoietic microRNA-126 protects against renal ischemia/reperfusion injury by promoting vascular integrity. J Am Soc Nephrol 25: 1710–1722, 2014. doi: 10.1681/ASN.2013060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bracken CP, Khew-Goodall Y, Goodall GJ. Network-Based Approaches to Understand the Roles of miR-200 and Other microRNAs in Cancer. Cancer Res 75: 2594–2599, 2015. doi: 10.1158/0008-5472.CAN-15-0287. [DOI] [PubMed] [Google Scholar]
- 10.Brennan EP, Nolan KA, Börgeson E, Gough OS, McEvoy CM, Docherty NG, Higgins DF, Murphy M, Sadlier DM, Ali-Shah ST, Guiry PJ, Savage DA, Maxwell AP, Martin F, Godson C; GENIE Consortium . Lipoxins attenuate renal fibrosis by inducing let-7c and suppressing TGFβR1. J Am Soc Nephrol 24: 627–637, 2013. doi: 10.1681/ASN.2012060550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bridge G, Monteiro R, Henderson S, Emuss V, Lagos D, Georgopoulou D, Patient R, Boshoff C. The microRNA-30 family targets DLL4 to modulate endothelial cell behavior during angiogenesis. Blood 120: 5063–5072, 2012. doi: 10.1182/blood-2012-04-423004. [DOI] [PubMed] [Google Scholar]
- 12.Cantaluppi V, Gatti S, Medica D, Figliolini F, Bruno S, Deregibus MC, Sordi A, Biancone L, Tetta C, Camussi G. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int 82: 412–427, 2012. doi: 10.1038/ki.2012.105. [DOI] [PubMed] [Google Scholar]
- 13.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res 65: 6029–6033, 2005. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 14.Chau BN, Xin C, Hartner J, Ren S, Castano AP, Linn G, Li J, Tran PT, Kaimal V, Huang X, Chang AN, Li S, Kalra A, Grafals M, Portilla D, MacKenna DA, Orkin SH, Duffield JS. MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci Transl Med 4: 121ra18, 2012. doi: 10.1126/scitranslmed.3003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen BF, Suen YK, Gu S, Li L, Chan WY. A miR-199a/miR-214 self-regulatory network via PSMD10, TP53 and DNMT1 in testicular germ cell tumor. Sci Rep 4: 6413, 2014. doi: 10.1038/srep06413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen HY, Zhong X, Huang XR, Meng XM, You Y, Chung AC, Lan HY. MicroRNA-29b inhibits diabetic nephropathy in db/db mice. Mol Ther 22: 842–853, 2014. doi: 10.1038/mt.2013.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Zmijewska A, Zhi D, Mannon RB. Cyclosporine-mediated allograft fibrosis is associated with micro-RNA-21 through AKT signaling. Transpl Int 28: 232–245, 2015. doi: 10.1111/tri.12471. [DOI] [PubMed] [Google Scholar]
- 18.Chen L, Wang J, Wang B, Yang J, Gong Z, Zhao X, Zhang C, Du K. MiR-126 inhibits vascular endothelial cell apoptosis through targeting PI3K/Akt signaling. Ann Hematol 95: 365–374, 2016. doi: 10.1007/s00277-015-2567-9. [DOI] [PubMed] [Google Scholar]
- 19.Chen PY, Qin L, Barnes C, Charisse K, Yi T, Zhang X, Ali R, Medina PP, Yu J, Slack FJ, Anderson DG, Kotelianski V, Wang F, Tellides G, Simons M. FGF regulates TGF-β signaling and endothelial-to-mesenchymal transition via control of let-7 miRNA expression. Cell Reports 2: 1684–1696, 2012. doi: 10.1016/j.celrep.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X, Song M, Chen W, Dimitrova-Shumkovska J, Zhao Y, Cao Y, Song Y, Yang W, Wang F, Xiang Y, Yang C. MicroRNA-21 Contributes to Liver Regeneration by Targeting PTEN. Med Sci Monit 22: 83–91, 2016. doi: 10.12659/MSM.896157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng X, Gao W, Dang Y, Liu X, Li Y, Peng X, Ye X. Both ERK/MAPK and TGF-Beta/Smad signaling pathways play a role in the kidney fibrosis of diabetic mice accelerated by blood glucose fluctuation. J Diabetes Res 2013: 463740, 2013. doi: 10.1155/2013/463740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiang Y, Zhou X, Wang Z, Song Y, Liu Z, Zhao F, Zhu J, Xu H. Expression levels of microRNA-192 and -215 in gastric carcinoma. Pathol Oncol Res 18: 585–591, 2012. doi: 10.1007/s12253-011-9480-x. [DOI] [PubMed] [Google Scholar]
- 23.Chou CH, Chang NW, Shrestha S, Hsu SD, Lin YL, Lee WH, Yang CD, Hong HC, Wei TY, Tu SJ, Tsai TR, Ho SY, Jian TY, Wu HY, Chen PR, Lin NC, Huang HT, Yang TL, Pai CY, Tai CS, Chen WL, Huang CY, Liu CC, Weng SL, Liao KW, Hsu WL, Huang HD. miRTarBase 2016: updates to the experimentally validated miRNA-target interactions database. Nucleic Acids Res 44, D1: D239–D247, 2016. doi: 10.1093/nar/gkv1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung AC, Huang XR, Meng X, Lan HY. miR-192 mediates TGF-beta/Smad3-driven renal fibrosis. J Am Soc Nephrol 21: 1317–1325, 2010. doi: 10.1681/ASN.2010020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung AC, Lan HY. MicroRNAs in renal fibrosis. Front Physiol 6: 50, 2015. doi: 10.3389/fphys.2015.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung AC, Yu X, Lan HY. MicroRNA and nephropathy: emerging concepts. Int J Nephrol Renovasc Dis 6: 169–179, 2013. doi: 10.2147/IJNRD.S37885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature 454: 56–61, 2008. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. Smad proteins bind a conserved RNA sequence to promote microRNA maturation by Drosha. Mol Cell 39: 373–384, 2010. doi: 10.1016/j.molcel.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Declèves AE, Sharma K. Novel targets of antifibrotic and anti-inflammatory treatment in CKD. Nat Rev Nephrol 10: 257–267, 2014. doi: 10.1038/nrneph.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denby L, Ramdas V, Lu R, Conway BR, Grant JS, Dickinson B, Aurora AB, McClure JD, Kipgen D, Delles C, van Rooij E, Baker AH. MicroRNA-214 antagonism protects against renal fibrosis. J Am Soc Nephrol 25: 65–80, 2014. doi: 10.1681/ASN.2013010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deshpande SD, Putta S, Wang M, Lai JY, Bitzer M, Nelson RG, Lanting LL, Kato M, Natarajan R. Transforming growth factor-β-induced cross talk between p53 and a microRNA in the pathogenesis of diabetic nephropathy. Diabetes 62: 3151–3162, 2013. doi: 10.2337/db13-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dey N, Ghosh-Choudhury N, Kasinath BS, Choudhury GG. TGFβ-stimulated microRNA-21 utilizes PTEN to orchestrate AKT/mTORC1 signaling for mesangial cell hypertrophy and matrix expansion. PLoS One 7: e42316, 2012. doi: 10.1371/journal.pone.0042316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du X, Shimizu A, Masuda Y, Kuwahara N, Arai T, Kataoka M, Uchiyama M, Kaneko T, Akimoto T, Iino Y, Fukuda Y. Involvement of matrix metalloproteinase-2 in the development of renal interstitial fibrosis in mouse obstructive nephropathy. Lab Invest 92: 1149–1160, 2012. doi: 10.1038/labinvest.2012.68. [DOI] [PubMed] [Google Scholar]
- 34.Duisters RF, Tijsen AJ, Schroen B, Leenders JJ, Lentink V, van der Made I, Herias V, van Leeuwen RE, Schellings MW, Barenbrug P, Maessen JG, Heymans S, Pinto YM, Creemers EE. miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling. Circ Res 104: 170–178, 2009. doi: 10.1161/CIRCRESAHA.108.182535. [DOI] [PubMed] [Google Scholar]
- 35.Eskildsen TV, Jeppesen PL, Schneider M, Nossent AY, Sandberg MB, Hansen PB, Jensen CH, Hansen ML, Marcussen N, Rasmussen LM, Bie P, Andersen DC, Sheikh SP. Angiotensin II regulates microRNA-132/-212 in hypertensive rats and humans. Int J Mol Sci 14: 11190–11207, 2013. doi: 10.3390/ijms140611190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Falke LL, Gholizadeh S, Goldschmeding R, Kok RJ, Nguyen TQ. Diverse origins of the myofibroblast—implications for kidney fibrosis. Nat Rev Nephrol 11: 233–244, 2015. doi: 10.1038/nrneph.2014.246. [DOI] [PubMed] [Google Scholar]
- 37.Falke LL, van Vuuren SH, Kazazi-Hyseni F, Ramazani F, Nguyen TQ, Veldhuis GJ, Maarseveen EM, Zandstra J, Zuidema J, Duque LF, Steendam R, Popa ER, Kok RJ, Goldschmeding R. Local therapeutic efficacy with reduced systemic side effects by rapamycin-loaded subcapsular microspheres. Biomaterials 42: 151–160, 2015. doi: 10.1016/j.biomaterials.2014.11.042. [DOI] [PubMed] [Google Scholar]
- 38.Fang Y, Yu X, Liu Y, Kriegel AJ, Heng Y, Xu X, Liang M, Ding X. miR-29c is downregulated in renal interstitial fibrosis in humans and rats and restored by HIF-α activation. Am J Physiol Renal Physiol 304: F1274–F1282, 2013. doi: 10.1152/ajprenal.00287.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feng Y, LoGrasso PV, Defert O, Li R. Rho Kinase (ROCK) Inhibitors and Their Therapeutic Potential. J Med Chem 59: 2269–2300, 2016. doi: 10.1021/acs.jmedchem.5b00683. [DOI] [PubMed] [Google Scholar]
- 40.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell 15: 272–284, 2008. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frost RJ, Olson EN. Control of glucose homeostasis and insulin sensitivity by the Let-7 family of microRNAs. Proc Natl Acad Sci USA 108: 21075–21080, 2011. doi: 10.1073/pnas.1118922109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gomez IG, MacKenna DA, Johnson BG, Kaimal V, Roach AM, Ren S, Nakagawa N, Xin C, Newitt R, Pandya S, Xia TH, Liu X, Borza DB, Grafals M, Shankland SJ, Himmelfarb J, Portilla D, Liu S, Chau BN, Duffield JS. Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J Clin Invest 125: 141–156, 2015. doi: 10.1172/JCI75852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomez IG, Nakagawa N, Duffield JS. MicroRNAs as novel therapeutic targets to treat kidney injury and fibrosis. Am J Physiol Renal Physiol 310: F931–F944, 2016. doi: 10.1152/ajprenal.00523.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 10: 593–601, 2008. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 45.Hallan SI, Øvrehus MA, Romundstad S, Rifkin D, Langhammer A, Stevens PE, Ix JH. Long-term trends in the prevalence of chronic kidney disease and the influence of cardiovascular risk factors in Norway. Kidney Int 90: 665–673, 2016. doi: 10.1016/j.kint.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 46.Huang Y, Liu Y, Li L, Su B, Yang L, Fan W, Yin Q, Chen L, Cui T, Zhang J, Lu Y, Cheng J, Fu P, Liu F. Involvement of inflammation-related miR-155 and miR-146a in diabetic nephropathy: implications for glomerular endothelial injury. BMC Nephrol 15: 142, 2014. doi: 10.1186/1471-2369-15-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jaber BL, Madias NE. Progression of chronic kidney disease: can it be prevented or arrested? Am J Med 118: 1323–1330, 2005. doi: 10.1016/j.amjmed.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 48.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, Persson R, King BD, Kauppinen S, Levin AA, Hodges MR. Treatment of HCV infection by targeting microRNA. N Engl J Med 368: 1685–1694, 2013. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 49.Jenkins RH, Martin J, Phillips AO, Bowen T, Fraser DJ. Pleiotropy of microRNA-192 in the kidney. Biochem Soc Trans 40: 762–767, 2012. doi: 10.1042/BST20120085. [DOI] [PubMed] [Google Scholar]
- 50.Jenkins RH, Martin J, Phillips AO, Bowen T, Fraser DJ. Transforming growth factor β1 represses proximal tubular cell microRNA-192 expression through decreased hepatocyte nuclear factor DNA binding. Biochem J 443: 407–416, 2012. doi: 10.1042/BJ20111861. [DOI] [PubMed] [Google Scholar]
- 51.Jiang L, Qiu W, Zhou Y, Wen P, Fang L, Cao H, Zen K, He W, Zhang C, Dai C, Yang J. A microRNA-30e/mitochondrial uncoupling protein 2 axis mediates TGF-β1-induced tubular epithelial cell extracellular matrix production and kidney fibrosis. Kidney Int 84: 285–296, 2013. doi: 10.1038/ki.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin Z, Selaru FM, Cheng Y, Kan T, Agarwal R, Mori Y, Olaru AV, Yang J, David S, Hamilton JP, Abraham JM, Harmon J, Duncan M, Montgomery EA, Meltzer SJ. MicroRNA-192 and -215 are upregulated in human gastric cancer in vivo and suppress ALCAM expression in vitro. Oncogene 30: 1577–1585, 2011. doi: 10.1038/onc.2010.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kato M, Arce L, Wang M, Putta S, Lanting L, Natarajan R. A microRNA circuit mediates transforming growth factor-β1 autoregulation in renal glomerular mesangial cells. Kidney Int 80: 358–368, 2011. doi: 10.1038/ki.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kato M, Dang V, Wang M, Park JT, Deshpande S, Kadam S, Mardiros A, Zhan Y, Oettgen P, Putta S, Yuan H, Lanting L, Natarajan R. TGF-β induces acetylation of chromatin and of Ets-1 to alleviate repression of miR-192 in diabetic nephropathy. Sci Signal 6: ra43, 2013. doi: 10.1126/scisignal.2003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kato M, Wang L, Putta S, Wang M, Yuan H, Sun G, Lanting L, Todorov I, Rossi JJ, Natarajan R. Post-transcriptional up-regulation of Tsc-22 by Ybx1, a target of miR-216a, mediates TGF-beta-induced collagen expression in kidney cells. J Biol Chem 285: 34004–34015, 2010. doi: 10.1074/jbc.M110.165027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kato M, Zhang J, Wang M, Lanting L, Yuan H, Rossi JJ, Natarajan R. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci USA 104: 3432–3437, 2007. doi: 10.1073/pnas.0611192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khwaja A, El Kossi M, Floege J, El Nahas M. The management of CKD: a look into the future. Kidney Int 72: 1316–1323, 2007. doi: 10.1038/sj.ki.5002489. [DOI] [PubMed] [Google Scholar]
- 58.Kok HM, Falke LL, Goldschmeding R, Nguyen TQ. Targeting CTGF, EGF and PDGF pathways to prevent progression of kidney disease. Nat Rev Nephrol 10: 700–711, 2014. doi: 10.1038/nrneph.2014.184. [DOI] [PubMed] [Google Scholar]
- 59.Kriegel AJ, Fang Y, Liu Y, Tian Z, Mladinov D, Matus IR, Ding X, Greene AS, Liang M. MicroRNA-target pairs in human renal epithelial cells treated with transforming growth factor beta 1: a novel role of miR-382. Nucleic Acids Res 38: 8338–8347, 2010. doi: 10.1093/nar/gkq718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kriegel AJ, Liu Y, Cohen B, Usa K, Liu Y, Liang M. MiR-382 targeting of kallikrein 5 contributes to renal inner medullary interstitial fibrosis. Physiol Genomics 44: 259–267, 2012. doi: 10.1152/physiolgenomics.00173.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 11: 597–610, 2010. [DOI] [PubMed] [Google Scholar]
- 62.Krupa A, Jenkins R, Luo DD, Lewis A, Phillips A, Fraser D. Loss of MicroRNA-192 promotes fibrogenesis in diabetic nephropathy. J Am Soc Nephrol 21: 438–447, 2010. doi: 10.1681/ASN.2009050530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lai JY, Luo J, O’Connor C, Jing X, Nair V, Ju W, Randolph A, Ben-Dov IZ, Matar RN, Briskin D, Zavadil J, Nelson RG, Tuschl T, Brosius FC III, Kretzler M, Bitzer M. MicroRNA-21 in glomerular injury. J Am Soc Nephrol 26: 805–816, 2015. doi: 10.1681/ASN.2013121274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lan W, Chen S, Tong L. MicroRNA-215 Regulates Fibroblast Function: Insights from a Human Fibrotic Disease. Cell Cycle 14: 1973–1984, 2015. doi: 10.1080/15384101.2014.998077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, Kauppinen S, Ørum H. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science 327: 198–201, 2010. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee SY, Kim SI, Choi ME. Therapeutic targets for treating fibrotic kidney diseases. Transl Res 165: 512–530, 2015. doi: 10.1016/j.trsl.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li C, Song L, Zhang Z, Bai XX, Cui MF, Ma LJ. MicroRNA-21 promotes TGF-β1-induced epithelial-mesenchymal transition in gastric cancer through up-regulating PTEN expression. Oncotarget 7: 66989–67003, 2016. doi: 10.18632/oncotarget.11888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li L, Chen XP, Li YJ. MicroRNA-146a and human disease. Scand J Immunol 71: 227–231, 2010. doi: 10.1111/j.1365-3083.2010.02383.x. [DOI] [PubMed] [Google Scholar]
- 69.Li LM, Hou DX, Guo YL, Yang JW, Liu Y, Zhang CY, Zen K. Role of microRNA-214-targeting phosphatase and tensin homolog in advanced glycation end product-induced apoptosis delay in monocytes. J Immunol 186: 2552–2560, 2011. doi: 10.4049/jimmunol.1001633. [DOI] [PubMed] [Google Scholar]
- 70.Li R, Chung AC, Dong Y, Yang W, Zhong X, Lan HY. The microRNA miR-433 promotes renal fibrosis by amplifying the TGF-β/Smad3-Azin1 pathway. Kidney Int 84: 1129–1144, 2013. doi: 10.1038/ki.2013.272. [DOI] [PubMed] [Google Scholar]
- 71.Lin CL, Lee PH, Hsu YC, Lei CC, Ko JY, Chuang PC, Huang YT, Wang SY, Wu SL, Chen YS, Chiang WC, Reiser J, Wang FS. MicroRNA-29a promotion of nephrin acetylation ameliorates hyperglycemia-induced podocyte dysfunction. J Am Soc Nephrol 25: 1698–1709, 2014. doi: 10.1681/ASN.2013050527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lino Cardenas CL, Henaoui IS, Courcot E, Roderburg C, Cauffiez C, Aubert S, Copin MC, Wallaert B, Glowacki F, Dewaeles E, Milosevic J, Maurizio J, Tedrow J, Marcet B, Lo-Guidice JM, Kaminski N, Barbry P, Luedde T, Perrais M, Mari B, Pottier N. miR-199a-5p Is upregulated during fibrogenic response to tissue injury and mediates TGFbeta-induced lung fibroblast activation by targeting caveolin-1. PLoS Genet 9: e1003291, 2013. doi: 10.1371/journal.pgen.1003291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu XJ, Hong Q, Wang Z, Yu YY, Zou X, Xu LH. MicroRNA21 promotes interstitial fibrosis via targeting DDAH1: a potential role in renal fibrosis. Mol Cell Biochem 411: 181–189, 2016. doi: 10.1007/s11010-015-2580-2. [DOI] [PubMed] [Google Scholar]
- 74.Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 7: 684–696, 2011. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y, Taylor NE, Lu L, Usa K, Cowley AW Jr, Ferreri NR, Yeo NC, Liang M. Renal medullary microRNAs in Dahl salt-sensitive rats: miR-29b regulates several collagens and related genes. Hypertension 55: 974–982, 2010. doi: 10.1161/HYPERTENSIONAHA.109.144428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Long J, Wang Y, Wang W, Chang BH, Danesh FR. MicroRNA-29c is a signature microRNA under high glucose conditions that targets Sprouty homolog 1, and its in vivo knockdown prevents progression of diabetic nephropathy. J Biol Chem 286: 11837–11848, 2011. doi: 10.1074/jbc.M110.194969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lv G, Shao S, Dong H, Bian X, Yang X, Dong S. MicroRNA-214 protects cardiac myocytes against H2O2-induced injury. J Cell Biochem 115: 93–101, 2014. doi: 10.1002/jcb.24636. [DOI] [PubMed] [Google Scholar]
- 78.Lv LL, Cao YH, Ni HF, Xu M, Liu D, Liu H, Chen PS, Liu BC. MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. Am J Physiol Renal Physiol 305: F1220–F1227, 2013. doi: 10.1152/ajprenal.00148.2013. [DOI] [PubMed] [Google Scholar]
- 79.Macconi D, Tomasoni S, Romagnani P, Trionfini P, Sangalli F, Mazzinghi B, Rizzo P, Lazzeri E, Abbate M, Remuzzi G, Benigni A. MicroRNA-324-3p promotes renal fibrosis and is a target of ACE inhibition. J Am Soc Nephrol 23: 1496–1505, 2012. doi: 10.1681/ASN.2011121144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Malaga-Dieguez L, Bouhassira D, Gipson D, Trachtman H. Novel therapies for FSGS: preclinical and clinical studies. Adv Chronic Kidney Dis 22: e1–e6, 2015. doi: 10.1053/j.ackd.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McClelland AD, Herman-Edelstein M, Komers R, Jha JC, Winbanks CE, Hagiwara S, Gregorevic P, Kantharidis P, Cooper ME. miR-21 promotes renal fibrosis in diabetic nephropathy by targeting PTEN and SMAD7. Clin Sci (Lond) 129: 1237–1249, 2015. doi: 10.1042/CS20150427. [DOI] [PubMed] [Google Scholar]
- 82.Morishita Y, Imai T, Yoshizawa H, Watanabe M, Ishibashi K, Muto S, Nagata D. Delivery of microRNA-146a with polyethylenimine nanoparticles inhibits renal fibrosis in vivo. Int J Nanomedicine 10: 3475–3488, 2015. doi: 10.2147/IJN.S82587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morizane R, Fujii S, Monkawa T, Hiratsuka K, Yamaguchi S, Homma K, Itoh H. miR-34c attenuates epithelial-mesenchymal transition and kidney fibrosis with ureteral obstruction. Sci Rep 4: 4578, 2014. doi: 10.1038/srep04578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murphy D, McCulloch CE, Lin F, Banerjee T, Bragg-Gresham JL, Eberhardt MS, Morgenstern H, Pavkov ME, Saran R, Powe NR, Hsu CY; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team . Trends in Prevalence of Chronic Kidney Disease in the United States. Ann Intern Med 165: 473–481, 2016. doi: 10.7326/M16-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murphy SR, Dahly-Vernon AJ, Dunn KM, Chen CC, Ledbetter SR, Williams JM, Roman RJ. Renoprotective effects of anti-TGF-β antibody and antihypertensive therapies in Dahl S rats. Am J Physiol Regul Integr Comp Physiol 303: R57–R69, 2012. doi: 10.1152/ajpregu.00263.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nagai T, Kanasaki M, Srivastava SP, Nakamura Y, Ishigaki Y, Kitada M, Shi S, Kanasaki K, Koya D. N-acetyl-seryl-aspartyl-lysyl-proline inhibits diabetes-associated kidney fibrosis and endothelial-mesenchymal transition. BioMed Res Int 2014: 696475, 2014. doi: 10.1155/2014/696475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nikolic I, Plate KH, Schmidt MH. EGFL7 meets miRNA-126: an angiogenesis alliance. J Angiogenes Res 2: 9, 2010. doi: 10.1186/2040-2384-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nitta K, Shi S, Nagai T, Kanasaki M, Kitada M, Srivastava SP, Haneda M, Kanasaki K, Koya D. Oral administration of N-acetyl-seryl-aspartyl-lysyl-proline ameliorates kidney disease in both type 1 and type 2 diabetic mice via a therapeutic regimen. BioMed Res Int 2016: 9172157, 2016. doi: 10.1155/2016/9172157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oba S, Kumano S, Suzuki E, Nishimatsu H, Takahashi M, Takamori H, Kasuya M, Ogawa Y, Sato K, Kimura K, Homma Y, Hirata Y, Fujita T. miR-200b precursor can ameliorate renal tubulointerstitial fibrosis. PLoS One 5: e13614, 2010. doi: 10.1371/journal.pone.0013614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oh J, Takahashi R, Kondo S, Mizoguchi A, Adachi E, Sasahara RM, Nishimura S, Imamura Y, Kitayama H, Alexander DB, Ide C, Horan TP, Arakawa T, Yoshida H, Nishikawa S, Itoh Y, Seiki M, Itohara S, Takahashi C, Noda M. The membrane-anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell 107: 789–800, 2001. doi: 10.1016/S0092-8674(01)00597-9. [DOI] [PubMed] [Google Scholar]
- 91.Park JT, Kato M, Lanting L, Castro N, Nam BY, Wang M, Kang SW, Natarajan R. Repression of let-7 by transforming growth factor-β1-induced Lin28 upregulates collagen expression in glomerular mesangial cells under diabetic conditions. Am J Physiol Renal Physiol 307: F1390–F1403, 2014. doi: 10.1152/ajprenal.00458.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park JT, Kato M, Yuan H, Castro N, Lanting L, Wang M, Natarajan R. FOG2 protein down-regulation by transforming growth factor-β1-induced microRNA-200b/c leads to Akt kinase activation and glomerular mesangial hypertrophy related to diabetic nephropathy. J Biol Chem 288: 22469–22480, 2013. doi: 10.1074/jbc.M113.453043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Patel V, Noureddine L. MicroRNAs and fibrosis. Curr Opin Nephrol Hypertens 21: 410–416, 2012. doi: 10.1097/MNH.0b013e328354e559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Putta S, Lanting L, Sun G, Lawson G, Kato M, Natarajan R. Inhibiting microRNA-192 ameliorates renal fibrosis in diabetic nephropathy. J Am Soc Nephrol 23: 458–469, 2012. doi: 10.1681/ASN.2011050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qin W, Chung AC, Huang XR, Meng XM, Hui DS, Yu CM, Sung JJ, Lan HY. TGF-β/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J Am Soc Nephrol 22: 1462–1474, 2011. doi: 10.1681/ASN.2010121308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Remenyi J, Hunter CJ, Cole C, Ando H, Impey S, Monk CE, Martin KJ, Barton GJ, Hutvagner G, Arthur JS. Regulation of the miR-212/132 locus by MSK1 and CREB in response to neurotrophins. Biochem J 428: 281–291, 2010. doi: 10.1042/BJ20100024. [DOI] [PubMed] [Google Scholar]
- 97.Schober A, Nazari-Jahantigh M, Wei Y, Bidzhekov K, Gremse F, Grommes J, Megens RT, Heyll K, Noels H, Hristov M, Wang S, Kiessling F, Olson EN, Weber C. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med 20: 368–376, 2014. doi: 10.1038/nm.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shi S, Yu L, Zhang T, Qi H, Xavier S, Ju W, Bottinger E. Smad2-dependent downregulation of miR-30 is required for TGF-β-induced apoptosis in podocytes. PLoS One 8: e75572, 2013. doi: 10.1371/journal.pone.0075572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shin HY, Kang HT. Recent trends in the prevalence of chronic kidney disease in Korean adults: Korean National Health and Nutrition Examination Survey from 1998 to 2013. J Nephrol 29: 799–807, 2016. doi: 10.1007/s40620-016-0280-y. [DOI] [PubMed] [Google Scholar]
- 100.Sirsi S, Borden M. Microbubble compositions, properties and biomedical applications. Bubble Sci Eng Technol 1: 3–17, 2009. doi: 10.1179/175889709X446507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stefoni S, Cianciolo G, Baraldi O, Iorio M, Angelini ML. Emerging drugs for chronic kidney disease. Expert Opin Emerg Drugs 19: 183–199, 2014. doi: 10.1517/14728214.2014.900044. [DOI] [PubMed] [Google Scholar]
- 102.Sun L, Zhang D, Liu F, Xiang X, Ling G, Xiao L, Liu Y, Zhu X, Zhan M, Yang Y, Kondeti VK, Kanwar YS. Low-dose paclitaxel ameliorates fibrosis in the remnant kidney model by down-regulating miR-192. J Pathol 225: 364–377, 2011. doi: 10.1002/path.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun X, Jiang S, Liu J, Wang H, Zhang Y, Tang SC, Wang J, Du N, Xu C, Wang C, Qin S, Zhang J, Liu D, Zhang Y, Li X, Wang J, Dong J, Wang X, Xu S, Tao Z, Xu F, Zhou J, Wang T, Ren H. MiR-208a stimulates the cocktail of SOX2 and β-catenin to inhibit the let-7 induction of self-renewal repression of breast cancer stem cells and formed miR208a/let-7 feedback loop via LIN28 and DICER1. Oncotarget 6: 32944–32954, 2015. doi: 10.18632/oncotarget.5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sun Y, Koo S, White N, Peralta E, Esau C, Dean NM, Perera RJ. Development of a micro-array to detect human and mouse microRNAs and characterization of expression in human organs. Nucleic Acids Res 32: e188, 2004. doi: 10.1093/nar/gnh186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tang O, Chen XM, Shen S, Hahn M, Pollock CA. MiRNA-200b represses transforming growth factor-β1-induced EMT and fibronectin expression in kidney proximal tubular cells. Am J Physiol Renal Physiol 304: F1266–F1273, 2013. doi: 10.1152/ajprenal.00302.2012. [DOI] [PubMed] [Google Scholar]
- 106.Tao L, Bei Y, Chen P, Lei Z, Fu S, Zhang H, Xu J, Che L, Chen X, Sluijter JP, Das S, Cretoiu D, Xu B, Zhong J, Xiao J, Li X. Crucial role of miR-433 in regulating cardiac fibrosis. Theranostics 6: 2068–2083, 2016. doi: 10.7150/thno.15007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thornton JE, Chang HM, Piskounova E, Gregory RI. Lin28-mediated control of let-7 microRNA expression by alternative TUTases Zcchc11 (TUT4) and Zcchc6 (TUT7). RNA 18: 1875–1885, 2012. doi: 10.1261/rna.034538.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Titze-de-Almeida R, David C, Titze-de-Almeida SS. The race of 10 synthetic RNAi-based drugs to the pharmaceutical market. Pharm Res 34: 1339–1363, 2017. doi: 10.1007/s11095-017-2134-2. [DOI] [PubMed] [Google Scholar]
- 109.Tolonen AM, Magga J, Szabó Z, Viitala P, Gao E, Moilanen AM, Ohukainen P, Vainio L, Koch WJ, Kerkelä R, Ruskoaho H, Serpi R. Inhibition of Let-7 microRNA attenuates myocardial remodeling and improves cardiac function postinfarction in mice. Pharmacol Res Perspect 2: e00056, 2014. doi: 10.1002/prp2.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Trionfini P, Benigni A, Remuzzi G. MicroRNAs in kidney physiology and disease. Nat Rev Nephrol 11: 23–33, 2015. doi: 10.1038/nrneph.2014.202. [DOI] [PubMed] [Google Scholar]
- 111.Ucar A, Vafaizadeh V, Jarry H, Fiedler J, Klemmt PA, Thum T, Groner B, Chowdhury K. miR-212 and miR-132 are required for epithelial stromal interactions necessary for mouse mammary gland development. Nat Genet 42: 1101–1108, 2010. doi: 10.1038/ng.709. [DOI] [PubMed] [Google Scholar]
- 112.Wakino S, Kanda T, Hayashi K. Rho/Rho kinase as a potential target for the treatment of renal disease. Drug News Perspect 18: 639–643, 2005. doi: 10.1358/dnp.2005.18.10.959578. [DOI] [PubMed] [Google Scholar]
- 113.Wang B, Jha JC, Hagiwara S, McClelland AD, Jandeleit-Dahm K, Thomas MC, Cooper ME, Kantharidis P. Transforming growth factor-β1-mediated renal fibrosis is dependent on the regulation of transforming growth factor receptor 1 expression by let-7b. Kidney Int 85: 352–361, 2014. doi: 10.1038/ki.2013.372. [DOI] [PubMed] [Google Scholar]
- 114.Wang B, Koh P, Winbanks C, Coughlan MT, McClelland A, Watson A, Jandeleit-Dahm K, Burns WC, Thomas MC, Cooper ME, Kantharidis P. miR-200a Prevents renal fibrogenesis through repression of TGF-β2 expression. Diabetes 60: 280–287, 2011. doi: 10.2337/db10-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang J, Gao Y, Ma M, Li M, Zou D, Yang J, Zhu Z, Zhao X. Effect of miR-21 on renal fibrosis by regulating MMP-9 and TIMP1 in kk-ay diabetic nephropathy mice. Cell Biochem Biophys 67: 537–546, 2013. doi: 10.1007/s12013-013-9539-2. [DOI] [PubMed] [Google Scholar]
- 116.Wang LP, Fan SJ, Li SM, Wang XJ, Gao JL, Yang XH. Protective role of ACE2-Ang-(1-7)-Mas in myocardial fibrosis by downregulating KCa3.1 channel via ERK1/2 pathway. Pflugers Arch 468: 2041–2051, 2016. doi: 10.1007/s00424-016-1875-9. [DOI] [PubMed] [Google Scholar]
- 117.Wang S, Li B, Li C, Cui W, Miao L. Potential renoprotective agents through inhibiting CTGF/CCN2 in diabetic nephropathy. J Diabetes Res 2015: 962383, 2015. doi: 10.1155/2015/962383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang V, Vilme H, Maciejewski ML, Boulware LE. The economic burden of chronic kidney disease and end-stage renal disease. Semin Nephrol 36: 319–330, 2016. doi: 10.1016/j.semnephrol.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 119.Wang W, Huang XR, Li AG, Liu F, Li JH, Truong LD, Wang XJ, Lan HY. Signaling mechanism of TGF-beta1 in prevention of renal inflammation: role of Smad7. J Am Soc Nephrol 16: 1371–1383, 2005. doi: 10.1681/ASN.2004121070. [DOI] [PubMed] [Google Scholar]
- 120.Wang X, Shen E, Wang Y, Li J, Cheng D, Chen Y, Gui D, Wang N. Cross talk between miR-214 and PTEN attenuates glomerular hypertrophy under diabetic conditions. Sci Rep 6: 31506, 2016. doi: 10.1038/srep31506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu C, Lv C, Chen F, Ma X, Shao Y, Wang Q. The function of miR-199a-5p/Klotho regulating TLR4/NF-κB p65/NGAL pathways in rat mesangial cells cultured with high glucose and the mechanism. Mol Cell Endocrinol 417: 84–93, 2015. doi: 10.1016/j.mce.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 122.Wu J, Zheng C, Fan Y, Zeng C, Chen Z, Qin W, Zhang C, Zhang W, Wang X, Zhu X, Zhang M, Zen K, Liu Z. Downregulation of microRNA-30 facilitates podocyte injury and is prevented by glucocorticoids. J Am Soc Nephrol 25: 92–104, 2014. doi: 10.1681/ASN.2012111101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wühl E, Schaefer F. Therapeutic strategies to slow chronic kidney disease progression. Pediatr Nephrol 23: 705–716, 2008. doi: 10.1007/s00467-008-0789-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xu J, Ai Q, Cao H, Liu Q. MiR-185-3p and miR-324-3p predict radiosensitivity of nasopharyngeal carcinoma and modulate cancer cell growth and apoptosis by targeting SMAD7. Med Sci Monit 21: 2828–2836, 2015. doi: 10.12659/MSM.895660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu X, Kriegel AJ, Jiao X, Liu H, Bai X, Olson J, Liang M, Ding X. miR-21 in ischemia/reperfusion injury: a double-edged sword? Physiol Genomics 46: 789–797, 2014. doi: 10.1152/physiolgenomics.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xu X, Zhu Y, Liang Z, Li S, Xu X, Wang X, Wu J, Hu Z, Meng S, Liu B, Qin J, Xie L, Zheng X. c-Met and CREB1 are involved in miR-433-mediated inhibition of the epithelial-mesenchymal transition in bladder cancer by regulating Akt/GSK-3β/Snail signaling. Cell Death Dis 7: e2088, 2016. doi: 10.1038/cddis.2015.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang HC, Fogo AB. Mechanisms of disease reversal in focal and segmental glomerulosclerosis. Adv Chronic Kidney Dis 21: 442–447, 2014. doi: 10.1053/j.ackd.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang HC, Liu SJ, Fogo AB. Kidney regeneration in mammals. Nephron, Exp Nephrol 126: 50–53, 2014. doi: 10.1159/000360661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yu J, Mao S, Zhang Y, Gong W, Jia Z, Huang S, Zhang A. MnTBAP therapy attenuates renal fibrosis in mice with 5/6 nephrectomy. Oxid Med Cell Longev 2016: 7496930, 2016. doi: 10.1155/2016/7496930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zeisberg M, Müller GA. Mechanistic insights into the antifibrotic activity of aliskiren in the kidney. Hypertens Res 35: 266–268, 2012. doi: 10.1038/hr.2011.226. [DOI] [PubMed] [Google Scholar]
- 131.Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, Hristov M, Köppel T, Jahantigh MN, Lutgens E, Wang S, Olson EN, Schober A, Weber C. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal 2: ra81, 2009. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 132.Zheng CZ, Shu YB, Luo YL, Luo J. The role of miR-146a in modulating TRAF6-induced inflammation during lupus nephritis. Eur Rev Med Pharmacol Sci 21: 1041–1048, 2017. [PubMed] [Google Scholar]
- 133.Zhong X, Chung AC, Chen HY, Meng XM, Lan HY. Smad3-mediated upregulation of miR-21 promotes renal fibrosis. J Am Soc Nephrol 22: 1668–1681, 2011. doi: 10.1681/ASN.2010111168. [DOI] [PMC free article] [PubMed] [Google Scholar]