Abstract
Norepinephrine (NE) can dynamically modulate excitability and functional connectivity of neural circuits in response to changes in external and internal states. Regulation by NE has been demonstrated extensively in mammalian sensory cortices, but whether NE-dependent modulation in sensory cortex alters response properties in downstream sensorimotor regions is less clear. Here we examine this question in male zebra finches, a songbird species with complex vocalizations and a well-defined neural network for auditory processing of those vocalizations. We test the hypothesis that NE modulates auditory processing and encoding, using paired extracellular electrophysiology recordings and pattern classifier analyses. We report that a NE infusion into the auditory cortical region NCM (caudomedial nidopallium; analogous to mammalian secondary auditory cortex) enhances the auditory responses, burst firing, and coding properties of single NCM neurons. Furthermore, we report that NE-dependent changes in NCM coding properties, but not auditory response strength, are transmitted downstream to the sensorimotor nucleus HVC. Finally, NE modulation in the NCM of males is qualitatively similar to that observed in females: in both sexes, NE increases auditory response strengths. However, we observed a sex difference in the mechanism of enhancement: whereas NE increases response strength in females by decreasing baseline firing rates, NE increases response strength in males by increasing auditory-evoked activity. Therefore, NE signaling exhibits a compensatory sex difference to achieve a similar, state-dependent enhancement in signal-to-noise ratio and coding accuracy in males and females. In summary, our results provide further evidence for adrenergic regulation of sensory processing and modulation of auditory/sensorimotor functional connectivity.
NEW & NOTEWORTHY This study documents that the catecholamine norepinephrine (also known as noradrenaline) acts in the auditory cortex to shape local processing of complex sound stimuli. Moreover, it also enhances the coding accuracy of neurons in the auditory cortex as well as in the downstream sensorimotor cortex. Finally, this study shows that while the sensory-enhancing effects of norepinephrine are similar in males and females, there are sex differences in the mode of action.
Keywords: birdsong, NCM, neuromodulator, nidopallium, noradrenaline
INTRODUCTION
Neuromodulators can dynamically shift the excitability of neurons and functional connectivity of neural circuits in response to changes in environmental context and internal state (Aston-Jones and Cohen 2005; Bargmann 2012). Norepinephrine (NE), in particular, modulates neural circuits that govern attention, vigilance, and sensory plasticity (Aston-Jones and Bloom 1981; Devilbiss and Waterhouse 2004; Eckmeier and Shea 2014; Hermans et al. 2011; Ikeda et al. 2015; Kasamatsu et al. 1979; Linster and Cleland 2016; Waterhouse and Woodward 1980). Notably, the auditory system is shaped by the actions of NE at all levels, spanning the peripheral and central nervous systems. In mammalian auditory cortex, NE generally enhances the neuronal responses to pure tones and simple calls, as well as the precision tuning of receptive fields (Foote et al. 1975; Hurley et al. 2004; Manunta and Edeline 1999, 2004; Martins and Froemke 2015; Salgado et al. 2011, 2012). However, it is less clear how NE regulates the processing of complex auditory stimuli, and whether this modulation impacts downstream circuits for sensorimotor integration.
Songbirds provide a key neurobiological system to address auditory processing and auditory-motor integration. They have quantifiable and complex vocal behaviors and a well-defined neural network for singing and song processing (Fig. 1, A and B) (Castelino and Schmidt 2010). In zebra finches, only males produce song and the sensorimotor nucleus HVC is larger in males than in females (Nottebohm and Arnold 1976). HVC is critical for song production and is a key node for auditory-motor integration in the songbird brain.
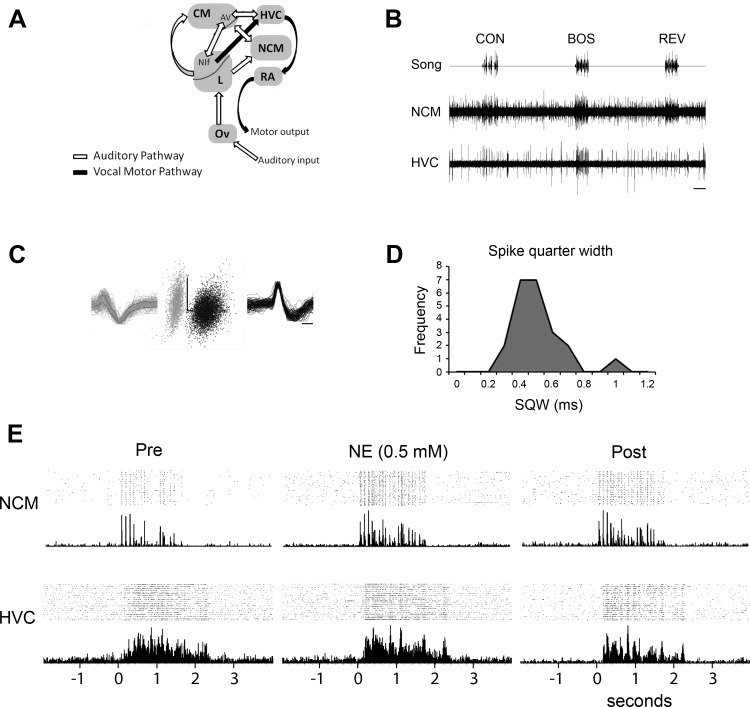
Fig. 1.
Single-unit recording in the auditory and vocal motor pathways of the songbird brain. A: auditory and motor pathways of the song system intersect at the sensorimotor nucleus HVC. B: paired multiunit neural activity from NCM and HVC in response to stimuli (CON, conspecific song; BOS, bird’s own song; REV, reverse BOS). Note the high selectivity for BOS in HVC and the lack of strong selectivity in NCM. C: sorted single units from NCM, with examples of average waveforms and 100-waveform overlays (left and right) and PCA clusters (center). D: neurons in our recorded population of NCM show a unimodal distribution for spike quarter widths (SQW). E: rasters and peristimulus time histograms of activity in NCM and HVC during BOS stimulus playback across 3 retrodialysis treatment conditions. CM, caudal mesopallium; Ov, nucleus ovoidalis; RA, robust nucleus of the arcopallium.
Auditory information first reaches the songbird pallium via the thalamorecipient field L (analogous to mammalian primary auditory cortex). Field L projects to two reciprocally connected regions of the caudal pallium, the caudomedial mesopallium (CMM) and the caudomedial nidopallium (NCM) (analogous to mammalian secondary auditory cortex), which in turn provide auditory signals to nucleus interface (NIf). HVC receives major auditory input from both NIf and CMM (via nucleus avalanche, Av). This ascending interconnected network helps transform the spectral and temporal content of song into sensorimotor-relevant information and motor output (Lewandowski et al. 2013; Schneider and Woolley 2013; Theunissen et al. 2008). Importantly, modulators like NE and acetylcholine can directly regulate the activity of HVC and its synaptic targets (Dave et al. 1998; Shea et al. 2010; Shea and Margoliash 2003). The feedforward architecture of the song network also suggests that modulation of upstream regions, including NCM and NIf, can drive changes in the processing of sensorimotor HVC.
In songbirds, NE has been associated with auditory processing within several nuclei in the song system network (Cardin and Schmidt 2004a; Castelino and Schmidt 2010; Lynch et al. 2012; Pawlisch et al. 2011; Sockman and Salvante 2008; Velho et al. 2012). In particular, infusion of NE into NIf in adult male zebra finches acts in a dose-dependent manner on auditory representations in both NIf and downstream HVC neurons (Cardin and Schmidt 2004a). In NCM, song-responsive neurons express α-adrenergic receptors and auditory-evoked gene induction is disrupted with local α-adrenergic antagonism (Velho et al. 2012). NE infused directly into NCM enhanced local auditory-evoked electrophysiological responses in adult female zebra finches by lowering baseline firing rates. This treatment also enhanced the coding properties of single neurons in NCM in females (Ikeda et al. 2015). Together, these observations lead to the prediction that NE influences activity in the NCM in males, causing changes in activity in downstream regions during auditory processing, sensorimotor integration, and singing. However, it is not known whether NE regulates audition in the NCM of male zebra finches. Another class of neuromodulators, the neuroestrogens, can influence NCM neurons and lead to changes in selective auditory representations of bird’s own song (BOS) in the downstream HVC (Remage-Healey and Joshi 2012). Moreover, the cross-coherence in firing between NCM and HVC is modest but evident (Soyman and Vicario 2017). Taken together, these studies suggest that NE could act in the NCM of male zebra finches to influence the selectivity of auditory-evoked responses downstream in sensorimotor HVC.
In the present study, using anesthetized adult male zebra finches, we test two novel hypotheses: 1) NE changes auditory-evoked activity in NCM similar to its mechanism in female zebra finches and 2) NE infusion in NCM changes auditory responses downstream in HVC. We employ a pattern classifier analysis to assess the influence of NE in NCM on the coding accuracy of single neurons both in NCM and in downstream HVC. Our results show that NE treatment in NCM enhances auditory responses locally and influences the coding properties of neurons downstream in HVC. Enhancement of auditory responses in NCM is similar to that in female zebra finches but occurs by enhancing stimulus-evoked firing rather than decreasing baseline firing. These results demonstrate a clear sex difference in NE-dependent auditory processing in the avian brain and provide further evidence of dynamic NE modulation of functional connectivity between brain regions.
METHODS
Subjects.
Animals were adult male zebra finches (at least 120 days old; total n = 19) from the breeding colony at the University of Massachusetts Amherst (14:10-h light-dark cycle, group housed in mixed-sex aviaries). Spontaneous song bouts were continuously recorded and sorted with the use of Song Analysis Pro for playback stimuli (Cardin and Schmidt 2004a; Remage-Healey and Joshi 2012; Tchernichovski et al. 2000). Subjects were housed individually for up to 24 h (or subsequently with a female companion for up to 48 h if males did not initially produce song) in sound-attenuation chambers until song was recorded. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Massachusetts Amherst.
Surgery.
Before the start of surgical procedures, birds were starved for 30 min to prevent aspiration occlusion during surgery. Craniotomy to expose the caudal forebrain was carried out on birds anesthetized systemically with 30–45 µl of Equithesin (depending on the animal’s weight) and with 10 µl of 2% lidocaine under the scalp. Locations of NCM (1.2 mm rostral, 0.8 mm medial/lateral) and HVC (2.4 mm medial/lateral) were stereotaxically marked on the skull with reference to the caudal bifurcation of the midsagittal sinus. The 1.2 mm skull marking position was used as a rostral border for inserting the probe 200–500 μm caudally in NCM, and the recording electrode was positioned caudally to the probe. A metal head post was secured to the base of the bird’s beak with dental cement to stabilize recordings. A short length of silver wire was also implanted near the base of the skull to serve as a reference ground. Birds were monitored for 24 h after surgery, and experiments were conducted after this recovery interval.
Electrophysiology.
Before electrophysiology recording and retrodialysis, birds were anesthetized with 90–100 µl of 20% urethane (3 successive 30-µl injections with ~40-min interinjection intervals) and a small fenestra of skull and dura mater overlying the regions NCM and HVC was removed. Inside a sound-attenuation chamber (Industrial Acoustics), birds were stabilized on a head post anchor stage and kept warm with a DC heating pad (FHC Neurocraft). A microdialysis probe (CMA 7 with 1-mm membrane; Microdialysis Probe, CMA Microdialysis) prefilled with aCSF (in mM: 199 NaCl, 26.2 NaHCO3, 2.5 KCl, 1 NaH2PO4, 1.3 MgSO4, 2.5 CaCl2, and 11 glucose with 1% BSA, pH 7.4–7.6) was advanced into NCM (~1.4 mm ventral to the brain surface) with a motorized micromanipulator. After a minimum 20-min period after implantation, extracellular electrodes (Carbostar-1) were advanced into NCM and HVC with separate motorized micromanipulators (Warner Instruments). Experiments did not begin until dialysate flow was stabilized at 2.0 µl/min.
Dual multiunit extracellular recordings (Fig. 1B), combined with retrodialysis, from ipsilateral NCM and HVC were obtained. All experiments took place inside a sound-attenuation booth (Industrial Acoustics) on an air table (TMC). Sound stimuli for n = 14 animals included the male’s own song (BOS), reverse BOS (REV), three different conspecific songs (CON1, CON2, CON3), and white noise (WN). All stimuli (~2 s in duration, interstimulus interval 10 ± 2 s) were band-pass filtered (300 Hz to 15 kHz, scientific) and presented in random sequence for 20 repetitions at ~70 dB. The first recording period consisted of 30 min of aCSF retrodialysis to determine pretreatment responsiveness, followed by a period of 30 min of NE (0.5 mM) retrodialysis, followed by a washout period of 30 min of aCSF. For each perfusion solution switch, 20 min of flow was allowed before the start of each recording period to allow delivery and/or washout. In a separate set of control animals (n = 5), aCSF alone was retrodialyzed for three successive periods as above, and animals were presented with four stimuli (CON1, CON2, REV, and WN) as above. All multiunit recordings were amplified, band-pass filtered (300–5,000 Hz; AM Systems), and digitized at 20 kHz (Micro 1401, Spike2 software; Cambridge Electronic Design). At the end of each experimental session, recording sites were lesioned electrolytically for later histological verification.
At the end of electrophysiological recordings, subjects were euthanized and brains were extracted and fixed in a 20% sucrose formalin solution. Brains were then frozen, sectioned sagittally from right to left at a thickness of 40 µm with a cryostat (Leica), and mounted on gelatinized Superfrost glass microscope slides. Mounted sections were Nissl stained and coverslipped before viewing under a microscope for probe and electrolytic lesion confirmation in NCM and HVC. One animal in the separate control experiment received 30 min of retrodialysis of Alexa 488 (0.5 mM; equimolar to the NE used in other experiments) to examine the extent of diffusion/spread from the probe within the auditory forebrain. This animal was immediately perfused after the treatment and processed for brain sectioning as above, and sections were viewed and photographed with a confocal microscope (NA1; Nikon, Tokyo, Japan) with NIS-Elements imaging software (Ar; RRID: SCR_002776).
Data analysis.
With Spike2 software (Cambridge Electronic Design), multiunit recordings were analyzed and sorted into single units by principal components analysis (PCA) (Fig. 1C). Single units were isolated by sorting spikes based on waveform shape in Spike2 and confirmed by both PCA and an interspike interval of >1 ms [average number of spikes per unit assignment = 3,679.5 ± 450.2; for all NCM units 0% of interstimulus intervals (ISIs) occurred in the 1-ms bin]. For each PCA we quantified the J3 statistic, which is expressed as a ratio of the variance within a PCA cluster to the variance between two identified clusters (see, e.g., Friend et al. 2015). Our single units had J3 values ranging from 2.39 to 6.27 (mean ± SE = 4.08 ± 0.64), reflecting excellent isolation in PC space (J3 values > 2 are considered to reflect well-isolated single units; Friend et al. 2015; Pais-Vieira et al. 2013; Wolff et al. 2014). Spike waveform quarter width durations (SQW; the duration of the waveform at 25% of the highest absolute peak value) and peak-to-peak durations were computed from the averaged waveforms. Auditory z scores were calculated for each stimulus and condition to determine selectivity of auditory-evoked activity for each stimulus (Ikeda et al. 2015; Remage-Healey and Joshi 2012). z scores were calculated as the difference between the mean response during the stimulus and the baseline (spontaneous activity preceding playback) periods divided by the standard deviation of the difference between the two conditions (Ikeda et al. 2015; Remage-Healey and Joshi 2012).
Signal-to-noise ratios, a measure of stimulus detection, were also computed as the ratio between the number of spikes per second during the presentation of stimuli and the number of spikes per second during baseline firing (Ikeda et al. 2015; Manunta and Edeline 1999). To further investigate changes in firing state during NE treatment we also evaluated burst firing, with all bursts computed as the occurrence of three or more action potentials separated by ≤10 ms (Kao et al. 2008; Remage-Healey et al. 2010). Stimulus-specific adaptation (SSA), which has been reported in NCM (Chew et al. 1995; Kozlov and Gentner 2014; Ono et al. 2016; Yoder et al. 2015), was also measured by computing the average root mean square in response to BOS of the multiunit traces across stimulus repetitions.
Statistical analyses were run with OriginPro (OriginLab) and Python 3 libraries. Data were analyzed by two-way mixed-effects ANOVA (ME-ANOVA; repeated factor: treatment, nonrepeated factor: stimulus). We reasoned that the factor stimulus should be treated as a nonrepeated factor because of the randomized playback design of our study (i.e., we had no reason to assume that a cell’s responsiveness to one stimulus would be carried over to the other stimuli, particularly within our interleaved playback design). When Mauchly’s sphericity test showed aspherical data we employed a Greenhouse-Geisser correction (GGc), and the corrected degrees of freedom for each test are reflected in results. Tukey tests were used in post hoc comparisons. For single-factor analyses, normality was assessed by Shapiro-Wilk tests followed by Friedman’s tests because normality could not be assumed. Regression analyses were performed and plotted with packages (SciPy, NumPy, Matplotlib) for Python language. Statistical significance was admitted when P < 0.05.
Pattern classifier.
A custom pattern classifier algorithm was coded in Python and used to determine how auditory-evoked spike count and timing of single units contributed to stimulus discrimination in our neuron population (Caras et al. 2015). For each neuron, responses to the six different stimuli were compared iteratively. At the start of each run of the classifier, one trial of each stimulus was pseudorandomly selected as template (6 templates). The other 19 trials of each stimulus (114 trials total) were compared one at a time to the templates with the two similarity measures described below. This procedure was repeated 1,000 times to generate confusion matrices (see Fig. 5), in which the diagonal band represents the classification accuracy.
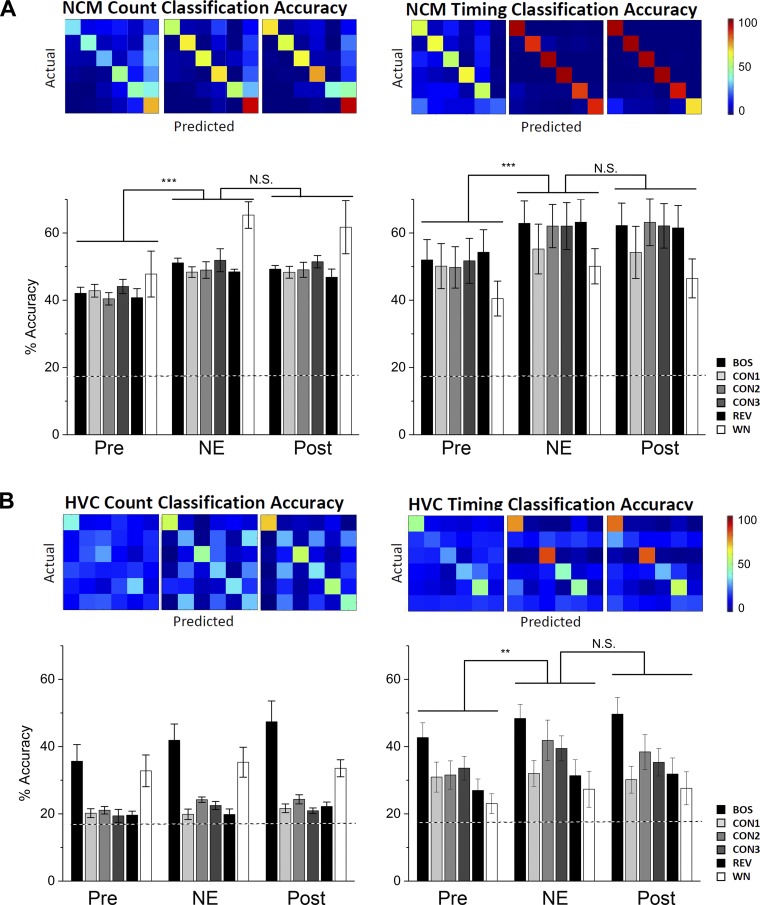
Fig. 5.
Pattern classification accuracy in NCM and HVC are altered by NE treatment in NCM. Representative confusion matrices depict the relative match between the presented stimulus on the vertical axis (“actual”) and the stimulus as predicted by the algorithm (“predicted”) on the horizontal axis. Data are means ± SE. **P < 0.01, ***P < 0.001. N.S., not significant. A: NE treatment in NCM increased count and timing accuracy in NCM. B: NE treatment in NCM increased timing accuracy in HVC and tended to increase HVC count accuracy. Dotted lines show chance level for classifier accuracy (i.e., randomized trial accuracy for 1 of 6 stimuli = 16.7%).
Spike count accuracy was determined by comparing the number of spikes during the stimulus presentation. Spikes were binned before comparison, and the optimal bin size was employed as a variable for each cell, i.e., the classifier was run 1,000 times with each bin size (1, 2, 4, 8, 16, 32, 64, 128, 256, 512, 1,024, 1,700 ms; the latter was the total duration of the shortest stimulus used) and the one that yielded the highest accuracy was selected. When a template was compared to a trial, spike counts were subtracted bin by bin and the distances were squared and summed to provide a total distance measure. The stimulus type of the template that yielded the shortest distance was considered the predicted stimulus for the trial in analysis. Ties were resolved pseudorandomly.
Spike timing accuracy was determined by correlating Gaussian-filtered signals during stimulus presentation. The standard deviation (σ) of the filter was employed as a variable for each cell, i.e., the classifier was run 1,000 times with each σ value of 1, 2, 4, 8, 16, 32, 64, 128, and 256 ms. The filter that yielded the highest accuracy score was used for that cell. For HVC units, only accuracy to BOS was used for the calculation of best σ, while for NCM all stimuli were averaged (this was also true for the best bin calculations in the count classifier). Templates and trials were correlated with the Rcorr method (Caras et al. 2015; Schreiber et al. 2003):
where represents the vectors of the trial and the template responses after filtering, which are dot-multiplied and then divided by the product of their lengths. This calculation returns a value between 0 and 1, which represents total dissimilarity or total similarity, respectively. The stimulus type of the template that provided the highest Rcorr (trial, template) value was considered the predicted stimulus for the trial in analysis. Therefore, percent accuracy scores were generated by how well the firing pattern, either spike count or timing, of each individual cell was predictive of the auditory stimulus. Figure 5 shows confusion matrices in which percent accuracy is presented in terms of predicted and actual stimulus classification in a heat map. In a confusion matrix, values along the diagonal represent an overall accuracy score.
RESULTS
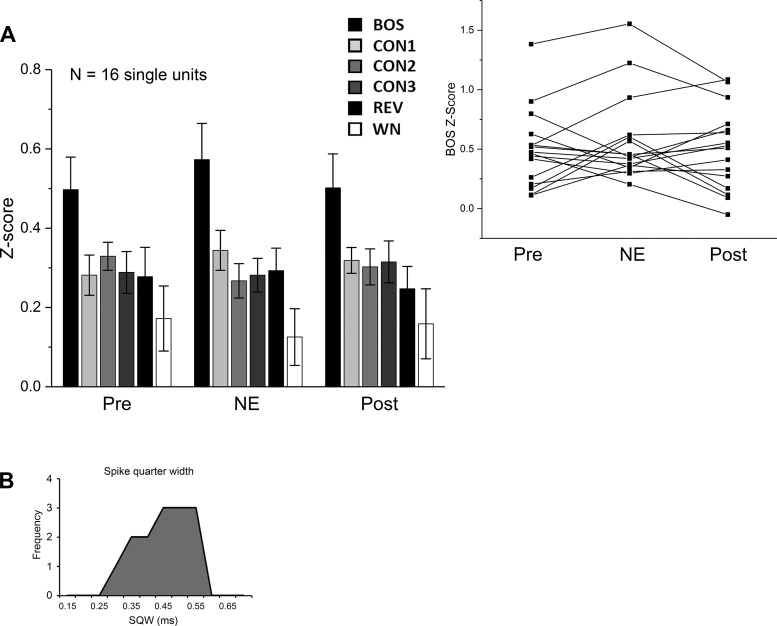
Experiments were conducted on 14 male zebra finches (11 recordings were obtained from NCM sites, and 8 were obtained from HVC sites). With Spike2 single-unit sorting and PCA criteria, high-amplitude spiking activity from 22 single units in NCM and 16 single units in HVC met our criteria for inclusion. ISIs had 0% of NCM spikes and 0.043% of HVC spikes occurring in the first millisecond (i.e., within the refractory period). The distribution of SQW for our NCM single units was unimodal (Fig. 1D), as was the distribution of peak-to-peak durations (data not illustrated). The distribution of SQW for our HVC single units was also unimodal (see Fig. 4B). As in our previous study with females (Ikeda et al. 2015), most subjects were tested in the left hemisphere; one subject was tested in both the left and right hemispheres, and one subject was only tested in the right hemisphere. Upon examination of right hemisphere recordings, we did not observe any outstanding differences between auditory evoked responses in the left and right hemispheres, and both hemispheres were combined for analysis. Hemispheric differences in auditory processing that have been documented previously in NCM (e.g., Moorman et al. 2012; Phan and Vicario 2010) may be more pronounced in awake or awake-restrained playback conditions. Histological verification confirmed microdialysis probe and electrode placements in all NCM and HVC targeted sites included in the study.
Fig. 4.
NE treatment in NCM does not alter auditory-evoked responses or selectivity in HVC. A: z-score data are means ± SE; n = 16 cells. Inset: individual trajectory plots show responses to BOS for each single unit. B: neurons in HVC show a unimodal distribution for spike quarter width (SQW) measurements.
NE treatment in NCM enhances local auditory-evoked activity for all stimuli.
NCM, a secondary auditory cortical region in the zebra finch pallium, characteristically responded to all auditory stimuli by increasing neural firing activity. On average, NE treatment locally in NCM caused a robust enhancement of auditory-evoked activity to all stimuli (Fig. 2A) as measured by the auditory z score of 22 single units. Indeed, the overwhelming majority (20 of 22) of single units exhibited enhancement of auditory-evoked activity after NE treatment.
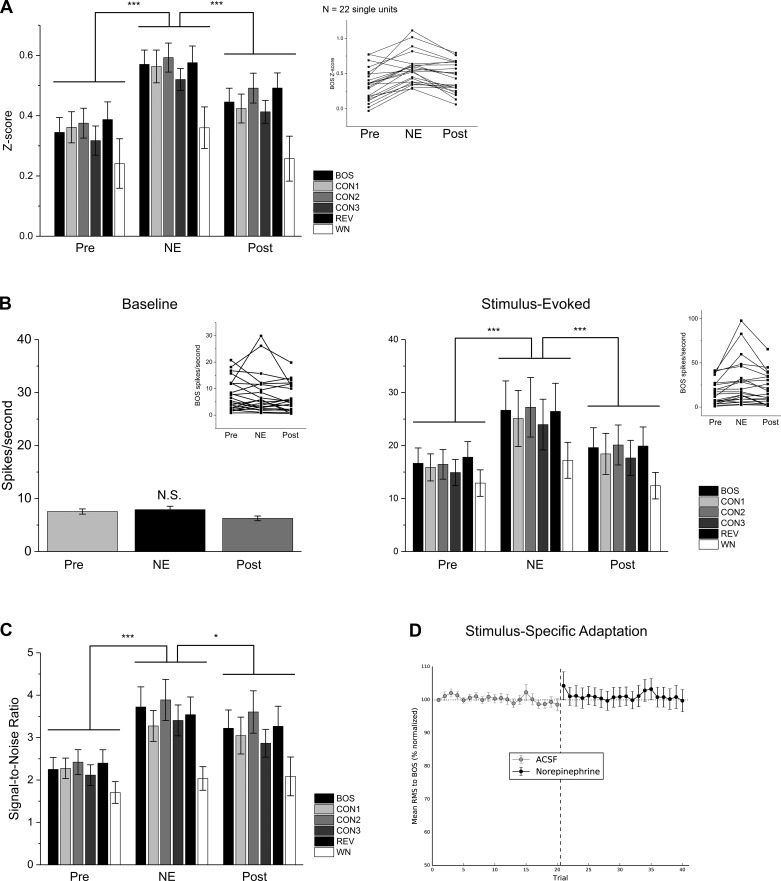
Fig. 2.
Local auditory-evoked activity is enhanced by NE treatment in NCM. Data are means ± SE; n = 22 cells. *P < 0.05, ***P < 0.001. A: auditory response strength (z score) is enhanced during NE treatment. Inset: individual trajectory plots show responses to BOS for each single unit. B: as demonstrated by firing rates before (baseline) and during the presentation of auditory stimuli (stimulus evoked), the NE-dependent enhancement of auditory responses is mediated by an increase in stimulus-evoked firing rates. Inset: individual trajectory plots show spikes/second at baseline and during stimulus presentation. C: local NE treatment in NCM also increases auditory signal-to-noise ratios. D: NE shows no effect on the rate of stimulus-specific adaptation in NCM.
A two-way ME-ANOVA yielded a significant NE simple treatment effect on auditory z scores [Fig. 2A; main effect treatment (GGc): F(1.751, 220.637) = 82.277, P < 0.001; main effect stimulus: F(5, 126) = 2.239, P = 0.054; treatment × stimulus interaction (GGc): F(8.755, 220.637) = 0.795, P = 0.618]. Post hoc Tukey tests demonstrated significant increases in z score in response to NE [Fig. 2A; Pre vs. NE: t(252) = 18.081, P < 0.001; NE vs. Post: t(252) = 10.324, P < 0.001].
NE-dependent enhancement in NCM z scores is mediated by an increase in bursting and stimulus-evoked firing rates.
From the same single units isolated in NCM, we analyzed neuronal firing rates at baseline and during the presentation of stimuli to determine the source of the enhancement of auditory z scores in NCM. At baseline (i.e., spontaneous activity preceding playback), there was no overall significant change in spikes per second among treatment conditions [Fig. 2B; Friedman test, χ2(2) = 5.182; P = 0.075]. Because this test approached significance, we performed post hoc tests to determine any NE-dependent changes in baseline firing rates. However, these pairwise tests were not significant [Wilcoxon paired sample test; Pre vs. NE: W = 139, P = 0.697; NE vs. Post: W = 170, P = 0.163]. For evoked activity, a significant difference in neuron firing rates between treatment conditions was detected [Fig. 2B; ME-ANOVA, main effect treatment (GGc): F(1.334, 168.070) = 40.128, P < 0.001; main effect stimulus: F(5, 126) = 0.578, P = 0.717; treatment × stimulus interaction (GGc): F(6.670, 168.070) = 0.438, P = 0.812]. Tukey post hoc tests indicated a significant increase in firing rate with NE treatment [Fig. 2B; Pre vs. NE: t(252) = 12.209, P < 0.001; NE vs. Post: t(252) = 9.034, P < 0.001]. Therefore, the local enhancement of auditory-evoked responses in NCM by NE treatment was due to a mechanism that increased neuronal firing rates during the presentation of a stimulus and not a change in baseline firing states. Interestingly, this increase in firing rate was accompanied by an increase in the occurrence of bursts (mean ± SE bursts Pre: 205.4 ± 52.5; NE: 300.5 ± 82.3; P = 0.036 for paired t-test) but no change in the average burst duration (Pre: 15.38 ± 2.25 ms; NE: 12.91 ± 3.66 ms; P = 0.353). In summary, the enhancing effect of NE on audition was restricted to stimulus-evoked activity (Fig. 2B). These results diverge from the effects of NE in the NCM of female zebra finches, in which NE locally enhances auditory responses via a suppression of baseline firing rates and no change in auditory-evoked rates (Ikeda et al. 2015).
We also measured SSA to auditory stimuli, since NCM neurons in awake animals are known to exhibit SSA (Chew et al. 1995; Yoder et al. 2015) and NE has been shown to alter sensory learning in other systems (Eckmeier and Shea 2014; Linster and Cleland 2016). In anesthetized recordings from NCM, SSA is typically reported after 100–1,000 repetitions of individual stimuli (Kozlov and Gentner 2014; Ono et al. 2016). Consistent with these findings, in our recordings from NCM in anesthetized subjects we did not observe SSA during treatment with aCSF or NE. Specifically, the slopes of the change in response for auditory units (i.e., rate of habituation) were not significantly different from zero for either treatment period [Fig. 2D; ACSF: t(8) = −1.053, P = 0.323; NE: t(8) = −0.862, P = 0.414].
Signal-to-noise ratio, calculated from baseline and stimulus presentation neuron firing rates, is another measure of stimulus detection and is typically enhanced by NE treatment in auditory cortex (Ikeda et al. 2015; Manunta and Edeline 1999). In NCM neurons, we found a significant effect of NE treatment on signal-to-noise ratio [Fig. 2C; ME-ANOVA, main effect treatment: F(2, 252) = 51.742, P < 0.001; main effect stimulus: F(5, 126) = 1.905, P = 0.098; treatment × stimulus interaction: F(10, 252) = 1.279, P = 0.242], with an increase in signal-to-noise ratios in response to NE treatment [Fig. 2C; Tukey test, Pre vs. NE: t(252) = 13.884, P < 0.001; NE vs. Post: t(252) = 3.679, P = 0.026]. This is consistent with the effects of NE treatment on NCM neurons in female zebra finches (Ikeda et al. 2015). In summary, both auditory z scores and auditory signal-to-noise ratios were enhanced by NE in NCM.
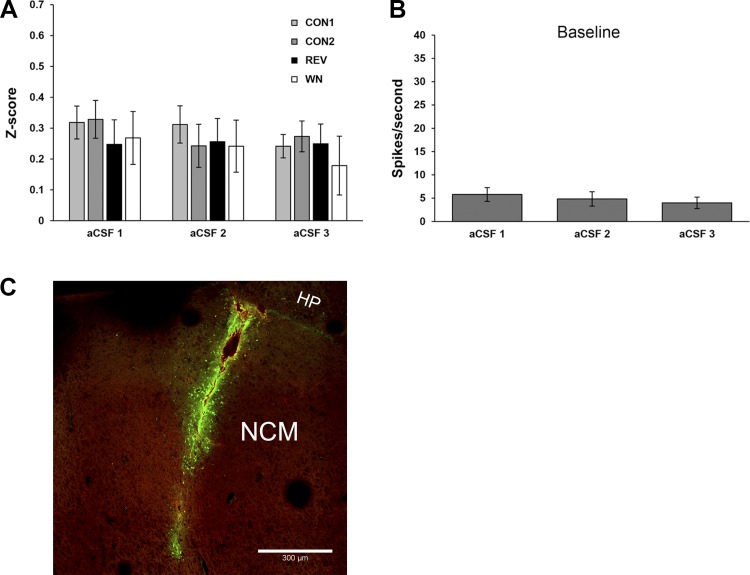
We next considered the hypothesis that the observed changes in the baseline and evoked activity of NCM neurons occur as a result of insertion of a microdialysis probe in the brain (i.e., in a nonspecific manner independent of NE). To address this hypothesis, in a separate set of animals (n = 5 males; n = 10 single units) we retrodialyzed aCSF alone for three successive periods, following the same experimental paradigm as above for Pre, NE, and washout periods. As shown in Fig. 3, we observed no significant changes across the three periods of aCSF retrodialysis in baseline firing rate [Fig. 3B; Friedman test, χ2(2) = 3.15, P = 0.207] or auditory z scores [Fig. 3A; ME-ANOVA, main effect treatment: F(2,8) = 1.243, P = 0.338; main effect stimulus: F(3,7) = 0.778, P = 0.542; treatment × stimulus interaction: F(6,4) = 2.198, P = 0.233]. These results are consistent with the interpretation that the rapid modulatory effects we observed during and after retrodialysis of NE above are not due to global changes over time in NCM physiology that occur during aCSF infusion alone. Importantly, the spread of equimolar Alexa 488 from the probe was within a ~300-µm band surrounding the 1-mm probe lesion site, indicating that the diffusion of water-soluble compounds like NE is restricted within NCM during the 30-min treatment period.
Fig. 3.
Testing the specificity of retrodialyzed NE treatments. A and B: local treatment with vehicle (aCSF) in 3 successive recording periods does not lead to changes in NCM auditory response strength (z score; A), or changes in NCM baseline firing rates (B). Data are means ± SE; n = 10 cells. C: photomicrograph showing that retrodialysis of equimolar Alexa 488 dissolved in aCSF for 30 min is associated with diffusion of fluorophore ~300–400 μm from the probe membrane in NCM. Green, Alexa 488; red channel is also shown to illustrate the low level of gliotic/red blood cell autofluorescence following acute probe implantation. HP, hippocampus.
NE treatment in NCM does not enhance auditory-evoked responses or selectivity downstream in HVC.
Neurons in HVC, a sensorimotor integration nucleus in the caudal forebrain, exhibit highly selective responses to BOS. There are indirect connections between NCM and HVC, and prior studies have documented neuromodulatory influences of events in both NIf and NCM on downstream representations in HVC (Cardin and Schmidt 2004a; Pawlisch and Remage-Healey 2015; Remage-Healey and Joshi 2012). We therefore predicted that the selectivity for BOS in HVC would be enhanced by NE acting in NCM. Auditory z scores for 16 isolated single units from HVC were analyzed while NE was delivered to NCM via retrodialysis.
Contrary to our prediction, despite the effects of NE on NCM neurons, no significant treatment effect was detected in HVC for auditory-evoked responses or selectivity [Fig. 4A; ME-ANOVA, main effect treatment (GGc): F(1.809, 162.782) = 0.048, P = 0.941; main effect stimulus: F(5, 90) = 5.059, P < 0.001; stimulus × treatment interaction: F(9.043, 162.782) = 0.581, P = 0.812].
NE in NCM shapes timing coding in NCM and in HVC.
One drawback of our analyses of raw changes in z score and signal-to-noise ratios (each of which sums the firing rates across the entire 2-s stimulus presentation window) is that they overlook changes in rate- and time-based coding of neurons that can occur with subsecond precision in NCM and HVC. A pattern classifier was therefore used to assess whether the rate and time coding properties of NCM and HVC neurons were influenced by NE treatment (Caras et al. 2015; Ikeda et al. 2015). This was measured by assessing both spike count accuracy and spike timing accuracy in our population of neurons.
In NCM, ME-ANOVA revealed a significant effect of NE treatment on count accuracy [Fig. 4A; main effect treatment (GGc): F(1.422, 162.125) = 27.738, P < 0.001; main effect stimulus: F(5, 114) = 0.771, P = 0.573; treatment × stimulus interaction: F(7.111, 162.125) = 0.860, P = 0.541]. Post hoc Tukey tests showed that NE increased count accuracy from Pre to NE, which remained enhanced in the Post period [Fig. 4A; Pre vs. NE: t(228) = 9.701; P < 0.001; NE vs. Post: t(228) = 1.296; P = 0.631]. Similarly, in the timing accuracy analyses, NE treatment in NCM changed timing coding [Fig. 4A; ME-ANOVA, main effect treatment: F(1.441, 164.234) = 23.797, P < 0.001; main effect stimulus: F(5, 114) = 0.869, P = 0.504; treatment × stimulus interaction: F(7.203, 164.234) = 0.490, P = 0.846]. Post hoc Tukey tests showed that NE increased timing accuracy from Pre to NE, which remained enhanced in the Post period [Fig. 4B; Pre vs. NE: t(228) = 8.867, P < 0.001; NE vs. Post: t(228) = 0.909, P = 0.797]. Therefore, the robust increase in z score by NE is associated with a clear enhancement in the time and count coding accuracy of NCM neurons. We note that even though the effects on auditory-evoked activity return to pretreatment levels after treatment, the effects on coding accuracy persist into the washout period.
NE treatment in NCM changed HVC count accuracy [Fig. 4B; ME-ANOVA, main effect treatment: F(1.791, 161.188) = 5.713, P = 0.005; main effect stimulus: F(5, 90) = 12.279, P < 0.001; treatment × stimulus interaction: F(8.955, 161.188) = 1.443, P = 0.174]. Post hoc Tukey tests detected an increasing trend from Pre to NE [t(180) = 3.254, P = 0.058; NE vs. Post: t(180) = 1.405, P = 0.582]. Similarly, NE treatment in NCM changed timing accuracy in HVC cells [Fig. 4B; ME-ANOVA, treatment main effect (GGc): F(1.612, 145.095) = 5.919, P = 0.006; main effect stimulus F(5, 90) = 3.487, P = 0.006; treatment × stimulus interaction: F(8.061, 145.095) = 0.504, P = 0.853]. Post hoc tests detected an increase in timing accuracy from Pre to NE and no return to baseline in Post [Fig. 4B; Tukey test, Pre vs. NE: t(180) = 4.651, P = 0.003; NE vs. Post: t(180) = 1.086, P = 0.723]. Therefore, similar to the local actions on NCM coding, NE treatment in NCM enhanced time coding of single neurons downstream in HVC. Importantly, this enhancement lasted beyond the treatment with NE and was not associated with changes in z score in HVC.
The bin size that yielded the highest count accuracy (see methods) did not change significantly in either NCM [Friedman’s test; χ2(2) = 0.743, P = 0.690] or HVC [Friedman’s test; χ2(2) = 0.514, P = 0.773] nor did the Gaussian filter’s standard deviation (best σ; see methods) for the timing accuracy [NCM: Friedman’s test; χ2(2) = 0.703, P = 0.704; HVC: Friedman’s test; χ2(2) = 1.762, P = 0.414]. During trial 1 (aCSF), the best bin size ranged in NCM between 1 and 1,024 ms (median = 32 ms), and in HVC between 16 and 1,700 ms (median = 768 ms). HVC cells had a significantly higher best bin size than NCM (Mann-Whitney test: U = 30.500, P < 0.001). The best σ ranged in NCM between 2 and 32 ms (median = 8 ms) and in HVC between 2 and 64 (median = 4 ms), and there was no difference between the two nuclei (Mann-Whitney test: U = 122.5, P = 0.222).
Together, these results indicate that while NE treatment in NCM did not lead to changes in the auditory z scores in HVC neurons (Fig. 4A) the coding properties in HVC were in fact sensitive to NE treatment in NCM (Fig. 5B).
DISCUSSION
Our study has three major findings. First, we report that NE enhances single-unit responses to song and burst firing in the male songbird auditory cortex (NCM), and that this modulation in NCM also shapes the coding properties in the downstream sensorimotor region HVC. Second, we find that although the NE-dependent increase in signal-to-noise ratio of single auditory neurons in the NCM of males is similar to prior findings in females, it is achieved via modulation of evoked firing as opposed to changes in baseline firing rates. Finally, we observe that coding properties in both NCM and HVC are enhanced by the actions of NE in NCM.
In female zebra finches, NE causes a suppression of baseline firing rates in NCM, via α2-receptor activation, and no effect on auditory-evoked firing (Ikeda et al. 2015). This is likely mediated by a direct effect on inhibitory neurons, consistent with the mechanism of enhanced inhibition by NE in a variety of sensory cortices in mammals (Salgado et al. 2016). Here we show that the effects of NE in males are markedly different from the effects in females. Namely, NE enhances auditory-evoked firing rates in the NCM of male zebra finches, yet it leaves baseline firing rates unchanged. These results suggest that, while the end result is the same in males and females (i.e., a local NE-dependent increase in auditory coding in NCM), there is a sex difference in the mechanism of action of NE in males vs. females. One possible explanation for these findings is that NE acts on different NCM neuronal classes/subclasses in males vs. females. Subclasses of NCM neuron carry different amounts of information and are most likely organized into a feedforward inhibitory microcircuit (see, e.g., Schneider and Woolley 2013). Our results suggest that adrenergic receptors are predominantly expressed in tonic inhibitory neurons in females (inhibited by NE), while in males adrenergic receptors are expressed in excitatory neurons (excited by NE) and/or in stimulus-responsive inhibitory interneurons (inhibited by NE) in the NCM, possibilities that await formal experimental tests. However, our population of NCM neurons showed a unimodal distribution of spike widths (Fig. 1D), indicating that NE modulates NCM neurons with a range of spike waveforms. Waveform duration has been used to segregate NCM neurons into putative projection neurons (broad spiking) vs. inhibitory interneurons (narrow spiking) (Ono et al. 2016; Schneider and Woolley 2013; Yanagihara and Yazaki-Sugiyama 2016), yet our recorded population bridges the typical 0.35- to 0.5-ms “dip” in waveform duration distribution to segregate NCM waveforms into these categories. We therefore do not have evidence supporting the hypothesis that NE modulates a subclass of NCM neurons, and we note that 20 of 22 single units in NCM exhibited an increase in z scores during NE treatment.
Despite this potential sex difference in mechanism, NE appears to achieve the same magnitude of enhanced auditory coding (~1.5-fold increase in z scores) in the NCM of males (this study) and females (Ikeda et al. 2015). Therefore, the sex difference in the mechanism of action (i.e., altered baseline in females, altered evoked activity in males) could in fact represent a compensatory sex difference. There is growing appreciation of a category of mechanisms that may counteract intrinsic sex differences in neural circuit morphology and/or neurochemistry to achieve similar neural and/or behavioral outcomes in males and females (De Vries 2004; Krentzel and Remage-Healey 2015; McCarthy et al. 2012). In mammalian cortex, microdialysis studies show that dopamine and serotonin levels differ between males and females, whereas NE is similar between the sexes (Staiti et al. 2011). Most studies of catecholamine-synthesis enzyme expression and levels in the songbird auditory forebrain have been conducted on females (Matragrano et al. 2011, 2012; Velho et al. 2012). When directly compared, males have elevated expression of tyrosine hydroxylase in the caudal forebrain song nuclei (Appeltants et al. 2001). Notably, adrenergic receptor expression is higher in song system nuclei (HVC, RA, lMAN, area X), but not the preoptic area, of males compared with female zebra finches (Castelino and Schmidt 2010; Riters and Ball 2002), yet sex differences in the auditory forebrain are not well characterized to date. Finally, no sex differences in dopamine-β-hydroxylase fiber density were observed in a relatively limited sample in the NCM (Mello et al. 1998). Our physiology results indicate that the role of NE on auditory processing is similar in male and female songbirds and support the notion of sex differences in the neural receptor distribution and/or neuronal subtype in NCM.
The actions of NE on NCM neurons at times persisted beyond the treatment period, into the “washout” phase. Mean z scores and signal-to-noise ratio did not fully return to pretreatment levels (Fig. 2, A and C), and this was also observed for mean coding accuracy in both NCM and HVC. The absence of a washout in these cases in vivo is not incompatible with persistent effects of neuromodulators such as NE. In fact, NE is known to have long-lasting effects after single dose administration (30 min to multiple hours) on neural end points such as spiking activity in the medial nucleus of the trapezoid body (Kimura et al. 1979), LTP in the hippocampus (Sarvey et al. 1989; Stanton and Sarvey 1985), and synaptic plasticity in several cortical regions (reviewed in Salgado et al. 2016). These effects depend on second messenger pathways and are sensitive to protein synthesis blockade, consistent with long-term NE-dependent modulation of cell signaling events in neurons. We also observed in our previous study on females using a very similar design that coding accuracy did not return fully to pretreatment levels after NE treatment (Ikeda et al. 2015). We therefore believe that our observations of a lack of a full return to baseline in the “post” recording period following NE treatment is consistent with longer-term plasticity in cortical sensory neurons.
Our data provide evidence that NE in NCM does not influence the overall auditory-evoked responses within HVC for any stimulus class. Therefore, despite the robust effects of NE in NCM, the transmission of similarly rapid neuroestrogen effects in NCM into HVC (Pawlisch and Remage-Healey 2015; Remage-Healey and Joshi 2012), as well as the transmission of NE effects from NIf into HVC (Cardin and Schmidt 2004a), there was no systematic effect of NE in NCM on the auditory-evoked responses of our population of HVC neurons. This is consistent with relatively weak cross-coherence between HVC and NCM, in terms of multiunit firing rates (Soyman and Vicario 2017). The role of behavioral state in HVC responses to song has been well documented (e.g., Cardin and Schmidt 2004a, 2004b; Schmidt and Konishi 1998), and in particular the suppression of auditory responses in HVC in awake birds calls into question the significance of observations about HVC response properties under anesthesia. However, the absence of auditory-driven spiking activity in awake HVC does not by itself indicate that auditory information does not reach the nucleus, since subthreshold synaptic activity can be driven by auditory stimuli in HVC (Mooney 2009; Rosen and Mooney 2003). Therefore, our findings open up new avenues for observing catecholamine actions—in the awake state—in upstream auditory regions that may modulate sensorimotor regions like HVC.
Inspection of our single-unit trajectory plots showed that the auditory-evoked responses in HVC appeared to be heterogeneous in response to NE in NCM. We therefore further analyzed the response patterns of single neurons based on rate and temporal coding. The accuracy of our pattern classifier analyses was enhanced by NE treatment in NCM for both NCM and HVC single units. Together, these findings indicate that the NE-dependent alterations in evoked activity in NCM are transmitted downstream to HVC in the form of enhanced temporal coding. Shifts in responsiveness and coding properties along the auditory-auditory/motor pathway as seen here are similar to the behavioral state-dependent changes in the representations of sensory stimuli in the songbird brain (e.g., Cardin and Schmidt 2004a, 2004b). In summary, NE enhances auditory responses and encoding in NCM as well as coding accuracy downstream in HVC. The route of transmission of this modulated state from NCM to HVC is currently unknown, but prior work implicates the interface nucleus NIf in similar phenomena for NE- and neuroestrogen-dependent modulation of HVC (Cardin and Schmidt 2004a; Pawlisch and Remage-Healey 2015). Collectively, our findings are consistent with the hypothesis that NE modulation in NCM achieves an end point in males similar to that in females, yet via a divergent mechanism, and that the auditory coding properties of HVC neurons are altered by modulatory events in NCM.
GRANTS
This research was supported by National Institute of Neurological Disorders and Stroke Grant R01 NS-082179, a UMass Commonwealth Honors College Undergraduate Research Assistant Fellowship (V. Lee), and the Coordination for Development of Higher Education Personnel (CAPES-Brazil 13640/13-5, M. Macedo-Lima).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.L., B.A.P., and L.R.-H. conceived and designed research; V.L. and B.A.P. performed experiments; V.L., B.A.P., M.M.-L., and L.R.-H. analyzed data; V.L., B.A.P., M.M.-L., and L.R.-H. interpreted results of experiments; V.L., B.A.P., and M.M.-L. prepared figures; V.L., B.A.P., and L.R.-H. drafted manuscript; V.L., B.A.P., M.M.-L., and L.R.-H. edited and revised manuscript; V.L., B.A.P., M.M.-L., and L.R.-H. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address for B. A. Pawlisch: Department of Biology, Adrian College, 110 S Madison St., Adrian, MI 49221.
REFERENCES
- Appeltants D, Ball GF, Balthazart J. The distribution of tyrosine hydroxylase in the canary brain: demonstration of a specific and sexually dimorphic catecholaminergic innervation of the telencephalic song control nuclei. Cell Tissue Res 304: 237–259, 2001. doi: 10.1007/s004410100360. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci 1: 887–900, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J Comp Neurol 493: 99–110, 2005. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- Bargmann CI. Beyond the connectome: how neuromodulators shape neural circuits. BioEssays 34: 458–465, 2012. doi: 10.1002/bies.201100185. [DOI] [PubMed] [Google Scholar]
- Caras ML, Sen K, Rubel EW, Brenowitz EA. Seasonal plasticity of precise spike timing in the avian auditory system. J Neurosci 35: 3431–3445, 2015. doi: 10.1523/JNEUROSCI.3407-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Schmidt MF. Noradrenergic inputs mediate state dependence of auditory responses in the avian song system. J Neurosci 24: 7745–7753, 2004a. doi: 10.1523/JNEUROSCI.1951-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Schmidt MF. Auditory responses in multiple sensorimotor song system nuclei are co-modulated by behavioral state. J Neurophysiol 91: 2148–2163, 2004b. doi: 10.1152/jn.00918.2003. [DOI] [PubMed] [Google Scholar]
- Castelino CB, Schmidt MF. What birdsong can teach us about the central noradrenergic system. J Chem Neuroanat 39: 96–111, 2010. doi: 10.1016/j.jchemneu.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew SJ, Mello C, Nottebohm F, Jarvis E, Vicario DS. Decrements in auditory responses to a repeated conspecific song are long-lasting and require two periods of protein synthesis in the songbird forebrain. Proc Natl Acad Sci USA 92: 3406–3410, 1995. doi: 10.1073/pnas.92.8.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave AS, Yu AC, Margoliash D. Behavioral state modulation of auditory activity in a vocal motor system. Science 282: 2250–2254, 1998. doi: 10.1126/science.282.5397.2250. [DOI] [PubMed] [Google Scholar]
- De Vries GJ. Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology 145: 1063–1068, 2004. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- Devilbiss DM, Waterhouse BD. The effects of tonic locus ceruleus output on sensory-evoked responses of ventral posterior medial thalamic and barrel field cortical neurons in the awake rat. J Neurosci 24: 10773–10785, 2004. doi: 10.1523/JNEUROSCI.1573-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmeier D, Shea SD. Noradrenergic plasticity of olfactory sensory neuron inputs to the main olfactory bulb. J Neurosci 34: 15234–15243, 2014. doi: 10.1523/JNEUROSCI.0551-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote SL, Freedman R, Oliver AP. Effects of putative neurotransmitters on neuronal activity in monkey auditory cortex. Brain Res 86: 229–242, 1975. doi: 10.1016/0006-8993(75)90699-X. [DOI] [PubMed] [Google Scholar]
- Friend DM, Kemere C, Kravitz AV. Quantifying recording quality in in vivo striatal recordings. Curr Protoc Neurosci 70: 6.28.1–6.28.9, 2015. doi: 10.1002/0471142301.ns0628s70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans EJ, van Marle HJ, Ossewaarde L, Henckens MJ, Qin S, van Kesteren MT, Schoots VC, Cousijn H, Rijpkema M, Oostenveld R, Fernández G. Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science 334: 1151–1153, 2011. doi: 10.1126/science.1209603. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Devilbiss DM, Waterhouse BD. A matter of focus: monoaminergic modulation of stimulus coding in mammalian sensory networks. Curr Opin Neurobiol 14: 488–495, 2004. doi: 10.1016/j.conb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Ikeda MZ, Jeon SD, Cowell RA, Remage-Healey L. Norepinephrine modulates coding of complex vocalizations in the songbird auditory cortex independent of local neuroestrogen synthesis. J Neurosci 35: 9356–9368, 2015. doi: 10.1523/JNEUROSCI.4445-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao MH, Wright BD, Doupe AJ. Neurons in a forebrain nucleus required for vocal plasticity rapidly switch between precise firing and variable bursting depending on social context. J Neurosci 28: 13232–13247, 2008. doi: 10.1523/JNEUROSCI.2250-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamatsu T, Pettigrew JD, Ary M. Restoration of visual cortical plasticity by local microperfusion of norepinephrine. J Comp Neurol 185: 163–181, 1979. doi: 10.1002/cne.901850110. [DOI] [PubMed] [Google Scholar]
- Kimura K, Kimura Y, Ohata K, Takagi H. Effects of intraventricularly administered serotonin, noradrenaline, dopamine and metaraminol on the reserpine-induced spikes recorded from the medial nucleus trapezoides in rabbits. Jpn J Pharmacol 29: 33–39, 1979. doi: 10.1254/jjp.29.33. [DOI] [PubMed] [Google Scholar]
- Kozlov AS, Gentner TQ. Central auditory neurons display flexible feature recombination functions. J Neurophysiol 111: 1183–1189, 2014. doi: 10.1152/jn.00637.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentzel AA, Remage-Healey L. Sex differences and rapid estrogen signaling: a look at songbird audition. Front Neuroendocrinol 38: 37–49, 2015. doi: 10.1016/j.yfrne.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski B, Vyssotski A, Hahnloser RH, Schmidt M. At the interface of the auditory and vocal motor systems: NIf and its role in vocal processing, production and learning. J Physiol Paris 107: 178–192, 2013. doi: 10.1016/j.jphysparis.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Cleland TA. Neuromodulation of olfactory transformations. Curr Opin Neurobiol 40: 170–177, 2016. doi: 10.1016/j.conb.2016.07.006. [DOI] [PubMed] [Google Scholar]
- Lynch KS, Diekamp B, Ball GF. Colocalization of immediate early genes in catecholamine cells after song exposure in female zebra finches (Taeniopygia guttata). Brain Behav Evol 79: 252–260, 2012. doi: 10.1159/000337533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manunta Y, Edeline JM. Effects of noradrenaline on frequency tuning of auditory cortex neurons during wakefulness and slow-wave sleep. Eur J Neurosci 11: 2134–2150, 1999. doi: 10.1046/j.1460-9568.1999.00633.x. [DOI] [PubMed] [Google Scholar]
- Manunta Y, Edeline JM. Noradrenergic induction of selective plasticity in the frequency tuning of auditory cortex neurons. J Neurophysiol 92: 1445–1463, 2004. doi: 10.1152/jn.00079.2004. [DOI] [PubMed] [Google Scholar]
- Martins AR, Froemke RC. Coordinated forms of noradrenergic plasticity in the locus coeruleus and primary auditory cortex. Nat Neurosci 18: 1483–1492, 2015. doi: 10.1038/nn.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matragrano LL, Beaulieu M, Phillip JO, Rae AI, Sanford SE, Sockman KW, Maney DL. Rapid effects of hearing song on catecholaminergic activity in the songbird auditory pathway. PLoS One 7: e39388, 2012. doi: 10.1371/journal.pone.0039388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matragrano LL, Sanford SE, Salvante KG, Sockman KW, Maney DL. Estradiol-dependent catecholaminergic innervation of auditory areas in a seasonally breeding songbird. Eur J Neurosci 34: 416–425, 2011. doi: 10.1111/j.1460-9568.2011.07751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex differences in the brain: the not so inconvenient truth. J Neurosci 32: 2241–2247, 2012. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Pinaud R, Ribeiro S. Noradrenergic system of the zebra finch brain: immunocytochemical study of dopamine-beta-hydroxylase. J Comp Neurol 400: 207–228, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- Mooney R. Neural mechanisms for learned birdsong. Learn Mem 16: 655–669, 2009. doi: 10.1101/lm.1065209. [DOI] [PubMed] [Google Scholar]
- Moorman S, Gobes SM, Kuijpers M, Kerkhofs A, Zandbergen MA, Bolhuis JJ. Human-like brain hemispheric dominance in birdsong learning. Proc Natl Acad Sci USA 109: 12782–12787, 2012. doi: 10.1073/pnas.1207207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the songbird brain. Science 194: 211–213, 1976. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- Ono S, Okanoya K, Seki Y. Hierarchical emergence of sequence sensitivity in the songbird auditory forebrain. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 202: 163–183, 2016. doi: 10.1007/s00359-016-1070-7. [DOI] [PubMed] [Google Scholar]
- Pais-Vieira M, Lebedev MA, Wiest MC, Nicolelis MA. Simultaneous top-down modulation of the primary somatosensory cortex and thalamic nuclei during active tactile discrimination. J Neurosci 33: 4076–4093, 2013. doi: 10.1523/JNEUROSCI.1659-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlisch BA, Remage-Healey L. Neuroestrogen signaling in the songbird auditory cortex propagates into a sensorimotor network via an “interface” nucleus. Neuroscience 284: 522–535, 2015. doi: 10.1016/j.neuroscience.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlisch BA, Stevenson SA, Riters LV. α1-Noradrenergic receptor antagonism disrupts female songbird responses to male song. Neurosci Lett 496: 20–24, 2011. doi: 10.1016/j.neulet.2011.03.078. [DOI] [PubMed] [Google Scholar]
- Phan ML, Vicario DS. Hemispheric differences in processing of vocalizations depend on early experience. Proc Natl Acad Sci USA 107: 2301–2306, 2010. doi: 10.1073/pnas.0900091107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci USA 107: 3852–3857, 2010. doi: 10.1073/pnas.0906572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Joshi NR. Changing neuroestrogens within the auditory forebrain rapidly transform stimulus selectivity in a downstream sensorimotor nucleus. J Neurosci 32: 8231–8241, 2012. doi: 10.1523/JNEUROSCI.1114-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV, Ball GF. Sex differences in the densities of alpha2-adrenergic receptors in the song control system, but not the medial preoptic nucleus in zebra finches. J Chem Neuroanat 23: 269–277, 2002. doi: 10.1016/S0891-0618(02)00005-4. [DOI] [PubMed] [Google Scholar]
- Rosen MJ, Mooney R. Inhibitory and excitatory mechanisms underlying auditory responses to learned vocalizations in the songbird nucleus HVC. Neuron 39: 177–194, 2003. doi: 10.1016/S0896-6273(03)00357-X. [DOI] [PubMed] [Google Scholar]
- Salgado H, Garcia-Oscos F, Martinolich L, Hall S, Restom R, Tseng KY, Atzori M. Pre- and postsynaptic effects of norepinephrine on γ-aminobutyric acid-mediated synaptic transmission in layer 2/3 of the rat auditory cortex. Synapse 66: 20–28, 2012. doi: 10.1002/syn.20979. [DOI] [PubMed] [Google Scholar]
- Salgado H, Garcia-Oscos F, Patel A, Martinolich L, Nichols JA, Dinh L, Roychowdhury S, Tseng KY, Atzori M. Layer-specific noradrenergic modulation of inhibition in cortical layer II/III. Cereb Cortex 21: 212–221, 2011. doi: 10.1093/cercor/bhq081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado H, Treviño M, Atzori M. Layer- and area-specific actions of norepinephrine on cortical synaptic transmission. Brain Res 1641: 163–176, 2016. doi: 10.1016/j.brainres.2016.01.033. [DOI] [PubMed] [Google Scholar]
- Sarvey JM, Burgard EC, Decker G. Long-term potentiation: studies in the hippocampal slice. J Neurosci Methods 28: 109–124, 1989. doi: 10.1016/0165-0270(89)90016-2. [DOI] [PubMed] [Google Scholar]
- Schmidt MF, Konishi M. Gating of auditory responses in the vocal control system of awake songbirds. Nat Neurosci 1: 513–518, 1998. doi: 10.1038/2232. [DOI] [PubMed] [Google Scholar]
- Schneider DM, Woolley SM. Sparse and background-invariant coding of vocalizations in auditory scenes. Neuron 79: 141–152, 2013. doi: 10.1016/j.neuron.2013.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S, Fellous JM, Whitmer D, Tiesinga P, Sejnowski TJ. A new correlation-based measure of spike timing reliability. Neurocomputing 52-54: 925–931, 2003. doi: 10.1016/S0925-2312(02)00838-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea SD, Koch H, Baleckaitis D, Ramirez JM, Margoliash D. Neuron-specific cholinergic modulation of a forebrain song control nucleus. J Neurophysiol 103: 733–745, 2010. doi: 10.1152/jn.00803.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea SD, Margoliash D. Basal forebrain cholinergic modulation of auditory activity in the zebra finch song system. Neuron 40: 1213–1226, 2003. doi: 10.1016/S0896-6273(03)00723-2. [DOI] [PubMed] [Google Scholar]
- Sockman KW, Salvante KG. The integration of song environment by catecholaminergic systems innervating the auditory telencephalon of adult female European starlings. Dev Neurobiol 68: 656–668, 2008. doi: 10.1002/dneu.20611. [DOI] [PubMed] [Google Scholar]
- Soyman E, Vicario DS. Principles of auditory processing differ between sensory and premotor structures of the songbird forebrain. J Neurophysiol 117: 1266–1280, 2017. doi: 10.1152/jn.00462.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiti AM, Morgane PJ, Galler JR, Grivetti JY, Bass DC, Mokler DJ. A microdialysis study of the medial prefrontal cortex of adolescent and adult rats. Neuropharmacology 61: 544–549, 2011. doi: 10.1016/j.neuropharm.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton PK, Sarvey JM. The effect of high-frequency electrical stimulation and norepinephrine on cyclic AMP levels in normal versus norepinephrine-depleted rat hippocampal slices. Brain Res 358: 343–348, 1985. doi: 10.1016/0006-8993(85)90981-3. [DOI] [PubMed] [Google Scholar]
- Tchernichovski O, Nottebohm F, Ho CE, Pesaran B, Mitra PP. A procedure for an automated measurement of song similarity. Anim Behav 59: 1167–1176, 2000. doi: 10.1006/anbe.1999.1416. [DOI] [PubMed] [Google Scholar]
- Theunissen FE, Amin N, Shaevitz S, Woolly SM, Fremouw T, Hauber ME. Song selectivity and the songbird brain. In: Neuroscience of Birdsong, edited by Zeigler P, Marler P. New York: Cambridge Univ. Press, 2008, p. 157–173. [Google Scholar]
- Velho TA, Lu K, Ribeiro S, Pinaud R, Vicario D, Mello CV. Noradrenergic control of gene expression and long-term neuronal adaptation evoked by learned vocalizations in songbirds. PLoS One 7: e36276, 2012. doi: 10.1371/journal.pone.0036276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse BD, Woodward DJ. Interaction of norepinephrine with cerebrocortical activity evoked by stimulation of somatosensory afferent pathways in the rat. Exp Neurol 67: 11–34, 1980. doi: 10.1016/0014-4886(80)90159-4. [DOI] [PubMed] [Google Scholar]
- Wolff SB, Gründemann J, Tovote P, Krabbe S, Jacobson GA, Müller C, Herry C, Ehrlich I, Friedrich RW, Letzkus JJ, Lüthi A. Amygdala interneuron subtypes control fear learning through disinhibition. Nature 509: 453–458, 2014. doi: 10.1038/nature13258. [DOI] [PubMed] [Google Scholar]
- Yanagihara S, Yazaki-Sugiyama Y. Auditory experience-dependent cortical circuit shaping for memory formation in bird song learning. Nat Commun 7: 11946, 2016. doi: 10.1038/ncomms11946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KM, Phan ML, Lu K, Vicario DS. He hears, she hears: are there sex differences in auditory processing? Dev Neurobiol 75: 302–314, 2015. doi: 10.1002/dneu.22231. [DOI] [PMC free article] [PubMed] [Google Scholar]