Abstract
Objectives
Cellular energy metabolism is important for the function of all tissues, including cartilage. Recent studies indicate that superficial and deep subpopulations of articular chondrocytes (ACs) have distinct metabolic profiles. At the cellular and molecular level, osteoarthritis (OA) is characterised by alteration from a healthy homoeostatic state towards a catabolic state. Several molecular pathways, including transforming growth factor beta (TGF-β) and bone morphogenetic protein (BMP) signalling, have been identified as critical players in the pathogenesis and progression of OA. However, the manner in which these factors influence cellular energy metabolism in ACs is not well understood. This study investigates the effect of TGF-β or BMP signalling on energy metabolism in human articular chondrocytes (hACs).
Methods
ACs were isolated from residual macroscopically full thickness and intact cartilage from the femoral condyle of human samples obtained from patients with OA. ACs were treated with Vehicle (control), TGF-β1 or BMP2 for 48–72 hours. Metabolic assays were performed to determine glucose consumption, lactate production and adenosine triphosphate (ATP) production, whereas the mitochondrial stress test was performed to determine oxygen consumption rate. Protein was isolated to assess translational activity and was evaluated using Western blot.
Results
We showed that TGF-β1, known to maintain chondrocyte homoeostasis, stimulated glycolysis by upregulating key glycolytic factors, such as glucose transporter 1 (Glut1) and hexokinase II, while reducing oxidative phosphorylation in hACs. In contrast, BMP2 enhanced mitochondrial metabolism and oxidative phosphorylation and had a minimal effect on key glycolytic regulators.
Conclusions
Our data revealed distinct metabolic programs induced by TGF-β1 and BMP2 in hACs, suggesting that the regulation of cellular metabolism may represent a new mechanism underlying the pathogenesis of OA.
The translational potential of this article
The findings define the regulation of energy metabolism as a potential novel therapeutic approach for the treatment of OA.
Keywords: Articular chondrocytes, BMP2, Metabolism, Osteoarthritis, TGF-β
Introduction
Osteoarthritis (OA) is the most common degenerative joint disorder, clinically characterised by articular cartilage degeneration, subchondral bone sclerosis, synovitis, osteophyte formation and pain [1]. OA is the leading cause of physical disability and is projected to affect 78.4 million adults in the United States by 2040 [2]. Despite many risk factors identified, including ageing, gender, obesity, joint biomechanics and inherent genetic and epigenetic alterations, the pathogenic mechanism of OA remains unclear. As a result, there is currently no disease-modifying OA treatment except pain relief medication and surgical joint replacement as an ultimate therapeutic option [3], [4].
Although OA is nowadays considered as a whole joint disease, articular cartilage degeneration remains to be the primary concern in OA. In healthy articular cartilage, matrix turnover remains at a relatively low rate, and articular chondrocytes (ACs) resist proliferation and terminal differentiation. During the progression of OA, genes associated with hypertrophy are upregulated, and cells acquire hypertrophy-like phenotypes with enhanced catabolism and subsequent extracellular matrix degradation [5]. Previous studies have identified TGF-β signalling as a critical regulator for articular cartilage maintenance and chondrocyte homoeostasis, and ablation of TGF-β–related molecules leads to chondrocyte hypertrophic differentiation and spontaneous OA in mice [6], [7], [8], [9], [10], [11]. In contrast to the chondroprotective factor TGF-β, activation of the BMP pathway induces hypertrophic maturation in ACs [12], [13], [14], [15], [16], [17]. Despite the known differences in cell differentiation, it is unclear whether the cellular metabolism is differentially regulated by these pathways, which is essential for cartilage physiology and OA pathogenesis.
Increasing evidence suggests that OA is a metabolic disorder, and a shift in energy metabolism from a regulatory resting state to a highly metabolically active state is believed to occur in articular cartilage with OA [18], [19], [20], [21], [22]. ACs utilise glucose as the main energy source and as a precursor for glycosaminoglycan synthesis. Unlike most cell types, ACs mainly utilise glycolysis for their energy production due to the low oxygen tension in such avascular tissue [23]. Interestingly, deep/hypertrophic cells have greater levels of oxygen consumption and oxidative phosphorylation than superficial/resting cells [24]. The intrinsic metabolic differences expressed by chondrocyte subpopulations within articular cartilage likely reflect the distinct metabolic states of chondrocytes under healthy and diseased conditions.
In this study, we compared the metabolic programs of TGF-β1– and BMP2-treated human articular chondrocytes (hACs) isolated from patients with OA. Our data demonstrated that TGF-β1 dramatically enhanced aerobic glycolysis by upregulating key glycolytic regulators. In contrast to the reduced oxidative metabolism observed after TGF-β1 treatment, BMP2 treatment decreased glucose metabolism and reprogrammed energy metabolism towards an oxidative state. Therefore, TGF-β, which promotes a healthy articular chondrocyte phenotype, and BMP2, which promotes chondrocyte maturation, have distinct effects on cell energy metabolism in hACs.
Materials and methods
Human cartilage tissue
An Institutional Review Board–approved protocol was executed at the Washington University School of Medicine to collect articular cartilage from patients (n = 4) undergoing total knee arthroplasty. Patients provided written informed consent before their participation in the study. Human articular chondrocyte isolation was approved by the Washington University Human Research Protection Office.
Human articular chondrocyte isolation and culture
The femoral condyles from patients with OA were used for chondrocyte isolation. The residual macroscopically full thickness region of articular cartilage was shaved from the underlying subchondral bone using a disposable scalpel and placed in 1X PBS (phophate-buffered saline). The sections were further diced into 2-mm fragments, washed with 1X PBS and then digested in a sterile 125 ml spinner flask at 37°C for approximately 16 hours. The digestion buffer consisted of sterile filtered DMEM (31053028; Thermo Fisher Scientific, Waltham, MA, USA), 10% foetal bovine serum (Gibco, 10082147, Waltham, MA, USA), 1% penicillin/streptomycin (P/S), 1% l-glutamine (Thermo Fisher Scientific, 25030081, Waltham, MA, USA), 0.025% pronase (Roche, 11459643001, Indianapolis, IN. USA), and 0.025% collagenase P (Roche, 11213865001, Indianapolis, IN. USA). After digestion, the cells were filtered through a 70 μm cell strainer (catalogue 10199-656; VWR International, Radnor, PA, USA), centrifuged and washed two times with 1x PBS. The cells were resuspended in full media (DMEM, Dulbecco's Modified Eagle Media, 10% foetal bovine serum, 1% P/S and 1% l-glutamine) and plated at 10 × 104 cells/cm2 of growth area in various sizes of multiple well plates according to experimental designs. After 24–28 hours, the chondrocytes were treated with vehicle, BMP2 (100 ng/ml) (355-BM-010; R&D Systems, Inc., Minneapolis, MN, USA) or TGF-β1 (5 ng/ml) (240-B-002/CF; R&D Systems, Inc.) for 48–72 hours. The following metabolic assays were performed independently with the cell cultures from individual patient (n = 4).
Metabolic assays and analyses
After treatment with vehicle, BMP2 (100 ng/ml) or TGF-β1 (5 ng/ml), aliquots of the culture media and the cell cultures were analysed for glucose consumption, lactate production and ATP production. Extracellular glucose concentration was measured using the Glucose (HK) Assay Kit (GAHK-20; Sigma-Aldrich, St. Louis, MO, USA), and glucose consumption during the period of treatment was calculated by subtracting the glucose levels in the media before and after treatment. For extracellular lactate measurement, the l-Lactate Assay Kit (120001100A; Eton Biosciences, Inc., San Diego, CA, USA) was used, and lactate production within the time of treatment was obtained by subtracting the lactate levels in the media before and after treatment. In addition, intracellular ATP levels were measured using the CellTiter-Glo 2.0 Assay (G9242; Promega, Madison, WI, USA). DNA contents were evaluated with Hoechst 33342 Solution (62249; Thermo Fisher Scientific). Glucose consumption, lactate production and ATP production within certain amount of time were all normalised by DNA contents in each well accordingly. All assays aforementioned were performed according to the manufacturer's instructions.
Oxygen consumption rate measurement with Seahorse XF cell mito stress test
hACs were plated in XF96 seahorse plate at 40,000 cells per well. Culture and treatment regimens were followed as described previously. After treatment, cells were switched to Seahorse XF base medium (103335-100, Agilent Technologies, Santa Clara, CA, USA) supplemented with 5.5 mM glucose and 2 mM GlutaMAX and further incubated in CO2-free incubator for 1 hour. Oligomycin, FCCP (carbonyl cyanide p-trifluoromethoxyphenylhydrazone) and antimycin A/rotenone were prepared in XF assay medium with final concentration of 1 μM, 0.5 μM and 1 μM, respectively, from Seahorse XF Cell Mito Stress Test Kit (103015-100; Agilent Technologies) and were serially injected to measure OCR of cells in the XF96 plate. At the end of assays, DNA contents were measured as aforementioned for normalisation of OCR.
Western blot
Protein extracts from hAC cultures were prepared in RIPA buffer (radioimmunoprecipitation assay buffer) (89900, Thermo Fisher Scientific) supplemented with phosphatase and proteinase inhibitors (78840, Thermo Fisher Scientific). Protein was fractioned in a precast polyacrylamide gel (456–1084; Bio-Rad Laboratories, Inc., Hercules, Ca, USA), transferred to a polyvinylidene difluoride (PVDF) membrane with Trans-Blot Turbo Mini PVDF Transfer Packs (1704156; Bio-Rad Laboratories, Inc.) using the Trans-Blot Turbo Transfer System (Bio-Rad Laboratories, Inc.) and detected with the following primary antibodies: Glut1 (1239S; Cell Signalling Technology, Danvers, MA, USA), Hexokinase I (HKI) (2024T; Cell Signalling Technology), Hexokinase II (HKII) (2867T; Cell Signalling Technology), Lactate dehydrogenase A (LDHA) (2012; Cell Signalling Technology) and β-actin (A2228; Sigma-Aldrich).
Statistics
Data generated from the metabolic assays and mitochondria stress tests are presented as mean ± standard deviation. The metabolic data were analysed by calculating the relative change versus the control, and comparisons between two groups were analysed using two-tailed, unpaired Student t test. A p value < 0.05 was considered statistically significant. All analyses and graphs were performed using the R environment (http://www.r-project.org/).
Results
TGF-β1 stimulates glucose consumption and lactate production while reducing ATP production in hACs with OA, whereas BMP2 exhibits opposite effects
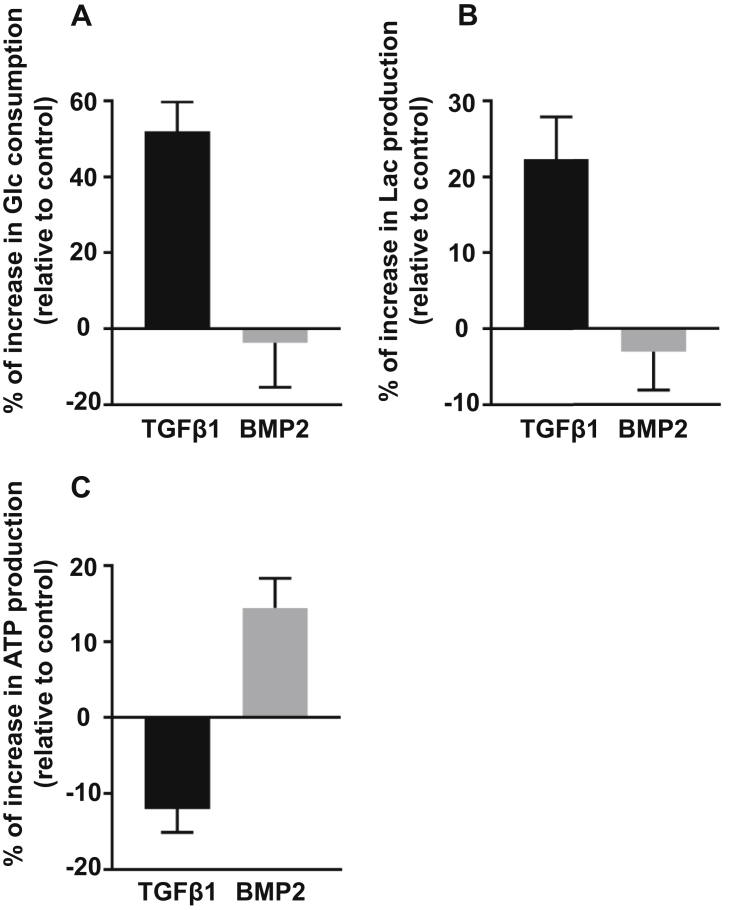
To evaluate potential changes in cellular metabolism after TGF-β1 or BMP2 treatment, we firstly examined the metabolic profiles of hACs including glucose consumption, lactate production and ATP production within 48–72 hours of treatment (Fig. 1). TGF-β1 markedly increased glucose consumption relative to the control by more than 50%, although the cell number, as assayed by DNA contents (data not shown), was slightly increased by TGF-β1 treatment during this time period. Compared with TGF-β1, BMP2 exhibited no obvious effect on glucose consumption, with the amount of glucose consumed by hACs trended less relative to the control. Consistent with the changes of glucose consumption, lactate production was enhanced after TGF-β1 treatment but reduced in BMP2 treated cells. Notably, a decrease in ATP production was observed in chondrocytes with TGF-β1 treatment. In contrast, BMP2 treated cells showed a marked increase in ATP production. Collectively, these data suggest that in hACs, TGF-β1 stimulates glycolysis even under normoxic condition. In contrast to TGF-β1, BMP2 drives glucose metabolism towards a more oxidative process, without affecting total glucose uptake.
Figure 1.
(A) hACs were isolated from the cartilage of patients with OA and then treated with TGF-β1 (5 ng/ml) or BMP2 (100 ng/ml) for 48–72 hours. Metabolic analysis of hACs with OA treated with BMP2 and TGF-β1 relative to the control shows that glucose consumption increased by 48.1% (p < 0.001) in TGF-β1–treated cells and decreased by 5.2% (p = 0.48) in BMP2-treated cells. (B) hACs were isolated from the cartilage of patients with OA and then treated with TGF-β1 (5 ng/ml) or BMP2 (100 ng/ml) for 48–72 hours. Metabolic analysis of hACs with OA treated with BMP2 and TGF-β1 relative to the control shows that lactate production increased by 19.3% (p < 0.001) in TGF-β1–treated cells and decreased by 2.8% (p = 0.34) in BMP2-treated cells. (C) hACs were isolated from the cartilage of patients with OA and then treated with TGF-β1 (5 ng/ml) or BMP2 (100 ng/ml) for 48–72 hours. Metabolic analysis of hACs with OA treated with BMP2 and TGF-β1 relative to the control shows that ATP production decreased by 12.4% (p < 0.001) in TGF-β1–treated cells and increased by 14.0% (p < 0.001) in BMP2-treated cells. Data are expressed as means ± SD of four independent experiments (*p < 0.05 by two-tailed t test).
Glc = glucose; hACs = human articular chondrocytes; Lac = lactate; OA = osteoarthritis; SD = standard deviation.
TGF-β1 induces aerobic glycolysis in hACs with OA
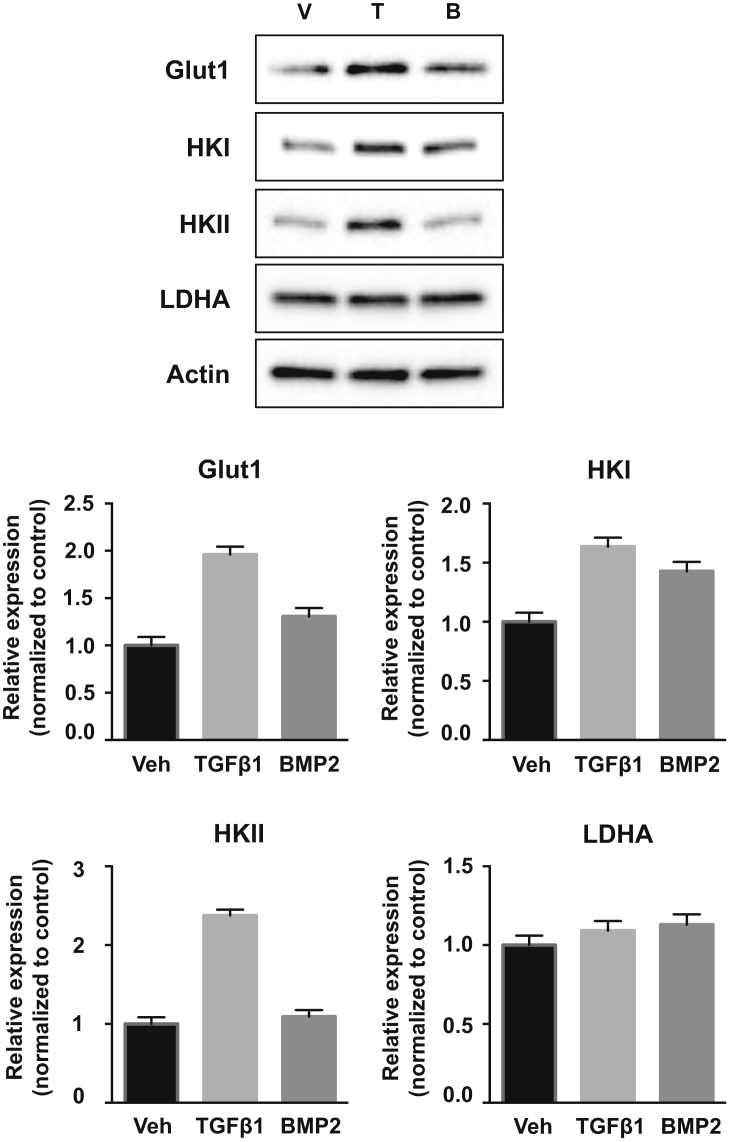
We then assessed the molecular basis for the metabolic changes induced by TGF-β1 or BMP2. Western blot analyses were performed to examine the expression levels of Glut1, the most responsive glucose transporter to both anabolic and catabolic stimuli in hACs [25], [26], [27], [28], [29] and HKII, a hexokinase isoform generally associated with aerobic glycolysis [30], [31], in hACs (Fig. 2). Glut1 and HKII were markedly elevated by more than two folds on TGF-β1 treatment. Interestingly, the expression of HKI, the other hexokinase isoform, was also increased in TGF-β1–treated cells, but with less induction compared with HKII. In contrast, BMP2 did not induce HKII, but resulted in small increases in Glut1 and HKI. LDHA, which catalyses the conversion of pyruvate to lactate, was unchanged by either TGF-β1 or BMP2 treatment. Therefore, TGF-β1 increases the protein levels of key glycolytic regulators to stimulate aerobic glycolysis, whereby BMP2 exhibited no obvious effect.
Figure 2.
Protein expression of glycolytic regulators in hACs on different treatments. Representative Western blots and quantifications of glycolytic proteins in cell cultures treated with vehicle (control), TGF-β1 (5 ng/ml) or BMP2 (100 ng/ml) for 72 h. TGF-β1–treated cells have increased protein expression of Glut1, HKI and HKII compared with the control. BMP2-treated cells have slightly increased protein expression of Glut1 and HKI, with HKII unchanged, compared with the control. Data are means ± SD of four independent experiments (*p < 0.05 by two-tailed t test).
hACs = human articular chondrocytes; HKI = hexokinase I; HKII = hexokinase II; SD = standard deviation.
BMP2 promotes oxidative phosphorylation in hACs with OA
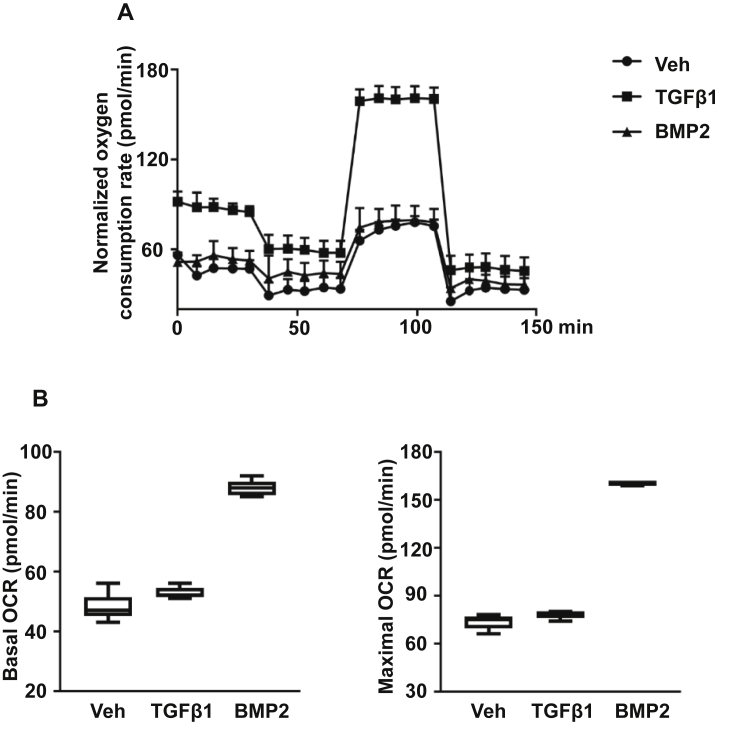
Because a decreasing trend in glucose consumption and lactate production, but an apparent increase in ATP production (Fig. 1) were noticed in chondrocytes treated with BMP2, we next sought to determine if BMP2 promotes mitochondrial oxidative metabolism by directly comparing the mitochondrial oxidative activity under different treatments. Relative to the control, BMP2-treated cells exhibited significantly elevated OCR using an extracellular flux analyser, with nearly twofold increase in both basal and maximal mitochondrial respiration (Fig. 3). By contrast, basal and maximal OCR in TGF-β1 treated cells did not differ from that of the control. Therefore, BMP2 promoted mitochondrial oxidative metabolism in hACs. In contrast, TGF-β1 did not alter the low level of mitochondrial energy metabolism, but markedly stimulated glycolysis.
Figure 3.
(A) hACs were treated with Vehicle (Control), TGF-β1 (5 ng/ml), or BMP2 (100 ng/ml) for 72 h and mitochondrial respiration was represented by the OCR (pMoles/min) and measured by the Seahorse XF Extracellular Flux Analyser. OCR was measured approximately every 8 minutes over the course of the 145-minute mitochondrial stress test. Basal respiration was measured over the first 30 minutes before the addition of any treatment, and maximal respiration was measured after FCCP treatment for 30 minutes until Antimycin A + Rotenone treatment was given to inhibit mitochondrial respiration. (B) Boxplots of basal and maximal respiration of cell cultures by treatment. The mean basal OCR of control, TGF-β1– and BMP2-treated cells was 47.3 pMoles/min, 53.2 pMoles/min and 87.8 pMoles/min, respectively. Mean basal OCR of BMP2-treated cells was significantly higher than that of the control. The mean maximal OCR of control, TGF-β1– and BMP2-treated cells was 73.6 pMoles/min, 77.9 pMoles/min and 160.2 pMoles/min, respectively. Mean maximal OCR of BMP2-treated cells was significantly higher than that of the control. Data are shown as means ± SD of four independent experiments (*p < 0.05 by two-tailed t test).
OA = osteoarthritis; OCR = oxygen consumption rate; SD = standard deviation.
Discussion and conclusions
In this study, we provide evidence that TGF-β and BMP signalling regulates energy metabolism in primary hACs. Specifically, TGF-β1 increases key glycolytic regulators, e.g., Glut1 and HKII, to stimulate glycolysis and increase lactate production. However, despite the remarkable increase of glucose consumption, oxidative phosphorylation in TGF-β1–treated cells is unchanged. In contrast, BMP2 promotes mitochondrial oxidative metabolism with no obvious effect on glucose consumption and with limited effect on the expression of key glycolytic proteins.
The present study further expands the repertoire of cellular processes that are regulated by TGF-β and BMP signalling in ACs. These two pathways are known to have opposite effects on chondrocyte extracellular matrix anabolism/catabolism and cell differentiation in ACs. TGF-β signalling has been identified as a critical maintenance factor for chondrocyte homoeostasis and articular cartilage integrity by inhibiting chondrocyte hypertrophic differentiation [6], [9]. Ablation of TGF-β signalling components in chondrocytes leads to progressive postnatal OA-like pathologies [7], [8], [11]. By contrast, BMP signalling has deleterious effects on articular cartilage and contributes to OA progression due to its effect on cell differentiation [12], [13], [14], [16], [17]. In addition, it has been suggested that TGF-β/BMP signalling must be delicately balanced and interventions that skew this balance towards procatabolic activities may result in a loss of articular cartilage. Indeed, alterations in the balance of TGF-β/BMP signalling, favouring BMP pathway signalling, has been implicated as a potential cause for enhanced catabolism and OA in mice and humans [15], [32]. However, the mechanisms through which TGF-β/BMP signalling regulates cell differentiation remain to be further elucidated, in particular, from the viewpoint of the role of cellular metabolism.
Cellular energy metabolism is critical for cartilage homoeostasis and is drastically altered in OA [18]. Under normal conditions, chondrocytes favour glycolysis more than oxidative phosphorylation for ATP production and maintain a stable extracellular matrix through a balance of anabolic and catabolic processes [33]. An increase in oxidative phosphorylation in chondrocytes may reflect an imbalance towards catabolic pathways and the development of disease. We show in this study that TGF-β1 dramatically increases glucose uptake and lactate production in hACs under normoxic conditions [34], a phenomenon that was originally described as “Warburg effect”, whereby tumour cells are thought to highly utilise aerobic glycolysis to allow diversion of glycolytic intermediates to biomass synthesis to accommodate rapid cellular proliferation [35]. ACs, however, barely proliferate in healthy cartilage. It is therefore interesting to speculate that, on the one hand, the increased glucose uptake and glycolysis may be required for sufficient provision of the enhanced anabolic activities by TGF-β1. On the other hand, limited oxidative metabolism may help prevent oxidative stress by reducing excessive production of reactive oxygen species [36]. Notably, we observed a potent induction of oxidative phosphorylation in BMP2-treated cells. The metabolic similarity between BMP2-induced cells and deep/hypertrophic cells in articular cartilage under physiological conditions [24] likely reveals that the metabolic reprogramming occurs during OA progression. Future studies are needed to investigate the functional outcomes of the metabolic reprogramming by these pathways and, in particular, how the metabolic reprogramming contributes to cell differentiation and the pathogenesis of OA.
Current findings with respect to its therapeutic potential of TGF-β are controversial since it can play both protective and deleterious roles in OA [37]. These apparently paradoxical roles of TGF-β signalling are explained in part by multiple intracellular signalling cascades activated by TGF-β proteins which are highly dependant on the cellular context. Our laboratory has recently demonstrated that TGF-β1 induced absolutely the same metabolic reprogramming in young/normal murine ACs as seen in aged/OA hACs (unpublished data), implicating a general role of TGF-β signalling in the regulation of cellular metabolism of ACs regardless of age and disease state. Future studies that elucidate the full mechanisms by which TGF-β signalling promotes glycolysis and maintains metabolic homoeostasis will provide important implications for understanding OA pathogenesis. One limitation in this study is the source of hACs which were isolated from patients with OA due to the limited availability of normal cartilage tissue. For future work, healthy articular cartilage can be obtained from patients undergoing surgery for either amputation or bone cancer or from cadavers with nondiseased joints with hopes of extrapolating more accurate and insightful information. Overall, our study revealed distinct metabolic programs by the activation of TGF-β and BMP signalling in hACs with OA, suggesting metabolism reprogramming as a possible mechanism of regulating hypertrophic cell differentiation in ACs.
In terms of clinical/translational potential, we believe that OA pathogenesis may be based on the difference of glucose utilisation between normal and diseased chondrocytes. The molecular mechanism can be further delineated to define essential downstream target genes and pathways in ACs. This will provide insights into the regulation of energy metabolism and lead to novel therapeutic approaches for effective management of OA by regulating cell metabolism.
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
Author contributions
Cuicui Wang, Richard M. Silverman, Jie Shen and Regis J. O'Keefe performed study design, analysis and interpretation of data and drafting of manuscript. Study conduct and data collection were performed by Cuicui Wang and Richard M. Silverman.
Acknowledgements
This work was supported by grants from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR069605 to RJO and T32 AR060719 to CW).
References
- 1.Felson D.T. Clinical practice. Osteoarthritis of the knee. N Engl J Med. 2006;354:841–848. doi: 10.1056/NEJMcp051726. [DOI] [PubMed] [Google Scholar]
- 2.Hootman J.M., Helmick C.G., Barbour K.E. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitation among US adults, 2015–2040. Arthr Rheumatol. 2016;68:1582–1587. doi: 10.1002/art.39692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martel-Pelletier J., Barr A.J., Cicuttini F.M. Osteoarthritis. Nat Rev Dis Primers. 2016;2:16072. doi: 10.1038/nrdp.2016.72. [DOI] [PubMed] [Google Scholar]
- 4.Shen J., Abu-Amer Y., O'Keefe R.J. Inflammation and epigenetic regulation in osteoarthritis. Connect Tissue Res. 2017;58:49–63. doi: 10.1080/03008207.2016.1208655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mueller M.B., Tuan R.S. Anabolic/Catabolic balance in pathogenesis of osteoarthritis: identifying molecular targets. PM R. 2011;3:S3–S11. doi: 10.1016/j.pmrj.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Blaney Davidson E.N., van der Kraan P.M., van den Berg W.B. TGF-beta and osteoarthritis. Osteoarthr Cartil. 2007;15:597–604. doi: 10.1016/j.joca.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Shen J., Li S., Chen D. TGF-beta signaling and the development of osteoarthritis. Bone Res. 2014;2 doi: 10.1038/boneres.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen J., Li J., Wang B. Deletion of the transforming growth factor beta receptor type II gene in articular chondrocytes leads to a progressive osteoarthritis-like phenotype in mice. Arthr Rheum. 2013;65:3107–3119. doi: 10.1002/art.38122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serra R., Johnson M., Filvaroff E.H. Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol. 1997;139:541–552. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaney Davidson E.N., Scharstuhl A., Vitters E.L. Reduced transforming growth factor-beta signaling in cartilage of old mice: role in impaired repair capacity. Arthr Res Ther. 2005;7:R1338–R1347. doi: 10.1186/ar1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang X., Chen L., Xu X. TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol. 2001;153:35–46. doi: 10.1083/jcb.153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Kraan P.M., Blaney Davidson E.N., van den Berg W.B. Bone morphogenetic proteins and articular cartilage: to serve and protect or a wolf in sheep clothing's? Osteoarthr Cartil. 2010;18:735–741. doi: 10.1016/j.joca.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Yoon B.S., Lyons K.M. Multiple functions of BMPs in chondrogenesis. J Cell Biochem. 2004;93:93–103. doi: 10.1002/jcb.20211. [DOI] [PubMed] [Google Scholar]
- 14.Zuscik M.J., Baden J.F., Wu Q. 5-azacytidine alters TGF-beta and BMP signaling and induces maturation in articular chondrocytes. J Cell Biochem. 2004;92:316–331. doi: 10.1002/jcb.20050. [DOI] [PubMed] [Google Scholar]
- 15.Blaney Davidson E.N., Remst D.F., Vitters E.L. Increase in ALK1/ALK5 ratio as a cause for elevated MMP-13 expression in osteoarthritis in humans and mice. J Immunol. 2009;182:7937–7945. doi: 10.4049/jimmunol.0803991. [DOI] [PubMed] [Google Scholar]
- 16.Brescia A.C., Simonds M.M., McCahan S.M. The role of transforming growth factor beta signaling in fibroblast-like synoviocytes from patients with oligoarticular juvenile idiopathic arthritis: dysregulation of transforming growth factor beta signaling, including overexpression of bone morphogenetic protein 4, may lead to a chondrocyte phenotype and may contribute to bony hypertrophy. Arthr Rheumatol. 2014;66:1352–1362. doi: 10.1002/art.38336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bobacz K., Sunk I.G., Hayer S. Differentially regulated expression of growth differentiation factor 5 and bone morphogenetic protein 7 in articular cartilage and synovium in murine chronic arthritis: potential importance for cartilage breakdown and synovial hypertrophy. Arthr Rheum. 2008;58:109–118. doi: 10.1002/art.23145. [DOI] [PubMed] [Google Scholar]
- 18.Mobasheri A., Rayman M.P., Gualillo O. The role of metabolism in the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2017;13:302–311. doi: 10.1038/nrrheum.2017.50. [DOI] [PubMed] [Google Scholar]
- 19.Mobasheri A. Glucose: an energy currency and structural precursor in articular cartilage and bone with emerging roles as an extracellular signaling molecule and metabolic regulator. Front Endocrinol (Lausanne) 2012;3:153. doi: 10.3389/fendo.2012.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sellam J., Berenbaum F. Is osteoarthritis a metabolic disease? Joint Bone Spine. 2013;80:568–573. doi: 10.1016/j.jbspin.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Kluzek S., Newton J.L., Arden N.K. Is osteoarthritis a metabolic disorder? Br Med Bull. 2015;115:111–121. doi: 10.1093/bmb/ldv028. [DOI] [PubMed] [Google Scholar]
- 22.June R.K., Liu-Bryan R., Long F. Emerging role of metabolic signaling in synovial joint remodeling and osteoarthritis. J Orthop Res. 2016;34:2048–2058. doi: 10.1002/jor.23420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajpurohit R., Koch C.J., Tao Z. Adaptation of chondrocytes to low oxygen tension: relationship between hypoxia and cellular metabolism. J Cell Physiol. 1996;168:424–432. doi: 10.1002/(SICI)1097-4652(199608)168:2<424::AID-JCP21>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 24.Heywood H.K., Knight M.M., Lee D.A. Both superficial and deep zone articular chondrocyte subpopulations exhibit the Crabtree effect but have different basal oxygen consumption rates. J Cell Physiol. 2010;223:630–639. doi: 10.1002/jcp.22061. [DOI] [PubMed] [Google Scholar]
- 25.Richardson S., Neama G., Phillips T. Molecular characterization and partial cDNA cloning of facilitative glucose transporters expressed in human articular chondrocytes; stimulation of 2-deoxyglucose uptake by IGF-I and elevated MMP-2 secretion by glucose deprivation. Osteoarthr Cartil. 2003;11:92–101. doi: 10.1053/joca.2002.0858. [DOI] [PubMed] [Google Scholar]
- 26.Mobasheri A., Neama G., Bell S. Human articular chondrocytes express three facilitative glucose transporter isoforms: GLUT1, GLUT3 and GLUT9. Cell Biol Int. 2002;26:297–300. doi: 10.1006/cbir.2001.0850. [DOI] [PubMed] [Google Scholar]
- 27.Rosa S.C., Goncalves J., Judas F. Impaired glucose transporter-1 degradation and increased glucose transport and oxidative stress in response to high glucose in chondrocytes from osteoarthritic versus normal human cartilage. Arthr Res Ther. 2009;11:R80. doi: 10.1186/ar2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shikhman A.R., Brinson D.C., Lotz M.K. Distinct pathways regulate facilitated glucose transport in human articular chondrocytes during anabolic and catabolic responses. Am J Physiol Endocrinol Metab. 2004;286:E980–E985. doi: 10.1152/ajpendo.00243.2003. [DOI] [PubMed] [Google Scholar]
- 29.Shikhman A.R., Brinson D.C., Valbracht J. Cytokine regulation of facilitated glucose transport in human articular chondrocytes. J Immunol. 2001;167:7001–7008. doi: 10.4049/jimmunol.167.12.7001. [DOI] [PubMed] [Google Scholar]
- 30.Gershon T.R., Crowther A.J., Tikunov A. Hexokinase-2-mediated aerobic glycolysis is integral to cerebellar neurogenesis and pathogenesis of medulloblastoma. Cancer Metab. 2013;1:2. doi: 10.1186/2049-3002-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolf A., Agnihotri S., Micallef J. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med. 2011;208:313–326. doi: 10.1084/jem.20101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen J., Wang C., Li D. DNA methyltransferase 3b regulates articular cartilage homeostasis by altering metabolism. JCI Insight. 2017;2 doi: 10.1172/jci.insight.93612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin J.A., Martini A., Molinari A. Mitochondrial electron transport and glycolysis are coupled in articular cartilage. Osteoarthr Cartil. 2012;20:323–329. doi: 10.1016/j.joca.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warburg O., Wind F., Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardin J.A., Cobelli N., Santambrogio L. Consequences of metabolic and oxidative modifications of cartilage tissue. Nat Rev Rheumatol. 2015;11:521–529. doi: 10.1038/nrrheum.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldring M.B., Berenbaum F. Emerging targets in osteoarthritis therapy. Curr Opin Pharmacol. 2015;22:51–63. doi: 10.1016/j.coph.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]