Abstract
The double-drift stimulus (a drifting Gabor with orthogonal internal motion) generates a large discrepancy between its physical and perceived path. Surprisingly, saccades directed to the double-drift stimulus land along the physical, and not perceived, path (Lisi M, Cavanagh P. Curr Biol 25: 2535−2540, 2015). We asked whether memory-guided saccades exhibited the same dissociation from perception. Participants were asked to keep their gaze centered on a fixation dot while the double-drift stimulus moved back and forth on a linear path in the periphery. The offset of the fixation was the go signal to make a saccade to the target. In the visually guided saccade condition, the Gabor kept moving on its trajectory after the go signal but was removed once the saccade began. In the memory conditions, the Gabor disappeared before or at the same time as the go-signal (0- to 1,000-ms delay) and participants made a saccade to its remembered location. The results showed that visually guided saccades again targeted the physical rather than the perceived location. However, memory saccades, even with 0-ms delay, had landing positions shifted toward the perceived location. Our result shows that memory- and visually guided saccades are based on different spatial information.
NEW & NOTEWORTHY We compared the effect of a perceptual illusion on two types of saccades, visually guided vs. memory-guided saccades, and found that whereas visually guided saccades were almost unaffected by the perceptual illusion, memory-guided saccades exhibited a strong effect of the illusion. Our result is the first evidence in the literature to show that visually and memory-guided saccades use different spatial representations.
Keywords: memory-guided saccades, visually guided saccades, double-drift illusion, action-perception dissociation
INTRODUCTION
When a single Gabor seen in peripheral vision moves back and forth along a linear trajectory and its internal motion drifts in an orthogonal direction (a double-drift stimulus), the perceived orientation of the path can deviate by 45° or more from its physical path (Kwonet al. 2015; Lisi and Cavanagh 2015; Shapiro et al. 2010; Tse and Hsieh 2006; see Fig. 1). This double-drift illusion thus exhibits a very large distortion between the physical and perceived paths. Recently, Lisi and Cavanagh (2015) found that saccadic eye movements directed to the double-drift stimulus targeted locations along their physical rather than perceived trajectories, providing strong evidence for a dissociation between perception and saccadic eye movements. In the current study, we asked if memory-guided saccades would exhibit the same dissociation from perception.
Fig. 1.
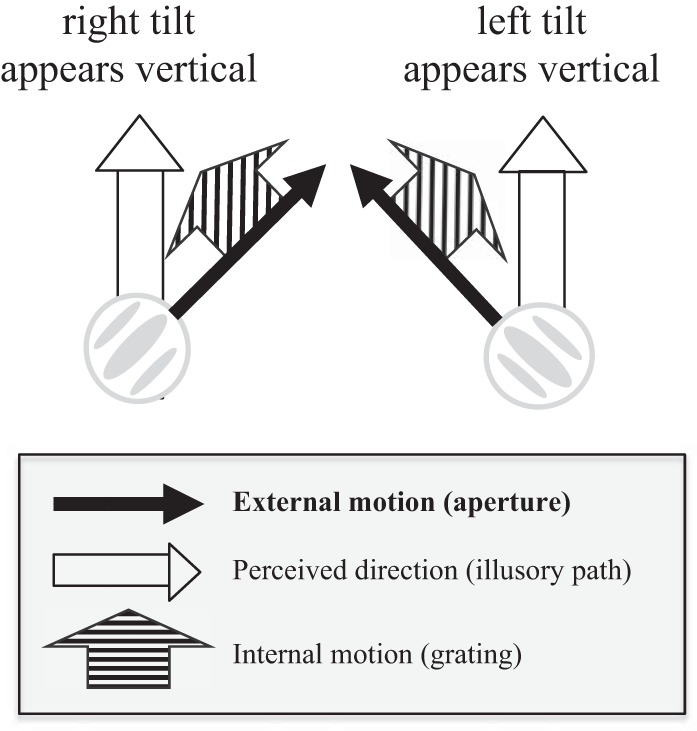
The two double-drift stimuli with tilted paths that appear vertical due to the addition of internal motion.
Although the explanatory mechanisms of the double-drift illusion are still not completely clear, a common idea is that the two motion vectors, the external direction of the aperture and the internal direction of the sinewave carrier, combine to produce an illusory direction. The apparent location of the stimulus is then extrapolated along this illusory direction, shifting farther and farther away from the physical location. According to Lisi and Cavanagh (2015, 2017) the different responses of saccades and perception to the double-drift stimulus result from the differences in the temporal interval over which this motion-induced position error accumulates: in perception it would accumulate over a long interval (possibly up to 1,500 ms), whereas in the saccadic system the extrapolation is thought to cover a much shorter temporal interval, no longer than the latency of the saccade (de Brouwer et al. 2002; Etchells et al. 2010), resulting in a smaller position error.
The difference between the saccade and perceptual results might be attributed to the difference in response modes: in the initial experiment (Lisi and Cavanagh 2015), the perceptual effect was measured as a change in motion direction, whereas the saccade required an action toward a position target. However, Lisi and Cavanagh (2015) demonstrated in a second experiment that the perceptual effect was indeed based on a position shift and then also showed (Lisi and Cavanagh 2017) that the lack of effect in the saccade case was not a general loss for any action toward the target position: pointing responses were significantly more influenced by the illusion than saccades. There appears to be something specific to the rapid programming of saccades that limits the time window over which the past sensory history influences the estimate of target location.
The study by Lisi and Cavanagh (2015) focused on interceptive, visually guided saccades, leaving open the question of what would happen when there is no current input available as in the case of a memory saccade (i.e., the target is removed from view before the action is initiated). Memory-guided saccades rely on information stored in memory to guide the eyes toward the remembered location when there is no visual stimulus. Movements directed to a remembered location of an object do show differences in dynamics and accuracy compared with visually guided saccades (Becker and Fuchs 1969; Gnadt et al. 1991; Smit et al. 1987; White et al. 1994). Furthermore, the neural systems generating saccades to remembered locations are to some degree independent from those generating visually guided saccades (e.g., Funahashi et al. 1989; Hikosaka and Wurtz 1985).
Wong and Mack (1981) were the first to hypothesize that saccade programming could be based on perceptual coordinates (which may differ from retinal coordinates in some instances), but only for position information stored in memory. The underlying assumption is that memory for visual location is encoded in perceptual coordinates and that when saccades are memory-guided, the saccadic target has no simultaneous conflicting, retinal information. Wong and Mack never tested their hypothesis, but there is supporting evidence from experiments with grasping movements. For example, Westwood and Goodale (2003) used a size-contrast illusion to assess the contribution of perceptual mechanisms to the control of visually guided and memory-guided grasping movements. They found that the peak grip aperture was less affected by the perceptual size illusion when the target array was visible compared with when the target array was occluded from view. They argued that perceptual mechanisms are necessary for the control of memory-guided action. According to them, this is because the dedicated visuomotor mechanisms of the dorsal stream require direct visual input and have only a brief memory. When an action is memory-guided, its control must access a stored representation of the target, and this stored representation cannot be provided by the visuomotor mechanisms in the dorsal pathway. Thus the stored representation available for the delayed grasp would be provided by the perceptual mechanisms in the ventral pathway, that is, the very mechanisms that lead to perception (see also Goodale et al. 1994; Post and Welch 1996; Hu et al. 1999; Westwood et al. 2000a, 2000b; for a review see Carey 2001; for an alternative point of view see Franz et al. 2000).
Together, these results suggest visually guided and memory-guided actions may not rely on the same sources of information. Two studies have tested this hypothesis in the context of saccadic eye movements using the Müller-Lyer illusion (de Brouwer et al. 2014, 2016). In these studies, de Brouwer and colleagues found no difference in the size of the illusion between memory-guided (0.8-s delay) and visually guided saccades to a briefly presented Müller-Lyer figure. They later confirmed this result with the duration of the delay increasing from 0 to 1.8 s. From their results they suggested that the absence of an increase in illusion effects on memory-guided saccades suggests that the same representation is used, independently of any delay. This is reasonable given that there is no proposal that the representation of the Müller-Lyer figure would be changing over time other than through the inevitable degradation of precision with delay.
The evidence that visually and memory-guided saccades use the same spatial representations of the target can best be challenged using a changing stimulus, one that may reveal different extents of temporal integration for visual and memory representations. To this aim, we conducted an experiment similar to the one carried out by Lisi and Cavanagh (2015) with the addition of a memory delay between the disappearance of the stimulus and the go signal to execute the saccade. Participants thus had to execute the saccades toward the remembered location of the double-drift stimulus. We tested different memory delay durations. As a control, our experiment also included trials without a memory delay in which saccades were visually guided. Our hypothesis was that the distribution of landing positions for visually guided saccades would be aligned with the physical path (as shown by Lisi and Cavanagh 2015), whereas for memory-guided saccades, landing positions would be more aligned with the perceived path.
METHODS
Participants
Participants were 10 volunteers (6 women, including 1 author; mean age = 27.2 yr, SD = 6.7 yr). All observers reported having normal or corrected-to-normal vision. Informed consent was obtained in writing in before participation, and the protocol for the study was approved by the Université Paris Descartes Review Board, CERES, in accordance with French regulations and the Declaration of Helsinki. All (except the author) were naive to the specific purpose of the experiment.
Setup
Participants sat in a quiet, dark room. We recorded the right-eye gaze position with an SR Research Eyelink 1000 desktop-mounted eye tracker, at a sampling rate of 1 kHz. Participant’s head was positioned on a chin rest, with an adjustable forehead rest, 54 cm in front of a gamma linearized Compaq P1220 CRT screen (vertical refresh rate 120 Hz) that was used to present stimuli. An Apple computer running MATLAB (MathWorks) with the Psychophysics and Eyelink toolboxes (Brainard 1997; Cornelissen et al. 2002; Pelli 1997) controlled stimulus presentation and response collection.
Stimuli
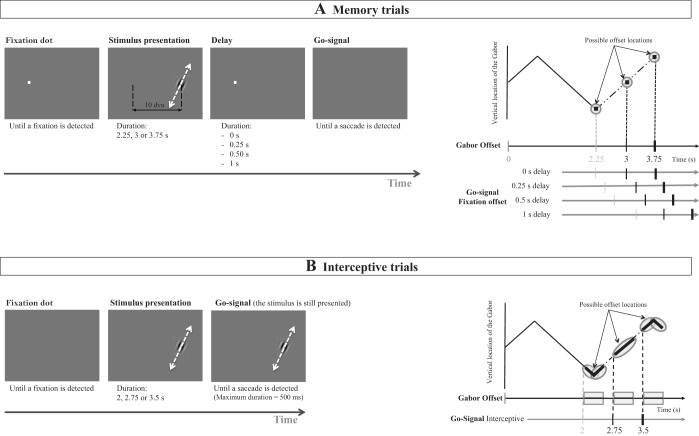
In both the perceptual and saccade conditions, the stimulus was a Gabor pattern (sinusoidal luminance modulations within a Gaussian contrast envelope) with a spatial frequency of 2 cycles/dva (cycles per degree of visual angle) and 100% contrast. The standard deviation of the contrast envelope was 0.1 dva. The Gabor moved back and forth along a linear path 3 dva in length, with a speed of 2 dva/s (external motion). The sinusoidal grating had the same orientation as the motion path and drifted in an orthogonal direction with a temporal frequency of 3Hz and a speed of 1.5 dva/s (internal motion), reversing its direction in synchrony with path reversals at the two end points (every 1.5 s). The combination of internal and external motion can make a tilted path appear vertical (see Fig. 1): a right-tilted path can appear vertical if the internal motion is to the left while the Gabor moves upward (and to the right when it moves downward), and vice versa for a left-tilted path (see Movie S1 in Lisi and Cavanagh 2015). The stimulus was presented on a uniform gray background (5.3 cd/m2), and the midpoint of the trajectory was placed at 10 dva from fixation to the right on the horizontal midline (see Fig. 2).
Fig. 2.
Procedure used in the memory (A) or interceptive (B) trials. Left, the general procedure. In memory trials, the stimulus had already disappeared at the time when the go signal was given (i.e., the removal of the fixation point), whereas in the interceptive saccade trials, the stimulus was still present. Right, the vertical location of the target as a function of stimulus time presentation. In memory trials, the stimulus could be presented for 2.25, 3, or 3.75 s, leading to 3 possible offset locations: the 2 extremities or the center of the path. Following Gabor offset, the go- signal was given after a delay varying from 0 to 1 s. In interceptive trials, the stimulus remained presented for 500 ms after the go signal. Participants thus had 500 ms to initiate their saccades to intercept the stimulus. As soon as the saccade was detected, the Gabor was removed, and this could happen at any point in time during the 500-ms interval. The go signal was given 250 ms before the Gabor reached 1 of the 2 extremities or the center of the path. The Gabor was exactly at 1of these 3 possible locations when participants initiated their saccades with a latency of 250 ms.
Part 1: Perceptual Task
Procedure and design.
The aim of the perceptual task was to measure the orientation of the Gabor’s physical path that was perceived as vertical for each participant. We used the same perceptual task as Lisi and Cavanagh (2015). We presented Gabor patterns moving along paths with different orientations, and participants were asked to judge the left/right tilt of the motion path. The stimulus was displayed until participants provided a response by pressing on the left or right arrow key. Gaze position was recorded and monitored online with the eye tracker, and trials in which the participant shifted gaze away from the fixation point or blinked before giving the response were immediately aborted and repeated at the end of the block. The physical orientation of the path was adjusted by means of multiple interleaved QUEST staircases (Watson and Pelli 1983) that converged to a 50% proportion of “right” tilt responses. Trials with left and right tilt were randomly interleaved. Each participant performed 2 sessions of 240 trials each, divided into 6 blocks.
Data analysis.
For each participant and condition, the point of subjective verticality of the physical trajectory was computed as the orientation corresponding to the 0.5 level of a cumulative Gaussian psychometric function, fitted by maximum likelihood on the proportion of “right” tilt responses (i.e., the orientation that would yield 50% “left” and 50% “right” tilt responses).
We thus obtained for each participant the physical left-tilted and right-tilted orientations of the Gabor’s physical path that were perceived as vertical.
Part 2: Saccade Task
Procedure and design.
The aim of the saccade task was to measure the influence of the removal of the Gabor stimulus before the execution of the saccade (memory conditions) on the landing position of the first saccade. The saccade task comprised five sessions. Among the five sessions: 1) four contained memory trials in which participants were asked to saccade to the last seen position of the target and where the Gabor disappeared at the same time as (delay 0 s) or before (delays 0.25, 0.5, and 1 s) the fixation offset, and 2) one contained visually guided (i.e., interceptive) trials in which participants were asked to saccade to the moving Gabor (i.e., to intercept it) and where the Gabor remained present and in motion after fixation offset until a saccade was detected (and for a maximum duration of 500 ms). The exact procedures used for memory and interceptive trials are detailed in the next two paragraphs and in Fig. 2. Each delay was presented in a separate session. The order of the five sessions was counterbalanced across participants using a Latin square. Each session lasted on1 h and included 480 trials divided into 10 blocks. However, note that we also ran a control experiment with interleaved memory- and visually guided trials to ensure that the presentation of the different delays in separate sessions had no influence in the results obtained (see appendix a).
In the saccade task, each participant was presented only the orientations of the motion path that corresponded to perceived verticality of the motion path (as measured in the perceptual task). In each block, the orientation of the physical path could be right-tilted or left-tilted and the internal motion could be absent (control condition) or present (double-drift condition): this yielded a total of 120 repetitions per condition. The different conditions were randomly interleaved in each block. During the saccade task, gaze position was recorded at 1 kHz and monitored online; trials in which participants shifted gaze or blinked before the disappearance of the fixation dot were aborted and repeated within the same block.
memory trials.
In the four blocks of memory-guided saccade trials, each trial started when the participant fixated on a black dot (a circle of 0.2 dva in diameter). The position of the fixation dot was jittered horizontally and vertically from trial to trial according to two Gaussian distributions (SD = 0.2 dva) centered on (−4, 0) relative to screen center. After a random interval of 400–600 ms, the Gabor appeared in the central position of its motion path, 10 dva to the right of the fixation point, and started moving upward or downward. During stimulus presentation, the fixation dot remained on the screen and participants were asked to keep their eyes on it. The Gabor drifted for 2.25, 3, or 3.75 s, leading to three possible offset locations: the two extremities or the center of the path (see Fig. 2A, right). Participants were then asked to saccade to this offset location (i.e., to the position where the Gabor target was last seen and removed) as soon as the go signal instructed them to do so, 0, 250, 500, or 1,000 ms later. The go signal was the removal of the fixation point. Each delay was presented in a separate session. In all conditions, the actual delay between go signal and saccade was the sum of the experimenter-defined delay and the saccade latency on that trial (mean latency in the memory trials = 215 ms, SD across participants = 46 ms). In zero-delay condition, the actual delay was therefore equal to saccade latency. The general procedure used for the memory trials is summarized in Fig. 2A.
interceptive trials.
The procedure (summarized in Fig. 2B) was almost identical to that for the memory-guided saccade trials, except that the Gabor did not disappear before or concurrently with the go signal. Instead, the go signal was given and the Gabor continued drifting until gaze position was detected outside a circular area with 2 dva of the radius around the fixation and for a maximum duration of 500 ms. Participants thus had 500 ms to initiate their saccades to intercept the stimulus. As soon as the saccade was detected, the Gabor was removed, and this could happen at any point in time during the 500-ms interval. The go signal was given 250 ms before the Gabor reached one of the two end points of its path or the center of the path so that the Gabor was exactly at one of these three possible locations when participants initiated their saccades with a latency of 250 ms. The 250-ms value had been chosen a priori to approximately match the mean saccade latency of the participants in this session. We observed a posteriori that it was an appropriate estimation (mean across participants = 249 ms; SD across participants = 35 ms). This was done to have a duration of presentation of the Gabor in the interceptive trials that corresponds on average to the duration of presentation in the memory condition and thus have interceptive saccades targeting approximately the same locations as in memory trials (see Fig. 2, right). As soon as a saccade was detected, the Gabor was removed so that participants received no feedback about the accuracy of their saccades. Participants were instructed to execute a saccade as soon as the fixation point disappeared, to intercept the moving Gabor.
Data analysis.
The eye-position signal was reanalyzed offline using a saccade-fixation algorithm based on two-dimensional eye velocity (Engbert and Mergenthaler 2006). Only the first saccade that followed the go signal (i.e., the disappearance of the fixation dot) was considered for analysis. Trials were excluded from the analysis when no saccade was detected after the go signal, a blink occurred before the saccade, the first saccade had an amplitude less than 1°, an eye-tracker sampling error occurred, or saccade latency was shorter than 100 ms or longer than 500 ms. This resulted in a rejection of 13.3% of the trials.
saccade amplitude.
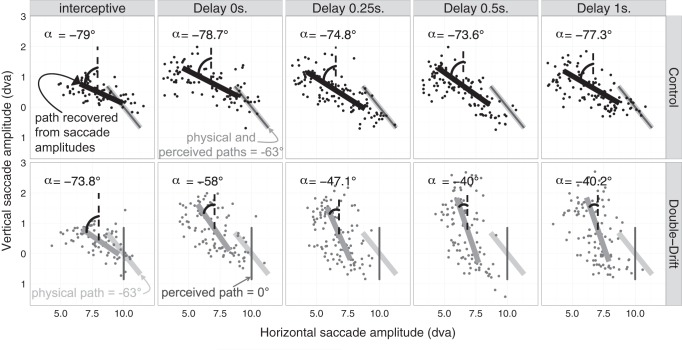
We analyzed horizontal and vertical saccade amplitudes (the differences in the horizontal and vertical coordinates of saccade offset and onset positions) to recover the landing position of the saccades in each condition. For each participant we fitted a multivariate linear model with the horizontal and vertical saccade amplitudes as dependent variables (e.g., see Fig. 3). The models included as linear predictors the horizontal and vertical coordinates of the Gabor at the moment of its disappearance (i.e., the offset location of the Gabor) together with the condition (with vs. without internal motion) and the interactions between condition and Gabor coordinates. We fitted this multivariate model for each participant, each delay, and each orientation of the physical path and then used the fitted model to generate horizontal and vertical amplitudes of saccades for all points along the path of the Gabor. We then computed a linear regression of the vertical on the horizontal predicted saccade amplitudes and derived the angle of deviation from vertical from the regression slope (e.g., see Fig. 3). We used this two-step approach because separating the noise in the vertical vs. horizontal dimensions gives a better match to the typically larger variability of saccade landings along the radial than tangential axis (Deubel 1987; van Opstal and van Gisbergen 1989). Finally, the difference between the angle of the recovered path in the control condition and the double-drift condition was calculated for each participant and each delay, but independently of the right vs. left orientation of the tilt, because, as revealed by an analysis of variance (ANOVA) with a 2 (orientation of the tilt) × 5 (delay) within-subject design, there was no significant difference between the two orientations [left-tilted: mean = 16.7°, SE = 1.9°; right-tilted: mean = 18.9°, SE = 2.03°; F(1,9) = 2.42, P = 0.15] and no interaction between the orientation of the tilt and delay [F(4,36) = 1.47, P = 0.23]. Thus we first calculated the mean difference between the control and double-drift condition for the left- and right-tilted paths and then averaged the two values for each participant and each delay. This difference was taken as a dependent variable in the statistical analyses; the larger the difference, the larger the effect of the internal motion on the orientation of the trajectory targeted by the saccades.
Fig. 3.
Horizontal and vertical saccade amplitudes for one representative participant are plotted along with the fitted value of the multivariate linear model. Top, results for the control condition (with no internal motion); bottom, results for the double-drift condition (where the physical path was tilted to the left and the perceived path was vertical). Panels from left to right correspond to the different delays starting with the interceptive condition. The angle of the deviation of the recovered path from vertical (α) is indicated in each graph. For this participant, the orientation of the double-drift path that appeared vertical in the perceptual test was −63°. This was then the path orientation presented in both the control and double-drift saccade conditions shown. In the control condition, the angle of the recovered path is similar for each delay (varying from −73.6° to −79°) and is relatively close to the real angle of the physical path (−63°). In the double-drift condition, there is a difference between the interceptive condition and the 4 other conditions with a memory delay. In the interceptive condition, the angle of the recovered path (−73.8°) is also close to the angle of the physical path (−63°), whereas in the memory conditions, the angle of the recovered path (varying from −58° to −40°) is closer to vertical.
We performed the following statistical analyses. We first ran a one-way ANOVA with a 5-condition (delay) within-subject design and then tested whether each condition (interceptive, 0-s delay, 0.25-s delay, 0.5-s delay, and 1-s delay) differed from 0 by using five paired t-tests that were corrected for multiple comparisons with a Bonferroni correction (i.e., the P value was multiplied by the number of comparisons; in this case, 5). These five comparisons indicated whether or not the difference between the control and double-drift condition was significant for each delay. Second, we performed a separate analysis for the four memory saccade conditions by testing the effect of the delay. To do this, we ran an ANOVA using a within-subject design including the delay as a continuous factor. Finally, to determine whether the absence of the stimulus during saccade programming was enough to induce a difference between the control and the double-drift condition, we tested the difference between the interceptive and the zero-delay conditions.
saccade latency.
We wanted to ensure that any difference observed on saccade amplitude (and thus on the angle of the recovered path) between the two internal motion conditions (control vs. double-drift) was not due to a difference in latencies. To do so, we ran a two-way ANOVA with a 5 (delay) × 2 (internal motion) within-subject design.
RESULTS
Results of the Perceptual Task
For the 10 participants, the orientations of the physical path that were perceived as vertical strongly deviated from 0 (with 0 corresponding to physical vertical). The mean right tilt that was perceived as vertical was 49.2° (range from 38° to 58°), and the mean left tilt that was perceived as vertical was −57.6° (range from −70° to −42°), revealing a dramatic influence of the internal motion of the perceived orientation of the trajectory.
Results of the Saccade Task
Saccade latency.
The ANOVA revealed an effect of the delay [F(4,36) = 14.23, P < 0.001]. This indicates that there were some differences in saccade latency across the memory delay. In particular, we found that saccade latencies were longer in the interceptive (mean = 249 ms, SE = 7.57 ms) and the zero-delay conditions (mean = 265 ms, SE = 11.21) compared with the other delays (delay 0.25 s: mean = 197 ms, SE = 5.5 ms; delay 0.5 s: mean = 188 ms, SE = 6.5 ms; delay 1 s: mean = 208 ms, SE = 6.1 ms). However, the most important result is that the ANOVA did not reveal any effect of the internal motion [F(1,9) = 4.7, P = 0.06] or interaction between the two [F(4, 36) = 0.49, P = 0.75], thus excluding latency as a potential explanatory factor for any difference between control and double-drift conditions in the distributions of saccadic end points.
Angle of the path recovered from saccade amplitude.
Results obtained in the saccade task are presented in Figs. 3 and 4. Figure 3 presents the results from one representative participant for only one orientation of the tilt (left-tilted path) and shows how the angle of the recovered path evolved with the delay in the two motion conditions: control (without internal motion) vs. double-drift (with internal motion). Figure 4 presents the mean difference across participants between the angle of the recovered path in the control and the double-drift conditions for the different delays. The one-way ANOVA with a 5-condition (delay) within-subject design revealed an effect of the delay [F(4,36) = 6.89, P < 0.001].
Fig. 4.
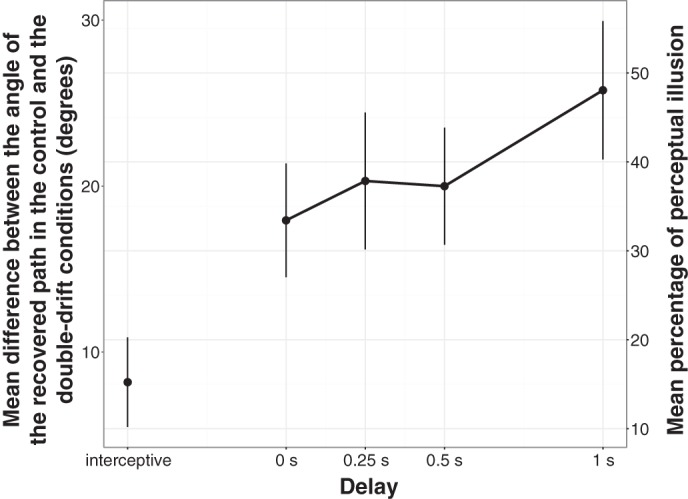
Mean difference between the angle of the recovered path in the control and the double-drift conditions as a function of the delay. The right y-axis shows the result as a percentage of the angular difference between the perceived path and the physical path. A full perceptual illusion would correspond to an average deviation between the 2 paths of 52°. Error bars represent SE.
The difference between the control and the double-drift condition was the smallest in the interceptive condition (7.68° on average, SE = 2.21°). However, it was significant [t(9) = 3.296; Bonferroni corrected P < 0.05], contrary to the findings of Lisi and Cavanagh (2015). Furthermore, the four comparisons that tested whether each delay condition differed from zero were all significant [delay 0 s: mean = 17.9°, SE = 3.78°, t(9) = 4.54; delay 0.25 s: mean = 20.22°, SE = 4.19°, t(9) = 4.51; delay 0.5 s: mean = 19.85°, SE = 3.59°, t(9) = 5.27; delay 1 s: mean = 25.7°, SE = 4.38°, t(9) = 5.56; Bonferroni corrected P values always <0.01), indicating that the control condition differed systematically from the double-drift condition when saccades were memory-guided, regardless of the duration of the memory delay.
Figure 4 also shows that when a memory delay is added before the execution of the saccade, the difference between the control and the double-drift conditions becomes bigger. The planned comparison between the interceptive (mean = 7.68°, SE = 2.21°) and the zero-delay (mean = 17.9°, SE = 3.78°) conditions was significant [t(9) = 2.54, P < 0.05]. Finally, the ANOVA that was run to assess the effect of the delay on the difference between the angle of the recovered path in the control and the double-drift conditions revealed a linear effect of the delay [F(1,9) = 6.6, P < 0.05]. This indicated that the difference between the control and double-drift conditions, and thus the effect of the illusion, increased with delay. Taken together, these results indicate that if the stimulus is absent while the saccade is programmed (delays 0 to 1 s), the saccade landings in the double-drift condition differ from those in the interceptive condition, in the direction of the perceptual illusion. Furthermore, increasing the delay led to a greater deviation in the direction of the illusion.
DISCUSSION
The aim of this study was to compare the effect of the double-drift illusion on two types of saccades: visually guided saccades and memory-guided saccades. Lisi and Cavanagh (2015) recently showed that although the double-drift stimulus leads to a very large discrepancy between its physical and its perceived path, visually guided saccades directed toward it land along the physical, and not the perceived, path. In this study, we asked whether memory-guided saccades would exhibit the same dissociation from perception.
Several arguments support the prediction that whenever a visually guided action is immune from a perception illusion, the corresponding memory-guided action may be influenced by the illusion. Many authors have proposed that memory encodes the perceived location of the stimulus even when this does not correspond to its retinal location (Goodale et al. 1994; Hu et al. 1999; Westwood and Goodale 2003; Wong and Mack 1981). Thus, when the information specifying the position of a target is derived from memory, the eyes should be directed toward its perceived, and not retinal, location. However, this prediction has not been tested for saccades, and this study was designed to fill this gap. We conducted an experiment similar to the one carried out by Lisi and Cavanagh (2015) with the addition of a memory delay between the disappearance of the stimulus and the go signal to execute the saccade. Participants thus had to memorize the offset position of the double-drift during a delay varying from 0 to 1 s and then execute the saccade toward the remembered location where the double-drift stimulus disappeared. The variable delay tested whether the influence of the perceptual illusion, if any, changed with the retention interval. Our experiment also included trials without a memory delay in which participants were instructed to intercept the double-drift; i.e., saccades were visually guided. In this condition, we expected to replicate the results obtained by Lisi and Cavanagh (2015).
Consistent with this general hypothesis, we found a significant difference between visually guided and memory-guided saccades such that visually guided saccades landed closer to the physical path, whereas memory-guided saccades were shifted toward the perceived path, showing on average as much as 48% of the perceptual illusion for the condition with the largest effect. Furthermore, increasing the duration of the delay significantly increased the effect of the illusion (from 33% to 48%). Unexpectedly, visually guided saccades also showed a small effect of the illusion (~13%). The finding of a significant (although small) difference between visually guided saccades targeting control and double-drift stimuli, a 7.68° shift in the direction of the perceptual illusion, contrasts with the absence of a significant difference in Lisi and Cavanagh (2015). This effect is most likely due to the difference in our sampling of path locations. In our experiment, participants were asked to saccade to one of three locations, as opposed to one of six in the previous study, doubling the frequency of sampling points where the constant, nonaccumulating effect of internal motion at saccade onset (Lisi and Cavanagh 2015) could influence the orientation recovered from saccade landings (see appendix b).
In the following, we discuss the larger effect of the illusion for memory-guided saccades compared with visually guided saccades. We argue that the memory trace available to the oculomotor system is of lower accuracy and stability than that available in perceptual memory, explaining why the saccade program may access both to achieve better performance.
The main result of this study is that visually guided saccades differed from memory-guided saccades. Whereas visually guided saccades were much less sensitive to the illusory effect, memory-guided saccades showed a clear effect of the illusion, which was robust and statistically significant in all the delay conditions tested and reached on average 48% of the perceptual effect. Our results are in agreement with the general idea that movement control may be guided by perceptual memory when the target is no longer present (Carey 2001; Goodale et al. 1994; Hu et al. 1999; Post and Welch 1996; Westwood et al. 2000a, 2000b; Wong and Mack 1981). Until now, the evidence for this hypothesis has come from experiments with grasping movements. Our results thus provide evidence that this hypothesis is also valid for saccadic eye movements. Below, we speculate about the neurophysiological mechanisms that could account for our results.
Brain-imaging studies on memory-guided saccades have provided evidence that some neurons show a tonic level of discharge that persists after the offset of the visual target until the saccade is performed and could therefore support saccades to remembered target locations (for reviews, see e.g., Curtis 2006; Mackey et al. 2016). More precisely, neurons that showed persistent delay period activity (i.e., activity in absence of visual stimuli falling within their receptive fields) have been found in a small subset of regions, most notably the lateral intraparietal area (LIP) and the Frontal Eye fields (FEF). All these areas would be necessary for intact spatial working memory. Furthermore, they both have projections to the superior colliculus (SC; for a review, see White and Munoz 2011) so that they can send diverse delay activity signals (including ones related to memory) to the SC, where the signals may be used for saccade generation (e.g., see Sommer and Wurtz 2000). However, the question of what is actually being remembered or coded for by this delay activity still remains unanswered (Curtis 2006). This question is particularly relevant in the case of our experiment in which the target induces a mismatch between veridical (i.e., retinal) and perceived target location.
The present results, showing that memory-guided saccades exhibit an effect of the illusion, suggest that some of the remembered location originates with the perceptual representation of the target. Furthermore, because we found that the effect of the illusion was observed from the shortest delay, 0 ms, this suggests that the switch of spatial representation (between the retinal and the perceived location) is triggered by the absence of the stimulus during saccade programming. One explanation for this transition is that the “oculomotor memory” of the veridical/retinal location may be unreliable;, i.e., it might have a poor precision. Thus saccade-targeting tasks are based on the more veridical oculomotor representation to the extent that retinal information is available (the stimulus is present) when the saccade is initiated. However, as soon as the stimulus disappears, an alternative source of information, the remembered perceptual location is accessed for movement control because it is now more reliable than the oculomotor location memory. This information appears to be rapidly accessible and ready to be used by the saccadic system in agreement with the findings of Westwood et al. (2000b), who found that illusory-size effects on peak grip aperture emerged with extremely brief retention intervals (i.e., 0–450 ms).
Our results also suggest that in addition to being unreliable, the oculomotor memory decays over time as seen in a greater effect of the illusion with increasing delay duration. The decrease of tonic activity seen in oculomotor structures that occurs over the course of several hundred milliseconds after target disappearance might be responsible of these changes in memory saccade accuracy (Edelman and Goldberg 2001). Thus, as the ability of the oculomotor system to keep a memory trace of the veridical target location (i.e., the retinal location) decays over time, the targeting information would rely increasingly on the perceptual memory with longer delays. The absence of a full effect of the illusion even at 1-s delay suggests that the oculomotor memory of the veridical/retinal location decays relatively slowly, which is compatible with the time constant of decaying collicular activity following target disappearance (Edelman and Goldberg 2001). This residual oculomotor information would be combined with information stored in perceptual memory, possibly at the level of premotor areas for eye movements, which are known to be involved in the orienting of spatial attention (Casarotti et al. 2012; Moore and Fallah 2001) and consequently also in spatial working memory (Awh and Jonides 2001).
Conclusions
Overall, these results point to a difference in the spatial representation of the target used to program visually guided saccades as opposed to that used to program memory-guided saccades. Whereas visually guided saccades were almost unaffected by the internal motion of the Gabor, memory-guided saccades showed a bias consistent with the perceptual effect (although with a smaller amplitude). As recently proposed by Lisi and Cavanagh (2017), these results support the idea that there are two distinct spatial representations of the visual world. One map, used to generate visually guided saccadic eye movements, would represent the retinal locations of potential saccadic targets using only recent sensory signals. The other map supports conscious perception and would integrate sensory signals over a much longer temporal interval, producing the accumulating shift that dramatically changes the perceived path. Our present results suggest that the information on this second “perceptual” map can be accessed for memory-guided saccades when there is no retinal input during the programming of the saccade. It is not the sole source of location information, however, because the deviation from the physical path showed on average ~50% of the illusion strength, a value that suggests a mixing of the two representations. We propose that there is a memory of the target location in the saccade system that gets combined with that from the perceptual system for memory-guided saccades. Although we did not directly address this question, it is evident that the saccade system represents space in a mostly retinotopic coordinate frame (Golomb et al. 2008), whereas the perceptual system may use a range of reference frames (Bosco et al. 2015; Chang and Snyder 2010). Our evidence that the two systems combine for memory-guided saccades has implications for the way we conceptualize how the visual and oculomotor systems use different information for guiding actions toward a unified perceptual experience.
GRANTS
The research leading to these results received funding from the European Research Council under the European Union’s Seventh Framework Program (FP7/2007-2013)/ERC Grant Agreement No. AG324070 (to P. Cavanagh) and from the Department of Psychological and Brain Sciences of Dartmouth College.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.M., M.L., T.C., and P.C. conceived and designed research; D.M. performed experiments; D.M. and M.L. analyzed data; D.M., M.L., T.C., and P.C. interpreted results of experiments; D.M. prepared figures; D.M. drafted manuscript; D.M., M.L., T.C., and P.C. edited and revised manuscript; D.M., M.L., T.C., and P.C. approved final version of manuscript.
APPENDIX A: SUPPLEMENTAL EXPERIMENT
The supplemental experiment used the same general procedure as the main experiment but differed on two points. First, there were only two delay conditions: interceptive and 1-s delay. Second, the interceptive and memory trials were no longer presented in separate blocks during the saccade task, but were mixed within blocks.
Methods
Participants.
Participants were five volunteers (4 women, including 1 author; mean age = 28.8 yr, SD = 5.9 yr), three of whom had participated in the main experiment. All observers reported having normal or corrected-to-normal vision. Informed consent was obtained in writing in before participation, and the protocol for the study was approved by the Université Paris Descartes Review Board, CERES, in accordance with French regulations and the Declaration of Helsinki. All (except the author) were naive to the specific purpose of the experiment.
Setup and stimuli.
This supplemental experiment used exactly the same setup and stimuli as the main experiment (see methods, Setup and Stimuli).
Part 1: Perceptual task.
Only the two participants who had not participated in the main experiment performed the perceptual task. For the three other participants, we used the results of the perceptual task they ran for the main experiment to set the physical direction perceived as vertical. The procedure and the data analysis were the same as described in the main text (see methods, Part 1: Perceptual task).
Part 2: Saccade task–mixed design.
procedure and design.
The saccade task comprised interceptive trials and memory trials (1-s delay) randomly interleaved. The procedures used for the two types of trials were identical to the main experiment (for details, see methods, Part 2: Saccade task). The experiment lasted 2 h and included 960 trials divided into 2 sessions of 10 blocks. As in the main experiment, in the saccade task, each participant was presented only the orientations of the motion path that corresponded to perceived verticality of the motion path (as measured in the perceptual task). In each block, the orientation of the physical path could be right-tilted or left-tilted, the internal motion could be absent (control condition) or present (double-drift condition), and the fixation offset could occurs before (interceptive condition) or after (memory condition) the removal of the drifting Gabor. There were 120 repetitions of each condition. The different conditions were randomly interleaved in each block.
Data analysis.
data selection.
We applied the same selection criterion (see methods, Part 2: Saccade task) to the data. This resulted in a rejection of 8.9% of the trials.
saccade amplitude.
As in the main experiment, we fitted a multivariate linear model with the horizontal and vertical saccade amplitudes as dependent variables (for details see methods, Part 2: Saccade task) and then computed a linear regression to derive the angle of the deviation from vertical from the regression slope. Finally, the difference between the angle of the recovered path in the control condition and the double-drift condition was calculated for each participant and each delay, but independently of the right vs. left orientation of the tilt because, as revealed by an ANOVA with a 2 (orientation of the tilt) × 2 (delay) within-subject design, there was no significant difference between the two orientations [left-tilted: mean = 16.9°, SE = 5.25°; right-tilted: mean = 19.48°, SE = 6.58°; F(1,4) = 0.19, P = 0.68] and no interaction between the orientation of the tilt and delay [F(1,4) = 0.52, P = 0.51]. Thus we first calculated the mean difference between the control and double-drift condition for the left- and right-tilted paths and then averaged the two values for each participant and each delay. This difference was taken as a dependent variable in the statistical analyses.
We performed the following statistical analyses. We first ran a one-way ANOVA with a 2-condition (delay) within-subject design and then tested whether each condition (interceptive and 1-s delay) differed from zero by using two paired t-tests that were corrected for multiple comparisons with a Bonferroni correction (i.e., the P value was multiplied by the number of comparisons, in this case, 2). These two comparisons indicated whether or not the difference between the control and double-drift condition was significant for each delay.
saccade latency.
We wanted to ensure that any difference observed on saccade amplitude (and thus on the angle of the recovered path) between the two internal motion conditions (control vs. double-drift) was not due to a difference in latencies. To do so, we ran a two-way ANOVA with a 2 (delay) × 2 (internal motion) within-subject design.
Results
Perceptual task.
For the five participants, the orientations of the physical path that were perceived as vertical strongly deviated from zero (with 0 corresponding to physical vertical). The mean right tilt that was perceived as vertical was 56.2° (range from 50° to 60°), and the mean left tilt that was perceived as vertical was −51.9° (range from −58° to −41°).
Saccade task: mixed design.
saccade latency.
The ANOVA revealed an effect of the delay [F(1,4) = 47.89, P < 0.01]. This indicates that latency differed between the two delays. In particular, we found that saccade latency was longer in the interceptive (mean = 328 ms, SE = 13.6 ms) compared with the 1-s delay (mean = 216 ms, SE = 7.8 ms). However, as in the blocked design, the ANOVA did not reveal any effect of the internal motion [F(1,4) = 0.5, P = 0.52] or interaction between the two [F(1,4) = 0.40, P = 0.55], thus excluding again latency as a potential explanatory factor for any difference between control and double-drift conditions in the distributions of saccadic end points.
angle of the path recovered from saccade amplitude.
Results obtained in the saccade task that used a mixed design are presented in Figs. 5 and 6. Figure 5 presents the results from one representative participant for only one orientation of the tilt (right-tilted path) and shows how the angle of the recovered path differed with the delay in the two motion conditions: control (without internal motion) vs. double-drift (with internal motion). Figure 6 presents the mean difference across participants between the angle of the recovered path in the control and the double-drift conditions for the two different delays.
Fig. 5.
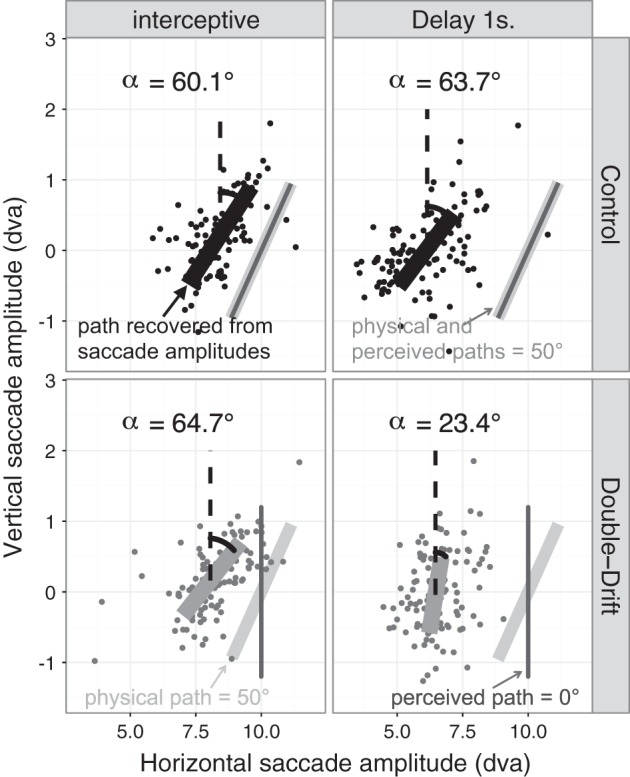
Results for one representative participant in the supplemental experiment that used a mixed design. Horizontal and vertical saccade amplitudes for one participant are plotted along with the fitted value of the multivariate linear model. Top, results for the control condition (with no internal motion); bottom, results for the double-drift condition (where the physical path was tilted to the left and the perceived path was vertical). Left panels correspond to the interceptive condition, and right panels correspond to the 1-s delay. The angle of the deviation of the recovered path from vertical (α) is indicated in each graph. For this participant, the orientation of the double-drift path that appeared vertical in the perceptual test was 50°. In the control condition, the angle of the recovered path is similar for the interceptive condition and the 1-s delay (60.1° and 63.7°, respectively) and is relatively close to the real angle of the physical path (50°). In the double-drift condition, there is a difference between the interceptive condition and the 1-s delay. In the interceptive condition, the angle of the recovered path (64.7°) is also close to the angle of the physical path (50°), whereas in the memory conditions, the angle of the recovered path (23.4°) is closer to vertical.
Fig. 6.
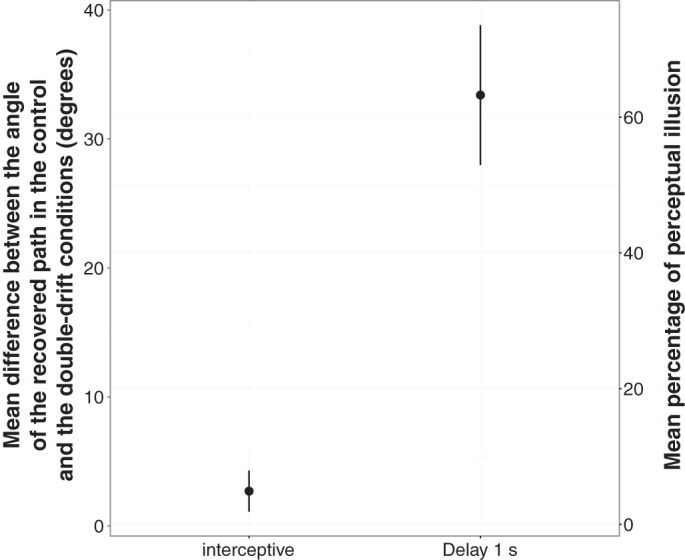
Mean difference between the angle of the recovered path in the control and the double-drift conditions as a function of the delay in the supplemental experience (that used a mixed design). The right y-axis shows the result as a percentage of the angular difference between the perceived path and the physical path. A full perceptual illusion would correspond to an average deviation between the 2 paths of 54°. Error bars represent SE.
The one-way ANOVA with a 2-condition (delay) within-subject design revealed an effect of the delay [F(1,4) = 40.49; P < 0.01].
The difference between the control and the double-drift condition was smaller in the interceptive condition (mean = 2.66°, SE = 1.65°) than in the 1-s delay condition (mean = 33.74°, SE = 5.11°). The first comparison that tested whether the interceptive condition differed from zero was not significant [t(4) = 1.48; Bonferroni corrected P = 0.43]. Nevertheless, the second comparison that tested whether the memory condition (1-s delay) differed from zero was strongly significant [t(4) = 5.99; Bonferroni corrected P < 0.01], indicating that the control condition differed from the double-drift condition when saccades were memory-guided but not when there were visually guided.
Conclusion
The results obtained in the main experiment using a blocked design were confirmed in this supplementary experiment using a mixed design.
APPENDIX B: ABOUT THE EFFECT OF THE ILLUSION ON VISUALLY GUIDED SACCADES
The finding of a small but significant difference between visually guided saccades targeting control and double-drift stimuli contrasts with the absence of a significant difference in Lisi and Cavanagh (2015). In this Appendix, we illustrate how a difference in the experimental paradigm may account for this difference in outcomes.
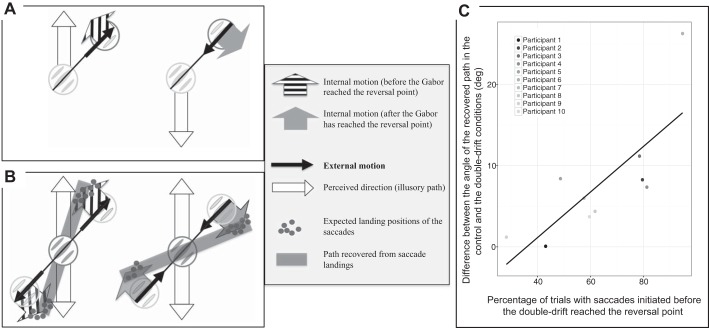
Whereas Lisi and Cavanagh (2015) found no differences in the orientation of the trajectories recovered from saccades made in the control and double-drift conditions, they also found that the landing positions were slightly shifted in the direction of the internal motion but that this local shift did not change or accumulate over time (see Supplemental Material in Lisi and Cavanagh 2015; see also Kerzel and Gegenfurtner 2005). This shift induced by the internal motion is a constant offset but can be in one direction when the Gabor is, say, moving up, and in the opposite direction when it is moving down. If the path is sampled at the ends of the path, this constant offset can bias the orientation of the path. These upper and lower end points of the trajectory correspond to the reversal points where the Gabor and its internal motion reversed their directions. Depending on when the participants initiate their saccades (i.e., before or after the Gabor reached the reversal point), the internal motion can be in two different directions, shifting the landing further or closer from the physical path (see Fig. 7A for an illustration). This shift can thus be in the same or opposite direction of the illusion depending on the timing of the saccade. More precisely, saccade landing positions should be shifted toward the perceived/illusory path when they were initiated before the Gabor reached the reversal point, and in the opposite direction when saccade were initiated after the Gabor has reached the reversal point (see Fig. 7B).
Fig. 7.
A: illustration of the direction of the internal motion as a function of the external motion of the Gabor. Before the Gabor reached the reversal point (left), the internal motion is in direction of the perceived path, i.e., in direction of the illusion. To the contrary, after the Gabor has reached the reversal point (right), the internal motion is in the opposite direction, i.e., away from the illusory path. B: illustration of the bias that occurred in our experiment. Depending on when the saccades were initiated (before or after the Gabor has reached the reversal point; left and right, respectively), the internal motion was in 2 possible directions. Saccade landings (gray points) were thus shifted in direction of the internal motion. This affects in turn the orientation of the path recovered from saccade landings. In our experiment, as participants initiated more often their saccades before the Gabor reached the reversal point (left), it biased the orientation toward the perceived path when triggering saccades only before the upper and lower end points. C: scatter plot representing the difference between the angle of the recovered path in the control and the double-drift conditions as a function of the percentage of trials with saccades initiated before the double-drift reached the reversal point in the visually guided saccades condition. Each dot corresponds to 1 participant. The black line corresponds to the regression line.
In the present experiment, the majority of trials to one or the other of the two end points (66%) were initiated before the Gabor reached the reversal point, biasing the orientation toward the perceived path. If this imbalance is the cause of the small effect found here for visually guided saccades, then the size of this effect should increase across participants with the proportion of saccades that were initiated before the Gabor reached the reversal point. This is what we found (see Fig. 7C): participants who had the stronger effect of the illusion in the visually guided saccades condition were also the participants who executed saccades more often before the Gabor reached the reversal point.
In Lisi and Cavanagh (2015), this issue concerning the reversal points was limited by sampling a larger number of offset locations so that only two of six corresponded to target locations close to the reversal points. In our present experiment, two of three sampled locations were at the end points. The bias was thus two times more evident in our experiment than in that of Lisi and Cavanagh (2015). This could explain why we found a small effect of the illusion for visually guided saccades that was not reported in the previous experiment.
To conclude, the small effect of internal motion on the orientation of the saccade landings is more likely due to our sampling of path locations rather than a change in the representation of the target path for saccades in the direction of the perceptual illusion.
REFERENCES
- Awh E, Jonides J. Overlapping mechanisms of attention and spatial working memory. Trends Cogn Sci 5: 119–126, 2001. doi: 10.1016/S1364-6613(00)01593-X. [DOI] [PubMed] [Google Scholar]
- Becker W, Fuchs AF. Further properties of the human saccadic system: eye movements and correction saccades with and without visual fixation points. Vision Res 9: 1247–1258, 1969. doi: 10.1016/0042-6989(69)90112-6. [DOI] [PubMed] [Google Scholar]
- Bosco A, Breveglieri R, Reser D, Galletti C, Fattori P. Multiple representation of reaching space in the medial posterior parietal area V6A. Cereb Cortex 25: 1654–1667, 2015. doi: 10.1093/cercor/bht420. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spat Vis 10: 433–436, 1997. doi: 10.1163/156856897X00357. [DOI] [PubMed] [Google Scholar]
- Carey DP. Do action systems resist visual illusions? Trends Cogn Sci 5: 109–113, 2001. doi: 10.1016/S1364-6613(00)01592-8. [DOI] [PubMed] [Google Scholar]
- Casarotti M, Lisi M, Umiltà C, Zorzi M. Paying attention through eye movements: a computational investigation of the premotor theory of spatial attention. J Cogn Neurosci 24: 1519–1531, 2012. doi: 10.1162/jocn_a_00231. [DOI] [PubMed] [Google Scholar]
- Chang SW, Snyder LH. Idiosyncratic and systematic aspects of spatial representations in the macaque parietal cortex. Proc Natl Acad Sci USA 107: 7951–7956, 2010. doi: 10.1073/pnas.0913209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen FW, Peters EM, Palmer J. The Eyelink Toolbox: eye tracking with MATLAB and the Psychophysics Toolbox. Behav Res Methods Instrum Comput 34: 613–617, 2002. doi: 10.3758/BF03195489. [DOI] [PubMed] [Google Scholar]
- Curtis CE. Prefrontal and parietal contributions to spatial working memory. Neuroscience 139: 173–180, 2006. doi: 10.1016/j.neuroscience.2005.04.070. [DOI] [PubMed] [Google Scholar]
- de Brouwer AJ, Brenner E, Medendorp WP, Smeets JB. Time course of the effect of the Muller-Lyer illusion on saccades and perceptual judgments. J Vis 14: 4, 2014. doi: 10.1167/14.1.4. [DOI] [PubMed] [Google Scholar]
- de Brouwer AJ, Brenner E, Smeets JB. Keeping a target in memory does not increase the effect of the Müller-Lyer illusion on saccades. Exp Brain Res 234: 977–983, 2016. doi: 10.1007/s00221-015-4520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brouwer S, Missal M, Barnes G, Lefèvre P. Quantitative analysis of catch-up saccades during sustained pursuit. J Neurophysiol 87: 1772–1780, 2002. doi: 10.1152/jn.00621.2001. [DOI] [PubMed] [Google Scholar]
- Deubel H. Adaptivity of gain and direction in oblique saccades. In: Eye Movements: From Physiology to Cognition, edited by O’Regan JK and Levy-Schoen A. Amsterdam: Elsevier Science, 1987. [Google Scholar]
- Edelman JA, Goldberg ME. Dependence of saccade-related activity in the primate superior colliculus on visual target presence. J Neurophysiol 86: 676–691, 2001. [DOI] [PubMed] [Google Scholar]
- Engbert R, Mergenthaler K. Microsaccades are triggered by low retinal image slip. Proc Natl Acad Sci USA 103: 7192–7197, 2006. doi: 10.1073/pnas.0509557103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchells PJ, Benton CP, Ludwig CJ, Gilchrist ID. The target velocity integration function for saccades. J Vis 10: 7, 2010. doi: 10.1167/10.6.7. [DOI] [PubMed] [Google Scholar]
- Franz VH, Gegenfurtner KR, Bülthoff HH, Fahle M. Grasping visual illusions: no evidence for a dissociation between perception and action. Psychol Sci 11: 20–25, 2000. doi: 10.1111/1467-9280.00209. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol 61: 331–349, 1989. [DOI] [PubMed] [Google Scholar]
- Gnadt JW, Bracewell RM, Andersen RA. Sensorimotor transformation during eye movements to remembered visual targets. Vision Res 31: 693–715, 1991. doi: 10.1016/0042-6989(91)90010-3. [DOI] [PubMed] [Google Scholar]
- Golomb JD, Chun MM, Mazer JA. The native coordinate system of spatial attention is retinotopic. J Neurosci 28: 10654–10662, 2008. doi: 10.1523/JNEUROSCI.2525-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodale MA, Jakobson LS, Keillor JM. Differences in the visual control of pantomimed and natural grasping movements. Neuropsychologia 32: 1159–1178, 1994. doi: 10.1016/0028-3932(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. J Neurophysiol 53: 266–291, 1985. [DOI] [PubMed] [Google Scholar]
- Hu Y, Eagleson R, Goodale MA. The effects of delay on the kinematics of grasping. Exp Brain Res 126: 109–116, 1999. doi: 10.1007/s002210050720. [DOI] [PubMed] [Google Scholar]
- Kerzel D, Gegenfurtner KR. Motion-induced illusory displacement reexamined: differences between perception and action? Exp Brain Res 162: 191–201, 2005. doi: 10.1007/s00221-004-2139-z. [DOI] [PubMed] [Google Scholar]
- Kwon OS, Tadin D, Knill DC. Unifying account of visual motion and position perception. Proc Natl Acad Sci USA 112: 8142–8147, 2015. doi: 10.1073/pnas.1500361112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisi M, Cavanagh P. Dissociation between the perceptual and saccadic localization of moving objects. Curr Biol 25: 2535–2540, 2015. doi: 10.1016/j.cub.2015.08.021. [DOI] [PubMed] [Google Scholar]
- Lisi M, Cavanagh P. Different spatial representations guide eye and hand movements. J Vis 17: 12, 2017. doi: 10.1167/17.2.12. [DOI] [PubMed] [Google Scholar]
- Mackey WE, Devinsky O, Doyle WK, Meager MR, Curtis CE. Human dorsolateral prefrontal cortex is not necessary for spatial working memory. J Neurosci 36: 2847–2856, 2016. doi: 10.1523/JNEUROSCI.3618-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Fallah M. Control of eye movements and spatial attention. Proc Natl Acad Sci USA 98: 1273–1276, 2001. doi: 10.1073/pnas.98.3.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10: 437–442, 1997. doi: 10.1163/156856897X00366. [DOI] [PubMed] [Google Scholar]
- Post RB, Welch RB. Is there dissociation of perceptual and motor responses to figural illusions? Perception 25: 569–581, 1996. doi: 10.1068/p250569. [DOI] [PubMed] [Google Scholar]
- Shapiro A, Lu Z-L, Huang C-B, Knight E, Ennis R. Transitions between central and peripheral vision create spatial/temporal distortions: a hypothesis concerning the perceived break of the curveball. PLoS One 5: e13296, 2010. doi: 10.1371/journal.pone.0013296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit AC, Van Gisbergen JAM, Cools AR. A parametric analysis of human saccades in different experimental paradigms. Vision Res 27: 1745–1762, 1987. doi: 10.1016/0042-6989(87)90104-0. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Composition and topographic organization of signals sent from the frontal eye field to the superior colliculus. J Neurophysiol 83: 1979–2001, 2000. [DOI] [PubMed] [Google Scholar]
- Tse PU, Hsieh PJ. The infinite regress illusion reveals faulty integration of local and global motion signals. Vision Res 46: 3881–3885, 2006. doi: 10.1016/j.visres.2006.06.010. [DOI] [PubMed] [Google Scholar]
- van Opstal AJ, van Gisbergen JA. Scatter in the metrics of saccades and properties of the collicular motor map. Vision Res 29: 1183–1196, 1989. doi: 10.1016/0042-6989(89)90064-3. [DOI] [PubMed] [Google Scholar]
- Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Percept Psychophys 33: 113–120, 1983. doi: 10.3758/BF03202828. [DOI] [PubMed] [Google Scholar]
- Westwood DA, Chapman CD, Roy EA. Pantomimed actions may be controlled by the ventral visual stream. Exp Brain Res 130: 545–548, 2000a. doi: 10.1007/s002219900287. [DOI] [PubMed] [Google Scholar]
- Westwood DA, Goodale MA. Perceptual illusion and the real-time control of action. Spat Vis 16: 243–254, 2003. doi: 10.1163/156856803322467518. [DOI] [PubMed] [Google Scholar]
- Westwood DA, Heath M, Roy EA. The effect of a pictorial illusion on closed-loop and open-loop prehension. Exp Brain Res 134: 456–463, 2000b. doi: 10.1007/s002210000489. [DOI] [PubMed] [Google Scholar]
- White BJ, Munoz DP. The superior colliculus. In: Oxford Handbook of Eye Movements, edited by Liversedge SP, Gilchrist ID, Everling S. Oxford: Oxford University Press, 2011, p. 195–213. [Google Scholar]
- White JM, Sparks DL, Stanford TR. Saccades to remembered target locations: an analysis of systematic and variable errors. Vision Res 34: 79–92, 1994. doi: 10.1016/0042-6989(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Wong E, Mack A. Saccadic programming and perceived location. Acta Psychol (Amst) 48: 123–131, 1981. doi: 10.1016/0001-6918(81)90054-8. [DOI] [PubMed] [Google Scholar]