Abstract
Brown adipose tissue (BAT) has been suggested to play an important role in lipid and glucose metabolism in rodents and possibly also in humans. In the current study, we used genetic and correlation analyses in the BXH/HXB recombinant inbred (RI) strains, derived from Brown Norway (BN) and spontaneously hypertensive rats (SHR), to identify genetic determinants of BAT function. Linkage analyses revealed a quantitative trait locus (QTL) associated with interscapular BAT mass on chromosome 4 and two closely linked QTLs associated with glucose oxidation and glucose incorporation into BAT lipids on chromosome 2. Using weighted gene coexpression network analysis (WGCNA) we identified 1,147 gene coexpression modules in the BAT from BXH/HXB rats and mapped their module eigengene QTLs. Through an unsupervised analysis, we identified modules related to BAT relative mass and function. The Coral4.1 coexpression module is associated with BAT relative mass (includes Cd36 highly connected gene), and the Darkseagreen coexpression module is associated with glucose incorporation into BAT lipids (includes Hiat1, Fmo5, and Sort1 highly connected transcripts). Because multiple statistical criteria were used to identify candidate modules, significance thresholds for individual tests were not adjusted for multiple comparisons across modules. In summary, a systems genetic analysis using genomic and quantitative transcriptomic and physiological information has produced confirmation of several known genetic factors and significant insight into novel genetic components functioning in BAT and possibly contributing to traits characteristic of the metabolic syndrome.
Keywords: brown adipose tissue, coexpression modules, quantitative trait locus, recombinant inbred strains, spontaneously hypertensive rat
INTRODUCTION
Metabolic syndrome is characterized by the clustering of several risk factors including insulin resistance in skeletal muscle and adipose tissue, dyslipidemia, Type 2 diabetes, and hypertension. Metabolic syndrome affects upwards of 25% of individuals in developed countries and is associated with increased risk for cardiovascular disease and for developing Type 2 diabetes (38). Normal cellular lipid homeostasis reflects a balance between processes that generate or deliver fatty acids and processes that utilize these molecules for energy homeostasis or storage in adipose tissue. Adipose tissue in mammals is categorized into white adipose tissue (WAT) and brown adipose tissue (BAT), although beige adipose tissue is now, also, an accepted category (27). WAT functions to store energy in the form of triglyceride-containing intracellular vacuoles as well as to secrete a host of hormones (adipokines) that regulate overall energy balance. Unlike WAT, BAT regulates thermogenesis upon environmental stresses, to maintain energy balance and to protect the organism from hypothermia. In addition, it has been shown that by activating BAT by short-term cold exposure, fatty acids are efficiently channeled into BAT through CD36 fatty acid transporter (9). Consequently, lipid clearance from plasma becomes more efficient, suggesting that BAT might be a master regulator of clearance of triglyceride-rich lipoproteins and blood lipid abundance. In addition, BAT was also found as to be a major organ for glucose disposal, as a large fraction of ingested glucose is channeled to BAT, where the glucose, just like lipids, is oxidized (10). These studies and recent findings that BAT is retained in humans into adulthood (84) and that its abundance is greatly diminished in obese individuals (83) increased interest to study BAT metabolism as a potential mechanism for reducing the risk factors for obesity and metabolic syndrome-related comorbidities (69).
In our current studies, we tested the hypothesis that genetically determined differences in the amount of BAT and its efficiency to metabolize glucose and lipids are related to groups of coexpressed genes (modules) whose expression is controlled from the same genomic locus as the biologic trait. To test this hypothesis, we combined linkage [quantitative trait locus (QTL)] and weighted gene coexpression network analysis (WGCNA) of genome-wide transcriptome data from BAT with measures of the physiological and biochemical parameters in BAT in the BXH/HXB recombinant inbred (RI) strains derived from spontaneously hypertensive rat (SHR) and Brown Norway (BN) progenitors (31, 57). These analyses identified three gene coexpression modules that significantly contribute to determining BAT mass or glucose incorporation into BAT lipids. Our analysis and results provide a “systems” perspective on the genetic components in BAT that can contribute to the development of the metabolic syndrome.
MATERIALS AND METHODS
Animals.
The SHR/OlaIpcv strain (referred to as the SHR) and the BN-Lx/Cub strain (referred to as the BN) are the parental strains from which the BXH/HXB RI strains were derived (31). SHR.BN-D2Rat171/D2Arb24 congenic strain (referred to as SHR-2 congenic) (58) and SHR.BN-Il6/Npy congenic strain (referred to as SHR-4 congenic (59) were used for functional validation of QTL identified on chromosomes 2 and 4. Biochemical and metabolic phenotypes in all strains were assessed in 3 mo old nonfasted male rats (n = 8 per strain). RNA microarray expression analyses in all strains were also assessed in 3 mo old nonfasted male rats (n = 4 per strain). All experiments were performed in agreement with the Animal Protection Law of the Czech Republic and were approved by the Ethics Committee of the Institute of Physiology of the Czech Academy of Sciences, Prague.
Calculation of BAT relative mass.
We used interscapular BAT for biochemical and gene expression analyses. Interscapular mass BAT was removed and weighed and relative mass was calculated as a ratio of BAT mass in grams per 100 g of body mass.
Basal and insulin-stimulated glucose utilization in BAT.
Following decapitation in the nonfasted state, interscapular BAT was dissected and incubated for 2 h in Krebs-Ringer bicarbonate buffer with 5 mmol/l glucose, 0.1 μCi [U-14C] glucose/ml and 2% bovine serum albumin, gaseous phase 95% O2 and 5% CO2 in the presence (250 μU/ml) or absence of insulin in the incubation media. Glucose oxidation was determined in BAT by measuring the incorporation of [U-14C] glucose into CO2. For measurement of incorporation of radiolabeled glucose into lipids, at the end of incubation, BAT was removed from media, rinsed in saline, and transferred into chloroform-methanol (2:1) where lipids were extracted and radioactivity was measured.
QTL analysis.
Phenotypic quantitative trait loci (pQTL) were identified using the qtl package (version 1.39-5) in R (12). The marker set used for QTL analysis in the BXH/HXB rats was derived from the single nucleotide polymorphisms (SNPs) genotyped by the STAR consortium (http://bbglab.irbbarcelona.org/funcSTAR//index.php) (80). The original marker set had 20,283 markers. After quality control and data cleaning including conversion to the RGSC 6.0/rn6 version of the rat genome, 10,486 informative markers remained. Marker regression was used with phenotypic strain means to identify pQTL(s) for each phenotype independently. Genome-wide P values (i.e., controlling for family-wise error rate for multiple testing across genomic markers) for QTL were determined using permutation (16) and 1.5 logarithm of odds (LOD) support intervals (40) were calculated for each significant (genome-wide P value <0.05) or suggestive QTL (genome-wide P value <0.63) (41).
BAT gene expression profiles in BXH/HXB RI strains.
Total RNA was extracted from BAT of 30 BXH/HXB RI strains and the SHR and BN progenitors (n = 4 per strain). The quality and concentration of RNA were determined using a NanoDrop 2000 spectrophotometer (Thermo Scientific). RNA integrity was analyzed in an Agilent Bioanalyzer 2100. We only included samples judged to have an intact RNA profile. The Affymetrix GeneChip Rat Gene 2.0 ST Array System (Affymetrix, Santa Clara, CA) was used for the microarray analysis following the standard protocol: 100 ng RNA was amplified with an Ambion WT Expression Kit (Applied Biosystems), 5.5 μg single-stranded cDNA was labeled and fragmented with GeneChip WT Terminal Labeling and Hybridization (Affymetrix) and hybridized on the chip according to the manufacturer’s procedure. The transcription data are MIAME compliant and deposited in the ArrayExpress database (E-MTAB-5292). In addition, the raw and processed transcription data are available for download on PhenoGen (https://phenogen.ucdenver.edu/PhenoGen/).
Relative RNA expression levels were estimated using robust multiple array average normalization (33). The broad sense heritability for each transcript was estimated using the Pearson R2 coefficient of determination from linear regression. Transcript probe clusters were retained if their heritability was at least 0.2. Expression analysis was executed using R, and transcript annotations were obtained from the Affymetrix ragene20 annotation data (package: ragene20sttranscriptcluster.db 8.4.0) within Bioconductor 3.3.
Gene coexpression network analysis in BAT.
An unsigned WGCNA based on Pearson correlation coefficients was executed to identify gene coexpression modules for the BXH/HXB RI panel. This was done using the WGCNA package (version 1.51) in R (39). Two parameters were altered from their default setting to allow for the identification of smaller modules: the minimum module size (set to 5) and the deepSplit parameter (set to 4). The model-fitting index proposed by Zhang and Horvath (91) was used to determine the appropriate soft thresholding power. Module eigengene (first principal component of the coexpression module) QTLs were identified using the same software, marker set, and methods as the phenotypic QTLs with the module eigengene as the quantitative trait. The coexpression module information for BAT is available for download from https://Phenogen.ucdenver.edu/PhenoGen/.
Identification of candidate modules.
To be considered a candidate coexpression module for a metabolic or physical phenotype 1) the module’s eigengene had to be significantly correlated (P < 0.05) with the phenotype, 2) the module’s eigengene had to have a significant (genome-wide P < 0.05) module eigengene QTL, and 3) the peak position of the module eigengene QTL had to fall within the 1.5 LOD support interval of a phenotypic QTL for the same phenotype with which it is correlated. Finally, to protect against associations due solely to colocalization, we examined partial correlations for genes in coexpression modules controlling for its module eigengene QTL using the ppcor package (version 1.0) in R (35). For purposes of examining connectivity between genes both before (and after) partial correlation, an edge represents a pairwise correlation between two genes that is either > 0.5 (0.3 for partial correlations) or < −0.5 (−0.3 for partial correlations). Candidate modules had to retain at least 50% of their edges after controlling for the module eigengene QTL.
Characterization of coexpression modules.
Intramodular connectivity was estimated as the sum of the pair-wise correlation coefficients (absolute values) of a gene with all the other genes within the module. The hub gene is the gene with the highest intramodular connectivity based on partial correlations that control for the module eigengene. If a network had unannotated genes, these transcript clusters were investigated using Ensembl (http://uswest.ensembl.org//useast.ensembl.org/index.html?redirectsrc=//uswest.ensembl.org%2Findex.html) and/or the University of California Santa Cruz databases (https://genome.ucsc.edu/). Probe sequences were aligned to the rat genome with BLAST-like alignment tool (BLAT) from the UCSC genome browser. BLAT results had to match 100%, and RefSeq annotations were used if they were available in the rat. If rat annotation was not available, the orthologous mouse annotations were used. Coexpression modules were visualized in Cytoscape 3.4.0 (74).
Strains used for functional confirmation of QTL regions.
To confirm the biologic importance of the identified, overlapping QTLs for phenotype and the module eigengene QTL, we measured glucose oxidation and incorporation into BAT lipids in congenic SHR-2 rats (11). This congenic strain contains the region on Ch. 2 from 116.1 Mb to 228.7 Mb from the BN strain on an SHR background. This region was shown by our studies (see results) to contain the full LOD support interval for the glucose incorporation into BAT QTL and for the glucose oxidation QTL. To examine the importance of the Ch. 4 locus for relative BAT mass (see results) we used the SHR-4 congenic strain (SHR, BN-I16/Npy) (59). The SHR-4 congenic strain contains the interval Ch 4:4.8–83.9 Mb from the BN strain on the SHR background. The rats of the congenic strains and their respective controls were phenotyped by the methods described for the RI strains (see below).
RESULTS
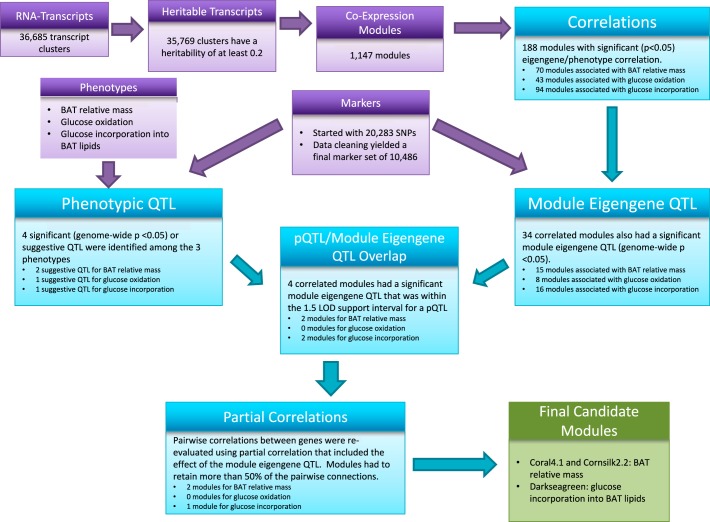
The steps and results of the analysis in the BXH/HXB RI panel are depicted in a flow chart (Fig. 1).
Fig. 1.
Analysis flow chart. BAT, brown adipose tissue; QTL, quantitative trait locus; LOD, logarithm of odds.
Metabolic BAT phenotypes.
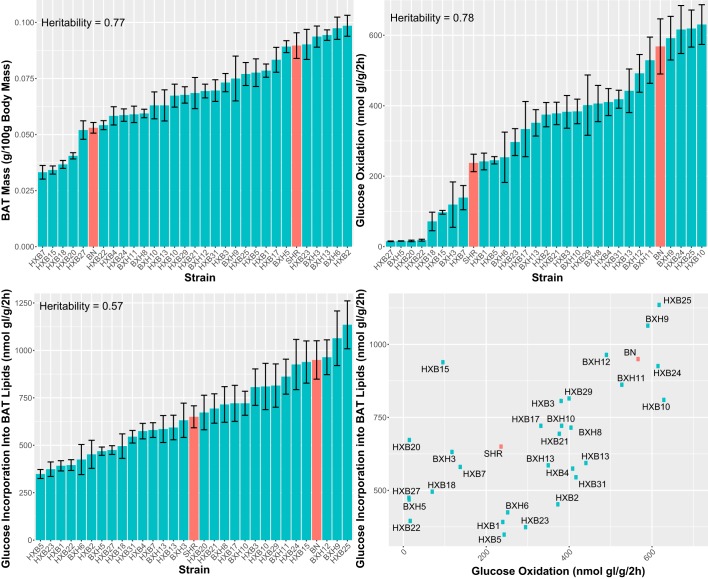
Relative mass of the BAT compared with total body mass was calculated for eight rats per strain. Likewise, glucose oxidation and glucose incorporation in the BAT lipids was measured on eight rats per strain. All three traits were highly heritable (BAT relative mass = 77%, glucose oxidation = 78%, glucose incorporation into BAT lipids = 57%). Four strains had extremely low values for glucose oxidation (Fig. 2). Glucose oxidation and glucose incorporation into BAT lipids were significantly correlated with each other (Spearman rank correlation coefficient = 0.60, P < 0.01), but BAT relative mass was not significantly correlated with either of the other two phenotypes (P > 0.2 for both phenotypes).
Fig. 2.
Metabolic phenotypes in the BXH/HXB recombinant inbred (RI) panel. The blue bars/dots represent the mean phenotypic values for the RI strains and the red bars/dots represent the mean phenotypic values for the parental strains (SHR/Ola and BN-Lx/Cub). Error bars represent SE across biological replicates within each strain.
Phenotypic QTL analysis.
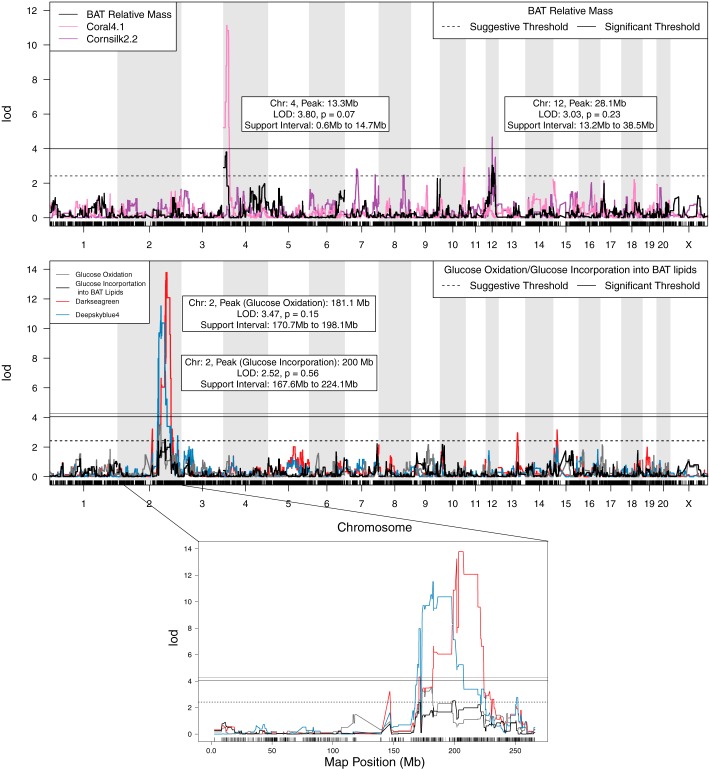
We identified two suggestive QTLs for relative BAT mass, one suggestive QTL for glucose oxidation in BAT and one suggestive QTL for glucose incorporation into BAT lipids. QTL for glucose oxidation and glucose incorporation into BAT lipids had 1.5 LOD support intervals that overlapped on chromosome 2 (Fig. 3). BAT relative mass did not have overlapping QTL with either of the other two phenotypes.
Fig. 3.
QTLs for metabolic phenotypes from brown adipose tissue (BAT) and associated module eigengene QTL. The LOD score is plotted along the y-axis, and the physical location of SNPs are plotted along the x-axis. The solid horizontal line represents the empirical threshold for genome-wide significance (P = 0.05), and the dotted horizontal line represents the empirical threshold for suggestive QTL (P = 0.63) for the respective physiological QTL. Top: the QTL for BAT relative mass (black) and the QTL for the 2 coexpression modules associated with BAT relative mass: Coral4.1 (pink) and Cornsilk2.2 (purple). Bottom: the QTL for glucose oxidation (gray), glucose incorporation into BAT lipids (black), and the 2 coexpression modules associated with glucose oxidation and/or glucose incorporation into BAT lipids: Darkseagreen and Deepskyblue4 (blue). Both the Darkseagreen module and the Deepskyblue4 module were associated with glucose incorporation, and their eigengene QTLs overlap the glucose incorporation QTL. Only the Deepskyblue4 modules was also associated with glucose oxidation and had a module eigengene QTL that overlapped the glucose oxidation QTL on chromosome 2. The Deepskyblue4 module was later removed from the set of candidate modules because its connectivity is not retained after partial correlations.
RNA expression.
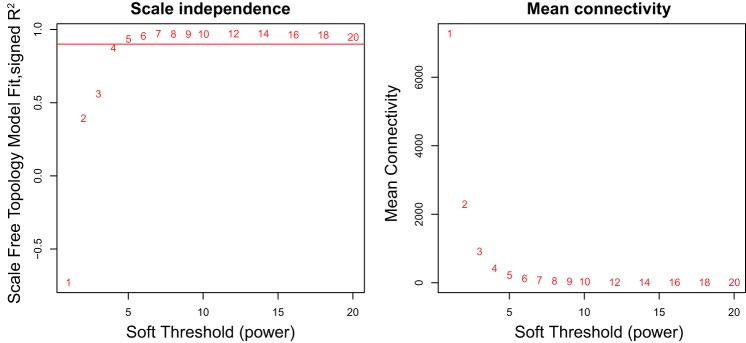
We started with 36,685 transcripts and 35,769 had heritabilities above the 0.2 threshold. Arrays were examined for quality, and there were no significant outliers (Fig. 4). A soft thresholding power of 7 was sufficient for WGCNA (Fig. 5). WGCNA resulted in 1,147 coexpression modules. Median module size was 7 with a range of module sizes from 5 to 4,981. There were 3,778 (10.6%) transcripts that were not assigned to a module.
Fig. 4.
A hierarchical clustering of samples to identify outliers. Height is defined as 1 minus the correlation Pearson coefficient. Labels describe the individual rats starting with the RI strain name. The first letter of the strain name describes the breeding mother (H = SHR strain, B = BN-Lx strain), X means crossed, and the last letter describes the father. The number at the end of the strain name signifies a unique strain i.e., genotype. The number after the underscore mark denotes the biological replicate.
Fig. 5.
Determining soft thresholding parameter for scale-free network in BAT expression. These 2 graphics are used to determine the appropriate soft thresholding parameter to use with weighted gene coexpression network analysis (WGCNA) to ensure that the relationships between genes are described using a scale-free network. On the left is the R2 value that ranges from 0 to 1 and quantifies how well the data resemble a scale-free network when different parameters (x-axis) are chosen. The red horizontal line represents an R2 value of 0.90, the minimum value recommended. The graph on the right depicts the mean gene connectivity for different soft thresholding parameters. In a scale-free network, most genes have few connections. It is recommended that a soft thresholding parameter is chosen when the mean connectivity is small and relatively stable. A soft thresholding parameter of 7 was chosen for this data set.
Identification of candidate coexpression modules.
There were 188 coexpression modules with a significant correlation (P < 0.05) of eigengene values with at least one phenotype across the RI strains. BAT relative mass was correlated with 70 coexpression modules (15 of which had significant module eigengene QTLs), glucose oxidation with 43 coexpression modules (8 of which had significant module eigengene QTLs), and glucose incorporation into BAT lipids with 94 coexpression modules (16 of which had significant module eigengene QTLs). After the requirement of phenotypic QTL/module eigengene QTL overlaps, there were four candidate coexpression modules that remained (Fig. 3). There were two modules associated with BAT relative mass, and two modules associated with glucose incorporation into BAT lipids. Three of the four coexpression modules had at least 50% of their genes colocalized with the module eigengene QTL. After we calculated partial correlations, only three modules retained at least 50% of their edges (Table 1; Supplementary Table S1). (The online version of this article contains supplemental material.) The Cornsilk2.2 module QTL overlapped one of the QTLs for BAT relative mass; however, this module only contained transcripts that mapped to intronic areas of protein-coding genes or unannotated transcripts and, thus, was not, at the present time, amenable to exploration of its function.
Table 1.
Candidate coexpression modules
| Coexpression Module | Genes in Module After Partial Correlation, n | Proportion of Variation in Gene Expression Explained by Module Eigengene | Associated Phenotype | Phenotypic QTL* | Correlation Coefficient | P Value | Module Eigengene QTL* |
|---|---|---|---|---|---|---|---|
| Cornsilk2.2 | 5 | 0.71 | BAT relative mass | Chr 12: 28.1 Mb (13.2–38.5) | 0.42 | 0.020 | Chr 12: 27.3 Mb (26.4–40.5) |
| Coral4.1 | 7 | 0.65 | BAT relative mass | Chr 4: 13.3 Mb (0.6–14.7) | −0.43 | 0.018 | Chr 4: 14.5 Mb (13.7–21.8) |
| Darkseagreen | 16 | 0.54 | glucose incorporation into BAT lipids | Chr 2: 200.0 Mb (167.6–224.1) | 0.56 | 0.001 | Chr 2: 205.3 Mb (200.5–207.7) |
Describes quantitative trait locus (QTL) peak location with 1.5 logarithm of odds support interval.
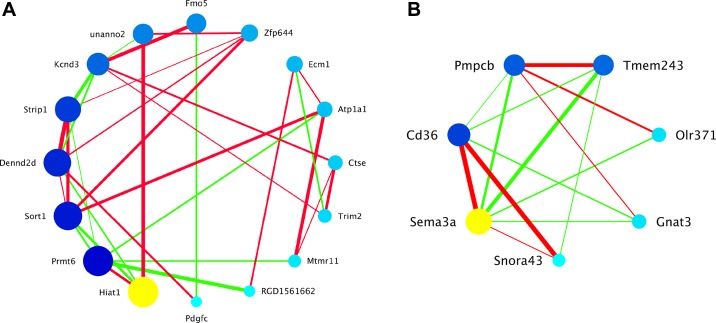
The Darkseagreen coexpression module (Fig. 6A) was associated with glucose incorporation into BAT lipids. Interestingly, although the QTL for the trait of glucose oxidation did not overlap the Darkseagreen module eigengene QTL, the correlation of the module eigengene with glucose oxidation was significant (P = 0.04). Hiat1, a newly recognized sugar transporter (49), was the hub gene for the Darkseagreen module, although its intramodular connectivity (i.e., the sum of its pairwise partial correlations within the module) was only 0.03 higher than protein arginine methyltransferase 6 (Prmt6). Darkseagreen had two unannotated transcripts. One was mapped to the intronic region of the homologous mouse Zinc finger protein 644 (Zfp644), and the other had no orthologs in mouse or human. The Coral4.1 coexpression module (Fig. 6B) was associated with BAT relative mass, and the Sema3a transcript was its hub (i.e., most connected) gene. RNA expression levels of Sema3a were positively correlated with BAT relative mass. Coral4.1 had 1 unannotated gene. The probe sequence for this unannotated transcript aligned to a region of the genome that, although not annotated in rat, was an orthologous region in mouse that contains the small nucleolar RNA43 (Snora43).
Fig. 6.
Candidate coexpression modules associated with metabolic phenotypes from brown adipose tissue (BAT). Genes within the candidate coexpression network are represented as circles (nodes) within the graphic. Circles are colored, ordered, and sized by intramodular connectivity calculated from partial correlation coefficients that have been adjusted for the module eigengene QTL. The yellow circle is the hub gene (i.e., most connected). Larger, darker nodes are more connected than smaller, lighter nodes. Edges indicate pair-wise correlations between connected genes. Thicker edges indicate correlations with higher magnitude than thinner edges (thickness is related to rank order but does not represent numerical values). Green edges are representative of negative correlations, and red edges are representative of positive correlations. Only pairwise partial correlation coefficients >0.3 or <−0.3 are shown as edges. A: Darkseagreen associated with glucose incorporation into BAT lipids. Hiat1 (yellow circle), hippocampus abundant transcript 1, is the hub gene, and it is negatively correlated with glucose incorporation into BAT lipids. B: Coral4.1 associated with relative mass of BAT. Sema3a (yellow circle), semaphorin 3A, is the hub gene, and it is positively correlated with BAT relative mass.
Function validation of the chromosome 2 locus in glucose oxidation and incorporation into BAT lipids.
On chromosome 2, the QTLs for glucose oxidation and glucose incorporation into BAT lipids overlap. The SHR-2 congenic rats with BN alleles in the chromosome 2:116.1–228.7 Mb region exhibited significantly higher glucose oxidation (mean difference = 174,; SE = 33, P value = 0.0001; Fig. 7A) and incorporation into BAT lipids (mean difference = 348, SE = 88, P value = 0.0017; Fig. 7B) when compared with the congenic-paired control. The introgression of the chromosome 2 region from BN rats into the SHR background produced rats (SHR-2) in which glucose oxidation and glucose incorporation into lipids were significantly higher than those found in the paired SHR control. The ratio of the phenotypic values between the BN and SHR rats derived from the regular breeding colony was found to be similar to the phenotypic ratios calculated for the SHR and the SHR-2 rats. It should be noted that the strain designated as the SHR paired control for the congenic and the SHR-2 has been isolated from the original SHR strain that has been bred in conjunction with the RI strains. The congenic control has been maintained in conjunction with the SHR-2 rats.
Fig. 7.
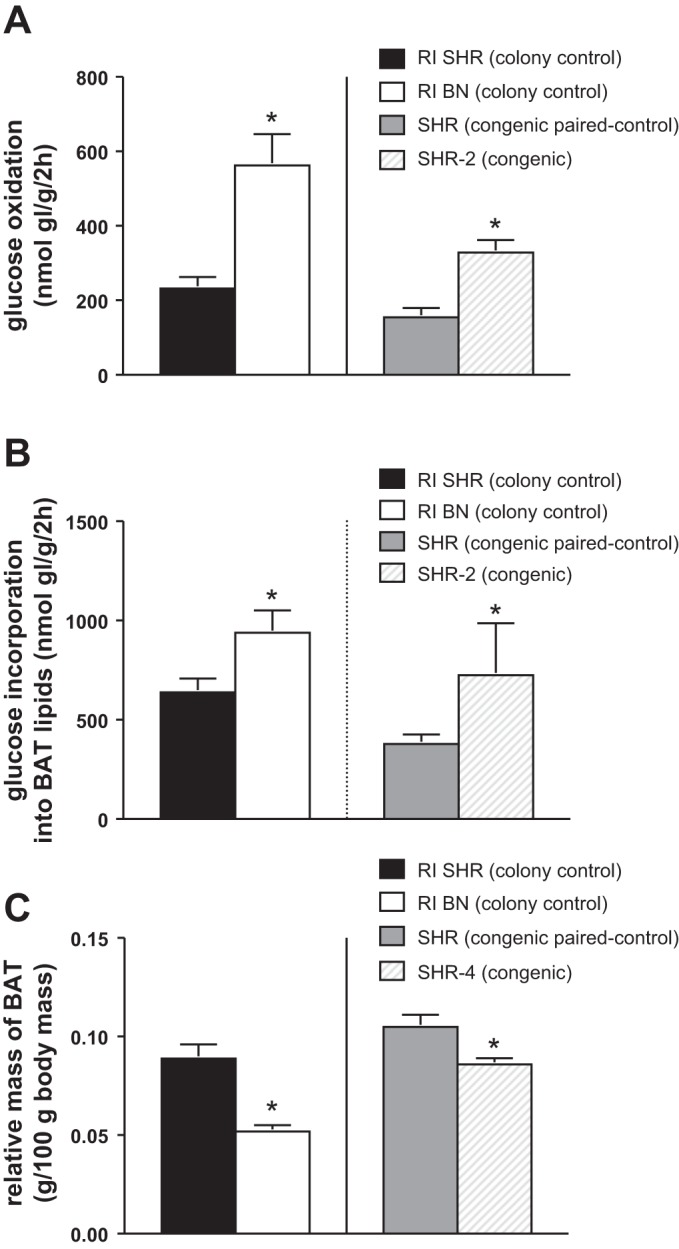
Comparison of BAT metabolic phenotypes in SHR-2 and SHR-4 congenic rats compared with paired controls and between SHR and BN from the BXH/HXB recombinant inbred colony. Glucose oxidation (A) and glucose incorporation into BAT lipids (B) were measured in the SHR-2 congenic rats (n = 10) and their SHR paired controls (n = 8) in addition to the BN and SHR strains maintained with the BXH/HXB colony. The SHR-2 congenic rats were generated by the introgression of the BN region Chr 2: 116.1−228.7 Mb onto an SHR background, which overlaps the QTLs for both glucose oxidation and glucose incorporation into BAT lipids. C: relative BAT mass was measured in the SHR-4 congenic rats (n = 8) and their SHR paired controls (n = 8) in addition to the BN and SHR strains maintained with the BXH/HXB colony. The SHR-4 congenic rats were generated by the introgression of the BN region Chr 4: 4.8–83.9 Mb onto an SHR background, which overlaps a QTL for relative BAT mass. Means and standard errors are displayed. *P < 0.01 compared with paired-control (SHR-2 and SHR-4) or BN strain (SHR from RI colony).
Functional validation of the chromosome 4 locus in relative BAT mass.
To confirm the biologic importance of the chromosome 4 region encompassing both the relative BAT mass QTL and the Darkseagreen coexpression module eigengene QTL, we measured relative BAT mass in SHR-4 congenic and its paired control strain. SHR-4 congenic rats had a significantly lower relative BAT mass than their paired SHR controls (mean difference = −0.019, SE = 0.005, P value = 0.0062; Fig. 7C). These differences resembled the differences in BAT relative mass noted between the SHR and the BN, RI colony rats with the introgression of the interval from the BN strain making the SHR more BN-like with regard to BAT-relative mass.
DISCUSSION
In our current work, we used a genetic genomics/phenomics approach, and we required that the candidate module be not only genetically correlated with the physiological phenotype but also controlled from the same genetic locus that contributes to variation in the physiological phenotype.
We examined gene expression in BAT, and three gene coexpression modules were identified as meeting criteria (see materials and methods and above) to be considered as contributors to a predisposition to a particular level of a quantitative trait (quantitative phenotype) related to BAT quantity and function. The physiological phenotypes were glucose incorporation into lipids and BAT relative mass, and the coexpression modules causing a predisposition to these phenotypes were Coral4.1, Cornsilk2.2, and Darkseagreen. Because of a lack of annotation for transcripts of the Cornsilk2.2 module, we were not able to characterize the function of this module. The examination of the relationship between phenotypic values and the expression levels of the module hub genes of Coral4.1 and Darkseagreen across strains suggests a bimodal distribution of gene expression for these hub genes (Fig. 8). Each of the phenotypes is, however, quantitatively distributed across the 30 strains of the BXH/HXB rats, indicating a polygenic contribution to each phenotype (Fig. 2).
Fig. 8.
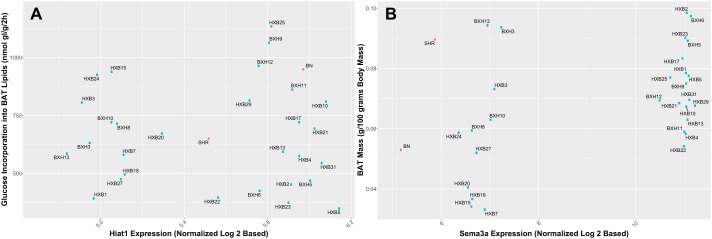
Distribution of hub genes from candidate modules and their association with the brown adipose tissue (BAT) metabolic phenotypes. The blue dots represent the mean phenotypic values for the RI strains, and the red dots represent the mean phenotypic values for the parental strains (SHR/Ola and BN-Lx/Cub). A: association between the hub gene of Darkseagreen (Hiat1) and glucose incorporation into BAT lipids. B: association between the hub gene of Coral4.1 (Sema3a) and BAT relative mass.
Due to our choice of parameters that generate modules with a smaller number of transcripts, standard functional enrichment analyses do not produce informative results in relation to phenotype. We have previously used a knowledge-based approach (28, 68) to find integrative information to relate the module members to each other and to their related phenotypes, and the same approach was used in the current work.
Functional relationship of the Darkseagreen module genes to glucose incorporation into BAT lipids.
Hiat1 was identified as the hub gene of the Darkseagreen module associated with glucose incorporation into BAT lipids (Fig. 6A). It again should be noted that the phenotype of glucose incorporation into BAT lipids is highly genetically correlated with several other metabolic syndrome phenotypes, particularly glucose oxidation in BAT (Fig. 2). Hiat1 is the acronym for hippocampus abundant gene transcript 1, which was named such because of its high expression in the hippocampus (49). The deduced amino acid sequence of this transcript indicated it to be a novel type of sugar transporter, and Northern hybridization indicated that it is expressed in several tissues other than brain (49). Our work demonstrates that Hiat1 (MFSD14A) is also expressed in BAT and is correlated with glucose incorporation into lipids in this tissue. Recent evidence indicates that Hiat1 expression can be modulated in brain by feeding mice a high-fat diet (43) and such studies may be of interest in BAT.
Within the Darkseagreen module, the sortilin (Sort1) expression levels are highly correlated with the expression of other genes in this module. Sortilin is a glycoprotein (92) that, among other functions, is a component of the insulin response vesicles that contain GLUT4 (glucose transporter), and sortilin participates in delivering the GLUT4-containing vesicles to the plasma membrane (76, 77). The knock-down of sortilin in 3T3-L1 adipocytes leads to decreases in GLUT4-containing vesicles and a decrease in insulin-regulated glucose uptake (76). Another member of the Darkseagreen module is Strip1 (striatin interacting protein 1). Strip1 is part of a scaffold complex (STRIPAK) that brings together certain kinases and phosphates (32). STK25 (YSK1) is a serine/threonine kinase and is part of the STRIPAK complex. STRIP1 localizes the STRIPAK complex primarily to the Golgi but may also be involved in vesicular trafficking of the complex to the plasma membrane (32). Stk25 (YSK1) knockdown in rat myoblasts enhances the expression of Glut1, Glut4, and hexokinase-2; enhances insulin stimulated glucose uptake; increases the expression of uncoupling protein 3; and increases lipid oxidation (54). Huang and Pollas (32) suggest that the STRIPAK complex may play an important role in glucose and lipid metabolism in diabetes relevant tissues, and as mentioned above STRIP1 is an integral part of the STRIPAK complex. The role of Fmo5 (flavin containing monooxygenase 5) in increasing glucose metabolism and insulin sensitivity in adipocytes has been recently uncovered through the creation of Fmo5 knockout (KO) mice (26, 72). In addition to the impact of Fmo5 disruption on glucose metabolism, the KO mice demonstrate an increased rate of free fatty acid oxidation, lower plasma glucose and cholesterol levels, and a “lean phenotype” even when fed a high-fat diet. Although the role of Fmo5 has not been specifically investigated in BAT, the phenotypes of the KO mice are certainly relevant to BAT function.
BAT is highly innervated with adrenergic neurons, and Trim2 (tripartite motif-containing 2), which is a member of the Nedd4 ubiquitin ligase (E3) family, plays a protective role for peripheral adrenergic neurons by ubiquitinating the excess microfilaments and leading to the microfilament degradation and clearance (44, 88). This function would protect the adrenergic innervation of BAT. Adrenergic stimulation of BAT promotes glucose uptake and glycolysis. Additionally the E3 ligases are important in ubiquitinating acetyl-CoA carboxylase and leading to its degradation (67). This degradation impairs the initial commitment of acetyl-CoA to the synthesis of fatty acids and thus reduces production of fatty acids from glucose (44). Our data cannot, however, distinguish the relative expression of Trim2 in BAT adrenergic neurons and BAT adipocytes. The protein products of Trim2 and Mtmr11 (myotubularin-related protein 11) (another member of the Darkseagreen module) are related in their function. Trim2 requires interaction with phosphoinositides [i.e., PI(4,5)P2] for its function (81). The product of Mtmr11 is a microtubulin-associated phosphatase that acts to dephosphorylate inositol trisphosphate at its 3 position (79). When acting on IP3, Mtmr11 would generate PI(4,5)P2 for modulating TRIM2 function.
Prmt6 codes for an arginine methyl transferase (19, 85), and one of the substrates for PRMT6 is GPS2 (30), which in the nucleus acts as a transcriptional repressor of several genes, including Pparg (13). PPAR-γ is a critical factor in differentiation of preadipocytes to BAT and maintenance of the BAT phenotype (7, 53). Another gene product of the Darkseagreen module is also a factor in the differentiation of preadipocytes to BAT. Pdgfc generates the platelet-derived growth factor C, and although PDGFC is expressed in adipose-derived stem cells (36), the source of PDGFC in BAT is primarily the endothelial cells of the blood vessels in BAT (73). It is of some interest that a product of the endothelial cells would be quantitatively correlated in its expression with genes expressed in the BAT cells, again indicating cross cell/tissue communication (73).
Another component of the Darkseagreen module is Kcnd3, which is a voltage-gated potassium channel. Our work is the first demonstration of the expression of this particular potassium channel (subfamily D, member-3) in BAT, but other voltage-sensitive potassium channels (82) have been shown to be important in the proliferation and differentiation of preadipocytes into BAT using human adipocytes. It is of interest that a voltage-sensitive potassium channel that could be modulated by long chain fatty acids has previously been reported in rat brown adipocytes (87), but its identity has remained unknown. Lysosome function in BAT is also evident in the coexpression profile of the Darkseagreen module. The Dennd2d (DENN domain containing 2D) product is an ARF (GDP-GTP exchange factor) that specifically controls the Rab9-dependent trafficking of the mannose 6-phosphate receptor to lysosomes (66, 89). The mannose 6-phosphate receptor shuttles newly synthesized lysosomal enzymes to lysosomes (e.g., lysosomal acid lipase) (29). The lysosomal acid lipase enzyme is a major contributor to hydrolysis of triglycerides and cholesterol esters and makes available the free fatty acids for oxidative metabolism. Another component of this module is Ctse coding for cathepsin E, which is an aspartic protease important in maintaining lysosome function. cathepsin E-deficient mice show a decrease in the development of BAT and a pathologic accumulation of triglycerides and cholesterol esters in lysosomes (34). Atp1a1 codes for the α-subunit of Na+K+ ATPase (65). Na+K+ ATPase has long been considered as an important contributor to thermogenesis (18), independent of mitochondrial uncoupling mechanisms. More recently, the Na+K+ ATPase, and particularly the α-subunit, has been shown to be a “docking station” for several important intracellular signaling molecules and acts as a participant in the signaling process (65). The α-subunit of Na+K+ ATPase preferentially binds to caveolin-1, and this interaction generates a pool of Na+K+ ATPase in caveolae. Some of the more relevant interactions of Na+K+ ATPase with regard to the Darkseagreen module are with Src and PLC in caveolae (65, 90). The activation of Src in this complex initiates the PLC-c signaling cascade leading to hydrolysis of inositol phosphate lipids to DAG and inositol [1,4,5-trisphosphate (PIP3)]. The conversion of PIP3 to PIP2 and PIP2 interaction with TRIM2 activity has been described above.
Finally, Ecm1, which codes for the extracellular matrix protein 1, is a member of the Darkseagreen module. The ECM1 protein participates in the signaling function of EGF/ERK to generate the Warburg effect (switch to primarily anaerobic metabolism of glucose). ECM1 induces the expression of Glut1 and Ldha in transformed BT-474 cells (42), and this may also occur in BAT. The Ldha product, lactate dehydrogenase, catalyzes an important step at the end of anaerobic glycolysis, leading to the production of lactate and NAD+ from pyruvate and NADH.
It needs to be stressed that the coexpression modules we identified are indicative of factors that cause a predisposition to various levels of glucose conversion into lipids and the relative mass of BAT across the BXH/HXB RI panel of rats. The components of the modules would act in a coordinated fashion to modulate phenotype given relevant environmental signals. When considering the relationship of the Darkseagreen module as a whole to the predisposition to the phenotype of glucose conversion into BAT lipids and the genetically correlated trait of glucose oxidation, one can discern several related processes that can interact to produce quantitative differences in glucose-related phenotypes. The control of cell surface expression of glucose transporters derived from Glut1 and Glut4 genes and increasing sensitivity to insulin is a notable characteristic of this module (function of Sort1, Strip1, and Ecm1 products), and the inclusion of Hiat1 as the hub gene in this module gives a novel context to the possible function of this postulated, novel, sugar transporter. Strip1 also is involved in Stk25 gene product function related to expression of uncoupling protein 3, which is located primarily to BAT and muscle (6). Further impact of the components of this module on glucose metabolism can be imputed from the involvement of FMO5, which increases glucose metabolism and insulin sensitivity in adipocytes and the function of TRIM2 in maintaining adrenergic innervation of BAT. Three components of the module play a significant role in the differentiation and maturation of BAT, PRMT6 acting to influence the transcription of Pparg, Pdgfc, and Kcnd3. The incorporation of carbons derived from glucose into lipids by components of the Darkseagreen module may be related to the degradation of acetyl-CoA carboxylase after the ubiquitination of this enzyme by TRIM2, the degradation event would reduce the conversion of acetyl-CoA to malonyl-CoA. Malonyl-CoA relieves the inhibition of carnitine palmitoyl-CoA transferase-1, leading to increases in mitochondrial fatty acid oxidation (46). This, in concert with induction of the Warburg effect by ECM1, and the increase of LDH expression by ECM1 would, in a complex and interactive fashion, impact the incorporation of glucose carbons into fatty acids in BAT. The other components of the Darkseagreen module that influence lipid metabolism and generation of lipids from carbohydrates are the Dennd2d and Ctse products that affect the function of lysosomes. Lysosomes have been shown to be the sites of “lipophagy” (86), which is an autophagic process for the degradation of cholesterol esters and triglycerides stored in multilocular small lipid droplets in BAT. The products of lipophagy are free fatty acids, which can be oxidized in mitochondria. Depending on the level of lipophagy within lysosomes, fatty acid production from this source can influence the de novo production of fatty acids from carbohydrate metabolites. In all, the Darkseagreen module balances glucose uptake, aerobic and anaerobic glucose metabolism, and production of fatty acids from degradation of stored lipids vs. de novo synthesis. This certainly is a complicated and speculative scheme of how the components of the Darkseagreen module may cause a predisposition to a quantitative control of the incorporation of glucose carbons into lipids, but the scheme produces a true appreciation of the interactive systems that can impact a phenotype.
Functional relationship of the Coral4.1 module genes to relative BAT mass.
The components of the Coral 4.1 module carry on the theme of lipid metabolism, but with relation to the phenotype of relative mass of BAT across the strains of the BXH/HXB panel (Fig. 6B). The Coral 4.1 module includes Cd36, a highly connected gene. The Cd36 gene in the parental SHR strain carries a deletion that is responsible for reduced long chain fatty acid uptake in tissues and plays an important role in predispositions to dyslipidemia, insulin resistance, and high blood pressure (2, 60, 61). It should be noted that our analysis pipeline, using an unsupervised approach for distinguishing among 1,147 modules identified in BAT, gave evidence that the Coral 4.1 module, of which Cd36 is included, causes a predisposition to the BAT relative mass phenotype. This is a relevant correlation to the previously assigned roles of Cd36 in physiological components of metabolic syndrome modeled in rats (2).
CD36 protein has long been known to act as a transporter of long chain fatty acids and oxidized low-density lipoproteins into various cell types, including BAT (10). Aitman et al. (2) were the first to bring attention to a deletion variant of Cd36 in SHR rats (one of the parental strains of the HXB/BXH RI panel) that is associated with defective fatty acid metabolism and hypertriglyceridemia in SHR rats. Cd36 can also transduce signals relevant to complications of obesity (2, 10). Genetic studies in humans have found that mutations in the CD36 gene are associated with components of metabolic syndrome and high concentrations of circulating lipids (22, 45). More recently, Cd36 has been linked to fat taste perception (20, 48). Cd36 expression in taste buds has been correlated with the propensity to consume fatty foods (66). Interestingly, Cd36 expression in the gastrointestinal track has also been linked to fatty acid sensitivity and obesity (63). The expression of Cd36 in other cell types, such as BAT, may thus predict that Cd36 may have a fat-sensing role as well as a fat uptake function in BAT. Another highly connected transcript within this module is Gnat3, which produces α-gustducin. α-Gustducin is an α-subunit of the G protein first identified as part of the taste transduction system for bitter taste receptors (23), and bitter-tasting compounds increased adrenergic signaling in BAT and expression of UCP-1 (51). A recent genetic association study of metabolic syndrome in Mexican Americans further implicated a region on human chromosome 7q, which contains CD36, as being associated with metabolic syndrome (22). Interestingly, two SNPs in this region, one in CD36 and one in the nearby GNAT3, displayed the strongest association with metabolic syndrome and together explained ~18% of the variance in the metabolic syndrome phenotype in this population (22). α-Gustducin activates a phosphodiesterase to decrease cellular cAMP levels (23), and the Gβγ-subunits of the gustducin-containing G protein can activate phospholipase-C β2 and IP3-mediated release of calcium from intracellular stores (23). The genetic association of CD36 and α-gustducin with metabolic syndrome in humans (22) and the association of Cd36 and Gnat3 expression in the Coral 4.1 module provide for a hypothesis that Cd36 and α-gustducin may both be involved with intracellular signaling in response to fatty acids in BAT. Data obtained with α-gustducin KO mice do indicate some of the end products (targets) that may be susceptible to gustducin instigated signaling in BAT. Avau et al. (5) showed that gustducin KO mice (α-gust−/−) fed a high-fat diet demonstrated a 2.4-fold increase in expression of UCP1 in intercapsular brown adipose tissue, coupled with a decrease (albeit not statistically significant) in the weight of the intercapsular BAT.
Heat production by BAT, in response to cold, is dependent on adrenergic signaling through the β3-adrenergic receptors expressed on BAT adipocytes (8). The agonist (NE) stimulation of the β3-adrenergic receptors is coupled to increases in intracellular cAMP, which results in BAT hyperplasia and angiogenesis in BAT. Overall, there is a pronounced expansion in BAT mass due to adrenergic stimulation during cold adaptation (64), and sympathetic innervation has been shown to be a necessary element in the maintenance of this phenotype (64). Sema3a, semaphorin-3A, is the hub gene for Coral4.1 module, and semaphorin-3A is involved in the growth and plasticity of the sympathetic innervation of various tissue (15), including BAT (8). Sema3a is expressed in BAT, and BAT was illustrated to secrete semaphorin in rats (24). When the rats were acclimated to cold, a change in semaphorin secretion was observed, leading the authors to suggest (24) that semaphorin plays a role in the plastic adjustment of BAT sympathetic innervation in response to functional demand. It should be noted that semaphorin-3A also plays an important role in angiogenesis (56). Both sympathetic innervation and angiogenesis are important components of increases in BAT mass (47). Our data also demonstrated that semaphorin-3A mRNA is expressed in BAT and it is related to BAT relative mass in concert with other components of the Coral 4.1 module. Pmpcb (peptidase, mitochondrial processing beta subunit) is also referred to as Mppb, and the protein product of this gene is a mitochondrial matrix-processing peptidase (52). MPPB has been shown to be necessary for proteolytic cleavage of full-length SIRT3 protein to its active 28 KDa form after mitochondrial targeting of the full-length SIRT3 (71). SIRT3 is a NAD+-dependent protein deacetylase that is exclusively localized in mitochondria (4), and BAT has been shown to have high levels of SIRT3. SIRT3-mediated deacetylation regulates global mitochondrial function including the tricarboxylic acid cycle, cytochrome-mediated oxidative phosphorylation, and fatty acid β-oxidation (25). In BAT, SIRT3 plays an important role in adaptive thermogenesis, and higher levels of SIRT3 can reduce the generation of reactive oxygen species while causing increased cellular respiration (25). In the absence of SIRT3 fatty acid oxidation intermediates and triglycerides accumulate in BAT, and this phenomenon has been clearly evident in several mouse models of genetically induced obesity (55). Although much is known regarding the function of SIRT3 in mitochondria, little is known about another product of a transcript that is part of the Coral 4.1 module. The Tmem243 (transmembrane protein 243) product has been referred to as MM-TRAG because of its mitochondrial localization and its increased expression in cells exposed to taxol (21). The increased expression of MM-TRAG has been suggested to lead to taxol resistance in human cancers (21), but the role of MM-TRAG in normal mitochondrial function and particularly in BAT mitochondria has not been investigated. The coexpression relationship of MM-TRAG with the other members of the Coral 4.1 module does, however, present some obvious functions that MM-TRAG may sustain (e.g., mitochondrial transport and/or respiration).
The Snora43 (small nucleolar RNA, H/ACA box 43) transcript codes for a small nucleolar RNA (snoRNA) with no currently known specific function. The snoRNAs, as a class, act as substrate (pre-rRNA) sequence recognition molecules that guide proteins to sites for o-methylation or the isomerization of uridine in ribosomal and transfer RNAs as well as some mRNAs (37, 50). Recently snoRNAs have been recognized as possible contributors to the developmental delay and morbid obesity that characterize the Prader-Willi syndrome (3, 14). The expression of Snora43 is, however, strongly correlated with Cd36 expression and may have a functional relationship to the expression of Cd36. The final member of the Coral 4.1 coexpression module is Olr371 (olfactory receptor 371), which generates a G protein-coupled odorant receptor. It is of interest to note the expression of an odorant receptor in BAT, since this may add another point of evidence that BAT may exhibit a sensory modality (5, 70). The expression of odorant receptors outside the olfactory epithelium has been previously demonstrated (62, 75). An olfactory receptor, Olf1393 has been shown to be expressed in the proximal tubule of the kidney and to be involved in the localization of the glucose transporter Sgtl1 in the luminal aspect of the proximal tubule (75), and a high-fat diet was shown to regulate the expression of three odorant receptors in the rat duodenum (62).
In all, the components of the Coral 4.1 module give the impression that they are participants in various intracellular and extracellular sensing and signaling functions in BAT. The overlap of the module QTL with the QTL for BAT relative mass would indicate that the function of the module constituents is in the expansion of BAT, including the participation of the module hub gene, Sema3a, in innervation of BAT by the sympathetic nervous system. The correlation between the expression of Cd36 and the mitochondrial matrix processing peptidase (Mppb) transcript is worth noting. It would be biologically relevant to couple production of an entity functioning to accumulate fatty acids and an entity that converts SIRT3 to its active form, which, in turn, promotes β-oxidation of fatty acids in the brown adipocyte.
The phenotypic QTLs for glucose oxidation and incorporation of glucose into lipids overlap (1.5 LOD support interval), and the interval is located between 167.6 and 224.1 Mb on chromosome 2. Previously, the SHR-2 congenic strain was created (11), in which the BN region Chr 2: 116.1−228.7 Mb was introgressed from the BN strain onto an SHR background. The introgressed interval covers the area of the QTLs for both glucose oxidation and glucose incorporation into lipid. When assessing the phenotypes of glucose oxidation or glucose carbon incorporation into lipids, we found the BN strain exhibited higher values than the SHR strain, and the congenic SHR-2 strain also exhibited higher values for both phenotypes compared with SHR (Fig. 7, A and B). The congenic strain allows one to capture the coding area of many of the genes whose expression values comprise the Darkseagreen module. In addition, since the expression of the most connected genes (Supplementary Table S1) in the module is controlled in cis, one would expect that the promoter/repressor and 3′-untranslated regions that control the steady-state levels of the mRNA would also be captured in the congenic.
Although the results with the SHR-2 congenic rats provide credence to the assertion that the interval on chromosome 2 and the components of the Darkseagreen module play an important role in determining glucose oxidation and glucose incorporation into lipids in BAT, one should note a caveat, i.e., there is clear transgression in phenotype values beyond the values displayed by SHR and BN strains (Fig. 2), and thus additional factors must play a role.
With regard to the phenotype of BAT relative mass and the relationship of the Coral 4.1 module to this phenotype, the literature has provided much evidence that the Cd36 gene product, which was highly connected within the Coral 4.1 module, is an integral component of BAT function. Thus, it may not be surprising that an association was found by our studies between the Coral 4.1 module with Cd36 as its hub gene and BAT relative mass. What is surprising and heartening is that this association became evident through a nonsupervised analytical process that started with 1,147 modules available for association with the phenotype. In addition, the systems (module) approach implicates both a novel function for Cd36 as a fat sensor, as well as its function as a transporter, and the involvement of BAT innervation through Sema3a as important components associated with BAT relative mass.
The phenotypic QTL for relative BAT mass lies within a region of chromosome 4 that was also associated with insulin action (1). Because of this region’s association with insulin action, the SHR-4 congenic strain was previously created (59), in which the BN region Chr 4: 4.8–83.9 Mb was introgressed from the BN strain onto an SHR background. The introgressed interval covers the area of the peak of the QTL for relative BAT mass and the majority of its 1.5 LOD support interval. When assessing the phenotype of relative BAT mass, we found the BN strain exhibited lower values than the SHR strain, and the congenic SHR-4 strain also exhibited lower values for both phenotypes compared with SHR (Fig. 7C). Like the congenic for chromosome 2, this congenic strain allows one to capture the coding area of many of the genes whose expression values comprise the Coral4.1 module.
The combination of a genomic association analysis based on DNA markers with a physiological phenotype across an RI panel of rats and the simultaneous association analysis of RNA coexpression modules in the phenotype-relevant tissue of the RI panel allows us to ascertain whether the same genomic regions contribute to the expression of the physiological phenotype and the expression of genes within the module as described by its first principal component (i.e., its module eigengene). If the same genomic region contains the QTL for both the phenotype and the module eigengene, one can postulate that the two are genetically related, or the coexpression module is an endophenotype (intermediate phenotype) of the measured physiological phenotype (78). In discussing the implications of gene coexpression analysis (17) we stress the predictive power of systems genetics (network analysis) in uncovering novel relationships between gene products and concerted contributions of gene expression modules/networks to complex traits. The results of the analysis of data we have generated on the characteristics of BAT (both transcriptional and physiological) are satisfying because our systems approach provided insight into BAT biology, but also because knowing the contribution of BAT to several aspects of metabolic syndrome has provided plausible BAT multigene correlates of metabolic syndrome.
It should be noted that the systems genetics approach that we employed is meant to be an initial step on the path to understanding the role of BAT in metabolism. As such, our methods and models were not designed to identify every biological entity involved. With an RI panel, we will only be able to identify genetic variants and their downstream consequences that are present in this population. By studying RNA expression levels rather than protein levels, we may miss genes/proteins that vary as a result of posttranslational modifications. By using microarrays as opposed to RNA-Seq, we are restricted to well-annotated genes and lack resolution at the individual splice-variant level. We used a genome-wide P value to control the family-wise error rate when testing multiple markers in our module eigengene QTL analysis. However, we did not adjust for the multiple testing across modules (module eigengene QTL or genetic correlations). This is a limitation of the study, as it will increase the chance of false positives for either test individually; however, the fact that candidate modules had to have a significant module eigengene QTL that overlapped a phenotypic QTL, as well as a significant correlation between the module eigengene and the strain phenotypic values, helps mitigate this concern. Overall, the work presented here has allowed us to derive biologically meaningful results, and these results will allow us and others to construct specific functional hypotheses for further studies.
GRANTS
This work was supported by Czech Science Foundation Grant 13-04420S to M. Pravenec. B. Tabakoff, L. Saba, and H. Smith derived support from National Institute on Alcohol Abuse and Alcoholism Grant R24AA-013162.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.P. and B.T. conceived and designed research; M.P., L.M.S., H. Strnad, L.K., H. Smith, and B.T. analyzed data; M.P., L.M.S., H. Smith, and B.T. interpreted results of experiments; M.P., L.M.S., H. Smith, and B.T. prepared figures; M.P., L.M.S., H. Smith, and B.T. drafted manuscript; M.P., L.M.S., and B.T. edited and revised manuscript; M.P. approved final version of manuscript; V.Z., V.L., P.M., J.S., M.S., H. Strnad, J.T., V.S., M.H., I.M., O.O., H.M., and L.K. performed experiments.
Supplemental Data
Summary of Genes in Co-Expression Candidate Modules
REFERENCES
- 1.Aitman TJ, Gotoda T, Evans AL, Imrie H, Heath KE, Trembling PM, Truman H, Wallace CA, Rahman A, Doré C, Flint J, Kren V, Zidek V, Kurtz TW, Pravenec M, Scott J. Quantitative trait loci for cellular defects in glucose and fatty acid metabolism in hypertensive rats. Nat Genet 16: 197–201, 1997. doi: 10.1038/ng0697-197. [DOI] [PubMed] [Google Scholar]
- 2.Aitman TJ, Glazier AM, Wallace CA, Cooper LD, Norsworthy PJ, Wahid FN, Al-Majali KM, Trembling PM, Mann CJ, Shoulders CC, Graf D, St Lezin E, Kurtz TW, Kren V, Pravenec M, Ibrahimi A, Abumrad NA, Stanton LW, Scott J. Identification of Cd36 (Fat) as an insulin-resistance gene causing defective fatty acid and glucose metabolism in hypertensive rats. Nat Genet 21: 76–83, 1999. doi: 10.1038/5013. [DOI] [PubMed] [Google Scholar]
- 3.Amri EZ, Scheideler M. Small non coding RNAs in adipocyte biology and obesity. Mol Cell Endocrinol 456: 87–94, 2017. doi: 10.1016/j.mce.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Ansari A, Rahman MS, Saha SK, Saikot FK, Deep A, Kim KH. Function of the SIRT3 mitochondrial deacetylase in cellular physiology, cancer, and neurodegenerative disease. Aging Cell 16: 4–16, 2017. doi: 10.1111/acel.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avau B, Bauters D, Steensels S, Vancleef L, Laermans J, Lesuisse J, Buyse J, Lijnen HR, Tack J, Depoortere I. The gustatory signaling pathway and bitter taste receptors affect the development of obesity and adipocyte metabolism in mice. PLoS One 10: e0145538, 2015. doi: 10.1371/journal.pone.0145538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azzu V, Jastroch M, Divakaruni AS, Brand MD. The regulation and turnover of mitochondrial uncoupling proteins. Biochim Biophys Acta 1797: 785–791, 2010. doi: 10.1016/j.bbabio.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bargut TC, Aguila MB, Mandarim-de-Lacerda CA. Brown adipose tissue: Updates in cellular and molecular biology. Tissue Cell 48: 452–460, 2016. doi: 10.1016/j.tice.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Bartness TJ, Vaughan CH, Song CK. Sympathetic and sensory innervation of brown adipose tissue. Int J Obes 34, Suppl 1: S36–S42, 2010. doi: 10.1038/ijo.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, Eychmüller A, Gordts PL, Rinninger F, Bruegelmann K, Freund B, Nielsen P, Merkel M, Heeren J. Brown adipose tissue activity controls triglyceride clearance. Nat Med 17: 200–205, 2011. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 10.Bartelt A, Heeren J. The holy grail of metabolic disease: brown adipose tissue. Curr Opin Lipidol 23: 190–195, 2012. doi: 10.1097/MOL.0b013e328352dcef. [DOI] [PubMed] [Google Scholar]
- 11.Bielavská E, Křen V, Musilová A, Zídek V, Pravenec M. Genome scanning of the HXB/BXH sets of recombinant inbred strains of the rat for quantitative trait loci associated with conditioned taste aversion. Behav Genet 32: 51–56, 2002. doi: 10.1023/A:1014407928865. [DOI] [PubMed] [Google Scholar]
- 12.Broman KW, Wu H, Sen S, Churchill GA. R/qtl: QTL mapping in experimental crosses. Bioinformatics 19: 889–890, 2003. doi: 10.1093/bioinformatics/btg112. [DOI] [PubMed] [Google Scholar]
- 13.Cardamone MD, Tanasa B, Chan M, Cederquist CT, Andricovich J, Rosenfeld MG, Perissi V. GPS2/KDM4A pioneering activity regulates promoter-specific recruitment of PPARγ. Cell Reports 8: 163–176, 2014. doi: 10.1016/j.celrep.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med 14: 10–26, 2012. doi: 10.1038/gim.0b013e31822bead0. [DOI] [PubMed] [Google Scholar]
- 15.Chilton JK. Molecular mechanisms of axon guidance. Dev Biol 292: 13–24, 2006. doi: 10.1016/j.ydbio.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 16.Churchill GA, Doerge RW. Empirical threshold values for quantitative trait mapping. Genetics 138: 963–971, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Civelek M, Lusis AJ. Systems genetics approaches to understand complex traits. Nat Rev Genet 15: 34–48, 2014. doi: 10.1038/nrg3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke RJ, Catauro M, Rasmussen HH, Apell HJ. Quantitative calculation of the role of the Na(+),K(+)-ATPase in thermogenesis. Biochim Biophys Acta 1827: 1205–1212, 2013. doi: 10.1016/j.bbabio.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Di Lorenzo A, Bedford MT. Histone arginine methylation. FEBS Lett 585: 2024–2031, 2011. doi: 10.1016/j.febslet.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dramane G, Akpona S, Besnard P, Khan NA. Cell mechanisms of gustatory lipids perception and modulation of the dietary fat preference. Biochimie 107: 11–14, 2014. doi: 10.1016/j.biochi.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Duan Z, Brakora KA, Seiden MV. MM-TRAG (MGC4175), a novel intracellular mitochondrial protein, is associated with the taxol- and doxorubicin-resistant phenotype in human cancer cell lines. Gene 340: 53–59, 2004. doi: 10.1016/j.gene.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Farook VS, Puppala S, Schneider J, Fowler SP, Chittoor G, Dyer TD, Allayee H, Cole SA, Arya R, Black MH, Curran JE, Almasy L, Buchanan TA, Jenkinson CP, Lehman DM, Watanabe RM, Blangero J, Duggirala R. Metabolic syndrome is linked to chromosome 7q21 and associated with genetic variants in CD36 and GNAT3 in Mexican Americans. Obesity (Silver Spring) 20: 2083–2092, 2012. doi: 10.1038/oby.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilbertson TA, Damak S, Margolskee RF. The molecular physiology of taste transduction. Curr Opin Neurobiol 10: 519–527, 2000. doi: 10.1016/S0959-4388(00)00118-5. [DOI] [PubMed] [Google Scholar]
- 24.Giordano A, Coppari R, Castellucci M, Cinti S. Sema3a is produced by brown adipocytes and its secretion is reduced following cold acclimation. J Neurocytol 30: 5–10, 2001. doi: 10.1023/A:1011916822633. [DOI] [PubMed] [Google Scholar]
- 25.Giralt A, Villarroya F. SIRT3, a pivotal actor in mitochondrial functions: metabolism, cell death and aging. Biochem J 444: 1–10, 2012. doi: 10.1042/BJ20120030. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez Malagon SG, Melidoni AN, Hernandez D, Omar BA, Houseman L, Veeravalli S, Scott F, Varshavi D, Everett J, Tsuchiya Y, Timms JF, Phillips IR, Shephard EA. The phenotype of a knockout mouse identifies flavin-containing monooxygenase 5 (FMO5) as a regulator of metabolic ageing. Biochem Pharmacol 96: 267–277, 2015. doi: 10.1016/j.bcp.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med 19: 1252–1263, 2013. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 28.Harrall KK, Kechris KJ, Tabakoff B, Hoffman PL, Hines LM, Tsukamoto H, Pravenec M, Printz M, Saba LM. Uncovering the liver’s role in immunity through RNA co-expression networks. Mamm Genome 27: 469–484, 2016. doi: 10.1007/s00335-016-9656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasanagic M, Waheed A, Eissenberg JC. Different pathways to the lysosome: Sorting out alternatives. Int Rev Cell Mol Biol 320: 75–101, 2015. doi: 10.1016/bs.ircmb.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Huang J, Cardamone MD, Johnson HE, Neault M, Chan M, Floyd ZE, Mallette FA, Perissi V. Exchange factor TBL1 and arginine methyltransferase PRMT6 cooperate in protecting G protein pathway suppressor 2 (GPS2) from proteasomal degradation. J Biol Chem 290: 19044–19054, 2015. doi: 10.1074/jbc.M115.637660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hübner N, Wallace CA, Zimdahl H, Petretto E, Schulz H, Maciver F, Mueller M, Hummel O, Monti J, Zídek V, Musilová A, Křen V, Causton H, Game L, Born G, Schmidt S, Müller A, Cook SA, Kurtz TW, Whittaker J, Pravenec M, Aitman TJ. Integrated transcriptional profiling and linkage analysis for identification of genes underlying disease. Nat Genet 37: 243–253, 2005. doi: 10.1038/ng1522. [DOI] [PubMed] [Google Scholar]
- 32.Hwang J, Pallas DC. STRIPAK complexes: structure, biological function, and involvement in human diseases. Int J Biochem Cell Biol 47: 118–148, 2014. doi: 10.1016/j.biocel.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res 31: e15, 2003. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kadowaki T, Kido MA, Hatakeyama J, Okamoto K, Tsukuba T, Yamamoto K. Defective adipose tissue development associated with hepatomegaly in cathepsin E-deficient mice fed a high-fat diet. Biochem Biophys Res Commun 446: 212–217, 2014. doi: 10.1016/j.bbrc.2014.02.089. [DOI] [PubMed] [Google Scholar]
- 35.Kim S. ppcor: An R package for a fast calculation to semi-partial correlation coefficients. Commun Stat Appl Methods 22: 665–674, 2015. doi: 10.5351/CSAM.2015.22.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim WS, Park HS, Sung JH. The pivotal role of PDGF and its receptor isoforms in adipose-derived stem cells. Histol Histopathol 30: 793–799, 2015. doi: 10.14670/HH-11-598. [DOI] [PubMed] [Google Scholar]
- 37.Kiss T. Small nucleolar RNAs: an abundant group of noncoding RNAs with diverse cellular functions. Cell 109: 145–148, 2002. doi: 10.1016/S0092-8674(02)00718-3. [DOI] [PubMed] [Google Scholar]
- 38.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 288: 2709–2716, 2002. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 39.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9: 559, 2008. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lander ES, Botstein D. Mapping mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics 121: 185–199, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lander E, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet 11: 241–247, 1995. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- 42.Lee KM, Nam K, Oh S, Lim J, Lee T, Shin I. ECM1 promotes the Warburg effect through EGF-mediated activation of PKM2. Cell Signal 27: 228–235, 2015. doi: 10.1016/j.cellsig.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Lekholm E, Perland E, Eriksson MM, Hellsten SV, Lindberg FA, Rostami J, Fredriksson R. Putative membrane-bound transporters MFSD14A and MFSD14B are neuronal and affected by nutrient availability. Front Mol Neurosci 10: 11, 2017. doi: 10.3389/fnmol.2017.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li JJ, Ferry RJ Jr, Diao S, Xue B, Bahouth SW, Liao FF. Nedd4 haploinsufficient mice display moderate insulin resistance, enhanced lipolysis, and protection against high-fat diet-induced obesity. Endocrinology 156: 1283–1291, 2015. doi: 10.1210/en.2014-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Love-Gregory L, Sherva R, Sun L, Wasson J, Schappe T, Doria A, Rao DC, Hunt SC, Klein S, Neuman RJ, Permutt MA, Abumrad NA. Variants in the CD36 gene associate with the metabolic syndrome and high-density lipoprotein cholesterol. Hum Mol Genet 17: 1695–1704, 2008. doi: 10.1093/hmg/ddn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Love-Gregory L, Abumrad NA. CD36 genetics and the metabolic complications of obesity. Curr Opin Clin Nutr Metab Care 14: 527–534, 2011. doi: 10.1097/MCO.0b013e32834bbac9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo X, Jia R, Luo XQ, Wang G, Zhang QL, Qiao H, Wang N, Yan JQ. Cold exposure differentially stimulates angiogenesis in BAT and WAT of mice: Implication in adrenergic activation. Cell Physiol Biochem 42: 974–986, 2017. doi: 10.1159/000478680. [DOI] [PubMed] [Google Scholar]
- 48.Martin C, Passilly-Degrace P, Gaillard D, Merlin JF, Chevrot M, Besnard P. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PLoS One 6: e24014, 2011. doi: 10.1371/journal.pone.0024014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsuo N, Kawamoto S, Matsubara K, Okubo K. Cloning of a cDNA encoding a novel sugar transporter expressed in the neonatal mouse hippocampus. Biochem Biophys Res Commun 238: 126–129, 1997. doi: 10.1006/bbrc.1997.7252. [DOI] [PubMed] [Google Scholar]
- 50.McMahon M, Contreras A, Ruggero D. Small RNAs with big implications: new insights into H/ACA snoRNA function and their role in human disease. Wiley Interdiscip Rev RNA 6: 173–189, 2015. doi: 10.1002/wrna.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morimoto-Kobayashi Y, Ohara K, Takahashi C, Kitao S, Wang G, Taniguchi Y, Katayama M, Nagai K. Matured hop bittering components induce thermogenesis in brown adipose tissue via sympathetic nerve activity. PLoS One 10: e0131042, 2015. doi: 10.1371/journal.pone.0131042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mossmann D, Meisinger C, Vögtle FN. Processing of mitochondrial presequences. Biochim Biophys Acta 1819: 1098–1106, 2012. doi: 10.1016/j.bbagrm.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 53.Nedergaard J, Petrovic N, Lindgren EM, Jacobsson A, Cannon B. PPARgamma in the control of brown adipocyte differentiation. Biochim Biophys Acta 1740: 293–304, 2005. doi: 10.1016/j.bbadis.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 54.Nerstedt A, Cansby E, Andersson CX, Laakso M, Stančáková A, Blüher M, Smith U, Mahlapuu M. Serine/threonine protein kinase 25 (STK25): a novel negative regulator of lipid and glucose metabolism in rodent and human skeletal muscle. Diabetologia 55: 1797–1807, 2012. doi: 10.1007/s00125-012-2511-7. [DOI] [PubMed] [Google Scholar]
- 55.Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO, Horvath TL, Sinclair DA, Pfluger PT, Tschöp MH. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev 92: 1479–1514, 2012. doi: 10.1152/physrev.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plein A, Fantin A, Ruhrberg C. Neuropilin regulation of angiogenesis, arteriogenesis, and vascular permeability. Microcirculation 21: 315–323, 2014. doi: 10.1111/micc.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pravenec M, Křen V, Landa V, Mlejnek P, Musilová A, Šilhavý J, Šimáková M, Zídek V. Recent progress in the genetics of spontaneously hypertensive rats. Physiol Res 63, Suppl 1: S1–S8, 2014. [DOI] [PubMed] [Google Scholar]
- 58.Pravenec M, Zídek V, Musilová A, Vorlícek J, Křen V, St Lezin E, Kurtz TW. Genetic isolation of a blood pressure quantitative trait locus on chromosome 2 in the spontaneously hypertensive rat. J Hypertens 19: 1061–1064, 2001. doi: 10.1097/00004872-200106000-00010. [DOI] [PubMed] [Google Scholar]
- 59.Pravenec M, Zídek V, Šimáková M, Křen V, Křenová D, Horký K, Jáchymová M, Miková B, Kazdová L, Aitman TJ, Churchill PC, Webb RC, Hingarh NH, Yang Y, Wang JM, Lezin EM, Kurtz TW. Genetics of Cd36 and the clustering of multiple cardiovascular risk factors in spontaneous hypertension. J Clin Invest 103: 1651–1657, 1999. doi: 10.1172/JCI6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pravenec M, Landa V, Zídek V, Musilová A, Křen V, Kazdová L, Aitman TJ, Glazier AM, Ibrahimi A, Abumrad NA, Qi N, Wang JM, St Lezin EM, Kurtz TW. Transgenic rescue of defective Cd36 ameliorates insulin resistance in spontaneously hypertensive rats. Nat Genet 27: 156–158, 2001. doi: 10.1038/84777. [DOI] [PubMed] [Google Scholar]
- 61.Pravenec M, Churchill PC, Churchill MC, Viklický O, Kazdová L, Aitman TJ, Petretto E, Hübner N, Wallace CA, Zimdahl H, Zídek V, Landa V, Dunbar J, Bidani A, Griffin K, Qi N, Maxová M, Křen V, Mlejnek P, Wang J, Kurtz TW. Identification of renal Cd36 as a determinant of blood pressure and risk for hypertension. Nat Genet 40: 952–954, 2008. doi: 10.1038/ng.164. [DOI] [PubMed] [Google Scholar]
- 62.Primeaux SD, Braymer HD, Bray GA. High fat diet differentially regulates the expression of olfactory receptors in the duodenum of obesity-prone and obesity-resistant rats. Dig Dis Sci 58: 72–76, 2013. doi: 10.1007/s10620-012-2421-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Primeaux SD, Braymer HD, Bray GA. CD36 mRNA in the gastrointestinal tract is differentially regulated by dietary fat intake in obesity-prone and obesity-resistant rats. Dig Dis Sci 58: 363–370, 2013. doi: 10.1007/s10620-012-2364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramseyer VD, Granneman JG. Adrenergic regulation of cellular plasticity in brown, beige/brite and white adipose tissues. Adipocyte 5: 119–129, 2016. doi: 10.1080/21623945.2016.1145846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reinhard L, Tidow H, Clausen MJ, Nissen P. Na(+),K (+)-ATPase as a docking station: protein-protein complexes of the Na(+),K (+)-ATPase. Cell Mol Life Sci 70: 205–222, 2013. doi: 10.1007/s00018-012-1039-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Riederer MA, Soldati T, Shapiro AD, Lin J, Pfeffer SR. Lysosome biogenesis requires Rab9 function and receptor recycling from endosomes to the trans-Golgi network. J Cell Biol 125: 573–582, 1994. doi: 10.1083/jcb.125.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qi L, Heredia JE, Altarejos JY, Screaton R, Goebel N, Niessen S, Macleod IX, Liew CW, Kulkarni RN, Bain J, Newgard C, Nelson M, Evans RM, Yates J, Montminy M. TRB3 links the E3 ubiquitin ligase COP1 to lipid metabolism. Science 312: 1763–1766, 2006. doi: 10.1126/science.1123374. [DOI] [PubMed] [Google Scholar]
- 68.Saba LM, Flink SC, Vanderlinden LA, Israel Y, Tampier L, Colombo G, Kiianmaa K, Bell RL, Printz MP, Flodman P, Koob G, Richardson HN, Lombardo J, Hoffman PL, Tabakoff B. The sequenced rat brain transcriptome–its use in identifying networks predisposing alcohol consumption. FEBS J 282: 3556–3578, 2015. doi: 10.1111/febs.13358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saito M. Brown adipose tissue as a therapeutic target for human obesity. Obes Res Clin Pract 7: e432–e438, 2013. doi: 10.1016/j.orcp.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 70.Sakamoto T, Takahashi N, Goto T, Kawada T. Dietary factors evoke thermogenesis in adipose tissues. Obes Res Clin Pract 8: e533–e539, 2014. doi: 10.1016/j.orcp.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 71.Schwer B, North BJ, Frye RA, Ott M, Verdin E. The human silent information regulator (Sir)2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase. J Cell Biol 158: 647–657, 2002. doi: 10.1083/jcb.200205057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scott F, Gonzalez Malagon SG, O’Brien BA, Fennema D, Veeravalli S, Coveney CR, Phillips IR, Shephard EA. Identification of flavin-containing monooxygenase 5 (FMO5) as a regulator of glucose homeostasis and a potential sensor of gut bacteria. Drug Metab Dispos 45: 982–989, 2017. doi: 10.1124/dmd.117.076612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seki T, Hosaka K, Lim S, Fischer C, Honek J, Yang Y, Andersson P, Nakamura M, Näslund E, Ylä-Herttuala S, Sun M, Iwamoto H, Li X, Liu Y, Samani NJ, Cao Y. Endothelial PDGF-CC regulates angiogenesis-dependent thermogenesis in beige fat. Nat Commun 7: 12152, 2016. doi: 10.1038/ncomms12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13: 2498–2504, 2003. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shepard BD, Cheval L, Peterlin Z, Firestein S, Koepsell H, Doucet A, Pluznick JL. A renal olfactory receptor aids in kidney glucose handling. Sci Rep 6: 35215, 2016. doi: 10.1038/srep35215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shi J, Kandror KV. Sortilin is essential and sufficient for the formation of Glut4 storage vesicles in 3T3-L1 adipocytes. Dev Cell 9: 99–108, 2005. doi: 10.1016/j.devcel.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 77.Shi J, Kandror KV. The luminal Vps10p domain of sortilin plays the predominant role in targeting to insulin-responsive Glut4-containing vesicles. J Biol Chem 282: 9008–9016, 2007. doi: 10.1074/jbc.M608971200. [DOI] [PubMed] [Google Scholar]
- 78.Sieberts SK, Schadt EE. Moving toward a system genetics view of disease. Mamm Genome 18: 389–401, 2007. doi: 10.1007/s00335-007-9040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Srivastava S, Li Z, Lin L, Liu G, Ko K, Coetzee WA, Skolnik EY. The phosphatidylinositol 3-phosphate phosphatase myotubularin- related protein 6 (MTMR6) is a negative regulator of the Ca2+-activated K+ channel KCa3.1. Mol Cell Biol 25: 3630–3638, 2005. doi: 10.1128/MCB.25.9.3630-3638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.STAR Consortium; Saar K, Beck A, Bihoreau MT, Birney E, Brocklebank D, Chen Y, Cuppen E, Demonchy S, Dopazo J, Flicek P, Foglio M, Fujiyama A, Gut IG, Gauguier D, Guigo R, Guryev V, Heinig M, Hummel O, Jahn N, Klages S, Kren V, Kube M, Kuhl H, Kuramoto T, Kuroki Y, Lechner D, Lee YA, Lopez-Bigas N, Lathrop GM, Mashimo T, Medina I, Mott R, Patone G, Perrier-Cornet JA, Platzer M, Pravenec M, Reinhardt R, Sakaki Y, Schilhabel M, Schulz H, Serikawa T, Shikhagaie M, Tatsumoto S, Taudien S, Toyoda A, Voigt B, Zelenika D, Zimdahl H, Hubner N. SNP and haplotype mapping for genetic analysis in the rat. Nat Genet 40: 560–566, 2008. doi: 10.1038/ng.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tsujita K, Itoh T, Kondo A, Oyama M, Kozuka-Hata H, Irino Y, Hasegawa J, Takenawa T. Proteome of acidic phospholipid-binding proteins: spatial and temporal regulation of Coronin 1A by phosphoinositides. J Biol Chem 285: 6781–6789, 2010. doi: 10.1074/jbc.M109.057018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vasconcelos LH, Souza IL, Pinheiro LS, Silva BA. Ion channels in obesity: Pathophysiology and potential therapeutic targets. Front Pharmacol 7: 58, 2016. doi: 10.3389/fphar.2016.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vijgen GH, Bouvy ND, Teule GJ, Brans B, Schrauwen P, van Marken Lichtenbelt WD. Brown adipose tissue in morbidly obese subjects. PLoS One 6: e17247, 2011. doi: 10.1371/journal.pone.0017247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Virtanen KA. The rediscovery of BAT in adult humans using imaging. Best Pract Res Clin Endocrinol Metab 30: 471–477, 2016. doi: 10.1016/j.beem.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 85.Wei H, Mundade R, Lange KC, Lu T. Protein arginine methylation of non-histone proteins and its role in diseases. Cell Cycle 13: 32–41, 2014. doi: 10.4161/cc.27353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wei P, Sun FD, Zuo LM, Qu J, Chen P, Xu LD, Luo SZ. Critical residues and motifs for homodimerization of the first transmembrane domain of the plasma membrane glycoprotein CD36. J Biol Chem 292: 8683–8693, 2017. doi: 10.1074/jbc.M117.779595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wilson SM, Lee SC, Shook S, Pappone PA. ATP and beta-adrenergic stimulation enhance voltage-gated K current inactivation in brown adipocytes. Am J Physiol Cell Physiol 279: C1847–C1858, 2000. doi: 10.1152/ajpcell.2000.279.6.C1847. [DOI] [PubMed] [Google Scholar]
- 88.Ylikallio E, Pöyhönen R, Zimon M, De Vriendt E, Hilander T, Paetau A, Jordanova A, Lönnqvist T, Tyynismaa H. Deficiency of the E3 ubiquitin ligase TRIM2 in early-onset axonal neuropathy. Hum Mol Genet 22: 2975–2983, 2013. doi: 10.1093/hmg/ddt149. [DOI] [PubMed] [Google Scholar]
- 89.Yoshimura S, Gerondopoulos A, Linford A, Rigden DJ, Barr FA. Family-wide characterization of the DENN domain Rab GDP-GTP exchange factors. J Cell Biol 191: 367–381, 2010. doi: 10.1083/jcb.201008051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yuan Z, Cai T, Tian J, Ivanov AV, Giovannucci DR, Xie Z. Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex. Mol Biol Cell 16: 4034–4045, 2005. doi: 10.1091/mbc.E05-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang B, Horvath S. A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol 4: Article17, 2005. doi: 10.2202/1544-6115.1128. [DOI] [PubMed] [Google Scholar]
- 92.Zhong LY, Cayabyab FS, Tang CK, Zheng XL, Peng TH, Lv YC. Sortilin: A novel regulator in lipid metabolism and atherogenesis. Clin Chim Acta 460: 11–17, 2016. doi: 10.1016/j.cca.2016.06.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of Genes in Co-Expression Candidate Modules