Abstract
A diverse range of effects of the intestinal microbiota on mucosal defense and injury has become increasingly clear over the past decade. Hydrogen sulfide (H2S) has emerged as an important mediator of many physiological functions, including gastrointestinal mucosal defense and repair. Hydrogen sulfide is produced by gastrointestinal tract tissues and by bacteria residing within the gut and can influence the function of a wide range of cells. The microbiota also appears to be an important target of hydrogen sulfide. H2S donors can modify the gut microbiota, and the gastrointestinal epithelium is a major site of oxidation of microbial-derived H2S. When administered together with nonsteroidal anti-inflammatory drugs, H2S can prevent some of the dysbiosis those drugs induce, possibly contributing to the observed prevention of gastrointestinal damage. Exogenous H2S can also markedly reduce the severity of experimental colitis and plays important roles in modulating epithelial cell-mucus-bacterial interactions in the intestine, contributing to its ability to promote resolution of inflammation and repair of tissue injury. In this paper we review recent studies examining the roles of H2S in mucosal defense, the possibility that H2S can damage the gastrointestinal epithelium, and effects of H2S on the gut microbiota and on mucus and biofilm interactions in the context of intestinal inflammation.
Keywords: bacteria, biofilm, colitis, epithelium, inflammatory bowel disease, intestine, microbiota, mucus, NSAID
INTRODUCTION
Over the past 15 years, our understanding of the roles of the gaseous mediator hydrogen sulfide (H2S) has grown considerably. It is now clear that H2S plays important roles in many physiological and pathophysiological processes (22, 44). Our laboratories have been particularly interested in the ability of H2S to act as a mediator of inflammation, homeostasis, and repair in the gastrointestinal (GI) tract. H2S is an important mediator of mucosal defense, affecting such basic processes as mucosal blood flow, bicarbonate and mucus secretion, and endothelial-leukocyte interactions (13, 19, 30, 49). Important roles for H2S have also been demonstrated in visceral pain (7, 8, 10, 11) and in resolution of inflammatory disorders such as colitis (14, 18, 23, 43). These observations have provided the impetus for several groups to design and develop novel H2S-releasing drugs that may be used to protect the GI tract, accelerate repair of GI damage, and reduce inflammation in diseases such as ulcerative colitis, Crohn’s disease, and nonsteroidal anti-inflammatory drug (NSAID)-induced gastroenteropathy (7, 21, 44).
The intestinal microbiome is a significant source of H2S, some of which permeates across the intestinal epithelium (15, 25, 35), providing an energy source for epithelial and other lamina propria cells (17, 29). Of course, diet can affect the amounts of H2S production. Organic polysulfides contained in garlic, onions, cruciferous vegetables (e.g., cabbage, cauliflower, kale, broccoli, etc.), and durian fruit can directly release H2S through interactions with protein thiols or intracellular thiols (e.g., glutathione). l-Cysteine is the main precursor for mammalian H2S production, and H2S synthesis can be modulated by dietary supplementation or restriction of this amino acid or of homocysteine (a precursor of l-cysteine).
Since there is a clear role for microbiota in the pathogenesis of many GI disorders, investigations began into the possible interactions of H2S with luminal bacteria. Many questions have arisen from these investigations: Can H2S damage the intestinal epithelium and/or promote GI cancer? Does H2S affect mucus secretion and/or function? Does H2S modulate the microbiota? Does H2S affect the interactions between microbiota and the epithelium?
Several recent studies have provided evidence that exogenous and endogenous H2S can significantly reduce the susceptibility of the GI mucosa to injury, and these effects appear to be produced, at least in part, through modulation of the enteric microbiota (4, 5, 30). Adverse, dysbiosis-inducing effects of some commonly used drugs appear to contribute significantly to the detrimental effects of those drugs in the GI tract but can be markedly attenuated by coadministration of H2S. Indeed, a novel class of NSAIDs has been developed to exploit these beneficial effects of H2S in countering the damaging effects of conventional NSAIDs (7), which are among the most widely used drugs.
IS LUMINAL H2S TOXIC TO THE INTESTINAL EPITHELIUM?
H2S is produced by a wide range of enteric bacteria, primarily of the γ-Proteobacteria genera [readers are referred to a comprehensive review by Linden (25)]. H2S concentrations in the cecum and rectum can reach 40 µM (36), and as much as 250 µM in the colon (1). There have been a wide range of studies using different types of transformed or nontransformed epithelial cells to determine their responsiveness to a range of concentrations of H2S, including concentrations well above 250 µM (25). Not surprisingly, there are discrepant findings from these studies, with no clear and consistent evidence for toxic effects of H2S on epithelial cell integrity and considerable variability in terms of the effects of H2S on epithelial cell proliferation (25). Linden (25) noted that H2S (250 µM) could increase DNA damage in colon cancer cells in vitro, but only when DNA repair was inhibited (1). Indeed, there are numerous studies demonstrating antiproliferative and chemopreventive effects of H2S in vitro and in vivo (9, 24, 32, 33, 46).
There has been speculation of potential links between bacterially derived H2S and inflammatory bowel disease (31). These may include genotoxic properties and the disruption of the mucus structure. In our laboratory, we have done extensive studies in which H2S donors were administered to rodents or dogs for periods of up to 2 wk but have not observed any evidence of tissue injury. Even with twice-daily administration of a garlic-derived H2S donor [diallyl disulfide (DADS)] for 5 days at a dose as high as 60 mmol/kg, there was no macroscopically or histologically detectable damage to the gastrointestinal epithelium, nor was there any mucosal inflammation (5). On the contrary, with administration of H2S in this fashion, the normal structure of mucus and microbiota was maintained (30). Benavides et al. (3) demonstrated that when they added DADS to a solution of glutathione, to mimic in vivo conditions, ~50% was rapidly converted to H2S.
On the other hand, a recent study of pediatric Crohn’s disease provided compelling evidence for a role of H2S in promoting damage in a subset of patients who had a genetic defect in mitochondrial oxidation of H2S (31). Interestingly, those patients also had substantially increased numbers of H2S-producing bacteria in their intestinal lumen (31). We observed a similar increase in H2S-producing intestinal bacteria in rat studies of the exacerbation of NSAID-enteropathy by proton pump inhibitors, which significantly worsened NSAID-induced enteropathy (42). Our microbiome analysis revealed that a major effect of both omeprazole and lansoprazole was to significantly increase the numbers of γ-Proteobacteria in the small intestine (4).
Several studies in recent years have clearly demonstrated that H2S is an important metabolic fuel for the epithelial cells that line the GI tract (17, 29). Colonic epithelial cells are particularly efficient in oxidizing H2S, producing ATP in the process (17, 29). The epithelium therefore functions both as a physical barrier against potentially harmful agents that might pass into the body from the lumen of the gut, as well as a metabolic barrier, oxidizing bacteria-derived H2S (41). A defect in this metabolic barrier function is what was observed in the pediatric Crohn’s disease patients mentioned in the preceding paragraph (31), which would allow H2S from luminal bacteria to gain access to the lamina propria.
PROTECTIVE AND REPARATIVE EFFECTS OF H2S
H2S has been shown to exert protective effects against GI injury induced by ethanol, NSAIDs, and ischemia-reperfusion, as well as promoting resolution of inflammation and repair of tissue damage (41). For example, H2S donors have been shown to promote resolution of colitis in several animal models (14, 18, 23, 43). Endogenous H2S production is markedly elevated at sites of mucosal injury, contributing significantly to promotion of healing (40, 43). Moreover, rates of oxidation of H2S are specifically and substantially reduced at sites of epithelial/mucosal injury (14). On the other hand, inhibition of H2S synthesis leads to increased susceptibility to mucosal injury, impairment of healing, increased proinflammatory cytokine expression, reduced expression of cyclooxygenase-2 (COX-2; and an associated reduction of PG synthesis), and increased mucosal granulocyte numbers (5, 14, 30, 43).
The protective effects of H2S in the GI tract have also been demonstrated in numerous studies of novel H2S-releasing NSAIDs. These drugs have been shown to cause negligible GI damage despite markedly suppressing prostaglandin synthesis in a wide range of animal models (39, 40). While NSAIDs impair healing of gastric ulcers, H2S-releasing NSAIDs significantly accelerate healing, and this is mimicked by administration of H2S donors (39, 40).
H2S, MUCUS, AND BIOFILMS
Intestinal bacteria are thought to contribute significantly to the pathogenesis of inflammatory bowel disease (IBD; ulcerative colitis and Crohn’s disease; 28), but clear evidence for clinical benefits of antibiotics or probiotics for treating these conditions is lacking. A “leaky” mucus layer that can permit bacterial invasion has been reported in humans with ulcerative colitis, as well as in murine models of colitis (20). A recent mouse study reported that antibiotics caused a thinning of the mucus layer by directly reducing mucus granule numbers, an effect that predisposed mice to further enteric infection with Citrobacter rodentium (48).
In nature, bacteria form biofilms that reduce their exposure to drugs and immune components (6). They are complex, multispecies bacterial colonies encapsulated in a self-secreted matrix of polysaccharides (10, 16). There is evidence that microbiota living as biofilms promote gut homeostasis (27, 37). We attempted to determine the changes in biofilms that occur during experimental colitis and the impact of reduced endogenous H2S synthesis vs. administering exogenous H2S (30). Fluorescent in situ hybridization (FISH) was used to examine the organization of gut microbiota in health and during colitis in rats and the role that H2S might play in modulating biofilm formation and stability.
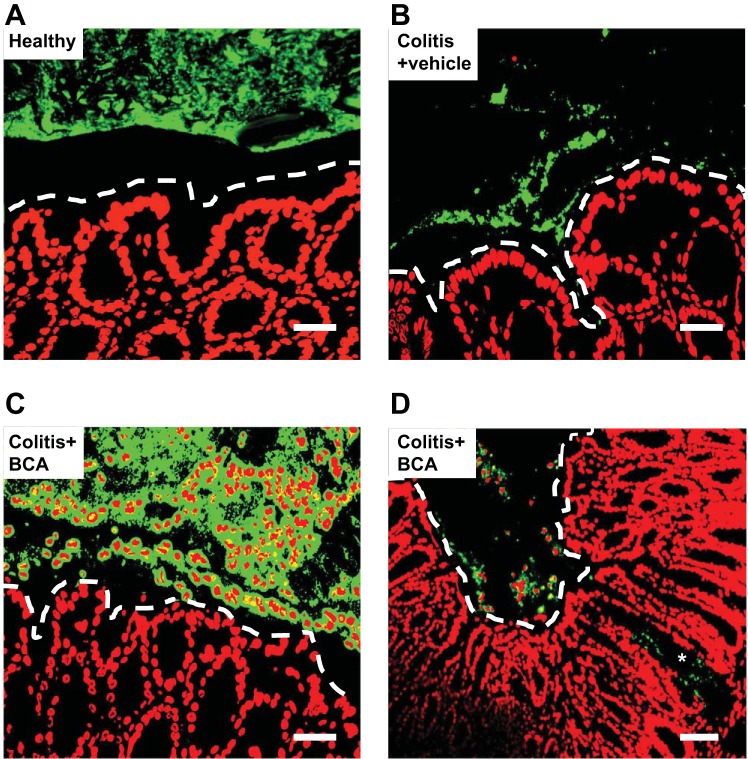
In both mice and rats, the microbiota is sandwiched between the sterile mucus layer and the luminal fecal content in a linear structure (Fig. 1). Within a week of induction of colitis with a hapten, the biofilm was severely disrupted in both mice and rats (30). The microbiota was disorganized and heterogeneous, with different size clusters of bacteria. Fragments of these dysbiotic microbiota biofilms were also in close contact with the host tissue, and there was clear evidence of translocation of bacteria into the lamina propria, in a manner reminiscent of what was recently reported in the intestine of patients with IBD (37). Extensive depletion of epithelial mucus granules was also evident.
Fig. 1.
Administration of an inhibitor of endogenous H2S synthesis in healthy mice and mice with hapten-induced colitis exacerbates inflammation and markedly alters the intestinal microbiota biofilm. Fluorescent in situ hybridization (FISH) was performed on colonic sections from C57BL/6 mice 7 days after induction of colitis with dinitrobenzene sulfonic acid. Representative images (from a total of 5 mice per group) are shown for healthy controls (A), colitis treated daily with vehicle (B), and colitis treated daily with β-cyanoalanine (BCA; 50 mg/kg; C and D). BCA is an inhibitor of H2S synthesis. Host nuclei are colored red while FISH-positive cells are green. Scale bars represent 25 µM (A and C) or 50 µM (B and D). Dashed lines indicate the limit of the mucosa. The asterisk denotes translocated bacteria in the lamina propria. DADS, diallyl disulfide. [From Motta et al. (30).]
Experiments with mice deficient of one of the key enzymes for synthesis of H2S, cystathionine γ-lyase (CSE), provided the initial indication that endogenous H2S played a role in regulating epithelial mucus production (30). In contrast to what was observed in wild-type mice, the CSE-deficient mice exhibited mild colonic inflammation with a thinner-than-normal inner mucus layer. Despite evidence of a linear biofilm, there were bacterial aggregates in close contact with the epithelium. Very similar effects were seen in rats treated with an inhibitor of CSE activity [β-cyanoalanine (BCA)]: the biofilm was fragmented, with bacteria in close contact with the colonic epithelium, and granulocytes were present within the biofilm and the lumen (Fig. 1). The depletion of epithelial mucus granules was much more evident in rats treated with the H2S synthesis inhibitor than in vehicle-treated rats. Taken together, the experiments with CSE-deficient mice and with rats treated with a CSE inhibitor suggest that CSE-derived H2S makes important contributions to the promotion of colonic microbiota biofilm formation and stability and contributes to enhanced mucus barrier function and epithelial integrity (30).
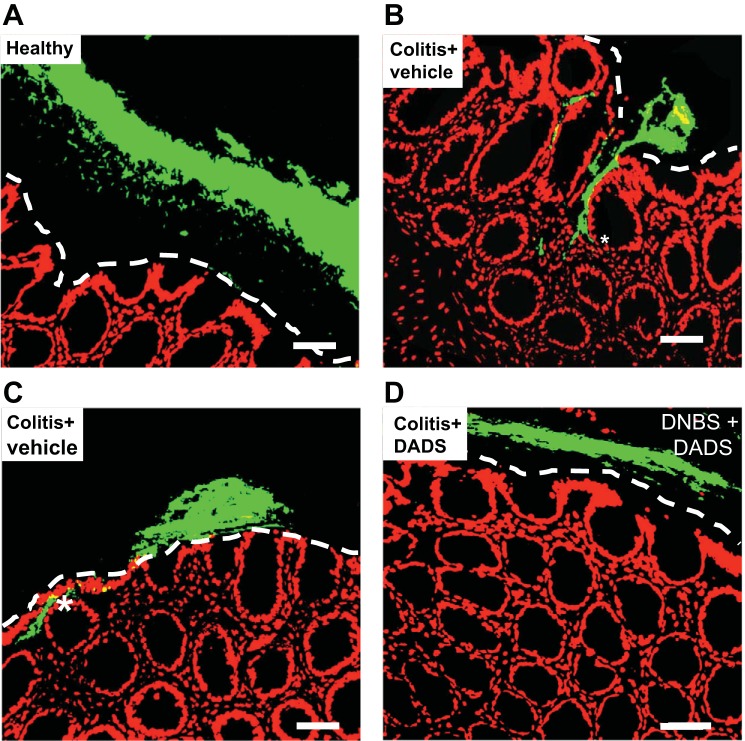
Additional evidence to support this hypothesis comes from studies of the effects of an H2S donor in experimental colitis. In rats with colitis, intracolonic administration of DADS (twice daily) for 1 wk resulted in a significant acceleration of the resolution of colitis, which included acceleration of the restoration of linear biofilm organization and reduced bacterial translocation (30). After DADS treatment, a clear mucus layer separated the epithelium from the microbiota biofilm (as observed in healthy controls; Fig. 2). When treatment with DADS was extended from 7 to 14 days, the mucus granule number per intestinal crypt increased significantly, to a level greater than that in healthy controls.
Fig. 2.
Administration of an H2S donor, diallyl disulfide (DADS), restored the biofilm in rats with hapten-induced colitis. After induction of colitis, groups of 4–5 rats each were treated daily with DADS (0.5 ml of 30 μM) or vehicle for 7 days. Fluorescent in situ hybridization was performed on colonic sections from the rats. The images shown are representative of what was observed in all rats receiving the treatments: healthy controls (A) and colitis treated daily with vehicle (B and C) or DADS (D). Scale bars represent 50 µM. Dashed lines indicate the limit of the mucosa. The asterisks denote translocated bacteria in the lamina propria. DNBS, dinitrobenzene sulfonic acid. [From Motta et al. (30).]
To further investigate the concept that H2S can enhance formation of biofilms, in vitro studies were performed with human-derived intestinal biofilms (30). These biofilms were exposed to various concentrations of H2S donors (NaHS or DADS). At concentrations of 1 and 10 µM, exposure to either of the H2S donors resulted in a higher metabolic activity (a reflection of increased numbers of bacteria in the biofilm) and increased biomass (likely due to increased quantity of cells and proteins within the biofilm).
CAN H2S MODIFY THE MICROBIOTA?
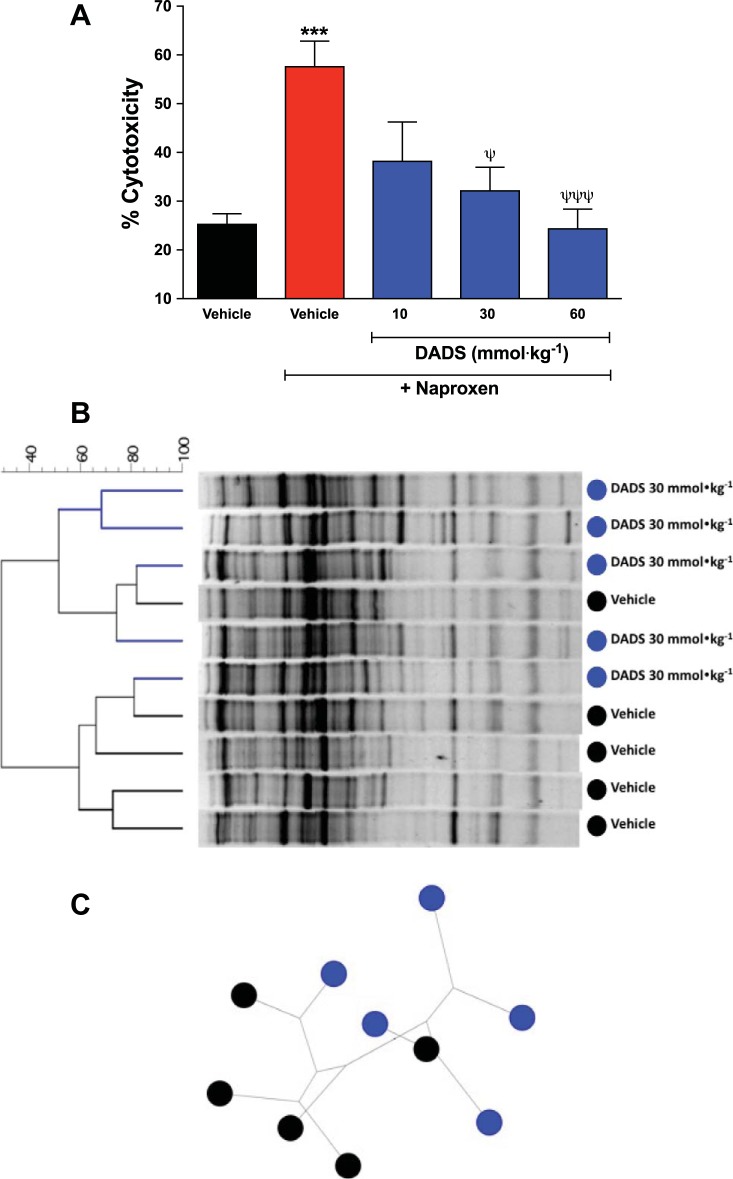
H2S donors derived from garlic have been shown to exert antimicrobial effects on planktonic gram-positive and gram-negative bacteria (12, 26, 34). We examined the effects of twice-daily administration of an H2S donor (DADS) to rats at doses of 10 or 30 mmol/kg. These doses of DADS were ineffective and effective, respectively, in reducing the severity of NSAID-induced damage in the rat stomach (21). We observed that administration of the higher doses of DADS resulted in significant shifts in the intestinal microbiota in rats (demonstrated via DNA extraction and denaturing gradient gel electrophoresis; Fig. 3). In the rats treated with the lower dose of DADS, the microbiota remained similar to that in vehicle-treated rats. None of the doses of DADS significantly changed the total numbers of aerobic or anaerobic bacteria in the jejunum (17).
Fig. 3.
A: naproxen treatment markedly increased the cytotoxic effects of bile on intestinal epithelial cells (IEC-6; ***P < 0.001). Bile collected from rats that had been cotreated with naproxen and an H2S donor [diallyl disulfide (DADS)] exhibited reduced cytotoxicity, in a dose-dependent manner (ψP < 0.05; ψψψP < 0.001). The rats were treated orally twice daily for 2 days with naproxen at 20 mg/kg and with vehicle or DADS. B: denaturing gradient gel electrophoresis analysis of intestinal microbiota samples from rats treated with naproxen (20 mg/kg) plus vehicle or DADS (30 mmol/kg). Treatment with DADS (blue) caused a marked shift in microbiota relative to vehicle-treated rats (black). C: using a resampling technique (majority unweighted-pair-group method with arithmetic mean algorithm), the dendrogram clustering observed in B was confirmed, indicating a robust difference in microbiota composition between groups (black, vehicle-treated rats; blue, DADs-treated rats). Each group consisted of five rats. Data were analyzed with Dunnett’s multiple-comparison test (cytotoxicity). [From Blackler et al. (5).]
Significant changes in the intestinal microbiota following administration of NSAIDs are well documented (45). Generally, NSAIDs given at doses that can cause significant enteropathy cause a shift in the microbiome toward more gram-negative organisms (38), and this contributes significantly to intestinal damage through TNF-α- and toll-like receptor 4 (TLR4)-dependent pathways (47). In contrast, administration of an H2S-releasing NSAID to rats resulted in significantly smaller shifts in the intestinal microbiome (4). It is not clear whether this is the cause or an effect of the greatly reduced intestinal injury with H2S-NSAID administration vs. conventional NSAID administration, but the observations from studies of other H2S donors suggest the former. One of the observed changes that may contribute significantly to the reduced intestinal damage observed with H2S-NSAIDs is a marked decrease in the cytotoxicity of bile compared with that from rats treated with a conventional NSAID (4). Secondary bile acids are much more cytotoxic than primary bile acids, and the conversion of primary to secondary bile acids is driven by bacterial enzymes (2). As shown in Fig. 3, a similar reduction in the cytotoxicity of bile was observed when rats were treated with an H2S donor at a dose that also caused a marked shift in intestinal microbiota (5).
SUMMARY AND FUTURE DIRECTIONS
There is substantial evidence that H2S, produced by enteric bacteria or host cells or administered exogenously, can affect intestinal microbiota in a variety of ways. As well as reducing GI inflammation and injury and promoting repair, exogenous and endogenous H2S can positively affect many aspects of bacterial-epithelial interactions. There is evidence suggesting potential for the use of H2S donors to favorably modulate the intestinal microbiota. Promotion of biofilm formation and integrity by H2S donors is an important aspect, particularly in the context of improved treatments for disorders such as ulcerative colitis and Crohn’s disease.
It is also becoming increasingly clear that in addition to probiotics and antibiotics, several classes of drugs can dramatically and rapidly alter the GI microbiota, including some of the most widely used drugs (e.g., NSAIDs, proton pump inhibitors, and histamine H2 receptor antagonists; 42). Studies in animals have provided proof-of-concept evidence that such detrimental changes can be reduced or prevented with H2S donors. Moreover, the linking of H2S-releasing groups to existing drugs is a promising approach to prevention of drug-induced dysbiosis and tissue injury, as has been demonstrated with the H2S-releasing NSAID, ATB-346 [2-(6-methoxynapthalen-2-yl)-propionic acid 4-thiocarbamoyl phenyl ester; 4, 5, 39].
GRANTS
J. L. Wallace’s research is supported by a grant from the Canadian Institutes of Health Research. A. G. Buret’s research is supported by grants from the National Science and Engineering Research Council and Crohn's Colitis Canada. J.-P. Motta was supported by an Alberta Innovates-Health Solutions Postdoctoral Fellowship and an AgreenSkills fellowship from the European Union’s Seventh Framework Programme under Grant Agreement FP7-609398.
DISCLOSURES
J. L. Wallace and A. G. Buret are founders of Antibe Therapeutics Incorporated, which is developing hydrogen sulfide-releasing anti-inflammatory drugs.
AUTHOR CONTRIBUTIONS
J.L.W., J.-P.M., and A.G.B. conceived and designed research; J.L.W., J.-P.M., and A.G.B. analyzed data; J.L.W., J.-P.M., and A.G.B. interpreted results of experiments; J.L.W., J.-P.M., and A.G.B. prepared figures; J.L.W. and A.G.B. drafted manuscript; J.L.W., J.-P.M., and A.G.B. edited and revised manuscript; J.L.W., J.-P.M., and A.G.B. approved final version of manuscript; J.-P.M. performed experiments.
REFERENCES
- 1.Attene-Ramos MS, Wagner ED, Plewa MJ, Gaskings HR. Evidence that hydrogen sulfide is a genotoxic agent. Mol Cancer Res 5: 455–459, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Begley M, Gahan CGM, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev 29: 625–651, 2005. doi: 10.1016/j.femsre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, Patel RP, Darley-Usmar VM, Doeller JE, Kraus DW. Hydrogen sulfide mediates the vasoactivity of garlic. Proc Natl Acad Sci USA 104: 17977–17982, 2007. doi: 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackler RW, De Palma G, Manko A, Da Silva GJ, Flannigan KL, Bercik P, Surette MG, Buret AG, Wallace JL. Deciphering the pathogenesis of NSAID enteropathy using proton pump inhibitors and a hydrogen sulfide-releasing NSAID. Am J Physiol Gastrointest Liver Physiol 308: G994–G1003, 2015. doi: 10.1152/ajpgi.00066.2015. [DOI] [PubMed] [Google Scholar]
- 5.Blackler RW, Motta JP, Manko A, Workentine M, Bercik P, Surette MG, Wallace JL. Hydrogen sulphide protects against NSAID-enteropathy through modulation of bile and the microbiota. Br J Pharmacol 172: 992–1004, 2015. doi: 10.1111/bph.12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buret AG, Reti K. Acute enteric infections alter commensal microbiota: new mechanisms in post-infectious intestinal inflammatory disorders. In: Old Herborn University Seminar Monograph. Persisting Consequences of Intestinal Infection, edited by Heidt PJ, Rusch V, Walker RI, Lange D, and Riddle MS. Herborn, Germany: Old Herborn University, 2014, vol. 27, p. 87–100. [Google Scholar]
- 7.Caliendo G, Cirino G, Santagada V, Wallace JL. Synthesis and biological effects of hydrogen sulfide (H2S): development of H2S-releasing drugs as pharmaceuticals. J Med Chem 53: 6275–6286, 2010. doi: 10.1021/jm901638j. [DOI] [PubMed] [Google Scholar]
- 8.Cenac N, Castro M, Desormeaux C, Colin P, Sie M, Ranger M, Vergnolle N. A novel orally administered trimebutine compound (GIC-1001) is anti-nociceptive and features peripheral opioid agonistic activity and hydrogen sulphide-releasing capacity in mice. Eur J Pain 20: 723–730, 2016. doi: 10.1002/ejp.798. [DOI] [PubMed] [Google Scholar]
- 9.Chattopadhyay M, Kodela R, Nath N, Dastagirzada YM, Velázquez-Martínez CA, Boring D, Kashfi K. Hydrogen sulfide-releasing NSAIDs inhibit the growth of human cancer cells: a general property and evidence of a tissue type-independent effect. Biochem Pharmacol 83: 715–722, 2012. doi: 10.1016/j.bcp.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 284: 1318–1322, 1999. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 11.Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Antonelli E, Roviezzo F, Morelli A, Cirino G, Wallace JL, Fiorucci S. Evidence that hydrogen sulfide exerts antinociceptive effects in the gastrointestinal tract by activating KATP channels. J Pharmacol Exp Ther 316: 325–335, 2006. doi: 10.1124/jpet.105.091595. [DOI] [PubMed] [Google Scholar]
- 12.Feng S, Eucker TP, Holly MK, Konkel ME, Lu X, Wang S. Investigating the responses of Cronobacter sakazakii to garlic-drived organosulfur compounds: a systematic study of pathogenic-bacterium injury by use of high-throughput whole-transcriptome sequencing and confocal micro-Raman spectroscopy. Appl Environ Microbiol 80: 959–971, 2014. doi: 10.1128/AEM.03460-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiorucci S, Antonelli E, Distrutti E, Rizzo G, Mencarelli A, Orlandi S, Zanardo R, Renga B, Di Sante M, Morelli A, Cirino G, Wallace JL. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology 129: 1210–1224, 2005. doi: 10.1053/j.gastro.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 14.Flannigan KL, Ferraz JG, Wang R, Wallace JL. Enhanced synthesis and diminished degradation of hydrogen sulfide in experimental colitis: a site-specific, pro-resolution mechanism. PLoS One 8: e71962, 2013. doi: 10.1371/journal.pone.0071962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flannigan KL, McCoy KD, Wallace JL. Eukaryotic and prokaryotic contributions to colonic hydrogen sulfide synthesis. Am J Physiol Gastrointest Liver Physiol 301: G188–G193, 2011. doi: 10.1152/ajpgi.00105.2011. [DOI] [PubMed] [Google Scholar]
- 16.Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol 8: 623–633, 2010. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 17.Goubern M, Andriamihaja M, Nübel T, Blachier F, Bouillaud F. Sulfide, the first inorganic substrate for human cells. FASEB J 21: 1699–1706, 2007. doi: 10.1096/fj.06-7407com. [DOI] [PubMed] [Google Scholar]
- 18.Hirata I, Naito Y, Takagi T, Mizushima K, Suzuki T, Omatsu T, Handa O, Ichikawa H, Ueda H, Yoshikawa T. Endogenous hydrogen sulfide is an anti-inflammatory molecule in dextran sodium sulfate-induced colitis in mice. Dig Dis Sci 56: 1379–1386, 2011. doi: 10.1007/s10620-010-1461-5. [DOI] [PubMed] [Google Scholar]
- 19.Ise F, Takasuka H, Hayashi S, Takahashi K, Koyama M, Aihara E, Takeuchi K. Stimulation of duodenal secretion by hydrogen sulphide in rats: relation to prostaglandins, nitric oxide and sensory neurones. Acta Physiol (Oxf) 201: 117–126, 2011. doi: 10.1111/j.1748-1716.2010.02152.x. [DOI] [PubMed] [Google Scholar]
- 20.Johansson ME, Gustafsson JK, Holmén-Larsson J, Jabbar KS, Xia L, Xu H, Ghishan FK, Carvalho FA, Gewirtz AT, Sjövall H, Hansson GC. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 63: 281–291, 2014. doi: 10.1136/gutjnl-2012-303207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kashfi K, Olson KR. Biology and therapeutic potential of hydrogen sulfide and hydrogen sulfide-releasing chimeras. Biochem Pharmacol 85: 689–703, 2013. doi: 10.1016/j.bcp.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimura H, Shibuya N, Kimura Y. Hydrogen sulfide is a signaling molecule and a cytoprotectant. Antioxid Redox Signal 17: 45–57, 2012. doi: 10.1089/ars.2011.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HJ, Lee HG, Choi KS, Surh YJ, Na HK. Diallyl trisulfide suppresses dextran sodium sulfate-induced mouse colitis: NF-κB and STAT3 as potential targets. Biochem Biophys Res Commun 437: 267–273, 2013. doi: 10.1016/j.bbrc.2013.06.064. [DOI] [PubMed] [Google Scholar]
- 24.Lee ZW, Zhou J, Chen CS, Zhao Y, Tan CH, Li L, Moore PK, Deng LW. The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLoS One 6: e21077, 2011. doi: 10.1371/journal.pone.0021077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linden DR. Hydrogen sulfide signaling in the gastrointestinal tract. Antioxid Redox Signal 20: 818–830, 2014. doi: 10.1089/ars.2013.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu X, Rasco BA, Jabal JM, Aston DE, Lin M, Konkel ME. Investigating antibacterial effects of garlic (Allium sativum) concentrate and garlic-derived organosulfur compounds on Campylobacter jejuni by using Fourier transform infrared spectroscopy, Raman spectroscopy, and electron microscopy. Appl Environ Microbiol 77: 5257–5269, 2011. doi: 10.1128/AEM.02845-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macfarlane S, McBain AJ, Macfarlane GT. Consequences of biofilm and sessile growth in the large intestine. Adv Dent Res 11: 59–68, 1997. doi: 10.1177/08959374970110011801. [DOI] [PubMed] [Google Scholar]
- 28.Macpherson A, Khoo UY, Forgacs I, Philpott-Howard J, Bjarnason I. Mucosal antibodies in inflammatory bowel disease are directed against intestinal bacteria. Gut 38: 365–375, 1996. doi: 10.1136/gut.38.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mimoun S, Andriamihaja M, Chaumontet C, Atanasiu C, Benamouzig R, Blouin JM, Tomé D, Bouillaud F, Blachier F. Detoxification of H(2)S by differentiated colonic epithelial cells: implication of the sulfide oxidizing unit and of the cell respiratory capacity. Antioxid Redox Signal 17: 1–10, 2012. doi: 10.1089/ars.2011.4186. [DOI] [PubMed] [Google Scholar]
- 30.Motta JP, Flannigan KL, Agbor TA, Beatty JK, Blackler RW, Workentine ML, Da Silva GJ, Wang R, Buret AG, Wallace JL. Hydrogen sulfide protects from colitis and restores intestinal microbiota biofilm and mucus production. Inflamm Bowel Dis 21: 1006–1017, 2015. doi: 10.1097/MIB.0000000000000345. [DOI] [PubMed] [Google Scholar]
- 31.Mottawea W, Chiang CK, Mühlbauer M, Starr AE, Butcher J, Abujamel T, Deeke SA, Brandel A, Zhou H, Shokralla S, Hajibabaei M, Singleton R, Benchimol EI, Jobin C, Mack DR, Figeys D, Stintzi A. Altered intestinal microbiota-host mitochondria crosstalk in new onset Crohn’s disease. Nat Commun 7: 13419, 2016. doi: 10.1038/ncomms13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagabhushan M, Line D, Polverini PJ, Solt DB. Anticarcinogenic action of diallyl sulfide in hamster buccal pouch and forestomach. Cancer Lett 66: 207–216, 1992. doi: 10.1016/0304-3835(92)90249-U. [DOI] [PubMed] [Google Scholar]
- 33.Paul-Clark M, Elsheikh W, Kirkby N, Chan M, Devchand P, Agbor TA, Flannigan KL, Cheadle C, Freydin M, Ianaro A, Mitchell JA, Wallace JL. Profound chemopreventative effects of a hydrogen sulfide-releasing NSAID in the APCMin/+ mouse model of intestinal tumorigenesis. PLoS One 11: e0147289, 2016. doi: 10.1371/journal.pone.0147289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ross ZM, O’Gara EA, Hill DJ, Sleightholme HV, Maslin DJ. Antimicrobial properties of garlic oil against human enteric bacteria: evaluation of methodologies and comparisons with garlic oil sulfides and garlic powder. Appl Environ Microbiol 67: 475–480, 2001. doi: 10.1128/AEM.67.1.475-480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen X, Carlström M, Borniquel S, Jädert C, Kevil CG, Lundberg JO. Microbial regulation of host hydrogen sulfide bioavailability and metabolism. Free Radic Biol Med 60: 195–200, 2013. doi: 10.1016/j.freeradbiomed.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suarez F, Furne J, Springfield J, Levitt M. Production and elimination of sulfur-containing gases in the rat colon. Am J Physiol Gastrointest Liver Physiol 274: G727–G733, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol 43: 3380–3389, 2005. doi: 10.1128/JCM.43.7.3380-3389.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uejima M, Kinouchi T, Kataoka K, Hiraoka I, Ohnishi Y. Role of intestinal bacteria in ileal ulcer formation in rats treated with a nonsteroidal antiinflammatory drug. Microbiol Immunol 40: 553–560, 1996. doi: 10.1111/j.1348-0421.1996.tb01108.x. [DOI] [PubMed] [Google Scholar]
- 39.Wallace JL, Caliendo G, Santagada V, Cirino G. Markedly reduced toxicity of a hydrogen sulphide-releasing derivative of naproxen (ATB-346). Br J Pharmacol 159: 1236–1246, 2010. doi: 10.1111/j.1476-5381.2009.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wallace JL, Dicay M, McKnight W, Martin GR. Hydrogen sulfide enhances ulcer healing in rats. FASEB J 21: 4070–4076, 2007. doi: 10.1096/fj.07-8669com. [DOI] [PubMed] [Google Scholar]
- 41.Wallace JL, Ferraz JG, Muscara MN. Hydrogen sulfide: an endogenous mediator of resolution of inflammation and injury. Antioxid Redox Signal 17: 58–67, 2012. doi: 10.1089/ars.2011.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wallace JL, Syer S, Denou E, de Palma G, Vong L, McKnight W, Jury J, Bolla M, Bercik P, Collins SM, Verdu E, Ongini E. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology 141: 1314–1322.e5, 2011. doi: 10.1053/j.gastro.2011.06.075. [DOI] [PubMed] [Google Scholar]
- 43.Wallace JL, Vong L, McKnight W, Dicay M, Martin GR. Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology 137: 569–578.e1, 2009. doi: 10.1053/j.gastro.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 44.Wallace JL, Wang R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat Rev Drug Discov 14: 329–345, 2015. doi: 10.1038/nrd4433. [DOI] [PubMed] [Google Scholar]
- 45.Wallace JL. Mechanisms, prevention and clinical implications of nonsteroidal anti-inflammatory drug-enteropathy. World J Gastroenterol 19: 1861–1876, 2013. doi: 10.3748/wjg.v19.i12.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wargovich MJ, Woods C, Eng VW, Stephens LC, Gray K. Chemoprevention of N-nitrosomethylbenzylamine-induced esophageal cancer in rats by the naturally occurring thioether, diallyl sulfide. Cancer Res 48: 6872–6875, 1988. [PubMed] [Google Scholar]
- 47.Watanabe T, Higuchi K, Kobata A, Nishio H, Tanigawa T, Shiba M, Tominaga K, Fujiwara Y, Oshitani N, Asahara T, Nomoto K, Takeuchi K, Arakawa T. Non-steroidal anti-inflammatory drug-induced small intestinal damage is Toll-like receptor 4 dependent. Gut 57: 181–187, 2008. doi: 10.1136/gut.2007.125963. [DOI] [PubMed] [Google Scholar]
- 48.Wlodarska M, Willing B, Keeney KM, Menendez A, Bergstrom KS, Gill N, Russell SL, Vallance BA, Finlay BB. Antibiotic treatment alters the colonic mucus layer and predisposes the host to exacerbated Citrobacter rodentium-induced colitis. Infect Immun 79: 1536–1545, 2011. doi: 10.1128/IAI.01104-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J 20: 2118–2120, 2006. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]