Abstract
Most cervical spinal cord injuries result in asymmetrical functional impairments in hand and arm function. However, the extent to which reach-to-grasp movements are affected in humans with incomplete cervical spinal cord injury (SCI) remains poorly understood. Using kinematics and electromyographic (EMG) recordings in hand and arm muscles we studied the different phases of unilateral self-paced reach-to-grasp movements (arm acceleration, hand opening and closing) to a small cylinder in the more and less affected arms of individuals with cervical SCI and in age-matched controls. We found that SCI subjects showed prolonged movement duration in both arms during arm acceleration, and hand opening and closing compared with controls. Notably, the more affected arm showed an additional increase in movement duration at the time to close the hand compared with the less affected arm. Also, the time at which the index finger and thumb contacted the object and the variability of finger movement trajectory were increased in the more compared with the less affected arm of SCI participants. Participants with prolonged movement duration during hand closing were those with more pronounced deficits in sensory function. The muscle activation ratio between the first dorsal interosseous and abductor pollicis brevis muscles decreased during hand closing in the more compared with the less affected arm of SCI participants. Our results suggest that deficits in movement kinematics during reach-to-grasp movements are more pronounced at the time to close the hand in the more affected arm of SCI participants, likely related to deficits in EMG muscle activation and sensory function.
NEW & NOTEWORTHY Humans with cervical spinal cord injury usually present asymmetrical functional impairments in hand and arm function. Here, we demonstrate for the first time that deficits in movement kinematics during reaching and grasping movements are more pronounced at the time to close the hand in the more affected arm of spinal cord injury. We suggest that this is in part related to deficits in muscle activation ratios between hand muscles and a decrease in sensory function.
Keywords: grasping, hand function, reaching, kinematics, electromyography, tetraplegia, arm movements, spinal cord injury
reach-to-grasp movements are impaired in animals (Schrimsher and Reier 1993; Stackhouse et al. 2008) and humans (Koshland et al. 2005; Laffont et al. 2007; Stahl et al. 2015) with cervical spinal cord injury (SCI). Animal models of SCI showed that the success rate of forelimb reach-to-grasp movements (Schrimsher and Reier 1993; Stackhouse et al. 2008) as well as precision grasping (Morris et al. 2011; Nakagawa et al. 2015) are altered after injury. Humans with cervical SCI showed a reduced ability to reach maximum hand aperture (Stahl et al. 2015), limited trunk stability (Kukke and Triolo 2004; Murphy et al. 2014), decreased interlimb coordination (Britten et al. 2017; Calabro and Perez 2016), and decreased activation of hand and wrist muscles (Koshland et al. 2005; Stahl et al. 2015) during reach-to-grasp movements compared with controls. However, the extent to which the different phases of reach-to-grasp movements are affected in humans with incomplete cervical SCI remain largely unknown.
Reach-to-grasp movements involve different kinematics phases including arm acceleration (the time between movement onset and peak arm velocity), hand opening (the time between hand opening onset and maximum aperture between the index finger and thumb), and closing (time between the maximum aperture and when the index finger and thumb grasp the object; Castiello 2005; Gentilucci et al. 1991; Marteniuk et al. 1990; Saling et al. 1998). Hand opening and closing involves progressive preshaping of the hand followed by a gradual closure of the hand, phases that are largely influenced by the properties of the objects (Castiello 2005). It is important to note that afferent inputs contribute to proper execution of reach-to-grasp movements (Carteron et al. 2016; Johansson and Flanagan 2009; Stone and Gonzalez 2015; Witney et al. 2004). In particular, the time to open or close the hand largely relies on cutaneous and proprioceptive information for online adjustments and error corrections (Gentilucci et al. 1994; Jeannerod 1984). SCI often disrupts the integrity of afferent axons projecting through the spinal cord dorsal columns to the brain (Ozdemir and Perez 2018). Electrophysiological studies showed that the amplitude and latency of somatosensory evoked potentials are altered in SCI participants compared with control subjects (Chabot et al. 1985). Individuals with cervical SCI show altered sensory function in hand muscles (Macklin et al. 2016, 2017) and an impaired ability to modulate physiological responses during grasping actions compared with control subjects (Bunday et al. 2014). Also, afferent input does not modulate corticospinal excitability in upper limb muscles to the same extent in SCI participants compared with controls (Bailey et al. 2015). Evidence showed that the demands for precise control during grasping gradually increase when approaching the object (Paulignan et al. 1991) and the degree of task difficulty increases the duration of a movement (Dohle et al. 2000). Indeed, impaired sensory function increases the time to close the hand during reach-to-grasp movements (Gentilucci et al. 1994). Thus we hypothesized that abnormalities in movement kinematics and electromyographic (EMG) activation will be pronounced during the hand closing phase of reach-to-grasp movements in humans with incomplete cervical SCI.
MATERIALS AND METHODS
Subjects.
Twenty individuals with SCI (mean age = 48.1 ± 15.9 yr, 3 women; Table 1) and 20 age-matched healthy controls (mean age = 42.7 ± 10.7 yr, 6 women, P = 0.4) participated in the study. All subjects gave written, informed consent to the experimental procedures, which were approved by the local ethics committee at the University of Miami. The study was performed in accordance with the Declaration of Helsinki. Individuals with SCI had a chronic (≥1 yr) cervical injury (C2–C7), an intact or impaired, but not absent, innervation in dermatomes C6 using the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) sensory scores, and residual hand and arm motor function. All SCI participants were able to reach and grasp with the index finger and thumb without compensatory trunk movements. Two of 20 SCI participants were categorized by the American Spinal Cord Injuries Association Impairment Scale (AIS) as AIS A (complete injury) due to the lack of sacral sparing (Marino et al. 2003), despite being able to exert voluntary force with hand and arm muscles. Eighteen participants were classified as incomplete AIS C and D (Table 1). The more and less affected arms in SCI participants was determined by averaging the maximum isometric voluntary contraction (MVC) quantified in all muscles tested, including the first dorsal interosseous (FDI), abductor pollicis brevis (APB), extensor digitorum communis (EDC) and flexor digitorum superficialis (FDS), and anterior deltoid (AD). Participants’ average level of MVCs in all muscles tested was different across groups (controls dominant arm = 0.59 ± 0.15 mV, controls nondominant arm = 0.58 ± 0.14 mV, SCI less affected arm = 0.39 ± 0.11 mV, SCI more affected arm = 0.26 ± 0.08 mV; F3,57 = 61.8, P < 0.001). MVCs were lower in the more affected arm of SCI subjects compared with the less affected arm (P < 0.001) and controls (P < 0.001). No differences were found in MVCs between the dominant and nondominant arm in controls (P = 0.5). Our determination of the “more affected arm” by MVCs was confirmed by the subjects own estimation of their more affected arm in 19/20 SCI subjects. In addition, in a subgroup of SCI participants (n = 15) the more and less affected arm was determined by averaging the values from the Jebsen Taylor Test (Jebsen et al. 1969). Here, subjects performed the following subtests with each arm: card turning, picking up small common objects, simulated feeding, moving light objects (i.e., empty cans), and moving heavy objects (i.e., 1-lb cans). Each task was performed on a wooden board and it was timed with a stopwatch. The time to complete the Jebsen Taylor Test was increased in the more affected arm (13.5 ± 7.7 s) compared with the less affected arm (9.2 ± 4.9 s, P = 0.01) of SCI participants. Note that the arm detected as the more affected arm by MVCs was also the arm with larger functional deficits in 13/15 SCI participants. Spared sensory function was assessed by the electrical perceptual threshold (EPT) on sensory key points in dermatomes C6 to C8 using constant-current square-wave electrical pulses (0.5-ms pulse width duration, 3-Hz stimulation frequency, DS7A, Digitimer) to measure the sensory threshold or minimally detectable electrical stimulus intensity (Macklin et al. 2016, 2017). The EPT is a sensitive tool to assess spared sensory function that complements clinical assessment of sensory function in humans with SCI likely reflecting activity in dorsal column pathways (Ozdemir and Perez 2017). This test was done to be able to relate changes in kinematics, EMG, and sensory function. EPT values were larger in the more (2.1 ± 0.9 mA, P < 0.001) and less (1.8 ± 0.8 mA, P = 0.001) affected arms of SCI participant compared with controls (1.1 ± 0.3 mA). Note that EPT values were also larger in the more affected compared with the less affected arm of SCI participants (P = 0.01).
Table 1.
Spinal cord injury participants
| Subject | Age, yr | Sex | Level | ASIA Score | Etiology | Time Postnjury, yr | MVC SCI Less Affected, mV | MVC SCI More Affected, mV |
|---|---|---|---|---|---|---|---|---|
| 1 | 40 | M | C3 | D | T | 2 | 0.50 (L) | 0.25 (R) |
| 2 | 70 | M | C5 | A | T | 54 | 0.46 (L) | 0.29 (R) |
| 3 | 33 | M | C6 | C | T | 15 | 0.37 (L) | 0.22 (R) |
| 4 | 62 | M | C4 | D | T | 2 | 0.42 (L) | 0.32 (R) |
| 5 | 45 | M | C7 | C | T | 6 | 0.34 (R) | 0.32 (L) |
| 6 | 55 | F | C4 | D | T | 5 | 0.39 (L) | 0.15 (R) |
| 7 | 57 | M | C4 | C | T | 5 | 0.22 (R) | 0.16 (L) |
| 8 | 23 | F | C3 | C | T | 7 | 0.38 (L) | 0.21 (R) |
| 9 | 56 | M | C5 | D | T | 13 | 0.30 (L) | 0.27 (R) |
| 10 | 36 | M | C7 | C | T | 2 | 0.52 (L) | 0.36 (R) |
| 11 | 73 | M | C4 | A | T | 22 | 0.43 (R) | 0.36 (L) |
| 12 | 31 | M | C3 | D | T | 9 | 0.59 (R) | 0.25 (L) |
| 13 | 33 | M | C5 | C | T | 1 | 0.49 (L) | 0.36 (R) |
| 14 | 51 | M | C3 | C | T | 15 | 0.24 (R) | 0.23 (L) |
| 15 | 36 | M | C2 | C | T | 3 | 0.29 (L) | 0.16 (R) |
| 16 | 64 | M | C5 | C | NT | 5 | 0.51 (L) | 0.44 (R) |
| 17 | 67 | M | C3 | D | T | 7 | 0.29 (L) | 0.15 (R) |
| 18 | 19 | M | C2 | C | T | 3 | 0.23 (R) | 0.19 (L) |
| 19 | 59 | M | C4 | D | T | 2 | 0.52 (R) | 0.31 (L) |
| 20 | 51 | F | C5 | C | T | 14 | 0.42 (L) | 0.18 (R) |
M, male; F, female; ASIA, American Spinal Injury Association impairment scale; T, traumatic; NT, nontraumatic; MVC, maximum voluntary contraction; L, left; R, right; Less Affected, More Affected, less and more impaired sides based on MVC.
Experimental setup.
Subjects were seated upright in a chair with both arms comfortably flexed at the elbow at 90° resting on a table top. The trunk was securely strapped to the chair with a harness to minimize compensatory trunk movements. At the start of the experiment participants performed 2–3 MVCs for 3–5 s with all muscles tested, separated by 1 min of rest. Subjects were asked to perform unilateral reaching and grasping movements to a small object. The object, affixed to a table top, was an cylinder with 10-mm diameter, which was placed at a distance that required subjects to maximally extend their arms without leaning forward and at a height that required subjects to flex the shoulder by ~90°. The height of the table was corrected for each subject’s arm length to ensure all subjects began the experiment with the arms positioned in the same configuration. For the starting position for each trial, forearms were placed on top of the testing table in the neutral position with the tip of the index finger and thumb touching each other, the elbow joint at ~90°. The instruction provided to the subjects was to reach and grasp at a comfortable self-paced speed, and they were not allowed to slide the hand/forearm on the table to perform the movement. An auditory Go cue (lasting 200 ms) playing a sound file of the word “Left” or “Right” was provided at the start of each trial by a computer positioned in front of the subject to indicate which task was needed to perform. Subjects were instructed to reach and grasp with the index finger and thumb and hold the cylinder for ~3 s until another auditory cue (“Return”) was provided. Subjects returned the arm to the initial position after the Return cue. The experiment consisted of two sessions, with 15 trials with the left arm and 15 trials with the right arm in each session.
Kinematic recordings.
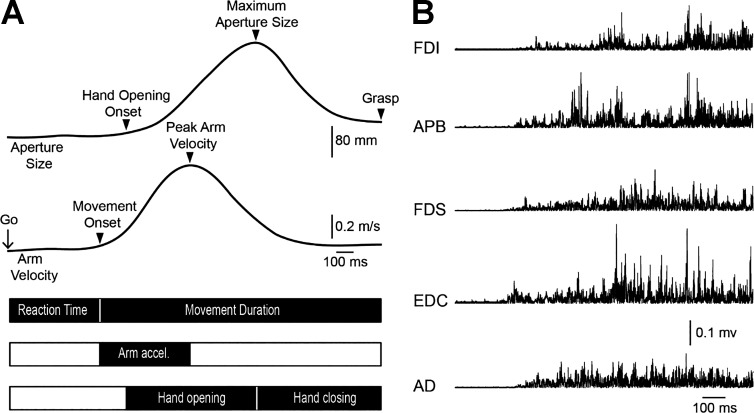
Reach-to-grasp movements were recorded using a OptiTrack motion analysis system with a sampling rate of 120 Hz. Reflective markers (4 mm) were placed on each arm on the following locations: tip of the thumb and index finger, top of the middle knuckle, inner side of the wrist, and dorsal side of the forearm. The spatial variables measured included 1) peak arm velocity, 2) maximum aperture measured relative to the final position of the fingers on the object to control for differences in marker placement, and 3) finger trajectory variability, defined as trial-to-trial variability measured by calculating the standard deviation of the index finger or thumb trajectory curvature across trials, in which the measure of trajectory curvature was the maximum perpendicular distance of trajectory’s point from a vector between start and end points. The temporal variables measured included 1) reaction time: time between auditory Go signal and movement onset; 2) total movement duration: time between movement onset and grasp; 3) arm acceleration: time between movement onset and peak arm velocity; 4) hand opening: time between hand opening onset and maximum aperture between the index finger and thumb; 5) hand closing: time between the maximum aperture between the index finger and thumb and grasping the object (Fig. 1A); and 6) finger contact: defined as the time between index finger and thumb object contact—a high value indicates that the index finger and thumb contacted the object at different times whereas a value close to zero indicates that the index finger and thumb contacted the object simultaneously. Consistent with previous studies (Calabro and Perez 2016), we compared three main phases of the reach-to-grasp movement: arm acceleration, hand opening, and hand closing.
Fig. 1.
Experimental setup. A: schematic of the experimental setup showing temporal and spatial variables measured during reach-to-grasp movements. Temporal variables measured included: reaction time (time between auditory Go signal and movement onset), Movement duration (time between movement onset and grasp), Arm acceleration [(Arm accel.) time between movement onset and peak arm velocity], Hand opening (time between hand opening onset and maximum aperture between the index finger and thumb), and Hand closing (time between the maximum aperture between the index finger and thumb and grasping the object). Spatial variables measured included peak arm velocity and maximum aperture. B: rectified electromyographic (EMG) activity in all muscles tested including the first dorsal interosseous (FDI), abductor pollicis brevis (APB), flexor digitorum superficialis (FDS), extensor digitorum communis (EDC), and anterior deltoid (AD) in a representative control subject.
EMG recordings.
EMG was recorded from the FDI, APB, EDC, FDS, and AD in both groups through surface electrodes secured to the skin over the belly of each muscle (Ag-AgCl, 10-mm diameter; Fig. 1B). The signals were amplified, filtered (20–1,000 Hz), and sampled at 2 kHz for off-line analysis (CED 1401 with Spike2 software, Cambridge Electronic Design, Cambridge, UK, off-line analysis in MATLAB, The MathWorks, Natick, MA). Mean rectified EMG activity was smoothed with a Gaussian smoothing kernel (σ = 20 ms), normalized to the MVC in each muscle and subject tested. Time courses were aligned to each kinematic event to determine the amount of EMG activity associated with each phase of the movement. To assess EMG coactivation patterns during hand opening we selected the EDC and FDS muscles because they contribute to extension and flexion of the interphalangeal, metacarpophalangeal, and wrist joints playing an important role during hand opening (Kendall et al. 1983). During hand closing, we selected the APB and FDI because both muscles contribute to the fine grading of forces during closing of the hand and grasping (Maier and Hepp-Reymond 1995). EMG coactivation patterns between EDC/FDS and APB/FDI muscle pairs were examined by calculating a ratio of the difference between mean rectified EMG activity over the sum of the mean EMG activity in the each muscle pair [(EDC−FDS)/(EDC+FDS) and (APB−FDI)/(APB+FDI) muscle activation ratio] over each phase of the movement (Stahl et al. 2015).
Data analysis.
Normal distribution was tested by the Shapiro-Wilk test and homogeneity of variances by the Levene’s test of equality and Mauchly’s test of sphericity. When normal distribution could not be assumed, data were log transformed. When sphericity could not be assumed, the Greenhouse-Geisser correction statistic was used. Since no differences were found in any of the kinematic variables measured in the left and right arms in control subjects, we only report data acquired from the dominant arm. One-way repeated-measures ANOVAs were performed to determine the effect of Side (controls dominant, SCI more affected arm, SCI less affected arm) on reaction time, peak arm velocity, maximum hand aperture, total movement duration, finger contact, finger trajectory variability, and EMG activity. Two-way repeated-measures ANOVA was performed to determine the effect of Phase (acceleration, hand opening, and hand closing) and Side on movement duration. We also tested the effect of Digit (index finger and thumb) and Side on finger contact time and finger trajectory. Paired t-tests were used to compare EMG activity across muscles on each group separately and the JJT scores across arms in SCI participants. Tukey post hoc analysis was used to test for significant comparisons. Pearson correlation analysis was used as needed corrected for multiple comparisons. Significance was set at P < 0.05. Group data are presented as means ± SD in the text.
RESULTS
Reaction time and total movement duration.
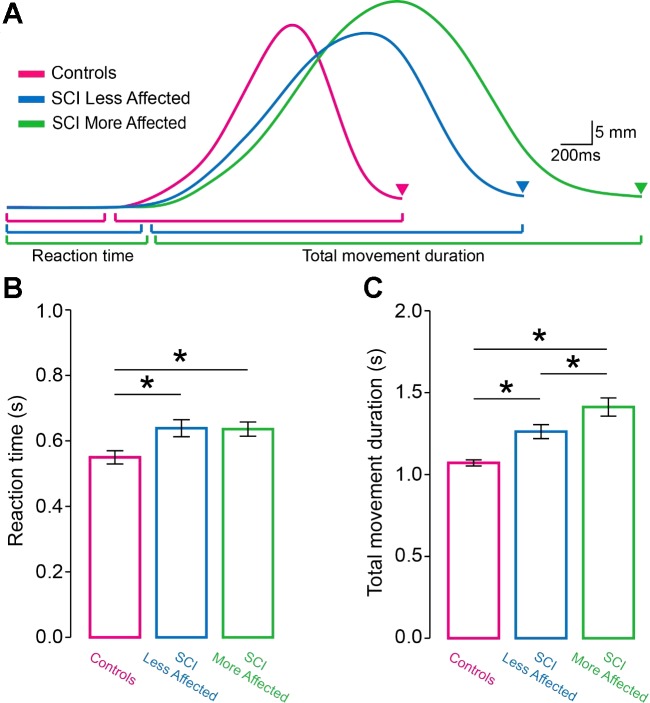
Figure 2A illustrates aperture size traces from representative participants. Total movement duration was prolonged in SCI subjects compared with controls. Note that total movement duration was also increased in the more affected compared with the less affected arm of SCI subjects.
Fig. 2.
Reaction time and total movement duration. A: raw traces showing hand aperture size of the dominant arm of controls (purple trace), and in the less affected (blue trace) and more affected (green trace) arm of spinal cord injury (SCI) participants during reach-to-grasp movements. Triangles indicate the time at which both the index finger and thumb contacted the object. Graphs show the group data (controls, n = 20 and SCI, n = 20). The abscissa shows all groups tested [controls (purple bar), SCI less affected arm (blue bar), and SCI more affected arm (green bar)]. The ordinate shows the reaction time (B) and total movement duration (C) expressed in seconds (s). Note that reaction time was shorter in controls compared with both arms of SCI participant. Also, note that the total movement duration was more prolonged in the more affected arm of SCI participants compared with controls and the less affected arm. Error bars indicate SEs. *P < 0.05.
Repeated-measures ANOVA showed an effect of Side on reaction time (F2,38 = 5.6, P = 0.007; Fig. 2B). Post hoc analysis revealed that reaction time was shorter in controls (549.5 ± 89.9 ms) compared with the more (638.7 ± 116.7 ms, P = 0.01) and less (635.8 ± 97.5 ms, P = 0.006) affected arm of SCI subjects. No differences were found in reaction time between arms of SCI subjects (P = 0.8). We also found an effect of Side on total movement duration (F2,38 = 21.9, P < 0.001; Fig. 2C). Total movement duration was prolonged in the more affected arm of SCI subjects (1,412.0 ± 249.2 ms) compared with controls (1,070.9 ± 83.2 ms, P < 0.001) and the less affected arm (1,261.9 ± 191.1 ms, P = 0.002). Total movement duration was prolonged in the less affected arm of SCI subjects compared with controls (P < 0.001).
Phases of the reaching and grasping movements.
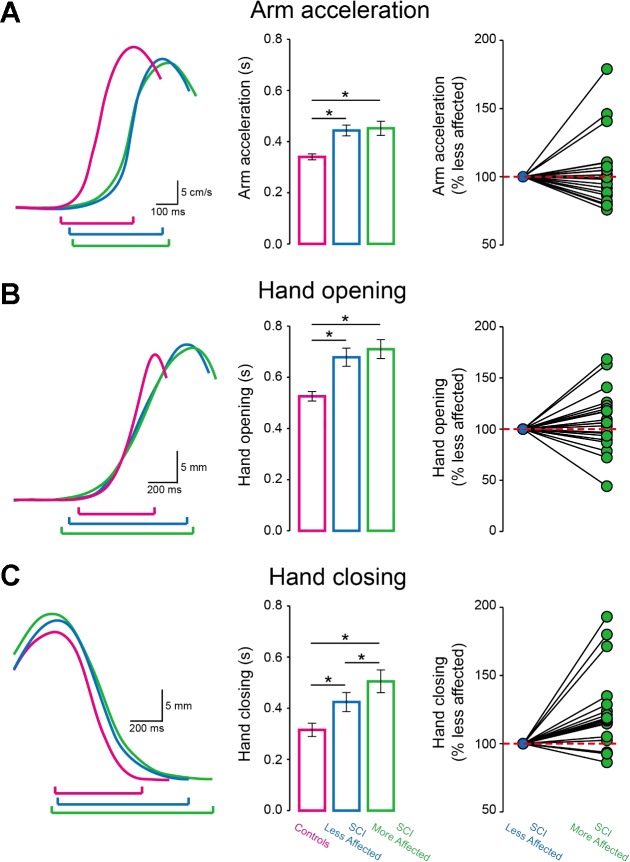
Figure 3, A–C (left column) illustrate arm velocity and aperture size traces from representative participants. Note that individuals with SCI showed prolonged movement duration arm acceleration, hand opening and closing compared with controls. Also, the more affected arm of SCI subjects showed an additional increase in movement duration during hand closing, but not during arm acceleration and hand opening, compared with the less affected arm.
Fig. 3.
Phases of the reach-to-grasp movement. Raw traces show arm velocity and aperture size of the dominant arm of controls (purple trace), and in the less affected (blue trace) and more affected (green trace) arm of SCI participants during different phases of reach-to-grasp movements. The graphs show group data (controls, n = 20 and SCI, n = 20). The abscissa shows the phases tested [Arm acceleration (A), Hand opening (B), and Hand closing (C) in controls (purple bars), SCI less affected arm (blue bars), and SCI more affected arm (green bars)]. The ordinate shows the movement duration in seconds (s) at each phase. The horizontal dotted lines represent the movement duration at each phase in SCI less affected arm. Note an additional increase in movement duration during hand closing, but not arm acceleration and hand closing in the more affected compared with the less affected arm of SCI participants. Error bars indicate SEs. *P < 0.05.
Repeated-measures ANOVA showed a significant effect of Phase (F1.3, 24.7 = 38.9, P < 0.001) and Side (F2, 38 = 24.9, P < 0.001) but not in their interaction (F4, 76 = 1.2, P = 0.3) on movement duration. Post hoc analysis showed that SCI subjects showed prolonged arm acceleration (controls = 340.2 ± 52.9 ms; SCI more affected arm = 452.1 ± 124.2 ms, P = 0.001; SCI less affected arm = 443.4 ± 94.5 ms, P < 0.001; Fig. 3A, middle column), hand opening (controls = 525.7 ± 82.7 ms; SCI more affected arm = 710.2 ± 165.3 ms, P < 0.001; SCI less affected arm = 678.5 ± 158.8 ms, P < 0.001; Fig. 3B, middle column) and hand closing (controls = 315.4 ± 115.4 ms; SCI more affected arm = 505.3 ± 199.1 ms, P = 0.005; SCI less affected arm = 424.3 ± 166.6 ms, P = 0.02; Fig. 3C, middle column) compared with controls in both arms. Note that the more affected arm of SCI subjects showed an additional increase in movement duration during hand closing compared with the less affected arm (P = 0.002) in the majority of SCI subjects (16/20; Fig. 3C, right column). However, no differences were found during arm acceleration (P = 0.7) and hand opening (P = 0.4) between both arms of SCI subjects.
Peak arm velocity and maximum aperture size.
Repeated-measures ANOVA showed an effect of Side on peak arm velocity (F2,38 = 17.5, P < 0.001). Post hoc testing showed that peak arm velocity was higher in controls (0.92 ± 0.09 m/s) compared with the more (0.75 ± 0.12 m/s, P < 0.001) and less (0.78 ± 0.10 m/s, P < 0.001) affected arm of SCI subjects. No differences were found between both arms of SCI subjects (P = 0.3). Maximum aperture size was similar between controls and both arms of SCI subjects (controls = 21.3 ± 6.1 mm, SCI more affected arm = 22.1 ± 9.2 mm, SCI less affected arm = 23.5 ± 7.1 mm; F2,38 = 0.5, P = 0.6).
Finger contact.
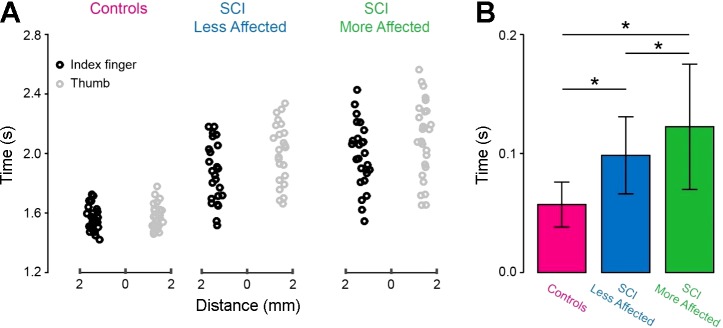
Figure 4A illustrates the time when the index finger and thumb contacted the object in representative participants. In SCI individuals, the time between index finger and thumb object contact was prolonged compared with controls in both arms. Note that the index finger is the first finger to contact the object in the majority of SCI (SCI more affected arm = 15/20; P = 0.02; SCI less affected arm = 13/20; P = 0.005) and control (15/20; P = 0.01; Fig. 4A) subjects.
Fig. 4.
Finger contact. A: the time when the index finger (black circles) and thumb (gray circles) contacted the object with the dominant arm of controls, and in the less affected and more affected arm of SCI subjects. B: the graphs show group data (controls, n = 20 and SCI, n = 20). The abscissa shows all groups tested [controls (purple bar), SCI less affected arm (blue bar), and SCI more affected arm (green bar)]. The ordinate shows the time between index finger and thumb contact with the object expressed in seconds (s) after the Go signal. Note that the time when the fingers contacted the object increased in the more affected arm of SCI participants compared with controls and the less affected arm. Error bars indicate SEs. *P < 0.05.
Repeated-measures ANOVA showed an effect of Side (F2,38 = 18.5, P < 0.001; Fig. 4B) on the time between finger contact. Post hoc analysis showed that the time between index finger and thumb object contact was higher in the more affected arm of SCI subjects (122.4 ± 52.6 ms) compared with controls (57.1 ± 18.9 ms, P < 0.001) and the less affected arm (98.5 ± 32.4 ms, P < 0.001). The time between finger contact was higher in the less affected arm of SCI subjects compared with controls (P = 0.02).
Finger trajectory variability.
Figure 5A illustrates index finger and thumb trajectories during the reach-to-grasp movement in representative subjects. Note that the more affected arm of SCI subjects showed higher trial-to-trial variability in index finger and thumb movement trajectories compared with controls and the less affected arm.
Fig. 5.
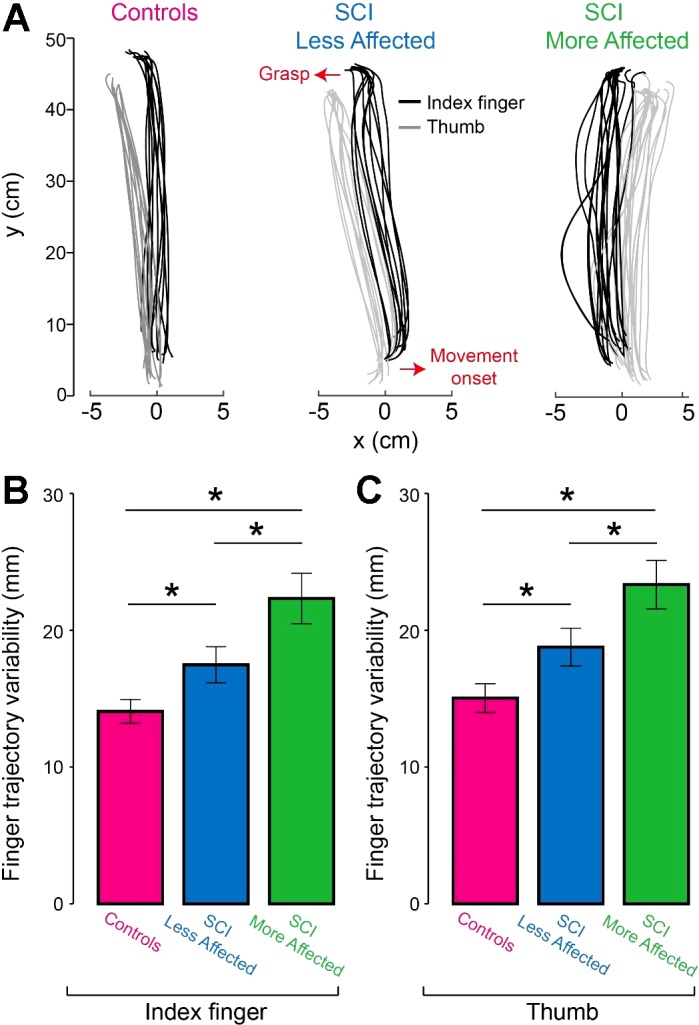
Finger trajectory variability. A: raw traces show index finger (black traces) and thumb (gray traces) trajectories of the dominant arm of controls, and in the less affected and more affected arm of SCI subjects. Arrows indicate movement onset and grasp. B and C: the graphs show group data (controls, n = 20 and SCI, n = 20). The abscissa shows the finger tested [index finger and thumb in controls (purple bars), SCI less affected arm (blue bars), and SCI more affected arm (green bars)]. The ordinate shows the finger trajectory variability in millimeters (mm) in the index finger and thumb. Note that the more affected arm of SCI participants showed higher variability in the index finger and thumb compared with controls and the less affected arm. *P < 0.05.
Repeated-measures ANOVA revealed a significant effect of Side (F1.5,28.1 = 17.0, P < 0.001), but not Digit (F1,19 = 1.3, P = 0.2) nor in their interaction (F1.4,27.3 = 0.04, P = 0.9) on finger trajectory variability. Post hoc analysis showed that finger trajectory variability was higher in the more affected arm of SCI subjects (22.3 ± 8.3 mm) compared with controls (14.1 ± 3.9 mm; P < 0.001) and the less affected arm (17.5 ± 5.9 mm, P = 0.03; Fig. 5B) in the index finger. Similarly, thumb trajectory variability was higher in the more affected arm of SCI subjects (23.3 ± 7.9 mm) compared with controls (15.0 ± 4.7 mm; P < 0.001) and the less affected arm (18.7 ± 6.2 mm; P = 0.04; Fig. 5C). Finger trajectory variability was higher in the less affected arm of SCI subjects compared with controls in the index finger (P = 0.03) and thumb (P = 0.03). Also note that finger trajectory variability was increased in the more affected arm compared with the less affected arm in the majority of SCI subjects (index finger = 18/20; thumb = 17/20).
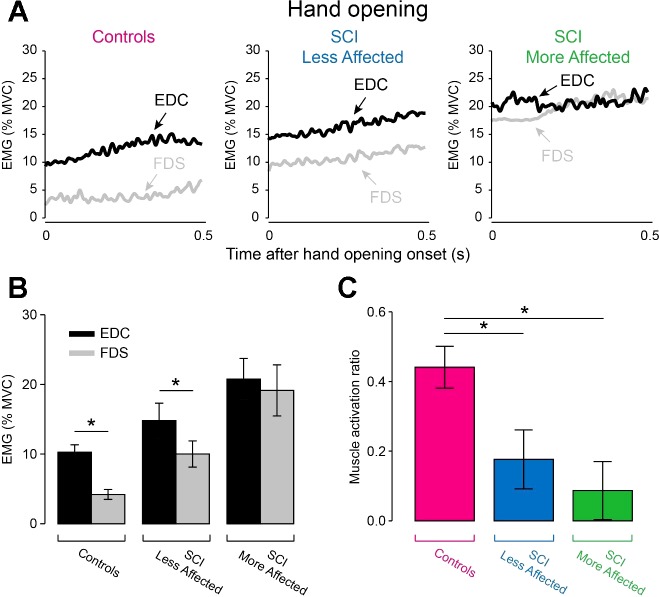
EMG activity.
Figure 6A illustrates EMG activity in EDC and FDS muscles during hand opening in representative participants. In this phase, we found that the EDC muscle was activated to a larger extent than the FDS muscle in controls (EDC = 10.3 ± 4.8% of MVC, FDS = 4.2 ± 3.2% of MVC, P < 0.001) and in the less affected arm of SCI subjects (EDC = 14.8 ± 11.2% of MVC, FDS = 10.0 ± 3.2% of MVC, P = 0.02) but not in the more affected arm of SCI subjects (EDC = 20.8 ± 13.3% of MVC, FDS = 19.1 ± 16.4% of MVC, P = 0.6; Fig. 6B). Repeated-measures ANOVA revealed a significant effect of Side (F2,38 = 6.8, P = 0.008; Fig. 6C) on EDC and FDS muscle activation ratio. Post hoc analysis showed that EDC and FDS muscle activation ratio was larger in controls (0.44 ± 0.27) compared with the more (0.09 ± 0.37, P = 0.001) and the less (0.18 ± 0.38, P = 0.01) affected arm of SCI participants. EDC and FDS muscle activation ratio was similar between both arms of SCI subjects (P = 0.4).
Fig. 6.
EMG activity during hand opening. A: mean rectified EMG activity expressed as a % of maximal voluntary contraction (MVC) in the EDC (black traces) and FDS (gray traces) in the dominant arm of controls, and in the less affected and more affected arm of SCI subjects measured during hand opening. Graphs show the group data (controls, n = 20 and SCI, n = 20) in EMG activity in EDC (black bars) and FDS (gray bars) muscles (B) and the muscle activation ratio between EDC and FDS muscles (C). In B, the abscissa shows all groups tested [controls (dominant arm), SCI less affected arm, and SCI more affected arm] and the ordinate shows the amount of EMG activity in EDC and FDS muscles expressed as a percentage of the MVC in each muscle (% MVC). In C, the graph shows the group data (controls, n = 20 and SCI, n = 20) muscle activation ratio between EDC and FDS and the abscissa shows all groups tested [controls (purple bar), SCI less affected arm (blue bar), and SCI more affected arm (green bar)] and the ordinate shows muscle activation ratio between EDC and FDS [calculated as a ratio of the difference between mean rectified EMG activity in the EDC (E) and FDS (F) over the sum of the mean E and F activity: (E−F)/(E+F)]. Error bars indicate SEs. *P < 0.05.
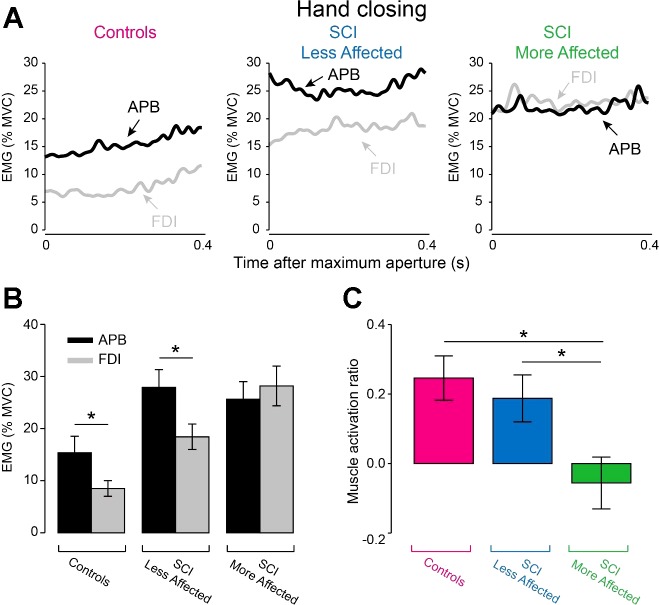
Figure 7A illustrates EMG activity in APB and FDI muscles during hand closing in representative participants. Here, EMG activity in the APB muscle was larger compared with FDI muscle in controls (APB = 15.4 ± 14.5% of MVC, FDI = 8.5 ± 6.7% of MVC, P = 0.01) and in the less affected arm of SCI subjects (APB = 27.9 ± 14.9% of MVC, FDI = 18.4 ± 10.8% of MVC, P = 0.01). However, the APB and FDI muscles were activated to a similar extent in the more affected arm of SCI subjects (APB = 25.6 ± 14.9% of MVC, FDI = 28.2 ± 16.5% of MVC, P = 0.5; Fig. 7B). Repeated-measures ANOVA revealed an effect of Side (F2,38 = 5.0, P = 0.01; Fig. 7C) on APB and FDI muscle activation ratio. Post hoc analysis showed that APB and FDI muscle activation ratio was smaller in the more affected arm of SCI participants (−0.06 ± 0.33) compared with controls (0.25 ± 0.29, P = 0.003) and the less affected arm of SCI participants (0.19 ± 0.30, P = 0.01). No differences were found in APB and FDI muscle activation ratio between the less affected arm of SCI subjects and controls (P = 0.5).
Fig. 7.
EMG activity during hand closing. A: mean rectified EMG activity expressed as a % of MVC in the APB (black traces) and FDI (gray traces) in the dominant arm of controls, and in the less affected and more affected arm of SCI subjects measured during hand closing. Graph shows the group data (controls, n = 20 and SCI, n = 20) in EMG activity in APB (black bars) and FDI (gray bars) muscles (B) and in muscle activation ratio between APB and FDI (C). In B, the abscissa shows all groups tested [controls (dominant arm), SCI less affected arm, and SCI more affected arm] and the ordinate shows the amount of EMG activity in APB and FDI muscles expressed as a percentage of the MVC in each muscle (% MVC). In C, the abscissa shows all groups tested [controls (purple bar), SCI less affected arm (blue bar), and SCI more affected arm (green bar)] and the ordinate shows muscle activation ratio between APB and FDI [calculated as a ratio of the difference between mean rectified EMG activity in the APB (E) and FDI (F) over the sum of the mean E and F activity: (E−F)/(E+F)]. Error bars indicate SEs. *P < 0.05.
Correlation analysis.
EPT values were positively correlated with movement duration during hand closing in the more affected (r = 0.57, P = 0.008; Fig. 8A) but not in the less affected (r = 0.33, P = 0.15; Fig. 8B) arm of SCI participants. Note that in the more affected arm SCI individuals with larger sensory deficits showed longer movement duration during hand closing, whereas no correlation was found between EPT values and movement duration during hand opening in the more affected (r = 0.23, P = 0.3) and the less affected (r = 0.18, P = 0.4) arm of SCI participants.
Fig. 8.
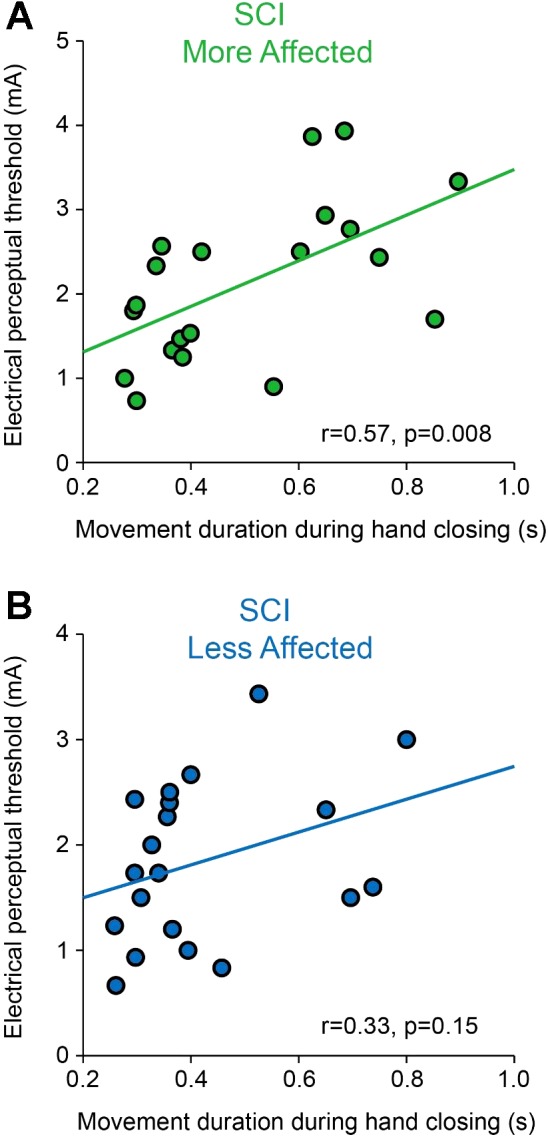
Correlation between electrical perceptual threshold (EPT) values and movement duration during hand closing. Graphs show a correlation analysis between EPT values and movement duration during hand closing in the less affected (A) and more affected (B) arm of SCI participants. Note that SCI subjects with larger sensory deficits were those who showed longer movement duration during hand closing.
DISCUSSION
Our findings indicate that humans with incomplete chronic cervical SCI show distinct kinematic deficits during the different phases of reach-to-grasp movements. We found that movement duration was prolonged during arm acceleration and hand opening and closing in both arms of SCI participants compared with controls. Notably, the more affected arm showed an additional increase in movement duration when closing the hand compared with the less affected arm and participants with longer movement duration were those with more pronounced deficits in sensory function. The degree of muscle activation between the first dorsal interosseous and abductor pollicis brevis muscle increased during hand closing in the more compared with the less affected arm of SCI participants. We conclude that deficits in movement kinematics and EMG activity are more pronounced in the more affected arm of individuals with SCI during the hand closing phase of reach-to-grasp movements.
Reach-to-grasp movements after SCI.
As in previous studies, we found that reaction time and total movement duration were increased and peak arm velocity was decreased during reach-to-grasp movements in SCI participants compared with controls (de los Reyes-Guzmán et al. 2010; Laffont et al. 2000; Mateo et al. 2013; Federico and Perez 2017). Here, for the first time we examined the extent to which the different phases of reach-to-grasp movements are affected in the more and less affected arm of humans with incomplete cervical SCI. First, we found that arm acceleration was prolonged to a similar extent in the more and less affected arm of SCI participants compared with control subjects. Prolonged movement duration during arm acceleration could be related to weakness in proximal arm muscles, resulting in an increased time to transport the hand to the target object. This is supported by evidence showing that SCI participants with weaknesses in the triceps brachii paralysis exhibit lower peak arm velocities (Gronley et al. 2000; Wierzbicka and Wiegner 1992). This is also consistent with our results showing that MVCs in proximal arm muscles were decreased in SCI participants compared with controls but to a similar extent in both arms. Second, we found that the time to open the hand was also increased, and to a similar extent, in both arms of SCI subjects compared with controls. It is less likely that the prolonged movement duration during hand opening was related to SCI subjects’ limited capabilities to scale the hand aperture in relation to the size of the object since we found no differences in maximum aperture size between groups. This is consistent with previous results showing that individuals with cervical SCI preserve the ability to modulate hand aperture to accurately reach for objects with different diameters (Stahl et al. 2015). It is possible that the decreased ability to coordinate activation of hand flexor and extensor muscles during hand opening in both arms of SCI participants contributed to the results. Our SCI participants exhibited an increased EMG activity in the FDS muscle during hand opening, resulting in a greater muscle activation ratio between EDC and FDS muscles compared with controls. This agrees with previous results showing an increased activation between flexor and extensor muscles at hand opening in individuals with cervical SCI during reach-to-grasp movements (Stahl et al. 2015).
An intriguing result is that the more affected arm of SCI participants showed an additional increase in movement duration during the closing of the hand compared with the less affected arm. This is interesting because one might expect that all phases of the reach-to-grasp movement might be affected to a similar extent in the more affected arm of SCI participants. Another expectation is that both hand opening and closing phases might be affected to a similar extent in the more affected arm, which was not the case in our investigation. During hand closing, we found that EMG activity in FDI muscle increased to a larger extent in the more affected compared with the less affected arm of SCI participants and controls. The larger activation of the FDI muscle resulted in greater activation ratio between APB and FDI muscles in the more affected arm at the time to close the hand. It is possible that a larger muscle activation ratio between finger muscles at the time to close the hand limited the ability to selectively control the finger muscles for rapidly closing of the hand contributing to an additional increase in movement duration. Importantly, note that the time between index finger and thumb object contact also increased in the more affected compared with the less affected arm of SCI participants. Thus this increase in time duration while grasping the object could also contribute to the increased movement duration during hand closing in our SCI participants. Evidence showed that impairments in sensory function increased the duration of hand closing during reach-to-grasp movements (Gentilucci et al. 1994). In agreement, we found that SCI participants with more pronounced sensory deficits in hand muscles were those individuals who showed prolonged movement duration during hand closing in the more affected compared with the less affected arm. This also agrees with evidence showing that cutaneous afferent inputs are largely involved in grasping manipulations (Datta et al. 1989; Johansson et al. 1994) and that in stroke patients the degree of sensory deficits is correlated with grasping function (Blennerhassett et al. 2007). Indeed, in deafferented patients the phase of the reach-to-grasp movement that is more affected is the closing of the hand (Gentilucci et al. 1994), supporting the interpretation of our results. We also found that finger movement trajectory variability increased to a larger extent in the more affected compared with the less affected arm of SCI participants and control subjects. The increases in finger trajectory variability could be related to deficits in trunk control (Reft and Hasan 2002; Cacho et al. 2011) and afferent inputs from the trunk movement (Adamovich et al. 2001). Although we provided trunk support to our SCI participants during testing these individuals had some difficulties with trunk stability which together with the more pronounced sensory deficits might have contributed to our results in the more affected arm. Deafferented patients are able to initiate flexion and extension arm movements of different amplitudes when visual feedback is provided while the trajectories and the end points of the movement continue to be altered (Sanes et al. 1985). The necessity of sensory information and in particular at the final phase of the reach-to-grasp movement agrees with the hypothesis that the final phase of the movement is mainly executed online (Woodsworth 1899). Cortical and subcortical descending pathways, as well as ascending sensory pathways, contribute to the control of reaching and grasping movements (Bawa et al. 2000; Gentilucci et al. 1994; Lemon 2008; Tazoe and Perez 2017). Transmission in some of these pathways is altered in humans with incomplete cervical SCI during static grasping behaviors (Baker and Perez 2017; Bunday et al. 2014). Thus the assessment of these neural sources during the different phases of reach-to-grasp movements might represent an area to consider during assessment of upper-limb function following SCI.
Functional consequences.
It is important to note that SCI participants showed asymmetrical kinematic and EMG muscle activation impairments in both arms. This is consistent with previous studies showing that individuals with cervical SCI typically present bilateral anatomical damage (Bunge et al. 1993; Kakulas 2004; Oxland et al. 2010). After SCI, animal models showed a significant damage of neuronal circuits crossing the injury epicenter (Oudega and Perez 2012) with corticospinal (Bareyre et al. 2004; Rosenzweig et al. 2010) and spinal interneurons (Fenrich et al. 2007; Fenrich and Rose 2009) sprouting extensively across the midline. In humans, the excitability of corticospinal projections to intrinsic hand muscles is disrupted during grasping actions following SCI (Bunday et al. 2014), suggesting that transmission in motor pathways involved in the fine control of hand muscles is impaired after SCI. Behavioral studies also showed that during bilateral arm movements the more affected arm of SCI subjects delays the motion of the less affected arm, resulting in large movement asymmetries between the arms during bilateral tasks (Calabro and Perez 2016). Our study extends previous findings and for the first time demonstrates asymmetric functional impairments during the closing of the hand during unilateral reach-to-grasp movements in humans with cervical SCI. Previous evidence showed that the object size might have a great impact in grasping actions during reach-to-grasp movements (Jeannerod 1981, 1984) and the ability to open the hand when grasping objects can be considerably affected in participants with cervical SCI. However, previous results demonstrated that the ability to scale hand aperture with object size is preserved in individuals with cervical SCI (Stahl et al. 2015), suggesting that is less likely that the object size contributed to our results. The implications of these kinematic and EMG deficits present during hand closing for hand rehabilitation after SCI remain to be determined but our results indicate that closing of the hand might represent a specific phase that needs to be reinforced to maximize the preservation of grasping function (Koshland et al. 2005) in humans with SCI. Since the demands for precise control during grasping gradually increase when approaching the object (Paulignan et al. 1991) and the degree of task difficulty increases the duration of a movement (Dohle et al. 2000) this information might be relevant to consider while training to enhance grasping function.
GRANTS
This research was supported by National Institute of Neurological Disorders and Stroke (NINDS) Grants R01NS076589-01 (M. A. Perez) and R01NS090622-01 (M. A. Perez); U.S. Department of Veterans Affairs (VA) Grants I01RX000815 (M. A. Perez) and I01RX001807 (M. A. Perez); and Craig H. Neilsen Foundation Grant 338132.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.L. and M.A.P. conceived and designed research; Y.L. and M.A.P. performed experiments; Y.L. and M.A.P. analyzed data; Y.L. and M.A.P. interpreted results of experiments; Y.L. and M.A.P. prepared figures; Y.L. and M.A.P. drafted manuscript; Y.L. and M.A.P. edited and revised manuscript; Y.L. and M.A.P. approved final version of manuscript.
REFERENCES
- Adamovich SV, Archambault PS, Ghafouri M, Levin MF, Poizner H, Feldman AG. Hand trajectory invariance in reaching movements involving the trunk. Exp Brain Res 138: 288–303, 2001. doi: 10.1007/s002210100694. [DOI] [PubMed] [Google Scholar]
- Bailey AZ, Mi YP, Nelson AJ. Short-latency afferent inhibition in chronic spinal cord injury. Transl Neurosci 6: 235–243, 2015. doi: 10.1515/tnsci-2015-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN, Perez MA. Reticulospinal contributions to gross hand function after human spinal cord injury. J Neurosci 37: 9778–9784, 2017. doi: 10.1523/JNEUROSCI.3368-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci 7: 269–277, 2004. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Bawa P, Chalmers GR, Jones KE, Søgaard K, Walsh ML. Control of the wrist joint in humans. Eur J Appl Physiol 83: 116–127, 2000. doi: 10.1007/s004210000270. [DOI] [PubMed] [Google Scholar]
- Blennerhassett JM, Matyas TA, Carey LM. Impaired discrimination of surface friction contributes to pinch grip deficit after stroke. Neurorehabil Neural Repair 21: 263–272, 2007. doi: 10.1177/1545968306295560. [DOI] [PubMed] [Google Scholar]
- Britten L, Coats R, Ichiyama R, Raza W, Jamil F, Astill S. Bimanual reach to grasp movements after cervical spinal cord injury. PLoS One 12: e0175457, 2017. doi: 10.1371/journal.pone.0175457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunday KL, Tazoe T, Rothwell JC, Perez MA. Subcortical control of precision grip after human spinal cord injury. J Neurosci 34: 7341–7350, 2014. doi: 10.1523/JNEUROSCI.0390-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge RP, Puckett WR, Becerra JL, Marcillo A, Quencer RM. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol 59: 75–89, 1993. [PubMed] [Google Scholar]
- Cacho EW, de Oliveira R, Ortolan RL, Varoto R, Cliquet A Jr. Upper limb assessment in tetraplegia: clinical, functional and kinematic correlations. Int J Rehabil Res 34: 65–72, 2011. doi: 10.1097/MRR.0b013e32833d6cf3. [DOI] [PubMed] [Google Scholar]
- Calabro FJ, Perez MA. Bilateral reach-to-grasp movement asymmetries after human spinal cord injury. J Neurophysiol 115: 157–167, 2016. doi: 10.1152/jn.00692.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carteron A, McPartlan K, Gioeli C, Reid E, Turturro M, Hahn B, Benson C, Zhang W. Temporary nerve block at selected digits revealed hand motor deficits in grasping tasks. Front Hum Neurosci 10: 596, 2016. doi: 10.3389/fnhum.2016.00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiello U. The neuroscience of grasping. Nat Rev Neurosci 6: 726–736, 2005. doi: 10.1038/nrn1744. [DOI] [PubMed] [Google Scholar]
- Chabot R, York DH, Watts C, Waugh WA. Somatosensory evoked potentials evaluated in normal subjects and spinal cord-injured patients. J Neurosurg 63: 544–551, 1985. doi: 10.3171/jns.1985.63.4.0544. [DOI] [PubMed] [Google Scholar]
- Datta AK, Harrison LM, Stephens JA. Task-dependent changes in the size of response to magnetic brain stimulation in human first dorsal interosseous muscle. J Physiol 418: 13–23, 1989. doi: 10.1113/jphysiol.1989.sp017826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de los Reyes-Guzmán A, Gil-Agudo A, Peñasco-Martín B, Solís-Mozos M, del Ama-Espinosa A, Pérez-Rizo E. Kinematic analysis of the daily activity of drinking from a glass in a population with cervical spinal cord injury. J Neuroeng Rehabil 7: 41, 2010. doi: 10.1186/1743-0003-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohle C, Ostermann G, Hefter H, Freund HJ. Different coupling for the reach and grasp components in bimanual prehension movements. Neuroreport 11: 3787–3791, 2000. doi: 10.1097/00001756-200011270-00039. [DOI] [PubMed] [Google Scholar]
- Federico P, Perez MA. Altered corticospinal function during movement preparation in humans with spinal cord injury. J Physiol 595: 233–245, 2017. doi: 10.1113/JP272266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenrich KK, Rose PK. Spinal interneuron axons spontaneously regenerate after spinal cord injury in the adult feline. J Neurosci 29: 12145–12158, 2009. doi: 10.1523/JNEUROSCI.0897-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenrich KK, Skelton N, MacDermid VE, Meehan CF, Armstrong S, Neuber-Hess MS, Rose PK. Axonal regeneration and development of de novo axons from distal dendrites of adult feline commissural interneurons after a proximal axotomy. J Comp Neurol 502: 1079–1097, 2007. doi: 10.1002/cne.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentilucci M, Castiello U, Corradini ML, Scarpa M, Umiltà C, Rizzolatti G. Influence of different types of grasping on the transport component of prehension movements. Neuropsychologia 29: 361–378, 1991. doi: 10.1016/0028-3932(91)90025-4. [DOI] [PubMed] [Google Scholar]
- Gentilucci M, Toni I, Chieffi S, Pavesi G. The role of proprioception in the control of prehension movements: a kinematic study in a peripherally deafferented patient and in normal subjects. Exp Brain Res 99: 483–500, 1994. doi: 10.1007/BF00228985. [DOI] [PubMed] [Google Scholar]
- Gronley JK, Newsam CJ, Mulroy SJ, Rao SS, Perry J, Helm M. Electromyographic and kinematic analysis of the shoulder during four activities of daily living in men with C6 tetraplegia. J Rehabil Res Dev 37: 423–432, 2000. [PubMed] [Google Scholar]
- Jeannerod M. Intersegmental coordination during reaching at natural visual objects. In: Attention and Performance IX, edited by Long J, Baddeley A. Hillsdale, NJ: Erlbaum, 1981, p. 153–168. [Google Scholar]
- Jeannerod M. The timing of natural prehension movements. J Mot Behav 16: 235–254, 1984. doi: 10.1080/00222895.1984.10735319. [DOI] [PubMed] [Google Scholar]
- Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Arch Phys Med Rehabil 50: 311–319, 1969. [PubMed] [Google Scholar]
- Johansson RS, Flanagan JR. Coding and use of tactile signals from the fingertips in object manipulation tasks. Nat Rev Neurosci 10: 345–359, 2009. doi: 10.1038/nrn2621. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Lemon RN, Westling G. Time-varying enhancement of human cortical excitability mediated by cutaneous inputs during precision grip. J Physiol 481: 761–775, 1994. doi: 10.1113/jphysiol.1994.sp020480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakulas BA. Neuropathology: the foundation for new treatments in spinal cord injury. Spinal Cord 42: 549–563, 2004. doi: 10.1038/sj.sc.3101670. [DOI] [PubMed] [Google Scholar]
- Kendall FP, McCreary EK, Kendall HO. Muscles, Testing and Function: Testing and Function. Baltimore, MD: Lippincott Williams and Wilkins, 1983. [Google Scholar]
- Koshland GF, Galloway JC, Farley B. Novel muscle patterns for reaching after cervical spinal cord injury: a case for motor redundancy. Exp Brain Res 164: 133–147, 2005. doi: 10.1007/s00221-005-2218-9. [DOI] [PubMed] [Google Scholar]
- Kukke SN, Triolo RJ. The effects of trunk stimulation on bimanual seated workspace. IEEE Trans Neural Syst Rehabil Eng 12: 177–185, 2004. doi: 10.1109/TNSRE.2004.827222. [DOI] [PubMed] [Google Scholar]
- Laffont I, Briand E, Dizien O, Combeaud M, Bussel B, Revol M, Roby-Brami A. Kinematics of prehension and pointing movements in C6 quadriplegic patients. Spinal Cord 38: 354–362, 2000. doi: 10.1038/sj.sc.3100999. [DOI] [PubMed] [Google Scholar]
- Laffont I, Hoffmann G, Dizien O, Revol M, Roby-Brami A. How do C6/C7 tetraplegic patients grasp balls of different sizes and weights? Impact of surgical musculo-tendinous transfers. Spinal Cord 45: 502–512, 2007. doi: 10.1038/sj.sc.3102047. [DOI] [PubMed] [Google Scholar]
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci 31: 195–218, 2008. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- Macklin RA, Bae J, Orell M, Anderson KD, Ellaway PH, Perez MA. Time-dependent discrepancies between assessments of sensory function after incomplete cervical spinal cord injury. J Neurotrauma 34: 1778–1786, 2017. doi: 10.1089/neu.2016.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklin RA, Brooke VJ, Calabro FJ, Ellaway PH, Perez MA. Discrepancies between clinical assessments of sensory function and electrical perceptual thresholds after incomplete chronic cervical spinal cord injury. Spinal Cord 54: 16–23, 2016. doi: 10.1038/sc.2015.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier MA, Hepp-Reymond MC. EMG activation patterns during force production in precision grip. I. Contribution of 15 finger muscles to isometric force. Exp Brain Res 103: 108–122, 1995. doi: 10.1007/BF00241969. [DOI] [PubMed] [Google Scholar]
- Marino RJ, Barros T, Biering-Sorensen F, Burns SP, Donovan WH, Graves DE, Haak M, Hudson LM, Priebe MM. International standards for neurological classification of spinal cord injury. J Spinal Cord Med 26, Suppl 1: S50–S56, 2003. doi: 10.1080/10790268.2003.11754575. [DOI] [PubMed] [Google Scholar]
- Marteniuk RG, Leavitt JL, MacKenzie CL, Athenes S. Functional relationships between grasp and transport components in a prehension task. Hum Mov Sci 9: 149–176, 1990. doi: 10.1016/0167-9457(90)90025-9. [DOI] [Google Scholar]
- Mateo S, Revol P, Fourtassi M, Rossetti Y, Collet C, Rode G. Kinematic characteristics of tenodesis grasp in C6 quadriplegia. Spinal Cord 51: 144–149, 2013. doi: 10.1038/sc.2012.101. [DOI] [PubMed] [Google Scholar]
- Morris R, Tosolini AP, Goldstein JD, Whishaw IQ. Impaired arpeggio movement in skilled reaching by rubrospinal tract lesions in the rat: a behavioral/anatomical fractionation. J Neurotrauma 28: 2439–2451, 2011. doi: 10.1089/neu.2010.1708. [DOI] [PubMed] [Google Scholar]
- Murphy JO, Audu ML, Lombardo LM, Foglyano KM, Triolo RJ. Feasibility of closed-loop controller for righting seated posture after spinal cord injury. J Rehabil Res Dev 51: 747–760, 2014. doi: 10.1682/JRRD.2013.09.0200. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Ninomiya T, Yamashita T, Takada M. Reorganization of corticospinal tract fibers after spinal cord injury in adult macaques. Sci Rep 5: 11986, 2015. doi: 10.1038/srep11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudega M, Perez MA. Corticospinal reorganization after spinal cord injury. J Physiol 590: 3647–3663, 2012. doi: 10.1113/jphysiol.2012.233189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxland TR, Bhatnagar T, Choo AM, Dvorak MF, Tetzlaff W, Cripton PA. Biomechanical aspects of spinal cord injury. In: Neural Tissue Biomechanics, edited by Bilston LE. Berlin: Springer, 2010, p. 159–180. doi: 10.1007/8415_2010_37. [DOI] [Google Scholar]
- Ozdemir RA, Perez MA. Effect of afferent input on sensory and motor cortex after human spinal cord injury. J Neurophysiol 119: 123–145, 2018. doi: 10.1152/jn.00354.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulignan Y, MacKenzie C, Marteniuk R, Jeannerod M. Selective perturbation of visual input during prehension movements. 1. The effects of changing object position. Exp Brain Res 83: 502–512, 1991. doi: 10.1007/BF00229827. [DOI] [PubMed] [Google Scholar]
- Reft J, Hasan Z. Trajectories of target reaching arm movements in individuals with spinal cord injury: effect of external trunk support. Spinal Cord 40: 186–191, 2002. doi: 10.1038/sj.sc.3101277. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Courtine G, Jindrich DL, Brock JH, Ferguson AR, Strand SC, Nout YS, Roy RR, Miller DM, Beattie MS, Havton LA, Bresnahan JC, Edgerton VR, Tuszynski MH. Extensive spontaneous plasticity of corticospinal projections after primate spinal cord injury. Nat Neurosci 13: 1505–1510, 2010. doi: 10.1038/nn.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saling M, Alberts J, Stelmach GE, Bloedel JR. Reach-to-grasp movements during obstacle avoidance. Exp Brain Res 118: 251–258, 1998. doi: 10.1007/s002210050279. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Mauritz KH, Dalakas MC, Evarts EV. Motor control in humans with large-fiber sensory neuropathy. Hum Neurobiol 4: 101–114, 1985. [PubMed] [Google Scholar]
- Schrimsher GW, Reier PJ. Forelimb motor performance following dorsal column, dorsolateral funiculi, or ventrolateral funiculi lesions of the cervical spinal cord in the rat. Exp Neurol 120: 264–276, 1993. doi: 10.1006/exnr.1993.1060. [DOI] [PubMed] [Google Scholar]
- Stackhouse SK, Murray M, Shumsky JS. Effect of cervical dorsolateral funiculotomy on reach-to-grasp function in the rat. J Neurotrauma 25: 1039–1047, 2008. doi: 10.1089/neu.2007.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl VA, Hayes HB, Buetefisch CM, Wolf SL, Trumbower RD. Modulation of hand aperture during reaching in persons with incomplete cervical spinal cord injury. Exp Brain Res 233: 871–884, 2015. doi: 10.1007/s00221-014-4163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone KD, Gonzalez CL. The contributions of vision and haptics to reaching and grasping. Front Psychol 6: 1403, 2015. doi: 10.3389/fpsyg.2015.01403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazoe T, Perez MA. Cortical and reticular contributions to human precision and power grip. J Physiol 595: 2715–2730, 2017. doi: 10.1113/JP273679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicka MM, Wiegner AW. Effects of weak antagonist on fast elbow flexion movements in man. Exp Brain Res 91: 509–519, 1992. doi: 10.1007/BF00227847. [DOI] [PubMed] [Google Scholar]
- Witney AG, Wing A, Thonnard JL, Smith AM. The cutaneous contribution to adaptive precision grip. Trends Neurosci 27: 637–643, 2004. doi: 10.1016/j.tins.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Woodsworth RS. The accuracy of voluntary movements. Psychol Res Monogr 3: 1–114, 1899. [Google Scholar]