Abstract
Inflammatory bowel diseases (IBD) are chronic inflammatory gastrointestinal diseases, primarily consisting of ulcerative colitis and Crohn’s disease. The complex nature of the disease, as well as the limited therapeutic options characterized by low efficiency and major side effects, highlights the importance of developing novel strategies of therapeutic intervention in IBD. Susceptibility loci related to IBD are present only in a small percentage of IBD patients, implying that epigenetic modifications could influence the pathogenesis of the disease. MicroRNAs (miRNAs) are small noncoding RNAs that regulate multiple molecular pathways involved in IBD pathobiology. MiRNA inhibitors targeting the IBD-activated miRNAs could have therapeutic value for IBD patients. This review provides an overview of the recent advances in miRNA biology related to IBD pathogenesis and the pharmacological development of miRNA-based therapeutics.
Keywords: antisense therapeutics, chemical modifications, inflammatory bowel disease, microRNA
INTRODUCTION
Inflammatory bowel disease (IBD) is a dynamic and multifactorial disease derived from alterations in the immune component, bacteria, genes, and environmental factors. Interestingly, IBD has had an increased incidence in the last few decades, particularly in developed countries (40), proposing the contribution of environmental and epigenetic alterations in IBD pathogenesis. Even though genome-wide association studies (GWAS) revealed 163 IBD susceptibility loci, the variance in disease risk remains poorly explained (22). Of note, maximal association signals are confined to noncoding genetic variation, while GWAS signals are significantly enriched with various epigenetic marks. In this regard, integrating gene expression with epigenetic data represents an improved tool for predicting IBD-related genes (37).
Epigenetics are heritable changes in gene activity and expression that occur without alteration in DNA sequence. DNA methylation, histone modifications, as well as noncoding RNAs, represent reversible epigenetic alterations that may serve as potential targets for IBD drug development (42, 53). Differential DNA methylation patterns have been observed in IBD patients in comparison to controls and are associated with cell-type-specific modifications (55). Importantly, a global methylation profile characteristic of ileal Crohn’s Disease (CD) has been described by Nimmo et al. (41), highlighting altered methylation in immune activation-related genes. In addition, histone 4 (H4) acetylation was increased in colonic biopsies from CD patients, suggesting that histone acetylation inhibitors could have therapeutic potential for these patients. Furthermore, miRNA signatures have been identified in IBD patients, and specific miRNAs have been correlated with IBD-related inflammatory pathways (10). Additionally, a recent study identified a long noncoding RNA (lncRNA) signature deregulated in ulcerative colitis (UC) patients and detected IFNG-AS1 as a novel regulator of interferon-γ inflammatory responses (43). These data suggest the potential importance of noncoding RNAs on IBD pathobiology.
All of the above-mentioned epigenetic modifications are targetable by therapeutic applications. However, compared with the others, miRNAs have the clearest and most direct mechanism of action and predictability of consequence when they are inhibited or overexpressed (33). One of the first studies of miRNA targeting in vivo found that intravenous injection of anti-miRNA oligonucleotides caused a systemic reduction of the specific miRNAs targeted that was long-lasting (25). Since then, many laboratories have studied the efficacy of targeting specific miRNAs in different disease contexts and have observed a variety of functional effects, resulting from the manipulation of miRNA expression, suggesting that the latter has a high potential for therapeutic applications.
The widespread impact of miRNAs on IBD-associated signaling pathways and biological functions provides the basis to investigate miRNA-based therapeutics for IBD patients. The focus of this review is to study the therapeutic potential of miRNA inhibitors for IBD patients. In the current state, IBD treatment exerts only limited efficacy and significant side effects. Thus, new approaches to therapeutic interventions in IBD are essential (14). The emerging study of the functions, targets, and therapeutic potential of different miRNAs is essential to develop novel drugs and biomarkers of drug response for IBD patients.
GENESIS OF miRNAS
MiRNAs are small (16–22 nucleotides), noncoding RNAs that are intronically, exonically, or intergenically located throughout the human genome. MiRNA biogenesis is a multistep process; initially, the nuclear primary miRNA transcripts are cleaved by the RNAse-3-type enzyme Drosha, resulting in stem-loop structured pre-miRNAs. Nuclear export and cleavage of the hairpin loop occur through Dicer. The remaining duplex is loaded in Argonaute, forming the RISC (RNA-induced silencing complex) complex. The guide strand leads the RISC to the mRNA target that is complementary in the 3′ untranslated region (3′UTR), leading to instability and inhibition of translation (4). The ability to target specific genes renders miRNAs as important regulators of gene expression (5). Aberrant expression of miRNAs in IBD has contributed to the hypothesis that miRNAs are mediators of the inflammatory response in IBD, consisting of novel potential therapeutic targets (48, 64).
IBD COLONIC TISSUE microRNA SIGNATURES
Numerous studies in the past 10 years have examined miRNA expression profiles in tissues derived from IBD patients (49). Comprehensive microarray profiling and/or quantitative PCR have been used to assess the differential miRNA expression levels in CD, UC, and non-IBD subjects. Different studies attempting to elucidate miRNA profiles in UC and/or CD often compare miRNA levels in different pairings, such as active vs. inactive disease, UC or CD samples vs. normal controls, or UC vs. CD samples, thus giving rise to variability in the results from different groups of the same tissue type. Concisely, studies have indicated a significant decrease in the levels of miR-124 (23), miR-4284 (24), miR-320 (45), miR-141 (19), miR-193a-3p (13), miR-200b (12), miR-10a (61), miR-19a (11), and miR-192 (60). Additionally, studies have specified increase in the levels of miR-21 (54), miR-301a (17), miR-665 (31), miR-155 (39), miR-133a (26), miR-210 (3), miR-31 (52), miR-124 (69), miR-223 (57), miR-214 (46), and miR-206 (62) in IBD patients. Throughout our review, we place emphasis on describing the role of miRNAs that have been functionally linked to IBD pathogenesis.
MiR-21, is elevated in both UC (54) and CD (59) patients and has been implicated in different proinflammatory functions, such as modulation of the T-cell responses (2) and attenuation of tight junction integrity (66). It should be underlined that knockout of miR-21 reduced the inflammatory responses and improved the survival rate in a dextran sodium sulfate (DSS)-induced fatal colitis mouse model (51). Furthermore, it has been shown that miR-31 is upregulated in both CD and UC patient colon tissue (50) and directly targets IL-25. Ultimately, the miRNA-31/IL-25 pathway is affecting Th1/Th17-mediated inflammatory responses in mouse colitis models (52). miR-141 was found decreased in both UC (8) and CD (19) patient colon tissue. It directly targets C-X-C motif chemokine (CXCL)5 and CXCL12β, and its downregulation in IBD appears to allow CXCL5 and CXCL12β to facilitate leukocyte recruitment and promote an inflammatory response. These data suggest that miR-21, miR-31, and miR-141 have a strong impact on both UC and CD pathogenesis and may serve as valuable targets for therapeutic intervention. These three miRNAs exemplify the functional significance of miRNAs’ dysregulation in IBD and illustrate the necessity for expanded research on the causal role of miRNA differential expression to specific disease-related phenotypes.
It is important to note that miRNA profiles vary between whole colonic tissues and isolated cell types of the colon. Seeing as many miRNA-centric IBD drugs tested in mice are administered intracolonically, these differences pose a limitation when assessing the specific effect of a therapy in vitro, as there is always the potential of non-cell-specific unforeseen side effects. However, in the grand scheme of an entire organism, direct injection of the synthetic oligonucleotides into the colon limits systemic side effects that could potentially eliminate therapeutic effectiveness and/or cause harmful consequences (6, 36).
IBD BLOOD microRNA SIGNATURES
Since miRNA signature identification in colonic tissues involves an invasive and costly diagnostic method, research has recently focused on the detection of serum or plasma circulating miRNA panels that could differentiate UC from CD and evaluate the therapeutic effectiveness of the current IBD drugs. Although there are few studies that have evaluated blood miRNAs in IBD patients, differential expression levels have been found in UC or CD serum samples (20, 47, 58), suggesting that circulating miRNAs in peripheral blood represent valuable diagnostic and/or therapeutic approaches for IBD treatment.
In support of this concept, various studies have described the impact of exosomal miRNAs on various disease pathologies, including IBD (44, 65, 67). Importantly, as reported by Yang et al. (65), improvement of 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colon fibrosis has been achieved through tissue-selective and vesicle-dependent transfer of miR-220b. Stability via binding to Argonaute proteins and the ability of encapsulation by exosomes confer important advantages on using serum miRNAs for IBD diagnostics.
A recent study by Viennois et al. (56) points out that serum miRNA signatures hold promise to accurately distinguish UC from controls. MiR-29b-3p, miR-122-5p, miR-192-5p, miR-194-5p, miR-375-3p, miR-150-5p, and miR-146a-3p were upregulated in the serum of IL10−/− mice. Interestingly, these miRNAs correlated with the response to antitumor necrosis factor-α (TNF-α) therapy and predicted UC human samples with 83.3% accuracy. Additional data support the hypothesis that peripheral blood miRNA expression patterns may form the basis of future IBD diagnostics. Specifically, miR-28-5p, miR-103-2, miR-151-5p, miR-340, and miR-532-3p were found significantly increased in UC compared with CD patients, distinguishing active IBD subtypes from each other and healthy controls. Eight miRNAs—miR-28, miR-103–2*, miR-149*, miR-151, miR-340*, miR-505*, miR-532, and miR-plus-E1153—distinguished active UC from CD samples. MiRplus-E1153 also distinguishes healthy control from UC active samples (58). A more recent study revealed miR-146a, miR-21, and miR-31 to be downregulated, while miR-19a, miR-101, miR142-5p, miR-223, miR-375, and miR-494 were activated in blood samples from UC relative to control subjects (50).
MiRNA profiling has been performed by several groups to uncover dysregulation of specific miRNAs, both in CD vs. non-IBD controls, as well as in specific CD-associated complications. It has been shown that miR-199a-5p, miR-362-3p, and miR-532-3p levels are elevated in CD patients’ blood compared with controls, while miR-149 and miRplus-F1065 are downregulated (58). Furthermore, blood serum miRNA profiling in CD patients revealed miRNAs that correlate with stricture formation revealed selective downregulation of miR-19a and miR-19b in the serum of CD patients having strictures (30).
Collectively, deregulated miRNA signatures propose that miRNAs may contribute to IBD pathobiology. However, it is important to perform miRNA profiling studies in large cohorts of well-characterized clinical samples to identify robust blood miRNA diagnostic and/or prognostic signatures. Toward this direction, miRNA expression studies in serum from IBD patients enrolled in longitudinal studies are needed to support the feasibility of using a panel that provides high sensitivity and specificity.
CHEMICALLY MODIFIED miRNA INHIBITORS FOR IBD TREATMENT
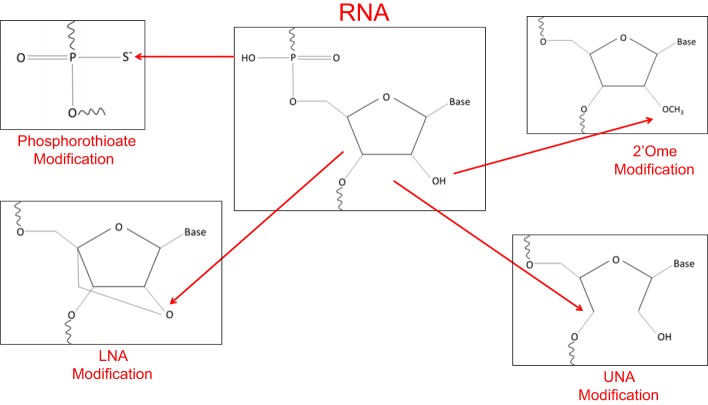
Recently, oligonucleotide-based strategies that block miRNA expression have been developed for therapeutic purposes in different human diseases. Antisense oligonucleotides (ASOs) can competitively block activated miRNAs in cancer, in a specific and efficient manner, resulting in activation of miRNA downstream effector genes (15). In an effort to strengthen the affinity for the target gene, confer nuclease resistance, achieve more selective and efficient cellular uptake, without activating the immune response, chemical modifications of anti-miRNA oligonucleotides (AMOs) are currently being developed (summarized in Fig. 1).
Fig. 1.
Schematic depiction of the relevant chemical modifications for in vivo application of anti-miRNA oligonucleotides.
AMOs are designed to have perfect complementarity with the miRNA, leading to firm binding and inactivation (27). However, DNA oligonucleotides are highly prone to degradation primarily due to the activity of nucleases, depending on the experimental context. To overcome this issue, the 2′-O-methyl RNA (2′-OMe) modification has been introduced, which has been shown to offer substantially higher binding affinity with RNAs, blocking nuclease degradation. However, 2′-OMe-modified RNAs are not completely resistant to nuclease degradation (38). On the other hand, phosphorothioate (PS) modifications in the backbone decrease the nuclease degradation activity, although simultaneously lowering binding affinity, which could be a drawback (29). As a next step, several groups constructed 2′-OMe PS-modified RNA oligonucleotides to block the exonuclease effects. Also, cholesterol was added to the 3′-end to facilitate delivery in animals; this chemical composition of AMO was called an “antagomir” and has been very effective to target tissues in mice without the use of any particles. However, complete PS modification of 2′-OMe oligonucleotides sufficiently lowers the melting temperature of the AMOs, such that potency is compromised (25, 35). The next major modification introduced was the use of locked nucleic acid (LNA) bases in AMOs. LNAs are bicyclic nucleic acids that “lock” the structure into a 3′-endo sugar conformation by tethering the 2′-O to the 4′-C via a methylene bridge. LNAs show increased hybridization affinity toward complementary single-stranded RNA molecules, thus offering increased nuclease resistance and conformational stability (27). Nonetheless, LNA bases may augment self-dimerization based on the context of the sequence, which can reduce AMO effectiveness. In contrast to LNA, which represents a highly rigid modification, unlocked nucleic acid, which lacks the C2′-C4′ bond normally found in ribonucleosides, is highly flexible, and can be used to modulate duplex characteristics (9).
Although the oligonucleotide technologies mentioned above have therapeutic potential, some synthetic oligonucleotides induce toxicities, due to either elicitation of an autoimmune response or specific chemical modifications causing issues. Although the 2′-OMe modification has little chemical toxicity (28), the risk of hepatotoxicity seems to apply to LNA ASOs (7). Future combinatorial studies will reinforce the efficacy and toxicity effects of all of these modifications for therapeutic purposes.
However, most of the chemically modified anti-miR oligonucleotides exhibit low tissue distribution when administered without a carrier. The factors contributing to decreased cellular uptake include 1) the drugs are taken up by the liver and kidney and rapidly excreted in urine, 2) biological instability in fluids or tissues, and 3) the negative charge preventing the compounds from entering cell membranes. To bypass the aforementioned hindrances, liposome-, microsphere-, hydrogel- and nanoparticle (polymer)-based methods are being explored (16, 32). Toward this direction, the development of viral vectors (1), engineered bacteria (63), or the concept of tagging nanoparticle-miRNA oligonucleotide complexes with antibodies that bind the desired target cell (68) could potentially offer a means for cell-specific delivery of miRNA therapies by minimizing off-target effects.
MicroRNA INHIBITORS IN MOUSE MODELS OF COLITIS
The previously referenced studies have clearly shown that miRNA deregulation is prevalent in IBD patients. The logical next step is to repair the erroneous miRNA expression by therapeutic increase or decrease, depending on the type of dysregulation. While miRNA inhibition is feasible through the chemical-modified oligonucleotides detailed in the previous section, miRNA replacement therapy is more challenging. Any type of synthetic miRNA mimic needs to be incorporated in a RISC complex to properly provide the biological function that was lost. With in vivo applications of synthetic miRNA oligonucleotides, complications lie in the correct delivery of the mimic without off-target side effects and potency of function before fast clearance. Furthermore, the potential of an inflammatory response deepens the difficulty of in vivo applications of miRNA replacement therapies (18). Thus, this review focuses on the potential of miRNA inhibitors as treatments for IBD and the different investigations related to their in vivo efficacy.
Mounting preclinical studies indicate potent activity of miRNA blockers in colitis mouse models (listed in Table 1), highlighting the therapeutic potential of anti-miRs in IBD. For animal studies, intracolonic injections have been performed as a means for topical delivery of miRNA inhibitors. As assessed by quantitative real-time-PCR, miR-301a expression levels were elevated in a TNBS-induced mouse colitis model compared with control mice, as well as in PBMCs and inflamed mucosal IBD tissues. Mechanistic studies revealed that miR-301a promotes Th17 cell differentiation through direct regulation of Smad Nuclear Interacting Protein 1. Furthermore, enema administration of a miR-301a inhibitor in TNBS-induced mice reduced the amount of proinflammatory cytokines in the inflamed colon (17). Interestingly, Law et al. (26) showed that colitis is regulated by miR-133α-afthiphilin direct interaction. Intracolonic administration of antisense-miR-133α before induction of TNBS- and DSS-induced colitis resulted in attenuation of colitis and inhibition of proinflammatory signals. Intracolonic LNA-anti-miR-210 administration also reduced colitis in response to TNBS-induced colitis in mice. Importantly, miR-210 expression was increased in tissue samples from UC patients. These findings provide evidence that miR-210 plays a proinflammatory role in the development of colitis (3). Alterations in miR-31 levels in the TNBS-induced colitis and IL-10 knockout mice could regulate the IL-12/23 pathway, resulting in improvement or worsening of colitis. Also, an IL-25 Ab blocked the therapeutic effects of the miR-31 inhibitor in mice (52). Inverse correlation between miR-124 and aryl hydrocarbon receptor (AHR) protein levels has been observed in colon tissues and intestinal epithelial cells of active CD patients. Notably, anti-miR-124 treatment of TNBS-induced colitis mice improved colitis scores, including decreased disease activity index and proinflammatory cytokine expression, through AHR modulation (69). We have previously shown that IL-6 upregulates STAT3-mediated transcription of miR-214 in colon tissues, resulting in the reduction of PDZ and LIM Domain 2 (PDLIM2) and Phosphatase and Tensin Homolog (PTEN) mRNA levels, increasing AKT phosphorylation, and activating NF-κB. To assess the therapeutic potential of miR-214 inhibition in UC, a chemically modified miR-214 inhibitor was developed; this inhibitor was highly efficient in suppressing miR-214 in human colonocytes in vitro and also reduced UC disease activity in DSS-induced experimental colitis. Collectively, in vitro, in vivo, and ex vivo data suggest the therapeutic potential of the miR-214 inhibitor in UC patients with active disease (46).
Table 1.
Summary of studies related to miRNA-based inhibition applied to in vivo mouse models of colitis
| miRNA | Gene Target(s) | Therapeutic Approach | Type of Administration | Mouse Model of Colitis | Biological Effect |
|---|---|---|---|---|---|
| miR-301a | SNIP1 | antisense oligonucleotide | intracolonic | TNBS | ↓ inflammatory response (17) |
| miR-665 | XBP1, ORMDL3 | antagomiR | intraperotineal injection | DSS | ↓ cell death and colitis severity score (31) |
| miR-155 | SHIP-1 | antagomiR | tail vein injection | DSS | ↓disease activity score (34) |
| miR-133a | AFTPH | LNA | intracolonic | DSS, TNBS | ↓ attenuated colitis development (26) |
| miR-210 | EFNA3 | LNA | intracolonic | TNBS | ↓ inflammatory response and colitis scores (3) |
| miR-31 | IL-25 | antisense oligonucleotide | intracolonic | TNBS | ↓ disease activity index and histological scores (52) |
| miR-124 | AHR | antisense oligonucleotide | intracolonic | TNBS | ↓inflammatory response (69) |
| miR-223 | CLDN8 | antagomiR | intraperotineal injection | TNBS | ↓intestinal permeability (57) |
| miR-214 | PTEN, PDLIM2 | LNA/PS | intracolonic | DSS | ↓disease activity index (46) |
| miR-206 | A3AR | antagomiR | tail vein injection | DSS | ↓disease activity index (62) |
DSS, dextran sodium sulfate; TNBS, 2,4,6-trinitrobenzenesulfonic acid.
Systemic administration of miRNA blockers in colitis mouse models has also been described in the literature. Specifically, gain-of-function and loss-of-function studies showed that ectopic expression of miR-665 promotes apoptosis under inflammatory stimuli by repressing the endoplasmic reticulum stress components X-Box Binding Protein 1 and ORMDL Sphingolipid Biosynthesis Regulator 3. Importantly, intraperitoneal injection of antagomiR-665 markedly impaired DSS-induced colitis in vivo, as evidenced by decreased weight loss, colon shortening, colitis, and cell death (31). Another study revealed that miR-223 interacts with the IL-23 pathway by targeting Claudin-8 (CLDN8), which is involved in the structure of tight junctions. Intraperitoneal injection of antagomiR-223 reactivated CLDN8 and decreased intestinal permeability in colitis mice colons (57). It has been recently shown that miR-155 directly binds to the 3′-UTR of inositol polyphosphate-5-phosphatase D, leading to a significant decrease in SHIP-1 expression in primary bone marrow-derived macrophages. Mouse studies revealed that a miR-155 inhibitor administered by tail vein injection reduced DSS-induced colitis by increasing SHIP-1 and decreasing AKT activity (34). Furthermore, a recent study indicated that miR-206 increases DSS-induced colitis severity through direct inhibition of the adenosine A3 receptor (A3AR) and secondarily via reduction of NF-κB/IL-8 signaling. Tail veil injection of a miR-206 inhibitor blocked this phenotype, suggesting that miR-206 has a proinflammatory function through suppression of A3AR expression and activation of NF-κB pathway (45).
CONCLUSIONS
Given the comprehensive role of miRNAs as key regulators of IBD pathogenesis and the inefficiency of the currently available IBD treatments, exploring the potential of miRNA-based therapeutics is an emerging future goal. Numerous studies have unraveled dysregulated miRNA signatures in IBD patients, along with detailed targets and regulatory functions of specific miRNAs. Furthermore, the in vivo effectiveness of miRNA inhibitors has been evaluated for a variety of specific miRNAs, many of which show great promise.
The clinical perspective of using blockers of miRNA expression is challenging due to limitations, including multiple genes and/or tissues being targeted simultaneously by a single miRNA inhibitor. As a consequence, suboptimal side effects could occur by affecting numerous physiological pathways in many different cell types. It is important to identify the IBD miRNA/gene networks to evaluate the implications of inhibiting a specific miRNA in vivo. Further assessment has to be done on the sustainability of miRNA inhibitors in the long term, particularly regarding systemic effects.
It is conceivable that the full potential of miRNA-based therapeutic agents will not be achieved until effective approaches for targeted and efficient delivery to cells and tissues are established. However, quite encouraging results arise from two completed Phase I trials (NCT00688012 and NCT00979927) conducted by Santaris Pharma related to a LNA-modified oligonucleotide that specifically inhibits the endogenous miR-122, a liver-specific miRNA required for the infection of hepatitis C virus, showing that the drug is well tolerated and safe.
Conclusively, miRNAs are a promising area of research for the development of IBD therapeutics. Assessing the relevance of in vitro studies with functional research studies in vivo is critical to support the clinical translation of miRNA-based therapeutic approaches. As clinical trials using miRNA inhibitors have been launched in hepatitis C patients and are accompanied with effectiveness and minimal side effects (21), the possibility of using miRNA inhibitors in the clinical setting has a foundation that may successfully expand to other diseases, including IBD.
GRANTS
This study was supported by National Institutes of Health (NIH) Grant RO1 DK-11003 (to D. Illiopoulos), the Broad Medical Foundation for IBD Research (to D. Illiopoulos and C. Pothoulakis), NIH Grant RO1 DK60729 (to C. Pothoulakis), and the Blinder Foundation for Crohn’s Disease (to C. Pothoulakis).
DISCLOSURES
Dimitrios Iliopoulos and Charalabos Pothoulakis are cofounders of Algorithm Therapeutics, Inc., a biotech company developing IBD therapeutics.
AUTHOR CONTRIBUTIONS
A.S. and D.I. drafted manuscript; A.S., M.K., C.P., and D.I. edited and revised manuscript; A.S., M.K., C.P., and D.I. approved final version of manuscript.
REFERENCES
- 1.Aagaard L, Rossi JJ. RNAi therapeutics: principles, prospects and challenges. Adv Drug Deliv Rev 59: 75–86, 2007. doi: 10.1016/j.addr.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ando Y, Mazzurana L, Forkel M, Okazaki K, Aoi M, Schmidt PT, Mjösberg J, Bresso F. Downregulation of microRNA-21 in colonic CD3+ T cells in UC remission. Inflamm Bowel Dis 22: 2788–2793, 2016. doi: 10.1097/MIB.0000000000000969. [DOI] [PubMed] [Google Scholar]
- 3.Bakirtzi K, Law IK, Xue X, Iliopoulos D, Shah YM, Pothoulakis C. Neurotensin promotes the development of colitis and intestinal angiogenesis via HIF-1α-miR-210 signaling. J Immunol 196: 4311–4321, 2016. doi: 10.4049/jimmunol.1501443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297, 2004. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 136: 215–233, 2009. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bumcrot D, Manoharan M, Koteliansky V, Sah DW. RNAi therapeutics: a potential new class of pharmaceutical drugs. Nat Chem Biol 2: 711–719, 2006. doi: 10.1038/nchembio839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burdick AD, Sciabola S, Mantena SR, Hollingshead BD, Stanton R, Warneke JA, Zeng M, Martsen E, Medvedev A, Makarov SS, Reed LA, Davis JW II, Whiteley LO. Sequence motifs associated with hepatotoxicity of locked nucleic acid—modified antisense oligonucleotides. Nucleic Acids Res 42: 4882–4891, 2014. doi: 10.1093/nar/gku142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai M, Chen S, Hu W. MicroRNA-141 is involved in ulcerative colitis pathogenesis via aiming at CXCL5. J Interferon Cytokine Res 37: 415–420, 2017. doi: 10.1089/jir.2017.0019. [DOI] [PubMed] [Google Scholar]
- 9.Campbell MA, Wengel J. Locked vs. unlocked nucleic acids (LNA vs. UNA): contrasting structures work towards common therapeutic goals. Chem Soc Rev 40: 5680–5689, 2011. doi: 10.1039/c1cs15048k. [DOI] [PubMed] [Google Scholar]
- 10.Chapman CG, Pekow J. The emerging role of miRNAs in inflammatory bowel disease: a review. Therap Adv Gastroenterol 8: 4–22, 2015. doi: 10.1177/1756283X14547360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen B, She S, Li D, Liu Z, Yang X, Zeng Z, Liu F. Role of miR-19a targeting TNF-α in mediating ulcerative colitis. Scand J Gastroenterol 48: 815–824, 2013. doi: 10.3109/00365521.2013.800991. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Xiao Y, Ge W, Zhou K, Wen J, Yan W, Wang Y, Wang B, Qu C, Wu J, Xu L, Cai W. miR-200b inhibits TGF-β1-induced epithelial-mesenchymal transition and promotes growth of intestinal epithelial cells. Cell Death Dis 4: e541, 2013. doi: 10.1038/cddis.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai X, Chen X, Chen Q, Shi L, Liang H, Zhou Z, Liu Q, Pang W, Hou D, Wang C, Zen K, Yuan Y, Zhang CY, Xia L. MicroRNA-193a-3p reduces intestinal inflammation in response to microbiota via down-regulation of colonic PepT1. J Biol Chem 290: 16,099–16,115, 2015. doi: 10.1074/jbc.M115.659318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol 13: 13–27, 2016. doi: 10.1038/nrgastro.2015.186. [DOI] [PubMed] [Google Scholar]
- 15.Esau CC. Inhibition of microRNA with antisense oligonucleotides. Methods 44: 55–60, 2008. doi: 10.1016/j.ymeth.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov 9: 775–789, 2010. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He C, Shi Y, Wu R, Sun M, Fang L, Wu W, Liu C, Tang M, Li Z, Wang P, Cong Y, Liu Z. miR-301a promotes intestinal mucosal inflammation through induction of IL-17A and TNF-α in IBD. Gut 65: 1938–1950, 2016. doi: 10.1136/gutjnl-2015-309389. [DOI] [PubMed] [Google Scholar]
- 18.Henry JC, Azevedo-Pouly AC, Schmittgen TD. MicroRNA replacement therapy for cancer. Pharm Res 28: 3030–3042, 2011. doi: 10.1007/s11095-011-0548-9. [DOI] [PubMed] [Google Scholar]
- 19.Huang Z, Shi T, Zhou Q, Shi S, Zhao R, Shi H, Dong L, Zhang C, Zeng K, Chen J, Zhang J. miR-141 regulates colonic leukocytic trafficking by targeting CXCL12β during murine colitis and human Crohn’s disease. Gut 63: 1247–1257, 2014. doi: 10.1136/gutjnl-2012-304213. [DOI] [PubMed] [Google Scholar]
- 20.Iborra M, Bernuzzi F, Correale C, Vetrano S, Fiorino G, Beltrán B, Marabita F, Locati M, Spinelli A, Nos P, Invernizzi P, Danese S. Identification of serum and tissue micro-RNA expression profiles in different stages of inflammatory bowel disease. Clin Exp Immunol 173: 250–258, 2013. doi: 10.1111/cei.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, Persson R, King BD, Kauppinen S, Levin AA, Hodges MR. Treatment of HCV infection by targeting microRNA. N Engl J Med 368: 1685–1694, 2013. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 22.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Büning C, Cohain A, Cichon S, D’Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H, Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH; International IBD Genetics Consortium (IIBDGC) . Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491: 119–124, 2012. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koukos G, Polytarchou C, Kaplan JL, Morley-Fletcher A, Gras-Miralles B, Kokkotou E, Baril-Dore M, Pothoulakis C, Winter HS, Iliopoulos D. MicroRNA-124 regulates STAT3 expression and is down-regulated in colon tissues of pediatric patients with ulcerative colitis. Gastroenterology 145: 842–852.e2, 2013. doi: 10.1053/j.gastro.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koukos G, Polytarchou C, Kaplan JL, Oikonomopoulos A, Ziring D, Hommes DW, Wahed R, Kokkotou E, Pothoulakis C, Winter HS, Iliopoulos D. A microRNA signature in pediatric ulcerative colitis: deregulation of the miR-4284/CXCL5 pathway in the intestinal epithelium. Inflamm Bowel Dis 21: 996–1005, 2015. doi: 10.1097/MIB.0000000000000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krützfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with ‘antagomirs’. Nature 438: 685–689, 2005. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 26.Law IK, Bakirtzi K, Polytarchou C, Oikonomopoulos A, Hommes D, Iliopoulos D, Pothoulakis C. Neurotensin—regulated miR-133α is involved in proinflammatory signalling in human colonic epithelial cells and in experimental colitis. Gut 64: 1095–1104, 2015. doi: 10.1136/gutjnl-2014-307329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lennox KA, Behlke MA. Chemical modification and design of anti-miRNA oligonucleotides. Gene Ther 18: 1111–1120, 2011. doi: 10.1038/gt.2011.100. [DOI] [PubMed] [Google Scholar]
- 28.Lennox KA, Owczarzy R, Thomas DM, Walder JA, Behlke MA. Improved performance of anti-miRNA oligonucleotides using a novel non-nucleotide modifier. Mol Ther Nucleic Acids 2: e117, 2013. doi: 10.1038/mtna.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lennox KA, Sabel JL, Johnson MJ, Moreira BG, Fletcher CA, Rose SD, Behlke MA, Laikhter AL, Walder JA, Dagle JM. Characterization of modified antisense oligonucleotides in Xenopus laevis embryos. Oligonucleotides 16: 26–42, 2006. doi: 10.1089/oli.2006.16.26. [DOI] [PubMed] [Google Scholar]
- 30.Lewis A, Mehta S, Hanna LN, Rogalski LA, Jeffery R, Nijhuis A, Kumagai T, Biancheri P, Bundy JG, Bishop CL, Feakins R, Di Sabatino A, Lee JC, Lindsay JO, Silver A. Low serum levels of microRNA-19 are associated with a stricturing Crohn’s disease phenotype. Inflamm Bowel Dis 21: 1926–1934, 2015. doi: 10.1097/MIB.0000000000000443. [DOI] [PubMed] [Google Scholar]
- 31.Li M, Zhang S, Qiu Y, He Y, Chen B, Mao R, Cui Y, Zeng Z, Chen M. Upregulation of miR-665 promotes apoptosis and colitis in inflammatory bowel disease by repressing the endoplasmic reticulum stress components XBP1 and ORMDL3. Cell Death Dis 8: e2699, 2017. doi: 10.1038/cddis.2017.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Z, Rana TM. Therapeutic targeting of microRNAs: current status and future challenges. Nat Rev Drug Discov 13: 622–638, 2014. doi: 10.1038/nrd4359. [DOI] [PubMed] [Google Scholar]
- 33.Ling H. Non-coding RNAs: therapeutic strategies and delivery systems. Adv Exp Med Biol 937: 229–237, 2016. doi: 10.1007/978-3-319-42059-2_12. [DOI] [PubMed] [Google Scholar]
- 34.Lu ZJ, Wu JJ, Jiang WL, Xiao JH, Tao KZ, Ma L, Zheng P, Wan R, Wang XP. MicroRNA-155 promotes the pathogenesis of experimental colitis by repressing SHIP-1 expression. World J Gastroenterol 23: 976–985, 2017. doi: 10.3748/wjg.v23.i6.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma L, Reinhardt F, Pan E, Soutschek J, Bhat B, Marcusson EG, Teruya-Feldstein J, Bell GW, Weinberg RA. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat Biotechnol 28: 341–347, 2010. doi: 10.1038/nbt.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marafini I, Di Fusco D, Calabrese E, Sedda S, Pallone F, Monteleone G. Antisense approach to inflammatory bowel disease: prospects and challenges. Drugs 75: 723–730, 2015. doi: 10.1007/s40265-015-0391-0. [DOI] [PubMed] [Google Scholar]
- 37.McGovern DP, Kugathasan S, Cho JH. Genetics of inflammatory bowel diseases. Gastroenterology 149: 1163–1176.e2, 2015. doi: 10.1053/j.gastro.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meister G, Landthaler M, Dorsett Y, Tuschl T. Sequence-specific inhibition of microRNA- and siRNA-induced RNA silencing. RNA 10: 544–550, 2004. doi: 10.1261/rna.5235104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Min M, Peng L, Yang Y, Guo M, Wang W, Sun G. MicroRNA-155 is involved in the pathogenesis of ulcerative colitis by targeting FOXO3a. Inflamm Bowel Dis 20: 652–659, 2014. doi: 10.1097/MIB.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 40.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142: 46–54.e42, 2012. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Nimmo ER, Prendergast JG, Aldhous MC, Kennedy NA, Henderson P, Drummond HE, Ramsahoye BH, Wilson DC, Semple CA, Satsangi J. Genome-wide methylation profiling in Crohn’s disease identifies altered epigenetic regulation of key host defense mechanisms including the Th17 pathway. Inflamm Bowel Dis 18: 889–899, 2012. doi: 10.1002/ibd.21912. [DOI] [PubMed] [Google Scholar]
- 42.Ozanne SE, Constância M. Mechanisms of disease: the developmental origins of disease and the role of the epigenotype. Nat Clin Pract Endocrinol Metab 3: 539–546, 2007. doi: 10.1038/ncpendmet0531. [DOI] [PubMed] [Google Scholar]
- 43.Padua D, Mahurkar-Joshi S, Law IKM, Polytarchou C, Vu JP, Pisegna JR, Shih D, Iliopoulos D, Pothoulakis C. A long noncoding RNA signature for ulcerative colitis identifies IFNG-AS1 as an enhancer of inflammation. Am J Physiol Gastrointest Liver Physiol 311: G446–G457, 2016. doi: 10.1152/ajpgi.00212.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pan S, Yang X, Jia Y, Li Y, Chen R, Wang M, Cai D, Zhao R. Intravenous injection of microvesicle-delivery miR-130b alleviates high-fat diet-induced obesity in C57BL/6 mice through translational repression of PPAR-γ. J Biomed Sci 22: 86, 2015. doi: 10.1186/s12929-015-0193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pierdomenico M, Cesi V, Cucchiara S, Vitali R, Prete E, Costanzo M, Aloi M, Oliva S, Stronati L. NOD2 is regulated by Mir-320 in physiological conditions but this control is altered in inflamed tissues of patients with inflammatory bowel disease. Inflamm Bowel Dis 22: 315–326, 2016. doi: 10.1097/MIB.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 46.Polytarchou C, Hommes DW, Palumbo T, Hatziapostolou M, Koutsioumpa M, Koukos G, van der Meulen-de Jong AE, Oikonomopoulos A, van Deen WK, Vorvis C, Serebrennikova OB, Birli E, Choi J, Chang L, Anton PA, Tsichlis PN, Pothoulakis C, Verspaget HW, Iliopoulos D. MicroRNA214 Is Associated With Progression of Ulcerative Colitis, and Inhibition Reduces Development of Colitis and Colitis-Associated Cancer in Mice. Gastroenterology 149: 981–992.e911, 2015. doi: 10.1053/j.gastro.2015.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polytarchou C, Oikonomopoulos A, Mahurkar S, Touroutoglou A, Koukos G, Hommes DW, Iliopoulos D. Assessment of Circulating MicroRNAs for the Diagnosis and Disease Activity Evaluation in Patients with Ulcerative Colitis by Using the Nanostring Technology. Inflamm Bowel Dis 21: 2533–2539, 2015. doi: 10.1097/MIB.0000000000000547. [DOI] [PubMed] [Google Scholar]
- 48.Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov 16: 203–222, 2017. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 49.Schaefer JS. MicroRNAs: how many in inflammatory bowel disease? Curr Opin Gastroenterol 32: 258–266, 2016. doi: 10.1097/MOG.0000000000000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schaefer JS, Attumi T, Opekun AR, Abraham B, Hou J, Shelby H, Graham DY, Streckfus C, Klein JR. MicroRNA signatures differentiate Crohn’s disease from ulcerative colitis. BMC Immunol 16: 5, 2015. doi: 10.1186/s12865-015-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi C, Liang Y, Yang J, Xia Y, Chen H, Han H, Yang Y, Wu W, Gao R, Qin H. MicroRNA-21 knockout improve the survival rate in DSS induced fatal colitis through protecting against inflammation and tissue injury. PLoS One 8: e66814, 2013. doi: 10.1371/journal.pone.0066814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi T, Xie Y, Fu Y, Zhou Q, Ma Z, Ma J, Huang Z, Zhang J, Chen J. The signaling axis of microRNA-31/interleukin-25 regulates Th1/Th17-mediated inflammation response in colitis. Mucosal Immunol 10: 983–995, 2017. doi: 10.1038/mi.2016.102. [DOI] [PubMed] [Google Scholar]
- 53.Talbert PB, Henikoff S. Spreading of silent chromatin: inaction at a distance. Nat Rev Genet 7: 793–803, 2006. doi: 10.1038/nrg1920. [DOI] [PubMed] [Google Scholar]
- 54.Thorlacius-Ussing G, Schnack Nielsen B, Andersen V, Holmstrøm K, Pedersen AE. Expression and Localization of miR-21 and miR-126 in Mucosal Tissue from Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis 23: 739–752, 2017. doi: 10.1097/MIB.0000000000001086. [DOI] [PubMed] [Google Scholar]
- 55.Ventham NT, Kennedy NA, Adams AT, Kalla R, Heath S, O’Leary KR, Drummond H, Wilson DC, Gut IG, Nimmo ER, Satsangi J IBD BIOM consortium; IBD CHARACTER consortium . Integrative epigenome-wide analysis demonstrates that DNA methylation may mediate genetic risk in inflammatory bowel disease. Nat Commun 7: 13507, 2016. doi: 10.1038/ncomms13507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Viennois E, Zhao Y, Han MK, Xiao B, Zhang M, Prasad M, Wang L, Merlin D. Serum miRNA signature diagnoses and discriminates murine colitis subtypes and predicts ulcerative colitis in humans. Sci Rep 7: 2520, 2017. doi: 10.1038/s41598-017-02782-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang H, Chao K, Ng SC, Bai AH, Yu Q, Yu J, Li M, Cui Y, Chen M, Hu JF, Zhang S. Pro-inflammatory miR-223 mediates the cross-talk between the IL23 pathway and the intestinal barrier in inflammatory bowel disease. Genome Biol 17: 58, 2016. doi: 10.1186/s13059-016-0901-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu F, Guo NJ, Tian H, Marohn M, Gearhart S, Bayless TM, Brant SR, Kwon JH. Peripheral blood microRNAs distinguish active ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis 17: 241–250, 2011. doi: 10.1002/ibd.21450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu F, Zhang S, Dassopoulos T, Harris ML, Bayless TM, Meltzer SJ, Brant SR, Kwon JH. Identification of microRNAs associated with ileal and colonic Crohn’s disease. Inflamm Bowel Dis 16: 1729–1738, 2010. doi: 10.1002/ibd.21267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu F, Zikusoka M, Trindade A, Dassopoulos T, Harris ML, Bayless TM, Brant SR, Chakravarti S, Kwon JH. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology 135: 1624–1635.e24, 2008. doi: 10.1053/j.gastro.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 61.Wu W, He C, Liu C, Cao AT, Xue X, Evans-Marin HL, Sun M, Fang L, Yao S, Pinchuk IV, Powell DW, Liu Z, Cong Y. miR-10a inhibits dendritic cell activation and Th1/Th17 cell immune responses in IBD. Gut 64: 1755–1764, 2015. doi: 10.1136/gutjnl-2014-307980. [DOI] [PubMed] [Google Scholar]
- 62.Wu W, He Y, Feng X, Ye S, Wang H, Tan W, Yu C, Hu J, Zheng R, Zhou Y. MicroRNA-206 is involved in the pathogenesis of ulcerative colitis via regulation of adenosine A3 receptor. Oncotarget 8: 705–721, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiang S, Fruehauf J, Li CJ. Short hairpin RNA-expressing bacteria elicit RNA interference in mammals. Nat Biotechnol 24: 697–702, 2006. doi: 10.1038/nbt1211. [DOI] [PubMed] [Google Scholar]
- 64.Xu XM, Zhang HJ. miRNAs as new molecular insights into inflammatory bowel disease: Crucial regulators in autoimmunity and inflammation. World J Gastroenterol 22: 2206–2218, 2016. doi: 10.3748/wjg.v22.i7.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang J, Zhou CZ, Zhu R, Fan H, Liu XX, Duan XY, Tang Q, Shou ZX, Zuo DM. Microvesicles shuttled miR-200b attenuate experimental colitis associated intestinal fibrosis by inhibiting the development of EMT. J Gastroenterol Hepatol 32: 1966–1974, 2017. doi: 10.1111/jgh.13797. [DOI] [PubMed] [Google Scholar]
- 66.Yang Y, Ma Y, Shi C, Chen H, Zhang H, Chen N, Zhang P, Wang F, Yang J, Yang J, Zhu Q, Liang Y, Wu W, Gao R, Yang Z, Zou Y, Qin H. Overexpression of miR-21 in patients with ulcerative colitis impairs intestinal epithelial barrier function through targeting the Rho GTPase RhoB. Biochem Biophys Res Commun 434: 746–752, 2013. doi: 10.1016/j.bbrc.2013.03.122. [DOI] [PubMed] [Google Scholar]
- 67.Yu X, Odenthal M, Fries JW. Exosomes as miRNA carriers: formation-function-future. Int J Mol Sci 17: 2028, 2016. doi: 10.3390/ijms17122028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao X, Pan F, Holt CM, Lewis AL, Lu JR. Controlled delivery of antisense oligonucleotides: a brief review of current strategies. Expert Opin Drug Deliv 6: 673–686, 2009. doi: 10.1517/17425240902992894. [DOI] [PubMed] [Google Scholar]
- 69.Zhao Y, Ma T, Chen W, Chen Y, Li M, Ren L, Chen J, Cao R, Feng Y, Zhang H, Shi R. MicroRNA-124 promotes itestinal inflammation by targeting aryl hydrocarbon receptor in Crohn’s disease. J Crohn’s Colitis 10: 703–712, 2016. doi: 10.1093/ecco-jcc/jjw010. [DOI] [PubMed] [Google Scholar]