Abstract
The inducible heat shock protein 70 (Hsp70) is both cytoprotective and immunomodulatory, potentially accounting for its critical role in maintaining gastrointestinal homeostasis. When levels are reduced in conditions like inflammatory bowel diseases (IBD), loss of function contributes to the severity and chronicity of these diseases, although through which cell types and mechanisms remains unclear. Here, the role of Hsp70-mediated intestinal epithelial protection and immune regulation in experimental colitis was examined by using a villin promoter-driven Hsp70 transgene in the 2,4,6-trinitrobenzene sulfonic acid (TNBS) and dextran sodium sulfate (DSS) models and in IL-10/Hsp70 double knockout (IL10−/−/Hsp70−/−) mice. In addition, Hsp70-mediated IL-10 production and immune protection were investigated using a CD45RBhigh transfer model and measuring colonic and immune cell cytokine expression during colitis. We found that the epithelial-specific expression of Hsp70 transgene attenuated DSS-induced colitis in Hsp70−/− mice by protecting tight junctions (TJ) and their interaction with the TJ-associated protein ZO-1. In the TNBS colitis and CD45RBhigh model, Hsp70 carried out its intracellular anti-inflammatory function by maintaining IL-10 production. Impaired ERK phosphorylation, but not p38 or JNK phosphorylation pathways, was associated with decreased IL-10 production in Hsp70-deficient cells. Together, these actions can be leveraged in the context of cellular specificity to develop complementary strategies that can lead to reduction in mucosal injury and immune activation in colonic colitis development.
NEW & NOTEWORTHY Using four different experimental colitis models, we filled an important gap in knowledge by defining essential roles of intracellular heat shock protein 70 in different cell types in maintaining intestinal integrity and immune regulation. These findings are relevant to human inflammatory bowel diseases and represent potential avenues for developing therapeutic strategies, not only to counter the destructive processes of inflammation but also to promote tissue healing and prevent complications frequently associated with chronic intestinal inflammation.
Keywords: gastrointestinal homeostasis, heat shock protein, inflammatory bowel diseases
INTRODUCTION
Inducible intestinal heat shock proteins (Hsps) such as Hsp70 play essential roles in maintaining mucosal homeostasis, although it is still unclear what processes and cell types mediate these actions. While most of these proteins are only expressed under conditions of stress, the constitutive expression of Hsp70 in the colonic epithelium appears to be largely maintained by microbial stimuli, including toll-like receptor (TLR) ligands, short-chain fatty acids, and small bioactive microbial peptides (1, 14, 16, 18, 40). Hsp70 knockout (KO) mice (where Hsp70.1 and Hsp70.3 are specifically targeted), for example, are highly susceptible to chemically and genetically induced experimental colitis (17). In Hsp70−/− mice, dextran sodium sulfate (DSS) causes a chronic and severe colitis, whereas most wild-type mice will spontaneously recover. Ironically, colonic mucosal expression of Hsp70 is significantly decreased in both human and experimental colitis, which in the latter appears to be mediated through translational downregulation involving the 3′-untranslated region of the Hsp70 transcript (15). Forced expression of Hsp70 in intestinal epithelial cell through a villin promoter-driven UTR-less Hsp70 transgene partially mitigates the mucosal injury induced by DSS, suggesting that Hsp70 in this cell type can have therapeutic benefit (17). Finally, another illustration of the importance of Hsp70 in the gut is the accelerated development of colon cancer in Hsp70/IL-10 double KO mice (38).
Collectively, these findings implicate essential roles of Hsp70 in the intestinal mucosa relevant to states of health, but also to the development of inflammatory diseases of the bowel. In the present study, we examined the possibility that Hsp70 in different gut mucosal cell types has distinct roles and mechanisms of action that are important for mucosal cytoprotection and immune regulation, when compromised in conditions of immune and inflammatory stress, contribute to the severity and duration of disease. We utilized four different experimental colitis models in this study. Two important protective functions of Hsp70 were elucidated in promoting intestinal homeostasis and limiting inflammation-associated mucosal injury. Epithelial specific expression of Hsp70 protected Hsp70−/− mice from DSS-induced colitis and decreased the penetrance of colitis in IL10−/−/Hsp70−/− mice. Diminished production of IL-10 in Hsp70−/− mice was associated with impaired ERK phosphorylation in immunocytes and rendered mice sensitive to 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis. The finding that Treg cells from Hsp70−/− mice were not able to rescue CD45RBhigh-induced colitis in Rag−/− mice further demonstrates that the intracellular anti-inflammatory function of Hsp70 requires IL-10 production.
MATERIALS AND METHODS
Animals.
Conventionalized wild-type (WT) C57BL/6J, Hsp70.1 and 70.3 deficient (Hsp70−/−), villin transgenic (Hsp70V-TG), Rag-deficient (Rag−/−), IL-10-deficient (IL10−/−) mice were maintained on a 12:12-h light-dark cycle and allowed free access to food and water in the animal facility at University of Chicago. Laboratories are housed separately in the animal care facility. All animal studies were approved by the Institutional Animal Care and Use Committee at the University of Chicago.
2,4,6-Trinitrobenzene sulfonic acid-induced colitis.
Mice were presensitized by back injection of a mixture of acetone-olive oil-2,4,6-trinitrobenzene sulfonic acid (TNBS; Sigma Chemical, St. Louis, MO) 7 days before the administration of TNBS. On the day of experiment, mice were anesthetized, and 2 mg of TNBS was rectally introduced by using a 3.5F catheter. Weight was measured on the following day and daily, and tissue collection was performed on day 7. Histological grading of intestinal inflammation was performed as described previously (4).
DSS-induced colitis.
Mice were given 2% DSS (ICN Chemicals, Costa Mesa, CA) in drinking water for 7 days. Weight changes, diarrhea, bloody stool, and overall health were monitored over time. Mice were euthanized with sevoflurane, and tissues were collected on day 10. Histological grading of intestinal inflammation was performed as previously described (17).
FITC-Dextran Permeability Assay.
Intestinal permeability was assessed by luminal enteral administration of FITC-dextran 10,000 (Sigma) (9). All mice were gavaged with FITC-dextran (40 mg/100 g body wt) 4 h before blood collection. Whole blood was obtained by retroorbital puncture, and FITC-dextran measurements were performed in duplicate by fluorometry. Dilutions of FITC-dextran in PBS were used as a standard curve, and absorption of 100 μl of serum or standard was measured in a fluorometer at 488 nm.
Immunofluorescence staining.
Sections were prepared as described for immunohistochemistry staining (17, 23). The specimens were blocked and then followed by overnight incubation with rabbit anti-ZO-1 (Invitrogen, Grand Island, NY). Sections were washed and then incubated with Cy2-anti-mouse and Cy5 anti-rabbit (Jackson ImmunoResearch, West Grove, PA). Nuclear stating was performed using 4′,6-diamidino-2-phenylindole (DAPI; Molecular Probes, Eugene, OR). Confocal microscopy was performed using a Leica SP2AOBS system (Leica, Wetzlar, Germany) at the Light Microscopy Core Center of the University of Chicago.
CD45RB adoptive transfer colitis.
Splenocytes were collected from Hsp70−/− and WT mice. CD4+ cells were isolated by MACS system (Miltenyi Biotec) by positive selection according to the manufacturer’s instruction. CD4+ T cells were further sorted to collect CD45RBhigh and CD45RBlow using FACS Vantage. The purity of MACS- and FACS-sorted cells was >90% and >99%, respectively. The equal numbers of CD45RBhigh cells (5×105) from WT mice were intraperitoneally injected into male Rag−/− mice. The mice were weighed once a week to monitor colitis development. Two weeks after CD45RBhigh cell transfer, 5×105 CD45RBlow cells from WT and Hsp70−/− mice were intraperitoneally injected into Rag−/− mice respectively. The mice were followed for another 10 wk before the tissue collection. Histological grading of intestinal inflammation for adoptive transfer colitis and IL-10-deficient spontaneous colitis was performed as described previously (12).
Cell isolation and in vitro stimulation.
Spleens and mesenteric lymph nodes were collected from 8-wk-old WT and Hsp70−/− mice, and then splenocytes and lymphocytes were isolated by sifting through 70-μm cell strainers. B and T cells were purified by using EasySep T cell negative selection kits according to the manufacturer’s instructions. Macrophages as well as dendritic cells were purified by using an EasySep cell-positive selection kit according to the manufacturer’s instructions. The isolated cells were resuspended and cultured in complete medium. Purified macrophage and dendritic cells (1 × 106) were incubated with LPS (100 ng/ml) for 16 h. Purified B cells and CD4+ T cells were incubated with PMA-ionomycin (50 ng/μg) for 24 h. Supernatants were harvested and stored at −80°C until analysis for cytokine levels.
Western blot.
Mouse mucosal scraping samples from colons and cells harvested from cultures were homogenized in cell lysis buffer (Cell Signaling Technology, Danvers, MA). Protein concentration determination and immunoblotting were performed as previously described (16). Briefly, 20 μg of protein was separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and blotted with primary antibodies and secondary antibodies (Cell Signaling Technology). Quantification of images was performed by scanning densitometry and using NIH Image J 1.54 software (National Institutes of Health, Bethesda, MD).
Quantitative RT-PCR.
Total RNA was extracted from mouse colonic mucosal scraping by TRIzol (Invitrogen) according to the manufacturer's instructions. Complementary DNA was synthesized using SuperScript II (Invitrogen) and random hexanucleotide primers. Primers used for Hsp70 were forward 5′-TGGTGCAGTCCGACATGAAG-3′, reverse 5′-GCTGAGAGTCGTTGAAGTAGGC-3′; IL-10 forward 5′-GCTCTTACTGACTGGCATGAG-3′, reverse 5′-CGCAGCTCTAGGAGCATGTG-3′; and GAPDH forward 5′-GGCAAATTCAACGGCACAGT-3′ and reverse 5′-AGATGGTGATGGGCTTCCC-3′. Real-time PCR was performed in an iCycler (Bio-Rad, Hercules, CA) using iQSYBR Green PCR Supermix (Bio-Rad). A two-step quantification cycling protocol was used. The CT value is defined as the cycle number at which the fluorescence crosses a fixed threshold above the baseline. As a relative quantification, the relative gene expression ratio changes were measured using ΔCT method as described previously (16).
Mononuclear cells preparation and flow cytometry.
Lymphocytes were isolated from mesentery lymph node and spleen as described previously (12). Cells were assessed by surface marker staining using specific antibodies to CD4 and CD45RB (BD Biosciences). Cells were permeabilized with the CytoFix/CytoPerm kit (BD Biosciences) for intracytoplasmic detection of IL-10 and IFNγ cytokines (eBioscience). Flow cytometry analysis was performed with a FACsCanto (BD Biosciences).
ELISA.
Cytokines in colonic tissue, mesenteric lymph node and cell culture supernatant (as listed in experimental method), were analyzed at different conditions using mouse ELISA kits from eBioscience.
Statistical analyses.
Results are reported as the means ± SD. Pairwise comparisons were made using the one- or two-tailed unpaired Student’s t-test, while one-way ANOVA with Bonferroni’s post hoc testing was used for multiple comparisons. Calculations were performed using GraphPad Prism software. A P value of less than 0.05 was considered as statistical significant.
RESULTS
DSS- and TNBS-induced colitis models.
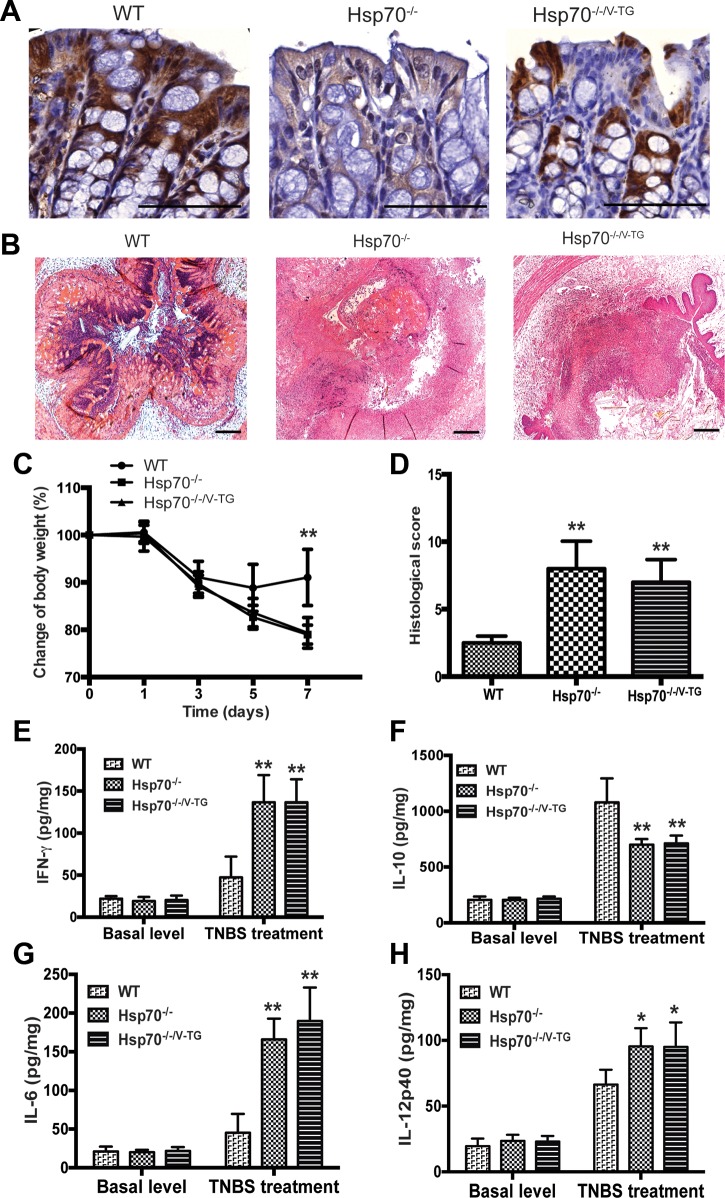
We crossed Hsp70−/− mice with villin promoter-driven Hsp70 transgenic mice (Hsp70V-TG) to obtain Hsp70−/−/V-TG mice in which Hsp70 was expressed only in intestinal epithelial cells but not in lamina propria. As shown in Fig. 1A, expression of Hsp70 could be detected in both epithelium and lamina propria by immunostaining in WT mice, but not in Hsp70−/− mice. In Hsp70−/−/V-TG mice, Hsp70 was expressed only in the intestinal epithelium and not by any other cell types.
Fig. 1.
Epithelial specific expression of heat shock protein-70 (Hsp70) cannot protect Hsp70−/− mouse from 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis. A: immunostaining of Hsp70 in mouse colon (dark brown staining). Hsp70 expression was observed only in epithelium and not in lamina propria cells of Hsp70−/−/V-TG mice. Scale bars represent 100 μm. B: representative colonic histological features of TNBS-induced colitis in WT, Hsp70−/−, and Hsp70−/−/V-TG mice. There were extensive inflammatory cell infiltration and marked colonic mucosal destruction in Hsp70−/− and Hsp70−/−/V-TG mice. Scale bars represent 50 μm. C and D: TNBS induces severe colitis in Hsp70−/− and Hsp70−/−/V-TG mice, but not in WT mice. Hsp70−/− and Hsp70−/−/V-TG mice lost significantly more body weight and had significantly higher histological scores than WT mice. No differences were found between Hsp70−/− and Hsp70−/−/V-TG mice. E–H: colonic cytokine expression was measured with and without TNBS treatment. The highest levels of the proinflammatory cytokines IFNγ, IL-12p40, and IL-6 were found in the colonic mucosa of Hsp70−/− and Hsp70−/−/V-TG mice with active colitis. In contrast, IL-10 levels were significantly lower in Hsp70−/− and Hsp70−/−/V-TG mice after TNBS challenge. No differences of cytokine levels were found between Hsp70−/− and Hsp70−/−/V-TG mice. Bars represent means ± SD. Data were analyzed by one-way ANOVA with Bonferroni’s multiple comparisons test. *P < 0.05, **P < 0.01; n ≥ 6 in each group.
To test the protective role of Hsp70 in experimental colitis, these mice were challenged with TNBS and DSS separately. The TNBS-induced colitis model exemplifies a Th1-mediated mucosal inflammation that previous studies have shown can be mitigated by IL-10 (21, 24). We hypothesized that if intracellular Hsp70 affects host immune function, the gene-targeted deletion of Hsp70 would render mice more susceptible to TNBS-induced colitis and that epithelial-specific expression of Hsp70 would be ineffective in preventing this outcome. Three groups of mice were first treated with TNBS rectally and followed overtime. As shown in Fig. 1, B–D, TNBS treatment resulted in severe colitis in both Hsp70−/− and Hsp70−/−/V-TG mice, characterized by extensive leukocytic infiltration, marked colonic mucosal destruction, and mucosal edema (Fig. 1B). These mice experienced significant weight loss and had high histological colitis scores during the acute phase of TNBS-induced colitis compared with WT mice. Moreover, compared with Hsp70−/− mice, no significant differences of weight loss and tissue damage were found in Hsp70−/−/V-TG mice (Fig. 1, C and D). These data support the notion that systemic expression instead of epithelial expression of Hsp70 is necessary to protect against Th1-mediated colitis. Consistent with histological observations, the highest levels of proinflammatory cytokines (IFNγ, IL-12p40, and IL-6) were observed in the colon of Hsp70−/− and Hsp70−/−/V-TG mice. Interestingly, mucosal IL-10 expression appeared to be inversely correlated with the presence of intracellular Hsp70 in these mice. In addition, no significant differences of IL-10 production in colonic tissue homogenates were found between Hsp70−/− and Hsp70−/−/V-TG mice (Fig. 1, E–H). Using the flow cytometry, we also found that there were increased IFNγ and decreased IL-10 expressions in CD4+ T cells from mesenteric lymph nodes that lacked Hsp70 expression, i.e., collected from Hsp70−/− and Hsp70−/−/V-TG mice compared with those from WT mice (data not shown).
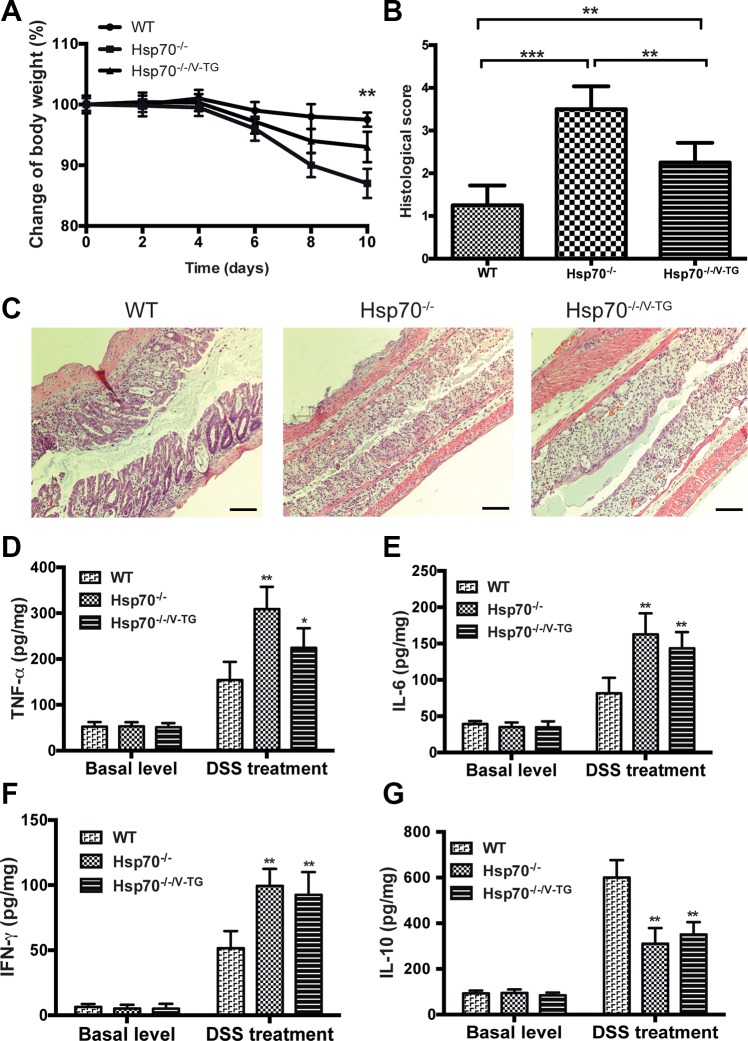
However, epithelial-specific expression of Hsp70 revealed significant protection from DSS-induced colitis, which is believed to initially arise from stress and injury of gut epithelial cells with associated loss of barrier function (21, 33). These findings were consistent with what we reported previously; i.e., Hsp70−/− mice significantly lost body weight and developed more severe colitis compared with WT mice (Fig. 2) (17). However, Hsp70−/−/V-TG mice lost less body weight and were less sensitive to DSS-induced damage than Hsp70−/− mice (Fig. 2, A–C), indicating that epithelial-specific expression of Hsp70 rescues Hsp70−/− mice from DSS injury. It is notable that the protection conferred by the epithelial-specific expression of Hsp70 was less than that observed in WT mice; i.e., Hsp70−/−/V-TG mice still had significant tissue damage and inflammation (Fig. 2, A–C). Combined with the finding of cytokine expression profile from TNBS model, these data raise the possibility that Hsp70 has other mechanisms and targets of action in maintaining host mucosal homeostasis besides just protecting the colonic epithelial cells.
Fig. 2.
Epithelial specific expression of Hsp70 protects Hsp70−/− mice from dextran sodium sulfate (DSS)-induced colitis. A and B: DSS induces severe colitis in Hsp70−/− mice, but not in WT and Hsp70−/−/V-TG mice. Compared with Hsp70−/− mice, Hsp70−/−/V-TG mice exhibited less body weight loss and had significantly lower histological colitis scores. However, Hsp70−/−/V-TG mice still had more body weight loss and higher colitis score than WT mice. C: representative colonic histological features of DSS-induced colitis in WT, Hsp70−/−, and Hsp70−/−/V-TG mice. All 3 groups exhibited mucosal destruction and inflammatory cell infiltration after DSS treatment. Scale bars represent 50 μm. D–G: cytokine levels measured in colonic mucosa among the 3 groups of mice with and without DSS treatment. No significant differences in cytokine levels were detected among WT, Hsp70−/−, and Hsp70−/−/V-TG mice at baseline. After DSS treatment, both Hsp70−/− and Hsp70−/−/V-TG mice showed significantly elevated IFNγ, TNFα, and IL-6 compared with WT mice. No differences of IFNγ, IL-10, and IL-6 were detected between Hsp70−/− and Hsp70−/−/V-TG mice. TNFα was significantly lower in Hsp70−/−/V-TG compared with that in Hsp70−/−. IL-10 was significantly lower in Hsp70−/− and Hsp70−/−/V-TG mice compared with that in WT mice after DSS treatment. Bars represent means ± SD. Data were analyzed by one-way ANOVA with Bonferroni’s multiple comparisons test. *P < 0.05, **P < 0.01, ***P < 0.001; n ≥ 6 in each group.
Similar to what was done in TNBS-treated mice, cytokine levels were measured in colonic tissues in the three groups of mice. Under the basal level, no differences in cytokine expressions were noted among the groups. However, after DSS treatment, significantly higher levels of the proinflammatory cytokines TNFα, IFNγ, and IL-6 were found in Hsp70−/− and Hsp70−/−/V-TG mice compared with WT mice (Fig. 2, D–G). On the other hand, IL-10 levels were significantly lower in both Hsp70−/− and Hsp70−/−/V-TG mice compared with that in WT mice (Fig. 2G). Only TNFα was significantly lower in Hsp70−/−/V-TG mice compared with that in Hsp70−/− mice, whereas the expression of the other three cytokines was not significantly different between Hsp70−/− mice and Hsp70−/−/V-TG mice. These data support that Hsp70 has a role in modulating immune cell IL-10 production an when inhibited by active inflammation, augments the immune dysfunction associated with experimental colitis.
Immunofluorescence staining and immunoprecipitation.
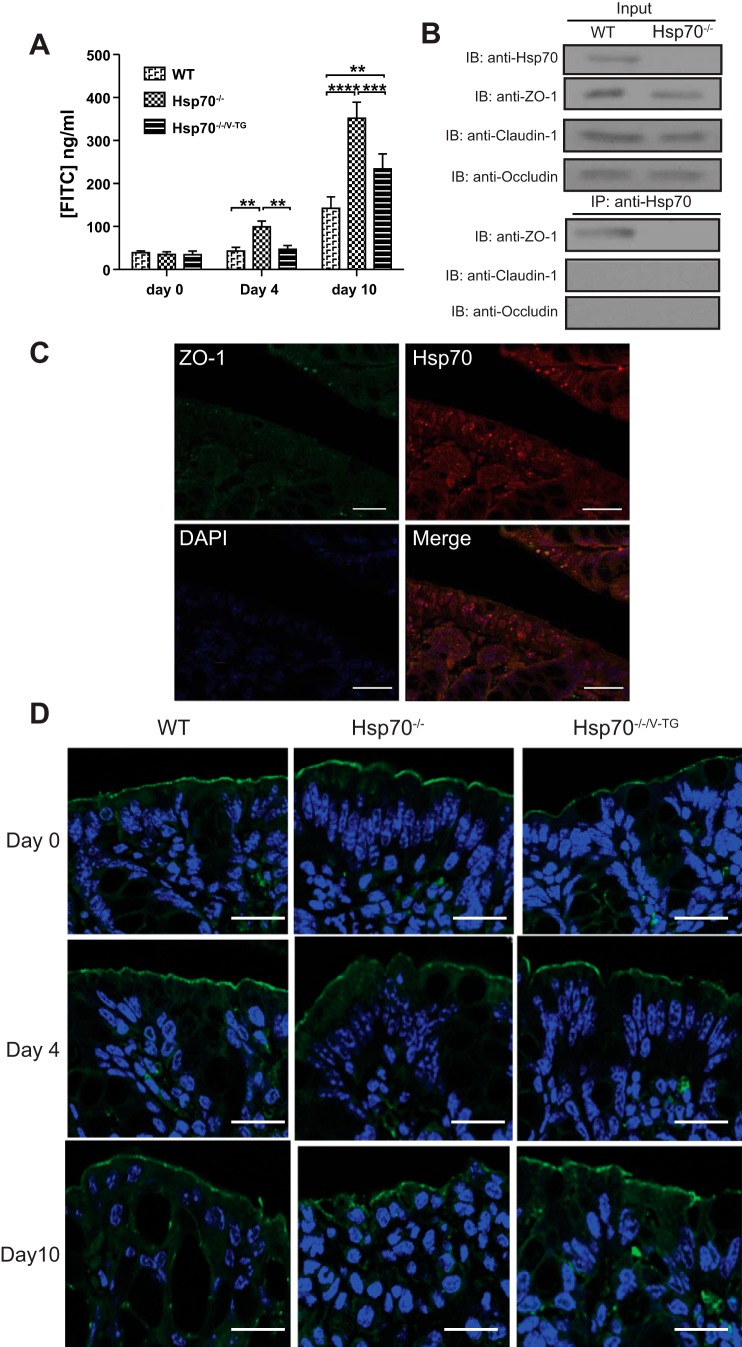
Part of the epithelial specific-protection provided by Hsp70 is through the maintenance of the integrity of tight junctional complexes (28, 30). To examine whether this could be solely through intestinal epithelial Hsp70, intestinal barrier function was assessed in DSS-treated Hsp70−/− and Hsp70−/−/V-TG mice (Fig. 3). Under basal conditions, no differences in epithelial permeability could be detected in the three mouse groups by using the FITC-dextran absorption assay. After DSS treatment, there was a significant increased uptake of FITC-dextran in Hsp70−/− mice on day 4 (when no histological tissue damage is observed) and day 10 (when both gross and microscopic inflammation are observed) compared with that in both WT and Hsp70−/−/V-TG mice. Differences in uptake of FITC-dextran were only observed between WT and Hsp70−/−/V-TG mice at day 10, but not at day 4 (Fig. 3A). Thus, the epithelial-specific expression of Hsp70 appears to play an important role in maintaining the tight junction and colonic epithelial integrity. However, the improvement in barrier function in the Hsp70−/−/V-TG mice still did not approach that of WT mice. The expression pattern of ZO-1 in the colonic epithelium was then examined by immunofluorescence and found to be significantly disrupted and irregular in Hsp70−/− mice compared with WT mice (32). These changes were much less in the presence of intestinal epithelial Hsp70 (Fig. 3D). Although increased expression of proinflammatory cytokines TNFα and IFNγ in Hsp70−/− mice could partially contribute to the dysfunction of ZO-1, forced expression of Hsp70 in the epithelium was able to ameliorate severe of colitis and also the integrity of ZO-1. Using an immunoprecipitation (IP) approach, colonic Hsp70 appeared to associate with the tight junction protein ZO-1 but not with other tight junction proteins such as claudin-1 and occludin (Fig. 3B). Colocalization of Hsp70 and ZO-1 was further confirmed by immunofluorescence staining (Fig. 3C). These data, along with our previous findings, demonstrate the importance of intestinal epithelial Hsp70 in mitigating experimental colitis through interactions with tight-junction protein ZO-1 to maintain its structural and functional integrity.
Fig. 3.
Hsp70 maintains tight junction through interaction with ZO-1. A: increased colonic permeability is detected in Hsp70−/− mice after DSS treatment. At days 4 and 10, Hsp70−/− mice exhibited significantly higher colonic leakage than both WT and Hsp70−/−/Villin TG mice. There was no significant difference of colonic permeability between WT and Hsp70−/−/V-TG mice at day 4, but difference was significant at day 10 after DSS treatment. B: immunoprecipitation shows Hsp70 binds to ZO-1 directly. C: colocalization of Hsp70 and ZO-1. Hsp70 is stained in red and ZO-1 is stained in green. Scale bars represent 100 μm. D: expression pattern of ZO-1 in colonic epithelium was characterized and found to be significantly disrupted and irregular in Hsp70−/− mice compared with those in WT and IL10−/−/Hsp70−/−/V-TG mice. ZO-1 is stained in green and cell nucleus in blue. Scale bars represent 50 μm. Bars represent means ± SD. Data were analyzed by one-way ANOVA with Bonferroni’s multiple comparisons test. **P < 0.01, ***P < 0.001, ****P < 0.0001; n ≥ 6 in each group.
CD4+CD45RBhigh adoptive transfer.
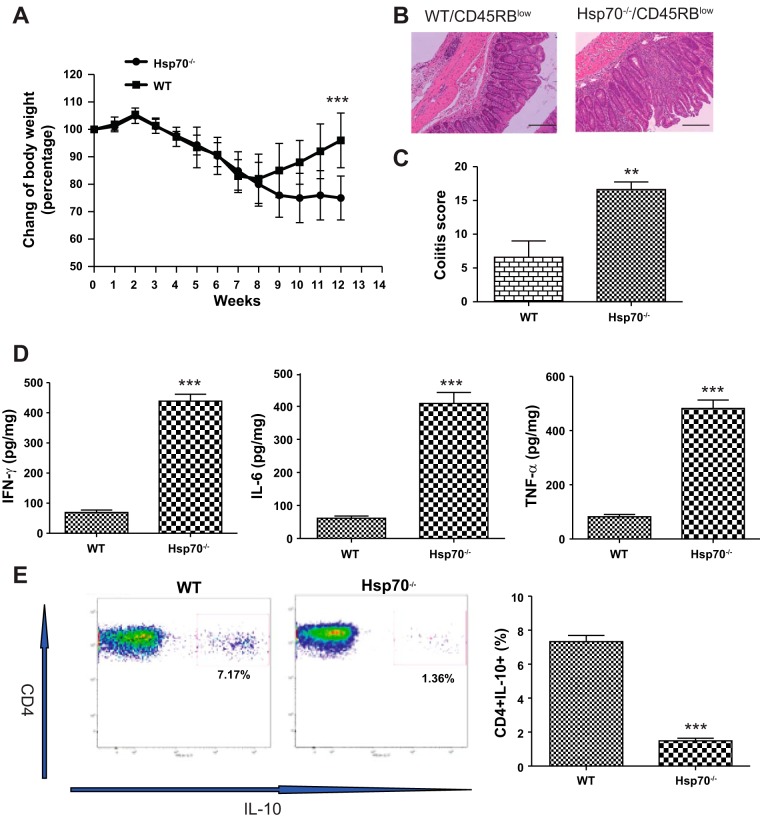
IL-10 plays an essential role in mediating the ability of Treg cells in inhibiting the development of experimental colitis (5, 8). CD45RBlow CD4+ cells from IL10−/− mice that are incapable of synthesizing IL-10, are unable to inhibit colitis induced by transfer of CD45RBhigh cells to Rag−/− mice (5). Using the same model, we sought to determine whether the Hsp70-deficient T cells were defective in their ability to produce IL-10 and unable to rescue these mice from the development of colitis. Colitis was induced in Rag−/− mice by intraperitoneal injection of CD45RBhigh cells harvested from WT mouse donors. Two weeks later, these male mice were divided into two groups that were given CD45RBlow CD4+ T cells from either WT or Hsp70−/− mice. Ten weeks later, mice receiving CD45RBlow CD4+ T cells from Hsp70−/− donors developed severe colitis (Fig. 4, A–C) that was associated with significantly elevated levels of the proinflammatory cytokines IFNγ, TNFα, and IL-6 in the colon (Fig. 4D) and decreased levels of IL-10 production in CD4+ T cells collected from mesenteric lymph node (Fig. 4E). In contrast, mice receiving WT CD45RBlow cells did not develop disease or showed only mild inflammation along with far less tissue damage. These results strongly suggest that Hsp70 in Treg cells is immunomodulatory and essential for the production of IL-10.
Fig. 4.
CD4+CD45RBhigh adoptive transfer colitis. A: change of body weight over 12-wk period after CD45RBhigh adoptive transfer. Equal numbers of CD45RBhigh cells from WT mice were intraperitoneally injected into Rag−/− mice. Two weeks later, mice were divided into 2 groups randomly, and equal amounts of CD45RBlow cells from WT and Hsp70−/− mice were intraperitoneally injected into Rag−/− mice, respectively. Mice were followed for another 10 wk before tissue collection. B: representative colonic histological features of CD4+CD45RBhigh adoptive transfer colitis from 2 groups. Scale bars represent 50 μm. C: Rag−/− mice receiving CD45RBlow cells from Hsp70−/− mice have significant higher colitis scores than mice receiving CD45RBlow cells from WT mice. D: cytokine levels between 2 groups of mice. Mice receiving CD45RBlow cells from Hsp70−/− mice had significant higher IFNγ, TNFα, and IL-6. E: IL-10 expression in CD4+ T cells collected from Rag−/− mice after adoptive transfer. Mesenteric lymph nodes were collected from mice, and IL-10 expression in CD4+ T cells was measured by flow cytometry. Significantly lower expression of IL-10 production was observed in Rag−/− mice receiving CD45RBlow cells from Hsp70−/− mice. Bars represent means ± SD. Data were analyzed by 2-tailed Student’s t-tests. **P < 0.01, ***P < 0.001; n = 6 in each group.
Western blot of ERK-phosphorylation pathway.
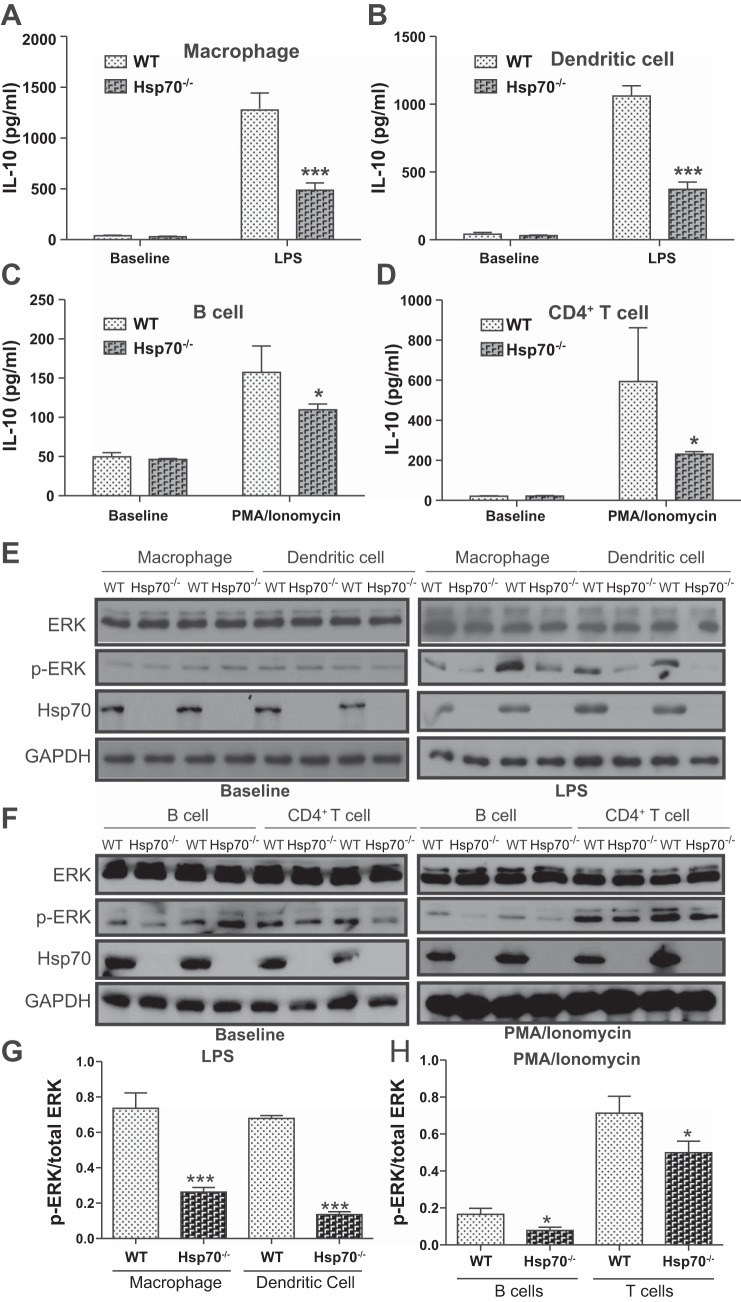
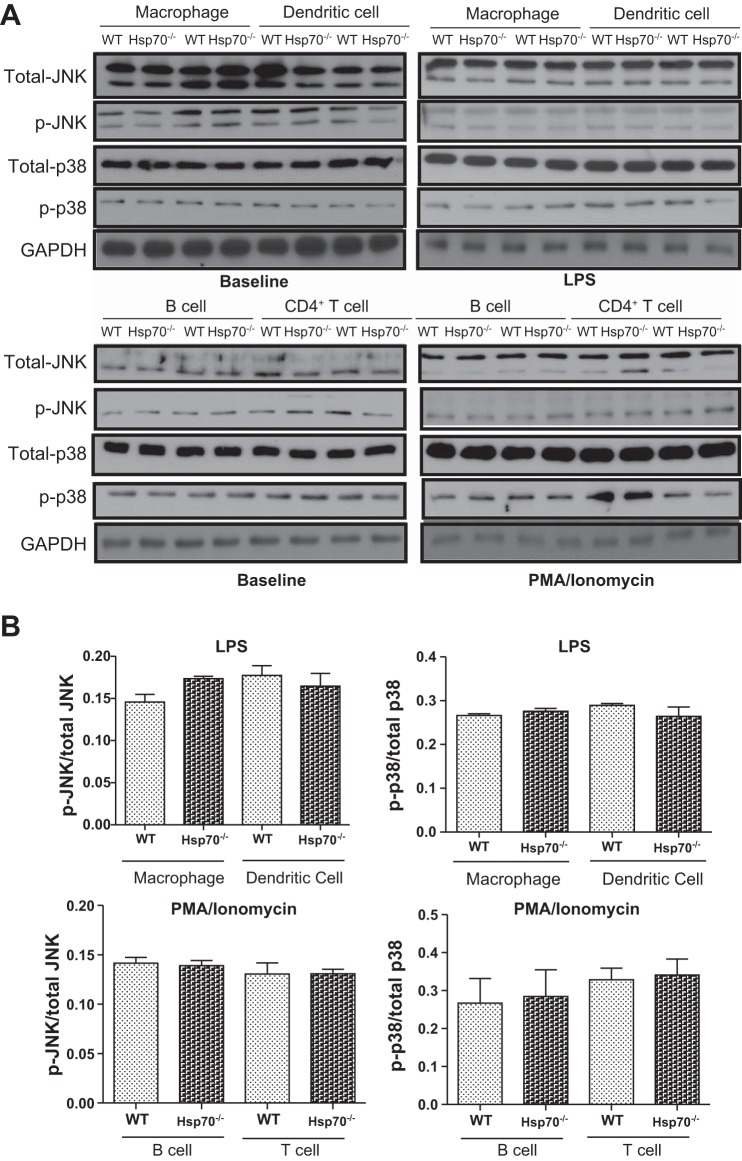
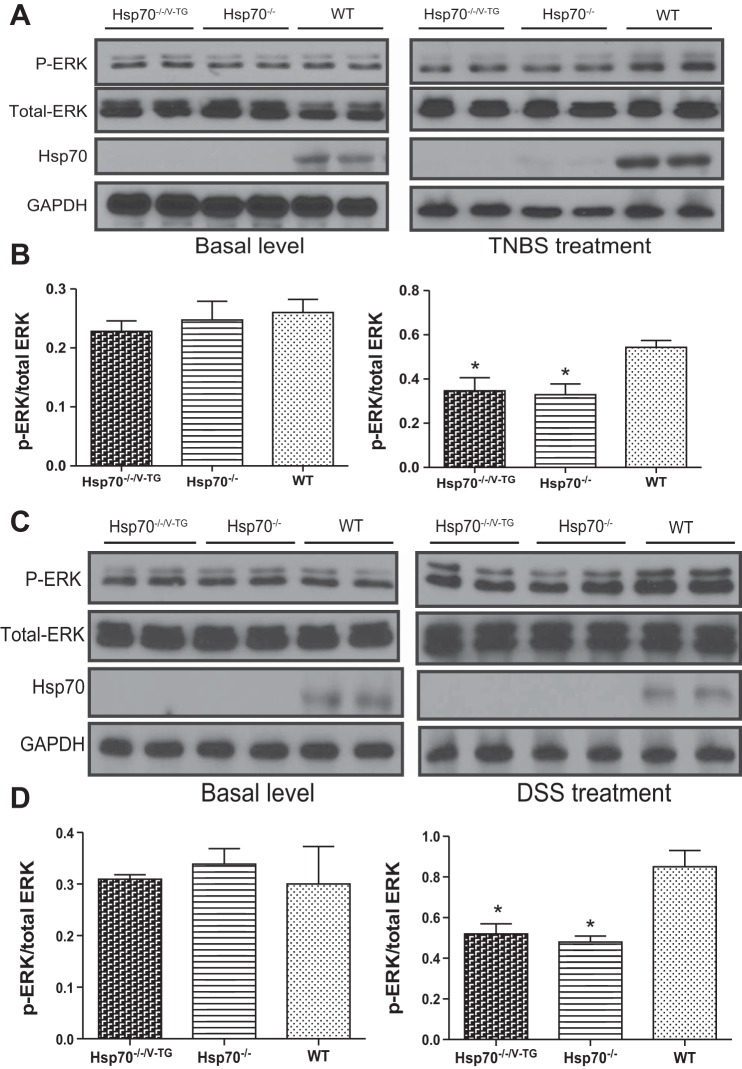
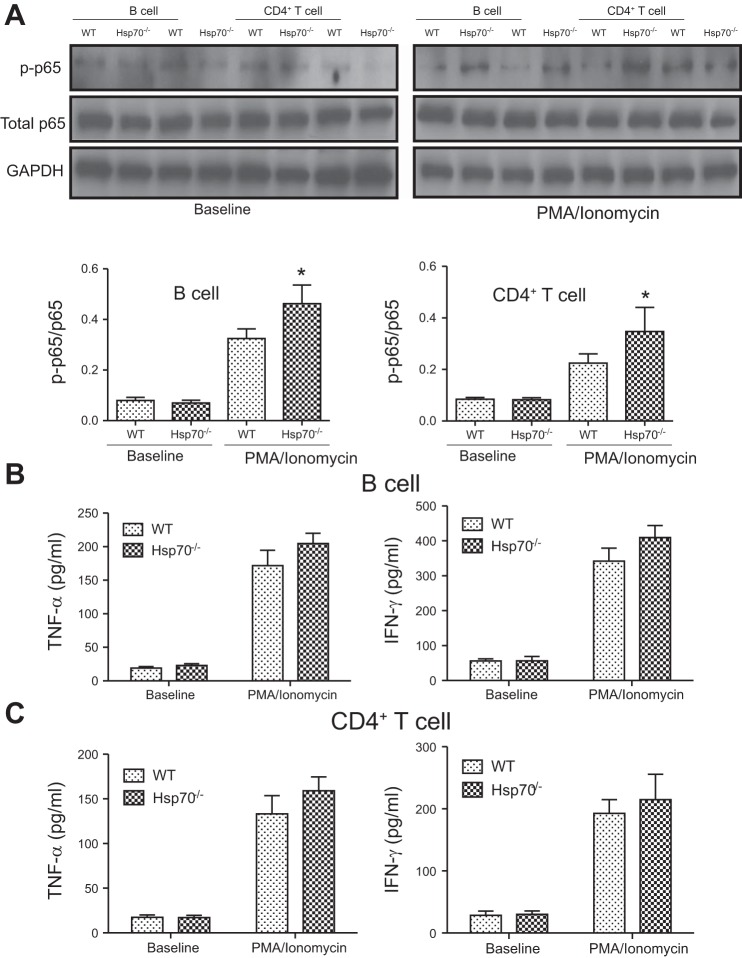
Previous reports have shown that extracellular Hsp70 can regulate disease-associated inflammatory responses by affecting IL-10 production (6, 7, 39). However, our data showed that intracellular Hsp70 was essential to IL-10 biosynthesis. IL-10 levels were measured in both innate and adaptive immune cells isolated from WT and Hsp70−/− mice. Hsp70 expression could be detected under basal conditions in all WT cells, with relatively higher levels in macrophage and dendritic cell (DC), and less in CD4+ T and B cells (data not shown). These cells were then subjected to in vitro stimulation with either mitogen or LPS. The induced expression of IL-10 by these cells was then compared between the two groups. As shown in Fig. 5, A–D, the induced levels of IL-10 were significantly greater in all cell types examined from WT mice, but much less in cells from Hsp70−/− mice by ELISA analysis. These differences were associated with significantly lower phosphorylation levels of ERK in Hsp70-deficient cells compared with WT cells (Fig. 5, E–H). In contrast, p38 and JNK phosphorylation were not affected by gene-targeted deletion of Hsp70 deficiency (Fig. 6). Moreover, these differences were also observed in both TNBS and DSS models, in which there was decreased phosphorylation of ERK in mesenteric lymph nodes from both Hsp70−/− and Hsp70−/−/V-TG mice compared with that in WT mice (Fig. 7). In addition, we also examined NF-κB phosphorylation and inflammatory cytokine expression in B and T cells. There was a significant increase of NF-κB phosphorylation in B and T cells from Hsp70−/− mice after stimulation but not at baseline compared with that in cells from WT. There was also a trend of elevated expression of TNFα and IFNγ in B and T cells collected from Hsp70−/− mice after stimulation, but the differences were not significant (Fig. 8). These data support the notion that intracellular Hsp70 is essential to the regulation of IL-10, mostly likely through the interaction with ERK phosphorylation.
Fig. 5.
Hsp70 regulates IL-10 production through ERK-phosphorylation. (A–D) In vitro stimulation assay. IL-10 expression between immune cells from WT and Hsp70−/− mice was examined before and after stimulation. IL-10 level was significant lower in macrophage, dendritic cells, B cells and CD4+ T cells from Hsp70−/− mice after stimulation (macrophage and dendritic cell were stimulated with LPS for 16 h; B cell and CD4+ T cell were stimulated with PMA/ionomycin for 24 h), but no differences in IL-10 were found between the two groups at baseline. (E) Western blot of ERK phosphorylation in macrophage and dendritic cells before and after LPS stimulation. ERK phosphorylation was decreased in cells from Hsp70−/− mice after stimulation. (F) Western blot of ERK phosphorylation in B and CD4+ T cells before and after PMA/ionomycin stimulation. ERK phosphorylation was decreased in cells from Hsp70−/− mice after stimulation. (G and H) Densitometry measurements show ERK phosphorylation is significantly decreased in macrophage, dendritic cells, B cells and CD4+ T cells from Hsp70−/− mice after LPS and PMA/ionomycin stimulation, respectively. Bars represent mean ± SD. Data were analyzed by 2-tailed Student’s t tests. * P < 0.05, *** P < 0.001, n = 3 and experiment was performed by two times independently.
Fig. 6.
Expression of JNK and p38 in cells after stimulation. A: Western blot was used to compare expression of total and phosphorylation JNK and p38 in macrophage, dendritic cells, B cells, and CD4+ T cells before and after stimulation. B: densitometry of Western blot shows that phosphorylation of p38 and JNK was not affected by Hsp70 deficiency. Bars represent means ± SD. Data were analyzed by 2-tailed Student’s t tests; n = 3 in each group and experiment was performed 2 times independently.
Fig. 7.
ERK phosphorylation in mesenteric lymph nodes in TNBS- and DSS-treated mice. Mesenteric lymph nodes from mice with and without TNBS and DSS treatment were collected, respectively. ERK phosphorylation status was compared among groups by Western blot. No difference of ERK phosphorylation was detected in mice without colitis, but significantly lower levels of ERK phosphorylation were detected in mesenteric lymph nodes from Hsp70−/− and Hsp70−/−/V-TG mice compared with those from WT mice after TNBS (A and B) and DSS treatments (C and D), respectively. Bars represent means ± SD. Data were analyzed by one-way ANOVA with Bonferroni’s multiple comparisons test. *P < 0.05; n = 6 in each group, and experiment was performed 2 times independently.
Fig. 8.
NF-κB phosphorylation and expression of proinflammatory cytokines in B and T cells. A: NF-κB phosphorylation status in B and CD4+ T cells at baseline and after stimulation was examined by comparing p65 phosphorylation. Densitometry measurements showed NF-κB phosphorylation was significantly increased in cells collected from Hsp70−/− mice compared with that in WT cells after stimulation. B: TNFα and IFNγ expression in B cells at baseline and after stimulation. No significant difference was detected between Hsp70−/− and WT. C: TNFα and IFNγ expression in CD4+ T cells at baseline and after stimulation. No significant difference was detected between Hsp70−/− and WT. Bars represent means ± SD. Data were analyzed by Student’s t-tests. *P < 0.05.
IL10/Hsp70 double knockout mouse model.
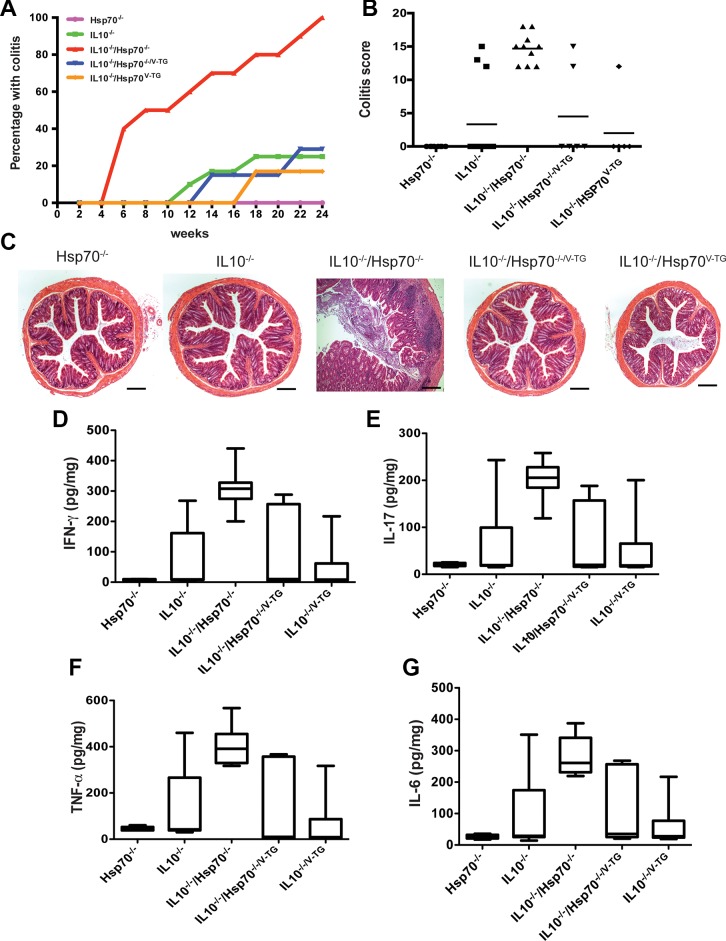
The IL10−/− mouse strain is a chronic, T cell-mediated, genetic model of experimental colitis that has been used extensively by many investigators (10). In our facility, only ~20–30% of these mice developed colitis spontaneously (Fig. 9A) (12). To assess whether Hsp70 plays a role in the development and/or progression of chronic colitis, Hsp70−/− mice were bred with IL10−/− mice to obtain IL10/Hsp70 double knockout (IL10−/−/Hsp70−/−) mice. These mice were followed for up to 6 mo of age, and the penetrance of spontaneous colitis was measured by histology, disease index, and colonic mucosal cytokine levels. IL10−/−/Hsp70−/− mice exhibited earlier onset of colitis with a 100% penetrance (10/10) by 6 mo of age (Fig. 9, A–C). To determine the relative importance of intestinal and nonintestinal epithelial Hsp70 in this model, the villin-promoter Hsp70 transgene was also introduced into IL10−/−/Hsp70−/− mice. As shown in Fig. 9, The IL10−/−/Hsp70−/−/V-TG mice showed the same disease progression and penetrance of colitis (2/7) as IL10−/− mice (3/12). Compared with IL10−/− mice that did not develop colitis, IL10−/−/Hsp70−/− mice with colitis showed significantly elevated TNFα, IFNγ, IL-6, and IL-17 (Fig. 9, D–G). More importantly, the pattern of cytokine production in IL10−/−/Hsp70−/− mice with colitis was similar to those in IL10−/− and IL10−/−/Hsp70−/−/V-TG mice that also developed colitis (Fig. 9, D–G). Thus, these data suggest that an important role for intestinal epithelial Hsp70 in maintaining intestinal homeostasis in the IL-10−/− model. They further illustrate that Hsp70 plays an anti-inflammatory role mediated through IL-10 production, as the inflammatory cytokines patterns between IL10−/− and IL10−/− /Hsp70−/− mice with colitis were very similar.
Fig. 9.
IL10/Hsp70 double knockout mice have increased penetrance of colitis. A: penetrance of spontaneous colitis in 6 mo period. Hsp70−/− mice were bred with IL10−/− mice to obtain IL10−/−/Hsp70−/− mice that had 100% penetrance of spontaneous colitis (10/10) at 6 mo old. Epithelial specific expression of Hsp70 in IL10−/−/Hsp70−/− double knockout mice could rescue the phenotype as IL10−/−/Hsp70−/−/V-TG mice and had the similar penetrance of colitis (2/7) as IL10−/− mice (3/12). B: colitis scores compared among groups at 6 mo of age. C: representative colonic histological features of spontaneous colitis. Scale bars represent 50 μm. D–G: colonic mucosal TNFα, IFNγ, IL-6, and IL-17 were increased in those mice with colitis compared with mice without colitis from different groups. The level was not different between IL10−/−/Hsp70−/− mice with colitis and IL10−/− mice with colitis. Box plot diagram shows minimum and maximum of all of cytokine expression; n ≥ 7 in each group, and data were analyzed by one-way ANOVA with Bonferroni’s multiple comparison tests.
DISCUSSION
Inducible Hsp70, a highly conserved protein and molecular chaperone, is a multifunctional protein that is expressed in many cell types (13, 20, 40). Several studies have shown a link between IBD and the downregulation of colonic Hsp70 in human IBD and experimental colitis (41, 42). In the context of diseases like IBD, the relative roles of Hsp70 in mediating epithelial cytoprotection and immune functions are poorly defined, in large part due to the unavailability of suitable model systems that can discriminate between these processes.
There is widely expressed Hsp70 in epithelium and lamina propria along the colon, as Hsp70 is a stress protein and can be induced by intestinal microbial signals (16, 23, 29, 37). Here, we established a mouse model Hsp70−/−/V-TG mouse in which Hsp70 is expressed only in the intestinal epithelium. We have shown that Hsp70−/−/V-TG could decrease the sensitivity of Hsp70−/− mice to DSS-induced colitis through maintaining tight junction ZO-1 integrity (Fig. 10). This epithelium-specific protection was further confirmed by using the IL10−/−/Hsp70−/− model. Compared with IL10−/− mice, those have only 20–30% of penetrance of colitis, IL10−/−/Hsp70−/−mice had compromised epithelial tight junction and had 100% colitis. As IL-10 production is absent in IL10−/− mice, the increased penetrance of colitis in IL10−/−/Hsp70−/−mice was attributed to the lack of expression of epithelial Hsp70. However, forced expression of intestinal epithelial Hsp70 restored barrier function, because IL10−/−/Hsp70−/−/V-TG mice exhibited improved phenotype and same rates of spontaneous colitis as IL10−/− mice. This is the first time using an animal model to address the epithelium-specific protection of Hsp70. Our results further highlight the significance of Hsp70-mediated barrier protection during IBD.
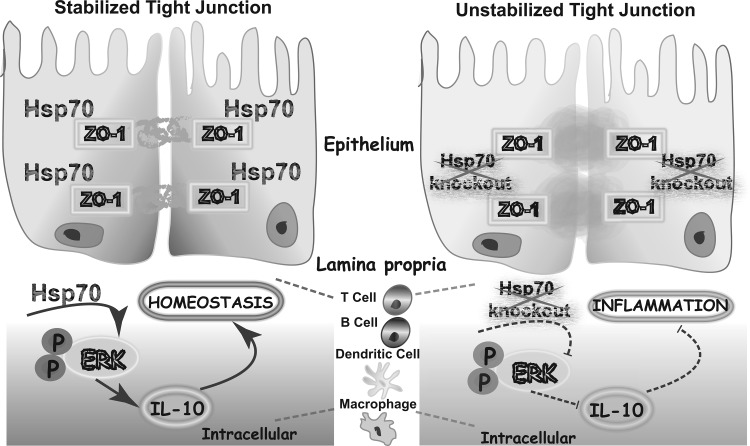
Fig. 10.
Intracellular Hsp70 plays dual protective functions during colitis.
Both epithelial barrier dysfunction and immune dysregulation are major contributors to the pathogenesis of IBD (3, 11, 27). In addition to its epithelial cytoprotection, Hsp70 has anti-inflammatory features through maintaining IL-10 production during colonic colitis (Fig. 10). In the previous reports, it was hypothesized that the Hsp70 soluble form was able to bind to cell surface receptors and signaling through TLR2 via ERK to induce IL-10 secretion (6). However, before our work, little was known about the role of intracellular Hsp70 in immune regulation. Another reason leading us to focus on the intracellular immunoregulation of Hsp70 was the availability of the Hsp70−/− mouse and the observation that Hsp70−/− mice have decreased production of IL-10 during colitis. Lack of sufficient induction of IL-10 during experimental colitis was observed in both Hsp70−/− and Hsp70−/−/V-TG mice after DSS and TNBS treatments. Because DSS is directly toxic to gut epithelial cells of mucosa and disrupts the integrity of the mucosal barrier (33), epithelial-specific expression of Hsp70 can ameliorate DSS-induced colitis phenotype of Hsp70−/− mice. However, it could not rescue TNBS-induced colitis because TNBS colitis is mediated by the Th1 response (21). Moreover, there are no differences in IFNγ and IL-10 production during TNBS colitis in both Hsp70−/− and Hsp70−/−/V-TG mice. These results suggest that susceptibility to colitis in Hsp70−/− mice is due to impaired intracellular Hsp70-dependent IL-10 production and the increased production of proinflammatory cytokines.
More importantly, results from a rescue study using a CD45RBhigh transfer model confirmed our finding that intracellular Hsp70 regulates IL-10, as the ability of CD4+ regulatory T cells to correct CD45RBhigh induced colitis is totally dependent on IL-10 generation (5, 8). Another implication of this model is to test whether the extracellular Hsp70 or intracellular Hsp70 is dominant in driving IL-10 production in vivo. There is a wide Hsp70 production in Rag−/− mice, which is expressed in colonic mucosa, macrophages, and dendritic cells. In theory, if extracellular Hsp70 is the major driver of IL-10 production, CD4+ regulatory T cells from both WT and Hsp70−/− mice can be stimulated to make IL-10 after transfer and then in turn to lessen CD45RBhigh induced colitis. In contrast, based on our data, CD4+ regulatory T cells from Hsp70−/− mice cannot rescue CD45RBhigh induced colitis, suggesting that intracellular Hsp70 is a primary driver of IL-10 production in immune cells. This is further supported by the ELISA data from both the DSS and TNBS colitis models, where we did not detect any significant differences in IL-10 in colonic mucosa between Hsp70−/− and Hsp70−/−/V-TG mice. Although these data are suggestive that intracellular Hsp70 has a strong immunodulatory role, we cannot rule out a role for extracellular Hsp70. Its effects can be diminished as a consequence of blockage of the intracellular Hsp70-involved signal pathway of IL-10 production in active colitis.
IL-10 is the key cytokine with anti-inflammatory properties and has an essential role in IBD by limiting the immune response and preventing damage to the gastrointestinal tract (19, 31, 36). Previous studies show that the MAPK cascade, and especially ERK activation, is associated with IL-10 expression regulation in all immune cells, given that IL-10 is downregulated in the presence of chemical inhibitors of ERK or in ERK-deficient mice (2, 35, 43). In addition, both ERK and p38 contribute IL-10 induction in macrophages and dendritic cells (25, 34). We found that immunocytes from Hsp70-deficient mice had significantly less IL-10 expression compared with cells from WT mice after either LPS or mitogen stimulation, although both groups had escalated IL-10 expression compared with their basal level after stimulation. Furthermore, ERK phosphorylation, but not p38 or JNK phosphorylation, was much diminished in cells with Hsp70 deficiency after stimulation, suggesting that Hsp70 regulates IL-10 production by specifically affecting the ERK phosphorylation pathway (Fig. 10). However, it is still not clear how Hsp70 affects ERK activation, because we do not find any direct bindings between ERK and Hsp70 by coimmunoprecipitation (data not shown). As Hsp70, a molecular chaperone, interacts with key regulators of many signal transduction pathways under stress (14), it is speculated that it may interact with either upstream kinases such as Raf/MEK or MAPK phosphatases to activate MAPK/ERK phosphorylation (22, 26). Moreover, Hsp70-regulated IL-10 production is immunocyte specific in this study because there are no differences in IL-10 production in colonic tissue between Hsp70−/− and Hsp70−/−/V-TG mice.
In conclusion, our studies have revealed new information about the pivotal roles of Hsp70 in experimental colitis. Intracellular Hsp70 can maintain the epithelial tight junction function by stabilizing the key tight junction-associated proteins like ZO-1. In immune cells, intracellular Hsp70 regulates IL-10 production by involvement of ERK phosphorylation. The loss of critical protection from intracellular Hsp70 has multiple profound negative consequences, rendering the mucosa highly susceptible to inflammatory and immune stresses during IBD. Hence, strategies to induce Hsp70 in colonic mucosa in the presence of inflammation, e.g., probiotics, glucocorticoids, and short-chain fatty acids, should be considered in future clinical therapy. Furthermore, studies of immune cell-specific Hsp70 transgenic mice will help us understand more about Hsp70-mediated host defense mechanisms under IBD.
GRANTS
This work was supported by the National Institute of Diabetes, Digestive and Kidney Diseases Grants DK-42086 (to E. B. Chang), DK-47722 (to E. B. Chang), DK-097268 (to E. B. Chang), 1F32-DK-082104 (to J. S. Messer), and P30-DK-42086 (to J. S. Messer). Support was also provided by grants from the Gastrointestinal Research Foundation, Chicago, IL, and the Crohn’s and Colitis Foundation of America (to J. S. Messer and Y. Wang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.W., V.A.L., and E.B.C. conceived and designed research; Y.W., F.L., X.Z., and Y.T. performed experiments; Y.W. and F.L. analyzed data; Y.W., F.L., X.Z., Y.T., and E.B.C. interpreted results of experiments; Y.W. and F.L. prepared figures; Y.W., X.Z., V.A.L., J.S.M., and E.B.C. drafted manuscript; Y.W., V.A.L., S.D., J.S.M., and E.B.C. edited and revised manuscript; E.B.C. approved final version of manuscript.
REFERENCES
- 1.Afrazi A, Sodhi CP, Good M, Jia H, Siggers R, Yazji I, Ma C, Neal MD, Prindle T, Grant ZS, Branca MF, Ozolek J, Chang EB, Hackam DJ. Intracellular heat shock protein-70 negatively regulates TLR4 signaling in the newborn intestinal epithelium. J Immunol 188: 4543–4557, 2012. doi: 10.4049/jimmunol.1103114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agrawal A, Dillon S, Denning TL, Pulendran B. ERK1−/− mice exhibit Th1 cell polarization and increased susceptibility to experimental autoimmune encephalomyelitis. J Immunol 176: 5788–5796, 2006. doi: 10.4049/jimmunol.176.10.5788. [DOI] [PubMed] [Google Scholar]
- 3.Al-Ghadban S, Kaissi S, Homaidan FR, Naim HY, El-Sabban ME. Cross-talk between intestinal epithelial cells and immune cells in inflammatory bowel disease. Sci Rep 6: 29783, 2016. doi: 10.1038/srep29783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antoniou E, Margonis GA, Angelou A, Pikouli A, Argiri P, Karavokyros I, Papalois A, Pikoulis E. The TNBS-induced colitis animal model: An overview. Ann Med Surg (Lond) 11: 9–15, 2016. doi: 10.1016/j.amsu.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med 190: 995–1004, 1999. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borges TJ, Lopes RL, Pinho NG, Machado FD, Souza AP, Bonorino C. Extracellular Hsp70 inhibits pro-inflammatory cytokine production by IL-10 driven down-regulation of C/EBPβ and C/EBPδ. Int J Hyperthermia 29: 455–463, 2013. doi: 10.3109/02656736.2013.798037. [DOI] [PubMed] [Google Scholar]
- 7.Borges TJ, Wieten L, van Herwijnen MJ, Broere F, van der Zee R, Bonorino C, van Eden W. The anti-inflammatory mechanisms of Hsp70. Front Immunol 3: 95, 2012. doi: 10.3389/fimmu.2012.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciucci T, Ibáñez L, Boucoiran A, Birgy-Barelli E, Pène J, Abou-Ezzi G, Arab N, Rouleau M, Hébuterne X, Yssel H, Blin-Wakkach C, Wakkach A. Bone marrow Th17 TNFα cells induce osteoclast differentiation, and link bone destruction to IBD. Gut 64: 1072–1081, 2015. doi: 10.1136/gutjnl-2014-306947. [DOI] [PubMed] [Google Scholar]
- 9.Coburn LA, Gong X, Singh K, Asim M, Scull BP, Allaman MM, Williams CS, Rosen MJ, Washington MK, Barry DP, Piazuelo MB, Casero RA Jr, Chaturvedi R, Zhao Z, Wilson KT. L-arginine supplementation improves responses to injury and inflammation in dextran sulfate sodium colitis. PLoS One 7: e33546, 2012. doi: 10.1371/journal.pone.0033546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson NJ, Fort MM, Müller W, Leach MW, Rennick DM. Chronic colitis in IL-10−/− mice: insufficient counter regulation of a Th1 response. Int Rev Immunol 19: 91–121, 2000. doi: 10.3109/08830180009048392. [DOI] [PubMed] [Google Scholar]
- 11.de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol 13: 13–27, 2016. doi: 10.1038/nrgastro.2015.186. [DOI] [PubMed] [Google Scholar]
- 12.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 487: 104–108, 2012. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans CG, Chang L, Gestwicki JE. Heat shock protein 70 (hsp70) as an emerging drug target. J Med Chem 53: 4585–4602, 2010. doi: 10.1021/jm100054f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 61: 243–282, 1999. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- 15.Hu S, Ciancio MJ, Lahav M, Fujiya M, Lichtenstein L, Anant S, Musch MW, Chang EB. Translational inhibition of colonic epithelial heat shock proteins by IFN-gamma and TNF-alpha in intestinal inflammation. Gastroenterology 133: 1893–1904, 2007. doi: 10.1053/j.gastro.2007.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu S, Wang Y, Lichtenstein L, Tao Y, Musch MW, Jabri B, Antonopoulos D, Claud EC, Chang EB. Regional differences in colonic mucosa-associated microbiota determine the physiological expression of host heat shock proteins. Am J Physiol Gastrointest Liver Physiol 299: G1266–G1275, 2010. doi: 10.1152/ajpgi.00357.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu S, Zhu X, Triggs JR, Tao Y, Wang Y, Lichtenstein L, Bissonnette M, Musch MW, Chang EB. Inflammation-induced, 3'UTR-dependent translational inhibition of Hsp70 mRNA impairs intestinal homeostasis. Am J Physiol Gastrointest Liver Physiol 296: G1003–G1011, 2009. doi: 10.1152/ajpgi.00027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwashita Y, Sakiyama T, Musch MW, Ropeleski MJ, Tsubouchi H, Chang EB. Polyamines mediate glutamine-dependent induction of the intestinal epithelial heat shock response. Am J Physiol Gastrointest Liver Physiol 301: G181–G187, 2011. doi: 10.1152/ajpgi.00054.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawachi S, Jennings S, Panes J, Cockrell A, Laroux FS, Gray L, Perry M, van der Heyde H, Balish E, Granger DN, Specian RA, Grisham MB. Cytokine and endothelial cell adhesion molecule expression in interleukin-10-deficient mice. Am J Physiol Gastrointest Liver Physiol 278: G734–G743, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Khandia R, Munjal AK, Iqbal HM, Dhama K. Heat shock proteins: therapeutic perspectives in inflammatory disorders. Recent Pat Inflamm Allergy Drug Discov 10: 94–104, 2017. doi: 10.2174/1872213X10666161213163301. [DOI] [PubMed] [Google Scholar]
- 21.Kiesler P, Fuss IJ, Strober W. Experimental Models of Inflammatory Bowel Diseases. Cell Mol Gastroenterol Hepatol 1: 154–170, 2015. doi: 10.1016/j.jcmgh.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J 351: 289–305, 2000. doi: 10.1042/bj3510289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liedel JL, Guo Y, Yu Y, Shiou SR, Chen S, Petrof EO, Hu S, Musch MW, Claud EC. Mother’s milk-induced Hsp70 expression preserves intestinal epithelial barrier function in an immature rat pup model. Pediatr Res 69: 395–400, 2011. doi: 10.1203/PDR.0b013e3182114ec9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsay J, Van Montfrans C, Brennan F, Van Deventer S, Drillenburg P, Hodgson H, Te Velde A, Sol Rodriguez Pena M. IL-10 gene therapy prevents TNBS-induced colitis. Gene Ther 9: 1715–1721, 2002. doi: 10.1038/sj.gt.3301841. [DOI] [PubMed] [Google Scholar]
- 25.Liu BS, Cao Y, Huizinga TW, Hafler DA, Toes RE. TLR-mediated STAT3 and ERK activation controls IL-10 secretion by human B cells. Eur J Immunol 44: 2121–2129, 2014. doi: 10.1002/eji.201344341. [DOI] [PubMed] [Google Scholar]
- 26.Maik-Rachline G, Seger R. The ERK cascade inhibitors: Towards overcoming resistance. Drug Resist Updat 25: 1–12, 2016. doi: 10.1016/j.drup.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Merga Y, Campbell BJ, Rhodes JM. Mucosal barrier, bacteria and inflammatory bowel disease: possibilities for therapy. Dig Dis 32: 475–483, 2014. doi: 10.1159/000358156. [DOI] [PubMed] [Google Scholar]
- 28.Musch MW, Ciancio MJ, Sarge K, Chang EB. Induction of heat shock protein 70 protects intestinal epithelial IEC-18 cells from oxidant and thermal injury. Am J Physiol 270: C429–C436, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Musch MW, Petrof EO, Kojima K, Ren H, McKay DM, Chang EB. Bacterial superantigen-treated intestinal epithelial cells upregulate heat shock proteins 25 and 72 and are resistant to oxidant cytotoxicity. Infect Immun 72: 3187–3194, 2004. doi: 10.1128/IAI.72.6.3187-3194.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musch MW, Sugi K, Straus D, Chang EB. Heat-shock protein 72 protects against oxidant-induced injury of barrier function of human colonic epithelial Caco2/bbe cells. Gastroenterology 117: 115–122, 1999. doi: 10.1016/S0016-5085(99)70557-3. [DOI] [PubMed] [Google Scholar]
- 31.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol 29: 71–109, 2011. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 32.Poritz LS, Garver KI, Green C, Fitzpatrick L, Ruggiero F, Koltun WA. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J Surg Res 140: 12–19, 2007. doi: 10.1016/j.jss.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 33.Randhawa PK, Singh K, Singh N, Jaggi AS. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J Physiol Pharmacol 18: 279–288, 2014. doi: 10.4196/kjpp.2014.18.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanin DE, Prendergast CT, Mountford AP. IL-10 production in macrophages is regulated by a TLR-driven CREB-mediated mechanism that is linked to genes involved in cell metabolism. J Immunol 195: 1218–1232, 2015. doi: 10.4049/jimmunol.1500146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol 10: 170–181, 2010. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 36.Sun X, Yang H, Nose K, Nose S, Haxhija EQ, Koga H, Feng Y, Teitelbaum DH. Decline in intestinal mucosal IL-10 expression and decreased intestinal barrier function in a mouse model of total parenteral nutrition. Am J Physiol Gastrointest Liver Physiol 294: G139–G147, 2008. doi: 10.1152/ajpgi.00386.2007. [DOI] [PubMed] [Google Scholar]
- 37.Tao Y, Drabik KA, Waypa TS, Musch MW, Alverdy JC, Schneewind O, Chang EB, Petrof EO. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol 290: C1018–C1030, 2006. doi: 10.1152/ajpcell.00131.2005. [DOI] [PubMed] [Google Scholar]
- 38.Tao Y, Messer JS, Goss KH, Hart J, Bissonnette M, Chang EB. Hsp70 exerts oncogenic activity in the Apc mutant Min mouse model. Carcinogenesis 37: 731–739, 2016. doi: 10.1093/carcin/bgw056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Molle W, Wielockx B, Mahieu T, Takada M, Taniguchi T, Sekikawa K, Libert C. HSP70 protects against TNF-induced lethal inflammatory shock. Immunity 16: 685–695, 2002. doi: 10.1016/S1074-7613(02)00310-2. [DOI] [PubMed] [Google Scholar]
- 40.Wang S, Diller KR, Aggarwal SJ. Kinetics study of endogenous heat shock protein 70 expression. J Biomech Eng 125: 794–797, 2003. doi: 10.1115/1.1632522. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Whittall T, McGowan E, Younson J, Kelly C, Bergmeier LA, Singh M, Lehner T. Identification of stimulating and inhibitory epitopes within the heat shock protein 70 molecule that modulate cytokine production and maturation of dendritic cells. J Immunol 174: 3306–3316, 2005. doi: 10.4049/jimmunol.174.6.3306. [DOI] [PubMed] [Google Scholar]
- 42.Wieten L, van der Zee R, Goedemans R, Sijtsma J, Serafini M, Lubsen NH, van Eden W, Broere F. Hsp70 expression and induction as a readout for detection of immune modulatory components in food. Cell Stress Chaperones 15: 25–37, 2010. doi: 10.1007/s12192-009-0119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yi AK, Yoon JG, Yeo SJ, Hong SC, English BK, Krieg AM. Role of mitogen-activated protein kinases in CpG DNA-mediated IL-10 and IL-12 production: central role of extracellular signal-regulated kinase in the negative feedback loop of the CpG DNA-mediated Th1 response. J Immunol 168: 4711–4720, 2002. doi: 10.4049/jimmunol.168.9.4711. [DOI] [PubMed] [Google Scholar]