Abstract
Coronary microvascular function and blood flow responses during acute exercise are impaired in the aged heart but can be restored by exercise training. Coronary microvascular resistance is directly dependent on vascular smooth muscle function in coronary resistance arterioles; therefore, we hypothesized that age impairs contractile function and alters the phenotype of vascular smooth muscle in coronary arterioles. We further hypothesized that exercise training restores contractile function and reverses age-induced phenotypic alterations of arteriolar smooth muscle. Young and old Fischer 344 rats underwent 10 wk of treadmill exercise training or remained sedentary. At the end of training or cage confinement, contractile responses, vascular smooth muscle proliferation, and expression of contractile proteins were assessed in isolated coronary arterioles. Both receptor- and non-receptor-mediated contractile function were impaired in coronary arterioles from aged rats. Vascular smooth muscle shifted from a differentiated, contractile phenotype to a secretory phenotype with associated proliferation of smooth muscle in the arteriolar wall. Expression of smooth muscle myosin heavy chain 1 (SM1) was decreased in arterioles from aged rats, whereas expression of phospho-histone H3 and of the synthetic protein ribosomal protein S6 (rpS6) were increased. Exercise training improved contractile responses, reduced smooth muscle proliferation and expression of rpS6, and increased expression of SM1 in arterioles from old rats. Thus age-induced contractile dysfunction of coronary arterioles and emergence of a secretory smooth muscle phenotype may contribute to impaired coronary blood flow responses, but arteriolar contractile responsiveness and a younger smooth muscle phenotype can be restored with late-life exercise training.
NEW & NOTEWORTHY Aging impairs contractile function of coronary arterioles and induces a shift of the vascular smooth muscle toward a proliferative, noncontractile phenotype. Late-life exercise training reverses contractile dysfunction of coronary arterioles and restores a young phenotype to the vascular smooth muscle.
Keywords: adiponectin, contractile phenotype, secretory phenotype, vascular smooth muscle, vasoconstriction
INTRODUCTION
Coronary blood flow regulation is achieved by multiple regulatory responses of the coronary resistance vasculature, including myogenic vasoconstriction and flow-induced and metabolic vasodilation (28, 51). Previous work from our laboratory (13) indicates that age impairs coronary blood flow responses to acute exercise, and this reduction of functional hyperemia is accompanied by diminished vasodilatory and contractile responses of coronary resistance arterioles (11, 17, 24, 35). Age-induced loss of vasodilatory function in coronary arterioles is largely attributable to increased oxidant stress and loss of nitric oxide-mediated vasodilation that can be restored with exercise training (17, 18, 24, 36). Similarly, Hanna et al. (11) reported that exercise training reversed age-induced impairment of myogenic responsiveness and attenuated age-related remodeling in coronary arterioles of aged rats; however, the cellular mechanisms that underlie age- and exercise training-induced alterations of vascular smooth muscle function in coronary arterioles have not been identified.
Vascular smooth muscle remodeling, involving cell proliferation and hypertrophy, is well-documented in large conduit arteries with age and disease (45); however, the impact of aging on vascular smooth muscle proliferation in coronary arterioles has not been investigated. Similarly, the vascular smooth muscle of aged large arteries has been shown to convert to a phenotype characterized by increased secretion of proteins and molecules that promote vascular degeneration through chronic inflammation and extracellular matrix degradation (45, 49); however, whether a phenotypic switch occurs in the vascular smooth muscle of aged coronary arterioles is not known. Dedifferentiated vascular smooth muscle cells lose myofilaments and their contractile ability while also exhibiting a high rate of proliferation and production of extracellular matrix proteins (42, 45), alterations that could contribute to age-induced contractile dysfunction in the coronary microvasculature (11). In this study, we investigated whether the vascular smooth muscle of aging coronary arterioles transitions to a secretory phenotype that manifests in contractile dysfunction. Both in vivo and in vitro studies have demonstrated that adiponectin inhibits proliferation and promotes differentiation and maintenance of a contractile phenotype in vascular smooth muscle (3, 6, 8); therefore, we also investigated whether age and/or exercise training alter adiponectin signaling in coronary arterioles.
METHODS
Animals.
Young (3–4 mo) and old (20–21 mo) male Fisher 344 rats were obtained from the National Institute on Aging. All rats were housed in a temperature/light-controlled environment and given access to standard rat chow and water ad libitum. Rats were randomly assigned to young sedentary (n = 44), young exercise-trained (n = 30), old sedentary (n = 39), or old exercise-trained (n = 29) groups. All animal procedures were approved by the Institutional Animal Care and Use Committees at University of Florida and Florida State University and conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (8th ed., 2011).
Exercise training.
Rats were habituated to treadmill exercise by walking on the treadmill at 15 m/min (0° incline), 5 min/day, for 3 days. Exercise-trained rats underwent 10–12 wk of treadmill exercise training. Exercise-trained rats exercised on the treadmill at 15 m/min (15° incline), 5 days/wk, for 10–12 wk. The duration of exercise was increased during the initial 4 wk until a 60-min duration was reached. The rats continued to exercise 5 days/wk for 60 min/day for the remainder of the 10- to 12-wk training period (11). Only rats that completed the entire 10-wk training protocol were studied. Exercise-trained rats were euthanized ≥24 h after the last exercise bout to exclude acute effects of exercise (37). To determine the efficacy of the training protocol, the soleus muscle was stored at −80°C for determination of citrate synthase activity, a mitochondrial enzyme and marker of muscle oxidative potential, according to the method of Srere (38).
Body composition.
Body composition was assessed using time-domain nuclear magnetic resonance with a lean fat analyzer (Bruker Optics, The Woodlands, TX) at 10 wk. The method was described previously (41). Two measurements were performed from each animal, and the average was reported.
Microvessel preparation.
Rats were weighed and anesthetized with 3% isoflurane-O2 mix and killed by excision of the heart, which was immediately placed in cold (4°C), filtered physiological saline solution (PSS) containing 145 mM NaCl, 4.7 mM KCl, 2.0 mM CaCl2, 1.17 mM MgSO4, 1.2 mM NaH2PO4, 5.0 mM glucose, 2.0 mM pyruvate, 0.02 mM EDTA, 3.0 mM MOPS buffer, and 1% bovine serum albumin. Coronary resistance arterioles were isolated from the left anterior descending artery distribution. In some arterioles, the endothelium was removed by passing 5 ml of air through the lumen without causing damage to vascular smooth muscle. Arterioles were then cannulated on pipettes and pressurized to 60 cmH2O (corresponding to an intraluminal pressure of 40–50 mmHg) in a Lucite chamber that contained warm (37°C), filtered PSS. The chamber was then placed on an inverted microscope equipped with a video camera and micrometer to measure intraluminal diameter. Coronary arterioles with no leaks were warmed to 37°C and allowed to equilibrate for ~1 h or until developing ≥15% spontaneous tone. The bathing solution was changed every 20 min during the course of each experiment. Lack of vasodilation to 50 µM acetylcholine confirmed removal of the endothelium. At the end of all experiments, arterioles were placed in Ca2+-free PSS with 100 µM sodium nitroprusside to determine maximal diameter. Development of spontaneous tone was expressed as the percentage constriction relative to maximal diameter and calculated as follows:
where Dmax is the maximal inner diameter recorded at a pressure of 60 cmH2O under Ca2+-free conditions and Ds is the steady-tone baseline diameter.
Evaluation of vasoconstrictor responsiveness.
To determine receptor-independent and -dependent vasoconstrictor responsiveness, concentration-response curves to K+ and the thromboxane analog, U46619 (1e−9 to 1e−4 M), were assessed. Responses to U46619 were assessed in both denuded and intact coronary arterioles. Reponses to depolarizing concentrations of K+ (10–100 mM, isotonic substitution for Na+) were assessed in denuded coronary arterioles as previously described (35).
Immunohistochemistry.
To determine whether aging and exercise training alter smooth muscle cell phenotype, coronary arterioles were cannulated, pressurized to 60 cmH2O, warmed to 37°C, incubated in Ca2+-free PSS and 100 µM sodium nitroprusside for 1 h, and then fixed in 50% Bouin solution. Fixed arterioles were frozen in optimum cutting temperature compound and stored at −80°C until use. Five-micrometer sections of coronary arterioles were cut on a cryostat. After washing with PSS, arteriolar sections were blocked using 0.3% Triton X-100 and 10% normal donkey serum at room temperature for 1 h and then incubated with primary antibodies against smooth muscle α-actin (1:50; cat. no. ab18147; Abcam) and phospho-histone H3 (PHH3; 1:100; #9701; Cell Signaling Technology) at 4°C for overnight. After washing, species-specific anti-IgG (Texas Red, cat. no. ab7059, Abcam; FITC, cat. no. ab6785, Abcam) were added at a dilution of 1:100 for 1 h at room temperature. After final washing and blotting of any excess PSS, mounting media containing 4′,6′-diamidino-2-phenylindole (DAPI; cat. no. P36962; Thermo Fisher Scientific) was applied to identify TA-rich regions of DNA in nuclei, and images were obtained using a fluorescence microscope (BX43; Olympus, Tokyo, Japan). The area of smooth muscle α-actin staining was determined via color threshold using MATLAB (MathWorks, Natick, MA). DAPI-stained nuclei were identified by a MATLAB program based on a threshold value for intensity and size. Nuclei specifically within the smooth muscle cross-sectional area (smooth muscle nuclei) were identified by superimposing DAPI and smooth muscle-stained images. Proliferating smooth muscle nuclei were determined based on positive staining for PHH3 via color threshold. Proliferating state was assessed by calculating the ratio of PHH3-positive smooth muscle nuclei to total number of smooth muscle nuclei. The average of the values obtained from analysis of four consecutive cross-sections per vessel was calculated and used for statistical analysis.
Adiponectin receptor 1.
To determine whether age and exercise training alter expression of adiponectin receptor 1 subtype in coronary arterioles, 5-µm frozen cross-sections of arterioles were thawed and washed with phosphate-buffered saline before adding the blocking solution of 0.3% Triton X-100 and 10% normal donkey serum at room temperature for 1 h. Then, the arterioles were incubated with primary antibodies against adiponectin receptor 1 (Anti-Adiponectin Receptor 1; 1:50; cat. no. ab126611; Abcam) at 4°C overnight. After PBS washes, species-specific anti-IgG (FITC; cat. no. ab6785; Abcam) was added at a dilution of 1:100 for 1 h at room temperature. After washing, DAPI (cat. no. P36962; Thermo Fisher Scientific) was added, and images were obtained using a fluorescence microscope (BX43; Olympus). To exclude adventitial staining, a region of interest for adiponectin receptor 1 staining was established manually using ImageJ after isolating the images for green fluorescence only. For each section, the average pixel intensity in the region of interest was obtained (ImageJ). Background subtraction was performed by subtracting the average pixel intensity obtained in an adjacent section from the same vessel that had treated in the same manner as the stained sections but in the absence of primary or secondary antibody. The average of the values obtained from analysis of three to five cross-sections per vessel was calculated and used for statistical analysis.
Immunoblotting.
The differentiation state of smooth muscle cells was determined by assessing expression of smooth muscle-specific contractile proteins smooth muscle myosin heavy chain isoforms 1 (SM1) and 2 (SM2) in coronary arterioles (20, 22). The levels of downstream effectors of adiponectin, AMP-activated kinase (2, 6, 8, 14, 29, 32, 39) and ribosomal protein S6 (12, 33), were assessed. Coronary arterioles (n = 5/rat) branching from the left anterior descending artery were dissected and stored at −80°C until use. Pooled vessels from each rat were lysed in 25 µl of 1× sample buffer (62.5 mM Tris, pH 6.8, 2% SDS, 6 M urea, 160 mM DTT, 0.1% bromophenol blue) and loaded (10-µg total protein) to 10% SDS-polyacrylamide gel. Proteins then were transferred to Hybond ECL membranes (GE Healthcare, Pittsburgh, PA). After 1-h blocking in 5% nonfat dry milk in Tris-buffered saline with 0.1% Tween 20, membranes were incubated at 4°C overnight with primary antibodies for the following proteins: SM1 (0.6 µg/ml), SM2 (0.6 µg/ml), S6 ribosomal protein (1:250; #2217; Cell Signaling Technology), phospho-S6 ribosomal protein (1:250; #2215; Cell Signaling Technology), AMP-activated kinase-α (AMPK; 1:250; #2532; Cell Signaling Technology), and β-actin (1:250; #4967; Cell Signaling Technology). After washing, membranes were incubated with anti-rabbit horseradish peroxide-conjugated secondary antibody (1:2,000; #7074; Cell Signaling Technology) for 1.5 h at room temperature. Peroxidase activity was determined using SuperSignal West Femto (Pierce), and images were analyzed using ImageJ. Loading differences were normalized by expressing all data as relative densitometry units of the protein of interest vs. β-actin.
Adiponectin ELISA.
To determine whether aging and exercise training alter global circulating level of adiponectin, ELISA (cat. no. 22-ADPRT-E01; ALPCO) was used for quantitative determination of adiponectin in rat serum. Blood was collected in serum separator tubes from the tail vein at the time of death. Serum was obtained by centrifuge at 4°C for 5 min and kept in −80°C until use. ELISA was performed following manufacturer’s instruction, and the plate was read on SpectraMax 190 (Molecular Devices).
Data analysis and statistics.
Vasoconstrictor responses were expressed as percentage constriction according to the following formula:
where Db is the baseline diameter immediately before addition of the first dose of vasoconstrictor agonist and Ds is the steady-state diameter measured after addition of each dose. Vessel responses to U46619 and K+ were evaluated using three-way ANOVA (age, exercise training, and dose/time) with repeated measurement (dose/time). Group differences in animal and vessel characteristics, body composition, circulating adiponectin, and values obtained from immunohistochemical and immunoblot analyses were assessed by two-way ANOVA (age and exercise training). Post hoc analyses were performed using Bonferroni test for pairwise comparisons if either significant main effects or significant interactions were found. Statistical significance was defined as P ≤ 0.05. All data are presented as means ± SE.
RESULTS
Animal and vessel characteristics.
Body weight increased with age (Table 1). Exercise training reduced body weight in both young and old rats. Both heart weight and left ventricular weight increased with age (Table 1). Heart weight-to-body weight ratio increased with exercise training in young and old rats. Exercise training increased soleus muscle citrate synthase activity by 20.9% in young rats [23.2 ± 1.1 vs. 19.1 ± 0.5 µmol·min−1·g−1; young exercise-trained group (YET) vs. young sedentary group (YSED)] and by 30.3% [18.5 ± 0.9 vs. 13.6 ± 0.7 µmol·min−1·g−1; old exercise-trained group (OET) vs. old sedentary group (OSED)] in old rats, confirming the efficacy of the exercise training regimen, as previously demonstrated (11, 36). Coronary arterioles that were <150 µm in diameter were isolated from the left ventricular free wall in all rats, and there were no significant differences in arteriolar diameters between groups. Spontaneous tone, assessed before addition of any contractile agents, did not differ between arterioles from young and old rats. Exercise training enhanced spontaneous tone in both young and old rats, consistent with our (11) previously reported observations.
Table 1.
Animal and coronary vessel characteristics
| Sedentary | Exercise Training | |
|---|---|---|
| Body weight, g | ||
| Young | 379 ± 5 (44) | 365 ± 4† (30) |
| Old | 448 ± 6* (39) | 415 ± 5*† (29) |
| Heart weight, mg | ||
| Young | 900 ± 10 (45) | 904 ± 15 (28) |
| Old | 1,138 ± 25* (39) | 1,092 ± 26* (29) |
| Left ventricular weight, mg | ||
| Young | 742 ± 8 (43) | 721 ± 12 (32) |
| Old | 914 ± 20* (36) | 858 ± 17*† (25) |
| HW/BW, mg/g | ||
| Young | 2.38 ± 0.03 (38) | 2.49 ± 0.03† (22) |
| Old | 2.55 ± 0.04* (35) | 2.65 ± 0.03*† (23) |
| LV/BW, mg/g | ||
| Young | 1.97 ± 0.04 (40) | 1.99 ± 0.03 (23) |
| Old | 2.06 ± 0.03* (32) | 2.12 ± 0.03* (24) |
| Maximal diameter, µm | ||
| Young | 135 ± 4 (17) | 128 ± 7 (15) |
| Old | 130 ± 5 (15) | 139 ± 5 (14) |
| Spontaneous tone, % | ||
| Young | 27.2 ± 1.8 (17) | 44.2 ± 6.1† (15) |
| Old | 26.9 ± 2.1 (15) | 42.0 ± 6.2† (13) |
Values are means ± SE; n (in parentheses), number of rats in each group; HW/BW, heart weight-to-body weight ratio; LV/BW, left ventricle weight-to-body weight ratio.
Effect of age (P ≤ 0.05).
Effect of exercise training (P ≤ 0.05).
Vasoconstrictor responses to U46619.
To determine initially the effects of aging and exercise training on contractile function of coronary arterioles, we assessed vasoconstrictor responses to the thromboxane analog, U46619, in intact coronary arterioles. Aging abolished vasoconstrictor responses to U46619 (Fig. 1A). Exercise training completely restored vasoconstrictor responses to U46619 in intact coronary arterioles from old rats, whereas contractile responsiveness to U46619 was reduced after exercise training in intact arterioles from young rats (Fig. 1A). To assess specifically contractile responses of the vascular smooth muscle, we evaluated contractile responses to U46619 in coronary arterioles denuded of endothelium. Removal of the endothelium reduced contractile responses to U46619 in arterioles from YSED rats but did not affect contractile responses in arterioles from OSED rats (Fig. 1, A vs. B). As in intact arterioles, aging nearly eliminated contractile responses to U46619 (Fig. 1B), and exercise training reversed the age-induced loss of U46619-mediated contractile responses of smooth muscle (Fig. 1B). In denuded arterioles from young rats, exercise training tended to reduce responsiveness to low concentrations of U46619, but the overall concentration-response relationship was not different between arterioles from YSED and YET rats. These data are consistent with an age-induced reduction of contractile responses in arteriolar smooth muscle and a training-induced reversal of this loss of contractile function.
Fig. 1.
Vasoconstriction to the thromboxane analog, U46619, in intact (A) and denuded (B) coronary resistance arterioles from young sedentary (YSED), old sedentary (OSED), young exercise-trained (YET), and old exercise-trained (OET) rats. Values are means ± SE; n = no. of rats. *Effect of age (P ≤ 0.05). †Effect of exercise training (P ≤ 0.05).
Vasoconstrictor responses to KCl.
To assess the effects of age and exercise training on receptor-independent vasoconstriction of the smooth muscle in coronary arterioles, we evaluated constrictor responses to increasing concentrations of potassium chloride (KCl; substituted for sodium chloride to maintain osmolarity and ionic balance). Aging decreased vasoconstrictor responses to KCl in denuded coronary arterioles from OSED rats relative to YSED rats (Fig. 2). In arterioles from old rats, exercise training restored vasoconstriction to KCl to a level not different from that of arterioles from YSED rats. In arterioles from young rats, exercise training did not alter responsiveness to KCl. These data further indicate that aging reduces contractile function of coronary arterioles, independent of membrane receptor signaling.
Fig. 2.

Vasoconstriction to potassium chloride (KCl) in denuded coronary resistance arterioles from young sedentary (YSED), old sedentary (OSED), young exercise-trained (YET), and old exercise-trained (OET) rats. Values are means ± SE; n = no. of rats. *Effect of age (P ≤ 0.05). †Effect of exercise training (P ≤ 0.05).
Vascular smooth muscle cell phenotype.
With age, vascular smooth muscle in coronary arterioles displayed a synthetic, dedifferentiated phenotype. Smooth muscle myosin, SM1, decreased in coronary arterioles with age (Fig. 3A). Exercise training increased SM1 protein levels in arterioles from old rats, although a significant difference persisted between SM1 proteins in arterioles from YET and OET rats. SM2 levels were similar among all four groups of rats (Fig. 3B). Age increased levels of the synthetic protein ribosomal protein S6 (rpS6) in coronary arterioles (Fig. 4A). Exercise training decreased the level of rpS6 in arterioles from old rats (Fig. 4A). Phosphorylation of rpS6 also increased with age but was reduced by exercise training in arterioles from old rats (Fig. 4B). Exercise training did not change total or phosphorylated rpS6 in arterioles from young rats.
Fig. 3.

Expression of contractile proteins in denuded coronary arterioles from YSED, OSED, YET, and OET rats. A: vascular smooth muscle heavy chain isoform 1 (SM1). B: vascular smooth muscle heavy chain isoform 2 (SM2). Values are means ± SE; n = no. of rats. *Effect of age (P ≤ 0.05). †Effect of exercise training (P ≤ 0.05).
Fig. 4.

Effects of age and exercise training on levels of total (A) and phosphorylated (p-; B) ribosomal protein S6 (rpS6) in denuded coronary arterioles from YSED, OSED, YET, and OET rats. Values are means ± SE; n = no. of rats. *Effect of age (P ≤ 0.05). **Effect of age (P = 0.058). †Effect of exercise training (P ≤ 0.05).
Phenotype of vascular smooth muscle cells.
Figure 5A shows representative cross-sections of coronary arterioles demonstrating DAPI-stained nuclei (a, e, i, and m), smooth muscle α-actin staining (b, f, j, and n), and phospho-histone H3-stained nuclei (PHH3; c, g, k, and o). Aging reduced the number of nuclei per cross-sectional unit of vascular smooth muscle (Fig. 5B). After exercise training, the age-related difference in number of nuclei in the media of the arteriolar wall was no longer detectable (Fig. 5B). Aging increased smooth muscle cell proliferation (Fig. 5C). Exercise training decreased smooth muscle cell proliferation in arterioles from old but not young rats (Fig. 5C).
Fig. 5.
Immunohistochemical analysis of vascular smooth muscle phenotype in coronary arterioles. A: representative cross-sections for YSED (a–d), OSED (e–h), YET (i–l), and OET (m–p). Merged images (d, h, l, and p) include blue DAPI-stained nuclei (DAPI; a, e, i, and m), red smooth muscle α-actin (SM actin; b, f, j, and n), and green phospho-histone H3-positive nuclei (PHH3; c, g, k, and o). Scale bar corresponds to 100 µm. B: smooth muscle nuclei/cross-sectional area. C: smooth muscle proliferation. %PHH pos, percentage positively stained for PHH3. Values are means ± SE; n = no. of rats. *Effect of age (P ≤ 0.05). †Effect of exercise training (P ≤ 0.05).
Body composition, serum adiponectin, AMPK, and R1 adiponectin receptor subtype.
During the 10-wk training period, YSED rats gained weight, whereas OSED rats lost weight (Fig. 6A). Compared with YSED rats, YET gained significantly less weight (Fig. 6A). OET rats lost significantly more weight compared with OSED in the 10-wk training period (Fig. 6A). Fat mass and percentage of fat mass increased with age (Fig. 6, B and C). Absolute lean mass increased with age (Fig. 6D), but percentage of lean mass decreased with age (Fig. 6E). Exercise training reduced the percentage of fat mass (Fig. 6C) and increased the percentage of lean mass in both young and old rats (Fig. 6E).
Fig. 6.
Effects of age and exercise training on body weight, fat mass, and lean mass. A: weekly changes in body weight in YSED, OSED, YET, and OET rats. Absolute body fat mass (B) and percentage of body fat (C) in YSED, OSED, YET, and OET rats are shown. Absolute lean mass (B) and percentage of lean mass (C) in YSED, OSED, YET, and OET rats are also shown. Values are means ± SE; n = no. of rats. *Effect of age (P ≤ 0.05). †Effect of exercise training (P ≤ 0.05).
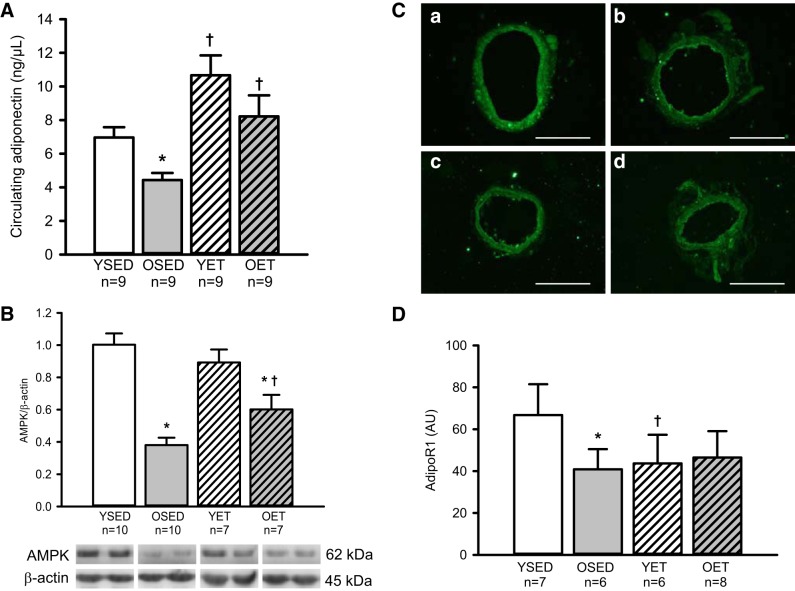
Age decreased circulating adiponectin (Fig. 7A). Exercise training increased circulating adiponectin in both young and old rats (Fig. 7A). Expression of AMPK, which is activated by adiponectin in vascular smooth muscle (8), was decreased by >50% in denuded coronary arterioles from aged rats (Fig. 7B). Exercise training increased AMPK levels in coronary arterioles from old rats Fig. 7B); however, the levels in arterioles from old exercise-trained rats did not reach those of arterioles from young rats. Exercise training did not alter AMPK levels in arterioles from young rats (Fig. 7B).
Fig. 7.
A: circulating adiponectin in YSED, OSED, YET, and OET rats. Expressions of AMPK protein (B) and adiponectin R1 receptor (C and D) in coronary arterioles from YSED, OSED, YET, and OET rats are shown. In B, representative cross-sections of AdipoR1 staining in coronary arterioles were from YSED (a), OSED (b), YET (c), and OET (d) rats. Scale bar corresponds to 100 µm. Values are means ± SE; n = no. of rats. *Effect of age (P ≤ 0.05). †Effect of exercise training (P ≤ 0.05). AU, arbitrary units.
Age decreased R1 adiponectin receptor subtype (AdipoR1) receptor levels (Fig. 7, C and D). Exercise training did not alter AdipoR1 levels in coronary arterioles from old rats. Surprisingly, exercise training decreased AdipoR1 levels in coronary arterioles from young rats (Fig. 7, C and D).
DISCUSSION
Our (11, 23, 35) previous work has shown that myogenic constriction and contractile responses to endothelin are impaired in coronary arterioles with advancing age. Our current findings confirm that contractile responses of coronary arterioles decline with age, but the loss of contractile function can be restored with late-life exercise training. Age reduces both thromboxane receptor-mediated vasoconstriction and receptor-independent constriction mediated by depolarizing concentrations of potassium chloride. Additionally, both receptor-dependent and receptor-independent vasoconstrictor responses were improved after late-life exercise training, suggesting that exercise training restores function of the intracellular contractile mechanisms in the vascular smooth muscle of coronary arterioles. These effects of age and exercise training on vasoconstrictor responses were also present in denuded arterioles, supporting the conclusion that late-life exercise training alters contractile function of arteriolar smooth muscle independent of changes in the endothelium. Importantly, our data indicate that an age-induced shift away from a differentiated, contractile smooth muscle phenotype toward a secretory, synthetic phenotype underlies contractile dysfunction of coronary arterioles, but this phenotypic shift can be reversed by late-life exercise training.
Here, we confirm our (11) previous work in which we found that exercise training reversed age-related thickening of the medial wall in coronary arterioles and ameliorated myogenic responses of coronary arterioles from aged rats. We (11) reported that age induces remodeling of the coronary arteriolar wall as evidenced by an increase in wall-to-lumen ratio that is accompanied by reduced wall stiffness but without a change in the collagen-to-elastin ratio. We now report that with age, the vascular smooth muscle of coronary arterioles undergoes a loss of contractile protein expression and an increase in expression of ribosomal protein S6, a key synthetic protein, suggesting that the increase in wall thickness that occurs with age in these arterioles results from a phenotypic switch in which the vascular smooth muscle cells lose contractile stiffness and become increasingly synthetic and hypertrophic. In large arteries, a similar phenotypic switch to a synthetic, secretory phenotype of vascular smooth muscle occurs with age and is accompanied by increased medial proliferation (10, 43, 44, 46); however, in large arteries, increased collagen secretion is a significant contributor to the age-related increase in wall stiffness (16). Importantly, in resistance arterioles that are critical for regulation of coronary blood flow distribution, the switch from a contractile to a synthetic phenotype in the vascular smooth muscle corresponds to a loss of functional contractility, possibly contributing to the microvascular dysfunction that occurs in the coronary circulation with age (13).
The previously reported medial thickening and reduction of stiffness in aged coronary arterioles (11) may result, in part, from a shift to a synthetic phenotype that lacks stiffness due to a reduction of contractile elements in the smooth muscle cells, per se (Fig. 3). The level of contractile protein SM1 was reduced in coronary arterioles by aging. Dedifferentiated vascular smooth muscle cells in a synthetic state demonstrate an extensive rough endoplasmic reticulum and a large Golgi complex, synthesizing extracellular matrix proteins and new proteins for cell proliferation (4). In addition to decreasing contractile protein expression, aging increased the levels of rpS6, an important regulator of protein synthesis and cell size (33), and also increased phosphorylation of rpS6 in the vascular smooth muscle of coronary arterioles. Late-life exercise training reversed these age-induced phenotypic changes in the smooth muscle and restored contractile function in aged arterioles. Indolfi et al. (15) demonstrated that exercise training reduced vascular smooth muscle proliferation and restenosis of common carotid arteries after balloon angioplasty and stenting; however, to our knowledge, this is the first report that exercise training can restore a differentiated, contractile phenotype in the smooth muscle of the aged vascular wall.
In our (11) previous work, the increase in wall-to-lumen ratio that occurred with age was reversed by exercise training. In humans, bicycle exercise training has been reported to induce a significant and gradual decrease in wall thickness and wall-to-lumen ratio in both the femoral and carotid arteries, suggesting that exercise training can lead to remodeling of the arterial wall through a systemic mechanism (40). It is possible that age- and exercise training-induced changes in blood pressure could contribute to vascular remodeling; however, we (13) have previously found that neither age nor exercise training altered blood pressure in Fischer 344 rats. Therefore, to investigate one potential systemic mechanism that may contribute to the age- and exercise training-induced phenotypic shifts in the vascular smooth muscle of coronary arterioles, we studied circulating adiponectin and its downstream effector, AMP-activated protein kinase (AMPK). Kim et al. (19) demonstrated that increased body weight and increased abdominal visceral fat were important predictors of decreased serum adiponectin concentration in nondiabetic, obese aged subjects. In both humans and in animal models, weight loss and exercise training increase circulating adiponectin (9, 21, 27, 47). This inverse relationship between body fat and circulating adiponectin was confirmed by our results in which body fat increased in old rats, whereas circulating adiponectin decreased with advancing age. In accordance with reports in the literature, we found that exercise training reduced body fat but increased circulating adiponectin in both young and old rats. Adiponectin is produced in various tissues, including adipose tissue, cardiac myocytes, aorta, and vascular smooth muscle (8, 25, 30, 39). Adiponectin has been reported to induce differentiation of vascular smooth muscle via AMPKα2-mediated inhibition of molecular target of rapamycin and its effector, S6 kinase-1, which phosphorylates rpS6 (8). Adiponectin attenuates transformation of vascular smooth muscle cells into osteoblast-like cells that contribute to vascular calcification and arterial plaque formation (50) through its action on the AMPK-molecular target of rapamycin signaling, and serum adiponectin levels negatively correlate with the degree of coronary artery calcification (26). We found that AMPK protein levels decreased in coronary arterioles with age, suggesting that the age-related reduction in systemic adiponectin as well as reduced AMPK signaling may contribute to the phenotypic changes that occurred in vascular smooth muscle of coronary arterioles with age.
Two adiponectin receptors, AdipoR1 and AdipoR2, as well as T-cadherin bind adiponectin and are expressed in the endothelium and vascular smooth muscle (1, 34). AdipoR1 is upregulated by exercise training in soleus muscle of obese rats (5). Exercise training-induced hippocampal neurogenesis has been linked to adiponectin signaling through AdipoR1 (48). In contrast, deficiency of AdipoR1 did not affect vascular recovery from peripheral ischemia, whereas mice deficient in AdipoR2 displayed a marked impairment of revascularization in hindlimb ischemia (31). Our results indicate that a decrease in AdipoR1 occurred with age in the wall of coronary arterioles; however, exercise training did not increase AdipoR1 in wall of coronary arterioles from old rats. Surprisingly, AdipoR1 levels decreased with exercise training in coronary arterioles from young rats. It is possible that these age- and exercise training-induced changes in AdipoR1 occur in conjunction with changes in AdipoR2, which we did not investigate. It is also possible that the high levels of circulating adiponectin that were found in young, exercise-trained rats leads to downregulation and/or altered distribution of AdipoR1 in the vascular wall. These arteriolar cross-sections contain both endothelium and vascular smooth muscle, and the levels of AdipoR1 in these respective cells may change in a directionally similar manner with age and exercise. Alternatively, the changes in AdipoR1 levels may be directionally opposite in endothelium and vascular smooth muscle and may be related to the reduction of endothelium-mediated constriction to U46619 that occurred with exercise training in arterioles from young, but not old, rats. Future work will need to address the role of local adiponectin signaling in arterioles and cell-specific adaptations of adiponectin signaling that occur in arterioles with age and exercise training.
In summary, age impairs contractile responses of coronary arterioles and induces a shift of the arteriolar smooth muscle toward a secretory, synthetic phenotype in which expression of contractile proteins is reduced. Late-life exercise training reverses contractile impairment and restores a younger, contractile phenotype to the smooth muscle of coronary arterioles. Reduction of circulating adiponectin and decreased local adiponectin signaling may contribute to phenotypic changes that occur in the smooth muscle of coronary arterioles with age and exercise training.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants R01-HL-077224 (J. M. Muller-Delp) and R01-HL-90937 (J. M. Muller-Delp) and National Aeronautics and Space Administration Space Biology Program Grant NNX14AQ57G (M. D. Delp).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M.M.-D. conceived and designed research; J.M.M.-D., K.H., B.C., T.R.L., J.A.B., D.B.A., H.E.M., M.R.C., and A.D.H. performed experiments; J.M.M.-D., K.H., B.C., B.J.B., J.J.M., J.A.B., D.B.A., H.E.M., M.R.C., and A.D.H. analyzed data; J.M.M.-D., K.H., B.C., B.J.B., J.J.M., and M.D.D. interpreted results of experiments; J.M.M.-D., K.H., and B.C. prepared figures; J.M.M.-D. drafted manuscript; J.M.M.-D., K.H., B.C., B.J.B., J.J.M., M.D.D., T.R.L., J.A.B., D.B.A., H.E.M., M.R.C., and A.D.H. edited and revised manuscript; J.M.M.-D., K.H., B.C., B.J.B., J.J.M., M.D.D., T.R.L., J.A.B., D.B.A., H.E.M., M.R.C., and A.D.H. approved final version of manuscript.
REFERENCES
- 1.Adya R, Tan BK, Chen J, Randeva HS. Protective actions of globular and full-length adiponectin on human endothelial cells: novel insights into adiponectin-induced angiogenesis. J Vasc Res 49: 534–543, 2012. doi: 10.1159/000338279. [DOI] [PubMed] [Google Scholar]
- 2.Aikawa M, Sivam PN, Kuro-o M, Kimura K, Nakahara K, Takewaki S, Ueda M, Yamaguchi H, Yazaki Y, Periasamy M. Human smooth muscle myosin heavy chain isoforms as molecular markers for vascular development and atherosclerosis. Circ Res 73: 1000–1012, 1993. doi: 10.1161/01.RES.73.6.1000. [DOI] [PubMed] [Google Scholar]
- 3.Arita Y, Kihara S, Ouchi N, Maeda K, Kuriyama H, Okamoto Y, Kumada M, Hotta K, Nishida M, Takahashi M, Nakamura T, Shimomura I, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation 105: 2893–2898, 2002. doi: 10.1161/01.CIR.0000018622.84402.FF. [DOI] [PubMed] [Google Scholar]
- 4.Chamley-Campbell J, Campbell GR, Ross R. The smooth muscle cell in culture. Physiol Rev 59: 1–61, 1979. [DOI] [PubMed] [Google Scholar]
- 5.Chang SP, Chen YH, Chang WC, Liu IM, Cheng JT. Increase of adiponectin receptor gene expression by physical exercise in soleus muscle of obese Zucker rats. Eur J Appl Physiol 97: 189–195, 2006. doi: 10.1007/s00421-006-0163-3. [DOI] [PubMed] [Google Scholar]
- 6.Ding M, Carrão AC, Wagner RJ, Xie Y, Jin Y, Rzucidlo EM, Yu J, Li W, Tellides G, Hwa J, Aprahamian TR, Martin KA. Vascular smooth muscle cell-derived adiponectin: a paracrine regulator of contractile phenotype. J Mol Cell Cardiol 52: 474–484, 2012. doi: 10.1016/j.yjmcc.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding M, Xie Y, Wagner RJ, Jin Y, Carrao AC, Liu LS, Guzman AK, Powell RJ, Hwa J, Rzucidlo EM, Martin KA. Adiponectin induces vascular smooth muscle cell differentiation via repression of mammalian target of rapamycin complex 1 and FoxO4. Arterioscler Thromb Vasc Biol 31: 1403–1410, 2011. doi: 10.1161/ATVBAHA.110.216804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garekani ET, Mohebbi H, Kraemer RR, Fathi R. Exercise training intensity/volume affects plasma and tissue adiponectin concentrations in the male rat. Peptides 32: 1008–1012, 2011. doi: 10.1016/j.peptides.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 10.Gennaro G, Ménard C, Giasson E, Michaud SE, Palasis M, Meloche S, Rivard A. Role of p44/p42 MAP kinase in the age-dependent increase in vascular smooth muscle cell proliferation and neointimal formation. Arterioscler Thromb Vasc Biol 23: 204–210, 2003. doi: 10.1161/01.ATV.0000053182.58636.BE. [DOI] [PubMed] [Google Scholar]
- 11.Hanna MA, Taylor CR, Chen B, La HS, Maraj JJ, Kilar CR, Behnke BJ, Delp MD, Muller-Delp JM. Structural remodeling of coronary resistance arteries: effects of age and exercise training. J Appl Physiol (1985) 117: 616–623, 2014. doi: 10.1152/japplphysiol.01296.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hedin U, Thyberg J. Plasma fibronectin promotes modulation of arterial smooth-muscle cells from contractile to synthetic phenotype. Differentiation 33: 239–246, 1987. doi: 10.1111/j.1432-0436.1987.tb01563.x. [DOI] [PubMed] [Google Scholar]
- 13.Hotta K, Chen B, Behnke BJ, Ghosh P, Stabley JN, Bramy JA, Sepulveda JL, Delp MD, Muller-Delp JM. Exercise training reverses age-induced diastolic dysfunction and restores coronary microvascular function. J Physiol 595: 3703–3719, 2017. doi: 10.1113/JP274172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Igata M, Motoshima H, Tsuruzoe K, Kojima K, Matsumura T, Kondo T, Taguchi T, Nakamaru K, Yano M, Kukidome D, Matsumoto K, Toyonaga T, Asano T, Nishikawa T, Araki E. Adenosine monophosphate-activated protein kinase suppresses vascular smooth muscle cell proliferation through the inhibition of cell cycle progression. Circ Res 97: 837–844, 2005. doi: 10.1161/01.RES.0000185823.73556.06. [DOI] [PubMed] [Google Scholar]
- 15.Indolfi C, Torella D, Coppola C, Curcio A, Rodriguez F, Bilancio A, Leccia A, Arcucci O, Falco M, Leosco D, Chiariello M. Physical training increases eNOS vascular expression and activity and reduces restenosis after balloon angioplasty or arterial stenting in rats. Circ Res 91: 1190–1197, 2002. doi: 10.1161/01.RES.0000046233.94299.D6. [DOI] [PubMed] [Google Scholar]
- 16.Jiang L, Zhang J, Monticone RE, Telljohann R, Wu J, Wang M, Lakatta EG. Calpain-1 regulation of matrix metalloproteinase 2 activity in vascular smooth muscle cells facilitates age-associated aortic wall calcification and fibrosis. Hypertension 60: 1192–1199, 2012. doi: 10.1161/HYPERTENSIONAHA.112.196840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang LS, Chen B, Reyes RA, Leblanc AJ, Teng B, Mustafa SJ, Muller-Delp JM. Aging and estrogen alter endothelial reactivity to reactive oxygen species in coronary arterioles. Am J Physiol Heart Circ Physiol 300: H2105–H2115, 2011. doi: 10.1152/ajpheart.00349.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang LS, Reyes RA, Muller-Delp JM. Aging impairs flow-induced dilation in coronary arterioles: role of NO and H2O2. Am J Physiol Heart Circ Physiol 297: H1087–H1095, 2009. doi: 10.1152/ajpheart.00356.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim C, Park J, Park J, Kang E, Ahn C, Cha B, Lim S, Kim K, Lee H. Comparison of body fat composition and serum adiponectin levels in diabetic obesity and non-diabetic obesity. Obesity (Silver Spring) 14: 1164–1171, 2006. doi: 10.1038/oby.2006.133. [DOI] [PubMed] [Google Scholar]
- 20.Kuro-o M, Nagai R, Nakahara K, Katoh H, Tsai RC, Tsuchimochi H, Yazaki Y, Ohkubo A, Takaku F. cDNA cloning of a myosin heavy chain isoform in embryonic smooth muscle and its expression during vascular development and in arteriosclerosis. J Biol Chem 266: 3768–3773, 1991. [PubMed] [Google Scholar]
- 21.Lang HF, Chou CY, Sheu WH, Lin JY. Weight loss increased serum adiponectin but decreased lipid levels in obese subjects whose body mass index was lower than 30 kg/m2. Nutr Res 31: 378–386, 2011. doi: 10.1016/j.nutres.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 149: 274–293, 2012. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LeBlanc AJ, Chen B, Dougherty PJ, Reyes RA, Shipley RD, Korzick DH, Muller-Delp JM. Divergent effects of aging and sex on vasoconstriction to endothelin in coronary arterioles. Microcirculation 20: 365–376, 2013. doi: 10.1111/micc.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeBlanc AJ, Shipley RD, Kang LS, Muller-Delp JM. Age impairs Flk-1 signaling and NO-mediated vasodilation in coronary arterioles. Am J Physiol Heart Circ Physiol 295: H2280–H2288, 2008. doi: 10.1152/ajpheart.00541.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee S, Park Y, Dellsperger KC, Zhang C. Exercise training improves endothelial function via adiponectin-dependent and independent pathways in type 2 diabetic mice. Am J Physiol Heart Circ Physiol 301: H306–H314, 2011. doi: 10.1152/ajpheart.01306.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maahs DM, Ogden LG, Kinney GL, Wadwa P, Snell-Bergeon JK, Dabelea D, Hokanson JE, Ehrlich J, Eckel RH, Rewers M. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation 111: 747–753, 2005. doi: 10.1161/01.CIR.0000155251.03724.A5. [DOI] [PubMed] [Google Scholar]
- 27.Moghadasi M, Mohebbi H, Rahmani-Nia F, Hassan-Nia S, Noroozi H, Pirooznia N. High-intensity endurance training improves adiponectin mRNA and plasma concentrations. Eur J Appl Physiol 112: 1207–1214, 2012. doi: 10.1007/s00421-011-2073-2. [DOI] [PubMed] [Google Scholar]
- 28.Muller JM, Davis MJ, Chilian WM. Integrated regulation of pressure and flow in the coronary microcirculation. Cardiovasc Res 32: 668–678, 1996. doi: 10.1016/S0008-6363(96)00111-3. [DOI] [PubMed] [Google Scholar]
- 29.Nagata D, Takeda R, Sata M, Satonaka H, Suzuki E, Nagano T, Hirata Y. AMP-activated protein kinase inhibits angiotensin II-stimulated vascular smooth muscle cell proliferation. Circulation 110: 444–451, 2004. doi: 10.1161/01.CIR.0000136025.96811.76. [DOI] [PubMed] [Google Scholar]
- 30.Ohashi K, Ouchi N, Matsuzawa Y. Anti-inflammatory and anti-atherogenic properties of adiponectin. Biochimie 94: 2137–2142, 2012. doi: 10.1016/j.biochi.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Parker-Duffen JL, Nakamura K, Silver M, Zuriaga MA, MacLauchlan S, Aprahamian TR, Walsh K. Divergent roles for adiponectin receptor 1 (AdipoR1) and AdipoR2 in mediating revascularization and metabolic dysfunction in vivo. J Biol Chem 289: 16200–16213, 2014. doi: 10.1074/jbc.M114.548115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rivard A, Principe N, Andrés V. Age-dependent increase in c-fos activity and cyclin A expression in vascular smooth muscle cells. A potential link between aging, smooth muscle cell proliferation and atherosclerosis. Cardiovasc Res 45: 1026–1034, 2000. doi: 10.1016/S0008-6363(99)00385-5. [DOI] [PubMed] [Google Scholar]
- 33.Ruvinsky I, Sharon N, Lerer T, Cohen H, Stolovich-Rain M, Nir T, Dor Y, Zisman P, Meyuhas O. Ribosomal protein S6 phosphorylation is a determinant of cell size and glucose homeostasis. Genes Dev 19: 2199–2211, 2005. doi: 10.1101/gad.351605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen X, Li H, Li W, Wu X, Ding X. Pioglitazone prevents hyperglycemia induced decrease of AdipoR1 and AdipoR2 in coronary arteries and coronary VSMCs. Mol Cell Endocrinol 363: 27–35, 2012. doi: 10.1016/j.mce.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Shipley RD, Muller-Delp JM. Aging decreases vasoconstrictor responses of coronary resistance arterioles through endothelium-dependent mechanisms. Cardiovasc Res 66: 374–383, 2005. doi: 10.1016/j.cardiores.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Sindler AL, Reyes R, Chen B, Ghosh P, Gurovich AN, Kang LS, Cardounel AJ, Delp MD, Muller-Delp JM. Age and exercise training alter signaling through reactive oxygen species in the endothelium of skeletal muscle arterioles. J Appl Physiol (1985) 114: 681–693, 2013. doi: 10.1152/japplphysiol.00341.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol 556: 947–958, 2004. doi: 10.1113/jphysiol.2003.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srere PA. Citrate synthase. In: Methods in Enzymology. New York: Elsevier, 1969, p. 3–11. [Google Scholar]
- 39.Tao L, Wang Y, Gao E, Zhang H, Yuan Y, Lau WB, Chan L, Koch WJ, Ma XL. Adiponectin: an indispensable molecule in rosiglitazone cardioprotection following myocardial infarction. Circ Res 106: 409–417, 2010. doi: 10.1161/CIRCRESAHA.109.211797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thijssen DH, Dawson EA, van den Munckhof IC, Birk GK, Timothy Cable N, Green DJ. Local and systemic effects of leg cycling training on arterial wall thickness in healthy humans. Atherosclerosis 229: 282–286, 2013. doi: 10.1016/j.atherosclerosis.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 41.Tinsley FC, Taicher GZ, Heiman ML. Evaluation of a quantitative magnetic resonance method for mouse whole body composition analysis. Obes Res 12: 150–160, 2004. doi: 10.1038/oby.2004.20. [DOI] [PubMed] [Google Scholar]
- 42.Vazquez-Padron RI, Lasko D, Li S, Louis L, Pestana IA, Pang M, Liotta C, Fornoni A, Aitouche A, Pham SM. Aging exacerbates neointimal formation, and increases proliferation and reduces susceptibility to apoptosis of vascular smooth muscle cells in mice. J Vasc Surg 40: 1199–1207, 2004. doi: 10.1016/j.jvs.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 43.Wang M, Fu Z, Wu J, Zhang J, Jiang L, Khazan B, Telljohann R, Zhao M, Krug AW, Pikilidou M, Monticone RE, Wersto R, Van Eyk J, Lakatta EG. MFG-E8 activates proliferation of vascular smooth muscle cells via integrin signaling. Aging Cell 11: 500–508, 2012. doi: 10.1111/j.1474-9726.2012.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang M, Jiang L, Monticone RE, Lakatta EG. Proinflammation: the key to arterial aging. Trends Endocrinol Metab 25: 72–79, 2014. doi: 10.1016/j.tem.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang M, Khazan B, Lakatta EG. Central arterial aging and angiotensin II signaling. Curr Hypertens Rev 6: 266–281, 2010. doi: 10.2174/157340210793611668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang M, Zhang J, Jiang LQ, Spinetti G, Pintus G, Monticone R, Kolodgie FD, Virmani R, Lakatta EG. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension 50: 219–227, 2007. doi: 10.1161/HYPERTENSIONAHA.107.089409. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, You T, Murphy K, Lyles MF, Nicklas BJ. Addition of exercise increases plasma adiponectin and release from adipose tissue. Med Sci Sports Exerc 47: 2450–2455, 2015. doi: 10.1249/MSS.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yau SY, Li A, Hoo RL, Ching YP, Christie BR, Lee TM, Xu A, So KF. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc Natl Acad Sci USA 111: 15810–15815, 2014. doi: 10.1073/pnas.1415219111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yin H, Pickering JG. Cellular senescence and vascular disease: novel routes to better understanding and therapy. Can J Cardiol 32: 612–623, 2016. doi: 10.1016/j.cjca.2016.02.051. [DOI] [PubMed] [Google Scholar]
- 50.Zhan JK, Wang YJ, Wang Y, Tang ZY, Tan P, Huang W, Liu YS. Adiponectin attenuates the osteoblastic differentiation of vascular smooth muscle cells through the AMPK/mTOR pathway. Exp Cell Res 323: 352–358, 2014. doi: 10.1016/j.yexcr.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 51.Zhang CH, Rogers PA, Merkus D, Muller-Delp JM, Tiefenbacher CP, Potter B, Knudson JD, Rocic P, Chilian WM. Regulation of coronary microvascular resistance in health and disease. In: Handbook of Physiology. Microcirculation (2nd ed.). San Diego, CA; Burlington, MA; London; Amsterdam: Academic Press, 2008, p. 521–549. doi: 10.1016/B978-0-12-374530-9.00014-0. [DOI] [Google Scholar]