Abstract
Pituitary adenylate cyclase activating polypeptide (PACAP, Adcyap1) activation of PAC1 receptors (Adcyap1r1) significantly increases excitability of guinea pig cardiac neurons. This modulation of excitability is mediated in part by plasma membrane G protein-dependent activation of adenylyl cyclase and downstream signaling cascades. However, additional mechanisms responsible for the enhanced excitability are activated following internalization of the PAC1 receptor and endosomal signaling. Src family kinases play critical roles mediating endocytosis of many trophic factor and G protein-coupled receptors. The present study investigated whether Src family kinases also support the PACAP-induced PAC1 receptor internalization, phosphorylation of ERK, and enhanced neuronal excitability. Using human embryonic kidney cells stably expressing a green fluorescent protein-tagged PAC1 receptor, treatment with the Src family kinase inhibitor PP2 (10 µM) markedly reduced the PACAP-induced PAC1 receptor internalization, and in parallel, both PP2 and Src inhibitor 1 (Src-1, 2 µM) reduced ERK activation determined by Western blot analysis. In contrast, Src family kinase inhibitors did not eliminate a PACAP-induced rise in global calcium generated by inositol (1,4,5)-trisphosphate-induced release of calcium from endoplasmic reticulum stores. From confocal analysis of phosphorylated ERK immunostaining, PP2 treatment significantly attenuated PACAP activation of ERK in neurons within cardiac ganglia whole mount preparations. Intracellular recordings demonstrated that PP2 also significantly blunted a PACAP-induced increase in cardiac neuron excitability. These studies demonstrate Src-related kinase activity in PAC1 receptor internalization, activation of MEK/ERK signaling, and regulation of neuronal excitability. The present results provide further support for the importance of PAC1 receptor endosomal signaling as a key mechanism regulating cellular function.
Keywords: autonomic neuron, neuronal excitability, Src family kinases
INTRODUCTION
Neurally released or exogenously applied pituitary adenylate cyclase activating polypeptide (PACAP, Adcyap1) activation of G protein-coupled PAC1 receptors (Adcyap1r1) significantly increases excitability of guinea pig cardiac neurons (2, 29). Some mechanisms contributing to the modulation of cardiac neuron excitability by PACAP are mediated by plasma membrane G protein-dependent activation of adenylyl cyclase (AC) and downstream signaling cascades (30–32, 34). However, other mechanisms underlying the increase in excitability appear to be mediated by internalization of the PAC1 receptor and activation of endosomal signaling (20, 22, 25). Evidence for this latter mechanism is derived from observations that treatment with inhibitors of clathrin-mediated endocytosis such as the clathrin inhibitor Pitstop 2 (36) or the dynamin I/II inhibitor dynasore (17) essentially eliminates the PACAP-stimulated increase in excitability (22). Reducing ambient temperature also markedly blunts the PACAP-induced PAC1 receptor endocytosis and the increase in neuronal excitability (22).
Activation of MEK/ERK signaling contributes to the PACAP-induced increase in cardiac neuron excitability (30). Furthermore, blockade of clathrin-mediated endocytosis with either Pitstop 2 or dynasore significantly diminishes PACAP-stimulated ERK phosphorylation (pERK) in cardiac neurons (7), indicating that recruitment of signaling cascades downstream of PAC1 receptor/endosomal formation regulates conductances that determine cardiac neuron excitability.
Mechanisms mediating the internalization of many G protein-coupled receptors (GPCRs) have been investigated extensively (8, 9, 14, 16, 18, 21, 28). Following GPCR activation, G protein-coupled receptor kinases (GRKs) phosphorylate GPCRs to foster separation of the receptor from its G protein partners and initiate recruitment of accessory proteins, including β-arrestins and Src family kinases, that in turn recruit clathrin, and direct translocation of the receptor to a clathrin-coated pit (16, 27, 37). Src kinases also mediate phosphorylation of the GTPase dynamin II, a protein responsible for the scission of clathrin-coated vesicles (1, 27). From many prior studies, it is evident that activation of Src family kinases can play a number of critical roles supporting GPCR endocytosis.
The present study was done to establish the role of Src family kinases in PACAP-induced PAC1 receptor internalization/endosomal signaling, leading to phosphorylation of ERK and increased neuronal excitability. The study utilizes two distinct experimental cell systems, a human embryonic kidney cell line stably expressing a green fluorescent protein (GFP)-tagged PAC1 Hop1 receptor (HEK PAC1R-EGFP cells) and neurons in guinea pig cardiac ganglia whole mounts (19, 22). Our results demonstrate that treatment with Src family kinase inhibitors blunt PACAP-induced PAC1 receptor endocytosis, pERK generation and increased neuronal excitability. These studies demonstrate that Src-related kinase activity supports PAC1 receptor internalization and provide further support for the critical role of PAC1 receptor endosomal signaling in regulating cardiac neuron excitability.
METHODS
Animals.
Intracellular recording and some imaging experiments were performed using cardiac ganglia whole mount preparations from Hartley guinea pigs (either sex, 250–350 g), following animal protocols approved by the Institutional Animal Care and Use Committees of the University of Vermont, the University of California, Los Angeles, and Ithaca College. Approved procedures also followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The guinea pigs were euthanized by isoflurane overdose and exsanguination. Hearts were quickly removed and placed in cold Krebs solution (in mM: 121 NaCl, 5.9 KCl, 2.5 CaCl2, 1.2 MgCl2, 25 NaHCO3, 1.2 NaH2PO4, 8 glucose; pH 7.4 maintained by 95% O2–5% CO2 aeration). The cardiac ganglia, located on the epicardium of the atria, were then removed to form a whole mount preparation suitable for intracellular recording from visually identified cells and for confocal analysis of immunolabeled neurons (7, 22, 30).
Cell culture.
Stable HEK PAC1R-EGFP cell lines maintained in DMEM/F-12 medium containing 8% fetal bovine serum were used to monitor PACAP-induced PAC1 receptor internalization and increase in phosphorylated ERK as previously described (19, 22). In brief, for analysis of receptor internalization, cells were seeded onto sterile 15-mm round glass coverslips. Upon 70% confluency, control and test cultures (cultures treated with different inhibitors for 15 min) were treated with PACAP (along with inhibitors when appropriate) for 20 min at 37°C, fixed with 4% paraformaldehyde for 15 min, and mounted on slides. Images were collected with a Nikon C2 confocal system with an Apo 60x oil λS DIC N2 objective with 1.4 numerical aperture (NA). Z-series at 0.1-μm step size were taken at 1,024 × 1,024 resolution through the cells using the 488-nm laser line, with the green detector collecting signal at 525/36 nm.
Chemicals.
All drugs were obtained from commercial sources: PACAP27 (American Peptide, Sunnyvale, CA, or Bachem, Torrance, CA); Bim1 (bisindoylmaleimide I, PP1 (1-(1,1-dimethylethyl)-3-(4-methylphenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine), Src inhibitor (Src-1; 6,7-dimethoxy-N-(4-phenoxyphenyl)-4-quinazolinamine) (Calbiochem EMD Biosciences, La Jolla, CA); Pitstop 2 (N-[5-(4-bromobenzylidene)-4-oxo-4,5-dihydro-1,3-thiazol-2-yl]naphthalene-1-sulfonamide), PP2 (1-tert-butyl-3-(4-chlorophenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine), PP3 (4-amino-1-phenyl-1H-pyrazolo[3,4-d]pyrimidine) (Abcam Biochemicals, Cambridge, UK). Pitstop 2, PP1, PP2, PP3, Src-1, and Bim1 were prepared as DMSO stocks, diluted, and added directly to the bath solution. The final concentration of DMSO was ≤0.2%. PACAP was prepared as a stock in distilled water and diluted with physiological solution just before use.
Western blots for phosphorylated ERK in HEK PAC1R-EGFP cells.
Control and treated cultures were extracted as previously described (19) with 75 µl of RIPA buffer (50 mM Tris·HCl, pH 8.0, 120 mM NaCl, 5 mM EDTA, 1% NP-40, 0.1% SDS) containing 0.3 mg/ml phenylmethylsulfonylfluoride, protease inhibitors (16 µg/ml benzamidine, 2 µg/ml leupeptin, 50 µg/ml lima bean trypsin inhibitor, 2 µg/ml pepstatin A) and phosphatase inhibitor mix (5 mM EDTA, 5 mM EGTA, 1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 50 mM sodium fluoride). Total protein concentrations were determined using the Coomassie Plus reagent (Pierce Technology, Rockford, IL). For Western analyses, protein samples (30 µg) were fractionated on 4–12% lithium dodecyl sulfate polyacrylamide gels, transferred onto Immobilon-FL polyvinylidene difluoride membranes (Millipore, Billerica, MA), blocked, and incubated with pan or phospho-specific ERK1/2 antisera (Cell Signaling Technology, Beverly, MA) for quantitative infrared imaging (Li-Cor Biosciences, Lincoln, NE).
Intracellular recordings from cardiac ganglia neurons.
Intracellular recordings from cardiac neurons followed methods described previously (2, 22, 29). Cardiac ganglia preparations were superfused continuously (6–7 ml/min) with Krebs solution containing 10 mM NaHEPES (32–35°C), and individual neurons were impaled using 2 M KCl-filled microelectrodes (60–120 MΩ). Membrane voltage was recorded using a Multiclamp 700B amplifier coupled with a Digidata 1550B data acquisition system and pCLAMP 10 software (Molecular Devices, Sunnyvale, CA). Depolarizing current steps (0.1–0.5 nA amplitude, 500-ms or 1-s duration) were applied to characterize neuron excitability. Commonly, ~90% of the cardiac neurons exhibit a phasic firing pattern in response to 500- or 1,000-ms depolarizing steps of increasing magnitude (22). However, PACAP significantly increases the number of action potentials elicited by the depolarizing current steps so the response shifts from a phasic action potential firing pattern to a multiple (>5 action potentials/step) firing pattern. Results with both the 500-ms and 1-s duration depolarizing steps were used to quantify the shift in excitability by PACAP. The degree of change in excitability was determined by plotting the number of action potentials generated with the 1-s duration step at each stimulus intensity.
Hyperpolarizing current steps (500 ms) of increasing amplitude were used to test for 1) rectification in the current-induced hyperpolarization, which occurs when the hyperpolarization-activated current Ih is initiated (23); and 2) a transient hyperpolarization-induced rebound depolarization, which is a characteristic feature of activation of the low voltage-activated calcium current, IT (30).
Immunostaining and confocal imaging of cardiac neurons.
The immunostaining of cardiac neurons in the ganglia whole mount preparations followed procedures similar to those reported previously (7, 12, 30). Following different experimental treatments at 37°C, cardiac ganglia whole mounts were fixed in a 2% paraformaldehyde and 0.2% picric acid solution for 2 h at 4°C, washed in PBS, treated with ice-cold methanol for 10 min, and then kept in blocking solution for 30 min before incubation overnight in 1:1,000 rabbit anti-phosphoERK1/2 (D13.14.4E, Cell Signaling Technology) for visualization with Cy3-conjugated donkey anti-rabbit IgG (1:500; Jackson ImmunoResearch, West Grove, PA). The guinea pig cardiac ganglia whole mounts were mounted on glass slides, coverslipped, and imaged with a Nikon/Yokogawa CSU W1 Spinning Disk confocal microscope using a Nikon Apo LWD 25X/1.10NA objective lens. Excitation was accomplished with a 100 mW 561 nm solid state laser, and emission was collected from 590 nm to 650 nm as 16-bit Nikon ND2 image files. Z-series (0.4 µm step) were taken on five random ganglia in each sample and regions of interest (ROI) generated for individual neurons at the axial midpoint adjacent to the nucleus and avoiding the cell membrane. Specifically, a single Z-slice was selected through the middle of the cell and a unique circular ROI was generated for each cell cytoplasm depending on cell size. All hardware settings were carefully maintained across samples and cross-checked by reviewing data header files. Data collection and analysis were all performed using Nikon Elements 4.30.10 (Build 1021). For each experimental condition, data were averaged from neurons in multiple ganglia from at least two whole mount preparations.
Intracellular Ca2+ measurements.
Measurements of PACAP-induced elevations of intracellular calcium using the calcium-sensitive dye Fura-2 AM (Life Technologies, Carlsbad, CA) were similar to those published previously (19). HEK PAC1R-EGFP cells were maintained in a physiological solution containing (in mM) 121 NaCl, 5.9 KCl, 2.5 CaCl2, 1.2 MgCl2, 25 NaHCO3, 1.2 NaH2PO4, 10 NaHEPES buffer and 8 glucose, pH 7.4. The cells were incubated in 4 μM Fura-2 AM along with 0.02% pluronic acid (Life Technologies) for 30 min at room temperature, followed by a 30 min wash in dye-free physiological solution at 37°C. Change in the F340/F380 ratio of Fura-2 AM fluorescence was used to indicate changes in intracellular calcium and was monitored using a PTI monochromator system coupled to a Nikon Eclipse E600 FN equipped with a 60X 1.00NA water dipping physiology lens. The PACAP-induced changes in Fura-2 AM fluorescence were compared in control cells and cells pretreated with two inhibitors of Src family kinases, PP1 and PP2 (both at 10 µM). The data are expressed as the change in the ratio F340/F380 as a function of time. The F340/F380 ratio is not a direct measure of calcium concentration but can be used as a means of illustrating changes in intracellular calcium levels.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism statistical software (version 5.4; La Jolla, CA). Data are presented as means ± SE. When changes in the excitability curve were evaluated, differences in mean action potential numbers elicited by 1-s depolarizing voltage steps at each current magnitude (0.1 to 0.5 nA) were tested for significance using an unpaired Student’s t-test. The χ2-test was used to determine differences between percentages of multiple firing cells in different conditions. In Fig. 5B, the significance of change in mean pERK immunoreactivity (pERK-IR) values was determined using a one-way ANOVA followed by a Tukey’s post hoc test. Values were considered statistically significant at P < 0.05.
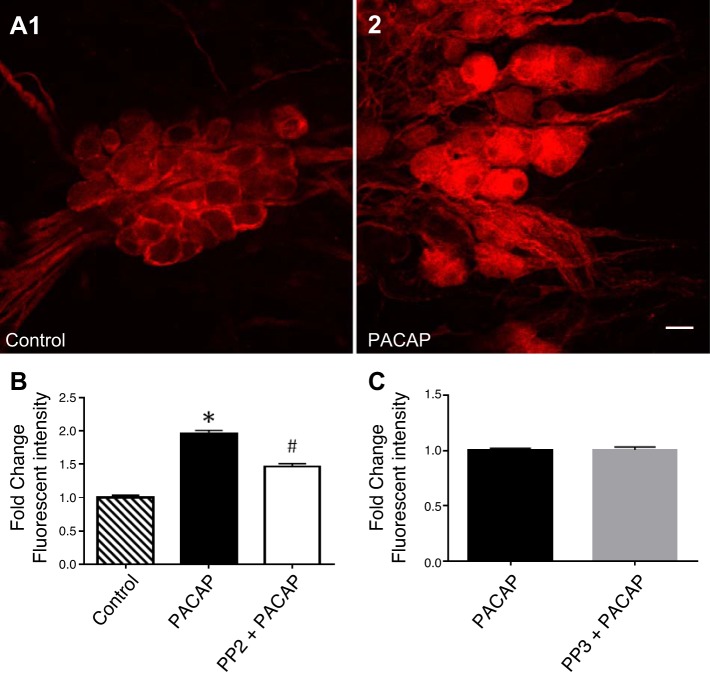
Fig. 5.
Src inhibition decreases the PACAP-induced pERK-IR in guinea pig cardiac neurons. A: confocal images (~1 µm optical sections) of cardiac ganglia neurons processed for pERK immunoreactivity and visualization using a Cy3 conjugated secondary antisera. A1: a control cardiac ganglia preparation maintained at 37°C before fixation. A2: a different cardiac ganglia preparation exposed to 25 nM PACAP at 37°C for 20 min before fixation. Calibration bar, 20 µm. B: averaged fluorescence intensity/area for the control cells, cells exposed to 25 nM PACAP for 20 min and cells pretreated with 10 µM PP2 for 15 min and then exposed to PP2 + PACAP for 20 min. Results are expressed as fold change above control. *pERK-IR is significantly different from control (P = 0.0001). #Significantly different from PACAP treatment and control (for both P = 0.0001). C: averaged fluorescence intensity/area for the cells exposed to 25 nM PACAP for 20 min and cells pretreated with the inactive analog PP3 (10 µM) for 15 min and then exposed to PP3 + PACAP for 20 min. Results are expressed as fold change normalized to PACAP alone. Values are not significantly different (P = 0.83). In B and C, data taken from 3 cardiac ganglia whole mount preparations for each condition.
RESULTS
Src family kinases blunt PACAP-induced PAC1 receptor internalization.
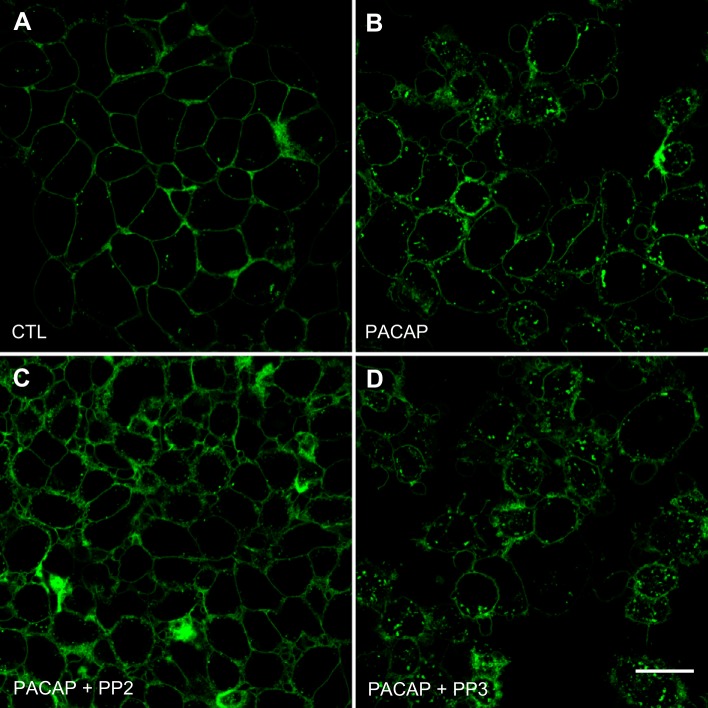
The initial experiments in this study used a stable HEK 293 PAC1R-EGFP cell line (HEK PAC1R EGFP cells) to test whether inhibition of Src family kinases suppressed PACAP-induced PAC1 internalization (19, 22). Confocal images indicated that, before PACAP exposure, the PAC1 receptor was located primarily on the cell surface, as shown by the green fluorescence surrounding each cell (Fig. 1A). However, after a 20-min treatment with 25 nM PACAP at 37°C, the EGFP-PAC1Rs were internalized via endocytic vesicles to form a punctate appearance in the cytoplasm (Fig. 1B). This change in receptor location was comparable to that reported earlier (19, 22).
Fig. 1.
Confocal images demonstrate that PAC1 receptor endocytosis is decreased by pretreatment with Src inhibitor PP2, but not its inactive analog PP3. PAC1 receptors tagged with EGFP are expressed predominantly on the cell surface of untreated control (CTL) cells (A). After 25 nM PACAP exposure for 20 min, internalized GFP-PAC1 receptors are present in numerous endocytic vesicles (B). When cultures were pretreated for 15 min with 10 µM PP2, 25 nM PACAP exposure for 20 min did not initiate EGFP-PAC1 receptor endocytosis (C). When cultures were pretreated for 15 min with 10 µM PP3 and then exposed to PACAP, EGFP-PAC1 receptor endocytosis was evident (D). Each panel is a projection of three confocal slices through the midpoint of the cell. Calibration bar, 20 μm.
In subsequent experiments, the HEK PAC1R-EGFP cells were pretreated with the Src family kinase inhibitor PP2 (10 µM) for 15 min and then exposed to 25 nM PACAP + PP2 for 20 min. All treatments were done at 37°C. PP2 pretreatment markedly reduced the PACAP-induced PAC1R internalization so most of the receptors remained at the cell surface (Fig. 1C). This observation is similar to that noted previously when the HEK PAC1R-EGFP cells were pretreated with either the clathrin inhibitor Pitstop 2 or the dynamin I/II inhibitor dynasore before exposure to PACAP (22). In contrast, pretreatment with 10 µM PP3, an inactive analog of PP2, did not blunt the PACAP-induced PAC1 receptor internalization (Fig. 1D). Collectively, these results demonstrate that PP2 blunts the PACAP-induced endocytosis of the PAC1 receptor.
Inhibition of the Src family kinases reduces PACAP-induced ERK phosphorylation in the HEK PAC1R-EGFP cells.
Previously, we used the HEK PAC1R-EGFP cells to establish that PAC1R endocytosis contributed to the total ERK phosphorylation induced by PACAP (19). Given that PP2 blunted PAC1 receptor internalization, inhibition of Src family kinases should also reduce PACAP-induced ERK phosphorylation. To test this prediction, different HEK PAC1R-EGFP cell cultures were pretreated for 15 min with two Src family inhibitors, PP2 (10 µM) or a more specific inhibitor Src-1 (2 µM), and then exposed to 25 nM PACAP + either PP2 or Src-1 for 15 min. A third group of HEK PAC1R-EGFP cells was pretreated with 10 µM PP3, the inactive analog of PP2, and then exposed to PACAP + PP3. A fourth group of cells was exposed to only PACAP. All treatments were done with the cultures maintained at 37°C. As evident from the Western blot analysis, pretreatment with the Src family kinase inhibitors significantly blunted the ERK phosphorylation (Fig. 2, A and B). The inhibition by both PP2 and Src-1 was 50–70%, whereas pretreatment with PP3 did not significantly decrease pERK levels (Fig. 2, A and B).
Fig. 2.
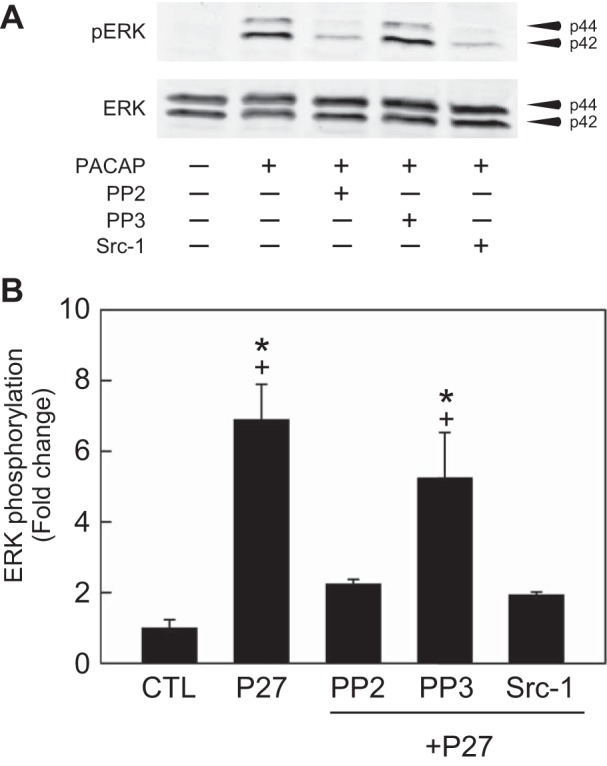
Src family inhibitors suppress PACAP-induced ERK phosphorylation. A: representative Western blot analysis. PAC1 receptor cell cultures were treated with PACAP (P27, 25 nM) alone for 15 min. Other cell cultures were pretreated with Src family inhibitors PP2 (10 µM) or Src-1 (2 µM), or with the inactive PP2 analog PP3 (10 µM) for 15 min before exposure to PACAP in the presence of Src compounds for an additional 15 min. B: PACAP stimulated ERK activation ~7-fold at 15 min. Following pretreatment with PP2 or Src-1, the PACAP-induced ERK phosphorylation was significantly reduced >50%. In contrast, pretreatment with PP3 did not significantly reduce the PACAP-induced ERK phosphorylation. All data were normalized to total ERK levels in the same samples. Data represent mean fold change ± SE; n = 3 independent experiments. *Different from CTL. +Different from P27 following pretreatment with PP2 or Src-1; all at P < 0.05.
Two mechanisms contribute to the total PACAP-induced ERK phosphorylation in the HEK PAC1R-EGFP cells, internalization/endosomal signaling and activation of the PLC/DAG/PKC cascade (19). Another series of experiments was done to establish that the effect of the Src family kinase inhibitors on ERK phosphorylation was distinct from inhibition of PLC/PKC signaling. Cultures were pretreated with either PP2 (10 µM) or the PKC inhibitor Bim1 (1 µM) and then exposed to PACAP + PP2 or Bim1 for 15 min (Fig. 3A). As noted previously, PP2 blunted pERK generation by ~50% and Bim1 reduced the PACAP-induced ERK phosphorylation by ~40% (Fig. 3, A and B). Other cultures were pretreated with both Bim1 and PP2 and then exposed to 25 nM PACAP + both inhibitors. In the presence of both inhibitors, the PACAP-induced level of pERK was not different from control levels of pERK seen in the absence of PACAP exposure (Fig. 3B). These observations indicate that the PP2 and Bim1 inhibition involves two distinct but complementary pathways contributing to the PACAP-induced ERK phosphorylation; results similar to those obtained with cotreatment of the clathrin inhibitor Pitstop 2 and Bim1 (19).
Fig. 3.
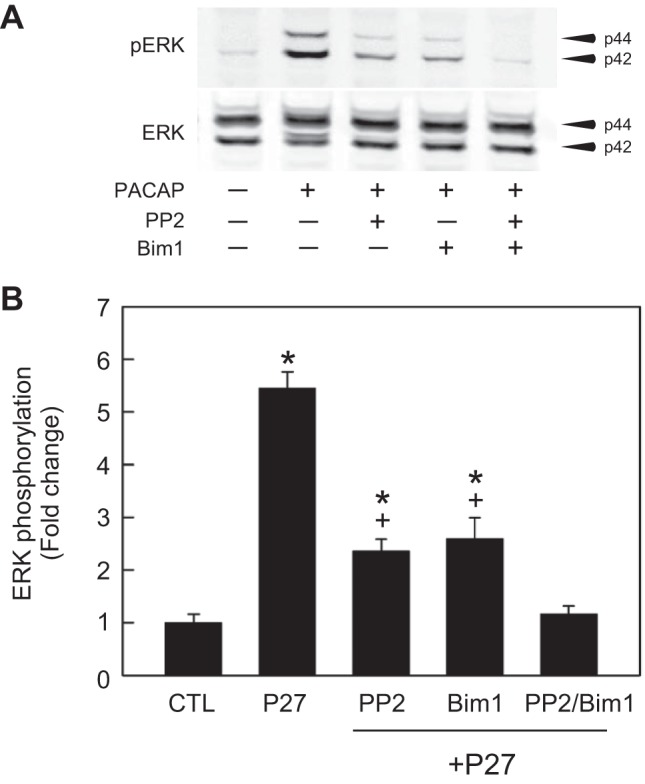
Combined Src and PKC inhibition blocks PACAP activation of ERK. A: representative Western blot analysis. Different PAC1 receptor cell cultures were treated with PACAP (P27, 25 nM) alone for 15 min or with PACAP + an inhibitor after 15 min pretreatment with PP2 (10 µM) or with the PKC inhibitor Bim1 (1 µM) or with both PP2 + Bim 1. B: PACAP (P27, 25 nM 15 min)-stimulated ERK activation ~5.5-fold; an effect attenuated by ~50% after a 15 min pretreatment of PAC1 receptor cells with either the Src inhibitor PP2 (10 μM) or the PKC inhibitor Bim 1 (1 μM) for 15 min. In the presence of both inhibitors, the PACAP-induced increase in phosphorylated ERK was diminished to control levels. All data were normalized to total ERK levels in the same samples. Data represent mean fold change ± SE; n = 3. *Different from CTL. +Different from P27; all at P < 0.05.
Inhibition of Src family kinases does not eliminate the PACAP-induced transient rise in intracellular Ca2+.
The PAC1 receptor can engage Gαq to activate PLC resulting in the generation of DAG/PKC and inositol (1,4,5)-trisphosphate (IP3) signaling (19). PACAP/PAC1 generation of IP3 initiates Ca2+ release from IP3 receptors in the endoplasmic reticulum (19). Additional studies tested whether treatment with the Src family kinase inhibitors, PP1 or PP2, affected IP3-induced Ca2+ release from Ca2+ stores in the endoplasmic reticulum. The initial Ca2+ imaging experiments were done with the HEK PAC1R-EGFP cells maintained at room temperature (~24°C), a condition that virtually eliminates any PACAP-induced endocytosis over the course of the experiment while PACAP-induced release of Ca2+ remains (19). However, pretreatment with the inhibitor was done with the cultures maintained at 37°C. Using Fura-2-loaded HEK PAC1R-EGFP cells, PACAP (25 nM) elicited a transient rise in intracellular Ca2+ as reported previously (Fig. 4A). Pretreatment with inhibitors of Src family kinases, either 10 µM PP1 or 10 µM PP2, did not blunt the 25 nM PACAP-induced rise in intracellular Ca2+ (Fig. 4B, data with PP1 pretreatment shown). Because kinase action can be reduced at room temperature (6, 19), the effect of PP2 on the PACAP-induced rise in intracellular calcium was also tested with the cells maintained at 34°C. Again, as seen in Fig. 4C, PACAP elicited a significant rise in intracellular calcium in HEK PAC1R-EGFP cells maintained at warm temperatures. We attribute the difference in latency between switching to the PACAP solution and beginning of the rise in calcium to differences in perfusion rate between experiments. Together, these results indicate that activation of PLC/DAG/IP3 signaling was not Src kinase dependent.
Fig. 4.
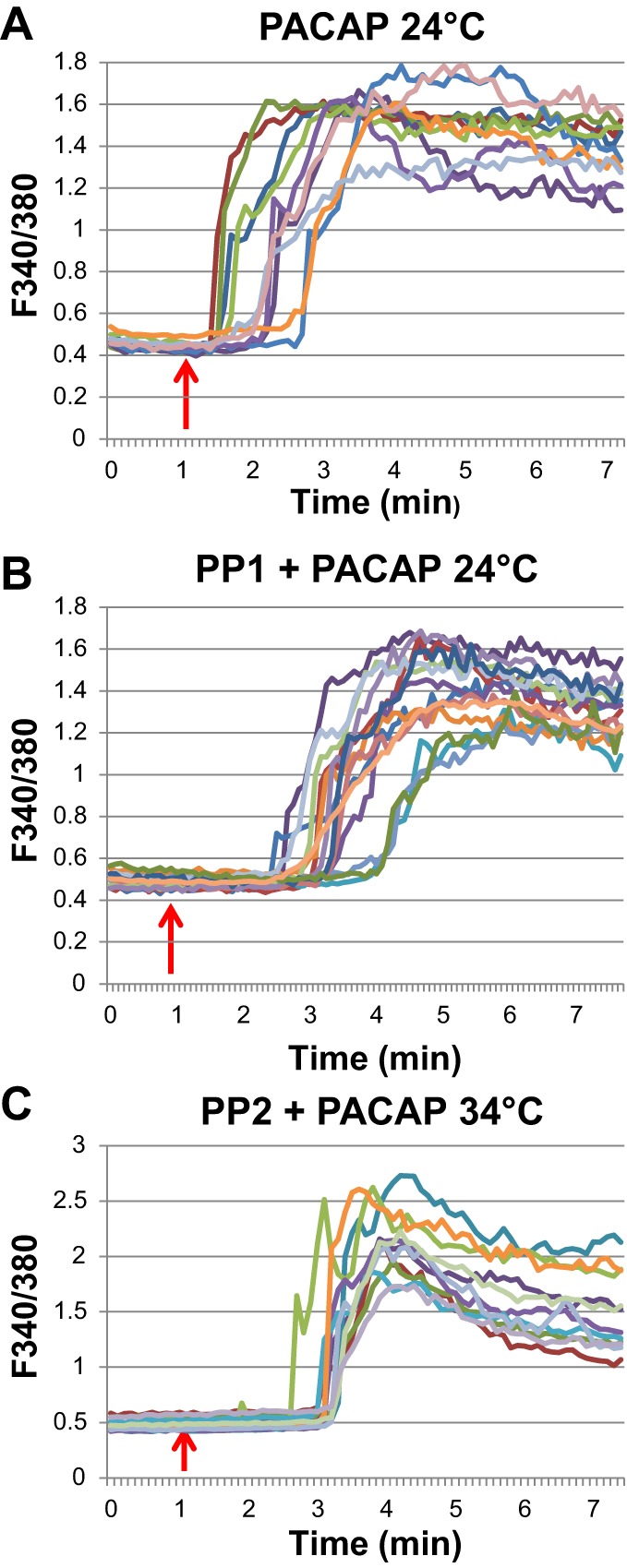
The PACAP-induced rise in intracellular Ca2+ was not eliminated by inhibition of Src family kinases. A: traces illustrating the transient rise in intracellular Ca2+ during PACAP treatment of PAC1 receptor cells at room temperature (~24°C). B: pretreatment at 37°C for 15 min with the Src family inhibitor PP1 (10 µM) had no effect on the PACAP-induced rise in intracellular calcium measured at 24°C. C: PACAP also elicited a marked rise in intracellular calcium recorded at 34°C following a 15-min pretreatment at 37°C with 10 µM PP2. Each trace in all panels is from an individual cell; the arrows indicate approximate initiation of 25 nM PACAP addition to the recording bath. Following the rapid rise in intracellular Ca2+ in PACAP, the signal decayed slowly toward a steady lower plateau (not shown).
PP2 reduces PACAP-stimulated ERK phosphorylation in guinea pig cardiac neurons.
In earlier studies, we demonstrated that PACAP activates the MEK/ERK signaling cascade in guinea pig cardiac neurons by quantifying changes in phosphorylated ERK immunoreactivity (pERK-IR) by confocal microscopy (7, 30). Different cardiac ganglia whole mount preparations were used to test whether PP2 affected PACAP-induced pERK generation in the cardiac neurons. As reported previously, in control cardiac ganglia neurons, maintained at 37°C, for ~20 min, basal pERK-IR was evident in cardiac neurons, Schwann cells and satellite cells (Fig. 5A1). After a 20-min exposure to 20 nM PACAP at 37°C, pERK-IR increased approximately twofold (Fig. 5, A2 and B). In cardiac ganglia whole mounts pretreated with 10 µM PP2 for 20 min and then exposed for 20 min to 20 nM PACAP + PP2 (both treatments at 37°C), pERK-IR was ~50% less than that produced by PACAP alone (Fig. 5B). This result is consistent with a component of pERK generation in cardiac neurons mediated by PAC1 internalization/endosomal signaling (7).
In a separate series of experiments, we tested whether PP3, the inactive analog of PP2, also affected the PACAP-induced activation of ERK. As evident from inspection of Fig. 5C, pretreatment with PP3 had no effect on the PACAP-induced increase in cardiac neuron pERK-IR.
Pretreatment with the Src family kinase inhibitor PP2 suppresses the PACAP induced increase in cardiac neuron excitability without suppressing the hyperpolarization -induced rectification or rebound depolarization.
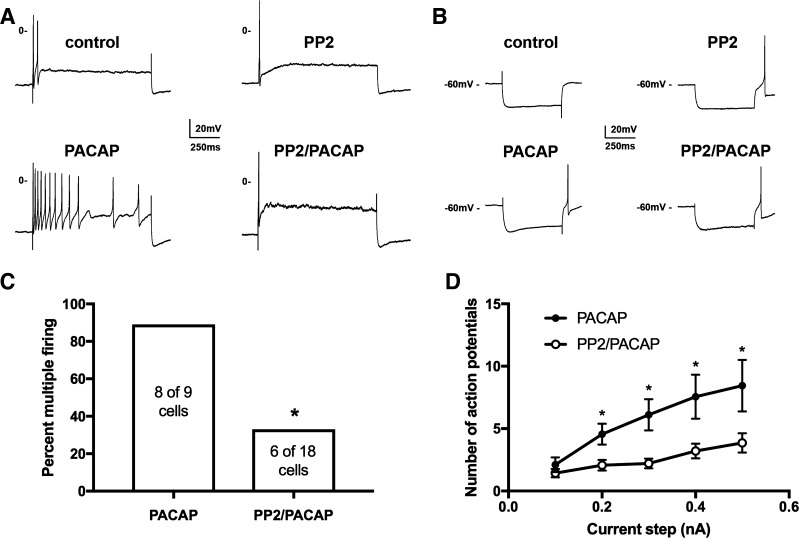
Exogenously applied and neurally released PACAP increases excitability of cardiac neurons as assessed from an increase in the number of action potentials elicited by suprathreshold depolarizing steps (2, 29). Our prior studies have demonstrated that this effect of PACAP on excitability is significantly depressed by conditions that inhibit PACAP-induced PAC1 endocytosis and recruitment of endosomal directed signaling cascades such as low temperature, treatment with the clathrin inhibitor Pitstop 2 or exposure to the dynamin I/II inhibitor dynasore (20, 22, 25). Because the Src family kinase inhibitor blunted PACAP-induced PAC1 internalization and pERK generation, it would be expected that Src inhibitors would also depress the increased neuronal excitability by PACAP. This prediction was tested using intracellular recordings from neurons in whole mount cardiac ganglia preparations. For these experiments, recordings were made from two groups of cardiac neurons that were exposed to 20 nM PACAP; control cells, and cells that were pretreated for at least 15 min with 10 µM PP2. The experimental protocol was to initially obtain intracellular recordings from the same cell before and then during PACAP application. After that, excitability was tested from other cardiac neurons from the same ganglia preparation over time in PACAP. As shown in Fig. 6A, cardiac neuron excitability was markedly enhanced, as evident from the shift of 2 action potentials to 12 action potentials elicited by a 1-s, 0.3-nA depolarizing step before and during exposure to 20 nM PACAP. PACAP increased excitability in 8 of 9 cells from 5 whole mount preparations (89%, Fig. 6C). In contrast, when whole mount preparations were pretreated with 10 µM PP2 and then exposed to PACAP + PP2, excitability was increased in only 6 of 18 cells from 7 whole mount preparations (33%, using data derived with 4 cells stimulated with 500-ms and 14 cells with 1-s duration steps; Fig. 6C). An example recording from a 10 µM PP2-pretreated cell before and during exposure to 20 nM PACAP + PP2 also is shown in Fig. 6A. Note that in this example, 1 action potential was elicited by a 1-s, 0.3-nA depolarizing step in PP2 alone and 1 action potential was also elicited by an identical depolarizing step during exposure to PACAP + PP2.
Fig. 6.
Src family inhibitor PP2 pretreatment suppresses the PACAP-induced increase in excitability without inhibiting rectification in current-induced hyperpolarizations. A: example traces illustrating the increase in excitability by 20 nM PACAP in a control cell (left traces) and example traces from a second cell illustrating the suppression of the PACAP-induced increase in excitability following pretreatment with 10 µM PP2 (right traces). B: in the control cell before PACAP, there was minimal rectification in the current-induced hyperpolarization and no posthyperpolarization-induced action potential. During 20 nM PACAP, the rectification became more evident and an action potential was elicited at the termination of the hyperpolarization. In the 10 µM PP2-pretreated cell before PACAP, there was no evidence of rectification in the hyperpolarizing step, but an action potential was initiated, when the hyperpolarization was terminated. In contrast, during PACAP application, rectification was evident in the current-induced hyperpolarization and an action potential was initiated after termination of the hyperpolarization. C: the percentage of cells exhibiting multiple firing (>5 action potentials) in PACAP alone (8 of 9) was significantly greater than when exposed to PACAP after pretreatment to 10 µM PP2 (6 of 18) (*P = 0.02, χ2). D: averaged excitability curves show that PP2 greatly suppressed the PACAP-induced increase in excitability. Asterisks indicate that the number of action potentials generated at each current step was significantly greater in PACAP (n = 9) than in PP2 and PACAP (n = 14; unpaired t-test, P = 0.008 for 0.2 nA: P = 0.002 for 0.3 nA; P = 0.013 for 0.4 nA; P = 0.024 for 0.5 nA).
Averaged excitability curves were created by plotting the number of action potentials elicited by depolarizing steps of increasing intensity (0.1 to 0.5 nA) to compare the change in excitability produced by PACAP in control cells and cells pretreated with PP2. For this comparison, only results obtained with the 1-s duration steps were used. As presented in Fig. 6D, PP2 significantly (P < 0.05) decreased the number of action potentials elicited by all depolarizing steps greater in magnitude than 0.1 nA. All of the observations indicate that PP2 produced a marked depression of the PACAP-induced increase in cardiac neuron excitability.
Multiple mechanisms can contribute to the PACAP modulation of cardiac neuron excitability, including an enhancement of the hyperpolarization-activated, cAMP modulated nonselective cation current Ih and the low voltage-activated calcium current IT (23, 25, 32, 33). The nonselective cation current Ih is evident as an increased rectification in the hyperpolarization induced by constant current injection, and IT contributes to the hyperpolarization-induced depolarization that follows the termination of the hyperpolarizing step. In the traces shown in Fig. 6B, the rectification in the hyperpolarization to ~−90 mV (Control) noticeably increased during exposure to PACAP (PACAP). This is consistent with a PACAP activation of adenylyl cyclase and increase in cytoplasmic cAMP concentration, which in turn produces a positive shift in the activation curve for Ih (23). In this cell, there was no obvious post hyperpolarization-induced depolarization before exposure to PACAP, but during PACAP application, a prominent hyperpolarization-induced depolarization was evident and it depolarized the cell sufficiently to elicit an action potential. In the example traces in Fig. 6B obtained from the PP2-pretreated cell, there was no rectification noted before PACAP application (PP2), but during exposure to PACAP, rectification became more evident, indicating an enhancement of Ih by PACAP (PP2/PACAP). In addition, in the PP2-pretreated cell, there was a hyperpolarization-induced depolarization, which elicited an action potential before and after PACAP exposure. Taken together, these observations, which are representative of results obtained from multiple recordings, indicated that PP2 did not affect the PACAP modulation of either Ih or IT.
DISCUSSION
Observations derived from this study demonstrate that Src family kinase inhibitors 1) blunt the PACAP-induced PAC1 receptor internalization, 2) diminish PACAP-induced phosphorylation of ERK, and 3) suppress the PACAP-induced increase in neuronal excitability.
Pretreatment with the Src family inhibitors PP1 and PP2 had no marked effect on the PACAP/PAC1 receptor-induced activation of PLC/IP3 signaling and activation of IP3 receptors, leading to a rise in intracellular calcium. This result indicated that signaling events downstream of PACAP/PAC1 receptor interactions that were blunted by Src family kinase inhibitors likely were not related to disruption of membrane delimited signaling.
In addition to membrane delimited G protein-dependent signaling, GPCRs undergo a sequence of steps leading to receptor internalization and endosomal signaling (4, 5, 11, 13, 35). GPCR endocytosis had initially been associated with receptor desensitization and recycling pathways, but it is now recognized to represent a mechanism supporting sustained second messenger generation via signaling endosomes (4, 5, 11, 13, 15, 35). After GPCR ligand binding and subsequent conformational change, different kinases have the potential to phosphorylate specific intracellular sites on the receptor. G protein receptor kinase (GRK) phosphorylation of serine/threonine clusters appears to be one central mechanism facilitating β-arrestin recruitment. The binding of β-arrestin to GPCRs facilitates the recruitment of other accessory proteins to initiate GPCR translocation to clathrin-coated pits and then endocytosis. Src nonreceptor tyrosine kinases are one component of the activated β-arrestin signaling platform. Src family kinases can regulate clathrin-mediated endocytosis by dynamin phosphorylation and assembly (24), phosphorylation of β2-adaptin subunit of the AP-2 complex (38), and regulation of GRKs (26). Thus, Src family kinases facilitate creation of the clathrin coat needed to initiate endocytosis and then for vesicular scission by dynamin I/II. The role of Src kinase in PAC1 receptor internalization/endosomal signaling was tested as an approach independent of direct clathrin or dynamin inhibition. Our results demonstrate that the Src inhibitors PP2 and Src-1 attenuated PAC1 receptor endocytosis and ERK signaling in parallel, whereas the inactive inhibitor analog PP3 had no apparent effects.
Having shown that inhibition of Src family kinases blunted PAC1 endocytosis in the HEK PAC1R-EGFP cells, we tested whether treatment with PP2 would blunt the PACAP-induced activation of cardiac neuron MEK/ERK signaling and block the PACAP-induced shift in excitability in cardiac neurons. As seen with the HEK PAC1R-EGFP cells, PP2, but not PP3, reduced the PACAP generation of pERK. The suppression by PP2 was to an extent similar to that noted previously for the clathrin inhibitor Pitstop 2 (7). In addition, also like Pitstop 2 and the dynamin inhibitor dynasore (22), PP2 significantly blunted the PACAP-induced increase in cardiac neuron excitability.
We have suggested that PP2 reduces PACAP-induced pERK generation primarily by suppressing PACAP-induced PAC1 receptor internalization/endosomal signaling leading to the recruitment of MEK. However, like many kinase inhibitors, PP2 can have off-target actions. For instance, Brandvold et al. (3) have reported that PP2 can inhibit a number of other kinases with comparable efficacy. Of note to our results is a potential inhibition of B-raf, which is an intermediate in a proposed Rapgef2 pathway mediating cAMP-dependent, PKA-independent, ERK activation in endocrine and neuronal cell lines (10). This is not a concern in our experiments using the HEK PAC1R-EGFP cells as nonneuronal cells do not express Rapgef2 (10), supporting the view that PACAP generation of pERK in these cells is mediated by a combination of PLC/PKC and PAC1 receptor internalization/endosomal signaling (19). However, the proposed cAMP/Rapgef2/B-raf pathway might contribute to the PACAP-induced activation of ERK in the cardiac neurons. Although certainly possible, earlier results indicate that the PACAP-induced increase in cardiac neuron pERK could be accounted for by recruitment of MEK/ERK through combined AC/cAMP/PKA and PAC1 internalization/endosomal signaling (7). Furthermore, given that PP2 and Pitstop 2 diminish pERK generation by the same extent, we suggest that the major effect of PP2 on PACAP-induced pERK generation in the cardiac neurons is related to an inhibitory effect on PACAP-induced PAC1 receptor internalization.
We also suggest that the PP2 suppression of PACAP enhanced excitability was not due to a potential inhibition of the AC/cAMP/Rapgef2/B-raf signaling pathway. Recently, we reported that the potent AC activator forskolin increased cardiac neuron excitability, but this effect was not inhibited by PP2 (12), a result indicating that activation of the cAMP/Rapgef2/B-raf signaling cascade did not potentially contribute to the forskolin-induced increase in neuronal excitability. Given that PACAP and forskolin increase cAMP levels to a comparable extent, it would be expected that the increase in cardiac neuron excitability by both drugs would be blunted by PP2, if a major site of PP2 action was on the cAMP/Rapgef2/B-raf signaling pathway.
In conclusion, inhibition of Src family kinase activity blocked PAC1 receptor internalization in parallel with attenuated ERK signaling and PACAP-enhanced neuronal excitability. Together, the present observations provide additional support for the hypothesis that PAC1 receptor internalization/endosomal signaling is a critical mechanism supporting the PACAP modulation of neuronal excitability.
GRANTS
This work was supported in part by National Institutes of Health (NIH) National Institute of General Medical Sciences (NIGMS) Grant P30 GM103498/National Center for Research Resources (NCRR) Grant P30 RR032135 (to R. L. Parsons) and by NIH/Department of Health and Human Services (DHHS) Grant S10 OD017969-01 (to R. L. Parsons).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
V.M. and R.L.P. conceived and designed research; J.D.T., T.A.C., T.R.B., B.M.G., A.K.L., J.C.H., L.A.M., V.M., and R.L.P. performed experiments; J.D.T., T.A.C., T.R.B., B.M.G., A.K.L., J.C.H., L.A.M., V.M., and R.L.P. analyzed data; J.D.T., T.A.C., T.R.B., B.M.G., J.C.H., L.A.M., V.M., and R.L.P. interpreted results of experiments; J.D.T., T.A.C., L.A.M., and V.M. prepared figures; J.D.T., T.A.C., T.R.B., B.M.G., J.C.H., V.M., and R.L.P. edited and revised manuscript; J.D.T., T.A.C., T.R.B., B.M.G., A.K.L., J.C.H., L.A.M., V.M., and R.L.P. approved final version of manuscript; V.M. and R.L.P. drafted manuscript.
REFERENCES
- 1.Auciello G, Cunningham DL, Tatar T, Heath JK, Rappoport JZ. Regulation of fibroblast growth factor receptor signalling and trafficking by Src and Eps8. J Cell Sci 126: 613–624, 2013. doi: 10.1242/jcs.116228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braas KM, May V, Harakall SA, Hardwick JC, Parsons RL. Pituitary adenylate cyclase-activating polypeptide expression and modulation of neuronal excitability in guinea pig cardiac ganglia. J Neurosci 18: 9766–9779, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandvold KR, Steffey ME, Fox CC, Soellner MB. Development of a highly selective c-Src kinase inhibitor. ACS Chem Biol 7: 1393–1398, 2012. doi: 10.1021/cb300172e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calebiro D, Nikolaev VO, Gagliani MC, de Filippis T, Dees C, Tacchetti C, Persani L, Lohse MJ. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol 7: e1000172, 2009. doi: 10.1371/journal.pbio.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calebiro D, Nikolaev VO, Persani L, Lohse MJ. Signaling by internalized G-protein-coupled receptors. Trends Pharmacol Sci 31: 221–228, 2010. doi: 10.1016/j.tips.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Chemin J, Mezghrani A, Bidaud I, Dupasquier S, Marger F, Barrère C, Nargeot J, Lory P. Temperature-dependent modulation of CaV3 T-type calcium channels by protein kinases C and A in mammalian cells. J Biol Chem 282: 32710–32718, 2007. doi: 10.1074/jbc.M702746200. [DOI] [PubMed] [Google Scholar]
- 7.Clason TA, Girard BM, May V, Parsons RL. Activation of MEK/ERK signaling by PACAP in guinea pig cardiac neurons. J Mol Neurosci 59: 309–316, 2016. doi: 10.1007/s12031-016-0766-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delom F, Fessart D. Role of phosphorylation in the control of clathrin-mediated internalization of GPCR. Int J Cell Biol 2011: 246954, 2011. doi: 10.1155/2011/246954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Fiore PP, von Zastrow M. Endocytosis, signaling, and beyond. Cold Spring Harb Perspect Biol 6: a016865, 2014. doi: 10.1101/cshperspect.a016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emery AC, Eiden MV, Mustafa T, Eiden LE. Rapgef2 connects GPCR-mediated cAMP signals to ERK activation in neuronal and endocrine cells. Sci Signal 6: ra51, 2013. doi: 10.1126/scisignal.2003993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrandon S, Feinstein TN, Castro M, Wang B, Bouley R, Potts JT, Gardella TJ, Vilardaga JP. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol 5: 734–742, 2009. doi: 10.1038/nchembio.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardwick JC, Clason TA, Tompkins JD, Girard BM, Baran CN, Merriam LA, May V, Parsons RL. Recruitment of endosomal signaling mediates the forskolin modulation of guinea pig cardiac neuron excitability. Am J Physiol Cell Physiol 313: C219–C227, 2017. doi: 10.1152/ajpcell.00094.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irannejad R, Tomshine JC, Tomshine JR, Chevalier M, Mahoney JP, Steyaert J, Rasmussen SG, Sunahara RK, El-Samad H, Huang B, von Zastrow M. Conformational biosensors reveal GPCR signalling from endosomes. Nature 495: 534–538, 2013. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jalink K, Moolenaar WH. G protein-coupled receptors: the inside story. BioEssays 32: 13–16, 2010. doi: 10.1002/bies.200900153. [DOI] [PubMed] [Google Scholar]
- 15.Luttrell LM, Gesty-Palmer D, Sibley DR. Beyond desensitization: physiological relevance of arrestin-dependent signaling. Pharmacol Rev 62: 305–330, 2010. doi: 10.1124/pr.109.002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science 283: 655–661, 1999. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 17.Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell 10: 839–850, 2006. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Magalhaes AC, Dunn H, Ferguson SS. Regulation of GPCR activity, trafficking and localization by GPCR-interacting proteins. Br J Pharmacol 165: 1717–1736, 2012. doi: 10.1111/j.1476-5381.2011.01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.May V, Buttolph TR, Girard BM, Clason TA, Parsons RL. PACAP-induced ERK activation in HEK cells expressing PAC1 receptors involves both receptor internalization and PKC signaling. Am J Physiol Cell Physiol 306: C1068–C1079, 2014. doi: 10.1152/ajpcell.00001.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.May V, Parsons RL. G protein-coupled receptor endosomal signaling and regulation of neuronal excitability and stress responses: signaling options and lessons from the PAC1 receptor. J Cell Physiol 232: 698–706, 2017. doi: 10.1002/jcp.25615. [DOI] [PubMed] [Google Scholar]
- 21.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 12: 517–533, 2011. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- 22.Merriam LA, Baran CN, Girard BM, Hardwick JC, May V, Parsons RL. Pituitary adenylate cyclase 1 receptor internalization and endosomal signaling mediate the pituitary adenylate cyclase activating polypeptide-induced increase in guinea pig cardiac neuron excitability. J Neurosci 33: 4614–4622, 2013. doi: 10.1523/JNEUROSCI.4999-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merriam LA, Barstow KL, Parsons RL. Pituitary adenylate cyclase-activating polypeptide enhances the hyperpolarization-activated nonselective cationic conductance, Ih, in dissociated guinea pig intracardiac neurons. Regul Pept 123: 123–133, 2004. doi: 10.1016/j.regpep.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 24.Miller WE, Maudsley S, Ahn S, Khan KD, Luttrell LM, Lefkowitz RJ. β-Arrestin 1 interacts with catalytic domain of the tyrosine kinase c-Src. J Biol Chem 275: 11312–11319, 2000. doi: 10.1074/jbc.275.15.11312. [DOI] [PubMed] [Google Scholar]
- 25.Parsons RL, Tompkins JD, Hardwick JC, Merriam LA, Girard BM, May V. Multiple mechanisms contribute to the PAC1 modulation of parasympathetic cardiac neuron excitability Pituitary Adenylate Cyclase Activating Polypeptide—PACAP, edited by Reglodi D, Tamas A. New York: Springer; 2016, p. 205–225. [Google Scholar]
- 26.Penela P, Elorza A, Sarnago S, Mayor F Jr. β-arrestin- and c-Src-dependent degradation of G-protein-coupled receptor kinase 2. EMBO J 20: 5129–5138, 2001. doi: 10.1093/emboj/20.18.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reinecke J, Caplan S. Endocytosis and the Src family of non-receptor tyrosine kinases. Biomol Concepts 5: 143–155, 2014. doi: 10.1515/bmc-2014-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scita G, Di Fiore PP. The endocytic matrix. Nature 463: 464–473, 2010. doi: 10.1038/nature08910. [DOI] [PubMed] [Google Scholar]
- 29.Tompkins JD, Ardell JL, Hoover DB, Parsons RL. Neurally released pituitary adenylate cyclase-activating polypeptide enhances guinea pig intrinsic cardiac neurone excitability. J Physiol 582: 87–93, 2007. doi: 10.1113/jphysiol.2007.134965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tompkins JD, Clason TA, Hardwick JC, Girard BM, Merriam LA, May V, Parsons RL. Activation of MEK/ERK signaling contributes to the PACAP-induced increase in guinea pig cardiac neuron excitability. Am J Physiol Cell Physiol 311: C643–C651, 2016. doi: 10.1152/ajpcell.00164.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tompkins JD, Hardwick JC, Locknar SA, Merriam LA, Parsons RL. Ca2+ influx, but not Ca2+ release from internal stores, is required for the PACAP-induced increase in excitability in guinea pig intracardiac neurons. J Neurophysiol 95: 2134–2142, 2006. doi: 10.1152/jn.01077.2005. [DOI] [PubMed] [Google Scholar]
- 32.Tompkins JD, Lawrence YT, Parsons RL. Enhancement of Ih, but not inhibition of IM, is a key mechanism underlying the PACAP-induced increase in excitability of guinea pig intrinsic cardiac neurons. Am J Physiol Regul Integr Comp Physiol 297: R52–R59, 2009. doi: 10.1152/ajpregu.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tompkins JD, Merriam LA, Girard BM, May V, Parsons RL. Nickel suppresses the PACAP-induced increase in guinea pig cardiac neuron excitability. Am J Physiol Cell Physiol 308: C857–C866, 2015. doi: 10.1152/ajpcell.00403.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tompkins JD, Parsons RL. Identification of intracellular signaling cascades mediating the PACAP-induced increase in guinea pig cardiac neuron excitability. J Mol Neurosci 36: 292–298, 2008. doi: 10.1007/s12031-008-9086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vilardaga JP, Jean-Alphonse FG, Gardella TJ. Endosomal generation of cAMP in GPCR signaling. Nat Chem Biol 10: 700–706, 2014. doi: 10.1038/nchembio.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Kleist L, Stahlschmidt W, Bulut H, Gromova K, Puchkov D, Robertson MJ, MacGregor KA, Tomilin N, Pechstein A, Chau N, Chircop M, Sakoff J, von Kries JP, Saenger W, Kräusslich HG, Shupliakov O, Robinson PJ, McCluskey A, Haucke V. Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell 146: 471–484, 2011. doi: 10.1016/j.cell.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 37.Wang Q, Lu R, Zhao J, Limbird LE. Arrestin serves as a molecular switch, linking endogenous α2-adrenergic receptor to SRC-dependent, but not SRC-independent, ERK activation. J Biol Chem 281: 25948–25955, 2006. doi: 10.1074/jbc.M605415200. [DOI] [PubMed] [Google Scholar]
- 38.Zimmerman B, Simaan M, Lee MH, Luttrell LM, Laporte SA. c-Src-mediated phosphorylation of AP-2 reveals a general mechanism for receptors internalizing through the clathrin pathway. Cell Signal 21: 103–110, 2009. doi: 10.1016/j.cellsig.2008.09.013. [DOI] [PubMed] [Google Scholar]