Abstract
Gestational potassium retention, most of which occurs during late pregnancy, is essential for fetal development. The purpose of this study was to examine mechanisms underlying changes in potassium handling by the kidney and colon in pregnancy. We found that potassium intake and renal excretion increased in late pregnancy while fecal potassium excretion remained unchanged and that pregnant rats exhibited net potassium retention. By quantitative PCR we found markedly increased H+-K+-ATPase type 2 (HKA2) mRNA expression in the cortex and outer medullary of late pregnant vs. virgin. Renal outer medullary potassium channel (ROMK) mRNA was unchanged in the cortex, but apical ROMK abundance (by immunofluorescence) was decreased in pregnant vs. virgin in the distal convoluted tubule (DCT) and connecting tubule (CNT). Big potassium-α (BKα) channel-α protein abundance in intercalated cells in the cortex and outer medullary collecting ducts (by immunohistochemistry) fell in late pregnancy. In the distal colon we found increased HKA2 mRNA and protein abundance (Western blot) and decreased BKα protein with no observed changes in mRNA. Therefore, the potassium retention of pregnancy is likely to be due to increased collecting duct potassium reabsorption (via increased HKA2), decreased potassium secretion (via decreased ROMK and BK), as well as increased colonic reabsorption via HKA2.
Keywords: big potassium channel, BK, H+-K+-ATPase type 2, HKA2, renal outer medullary potassium channel, ROMK
INTRODUCTION
It is well known that renal sodium retention begins early in normal pregnancy and increases exponentially throughout gestation, resulting in the maternal plasma volume expansion that is critical for optimal fetal development. This process is driven by activation of the renin, angiotensin, and aldosterone system (33). The fetus also requires a large amount of potassium for normal development. To accommodate this need, the normal pregnant rat accumulates potassium over the course of gestation, most of which is retained during late pregnancy and the majority resides within cells of the fetus and placenta (5). Remarkably, gestational potassium retention occurs in the face of high circulating aldosterone (4, 11), decreased renal sodium chloride cotransporter (NCC) abundance/phosphorylation (32), and increased renal epithelial sodium channel (ENaC) activity (35), all of which are potent kaliuretic factors in nonpregnant animals.
Control of potassium balance is quite complex and involves integration of gut absorption and renal excretion, the “external” regulatory processes, as well as sequestration/release of potassium from tissues, particularly liver and skeletal muscle (15, 38). Potassium secretion and reabsorption from gut and kidney are determined by the abundance and activity of potassium channels and transporters that control the gestational potassium retention. The renal outer medullary potassium channel (ROMK) represents an important and highly regulated renal K+ secretory pathway (16, 30). The calcium-gated big potassium (BK) channel is also present in renal tubular epithelium and contributes to renal potassium secretion and control of potassium homeostasis (7). The major potassium reabsorptive transporters are the H+-K+-ATPase type 1 (HKA1) and H+-K+-ATPase type 2 (HKA2), which are present in the collecting duct (29, 36). HKA2 and BK are also present and functional in the distal colon where, as with kidney, they promote potassium retention and removal, respectively (6, 23).
The renal ROMK transcript is reported to be unchanged during pregnancy in whole rat kidney (19) but reduced in rat kidney cortex (1). There is no information on either epithelial ROMK protein levels/distribution or on the BK channel in the kidney during pregnancy. An increase in HKA2 transcript, protein, and activity occurs in the kidney of the normal pregnant mouse in association with decreased urinary potassium excretion, whereas mice with global HKA2 knockout exhibit both renal and colonic potassium losses and impaired pregnancies (22). Because much of the renal physiology of pregnancy has been studied in the rat, we conducted the present study to investigate ROMK, BK, HKA1, and HKA2 in the kidney and BK and HKA2 in the colon of the gravid rat. Our goal was to test the hypothesis that the potassium secretory channels (ROMK and/or BK) are downregulated and/or the potassium reabsorptive transporters are upregulated in the kidney and colon of the pregnant rat, permitting the accumulation of potassium in late gestation. We conducted balance experiments and screened [by quantitative (q)PCR] for the major apical potassium channels/transporters in the renal cortex and medulla as well as the distal colon. We then examined the protein levels/distribution [by Western blot/immunohistochemistry (IHC)] of channels and transporters to determine which changed in pregnancy. All measurements were made in virgin and age-matched late pregnant rats (when most of the gestational potassium is retained).
METHODS
Animal experiments were carried out using a total of 19 female Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN). Animals were maintained in the University of Florida animal facility in compliance with institutional guidelines and the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. All animal protocols were approved by the University of Florida’s Institutional Animal Care and Use Committee, and experiments were carried out according to institutional guidelines. Rats were maintained on a normal 12:12-h light-dark cycle and were given ad libitum access to water and Harlan Teklad LM-485 Mouse/Rat Diet (0.3% sodium and 0.8% potassium). Rats destined to become pregnant were placed with a fertile male, and day 1 of pregnancy was designated as the day that sperm was present in vaginal smears. Rat gestation is ~22 days. Group 1 rats [virgin (n = 6) and late pregnant (day 19–21; n = 6)] were used for determination of electrolyte balance, mRNA by qPCR, and protein abundance by Western blotting, and qroup 2 rats [virgin (n = 3) and late pregnant (days 18–20; n = 3 for BKα and n = 4 for ROMK)] were used for immunolocalization by IHC. Some of the animals used in this study are common to those used in our recent NCC and pendrin studies (32, 34). Animals were euthanized by exsanguination under 4% isoflurane (Baxter Healthcare, Deerfield, IL) anesthesia.
Metabolic cage studies.
Metabolic cage studies for determination of electrolyte balance were performed in Group 1 virgin (n = 6) and late pregnant (days 19–21; n = 6) rats. Rats were acclimated to the metabolic cages for 24 h before the 48-h collection period. Rats were given ad libitum access to water and powdered rat chow (Harlan Teklad LM-485; 0.3% sodium, 0.8% potassium; Harlan Teklad, Madison, WI). Food intake, urine volume, and fecal output were measured gravimetrically. Fecal samples were digested in nitric acid and processed as described previously (20). Urine and digested feces were analyzed by flame photometry for potassium. After the collection period, rats were removed from the metabolic cages and euthanized under isoflurane anesthesia, and the kidneys were perfused with cold PBS and separated into cortex and outer and inner medulla. The dissected kidneys and colon were then frozen in liquid nitrogen and maintained at −80°C until qPCR and Western blotting analysis.
RNA isolation and qPCR.
RNA was isolated using Direct-zol RNA MiniPrep kit with on column DNA digestion (Zymo Research) according to the manufacturer’s instructions. RNA (2 μg) samples were used as template for reverse transcription with High Capacity cDNA Reverse Transcription Kit [Applied Biosystems (AB)]. The resulting cDNAs (20 ng) were then used as template in real-time qPCR reactions (AB) to evaluate changes in H+-ATPase encoded by the Atp6v1b2 gene (AB Cat. No. Rn00589512_m1), H+/K+-ATPase type 1 (HKA1), encoded by the Atp4a gene (AB Cat. No. Rn01476803_m1), H+/K+-ATPase type 2 (HKA2), encoded by the Atp12a gene (AB Cat. No. Rn00591698_m1), ROMK (Kir1.1), encoded by the Kcnj1 gene (AB Cat. No. Rn00566732_m1) (Kcnj1 primer/probe set does not distinguish between ROMK splice variants), and BK α-subunit encoded by the KCNMA1 gene (AB Cat. No. Rn00582881_m1). Cycle threshold (CT) values were normalized against GAPDH (AB Cat. No. Rn01775763_g1), and relative quantification was performed using the ΔΔCt method (18). Fold change values were calculated as the change in mRNA expression levels relative to the virgin control. TaqMan primer/probe sets were purchased from AB.
Tissue homogenate preparation.
Kidneys were dissected into cortex and outer medulla, and the distal colon was removed and cleaned. Tissues were snap frozen in liquid nitrogen and stored at −80°C until homogenization. Tissues were homogenized in ice-cold homogenization buffer (5% sorbitol containing 25 mM histidine-imidazole (pH 7.5), 100 mM Na2EDTA, 20 mg/ml aprotinin, 167 mM PMSF, and a phosphatase inhibitor cocktail (Sigma P0044). After homogenization, samples were centrifuged at 2,000 g for removal of debris, and then the supernatants were spun at 15,000 g and the pellet was resuspended in homogenization buffer for generation of the renal cortex membrane fraction. Protein concentration of the homogenates was determined by BCA assay (Pierce Thermo, Rockford, IL). All samples were solubilized at 60°C for 15 min in a Laemmli sample buffer and stored at −80°C.
Quantitative immunoblotting and reagents.
BK alpha protein abundance was determined in kidney cortex (90 μg loading), kidney outer medulla (30 μg loading), and distal colon (21 μg). Samples were loaded on 10% polyacrylamide gels and separated by gel electrophoresis at a constant voltage of 100 V for 15 min followed by 140 V for 90 min and transferred to nitrocellulose membranes. Membranes were incubated overnight with anti-BKα primary antibody (1:1,000 dilution; 75–022; NeuroMab). Blots were then incubated with a goat anti-mouse IgG-horseradish peroxidase secondary antibody (1:10,000 dilution; sc-2005; Santa Cruz Biotechnology). HKA2 antibody (6) specificity was confirmed by peptide absorption (Fig. 1). Several nonspecific background bands were visible with the HKA2 antibody; however, the specific band at 100 kDa was visible only in colon and ablated when the HKA2 antibody was preincubated with immunizing peptide. Despite multiple attempts with differing protein and/or antibody quantities, we could not detect HKA2 protein in kidney tissue. Therefore, only colon HKA2 protein abundance was determined by Western blotting (using 30 μg of distal colon). Samples were loaded on 7.5% polyacrylamide gels and separated by gel electrophoresis at a constant voltage of 100 V for 15 min followed by 140 V for 90 min and transferred to nitrocellulose membranes. Membranes were incubated overnight with anti-HKA2 primary antibody (1:2,000) (6). Blots were then incubated with a goat anti-rabbit IgG-horseradish peroxidase secondary antibody (1:15,000 dilution; sc-2004; Santa Cruz Biotechnology). Bands of interest were visualized using enhanced chemiluminescence reagent (Supersignal West Pico; Thermo Scientific, Rockford, IL) and quantified by densitometry (Quantity One Analysis software; Bio-Rad). Densitometry was normalized to Ponceau staining (Sigma) and virgin controls, with the mean for the virgin control group being set as 100%.
Fig. 1.
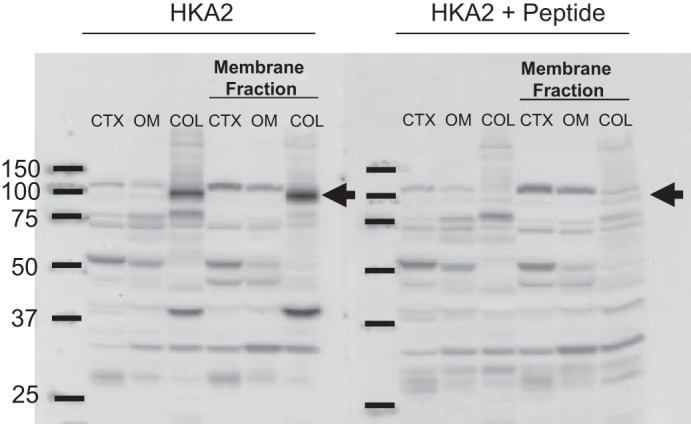
H+-K+-ATPase type 2 (HKA2) antibody specificity was confirmed by peptide absorption. The specific band at 100 kDa was visible only in colon (COL) in both total tissue homogenate and membrane fraction and was greatly reduced when the HKA2 antibody was preincubated with immunizing peptide. Only nonspecific background bands, unaffected by immunizing peptide, were visible in the whole renal cortex (CTX), membrane fraction of the renal CTX, and outer medulla (OM) of virgin (V) rats. Samples were from a virgin rat and were all loaded at 50 µg of protein.
Tissue preparation for immunofluorescence.
Group 2 rats consisted of virgins (n = 3) and 18- to 20-day late pregnant (n = 3–4). Rats were anesthetized with inhalant isoflurane and the kidneys were perfused via abdominal aortic cannula, first rinsed blood free with PBS (pH 7.4) followed by periodate-lysine-2% paraformaldehyde for 6 min, at a controlled pressure of 140 mmHg. The perfused kidneys were then cut transversely into several 2- to 4-mm-thick slices, immersed ∼24 h at 4°C in the same fixative, and then placed in PBS at 4°C. Kidney samples from each animal were embedded in polyester wax [polyethylene glycol 400 distearate (Polysciences, Warrington, PA) and 10% 1-hexadecanol], and 3-μm-thick sections were cut and mounted on triple chrome-alum-gelatin-coated glass slides (28).
Immunofluorescence.
To evaluate ROMK in the late distal tubule (DCT2) and connecting tubule (CNT), where apical ROMK abundance is primarily regulated to control potassium excretion (30), ROMK was colabeled with anti-calbindin and anti-aquaporin-2 (AQP2) antibodies (DCT2, calbindin positive, AQP2 negative; CNT, calbindin positive, weak AQP positive). The following antibodies were used: anti-rabbit ROMK R-80 [Welling laboratory mouse (30)], anti-calbindin D 28kDa (IHC 1:600; c-9848; Sigma-Aldrich), and chicken anti-AQP2 (IHC 1:100; gift from Dr. James Wade).
Antibodies were diluted in PBS containing 1%BSA, 0.2% fish gelatin, 0.1% Tween 20, and 0.2% sodium azide, and incubated overnight in a humid chamber at 4°C. After being thoroughly washed in a high-salt wash (incubation medium plus added sodium chloride at 0.5 M), antibodies were detected with Alexa Fluor-conjugated secondary antibodies. Unconjugated secondary antibodies from Jackson Laboratories were coupled to the respective fluorophores using kits from Invitrogen.
Images were obtained with Zeiss 510 laser scanning confocal microscope using a ×40 oil-immersion lens. Images from different groups were acquired with identical laser power, pinhole, and contrast settings and within the linear range of the photo detector.
Quantitative analysis of images.
Quantitative analysis of apical ROMK was performed using Improvision Volocity 5 by a trained investigator who was blind as to the identity of the groups. Line scans were measured over a 4- to 6-μm distance from the tubule lumen into the cytoplasm at resolution increments (0.4 μm) as reported previously (14). Apical ROMK intensity was assessed as the peak apical membrane-delimited pixel intensity within 1 μm of the lumen minus the local cytoplasmic intensity (2–3 μM from the lumen). At least 30 DCT2 and 30 CNT cells per rat were measured and averaged. Average measurements from three virgin and four late pregnant rats were evaluated for statistical difference by t-test using GraphPad PRISM version 6.
Immunohistochemistry.
Immunolocalization of the BKα protein was accomplished using immunoperoxidase, as follows: sections were dewaxed in ethanol, rehydrated, heated in Trilogy (Cell Marque 920P-10, Rocklin, CA) to 88°C for 30 min and then to 96°C for 30 min, cooled for 30 min, and rinsed in glass-distilled water. Endogenous peroxidase activity was quenched by incubating the sections in 3% H2O2 in distilled water for 45 min. Sections were blocked for 15 min with Serum-Free Protein Block (DakoCytomation X0909) and then incubated at 4°C overnight with BKα (1:5,000; Neuromab 75–022) primary antibody diluted in Dako antibody diluent (S0809). Sections were washed in PBS; incubated for 30 min with polymer-linked, peroxidase-conjugated goat anti-mouse IgG (ImmPRESS MP-7422; Vector Laboratories, Burlingame, CA); washed again with PBS; and then exposed to diaminobenzidine (Vector DAB substrate kit SK-4100) for 5 min. Sections were washed in distilled water, dehydrated with xylene, mounted, and observed by light microscopy. Comparisons of labeling were made only between sections of the same thickness from the same IHC experiment. Sections were examined on a Leica DM2000 microscope and photographed using a Leica DFC425 digital camera and Leica DFC Twain Software and LAS application suite (Leica Microsystems, Buffalo Grove, IL).
Quantative analysis of IHC.
Quantitation was determined as previously described (28). All tissues were prepared and stained using identical procedures and were viewed at the same time using identical settings. Briefly, high-resolution digital micrographs were taken of defined tubular segments. Freely available software (National Institutes of Health ImageJ, version 1.48v) quantified pixel intensity from outlining of the perimeter of the whole cell of interest. These data were then analyzed using custom software. The individual performing the microscopy, photography, and quantitative analysis was blinded to the pregnancy status of the animal.
Double immunolabeling procedure.
To distinguish cell types, tissue sections were colabeled with AQP2 (1:50,000, M. Knepper) using Vector SG as the chromogen to produce a blue color alongside primary label of BKα (1:5,000, Neuromab 75 022) with DAB. The above IHC procedure was repeated with a second primary AQP2 antibody right after the first primary BKα antibody DAB stain reveal. Sections were then incubated in 3% H2O2 in methanol for 45 min to further quench endogenous peroxidase activity. Blocking reagent, diluent, rinses, and time were kept the same as above except polymer-linked peroxidase anti-rabbit IgG (ImmPRESS MP-MP-7401; Vector Laboratories, Burlingame, CA) was substituted for host animal antibody compatibility with AQP2. Vector SG blue stain was also substituted for easy distinction between DAB stain. Sections were then washed with glass-distilled water, dehydrated, mounted, and observed by light microscopy.
Statistics.
Results are presented as means ± SE. Statistical analyses were performed using an unpaired t-test, and P < 0.05 was considered statistically significant.
RESULTS
Electrolyte measurements.
As shown in Table 1, potassium intake, plasma potassium, and urinary potassium increased in late gestation while fecal potassium output remained unchanged. Despite the increase in renal potassium excretion, the net result was that in late pregnancy potassium intake exceeded urinary and fecal excretion, indicating net retention.
Table 1.
Potassium balance measurements
| Virgin (n = 6) | Late Pregnant (n = 6) | |
|---|---|---|
| Food intake, g/24 h | 16.7 ± 0.8 | 25.5 ± 0.8* |
| K+ intake, mEq/24 h | 3.42 ± 0.15 | 5.21 ± 0.16* |
| Urine volume, ml/24 h | 16.5 ± 2.0 | 21.2 ± 1.5 |
| UKV, mEq/24 h | 3.13 ± 0.18 | 4.62 ± 0.14* |
| Fecal weight, g/24 h | 3.3 ± 0.3 | 4.8 ± 0.2* |
| Fecal K+ excretion, mEq/24 h | 0.23 ± 0.03 | 0.22 ± 0.04 |
| K+ retention, mEq/24 h | 0.06 ± 0.05 | 0.37 ± 0.16* |
| Plasma K+, mEq/l | 3.4 ± 0.3 | 4.4 ± 0.4* |
Data are presented as means ± SE. K+ balance = K+ intake – (UKV + fecal K+ excretion), where UKV is urinary potassium.
P < 0.05 vs. virgin, by unpaired t-test.
Transcript expression and protein abundance of apical potassium channels/transporters.
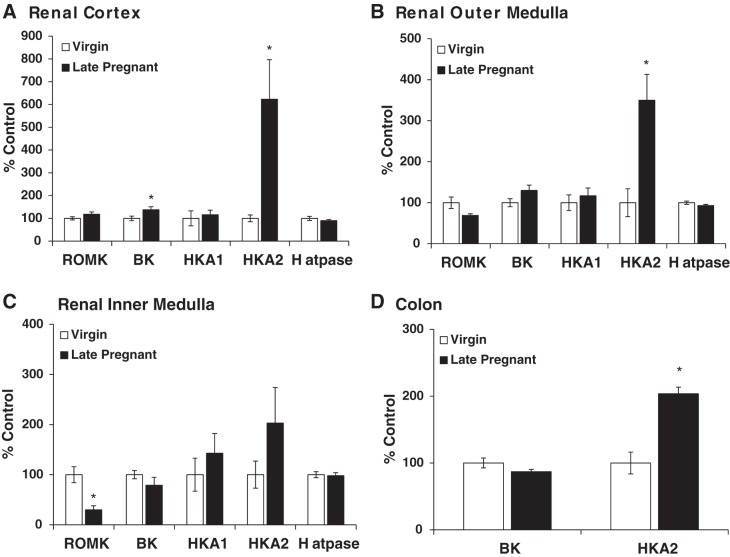
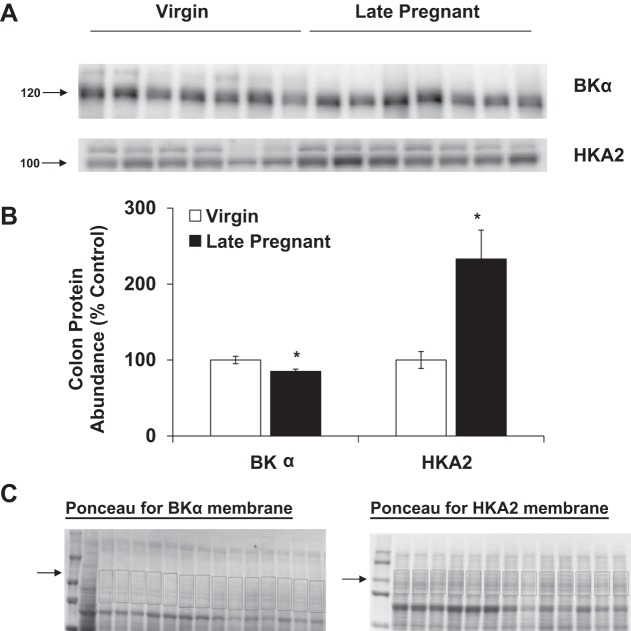
The mRNA expression of the H+-ATPase B2 subunit, HKA1, HKA2, BK, and ROMK wsd evaluated in the renal cortex and outer and inner medulla of virgin and late pregnant rats by qPCR. As shown in Fig. 2, we found no difference in H+-ATPase B2 subunit mRNA expression in any region of the kidney in pregnancy whereas markedly increased HKA2 expression was seen in both cortex and outer medulla of pregnant vs. virgin rats. H+-ATPase expression was measured to assess whether all renal H+ secretory mechanisms were altered by pregnancy or whether the changes were restricted to the potassium reabsorbing transporters, as found. The mRNA expression of HKA2 was also increased in the colon of late pregnant rats compared with virgins (Fig. 2D). Of note, HKA2 transcript expression in the distal colon (Virgin CT = 23.4 ± 0.3) was ~1,000-fold higher than in the kidney cortex (Virgin CT = 33.1 ± 0.2), outer medulla (Virgin CT = 33.0 ± 0.7), and inner medulla (Virgin CT = 35.1 ± 0.5). As discussed in methods we were unable to detect the HKA2 protein in any location in the kidney (Fig. 1), possibly due to the low abundance. HKA2 protein was detectable in distal colon, and as summarized in Fig. 3, there was an increase in HKA2 protein in pregnancy.
Fig. 2.
Relative expression of H-ATPase, H+-K+-ATPase type 1 (HKA1), H+/K+-ATPase type 2 (HKA2), and renal outer medullary potassium channel (ROMK) (Kir1.1) mRNA in the renal cortex (A), outer medulla (B), inner medulla (C), and colon (D) of virgin (n = 6) and late pregnant (n = 6) rats. BK, big potassium channel. Real-time quantitative RT-PCR (qPCR) was performed to evaluate mRNA expressions. All samples were normalized to GAPDH. An unpaired t-test was performed. Data are presented as means ± SE. *P < 0.05 vs. virgin.
Fig. 3.
Protein abundance of HKA2 and BKα in distal colon of virgin (n = 6–7) and late pregnant rats (n = 7). Band densities were normalized to virgin controls with virgins set at 100% (A) and summarized as bar graphs (B). An unpaired t-test was performed, and data are presented as means ± SE. *P < 0.05 vs. virgin. C: Ponceau-stained membranes with area selected for normalization.
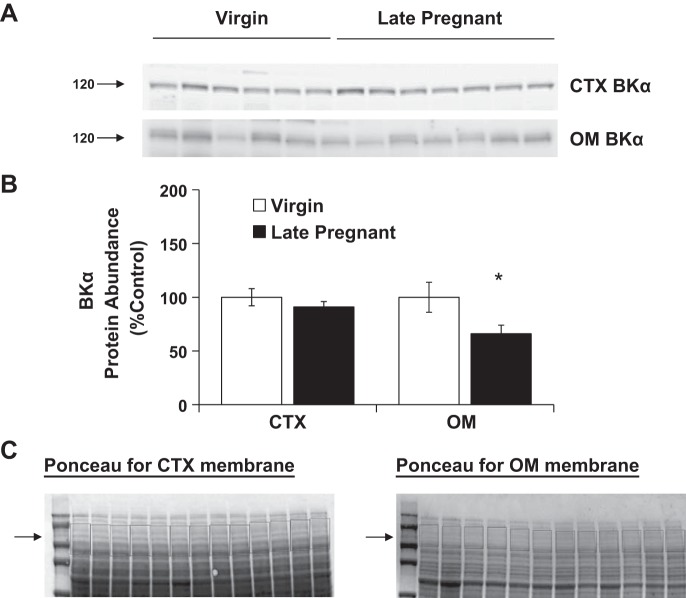
Total ROMK mRNA was unchanged in the cortex and decreased in the inner medulla of late pregnant rats vs. virgins. BKα mRNA expression was increased in the cortex of late pregnant rats but was unchanged in the renal medulla and distal colon compared with virgins (Fig. 2). BKα protein abundance was unchanged in the renal cortex and decreased in the outer medulla of pregnant rats (Fig. 4). There was also a decrease in BKα protein abundance in colon during late pregnancy (Fig. 3).
Fig. 4.
Protein abundance of BKα in kidney cortex and outer medulla of virgin (n = 6) and late pregnant rats (n = 6–7). Band densities were normalized to virgin controls with virgins set at 100% (A) and summarized as bar graphs (B). An unpaired t-test was performed, and data are presented as means ± SE. *P < 0.05 vs. virgin. C: Ponceau-stained membranes with area selected for normalization.
ROMK immunofluorescence.
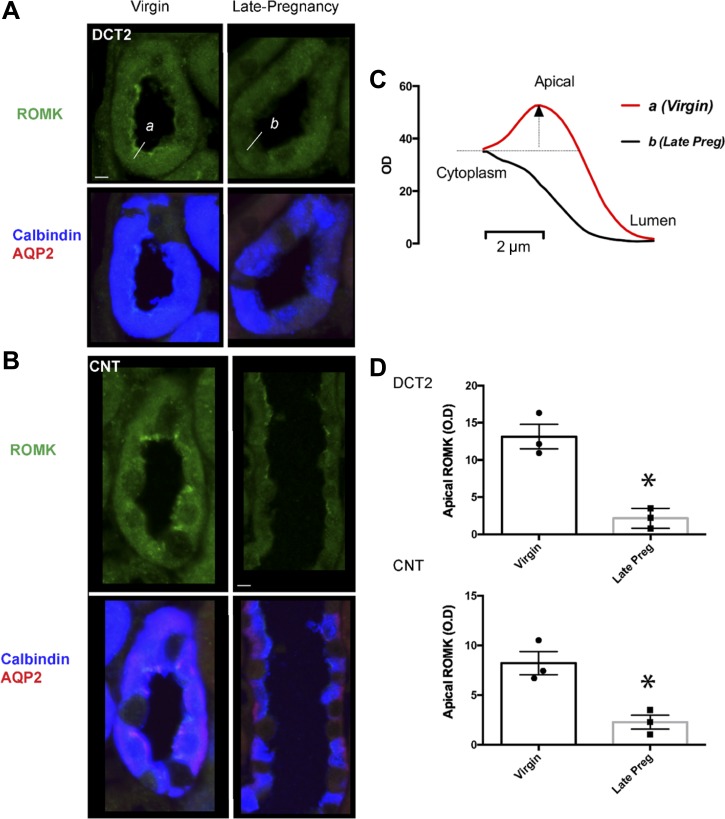
Since ROMK is strongly expressed in the thick ascending limb, measures of regional abundance by Western blot would be difficult to interpret. Therefore, we used immunofluorescence to assess changes in ROMK protein specifically in the DCT2 and CNT where apical ROMK abundance is primarily regulated (30). Apical labeling of ROMK in the cortical collecting duct and medulla was weak in virgin and pregnant rats, and we could not quantify any effect of pregnancy (data not shown). In comparison to the strong apical membrane ROMK labeling in the DCT2 and CNT of virgin rats, ROMK was largely absent on the apical membrane in pregnant rats (Fig. 5, A and B). Quantitative measurements of ROMK pixel intensity across the apical membrane and cytoplasm in multiple cells confirmed that the decrease in ROMK abundance at the apical membrane in late pregnant rats was statistically significant (Fig. 5, C and D).
Fig. 5.
Immunolocalization of ROMK. Apical ROMK in the aldosterone-sensitive distal nephron diminishes in pregnancy. Representative images of ROMK in the late distal convoluted tubule (DCT2; A) and connecting tubule (CNT; B) of virgin and late pregnant rats. DCT2 was identified by the presence of calbindin and absence of aquaporin 2 (AQP2); CNT was identified by the presence of calbindin and AQP2. Bar = 5 μM. C: representative line scan measurements of ROMK pixel intensity (shown for the cells on the left, labeled a and b. Apical membrane delimited ROMK pixel intensity is taken as peak pixel intensity within 1 μm of the lumen minus the local cytoplasmic intensity (within 2–3 μM from the lumen). D: summary data of apical membrane delimited ROMK pixel intensity in DCT2 and CNT of virgin (n = 3) and late pregnant rats (n = 4). Bar graphs are means ± SE of n = 3–4 rats; individual data points are average intensity of >30 cells from each animal. *P < 0.05 vs virgin by unpaired t-test.
BKα immunolocalization.
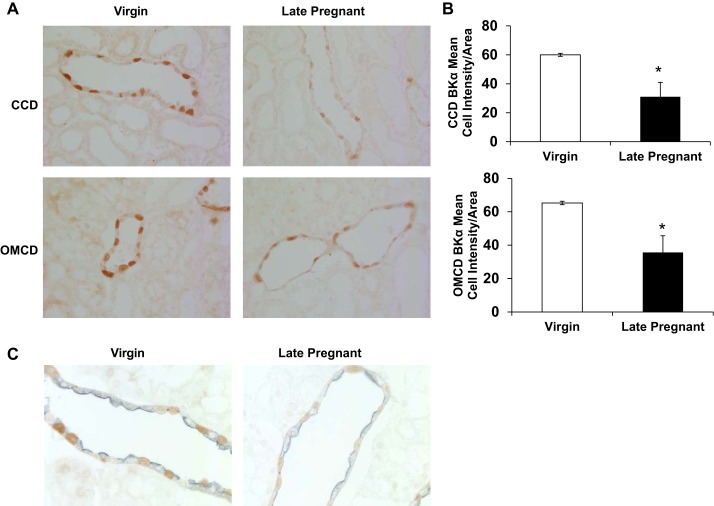
As shown in Fig. 6A, abundance of BKα was decreased in intercalated cells of late pregnant rats cortical collecting duct compared with virgin controls (n = 3 per group; P < 0.05 by t-test). There was also a significant decrease in BKα channel in the outer medullary collecting duct (Fig. 6B). BKα cell type localization was confirmed by BKα/AQP2 double labeling (Fig. 6C). As shown, the AQP2 (blue label) did not colocalize with BKα (brown label) in the renal collecting duct in neither virgin nor late pregnant rats.
Fig. 6.
Immunolocalization of BKα in intercalated cells of the cortical collecting duct (CCD) and outer medullary collecting duct (OMCD) of virgin and late pregnant rats. A: representative images. B: immunoreactivity expression as determined by pixel intensity was determined in the whole cell and normalized to cell area and summarized as bar graphs for virgin (n = 3) and late pregnant (n = 3) rats. An unpaired t-test was performed. Data presented as means ± SE. *P < 0.05 vs. virgin. C: representative images of BKα (brown)/AQP2 (blue) dual labeling of renal collecting duct.
DISCUSSION
The main finding of this study is that late pregnancy in the rat is associated with synergistic decreases in the cellular expression of potassium secretory channels, ROMK and BKα, with increases in the reabsorptive transporter HKA2 mRNA expression in the kidney. There is also a decrease in BKα as well as increased HKA2 in the distal colon. The combination of changes in the kidney and colon is consistent with potassium retention.
The classical explanation for the mechanism of potassium retention in pregnancy, despite high aldosterone levels, has been that progesterone inhibits aldosterone-mediated kaliuresis through mineralocorticoid receptor (MR) antagonism. This was based on early studies suggesting that administered progesterone inhibits the antinatriuretic action of administered mineralocorticoids (17). Also, in vitro studies indicated that progesterone competes with aldosterone for occupancy of the MR (2, 21), although in vivo, progesterone is a much less potent competitor (21).
It is now well recognized, however, that MR-mediated sodium retention is critical for maternal plasma volume expansion and normal fetal development (3, 26, 31), making it unlikely that progesterone is an effective antagonist to the MR-mediated sodium retention during pregnancy. This was addressed in carefully controlled clinical studies by Ehrlich and Lindheimer (10), in which synthetic mineralocorticoids were administered for several days to normal pregnant women in the third trimester. The administered mineralocorticoids caused additional renal sodium retention, while a natriuresis followed discontinuation of the drug. In contrast, administered mineralocorticoids had no effect on potassium balance in these normal pregnant women. Furthermore, normal men who received synthetic mineralocorticoids, “escaped” from the sodium retaining effects after several days but maintained kaliuresis. When progesterone was also given, there was no effect on sodium excretion, but significant potassium retention occurred despite continued MR stimulation (10), suggesting that in pregnancy, progesterone might be controlling potassium excretion by some non-MR-dependent action.
We now know that progesterone can have direct effects on potassium transport, specifically increasing potassium reabsorption via apical HKA2 in the collecting duct. In male mice, low dietary potassium intake, which suppresses aldosterone release, leads to marked stimulation of adrenal progesterone release; this finding was replicated in ovariectomized female mice and in normal men (8). When the male mice on low potassium intake were adrenalectomized and unable to raise progesterone levels, they exhibited increases in urinary potassium excretion and enhanced potassium wasting (8). In this same study, progesterone increased mRNA of the “colonic” HKA2 in cultured collecting duct cells and kidney, an effect abolished by a progesterone receptor antagonist. Finally, dietary potassium restriction led to increased plasma progesterone in both wild-type and HKA2 knockout mice, but only the wild types were able to reduce potassium excretion. This study makes a strong case for a role for progesterone in the control of potassium balance by regulation of HKA2 (8). This work agrees with observations in the pregnant HKA2 knockout mouse, in which both renal and colonic potassium wasting occur, leading to inadequate potassium retention and decreases in viable pregnancies and in litter number (22). In addition, MR stimulation by DOCA administration directly increases HKA2 in the mouse collecting duct (13), which suggests that the high plasma aldosterone of normal pregnancy may reinforce the progesterone-mediated HKA2 stimulation.
In the present study, we observed markedly increased HKA2 mRNA in the renal cortex and outer medulla as well as the distal colon, in late pregnancy. Of note, the relative amount of HKA2 transcript was ~1,000 times greater in the colon than in the kidney, which may explain why we were unable to detect HKA2 in the kidney at the protein level. The greater abundance of HKA2 in the colon was detectable by Western blotting, and increased significantly in late pregnancy. As reported previously (5), we observed an increased urinary potassium excretion in late pregnancy and no change in fecal potassium excretion, which because of the increased intake, indicated increased colonic potassium reabsorption leading to net potassium retention.
In the healthy, nonpregnant human and rat, potassium intake exceeds balance requirements and the kidney plays an important role by excreting excess potassium. The majority of filtered potassium is reabsorbed before the distal nephron, and the apical potassium channel ROMK plays a key role in distal potassium secretion, which is important for maintenance of potassium balance (16). In the distal nephron, most ROMK protein is found in DCT2 and CNT and it is now accepted that this is the primary regulated site of ROMK-mediated potassium secretion and excretion (9, 30). Despite no change in cortical ROMK transcript, we observed marked decreases in apical ROMK localization in DCT2 and CNT of late pregnant rats compared with virgins, which is consistent with decreased potassium secretion from these segments. This occurs despite the increased plasma aldosterone during high potassium intake. In addition to aldosterone, a moderate rise in dietary potassium intake increased apical ROMK in the aldosterone synthase knockout mouse (27). Therefore, although the mechanism is currently unknown, some factor in the pregnancy milieu inhibits these two potent kaliuretic signals.
We also assessed the expression, protein abundance, and cellular localization of the renal BKα subunit. In the cortex, BKα transcript abundance was increased and was unchanged in the medulla (Fig. 2), while protein abundance was unchanged in the cortex and decreased in the medulla (Fig. 4). Since BK channels are widely distributed in many cell types (12) we also used IHC to determine the BKα protein abundance in intercalated cells of the cortex and outer medulla and found a reduction (~50%) of intercalated cell BKα abundance in both regions in pregnant rats (Fig. 6). Of note, renal tubular BK channel activity is enhanced by high aldosterone levels in nonpregnant rodents fed low sodium, high potassium diet (7). Aldosterone also increases colonic BK-mediated potassium secretion in nonpregnant mice (24). Thus some influence of normal pregnancy overcomes the potassium wasting action of high aldosterone on both ROMK and BK. Although there is little information on possible actions of progesterone on renal potassium channels, progesterone reduces potassium channel activity in Xenopus oocytes (37) and in Madin-Darby canine kidney cells (25). Thus progesterone’s effects on renal potassium channels warrant future study.
Figure 7 summarizes the regulation of potassium homeostasis. The regulated processes of gut absorption in nonpregnant animals (open arrows), exchange between the small extracellular pool and large intracellular (tissue) pool of potassium, and exchange within the kidney (filtration/secretion/reabsorption) are shown. In normal pregnancy (black arrows), there is an additional compartment consisting of the fetoplacental unit. Here, potassium is sequestered, but not exchanged, with the maternal potassium pools; thus this component of the retained potassium becomes invisible to maternal potassium regulatory processes. During the process of rapid fetal growth and development that occurs in late gestation, potassium is shunted out of the maternal pool, equivalent to the development of a third “excretory” path. The maternal mechanisms of potassium homeostasis have to integrate this rapid “disappearance” of potassium to the fetoplacental unit, into the “external” regulatory processes (of gut absorption and renal excretion), as well as partitioning potassium between the small extracellular pool and the tissues (particularly liver and skeletal muscle) (15, 38). Exactly how maternal potassium balance is being “sensed” during normal pregnancy remains an enigma.
Fig. 7.
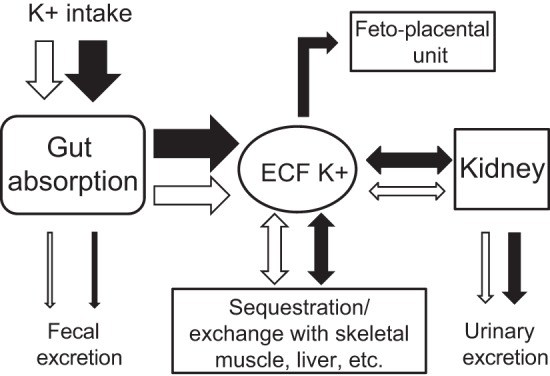
Summary of potassium homeostasis in pregnancy. The regulated processes of gut absorption, exchange between the small extracellular pool and large intracellular (tissue) pool of potassium, and exchange within the kidney (filtration/secretion/reabsorption) are shown in blue. Shown in black are adaptations of normal pregnancy, including an additional compartment (fetoplacental unit).
In conclusion, late pregnancy is associated with a decrease in the apical localization of the renal secretory channel ROMK, decreased intercalated cell and colonic BKα abundance, and an increase in the renal and colonic reabsorptive transporter HKA2. These adaptations in the potassium transporter profile are consistent with net potassium retention and likely necessary for the required increase in potassium delivery to the fetoplacental unit.
GRANTS
This work was supported by the Robert and Mary Cade Professorship of Physiology and National Institutes of Health Grants T32-HL-083810, R01-HD-041571, and R01-DK-56843 (to C. Baylis); R01-DK-63049, R01-DK-54231, and R01-DK-093501 (to P. Welling); DK-085193 and the American Society for Nephrology (ASN) Foundation for Kidney Research (to M. Gumz); and T32-HL-083810 and an ASN Career Development award (to C. West).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.A.W., C.B., and M.L.G. conceived and designed research; C.A.W., P.A.W., D.A.W., R.A.C., K.-Y.C., and C.C. performed experiments; C.A.W., P.A.W., D.A.W., K.-Y.C., and C.B. analyzed data; C.A.W., P.A.W., D.A.W., J.W.V., C.B., and M.L.G. interpreted results of experiments; C.A.W., P.A.W., D.A.W., and C.B. prepared figures; C.A.W. and C.B. drafted manuscript; C.A.W., P.A.W., C.C., T.D.D., J.W.V., C.B., and M.L.G. edited and revised manuscript; C.A.W., P.A.W., D.A.W., R.A.C., K.-Y.C., C.C., T.D.D., J.W.V., C.B., and M.L.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank I. David Weiner for developing the software for the quantitative IHC. We also thank Richard Smith for expert technical assistance as well as the University of Florida College of Medicine Electron Microscopy Core for preparing the tissues for IHC.
REFERENCES
- 1.Abreu N, Tardin JC, Boim MA, Campos RR, Bergamaschi CT, Schor N. Hemodynamic parameters during normal and hypertensive pregnancy in rats: evaluation of renal salt and water transporters. Hypertens Pregnancy 27: 49–63, 2008. doi: 10.1080/10641950701825887. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal MK, Paillard J. Paradoxical nature of mineralocorticoid receptor antagonism by progestins. Biochem Biophys Res Commun 89: 77–84, 1979. doi: 10.1016/0006-291X(79)90945-8. [DOI] [PubMed] [Google Scholar]
- 3.Barron W, Brandt CN, Lindheimer MD. Role of adrenal mineralocorticoid in volume homeostasis and pregnancy performance in the rat. Hypertens Pregnancy 12: 53–69, 1993. doi: 10.3109/10641959309031053. [DOI] [Google Scholar]
- 4.Brochu M, Lehoux JG, Picard S. Effects of gestation on enzymes controlling aldosterone synthesis in the rat adrenal. Endocrinology 138: 2354–2358, 1997. doi: 10.1210/endo.138.6.5198. [DOI] [PubMed] [Google Scholar]
- 5.Churchi-l SE, Bengele HH, Alexander EA. Sodium balance during pregnancy in the rat. Am J Physiol Regul Integr Comp Physiol l 239: R143–R148, 1980. [DOI] [PubMed] [Google Scholar]
- 6.Codina J, Delmas-Mata JT, DuBose TD Jr. The alpha-subunit of the colonic H+,K+-ATPase assembles with beta1-Na+,K+-ATPase in kidney and distal colon. J Biol Chem 273: 7894–7899, 1998. doi: 10.1074/jbc.273.14.7894. [DOI] [PubMed] [Google Scholar]
- 7.Cornelius RJ, Wen D, Li H, Yuan Y, Wang-France J, Warner PC, Sansom SC. Low Na, high K diet and the role of aldosterone in BK-mediated K excretion. PLoS One 10: e0115515, 2015. doi: 10.1371/journal.pone.0115515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elabida B, Edwards A, Salhi A, Azroyan A, Fodstad H, Meneton P, Doucet A, Bloch-Faure M, Crambert G. Chronic potassium depletion increases adrenal progesterone production that is necessary for efficient renal retention of potassium. Kidney Int 80: 256–262, 2011. doi: 10.1038/ki.2011.15. [DOI] [PubMed] [Google Scholar]
- 9.Ellison DH, Terker AS, Gamba G. Potassium and its discontents: new insight, new treatments. J Am Soc Nephrol 27: 981–989, 2016. doi: 10.1681/ASN.2015070751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehrlich EN, Lindheimer MD. Effect of administered mineralocorticoids or ACTH in pregnant women. Attenuation of kaliuretic influence of mineralocorticoids during pregnancy. J Clin Invest 51: 1301–1309, 1972. doi: 10.1172/JCI106926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garland HO, Atherton JC, Baylis C, Morgan MR, Milne CM. Hormone profiles for progesterone, oestradiol, prolactin, plasma renin activity, aldosterone and corticosterone during pregnancy and pseudopregnancy in two strains of rat: correlation with renal studies. J Endocrinol 113: 435–444, 1987. doi: 10.1677/joe.0.1130435. [DOI] [PubMed] [Google Scholar]
- 12.Ge L, Hoa NT, Wilson Z, Arismendi-Morillo G, Kong XT, Tajhya RB, Beeton C, Jadus MR. Big Potassium (BK) ion channels in biology, disease and possible targets for cancer immunotherapy. Int Immunopharmacol 22: 427–443, 2014. doi: 10.1016/j.intimp.2014.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenlee MM, Lynch IJ, Gumz ML, Cain BD, Wingo CS. Mineralocorticoids stimulate the activity and expression of renal H+,K+-ATPases. J Am Soc Nephrol 22: 49–58, 2011. doi: 10.1681/ASN.2010030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimm PR, Lazo-Fernandez Y, Delpire E, Wall SM, Dorsey SG, Weinman EJ, Coleman R, Wade JB, Welling PA. Integrated compensatory network is activated in the absence of NCC phosphorylation. J Clin Invest 125: 2136–2150, 2015. doi: 10.1172/JCI78558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gumz ML, Rabinowitz L, Wingo CS. An Integrated View of Potassium Homeostasis. N Engl J Med 373: 60–72, 2015. doi: 10.1056/NEJMra1313341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hebert SC, Desir G, Giebisch G, Wang W. Molecular diversity and regulation of renal potassium channels. Physiol Rev 85: 319–371, 2005. doi: 10.1152/physrev.00051.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landau RL, Lugibihl K. Inhibition of the sodium-retaining influence of aldosterone by progesterone. J Clin Endocrinol Metab 18: 1237–1245, 1958. doi: 10.1210/jcem-18-11-1237. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Lundgren DW, Moore JJ, Chang SM, Collins PL, Chang AS. Gestational changes in the uterine expression of an inwardly rectifying K+ channel, ROMK. Proc Soc Exp Biol Med 216: 57–64, 1997. doi: 10.3181/00379727-216-44156. [DOI] [PubMed] [Google Scholar]
- 20.Lynch IJ, Welch AK, Gumz ML, Kohan DE, Cain BD, Wingo CS. Effect of mineralocorticoid treatment in mice with collecting duct-specific knockout of endothelin-1. Am J Physiol Renal Physiol 309: F1026–F1034, 2015. doi: 10.1152/ajprenal.00220.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myles K, Funder JW. Progesterone binding to mineralocorticoid receptors: in vitro and in vivo studies. Am J Physiol Endocrinol Physiol 270: E601–E607, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Salhi A, Lamouroux C, Pestov NB, Modyanov NN, Doucet A, Crambert G. A link between fertility and K+ homeostasis: role of the renal H,K-ATPase type 2. Pflugers Arch 465: 1149–1158, 2013. doi: 10.1007/s00424-013-1252-x. [DOI] [PubMed] [Google Scholar]
- 23.Sorensen MV, Matos JE, Praetorius HA, Leipziger J. Colonic potassium handling. Pflügers Arch 459: 645–656, 2010. doi: 10.1007/s00424-009-0781-9. [DOI] [PubMed] [Google Scholar]
- 24.Sørensen MV, Matos JE, Sausbier M, Sausbier U, Ruth P, Praetorius HA, Leipziger J. Aldosterone increases KCa1.1 (BK) channel-mediated colonic K+ secretion. J Physiol 586: 4251–4264, 2008. doi: 10.1113/jphysiol.2008.156968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steidl M, Pinggera G, Ritter M, Lang F. Progesterone inhibits K conductance in plasma membrane of cultured renal epitheloid MDCK cells. Am J Physiol Endocrinol Metab 260: E743–E750, 1991. [DOI] [PubMed] [Google Scholar]
- 26.Todkar A, Di Chiara M, Loffing-Cueni D, Bettoni C, Mohaupt M, Loffing J, Wagner CA. Aldosterone deficiency adversely affects pregnancy outcome in mice. Pflügers Arch 464: 331–343, 2012. doi: 10.1007/s00424-012-1145-4. [DOI] [PubMed] [Google Scholar]
- 27.Todkar A, Picard N, Loffing-Cueni D, Sorensen MV, Mihailova M, Nesterov V, Makhanova N, Korbmacher C, Wagner CA, Loffing J. Mechanisms of renal control of potassium homeostasis in complete aldosterone deficiency. J Am Soc Nephrol 26: 425–438, 2015. doi: 10.1681/ASN.2013111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verlander JW, Chu D, Lee HW, Handlogten ME, Weiner ID. Expression of glutamine synthetase in the mouse kidney: localization in multiple epithelial cell types and differential regulation by hypokalemia. Am J Physiol Renal Physiol 305: F701–F713, 2013. doi: 10.1152/ajprenal.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verlander JW, Moudy RM, Campbell WG, Cain BD, Wingo CS. Immunohistochemical localization of H-K-ATPase α2c-subunit in rabbit kidney. Am J Physiol Renal Physiol 281: F357–F365, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Wade JB, Fang L, Coleman RA, Liu J, Grimm PR, Wang T, Welling PA. Differential regulation of ROMK (Kir1.1) in distal nephron segments by dietary potassium. Am J Physiol Renal Physiol 300: F1385–F1393, 2011. doi: 10.1152/ajprenal.00592.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.West CA, Han W, Li N, Masilamani SM. Renal epithelial sodium channel is critical for blood pressure maintenance and sodium balance in the normal late pregnant rat. Exp Physiol 99: 816–823, 2014. doi: 10.1113/expphysiol.2013.076273. [DOI] [PubMed] [Google Scholar]
- 32.West CA, McDonough AA, Masilamani SM, Verlander JW, Baylis C. Renal NCC is unchanged in the mid pregnant rat and decreased in the late pregnant rat despite avid renal Na+ retention. Am J Physiol Renal Physiol 309: F63–F70, 2015. doi: 10.1152/ajprenal.00147.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.West CA, Sasser JM, Baylis C. The enigma of continual plasma volume expansion in pregnancy: critical role of the renin-angiotensin-aldosterone system. Am J Physiol Renal Physiol 311: F1125–F1134, 2016. doi: 10.1152/ajprenal.00129.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.West CA, Verlander JW, Wall SM, Baylis C. The chloride-bicarbonate exchanger pendrin is increased in the kidney of the pregnant rat. Exp Physiol 100: 1177–1186, 2015. doi: 10.1113/EP085396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.West C, Zhang Z, Ecker G, Masilamani SM. Increased renal α-epithelial sodium channel (ENAC) protein and increased ENAC activity in normal pregnancy. Am J Physiol Regul Integr Comp Physiol 299: R1326–R1332, 2010. doi: 10.1152/ajpregu.00082.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wingo CS, Madsen KM, Smolka A, Tisher CC. H-K-ATPase immunoreactivity in cortical and outer medullary collecting duct. Kidney Int 38: 985–990, 1990. doi: 10.1038/ki.1990.302. [DOI] [PubMed] [Google Scholar]
- 37.Wong CM, Tsang SY, Yao X, Chan FL, Huang Y. Differential effects of estrogen and progesterone on potassium channels expressed in Xenopus oocytes. Steroids 73: 272–279, 2008. doi: 10.1016/j.steroids.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Youn JH, McDonough AA. Recent advances in understanding integrative control of potassium homeostasis. Annu Rev Physiol 71: 381–401, 2009. doi: 10.1146/annurev.physiol.010908.163241. [DOI] [PMC free article] [PubMed] [Google Scholar]