Abstract
We investigated the effects of meal ingestion on intramyofibrillar (IMF) and subsarcolemmal (SS) ceramide metabolism in volunteers ranging from lean to obese. Thirty-eight women and men underwent a steady-state meal ingestion protocol that included a 6.5-h infusion of [U-13C]palmitate and muscle biopsies 1.5 and 6.5 h after starting the tracer infusion. We measured IMF and SS sphingolipid concentrations and the contribution of plasma palmitate to intramyocellular C16:0 ceramide by use of LC-MS-MS. In response to meal ingestion SS C24 ceramide concentrations, but not C14-C20 concentrations, increased significantly. IMF ceramide concentrations did not change. The increases in SS C24 ceramides were negatively related to parameters of insulin resistance. The fractional contribution of plasma palmitate to intramyocellular C16:0 ceramides in both IMF and SS fractions was inversely related to overweight status (β = –0.432, P = 0.0095 and β = –0.443, P = 0.0058, respectively). These data indicate that meal ingestion has differing effects on SS ceramide subspecies and suggest that the fractional de novo synthesis of intramyocellular ceramide from plasma palmitate in the postprandial condition is reduced in those who are overweight.
Keywords: insulin resistance, isotopic tracers, meals, obesity, skeletal muscle
INTRODUCTION
Skeletal muscle is exposed to wide variations in free fatty acids (FFA) in daily life. Under postprandial conditions, insulin suppresses adipose tissue lipolysis, thus reducing the delivery of FFA to skeletal muscle. However, the adipose tissue insulin resistance in some types of obesity results in greater skeletal muscle exposure to FFA in the postprandial condition (16, 36), likely contributing to obesity-related metabolic complications (5, 20). Given the amount of time humans spend in the postprandial state, it is important to elucidate the fate of plasma FFA within skeletal muscle in this condition. It is possible the excess accumulation of plasma-derived intramyocellular fatty acids in muscle could be channeled toward metabolic signaling molecules (28).
Ceramides are important regulators of a variety of cellular processes (10, 28). Emerging evidence from experimental studies indicates that ceramide plays a causal role in mediating impaired insulin signaling in skeletal muscle (6, 9). However, there have been inconsistent findings regarding the association between intramyocellular ceramide concentrations and insulin resistance in humans. Several studies have shown that intramyocellular ceramides are increased in obese insulin-resistant humans (2, 40), whereas others have not (19, 39). As one of its possible explanations, it has been suggested that subcellular location of intramyocellular lipid might play a pivotal role in dictating insulin signaling in skeletal muscle (32). Recently, we reported that ceramide species in subsarcolemmal (SS) fraction are more strongly related to insulin resistance than intramyofibrillar (IMF) ceramide species in postabsorptive humans (11).
Because the serine palmitoyltransferase, the rate-limiting enzyme in de novo ceramide synthesis, is largely influenced by substrate availability (17), increased FFA availability might contribute to increased concentrations of intramyocellular ceramides in obesity. We found that FFA are readily incorporated into intramyocellular ceramides in postabsorptive humans (11). The current studies were conducted to evaluate the effects of meal ingestion on subcellular ceramide concentrations and the contribution of plasma FFA to subcellular ceramide synthesis. We also evaluated the relationships between markers of insulin resistance/adiposity and the incorporation of plasma FFA to intramyocellular C16:0 ceramides.
METHODS
This study was approved by the Mayo Clinic Institutional Review Board. Informed, written consent was obtained from all volunteers.
Participants
Healthy participants receiving no medications known to affect fatty acid metabolism, including oral contraceptives, participated in the study. Nine nonobese and 12 obese premenopausal women, as well as nibe nonobese and eught obese men participated in this study. They had been weight stable for >3 mo before the study. Blood tests were done to ensure normal liver and kidney function, normal electrolytes, nondiabetic status, and absence of anemia. Twelve volunteers in this study had also participated in a previous study of intramyocellular ceramide metabolism (11).
Study Protocol
The experimental design of the present study is provided in Fig. 1. Participants received isoenergetic meals (50% carbohydrate, 35% fat, and 15% protein) from the Mayo Clinic Clinical Research Unit (CRU) metabolic kitchen for 5 days before the study. Volunteers were admitted to the CRU at 1700 the day before the experiments and consumed a meal at 1800. At ~0545 the next day, a forearm vein catheter was inserted and kept patent with a controlled infusion of 0.45% NaCl. A second catheter was placed in retrograde fashion in a hand vein for collecting arterialized blood by using the heated hand vein technique. A baseline blood sample for background palmitate enrichment was collected, and then a continuous infusion of [U-13C]palmitate (0.6–1.2 nmol·kg FFM−1·min−1; Cambridge Isotope Laboratories, Andover, MA) was started at 0630. After 30 min for isotopic equilibration, a series of four blood samples were collected at 10-min intervals, then at 30-min intervals for the next hour, and then hourly until 1330 to measure palmitate kinetics.
Fig. 1.
Schematic study design. Time denotes clock time. FFM, fat-free mass.
At 0620, the participants began consuming small portions of a fat-free smoothie containing fat-free frozen yogurt, skim milk, Beneprotein (Nestlé Nutrition), Polycose (Abbott Nutrition), and frozen unsweetened strawberries. Portions were given at 20-min intervals and continued until 1330. The total energy consumed during this time interval was equal to 70% of each individual’s measured daily resting energy expenditure. The smoothie provided 30% of energy as protein and 70% as carbohydrate. This feeding paradigm was designed to provide a relatively continuous nutrient intake and thus create suppressed, steady-state rates of FFA release and uptake. The nonfat nature of the food eliminated chylomicron production and thereby delivery of meal-derived fatty acids into the plasma FFA pool. The volunteers rested throughout this protocol.
Muscle biopsies were collected at 0830 (first biopsy) and1330 h (second biopsy) from the vastus lateralis under local anesthesia (2% lidocaine: 8.9% sodium bicarbonate, 3:1) using sterile technique. The volunteers remained in bed in between the two biopsies, but were asked to move both legs every 15min to avoid immobility. The muscle tissue was immediately washed of blood using an ice-cold normal saline solution, dissected of all visible adipose tissue, further rinsed of lipid droplets, and saved immediately in liquid N2. Samples were stored at −80◦C until analysis.
Body composition measurements.
Total and regional fat masses were assessed with dual-energy X-ray absorptiometry (DEXA; Lunar Radiation, Madison, WI). Leg fat mass was considered LBSQ fat. Visceral fat mass was estimated using a combination of single-slice CT (L2–L3 interspace) and DEXA-measured abdominal fat (21). Total body fat minus visceral and LBSQ fat masses was UBSQ fat mass.
Assays
Plasma analyses.
A Beckman instrument (Fullerton, CA) was used to measure plasma glucose concentrations. Plasma palmitate concentrations were measured using HPLC (30), and enrichments were measured using LC-MS (35). Plasma triglyceride (TG) concentrations were measured by a microfluorometric method (18).
Muscle analyses.
The frozen samples were dissected free of extramyocellular fat while kept at 0°C on dry ice (15). Approximately 100 mg of cleaned muscle was pulverized into a fine powder using a stainless steel mortar and pestle also on dry ice (15). We separated the samples into subsarcolemmal (SS) and intramyofibrillar (IMF) fractions. The SS fraction also contains the “membrane fraction,” as well as cytosolic compounds. The two muscle fractions were obtained by homogenizing the sample in 1,000 µl of homogenization buffer consisting of 0.25 M sucrose (S 5–500), 25 mM KCl, 50 mM Tris (T 87602), 0.5 mM EDTA (E 4884), pH 7.4, at a tissue-to-buffer ratio of 1:10. The sample (800 µl of homogenate) was then centrifuged for 10 min (4°C) at 2,000 rpm. The supernatant was taken as the SS fraction and the pellet as the IMF fraction. The supernatant containing the SS fraction was transferred to a new vial, from which we took 3 µl to measure protein concentration. We then added 10 µl of the internal standard mix (17C Sph, 17C S1P, and 17C-Cer), vortexed the sample and extracted the compounds of interest with isopropanol-water-ethyl acetate at a ratio of 30:10:60. For the pellet containing the IMF fraction we added 800 µl of homogenization buffer to resuspend the pellet and then processed the sample as outlined above.
Intramyocellular ceramide concentrations and enrichment were measured by liquid chromatography-tandem mass spectrometry as previously described (8). The concentration standard curve included the following ceramide species: C14:0-Cer, C16:0-Cer, C18:1-Cer, C18:0-Cer, C20:0-Cer, C24:0-Cer, and C24:1-Cer. Subsequently to the analysis of these samples we learned that very-long-chain ceramides (C20 and longer) and the shorter chain ceramide are differentially extracted, an issue that is overcome by inclusion of a 17C-Cer C24:0 internal standard. Unfortunately, we didn’t have the 17C-Cer C24:0 internal standard at the time we processed these samples, and because of this we systematically overestimated long-chain ceramide concentrations.
Calculations and Statistical Analysis
Plasma palmitate flux (rate of appearance and disappearance) was calculated as previously described (35). The M+16 enrichment in 16C ceramide was divided by the average plasma palmitate M+16 enrichment to estimate the fraction of intramyocellular ceramide that was derived from newly arrived plasma palmitate via the de novo synthesis pathway (8).
Data are represented as means ± SD or median (interquartile range) unless otherwise remarked. Differences in the variables were made using the Wilcoxon signed rank test or paired t-test. The change in intramyocellular ceramide concentrations was calculated as the data from the second biopsy minus that from the first biopsy. Spearman’s rank correlation or Pearson correlation analysis was used to evaluate the associations between the measurements or between the changes in ceramide concentrations and metabolic and body fat distribution. Only factors that were correlated with these changes with a P value of <0.05 were used for subsequent multivariate regression analyses. To assess statistical significance with three different groups of ceramide species according to acyl chain lengths (medium chain, long chain, and very long chain), the Bonferroni correction was utilized for three comparisons, with a significant P value being set at a value of <0.017. For statistical significance other than relations with ceramide species, an α-level of 0.05 was used. We performed stepwise multiple linear regression analysis to examine the independent factors predicting fractional contribution of plasma palmitate to intramyocellular 16:0 ceramide and the changes in intramyocellular ceramide species during the postprandial protocol. Parameters with skewed distributions were log-transformed before analysis. We used a variance inflation factor to identify collinearity. A variance inflation factor of <10 was included in the model. Statistical analyses were conducted using SPSS software (SPSS version 20.0, SPSS, Chicago, IL).
RESULTS
Characteristics of Study Subjects
The characteristics of the study subjects are summarized in Table 1. Plasma palmitate concentrations were suppressed to an average of ~30 μmol/l, consistent with the prandial insulin concentrations. Plasma palmitate concentrations and enrichments/flux were relatively stable between 0 and 90 min (before the first biopsy) and between 150–330 min (before the second biopsy), as well as between 0 and 330 min. As expected, obese participants had greater concentrations of glucose and insulin, higher percent body fat, and total fat mass than nonobese participants. There were no significant differences in plasma palmitate concentrations and enrichments/flux between nonobese and obese volunteers, although this was in the context of postprandial insulin concentrations that were over twice that of the nonobese volunteers. Plasma glucose, insulin, palmitate, and VLDL-triglyceride concentrations during the study are provided in Supplementary Fig. S1 (accessible online only).
Table 1.
Characteristics of study participants
| Variables | Total | Nonobese | Obese |
|---|---|---|---|
| N | 38 | 18 | 20 |
| Age, yr | 34 ± 9 | 34 ± 10 | 34 ± 9 |
| BMI, kg/m2 | 28.6 ± 5.9 | 23.0 ± 1.8 | 33.5 ± 3.2‡ |
| Fasting glucose, mg/dl | 91 ± 10 | 86 ± 6 | 96 ± 11† |
| Fasting insulin, μIU/ml | 7 ± 4 | 5 ± 2 | 9 ± 4‡ |
| Postprandial glucose, mg/dl | 108 ± 10 | 104 ± 8 | 111 ± 10* |
| Postprandial insulin, μIU/ml | 19 ± 14 | 11 ± 7 | 25 ± 15‡ |
| HOMA-IR | 1.7 ± 1.0 | 1.0 ± 0.4 | 2.2 ± 0.9‡ |
| Total cholesterol, mg/dl | 180 ± 35 | 176 ± 38 | 183 ± 33 |
| HDL-cholesterol, mg/dl | 58 ± 17 | 64 ± 18 | 53 ± 14 |
| Triglyceride, mg/dl | 89 ± 53 | 75 ± 32 | 101 ± 64 |
| Body fat, % | 33.9 ± 11.2 | 25.5 ± 8.7 | 41.0 ± 7.6‡ |
| Total body fat, kg | 28.4 ± 12.3 | 16.9 ± 5.0 | 38.2 ± 6.7‡ |
| FFM, kg | 53.4 ± 11.4 | 50.8 ± 12.0 | 55.7 ± 10.6 |
| UBSQ fat, kg | 15.5 ± 7.2 | 9.0 ± 3.1 | 20.9 ± 4.8‡ |
| LBSQ fat, kg | 10.2 ± 4.3 | 6.6 ± 2.0 | 13.4 ± 3.0‡ |
| Visceral fat, kg | 2.7 ± 2.1 | 1.3 ± 0.7 | 4.0 ± 2.1‡ |
| Plasma palmitate, μmol/l (0–90 min) | 30 ± 21 | 26 ± 12 | 34 ± 27 |
| Plasma palmitate turnover, μmol/min (0–90 min) | 70 ± 38 | 65 ± 31 | 74 ± 43 |
| Plasma [U-13C]palmitate enrichment (0–90 min) | 0.064 ± 0.035 | 0.064 ± 0.035 | 0.064 ± 0.036 |
| Plasma palmitate, μmol/l (150–330 min) | 32 ± 24 | 28 ± 19 | 35 ± 27 |
| Plasma palmitate turnover, μmol/min (150–330 min) | 63 ± 36 | 59 ± 28 | 66 ± 43 |
| Plasma [U-13C]palmitate enrichment (150–330 min) | 0.072 ± 0.040 | 0.072 ± 0.037 | 0.072 ± 0.042 |
Data are shown as means ± SD. Postprandial insulin is an average concentration of plasma insulin during the postprandial condition. For the entire group, plasma palmitate enrichment increased significantly from the time interval 0–90 min to 150–330 min, P = 0.012. BMI, body mass index; HOMA-IR, homeostasis model assessment of insulin resistance; FFM, fat-free mass; HDL, high-density lipoprotein; LBSQ, lower body subcutaneous; UBSQ, upper body subcutaneous.
vs. nonobese, P < 0.05;
vs. nonobese, P < 0.01,
vs. nonobese, P < 0.001.
Changes in Subcellular Ceramide Concentrations During the Postprandial Condition
There was no statistically significant interaction between obesity status and any of the covariates for intramyocellular ceramide subspecies concentrations (P > for interaction > 0.05). The changes in ceramide concentrations in SS fraction differed between subspecies (Table 2). SS C24:1 and C24:0 ceramide concentrations increased significantly, whereas SS C16:0, C18:1 and C18:0 ceramide concentrations tended (P values of 0.035–0.062) to decrease. Intramyofibrillar ceramide species concentrations remained stable between the first and second biopsy.
Table 2.
Intramyocellular sphingolipid concentrations during the postprandial protocol
| Postprandial Period |
|||
|---|---|---|---|
| 1.5 h | 6.5 h | P Value | |
| SS fraction, pmol/mg protein | |||
| Sphingosine | 0.85 (0.58–1.25) | 0.83 (0.65–1.18) | 0.994 |
| Sphinganine | 0.81 ± 0.42 | 0.81 ± 0.54 | 0.993 |
| Total Cer | 73.8 ± 28.0 | 80.1 ± 26.0 | 0.262 |
| C14:0 Cer | 0.27 ± 0.12 | 0.25 ± 0.12 | 0.202 |
| C16:0 Cer | 9.5 ± 5.2 | 8.0 ± 2.8 | 0.062 |
| C18:1 Cer | 5.3 (4.0–7.2) | 4.9 (3.4–6.7) | 0.056 |
| C18:0 Cer | 35.0 ± 12.0 | 30.8 ± 10.5 | 0.035 |
| C20:0 Cer | 0.99 ± 0.27 | 0.96 ± 0.41 | 0.608 |
| C24:1 Cer | 4.2 ± 3.5 | 7.5 ± 5.3 | 0.0016 |
| C24:0 Cer | 17.0 ± 12.8 | 27.5 ± 12.7 | 0.0016 |
| IMF fraction, pmol/mg protein | |||
| Sphingosine | 0.69 (0.51–1.03) | 0.74 (0.47–1.57) | 0.592 |
| Sphinganine | 0.71 ± 0.42 | 0.73 ± 0.43 | 0.735 |
| Total Cer | 44.8 (37.0–54.9) | 46.1 (35.6–55.9) | 0.483 |
| C14:0 Cer | 0.31 ± 0.15 | 0.34 ± 0.24 | 0.233 |
| C16:0 Cer | 5.5 ± 2.0 | 5.8 ± 3.1 | 0.414 |
| C18:1 Cer | 5.5 (4.3–7.6) | 5.4 (3.9–8.0) | 0.922 |
| C18:0 Cer | 18.7 ± 8.7 | 19.1 ± 9.4 | 0.769 |
| C20:0 Cer | 0.10 (0.07–0.24) | 0.09 (0.07–0.21) | 0.492 |
| C24:1 Cer | 1.8 (1.4–2.5) | 1.7 (1.4–2.4) | 0.115 |
| C24:0 Cer | 14.0 (9.8–15.4) | 13.0 (10.1–17.9) | 0.053 |
Data are shown as means ± SD or median (interquartile range). Analyzed by paired t-test or Wilcoxon signed rank test. SS, subsarcolemmal; IMF, intramyofibrillar; Cer, ceramide.
Relationship Among Metabolic Parameters, Body Fat Distribution, and Changes in Intramyocellular Subcellular Ceramide Concentrations
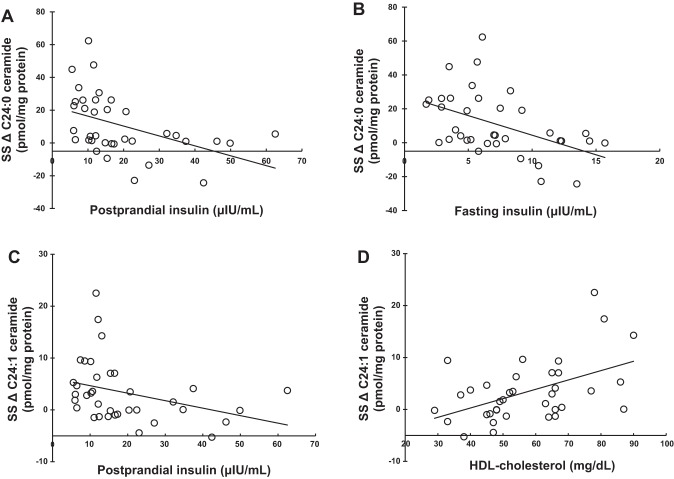
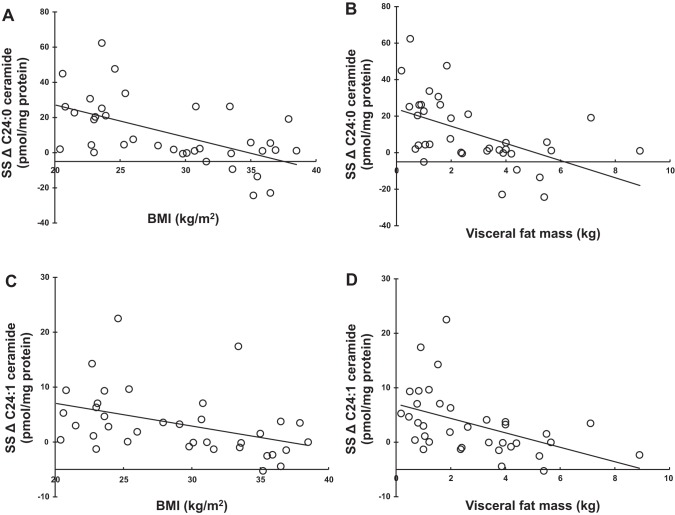
We examined whether body composition or metabolic factors were related to the variability in the increase in SS C24:1 and C24:0 ceramide concentrations. Fasting and postprandial insulin concentrations were negatively correlated with the change in SS C24 ceramide concentrations (Table 3 and Fig. 2, A–C), as were measurements of adiposity including BMI and visceral fat mass (Table 4 and Fig. 3, A–D). Fat mass also correlated negatively with the change in SS C24:0 ceramide concentrations (ρ = –0.411, P = 0.0116). Although UBSQ and LBSQ fat masses correlated negatively with the changes in SS C24:1 and C24:0 ceramide as well, the P value did not reach the levels we selected for statistical significance. In summary, greater amounts of body fat and hyperinsulinemia were associated with lesser prandial increases in SS C24 ceramide concentrations. More body fat and greater hyperinsulinemia were also associated with lesser prandial increases in total amounts of ceramide species in the SS fraction (largely driven by changes in SS C24 ceramide concentrations).
Table 3.
Relationships between metabolic parameters and postprandial changes in intramyocellular ceramide concentrations
| Fasting Insulin |
Postprandial Insulin |
HDL-Chol |
||||
|---|---|---|---|---|---|---|
| ρ | P value | ρ | P value | ρ | P value | |
| IMF fraction | ||||||
| ΔTotal Cer | −0.221 | 0.188 | −0.268 | 0.109 | 0.285 | 0.087 |
| ΔC14:0 Cer | −0.289 | 0.083 | −0.307 | 0.065 | 0.007 | 0.968 |
| ΔC16:0 Cer | −0.257 | 0.125 | −0.222 | 0.186 | 0.188 | 0.264 |
| ΔC18:1 Cer | −0.231 | 0.169 | −0.241 | 0.151 | 0.014 | 0.934 |
| ΔC18:0 Cer | −0.023 | 0.894 | −0.083 | 0.627 | 0.174 | 0.304 |
| ΔC20:0 Cer | −0.069 | 0.686 | −0.093 | 0.582 | 0.350 | 0.034 |
| ΔC24:1 Cer | −0.174 | 0.304 | −0.261 | 0.119 | 0.332 | 0.044 |
| ΔC24:0 Cer | −0.196 | 0.246 | −0.270 | 0.106 | 0.395 | 0.0155 |
| SS fraction | ||||||
| ΔTotal Cer | −0.416 | 0.010 | −0.529 | 0.0008 | 0.269 | 0.107 |
| ΔC14:0 Cer | −0.142 | 0.401 | −0.077 | 0.649 | −0.287 | 0.085 |
| ΔC16:0 Cer | −0.272 | 0.104 | −0.190 | 0.259 | 0.012 | 0.946 |
| ΔC18:1 Cer | 0.116 | 0.493 | 0.088 | 0.603 | −0.369 | 0.025 |
| ΔC18:0 Cer | −0.132 | 0.435 | −0.085 | 0.615 | −0.085 | 0.617 |
| ΔC20:0 Cer | −0.187 | 0.269 | −0.162 | 0.338 | 0.163 | 0.335 |
| ΔC24:1 Cer | −0.329 | 0.047 | −0.463 | 0.0039a | 0.434 | 0.0072a |
| ΔC24:0 Cer | −0.430 | 0.0078a | −0.553 | 0.0004a | 0.376 | 0.022 |
ρ represents the Spearman’s rank correlation coefficient. Intramyocellular ceramides are normalized to mg protein. The change (Δ) in intramyocellular ceramide concentrations was calculated as the data from the second biopsy minus that from the first biopsy.
Statistically significant association after modified adjustment for multiple species comparisons.
Fig. 2.
Relationships between metabolic parameters and the postprandial changes of intramyocellular very-long-chain ceramide species concentrations. A: correlation between postprandial insulin concentration and the change in subsarcolemmal (SS) C24:0 ceramide concentration (ρ = –0.553, P = 0.0004). B: correlation between fasting insulin concentration and the change in SS C24:0 ceramide concentration (ρ = –0.430, P = 0.0078). C: correlation between postprandial insulin concentration and the change in SS C24:1 ceramide concentration (ρ = –0.463, P = 0.0039). D: correlation between plasma HDL-cholesterol concentration and the change in SS C24:1 ceramide concentration (ρ = 0.434, P = 0.0072). ρ represents the Spearman’s rank correlation coefficient. Intramyocellular ceramide concentrations are normalized to per milligram of protein. The change (Δ) in intramyocellular ceramide concentrations was calculated as the data from the second biopsy minus that from the first biopsy.
Table 4.
Relationships between body fat and fat distribution and postprandial changes in intramyocellular ceramide concentrations
| BMI |
Visceral Fat Mass |
|||
|---|---|---|---|---|
| Ρ | P value | ρ | P value | |
| IMF fraction | ||||
| ΔTotal Cer | −0.170 | 0.315 | −0.213 | 0.206 |
| ΔC14:0 Cer | −0.143 | 0.397 | −0.295 | 0.076 |
| ΔC16:0 Cer | −0.137 | 0.418 | −0.285 | 0.088 |
| ΔC18:1 Cer | −0.122 | 0.473 | −0.160 | 0.345 |
| ΔC18:0 Cer | −0.011 | 0.949 | 0.044 | 0.797 |
| ΔC20:0 Cer | 0.004 | 0.982 | −0.004 | 0.981 |
| ΔC24:1 Cer | −0.078 | 0.648 | −0.208 | 0.216 |
| ΔC24:0 Cer | −0.086 | 0.511 | −0.242 | 0.148 |
| SS fraction | ||||
| ΔTotal Cer | −0.502 | 0.002 | −0.580 | 0.0002 |
| ΔC14:0 Cer | −0.010 | 0.954 | −0.100 | 0.555 |
| ΔC16:0 Cer | −0.233 | 0.166 | −0.306 | 0.066 |
| ΔC18:1 Cer | 0.023 | 0.890 | 0.056 | 0.742 |
| ΔC18:0 Cer | −0.072 | 0.671 | −0.110 | 0.517 |
| ΔC20:0 Cer | −0.133 | 0.431 | −0.290 | 0.082 |
| ΔC24:1 Cer | −0.461 | 0.0041a | −0.545 | 0.0005a |
| ΔC24:0 Cer | −0.439 | 0.0065a | −0.543 | 0.0005a |
ρ represents the Spearman’s rank correlation coefficient. Intramyocellular ceramides are normalized to mg protein. The change (Δ) in intramyocellular ceramide concentrations was calculated as the data from the second biopsy minus that from the first biopsy.
Statistically significant association after the modified adjustment for multiple species comparisons.
Fig. 3.
Relationships between metabolic parameters and the postprandial changes of intramyocellular very-long-chain ceramide species concentrations. A: correlation between body mass index (BMI) and the change in SS C24:0 ceramide concentration (ρ = –0.439, P = 0.0065). B: correlation between visceral fat mass and the change in SS C24:0 ceramide concentration (ρ = –0.543, P = 0.0005). C: correlation between BMI and the change in SS C24:1 ceramide concentration (ρ = –0.461, P = 0.0041). D: correlation between visceral fat mass and the change in SS C24:1 ceramide concentration (ρ = –0.545, P = 0.0005). ρ represents the Spearman’s rank correlation coefficient. Intramyocellular ceramide concentrations are normalized to per milligram of protein. The change (Δ) in intramyocellular ceramide concentrations was calculated as the data from the second biopsy minus that from the first biopsy.
We found no significant correlation between the insulin concentrations and the postprandial changes of IMF ceramide species concentrations. Plasma HDL-cholesterol concentration correlated positively with the changes in SS C24:1 ceramide concentrations (Fig. 2D).
We then investigated whether body composition or metabolic factors were the best predictors of the changes in SS C24 ceramide concentrations. Because we had only 38 observations, we elected to evaluate anthropometric and metabolic variables separately and then test which of those two types of variables better explain the changes in SS C24 ceramides. We used multivariate regression analysis with the changes in SS C24 ceramide concentrations as the dependent factor and BMI and visceral fat as independent factors. We repeated this analysis using fasting insulin concentrations, postprandial insulin concentrations, and HDL-cholesterol concentrations as independent factors. Of the anthropometric variables, BMI was the only independent predictor of the changes in SS C24:0 ceramide concentrations. Of the metabolic variables, fasting insulin concentration was the strongest predictor. When these two were included in a multivariate model, BMI was the only independent predictor of the changes in SS C24:0 ceramide concentrations (β = –0.516, P = 0.0011).
Fractional Contribution of De Novo Synthesis of Plasma Palmitate to Intramyocellular Ceramides
Plasma [U-13C]palmitate enrichments increased by an average of ~0.008 (16%, P = 0.012) between the first and second biopsies (Table 1). Because of the relatively modest, albeit statistically significant, change in plasma palmitate enrichments between the first and second biopsies, we used the overall average palmitate enrichments to calculate the fractional contribution of plasma palmitate to de novo C16:0 ceramides (Table 5).
Table 5.
Intramyocellular [13C16]16:0 ceramide enrichment and fractional contributions of plasma palmitate to intramyocellular ceramide during continuous intravenous infusion of [U-13C]palmitate
| Postprandial Period |
|||
|---|---|---|---|
| Time from baseline | 1.5 h | 6.5 h | P Value |
| Plasma [U-13C]palmitate enrichment | 0.064 ± 0.035 | 0.068 ± 0.036 | |
| [13C16]16:0 ceramide enrichment | |||
| SS fraction | 0.009 (0.000–0.015) | 0.014 (0.008–0.021) | 0.0024 |
| IMF fraction | 0.002 (0.000–0.005) | 0.004 (0.000–0.007) | 0.0025 |
| Fractional contribution of plasma palmitate to ceramide, % | |||
| SS fraction | 9.4 (0.0–23.5)* | 19.9 (5.8–39.5)† | 0.0043 |
| IMF fraction | 4.1 (0.0–8.7) | 7.1 (0.0–15.2) | 0.0030 |
Data are shown as means ± SD or median (interquartile range). Plasma [U-13C]palmitate enrichments at 1.5 h and 6.5 h represent mean values until the first biopsy and the mean values from time 0 until the second biopsy, respectively. Analyzed by Wilcoxon signed rank test.
Significantly different from fractional contribution of plasma palmitate in IMF at the same time point (P = 0.0143);
Significantly different from fractional contribution of plasma palmitate in IMF at the same time point (P = 0.0002).
During the continuous [U-13C]palmitate infusion, the C16:0 ceramide enrichment increased significantly in both IMF and SS (Table 5). The fractional contribution of plasma palmitate to intramyocellular C16:0 ceramide was greater in the SS than in the IMF fraction at the time of both the first and second muscle biopsies (Table 5). We used the data from the second biopsy to calculate the fractional contribution of plasma palmitate to intramyocellular C16:0 ceramide for subsequent analyses.
We evaluated whether the fraction of intramyocellular de novo C16:0 ceramide synthesis derived from plasma palmitate was related to plasma palmitate concentrations, the size of the C16:0 ceramide pool, or the metabolic parameters in postprandial conditions. There were no significant relationships between the fractional contributions of plasma palmitate to de novo intramyocellular C16:0 and plasma palmitate concentrations (Table 6) or the intramyocellular C16:0 ceramide concentrations (γ = 0.095, P = 0.586 for IMF; γ = –0.140, P = 0.431 for SS).
Table 6.
Relationship between fractional incorporation of plasma palmitate into intramyocellular 16:0 ceramide and clinical parameters
| IMF 13C16 16:0 Cer |
SS 13C16 S16:0 Cer |
|||
|---|---|---|---|---|
| γ | P value | γ | P value | |
| Age | –0.307 | 0.073 | 0.329 | 0.050 |
| BMI | –0.432 | 0.010 | –0.417 | 0.014 |
| Fasting glucose | –0.140 | 0.438 | –0.017 | 0.925 |
| Postprandial glucose | –0.053 | 0.764 | –0.061 | 0.732 |
| HOMA-IR | –0.066 | 0.715 | –0.048 | 0.795 |
| Fasting insulin | –0.041 | 0.815 | –0.068 | 0.701 |
| Postprandial insulin | –0.141 | 0.419 | –0.133 | 0.452 |
| Plasma triglycerides | –0.037 | 0.836 | –0.059 | 0.744 |
| Fat mass | –0.335 | 0.049 | –0.328 | 0.059 |
| UBSQ fat mass | –0.311 | 0.069 | –0.344 | 0.046 |
| LBSQ fat mass | –0.290 | 0.092 | –0.252 | 0.151 |
| Visceral fat mass | –0.273 | 0.113 | –0.194 | 0.272 |
| Plasma palmitate (0–90 min) | 0.050 | 0.777 | –0.092 | 0.603 |
| Plasma palmitate (150–330 min) | –0.001 | 0.998 | –0.079 | 0.657 |
Fractional contribution of plasma palmitate to intramyocellular ceramide was at the time of the second biopsy.
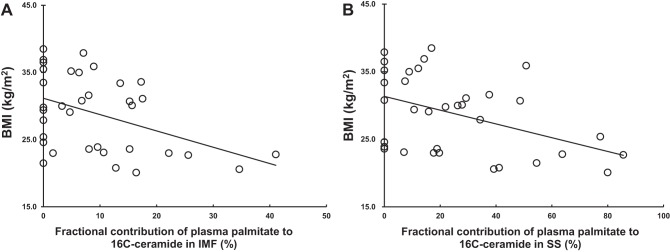
For both the SS and IMF, the fractional contribution of plasma palmitate to intramyocellular C16:0 ceramide was negatively correlated with indexes of adiposity, especially BMI (Table 6 and Fig. 4).
Fig. 4.
Relationships between body fat and the fractional contribution of plasma palmitate to intramyocellular 13C16 16:0 ceramide. A: correlation between BMI and fractional contribution of plasma palmitate to 16C-ceramide in intramyofibrillar (IMF) fraction (γ = –0.432, P = 0.010). B: correlation between BMI and fractional contribution of plasma palmitate to 16C-ceramide in SS fraction (γ = –0.417, P = 0.014).
To evaluate the factors relating to fractional contribution of plasma palmitate to intramyocellular C16:0 ceramide, multivariate regression analyses were performed with age, BMI, and UBSQ fat mass as independent variables and fractional contribution of plasma palmitate to intramyocellular ceramide as the dependent variable. BMI and age were independent predictors of the fractional contribution of plasma palmitate to SS 16:0 ceramide (β = –0.443, P = 0.0058 and β = 0.370, P = 0.0189, respectively). BMI was an independent predictor of the fractional contribution of plasma palmitate to IMF C16:0 ceramide (β = –0.432, P = 0.0095).
DISCUSSION
Because of the importance of ceramides to muscle metabolic performance (7, 10, 38) and possible contributors to insulin resistance in obesity (1), we investigated the interactions between meal ingestion and obesity on intramyocellular ceramides. Specifically, we measured the concentrations of SS and IMF ceramide species as well as the fractional contribution of plasma palmitate to SS and IMF 16:0 ceramide, using stable-isotope approaches. We examined whether metabolic and anthropometric parameters are associated with the changes in intramyocellular ceramides and the incorporation of plasma palmitate into C16:0 ceramide in the postprandial state. Our primary findings were that 1) in response to meal ingestion SS C24 ceramide concentrations, but not C14–C20 concentrations, increased significantly; 2) the change in SS C24 ceramide concentrations was significantly and negatively related to parameters of overweight and insulin resistance; and 3) the fractional contribution of plasma palmitate to intramyocellular C16:0 ceramides in both IMF and SS fractions was inversely related to BMI.
Previous studies of how diet changes intramyocellular ceramide contents typically involved longer-term high-fat feeding in animal models (reviewed in Ref. 10); increases in intramyocellular ceramide accrual are typically reported. We found no reports of the effects of acute meal ingestion on de novo synthesis of intramyocellular ceramides in humans. In the present study, we found that during the ingestion of a nonfat meal the C16:0 ceramide enrichments in both IMF and SS increased over the 6.5 h of the palmitate tracer infusion. The average enrichments approximately doubled from the first 1.5 h to the last 5 h of the tracer infusion, suggesting that de novo incorporation of plasma palmitate to ceramides in both IMF and SS fractions is not directly proportional to the duration of the tracer infusion under meal ingestion conditions. In addition, the greater C16:0 ceramide enrichments (~3–4 times) in SS than in IMF after 6.5 h of the tracer infusion may indicate that SS depends more on plasma FFA for de novo ceramide synthesis. Alternatively, IMF ceramides may be derived from those synthesized in SS, and the equilibration between the two pools may be slow. Interestingly, the fractional contribution of plasma palmitate to ceramide in both the SS and IMF pools during meal ingestion were relatively similar to those observed after an overnight fast condition (~9% in IMF and ~18% in SS, respectively) (11).
In the present study, plasma palmitate concentrations were approximately one-fourth of those we found in the fasting conditions (11). We might have expected that as a result of the lower palmitate concentrations the fraction of intramyocellular ceramides derived from de novo synthesis using plasma palmitate would be less than what we observed in fasting conditions. The similar fractional contribution of plasma palmitate to C16:0 ceramide under fed and fasting conditions has two possible explanations. The first is that muscle fatty acid transport and ceramide synthesis enzymes are regulated in such a manner that plasma FFA contribute consistently to muscle ceramide content over a wide range of FFA concentrations. The other possibility is that intramyocellular ceramide pool turnover increases during meal ingestion such that a lesser absolute C16:0 ceramide synthesis rate results in a similar fractional contribution of plasma FFA to that seen in the postabsorptive condition. Consistent with that hypothesis, SS C16:0 ceramide concentrations tended to decrease from the first to the second biopsy.
If the intramyocellular ceramide turnover does increase in response to meal ingestion, this response appears to be blunted in obesity. This might explain the surprising negative relationship between indexes of adiposity, especially upper body subcutaneous fat mass, and the fractional contribution of plasma palmitate to both IMF and SS C16:0 ceramide. It is reasonably well established that FFA oversupply can lead to greater intramyocellular ceramides (1). Because some types of obesity are associated with higher postprandial FFA concentrations, we hypothesized that the increased FFA would drive greater intramyocellular de novo ceramide synthesis in obesity (1). Indeed, previous studies showed that hyperinsulinemia/insulin resistance was positively related to serine palmitoyltransferase gene expression (37) and intramyocellular ceramide content (11). Unfortunately, we can only measure the fractional contribution of FFA to de novo ceramide synthesis, and thus we cannot exclude confounding effects of differential changes in C16:0 ceramide turnover between lean and obese humans.
Although FFA released from chylomicron- and VLDL-triglyceride hydrolysis are taken up by muscle in the postprandial state (22, 23), adipose tissue is the main site for clearance of fatty acids released from circulating triglycerides (5). Skeletal muscle takes up FFA in the postabsorptive state (12, 20) and switches the predominant fuel source from fatty acids to glucose in the postprandial state (13). We have found that a relatively large proportion of plasma palmitate is stored in upper and lower body subcutaneous adipose tissue depots in the fed condition (26, 27). It is possible that the greater shunting of plasma FFA into adipose tissue during feeding in obese adults results in lesser FFA availability for muscle uptake and de novo synthesis of ceramides. Other possible explanations include an exaggerated redirection of FFA toward other intramyocellular fatty acid pools during meal ingestion in obesity or reduced muscle delivery of FFA to muscle due to impaired vascular recruitment to skeletal muscle (24).
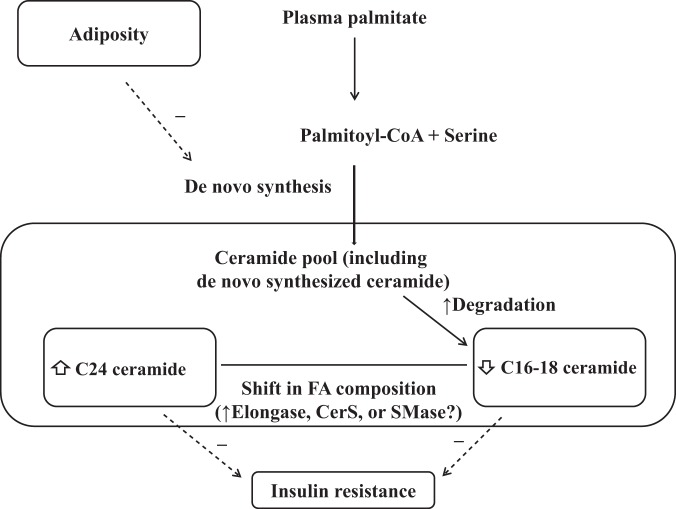
The differing postprandial changes in intramyocellular ceramide subspecies concentrations (Table 2) deserve comment. Accumulating evidence suggests that specific types of muscle ceramide subspecies are associated with insulin resistance (14). An inverse relationship between insulin sensitivity and the C16:0 and C18:0 ceramide concentration in human skeletal muscle cell homogenate has been reported (4, 41). We found that SS C16–C18 ceramide, but not SS C24 ceramide, were related to markers of insulin resistance (11). Recent studies reported beneficial effects of C24 ceramide on cellular stress and insulin sensitivity (31, 34). The inverse relationships between the increase in C24 ceramide and insulin concentrations and adiposity indexes that we observed supports the concept that C24 ceramides are negatively linked to insulin resistance. Therefore, differing postprandial changes in SS ceramide subspecies might suggest compensatory responses against metabolic deterioration in response to meal ingestion, as depicted in Fig. 5, to the extent that specific types of ceramides subspecies might be differently and causally related to insulin resistant state.
Fig. 5.
Proposed ceramide regulations in intramyocellular SS fraction during meal ingestion. In postprandial conditions, there are differing effects on SS ceramide subspecies, and the de novo synthesis of intramyocellular ceramides from plasma palmitate is reduced in the overweight condition.
Although the mechanism for different changes in ceramide subspecies concentrations during meal ingestion has not been fully clarified, several factors might account for the preferential increase in very long acyl chain ceramides in SS fraction. Ceramide synthase-2 catalyzes preferentially the conjugation of a second acyl-CoA of very long chain length, resulting in the formation of very long chain ceramides such as C24 ceramide (10). In addition, very long chain fatty acids are derived from long chain fatty acids by elongation (25). In vitro studies showed that elongase-1 plays a major role in generation of C24 ceramides via ceramide synthase-2 (25, 33). In addition, the increase in SS C24 ceramide concentrations might be via turnover of sphingomyelin from cell membrane. Sphingomyelin can be degraded to ceramide by neutral sphingomyelinase-2 with a preference for C24 subspecies, causing the selective increase in C24 ceramides (29).
The present study has some limitations. The participants in the present study consumed fat-free food in a continuous-feeding paradigm, which does not reflect ordinary dietary patterns. However, this feeding paradigm circumvents the confounding entrance of chylomicron-derived fatty acids into the plasma FFA pool, eliminates the problem of direct (muscle LPL-mediated) contribution of chylomicron fatty acids to ceramides, and also creates steady-state or near-steady-state FFA kinetics. Another limitation is that the first muscle biopsy was obtained after 1.5 h of tracer infusion, which might have attenuated the changes in intramyocellular ceramide concentrations from a true fasting level. Finally, we acknowledge that we systematically overestimated the intramyocellular concentrations of C20–C24 ceramides as a result of not including a species-specific internal standard. However, because all samples were analyzed together and the change in C24 ceramide concentrations was a paired analysis, our conclusions regarding the specificity of the change in ceramide species is correct. Plasma palmitate that is taken up by the liver will be released into the circulation as labeled VLDL-triglycerides and might be released as labeled ceramides in lipoprotein particles, either of which could potentially contribute to skeletal muscle ceramides. To address this, we measured the time course of enrichment in VLDL-triglyceride [U-13C]palmitate in seven of the participants in these studies. The enrichment gradually increased so that at the time of the second muscle biopsy the enrichment in VLDL-triglyceride palmitate had reached one-fifth to one-half that of plasma palmitate. Because only ~0.2–0.3% of plasma VLDL-triglycerides are taken up by muscle (3) [as opposed to ~10% of FFA (23)] it seems unlikely that VLDL-triglyceride fatty acids contribute to intramyocellular ceramides. We also analyzed a limited number of plasma samples to understand whether the [U-13C]palmitate is incorporated into circulating C16:0 ceramide; we could not detect any enrichment. These data suggest that FFA palmitate was the primary if not only source of circulating palmitate we found in muscle ceramides. Unfortunately, we measured the fractional contribution of plasma palmitate only to intramyocellular C16:0 ceramide, whereas if we had been able to purchase enriched standards for all of the species (labeled with [U-13C]palmitate in the sphingoid base), we could have estimated the fractional contribution of plasma palmitate to all the ceramide species. Acquiring custom-synthesized enriched standards of all ceramide species will be very helpful in gaining more from future studies of muscle ceramide metabolism. Despite these limitations, we believe our data provide important information regarding subcellular ceramide metabolism in human skeletal muscle.
In summary, the present study provides novel, fundamental information regarding postprandial ceramide regulation in skeletal muscle in humans, suggesting important differences between various ceramide species with regard to metabolic responses. Future studies that can examine the de novo synthesis of longer-chain ceramides from plasma fatty acids will be necessary to determine the underlying mechanisms for these changes. Finally, our findings were contrary to our hypothesis that greater amounts of body fat result in increased de novo intramyocellular ceramide synthesis from plasma FFA under all conditions.
GRANTS
These studies were supported by National Institutes of Health grants 1UL1 RR-024150, DK-45343, DK-40484, and DK-50456.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.O.C., A.U.B.-Z., K.C.H., and M.D.J. analyzed data; J.O.C. drafted manuscript; J.O.C., C.K., A.U.B.-Z., K.C.H., and M.D.J. edited and revised manuscript; J.O.C. and M.D.J. approved final version of manuscript; C.K. and M.D.J. conceived and designed research; C.K. performed experiments.
ACKNOWLEDGMENTS
We are indebted to the research volunteers for their participation. We are also grateful to Barbara Norby and Carley Vrieze for assistance with nursing care and muscle biopsies and for assay development and performance to Debra Harteneck, Darlene Lucas, and Lendia Zhou.
REFERENCES
- 1.Aburasayn H, Al Batran R, Ussher JR. Targeting ceramide metabolism in obesity. Am J Physiol Endocrinol Metab 311: E423–E435, 2016. doi: 10.1152/ajpendo.00133.2016. [DOI] [PubMed] [Google Scholar]
- 2.Adams JM II, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53: 25–31, 2004. doi: 10.2337/diabetes.53.1.25. [DOI] [PubMed] [Google Scholar]
- 3.Andersen IR, Søndergaard E, Sørensen LP, Nellemann B, Gormsen LC, Jensen MD, Nielsen S. Increased VLDL-TG Fatty Acid Storage in Skeletal Muscle in Men With Type 2 Diabetes. J Clin Endocrinol Metab 102: 831–839, 2017. [DOI] [PubMed] [Google Scholar]
- 4.Bergman BC, Brozinick JT, Strauss A, Bacon S, Kerege A, Bui HH, Sanders P, Siddall P, Wei T, Thomas MK, Kuo MS, Perreault L. Muscle sphingolipids during rest and exercise: a C18:0 signature for insulin resistance in humans. Diabetologia 59: 785–798, 2016. doi: 10.1007/s00125-015-3850-y. [DOI] [PubMed] [Google Scholar]
- 5.Bickerton AS, Roberts R, Fielding BA, Hodson L, Blaak EE, Wagenmakers AJM, Gilbert M, Karpe F, Frayn KN. Preferential uptake of dietary Fatty acids in adipose tissue and muscle in the postprandial period. Diabetes 56: 168–176, 2007. doi: 10.2337/db06-0822. [DOI] [PubMed] [Google Scholar]
- 6.Bikman BT, Summers SA. Ceramides as modulators of cellular and whole-body metabolism. J Clin Invest 121: 4222–4230, 2011. doi: 10.1172/JCI57144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blachnio-Zabielska AU, Koutsari C, Tchkonia T, Jensen MD. Sphingolipid content of human adipose tissue: relationship to adiponectin and insulin resistance. Obesity (Silver Spring) 20: 2341–2347, 2012. doi: 10.1038/oby.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blachnio-Zabielska AU, Persson X-MT, Koutsari C, Zabielski P, Jensen MD. A liquid chromatography/tandem mass spectrometry method for measuring the in vivo incorporation of plasma free fatty acids into intramyocellular ceramides in humans. Rapid Commun Mass Spectrom 26: 1134–1140, 2012. doi: 10.1002/rcm.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blouin CM, Prado C, Takane KK, Lasnier F, Garcia-Ocana A, Ferré P, Dugail I, Hajduch E. Plasma membrane subdomain compartmentalization contributes to distinct mechanisms of ceramide action on insulin signaling. Diabetes 59: 600–610, 2010. doi: 10.2337/db09-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab 15: 585–594, 2012. doi: 10.1016/j.cmet.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Chung JO, Koutsari C, Blachnio-Zabielska AU, Hames KC, Jensen MD. Intramyocellular ceramides – sub-cellular concentrations and fractional de novo synthesis in postabsorptive humans. Diabetes 66: 2082–2091, 2017. doi: 10.2337/db17-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckel RH. Lipoprotein lipase. A multifunctional enzyme relevant to common metabolic diseases. N Engl J Med 320: 1060–1068, 1989. [DOI] [PubMed] [Google Scholar]
- 13.Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, DeFronzo RA. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest 84: 205–213, 1989. doi: 10.1172/JCI114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grösch S, Schiffmann S, Geisslinger G. Chain length-specific properties of ceramides. Prog Lipid Res 51: 50–62, 2012. doi: 10.1016/j.plipres.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Guo Z, Mishra P, Macura S. Sampling the intramyocellular triglycerides from skeletal muscle. J Lipid Res 42: 1041–1048, 2001. [PubMed] [Google Scholar]
- 16.Guo Z, Hensrud DD, Johnson CM, Jensen MD. Regional postprandial fatty acid metabolism in different obesity phenotypes. Diabetes 48: 1586–1592, 1999. doi: 10.2337/diabetes.48.8.1586. [DOI] [PubMed] [Google Scholar]
- 17.Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev 29: 381–402, 2008. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Humphreys SM, Fisher RM, Frayn KN. Micro-method for measurement of sub-nanomole amounts of triacylglycerol. Ann Clin Biochem 27: 597–598, 1990. doi: 10.1177/000456329002700613. [DOI] [PubMed] [Google Scholar]
- 19.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 51: 2005–2011, 2002. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 20.Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab 93, Suppl 1: s57–s63, 2008. doi: 10.1210/jc.2008-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen MD, Kanaley JA, Reed JE, Sheedy PF. Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr 61: 274–278, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Kanaley JA, Shadid S, Sheehan MT, Guo Z, Jensen MD. Hyperinsulinemia and skeletal muscle fatty acid trafficking. Am J Physiol Endocrinol Metab 305: E540–E548, 2013. doi: 10.1152/ajpendo.00143.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanaley JA, Shadid S, Sheehan MT, Guo Z, Jensen MD. Relationship between plasma free fatty acid, intramyocellular triglycerides and long-chain acylcarnitines in resting humans. J Physiol 587: 5939–5950, 2009. doi: 10.1113/jphysiol.2009.180695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keske MA, Clerk LH, Price WJ, Jahn LA, Barrett EJ. Obesity blunts microvascular recruitment in human forearm muscle after a mixed meal. Diabetes Care 32: 1672–1677, 2009. doi: 10.2337/dc09-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kihara A. Very long-chain fatty acids: elongation, physiology and related disorders. J Biochem 152: 387–395, 2012. doi: 10.1093/jb/mvs105. [DOI] [PubMed] [Google Scholar]
- 26.Koutsari C, Mundi MS, Ali AH, Jensen MD. Storage rates of circulating free fatty acid into adipose tissue during eating or walking in humans. Diabetes 61: 329–338, 2012. doi: 10.2337/db11-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koutsari C, Snozek CL, Jensen MD. Plasma NEFA storage in adipose tissue in the postprandial state: sex-related and regional differences. Diabetologia 51: 2041–2048, 2008. doi: 10.1007/s00125-008-1126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsen PJ, Tennagels N. On ceramides, other sphingolipids and impaired glucose homeostasis. Mol Metab 3: 252–260, 2014. doi: 10.1016/j.molmet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marchesini N, Osta W, Bielawski J, Luberto C, Obeid LM, Hannun YA. Role for mammalian neutral sphingomyelinase 2 in confluence-induced growth arrest of MCF7 cells. J Biol Chem 279: 25101–25111, 2004. doi: 10.1074/jbc.M313662200. [DOI] [PubMed] [Google Scholar]
- 30.Miles JM, Ellman MG, McClean KL, Jensen MD. Validation of a new method for determination of free fatty acid turnover. Am J Physiol 252: E431–E438, 1987. [DOI] [PubMed] [Google Scholar]
- 31.Montgomery MK, Brown SH, Lim XY, Fiveash CE, Osborne B, Bentley NL, Braude JP, Mitchell TW, Coster AC, Don AS, Cooney GJ, Schmitz-Peiffer C, Turner N. Regulation of glucose homeostasis and insulin action by ceramide acyl-chain length: A beneficial role for very long-chain sphingolipid species. Biochim Biophys Acta 1861: 1828–1839, 2016. doi: 10.1016/j.bbalip.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen J, Mogensen M, Vind BF, Sahlin K, Højlund K, Schrøder HD, Ortenblad N. Increased subsarcolemmal lipids in type 2 diabetes: effect of training on localization of lipids, mitochondria, and glycogen in sedentary human skeletal muscle. Am J Physiol Endocrinol Metab 298: E706–E713, 2010. doi: 10.1152/ajpendo.00692.2009. [DOI] [PubMed] [Google Scholar]
- 33.Ohno Y, Suto S, Yamanaka M, Mizutani Y, Mitsutake S, Igarashi Y, Sassa T, Kihara A. ELOVL1 production of C24 acyl-CoAs is linked to C24 sphingolipid synthesis. Proc Natl Acad Sci USA 107: 18439–18444, 2010. doi: 10.1073/pnas.1005572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park JW, Park WJ, Kuperman Y, Boura-Halfon S, Pewzner-Jung Y, Futerman AH. Ablation of very long acyl chain sphingolipids causes hepatic insulin resistance in mice due to altered detergent-resistant membranes. Hepatology 57: 525–532, 2013. doi: 10.1002/hep.26015. [DOI] [PubMed] [Google Scholar]
- 35.Persson X-MT, Blachnio-Zabielska AU, Jensen MD. Rapid measurement of plasma free fatty acid concentration and isotopic enrichment using LC/MS. J Lipid Res 51: 2761–2765, 2010. doi: 10.1194/jlr.M008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roust LR, Jensen MD. Postprandial free fatty acid kinetics are abnormal in upper body obesity. Diabetes 42: 1567–1573, 1993. doi: 10.2337/diab.42.11.1567. [DOI] [PubMed] [Google Scholar]
- 37.Samad F, Hester KD, Yang G, Hannun YA, Bielawski J. Altered adipose and plasma sphingolipid metabolism in obesity: a potential mechanism for cardiovascular and metabolic risk. Diabetes 55: 2579–2587, 2006. doi: 10.2337/db06-0330. [DOI] [PubMed] [Google Scholar]
- 38.Schmitz-Peiffer C. Targeting ceramide synthesis to reverse insulin resistance. Diabetes 59: 2351–2353, 2010. doi: 10.2337/db10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skovbro M, Baranowski M, Skov-Jensen C, Flint A, Dela F, Gorski J, Helge JW. Human skeletal muscle ceramide content is not a major factor in muscle insulin sensitivity. Diabetologia 51: 1253–1260, 2008. doi: 10.1007/s00125-008-1014-z. [DOI] [PubMed] [Google Scholar]
- 40.Straczkowski M, Kowalska I, Baranowski M, Nikolajuk A, Otziomek E, Zabielski P, Adamska A, Blachnio A, Gorski J, Gorska M. Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia 50: 2366–2373, 2007. doi: 10.1007/s00125-007-0781-2. [DOI] [PubMed] [Google Scholar]
- 41.Straczkowski M, Kowalska I, Nikolajuk A, Dzienis-Straczkowska S, Kinalska I, Baranowski M, Zendzian-Piotrowska M, Brzezinska Z, Gorski J. Relationship between insulin sensitivity and sphingomyelin signaling pathway in human skeletal muscle. Diabetes 53: 1215–1221, 2004. doi: 10.2337/diabetes.53.5.1215. [DOI] [PubMed] [Google Scholar]