Abstract
Cellular, molecular, and ultrastructural nephron changes associated with ischemia-reperfusion injury-induced acute kidney injury (IRI-AKI) are not completely understood. Here, a multidisciplinary study was used to identify nephron changes in a mouse model of IRI-AKI. Histological analyses indicated distended Bowman’s glomerular spaces and proximal and distal tubules. Increased filtrate volume in nephrons was caused by reduced water reabsorption by severely damaged proximal tubule brush borders and blocked flow of filtrate into collecting tubules by mucoprotein casts in distal tubules. Immunohistochemistry revealed protein AKI biomarkers in proximal tubules and glomeruli but not in distal tubules. Nuclear magnetic resonance spectroscopy revealed several metabolites that increased such as valine, alanine, and lactate. Other metabolites such as trigonelline, succinate, 2-oxoisocaproate, and 1- methyl-nicotinamide decreased or were absent in urine following IRI due to altered kidney function or metabolism. Urinary glucose increased due to reduced reabsorption by damaged proximal tubule brush borders. Scanning electron microscopy revealed flattening of podocytes and pedicals surrounding glomerular capillaries, and transmission electron microscopy (TEM) revealed effacement of podocyte pedicals, both consistent with increased hydrostatic pressure in nephrons following IRI-AKI. TEM revealed shortened proximal tubule microvilli in IRI kidneys with diminished lamina propia. TEM showed dramatic loss of mitochondria in distal tubule epithelia of IRI kidneys and emergence of multivesicular bodies of endosomes indicating ongoing cellular death. Collectively, the data define ultrastructural changes to nephrons and altered kidney metabolism associated with IRI-AKI.
Keywords: acute kidney injury, ischemia-reperfusion injury, NMR spectroscopy, scanning electron microscopy, transmission electron microscopy
INTRODUCTION
Acute kidney injury (AKI) is caused by kidney damage due to sepsis, respiratory failure, heart failure, trauma, major surgery, burns, toxic insult caused by medications, and contrast agents used for imaging (3, 8, 11, 22, 25, 44, 49). AKI accounts for 1% of all hospital admissions, complicates 7% of hospitalizations (>1 million patients) in the United States annually), and is present in up to 20% of critically ill patients (23, 36). AKI is associated with significantly increased mortality, hospital stays, renal replacement therapy, and cost (9, 12, 40). Overall, AKI-associated mortality is estimated at 45–70% (4), AKI-associated mortality in intensive care unit patients requiring renal replacement therapy is 50–60% (19), and >2 million die from AKI each year (30, 39). Excess AKI-associated costs in the United States are estimated at $10 billion (9, 41) and £1.02 billion in England (21). AKI is associated with increased risk for developing chronic kidney disease (25).
AKI renal etiology results from tubular, glomerular, interstitial, and vascular damage (4). AKI caused by damage to tubules is referred to as acute tubular necrosis. The S3 segment of the proximal tubule, i.e., the descending straight segment in the medulla, is considered the most important site of damage; however, this remains controversial (4). Acute tubular necrosis is the most common form of AKI in hospitalized patients (14, 47).
Multiple protein AKI biomarkers have been identified. Neutrophil gelatinase-associated lipocalin (NGAL) increases in urine and serum of adult and pediatric patients (27) due to increased NGAL expression by nephrons in response to renal epithelial injury (31). Kidney injury molecule 1 (Kim-1) is a type 1 transmembrane glycoprotein upregulated in postischemic rat kidney (20) that is not expressed in normal human kidney (7). Serum cystatin C enables detection of AKI 1–2 days earlier than serum creatinine (13, 16, 38) and is an effective predictor of AKI in newborns (1). Askenazi et al. (2) demonstrated that baseline values of these AKI biomarkers vary with gestational age in preterm infants, complicating their use for diagnosis. Protein AKI biomarkers have been recently reviewed (5, 34, 35).
Mice have been used to model ischemic AKI since 1990 (18). Technical aspects and guidelines for use of this model have been recently reviewed (42). Metabolic profiling can provide important insight into changes in kidney function associated with AKI, e.g., Niemann and Serkova (32) have presented an excellent review summarizing biochemical mechanisms of nephrotoxicity providing a framework for interpreting ischemic- and hypoxic-induced AKI.
Here, we used a well-established mouse model for ischemia-reperfusion injury-induced acute kidney injury (IRI-AKI) to investigate nephron changes in response to IRI. Histology, immunohistochemistry, nuclear magnetic resonance (NMR) spectroscopy, scanning electron microscopy (SEM), and transmission electron microscopy (TEM) were used to study structural and cellular changes to nephrons responsible for altered kidney function. NMR data indicated major changes in kidney metabolism. TEM of distal tubule epithelia revealed extensive loss of mitochondria critical to distal tubule function. Integrated data analysis led to a model that successfully explained all data collected by the individual techniques used in the study.
MATERIALS AND METHODS
Mouse Model of Renal IRI
A murine model of renal IRI was applied to male Swiss-Webster mice (Taconic Farms, Germantown, NY) weighing 25–30 g as previously described (28, 29, 37). Mice were anesthetized with sodium pentobarbital (50 mg/kg intraperitoneally) and placed on a warming table to maintain a rectal temperature of 37°C while anesthesia was maintained using 2% isoflurane gas. Following a 2.5-cm abdominal incision, renal pedicles were occluded with a nontraumatic vascular clamp for 30 min, during which time the kidneys were kept warm and moist. The clamps were then removed, the kidneys were observed for return of blood flow, and the incision was sutured. Mice were allowed to recover in a warm cage, and urine was collected 24 h following injury. Mice were reanesthetized 24 h after reperfusion, the abdominal cavity was opened, and blood was obtained via a puncture of the inferior vena cava for measurement of serum creatinine by the quantitative colorimetric assay kit (Sigma, St. Louis, MO) and via cardiac puncture for NMR analysis. The mice were euthanized and both kidneys were harvested and stored. Fixed kidney tissue was paraffin embedded and sectioned (5 µm). The study was approved by the Institutional Animal Care and Use Committee of Cincinnati Children’s Hospital Medical Center.
Urinary NGAL and Serum Creatinine Assays
Mice were placed in metabolic cages (Nalgene, Rochester, NY), and urine was collected 3 days before and 1 day post-IRI. The concentrations of urinary creatinine (mg/ml), serum creatinine (mg/dl), and urinary NGAL (ng/ml) were determined using quantitative colorimetric assay kits (Sigma) (28, 29) and are reported in Table 1.
Table 1.
Study details and summary of clinical parameters of AKI in Swiss Webster mice following IRI
| Data | Control | IRI | P Value |
|---|---|---|---|
| n (samples) | 17 | 17 | |
| S-Cr, mg/dl | 0.29 ± 0.06 | 1.93 ± 0.06 | 0.001 |
| U-NGAL, ng/ml | 100.4 ± 116 | 69,834 ± 79,301 | 0.002 |
| U-Cr, mg/ml | 0.56 ± 0.13 | 0.09 ± .034 | 0.001 |
| NGAL/Cr ratio, ng/mg | 182.3 ± 230 | 993,803 ± 144,6768 | 0.012 |
Values are means ± SD. AKI, acute kidney injury; IRI, ischemia-reperfusion; S-Cr, serum creatinine; U-NGAL, urinary Neutrophil gelatinase-associated lipocalin; U-Cr, urinary creatinine; NGAL/Cr ratio, NGAL to creatinine ratio.
Histology
Kidney sagittal sections (5 μm) were examined after hematoxylin and eosin and periodic acid-Schiff (PAS) staining. Micrographs were taken using an Olympus AX-70 light microscope (Photometrics, Tucson AZ).
Immunohistochemistry
NGAL.
Frozen kidney sections were permeabilized with 0.2% Triton X-100 in PBS for 10 min, blocked with goat serum for 1 h, and incubated with primary antibody to NGAL (1:500 dilution) for 1 h. Slides were then exposed for 30 min in the dark to secondary antibodies conjugated with Cy5 (Amersham, Arlington Heights, IL).
KIM-1.
Mouse kidney sections were deparaffinized, and endogenous peroxidase activity was ablated by incubation in 0.5% hydrogen peroxide in methanol for 10 min. Sections were heated with a microwave oven in 0.1 mol/l sodium citrate buffer, pH 6.0 for 10 min, and blocked with 2.5% normal horse serum (Vector Laboratories, Burlingame, CA) at room temperature for 1 h. The primary rabbit anti-mouse antibody KIM was then added to sections and incubated overnight at 4°C with 50 μl of antibody at 5 μg/ml in blocking solution. After application of primary antibody, KIM-1 was detected using an ImmPRESS reagent kit (Vector Laboratories) with DAB Substrate (Vector Laboratories) kit for peroxidase staining. Sections were counterstained with hematoxylin.
Cystatin C.
Mouse kidney sections were deparaffinized and endogenous peroxidase activity was ablated by incubation in 0.5% hydrogen peroxide in methanol for 10 min. Sections were heated with a microwave oven in 0.1 mol/l sodium citrate buffer, pH 6.0 for 10 min, and blocked with 2.5% normal horse serum (Vector Laboratories) at room temperature for 1 h. Primary antibody cystatin C (1/250; rabbit anti-mouse antibody; Abcam, Cambridge, MA) was then added to the sections and incubated overnight at 4°C in blocking solution. After application of the primary antibody, cystatin C was detected using the ImmPRESS reagent kit (Vector Laboratories) with DAB Substrate (Vector Laboratories) kit for peroxidase staining. Sections were counterstained with hematoxylin.
Preparation of Biological Fluids for NMR Analysis
Urine samples were stored and prepared for NMR analysis as previously described by Romick-Rosendale et al. (33). Serum samples were stored at −80°C following collection. Samples were thawed on ice before preparation for NMR analysis. Serum samples were prepared with D2O, EDTA, and trimethylsilylpropanoic acid (TSP) to a final concentration of 10%, 10 mM and 10 mM, respectively. Kidney tissue extract samples were prepared using the following steps. A small piece was cut from the snap frozen kidney tissue samples, weighed, immediately homogenized using a bead beater (Precellys, Montigny-le-Bretonneux, France), and extracted using methanol:chloroform:water (1:1:1). The hydrophilic samples were lyophilized and stored −80°C. Tissue extracts were reconstituted using a phosphate buffer described previously (33), which contained D2O, and TSP to a final concentration of 10% and 10 mM, respectively.
NMR Data Collection
NMR spectra were recorded on a Bruker AVANCE III spectrometer operating at 850.1 MHz (Bruker, Billerica, MA). Experiments were conducted at 298 K using 5-mm NMR tubes (Norell, Morganton, NC). Standard 1H one-dimensioanl (1D) presaturation (zgpr) and 1D 1H CPMG-presat (cpmgpr1d) experiments were recorded for urine, serum and hydrophilic kidney tissue extract samples and processed as previously reported (33). 1D 1H data were collected using a spectral width of 20.0 ppm, 64-K points, an acquisition time of 1.87 s, and on-resonance presaturation for solvent suppression during a 4-s recycle delay and 128 echo loops and 64 transients. A short 1-min zgpr experiment was used to screen the samples and to check the shimming. The 90° pulse widths, measured for each sample using the automatic pulse calculation experiment (pulsecal) in TopSpin 2.1.1 (Bruker BioSpin), were between 13 and 14 μs. Standard two-dimensional (2D) 1H-1H TOCSY and 1H-13C HSQC experiments from the TopSpin 2.1.1. were used for data collection. 1H-13C HSQC spectra were recorded at 298 K using the HSQC (hsqcetgpsi) pulse sequence with 128 scans and a 1-s recycle delay with acquisition times of 0.086 s (F2) and 0.0074 s (F1). For the aliphatic 1H-13C HSQC, spectral widths of 14 ppm (F2) and 80 ppm (F1), centered at 4.7 and 50 ppm, respectively, were used with a J-coupling constant of 146 Hz. For the aromatic 1H-13C HSQC, spectra widths of 14 ppm (F2) and 30 ppm (F1), centered at 4.7 and 125 ppm, respectively, were used, with a J-coupling constant of 165 Hz. The total acquisition time for each experiment was 5 h. The data were processed with Topspin 3.2 (Bruker BioSpin). Free induction decays were apodized using a 90°-shifted qsine window function, zero filled to twice the number of acquired points, Fourier transformed, and subjected to automated phase and baseline correction. The 2D 1H-1H TOCSY was collected using the TOCSY (mlevgpph19) pulse sequence at 298 K, 32 scans, a 1.5-s recycle delay with acquisition times of 0.17 s (F2) and 0.042 s (F1), and a TOCSY mixing time of 0.06 s. Spectral widths were set to 10 ppm for both the direct and indirect dimension.
Principal Components Analysis, Partial Least Squares-Discriminant Analysis, and Statistical Significance Analysis
All apparent spectral resonances were manually bucketed using AMIX. Spectra were not scaled or normalized before principal component analysis (PCA) or partial least squares-discriminant analysis (PLS-DA). PCA analysis was performed using the AMIX v.3.9 (Analysis of MIXtures software, Bruker Biospin) software. PCA loadings were color-coded based on paired t-test P values as described by Goodpaster et al. (15). The critical value α was 0.05 and the Bonferroni corrected α-value of 2.7E-4 was used was used for analysis of the Welch’s t-test results. PLS-DA was performed using the SIMCA-P ver. 11.0 software package (Umetrics, Umea, Sweden).
Identification and Quantification of Metabolites
Resonances from 1D NMR spectra were assigned using Bruker AMIX v.3.9 (Analysis of MIXtures software, Bruker Biospin) and Chenomx v.8.1 (Chenom, Alberta, Canada) with the HMDB (45) metabolite package. Metabolite assignments were confirmed and some additional unambiguous assignments were made from analysis of 2D NMR spectra (TOCSY, HSQC) using the COLMAR software (http://spin.ccic.ohio-state.edu/index.php/hsqc/index) (6). Collectively, 31 metabolites were identified from 85 assigned buckets. An additional 79 significant buckets were unassigned and 22 unassigned buckets were not significant. Urine metabolite concentrations were measured relative to the TSP internal standard concentration. The molar concentrations of identified metabolites were determined in Chenomx, which bases the concentration calculations on comparison of the metabolite peak areas to the integrated area of the TSP peak of known concentration (in this case 10 mM). The resulting concentration estimates of the urine metabolites are reported in Table 2. Urine metabolites were quantified from CPMG spectra. The same approach was used to quantify the serum metabolites. In the case of serum, the peak area of the TSP internal standard was compared with the TSP peak area measured in a control sample in the absence of serum using the identical NMR experiment and acquisition parameters. If the integrated peak area of the TPS internal standard in the serum sample was smaller than in the control sample (which can be caused by TSP binding to serum proteins), the metabolite concentration determined by Chenomx (based on the reduced area of the internal TSP standard) was corrected by scaling the its value by the (control TSP peak area/serum TSP peak area) ratio. The kidney tissue extract samples were not quantified since they were only used for qualitative analysis.
Table 2.
Metabolites that exhibited different concentrations in control and IRI urine samples
| Control Mice (pre-IRI) |
Study Mice (post-IRI) |
||||||
|---|---|---|---|---|---|---|---|
| Metabolite, ppm | Multiplet Structurea | P Value | Concentration, mM | SD | Concentration, mM | SD | |
| 1-Methyl-nicotinamide | |||||||
| 4.48 | s | 1.77E−14 | 1.09E−01 | 2.80E−02 | 1.12E−02 | 2.36E−02 | 13/17 AB |
| 8.18 | m | ABb | AB | AB | AB | AB | |
| 8.89 | d | 1.83E−09 | 7.75E−02 | 2.53E−02 | 9.98E−03 | 2.33E−02 | |
| 8.96 | d | 3.42E−10 | 8.10E−02 | 2.42E−02 | 1.79E−02 | 2.90E−02 | |
| 9.272 | s | 9.28E+00 | 7.84E−02 | 3.20E−02 | 3.70E−02 | 5.89E−02 | |
| 2-Oxoglutarate | |||||||
| 2.42 | t | 2.38E−06 | 3.62E+00 | 1.95E+00 | 1.25E−01 | 3.55E−01 | 15/17 AB |
| 2.99 | t | 9.15E−10 | 4.01E+00 | 2.16E+00 | 3.31E−02 | 3.58E−01 | |
| 2-Oxoisocaproate | |||||||
| 0.941 | d | 2.77E−12 | 8.53E−01 | 2.32E−01 | AB | 6.97E−02 | 13/17 AB |
| 2.101 | m | AB | AB | AB | 3.65E−03 | AB | |
| 2.617 | d | 2.18E−05 | 7.27E−01 | 1.74E−01 | **c | 1.50E−02 | |
| Cis-aconitate | |||||||
| 3.124 | m | 2.62E−10 | 9.59E−01 | 1.21E−01 | ** | ** | |
| 5.71 | s | 1.33E−19 | 9.03E−01 | 1.43E−01 | 1.98E−01 | 1.66E−01 | |
| Citrate | |||||||
| 2.52 | d | 1.83E−07 | 5.84E+00 | 1.98E+00 | 3.98E+00 | 3.84E+00 | |
| 2.65 | d | 1.47E−06 | 4.27E+00 | 2.06E+00 | 6.94E−01 | 3.45E+00 | |
| Creatinine | |||||||
| 3.049 | s | ** | 1.86E+00 | 6.56E−01 | 6.77E−01 | 3.36E−01 | |
| 4.052 | s | 1.45E−19 | 1.74E+00 | 3.03E−01 | ** | 3.20E−01 | |
| Creatinine phosphate | |||||||
| 3.046 | s | ** | ** | ** | 1.93E+00 | ** | |
| 3.96 | s | 6.80E−10 | 2.59E+00 | 4.69E−01 | 1.35E+00 | 1.07E+00 | |
| Ethanol | |||||||
| 1.17 | d | 6.63E−03 | 1.35E+00 | 4.52E−01 | ** | 9.23E−01 | |
| 3.64 | q | ** | ** | ** | AB | ** | |
| Galactaric acid | |||||||
| 3.94 | s | ** | ** | ** | ** | ** | 8/17 AB |
| 4.25 | s | 7.17E−15 | 1.20E+00 | 1.02E+00 | 1.19E−01 | 1.39E−01 | |
| Guanine | |||||||
| 7.66 | S | 1.29E−09 | 3.49E+00 | 9.81E−01 | 1.55E−02 | 1.20E+00 | 7/17 AB |
| Hippurate | |||||||
| 3.97 | d | ** | 1.91E+00 | 4.00E−01 | 7.47E−02 | 6.39E−02 | 5/17 AB |
| 7.551 | t | 1.33E−15 | 2.18E+00 | 3.95E−01 | ** | 1.09E−01 | |
| 7.638 | t | ** | ** | ** | 1.25E−01 | ** | |
| 7.837 | d | 9.47E−15 | 2.28E+00 | 4.14E−01 | AB | 1.59E−01 | |
| 8.51 | AB | AB | AB | 1.88E+00 | AB | ||
| Malonate | |||||||
| 3.108 | s | 4.98E−04 | 4.73E−01 | 1.33E−01 | 3.03E−01 | 1.49E−01 | |
| Nicotinomide-n-oxide | |||||||
| 7.64 | t | 8.87E−15 | ** | ** | AB | AB | |
| 7.74 | m | ** | 1.97E−01 | 6.42E−02 | AB | AB | |
| 8.12 | m | 1.03E−11 | 1.73E−01 | 4.67E−02 | AB | AB | |
| 8.4 | AB | AB | AB | AB | AB | ||
| 8.49 | m | 4.20E−11 | 1.65E−01 | 5.02E−02 | AB | AB | |
| 8.743 | m | 1.18E−16 | 1.58E−01 | 3.90E−02 | 2.53E−01 | AB | |
| n-Nitrosodimethylamine | |||||||
| 3.161 | s | 5.88E−15 | 6.42E−01 | 1.14E−01 | ** | 1.51E−01 | |
| 3.803 | s | 6.09E−05 | 7.03E−01 | 1.45E−01 | 2.04E−01 | ** | |
| Putrescine | |||||||
| 1.772 | m | 1.19E−07 | 1.33E+00 | 8.21E−01 | 2.61E−01 | 2.57E−01 | |
| 3.06 | m | 4.10E−04 | 1.08E+00 | 8.05E−01 | 2.70E−01 | 3.00E−01 | |
| Sarcosine | |||||||
| 2.724 | s | 5.66E−14 | 1.01E+00 | 2.36E−01 | ** | 1.36E−01 | |
| 3.6 | s | ** | 1.01E+00 | 1.82E−01 | 2.07E−01 | ** | |
| Succinate | |||||||
| 2.411 | s | 3.96E−09 | 1.79E+00 | 6.81E−01 | 2.07E−01 | 8.80E−02 | |
| Taurine | |||||||
| 3.25 | t | ** | 1.25E+01 | 3.42E+00 | 1.31E+01 | 5.07E+00 | |
| 3.42 | t | 2.75E−02 | 1.43E+01 | 4.54E+00 | AB | 7.07E+00 | |
| Trigonelline | |||||||
| 4.443 | s | 2.93E−15 | 4.50E−01 | 5.80E−02 | 5.42E−02 | AB | |
| 8.086 | t | 5.92E−12 | 5.10E−01 | 7.97E−02 | AB | 6.46E−02 | |
| 8.78 | AB | AB | AB | 7.23E−02 | AB | ||
| 8.84 | dd | 2.09E−20 | 4.49E−01 | 5.98E−02 | AB | 7.02E−02 | |
| 9.0736 | AB | AB | AB | 7.70E−02 | AB | ||
| 9.125 | m | 2.16E−20 | 5.32E−01 | 6.84E−02 | 1.05E+00 | 6.23E−02 | |
| Trimethlyamine N-oxide | |||||||
| 3.26 | s | 3.46E−11 | 1.79E+00 | 1.39E+00 | 1.05E+00 | 5.63E−01 | |
| Trimethylamine | |||||||
| 2.88 | s | 1.96E−06 | 4.00E+00 | 2.15E+00 | 1.79E−01 | 3.38E−01 | |
| Uracil | |||||||
| 5.79 | d | 2.88E−12 | 3.26E−01 | 8.64E−02 | 1.17E−01 | 8.05E−02 | |
| 7.52 | d | AB | AB | AB | AB | AB | |
| Alanine | |||||||
| 1.48 | d | 4.88E−11 | 8.06E−02 | 2.80E−02 | 4.44E−01 | 3.17E−01 | |
| 3.8 | q | ** | ** | ** | ** | ** | |
| Lactate | |||||||
| 1.33 | d | 1.37E−11 | 4.08E−01 | 1.95E−01 | 1.72E+00 | 1.20E+00 | |
| 4.1 | q | ** | ** | ** | ** | ** | |
| Valine | |||||||
| 1 | d | 6.12E−03 | 4.96E−02 | 1.60E−02 | 4.18E−01 | 3.16E−01 | |
| 1 | d | 6.12E−03 | AB | AB | ** | 3.16E−01 | |
| 2.3 | m | ** | ** | ** | ** | ** | |
| 3.6 | d | ** | ** | ** | 2.75E−01 | ** | |
| Leucine | |||||||
| 0.9 | t | 2.77E−12 | AB | AB | ** | 2.02E−01 | |
| 1.7 | m | ** | ** | ** | ** | ** | |
| 3.7 | m | ** | ** | ** | 7.88E+00 | ** | |
| Glucose | |||||||
| 3.72 | m | 1.39E−09 | ** | ** | 7.86E+00 | 4.47E+00 | |
| 3.82 | m | 8.12E−04 | ** | ** | 7.59E+00 | 4.20E+00 | |
| 3.889 | d | 2.39E−06 | ** | ** | 7.78E+00 | 4.48E+00 | |
| 4.63 | d | 3.21E−08 | 7.53E−01 | 4.57E−01 | 7.97E+00 | 4.48E+00 | |
| 5.223 | d | 2.66E−08 | 6.37E−01 | 3.89E−01 | 7.97E+00 | 4.32E+00 | |
NMR peak multiplet structure: s, singlet; d, doublet; t, triplet; q, quartet; dd, doublet of doublets; m, complex multiplet.
AB indicates that the peak was absent in the spectra.
Double asterisks indicate that the peaks were overlapped and a reliable P value or average concentration could not be calculated.
SEM
Kidney tissues were fixed in 2.5% glutaraldehyde-2% paraformaldehyde in 0.1 M cacodylate buffer, followed by 1% OsO4 in 0.1 M cacodylate buffer at pH 7.4. Samples were rinsed using 0.1 M cacodylate buffer and stained using uranyl acetate. Following multiple rinse cycles with double distilled water, tissue samples were dehydrated in a graded series of acetone, frozen, and fractured. Samples were thawed, critical-point dried, gold sputter coated, and observed with a Zeiss Supra 35 FESEM at 10 KV. SEM was conducted on a Zeiss Supra 35 FESEM at 10 KV.
TEM
Kidney tissues were fixed in 2.5% glutaraldehyde-2% paraformaldehyde in 0.1 M cacodylate buffer followed 1% OsO4 in 0.1 M cacodylate buffer at pH 7.4. Samples were rinsed using 0.1 M cacodylate buffer and stained using uranyl acetate. Following multiple rinse cycles with double distilled water, samples were dehydrated in a graded series of acetone and embedded in Spurr's resin for ultrathin sectioning. Toluidine blue sections were cut to visualize glomeruli and, once localized, marked to allow blocks to be trimmed for ultrathin sectioning of this region. Ultrathin sections were cut to ~90-nm thickness with a Reichert-Jung Ultracut S Ultramicrotome (Reichert-jung, Wein, Austria). Sections were stained with uranyl acetate and lead citrate and observed with a JEOL 1200EX II TEMSCAN (Tokyo, Japan) at 80 KV. TEM was performed with a JEOL 1200EX II TEMSCAN at 80 KV.
RESULTS
Clinical Assays for AKI
Mouse group details are in Table 1. Urine samples were collected from 17 mice for 3 days before IRI and 24 h after IRI. Serum creatinine, urinary NGAL, urinary NGAL/urinary creatinine ratios, and urinary creatinine levels confirmed AKI 24 h post-IRI (Table 1).
Histological and Immunohistochemical Analysis of IRI of the Cortex of the Kidney
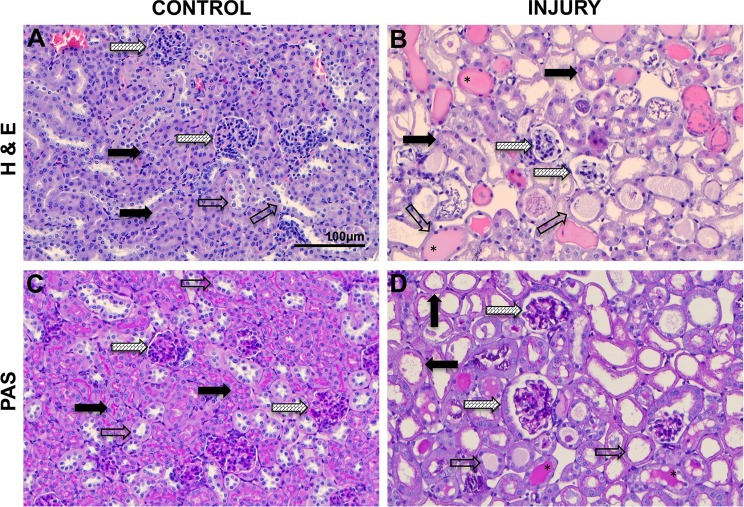
Hematoxylin and eosin staining of control cortical kidney tissue (Fig. 1A) showed dense packing of renal corpuscles and proximal and distal tubules. Normal renal corpuscles exhibited Bowman’s capsules fitted tightly around glomerular capillary bundles with thin Bowman’s spaces (Fig. 1A). Normal proximal tubule lumina were barely detectable, appearing as narrow spaces between proximal tubule epithelia (Fig. 1A). Distal tubules had wider lumina compared with proximal tubules, appearing as large white paths along distal tubules or open white spaces in distal tubule cross sections (Fig. 1A). In IRI kidneys, renal corpuscles exhibited compressed glomerular capillary bundles with widened Bowman’s capsule spaces (Fig. 1B). Proximal tubules were significantly distended and contained debris in lumina (Fig. 1B). Distal tubules were distended, contained debris, and, in many cases, exhibited mucoprotein casts (Fig. 1B). Increased intertubular spaces were evident (Fig. 1B). PAS staining revealed brush borders in normal proximal tubules that stained bright pink providing strong contrast with distal tubules that lacked brush borders (Fig. 1C). PAS staining of IRI kidneys revealed a dramatic reduction of proximal tubule epithelial brush borders, which appeared as thin ragged remnant layers (Fig. 1D).
Fig. 1.
Histological analysis of cortical tissue in control mice and mice subjected ischemia-reperfusion injury (IRI) of the kidney. A: control hematoxylin and eosin (H&E) staining showing normal proximal tubules (solid arrows), distal tubules (open arrows), and glomeruli (hatched arrows). B: IRI H&E-stained kidney tissue showing normal proximal tubules solid arrows), distal tubules (open arrows), and glomeruli (hatched arrows). *Mucoprotein casts in distal tubules. C: control periodic acid-Schiff (PAS) staining showing normal proximal tubules with bright pink brush borders (solid arrows), distal tubules (open arrows), and glomeruli (hatched arrows). D: IRI PAS-stained kidney tissue showing proximal tubules (solid arrows), distal tubules (open arrows), and glomeruli (hatched arrows). All images were captured at the same magnification.
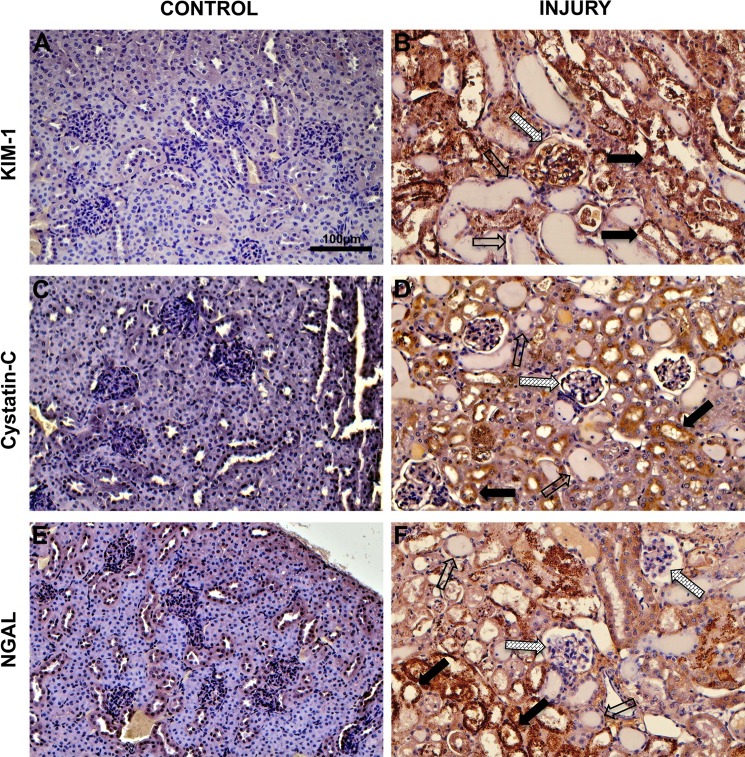
Immunohistochemical staining was used to examine cortical tissue distributions of KIM-1, cystatin C, and NGAL. Control tissue exhibited only faint nonspecific KIM-1 staining (Fig. 2A). In contrast, IRI kidneys exhibited strong KIM-1 staining in glomerular capillary endothelia and proximal tubule epithelia, in addition to debris in proximal tubule lumina (Fig. 2B). Distal tubule epithelia and casts within distal tubule lumina did not stain for KIM-1 (Fig. 2B). Normal cortical kidney tissue did not stain for cystatin C (Fig. 2C). In IRI kidneys, strong cystatin C staining was observed in proximal tubule epithelia and in debris within proximal tubule lumina, but the endothelia of glomerular capillary bundles did not stain for cystatin C (Fig. 2D). Distal tubule epithelia also exhibited negative cystatin C staining (Fig. 2D). Finally, normal cortical tissue exhibited weak nonspecific NGAL staining (Fig. 2E) whereas NGAL staining was strong in proximal tubule epithelia and in debris within proximal tubule lumina but NGAL staining was negative in glomerular capillary endothelia and in distal tubule epithelia (Fig. 2F).
Fig. 2.
Immunohistochemical analysis of cortical tissue in control mice and mice subjected ischemia reperfusion injury of the kidney. A; control cortical tissue probed with kidney injury molecule 1 (KIM-1). B: IRI cortical tissue probed with KIM-1. C: control cortical tissue probed with cystatin C. D: IRI cortical tissue probed with cystatin C. E: control cortical tissue probed with neutrophil gelatinase-associated lipocalin (NGAL). F: IRI cortical tissue probed with NGAL. In IRI images, proximal tubules (solid arrows), distal tubules (open arrows), and glomeruli (hatched arrows) are indicated.
Insights Regarding Altered Nephron Function from 1H NMR-Based Metabolic Profiling of Urine, Plasma, and Kidney Tissue Extracts
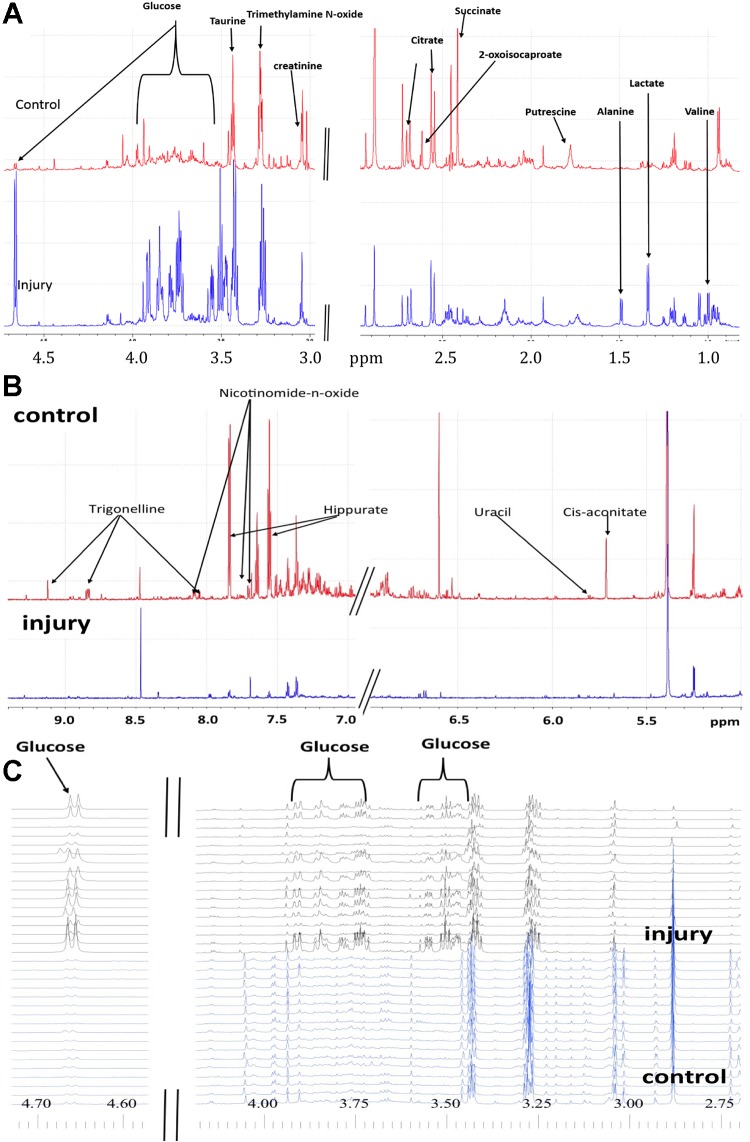
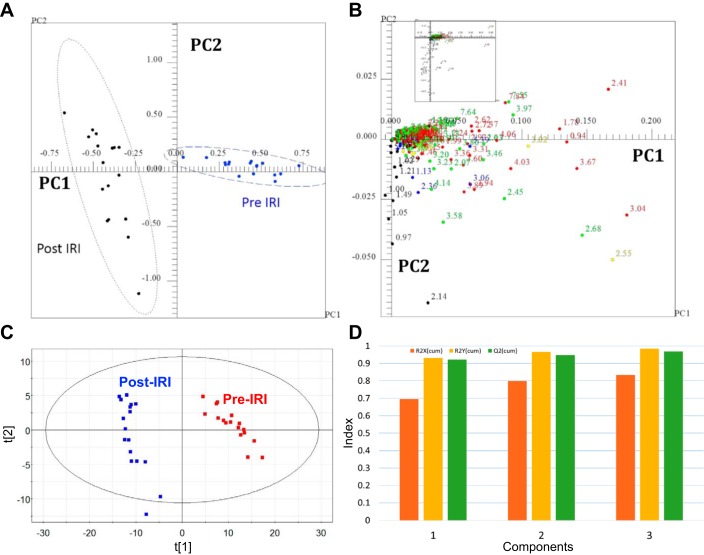
1H NMR spectra of urine revealed that the concentrations of many metabolites before IRI changed dramatically after IRI (Fig. 3). These differences caused strong cluster separation in PCA scores plots of urines collected before and after IRI (Fig. 4A), and the NMR peaks responsible for separation in the scores plot were identified in the PCA loadings plot (Fig. 4B). Statistical analysis indicated significant changes for >150 resonances between pre-IRI and post-IRI spectra (Table 2). Cis-aconitate, citrate, creatine, phosphocreatine, putrescine, sarcosine succinate, taurine, n-nitrosodimethylamine, trimethylamine, uracil, and trimethylamine N-oxide, galactric acid, guanine and hippurate all had lower urine concentrations following IRI (Table 3). Nicotinomide-n-oxide, trigonelline, 2-oxoglutarate, and 2-oxoisocaproate were absent in urine following injury (Table 3). Glucose, lactate, alanine, valine, and leucine had higher urine concentrations following IRI (Table 3). The 1H NMR spectra of the urine samples were independently evaluated using PLS-DA, which indicated clear separation of control and IRI urines (Fig. 4C). PLS-DA cross validation indicated excellent quality data based on the R2X and R2Y values, and the predictive power of the model was also excellent based on a cumulative Q2 value = 0.969 (Fig. 4D). The PLS-DA VIP (variable importance in the projection) scores were consistent with the list of statistically significant metabolites identified using the P-value analysis, i.e., no new metabolites were identified as significant or missing based on the VIP scores.
Fig. 3.
Differences in urine NMR spectra illustrating changes in metabolite concentrations as a result of IRI. Representative spectra in the aliphatic regions of control (A, top) and IRI (A, bottom) urine samples, the aromatic region of control (B, top) and IRI (B, bottom) urine samples, and stacked NMR spectra in the aliphatic region of control (C, bottom) and IRI (C, top) urine sample samples highlighting the difference in glucose signals.
Fig. 4.
Two-dimensional principal component analysis (PCA) scores and loadings plots for comparison of urine samples from post-IRI mice and control mice. A: PCA scores plot for comparison of urines from control mice and IRI mice. Each point in the scores plot represents the NMR spectrum of a mouse projected onto the 2-dimensional space defined by the 1st 2 principal components. B: the loadings plot corresponding to the scores plot shown in A. The buckets (points) in the loadings plot are color-coded according to bucket P value: P > 0.00027027 (black); zone 1: 1e-005–2.7e-004 (blue); zone 2: 1e-007–1e-005 (green); zone 3: 1e-009–1e-007 (yellow); and zone 4: 1e-020–1e-009 (red). The Bonferroni corrected α-value was 2.7e-004. 163 of 186 resonances were significant by α = 0.05 with 145 of those resonances being significant by the Bonferroni corrected α-value of 2.7E-4. Twenty-three metabolites were significantly different in urine following IRI. C: PLS-DA scores plot for comparison of urines from control mice (Pre) and IRI mice (Post). D: graph of the cumulative R2X, R2Y, and Q2 values obtained from cross validation of the data using repeated calculations withholding 1/7 of the data.
Table 3.
Summary of changes in urine metabolite concentrations following IRI to the kidney
| Increased | Decreased | Absent | |
|---|---|---|---|
| Alanine | Creatinine | 1- Methyl-nicotinamide | (13/17)a |
| Lactate | Creatinine phosphate | 2-Oxoglutarate | (15/17) |
| Valine | Malonate | 2-Oxoisocaproate | (13/17) |
| Leucine | Nicotinomide-n-oxide | Hippurate | (7/17) |
| Glucose | N-nitrosodimethylamine | Guanine | (5/17) |
| Putrescine | |||
| Dimethyl sulfone* | Sarcosine | ||
| Taurine | |||
| Trigonelline | |||
| Trimethlyamine N-oxide | |||
| Uracil | |||
| Cis-aconitate | |||
| Galactaric acid | |||
| Succinate | |||
| Dimethylamine** | |||
| Fumaric acid* | |||
| Methylamine** | |||
| Methylguanidine** | |||
| Threo isocitric acid** | |||
| Indoxyl sulfate** |
The first number indicates the number of spectra in which the metabolite was absent, and the second number indicates the total number of spectra measured.
Confirmed using COLMAR with minimal overlap.
Confirmed using COLMAR with significant overlap.
NMR spectral complexity generally decreased following IRI, especially in the aromatic region (Fig. 3B). Severe nephron distension following IRI (Fig. 1B) potentially explained decreased complexity due to dilution of metabolites beyond detection. To test this possibility, NMR data were recollected for IRI samples using a ×100 longer time, however, spectral complexity did not change ruling out dilution as an explanation. Decreased complexity in post-IRI samples could have reflected changes in eating behavior following surgery or IRI. To test these possibilities, additional control experiments were conducted including sham surgeries followed by 1) fasting mice for 24 h after surgery, 2) letting mice eat for 3 h then fasting for remaining 21 h after surgery, or 3) letting mice eat ab libitum following surgery. No sham surgery mice groups exhibited loss of urine spectra complexity ruling out changes in eating behavior as an explanation. NMR spectroscopy of plasma collected 24 h post-IRI indicated no new metabolites post-IRI except for creatinine (Table 4). Metabolites observed in IRI mice were generally also observed control mice plasma but at lower concentrations. Altered glomerular filtration could not explain absence or decreased of metabolites in urine in IRI mice since they did not accumulate in blood (Table 4). Metabolic profiling of kidney tissue extracts indicated no new metabolites following IRI ruling out catastrophic rupture of kidney tubules or renal corpuscles following IRI. A striking feature obtained from the NMR analysis was a significant increase in glucose in urine following IRI (Fig. 3C). This indicated that blood glucose was efficiently filtered by glomeruli but not reabsorbed by damaged proximal brush borders.
Table 4.
List of metabolites that exhibited different concentrations in control and IRI serum samples
| Preserum |
Postserum |
||||||
|---|---|---|---|---|---|---|---|
| Metabolite, ppm | P Value | Fold Changea | mM | SD | mM | SD | |
| Alanine | |||||||
| 1.50 | 2.78E−03 | 1.42 | 4.14E−01 | 4.60E−01 | 1.51E−01 | 1.35E−01 | |
| 3.80 | **b | ** | ** | ** | ** | ** | ov |
| Citrate | |||||||
| 2.56 | 8.45E−02 | −1.15 | 2.92E−01 | 2.92E−01 | 3.68E−01 | 4.06E−01 | |
| 2.68 | 1.24E−02 | −1.90 | 1.92E−01 | 2.62E−01 | −2.32E−01 | 3.67E−01 | |
| Creatinine | |||||||
| 3.06 | 6.96E−04 | −3.19 | 1.07E−01 | 5.98E−02 | 3.25E−01 | 2.20E−01 | |
| 4.05 | 1.70E−02 | −2.08 | ** | ** | ** | ** | ov |
| Glucose | |||||||
| 3.26 | 9.30E−01 | −1.01 | ** | ** | ** | ** | ov |
| 3.43 | 7.82E−01 | −1.03 | 4.43E+00 | 3.07E+00 | 3.62E+00 | 2.75E+00 | |
| 3.51 | 2.04E−01 | 1.16 | 4.29E+00 | 3.59E+00 | 3.19E+00 | 2.34E+00 | |
| 3.56 | 8.21E−01 | 1.02 | ** | ** | ** | ** | ov |
| 3.75 | 8.52E−01 | 1.02 | ** | ** | ** | ** | ov |
| 3.79 | 6.15E−01 | −1.05 | 4.03E+00 | 3.59E+00 | 3.03E+00 | 2.75E+00 | |
| 3.85 | 8.20E−01 | −1.03 | ** | ** | ** | ** | ov |
| 3.93 | 6.82E−01 | −1.04 | ** | ** | ** | ** | ov |
| 4.67 | 5.13E−01 | 1.07 | 4.34E+00 | 2.46E+00 | 3.35E+00 | 2.46E+00 | |
| 5.26 | 7.64E−01 | 1.03 | 4.04E+00 | 3.11E+00 | 3.65E+00 | 2.26E+00 | |
| Lactate | |||||||
| 1.35 | 9.38E−02 | 1.13 | 6.29E+00 | 6.04E+00 | 3.40E+00 | 2.35E+00 | |
| 4.11 | ** | ** | ** | ** | ** | ** | ov |
| Phenylalanine | |||||||
| 7.35 | 5.26E−05 | −1.71 | 7.45E−02 | 9.58E−02 | 5.86E−02 | 2.84E−02 | |
| 7.40 | 7.79E−03 | −1.43 | 7.54E−02 | 9.51E−02 | 5.76E−02 | 2.72E−02 | |
| 7.44 | 9.32E−01 | 1.03 | 7.38E−02 | 9.55E−02 | 5.58E−02 | 2.90E−02 | |
| 4 | ** | ** | ** | ** | ** | ** | ov |
| 3.3 | ** | ** | ** | ** | ** | ** | ov |
| 3.1 | ** | ** | ** | ** | ** | ** | ov |
| Tyrosine | |||||||
| 6.91 | 2.17E−05 | 1.65 | 1.07E+00 | 1.77E+00 | 2.80E−01 | 2.62E−01 | |
| 7.21 | 2.14E−03 | 1.35 | 1.08E+00 | 1.28E+00 | 2.88E−01 | 2.67E−01 | |
| 3.90 | ** | ** | ** | ** | ** | ** | ov |
| 3.20 | ** | ** | ** | ** | ** | ** | ov |
| 3.00 | ** | ** | ** | ** | ** | ** | ov |
| Valine | |||||||
| 1.01 | 2.58E−05 | 1.44 | 3.51E−01 | 4.72E−01 | 1.05E−01 | 5.42E−02 | |
| 1.06 | 6.01E−06 | 1.59 | 3.65E−01 | 4.67E−01 | 1.39E−01 | 5.70E−02 | |
| 3.20 | ** | ** | ** | ** | ** | ** | ov |
| 3.60 | ** | ** | ** | ** | ** | ** | ov |
Fold changes were calculated by control/IRI. Therefore, positive values indicate decreased concentration in IRI serum and negative values indicate increased concentration in IRI serum.
Double asterisks indicate that the peaks were overlapped (ov), and a reliable P value or fold change could not be calculated.
SEM and TEM Analysis of IRI to the Cortex of the Kidney
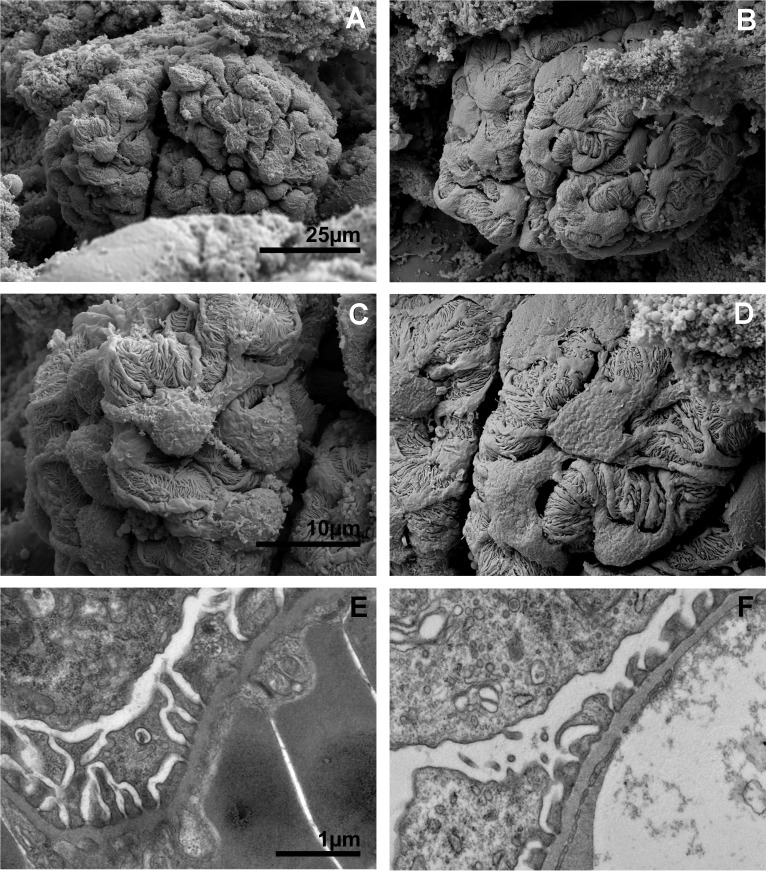
SEM images of normal renal corpuscles revealed glomerular capillary bundles structurally divided into multiple lobes or segments further organized into tightly packed serpentine paths of capillaries within each larger lobe or segment (Fig. 5A). Careful examination of glomerular structure following IRI revealed subtle but important changes. The highly complex, irregular, and rough surface topology of the podocytes observed in normal glomeruli (Fig. 5A) appeared smoothened and flattened in glomeruli following IRI (Fig. 5B). This feature was consistent with increased hydrostatic pressure within nephrons caused by dramatic increase in filtrate volume extending from distal tubules to the renal corpuscle. At higher magnification, flattening of control glomeruli podocytes and podocyte foot processes (Fig. 5C) was more evident after IRI (Fig. 5D). TEM of glomeruli revealed elongated and ordered podocyte pedicals in control tissues (Fig. 5E) that were shorter and more disorganized in IRI kidneys (Fig. 5F) consistent with flattening observed in SEM images.
Fig. 5.
Electron microscopy images of glomeruli from control and IRI mice. Scanning electron microscopy (SEM) of a control glomerulus (A) and an IRI glomerulus (B) at low magnification. C: high-magnification SEM of the surface of a control glomerulus showing the complex interdigitations of podocytes and their foot processes. D: high-magnification SEM of an IRI glomerulus podocyte foot process effacement following injury. E: transmission electron microscopy (TEM) of a control glomerulus podocyte foot processes. F: TEM of IRI glomerulus podocyte foot processes. A and B, C and D, and E and F were captured at the same magnifications.
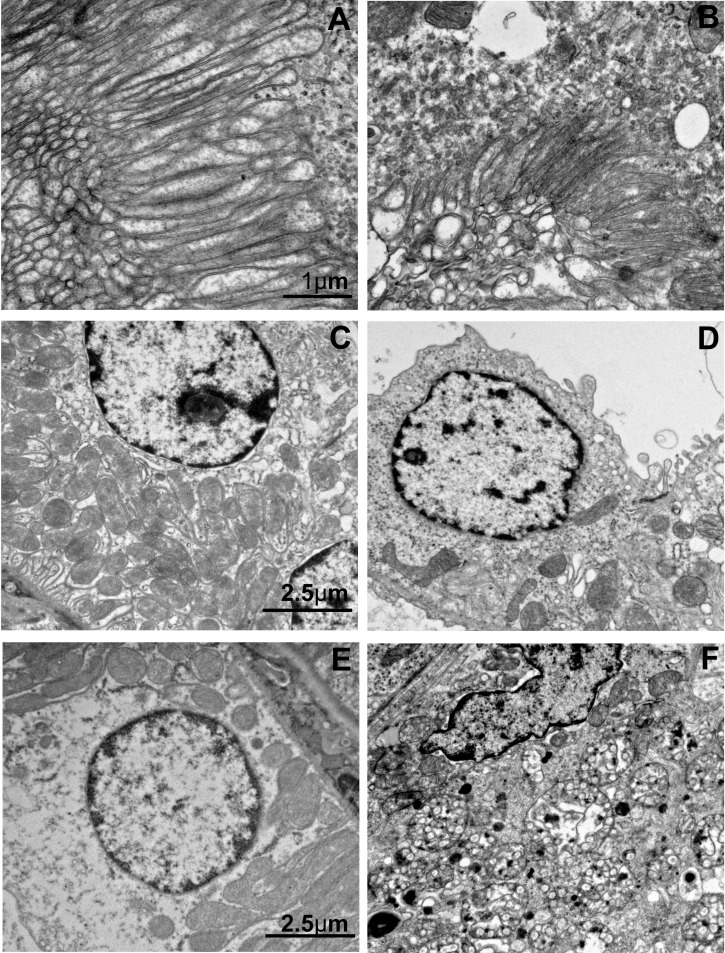
TEM of proximal tubules following IRI revealed normal brush borders in control mice with microvilli extending several microns in length with prominent lamina propia ~0.25-μm wide (Fig. 6A). In contrast, brush borders in proximal tubules of IRI mice were rare and difficult to detect and when observed displayed only remnants of original brush border structure with significantly shorter microvilli 1–2 μm in length with almost completely absent lamina propia (Fig. 6B).
Fig. 6.
TEM images of cortical proximal and distal tubule epithelia of control and IRI kidneys. A: TEM of microvilli of proximal tubule brush border in a control mouse. B: TEM of microvilli of a proximal tubule brush border in an IRI mouse. C: TEM of control distal tubule basement exhibiting basement labyrinth and organized mitochondria. D: TEM of IRI distal tubule basement labyrinth disruption and decrease and disorganization of mitochondria. E: TEM of control distal tubule epithelial cell showing absence of multivesicular bodies. F: TEM of IRI epithelial cell showing an abundance of multivesicular bodies. A and B, and C–F were captured at the same magnification.
TEM images of distal tubules of normal kidneys revealed an organized elongated basal membrane labyrinth pointing away from the basal membrane and reaching into the cytoplasm by ~1 μm (Fig. 6C). Densely packed mitochondria were evident in normal kidneys, intermingled with the basal membrane labyrinth and filling the cytoplasm between the basal membranes and nuclei (Fig. 6C). In sharp contrast, cellular membranes of distal tubule epithelial cells in IRI kidneys were poorly defined, basal lamina labyrinths were highly disorganized lying along the cell wall, and the densely packed mitochondria had almost entirely disappeared (Fig. 6D). At higher magnification, one could visualize densely packaged mitochondria in control kidneys (Fig. 6E) in comparison to sparse mitochondria in IRI kidneys, which showed emergence of multivesicular bodies typical of endosomes or autolysosomes (Fig. 6F).
DISCUSSION
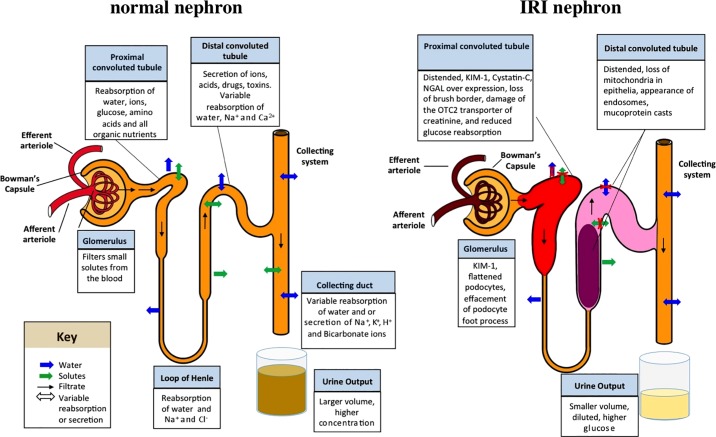
Histological examination of IRI kidneys revealed severely distended proximal and distal tubules and Bowman’s spaces of renal corpuscles. Proximal tubule brush border microvilli were destroyed. Distal tubules contained significant casts. Nephron distension appeared due to increased water retention spanning collecting tubules to renal corpuscles. Widespread tubular casts appeared to block free flow of filtrate into collecting tubules causing increased filtrate volume and reduced urine output. Distal tubular casts likely contained mucoproteins shed from proximal tubular microvilli epithelia damaged during IRI. Reduced water reabsorption by proximal tubule brush borders likely contributed to increased filtrate volume. A model summarizing structural changes accompanying kidney IRI damage is presented in Fig. 7.
Fig. 7.
Schematic model of a normal and IRI nephron. A: selected normal functions of the nephron are indicated. B: summary of changes to the nephron observed in this study. The red coloration of the glomerulus indicates elevated cystatin C; the widened proximal and distal tubules represent the distension observed in histology analyses; the red color of the proximal tubule indicates elevated KIM-1, cystatin C, and NGAL; the pink coloration in the distal tubule indicates presence of mucoprotein plugs; and the reduced and lighter color of urine in the collection vial indicates reduced urine output and dilution of the collected urine.
All protein AKI biomarkers were highly overexpressed in proximal tubule epithelia after IRI. Only KIM-1 was elevated in glomeruli after IRI. No AKI biomarkers were elevated in distal tubule epithelia 24 h post-IRI, despite changes in cellular structure and subcellular composition of distal tubule epithelia based on TEM, indicating localization of AKI protein biomarkers to specific cell types along the nephron.
Loss of glucose reuptake in proximal tubules due to damage to brush border epithelia caused increased urinary glucose. Decreased peak intensities for most metabolites were consistent with urine dilution following IRI. Increased filtrate volume caused dilution of metabolites. Reduced urine output composition reflected restricted passage of diluted filtrate across distal tubular casts (Fig. 7).
Nicotinamide-n-oxide, trigonelline (nicotinic acid metabolism byproduct), 2-oxoglutarate (key Kreb’s cycle intermediate), and 2-oxoisocaproate (involved in energy storage found in mitochondria) were absent in urine following IRI. These metabolites are associated with NADH usage or metabolism. Proximal tubule epithelia synthesize NAD+ from nicotinic acid, nicotinamide, and nicotinamide riboside taken up by proximal tubule brush borders (17). By-products of excess nicotinic acid, e.g., trigonelline, 1-methyl-nicotinamide, and nicotinamide-n-oxide, are normally secreted in urine. Destruction of brush borders following IRI would prevent absorption of nicotinic acid and abolish production of these byproducts of nicotinic acid metabolism.
Urinary hippurate decreased following IRI. Kidneys play a significant role in hippurate production and secretion, and kidney cortical cells are a primary location of hippurate synthesis (24). Hippurate synthesis takes place in mitochondria and dramatic loss of mitochondria in distal tubules could explain reduced hippurate in urine.
Trimethylamine-n-oxide, trimethylamine, and n-nitrosodimethylamine, which decreased in urine following IRI, are related to arginine and methylamine metabolism. Proximal tubule epithelia are a major source of arginine synthesis. Loss of proximal tubule brush border epithelia would reduce arginine synthesis in the kidneys and therefore creatine production. This would downregulate methylamine metabolism in the kidney (43, 46–48), resulting in decreased urinary concentrations of these metabolites.
Decreased creatinine in urine following IRI can be traced to loss of organic cation transporter OTC2 uptake of creatine in proximal tubules and subsequent secretion into tubule lumina by other transporters (10). Proximal tubule secretion is responsible for up to 40% of creatinine clearance from blood. Loss of OTC2 transporters in proximal tubules due to damage to brush borders would result in decreased creatinine clearance, reduced urinary creatinine, and elevated blood creatinine.
Decreased urinary putrescine following IRI was related to arginine metabolism. In the urea cycle, arginine is converted to ornithine, which is subsequently converted to putrescine by ornithine decarboxylase (26). Reduction in arginine biosynthesis in IRI kidneys would disrupt ornithine production and conversion to putrescine, leading to lower urinary putrescine.
Alanine, valine, and leucine, elevated in urine following IRI, are involved in anabolic and catabolic reactions that produce metabolites normally consumed to support mitochondrial metabolism. Loss of distal tubule epithelia mitochondria would reduce consumption of these substrates resulting in their accumulation and increase in filtrate and urine after IRI.
SEM data indicated nephron ultrastructural changes consistent with histology and NMR data. Smoothing of glomerular podocytes with simpler topography in IRI glomeruli was consistent with increased hydrostatic pressure caused by increased filtrate volume inferred from histology images of IRI mice and from diluted urine observed by NMR. TEM images of IRI glomeruli confirmed podocyte pedical effacement caused by increased hydrostatic pressure in Bowman’s spaces.
TEM images of IRI kidney proximal tubules revealed shortening of microvilli in remnant brush borders accompanied by almost complete loss of lamina propia. Two surprising results emerged from TEM of IRI kidney distal tubules. First, distal tubule epithelia in IRI kidneys exhibited almost complete loss of densely packed mitochondria in the cytoplasm that supports ATP synthesis required for active transport of Na+ and K+ ions, which controls filtrate osmotic pressure in distal nephrons. Second, ubiquitous multivesicular bodies of endosomes and autolysosomes were observed in IRI nephrons, effectively replacing dense mitochondria found in distal tubule epithelia of normal mice, indicating transition of cells progressing toward death.
In summary, combined use of histology and immunohistology, NMR based metabolic profiling, and SEM and TEM yielded substantial new insight into structural and functional changes that occur in the kidney in response to IRI-AKI, including novel insight into cellular responses of the distal tubules. These results establish hallmarks of IRI-AKI that can be used as benchmarks for future investigations of macroscopic, ultrastructural, cellular, and metabolic responses to other causes of AKI.
GRANTS
P. Devarajan is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant P50-DK-096418.
DISCLOSURES
M. A. Kennedy, T. Chihanga, J. D. Nicolson, H. Ruby, Q. Ma, and R. Edelmann declare no relationship with any companies that might have a financial interest in the information contained in this article. P. Devarajan declares that he serves as a consultant for BioPorto, Inc. and is a coinventor on submitted patents on the use of NGAL as a biomarker of kidney injury.
AUTHOR CONTRIBUTIONS
T.C., P.D., and M.A.K. conceived and designed research; T.C., Q.M., J.D.N., H.N.R., and R.E.E. performed experiments; T.C., Q.M., and M.A.K. analyzed data; T.C. and M.A.K. interpreted results of experiments; T.C. prepared figures; T.C., R.E.E., and M.A.K. drafted manuscript; T.C., Q.M., R.E.E., P.D., and M.A.K. edited and revised manuscript; T.C., P.D., and M.A.K. approved final version of manuscript.
REFERENCES
- 1.Askenazi DJ, Koralkar R, Hundley HE, Montesanti A, Parwar P, Sonjara S, Ambalavanan N. Urine biomarkers predict acute kidney injury in newborns. J Pediatr 161: 270–5.e1, 2012. doi: 10.1016/j.jpeds.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Askenazi DJ, Koralkar R, Levitan EB, Goldstein SL, Devarajan P, Khandrika S, Mehta RL, Ambalavanan N. Baseline values of candidate urine acute kidney injury biomarkers vary by gestational age in premature infants. Pediatr Res 70: 302–306, 2011. doi: 10.1203/PDR.0b013e3182275164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagshaw SM, George C, Bellomo R; ANZICS Database Management Committee . Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care 12: R47, 2008. doi: 10.1186/cc6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol 2: 1303–1353, 2012. doi: 10.1002/cphy.c110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett MR, Devarajan P. Proteomic analysis of acute kidney injury: biomarkers to mechanisms. Proteomics Clin Appl 5: 67–77, 2011. doi: 10.1002/prca.201000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bingol K, Li DW, Bruschweiler-Li L, Cabrera OA, Megraw T, Zhang F, Brüschweiler R. Unified and isomer-specific NMR metabolomics database for the accurate analysis of (13)C-(1)H HSQC spectra. ACS Chem Biol 10: 452–459, 2015. doi: 10.1021/cb5006382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14: 2199–2210, 2003. doi: 10.1097/01.ASN.0000079785.13922.F6. [DOI] [PubMed] [Google Scholar]
- 8.Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, Bittleman D, Cruz D, Endre Z, Fitzgerald RL, Forni L, Kane-Gill SL, Hoste E, Koyner J, Liu KD, Macedo E, Mehta R, Murray P, Nadim M, Ostermann M, Palevsky PM, Pannu N, Rosner M, Wald R, Zarbock A, Ronco C, Kellum JA; Acute Disease Quality Initiative Workgroup 16.. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 13: 241–257, 2017. doi: 10.1038/nrneph.2017.2. [DOI] [PubMed] [Google Scholar]
- 9.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 10.Ciarimboli G, Lancaster CS, Schlatter E, Franke RM, Sprowl JA, Pavenstädt H, Massmann V, Guckel D, Mathijssen RH, Yang W, Pui CH, Relling MV, Herrmann E, Sparreboom A. Proximal tubular secretion of creatinine by organic cation transporter OCT2 in cancer patients. Clin Cancer Res 18: 1101–1108, 2012. doi: 10.1158/1078-0432.CCR-11-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciccia E, Devarajan P. Pediatric acute kidney injury: prevalence, impact and management challenges. Int J Nephrol Renovasc Dis 10: 77–84, 2017. doi: 10.2147/IJNRD.S103785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant 23: 1970–1974, 2008. doi: 10.1093/ndt/gfm908. [DOI] [PubMed] [Google Scholar]
- 13.Delanaye P, Lambermont B, Chapelle JP, Gielen J, Gerard P, Rorive G. Plasmatic cystatin C for the estimation of glomerular filtration rate in intensive care units. Intensive Care Med 30: 980–983, 2004. doi: 10.1007/s00134-004-2189-5. [DOI] [PubMed] [Google Scholar]
- 14.Gill N, Nally JV Jr, Fatica RA. Renal failure secondary to acute tubular necrosis. Chest 128: 2847–2863, 2005. doi: 10.1378/chest.128.4.2847. [DOI] [PubMed] [Google Scholar]
- 15.Goodpaster AM, Romick-Rosendale LE, Kennedy MA. Statistical significance analysis of nuclear magnetic resonance-based metabonomics data. Anal Biochem 401: 134–143, 2010. doi: 10.1016/j.ab.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Herget-Rosenthal S, Marggraf G, Hüsing J, Göring F, Pietruck F, Janssen O, Philipp T, Kribben A. Early detection of acute renal failure by serum cystatin C. Kidney Int 66: 1115–1122, 2004. doi: 10.1111/j.1523-1755.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 17.Hershberger KA, Martin AS, Hirschey MD. Role of NAD+ and mitochondrial sirtuins in cardiac and renal diseases. Nat Rev Nephrol 13: 213–225, 2017. doi: 10.1038/nrneph.2017.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hörbelt M, Lee SY, Mang HE, Knipe NL, Sado Y, Kribben A, Sutton TA. Acute and chronic microvascular alterations in a mouse model of ischemic acute kidney injury. Am J Physiol Renal Physiol 293: F688–F695, 2007. doi: 10.1152/ajprenal.00452.2006. [DOI] [PubMed] [Google Scholar]
- 19.Hoste EA, Schurgers M. Epidemiology of acute kidney injury: how big is the problem? Crit Care Med 36, Suppl: S146–S151, 2008. doi: 10.1097/CCM.0b013e318168c590. [DOI] [PubMed] [Google Scholar]
- 20.Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem 273: 4135–4142, 1998. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 21.Kerr M, Bedford M, Matthews B, O’Donoghue D. The economic impact of acute kidney injury in England. Nephrol Dial Transplant 29: 1362–1368, 2014. doi: 10.1093/ndt/gfu016. [DOI] [PubMed] [Google Scholar]
- 22.Lameire N, Van Biesen W, Vanholder R. The changing epidemiology of acute renal failure. Nat Clin Pract Nephrol 2: 364–377, 2006. doi: 10.1038/ncpneph0218. [DOI] [PubMed] [Google Scholar]
- 23.Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, Liu KD, Mehta RL, Pannu N, Van Biesen W, Vanholder R. Acute kidney injury: an increasing global concern. Lancet 382: 170–179, 2013. doi: 10.1016/S0140-6736(13)60647-9. [DOI] [PubMed] [Google Scholar]
- 24.Lees HJ, Swann JR, Wilson ID, Nicholson JK, Holmes E. Hippurate: the natural history of a mammalian-microbial cometabolite. J Proteome Res 12: 1527–1546, 2013. doi: 10.1021/pr300900b. [DOI] [PubMed] [Google Scholar]
- 25.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A; Acute Kidney Injury Network . Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minois N, Carmona-Gutierrez D, Madeo F. Polyamines in aging and disease. Aging (Albany NY) 3: 716–732, 2011. doi: 10.18632/aging.100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231–1238, 2005. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 28.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534–2543, 2003. doi: 10.1097/01.ASN.0000088027.54400.C6. [DOI] [PubMed] [Google Scholar]
- 29.Mishra J, Mori K, Ma Q, Kelly C, Yang J, Mitsnefes M, Barasch J, Devarajan P. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 15: 3073–3082, 2004. doi: 10.1097/01.ASN.0000145013.44578.45. [DOI] [PubMed] [Google Scholar]
- 30.Murugan R, Kellum JA. Acute kidney injury: what’s the prognosis? Nat Rev Nephrol 7: 209–217, 2011. doi: 10.1038/nrneph.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nielsen BS, Borregaard N, Bundgaard JR, Timshel S, Sehested M, Kjeldsen L. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut 38: 414–420, 1996. doi: 10.1136/gut.38.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niemann CU, Serkova NJ. Biochemical mechanisms of nephrotoxicity: application for metabolomics. Expert Opin Drug Metab Toxicol 3: 527–544, 2007. doi: 10.1517/17425255.3.4.527. [DOI] [PubMed] [Google Scholar]
- 33.Romick-Rosendale LE, Goodpaster AM, Hanwright PJ, Patel NB, Wheeler ET, Chona DL, Kennedy MA. NMR-based metabonomics analysis of mouse urine and fecal extracts following oral treatment with the broad-spectrum antibiotic enrofloxacin (Baytril). Magn Reson Chem 47, Suppl 1: S36–S46, 2009. doi: 10.1002/mrc.2511. [DOI] [PubMed] [Google Scholar]
- 34.Siew ED, Ware LB, Ikizler TA. Biological markers of acute kidney injury. J Am Soc Nephrol 22: 810–820, 2011. doi: 10.1681/ASN.2010080796. [DOI] [PubMed] [Google Scholar]
- 35.Simsek A, Tugcu V, Tasci AI. New biomarkers for the quick detection of acute kidney injury. ISRN Nephrol 2013: 394582, 2012. doi: 10.5402/2013/394582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sirota JC, Klawitter J, Edelstein CL. Biomarkers of acute kidney injury. J Toxicol 2011: 328120, 2011. doi: 10.1155/2011/328120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P. Differential gene expression following early renal ischemia/reperfusion. Kidney Int 63: 1714–1724, 2003. doi: 10.1046/j.1523-1755.2003.00928.x. [DOI] [PubMed] [Google Scholar]
- 38.Trof RJ, Di Maggio F, Leemreis J, Groeneveld ABJ. Biomarkers of acute renal injury and renal failure. Shock 26: 245–253, 2006. doi: 10.1097/01.shk.0000225415.5969694.ce. [DOI] [PubMed] [Google Scholar]
- 39.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators . Acute renal failure in critically ill patients a multinational, multicenter study. JAMA 294: 813, 2005. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 40.Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol 3: 844–861, 2008. doi: 10.2215/CJN.05191107. [DOI] [PubMed] [Google Scholar]
- 41.Waikar SS, Winkelmayer WC. Chronic on acute renal failure: long-term implications of severe acute kidney injury. JAMA 302: 1227–1229, 2009. doi: 10.1001/jama.2009.1364. [DOI] [PubMed] [Google Scholar]
- 42.Wei Q, Dong Z. Mouse model of ischemic acute kidney injury: technical notes and tricks. Am J Physiol Renal Physiol 303: F1487–F1494, 2012. doi: 10.1152/ajprenal.00352.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei T, Zhao L, Jia J, Xia H, Du Y, Lin Q, Lin X, Ye X, Yan Z, Gao H. Metabonomic analysis of potential biomarkers and drug targets involved in diabetic nephropathy mice. Sci Rep 5: 11998, 2015. doi: 10.1038/srep11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams DM, Sreedhar SS, Mickell JJ, Chan JC. Acute kidney failure: a pediatric experience over 20 years. Arch Pediatr Adolesc Med 156: 893–900, 2002. doi: 10.1001/archpedi.156.9.893. [DOI] [PubMed] [Google Scholar]
- 45.Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, Djoumbou Y, Mandal R, Aziat F, Dong E, Bouatra S, Sinelnikov I, Arndt D, Xia J, Liu P, Yallou F, Bjorndahl T, Perez-Pineiro R, Eisner R, Allen F, Neveu V, Greiner R, Scalbert A. HMDB 3.0–the Human Metabolome Database in 2013. Nucleic Acids Res 41: D801–D807, 2013. doi: 10.1093/nar/gks1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev 80: 1107–1213, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Zapitelli M. Goldstein S. Acute Kidney Injury: General Aspects. Kiessling S. G, Sobel J, Somers M. J. G In: Pediatric Nephrology in the ICU. New York: Springer, 2009. [Google Scholar]
- 48.Zhao L, Dong M, Liao S, Du Y, Zhou Q, Zheng H, Chen M, Ji J, Gao H. Identification of key metabolic changes in renal interstitial fibrosis rats using metabonomics and pharmacology. Sci Rep 6: 27194, 2016. doi: 10.1038/srep27194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zuk A, Bonventre JV. Acute kidney injury. Annu Rev Med 67: 293–307, 2016. doi: 10.1146/annurev-med-050214-013407. [DOI] [PMC free article] [PubMed] [Google Scholar]