Abstract
Posttranslational modifications affect almost all proteins and are critical to a well-functioning and diverse proteome; however, many modifications remain relatively unknown and unstudied. This paper will give a perspective on the rapidly developing, novel posttranslational modification called succinylation. This modification may be implicated in numerous diseases, such as hepatic, cardiac, and pulmonary diseases. Although the influences of this modification still remain poorly understood, we are confident that further research into succinylation will provide an enhanced understanding of the complex machinery within the mitochondria, as well as the imposing consequences associated with its dysfunction.
Keywords: metabolism, mitochondria, modification, posttranslational, sirtuin 5, succinylation
INTRODUCTION
Research within the past decade has revealed an unexpectedly vast human proteome, one that the genome alone cannot account for. This leads to the conclusion that a single gene must be able to encode multiple proteins and that the proteins themselves must be modified to allow for this advanced diversity. There are two main mechanisms used to accomplish this proteome expansion: posttranscriptional modifications and posttranslational modifications (PTMs), the latter of which will be the focus of this paper. PTMs are a widespread mechanism used by cells to alter the structure, activity, stability, and localization of various proteins and are biologically efficient in regulating a variety of cellular functions (29). Some of the most powerful and diverse PTMs are covalent modifications, which are characterized by the addition of chemical groups to amino acid side chains.
Among the common amino acids involved in PTMs, lysine is one of the three that has a positively charged side chain when at physiological pH (7.4). Consequently, this makes lysine essential for the formation of protein structures and functions (35). The side chain of lysine can participate in hydrogen bonding as well as Van der Waals and electrostatic interactions with negatively charged structures. An example of this is the DNA-binding activity lysine has in the leucine zipper, a type of protein motif that serves as an important transcription factor (17). Additionally, lysine is known to have a crucial role in enzymatic reactions that require proton transfer and are acid-base catalyzed (16). With the extensive involvement lysine has in varying protein interactions, it comes as no surprise that a shift in its charge has drastic consequences on the functionality of the protein (8, 9, 14, 35). Specifically, acetylation of lysine residues, a well-studied topic, has been verified to influence intricate processes including enzymatic activity, transcriptional regulation, and protein degradation (7). Studies of acetylation have led research to focus on other acyl modifications of lysine residues, such as succinylation, malonylation, and glutarylation. This paper will explore and explain the relatively new, and compelling, modification called succinylation.
HISTORY AND DISCOVERIES OF SUCCINYLATION
The discovery of lysine succinylation stems from the extensive research that was conducted on acetylation. These studies sparked interest in the field and introduced a variety of new acylations, including succinylation. In 1961, succinylation was first used as a method of inhibiting antibody formation to ultimately test for erythematous responses in animals sensitive to dinitrophenyl-polyline (21). Then, a paper published in 1975 investigated the effects of lysine succinylation in ovalbumin. They discovered that succinylation via succinic anhydride caused a conformational shift in the protein and described a possible connection between succinylation and resistance to digestion by tryptic attack (10). In 2004, a research paper delved deeper into the binding of succinyl groups to the amino acid homoserine through homoserine trans-succinylase (26). They found that the lysines on the enzyme are indispensable for the binding of succinyl, inspiring others to examine the effects of lysine succinylation on different proteins (26).
Using affinity purification with an anti-succinyl lysine antibody in 2011, it was revealed that succinylation is a naturally occurring PTM on lysine residues in bacteria (35). Over the next four years, numerous studies discovered proteins that are targeted by succinylation, such as histones, the sites that are succinylated on proteins, and some of the succinylases involved with its regulation (4, 5, 8, 13, 19, 24, 31–33) (Table 1). Notably, these recent discoveries indicate that succinylation may be relevant in diseases associated with mitochondrial dysregulation, such as those concerning oxidative stress (6, 12, 30, 33). Even though the process of succinylation has garnered more attention in the past few years than any of the years prior, there is still uncertainty surrounding its role in physiology.
Table 1.
A selection of a few key mitochondrial proteins (alphabetically organized) that were found to be succinylated, as identified by Weinert et al. (31)
| 3-Hydroxy-3-methylglutaryl-CoA synthase 2 | Aspartate aminotransferase | Malate dehydrogenase |
| 60 kDa heat shock protein | Carnitine O-acetyltransferase | NADH dehydrogenase |
| Acetyl-CoA acetyltransferase | Carnitine O-plamitoyltransferase | Phosphoenolpyruvate carboxykinase |
| ADP/ATP translocase 1 | Glutamate dehydrogenase 1 | Pyruvate carboxylase |
| Aldehyde dehydrogenase | Isocitrate dehydrogenase | Superoxide dismutase |
COMPARISON OF SUCCINYLATION WITH OTHER PTMS
The field of PTMs is a broad spectrum, with various mechanisms and proteins involved; as a result, it can be difficult to grasp the complexity of a PTM. Here we will compare succinylation with some of the more well-known modifications, starting with the substrates utilized. Among the most well-studied PTMs in the field of proteomics is phosphorylation, which utilizes a small phosphoryl group typically derived from an ATP or a GTP. Acetylation, another well-studied PTM in the field, attaches an acetyl group obtained from the tricarboxylic acid (TCA) cycle metabolite acetyl coenzyme A (AcCoA) to a protein. Of the modifications that can be made through acetylation, the reversible addition of acetyl groups to an ε-amino group of a lysine residue is biologically significant (1, 7). Likewise, succinylation utilizes succinyl CoA (another metabolite of the TCA cycle) for its supply of succinyl groups. Coincidentally, succinylation is also a reversible process in which proteins are succinylated at ε-amino groups of lysine residues (24, 34).
Looking at the substrates more in depth reveals the drastic differences between the succinyl group and other covalent modifications added from various PTMs. The most noticeable of these differences is the comparative size of the succinate group, which was found to be approximately 100.02 Daltons (Da) (35). To put this in perspective, other small chemical groups added such as acetyl and dimethyl have masses of 42.0106 and 28.0313 Da, respectively (20, 35). This, along with the visual comparisons of the succinate chemical group with those of acetyl and methyl, led to the logical conclusion that the succinate group is far bulkier than either the acetyl group or the methyl group, and that this may cause it to have a larger impact on the structure and function of the proteins that it binds to.
The most distinguishing quality when analyzing the potency of a PTM is to determine the charge that the molecule imparts. Lysine, one of the fundamental residues that these groups attach to, has an innate positive charge at physiological pH. When the lysines in a protein are acetylated, the acetyl groups neutralize this natural positive charge of the protonated lysines, causing a shift in the structure of the protein and affecting the enzymatic properties of the protein. Comparatively, the addition of succinyl groups neutralizes the natural positive charge of lysine and imparts a negative charge in the form of a carboxylate group (4, 35). This is comparable to the same charge reversal that can be seen in the phosphorylation of proteins, a process which is well known to cause drastic effects on the chemical properties, structure, and function of the proteins it binds to (35). This allows us to make the reasonable assumption that succinylation may have the same potential impact as these other PTMs with respect to influencing protein functions.
Finally, the location of where these modifications occur will be examined and compared. Lysine acetylation is known to be evolutionarily conserved, and it is found in a wide range of life, from prokaryotes to eukaryotes. Acetylation is highly prevalent in the mitochondria, and it is particularly important in gluconeogenesis, glycolysis, the TCA and urea cycles, glycogen metabolism, and fatty acid metabolism (11, 36). Similarly, succinylation was first identified in Escherichia coli, and has now been identified in eukaryotes as well, especially in the mitochondria of eukaryotes (31, 35). While both modifications occur in the mitochondria, succinylation of proteins appears to be more prevalent in mechanisms involving cellular metabolism within the mitochondria, while acetylation appears to be more prevalent in the cytosol (33). Interestingly, succinylation and acetylation have been shown to share an impressive amount of binding sites: up to 27% of sites in humans, and up to 66% sites for E. coli, that are succinylated are also acetylated (31). This overlap, as well as the similar localization and participation in metabolic processes, leads to a question of interactions between succinylation and acetylation. The dynamic changes of acetylation and succinylation together in response to various forms of stimulation remain poorly characterized and understood, and more research needs to be performed to investigate any possible effects of cross talk between the two.
MECHANISM AND REGULATION OF SUCCINYLATION
At the time of writing this, an enzyme within the mitochondria that performs a lysine succinyl-transferase activity has not been established. This leads us to ponder the mechanism that allows the succinylation of an extensive, yet specific, range of proteins in the mitochondria that has been shown to occur (11, 24, 25). It is possible that nonenzymatic activities control the succinylation of proteins, and that the conditions of the mitochondria (abundance of succinyl CoA, pH, protein parameters, etc.) would be the main governing factor, though more research is needed to determine whether this is truly the case (28).
The regulation of reversible PTMs has become a readily studied topic, and its importance in normal physiological behavior is widely known. As for the regulation of succinylation, significant breakthroughs have been recently made in the form of the family of silent information regulators 2 (sirtuins, or sirt). These proteins are an evolutionarily conserved class of nicotinamide adenine dinucleotide (NAD+)-dependent deacylases (18, 23). These proteins have been especially distinguished for their deacetylase activities, but recent research has identified a sirtuin, Sirt5, that is localized in the mitochondria and has a weak deacetylase activity. Additionally, Sirt5 has been shown to contain potent desuccinylase activity on lysine residues and to be conserved across species (5, 22). This enzyme performs the catalytic removal of a succinyl group from a lysine residue on a protein using NAD+ as a cosubstrate, and it generates nicotinamide as well as 2′-o-succinyl-ADP-ribose as a result (24). A tyrosine located at residue position 102 and an arginine at residue position 105, both notable for their positive charges, are located within the catalytic pocket of Sirt5. These residues are necessary for the proper functioning of the desuccinylase activity of Sirt5, most likely due to their positive charges (5).
The potent desuccinylase activity of Sirt5 allows questions concerning control over succinylation to transition to the more readily researchable questions regarding regulation of Sirt5. One of the most direct ways of affecting enzyme kinetics is through manipulation of its substrates and products. An increase in Sirt5 activity will follow from an increase in NAD+ (substrate), an increase in nicotinamide (product) will result in a decrease in Sirt5 activity. Additionally, there are other compounds that are believed to hold an influence over Sirt5, including the complex interactions between Sirts, AMP-activated protein kinase (AMPK), and proliferator-activated receptor γ coactivator-1α (PGC-1α). PGC-1α, AMPK, and sirtuins are all regulators of cellular energy homeostasis and were shown to interact with one another both in vivo and in vitro (2). In this interaction, PGC-1α was found to upregulate Sirt5 on both the mRNA and protein expression levels, yet, AMPK was found to downregulate Sirt5 activity, a characteristic property unique to Sirt5 out of all the sirtuins (2). Finally, research has been performed to investigate the interactions between Sirt5 and protein kinase C epsilon (PKCε) in the context of cerebral ischemia. It was found that activated PKCε resulted in elevated Sirt5 levels as well as enhanced desuccinylase activities in the cortical mitochondria, thereby protecting the brain against metabolic and ischemic stress (15). These results together suggest that Sirt5, and succinylation as a whole, is critical in the regulation of the mitochondria and in energy metabolism. A greater understanding of the possible methods of succinylation regulation, both through activators and inhibitors alike, will lead to the development of new therapeutic treatments towards diseases that affect the mitochondria.
THERAPEUTIC RELEVANCE
The reversible succinylation of lysines is a recently identified posttranslational modification with largely unknown prevalence or biological function. Despite this, some studies have revealed the importance of succinylation and are laying the foundation for more research to be done in a faster and more efficient manner. An example of this is the realization that succinylation has been a highly conserved mechanism across prokaryotes as well as eukaryotic mitochondria (31, 33). A recent study has shown that Sirt5 can directly interact with cardiolipin, a molecule that is unique to the inner mitochondrial membrane and is vital for the formation of cristae (34). The study found the possibility that Sirt5 may localize to areas of the mitochondria that are high in cardiolipin as a way to desuccinylate the many proteins that accumulate around it (34). These findings suggest that clinical research examining mitochondrial succinylation is the next logical step in our pursuit of therapeutic treatments.
To further substantiate this, another study identified 1,190 sites on 252 proteins within the mitochondria of liver cells from mice and discovered that Sirt5 deficiency in these cells led to hypersuccinylation on 140 proteins (24). The study established the importance of desuccinylation activities in fatty acid β-oxidation and ketogenesis. Previously, it had been thought that concentrations of succinyl-CoA were the regulatory element in controlling the hypersuccinylation of the mitochondrial enzyme 3-hydroxy-3-methylglutaryl-CoA synthase 2 (HMGCS2); however, this study demonstrated the role that Sirt5 may play in ketogenesis through desuccinylating this enzyme (Fig. 1). Surprisingly, the levels of succinyl-CoA remained unchanged between fasting and fed states of mice, yet the loss of Sirt5 desuccinylase activities led to the hypersuccinylation of HMGCS2. This hypersuccinylation due to the loss of desuccinylase activity led to a decreased ketone body production during fasting (24). Succinylation has also been found in the heart, which has a much higher concentration of succinyl-CoA within its cells than any other organ. This supports the knowledge that the heart would need to prioritize energy production through the tricarboxylic acid cycle (27). With this information in mind, the next step was to determine the effects of Sirt5 knockout in mice. The study found that the Sirt5 knockout mice displayed decreased cardiac function and exhibited increased signs of cardiomyopathy as they aged (27). These findings support the establishment of Sirt5 as a global regulator of the succinylome.
Fig. 1.
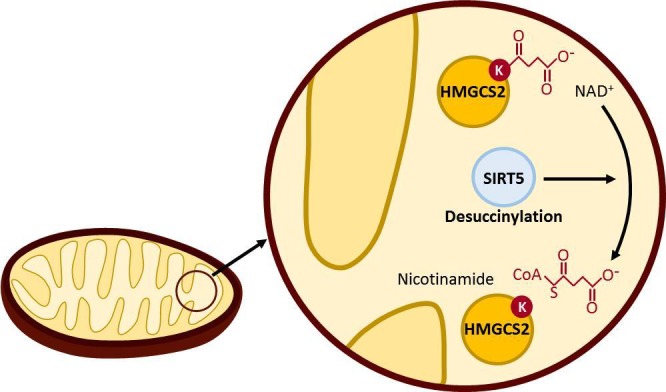
As explored by Rardin et al., Sirt5 influences HMGCS2, the rate-limiting protein of ketogenesis, through desuccinylation. The study found that the negative charge provided by a succinyl group strongly inhibits the function of HMGCS2 (24). Additionally, lysine residues near the substrate binding site were found to be hypersuccinylated. This is one of the many different proteins and pathways that may be influenced by succinylation.
To add to the wide range in which succinylation affects an organism, a paper published in July of 2017 explored the prevalence of succinylation in neurodegeneration disorders, such as Alzheimer’s disease (3). This study focused on the metabolically diminished nature of the disease and investigated various alterations to metabolic pathways and their effects on the succinylation of proteins. Ultimately, they determined that even brief metabolic perturbations (20 min) were enough to cause a drastic change in succinylation levels (3). In all of the conditions (except hypoxia) the cells were exposed to, the levels of succinylated proteins dropped anywhere between 28.9% and 57%, while during hypoxia the levels of succinylated proteins increased by 84.7% (3). Though the treatments used to inhibit metabolic processes (2-deoxyglucose, carboxyethyl ester of succinyl phosphonate, rotenone, iodoacetic acid, oligomycin, carbonyl cyanide-m-chlorophenyl hydrazine, and tyrphostin A9) lack specificity for their targets, the drastic changes in succinylated proteins affirm that succinylation is tremendously dynamic when faced with metabolic fluctuations. These results should be kept in mind when further research investigates the role of metabolism in the pathophysiology of disease processes (3).
CONCLUSIONS
As growing evidence demonstrates, succinylation is a highly important mechanism of protein regulation that necessitates further research. Despite the piqued interest within the past decade, the mechanisms associated with succinylation are still relatively unknown. The characteristic quality of performing an overall charge reversal on lysine residues indicates that this PTM may be just as important as more widely studied modifications, such as acetylation and phosphorylation (24, 27, 31, 34, 35). Additionally, the conservation of succinylation as a regulatory mechanism from prokaryotes to mitochondria exhibits the importance and significance of it in influencing the energy metabolism of the cell (16, 31, 33). The mitochondrial specificity of the most studied desuccinylase (Sirt5) implies that succinylation may be involved in many of the diseases in which mitochondrial dysfunction is a common factor, such as acute lung injury and age-related diseases (6, 12, 30). The current consensus is that more work needs to be performed to fully elucidate the cellular interactions that succinylation participates in. Future work should shift focus to the reversible succinylation of lysines, within the mitochondria, to better understand how the cell utilizes succinylation-mediated regulation. As this shift occurs, more research will focus on the involvement of the mitochondrial desuccinylase Sirt5 and how it affects the proteins involved in energy metabolism. A further look into the cross talk between Sirt5 and other proteins, which target the same lysine sites in proteins (such as Sirt3 for acetylation), will reveal the dynamic alterations in PTMs that may occur within the mitochondria (33). Prospective research in succinylation will develop a greater understanding of mitochondrial regulation, resulting in an advancement of our knowledge regarding the role of succinylation in physiology and pathology.
GRANTS
N. Kolliputi was funded by the American Heart Association National Scientist Development Grant 09SDG2260957, National Institutes of Health National Heart, Lung, and Blood Institute Grant R01 HL-105932, and the Joy McCann Culverhouse endowment to the Division of Allergy and Immunology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.A. drafted manuscript; M.B. edited and revised manuscript; R.L. and N.K. approved final version of manuscript.
REFERENCES
- 1.Audagnotto M, Dal Peraro M. Protein post-translational modifications: in silico prediction tools and molecular modeling. Comput Struct Biotechnol J 15: 307–319, 2017. doi: 10.1016/j.csbj.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buler M, Aatsinki SM, Izzi V, Uusimaa J, Hakkola J. SIRT5 is under the control of PGC-1α and AMPK and is involved in regulation of mitochondrial energy metabolism. FASEB J 28: 3225–3237, 2014. doi: 10.1096/fj.13-245241. [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Xu H, Potash S, Starkov A, Belousov VV, Bilan DS, Denton TT, Gibson GE. Mild metabolic perturbations alter succinylation of mitochondrial proteins. J Neurosci Res 95: 2244–2252, 2017. doi: 10.1002/jnr.24103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colak G, Xie Z, Zhu AY, Dai L, Lu Z, Zhang Y, Wan X, Chen Y, Cha YH, Lin H, Zhao Y, Tan M. Identification of lysine succinylation substrates and the succinylation regulatory enzyme CobB in Escherichia coli. Mol Cell Proteomics 12: 3509–3520, 2013. doi: 10.1074/mcp.M113.031567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, Kim J, Woo J, Kim JH, Choi BH, He B, Chen W, Zhang S, Cerione RA, Auwerx J, Hao Q, Lin H. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science 334: 806–809, 2011. doi: 10.1126/science.1207861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galam L, Failla A, Soundararajan R, Lockey RF, Kolliputi N. 4-Hydroxynonenal regulates mitochondrial function in human small airway epithelial cells. Oncotarget 6: 41508–41521, 2015. doi: 10.18632/oncotarget.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirschey MD, Shimazu T, Huang JY, Schwer B, Verdin E. SIRT3 regulates mitochondrial protein acetylation and intermediary metabolism. Cold Spring Harb Symp Quant Biol 76: 267–277, 2011. doi: 10.1101/sqb.2011.76.010850. [DOI] [PubMed] [Google Scholar]
- 8.Hirschey MD, Zhao Y. Metabolic Regulation by lysine malonylation, succinylation, and glutarylation. Mol Cell Proteomics 14: 2308–2315, 2015. doi: 10.1074/mcp.R114.046664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humphrey SJ, James DE, Mann M. Protein phosphorylation: a major switch mechanism for metabolic regulation. Trends Endocrinol Metab 26: 676–687, 2015. doi: 10.1016/j.tem.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Kidwai SA, Ansari AA, Salahuddin A. Effect of succinylation (3-carboxypropionylation) on the conformation and immunological activity of ovalbumin. Biochem J 155: 171–180, 1976. doi: 10.1042/bj1550171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L, Grishin NV, White M, Yang XJ, Zhao Y. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell 23: 607–618, 2006. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 12.Kolliputi N, Waxman AB. IL-6 cytoprotection in hyperoxic acute lung injury occurs via PI3K/Akt-mediated Bax phosphorylation. Am J Physiol Lung Cell Mol Physiol 297: L6–L16, 2009. doi: 10.1152/ajplung.90381.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li F, He X, Ye D, Lin Y, Yu H, Yao C, Huang L, Zhang J, Wang F, Xu S, Wu X, Liu L, Yang C, Shi J, He X, Liu J, Qu Y, Guo F, Zhao J, Xu W, Zhao S. NADP(+)-IDH mutations promote hypersuccinylation that impairs mitochondria respiration and induces apoptosis resistance. Mol Cell 60: 661–675, 2015. doi: 10.1016/j.molcel.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol 6: 838–849, 2005. doi: 10.1038/nrm1761. [DOI] [PubMed] [Google Scholar]
- 15.Morris-Blanco KC, Dave KR, Saul I, Koronowski KB, Stradecki HM, Perez-Pinzon MA. Protein kinase C epsilon promotes cerebral ischemic tolerance via modulation of mitochondrial Sirt5. Sci Rep 6: 29790, 2016. doi: 10.1038/srep29790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson DL, Nelson DL, Lehninger AL, Cox MM. Lehninger Principles of Biochemistry. New York: W.H. Freeman, 2008. [Google Scholar]
- 17.Nijhawan A, Jain M, Tyagi AK, Khurana JP. Genomic survey and gene expression analysis of the basic leucine zipper transcription factor family in rice. Plant Physiol 146: 333–350, 2008. doi: 10.1104/pp.107.112821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishida Y, Rardin MJ, Carrico C, He W, Sahu AK, Gut P, Najjar R, Fitch M, Hellerstein M, Gibson BW, Verdin E. SIRT5 regulates both cytosolic and mitochondrial protein malonylation with glycolysis as a major target. Mol Cell 59: 321–332, 2015. doi: 10.1016/j.molcel.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park J, Chen Y, Tishkoff DX, Peng C, Tan M, Dai L, Xie Z, Zhang Y, Zwaans BM, Skinner ME, Lombard DB, Zhao Y. SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol Cell 50: 919–930, 2013. doi: 10.1016/j.molcel.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parker CE, Mocanu V, Mocanu M, Dicheva N, Warren MR. Mass spectrometry for post-translational modifications. Neuroproteomics, edited by Alzate O. Boca Raton, FL: CRC, 2010. [PubMed] [Google Scholar]
- 21.Parker CW, Kern M, Eisen HN. Polyfunctional dinitrophenyl haptens as reagents for elicitation of immediate type allergic skin responses. J Exp Med 115: 789–801, 1962. doi: 10.1084/jem.115.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng C, Lu Z, Xie Z, Cheng Z, Chen Y, Tan M, Luo H, Zhang Y, He W, Yang K, Zwaans BM, Tishkoff D, Ho L, Lombard D, He T-C, Dai J, Verdin E, Ye Y, Zhao Y. The first identification of lysine malonylation substrates and its regulatory enzyme. Mol Cell Proteomics 10: M111.012658, 2011. doi: 10.1074/mcp.M111.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Philp A, Rowland T, Perez-Schindler J, Schenk S. Understanding the acetylome: translating targeted proteomics into meaningful physiology. Am J Physiol Cell Physiol 307: C763–C773, 2014. doi: 10.1152/ajpcell.00399.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rardin MJ, He W, Nishida Y, Newman JC, Carrico C, Danielson SR, Guo A, Gut P, Sahu AK, Li B, Uppala R, Fitch M, Riiff T, Zhu L, Zhou J, Mulhern D, Stevens RD, Ilkayeva OR, Newgard CB, Jacobson MP, Hellerstein M, Goetzman ES, Gibson BW, Verdin E. SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab 18: 920–933, 2013. doi: 10.1016/j.cmet.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rardin MJ, Newman JC, Held JM, Cusack MP, Sorensen DJ, Li B, Schilling B, Mooney SD, Kahn CR, Verdin E, Gibson BW. Label-free quantitative proteomics of the lysine acetylome in mitochondria identifies substrates of SIRT3 in metabolic pathways. Proc Natl Acad Sci USA 110: 6601–6606, 2013. doi: 10.1073/pnas.1302961110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosen R, Becher D, Büttner K, Biran D, Hecker M, Ron EZ. Probing the active site of homoserine trans-succinylase. FEBS Lett 577: 386–392, 2004. doi: 10.1016/j.febslet.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 27.Sadhukhan S, Liu X, Ryu D, Nelson OD, Stupinski JA, Li Z, Chen W, Zhang S, Weiss RS, Locasale JW, Auwerx J, Lin H. Metabolomics-assisted proteomics identifies succinylation and SIRT5 as important regulators of cardiac function. Proc Natl Acad Sci USA 113: 4320–4325, 2016. doi: 10.1073/pnas.1519858113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner GR, Payne RM. Widespread and enzyme-independent Nε-acetylation and Nε-succinylation of proteins in the chemical conditions of the mitochondrial matrix. J Biol Chem 288: 29036–29045, 2013. doi: 10.1074/jbc.M113.486753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walsh CT, Garneau-Tsodikova S, Gatto GJ Jr. Protein posttranslational modifications: the chemistry of proteome diversifications. Angew Chem Int Ed Engl 44: 7342–7372, 2005. doi: 10.1002/anie.200501023. [DOI] [PubMed] [Google Scholar]
- 30.Waxman AB, Kolliputi N. IL-6 protects against hyperoxia-induced mitochondrial damage via Bcl-2-induced Bak interactions with mitofusins. Am J Respir Cell Mol Biol 41: 385–396, 2009. doi: 10.1165/rcmb.2008-0302OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinert BT, Schölz C, Wagner SA, Iesmantavicius V, Su D, Daniel JA, Choudhary C. Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation. Cell Rep 4: 842–851, 2013. doi: 10.1016/j.celrep.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 32.Xie Z, Dai J, Dai L, Tan M, Cheng Z, Wu Y, Boeke JD, Zhao Y. Lysine succinylation and lysine malonylation in histones. Mol Cell Proteomics 11: 100–107, 2012. doi: 10.1074/mcp.M111.015875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu H, Chen X, Xu X, Shi R, Suo S, Cheng K, Zheng Z, Wang M, Wang L, Zhao Y, Tian B, Hua Y. Lysine acetylation and succinylation in HeLa cells and their essential roles in response to UV-induced stress. Sci Rep 6: 30212, 2016. doi: 10.1038/srep30212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Bharathi SS, Rardin MJ, Lu J, Maringer KV, Sims-Lucas S, Prochownik EV, Gibson BW, Goetzman ES. Lysine desuccinylase SIRT5 binds to cardiolipin and regulates the electron transport chain. J Biol Chem 292: 10239–10249, 2017. doi: 10.1074/jbc.M117.785022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Z, Tan M, Xie Z, Dai L, Chen Y, Zhao Y. Identification of lysine succinylation as a new post-translational modification. Nat Chem Biol 7: 58–63, 2011. doi: 10.1038/nchembio.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, Li Y, Shi J, An W, Hancock SM, He F, Qin L, Chin J, Yang P, Chen X, Lei Q, Xiong Y, Guan KL. Regulation of cellular metabolism by protein lysine acetylation. Science 327: 1000–1004, 2010. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]


