Abstract
The expression of apelin and its receptors (APJ) in central autonomic networks suggests that apelin may regulate gastrointestinal motor functions. In rodents, central administration of apelin-13 has been shown to inhibit gastric emptying; however, the mechanisms involved remain to be determined. Using male adult Sprague-Dawley rats, the aims of the present study were 1) to determine the expression of APJ receptor in the dorsal vagal complex (DVC), 2) to assess the effects of central application of apelin-13 into the DVC on gastric tone and motility, and 3) to investigate the neuronal pathways responsible for apelin-induced alterations. APJ receptor immunoreactivity was detected in gastric-projecting and choline acetyltransferase-positive neurons of the DVC. Microinjection of apelin-13 into the DVC significantly decreased gastric tone and motility in both corpus and antrum. The apelin-induced reduction in gastric tone and motility was prevented by surgical vagotomy or fourth ventricular application of the APJ receptor antagonist, [Ala13]apelin-13 (F13A). Systemic administration of the muscarinic receptor antagonist atropine, but not the nitric oxide synthase inhibitor nitro-l-arginine methyl ester (l-NAME), abolished the apelin-induced inhibitory responses. The present results indicate a central modulatory role of apelin in the vagal neurocircuitry that controls gastric motor functions via withdrawal of the tonically active cholinergic pathway.
NEW & NOTEWORTHY This is the first study investigating the effects induced by brain stem application of apelin-13 while monitoring gastric tone and motility in rats. We have found that gastric-projecting neurons of the dorsal vagal complex express apelin receptors (APJ), which mediate the inhibitory actions of apelin-13. The inhibitory effects of apelin were abolished by systemic preadministration of atropine, but not nitro-l-arginine methyl ester (l-NAME). Apelin seems to modulate gastric motility via withdrawal of the tonically active vagal cholinergic pathway.
Keywords: apelin, apelin receptor, brain stem, gastric motility, vagus
INTRODUCTION
Cleavage of the 77-amino acid precursor preproapelin produces, among others, the neuropeptide apelin, which is the endogenous ligand of the G protein-coupled receptor APJ (42). Among the cleavage products of preproapelin, including apelin-36, apelin-26, and apelin-12, the pyroglutamyl form of apelin-13 has been shown to have the highest affinity for APJ receptors in rats (13, 30). The expression of both apelin and APJ receptor has been detected in a variety of peripheral tissues including heart (24), lung (34), gastric mucosa (21), small and large intestine (49, 50), placenta (30), ovary (37), adipose tissue, thyroid and mammary glands (17), and costal cartilage (20) as well as central nervous system (35, 36).
Previous studies in mice have shown that central administration of apelin inhibits gastric emptying (GE) and gastrointestinal (GI) transit (27) and delays colonic transit (CT) (52). We have shown that intracerebroventricular (icv) application of apelin in Wistar rats induces similar gastroinhibitory effects (9). Furthermore, the restraint stress-induced delayed GE and accelerated CT were attenuated by icv administration of the APJ receptor antagonist, [Ala13]apelin-13 (F13A), suggesting that central apelin plays a relevant role in the stress-induced changes in GI motor functions (9).
The mRNA of APJ receptors has been detected in several forebrain and brain stem regions that are involved in sympathetic neuronal activity, including the paraventricular nucleus of the hypothalamus (PVN) (31, 35), subventricular organs (11), and rostral ventrolateral medulla (33). Furthermore, microinjection of apelin-13 into the rostral ventrolateral medulla was shown to increase heart rate, arterial pressure, and renal sympathetic nerve activity in rats (29, 39, 53, 54). Conversely, microinjection of apelin in the subfornical organ decreased both blood pressure and heart rate (11), raising the possibility that exogenous apelin may affect GI functions via sympathetic pathways. However, motor and secretory functions of the upper GI tract are predominantly under vagal control (45, 48). Vagal efferent fibers originate in the preganglionic cells of the dorsal motor nucleus of the vagus (DMV), which, together with the nucleus tractus solitarius (NTS) and area postrema, form the dorsal vagal complex (DVC). Vagal motor fibers originating in the DMV modulate gastric tone and motility via projections to excitatory cholinergic and/or inhibitory nonadrenergic-noncholinergic (NANC), mainly nitrergic, postganglionic myenteric neurons. Gastric relaxation, therefore, may occur as a consequence of either inhibition of the vagal cholinergic or excitation of the vagal NANC pathway (45, 48).
The presence of APJ receptors and the effects of central administration of apelin on vagal pathways have not been investigated. Thus the aims of the present study were to 1) determine the expression of APJ receptors in DVC, 2) assess the effects of brain stem application of apelin-13 on gastric tone and motility, and 3) investigate the neuronal pathways responsible for apelin-induced effects.
MATERIALS AND METHODS
Animals.
Experiments were performed on male, adult (250–300 g) Sprague-Dawley rats (Charles River, Kingston, NY), housed under a standard 12-h light-dark cycle and allowed ad libitum access to standard laboratory food. All experimental interventions and procedures were conducted with the approval of the Penn State College of Medicine Institutional Animal Care and Use Committee and according to National Institutes of Health regulations.
Retrograde tracing.
Under isoflurane anesthesia (5% for induction; 2.5% for maintenance in pure O2 at a flow rate of 200–400 ml/min), a midline abdominal laparotomy was performed, and the stomach was exposed, retracted slightly, and placed on a gauze. Twenty-five microliters of 4% suspension of Fast Blue fluorescent dye (Sigma-Aldrich, St. Louis, MO) diluted in distilled water were injected into the muscularis externa of the greater (3 injections; 5 µl each) and lesser curvature (2 injections; 5 µl each). The surgical area was washed with warm sterile saline solution, the laparotomy was closed, and the animals were allowed to recover for 10 days to allow retrograde transport of the fluorescent dye.
In vivo recording of gastric motility.
Following overnight fasting, the in vivo motility experiments were started at 0800 on each experimental day. The rats were anesthetized deeply with intraperitoneal thiobutabarbital (Inactin 120–150 mg/kg). Prior to surgical interventions, the adequate plane of anesthesia was confirmed with absence of the foot pinch withdrawal reflex. Rats were intubated with a tracheal catheter and underwent a laparotomy to expose the stomach, and two miniature strain gauges (6 × 8 mm; AT Engineering, Hershey, PA) were sutured onto the serosal surface of the anterior gastric antrum and corpus in alignment with the circular smooth muscle. The laparotomy was closed, and the wires of the strain gauge were exteriorized. The rats were then transferred to a stereotaxic frame (1730; David Kopf Instruments, Tujunga, CA). The rectal temperature was monitored and kept constant at 37 ± 1°C throughout the experiment using a heating pad and temperature controller (TCAT-2; Physitemp, Clifton, NJ). The fourth ventricle was exposed via blunt dissection, the meningeal membranes above the vagal trigone were dissected, and the exposed brain stem was covered with sterile prewarmed phosphate-buffered saline (PBS) during a 60-min period of stabilization. The strain gauges were connected to a direct current bridge amplifier (EXP-SG2; MDE Electronics, Walldorf, Germany), filtered (low-pass cutoff = 0.5 Hz; AT Engineering), and recorded using a computer-based data acquisition system and software (Axoscope; Molecular Devices, Sunnyvale, CA). Following a 1-h stabilization period, a glass micropipette (20–40-μm tip diameter) affixed to a stereotaxic holder was inserted into the left DVC (in mm, +0.4–0.6 rostrocaudal from calamus scriptorius, 0.2–0.4 mediolateral from midline, and 0.6−0.7 dorsoventral from brain stem surface). The gastric tone and motility were determined by calculating the mean value of the 5–10-min time period before and after the central drug applications. The drug-induced gastric effects were measured in 60–120-s alterations around the peak effect of motility and tone and reported as absolute changes over baseline as described previously (6, 41). Briefly, the gastric motility was calculated using the following formula:
where N is the number of motility peaks in a given force range and t is the time period during which gastric motility was measured. Assuming that a 0-mV signal equates to no motility, the grouping of peak-to-peak waves was reflective of N1 = 25–50 mg, N2 = 51–100 mg, N3 = 101–200 mg, and N4 ≥ 201 mg.
Drugs and intra-DVC application.
All drugs were freshly prepared in isotonic PBS on the day of experimentation. Apelin-13 or PBS was injected in a volume of 60 nl over 60 s using a picospritzer microinjector (Toohey, Fairfield, NJ), whereas the APJ receptor antagonist F13A was applied onto the surface of the fourth ventricle (10 nmol in 2 μl) by a micropipette. Similar doses of F13A were previously shown to prevent restraint stress-induced delayed GE in rats (9). Apelin-13 was prepared in PBS containing 1% 0.50-µm fluorescent microspheres (Fluoresbrite YO carboxylate microspheres, 18720-10; Polysciences, Warrington, PA). Atropine and nitro-l-arginine methyl ester (l-NAME) were freshly diluted in PBS from their stock solutions on experimental days. The intravenous doses of atropine (100 μg/kg) and l-NAME (10 mg/kg) were obtained from previous studies in rats with similar approaches (18, 19).
Experimental design.
The first group of rats (n = 5) received apelin-13 microinjections into the DVC (1–300 pmol/60 nl). A second group of rats underwent vagotomy (VGX; n = 5), as described previously (19). Briefly, before the apposition of the strain gauges, the subdiaphragmatic posterior (right) vagus was sectioned at the midlevel of the subdiaphragmatic esophagus. A 3-0 silk thread was tied around the left cervical vagus, and the trailing ends were passed through a 3-cm length of polyethylene (PE-240) tubing. Because we recorded from the anterior stomach, which is innervated by the anterior (left) gastric branch of the vagus, the response to microinjections in the left DVC was unaltered by the posterior subdiaphragmatic vagotomy. To achieve a complete vagotomy, the ligature around the left cervical vagus was pulled out through the tube. Following complete vagotomy, there was a clear reduction of tone and motility; however, each animal served as its own control.
After sectioning the subdiaphragmatic posterior vagus, apelin-13 (300 pmol/60 nl) was microinjected into the DVC. After 30 min, the left cervical vagus was severed, disconnecting the remaining vagal outflow to the stomach. Apelin-13 was microinjected again 30 min after the cervical VGX. In the third group of rats, 30 min after the first apelin-13 microinjection (30 pmol/60 nl) into the DVC, the APJ antagonist F13A (10 nmol in 2 μl) was applied onto the surface of the fourth ventricle (n = 5). Following 3–5 min, the intra-DVC microinjection of apelin-13 (30 pmol/60 nl) was repeated. In the fourth group, 30 min after the first apelin-13 microinjection (30 pmol/60 nl), the rats received intravenous drug treatments: 1) the muscarinic receptor antagonist atropine methyl nitrate (100 μg/kg iv) or 2) the nitric oxide synthase (NOS) inhibitor l-NAME (10 mg/kg iv), followed 3–5 min later by another intra-DVC microinjection of apelin-13 (30 pmol/60 nl) (n = 5).
After completion of the experiments, rats were euthanized by administration of a pneumothorax followed by transcardiac perfusion with saline and then 4% paraformaldehyde in PBS. The skull was opened, and brain stem tissues were removed and postfixed in 4% paraformaldehyde + 20% sucrose for 24–48 h. Subsequently, 50-μm-thick coronal sections were cut using a freezing sledge microtome (model H/I; Hacker Instruments, Fairfield, NJ). The slides were mounted on gelatin-coated slides, and the fluorescent microspheres present in the microinjection sites in the brain stem were identified using an E-400 fluorescent microscope (Nikon, Tokyo, Japan) equipped with a tetramethylrhodamine isothiocyanate filter.
Histology.
Immediately after cutting, the brain stem slices were kept in long-term storage buffer solution (0.1 M phosphate buffer, 30% sucrose, and 25% ethylene glycol) at −20°C. On the experimental day, following a slow thawing on ice, the slices were incubated in 0.2 M boric acid solution for 2 × 30 min at 60°C for antigen retrieval.
After cooling for 30 min at room temperature, the APJ receptor immunoreactivity was validated in the following ways: 1) negative staining; the negative control samples were stained using the same protocol by replacing the primary antibody with diluent (10% normal horse serum, 0.3% Triton X-100, and 0.01 M Tris), 2) a commercial blocking peptide (ab101728; Abcam, Cambridge, MA) for the neutralization of the antibody-antigen binding by preabsorption with the blocking peptide (before the staining protocol, the anti-APJ primary antibody was incubated with the blocking peptide overnight at 4°C in 0.1 M PBS at a ratio of 1:10), and 3) two different anti-APJ receptor antibodies (ab214369 and ab66218, 1:100 and 1:250 dilution, respectively; Abcam) for sequential staining in consecutive sections.
Once the selectivity of the antibodies was confirmed, the slices were processed for double-labeling immunofluorescence using rabbit anti-APJ receptor (ab66218, 1:250 dilution; Abcam) and goat anti-choline acetyltransferase (ChAT; AB144P, 1:1,000 dilution; Merck Millipore, Darmstadt, Germany). The diluent used for all antibodies was 0.1 M PBS (pH 7.4) containing 10% normal horse serum, 0.3% Triton X-100, and 0.01 M Tris. The sections were incubated with primary antibodies for 3 days at room temperature, washed in PBS containing 0.3% Triton X and 0.01 M Tris (30 min × 3), and incubated overnight at 4°C with secondary antibodies (donkey anti-rabbit conjugated with Alexa Fluor 546 for APJ receptor and donkey anti-goat conjugated with Alexa Fluor 488 for ChAT; 1:400 for both stainings; Invitrogen, Carlsbad, CA). After the slices were rinsed with PBS and mounted with Fluoromount-G (Southern Biotechnology, Birmingham, AL), the images were captured using a fluorescent microscope equipped with appropriate filters for secondary antibodies. All other chemicals were purchased from Sigma-Aldrich.
Statistics.
Data are expressed as means ± SE. For in vivo measurement of gastric motility and tone, the strain gauges were calibrated before the recording sessions. Each rat served as its own control; therefore the data were calculated as changes relative to the relevant baseline. One-way ANOVA followed by paired or unpaired t-test was used to determine significance among groups, as appropriate. ANOVA followed by Bonferroni correction was used to compare the differences in the median effective dose (ED50) values determining the dose dependency of apelin-induced effects. A P value <0.05 was considered to be statistically significant. The t-statistic, degrees of freedom (df), and P values are reported in results. All statistical analyses were performed using GraphPad Prism 5.0 software (GraphPad Software, La Jolla, CA).
RESULTS
APJ receptor is expressed in gastric-projecting ChAT-positive cells in the DVC.
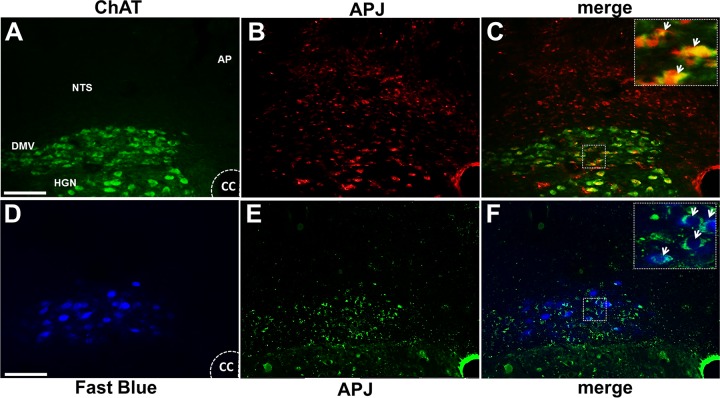
APJ-immunoreactive (APJ-IR) neurons were present in the cholinergic neurons of the DMV as well as in neurons of the NTS and hypoglossal nucleus (HGN). In particular, APJ-IR was detected on identified gastric-projecting efferent neurons of the DMV, which were labeled with the neuronal retrograde tracer Fast Blue (Fig. 1).
Fig. 1.
Apelin receptors (APJ) are present in the dorsal vagal complex. Representative micrographs showing colocalization of APJ receptors immunoreactive (red) with choline acetyltransferase (ChAT; green; A–C) or neuronal retrograde tracer Fast Blue (D–F; blue) in the intermediate dorsal vagal complex. Insets in C and F are enlargements of the boxes in their respective panels. Arrows indicate colocalized cells in dorsal motor nucleus of the vagus (DMV). AP, area postrema; CC, central canal; HGN, hypoglossal nucleus; NTS, nucleus tractus solitarius. Magnification ×40, scale bars represent 200 μm.
APJ-IR gastric-projecting neurons were distributed uniformly throughout the rostrocaudal extent of the DMV; however, the percentage of ChAT-IR neurons that colocalized APJ-IR was larger in the most caudal portions of the DMV. Results are summarized in Table 1.
Table 1.
APJ-positive cells among the ChAT-positive and gastric-projecting neurons in DMV
Values are percentages. Neurons immunoreactive to both apelin receptor (APJ) and choline acetyltransferase (ChAT) or to both APJ and Fast Blue were counted in caudal, intermediate, and rostral portions of the dorsal motor nucleus of the vagus (DMV; n = 3). For each DMV region, the immunoreactive cells were counted in five nonconsecutive sections obtained from each animal.
P < 0.05 vs. rostral;
P < 0.05 vs. intermediate DMV.
Microinjection of apelin-13 in the DVC decreases gastric tone and motility in a dose-dependent manner.
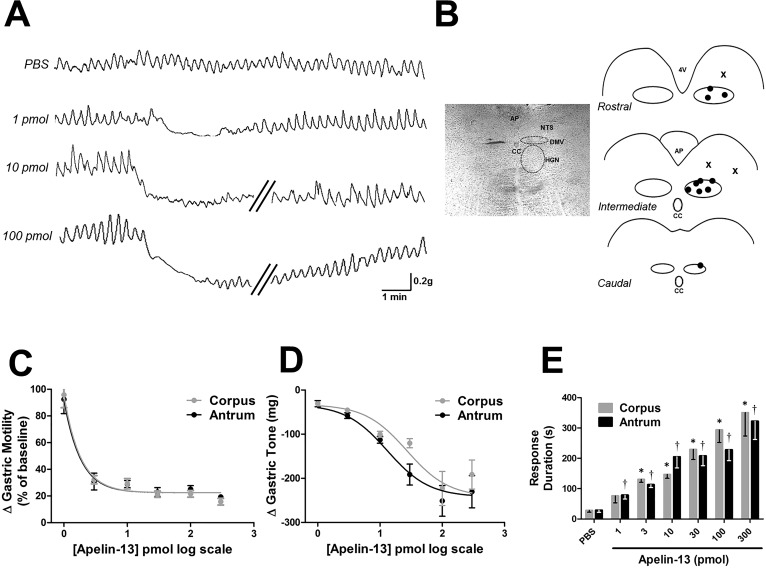
Microinjection of PBS did not induce any significant change in tone or motility either in antrum (96% ± 21.3, n = 4) or the corpus (96% ± 19.1, n = 4). The dose-response study was performed in 16 rats, in which each rat received different doses of apelin-13 at 30–60-min intervals (3–4 doses for each rat). Microinjection of apelin-13 decreased gastric tone and motility in a dose-dependent manner (1–300 pmol/60 nl; ED50 ≈ 27 pmol for both antrum and corpus). The duration of the apelin-induced inhibitory response was also dose dependent and lasted 1–6 min (Fig. 2).
Fig. 2.
Microinjection of apelin-13 into the dorsal vagal complex (DVC) induces a dose-dependent inhibition of gastric tone and motility. A: representative traces showing the response of the antrum to microinjections of 1–100 pmol apelin-13 in the DVC. Oblique parallel lines designate a 3–5-min time interval. B: schematic representation of microinjection sites. Left: approximate boundaries of dorsal motor nucleus of the vagus (DMV) and hypoglossal nucleus (HGN) are indicated with dashed curves in bright-field view. Right: ●, brain stem microinjection sites performed in caudal, intermediate, and rostral DVC. For simplicity, not all the injection sites are included. X, sites of injection outside the DMV that did not induce any gastric motor response. AP, area postrema; CC, central canal; NTS, nucleus tractus solitarius; 4V, fourth ventricle. C: graphic representation showing the dose-response curve for gastric motility following apelin-13 microinjection in the DVC. D: graphic representation showing the dose-response curve for gastric tone following apelin-13 microinjection in the DVC. E: graphic summary of the duration of dose-dependent alterations in gastric motor function following apelin-13 (1–300 pmol) microinjection (n = 16). The duration was defined as the postinjection time period in which contraction amplitude was accompanied by decreased tone. *P < 0.05 vs. PBS in corpus, †P < 0.05 vs. PBS in antrum.
These data indicate that brain stem microinjection of apelin-13 induces gastroinhibitory effects.
Apelin-13 modulation of DMV motoneurons is vagally dependent.
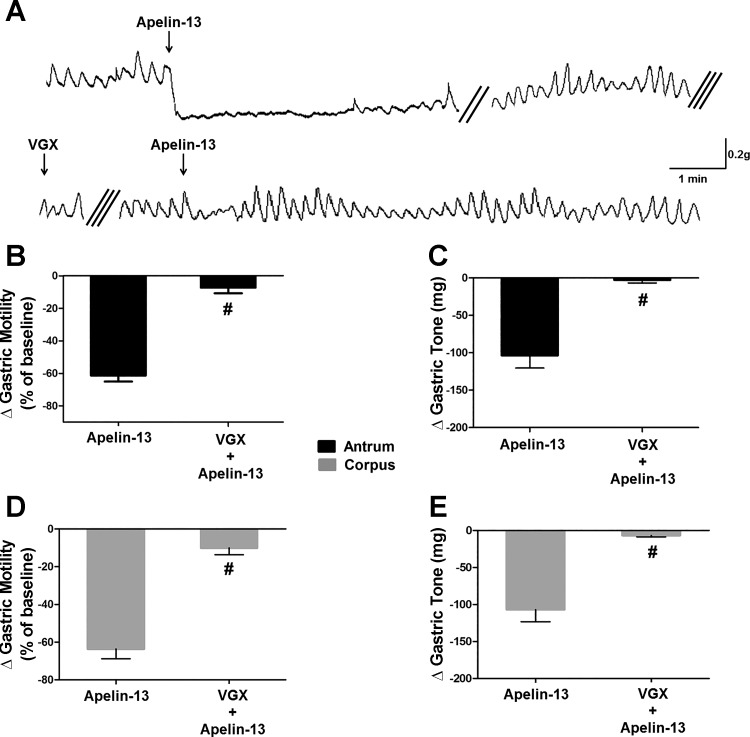
Five rats underwent posterior VGX, thus severing the vagal motor pathway originating in the right DMV (19). In these animals, microinjection of apelin-13 (30 pmol/60 nl) into the left DVC reduced gastric tone (corpus: −107 ± 16.3 mg, t = 6.3, df = 6, P < 0.05; antrum: −104 ± 16.8 mg, t = 5.6, df = 6, P < 0.05) and motility (corpus: −64 ± 5.7% of baseline, t = 6.9, df = 5, P < 0.05; antrum: −61 ± 3.4% of baseline, t = 6.5, df = 4, P < 0.05) similar to the responses observed in naive rats. Upon recovery to baseline levels, the left cervical vagus was severed, thus achieving a complete VGX. Following a 30-min recovery period, a second microinjection of apelin in the brain stem did not induce any inhibitory effect on either gastric tone or motility (tone: −6.7 ± 1.8 mg, t = 6.2, df = 6 and −2.6 ± 4.1 mg, t = 5.6, df = 6 in corpus and antrum, respectively; motility: −10.0 ± 3.9% of baseline, t = 11, df = 5 and −7.2 ± 3.5% of baseline, t = 12.9, df = 4 in corpus and antrum, respectively; Fig. 3). The loss of apelin-induced inhibitory effects in gastric tone and motility following complete VGX suggests that the effects of DVC administration of apelin-13 are mediated by vagal efferent pathways.
Fig. 3.
Gastroinhibitory effects of centrally administered apelin-13 are vagally mediated. A: original recording of antrum from a fasted rat in which the subdiaphragmatic posterior branch of the vagus was transected. Silk suture ligature was loosely affixed around the left cervical branch of vagus. Thirty minutes before the central apelin-13 application, the ligature was withdrawn, completing the surgical vagotomy (VGX). Double and triple oblique parallel lines designate a 3–5-min time interval spent for recovery and 30-min time periods before and after the surgical VGX, respectively. Graphic representation of the changes in gastric motility induced by 30 pmol of apelin-13 (B: antrum; D: corpus) before and after complete VGX. Graphic representation of the changes in gastric tone induced by 30 pmol of apelin-13 (C: antrum; E: corpus) before and after complete VGX. #P < 0.05 vs. 30 pmol of apelin-13, n = 5.
Apelin-induced inhibition is mediated by APJ receptor activation in the DMV.
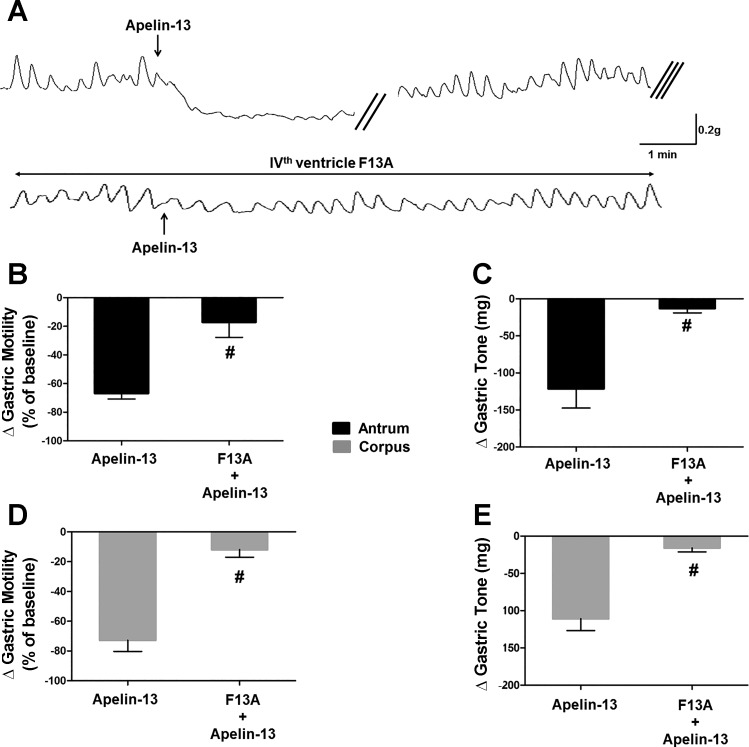
To investigate whether APJ receptor mediates the decreased gastric tone and motility, we conducted a series of experiments in which apelin-13 microinjections (30 pmol/60 nl) were performed before and after application of the APJ receptor antagonist F13A (10 nmol/2 µl) onto the floor of the fourth ventricle (n = 5). Microinjection of apelin-13 decreased gastric tone (corpus: −111 ± 6.0 mg, t = 7.5, df = 3, P < 0.05; antrum: −121 ± 26.1 mg, t = 4.5, df = 3, P < 0.05) and motility (corpus: −73 ± 7.4% of baseline, t = 7.9, df = 4, P < 0.05; antrum: −67 ± 3.9% of baseline, t = 7.5, df = 4, P < 0.05). Following a 30-min recovery period, the APJ antagonist F13A was applied to the fourth ventricle followed 2–3 min later by microinjection of apelin-13. F13A did not produce any significant change in motility or tone; however, it significantly attenuated the apelin-induced reduction in tone of corpus and antrum (−16 ± 4.7 mg, t = 4.5, df = 3 and −13 ± 5.5 mg, t = 3.8, df = 3 in corpus and antrum, respectively, n = 5, P < 0.05 vs. apelin alone) and motility (−12 ± 5.1% of baseline, t = 8.5, df = 4 and −17 ± 10.7% of baseline, t = 5.9, df = 4 in corpus and antrum, respectively, n = 5, P < 0.05 vs. apelin alone; Fig. 4).
Fig. 4.
Gastroinhibitory effects of centrally administered apelin-13 are apelin receptor (APJ) mediated. A: representative traces obtained from the antrum of a fasted rat in which the APJ receptor antagonist, [Ala13]apelin-13 (F13A) (10 nmol in 2 µl) was administered onto the surface of the fourth ventricle 2 min before the central apelin-13 application. Note that in the presence of F13A, the gastroinhibitory effects of administration of apelin-13 were attenuated significantly. The double oblique parallel lines designate a 3–5-min time interval, whereas the triple oblique parallel lines indicate the 30-min time period before the second apelin-13 application. Graphic representation of the changes in gastric motility induced by 30 pmol of apelin-13 before and after F13A (B: antrum; D: corpus). Graphic representation of the changes in gastric tone induced by 30 pmol of apelin-13 before and after F13A (C: antrum; E: corpus). #P < 0.05 vs. 30 pmol of apelin-13, n = 5.
These data indicate that the apelin-induced inhibition in gastric tone and motility is mediated by activation of APJ receptors in the DVC.
Apelin-induced inhibition is mediated by inhibition of the cholinergic postganglionic pathway.
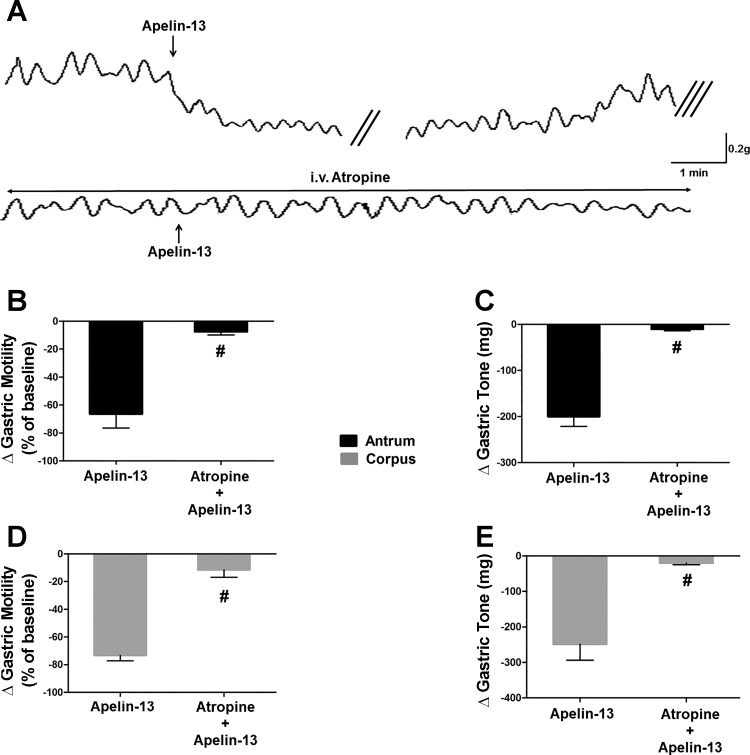
To investigate which vagal postganglionic pathway is involved in the apelin-induced gastroinhibition, in five rats, apelin-13 microinjections (30 pmol/60 nl) were performed before and after application of the muscarinic receptor antagonist atropine (100 μg/kg iv). Apelin-13 microinjection reduced gastric tone (corpus: −249 ± 45.1 mg, t = 5, df = 3, P < 0.05; antrum: −200 ± 21.8 mg, t = 6.9, df = 3, P < 0.05) and motility (corpus: −73 ± 3.8% of baseline, t = 6.3, df = 4, P < 0.05; antrum: −66 ± 10.2% of baseline, t = 4.2, df = 4, P < 0.05). Following a recovery period of 30 min, atropine was administered, followed 2–3 min later by DVC microinjection of apelin-13 (30 pmol/60 nl). Immediately after atropine administration the basal gastric tone and motility decreased slightly (not shown). Administration of atropine abolished the apelin-induced inhibition of tone in corpus and antrum (−20 ± 4.5 mg, t = 4.8, df = 4 and −10 ± 3.5 mg, t = 9.5, df = 4 in corpus and antrum, respectively, n = 5, P < 0.05 vs. apelin alone, P < 0.05 vs. control) and motility (−11 ± 5.3% of baseline, t = 13.3, df = 4 and −7 ± 2.3% of baseline, t = 6, df = 4 in corpus and antrum, respectively, n = 5, P < 0.05 vs. apelin alone, P < 0.05 vs. control; Fig. 5).
Fig. 5.
Gastroinhibitory effects of centrally administered apelin-13 are mediated by vagal cholinergic pathways. A: representative recording traces of antrum obtained from a fasted rat showing the gastric response to microinjection of apelin (30 pmol/60 nl) in the absence (upper trace) or presence (lower trace) of atropine (50 µg/kg iv). Note that pretreatment with atropine prevented the gastroinhibitory effects of apelin-13. The double oblique parallel lines designate a 3–5-min time interval, whereas the triple oblique parallel lines indicate the 30-min time interval before the administration of atropine. Graphic representation of the changes in gastric motility induced by 30 pmol of apelin-13 before and after atropine (B: antrum; D: corpus). Graphic representation of the changes in gastric tone induced by 30 pmol of apelin-13 before and after atropine (C: antrum; E: corpus). #P < 0.05 vs. 30 pmol of apelin-13, n = 5.
These results suggest that the apelin-induced inhibition in gastric tone and motility is mediated by withdrawal of cholinergic tone to postganglionic neurons.
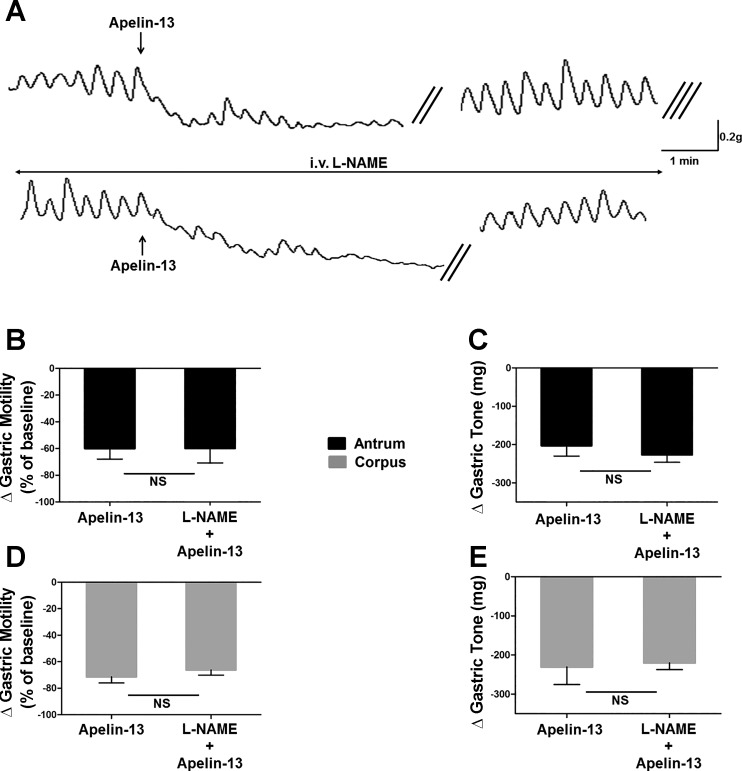
To investigate whether the nonadrenergic-noncholinergic (NANC) postganglionic pathway plays a role in the apelin-induced inhibitory effects, a series of experiments assessed the effects of DVC microinjections of apelin-13 (30 pmol/60 nl) before and after administration of the NOS inhibitor l-NAME (10 mg/kg iv). In five rats, microinjection of 30 pmol apelin-13 in the DVC decreased gastric tone (−231 ± 44.9 and −203 ± 27.3 mg in corpus and antrum, respectively) and motility (−71 ± 4.6% of baseline and 60 ± 7.9% of baseline in corpus and antrum, respectively). Following a recovery period of 30 min, l-NAME (10 mg/kg iv) was administered 2–3 min before the second microinjection of apelin-13. Following l-NAME administration, a slight increase was observed in gastric tone while no remarkable change in amplitudes of spontaneous contractions was observed. Unlike atropine treatment, however, l-NAME administration did not prevent apelin-induced decreased tone and motility either in the corpus or in the antrum (tone: −156 ± 43.0 and −159 ± 45.6 mg in corpus and antrum, respectively, n = 5; P > 0.05 vs. apelin alone; motility: −66 ± 3.9 and −60 ± 11.3% of baseline in corpus and antrum, respectively, n = 5; P > 0.05 vs. apelin alone; Fig. 6).
Fig. 6.
Gastroinhibitory effect of centrally administered apelin-13 is not mediated by the postganglionic nonadrenergic-noncholinergic pathway. A: representative recording traces of antrum obtained from a fasted rat illustrating the response to microinjection of apelin (30 pmol/60 nl) in the absence (upper trace) or presence (lower trace) of nitro-l-arginine methyl ester (l-NAME; 10 mg/kg iv). Note that pretreatment with l-NAME did not prevent the gastroinhibitory effects of apelin-13. The double oblique parallel lines designate a 3–5-min time interval, whereas the triple oblique parallel lines indicate the 30-min time interval before the administration of atropine. Graphic representation of the changes in gastric motility induced by 30 pmol of apelin-13 before and after l-NAME (B: antrum; D: corpus). Graphic representation of the changes in gastric tone induced by 30 pmol of apelin-13 before and after l-NAME (C: antrum; E: corpus); n = 5.
These results suggest that the postganglionic nitrergic pathway does not play a role in inhibitory effects of apelin-13 on either gastric tone or motility.
DISCUSSION
In this study investigating the neuronal pathways responsible for the gastroinhibitory effects of apelin, we have shown that 1) APJ receptors were expressed in a subpopulation of ChAT-positive and gastric-projecting preganglionic DMV neurons, 2) intra-DVC-injected apelin inhibited both gastric motility and tone via vagal pathways, and 3) the apelin-induced gastroinhibitory effects were mediated by vagal cholinergic, but not NANC, postganglionic pathways. Therefore our data suggest that apelin may play a regulatory role within brain stem neuronal circuitry by modulating the vagal motor output to the stomach.
Apelin-producing cells have been shown to reside within the hypothalamic PVN (9, 31), and PVN neurons have dense connections with premotor parasympathetic neurons of the DMV, as well as with viscerosensory NTS neurons (8). A previous study in rats detected apelin-positive fibers in lower brain stem structures, including the area postrema and NTS (36). It is possible, therefore, that DMV neurons also receive direct projections from hypothalamic apelinergic neurons. These projections would then impinge on a widespread and dense distribution of APJ receptors in cholinergic DMV motoneurons.
The mechanisms of release of endogenous apelin remain to be identified but involve responses to external stimuli such as stress (9) or hyperglycemia (1, 58) or are a response to food/meal-related signals such as activation of tension-sensitive (34a), chemosensitive (2, 32), or neuroendocrine activated afferent fibers (10).
Independently of the mechanisms of release, our data suggest a possible physiological role of apelin to modulate the efferent vagal signaling that regulates gastric motor functions. Our results indicate that apelin has a profound effect on brain stem vagal neurocircuits. The vagal efferent fibers originating in the preganglionic motoneurons of the DMV play a pivotal role in the regulation of upper gastric motor functions (45). Indeed, vagal efferent fibers modulate gastric tone and motility via projections to both excitatory cholinergic or inhibitory NANC postganglionic myenteric neurons; hence gastric relaxation may result from vagal modulatory inputs that either inhibit the cholinergic pathway or excite the NANC pathway (45). In our study, the gastroinhibition observed following intra-DVC microinjection of apelin-13 was abolished by atropine but was unaffected by l-NAME, suggesting that apelin-induced effects are mediated by inhibition of the excitatory cholinergic pathway subsequent to activation of APJ receptors expressed on gastric-projecting cholinergic DMV neurons.
In rats, nitric oxide synthase (NOS)-containing abdominal-projecting vagal preganglionic cell bodies are present mainly within the caudal and rostral regions of DMV (23, 38, 56), and microinjection of l-arginine into the DVC decreases intragastric pressure (23) via an excitatory effect on DMV neurons (46). In rodents, apelin is known to activate the l-arginine-NOS pathway in peripheral vasculature (22, 57), and more importantly, recent studies have shown that central administration of apelin induces hypothalamic NO release in mice (14, 16). Our data indicate that the gastric effects of apelin are mediated by withdrawal of cholinergic tone rather than release of NO; this observation would thus argue against the apelin-mediated effects on gastric tone and motility as being mediated by NO release in DMV. These data, however, do not exclude the possibility that brain stem application of apelin-13 may stimulate NOS-producing neurons in DVC through activation of APJ receptors to contribute, at least in part, to the apelin-induced modulation of subsets of vagal motoneurons devoted to functions other than modulation of gastric tone or motility.
Similarly, the spontaneous pacemaker activity of DMV neurons is well recognized; however, the firing rate of these neurons is also under the control of excitatory glutamatergic and inhibitory GABAergic synaptic projections, which arise mainly from adjacent neurons of the NTS (3, 12, 47). Indeed, the inhibitory GABAergic influence on DMV neurons has a profound role in regulating DMV neuronal activity and vagal efferent control of visceral functions. Although the effects of apelin on GABAergic inputs to DMV neurons were not investigated in the present study, it should be noted that a recent study in mice has established that the alterations in passive avoidance learning following central administration of apelin-13 were prevented by the GABA-A receptor antagonist bicuculline (43). Moreover, Lv et al. (27) have demonstrated that in mice, central (icv) injection of apelin-13 decreased GE rate, an effect that was significantly antagonized by both the APJ receptor antagonist F13A and naloxone, a competitive antagonist at µ-, δ-, and κ-opioid receptors. The APJ receptor has been shown previously to form a heterodimer with the κ-opioid receptor, leading to an increase in protein kinase C and a decrease in protein kinase A activity (25). More importantly, it has been demonstrated that within the NTS-DMV synapse, μ-opioid receptors are translocated via a cAMP/PKA-dependent mechanism on the presynaptic GABAergic terminals, thus potentially contributing to the vagally mediated inhibitory actions of opioid peptides (7). It is thus possible that the effects of exogenously administered apelin are due to a combination of effects on both the membranes of DMV neurons as well as GABAergic synapses impinging on them.
The wide topographical distribution of apelinergic neurons in the brain suggests multiple regulatory functions for apelin, including modulation of ingestive behaviors (36), for which vagal brain stem neurocircuits play a fundamental integrative role. Recently, it has been established that overnight fasting significantly reduced the expression of APJ receptor in nodose ganglia in mice and reduced gastroesophageal vagal mechanoreceptors responsiveness, suggesting that apelin plays a role in the regulation of food intake through vagal afferent pathways (34a). Indeed, apelin-induced anorexigenic effects were abolished by administration of the cholecystokinin receptor type-1 antagonist lorglumide, suggesting a modulatory role of apelin on second-order NTS neurons via a vagal afferent pathway (10). Accumulating evidence also suggests an anorexic effect of central administration of apelin in rodents (26, 40), implying that apelin may be released in response to the ingestion of a meal. Interestingly, a recent report indicated that the inhibitory effect of peripherally administered apelin-13 on GE and CT was abolished following afferent denervation by capsaicin (10). It should be noted, however, that peripheral capsaicin also induces significant damage to vagal efferent fibers, rendering these results difficult to interpret (5).
Importantly, recent evidence suggests that apelin also plays a regulatory role in glucose uptake by promoting the translocation of glucose transporters (GLUT4) to the plasma membrane of adipocytes (1, 58). In this regard, plasma apelin concentration could be a novel biomarker for predicting type 2 diabetes mellitus (28), since in diabetic patients, plasma apelin levels are negatively correlated with fasting blood glucose and homeostatic model assessment indexes (15, 28, 55). Following ingestion of a meal, glucose triggers vagally mediated gastroinhibitory effects, in part to stabilize excessive fluctuations in plasma glucose levels. The hyperglycemia-induced delay in GE reduces glucose absorption further to dampen the elevations in glycemic levels (4). Interestingly, apelin is known to stimulate the secretion of the insulin-releasing glucagon-like peptide 1 (GLP-1) in rats (51), and intra-DVC application of GLP-1 inhibits gastric tone and motility in rats (18, 19).
Finally, it has been shown that the corticotrophin-releasing factor (CRF) receptor antagonist α-helical CRF9–41 reversed the inhibitory effect on cumulative food intake induced by centrally administered apelin-13 in mice (26). Similarly, the apelin-13-induced inhibitory effect on gastric postprandial motility is abolished by central pretreatment of CRF antagonist in rats (9). Apelin may, therefore, play an important homeostatic role via interaction with brain stem vagal neurocircuits.
In summary, our present data demonstrate a potentially important role of the central apelinergic system and APJ receptors in the regulation of gastric tone and motility via modulation of vagal efferent signaling. Furthermore, apart from its putative physiological role in the postprandial state, malfunctioning apelinergic activity and/or APJ signaling in the brain stem may contribute to the clinical manifestations of disturbed vagal efferent signaling such as diabetic gastroparesis or stress.
GRANTS
This work was partly supported by Core Fulbright Visiting Scholar Program and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-55530.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.B. and R.A.T. conceived and designed research; M.B. performed experiments; M.B., O.S., and M.G. analyzed data; M.B. and R.A.T. interpreted results of experiments; M.B., O.S., and M.G. prepared figures; M.B., O.S., M.G., and R.A.T. drafted manuscript; M.B. and R.A.T. edited and revised manuscript; M.B. and R.A.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Cesare M. and Zoraide Travagli for support and encouragement and Drs. Kirsteen N. Browning and Yanyan Jiang for discussion and suggestions on earlier versions of the manuscript.
REFERENCES
- 1.Attané C, Daviaud D, Dray C, Dusaulcy R, Masseboeuf M, Prévot D, Carpéné C, Castan-Laurell I, Valet P. Apelin stimulates glucose uptake but not lipolysis in human adipose tissue ex vivo. J Mol Endocrinol 46: 21–28, 2011. doi: 10.1677/JME-10-0105. [DOI] [PubMed] [Google Scholar]
- 2.Azizi M, Iturrioz X, Blanchard A, Peyrard S, De Mota N, Chartrel N, Vaudry H, Corvol P, Llorens-Cortes C. Reciprocal regulation of plasma apelin and vasopressin by osmotic stimuli. J Am Soc Nephrol 19: 1015–1024, 2008. doi: 10.1681/ASN.2007070816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babic T, Browning KN, Travagli RA. Differential organization of excitatory and inhibitory synapses within the rat dorsal vagal complex. Am J Physiol Gastrointest Liver Physiol 300: G21–G32, 2011. doi: 10.1152/ajpgi.00363.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Browning KN. Modulation of gastrointestinal vagal neurocircuits by hyperglycemia. Front Neurosci 7: 217, 2013. doi: 10.3389/fnins.2013.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Browning KN, Babic T, Holmes GM, Swartz E, Travagli RA. A critical re-evaluation of the specificity of action of perivagal capsaicin. J Physiol 591: 1563–1580, 2013. doi: 10.1113/jphysiol.2012.246827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browning KN, Babic T, Toti L, Holmes GM, Coleman FH, Travagli RA. Plasticity in the brainstem vagal circuits controlling gastric motor function triggered by corticotropin releasing factor. J Physiol 592: 4591–4605, 2014. doi: 10.1113/jphysiol.2014.278192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Browning KN, Kalyuzhny AE, Travagli RA. Mu-opioid receptor trafficking on inhibitory synapses in the rat brainstem. J Neurosci 24: 7344–7352, 2004. doi: 10.1523/JNEUROSCI.1676-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Browning KN, Travagli RA. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr Physiol 4: 1339–1368, 2014. doi: 10.1002/cphy.c130055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bülbül M, İzgüt-Uysal VN, Sinen O, Birsen İ, Tanrıöver G. Central apelin mediates stress-induced gastrointestinal motor dysfunction in rats. Am J Physiol Gastrointest Liver Physiol 310: G249–G261, 2016. doi: 10.1152/ajpgi.00145.2015. [DOI] [PubMed] [Google Scholar]
- 10.Bülbül M, Sinen O, Birsen İ, Nimet İzgüt-Uysal V. Peripheral apelin-13 administration inhibits gastrointestinal motor functions in rats: the role of cholecystokinin through CCK1 receptor-mediated pathway. Neuropeptides 63: 91–97, 2017. doi: 10.1016/j.npep.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Dai L, Smith PM, Kuksis M, Ferguson AV. Apelin acts in the subfornical organ to influence neuronal excitability and cardiovascular function. J Physiol 591: 3421–3432, 2013. doi: 10.1113/jphysiol.2013.254144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis SF, Derbenev AV, Williams KW, Glatzer NR, Smith BN. Excitatory and inhibitory local circuit input to the rat dorsal motor nucleus of the vagus originating from the nucleus tractus solitarius. Brain Res 1017: 208–217, 2004. doi: 10.1016/j.brainres.2004.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Mota N, Reaux-Le Goazigo A, El Messari S, Chartrel N, Roesch D, Dujardin C, Kordon C, Vaudry H, Moos F, Llorens-Cortes C. Apelin, a potent diuretic neuropeptide counteracting vasopressin actions through inhibition of vasopressin neuron activity and vasopressin release. Proc Natl Acad Sci USA 101: 10464–10469, 2004. doi: 10.1073/pnas.0403518101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duparc T, Colom A, Cani PD, Massaly N, Rastrelli S, Drougard A, Le Gonidec S, Moulédous L, Frances B, Leclercq I, Llorens-Cortes C, Pospisilik JA, Delzenne NM, Valet P, Castan-Laurell I, Knauf C. Central apelin controls glucose homeostasis via a nitric oxide-dependent pathway in mice. Antioxid Redox Signal 15: 1477–1496, 2011. doi: 10.1089/ars.2010.3454. [DOI] [PubMed] [Google Scholar]
- 15.Erdem G, Dogru T, Tasci I, Sonmez A, Tapan S. Low plasma apelin levels in newly diagnosed type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 116: 289–292, 2008. doi: 10.1055/s-2007-1004564. [DOI] [PubMed] [Google Scholar]
- 16.Fournel A, Drougard A, Duparc T, Marlin A, Brierley SM, Castro J, Le-Gonidec S, Masri B, Colom A, Lucas A, Rousset P, Cenac N, Vergnolle N, Valet P, Cani PD, Knauf C. Apelin targets gut contraction to control glucose metabolism via the brain. Gut 66: 258–269, 2017. doi: 10.1136/gutjnl-2015-310230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habata Y, Fujii R, Hosoya M, Fukusumi S, Kawamata Y, Hinuma S, Kitada C, Nishizawa N, Murosaki S, Kurokawa T, Onda H, Tatemoto K, Fujino M. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim Biophys Acta 1452: 25–35, 1999. doi: 10.1016/S0167-4889(99)00114-7. [DOI] [PubMed] [Google Scholar]
- 18.Holmes GM, Browning KN, Tong M, Qualls-Creekmore E, Travagli RA. Vagally mediated effects of glucagon-like peptide 1: in vitro and in vivo gastric actions. J Physiol 587: 4749–4759, 2009. doi: 10.1113/jphysiol.2009.175067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmes GM, Tong M, Travagli RA. Effects of brain stem cholecystokinin-8s on gastric tone and esophageal-gastric reflex. Am J Physiol Gastrointest Liver Physiol 296: G621–G631, 2009. doi: 10.1152/ajpgi.90567.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosoya M, Kawamata Y, Fukusumi S, Fujii R, Habata Y, Hinuma S, Kitada C, Honda S, Kurokawa T, Onda H, Nishimura O, Fujino M. Molecular and functional characteristics of APJ. Tissue distribution of mRNA and interaction with the endogenous ligand apelin. J Biol Chem 275: 21061–21067, 2000. doi: 10.1074/jbc.M908417199. [DOI] [PubMed] [Google Scholar]
- 21.Izgüt-Uysal VN, Gemici B, Birsen I, Acar N, Üstünel I. The protective effect of apelin against water-immersion and restraint stress-induced gastric damage. J Physiol Sci 64: 279–289, 2014. doi: 10.1007/s12576-014-0317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia YX, Lu ZF, Zhang J, Pan CS, Yang JH, Zhao J, Yu F, Duan XH, Tang CS, Qi YF. Apelin activates l-arginine/nitric oxide synthase/nitric oxide pathway in rat aortas. Peptides 28: 2023–2029, 2007. doi: 10.1016/j.peptides.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 23.Krowicki ZK, Sharkey KA, Serron SC, Nathan NA, Hornby PJ. Distribution of nitric oxide synthase in rat dorsal vagal complex and effects of microinjection of nitric oxide compounds upon gastric motor function. J Comp Neurol 377: 49–69, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 24.Lee DK, Cheng R, Nguyen T, Fan T, Kariyawasam AP, Liu Y, Osmond DH, George SR, O’Dowd BF. Characterization of apelin, the ligand for the APJ receptor. J Neurochem 74: 34–41, 2000. doi: 10.1046/j.1471-4159.2000.0740034.x. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Chen J, Bai B, Du H, Liu Y, Liu H. Heterodimerization of human apelin and kappa opioid receptors: roles in signal transduction. Cell Signal 24: 991–1001, 2012. doi: 10.1016/j.cellsig.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Lv SY, Yang YJ, Qin YJ, Mo JR, Wang NB, Wang YJ, Chen Q. Central apelin-13 inhibits food intake via the CRF receptor in mice. Peptides 33: 132–138, 2012. doi: 10.1016/j.peptides.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Lv SY, Yang YJ, Qin YJ, Xiong W, Chen Q. Effect of centrally administered apelin-13 on gastric emptying and gastrointestinal transit in mice. Peptides 32: 978–982, 2011. doi: 10.1016/j.peptides.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 28.Ma WY, Yu TY, Wei JN, Hung CS, Lin MS, Liao YJ, Pei D, Su CC, Lu KC, Liu PH, Lin CH, Chuang LM, Kao HL, Lin JW, Chuang YJ, Li HY. Plasma apelin: a novel biomarker for predicting diabetes. Clin Chim Acta 435: 18–23, 2014. doi: 10.1016/j.cca.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 29.Masaki T, Yasuda T, Yoshimatsu H. Apelin-13 microinjection into the paraventricular nucleus increased sympathetic nerve activity innervating brown adipose tissue in rats. Brain Res Bull 87: 540–543, 2012. doi: 10.1016/j.brainresbull.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Medhurst AD, Jennings CA, Robbins MJ, Davis RP, Ellis C, Winborn KY, Lawrie KW, Hervieu G, Riley G, Bolaky JE, Herrity NC, Murdock P, Darker JG. Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J Neurochem 84: 1162–1172, 2003. doi: 10.1046/j.1471-4159.2003.01587.x. [DOI] [PubMed] [Google Scholar]
- 31.O’Carroll AM, Don AL, Lolait SJ. APJ receptor mRNA expression in the rat hypothalamic paraventricular nucleus: regulation by stress and glucocorticoids. J Neuroendocrinol 15: 1095–1101, 2003. doi: 10.1046/j.1365-2826.2003.01102.x. [DOI] [PubMed] [Google Scholar]
- 32.O’Carroll AM, Lolait SJ. Regulation of rat APJ receptor messenger ribonucleic acid expression in magnocellular neurones of the paraventricular and supraopric nuclei by osmotic stimuli. J Neuroendocrinol 15: 661–666, 2003. doi: 10.1046/j.1365-2826.2003.01044.x. [DOI] [PubMed] [Google Scholar]
- 33.O’Carroll AM, Lolait SJ, Harris LE, Pope GR. The apelin receptor APJ: journey from an orphan to a multifaceted regulator of homeostasis. J Endocrinol 219: R13–R35, 2013. doi: 10.1530/JOE-13-0227. [DOI] [PubMed] [Google Scholar]
- 34.O’Carroll AM, Selby TL, Palkovits M, Lolait SJ. Distribution of mRNA encoding B78/apj, the rat homologue of the human APJ receptor, and its endogenous ligand apelin in brain and peripheral tissues. Biochim Biophys Acta 1492: 72–80, 2000. doi: 10.1016/S0167-4781(00)00072-5. [DOI] [PubMed] [Google Scholar]
- 34a.O'Donnell TA, Kentish SJ, Healey K, Ratcliff K, Wittert GA, Page AJ. Apelin-13 effects on peripheral vagal afferent mechanosensitivity with feeding and fasting (Abstract). Gastroenterology 142, Suppl 1: S-57, 2012. doi: 10.1016/S0016-5085(12)60220-0. [DOI] [Google Scholar]
- 35.Pope GR, Roberts EM, Lolait SJ, O’Carroll AM. Central and peripheral apelin receptor distribution in the mouse: species differences with rat. Peptides 33: 139–148, 2012. doi: 10.1016/j.peptides.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reaux A, Gallatz K, Palkovits M, Llorens-Cortes C. Distribution of apelin-synthesizing neurons in the adult rat brain. Neuroscience 113: 653–662, 2002. doi: 10.1016/S0306-4522(02)00192-6. [DOI] [PubMed] [Google Scholar]
- 37.Schilffarth S, Antoni B, Schams D, Meyer HH, Berisha B. The expression of apelin and its receptor APJ during different physiological stages in the bovine ovary. Int J Biol Sci 5: 344–350, 2009. doi: 10.7150/ijbs.5.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sehirli US, Tuğtepe H, Verimli U, Kirazli Ö, Özkan M, Dağli ET. Expression of NADPH-d in the vagal nuclei of the chronic esophagitis model in rats. Turk J Med Sci 44: 243–248, 2014. doi: 10.3906/sag-1209-30. [DOI] [PubMed] [Google Scholar]
- 39.Seyedabadi M, Goodchild AK, Pilowsky PM. Site-specific effects of apelin-13 in the rat medulla oblongata on arterial pressure and respiration. Auton Neurosci 101: 32–38, 2002. doi: 10.1016/S1566-0702(02)00178-9. [DOI] [PubMed] [Google Scholar]
- 40.Sunter D, Hewson AK, Dickson SL. Intracerebroventricular injection of apelin-13 reduces food intake in the rat. Neurosci Lett 353: 1–4, 2003. doi: 10.1016/S0304-3940(03)00351-3. [DOI] [PubMed] [Google Scholar]
- 41.Swartz EM, Browning KN, Travagli RA, Holmes GM. Ghrelin increases vagally mediated gastric activity by central sites of action. Neurogastroenterol Motil 26: 272–282, 2014. doi: 10.1111/nmo.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, Kurokawa T, Onda H, Fujino M. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun 251: 471–476, 1998. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 43.Telegdy G, Adamik A, Jászberényi M. Involvement of neurotransmitters in the action of apelin-13 on passive avoidance learning in mice. Peptides 39: 171–174, 2013. doi: 10.1016/j.peptides.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 45.Travagli RA, Anselmi L. Vagal neurocircuitry and its influence on gastric motility. Nat Rev Gastroenterol Hepatol 13: 389–401, 2016. doi: 10.1038/nrgastro.2016.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Travagli RA, Gillis RA. Nitric oxide-mediated excitatory effect on neurons of dorsal motor nucleus of vagus. Am J Physiol Gastrointest Liver Physiol 266: G154–G160, 1994. [DOI] [PubMed] [Google Scholar]
- 47.Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol Gastrointest Liver Physiol 260: G531–G536, 1991. [DOI] [PubMed] [Google Scholar]
- 48.Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol 68: 279–305, 2006. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang G, Anini Y, Wei W, Qi X, OCarroll AM, Mochizuki T, Wang HQ, Hellmich MR, Englander EW, Greeley GH Jr. Apelin, a new enteric peptide: localization in the gastrointestinal tract, ontogeny, and stimulation of gastric cell proliferation and of cholecystokinin secretion. Endocrinology 145: 1342–1348, 2004. doi: 10.1210/en.2003-1116. [DOI] [PubMed] [Google Scholar]
- 50.Wang G, Kundu R, Han S, Qi X, Englander EW, Quertermous T, Greeley GH Jr. Ontogeny of apelin and its receptor in the rodent gastrointestinal tract. Regul Pept 158: 32–39, 2009. doi: 10.1016/j.regpep.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wattez JS, Ravallec R, Cudennec B, Knauf C, Dhulster P, Valet P, Breton C, Vieau D, Lesage J. Apelin stimulates both cholecystokinin and glucagon-like peptide 1 secretions in vitro and in vivo in rodents. Peptides 48: 134–136, 2013. doi: 10.1016/j.peptides.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Yang YJ, Lv SY, Xiu MH, Xu N, Chen Q. Intracerebroventricular administration of apelin-13 inhibits distal colonic transit in mice. Peptides 31: 2241–2246, 2010. doi: 10.1016/j.peptides.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 53.Yao F, Modgil A, Zhang Q, Pingili A, Singh N, O’Rourke ST, Sun C. Pressor effect of apelin-13 in the rostral ventrolateral medulla: role of NAD(P)H oxidase-derived superoxide. J Pharmacol Exp Ther 336: 372–380, 2011. doi: 10.1124/jpet.110.174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Q, Yao F, Raizada MK, O’Rourke ST, Sun C. Apelin gene transfer into the rostral ventrolateral medulla induces chronic blood pressure elevation in normotensive rats. Circ Res 104: 1421–1428, 2009. doi: 10.1161/CIRCRESAHA.108.192302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Shen C, Li X, Ren G, Fan X, Ren F, Zhang N, Sun J, Yang J. Low plasma apelin in newly diagnosed type 2 diabetes in Chinese people. Diabetes Care 32: e150, 2009. doi: 10.2337/dc09-1146. [DOI] [PubMed] [Google Scholar]
- 56.Zheng ZL, Rogers RC, Travagli RA. Selective gastric projections of nitric oxide synthase-containing vagal brainstem neurons. Neuroscience 90: 685–694, 1999. doi: 10.1016/S0306-4522(98)00586-7. [DOI] [PubMed] [Google Scholar]
- 57.Zhong JC, Yu XY, Huang Y, Yung LM, Lau CW, Lin SG. Apelin modulates aortic vascular tone via endothelial nitric oxide synthase phosphorylation pathway in diabetic mice. Cardiovasc Res 74: 388–395, 2007. doi: 10.1016/j.cardiores.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 58.Zhu S, Sun F, Li W, Cao Y, Wang C, Wang Y, Liang D, Zhang R, Zhang S, Wang H, Cao F. Apelin stimulates glucose uptake through the PI3K/Akt pathway and improves insulin resistance in 3T3-L1 adipocytes. Mol Cell Biochem 353: 305–313, 2011. doi: 10.1007/s11010-011-0799-0. [DOI] [PubMed] [Google Scholar]