Abstract
We explored predictions of a scheme that views position and force perception as a result of measuring proprioceptive signals within a reference frame set by ongoing efferent process. In particular, this hypothesis predicts force illusions caused by muscle vibration and mediated via changes in both afferent and efferent components of kinesthesia. Healthy subjects performed accurate steady force production tasks by pressing with the four fingers of one hand (the task hand) on individual force sensors with and without visual feedback. At various times during the trials, subjects matched the perceived force using the other hand. High-frequency vibration was applied to one or both of the forearms (over the hand and finger extensors). Without visual feedback, subjects showed a drop in the task hand force, which was significantly smaller under the vibration of that forearm. Force production by the matching hand was consistently higher than that of the task hand. Vibrating one of the forearms affected the matching hand in a manner consistent with the perception of higher magnitude of force produced by the vibrated hand. The findings were consistent between the dominant and nondominant hands. The effects of vibration on both force drift and force mismatching suggest that vibration led to shifts in both signals from proprioceptors and the efferent component of perception, the referent coordinate and/or coactivation command. The observations fit the hypothesis on combined perception of kinematic-kinetic variables with little specificity of different groups of peripheral receptors that all contribute to perception of forces and coordinates.
NEW & NOTEWORTHY We show that vibration of hand/finger extensors produces consistent errors in finger force perception. Without visual feedback, finger force drifted to lower values without a drift in the matching force produced by the other hand; hand extensor vibration led to smaller finger force drift. The findings fit the scheme with combined perception of kinematic-kinetic variables and suggest that vibration leads to consistent shifts of the referent coordinate and, possibly, of coactivation command to the effector.
Keywords: force drift, kinesthetic perception, motor control, referent coordinate, vibration
INTRODUCTION
The perception of mechanical variables typically associated with kinesthetic perception (coordinates and forces), and reporting on or reproducing those variables, may be viewed as a problem of measuring those variables based on available physiological signals. To measure any physical variable, one has to have a frame of reference (e.g., a point from which to measure) and a measuring device (e.g., a ruler). This basic idea has been developed within the hypothesis that the neural control of movement can be adequately described as specifying time patterns of spatial referent coordinates (RCs) for salient variables (Feldman 2015; Latash 2010). At the single-muscle level, this hypothesis is equivalent to the classical equilibrium-point hypothesis (Feldman 1966, 1986), and RC is equivalent to threshold of the stretch reflex (λ).
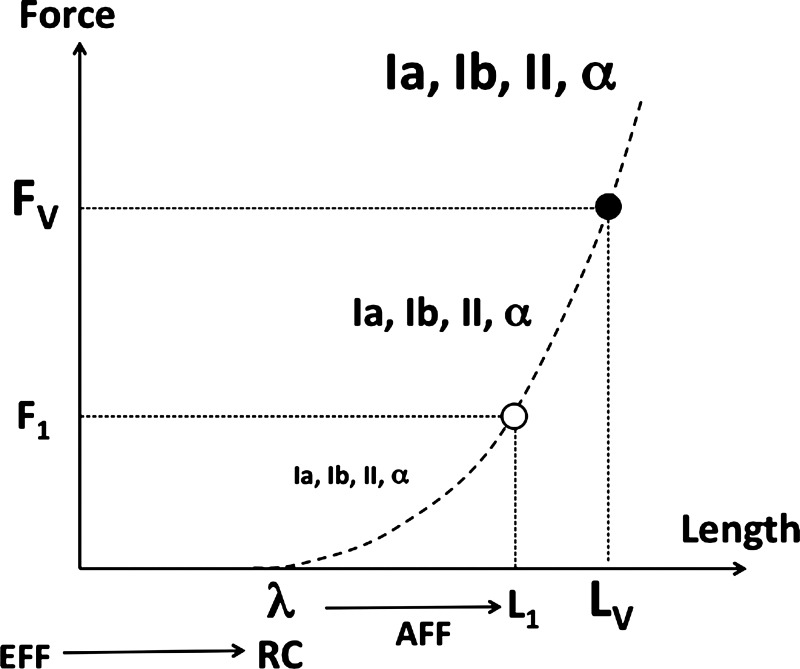
Figure 1 illustrates the perception of muscle length in static conditions within this framework. An efferent process is associated with setting a value of λ, which plays the role of RC. Afferent signals, in particular those from muscle spindles, carry information on the deviation of actual muscle length from λ. Deviations from λ along the characteristic lead to an increase in the activity of many groups of neurons, including α-motoneurons (due to the reflex action), primary and secondary spindle endings (due to an increase in muscle length), and Golgi tendon organs (due to an increase in muscle force). There is therefore an abundance of sources of neural signals that can be used to identify a point on the muscle characteristic and, as a result, allow perception of muscle length and force defined by an interaction between muscle and external forces on the force-length characteristic.
Fig. 1.
Illustration of the perception of muscle length (L1) and force (F1) in static conditions based on 2 components: efferent (EFF) and afferent (AFF). The efferent process is associated with setting a value of λ, which plays the role of a referent coordinate (RC). Signals from many sources increase monotonically along the force-length curve (dashed line). These include signals along Ia, Ib, and II afferents as well as those from α-motoneurons. Muscle vibration leads to an increase in the activity of the primary spindle afferents; this increased activity corresponds to a new point along the muscle characteristic (filled circle) corresponding to misperception of an increased length (LV) and an increased force (FV).
The scheme illustrated in Fig. 1 was originally developed to account for kinesthetic illusions induced by high-frequency, low-amplitude muscle vibration (Feldman and Latash 1982a). Commonly, muscle vibration leads to misperception of the current position of the joint spanned by the vibrated muscle; the joint is perceived as being at a new position corresponding to longer length of the muscle (Goodwin et al. 1972; Lackner and Taublieb 1984; Roll and Vedel 1982). Traditional interpretations of these effects have been based on the idea that vibration leads to very high activity of the spindle sensory endings (in particular, the primary endings sensitive to muscle length and velocity; Brown et al. 1967), which is interpreted by the brain as corresponding to muscle elongation. Note, however, that afferent signals by themselves may be unable to distinguish between different muscle length values (joint positions) as shown in direct recordings from afferent fibers during voluntary movements (Hulliger et al. 1982).
We explored two consequences of the scheme illustrated in Fig. 1. First, with the assumption that muscle vibration leads to an increase in the activity of the primary spindle afferents, this increased activity would correspond to a new point along the muscle characteristic (filled circle in Fig. 1) corresponding to misperception of an increased length and an increased force. This hypothesis (hypothesis 1) is drawn by assuming no changes in the efferent component, which we associate with RC (λ) shifts. Note that muscle vibration can lead to the tonic vibration reflex (TVR; DeGail et al. 1966; Hagbarth and Eklund 1966), which may involve changes in both λ and reflex inputs into the α-motoneuronal pool. We address the role of TVR and vibration-induced changes in efferent signals in more detail in the discussion. To our knowledge, only one group has investigated vibration-induced changes in force perception (Cafarelli and Kostka 1981); the authors concluded that these effects could not be linked to any specific afferent source and offered no general scheme to account for the observations. One of the main goals of the current study was to document and explore force illusions caused by muscle vibration in support of the suggested scheme for force and position perception.
Second, earlier studies suggested that vibration could also lead to shifts in the central (efferent) component of kinesthetic perception (Feldman and Latash 1982a). If this is true, vibration could be expected to lead to changes in unintentional force drifts caused by turning the visual feedback off (Shapkova et al. 2008; Slifkin et al. 2000; Vaillancourt and Russell 2002). Testing this hypothesis (hypothesis 2) also contributed to our understanding of causes of unintentional force drifts, phenomena that have received contrasting interpretations (Ambike et al. 2015; Parsa et al. 2016; Prodoehl et al. 2007; Vaillancourt and Russell 2002).
Measuring perceived force is nontrivial. In this study we opted to use the method of force matching by the contralateral effector (cf. Cafarelli and Bigland-Ritchie 1979; Cafarelli and Kostka 1981; Luu et al. 2011) to avoid possible effects of memory (potentially present in delayed matching by the same effector) or the use of nonlinear and drifting psychophysical scales. To explore the generality of vibration-induced force illusions, we studied force matching by both dominant and nondominant hand during accurate force production by the other hand.
METHODS
Subjects
Nine healthy young adult subjects (6 women; ages 21–30 yr) were recruited for the present study. All subjects were right-handed, as assessed by their reported hand use during daily activities such as eating and writing; subjects were also screened for neurological disorders and upper limb injuries that could interfere with their ability to perform experimental procedures. Before the experimental procedures began, all subjects provided written, informed consent. All protocols and procedures were approved by the Office of Research Protections at The Pennsylvania State University.
Apparatus
During experimental procedures, subjects used the index, middle, ring, and little fingers of both their right and left hands to perform isometric finger force production tasks. Subjects pressed on eight Nano-17 six-axis force/torque transducers (ATI Industrial Automation, Apex, NC) with all four fingers of both hands. The sensors’ top surfaces (which subjects’ fingers pressed against) were covered with 320-grit sandpaper. The sensors were mounted on slotted aluminum plates in two groups of four; the slotted plates allowed the sensors to be moved in the anterior-posterior direction to accommodate digits of different lengths. The sensors on each plate were spaced 3.0 cm from center to center in the medial-lateral direction. The sensors were powered and amplified by multisensor interface boxes (ATI Industrial Automation), which were factory calibrated for the individual sensors used. Data from the interface boxes were sampled at 1,000 Hz with two PCI-6225 16-bit analog-to-digital cards (National Instruments, Austin, TX). A custom application built in the LabVIEW programming environment logged data for offline analysis and provided visual feedback to the experimenter and (during some experimental conditions) to the subjects. Subjects viewed any available visual feedback on their performance via a 20-in. monitor placed 1 m from their head at eye level. The experimental setup is shown in Fig. 2.
Fig. 2.
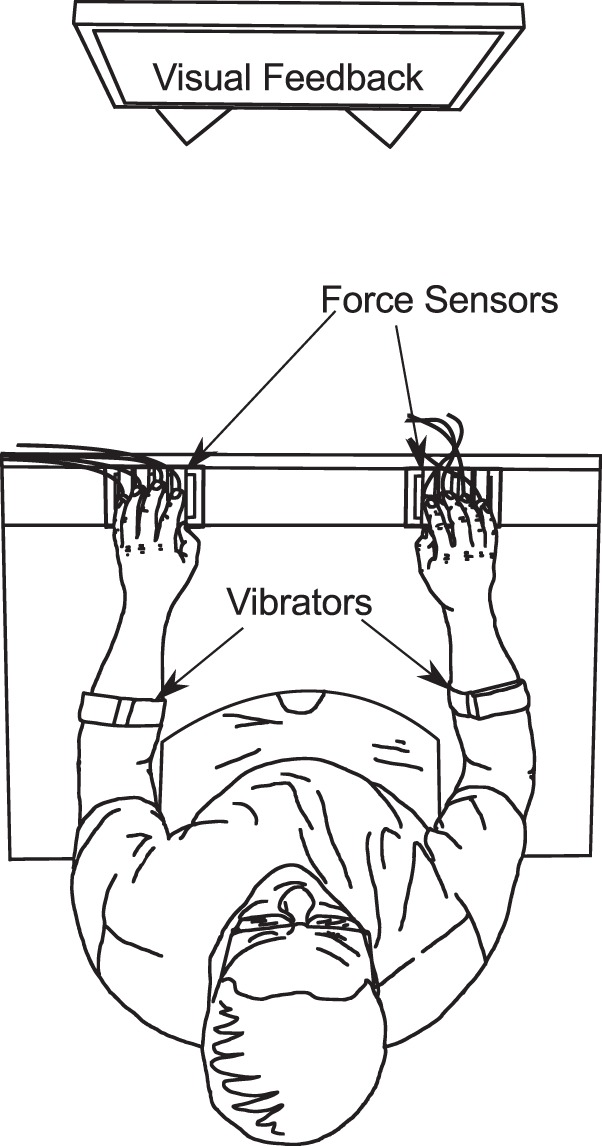
Illustration of the setup, showing the position of the subject, the location of the vibrators, and the monitor used for visual feedback.
During some experimental procedures, the extrinsic extensors of one or both hands were vibrated. Each subject wore two identical vibrators (VB 100; Dynatronic) during the entire experimental procedure, even when the vibrators were turned off. These vibrators were secured to the forearms with integrated elastic bands and controlled by a single control unit set for 80-Hz vibration. Each vibrator was placed over the muscle bellies of the hand/finger extensors on the proximal one-third of the forearm. Vibrators were turned on (as appropriate; see Procedure) for at least 10 s before subjects performed vibration-on conditions; after completion of such tasks, the vibrator(s) were turned off and at least 1 min elapsed before the beginning of the next experimental trial.
We carried out a pilot test to determine the extent of the propagation of vibration to the extrinsic finger flexors via the elastic bands. To do this, we placed a triaxial accelerometer against the vibrator while it was attached to the forearm. In a separate trial, we placed the accelerometer between the elastic band and the flexor muscles. In both conditions, the subject’s arm was free in the air, with moderate co-contraction to simulate muscle activity similar to that required by the task. The frequency of vibration was similar on both sides of the arm, but the extensors experienced accelerations approximately five times larger than the flexors. Note that this setup models a worst case scenario, because in the main experimental series, the forearms were held against the table, which we expected to attenuate vibrations transmitted by the elastic bands. As such, we believe our results reflect primarily the effect of extensor vibration.
Procedure
The experimental procedure consisted of three main parts: maximal voluntary contraction (MVC) trials; steady-state accurate force production tasks with and without visual feedback; and matching tasks. During all trials, subjects were instructed to rest their fingers gently on the sensors, and the force readings from the sensors were automatically zeroed at this force level; as such, only active downward forces were recorded. During all tasks, regardless of the hand(s) instructed to press, subjects kept both hands on the sensors and all forces produced by both hands were recorded.
MVC task.
These tests were performed at the beginning of the experimental procedure to select force levels for subsequent trials. During the MVC trials, subjects were instructed to use all four fingers of both hands to press as hard as possible and maintain this force level for a few seconds. Subjects repeated MVC task three times with at least 30 s of rest between repetitions; the highest MVC (largest total force by all 8 fingers) was taken as the MVC for the subsequent tasks. We used the two-hand, eight-finger MVC test rather than individual hand/finger tests to minimize testing time and fatigue and also because the tasks in the main experimental series were formulated at the hand level, not at the finger level.
Blind steady-state tasks.
After completing MVC tasks, and before they were exposed to visual feedback, subjects completed a series of steady-state force production tasks during which they received no visual feedback on their performance. These tasks were performed for the left and right hands individually. Starting with their fingers resting on the sensors, subjects were verbally instructed to smoothly increase the amount of force they were producing until they reached a predetermined level of force production (these levels were ~15 N for men and 10 N for women). As subjects approached the target force level, they were instructed to slow down the rate of force increase and, finally, to stop and hold the level of force they were producing for 30 s. These trials were repeated three times for each hand.
Force-matching tasks.
During the next series of tasks, subjects were instructed to press with one hand (the task hand) to move the cursor to a visual target that was set at 25% of the task hand’s contribution to MVCs; after 8 s, the cursor disappeared and subjects were instructed to continue producing the same force for the remainder of the trial (~30 s).
Before any experimental trials, subjects practiced moving the cursor to the target and stabilizing it there; three practice trials were provided for each hand. After practice trials were completed, baseline measures of force drift with and without vibration were obtained. For each hand, subjects performed three trials with vibration and three trials without vibration. The order of using different hand and vibration conditions was counterbalanced across subjects.
Furthermore, subjects used one hand (the task hand) for steady-state force production. As in the baseline trials, subjects received 8 s of visual feedback on the force produced by the task hand before feedback disappeared and they were instructed to keep on producing the same force. With the other hand (match hand), subjects were instructed to produce the force they believed the task hand was producing, using brief (3–4 s) episodes of force production. Subjects never received feedback on the force produced by the match hand. After visual feedback for task hand disappeared, subjects were verbally instructed to produce four matches during each trial. For each match, the experimenter instructed the subject to press, keep steady at the matching force level, and then to relax the match hand. There were ~4-s intervals between the end of one matching episode and the beginning of the next one. Each combination of task hand and vibration application was applied to the left and right hands for a total of eight trials. Each trial lasted ~40 s and rest episodes (in addition to the obligatory 60-s washout period for vibration) were provided as needed. This trial duration was selected to ensure measurable force drifts (if they took place); note that typical time exponents of force drift in earlier studies were within the range of 10–20 s (Ambike et al. 2015; Parsa et al. 2016; Vaillancourt and Russell 2002).
Analysis
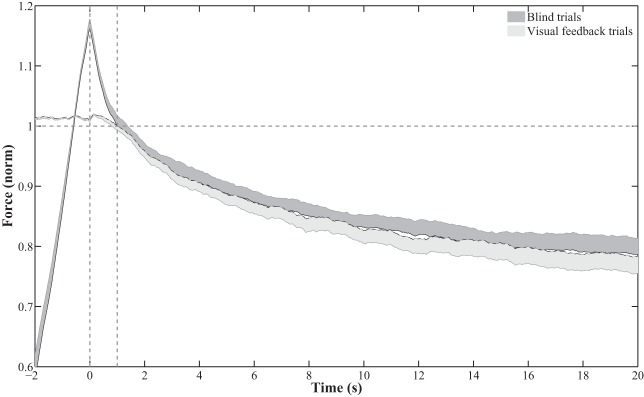
Data were processed offline using routines written in MATLAB (The MathWorks, Natick, MA). Before analysis, data were low-pass filtered using forward and reverse 2nd-order Butterworth filters, yielding a 4th-order filter with a cutoff frequency of 10 Hz. To characterize the changes in total force produced by a hand (FTOT) observed during continuous force production (in the steady-state tasks with and without visual feedback, as well as in the task hand during matching tasks), we computed an index of force drop over 20 s: ΔF20. ΔF20 was computed starting from 1 s after the subject was instructed to maintain his or her force level (in blind steady-state tasks) or after the time when visual feedback was removed (in tasks with visual feedback) until 20 s later. This definition of ΔF20 was chosen on the basis of the observation that FTOT dropped in some subjects very quickly at the beginning of the blind steady-state tasks. For statistical analysis, we used the factor “phase” to make comparisons of force production between the initial and final time periods. This force drop can be seen in Fig. 3 showing across-subjects performance in the blind steady-state task (solid trace, dark shading) and the baseline visual feedback tasks without vibration (dashed trace, light shading). This index was normalized by the initial force level, defined as the force 1 s after visual feedback was removed (in tasks with visual feedback) or 1 s after the subject was instructed to maintain his or her force level (in blind steady-state trials).
Fig. 3.
Total force (FTOT) produced by a hand under conditions without visual feedback (“blind trials”; solid line with dark shading) and with visual feedback presented over the first few seconds of the trial (“visual feedback trials”; dashed line with light shading). Time 0 shows the time when the subject was asked to stop increasing force in the blind trials and when the visual feedback was turned off in the trials with visual feedback. Mean data across subjects are shown with SE half-shading. FTOT was normalized by the force value observed 1 s after time 0. The task force is indicated by the horizontal dashed line. Analysis was performed starting 1 s after time 0 (second vertical dashed line).
To compare FTOT produced by the match hand (FMATCH) and the task hand (FTASK), FTOT was normalized to the target force (25% MVC of the task hand), yielding normalized force units (NFU). We identified each matching episode (4 episodes; statistical factor “episode”) by finding the beginning of the match when FMATCH exceeded 0.4 NFU of the task hand and the end of the match, when FMATCH fell below 0.4 NFU. We then computed the average difference between the task and match hand forces over a 1-s window centered at the middle of the matching episode: ΔFMT = FMATCH − FTASK. The midpoint of these two times was computed, and FMATCH and FTASK were averaged from 500 ms preceding this midpoint to 500 ms after this midpoint. Because four matches were performed in each trial, four values of ΔFMT were computed for each condition. In addition, to help explicate whether differences in ∆FMT resulted from drifts in performance by the task and/or match hands, we computed two additional quantities for each match: the differences in force production between each hand and the target force (defined as 1 NFU). These quantities are denoted by ∆FM25 for the difference between the match hand and the target and by ∆FT25 for the difference between the task hand and the target level.
Statistics
Unless otherwise stated, all data presented in the text and figures are means ± SE. We assume statistical significance when P < 0.05. To test the specific hypotheses, ANOVAs with repeated measures (using a mixed-model method) were used, as appropriate, to compare characteristics of FTOT produced by each hand. To determine whether there was a significant force drift, we performed a one-sample t-test comparing ΔF20 values with zero. To investigate the effect of visual feedback on ΔF20, we analyzed ΔF20 from the blind steady-state tasks and from the baseline, nonvibrated visual feedback tasks using the two-way ANOVA Hand (2 levels: right, left) × Visual Feedback (2 levels: yes, no). To assess whether the perceived level of force produced by the task hand drifted, we used the two-way ANOVA Hand × Episode (4 levels: first–fourth) to analyze ΔFMT. To investigate whether adding the matching activity to steady-state force production affected force drift in the steady-state task, we analyzed ΔF20 from matching tasks without vibration and baseline visual feedback tasks without vibration using the two-way ANOVA Hand × Matching (2 levels: yes, no) on ΔF20. To determine whether vibration affects ΔF20, we analyzed data obtained from the baseline visual feedback tasks using the three-way ANOVA Hand × Vibration (2 levels: yes, no) × Phase (2 levels: initial, final). Finally, to investigate the effects of vibration on perceived force production, we initially analyzed ΔFMT using the three-way ANOVA Hand × Episode × Vibration Combination (4 levels: no vibration/no vibration, vibration/no vibration, no vibration/vibration, vibration/vibration). Because the effect of hand (right and left) was found to be nonsignificant, we subsequently collapsed FMATCH across hands and used the targeted two-way ANOVA Vibration Combination × Episode.
Significant effects were further explored with pairwise contrasts with Bonferroni corrections. Normality of ANOVA model residuals was assessed with quantile-quartile plots. All statistical tests were run in SAS 9.4 (The SAS Institute, Cary, NC).
RESULTS
Steady-State Tasks With and Without Visual Feedback
During continuous force production without any visual feedback (blind steady-state tasks), subjects tended to show a precipitous drop in FTOT over ~1 s after they were verbally instructed to stop increasing force and maintain a steady level of FTOT (see Fig. 3). This phenomenon was not seen during tasks that started with visual feedback on FTOT. For comparisons of force drifts across conditions, the change in force, ΔF20 was calculated beginning 1 s after subjects were instructed to maintain a constant force level. In trials that started with visual feedback, the first 1 s after the removal of visual feedback was also excluded from analysis. Across hands and subjects, ΔF20 normalized by the initial force level showed no difference [F(1, 24) = 0.12, P > 0.05] between blind tasks (−0.219 ± 0.026) and tasks that began with visual feedback (−0.227 ± 0.026). Both values were significantly different from zero according to the t-test [blind tasks: t(17) = −8.48, P < 0.01; visual feedback task: t(17) = −8.85, P < 0.01].
Matching by the contralateral hand had no significant effect on the force drop seen in the task hand: there was no significant difference between ΔF20 (−0.224 ± 0.036 NFU) in tasks with force-matching episodes by the contralateral hand compared with that observed without matching [−0.214 ± 0.024 NFU; F(1, 24) = 0.09, P > 0.1]. There was no difference in ∆F20 between the dominant and nondominant hands [F(1, 24) = 0.05, P > 0.1].
Effects of Time on Matching Force
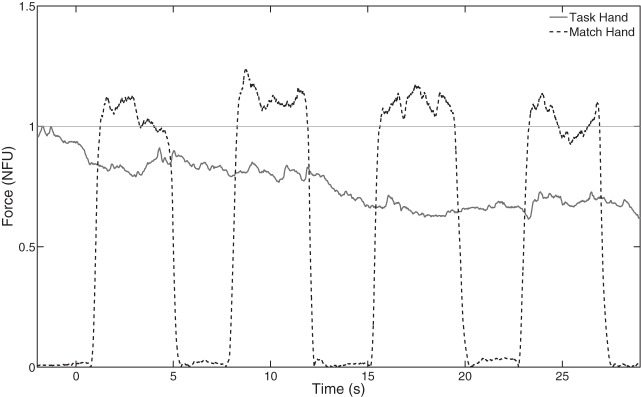
Although the force produced by the task hand decreased over time, the amount of force subjects produced by the contralateral hand (FMATCH) did not. Performance of a representative subject in a matching trial is shown in Fig. 4. Figure 5 illustrates the difference between FTASK of the task hand and its target value (∆FT25; gray bars) as well as the difference between FMATCH of the match hand and the target value (∆FM25; black bars) during the four matching episodes across subjects. Subjects consistently produced more force with the match hand than they were producing with the task hand. The two-way ANOVA Hand × Episode showed that ∆FM25 was unchanged across the four matching episodes [F(1, 56) = 0.29, P > 0.1]; these results were consistent regardless of which hand was the match hand and which hand was the task hand [F(1, 56) = 1.66, P > 0.05].
Fig. 4.
Typical trial by a representative subject under the force matching condition. No vibration was applied. Visual feedback was turned off at time 0. The horizontal line shows the task force level. Note the drift to lower force values in the task hand (solid line) and no consistent drift in the match hand that performed force-matching episodes (dashed line).
Fig. 5.
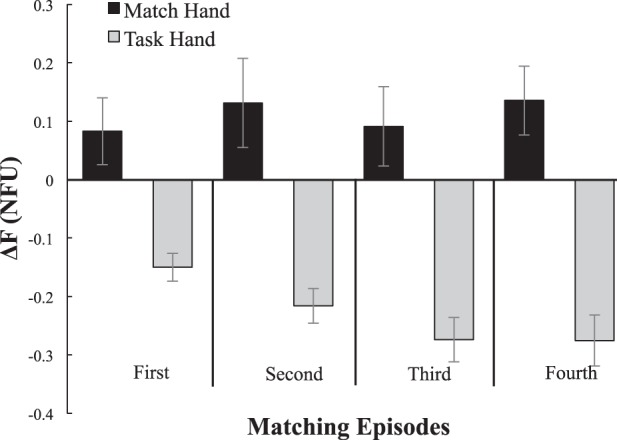
Difference between the hand force and the task force during the 4 force-matching episodes for the task hand (gray bars; ΔFT25) and match hand (black bars; ΔFM25). Mean data across subjects are shown with SE bars. Note the increasing magnitudes of the negative values of ΔFT25 and no changes in ΔFM25 over the 4 matching episodes.
A similar ANOVA on ∆FT25 confirmed the force drop with time [a significant effect of Episode: F(3, 56) = 15.34, P < 0.001] with no other effects. Pairwise comparisons showed that ∆FT25 was significantly larger than its initial level (indicating less force production) during the third and fourth episodes. Force production during the final episode was also significantly smaller than during the second episode.
Effects of Vibration on Steady-State Force Production
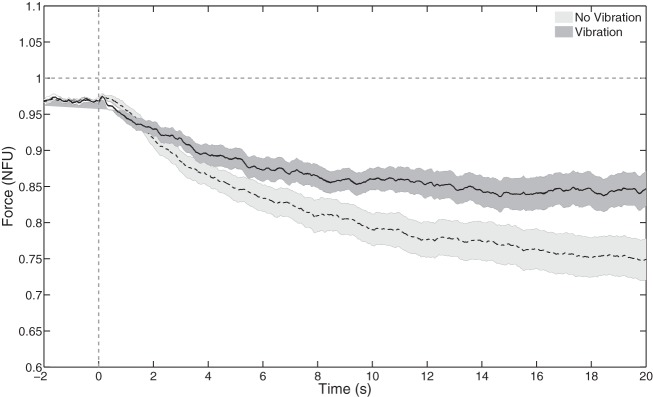
When vibration was applied to the task hand extensors, the drop in force after the visual feedback was turned off was reduced: ΔF20 was smaller with vibration (−0.09 ± 0.005 NFU) than without vibration (−0.214 ± 0.024 NFU). The two-way ANOVA Hand × Vibration confirmed the significant effect of Vibration [F(1, 24) = 24.2, P < 0.001] without other effects. These results are illustrated in Fig. 6, which shows the time profiles of FTASK averaged across the right and left hands and across subjects with (solid trace, dark shading) and without vibration (dashed trace, light shading).
Fig. 6.
Effect of vibration applied to the task hand on the force drift. Visual feedback was turned off at time 0. The horizontal line shows the task force level. Note the smaller force drift with vibration (solid line, dark shading) compared with the no-vibration condition (dashed line, light shading). Mean data across subjects are shown with SE shading.
Effects of Vibration of Force Matching
As previously described, subjects consistently produced more force with their match hands than with their task hands. However, the difference between the two forces (ΔFMT) depended on the application of vibration [effect of Vibration Condition: F(3, 264) = 34.37, P < 0.001], and pairwise contrasts confirmed the mentioned differences across conditions. When vibration was applied to the task hand only, the match hand showed larger forces (compared with the no-vibration case: 0.548 ± 0.062 vs. 0.339 ± 0.055 NFU, P < 0.001). In contrast, when vibration was applied to the match hand only, the match hand produced smaller forces (compared with the no-vibration case: 0.206 ± 0.051 vs. 0.339 ± 0.055 NFU, P < 0.001). Applying vibration to both hands simultaneously led to no significant differences from the no-vibration condition. These findings were consistent for trials with the right hand as the task hand and those with the left hand as the task hand.
There was a drift in ∆FTM toward higher magnitudes over the four episodes reflecting the drop in FTASK and no change in FMATCH (see also Fig. 5). This drift was confirmed by a significant effect of Episode [F(3, 264) = 3.95, P < 0.05] on ΔFMT. There were no significant interactions. These results are illustrated in Fig. 7, which shows ∆FMT values over the four vibration conditions and four matching episodes. Note the consistent increase in ΔFMT over the four episodes and the differences between the conditions with vibration applied to one of the hands only compared with the no-vibration condition.
Fig. 7.
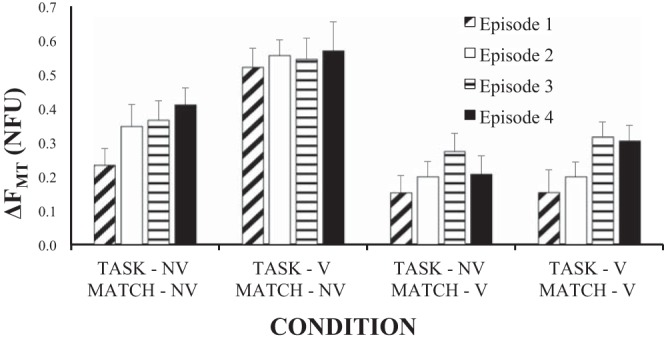
Difference between the forces produced by the match hand and by the task hand (ΔFMT) during the 4 episodes under the 4 vibration conditions. Mean data across subjects are shown with SE bars. Note the increase in ΔFMT from episode 1 to episode 4, the larger values when only the task hand was vibrated (second cluster of bars), and the smaller values when only the match hand was vibrated (third cluster of bars). V, vibration; NV, no vibration.
DISCUSSION
Our experiments demonstrate consistent effects of vibration on performance in force-matching tasks. These effects suggest that the central nervous system (CNS) consistently overestimated the force produced by the hand under vibration. Compared with trials without vibration, vibrating extensors of the hand performing the continuous force production task led to higher forces produced by the matching hand (suggesting overestimation of force produced by the task hand, in support of hypothesis 1). Similarly, vibrating the hand that performed the matching task led to lower forces produced by that hand, suggesting that the CNS overestimated the amount of force produced by the matching hand. These observations fit the scheme for kinesthetic perception described in the Introduction (see also Feldman and Latash 1982a) by demonstrating effects that amount to vibration-induced force illusions consistent with those reported by Cafarelli and Kostka (1981). They have important implications for such issues as interactions of efferent and afferent processes during kinesthetic perception and specificity of afferent systems.
Two additional important findings are 1) the presence of force drift in the hand performing a continuous force production task that was never defined by visual feedback (cf. Ambike et al. 2015; Shapkova et al. 2008; Vaillancourt and Russell 2002) and 2) the lack of a force drift in the matching hand even as the performance of the hand to be matched drifted. These findings suggest that the force drift originates not at the task level, but rather at an intermediate level where interactions between the hypothetical control variables (associated with time-varying RCs; Feldman 2015; Latash 2010) and feedback signals on current effector configuration take place. Note that we used relatively low levels of task force and a short trial duration, which were not expected to lead to muscle fatigue (cf. Ambike et al. 2015).
Finally, some of our observations do not easily fit any of the existing theoretical schemes. One of those findings is the smaller force drift observed during the vibration of hand/finger extensors (in support of hypothesis 2). Although we subsequently offer interpretations of these findings, the interpretations remain speculative and need to be tested in future studies.
Vibration Effects on Perception of Kinematic and Kinetic Variables
Traditionally, different peripheral sensory endings have been viewed as specific to particular mechanical variables (reviewed in Proske 2006). According to this doctrine, primary endings of muscle spindles have been viewed as sensors of muscle length and velocity, the secondary spindle endings have been viewed as sensors of muscle length, and Golgi tendon organs have been viewed as sensors of muscle force. This view has faced a number of problems, however. First, a number of studies have documented convergence of signals from different afferent systems on spinal interneurons (Jankowska 1979; Jankowska and McCrea 1983; Kistemaker et al. 2013; Lundberg 1979), which would seem to garble information from different proprioceptive subsystems. In addition, the sensitivity of spindle afferents to muscle length and velocity is modulated by γ-motoneurons, which are commonly coactivated with α-motoneurons. As a result, afferent signals are sensitive not only to salient mechanical variables (muscle length and velocity) but also to concurrent neural output to the muscle. This problem has been exemplified by results of a classical study (Hulliger et al. 1982) demonstrating that muscle spindle afferents are sensitive to passive joint motion but show unchanged levels of activity after a voluntary joint movement to a new position corresponding to a different muscle length (see also Stay et al. 2016).
Still another problem for accurate perception of body configuration is that muscle length is not the same as the length of the muscle-tendon unit. Salient elements of body configuration, such as joint angles and segment locations in space, are defined by the muscle-tendon unit length, not by muscle length, so signals related only to muscle length are only marginally useful for kinesthetic perception. A similar problem exists with signals from Golgi tendon organs that are sensitive to muscle force. The salient, behavior-specific variable for the body is joint moment of force, which depends on both muscle force and the lever arm of that force. Because muscle lever arms change with joint angle, signals from Golgi tendon organs by themselves are unable to inform the CNS on the joint moment.
One of the early attempts to address some of these problems invoked the notion of efferent copy (von Holst and Mittelstaedt 1950), which was posited as a copy of current neural output to the muscle. In this theory, the efferent copy was used to extract salient kinesthetic information from afferent signals and to define muscle reactions to joint motion. This concept, however, leads to a number of predictions that are obviously false, including the inability to relax muscles after a voluntary joint movement to a new position (for details see Feldman 2009, 2015). The basic idea of efferent processes taking part in kinesthetic perception remains valid, however, if the efferent process is associated not with output of α-motoneuronal pools, but rather with the specification of RC for the effector (see Introduction and Latash and Zatsiorsky 2016).
Figure 1 in the Introduction suggests that afferents from different peripheral sources may be nonspecific to mechanical variables that they seem to be particularly sensitive to (see also Luu et al. 2011; Proske and Gandevia 2012; Watson et al. 1984). In particular, when combined with efferent processes that specify a relation between muscle length and muscle force, spindle afferents can be used for force perception and afferents from Golgi tendon organs can be used as sources of sensory information on muscle length. Our results suggest that, in fact, muscle vibration affects perception of force produced by an effector system spanned by that muscle (see also Cafarelli and Kostka 1981). Note that contribution of muscle spindles to force perception has been suggested in earlier studies (Luu et al. 2011; Watson et al. 1984). In addition, effects of muscle vibration of activity of Golgi tendon organs also have been documented (Fallon and Macefield 2007). Our general scheme in Fig. 1 incorporates all these effects and offers a general conceptual framework for both unaffected and distorted perception of forces and coordinates.
The traditional interpretation of effects of vibration as produced by changes in afferent information from muscle spindles is inconsistent with the results of our study. Consider Fig. 8, which illustrates the task as being produced by two muscle groups, flexors (agonists) and extensors (antagonists). This illustration assumes a degree of coactivation of extensor muscles during flexor force production. This assumption is based on earlier studies providing evidence for substantial antagonist co-contraction during isometric steady-state tasks (e.g., Corcos et al. 1990; Ghez and Gordon 1987). Also, a biomechanical model of the finger action suggests that isometric force production by the fingertip has to be associated with substantial extensor action, including the action mediated by the extensor hand mechanism, to maintain finger posture in all the joints and prevent “buckling” (Li et al. 2000, 2001b). However, because we did not record electromyograms in the study, we cannot confirm the veracity of this assumption.
Fig. 8.
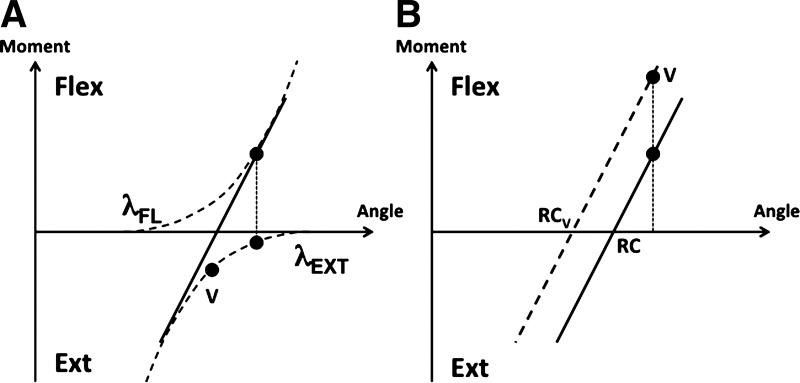
A: illustration of a task performed by 2 muscle groups, flexors (agonists) and extensors (antagonists). The thresholds of the 2 muscle characteristics are shown as λFL (flexor) and λEXT (extensor). The initial state is shown by the vertical dotted line connecting 2 black dots on the flexor and extensor characteristics. Vibration of extensors is expected to result in perception of larger values of both muscle length and force produced by those muscles (point V, corresponding to lower net torque and end-effector force). B: perception of higher force caused by vibration of antagonist muscles may be expected if the referent coordinate (RC) of the effector shifts to RCV. The slanted lines show the effector characteristics.
Vibration of extensors is expected to result in perception of larger values of both muscle length of and force produced by those muscles (point V on the extensor muscle stretch reflex characteristic in Fig. 8A). In our pressing task, extensors were antagonists; therefore, perception of increased extensor activity should result in the perception of decreased total force production compared with the no-vibration condition. As such, a traditional view of vibration-induced illusions would predict that when the hand performing the continuous force production task was vibrated, the matching hand (without vibration) would show lower force magnitudes. In fact, we observe the opposite effect (see Fig. 7). The traditional view would also predict that vibrating the hand performing the matching task would result in its force being underestimated and, therefore, that it would produce larger magnitudes of force to match the perceived force produced by the nonvibrated hand. Again, our experimental results are in opposition to this account (see Fig. 7).
Our observations suggest that muscle vibration leads to changes not only in the afferent component of kinesthetic perception but also in the efferent (RC) component. A similar conclusion was drawn in an earlier study based on analysis of vibration-induced effects on perception of joint position (Feldman and Latash 1982a). Moreover, our results suggest that effects of vibration on RC may overpower the assumed effects on afferent activity and define the experimentally observed behavioral patterns. In particular, under vibration of the extensors, RC value participating in kinesthetic perception appears to shift toward a more flexed coordinate (RCV in Fig. 8B), which corresponds to overestimation of the actual pressing force (point V). As described in more detail in Unintentional Force Drift and Effects of Vibration, vibration may also lead to shifts of the other basic command, the c-command (Feldman 2015), that defines overlap in the spatial range where both agonist and antagonist muscles can be activated simultaneously. Changing the c-command may results in a change in the slope of the torque-angle characteristic in Fig. 8, assuming that the task involves some agonist-antagonist co-contraction.
A number of earlier studies used the concept of “effort” to describe kinesthetic perception (Jones and Hunter 1983; Jones 2003; McCloskey et al. 1974). This concept is not well defined and, in most studies, was operationally associated with levels of muscle activation as measured by electromyography. We emphasize that muscle activation reflects both central control processes (λ in Fig. 1) and effects of reflex loops on α-motoneurons. Thus muscle activation cannot be seen as an adequate reflection of central neural processes implied by the word “effort.” In addition, there are no receptors for muscle activation. It seems to us less controversial to associate sense of effort with changes in the referent coordinate, such as λ for a single muscle (cf. Feldman and Latash 1982a; Feldman 2009).
Unintentional Force Drift and Effects of Vibration
Unintentional drifts in force produced without visual feedback are well documented (Slifkin et al. 2000; Vaillancourt and Russell 2002). Note that such drifts are observed over relatively short times (~10–20 s) and at relatively modest initial force magnitudes (~25% of MVC), which makes effects of fatigue unlikely. Early studies suggested that these phenomena reflected limitations of working memory (Vaillancourt and Russell 2002), supported by observations of a significantly faster and larger force drift in patients with Parkinson’s disease (Jo et al. 2016; Prodoehl et al. 2007; Vaillancourt et al. 2001), as well as by EEG and MRI studies (Poon et al. 2012; Vaillancourt et al. 2003). Moreover, one of those studies reported a change in the direction of force drift, from the typical downward drift to an upward drift, in patients with Parkinson’s disease induced by stimulation of the subthalamic nucleus (Prodoehl et al. 2007). These observations present strong evidence for a central mechanism involved in the force drift phenomena. This interpretation is also compatible with a body of literature describing connections between prefrontal and premotor cortices with the dorsolateral prefrontal cortex and posterior parietal cortex during tasks requiring memory in nonhuman primates (Goldman-Rakic 1988; Selemon and Goldman-Rakic 1988).
An explanation at a different conceptual level has been suggested on the basis of an assumption that the CNS manipulates parameters of physical laws to perform intentional actions (reviewed in Latash 2016). In particular, force production may be viewed as a consequence of defining a referent coordinate (RC) for the effector, which is different from its actual coordinate (AC). At present, mapping of RC to physiological structures has been suggested only for the control of individual muscles. At that level, RC is associated with the threshold of the tonic stretch reflex (λ in Fig. 8), which is modified by changes in subthreshold depolarization of the corresponding α-motoneuronal pool (Feldman 2015). The difference between RC and AC translates into active force toward RC. If the effector is released, it moves toward RC. If AC cannot move over a relatively long time (e.g., in isometric conditions), a natural drift of RC toward AC is expected (coined “RC-back-coupling”; Zhou et al. 2014), reducing the difference between RC and AC and moving the system toward a state with lower potential energy. If visual feedback is available, the subject corrects the drift and no net force change is observed. Although such an interpretation is not at odds with the concept of memory, which may encompass a number of central processes at various levels of the central nervous system, intuitive notions of memory may fail to capture some of the details of the observed behaviors, including the tendency of force to drift in only one direction (except the mentioned study with deep brain stimulation; Prodoehl et al. 2007) and the contrasting effects in the two hands of a person.
Our discussion has so far been limited to changes in RC (associated with the reciprocal command, r-command) without a change in the other basic command, which defines the spatial range where both muscle groups, agonist and antagonist, are activated, the so-called c-command (reviewed in Feldman 2015). The notion of RC-back-coupling implies possible drifts in both central commands. For example, after a quick joint motion, a slow drop in the activation level of the agonist and antagonist muscles is observed without a drift in the joint final position (reviewed in Gottlieb et al. 1989). This process likely reflects a drift in the c-command, whereas the r-command remains unchanged. A drift in the c-command can also lead to a drop in force, and indeed, such drifts have been reported recently in parallel with drifts in the r-command (Ambike et al. 2016b; Reschechtko and Latash 2017). In our experiment, we cannot distinguish between possible effects of drifts in the r- and c-commands and unite them under an umbrella term “RC-back-coupling.” Distinguishing the effects of the two commands may be an important issue because, as suggested earlier (Feldman 2015, 2016), changes in the c-command may not be accompanied by changes in perception of salient mechanical variables.
In a recent study (Solnik et al. 2017), subjects were asked to produce accurate force levels by pressing with a finger, memorize this force, and then reproduce it a few times over 30 s using brief force pulses spaced with periods of rest. No force drift to lower values was observed in that study, suggesting that the subjects did not forget the initial force level. In contrast, if the subjects maintained the force throughout the 30-s time interval, a significant force drop was seen. These observations are similar to our results with force matching by the contralateral effector. Indeed, a force drop was seen in the task hand, whereas the match hand showed no change in the force level with time (Fig. 5). These results suggest that, at a hypothetical task level of the control hierarchy, the task force is not forgotten and shows no drift when another effector tries to reproduce it. Overall, these observations suggest that force drift reflects loss of stability of force downstream from the task specification level (Parsa et al. 2016, 2017), although it is still likely to be of a central origin (including the basal ganglia; cf. Prodoehl et al. 2007).
We observed no significant effects of the matching on the force drift in the task hand: note that force matching typically led to small transient force changes in the task hand, which was instructed to keep producing the same force level (cf. Fig. 4). These transient unintentional force changes likely resulted from bilateral force effects demonstrated in many studies (Howard and Enoka 1991; Li et al. 2001a; Oda and Moritani 1994; Ohtsuki 1983).
It has been a puzzle why undisturbed somatosensory information (from sensory receptors in the fingertips and hand muscles) is unable to prevent rather large unintentional force drifts (on the order of 20–30%). The cutaneous and proprioceptive feedback from the fingers has been shown to play an important role in force estimation (Jones and Piateski 2006); however, this effect depends primarily on changes in finger configuration resulting from force production or the fingertips pressing against nonuniform surfaces. Given the low levels of force in our study and the fact that all fingers pressed against the same surfaces while subjects kept a standard hand configuration, we believe that these feedback effects were equivalent across subjects and conditions and, therefore, did not play a major role in the force time profiles observed in the experiment. It is possible that slowly adapting receptors, such as Merkel disks and Ruffini corpuscles, showed a slow drop in their firing rate during a constant external deformation of the fingertip (Iggo and Muir 1969). These effects could lead to perception of lower force magnitude and corrective actions by the task hand, leading to an increase in the actual force, which is opposite to what we observed in the experiment. The hypothesized perception of lower force could also lead to lower forces produced by the matching hand, again, an effect in the opposite direction to our experimental observations.
Earlier studies (Ambike et al. 2015, 2016a) suggested a hypothesis that using visual feedback to set a task conditioned the subjects to use that sensory modality for force corrections. According to this hypothesis, when visual feedback becomes unavailable, other sensory modalities are not ready to compensate for the drift. Our trials without any visual feedback (the blind trials) do not support this hypothesis: when the subjects performed pressing tasks while directed verbally by the experimenter to press to a particular force level, force drift was seen to lower force value and the magnitude of the force drift was not smaller than in more traditional trials that started with force production under visual feedback. We therefore conclude that force drift to lower magnitudes is a robust phenomenon that does not depend on whether visual feedback is used to set the initial force level.
Muscle vibration disturbs sensory feedback from the effector by making some sources of sensory information saturated (e.g., driving primary endings; Brown et al. 1967; Roll and Vedel 1982) and unreliable. Because of these effects, we expected force drift to become larger when the task hand was subjected to vibration. Our result was the opposite: the force drift became significantly smaller (Fig. 6). Surprisingly, under vibration of the extensor muscles, the subjects performed the task better, with smaller force deviations from the task level!
Could these effects be due to the tonic vibration reflex (TVR)? Muscle vibration is known to produce reflex activation of the muscle, the TVR (DeGail et al. 1966; Hagbarth and Eklund 1966). In our experiment, vibration of extensors could be expected to lead to additional reflex activation of the extensors (task antagonists) and hence to an additional drop in the pressing force. The experimental observation was opposite. During our experimental procedures, vibrators were turned on at least 10 s before trial onset. This was done to ensure that any effects mediated by the TVR would have reached a steady-state level (cf. Latash and Gurfinkel 1976) before visual feedback was turned off. The thought behind this manipulation is that subjects would become accustomed to stabilizing force production in the presence of TVR. Thus, if TVR contributions to force were underestimated centrally, these effects would have been corrected for during the early phase of the trial on the basis of visual feedback. However, it is possible that the effects of vibration continued to grow after visual feedback was removed or that the subjects’ perception of TVR contribution faded when visual feedback was removed; we cannot test either of these hypotheses, but they could have contributed to the observed decrease in force drift.
It is possible that vibration produced reflex activation not only of extensors but also of flexors. This could be due to a few factors, including mechanical spread of vibration. We tried to control for spread of vibration by the elastic bands (see methods) and feel confident that those effects were small. We could not, however, control for spread of vibration through the forearm tissues. In addition, reflex effects of vibration are not limited to autogenic effects but can lead to activation of antagonist muscles (Feldman and Latash 1982b; Latash and Gurfinkel 1976) and to reflex agonist-antagonist coactivation (Jones and Hunter 1985). Independently of the pattern of reflex effects of vibration, the presence of feedback early in the trial should have allowed the subjects to adjust their performance accordingly.
Another possible contributor to the reduced force drift under vibration of the extensors is the response of the system of γ-motoneurons. A mixture of excitatory and inhibitory effects of muscle vibration on γ-motoneurons has been reported (Fromm and Noth 1976). If inhibitory effects prevailed in our subjects and these effects continued to grow after the visual feedback had been turned off, this could potentially lead to the production of larger pressing force. As of now, no experimental data exist to check this hypothesis.
Within the RC-back-coupling scheme, a smaller drop in force means a smaller drift in the RC compared with trials without vibration. Thus vibration effectively pushes RC values into the direction of flexion. Note that vibration was applied throughout the trial, including the initial episode when subjects matched the target force with the help of visual feedback. During that interval, effects of vibration were corrected using the feedback. The effects became obvious after the feedback had been turned off. This interpretation fits the illustration suggested to interpret perceptual data illustrated in Fig. 8B. Thus both force-matching trials and trials without matching lead us to the same conclusion, that vibration induces a drift of RC away from the AC.
Concluding Comments
The main result of the study is the demonstration of consistent vibration-induced force perception effects. These observations fit our main hypothesis on combined perception of kinematic-kinetic variables with contributions from both peripheral sensory signals and ongoing efferent process interpreted as time shifts of RCs (cf. Feldman 2015). According to this hypothesis, there is little specificity of different groups of peripheral receptors, and all of them contribute to perception of salient mechanical variables such as forces and coordinates. Our data also show the complexity of the force drift phenomenon and support its origin at intermediate levels of the control hierarchy rather than at the level of task specification. The observed changes in force drifts under muscle vibration support the idea that vibration leads to shifts in RC in addition to the well-known reactions of spindle sensory endings.
GRANTS
The study was supported in part by National Institute of Neurological Disorders and Stroke Grant R21 NS095873.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.R., C.C., and M.L.L. conceived and designed research; S.R. and C.C. performed experiments; S.R., C.C., and M.L.L. analyzed data; S.R., C.C., and M.L.L. interpreted results of experiments; S.R., C.C., and M.L.L. prepared figures; S.R., C.C., and M.L.L. drafted manuscript; S.R., C.C., and M.L.L. edited and revised manuscript; S.R., C.C., and M.L.L. approved final version of manuscript.
REFERENCES
- Ambike S, Mattos D, Zatsiorsky VM, Latash ML. The nature of constant and cyclic force production: unintentional force-drift characteristics. Exp Brain Res 234: 197–208, 2016a. doi: 10.1007/s00221-015-4453-z. [DOI] [PubMed] [Google Scholar]
- Ambike S, Mattos D, Zatsiorsky VM, Latash ML. Unsteady steady-states: central causes of unintentional force drift. Exp Brain Res 234: 3597–3611, 2016b. doi: 10.1007/s00221-016-4757-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambike S, Zatsiorsky VM, Latash ML. Processes underlying unintentional finger-force changes in the absence of visual feedback. Exp Brain Res 233: 711–721, 2015. doi: 10.1007/s00221-014-4148-x. [DOI] [PubMed] [Google Scholar]
- Brown MC, Engberg I, Matthews PB. The relative sensitivity to vibration of muscle receptors of the cat. J Physiol 192: 773–800, 1967. doi: 10.1113/jphysiol.1967.sp008330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafarelli E, Bigland-Ritchie B. Sensation of static force in muscles of different length. Exp Neurol 65: 511–525, 1979. doi: 10.1016/0014-4886(79)90040-2. [DOI] [PubMed] [Google Scholar]
- Cafarelli E, Kostka CE. Effect of vibration on static force sensation in man. Exp Neurol 74: 331–340, 1981. doi: 10.1016/0014-4886(81)90173-4. [DOI] [PubMed] [Google Scholar]
- Corcos DM, Agarwal GC, Flaherty BP, Gottlieb GL. Organizing principles for single-joint movements. IV. Implications for isometric contractions. J Neurophysiol 64: 1033–1042, 1990. doi: 10.1152/jn.1990.64.3.1033. [DOI] [PubMed] [Google Scholar]
- De Gail P, Lance JW, Neilson PD. Differential effects on tonic and phasic reflex mechanisms produced by vibration of muscles in man. J Neurol Neurosurg Psychiatry 29: 1–11, 1966. doi: 10.1136/jnnp.29.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JB, Macefield VG. Vibration sensitivity of human muscle spindles and Golgi tendon organs. Muscle Nerve 36: 21–29, 2007. doi: 10.1002/mus.20796. [DOI] [PubMed] [Google Scholar]
- Feldman AG. Functional tuning of the nervous system with control of movement or maintenance of a steady posture. II. Controllable parameters of the muscle. Biophysics (Oxf) 11: 565–578, 1966. [Google Scholar]
- Feldman AG. Once more on the equilibrium-point hypothesis (λ model) for motor control. J Mot Behav 18: 17–54, 1986. doi: 10.1080/00222895.1986.10735369. [DOI] [PubMed] [Google Scholar]
- Feldman AG. New insights into action-perception coupling. Exp Brain Res 194: 39–58, 2009. doi: 10.1007/s00221-008-1667-3. [DOI] [PubMed] [Google Scholar]
- Feldman AG. Referent Control of Action and Perception: Challenging Conventional Theories in Behavioral Science. New York: Springer, 2015. [Google Scholar]
- Feldman AG. Active sensing without efference copy: referent control of perception. J Neurophysiol 116: 960–976, 2016. doi: 10.1152/jn.00016.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman AG, Latash ML. Afferent and efferent components of joint position sense; interpretation of kinaesthetic illusion. Biol Cybern 42: 205–214, 1982a. [DOI] [PubMed] [Google Scholar]
- Feldman AG, Latash ML. Inversions of vibration-induced senso-motor events caused by supraspinal influences in man. Neurosci Lett 31: 147–151, 1982b. doi: 10.1016/0304-3940(82)90107-0. [DOI] [PubMed] [Google Scholar]
- Fromm C, Noth J. Reflex responses of gamma motoneurones to vibration of the muscle they innervate. J Physiol 256: 117–136, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghez C, Gordon J. Trajectory control in targeted force impulses. I. Role of opposing muscles. Exp Brain Res 67: 225–240, 1987. doi: 10.1007/BF00248545. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Topography of cognition: parallel distributed networks in primate association cortex. Annu Rev Neurosci 11: 137–156, 1988. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Matthews PB. The contribution of muscle afferents to kinaesthesia shown by vibration induced illusions of movement and by the effects of paralysing joint afferents. Brain 95: 705–748, 1972. doi: 10.1093/brain/95.4.705. [DOI] [PubMed] [Google Scholar]
- Gottlieb GL, Corcos DM, Agarwal GC. Strategies for the control of voluntary movements with one mechanical degree of freedom. Behav Brain Sci 12: 189–210, 1989. doi: 10.1017/S0140525X00048238. [DOI] [Google Scholar]
- Hagbarth KE, Eklund G. Tonic vibration reflexes (TVR) in spasticity. Brain Res 2: 201–203, 1966. doi: 10.1016/0006-8993(66)90029-1. [DOI] [PubMed] [Google Scholar]
- Howard JD, Enoka RM. Maximum bilateral contractions are modified by neurally mediated interlimb effects. J Appl Physiol (1985) 70: 306–316, 1991. doi: 10.1152/jappl.1991.70.1.306. [DOI] [PubMed] [Google Scholar]
- Hulliger M, Nordh E, Vallbo AB. The absence of position response in spindle afferent units from human finger muscles during accurate position holding. J Physiol 322: 167–179, 1982. doi: 10.1113/jphysiol.1982.sp014030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A, Muir AR. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol 200: 763–796, 1969. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E. New observations on neuronal organization of reflexes from tendon organ afferents and their relation to reflexes evoked from muscle spindle afferents. In: Reflex Control of Posture and Movement, edited by Granit R and Pompeiano O. Amsterdam: Elsevier, 1979, p. 29–36. [DOI] [PubMed] [Google Scholar]
- Jankowska E, McCrea DA. Shared reflex pathways from Ib tendon organ afferents and Ia muscle spindle afferents in the cat. J Physiol 338: 99–111, 1983. doi: 10.1113/jphysiol.1983.sp014663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo HJ, Ambike S, Lewis MM, Huang X, Latash ML. Finger force changes in the absence of visual feedback in patients with Parkinson’s disease. Clin Neurophysiol 127: 684–692, 2016. doi: 10.1016/j.clinph.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LA. Perceptual constancy and the perceived magnitude of muscle forces. Exp Brain Res 151: 197–203, 2003. doi: 10.1007/s00221-003-1434-4. [DOI] [PubMed] [Google Scholar]
- Jones LA, Hunter IW. Effect of fatigue on force sensation. Exp Neurol 81: 640–650, 1983. doi: 10.1016/0014-4886(83)90332-1. [DOI] [PubMed] [Google Scholar]
- Jones LA, Hunter IW. Effect of muscle tendon vibration on the perception of force. Exp Neurol 87: 35–45, 1985. doi: 10.1016/0014-4886(85)90131-1. [DOI] [PubMed] [Google Scholar]
- Jones LA, Piateski E. Contribution of tactile feedback from the hand to the perception of force. Exp Brain Res 168: 298–302, 2006. doi: 10.1007/s00221-005-0259-8. [DOI] [PubMed] [Google Scholar]
- Kistemaker DA, Van Soest AJ, Wong JD, Kurtzer I, Gribble PL. Control of position and movement is simplified by combined muscle spindle and Golgi tendon organ feedback. J Neurophysiol 109: 1126–1139, 2013. doi: 10.1152/jn.00751.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner JR, Taublieb AB. Influence of vision on vibration-induced illusions of limb movement. Exp Neurol 85: 97–106, 1984. doi: 10.1016/0014-4886(84)90164-X. [DOI] [PubMed] [Google Scholar]
- Latash ML. Motor synergies and the equilibrium-point hypothesis. Mot Contr 14: 294–322, 2010. doi: 10.1123/mcj.14.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latash ML. Towards physics of neural processes and behavior. Neurosci Biobehav Rev 69: 136–146, 2016. doi: 10.1016/j.neubiorev.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latash ML, Gurfinkel VS. Tonic vibration reflex and position of the body. Fiziol Cheloveka 2: 593–598, 1976. [Google Scholar]
- Latash ML, Zatsiorsky VM. Biomechanics and Motor Control: Defining Central Concepts. New York: Academic, 2016. [Google Scholar]
- Li S, Danion F, Latash ML, Li ZM, Zatsiorsky VM. Bilateral deficit and symmetry in finger force production during two-hand multifinger tasks. Exp Brain Res 141: 530–540, 2001a. doi: 10.1007/s002210100893. [DOI] [PubMed] [Google Scholar]
- Li ZM, Zatsiorsky VM, Latash ML. Contribution of the extrinsic and intrinsic hand muscles to the moments in finger joints. Clin Biomech (Bristol, Avon) 15: 203–211, 2000. doi: 10.1016/S0268-0033(99)00058-3. [DOI] [PubMed] [Google Scholar]
- Li ZM, Zatsiorsky VM, Latash ML. The effect of finger extensor mechanism on the flexor force during isometric tasks. J Biomech 34: 1097–1102, 2001b. doi: 10.1016/S0021-9290(01)00061-6. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Multisensory control of spinal reflex pathways. In: Reflex Control of Posture and Movement, edited by Granit R and Pompeiano O. Amsterdam: Elsevier, 1979, p. 11–28. 10.1016/S0079-6123(08)60803-1 [DOI] [PubMed] [Google Scholar]
- Luu BL, Day BL, Cole JD, Fitzpatrick RC. The fusimotor and reafferent origin of the sense of force and weight. J Physiol 589: 3135–3147, 2011. doi: 10.1113/jphysiol.2011.208447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey DI, Ebeling P, Goodwin GM. Estimation of weights and tensions and apparent involvement of a “sense of effort”. Exp Neurol 42: 220–232, 1974. doi: 10.1016/0014-4886(74)90019-3. [DOI] [PubMed] [Google Scholar]
- Oda S, Moritani T. Maximal isometric force and neural activity during bilateral and unilateral elbow flexion in humans. Eur J Appl Physiol Occup Physiol 69: 240–243, 1994. doi: 10.1007/BF01094795. [DOI] [PubMed] [Google Scholar]
- Ohtsuki T. Decrease in human voluntary isometric arm strength induced by simultaneous bilateral exertion. Behav Brain Res 7: 165–178, 1983. doi: 10.1016/0166-4328(83)90190-0. [DOI] [PubMed] [Google Scholar]
- Parsa B, O’Shea DJ, Zatsiorsky VM, Latash ML. On the nature of unintentional action: a study of force/moment drifts during multifinger tasks. J Neurophysiol 116: 698–708, 2016. doi: 10.1152/jn.00180.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa B, Terekhov A, Zatsiorsky VM, Latash ML. Optimality and stability of intentional and unintentional actions: I. Origins of drifts in performance. Exp Brain Res 235: 481–496, 2017. doi: 10.1007/s00221-016-4809-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon C, Chin-Cottongim LG, Coombes SA, Corcos DM, Vaillancourt DE. Spatiotemporal dynamics of brain activity during the transition from visually guided to memory-guided force control. J Neurophysiol 108: 1335–1348, 2012. doi: 10.1152/jn.00972.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodoehl J, Corcos DM, Rothwell JC, Metman LV, Bakay RA, Vaillancourt DE. Effects of STN DBS on memory guided force control in Parkinson’s disease (June 2007). IEEE Trans Neural Syst Rehabil Eng 15: 155–165, 2007. doi: 10.1109/TNSRE.2007.896992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U. Kinesthesia: the role of muscle receptors. Muscle Nerve 34: 545–558, 2006. doi: 10.1002/mus.20627. [DOI] [PubMed] [Google Scholar]
- Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev 92: 1651–1697, 2012. doi: 10.1152/physrev.00048.2011. [DOI] [PubMed] [Google Scholar]
- Reschechtko S, Latash ML. Stability of hand force production: I. Hand level control variables and multi-finger synergies. J Neurophysiol 118: 3152–3164, 2017. doi: 10.1152/jn.00485.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll JP, Vedel JP. Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res 47: 177–190, 1982. doi: 10.1007/BF00239377. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci 8: 4049–4068, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapkova EY, Shapkova AL, Goodman SR, Zatsiorsky VM, Latash ML. Do synergies decrease force variability? A study of single-finger and multi-finger force production. Exp Brain Res 188: 411–425, 2008. doi: 10.1007/s00221-008-1371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifkin AB, Vaillancourt DE, Newell KM. Intermittency in the control of continuous force production. J Neurophysiol 84: 1708–1718, 2000. doi: 10.1152/jn.2000.84.4.1708. [DOI] [PubMed] [Google Scholar]
- Solnik S, Qiao M, Latash ML. Effects of visual feedback and memory on unintentional drifts in performance during finger-pressing tasks. Exp Brain Res 235: 1149–1162, 2017. doi: 10.1007/s00221-017-4878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay A, Allen TJ, Proske U. Position sense at the human elbow joint measured by arm matching or pointing. Exp Brain Res 234: 2787–2798, 2016. doi: 10.1007/s00221-016-4680-y. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Russell DM. Temporal capacity of short-term visuomotor memory in continuous force production. Exp Brain Res 145: 275–285, 2002. doi: 10.1007/s00221-002-1081-1. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Slifkin AB, Newell KM. Visual control of isometric force in Parkinson’s disease. Neuropsychologia 39: 1410–1418, 2001. doi: 10.1016/S0028-3932(01)00061-6. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Thulborn KR, Corcos DM. Neural basis for the processes that underlie visually guided and internally guided force control in humans. J Neurophysiol 90: 3330–3340, 2003. doi: 10.1152/jn.00394.2003. [DOI] [PubMed] [Google Scholar]
- Von Holst E, Mittelstaedt H. The reafference principle. In: The Behavioral Physiology of Animals and Man. The Collected Papers of Erich von Holst, translated by Martin R (1973). Coral Gables, FL: University of Miami Press, 1950, vol. 1, p. 139–173. [Google Scholar]
- Watson JD, Colebatch JG, McCloskey DI. Effects of externally imposed elastic loads on the ability to estimate position and force. Behav Brain Res 13: 267–271, 1984. doi: 10.1016/0166-4328(84)90169-4. [DOI] [PubMed] [Google Scholar]
- Zhou T, Solnik S, Wu YH, Latash ML. Equifinality and its violations in a redundant system: control with referent configurations in a multi-joint positional task. Mot Contr 18: 405–424, 2014. doi: 10.1123/mc.2013-0105. [DOI] [PubMed] [Google Scholar]