Abstract
Podocyte dysfunction and loss is an early event and a hallmark of proteinuric kidney diseases. A podocyte’s normal function is maintained via its unique cellular architecture that relies on an intracellular network of filaments, including filamentous actin (F-actin) and microtubules, that provides mechanical support. Damage to this filamentous network leads to changes in cellular morphology and results in podocyte injury, dysfunction, and death. Conversely, stabilization of this network protects podocytes and ameliorates proteinuria. This suggests that stabilization of podocyte architecture via its filamentous network could be a key therapeutic strategy for proteinuric kidney diseases. However, development of podocyte-directed therapeutics, especially those that target the cell’s filamentous network, is still lacking, partly because of unavailability of appropriate cellular assays for use in a drug discovery environment. Here, we describe a new high-content screening-based methodology and its implementation on podocytes to identify paullone derivatives as a novel group of podocyte-protective compounds. We find that three compounds, i.e., kenpaullone, 1-azakenpaullone, and alsterpaullone, dose dependently protect podocytes from puromycin aminonucleoside (PAN)-mediated injury in vitro by reducing PAN-induced changes in both the filamentous actin and microtubules, with alsterpaullone providing maximal protection. Mechanistic studies further show that alsterpaullone suppressed PAN-induced activation of signaling downstream of GSK3β and p38 mitogen-activated protein kinase. In vivo it reduced ADR-induced glomerular injury in a zebrafish model. Together, these results identify paullone derivatives as novel podocyte-protective agents for future therapeutic development.
Keywords: drug discovery, high-throughput screening, kenpaullone, phenotypic assay, podocytes
INTRODUCTION
Podocytes are terminally differentiated epithelial cells that form the filtration barrier in the kidney glomeruli (40). Podocytes have a unique cellular morphology that is held together via highly specialized intracellular filaments that are composed of filamentous actin (F-actin), intermediate filaments, and microtubules. Podocytes use a specialized subcellular structure, foot processes, to wrap themselves around glomerular capillaries. A network of interdigitating foot processes interact with neighboring podocytes and form filtration slits, also known as slit diaphragm (43). Damage or reorganization of the intracellular filaments changes the cellular architecture and results in podocyte injury, foot process effacement, and loss. Given their central role in glomerular filtration, podocyte injury and loss is an early event in the disease process and is a hallmark of glomerular diseases (62). Thus podocyte is a key cellular target for developing novel therapeutics for glomerular diseases (47). Additionally, as podocyte morphology is essential for its biological function and viability, strategies that decrease rearrangement of the cellular cytoskeleton to protect the cellular architecture and morphology have been the key focus of drug discovery efforts in the last few years (18, 35, 47). However, a key roadblock in drug discovery efforts has been a lack of cell-based high-throughput assays to quantitatively examine changes in cellular cytoskeleton and podocyte morphology. To address these shortcomings in the field, we recently described a novel, imaging microscopy-based high-content screening (HCS) assay for podocytes for use in a drug discovery environment (35).
Here, we describe a new methodology for improved analysis of the data from such HCS assays and use this methodology to identify additional podocyte-protective compounds from our previous chemical screen (50). We show that a new, multiparametric analysis of data from our screen with a library of 2,121 unique compounds revealed 65 compounds as potential hits. Among them, we found kenpaullone, a small molecule currently under preclinical development for neurological diseases (67), as a novel hit compound. Furthermore, using a group of commercially available analogs of kenpaullone, we describe our identification of 1-azakenpaullone and alsterpaullone as novel and highly efficacious podocyte-protective agents. We also present data from a number of in vitro and in vivo assays that define GSK-3β inhibition as the primary molecular mechanism of action of this group of compounds in podocytes. They also show that small-molecule mediate reduction in F-actin and microtubule rearrangements has a beneficial effect. The results presented here offer new insights about implementing podocyte cell-based assays in a drug discovery environment and also provide kenpaullone, 1-azakenpaullone, and alsterpaullone as novel candidates for future development as therapeutics for glomerular diseases.
MATERIALS AND METHODS
Reagents.
All cell culture reagents, including RPMI 1640, fetal bovine serum (FBS), and penicillin/streptomycin solution, were purchased from Life Technologies (Carlsbad, CA). Rat tail collagen I (cat. no. 354236) for coating on tissue culture plates and assay plates to promote the attachment of podocytes was purchased from BD Biosciences (San Jose, CA). For Western blotting, the anti-p38 (cat. no. 9212), anti-phospho-p38 (Thr180/Tyr182) (cat. no. 9215), anti-GAPDH (cat. no. 2118), anti-JNK (cat. no. 9258), and the anti-phospho-JNK (Thr183/Tyr185) (cat. no. 4668) antibodies were obtained from Cell Signaling Technology (Danvers, MA); the anti-phospho-p38 (cat. no. sc-7972) and the anti-pJNK (cat. no. sc-6254) antibodies were obtained from Santa Cruz Biotechnology (Dallas, TX); the anti-acetylated tubulin antibody (cat. no. T7451) was purchased from Sigma (St. Louis, MO); the anti-β-catenin antibody (cat. no. 610154) was obtained from BD Transduction (San Jose, CA); and the anti-GAPDH antibody (cat. no. MAB374) was obtained from Millipore (Billerica, MA). For immunostaining, the anti-paxillin antibody (cat. no. 04-581) was obtained from Millipore, the Alexa Fluor 568-labeled donkey anti-rabbit IgG (cat no. A-10042) and the Alexa Fluor 647-labeled donkey anti-mouse IgG (cat no. A-31571) were obtained from Life Technologies, and the Alexa-488-labeled donkey anti-rabbit IgG (cat no. 711-545-152) was obtained from Jackson ImmunoResearch (West Grove, PA). Paullone (cat. no. sc-208152) was purchased from Santa Cruz Biotechnology; kenpaullone (cat. no. 1398) was purchased from Tocris Bioscience (Minneapolis, MN); p38 mitogen-activated protein kinase (MAPK) inhibitor SB203580 (cat. no. tlrl-sb20) was obtained from InvivoGen (San Diego, CA); and puromycin aminonucleoside (cat. no. P7130), 1-azakenpaullone (cat. no. A3734), alsterpaullone (cat. no. A4847), GSK3 inhibitor SB216763 (cat. no. S3442), and anisomycin (cat. no. A9789) were purchased from Sigma-Aldrich.
Image quantification and analysis.
Podocyte HCS assay has been described previously (50). Briefly, after cell culture in multiwell plates and treatment with various agents, podocytes were fixed with a solution of 4% paraformaldehyde in phosphate buffered saline (PBS) for 15 min at room temperature and subsequently washed three times with PBS. Next, cells were permeabilized using a solution of 0.1% Triton X-100 in PBS for 15 min at room temperature and stained with CellMask Blue (Life Technologies) for 1 h at room temperature. Subsequently, after washing with PBS, images of cells were acquired using an Opera High Content Screening system (PerkinElmer) using a ×10 objective. Data were analyzed using Columbus data management and analysis system (PerkinElmer). Nuclear region was defined by Method B having the common threshold 0.55 with area >30 μm and signal intensity >2,900. Cellular cytoplasm was defined by Method A with 0.15 individual threshold. Partially shown cells in each field were excluded from further analysis. A multiparametric analysis approach was used to calculate 216 features based on the images with CellMask stain. Area, roundness, width, length, and ratio of width-to-length of nucleus and cells were measured. Also, texture structure was quantified using the Symmetry properties, Threshold compactness, Axial properties, Radial properties and profile (STAR) method (49).
High Content Profiler (PerkinElmer), a module for Spotfire data analytics software (TIBCO Software, Palo Alto, CA), was used for the analysis of the multiparametric HCS data derived by Columbus system. Median interplate data normalization (using median values of negative controls) was performed across all of the screen’s plates. This was followed by ensemble-based tree classification, which is a selection and classification method based on the ensemble of decision tree classifiers (8). This supervised classification method places the sample compounds into two predicted classes based on the positive [puromycin aminonucleoside (PAN) + mizoribine (MZR)] and negative (PAN) controls.
For quantification of acetylated tubulin intensity, data from the anti-acetylated tubulin-stained cells were analyzed using the Columbus data management and analysis system, as above. The signal intensity around the nuclear membrane was determined using Profile 4/5 and was normalized per cell by multiplying with total anti-acetylated tubulin signal intensity.
Mouse podocyte culture.
Immortalized murine podocytes were cultured according to published protocols with slight modification (39). Briefly, the cells were incubated in tissue culture medium (RPMI 1640 medium with 10% FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin) and mouse recombinant interferon-γ (cat. no. CR2041, Cell Sciences, Newburyport, MA), 50 U/ml for the first two passages and 20 U/ml for the later passages, in collagen-coated flasks at 33°C for proliferation. For differentiation, cells were thermo-shifted to 37°C and incubated without interferon-γ for 10–14 days. For assays in the 96-well plates, the cells were differentiated at 37°C in large (e.g., 75 or 150 cm2) tissue culture flasks for 7 days before seeding in the multiwell plates, and subsequently reseeded and incubated in multiwell plates for another 4 days.
Western blotting.
Cells were lysed in lysis buffer (20 mM Tris, 150 mM NaCl, 10 mM EDTA, 1% NP40, pH 7.5) containing protease inhibitors (cat. no. 11836153001, Roche Diagnostics, Indianapolis, IN) and phosphatase inhibitors (cat no. 78420, Thermo Fisher Scientific). LDS sample loading buffer and reducing agent (both from Thermo Fisher Scientific) were added to lysates, and the lysate was boiled at 95°C for 5 min. Proteins were separated by electrophoresis by using NuPAGE Novex 4–12% Bis-Tris protein gels (Thermo Fisher Scientific) and transferred to polyvinylidene difluoride membrane (Millipore). Subsequently, membranes were blocked with Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) and incubated with primary antibody diluents and fluorophore-conjugated secondary antibody diluents. After washing of the membranes, fluorescence was detected using the Odyssey CLx Imaging system (LI-COR Biosciences).
Fluorescent immunostaining of cells.
Cultured podocytes were fixed using a solution of 4% paraformaldehyde in PBS for 15 min at room temperature, followed by permeabilization using a solution of 0.1% Triton X-100 (Sigma-Aldrich) in PBS for 15 min at room temperature. Subsequently, the cells were blocked using 5% donkey serum (cat. no. D9663, Sigma) for 1 h. After blocking, cells were incubated with the desired primary and fluorophore-conjugated secondary antibodies, respectively. HCS CellMask Blue (cat. no. H32720, Invitrogen) and Alexa Fluor 568 phalloidin (cat. no. A12380, Invitrogen) were incubated for staining nucleus/cytoplasm and F-actin, respectively. Fluorescence images were acquired using a Zeiss 700 LSM confocal microscope (Zeiss, Thornwood, NY) or Opera XL (PerkinElmer).
mRNA expression quantification.
Total RNA was extracted from cultured podocytes by using RNeasy Mini Kit (cat. no. 74104, Qiagen) according to manufacturer’s instructions. Extracted RNA was reverse transcripted to cDNA with SuperScript III (cat. no. 18080-051, Life Technologies). Quantitative real-time PCR (qRT-PCR) was performed using Power SYBR Green Master Mix (cat. no. 4367659, Applied Biosystems) with the following primer sets: Desmin, GTGGATGCAGCCACTCTAGC and TTAGCCGCGATGGTCTCATAC; Cd80, CTGCAAAGGACTTCAGAAACCT and AGGCTTCACCTAGAGAACCGT; Actb, GGCTGTATTCCCCTCCATCG and CCAGTTGGTAACAATGCCATGT. The qRT-PCR reaction was performed using a CFX Connect real-time PCR detection system (Bio-Rad, Hercules, CA). Relative expression of Desmin and Cd80 was normalized to the expression level of β-actin mRNA.
Scratch wound-healing migration assay.
The scratch wound-healing migration assay was performed as previously described (50), with minor modifications. Briefly, mouse podocytes were differentiated at 37°C for 10–14 days. Subsequently, ~20,000 cells were seeded in each well of a 96-well plate and cultured for 6 h. Subsequently, a uniform scratch wound was created in the cell monolayer near the marked lines by sing a 10-μl pipette tip. Each well near the scratch wound in the monolayer was imaged at an initial time point by using the Opera HCS imaging system. Subsequently, the cells were incubated with various compounds at 37°C for 24 h, washed, fixed with 4% paraformaldehyde, and stained with CellMask Blue solution and reimaged. The images of pre- and posttreated cells were aligned to define the initial wound boundaries to quantify the number of cells that migrated into the area of the scratch wound.
Caspase 3/7 activity measurement.
Differentiated mouse podocytes were seeded in 96-well plates. After treatment with various agents for 24–48 h at 37°C, Caspase-Glo 3/7 working solution containing a luminogenic substrate (cat. no. G8090, Promega, Madison, WI) was added to each well. Subsequently, luminescence from each well was measured using EnSpire microplate reader (PerkinElmer).
Annexin V adhesion assay.
Differentiated mouse podocytes were treated for 72 h with various compounds according to concentrations presented in the figure legend of Fig. 6. Cells were detached using trypsin-EDTA (Life Technologies) and incubated with FITC-annexin V (cat. no. 640906, BioLegend, San Diego, CA) and propidium iodide (PI; cat. no. P3566, Life Technologies) for 15 min in annexin V binding buffer (cat. no. 422201, BioLegend). Flow cytometry was performed using BD FACS Canto II (BD Biosciences) to detect annexin V- and/or PI-bound cells. Apoptotic cells were defined as cells that were annexin V positive and PI negative.
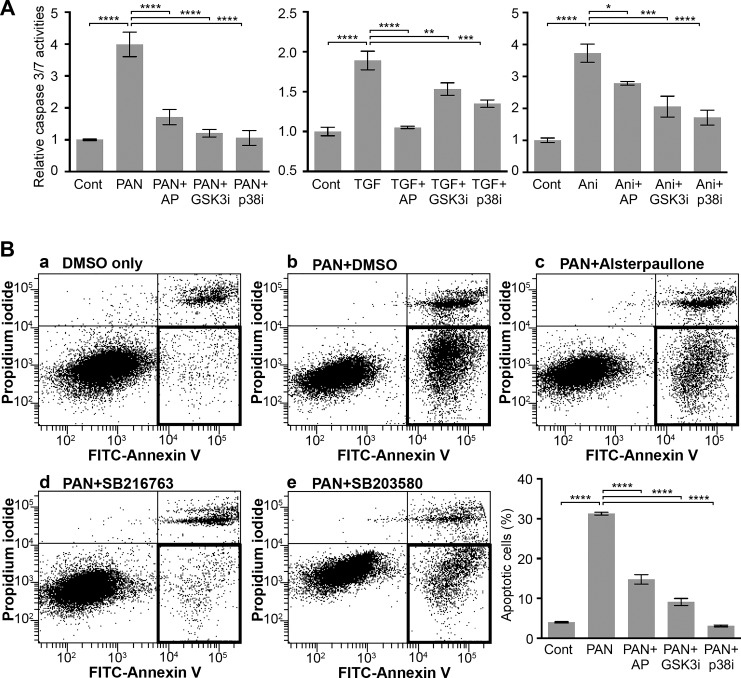
Fig. 6.
Alsterpaullone reduces apoptosis in PAN-treated podocytes. A: a bar graph showing activity level of caspase 3 and 7 in podocytes treated with various compounds. Mouse podocytes were treated with 30 µg/ml PAN in the absence or presence of 1 µM alsterpaullone (AP), 10 µM SB216763 (GSK3bi), or 20 µM SB203580 (p38i), and the level of caspase 3 and 7 activity was quantified in treated cells by using a luminogenic caspase-3/7 substrate. Vehicle DMSO-treated podocytes were used as control (Cont). Data shown are means ± SE (n = 4). One-way ANOVA with Dunnett’s multiple comparison test. *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.001. B: flow cytometry-based analyses of level of apoptosis in podocytes treated with various compounds. Mouse podocytes were treated with 30 µg/ml PAN in the presence of vehicle control (PAN+DMSO) or with various protective compounds: 1 µM alsterpaullone (AP), 10 µM SB216763 (GSK3bi), or 20 µM SB203580 (p38i) for 72 h, and the level of apoptosis was quantified in treated cells on staining with FITC-annexin V and propidium iodine (PI). Representative flow cytometry results are shown in panels a–e of B. Quantification of apoptotic cells (defined as cells that were annexin V positive and PI negative, highlighted as a darker rectangle) under each condition is presented as a bar graph. Data shown are means ± SE (n = 3). One-way ANOVA with Dunnett’s multiple comparison test was used. ****P < 0.001.
Zebrafish embryo-based glomerular injury assay.
Zebrafish studies were performed at the Medical University of South Carolina (MUSC) Zebrafish Core Facility. All animal work was performed under an IACUC-approved protocol from MUSC, protocol number 3364. Egg medium (E3) was used to grow zebrafish at 28.5°C. Adriamycin at a final concentration of 30.3 mg/l was used at 8 h postfertilization (hpf) in E3 growth medium to induce glomerular injury as described previously (25, 69). 1-Azakenpaullone (0.25 µM), alsterpaullone (0.25 µM), SB216763 (0.5 µM), or SB203580 (1 µM) were given in the medium at 8 hpf. Adriamycin was removed at 48 hpf, and the fish were kept in normal E3 medium for 72 hpf. The images were collected in a dissecting Leica microscope for the phenotypic analysis. Quantification of pericardial edema and whole body area were performed with images of zebrafish by using ImageJ software (National Institutes of Health, Bethesda, MD). Statistical analysis was performed using Prism 6 software (GraphPad, La Jolla, CA).
Statistical analysis.
For multiple comparisons, one-way ANOVA with Tukey’s or Dunnett’s multiple comparison test was used. A P value of <0.05 was considered statistically significant. Results are represented as means ± SE.
RESULTS
Identification of kenpaullone as a podocyte-protective compound from a podocyte-based HCS assay.
Confocal microscopy images of cells contain a large body of useful information about the state of the cells, including cell size, roundness and shape, nuclear size and shape, and cytoskeletal textures and intensity. Analysis and quantification of such parameters can be used to establish a cellular phenotype. Given that cellular morphology is essential for podocyte function and that aberrant changes in podocyte morphology associate with podocyte damage, we recently developed and described a novel HCS assay using podocyte morphology (35). In this assay, we cultured immortalized mouse podocytes in 96-well optical assay plates. We used puromycin aminonucleoside (PAN) treatment to induce podocyte damage in vitro and performed cotreatment with mizoribine (MZR) to protect podocytes from PAN damage (58), for use as a positive control in the assay. Subsequently, using a confocal microscopy-based automated imaging system (Opera HCS system, PerkinElmer), we imaged fluorescently labeled cells to establish quantitative parameters that distinguish changes in cellular phenotypes between cells treated with PAN and PAN+MZR, respectively. To identify novel podocyte-protective compounds, we utilized a library of 2,121 small-molecule compounds on PAN-treated podocytes and quantified single cellular parameters, such as cell roundness and the number of F-actin fibers per cell. From this assay, we previously reported on our identification of 24 unique podocyte-protective compounds, including the integrin β1 agonist compound pyrintegrin that we subsequently validated as a novel podocyte-protective agent in vitro and in vivo (35).
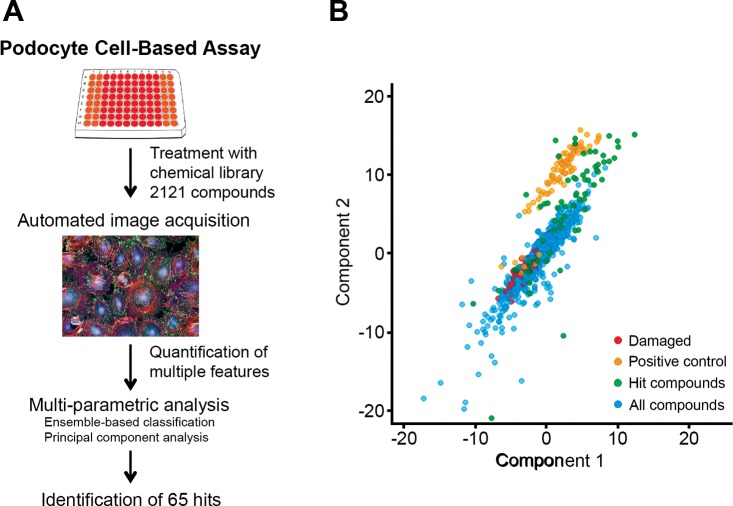
However, given that microscopy images from such HCS assays contain rich data, we wondered whether newer approaches could be used for multiparametric analysis of that data set to identify additional protective compounds. Toward that aim, here we describe our development of a multiparametric approach and its use in identification of additional compounds as hits from our screen (Fig. 1A). First, we performed analysis of the cellular images using multiple parameters [compared with analyzing only a single parameter before (35)] from the chemical screen with 2,121 compounds to calculate quantitative values for 216 cellular features, including area, roundness, width, length, and the width-to-length ratio of each cell and its nucleus using Columbus software (PerkinElmer). We further quantified texture structure using STAR method (49). Next, this multiparametric data set was analyzed using the High Content Profiler of Spotfire software (TIBCO Software). This module identified five features that showed high discrimination between PAN-damaged podocytes and the podocytes protected from injury with MZR cotreatment. These five measurement modules within the High Content Profiler directory were Cell Profile 1/5 SER Edge, 2/5 SER-Ridge, 4/5 SER-Ridge, 2/5 SER-Bright, and Cell Radial Mean Ratio SER Edge. This was followed by a supervised classification method, the ensemble-based tree classification, which is a feature selection and classification method based on the ensemble of decision tree classifiers (8), that placed data from the screen into two predicted classes based on the positive (PAN+MZR) and negative (PAN) controls. This methodology identified 65 compounds as potential hits in this assay (Table 1). To visually present this data, we generated the principle component analysis (PCA) visualization graph by using the first two principle components from all of the image analysis features (Fig. 1B). PCA is a statistical method that transforms a number of possibly correlated variables into a smaller number of uncorrelated ones, called principal components. The first principal component explains the maximum possible variability in the data, and each succeeding component accounts for as much of the remaining variability as possible. A common application of PCA is to find relationships in a multiparametric data set by projecting it into a new reduced space (usually two dimensional) that retains the main properties of the data. The two-dimensional PCA graph in Fig. 1B shows that PAN-damaged cells (red dots) are well segregated from the PAN+MZR-treated cells (positive control, yellow dots) in this multiparametric analysis. Here, the 65 hit compounds identified using this methodology (positive predicted class) are represented as dark green dots, while 783 negatively classified compounds are represented in blue. Known podocyte-protective compounds such as glucocorticoids (10) are included in the hits. Analysis of the list of hit compounds identified kenpaullone, a compound that is annotated as a GSK-3β inhibitor and is currently under preclinical development as a therapeutic for amyotrophic lateral sclerosis (ALS) (38, 67), as a top hit. Kenpaullone has not yet been studied in podocytes. Given that it is currently under preclinical development and thus has high translational potential, and since GSK-3β is a validated target in podocytes (5, 65, 66, 70), we decided to further investigate kenpaullone’s efficacy and its mechanism of action in podocytes (Table 1).
Fig. 1.
Multiparametric analysis of podocyte high-content screening (HCS) assay. A: schematic description of the assay showing a multiparametric approach for analyzing podocyte cell-based HCS data to identify new podocyte-protective compounds. B: principal component analysis (PCA) of podocyte HCS data. The x- and y-axes represent the first and second principal components with the largest variability obtained by PCA of the HCS multiparametric data set, respectively, on a relative scale. Each dot represents one sample or treatment. The injured puromycin aminonucleoside (PAN)-treated cells are colored red and are well segregated from the protected cells cotreated with mizoribine (MZR) (colored yellow). The 65 compounds identified as hits are represented as dark green dots (positive predicted class). The blue dots represents 783 negatively classified compounds.
Table 1.
List of hit compounds identified using multiparametric analysis
| Compound Name | Known Bioactivity |
|---|---|
| Kenpaullone | GSK-3β inhibitor |
| Agelasine | Cytotoxic, antineoplastic |
| Antimycin A | Antifungal, antiviral, interferes in cytochrome oxidation |
| Berberine chloride | Antiarrhythmic, α2 agonist, cholinesterase |
| Derrusnin | Mechanism of action unknown |
| Mundulone acetate | Mechanism of action unknown |
| Salicyl alcohol | Anesthetic (local), anti-inflammatory |
| 1R,2S-Phenylpropylamine | Decongestant |
| 2-Aminoguanidine hemisulfate | Nitric oxide synthase inhibitor |
| 3,6-Dimethoxyflavone | Mechanism of action unknown |
| 4,4′-Diisothiocyanostilbene-2,2′-sufonic acid sodium salt | ATP transport inhibitor, anion transport inhibitor, antiulcer |
| 5-Hydroxy-2′,4′,7,8-tetramethoxyflavone | Mechanism of action unknown |
| 6,4′-Dihydroxyflavone | Antihemorrhagic |
| 6α-Methylprednisolone acetate | Glucocorticoid |
| Aminoethylisothiourea dihydrobromide | Nitric oxide synthase inhibitor |
| Apotoxicarol | Mechanism of action unknown |
| Bisabolol | Mechanism of action unknown |
| Bromopride | Antiemetic |
| Budesonide | Antiinflammatory |
| Carapin-8 (9)-ene | Mechanism of action unknown |
| Carbetapentane citrate | Antitussive |
| Cinchonine | Antimalarial |
| Clenbuterol hydrochloride | Bronchodilator, β-2 adrenergic agonist |
| Coniferyl alcohol | Mechanism of action unknown |
| Curcumin | Antiinflammatory, antifungal, lipo/cyclooxygenase inhibitor |
| Desacetylcolforsin | Mechanism of action unknown |
| Embelin | Anthelmintic, oral contraceptive |
| Epiandrosterone | Mechanism of action unknown |
| Epoxy (1, 11)humulene | Mechanism of action unknown |
| Ethaverine hydrochloride | Antispasmodic |
| Fenoterol hydrobromide | β-Adrenergic agonist |
| Gardenin B | Mechanism of action unknown |
| Ginkgolide A | Antibacterial |
| Gossypin | Mechanism of action unknown |
| Halcinonide | Glucocorticoid, antiinflammatory |
| Hesperidin | Sweetener |
| Hexamethylquercetagetin | Mechanism of action unknown |
| Hinokitiol | Antifungal, metaloprotease inhibitor, DNA synthesis inhibitor |
| Isoliquiritigenin | Aldose reductase inhibitor, antineoplastic, antiinflammatory |
| Lawsone | Mechanism of action unknown |
| Lobeline hydrochloride | Antiasthmatic, respiratory stimulant |
| Lorglumide sodium | Cholecystokinin (CCK) receptor antagonist |
| Madecassic acid | Wound healing |
| Methoprene (S) | Ectoparasiticide |
| Mycophenolic acid | Antineoplastic |
| Nobiletin | Mechanism of action unknown |
| Piperine | Analeptic, antibacterial |
| Pridinol methanesulfonate | Anticholinergic |
| Pteryxin | Muscle relaxant |
| Quercetin tetramethyl (5,7,3′,4′) ether | Mechanism of action unknown |
| Racephedrine hydrochloride | Bronchodilator, decongestant |
| Resveratrol | Antifungal, antibacterial |
| Ritodrine hydrochloride | Muscle relaxant (smooth) |
| Sennoside B | Cathartic |
| Sinensetin | Mechanism of action unknown |
| Sulfamethoxazole | Antibacterial, antipneumocystis |
| Terbutaline hemisulfate | β-Adrenergic agonist, bronchodilator |
| Thiodiglycol | Antineoplastic, alkylating agent |
| Tolnaftate | Antifungal |
| Triamcinolone | Glucocorticoid |
| Triamcinolone acetonide | Antiinflammatory |
| Triptophenolide | Mechanism of action unknown |
Paullone derivatives protect podocytes by suppressing cytoskeletal changes and signaling downstream of GSK-3β.
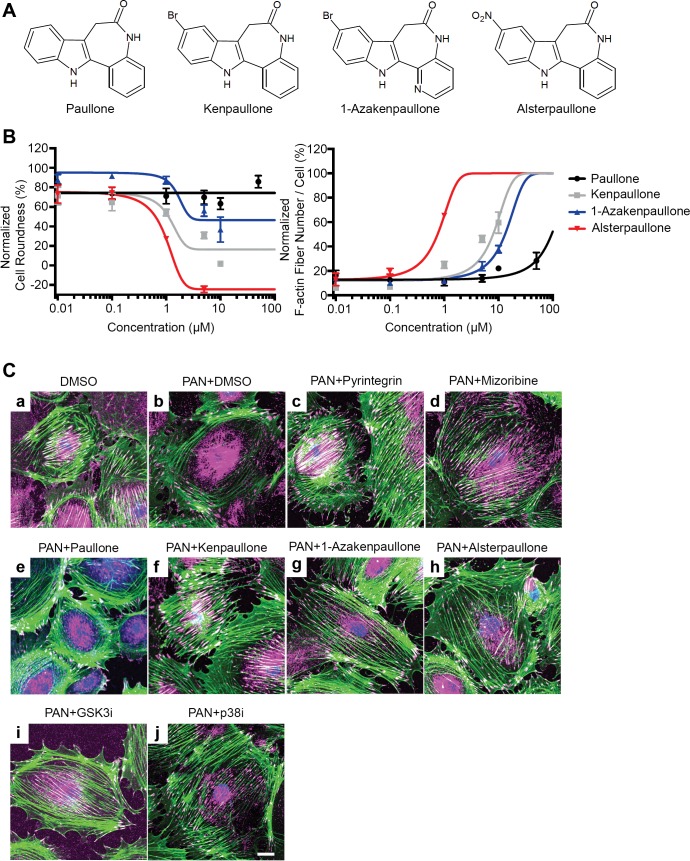
Kenpaullone belongs to the paullone family of small-molecule compounds (31). To study if other members of the paullone family have podocyte-protective activity, we obtained four different paullones—paullone, kenpaullone, 1-azakenpaullone, and alsterpaullone (Fig. 2A)—from commercial sources and tested them for their efficacy in protecting podocytes from PAN injury in vitro. We found that three compounds—alsterpaullone, kenpaullone, and 1-azakenpaulone—protected podocytes from PAN damage in a dose-dependent fashion. Each compound significantly reduced cell roundness and increased number of F-actin fibers per cell in podocytes (Fig. 2B and Supplementary Fig. S1). On the other hand, paullone showed no protective effect. As with published studies with motor neurons (38), kenpaullone, as well as alsterpaullone, and 1-azakenpaulone, showed dose-dependent protection of podocytes from injury. They showed optimal efficacy at concentrations <5 μM, whereas concentrations higher than 5 μM showed loss of efficacy due to compound precipitation. Confocal microscopy-based imaging confirmed the protection of F-actin stress fibers and focal adhesions in PAN-injured podocytes by alsterpaullone, kenpaullone, and 1-azakenpaullone (Fig. 2C), as compared with vehicle DMSO-treated cells. Mizoribine and our recently discovered agent, integrin β1 agonist pyrintegrin, also showed a high level of protection and served as positive controls. GSK-3β is a direct target of kenpaullone (4), suggesting GSK-3β inhibition as the primary mechanism behind the efficacy of these paullone derivatives in podocytes. GSK-3β is the predominant GSK-3 isoform in podocytes, and signaling downstream of GSK-3β is increased during podocyte injury (9, 42, 66, 70), resulting in increased oxidative stress, mitochondrial dysfunction, and podocyte apoptosis (9). To confirm that GSK-3β inhibition is protective in this PAN injury-based in vitro model, we utilized a known GSK-3β inhibitor, SB216763 (12), and found that it also protected podocytes from PAN injury (PAN+GSK3i, Fig. 2C). Finally, as the activation of p38 MAPK is a common upstream mechanism in podocyte injury, including PAN injury (29), we tested a p38 inhibitor, SB203580 (68), in this assay and found that it too protected podocytes in this assay by reducing F-actin cytoskeletal rearrangement and preventing the loss of focal adhesions (PAN+p38i, Fig. 2C).
Fig. 2.
Paullone derivatives protect podocytes from injury. A: chemical structures of paullone, kenpaullone, 1-azakenpaullone, and alsterpaullone. B: graphs showing that paullone derivatives protect podocytes from PAN injury in a dose-dependent fashion. Podocytes were cultured in 96-well optical plates and were treated with PAN (30 μg/ml) and an increasing concentration of the various compounds, as indicated, at 37°C for 48 h. Change in various cellular phenotypes was assessed using Opera HCS system after staining cells with CellMask Blue (to measure cell morphology), fluorescently labeled phalloidin (for the quantification of F-actin fibers), and anti-paxillin antibody (for the quantification of focal adhesions). Cell roundness and F-actin fiber number were determined using the Columbus software. Dose-response curves show the relative effects of increasing concentration of the various compounds on two different cellular parameters [cell morphology, as defined by cell roundness (left graph), and the number of F-actin fibers per cell (right graph) as compared with healthy podocytes, as well as PAN-injured podocytes]. X-axis represents increasing compound concentration, and y-axis shows relative quantification of each of the two parameters at a defined dose of PAN (30 μg/ml). Dose-response curves show that kenpaullone, 1-azakenpaullone, and alsterpaullone protect podocytes from PAN injury with varying degree of efficacy, with alsterpaullone being the most efficacious. Five hundred to one thousand cells were quantified in each assay well. Data shown are means ± SE (n = 4). Dashed blue lines represent the average relative values for the two parameters from podocytes treated with vehicle DMSO alone (control, healthy cells). The dashed red lines represent the average relative values for the two parameters from podocytes treated with PAN (30 μg/ml) (injured cells). C. paullone derivatives protect podocytes from PAN injury mediated F-actin reorganization. Representative fluorescence confocal microscopy images of mouse podocytes treated either with 15 µg/ml PAN alone (b, injured cells), or cotreated with 15µg/ml PAN and 10 µM paullone (c), 1 µM alsterpaullone (d), 5 µM kenpaullone (e), 10 µM 1-azakenpaullone (f), 1 µM pyrintegrin (g), 5 µg/ml MZR (h), 10 µM SB216763 (i, GSK3i), or 20 µM SB203580 (j, p38i) for 48 h. Control vehicle DMSO-treated cells are shown in panel a. Cells were stained with CellMask (blue), Alexa Fluor 488 phalloidin (green), and anti-paxillin antibody (magenta). Scale bar = 20 μm. GSK3i, GSK3 inhibitor; p38i, p38 inhibitor.
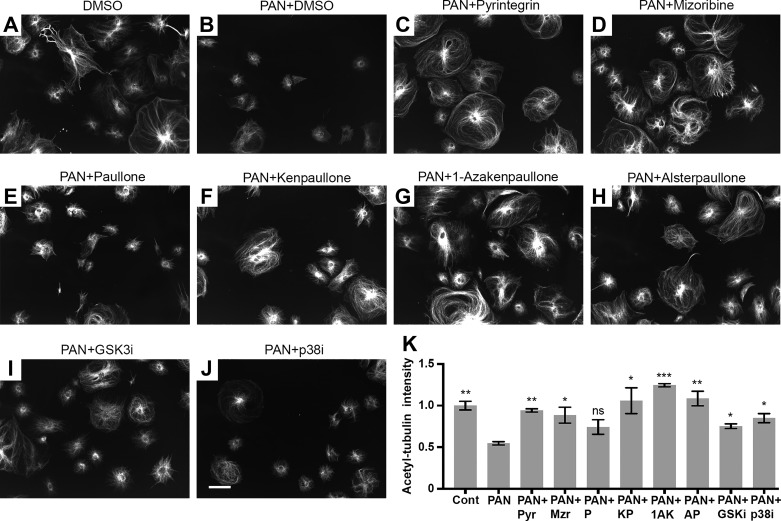
To maintain their cellular architecture, podocytes also utilize microtubules and intermediate filaments in the cell body and primary processes (43), in addition to the densely accumulated actin filaments in their foot processes. Microtubules are comprised of polymerized heterodimers of α- and β-tubulin. Immunofluorescence staining of immortalized murine podocytes with an anti-acetylated tubulin antibody showed that they radiate from the juxtanuclear region and extend toward peripheral area in healthy podocytes (Fig. 3A). To quantify, we measured signal intensity of acetylated tubulin around the nuclear membrane (Fig. 3K). As expected, PAN treatment induced microtubule rearrangement and significantly reduced the microtubule fiber density in podocytes (Fig. 3B). As with F-actin stabilization, the control compounds mizoribine and pyrintegrin, as well as GSK-3β inhibitor SB216763 and the p38 inhibitor SB203580 also showed protection of microtubules, whereas paullone did not. Notably, we found that kenpaullone, 1-azakenpaullone, and alsterpaullone also significantly protected podocytes from the PAN injury-mediated microtubule rearrangements (Fig. 3, F–H), suggesting that their effects extended to protecting the microtubule architecture in cells. Thus the newly discovered protective compounds help maintain podocyte cellular architecture and shape by targeting key intracellular structural elements. Collectively, these studies suggest that three paullone derivatives protect podocytes from PAN injury, although they do so with varying degrees of efficacy. Among them, alsterpaullone showed the strongest protective effect at the lowest concentration (<5 μM) (Fig. 2B). Therefore, we chose alsterpaullone for further mechanistic studies and in vivo experiments.
Fig. 3.
Paullone derivatives protect podocytes from PAN injury-mediated loss in microtubules. Representative fluorescence confocal microscopy images show acetylated tubulin expression in mouse podocytes treated either with 30 µg/ml PAN and DMSO vehicle control (B), or cotreated with 30 µg/ml PAN and 1 µM pyrintegrin (C), 5 µg/ml mizoribine (D), 10 µM paullone (E), 5 µM kenpaullone (F), 10 µM 1-azakenpaullone (G), 1 µM alsterpaullone (H), 10 µM SB216763 (I, GSK3i), or 20 µM SB203580 (J, p38i) for 48 h. Control vehicle DMSO-treated cells are shown in A. Cells were stained with CellMask and anti-acetylated tubulin antibody. Scale bar represents 100 μm and applies to all panels (A–J). GSK3i, GSK3 inhibitor; p38i, p38 inhibitor. Quantification of normalized acetylated tubulin signal intensity around nuclear membrane from each of these conditions is presented in K. Signal intensity was quantified using the Columbus software. The intensity values were normalized using the mean values from control cells. One-way ANOVA with Fisher’s LSD multiple comparison test was used. *P < 0.05; **P < 0.01; ***P < 0.005; ns = not significant as compared with the PAN-only (PAN) treated samples. Pyr, pyrintegrin; Mzr, mizoribine; P, paullone; KP, kenpaullone; AP, alsterpaullone; GSK3i, GSK3 inhibitor; p38i, p38 inhibitor.
Podocyte-protective effects of alsterpaullone.
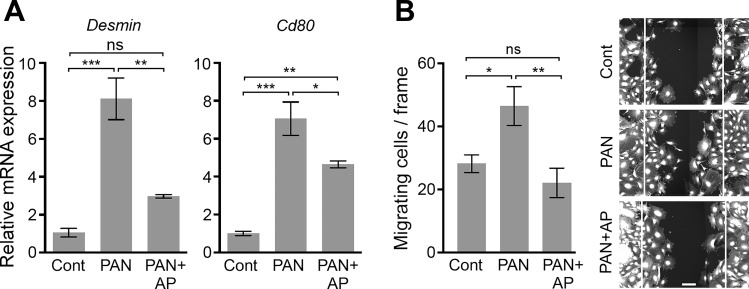
Podocyte injury results in gene expression changes, including significant upregulation in Desmin and Cd80 expression levels (16, 46, 52). Real-time quantitative RT-PCR of PAN-treated podocytes showed significantly increased mRNA expression of Desmin and Cd80, which was reversed upon cotreatment with alsterpaullone (Fig. 4A). Podocyte injury also results in increased cell motility (60). We found that PAN injury-mediated increase in podocyte migration and wound closure (3, 34, 35, 48) was also significantly reduced by cotreatment of cells with alsterpaullone, as measured using a scratch wound-healing assay (Fig. 4B). This suggests that alsterpaullone-dependent protection of cellular cytoskeleton results in a functional protection of podocytes.
Fig. 4.
Induction of podocyte damage markers is suppressed by alsterpaullone. A: mRNA expression of two known markers of podocyte damage is reduced by alsterpaullone. Graphs showing qRT-PCR-based measurement of Desmin and Cd80 in cultured mouse podocytes treated with either PAN (30 µg/ml) alone or with 30 µg/ml PAN and 1 µM alsterpaullone (AP). Data are expressed as fold change of mRNA levels in treated cells over the control vehicle-treated cells (normalized to 1). Data are means ± SE (n = 3). B: alsterpaullone reduces podocyte migration in scratch wound-healing migration assay. A bar graph showing the number of podocytes migrating toward the gap of scratch in a wound-healing assay (left). Scratch wounds were created in podocyte monolayers by using a sterile 10-μl pipette tip, and the monolayers were incubated in the absence (control, cont) or presence of PAN (30 µg/ml, PAN) at 37°C for 24 h. One set of wounded monolayers was cotreated with 1 µM alsterpaullone (PAN+AP). Subsequently, cells were fixed and stained with CellMask Blue, and the number of cells migrating inside the edge of the wounds was quantified for each condition. Representative images show cells after 24-h treatment (right). The white lines shown in the images represent the wound edges at the beginning of treatment. Scale bar represents 100 μm. The data presented in the graph are plotted as means ± SE (n ≥ 7). One-way ANOVA with Tukey’s multiple comparison test. *P < 0.05; **P < 0.01; ***P < 0.005; ns = not statistically significant.
Alsterpaullone suppresses PAN injury-mediated changes in β-catenin expression and JNK and p38 phosphorylation.
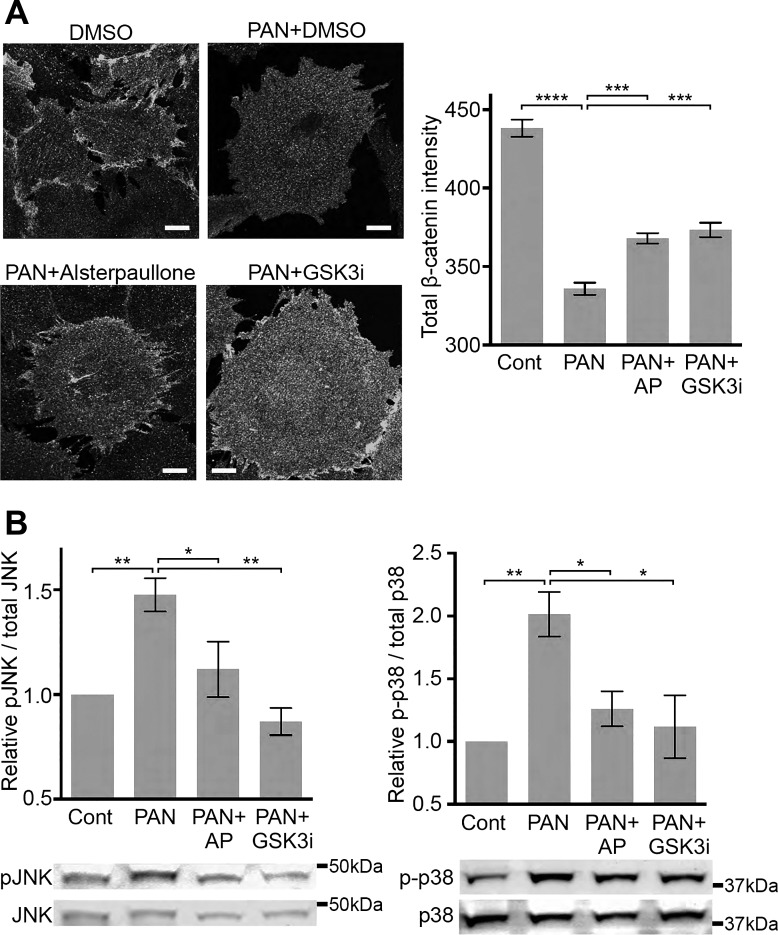
GSK-3β activation leads to degradation of β-catenin through a ubiquitin/proteasome-dependent pathway (1, 41), implicating that GSK-3β inhibition would protect β-catenin expression. As it is involved in the formation of cadherin-based adherens junctions, stabilization of β-catenin is highly beneficial in maintaining cytoskeletal interaction (22), epithelial integrity (6), and cell adhesion (57). Immunofluorescent staining of murine podocytes with anti-β-catenin antibody showed a significant reduction in β-catenin expression on PAN injury, as compared with the control cells, and that cotreatment with alsterpaullone or GSK3β inhibitor SB216763 resulted in significant protection against this PAN-induced loss of β-catenin to a similar extent (Fig. 5A). PAN injury also leads to increased activation of MAP kinases JNK and p38 via phosphorylation of JNK at Thr183/Tyr185 and of p38 at Thr180/Tyr182 (Fig. 5B) (15, 44a, 33, 56). Alsterpaullone and the GSK-3β inhibitor SB216763 significantly decreased phosphorylation of JNK and p38 in PAN-injured podocytes (Fig. 5B).
Fig. 5.
Alsterpaullone functions as a GSK-3β inhibitor and reduces JNK and p38 phosphorylation. A: alsterpaullone protects against PAN injury-mediated loss of β-catenin expression in mouse podocytes. Representative fluorescence confocal microscopy images of mouse podocytes treated with vehicle (DMSO, control), 30 µg/ml PAN (PAN+DMSO), cotreated with 30 µg/ml PAN and 1 µM alsterpaullone (PAN+Alsterpaullone), or cotreated with 30 µg/ml PAN and 10 µM SB216763 (PAN+GSK3i) for 48 h. Cells were immunostained with anti-β-catenin antibody and imaged using HCS Opera system and Zeiss 700 LSM confocal microscope. The images taken by Zeiss 700 LSM are shown as representative images. Scale bar = 20 μm. With the images taken by HCS Opera system, β-catenin expression was quantified by measuring fluorescence intensity of anti-β-catenin antibody staining per cell in each condition from 500 to 1,000 cells per well and is presented as a graph (right). Data are means ± SE (n = 4). One-way ANOVA with Dunnett’s multiple comparison test was used. ***P < 0.005; ****P < 0.001. B: alsterpaullone (AP) and GSK3β inhibitor SB216763 (GSK3i) protect against increase in phosphorylation of JNK (Thr183/Tyr185) and p38 (Thr180/Tyr182) in PAN-injured cells. Mouse podocytes were treated with 30 µg/ml PAN in the absence (Cont) or presence of 1 µM alsterpaullone or 10 µM SB216763 (GSK3bi), and cell lysates were analyzed by Western blot analysis for relative level of phosphorylated and total JNK and p38 proteins. Representative Western blot images are shown. Bar graphs show ratio of phosphorylated to total protein in the lysates. Data are means ± SE (n = 3). One-way ANOVA with Dunnett’s multiple comparison test was used. *P < 0.05; **P < 0.01.
Alsterpaullone reduces apoptosis in PAN-injured podocytes.
Given that both GSK-3β (42) and p38 signaling pathways regulate cellular apoptosis (59), and that alsterpaullone suppresses the GSK-3β signaling and reduces p38 phosphorylation, we wondered if part of alsterpaullone’s efficacy is due to inhibition of podocyte apoptosis, a common response to stressful stimuli in podocytes (40). As caspase 3 and 7 proteases are key players in apoptosis and their activity determines the level of cellular apoptosis (14, 51), we quantified caspase 3/7 activity by using a luminogenic caspase-3/7 substrate as a measure of podocyte apoptosis. Whereas PAN treatment significantly increased caspase 3/7 activity relative to healthy control cells, podocyte cotreatment with alsterpaullone, GSK3β inhibitor SB216763, or p38 inhibitor SB203580 significantly reduced caspase 3/7 activity (Fig. 6A), suggesting protection from apoptosis. Furthermore, an increase in podocyte apoptosis on treatment with additional damaging agents, such as TGF-β1 that is involved in podocyte damage in diabetic nephropathy (11, 23, 44, 50, 55, 63, 71) (Supplementary Fig. S2A) or with p38 agonist anisomycin that induces stress-mediated cellular apoptosis (54) (Supplementary Fig. S2B), was also similarly and significantly reduced by alsterpaullone, SB216763, and SB203580. We further confirmed this protective activity of alsterpaullone against apoptosis by using a flow cytometry-based annexin V affinity assay (Fig. 6B), where PAN injury resulted in apoptosis of ~30% of podocytes that was significantly reduced by alsterpaullone, SB216763, and SB203580 (Fig. 6B, panels c–e). There was no significant difference in necrotic cell number (PI-positive cells) between various treatment conditions. Together, these results suggest that alsterpaullone-mediated inhibition of GSK-3β suppresses downstream p38 activation and pro-apoptotic signaling in podocytes.
Alsterpaullone and 1-azakenapaullone protect against glomerular damage in zebrafish.
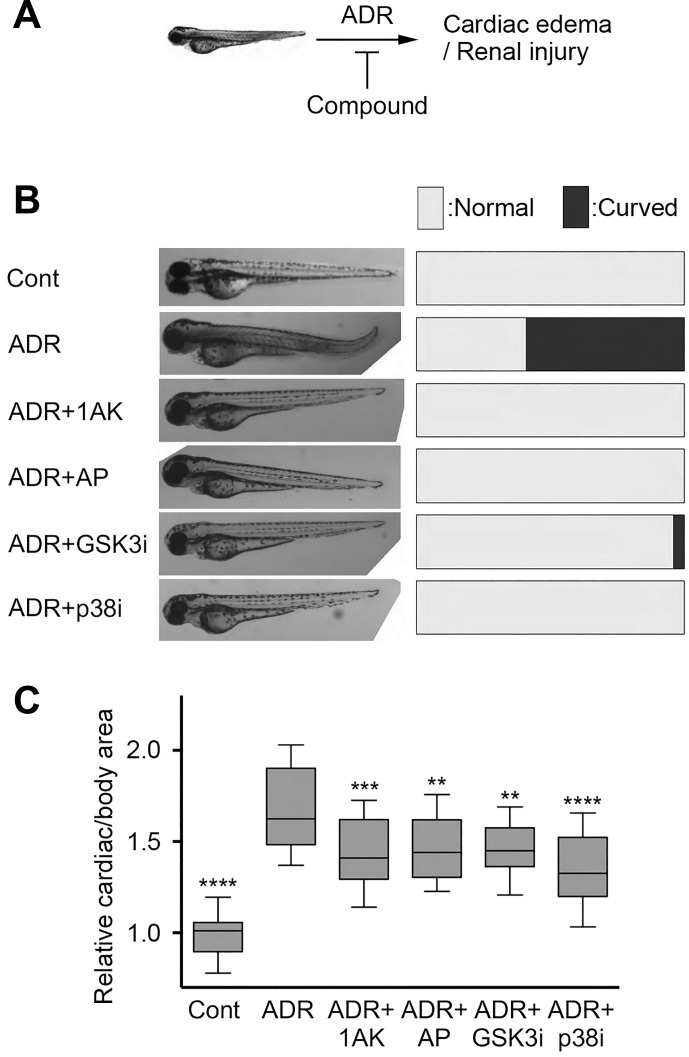
Adriamycin (ADR)-induced nephropathy is a well-established glomerular injury model. This anthracycline derivative antibiotic causes proteinuria and FSGS-like phenotype in rodents (36) and was shown to induce glomerular damage in zebrafish embryos (69). Phenotypic characterization of zebrafish embryos in this model includes analysis of body axis curvature, pericardial edema, and whole body edema (2, 13, 19, 30). Pericardial edema in zebrafish embryo is followed by loss of kidney function, because the pronephric kidney in these animals regulates osmolality (21, 27). As we recently showed, ADR-induced injury resulted in moderate curved body axis in animals (2). Treatment of zebrafish embryos with ADR resulted in approximately 60% of embryos with curved body axis, while none of the untreated embryos presented with curved body axis (Fig. 7A). We also found that cotreatment of embryos with either 1-azakenpaullone (0.25 µM), alsterpaullone (0.25 µM), SB216763 (0.5 µM), or SB203580 (1 µM) in the presence of ADR showed significant reduction in the curved body axis, with very few embryos (0 to 4%) showing curved body axis (Fig. 7A). We also estimated the level of pericardial edema by quantifying cardiac area per total body area as another measure of kidney dysfunction. This cardiac to total body area ratio was significantly increased in ADR-treated embryos (Fig. 7B). However, cotreatment with either 1-azakenpaullone, alsterpaullone, GSK-3β inhibitor SB216763, or p38 inhibitor SB203580 showed a significant reduction in the ratio of cardiac/total body area (Fig. 7B), suggesting protection from ADR-mediated kidney in vivo damage by these compounds.
Fig. 7.
Alsterpaullone reduces pericardial edema and glomerular injury in zebrafish. A: schematic representation of the zebrafish embryo-based in vivo assay to test efficacy of the novel compounds. Adriamycin (ADR) treatment induces pericardial edema and renal injury in zebrafish embryos, which can be studied by examining body curvature and the size of cardiac region. Compounds that protect renal function reduce the in vivo damage as defined by these measurements. B–C: representative images (left) and graphs showing quantification of level of body curvature (B) and edema (C) in zebrafish embryos treated as described in materials and methods. Control animals (Cont) were kept in the E3 media. Glomerular injury was induced by treatment with ADR, which resulted in significant increase in body curvature and edema (expressed as a ratio of the cardiac area over total body area). Treatment with alsterpaullone (0.25 µM), 1-azakenpaullone (0.25 µM), GSK3 inhibitor SB216763 (0.5 µM), or p38 inhibitor SB203580 (1 µM) reduced body curvature and edema/cardiac area per body area. Graph in B presents relative number of embryos displaying normal vs. curved bodies in each treatment group (n = 18–36/group). Graph in C shows 10–90 percentiles per treatment group, with mean and 95% confidence interval in the graph (n = 18–36/group). One-way ANOVA with Dunnett’s multiple comparison test was used. **P < 0.01; ***P < 0.005; ****P < 0.001.
DISCUSSION
Healthy podocytes are central for preserving a normal kidney function. Podocytes create a stable filtration barrier in the glomeruli by using their unique cellular shape that is maintained with the help of a number of intracellular molecular fibers. Chief among them are the F-actin and microtubules, which provide structural elements for podocytes’ cellular architecture. Rearrangement or damage of the integrity of these cytoskeletal structures, due to genetic, circulating exogenous, or endogenous factors, greatly alters the cellular morphology that results in foot processes effacement, a hallmark of glomerular diseases (Fig. 8) (45). Conversely, prevention of damage or rearrangement of F-actin and microtubule assemblies protects podocytes from injury and death in vitro and ameliorates proteinuria in vivo (24). Thus recent efforts have focused on identifying novel agents that protect podocyte cytoskeleton from injury as a promising approach to treating glomerular diseases. Toward that end, we recently described a first HCS-based phenotypic assay to discover novel podocyte-protective compounds from a chemical screen with a library of 2,121 unique compounds (35). Here, we extend our previous studies by performing an improved, multiparametric analysis of the imaging data set from our previous screen to identify a novel family of compounds as podocyte-protective agents. We found that an unbiased, multiparametric approach is able to identify 65 unique compounds as potential hits that protect podocytes from PAN injury-mediated damage in vitro. Among them, we found kenpaullone to be a top hit. Given that kenpaullone is currently under preclinical development as a therapeutic for neurological diseases (67), and that it has been shown to be an inhibitor of signaling by GSK-3β (31, 37), which is a validated target in podocytes (5, 65, 66, 70), we further investigated its mechanism of action in podocytes in vitro. We also studied additional commercially available analogs in the paullone family of compounds. We found that while kenpaullone and two other paullone derivatives (1-azakenpaullone and alsterpaullone) protected podocytes from PAN-induced injury in dose-dependent fashion, paullone was ineffective. Our studies also showed alsterpaullone to be the most potent out of these three derivatives in vitro. This is also in accordance with it being reported to be the most potent inhibitor of GSK-3β in literature, with an IC50 value of 4 nM, which compares favorably as compared with the IC50 values of kenpaullone (23 nM), 1-azakenpaullone (18 nM), and paullone (620 nM), as well as the selective GSK-3β inhibitor SB216763 (34 nM) (12, 31, 37). Emerging studies suggest that podocyte damage also results in reorganization of microtubules (24, 28, 61, 64), in addition to the changes in the F-actin cytoskeleton, that together significantly affect cell shape and motility. Our cell-based studies presented here further showed that these three paullone derivatives suppressed reorganization of the intracellular F-actin and microtubule assemblies. This further suggests that stabilization of such intracellular protein networks is feasible via small molecules, which could lead to newer strategies for maintaining a podocyte’s cellular architecture and shape.
Fig. 8.
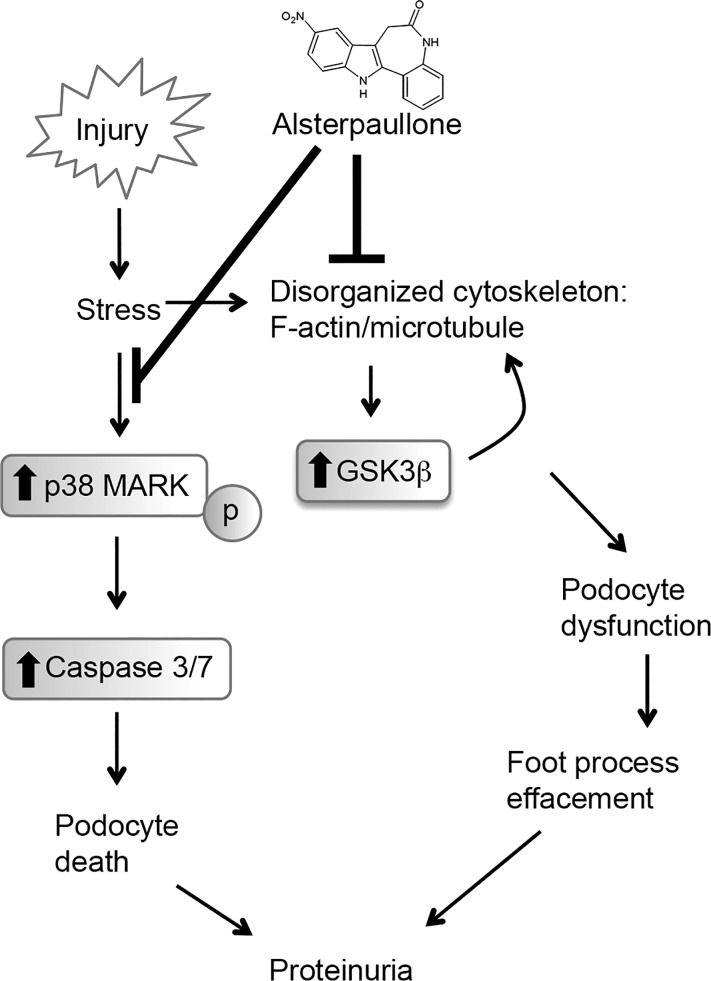
Working model: a schematic representation of the potential mechanisms behind alsterpaullone-mediated protection of podocytes from injury. Podocyte injury due to agents, such as puromycin aminonucleotide (PAN), results in stress-mediated activation of p38 MAP kinase and GSK3β. It also induces a reorganization of the cellular filamentous network comprising of F-actin and microtubules that affect the mechanical stability of the cell. Activation of p38 MAPK leads to induction of caspase 3/7-mediated apoptosis and podocyte loss. Activation of GSK-3β results in reduced β-catenin levels, further affecting the integrity of the podocyte cytoskeleton and increasing podocyte dynamics and dysfunction. Alsterpaullone (and other paullone derivatives) suppress signaling downstream of GSK-3β and p38, thereby reducing progressive loss in podocyte function and ameliorating glomerular dysfunction.
We also found that alsterpaullone treatment in vitro reduced mRNA expression of podocyte damage markers and cell motility that was induced in injured podocytes, providing new insights into its roles on regulating cell and cytoskeleton morphology. Furthermore, we found that alsterpaullone altered signaling downstream of GSK-3β and p38. PAN-mediated podocyte injury increased GSK-3β and p38 activity, which is consistent with previous reports suggesting that phosphorylation of GSK-3β and p38 is altered in podocytes in rodent models of glomerular injury and in patients, partly via GSK-3β-dependent modulation of focal adhesion dynamics and p38-mediated actin reorganization (5, 29, 65). Podocyte damage also reduced β-catenin protein expression that is regulated by GSK-3β (7, 20, 26). Additionally, JNK was activated in mouse podocytes damaged by PAN. It has been previously shown that GSK-3β activates JNK, which leads to increased apoptosis (53). Alsterpaullone and SB216763 increased β-catenin expression and reduced phosphorylation of JNK and apoptosis. These findings support previous findings that GSK-3β activation in injured podocytes is damaging to cells and that inhibition of GSK-3β protects podocytes. These studies, in concert with our findings, show the broad pathological and potentially therapeutic significance of GSK-3β inhibitors. Additionally, recent studies show that GSK-3β is linked to an aggressive form of amyotrophic lateral sclerosis (ALS), a neurodegenerative disease, and that a small-molecule screen identified kenpaullone (38, 67) and other GSK-3β inhibitors (67) as novel agents to rescue functional deficits in patient derived neuronal cells. This has initiated new efforts for developing kenpaullone as a potential therapeutic for ALS (67). Furthermore, our in vivo experiments using ADR-induced glomerular injury in a zebrafish as a model system showed that alsterpaullone and 1-azakenpaullone significantly reduced injury in the model organism. Together these results identify paullone derivatives as novel podocyte-protective agents and suggest alsterpaullone as a highly promising new agent for future therapeutic development for kidney diseases.
However, the in vitro and the zebrafish-based model systems used in the identification of compounds here have many limitations and are not predictive of clinical efficacy of these agents. Future studies using additional model systems, including human cell-based assays and murine models of glomerular injury, are needed.
In summary, we describe here further development of a podocyte cell-based HCS assay that is designed to carefully assess podocyte phenotype. We believe that the newer multiparametric approaches are well suited for data sets from imaging-based screening assays. Our identification of paullone derivatives as novel podocyte-protective compounds also sets the stage for their future development to treat proteinuric kidney diseases. Our studies also show that both F-actin and microtubule-based filamentous networks can be stabilized in podocytes to protect cells from injury and death (Figure 8). Future research will fully elucidate the underlying mechanism of action. Finally, we believe that the methodologies presented here will also be useful in phenotypic screens with other cell types.
GRANTS
This work was supported in part by National Institutes of Health Grants R01 DK-084195 and R01 HL-109582 (to V. Gupta), R01 DK-106512 and R01 DK-107984 (to V. Gupta and J. Reiser), and R01 DK-087956 (to D. Nihalani); the Nephcure Foundation; and with resources from the Rush University Medical Center.
DISCLOSURES
V. Gupta and J. Reiser are inventors on pending patents related to this study and have the potential for financial benefit from their future commercialization.
AUTHOR CONTRIBUTIONS
H.W.L., D.N., and V.G. conceived and designed research; H.W.L., E.A., M.M.A., and K.Q. performed experiments; H.W.L., E.A., M.M.A., K.Q., S.M., A.P., A.M., and D.N. analyzed data; H.W.L., E.A., K.Q., and D.N. interpreted results of experiments; H.W.L., E.A., M.M.A., K.Q., S.M., A.P., A.M., D.N., and V.G. prepared figures; H.W.L., E.A., J.R., D.N., and V.G. drafted manuscript; H.W.L., E.A., D.N., and V.G. edited and revised manuscript; H.W.L., E.A., M.M.A., K.Q., S.M., J.R., D.N., and V.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank M. Hafeez Faridi, Xiaobo Li, Terese Geraghty, Nicholas J. Tardi, Hatem Elshabrawy, and Tristan Hays for generous technical help and helpful discussions.
REFERENCES
- 1.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. β-Catenin is a target for the ubiquitin-proteasome pathway. EMBO J 16: 3797–3804, 1997. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arif E, Kumari B, Wagner MC, Zhou W, Holzman LB, Nihalani D. Myo1c is an unconventional myosin required for zebrafish glomerular development. Kidney Int 84: 1154–1165, 2013. doi: 10.1038/ki.2013.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asanuma K, Yanagida-Asanuma E, Faul C, Tomino Y, Kim K, Mundel P. Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat Cell Biol 8: 485–491, 2006. doi: 10.1038/ncb1400. [DOI] [PubMed] [Google Scholar]
- 4.Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J 371: 199–204, 2003. doi: 10.1042/bj20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bao H, Ge Y, Peng A, Gong R. Fine-tuning of NFκB by glycogen synthase kinase 3β directs the fate of glomerular podocytes upon injury. Kidney Int 87: 1176–1190, 2015. doi: 10.1038/ki.2014.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel MM, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol 120: 757–766, 1993. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boini KM, Amann K, Kempe D, Alessi DR, Lang F. Proteinuria in mice expressing PKB/SGK-resistant GSK3. Am J Physiol Renal Physiol 296: F153–F159, 2009. doi: 10.1152/ajprenal.90398.2008. [DOI] [PubMed] [Google Scholar]
- 8.Breiman L. Random forests. Mach Learn 45: 5–32, 2001. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 9.Brem AS, Gong R. Therapeutic targeting of aldosterone: a novel approach to the treatment of glomerular disease. Clin Sci (Lond) 128: 527–535, 2015. doi: 10.1042/CS20140432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caccamo A, Medina DX, Oddo S. Glucocorticoids exacerbate cognitive deficits in TDP-25 transgenic mice via a glutathione-mediated mechanism: implications for aging, stress and TDP-43 proteinopathies. J Neurosci 33: 906–913, 2013. doi: 10.1523/JNEUROSCI.3314-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang AS, Hathaway CK, Smithies O, Kakoki M. Transforming growth factor-β1 and diabetic nephropathy. Am J Physiol Renal Physiol 310: F689–F696, 2016. doi: 10.1152/ajprenal.00502.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee Cox L, Mills D, Brown MJ, Haigh D, Ward RW, Smith DG, Murray KJ, Reith AD, Holder JC. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol 7: 793–803, 2000. doi: 10.1016/S1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 13.Drummond IA, Majumdar A, Hentschel H, Elger M, Solnica-Krezel L, Schier AF, Neuhauss SC, Stemple DL, Zwartkruis F, Rangini Z, Driever W, Fishman MC. Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development 125: 4655–4667, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Duan H, Chinnaiyan AM, Hudson PL, Wing JP, He WW, Dixit VM. ICE-LAP3, a novel mammalian homologue of the Caenorhabditis elegans cell death protein Ced-3 is activated during Fas- and tumor necrosis factor-induced apoptosis. J Biol Chem 271: 1621–1625, 1996. doi: 10.1074/jbc.271.3.1621. [DOI] [PubMed] [Google Scholar]
- 15.Fleming Y, Armstrong CG, Morrice N, Paterson A, Goedert M, Cohen P. Synergistic activation of stress-activated protein kinase 1/c-Jun N-terminal kinase (SAPK1/JNK) isoforms by mitogen-activated protein kinase kinase 4 (MKK4) and MKK7. Biochem J 352: 145–154, 2000. doi: 10.1042/bj3520145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Floege J, Kriz W, Schulze M, Susani M, Kerjaschki D, Mooney A, Couser WG, Koch KM. Basic fibroblast growth factor augments podocyte injury and induces glomerulosclerosis in rats with experimental membranous nephropathy. J Clin Invest 96: 2809–2819, 1995. doi: 10.1172/JCI118351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta V, Reiser J. Stop that podocyte! Am J Physiol Renal Physiol 312: F373–F374, 2017. doi: 10.1152/ajprenal.00499.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He B, Ebarasi L, Hultenby K, Tryggvason K, Betsholtz C. Podocin-green fluorescence protein allows visualization and functional analysis of podocytes. J Am Soc Nephrol 22: 1019–1023, 2011. doi: 10.1681/ASN.2010121291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heikkilä E, Juhila J, Lassila M, Messing M, Perälä N, Lehtonen E, Lehtonen S, Sjef Verbeek J, Holthofer H. β-Catenin mediates adriamycin-induced albuminuria and podocyte injury in adult mouse kidneys. Nephrol Dial Transplant 25: 2437–2446, 2010. doi: 10.1093/ndt/gfq076. [DOI] [PubMed] [Google Scholar]
- 21.Hentschel DM, Park KM, Cilenti L, Zervos AS, Drummond I, Bonventre JV. Acute renal failure in zebrafish: a novel system to study a complex disease. Am J Physiol Renal Physiol 288: F923–F929, 2005. doi: 10.1152/ajprenal.00386.2004. [DOI] [PubMed] [Google Scholar]
- 22.Hülsken J, Birchmeier W, Behrens J. E-cadherin and APC compete for the interaction with β-catenin and the cytoskeleton. J Cell Biol 127: 2061–2069, 1994. doi: 10.1083/jcb.127.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iglesias-de la Cruz MC, Ziyadeh FN, Isono M, Kouahou M, Han DC, Kalluri R, Mundel P, Chen S. Effects of high glucose and TGF-β1 on the expression of collagen IV and vascular endothelial growth factor in mouse podocytes. Kidney Int 62: 901–913, 2002. doi: 10.1046/j.1523-1755.2002.00528.x. [DOI] [PubMed] [Google Scholar]
- 24.Jeruschke S, Jeruschke K, DiStasio A, Karaterzi S, Büscher AK, Nalbant P, Klein-Hitpass L, Hoyer PF, Weiss J, Stottmann RW, Weber S. Everolimus stabilizes podocyte microtubules via enhancing TUBB2B and DCDC2 expression. PLoS One 10: e0137043, 2015. doi: 10.1371/journal.pone.0137043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kari G, Rodeck U, Dicker AP. Zebrafish: an emerging model system for human disease and drug discovery. Clin Pharmacol Ther 82: 70–80, 2007. doi: 10.1038/sj.clpt.6100223. [DOI] [PubMed] [Google Scholar]
- 26.Kato H, Gruenwald A, Suh JH, Miner JH, Barisoni-Thomas L, Taketo MM, Faul C, Millar SE, Holzman LB, Susztak K. Wnt/β-catenin pathway in podocytes integrates cell adhesion, differentiation, and survival. J Biol Chem 286: 26003–26015, 2011. doi: 10.1074/jbc.M111.223164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiener TK, Selptsova-Friedrich I, Hunziker W. Tjp3/zo-3 is critical for epidermal barrier function in zebrafish embryos. Dev Biol 316: 36–49, 2008. doi: 10.1016/j.ydbio.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi N, Reiser J, Kriz W, Kuriyama R, Mundel P. Nonuniform microtubular polarity established by CHO1/MKLP1 motor protein is necessary for process formation of podocytes. J Cell Biol 143: 1961–1970, 1998. doi: 10.1083/jcb.143.7.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koshikawa M, Mukoyama M, Mori K, Suganami T, Sawai K, Yoshioka T, Nagae T, Yokoi H, Kawachi H, Shimizu F, Sugawara A, Nakao K. Role of p38 mitogen-activated protein kinase activation in podocyte injury and proteinuria in experimental nephrotic syndrome. J Am Soc Nephrol 16: 2690–2701, 2005. doi: 10.1681/ASN.2004121084. [DOI] [PubMed] [Google Scholar]
- 30.Kramer-Zucker AG, Wiessner S, Jensen AM, Drummond IA. Organization of the pronephric filtration apparatus in zebrafish requires Nephrin, Podocin and the FERM domain protein Mosaic eyes. Dev Biol 285: 316–329, 2005. doi: 10.1016/j.ydbio.2005.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kunick C, Lauenroth K, Leost M, Meijer L, Lemcke T. 1-Azakenpaullone is a selective inhibitor of glycogen synthase kinase-3β. Bioorg Med Chem Lett 14: 413–416, 2004. doi: 10.1016/j.bmcl.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 33.Lawler S, Fleming Y, Goedert M, Cohen P. Synergistic activation of SAPK1/JNK1 by two MAP kinase kinases in vitro. Curr Biol 8: 1387–1391, 1998. doi: 10.1016/S0960-9822(98)00019-0. [DOI] [PubMed] [Google Scholar]
- 34.Lee EY, Chung CH, Khoury CC, Yeo TK, Pyagay PE, Wang A, Chen S. The monocyte chemoattractant protein-1/CCR2 loop, inducible by TGF-β, increases podocyte motility and albumin permeability. Am J Physiol Renal Physiol 297: F85–F94, 2009. doi: 10.1152/ajprenal.90642.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee HW, Khan SQ, Faridi MH, Wei C, Tardi NJ, Altintas MM, Elshabrawy HA, Mangos S, Quick KL, Sever S, Reiser J, Gupta V. A podocyte-based automated screening assay identifies protective small molecules. J Am Soc Nephrol 26: 2741–2752, 2015. doi: 10.1681/ASN.2014090859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee VWS, Harris DCH. Adriamycin nephropathy: a model of focal segmental glomerulosclerosis. Nephrology (Carlton) 16: 30–38, 2011. doi: 10.1111/j.1440-1797.2010.01383.x. [DOI] [PubMed] [Google Scholar]
- 37.Leost M, Schultz C, Link A, Wu YZ, Biernat J, Mandelkow EM, Bibb JA, Snyder GL, Greengard P, Zaharevitz DW, Gussio R, Senderowicz AM, Sausville EA, Kunick C, Meijer L. Paullones are potent inhibitors of glycogen synthase kinase-3β and cyclin-dependent kinase 5/p25. Eur J Biochem 267: 5983–5994, 2000. doi: 10.1046/j.1432-1327.2000.01673.x. [DOI] [PubMed] [Google Scholar]
- 38.Liu M-L, Zang T, Zhang C-L. Direct lineage reprogramming reveals disease-specific phenotypes of motor neurons from human ALS patients. Cell Reports 14: 115–128, 2016. doi: 10.1016/j.celrep.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mundel P, Reiser J, Zúñiga Mejía Borja A, Pavenstädt H, Davidson GR, Kriz W, Zeller R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- 40.Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol 13: 3005–3015, 2002. doi: 10.1097/01.ASN.0000039661.06947.FD. [DOI] [PubMed] [Google Scholar]
- 41.Orford K, Crockett C, Jensen JP, Weissman AM, Byers SW. Serine phosphorylation-regulated ubiquitination and degradation of β-catenin. J Biol Chem 272: 24735–24738, 1997. doi: 10.1074/jbc.272.40.24735. [DOI] [PubMed] [Google Scholar]
- 42.Paeng J, Chang JH, Lee SH, Nam BY, Kang H-Y, Kim S, Oh HJ, Park JT, Han SH, Yoo T-H, Kang S-W. Enhanced glycogen synthase kinase-3β activity mediates podocyte apoptosis under diabetic conditions. Apoptosis 19: 1678–1690, 2014. doi: 10.1007/s10495-014-1037-5. [DOI] [PubMed] [Google Scholar]
- 43.Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 44.Phillips A, Janssen U, Floege J. Progression of diabetic nephropathy. Insights from cell culture studies and animal models. Kidney Blood Press Res 22: 81–97, 1999. doi: 10.1159/000025912. [DOI] [PubMed] [Google Scholar]
- 44a.Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem 270: 7420–7426, 1995. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 45.Reiser J, Altintas MM. Podocytes. F1000Res 5, 2016. doi: 10.12688/f1000research.7255.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, Rastaldi MP, Calvaresi N, Watanabe H, Schwarz K, Faul C, Kretzler M, Davidson A, Sugimoto H, Kalluri R, Sharpe AH, Kreidberg JA, Mundel P. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest 113: 1390–1397, 2004. doi: 10.1172/JCI20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reiser J, Gupta V, Kistler AD. Toward the development of podocyte-specific drugs. Kidney Int 77: 662–668, 2010. doi: 10.1038/ki.2009.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reiser J, Oh J, Shirato I, Asanuma K, Hug A, Mundel TM, Honey K, Ishidoh K, Kominami E, Kreidberg JA, Tomino Y, Mundel P. Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and α3 integrin. J Biol Chem 279: 34827–34832, 2004. doi: 10.1074/jbc.M401973200. [DOI] [PubMed] [Google Scholar]
- 49.Sailem HZ, Sero JE, Bakal C. Visualizing cellular imaging data using PhenoPlot. Nat Commun 6: 5825, 2015. doi: 10.1038/ncomms6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schiffer M, Bitzer M, Roberts ISD, Kopp JB, ten Dijke P, Mundel P, Böttinger EP. Apoptosis in podocytes induced by TGF-β and Smad7. J Clin Invest 108: 807–816, 2001. doi: 10.1172/JCI200112367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schlegel J, Peters I, Orrenius S, Miller DK, Thornberry NA, Yamin TT, Nicholson DW. CPP32/apopain is a key interleukin 1β converting enzyme-like protease involved in Fas-mediated apoptosis. J Biol Chem 271: 1841–1844, 1996. doi: 10.1074/jbc.271.4.1841. [DOI] [PubMed] [Google Scholar]
- 52.Schmid H, Henger A, Cohen CD, Frach K, Gröne H-J, Schlöndorff D, Kretzler M. Gene expression profiles of podocyte-associated molecules as diagnostic markers in acquired proteinuric diseases. J Am Soc Nephrol 14: 2958–2966, 2003. doi: 10.1097/01.ASN.0000090745.85482.06. [DOI] [PubMed] [Google Scholar]
- 53.Sluss HK, Barrett T, Dérijard B, Davis RJ. Signal transduction by tumor necrosis factor mediated by JNK protein kinases. Mol Cell Biol 14: 8376–8384, 1994. doi: 10.1128/MCB.14.12.8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stadheim TA, Kucera GL. c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for mitoxantrone- and anisomycin-induced apoptosis in HL-60 cells. Leuk Res 26: 55–65, 2002. doi: 10.1016/S0145-2126(01)00099-6. [DOI] [PubMed] [Google Scholar]
- 55.Sureshbabu A, Muhsin SA, Choi ME. TGF-β signaling in the kidney: profibrotic and protective effects. Am J Physiol Renal Physiol 310: F596–F606, 2016. doi: 10.1152/ajprenal.00365.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sutherland C, Leighton IA, Cohen P. Inactivation of glycogen synthase kinase-3β by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem J 296: 15–9, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taddei ML, Chiarugi P, Cirri P, Buricchi F, Fiaschi T, Giannoni E, Talini D, Cozzi G, Formigli L, Raugei G, Ramponi G. β-Catenin interacts with low-molecular-weight protein tyrosine phosphatase leading to cadherin-mediated cell-cell adhesion increase. Cancer Res 62: 6489–6499, 2002. [PubMed] [Google Scholar]
- 58.Takeuchi S, Hiromura K, Tomioka M, Takahashi S, Sakairi T, Maeshima A, Kaneko Y, Kuroiwa T, Nojima Y. The immunosuppressive drug mizoribine directly prevents podocyte injury in puromycin aminonucleoside nephrosis. Nephron, Exp Nephrol 116: e3–e10, 2010. doi: 10.1159/000314668. [DOI] [PubMed] [Google Scholar]
- 59.Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 23: 2838–2849, 2004. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- 60.Wei C, Möller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, Xie L, Henger A, Schmid H, Rastaldi MP, Cowan P, Kretzler M, Parrilla R, Bendayan M, Gupta V, Nikolic B, Kalluri R, Carmeliet P, Mundel P, Reiser J. Modification of kidney barrier function by the urokinase receptor. Nat Med 14: 55–63, 2008. doi: 10.1038/nm1696. [DOI] [PubMed] [Google Scholar]
- 61.Widmeier E, Tan W, Airik M, Hildebrandt F. A small molecule screening to detect potential therapeutic targets in human podocytes. Am J Physiol Renal Physiol 312: F157–F171, 2017. doi: 10.1152/ajprenal.00386.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- 63.Wu DT, Bitzer M, Ju W, Mundel P, Böttinger EP. TGF-β concentration specifies differential signaling profiles of growth arrest/differentiation and apoptosis in podocytes. J Am Soc Nephrol 16: 3211–3221, 2005. doi: 10.1681/ASN.2004121055. [DOI] [PubMed] [Google Scholar]
- 64.Xing C-Y, Saleem MA, Coward RJ, Ni L, Witherden IR, Mathieson PW. Direct effects of dexamethasone on human podocytes. Kidney Int 70: 1038–1045, 2006. doi: 10.1038/sj.ki.5001655. [DOI] [PubMed] [Google Scholar]
- 65.Xu W, Ge Y, Liu Z, Gong R. Glycogen synthase kinase 3β dictates podocyte motility and focal adhesion turnover by modulating paxillin activity: implications for the protective effect of low-dose lithium in podocytopathy. Am J Pathol 184: 2742–2756, 2014. doi: 10.1016/j.ajpath.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu W, Ge Y, Liu Z, Gong R. Glycogen synthase kinase 3β orchestrates microtubule remodeling in compensatory glomerular adaptation to podocyte depletion. J Biol Chem 290: 1348–1363, 2015. doi: 10.1074/jbc.M114.593830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang YM, Gupta SK, Kim KJ, Powers BE, Cerqueira A, Wainger BJ, Ngo HD, Rosowski KA, Schein PA, Ackeifi CA, Arvanites AC, Davidow LS, Woolf CJ, Rubin LL. A small molecule screen in stem-cell-derived motor neurons identifies a kinase inhibitor as a candidate therapeutic for ALS. Cell Stem Cell 12: 713–726, 2013. doi: 10.1016/j.stem.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Young PR, McLaughlin MM, Kumar S, Kassis S, Doyle ML, McNulty D, Gallagher TF, Fisher S, McDonnell PC, Carr SA, Huddleston MJ, Seibel G, Porter TG, Livi GP, Adams JL, Lee JC. Pyridinyl imidazole inhibitors of p38 mitogen-activated protein kinase bind in the ATP site. J Biol Chem 272: 12116–12121, 1997. doi: 10.1074/jbc.272.18.12116. [DOI] [PubMed] [Google Scholar]
- 69.Zennaro C, Mariotti M, Carraro M, Pasqualetti S, Corbelli A, Armelloni S, Li M, Ikehata M, Clai M, Artero M, Messa P, Boscutti G, Rastaldi MP. Podocyte developmental defects caused by adriamycin in zebrafish embryos and larvae: a novel model of glomerular damage. PLoS One 9: e98131, 2014. doi: 10.1371/journal.pone.0098131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou S, Wang P, Qiao Y, Ge Y, Wang Y, Quan S, Yao R, Zhuang S, Wang LJ, Du Y, Liu Z, Gong R. Genetic and pharmacologic targeting of glycogen synthase kinase 3β reinforces the Nrf2 antioxidant defense against podocytopathy. J Am Soc Nephrol 27: 2289–2308, 2016. doi: 10.1681/ASN.2015050565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ziyadeh FN. Mediators of diabetic renal disease: the case for tgf-β as the major mediator. J Am Soc Nephrol 15 Suppl 1: S55–S57, 2004. [DOI] [PubMed] [Google Scholar]