Abstract
Intramuscular triglyceride (IMTG) concentration is elevated in insulin-resistant individuals and was once thought to promote insulin resistance. However, endurance-trained athletes have equivalent concentration of IMTG compared with individuals with type 2 diabetes, and have very low risk of diabetes, termed the “athlete’s paradox.” We now know that IMTG synthesis is positively related to insulin sensitivity, but the exact mechanisms for this are unclear. To understand the relationship between IMTG synthesis and insulin sensitivity, we measured IMTG synthesis in obese control subjects, endurance-trained athletes, and individuals with type 2 diabetes during rest, exercise, and recovery. IMTG synthesis rates were positively related to insulin sensitivity, cytosolic accumulation of DAG, and decreased accumulation of C18:0 ceramide and glucosylceramide. Greater rates of IMTG synthesis in athletes were not explained by alterations in FFA concentration, DGAT1 mRNA expression, or protein content. IMTG synthesis during exercise in Ob and T2D indicate utilization as a fuel despite unchanged content, whereas IMTG concentration decreased during exercise in athletes. mRNA expression for genes involved in lipid desaturation and IMTG synthesis were increased after exercise and recovery. Further, in a subset of individuals, exercise decreased cytosolic and membrane di-saturated DAG content, which may help explain insulin sensitization after acute exercise. These data suggest IMTG synthesis rates may influence insulin sensitivity by altering intracellular lipid localization, and decreasing specific ceramide species that promote insulin resistance.
Keywords: IMCL, intramyocellular triglyceride, sphingolipid
INTRODUCTION
Intramuscular triglyceride (IMTG) concentration is inversely related to insulin-stimulated glucose disposal in rats (53), healthy humans (30, 40, 46), individuals with type 1 (13) and type 2 diabetes (15), and offspring of people with type 2 diabetes (25, 45). Although the literature supports a strong negative relationship between IMTG content and insulin sensitivity (13, 15, 30, 40, 45, 46), this relationship does not hold when endurance-trained athletes are included in the analysis (14, 55). Endurance athletes are very insulin sensitive and also have skeletal muscle IMTG concentration similar to that reported for people with type 2 diabetes, which is known as the “athlete’s paradox” (14, 55). IMTG can also be dissociated from insulin sensitivity by exercise training, which increases insulin sensitivity and has been reported to either not change (9, 38) or increase IMTG content (11, 23). This makes studying endurance athletes a useful tool to unraveling the relationships between skeletal muscle IMTG metabolism and insulin sensitivity.
In addition to data from athletes and exercise training, recent studies challenged the dogma that IMTG promotes insulin resistance. Women have greater IMTG content than men, yet are more insulin sensitive (24). Protection from fat-induced insulin resistance was reported with increased IMTG synthesis in muscle cell culture (33), a transgenic mouse model (34), following a single exercise bout in humans (50), and by inhibiting IMTG degradation in mice (21). Knocking out the ability to store intracellular triglyceride in hepatocytes induced insulin resistance on a high-fat diet (22). A new view has emerged where IMTG synthesis acts as a sink for storage of FFA transported into muscle and protects against the formation of lipid intermediates known to induce insulin resistance. This idea has been corroborated by our laboratory, showing that insulin-resistant men with prediabetes had lower rates of IMTG synthesis compared with obese men with normal glucose tolerance (43), and that endurance-trained athletes have rates of IMTG synthesis twice that of their lean, sedentary, age-matched counterparts (5). Together, these data suggest high rates of IMTG synthesis are protective against insulin resistance. However, it is not known if basal rates of IMTG synthesis differ in endurance-trained athletes relative to obese individuals with and without type 2 diabetes, and if IMTG synthesis impacts intracellular lipid intermediates thought to impact insulin sensitivity such as diacylglycerol and ceramide.
In addition to turnover at rest, IMTG can be used as a fuel for muscle contraction during exercise. During exercise, IMTG utilization is often (30, 52, 56, 60) but not always (29, 48) found in endurance-trained individuals, in part due to lower biopsy-to-biopsy variation in IMTG content in athletes (61). Interpretation of IMTG utilization during exercise is difficult due to differences in measurement methodology and population studied [see review (61)]. In contrast to data from athletes, IMTG degradation during exercise is not apparent in individuals with obesity, prediabetes, and type 2 diabetes (44, 57). Elevated serum FFA concentration in type 2 diabetes explained the lack of IMTG use during exercise in this population (57), and is supported by regulation of IMTG utilization by serum FFA during exercise (60). However, it is also possible that unchanged IMTG concentration during exercise in obesity and type 2 diabetes results from IMTG degradation that is matched by synthesis during exercise (16).
We performed this study to determine whether high rates of IMTG synthesis in endurance-trained athletes promote insulin sensitivity by altering intracellular lipid trafficking in skeletal muscle compared with obese individuals with and without type 2 diabetes. To achieve our aims, participants were studied at baseline, during a bout of acute exercise, and during recovery.
METHODS
Subjects.
We have previously published data from this cohort, including localized muscle DAG content, and the response to exercise and recovery for serum and muscle sphingolipids and phospholipids (1, 2, 4, 39). Fourteen obese sedentary controls (Ob), 15 individuals with type 2 diabetes (T2D), and 15 endurance-trained athletes were recruited for this study. Subjects gave written informed consent and were excluded if they had a body mass index (BMI) <20 kg/m2 or >25 kg/m2 for athletes, and BMI <28 kg/m2 or >40 kg/m2 for Ob and T2D. Subjects were excluded if they had fasting triglycerides >150 mg/dl, or liver, kidney, thyroid, or lung disease. Sedentary subjects were engaged in planned physical activity < 2 h/wk. Endurance-trained subjects were competitive cyclists, triathletes, and runners with average V̇o2max values of 47.8 ± 3.8 ml·kg−1·min−1, lactate thresholds of 78 ± 1.4% V̇o2max, and were training on average 12.3 ± 0.8 h/wk for the past 9.7 ± 2.1 yr for the purpose of competition. Individuals with type 2 diabetes were included in the study if they did not take insulin and/or thiazolidinediones. All other medications were washed out for 2 wk before metabolic testing. Control subjects and athletes were not taking medications. Subjects were weight stable in the 6 mo before the study. This study was approved by the Colorado Multiple Institution Review Board at the University of Colorado Denver.
Preliminary testing.
As previously reported, subjects reported to the Clinical Translational Research Center (CTRC) for screening procedures following a 12-h overnight fast, where they were given a health and physical examination, followed by a fasting blood draw (1, 2, 4, 39). Body composition was determined using Dual-Energy X-ray Absorptiometry (DEXA) analysis (Lunar DPX-IQ, Lunar, Madison, WI).
Insulin sensitivity.
Insulin sensitivity was determined during a second preliminary screening visit via an intravenous glucose tolerance test (IVGTT) using standard methods after an overnight fast (8), as previously reported (1, 2, 4, 39). Briefly, after baseline samples, intravenous glucose (0.3 g/kg) was infused over 1 min, followed by insulin at 0.03 U/kg, 20 min after glucose administration. Blood samples were then frequently sampled over 3 h, and whole body insulin sensitivity (Si) calculated using the Bergman minimal model (8) (Milennium Version, MINMOD, Los Angeles, CA). Following the IVGTT, all subjects performed a V̇o2max test on an electronically braked cycle ergometer (Lode Excalibur, Quinton Instruments, Seattle, WA). Respiratory gases were measured via indirect calorimetry (Parvo Medics, Sandy, UT).
Diet and exercise control.
All subjects were given a prescribed diet for 3 days before admission to the CTRC. Daily caloric requirement was estimated from the DEXA measurement of fat-free mass (FFM) using the equation: daily energy intake = 1.4 kcal/day × [372 + (23.9 × FFM)], and analysis of dietary records performed during the preliminary screening visit. As a percent of total energy intake, the composition of this diet was 55% carbohydrate, 30% fat, and 15% protein. The fat content of the diet was controlled with the composition of saturated, monounsaturated, and polyunsaturated fat in a 1:1:1 ratio. All subjects were asked to refrain from planned physical activity for 48 h before the muscle biopsy study.
Metabolic study.
As previously reported, subjects arrived in the morning after a 12-h overnight fast when an antecubital vein in one arm was cannulated for isotope infusion, and a retrograde dorsal hand vein in the contralateral side was catheterized for blood sampling via the heated hand technique (1, 2, 4, 39). To measure glucose and palmitate turnover and IMTG fractional synthesis rate (FSR), background blood samples were taken, followed by a primed (4.5 mg/kg) constant (0.03 mg·kg−1·min−1) infusion of [6,6-2H2]glucose, and a continuous infusion of [U-13C] palmitate (Isotec, Miamisburg, OH) (0.0174 µmol·kg−1·min−1). These infusions were continued throughout the study. Blood sampling and indirect calorimetry were performed during minutes 210, 220, 230, and 240 of infusion. A percutaneous needle biopsy was performed after 240 min of isotope infusion for lipidomic analysis. Muscle biopsies (~150 mg) were taken from midway between the greater trochanter of the femur and the patella from similar depth to minimize variance in muscle fiber composition which varies with depth and length in the vastus lateralis (31). Muscle was immediately flash-frozen in liquid nitrogen and stored at −80°C until analysis. Skeletal muscle samples were dissected free of extramuscular fat on ice as previously described (19).
After the resting muscle biopsy was taken, subjects exercised on a cycle ergometer for 1.5 h at a goal exercise intensity of 50% of V̇o2max. Isotope infusion rates were doubled during exercise to help maintain steady-state plasma enrichments. Exercise intensity was determined using indirect calorimetry performed five times during the exercise bout and exercise workload adjusted to maintain the relative intensity. Blood sampling was performed during minutes 60, 70, 80, and 90 of exercise. A percutaneous needle biopsy was performed immediately after exercise from the same site used during rest. Subjects then remained supine during 2 h of recovery after the exercise bout. Isotope infused rates were decreased back to resting levels during the start of recovery. Only water consumption was allowed as subjects remained fasted. Blood sampling and indirect calorimetry were performed during minutes 90, 100, 110, and 120 of the recovery period. A final percutaneous needle biopsy was performed after the 2 h of recovery from the contralateral leg.
Metabolite and hormone analyses.
Standard enzymatic assays were used to measure glucose (Olympus AU400e Chemistry analyzer, Olympus America, Center Valley, PA), lactate (Sigma kit no. 826, St. Louis, MO), and FFA (NEFA kit, Wako, TX). Insulin and glucagon were measured using an RIA (Diagnostic Systems Laboratories, Webster, TX).
Muscle lipid analysis.
Skeletal muscle samples were dissected free of extramuscular fat on ice, lyophilized, and processed as previously described to isolate intramuscular free fatty acids (FFA), IMTG, and DAG concentration, enrichment, and composition (6). The FFA, phospholipid, IMTG, and DAG fractions were converted to fatty acid methyl esters (FAME) and the stable isotope ratios of 13C in FAMEs were measured using a gas chromatography-combustion isotope ratio mass spectrometer (GC/C-IRMS) system (Thermo Electron, Bremen, Germany). Concentration and composition analysis was performed on an HP 6890 GC with a 30 m DB-23 capillary column, connected to a HP 5973 MS. Peak identities were determined by retention time and mass spectra compared with standards of known composition.
Plasma palmitate and glucose isotope analysis.
Plasma glucose derivatization and analysis were determined as previously described (3). Methylation and extraction of plasma palmitate were performed as previously described (41). Samples were run on an HP 6890 GC with a 30 m DB-23 capillary column, connected to a HP 5973 MS. Enrichments were calculated based on a standard curve of known enrichments, and corrected for variations in abundance (42). Peak identities were determined by retention time and mass spectra compared with standards of known composition.
Diacylglycerol lipidomics.
Measurement of diacylglycerol molecular species on cytosolic and membrane fractions were previously reported in a subset of these volunteers (4).
Ceramide lipidomics.
LC/ESI/MS/MS analysis of ceramide and other sphingolipids has been described previously by our laboratory (1).
Gene chip analysis.
Skeletal muscle biopsies for RNA extraction were lysed in TRIzol (Invitrogen) using a FastPrep instrument and Lysing Matrix D tubes (MP Biomedical). RNA was isolated using chloroform extraction and ethanol precipitation. The isolated RNA was further purified utilizing RNeasy columns (Qiagen) and was treated with DNase before elution into RNase-free water. RNA was then used for Affymetrix gene chip analysis according to the manufacturer instructions (10).
Western blotting.
To measure muscle protein expression, 20 µg of sample protein was run on an SDS-PAGE 8% Bis-Tris gel (Invitrogen, Carlsbad, CA), transferred to a polyvinylidene fluoride (PVDF) membrane, and blocked with 5% BSA. Primary antibodies were from Cell Signaling (SCD1 no. 2438, HSLser660 no. 4126, HSL total no. 4107, ATGL no. 2138, Danvers, MA). Horseradish peroxidase (HRP) conjugated secondary antibody and enhanced chemiluminescence (ECL) were used to visualize protein bands of interest. Intensity of protein bands was captured using an AlphaImager 3300, quantified using FluorChem software (Alpha Innotech, San Leandro, CA), and normalized to beta-actin or total protein content.
Calculations.
IMTG fractional synthesis rate was calculated as previously described for use with stable isotopes in humans (49). The enrichment of the skeletal muscle FFA pool measured after the 4 h infusion was used as the precursor pool from which TG were synthesized (18):
where EIMTG palm(t1) is enrichment of palmitate in the IMTG pool after 4 h of infusion, and EIMTG palm(t0) is enrichment of background palmitate in the IMTG pool. EFFA palm(t1) is the enrichment of skeletal muscle palmitate in the free fatty acid pool. Background enrichment of IMTG-palmitate was determined using enrichment of IMTG-stearate as previously described, eliminating the need for a baseline biopsy specifically for this purpose (17).
Palmitate rate of disappearance (Rd) was calculated using steady-state kinetics as previously described (62). Palmitate incorporation rate into IMTG was calculated as the product of IMTG FSR and palmitate pool size in IMTG. Fat-free mass was used to extrapolate skeletal muscle palmitate IMTG storage to the whole body using estimates for the contribution of skeletal muscle to fat-free mass in men and women (59).
Statistical analysis.
Data are presented as means ± SE. Differences in normally distributed data between groups were analyzed using a one-way ANOVA (SPSS, Chicago, IL). Nonnormally distributed data were log-transformed before analysis using a one-way ANOVA. When significant differences were detected, individual means were compared using Student's t-tests to determine differences between groups. Changes from rest and exercise, and rest and recovery were evaluated using paired t-tests. Relationships between measurements were determined using Pearson’s correlation coefficient. Gene transcription data were analyzed with a one-way ANOVA, with linear contrasts used to evaluate comparisons between groups. Differences in gene transcription during rest, exercise, and recovery were made using paired comparisons and corrected for three comparisons using Bonferroni resulting in a P value for significance at <0.017. An alpha level of 0.05 was used for statistical significance for all other comparisons.
RESULTS
Demographics as well as plasma substrates and hormones for this cohort have been reported previously (1, 2). Volunteers were matched for age (Ob 39.7 ± 1.6, T2D 42.5 ± 1.1, athletes 41.3 ± 0.86 yr) and sex (Ob 9/5, T2D 11/4, athletes 11/4, men/women) between groups, while athletes had significantly lower BMI compared with obese and T2D groups. Athletes had significantly greater insulin sensitivity (Ob 2.93 ± 0.2, T2D 2.0 ± 0.3, athletes 9.4 ± 1.0, mU·l−1·min−1, P < 0.0001) and V̇o2max (Ob 23.8 ± 2.5, T2D 18.7 ± 2.7, athletes 47.8 ± 3.8 ml·kg−1·min−1, P < 0.0001) compared with both Ob and T2D. We previously published the FFA and insulin concentration data for this cohort, but are including them here to assist with data interpretation regarding changes in muscle lipid metabolism (2). Plasma FFA concentration was not different between groups at rest (Ob 0.63 ± 0.04, T2D 0.66 ± 0.05, athletes 0.63 ± 0.04 mmol/l, P = 0.87), increased significantly in all groups during exercise (Ob 0.81 ± 0.06, T2D 0.77 ± 0.07, athletes 0.76 ± 0.06 mmol/l, P < 0.0001), and continued to significantly increase during recovery from exercise (Ob 1.0 ± 0.06, T2D 1.1 ± 0.07, athletes 1.0 ± 0.05 mmol/l, P < 0.0001). At rest, plasma insulin concentration was significantly greater in T2D compared with obese and athletes (Ob 45.1 ± 6.3, T2D 106.3 ± 18.1, athletes 24.3 ± 6.3 pmol/l, P < 0.0001). This difference continued during exercise, as insulin concentration significantly decreased only in obese individuals (Ob 32.6 ± 4.9, T2D 97.9 ± 18.1, athletes 21.5 ± 1.4 pmol/l, P < 0.0001). Insulin concentration remained significantly elevated in T2D compared with the other groups in recovery from exercise (Ob 36.8 ± 5.6, T2D 83.3 ± 14.6, athletes 25.0 ± 2.1 pmol/l, P < 0.0001).
Indirect calorimetry.
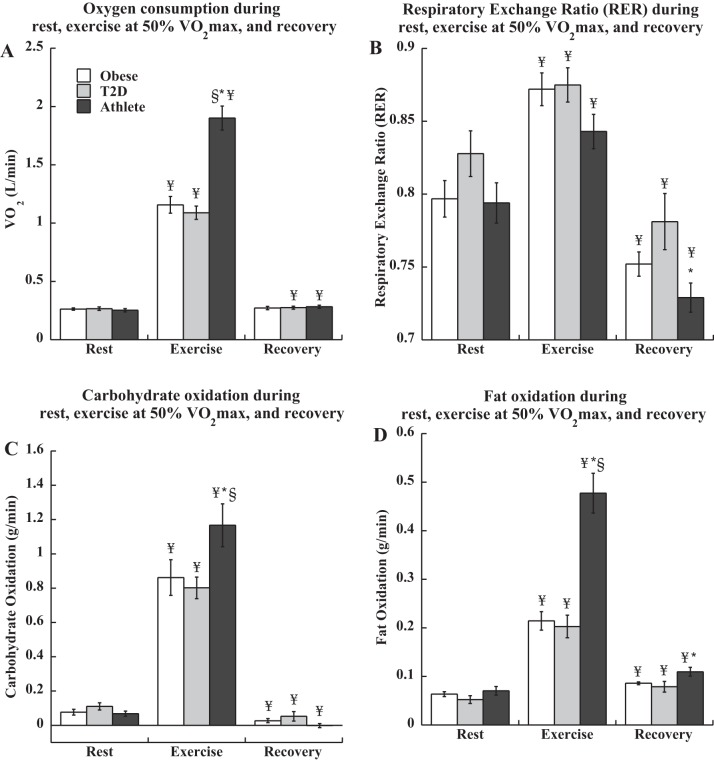
Resting oxygen consumption was not different between groups (Ob 0.26 ± 0.01, T2D 0.27 ± 0.02, athletes 0.25 ± 0.01 l/min), and significantly increased during exercise as expected (P < 0.0001, Fig. 1A). There were no significant differences in relative exercise intensity during exercise, (Ob 49.6 ± 0.70, T2D 49.8 ± 0.35, athletes 50.5 ± 0.41% V̇o2max) by design, but exercise V̇o2 was significantly greater in athletes compared with the other two groups (P < 0.0001). Recovery V̇o2 was significantly elevated compared with rest in T2D and athletes (P < 0.05), but not Ob (Ob 0.27 ± 0.01, T2D 0.28 ± 0.01, athletes 0.28 ± 0.01 l/min). Respiratory exchange ratio (RER) increased from rest to exercise, and was significantly lower in recovery compared with rest for all groups (P < 0.0001, Fig. 1B). There were no differences in RER between groups during rest or exercise, but recovery RER was significantly lower in athletes compared with T2D (P = 0.01). Carbohydrate and fat oxidation was increased during exercise compared with rest in all groups (P < 0.0001, Figs. 1, C and D), with a significant decrease in carbohydrate oxidation (P = 0.04) and increase in fat oxidation (P = 0.03) in recovery compared with rest in all groups. Athletes had significantly greater total carbohydrate (P = 0.03) and fat oxidation (P < 0.0001) during exercise compared with the other two groups, and greater fat oxidation in recovery compared with T2D (P = 0.02).
Fig. 1.
Oxygen consumption (A), respiratory exchange ratio (B), carbohydrate oxidation (C), and fat oxidation (D) during rest, exercise, and recovery in obese volunteers, individuals with type 2 diabetes, and endurance-trained athletes. *Significantly different from T2D, P < 0.05; §significantly different from obese, P < 0.05; ¥significantly different from rest, P < 0.05. Values are means ± SE.
Isotope kinetics.
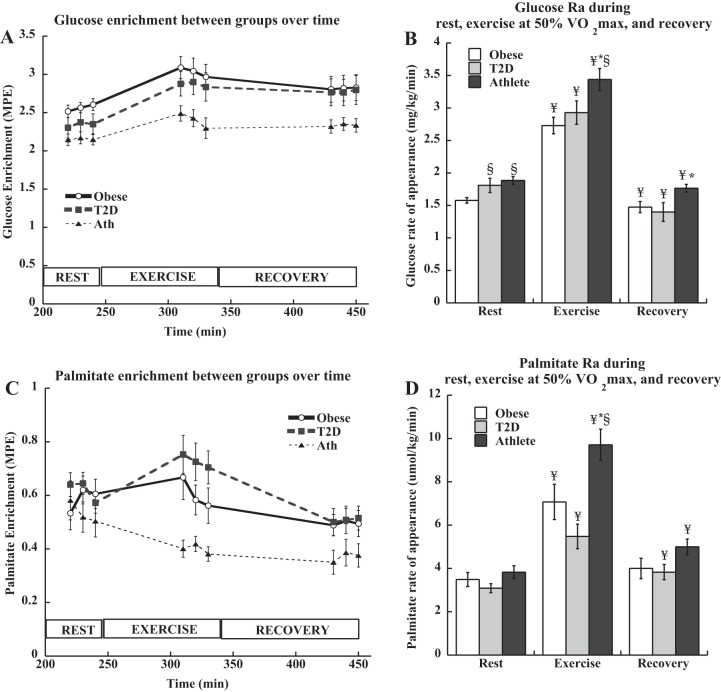
Glucose enrichments were stable during the study and are shown in Fig. 2A. There were no differences in resting glucose Ra between groups (P = 0.11, Fig. 2B). Glucose Ra increased during exercise in all groups (P < 0.0001), and was lower in recovery compared with resting rates (P < 0.05). Palmitate enrichments were stable during the study and are shown in Fig. 2C. Resting palmitate Ra was not different between groups, and significantly increased from rest to exercise in all groups (P = 0.0004, Fig. 2D). Exercise palmitate Ra was significantly higher in athletes compared with T2D (P = 0.001). Palmitate Ra decreased in recovery to values higher than rest in T2D and athletes (P = 0.004).
Fig. 2.
Plasma glucose enrichment (A), glucose rate of appearance (Ra) (B), palmitate enrichment (C), and palmitate rate of appearance (Ra) (D) during rest, exercise, and recovery in obese volunteers, individuals with type 2 diabetes, and endurance-trained athletes. *Significantly different from T2D, P < 0.05; §significantly different from obese, P < 0.05; ¥significantly different from rest, P < 0.05. Values are means ± SE.
Muscle lipid metabolism.
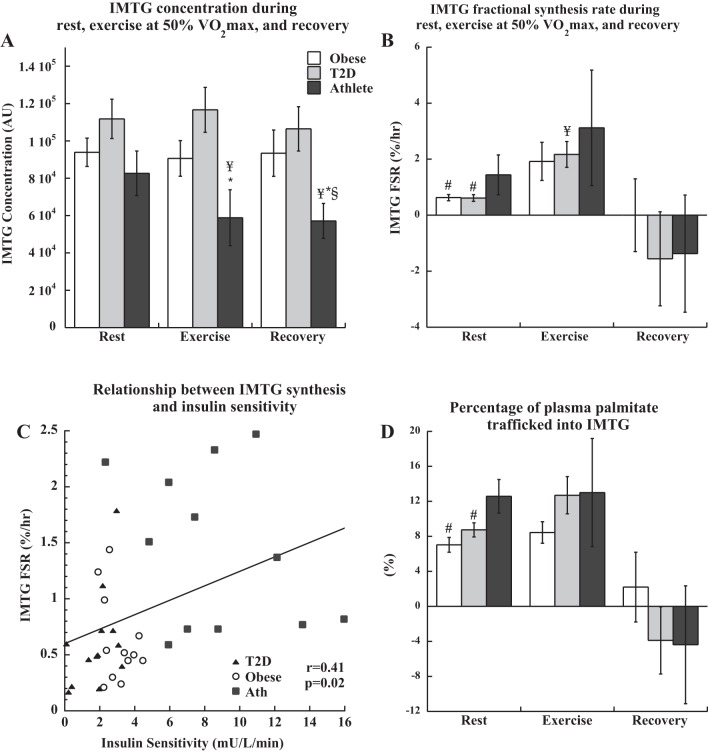
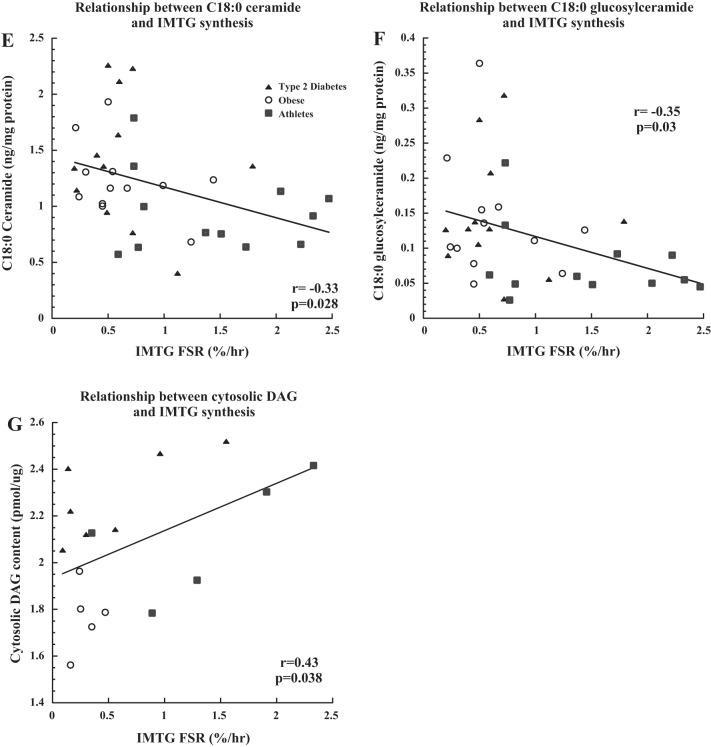
IMTG concentration was not significantly different between groups at rest, was not significantly different in Ob and T2D during exercise and recovery, but significantly decreased in athletes during exercise (P < 0.05) and remained low in recovery (Fig. 3A). There were no significant differences in DAG concentration after rest, exercise, or recovery between groups (P > 0.11 for all). Resting rates of IMTG synthesis were significantly greater in athletes compared with Ob and T2D (Fig. 3B). During exercise, there was a significant increase in IMTG FSR in all groups combined (P = 0.02), driven by an increase in T2D (P = 0.006) and a trend to increase in Ob (P = 0.08). IMTG FSR decreased significantly during recovery (P = 0.04) to values that were not significantly different from rest. There was a significant positive relationship between IMTG FSR at rest and insulin sensitivity (Fig. 3C, r = 0.41, P = 0.02). The muscle IMTG pool was a significant source of palmitate disposal during rest and exercise, with 6–12% of palmitate Rd trafficked through IMTG synthesis (Fig. 3D). Palmitate disposal into IMTG was significantly lower in Ob and T2D compared with athletes at rest (P = 0.01), with no significant differences between groups during exercise or recovery. Resting muscle sphingolipid content from this cohort have been previously published (2). There were no relationships between resting IMTG synthesis and total ceramide content, sphingosine, sphingosine-1-phosphate, dihydroceramide, glucosylceramide, and GM3 gangliosides. However, in all groups combined there were significant inverse relationships between IMTG FSR and C18:0 ceramide (r = −0.33, P = 0.03, Fig. 3E), C18:0 glucosylceramide (r = −0.35, P = 0.02, Fig. 3F), and C16:0 GM3 (r = −0.36, P = 0.03). Resting muscle localized DAG content from a subset of this cohort has been previously published (4). IMTG FSR was not significantly related to whole cell DAG concentration (P = 0.64) or membrane DAG content (P = 0.13); however, it was positively related to cytosolic DAG content (r = 0.43, P = 0.04, Fig. 3G).
Fig. 3.
IMTG concentration (A), fractional synthesis rate (B), relationship between IMTG FSR and insulin sensitivity (C), and percentage of whole body palmitate turnover trafficked into IMTG (D) during rest, exercise, and recovery in obese volunteers, individuals with type 2 diabetes, and endurance-trained athletes. The relationship between IMTG synthesis rate and C18:0 ceramide content (E), C18:0 glucosylceramide content (F), and cytosolic DAG content (G) are also shown. #Significantly different from athletes, P < 0.05; *significantly different from T2D, P < 0.05; §significantly different from obese, P < 0.05; ¥significantly different from rest, P < 0.05. Values are means ± SE.
Affymetrix Gene Chip Analysis.
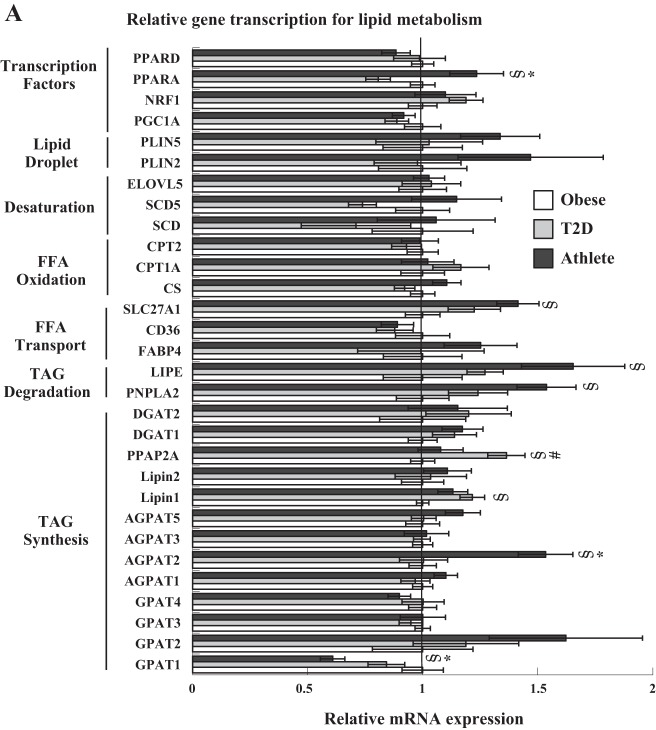
The expression of mRNA for various genes related to lipid metabolism is shown in Fig. 4A. Athletes had significantly greater mRNA expression of several genes, including peroxisome proliferator-activated receptor alpha (PPARα) and 1-acylglycerol-3-phosphate O-acyltransferase 2 (AGPAT2) compared with both groups, and fatty acid transport protein 1 (SLC27A1), hormone-sensitive lipase (LIPE), and patatin-like phospholipase domain containing 2 (PNPLA2) compared with Ob, with significantly lower expression of glycerol-3-phosphate acyltransferase 1 (GPAT1) compared with both groups. T2D showed increased expression of phosphatidic acid phosphatase type 2A (PPAP2A) compared with both groups, and Lipin1 compared with Ob. In all groups combined, the change in mRNA expression from rest to exercise showed a significant increase in peroxisome proliferator-activated receptor delta (PPARδ), ELOVL fatty acid elongase 5 (ELOVL5), glycerol-3-phosphate acyltransferase 2 and 3 (GPAT2 and 3), and a significant decrease in cluster of differentiation 36 (CD36), fatty acid binding protein 4 (FABP4), and GPAT1 expression. The change from rest to recovery showed a significant increase in peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1a), ELOVL5, Lipin1, and GPAT3 expression.
Fig. 4.
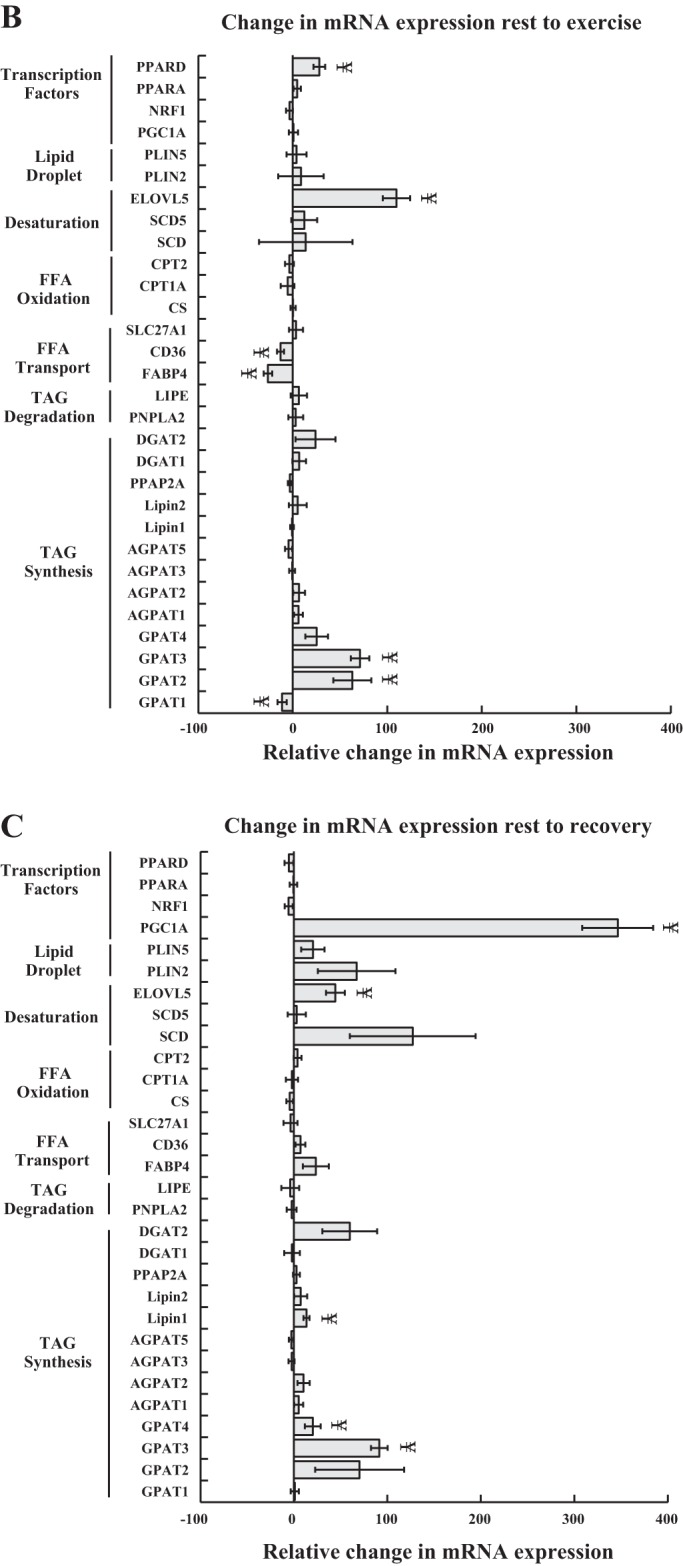
Gene transcription of transcription factors, proteins associated with the lipid droplet, and enzymes involved in lipid desaturation, oxidation, transport, and IMTG degradation and synthesis. mRNA expression is reported relative to the obese control group, which was set to a value of 1. Data are shown for each group at rest (A), and in all groups combined during the change from rest to exercise (B), and rest to recovery (C). #Significantly different from athletes, P < 0.05; *significantly different from T2D, P < 0.05; §significantly different from obese, P < 0.05; ¥significantly different from rest, P < 0.05. Values are means ± SE. PPARD, peroxisome proliferator-activated receptor delta; PPARA, peroxisome proliferator-activated receptor alpha; NRF1, nuclear respiratory receptor 1; PGC1A, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; PLIN5, perilipin 5; PLIN2, perilipin 2; ELOVL5, ELOVL Fatty Acid Elongase 5; SCD5, stearoyl-CoA desaturase 5; SCD, stearoyl-CoA desaturase; CPT2, carnitine palmitoyl transferase 2, CPT1A, carnitine palmitoyl transferase 1a, CS, citrate synthase, SLC27A1, fatty acid transport protein 1, CD36, cluster of differentiation 36, FABP4, fatty acid binding protein 4, LIPE, hormone-sensitive lipase, PNPLA2, Patatin-like phospholipase domain containing 2, a.k.a. adipose triglyceride lipase; DGAT2, diacylglycerol acyltransferase 2; DGAT1, diacylglycerol acyltransferase 1; PPAP2A, phosphatidic acid phosphatase type 2A; Lipin 2; Lipin 1; AGPAT, 1-acylglycerol-3-phosphate O-acyltransferase; GPAT, glycerol-3-phosphate acyltransferase.
Western analysis.
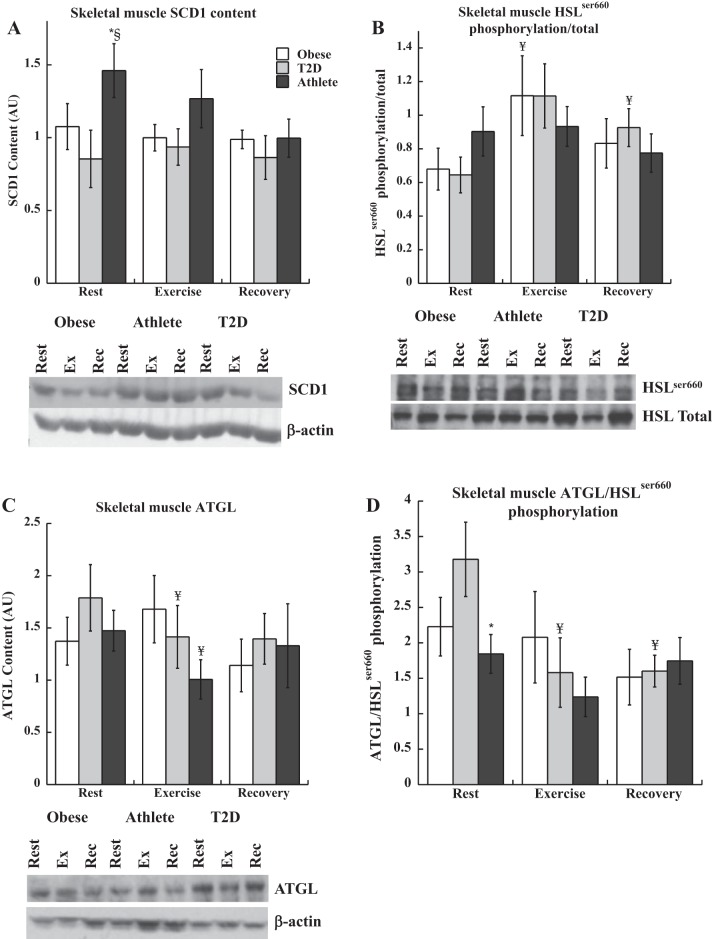
The content of many proteins was not different between groups and did not significantly change during exercise. These data are not shown, but include ERK1/2, PLIN2, PLIN5, and GOS2. Skeletal muscle protein content of stearoyl-CoA desaturase 1 (SCD1) was significantly higher at rest in athletes compared with the other groups (P = 0.002, Fig. 5A) but was not different between groups immediately after exercise or recovery. There were no changes in SCD1 content from rest to exercise or recovery in any group (P = 0.06 for athletes rest to exercise, and P = 0.08 for athlete rest to recovery). There was a significant positive relationship between IMTG synthesis and SCD1 content (P = 0.002). Hormone sensitive lipase (HSL) phosphorylation was not different between groups at rest, and increased significantly during exercise in Ob (P = 0.05), and tended to increase in T2D (P = 0.06, Fig. 5B). HSL phosphorylation was greater in recovery compared with rest for T2D (P = 0.03). ATGL content was not different at rest between groups, but decreased significantly during exercise in T2D (P = 0.03, Fig. 5C) and athletes (P = 0.002). The ratio of ATGL/HSLser660 phosphorylation was used as a relative estimate of DAG production from a mismatch between ATGL generation and clearance through HSL (Fig. 5D). This ratio was significantly increased in T2D compared with athletes (P = 0.03), and decreased significantly only in T2D during exercise (P = 0.02) and recovery (P = 0.01).
Fig. 5.
Skeletal muscle protein content and phosphorylation for SCD1 (A), HSLser660 phosphorylation (B), ATGL (C), and ATGL/HSLser660 phosphorylation (D) during rest, exercise, and recovery in obese volunteers, individuals with type 2 diabetes, and endurance-trained athletes. *Significantly different from T2D, P < 0.05, §significantly different from obese, P < 0.05, ¥significantly different from rest, P < 0.05. Values are means ± SE.
DISCUSSION
Intramuscular triglyceride has long been associated with insulin resistance, but recent studies suggest flux through this triglyceride depot is related to insulin sensitivity. This study examined IMTG synthesis rates in humans spanning a wide range of insulin sensitivities during rest, exercise, and recovery. We found resting IMTG synthesis rates were positively correlated with insulin sensitivity, cytosolic DAG accumulation in muscle, and inversely related to muscle C18:0 ceramide and glucosylceramide. Thus high rates of IMTG synthesis may promote cytosolic accumulation of DAG, which is a benign muscle storage location (4), as well as a decrease in specific sphingolipid species related to insulin resistance in humans (47, 54). These results suggest chronic endurance exercise training promotes high rates of IMTG synthesis, which alters intramuscular lipid partitioning, localization, and insulin sensitivity and may help explain the athlete’s paradox (14).
IMTG synthesis is related to insulin sensitivity in both animals and humans. Transgenic overexpression of DGAT1 recapitulates the athlete’s paradox by increasing insulin sensitivity and IMTG content, and decreasing DAG and ceramide (34). In humans, acute exercise prevents fatty acid-induced insulin resistance by increasing IMTG synthesis, and decreasing DAG and ceramide content (50). Acute exercise also increases SCD1, mGPAT, and DGAT1 content which helps explain the increase in IMTG synthesis. Previous studies by our laboratory showed high rates of IMTG synthesis was positively related to insulin sensitivity, and was greater in endurance-trained athletes compared with sedentary controls (5). The current data agree with these previous studies and suggest variation in rates of IMTG synthesis influence muscle lipid trafficking in humans, by altering localized DAG and accumulation of sphingolipid species associated with insulin resistance.
Greater rates of IMTG synthesis in athletes do not appear to result from differences in basal DGAT1 mRNA content. While AGPAT2 mRNA was upregulated in athletes, there were no other consistent differences in mRNA expression of enzymes for IMTG synthesis. Our data parallel a previous study that found higher IMTG content in obesity was not related to upregulation of the content or activity of enzymes for IMTG synthesis including GPAT and DGAT, or lower content of enzymes for IMTG degradation including ATGL and HSL (32). However, the latter study found increased CD36/FATP protein content in obesity, suggesting greater capacity for fatty acid transport may contribute to IMTG accumulation. Likewise, we found increased mRNA expression of fatty acid transport protein 1 (SLC27A1) in athletes which is consistent with increased fatty acid transport capacity promoting IMTG accumulation. Furthermore, we found increased SCD1 content in athletes, which colocalizes with DGAT2 (35). Therefore, increase channeling of unsaturated lipids into IMTG pools via SCD1 may help explain increased IMTG synthesis rates in athletes. Greater desaturation of lipids in recovery from exercise may explain decreased muscle lipid saturation reported in athletes, which has been linked with increased insulin sensitivity (5, 26). IMTG may also accumulate in athletes as an adaptation to acute exercise, as gene expression of enzymes for IMTG synthesis and lipid desaturation were upregulated immediately after exercise and following recovery. These data support the concept that IMTG accumulates in athletes in response to acute bouts of exercise, which promotes increased lipid desaturation and storage of unsaturated fatty acids into IMTG.
A similar proportion of plasma palmitate was channeled into IMTG synthesis during rest and exercise, with a greater proportion of palmitate uptake trafficked into IMTG synthesis in athletes. It is thought that plasma fatty acids taken up by muscle must first cross the IMTG pool before oxidation (28). Our data showing 7–12% of palmitate disposal into IMTG agree with Kanaley et al. (28) who showed 10–13% of plasma fatty acids are esterified into IMTG. Combined, these data indicate that skeletal muscle IMTG synthesis is a relatively minor sink for plasma fatty acid disposal, but our data suggest it plays an important role in regulating the fate of lipid uptake in skeletal muscle. IMTG synthesis correlated with increased cytosolic accumulation of DAG, and decreased accumulation of C18:0 ceramide and glucosylceramide, each of which would be expected to promote insulin sensitivity (4). These data suggest that IMTG synthesis rate plays an important role in dictating intracellular lipid trafficking, DAG localization, as well as ceramide species and concentration. Thus muscle lipid synthesis may be an interesting therapeutic target to modify muscle lipid accumulation and insulin sensitivity in humans.
Despite methodological differences, previous studies generally agree that IMTG is utilized as a fuel during exercise, especially in endurance-trained athletes, with little IMTG use in obese or T2D populations (52, 57, 60). Our study confirms these findings by directly comparing these three groups. Endurance-trained athletes had a significant decrease in IMTG content during exercise despite sustained rates of IMTG FSR, which points to a greater capacity for IMTG lipolysis compared with obese individuals with and without diabetes. The explanation for minimal IMTG use during exercise in obese and T2D humans has been explained by excessive systemic lipolysis and higher FFA concentrations in these populations (57). FFA regulation of IMTG utilization has also been observed during exercise, where IMTG use is inversely proportional to plasma FFA concentration (58, 60). However, our results differ from previous reports as FFA concentration was not different between groups, suggesting FFA concentration cannot explain group differences in IMTG utilization. However, it is likely that greater rates of O2 consumption in athletes are paralleled by greater blood flow, resulting in greater rates of FFA delivery. If true, greater FFA delivery did not prevent net degradation of IMTG during exercise in athletes. Further, IMTG content was unchanged during exercise in Ob and T2D, despite an increase in IMTG synthesis compared with rest. Therefore, IMTG degradation must have matched rates of synthesis and reveals turnover of this pool during exercise, despite no change in concentration, similar to earlier reports in sedentary men and women (16). The increase in IMTG FSR from rest to exercise was most pronounced in T2D, which may be due to a maintenance of greater plasma insulin concentration during exercise compared with Ob and athletes. Therefore, IMTG utilization during exercise does occur in obese and T2D individuals, but not on a net basis. Combined, these data point to intracellular regulation of IMTG synthesis and oxidation, rather than strictly FFA delivery, in determining IMTG utilization during exercise.
Regulation of IMTG utilization may occur via PLIN2/ADRP and PLIN5/OXPAT, which are members of a family of perilipin proteins that coat the lipid droplet. Knockdown of PLIN2/ADRP in rodents decreased lipid droplet formation and triacylglycerol storage, and increased FA oxidation and incorporation of palmitate into diacylglycerol and phospholipids (7). PLIN2 overexpression in vitro increased IMTG storage paralleled with improved insulin sensitivity. IMTG use during exercise appears to occur in PLIN2 containing lipid droplets (51). PLIN5 knockout in muscle depleted IMTG concentration in red fibers, increased IMTG lipolysis, and resulted in sphingolipid accumulation (36). PLIN5 limits IMTG lipolysis, and may promote mitochondrial oxidation of IMTG-derived FFA (37). Therefore, lack of IMTG use during exercise in Ob and T2D may be due to differences in PLIN2 and PLIN5 distribution or content in these populations. However, we did not find any differences in PLIN2 or PLIN5 mRNA between groups, nor changes during exercise. Therefore, based on differences in mRNA expression, PLIN2 and PLIN5 are not likely to explain alterations in IMTG use during exercise between groups.
We did not find any differences in resting DAG concentration between groups, despite data suggesting a mismatch between ATGL and HSL consistent with increased DAG accumulation at rest. These data suggest that IMTG-derived DAG production is a relatively minor contribution to whole muscle DAG concentration at rest. DAG can also be produced via de novo synthesis, conversion of ceramide to sphingomyelin, and phospholipid degradation. Our data suggest these other routes of DAG synthesis predominate in humans at rest.
There are several limitations in this project that should be addressed. We do not have a lean sedentary control group, which limits our ability to evaluate the impact of endurance exercise training compared with body habitus on outcomes in this study. Individuals fasted for 19–20 h by the end of the metabolic study, resulting in FFA concentrations that increased throughout the experiment. Our results may have changed if the fast were shorter or a meal provided before or after exercise. These changes would have decreased plasma FFA concentration, which would increase IMTG synthesis and turnover rates (20). There is 23% variability in IMTG content in skeletal muscle biopsies within an individual, which is less dramatic with endurance-trained subjects (60). Therefore lack of a change in IMTG use during exercise in obese and T2D individuals may simply reflect the change during exercise was less than the variability between biopsies. IMTG synthesis during exercise has been reported by others (16, 49), but has recently been called into question (27). Due to heterogeneity of muscle fiber utilization, not all muscle fibers are contracting during exercise. It is possible that IMTG synthesis during exercise may therefore reflect synthesis of IMTG in inactive fibers, and not IMTG synthesis in contracting muscle fibers. Muscle fiber type may also explain differences in IMTG FSR between groups if endurance-trained athletes have a greater proportion of type 1 muscle fibers. The postexercise muscle biopsy was taken from the same site as the resting muscle biopsy, so muscle inflammation may have influenced our exercise data. Further, these studies assume skeletal muscle contains homogeneous pools of IMTG that turn over at similar rates, when there may actually be multiple pools of IMTG turning over at different rates which will not be captured using these methods (12).
These results suggest basal rates of IMTG synthesis influence muscle DAG localization and ceramide accumulation, both of which can impact insulin sensitivity and help explain the athlete’s paradox. These data also indicate IMTG is turning over during exercise, and is used during exercise in obesity and type 2 diabetes despite no net change in IMTG content. Overall, enhancing IMTG turnover may be a promising therapeutic target to increase muscle insulin sensitivity.
GRANTS
This work was partially supported by National Institutes of Health General Clinical Research Center Grant RR-00036 and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants to B. C. Bergman (KO1-DK-066219, RO3-DK-084093) and L. Perreault (DK-064811).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.C.B., L.P., and R.H.E. conceived and designed research; B.C.B., L.P., A.S., S.D.B., A.A.K., K.H., J.T.B., D.M.H., M.C.P., H.H.B., P. Sanders, P. Siddall, T.W., M.K.T., M.S.K., and R.H.E. performed experiments; B.C.B., L.P., A.S., S.D.B., A.A.K., K.H., J.T.B., D.M.H., M.C.P., W.H., H.H.B., P. Sanders, P. Siddall, T.W., M.K.T., M.S.K., and R.H.E. analyzed data; B.C.B., L.P., A.S., S.D.B., A.A.K., K.H., J.T.B., D.M.H., M.C.P., W.H., and R.H.E. interpreted results of experiments; B.C.B. prepared figures; B.C.B. drafted manuscript; B.C.B., L.P., A.S., S.D.B., A.A.K., K.H., J.T.B., D.M.H., M.C.P., and R.H.E. edited and revised manuscript; B.C.B., L.P., A.S., S.D.B., A.A.K., K.H., J.T.B., D.M.H., M.C.P., W.H., H.H.B., P. Sanders, P. Siddall, T.W., M.K.T., M.S.K., and R.H.E. approved final version of manuscript.
REFERENCES
- 1.Bergman BC, Brozinick JT, Strauss A, Bacon S, Kerege A, Bui HH, Sanders P, Siddall P, Kuo MS, Perreault L. Serum sphingolipids: relationships to insulin sensitivity and changes with exercise in humans. Am J Physiol Endocrinol Metab 309: E398–E408, 2015. doi: 10.1152/ajpendo.00134.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergman BC, Brozinick JT, Strauss A, Bacon S, Kerege A, Bui HH, Sanders P, Siddall P, Wei T, Thomas MK, Kuo MS, Perreault L. Muscle sphingolipids during rest and exercise: a C18:0 signature for insulin resistance in humans. Diabetologia 59: 785–798, 2016. doi: 10.1007/s00125-015-3850-y. [DOI] [PubMed] [Google Scholar]
- 3.Bergman BC, Cornier MA, Horton TJ, Bessesen DH. Effects of fasting on insulin action and glucose kinetics in lean and obese men and women. Am J Physiol Endocrinol Metab 293: E1103–E1111, 2007. doi: 10.1152/ajpendo.00613.2006. [DOI] [PubMed] [Google Scholar]
- 4.Bergman BC, Hunerdosse DM, Kerege A, Playdon MC, Perreault L. Localisation and composition of skeletal muscle diacylglycerol predicts insulin resistance in humans. Diabetologia 55: 1140–1150, 2012. doi: 10.1007/s00125-011-2419-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergman BC, Perreault L, Hunerdosse DM, Koehler MC, Samek AM, Eckel RH. Increased intramuscular lipid synthesis and low saturation relate to insulin sensitivity in endurance-trained athletes. J Appl Physiol (1985) 108: 1134–1141, 2010. doi: 10.1152/japplphysiol.00684.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergman BC, Perreault L, Hunerdosse DM, Koehler MC, Samek AM, Eckel RH. Intramuscular lipid metabolism in the insulin resistance of smoking. Diabetes 58: 2220–2227, 2009. doi: 10.2337/db09-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosma M, Hesselink MK, Sparks LM, Timmers S, Ferraz MJ, Mattijssen F, van Beurden D, Schaart G, de Baets MH, Verheyen FK, Kersten S, Schrauwen P. Perilipin 2 improves insulin sensitivity in skeletal muscle despite elevated intramuscular lipid levels. Diabetes 61: 2679–2690, 2012. doi: 10.2337/db11-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther 5: 1003–1015, 2003. doi: 10.1089/152091503322641060. [DOI] [PubMed] [Google Scholar]
- 9.Bruce CR, Thrush AB, Mertz VA, Bezaire V, Chabowski A, Heigenhauser GJ, Dyck DJ. Endurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid content. Am J Physiol Endocrinol Metab 291: E99–E107, 2006. doi: 10.1152/ajpendo.00587.2005. [DOI] [PubMed] [Google Scholar]
- 10.Dalma-Weiszhausz DD, Warrington J, Tanimoto EY, Miyada CG. The affymetrix GeneChip platform: an overview. Methods Enzymol 410: 3–28, 2006. doi: 10.1016/S0076-6879(06)10001-4. [DOI] [PubMed] [Google Scholar]
- 11.Dubé JJ, Amati F, Stefanovic-Racic M, Toledo FG, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete’s paradox revisited. Am J Physiol Endocrinol Metab 294: E882–E888, 2008. doi: 10.1152/ajpendo.00769.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dyck DJ, Peters SJ, Glatz J, Gorski J, Keizer H, Kiens B, Liu S, Richter EA, Spriet LL, van der Vusse GJ, Bonen A. Functional differences in lipid metabolism in resting skeletal muscle of various fiber types. Am J Physiol Endocrinol Metab 272: E340–E351, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Ebeling P, Essén-Gustavsson B, Tuominen JA, Koivisto VA. Intramuscular triglyceride content is increased in IDDM. Diabetologia 41: 111–115, 1998. doi: 10.1007/s001250050875. [DOI] [PubMed] [Google Scholar]
- 14.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 86: 5755–5761, 2001. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- 15.Goodpaster BH, Theriault R, Watkins SC, Kelley DE. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism 49: 467–472, 2000. doi: 10.1016/S0026-0495(00)80010-4. [DOI] [PubMed] [Google Scholar]
- 16.Guo Z, Burguera B, Jensen MD. Kinetics of intramuscular triglyceride fatty acids in exercising humans. J Appl Physiol (1985) 89: 2057–2064, 2000. doi: 10.1152/jappl.2000.89.5.2057. [DOI] [PubMed] [Google Scholar]
- 17.Guo Z, Jensen MD. Determination of skeletal muscle triglyceride synthesis using a single muscle biopsy. Metabolism 51: 1198–1205, 2002. doi: 10.1053/meta.2002.34711. [DOI] [PubMed] [Google Scholar]
- 18.Guo Z, Jensen MD. Intramuscular fatty acid metabolism evaluated with stable isotopic tracers. J Appl Physiol (1985) 84: 1674–1679, 1998. doi: 10.1152/jappl.1998.84.5.1674. [DOI] [PubMed] [Google Scholar]
- 19.Guo Z, Mishra P, Macura S. Sampling the intramyocellular triglycerides from skeletal muscle. J Lipid Res 42: 1041–1048, 2001. [PubMed] [Google Scholar]
- 20.Guo Z, Zhou L. Fatty acids inhibit intramyocellular triglyceride synthesis and turnover acutely in high fat-fed obese rats. Horm Metab Res 38: 721–726, 2006. doi: 10.1055/s-2006-955093. [DOI] [PubMed] [Google Scholar]
- 21.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 312: 734–737, 2006. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 22.Hammond LE, Albright CD, He L, Rusyn I, Watkins SM, Doughman SD, Lemasters JJ, Coleman RA. Increased oxidative stress is associated with balanced increases in hepatocyte apoptosis and proliferation in glycerol-3-phosphate acyltransferase-1 deficient mice. Exp Mol Pathol 82: 210–219, 2007. doi: 10.1016/j.yexmp.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haus JM, Solomon TP, Lu L, Jesberger JA, Barkoukis H, Flask CA, Kirwan JP. Intramyocellular lipid content and insulin sensitivity are increased following a short-term low-glycemic index diet and exercise intervention. Am J Physiol Endocrinol Metab 301: E511–E516, 2011. doi: 10.1152/ajpendo.00221.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Høeg L, Roepstorff C, Thiele M, Richter EA, Wojtaszewski JF, Kiens B. Higher intramuscular triacylglycerol in women does not impair insulin sensitivity and proximal insulin signaling. J Appl Physiol (1985) 107: 824–831, 2009. doi: 10.1152/japplphysiol.91382.2008. [DOI] [PubMed] [Google Scholar]
- 25.Jacob S, Machann J, Rett K, Brechtel K, Volk A, Renn W, Maerker E, Matthaei S, Schick F, Claussen CD, Häring HU. Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes 48: 1113–1119, 1999. doi: 10.2337/diabetes.48.5.1113. [DOI] [PubMed] [Google Scholar]
- 26.Jocken JW, Goossens GH, Boon H, Mason RR, Essers Y, Havekes B, Watt MJ, van Loon LJ, Blaak EE. Insulin-mediated suppression of lipolysis in adipose tissue and skeletal muscle of obese type 2 diabetic men and men with normal glucose tolerance. Diabetologia 56: 2255–2265, 2013. doi: 10.1007/s00125-013-2995-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jordy AB, Kiens B. Regulation of exercise-induced lipid metabolism in skeletal muscle. Exp Physiol 99: 1586–1592, 2014. doi: 10.1113/expphysiol.2014.082404. [DOI] [PubMed] [Google Scholar]
- 28.Kanaley JA, Shadid S, Sheehan MT, Guo Z, Jensen MD. Relationship between plasma free fatty acid, intramyocellular triglycerides and long-chain acylcarnitines in resting humans. J Physiol 587: 5939–5950, 2009. doi: 10.1113/jphysiol.2009.180695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiens B, Richter EA. Utilization of skeletal muscle triacylglycerol during postexercise recovery in humans. Am J Physiol Endocrinol Metab 275: E332–E337, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Krssak M, Petersen KF, Bergeron R, Price T, Laurent D, Rothman DL, Roden M, Shulman GI. Intramuscular glycogen and intramyocellular lipid utilization during prolonged exercise and recovery in man: a 13C and 1H nuclear magnetic resonance spectroscopy study. J Clin Endocrinol Metab 85: 748–754, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Lexell J, Henriksson-Larsén K, Winblad B, Sjöström M. Distribution of different fiber types in human skeletal muscles: effects of aging studied in whole muscle cross sections. Muscle Nerve 6: 588–595, 1983. doi: 10.1002/mus.880060809. [DOI] [PubMed] [Google Scholar]
- 32.Li M, Paran C, Wolins NE, Horowitz JF. High muscle lipid content in obesity is not due to enhanced activation of key triglyceride esterification enzymes or the suppression of lipolytic proteins. Am J Physiol Endocrinol Metab 300: E699–E707, 2011. doi: 10.1152/ajpendo.00316.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Listenberger LL, Han X, Lewis SE, Cases S, Farese RV Jr, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA 100: 3077–3082, 2003. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu L, Zhang Y, Chen N, Shi X, Tsang B, Yu YH. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Invest 117: 1679–1689, 2007. doi: 10.1172/JCI30565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Man WC, Miyazaki M, Chu K, Ntambi J. Colocalization of SCD1 and DGAT2: implying preference for endogenous monounsaturated fatty acids in triglyceride synthesis. J Lipid Res 47: 1928–1939, 2006. doi: 10.1194/jlr.M600172-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Mason RR, Mokhtar R, Matzaris M, Selathurai A, Kowalski GM, Mokbel N, Meikle PJ, Bruce CR, Watt MJ. PLIN5 deletion remodels intracellular lipid composition and causes insulin resistance in muscle. Mol Metab 3: 652–663, 2014. doi: 10.1016/j.molmet.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mason RR, Watt MJ. Unraveling the roles of PLIN5: linking cell biology to physiology. Trends Endocrinol Metab 26: 144–152, 2015. doi: 10.1016/j.tem.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Meex RC, Schrauwen-Hinderling VB, Moonen-Kornips E, Schaart G, Mensink M, Phielix E, van de Weijer T, Sels JP, Schrauwen P, Hesselink MK. Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes 59: 572–579, 2010. doi: 10.2337/db09-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newsom SA, Brozinick JT, Kiseljak-Vassiliades K, Strauss AN, Bacon SD, Kerege AA, Bui HH, Sanders P, Siddall P, Wei T, Thomas M, Kuo MS, Nemkov T, D’Alessandro A, Hansen KC, Perreault L, Bergman BC. Skeletal muscle phosphatidylcholine and phosphatidylethanolamine are related to insulin sensitivity and respond to acute exercise in humans. J Appl Physiol (1985) 120: 1355–1363, 2016. doi: 10.1152/japplphysiol.00664.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, Jenkins AB, Storlien LH. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes 46: 983–988, 1997. doi: 10.2337/diab.46.6.983. [DOI] [PubMed] [Google Scholar]
- 41.Patterson BW, Zhao G, Elias N, Hachey DL, Klein S. Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res 40: 2118–2124, 1999. [PubMed] [Google Scholar]
- 42.Patterson BW, Zhao G, Klein S. Improved accuracy and precision of gas chromatography/mass spectrometry measurements for metabolic tracers. Metabolism 47: 706–712, 1998. doi: 10.1016/S0026-0495(98)90035-X. [DOI] [PubMed] [Google Scholar]
- 43.Perreault L, Bergman BC, Hunerdosse DM, Eckel RH. Altered intramuscular lipid metabolism relates to diminished insulin action in men, but not women, in progression to diabetes. Obesity (Silver Spring) 18: 2093–2100, 2010. doi: 10.1038/oby.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perreault L, Bergman BC, Hunerdosse DM, Playdon MC, Eckel RH. Inflexibility in intramuscular triglyceride fractional synthesis distinguishes prediabetes from obesity in humans. Obesity (Silver Spring) 18: 1524–1531, 2010. doi: 10.1038/oby.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, Vanzulli A, Testolin G, Pozza G, Del Maschio A, Luzi L. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes 48: 1600–1606, 1999. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- 46.Phillips DI, Caddy S, Ilic V, Fielding BA, Frayn KN, Borthwick AC, Taylor R. Intramuscular triglyceride and muscle insulin sensitivity: evidence for a relationship in nondiabetic subjects. Metabolism 45: 947–950, 1996. doi: 10.1016/S0026-0495(96)90260-7. [DOI] [PubMed] [Google Scholar]
- 47.Pinto SN, Silva LC, Futerman AH, Prieto M. Effect of ceramide structure on membrane biophysical properties: the role of acyl chain length and unsaturation. Biochim Biophys Acta 1808: 2753–2760, 2011. doi: 10.1016/j.bbamem.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 48.Roepstorff C, Steffensen CH, Madsen M, Stallknecht B, Kanstrup IL, Richter EA, Kiens B. Gender differences in substrate utilization during submaximal exercise in endurance-trained subjects. Am J Physiol Endocrinol Metab 282: E435–E447, 2002. doi: 10.1152/ajpendo.00266.2001. [DOI] [PubMed] [Google Scholar]
- 49.Sacchetti M, Saltin B, Osada T, van Hall G. Intramuscular fatty acid metabolism in contracting and non-contracting human skeletal muscle. J Physiol 540: 387–395, 2002. doi: 10.1113/jphysiol.2001.013912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest 117: 1690–1698, 2007. doi: 10.1172/JCI30566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shepherd SO, Cocks M, Tipton KD, Ranasinghe AM, Barker TA, Burniston JG, Wagenmakers AJ, Shaw CS. Preferential utilization of perilipin 2-associated intramuscular triglycerides during 1 h of moderate-intensity endurance-type exercise. Exp Physiol 97: 970–980, 2012. doi: 10.1113/expphysiol.2012.064592. [DOI] [PubMed] [Google Scholar]
- 52.Stellingwerff T, Boon H, Jonkers RA, Senden JM, Spriet LL, Koopman R, van Loon LJ. Significant intramyocellular lipid use during prolonged cycling in endurance-trained males as assessed by three different methodologies. Am J Physiol Endocrinol Metab 292: E1715–E1723, 2007. doi: 10.1152/ajpendo.00678.2006. [DOI] [PubMed] [Google Scholar]
- 53.Storlien LH, Jenkins AB, Chisholm DJ, Pascoe WS, Khouri S, Kraegen EW. Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes 40: 280–289, 1991. doi: 10.2337/diab.40.2.280. [DOI] [PubMed] [Google Scholar]
- 54.Turner N, Kowalski GM, Leslie SJ, Risis S, Yang C, Lee-Young RS, Babb JR, Meikle PJ, Lancaster GI, Henstridge DC, White PJ, Kraegen EW, Marette A, Cooney GJ, Febbraio MA, Bruce CR. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia 56: 1638–1648, 2013. doi: 10.1007/s00125-013-2913-1. [DOI] [PubMed] [Google Scholar]
- 55.van Loon LJ, Koopman R, Manders R, van der Weegen W, van Kranenburg GP, Keizer HA. Intramyocellular lipid content in type 2 diabetes patients compared with overweight sedentary men and highly trained endurance athletes. Am J Physiol Endocrinol Metab 287: E558–E565, 2004. doi: 10.1152/ajpendo.00464.2003. [DOI] [PubMed] [Google Scholar]
- 56.van Loon LJ, Koopman R, Stegen JH, Wagenmakers AJ, Keizer HA, Saris WH. Intramyocellular lipids form an important substrate source during moderate intensity exercise in endurance-trained males in a fasted state. J Physiol 553: 611–625, 2003. doi: 10.1113/jphysiol.2003.052431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Loon LJ, Manders RJ, Koopman R, Kaastra B, Stegen JH, Gijsen AP, Saris WH, Keizer HA. Inhibition of adipose tissue lipolysis increases intramuscular lipid use in type 2 diabetic patients. Diabetologia 48: 2097–2107, 2005. doi: 10.1007/s00125-005-1889-x. [DOI] [PubMed] [Google Scholar]
- 58.van Loon LJ, Thomason-Hughes M, Constantin-Teodosiu D, Koopman R, Greenhaff PL, Hardie DG, Keizer HA, Saris WH, Wagenmakers AJ. Inhibition of adipose tissue lipolysis increases intramuscular lipid and glycogen use in vivo in humans. Am J Physiol Endocrinol Metab 289: E482–E493, 2005. doi: 10.1152/ajpendo.00092.2005. [DOI] [PubMed] [Google Scholar]
- 59.Wang Z, Heo M, Lee RC, Kotler DP, Withers RT, Heymsfield SB. Muscularity in adult humans: proportion of adipose tissue-free body mass as skeletal muscle. Am J Hum Biol 13: 612–619, 2001. doi: 10.1002/ajhb.1099. [DOI] [PubMed] [Google Scholar]
- 60.Watt MJ, Heigenhauser GJ, Dyck DJ, Spriet LL. Intramuscular triacylglycerol, glycogen and acetyl group metabolism during 4 h of moderate exercise in man. J Physiol 541: 969–978, 2002. doi: 10.1113/jphysiol.2002.018820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watt MJ, Heigenhauser GJ, Spriet LL. Intramuscular triacylglycerol utilization in human skeletal muscle during exercise: is there a controversy? J Appl Physiol (1985) 93: 1185–1195, 2002. doi: 10.1152/japplphysiol.00197.2002. [DOI] [PubMed] [Google Scholar]
- 62.Wolfe R. Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetic Analysis. New York: Wiley-Liss, 1992. [Google Scholar]