Abstract
Acidic microenvironments commonly occur at sites of inflammation and bacterial infections. In the context of a Pseudomonas aeruginosa infection, we previously demonstrated that acidosis enhances the cellular proinflammatory interleukin (IL)-1β response in vitro. However, how pH alterations affect in vivo IL-1β responses and subsequent IL-1-driven inflammation during infection with P. aeruginosa is unclear. Here, we report that acidosis enhances in vivo IL-1β production and downstream IL-1 receptor-dependent responses during infection with P. aeruginosa in models of acute pneumonia and peritonitis. Importantly, we demonstrate that infection with P. aeruginosa within an acidic environment leads to enhanced production of a subset of proinflammatory cytokines, including chemokine (C-X-C) motif ligand 1, IL-6, and chemokine (C-C motif) ligand 2, and increased neutrophil recruitment. Furthermore, with the use of IL-1 receptor type 1-deficient mice, we identify the contribution of the IL-1 signaling pathway to the acidosis-enhanced inflammatory response and pathology. These data provide insights into the potential benefit of pH regulation during bacterial infections to control disease progression and immunopathology.
Keywords: acidosis, inflammation, Pseudomonas aeruginosa
INTRODUCTION
The gram-negative bacterium Pseudomonas aeruginosa is one of the leading causes of nosocomial infections that are associated with high mortality rates, such as ventilator-associated pneumonia (28, 59, 61). With its inherent ability to adapt to different environments, P. aeruginosa is able to acutely infect diverse tissues, including the human respiratory tract, burn wounds, and the cornea (24, 43, 54, 80). Moreover, P. aeruginosa is able to persist and establish chronic infections in immunocompromised tissues, such as the lungs of cystic fibrosis (CF) patients (21). A common feature among these disease processes is the pivotal role of innate immunity during P. aeruginosa infections (42). The innate host defense is extraordinarily effective at clearing acute infections in immunocompetent individuals. However, in susceptible individuals with chronic infections, the prolonged immune response can result in excessive and damaging inflammation that can lower the pH of the microenvironment and result in immune-mediated pathology. However, how local acidosis alters the immune pathology is poorly understood.
Physiological levels of acidosis have been reported in various models of inflammation (4, 16, 35, 37, 41, 44, 48, 50, 60). The lungs of CF patients are known to be more acidotic than those of healthy individuals, as reported by pH measurements of sputum, airway surface liquid, and exhaled breath condensates (12, 49, 55, 69, 73). The low pH in CF, which is due to loss of bicarbonate transport by the CF transmembrane conductance regulator and chronic inflammatory responses in the lung, might contribute to pathogenesis (8, 12, 23, 56, 69). Particularly, extracellular acidosis can alter immune cell functions, bactericidal responses (2, 6, 35, 41, 45, 50, 55, 74, 75), and in vitro production of proinflammatory cytokines (7, 17, 31, 57, 62). However, our understanding of the contributions of in vivo acidosis during bacterial infections is very limited.
Previously, we reported that interleukin (IL)-1β production by immune cells is enhanced by acidosis during infection with P. aeruginosa (76). IL-1β is predominantly produced by macrophages after recognition of P. aeruginosa by Toll-like receptors and activation of the NOD-like receptor CARD domain-containing protein 4 (NLRC4) inflammasome by the bacterial type III secretion system (T3SS) (22, 46, 47, 71, 79). Interestingly, IL-1β has been implicated in both protective and deleterious host responses to P. aeruginosa (13, 66, 79). Once secreted, IL-1β can bind to the IL-1 receptor type 1 (IL-1R1) and induce downstream signaling that elicits production of several proinflammatory products, such as IL-8 in humans or chemokine (C-X-C motif) ligand 1 (CXCL1)/KC in mice (40, 66). CXCL1 is a potent chemoattractant for neutrophils, which are vital for control and clearance of bacteria in respiratory infections of P. aeruginosa (15, 36, 42, 78, 80). Paradoxically, neutrophil-derived products (e.g., reactive oxygen species, proteases, and hydrolytic enzymes) are a major cause of the immune-mediated tissue damage observed during chronic inflammatory responses (68). In particular, neutrophil infiltration and activity can decrease the pH of the surrounding microenvironment below the physiological norm of 7.4, which can further amplify inflammatory responses (20, 37, 38, 41, 50). Consequently, this may provide a feedforward amplification of the IL-1β response, which is regulated by acidic pH (76). However, the role of acidosis in alteration of IL-1β production and downstream IL-1-dependent immune responses during in vivo bacterial infections remains unclear.
Since previous data support the idea that pH regulates cellular IL-1β production in vitro, the objective of the current study was to assess the pH-dependent IL-1β responses in vivo and to determine the impact on downstream immune effectors with respect to pathogenesis and immune pathology. We found that P. aeruginosa infection within an acidic environment leads to significantly elevated levels of IL-1β in vivo and that the consequent impact on IL-1R1 signaling results in elevation of a specific set of proinflammatory cytokines. This, in turn, results in increased chemotactic recruitment of neutrophils to the infection site. Moreover, we demonstrate that these pH-dependent responses can promote pulmonary damage, thereby providing insights into how extracellular acidosis can affect the pathology associated with bacterial infections.
MATERIALS AND METHODS
Mice.
Age-matched female mice (6 to 8 wk old) on the C57BL/6 background were obtained from Charles River Laboratories (Frederick, MD). IL-1R1−/− mice were purchased from Jackson Laboratory (Bar Harbor, ME). Studies were carried out in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with approval of the Dartmouth Institutional Animal Care and Use Committee.
Reagents.
HEPES was purchased from Sigma-Aldrich (St. Louis, MO); DuoSet ELISA kits for mouse IL-1β, CXCL1/KC, chemokine (C-C motif) ligand (CCL2)/monocyte chemoattractant protein 1 (MCP-1), IL-6, and myeloperoxidase (MPO) from R & D Systems (Minneapolis, MN); allophycocyanin (APC)-conjugated anti-mouse Ly6G antibody (clone 1A8) from BioLegend (San Diego, CA); CytoTox96 nonradioactive cytotoxicity assay from Promega (Madison, WI); fluorescein sodium salt (catalog no. 46960) from Fluka (Buchs, Switzerland); and HEPES pH 7.3 (catalog no. BP299-100), used for intraperitoneal pH measurements, from Fisher BioReagents (Pittsburgh, PA).
Bacteria.
P. aeruginosa strains on the PA14 background were obtained from Drs. G. O’Toole and D. Hogan (Geisel School of Medicine at Dartmouth). All strains have been previously described (76). Bacteria were cultured overnight at 37°C and subsequently subcultured for 2 h in Luria broth (LB). Bacterial concentrations were determined by optical density at 600 nm (OD600) and subsequently validated by plating on LB agar and counting colony-forming units (CFUs).
In vitro bacterial growth assay.
Subcultured wild-type (WT) PA14 (10 × 103 CFU) was incubated in PBS with 0.5 M HEPES buffer at pH 5.4 or 8.0 supplemented with 0.2% casamino acids and 1 mM MgSO4 for 45 min at 37°C and 5% CO2. After incubation, the medium was plated on LB agar, and CFUs were counted. Recovered bacteria are shown as the ratio of output to input (inoculum).
In vivo bacterial infection.
For pulmonary infection, mice were anesthetized with isoflurane gas delivered in 1 l/min oxygen gas ventilation and then infected intratracheally with 3 × 106 CFU of WT or popB PA14 in a total volume of 50 μl of sterile filtered 1 M HEPES buffer at pH 5.4 (acidic) or pH 8.0 (basic). At the indicated time points, mice were euthanized by CO2 inhalation, and bronchoalveolar lavage fluid (BALF) was collected using 800 μl of 5 mM EDTA in PBS and used as described previously for ELISA and fluorescence-activated cell sorting (FACS) (53). For analysis of infiltrating neutrophils, cells within the BALF were pelleted and surface-stained with APC-conjugated anti-mouse Ly6G antibody after Fc receptor block with monoclonal antibody 2.4G2. Gating to exclude bacteria and debris was done based on scatter controls for flow cytometry analysis. Recovered CFUs were determined by plating BALF on LB agar and counting CFUs.
For intraperitoneal infection, mice were injected intraperitoneally with 1 ml of 4% thioglycollate solution and 4 days later infected with 0.75 × 106 CFU, as previously described (76). For infection, sterile filtered 1 M HEPES buffer at pH 5.4, 7.3, or 8.0 was used. Mice were euthanized at 2 h postinfection, and intraperitoneal lavage was performed using 1 ml of PBS. Cell-free peritoneal lavage samples were analyzed for cytokines by ELISA. For intraperitoneal pH analysis, mice were euthanized at 30 min or 2 h postinfection, and intraperitoneal exudate was recovered without addition of PBS. The intraperitoneal pH was determined using a fluorescein-based fluorescence assay with a standard curve obtained using 100 mM phosphate buffer at 0.5-unit pH increments from pH 5.0 to 7.5 (76).
Luminex assay.
Cytokines were measured using the Millipore mouse cytokine multiplex kit (EMD Millipore, Billerica, MA), and the assay was carried out in DartLab, the Immune Monitoring and Flow Cytometry Shared Resource Laboratory (Geisel School of Medicine at Dartmouth). Cytokines that were outside the limit of detection or <10 pg/ml were not considered for the analysis.
Quantification of lung damage.
Pulmonary cell death, as measured by lactate dehydrogenase (LDH) release, was determined from cell-free BALF using the CytoTox96 kit, according to the manufacturer’s protocol. LDH units of pH 6.7 BALF were normalized to 1, and arbitrary units of LDH are shown relative to pH 6.7 BALF. To assess lung injury, total protein concentration in cell-free BALF was determined by OD280 compared against an IgG standard curve.
Statistical analyses.
Scatter plots with means obtained from multiple independent experiments with biological replicates are shown. Statistical analyses were performed using GraphPad Prism 7.
RESULTS
IL-1β response to P. aeruginosa is exacerbated within an acidic environment in vivo.
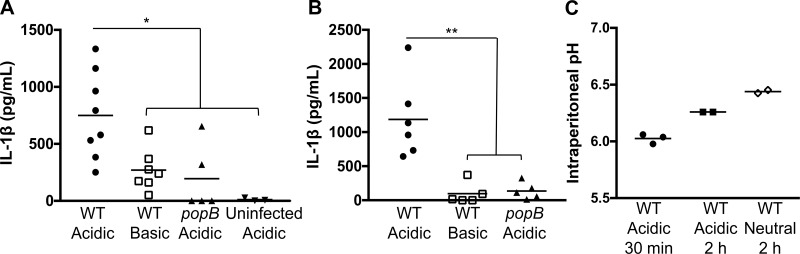
To investigate the effect of acidosis in a P. aeruginosa infection in vivo, we temporarily shifted the pH of the lung or peritoneum in established murine models of acute pneumonia and peritonitis (71, 79). To achieve this, mice were infected with the PA14 strain of P. aeruginosa in the presence of buffered medium that was either acidic (pH 5.4) or basic (pH 8.0). C57BL/6 mice infected intratracheally with WT PA14 at an acidic pH elicited robust pulmonary IL-1β production compared with mice infected at a basic pH at 3 h postinfection (Fig. 1A). However, infection with the popB mutant, which is deficient in T3SS activity, resulted in significantly lower levels of IL-1β in the BALF, even under acidic conditions, demonstrating that this response is dependent on bacterial T3SS activity (Fig. 1A) (76). Consistent with the previous observation, intratracheal instillation of the acidic buffer in the absence of bacteria did not induce pulmonary IL-1β production in mice, validating that the acidic buffer is not directly triggering IL-1β production (Fig. 1A). These results were recapitulated with a peritonitis model of infection in which IL-1β production was higher during an infection with WT PA14 in an acidified microenvironment than during an infection at basic pH or an infection with popB PA14 under acidic conditions at 2 h postinfection (Fig. 1B). To confirm that this buffering methodology impacted the in vivo pH and to assess the durability of the effect, we compared mice infected with P. aeruginosa in an acidic (pH 5.4) buffer with mice infected with P. aeruginosa in a neutral (pH 7.3) buffer to modify the pH at the site of infection. A fluorescein-based ex vivo assay (76) to measure pH revealed that the initial pH of 5.4 rapidly shifts to a pH of ~6.0 once inside the peritoneum, likely due to in vivo equilibration, but an acidic pH of ~6.3 is maintained for ≥2 h thereafter (Fig. 1C). In contrast, infection within a neutral buffer maintains the intraperitoneal pH at ~6.5, even 2 h after infection (Fig. 1C). The modest acidification, even in the presence of a neutral buffer, may reflect increases in metabolic activity in response to the infection. The intraperitoneal pH measurements confirm that our buffered infection model induces a transient and measurable alteration in the pH of the microenvironment. Therefore, in multiple infection models and anatomic locations, an infection with P. aeruginosa within an acidic environment leads to increased IL-1β production that is dependent on a functional bacterial T3SS.
Fig. 1.
Acidosis increases the P. aeruginosa-elicited IL-1β response in vivo. C57BL/6 mice were infected with P. aeruginosa strain PA14 [wild-type (WT)] or the PA14 popB isogenic mutant in an acidic (pH 5.4) or a basic (pH 8.0) buffer. Bacteria were instilled intratracheally [3 × 106 colony-forming units (CFU); A] or intraperitoneally (0.75 × 106 CFU; B). Mice instilled with the acidic pH buffer in the absence of bacteria (uninfected acidic) were used as a negative control. Bronchoalveolar and peritoneal lavage samples were collected at 3 and 2 h postinfection, respectively, and analyzed by ELISA for IL-1β. Data are derived from 3 independent experiments (n ≥ 5 for all test groups except uninfected acidic, in which n = 3). **P ≤ 0.001; *P ≤ 0.01 (by 1-way ANOVA with Dunnett’s post hoc analysis). C: pairs of C57BL/6 mice were infected intraperitoneally with 106 CFU of WT PA14 in an acidic (pH 5.4) or a neutral (pH 7.3) buffer. Mice were euthanized at 30 min or 2 h postinfection, and peritoneal exudates were collected. Intraperitoneal pH was measured using an ex vivo fluorescein-based assay.
P. aeruginosa infection within an acidified environment leads to a heightened inflammatory profile and increased neutrophil influx into the lungs.
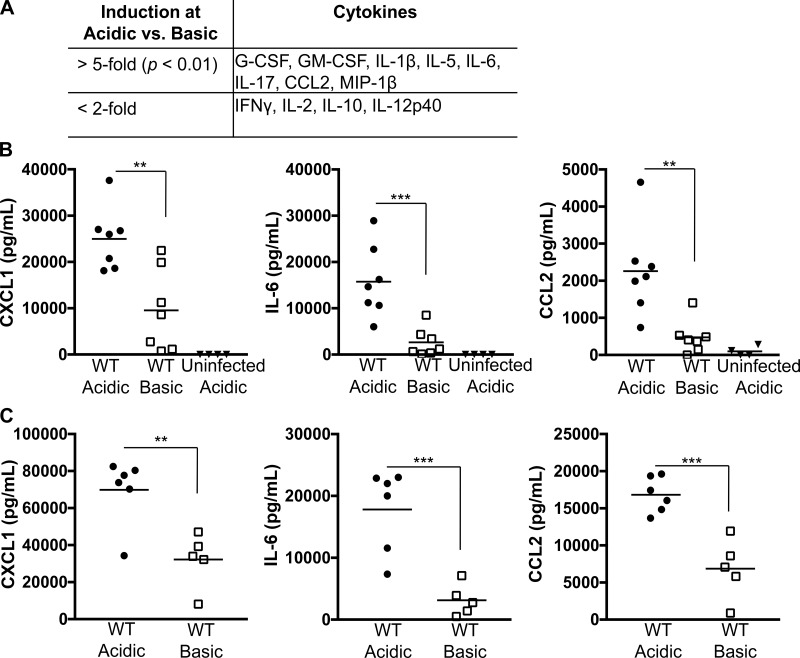
To determine the breadth of inflammatory responses altered by acidosis during a P. aeruginosa infection, we employed multiplex analyses of the BALF from C57BL/6 mice challenged with WT PA14 for 3 h under acidic or basic conditions. In validation of the results from Fig. 1, IL-1β was significantly induced during an acidic infection (Fig. 2A). Additionally, a specific subset of proinflammatory cytokines were elevated in the BALF from mice infected with P. aeruginosa in the presence of an acidic buffer (Fig. 2A). The cytokines induced by an acidic infection with WT PA14 are those that mediate chemotactic recruitment and survival of neutrophils during P. aeruginosa infections (3, 5, 26, 78). To further validate these results, we measured CXCL1, IL-6, and CCL2/MCP-1 protein levels by ELISA in the BALF at 3 h postinfection (Fig. 2B) and intraperitoneal lavage at 2 h postinfection (Fig. 2C) of C57BL/6 mice infected with WT PA14. Consistent with the multiplex analysis, there was a pH-dependent increase in IL-6 and CCL2 in both pulmonary and peritoneal infection models (Fig. 2). Intriguingly, CXCL1, the murine functional homolog of human IL-8, which was above the limit of detection for the multiplex analysis, also demonstrated a pH-dependent induction upon infection with WT PA14, with significantly higher levels at acidic pH in both infection models (Fig. 2). Thus a P. aeruginosa challenge under acidic conditions results in higher production of a distinct subset of inflammatory cytokines and chemokines in two established models of infection.
Fig. 2.
Cytokines that facilitate neutrophil recruitment are enhanced during infection with P. aeruginosa under acidic conditions. Bronchoalveolar (A and B) and peritoneal (C) lavage samples from C57BL/6 mice infected with WT PA14 in an acidic or a basic pH buffer were assessed by Luminex assay (A) or ELISA (B and C) for inflammatory cytokines. Mice were infected with 3 × 106 CFU for 3 h (A and B) or 0.75 × 106 CFU for 2 h (C). G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; CCL2, chemokine (C-C motif) ligand; MIP-1β, macrophage inflammatory protein-1β; CXCL1, chemokine (C-X-C motif) ligand 1. Data are derived from 3 independent experiments [n = 3 mice/pH group (A) and n ≥ 5 for all test groups except uninfected acidic, in which n = 4 (B and C)]. ***P ≤ 0.0005; **P ≤ 0.005 [by 1-way ANOVA with Sidak’s post hoc analysis (B) or unpaired Student’s t-test with Welch’s correction (A and C)].
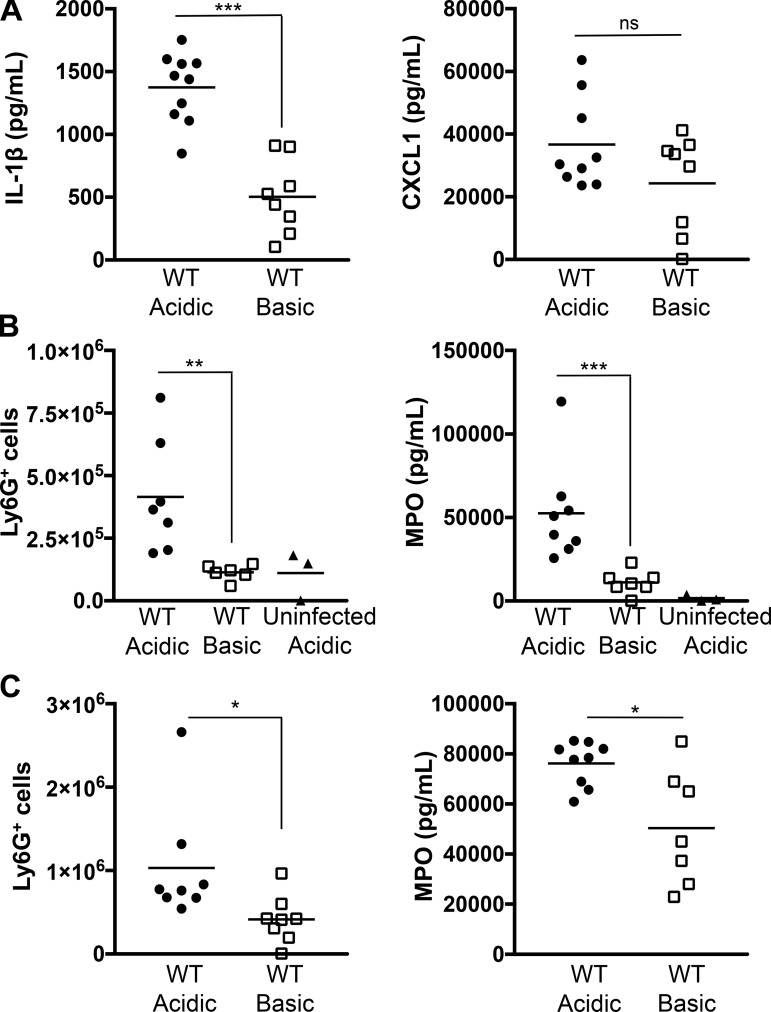
To assess the progression and durability of the acidosis-enhanced inflammatory response, BALF was collected 6 h after P. aeruginosa challenge and analyzed by ELISA for IL-1β and CXCL1 production. Cytokine levels were higher at 6 h than at 3 h postinfection for both buffered infections, demonstrating development of the inflammatory response (Figs. 2 and 3A). Notably, infection at an acidic pH continued to result in increased production of IL-1β compared with infection at basic pH at 6 h postinfection (Fig. 3A). Although production of CXCL1 did not reach significance between buffered conditions, there was a clear trend to lower levels at basic pH (Fig. 3A). This suggests that a P. aeruginosa infection at basic pH results in a delayed inflammatory response.
Fig. 3.
Acidosis enhances neutrophil recruitment in a pneumonia model of P. aeruginosa infection. C57BL/6 mice were infected intratracheally with 3 × 106 CFU of WT PA14 in an acidic or a basic pH buffer, and bronchoalveolar lavage samples were collected. A: IL-1β and CXCL1 production was analyzed by ELISA at 6 h postinfection. B and C: neutrophil recruitment and activation were determined at 3 h (B) or 6 h (C) postinfection by quantification of pulmonary Ly6G+ cells by flow cytometry and myeloperoxidase (MPO) levels by ELISA. Data are derived from 3 independent experiments (n ≥ 6 for all test groups except uninfected acidic, in which n = 3). ***P ≤ 0.0005; **P ≤ 0.005; *P ≤ 0.05; ns, not significant [by unpaired Student’s t-test with Welch’s correction (A and C) or 1-way ANOVA with Sidak’s post hoc analysis (B)].
CXCL1 plays a critical role in promoting neutrophil influx into the lungs of mice in response to bacterial challenge (15). Since an infection with P. aeruginosa at acidic pH resulted in higher production of CXCL1 in the BALF from mice, we hypothesized that, as a consequence, this would lead to increased pulmonary neutrophil recruitment. C57BL/6 mice were infected intratracheally with WT PA14 in buffered medium, and neutrophil recruitment from the BALF was quantified by flow cytometry at 3 and 6 h postinfection (Fig. 3, B and C). Acidosis enhanced the pulmonary recruitment of Ly6G+ neutrophils following infection with P. aeruginosa (Fig. 3, B and C). However, the presence of acidosis in the absence of infection did not result in significant recruitment of neutrophils at 3 h postinfection (Fig. 3B). To extend these studies to assess relative neutrophil activation, we employed the complementary approach of measuring MPO content within the BALF by ELISA at 3 and 6 h postinfection (Fig. 3, B and C). Instillation of the acidic buffer without bacteria did not induce appreciable MPO release (Fig. 3B). However, consistent with neutrophil recruitment, MPO levels were elevated upon infection, with significantly greater release of MPO under acidic conditions at 3 and 6 h postinfection (Fig. 3, B and C). Together, these findings demonstrate that chemotactic recruitment of neutrophils is increased in response to a P. aeruginosa infection at acidic pH.
Acidosis results in increased bacterial burden and T3SS-dependent pulmonary damage during P. aeruginosa infection.
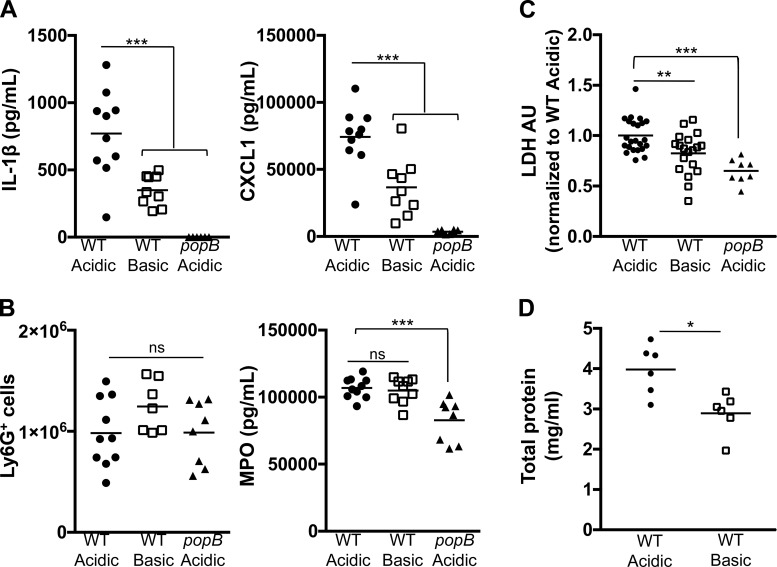
Based on the striking difference in cytokine production and neutrophil recruitment at early time points between an acidic- and a basic-buffered lung infection with P. aeruginosa, we further examined the longevity of the response and the sequence of outcomes following infection. BALF from C57BL/6 mice infected intratracheally with WT PA14 or popB PA14 in medium buffered to pH 5.4 or 8.0 was collected at 12 h postinfection and analyzed for inflammatory cytokine production (IL-1β and CXCL1), neutrophil recruitment (Ly6G+ cells), and neutrophil activity (MPO). Levels of IL-1β and CXCL1 were still higher within the BALF from mice with a WT PA14 infection at acidic than basic pH (Fig. 4A). However, while the number of neutrophils within the lungs was significantly elevated, they were comparable between both buffered infections (Fig. 4B). Consistent with this observation, MPO levels were not significantly different between pH groups (Fig. 4B). Interestingly, this was likewise observed during an infection with the popB mutant of PA14 under acidic conditions, where low levels of IL-1β and CXCL1, but elevated numbers of recruited neutrophils, were observed at 12 h postinfection (Fig. 4B). These data indicate that, over the course of a pulmonary infection, there is a temporal difference in the recruitment of neutrophils into the lungs, depending on the transient pH at which the infection occurs. Rapid neutrophil recruitment occurs during infection with an acidic pH; however, by 12 h postinfection, the differential is no longer observed.
Fig. 4.
Acidosis exacerbates type III secretion system-dependent pulmonary damage during infection with P. aeruginosa. C57BL/6 mice were infected intratracheally with 3 × 106 CFU of WT or popB PA14 in an acidic or a basic pH buffer for 12 h. Bronchoalveolar lavage samples were collected, and inflammatory cytokine production and neutrophil recruitment were assessed by ELISA (A and B) or fluorescence-activated cell sorting and lung damage was determined by lactate dehydrogenase (LDH) release [arbitrary units (AU); C] or total protein concentration (D). Data are derived from ≥3 independent experiments (n ≥ 7 for all test groups). ***P ≤ 0.0005; **P ≤ 0.005; *P ≤ 0.05; ns, not significant [by 1-way ANOVA with Dunnett’s post hoc analysis (A–C) or unpaired Student’s t-test with Welch’s correction (D)].
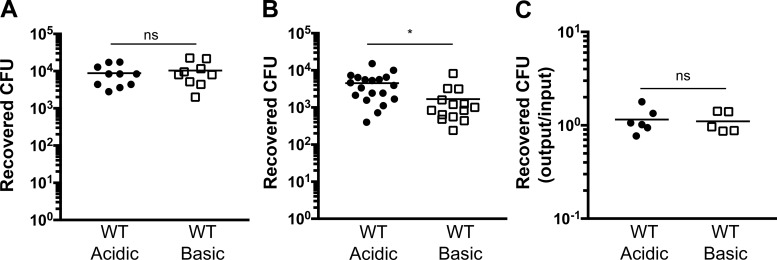
Based on clinical observations of neutrophil-mediated tissue damage during inflammatory responses (68), we assessed pulmonary damage as a downstream effect of the immediate pH-dependent response during infection with P. aeruginosa. To assess cell death in vivo, we infected mice with WT or popB PA14 and measured LDH release in the BALF at 12 h postinfection (Fig. 4C). There was a modest, but significant, decrease in pulmonary damage, as determined by LDH release, in mice infected with WT PA14 in a basic medium compared with an acidic medium (Fig. 4C). As expected, the level of lung injury was considerably lower in mice infected with popB PA14 at acidic pH than in mice infected under the same conditions with WT bacteria, demonstrating that bacterial T3SS activity contributes to the cell death (Fig. 4C). To confirm this analysis with an additional marker of lung injury, we quantified total protein concentration in the BALF at 12 h postinfection. An infection at acidic pH markedly increased the BALF protein concentration (Fig. 4D), further establishing that an acidified infection leads to increased pulmonary damage. To determine if the differences in lung damage correspond to the pulmonary bacterial burden, we infected mice intratracheally with WT PA14 and plated the BALF to count recovered CFUs at 3 and 12 h postinfection (Fig. 5, A and B). At 3 h postinfection, CFU recovery was comparable between both pH conditions (Fig. 5A). However, at 12 h postinfection, there was an increase in the number of CFUs recovered from the lungs of an acidified infection (Fig. 5B). Equal CFU loads early in the infection (Fig. 5A) and, as an additional control, similar bacterial recovery following in vitro bacterial culture within the acidic and basic media (Fig. 5C) suggest that the difference in bacterial burden at 12 h postinfection (Fig. 5B) is not due to pH-dependent differences in bacterial growth kinetics but, rather, that bacterial clearance is impaired during an infection at acidic pH. Together, these data indicate that acidosis intensifies inflammation by increasing inflammatory cytokine production, neutrophil recruitment, tissue damage, and bacterial burden during pulmonary P. aeruginosa infection. Moreover, these results suggest that there is a signaling cascade that is being amplified by acidosis.
Fig. 5.
P. aeruginosa infection at acidic pH leads to increased bacterial burden. A and B: C57BL/6 mice were infected intratracheally with 3 × 106 CFU of WT PA14 in an acidic or a basic pH buffer. Bronchoalveolar lavage samples were collected and plated, and recovered CFUs were counted at 3 h (A) and 12 h (B) postinfection. C: WT PA14 were incubated in an acidic or a basic pH buffer for 45 min, and bacterial recovery was assayed by CFU. Data are derived from ≥3 independent experiments (n ≥ 5 for all test groups). *P ≤ 0.05; ns, not significant (by unpaired Student’s t-test with Welch’s correction).
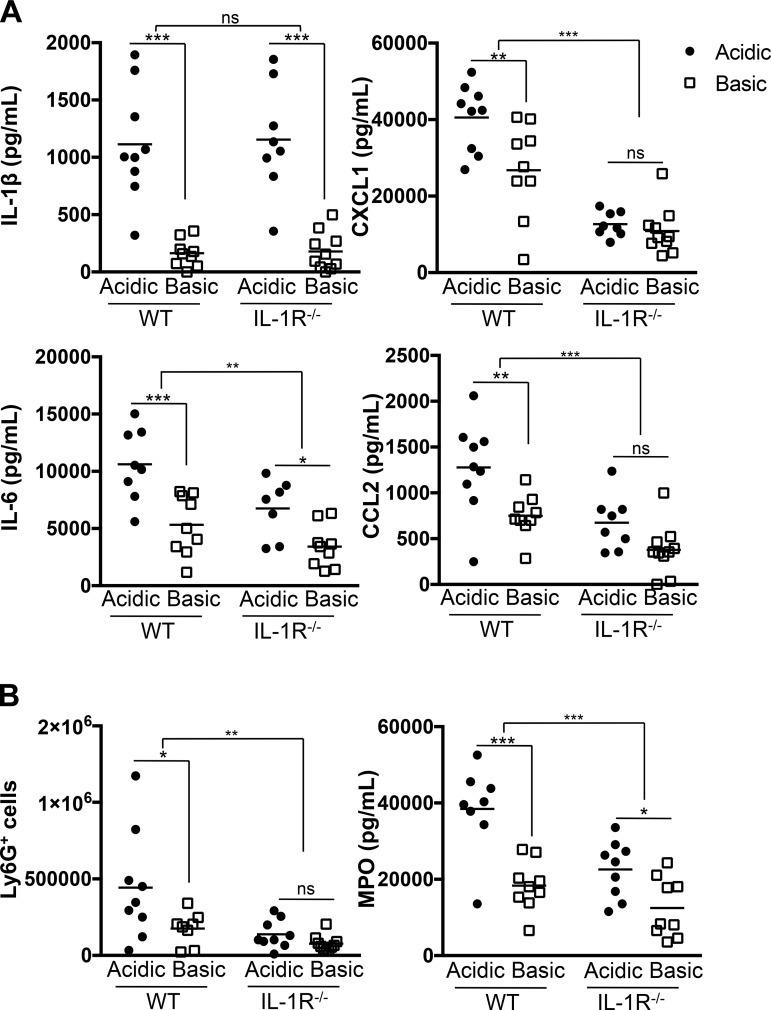
Pulmonary neutrophil recruitment enhanced by acidosis is dependent on IL-1R1 signaling.
Enhanced IL-1β production, and consequent signaling through IL-1R1, initiates a sequential cascade of downstream inflammatory responses. Therefore, since acidosis enhances the IL-1β output during in vivo infection, we next analyzed which of the pH-enhanced responses, including cytokine (CXCL1, IL-6, and CCL2) production, neutrophil recruitment, and neutrophil activation (Figs. 2 and 3), were dependent on, and downstream of, IL-1R1 signaling. C57BL/6 WT mice and IL-1R1−/− mice were challenged with WT PA14 intratracheally for 3 h, and BALF was analyzed for cytokine production and neutrophil recruitment. As previously demonstrated (Fig. 2), an infection under acidic conditions resulted in robust production of the proinflammatory cytokines IL-1β, CXCL1, IL-6, and CCL2 in WT mice (Fig. 6A). IL-1β levels were comparable between WT and IL-1R1−/− mice, which was anticipated, since IL-1R1−/− mice do not respond to IL-1β but are still able to produce it (Fig. 6A) (40). However, loss of IL-1R1 signaling led to significant decreases in CXCL1, IL-6, and CCL2. Furthermore, CXCL1 and CCL2 were not produced in a pH-dependent manner in IL-1R1−/− mice (Fig. 6A). The dependence on IL-1R1 signaling is similarly observed when neutrophil recruitment is assessed. Significantly fewer neutrophils are recruited into the lungs of IL-1R1−/− than WT mice infected under acidic pH (Fig. 6B). There is no difference between numbers of Ly6G+ neutrophils in the lungs of IL-1R1−/− mice, regardless of the pH of the buffer (Fig. 6B). These results demonstrate that IL-1R1 signaling is critical for acidosis-enhanced levels of proinflammatory cytokines and chemokines and pulmonary neutrophil recruitment during P. aeruginosa infection. Overall, infection with P. aeruginosa is significantly altered by the presence of acidic pH in the environment and results in an elevated inflammatory profile that induces increased neutrophil influx into the lungs in an IL-1R1-dependent manner. Furthermore, acidosis plays an important role in enhancing downstream pulmonary damage in a T3SS-dependent manner.
Fig. 6.
IL-1 receptor type 1 (IL-1R1) signaling is necessary for acidosis-enhanced pulmonary recruitment of neutrophils during P. aeruginosa infection. C57BL/6 and IL-1R1−/− mice were infected intratracheally with 3 × 106 CFU of WT PA14 in an acidic or a basic pH buffer. Bronchoalveolar lavage samples were collected at 3 h postinfection, and inflammatory cytokine production (A) and neutrophil recruitment (B) were assessed by ELISA and fluorescence-activated cell sorting, respectively. Data are derived from 5 independent experiments (for all test groups n ≥ 8). ***P ≤ 0.0005; **P ≤ 0.005; *P ≤ 0.05; ns, not significant (by 2-way ANOVA with Sidak’s post hoc analysis).
DISCUSSION
Several reports have demonstrated that acidic pH can enhance production of the proinflammatory cytokine IL-1β in vitro (17, 31, 57, 72). In support of this finding, we previously demonstrated that the IL-1β response is amplified by acidosis during in vitro cellular infection with P. aeruginosa (76). In contrast to some reports, acidosis was not sufficient to induce IL-1β release; rather, in the context of P. aeruginosa infection, it required functional T3SS activity by the bacteria and caspase-1 and NLRC4 expression by the host cells (76). Based on our previous in vitro findings, we hypothesized that an in vivo infection with P. aeruginosa at low pH will lead to enhanced IL-1β production and altered IL-1-driven responses.
To test this hypothesis, we infected mice with P. aeruginosa in an acidic (pH 5.4) or a basic (pH 8.0) buffer to modify the pH at the site of infection. A fluorescein-based ex vivo assay (76) confirms that acidic buffering of the infection model induces a transient and measurable alteration in the pH of the microenvironment. It is noteworthy that this ex vivo assay is limited with regard to absolute precision and our lack of ability to collect sufficient pulmonary exudate liquid (30), but it is possible that in vivo pH measurements utilizing microelectrodes or MRI may facilitate direct pulmonary measurements (12, 19, 32, 34, 39, 70). However, the finding that even transient fluxes in pH may alter inflammatory processes may have direct relevance to acute respiratory exacerbations, where increased bacterial colonization and neutrophilic inflammation inversely correlate with pH levels in measurements of exhaled breath condensates from patients with non-CF pulmonary disorders, such as asthma, chronic obstructive pulmonary disease, and bronchiectasis (29, 37). Interestingly, in CF, airway acidification is inherently present, leading to recent studies that focus on the role of pH in impairment of host defenses within CF airways (2, 55, 67). However, whether CF exacerbations further lower the pulmonary pH is controversial (9, 11, 49, 51, 63, 73).
Overall, in this report we demonstrate that IL-1β is produced in a pH-dependent manner in vivo in response to a pulmonary P. aeruginosa infection. Furthermore, through the use of transient pH flux in combination with the IL-1R1 genetic knockout mice, we are able to analyze the pH-dependent and -independent downstream responses and the temporal consequences of the enhanced IL-1 signaling. In both pulmonary and peritonitis in vivo infection models, we demonstrate that an acidic environment potentiates IL-1β production. In support of our in vitro findings (76), the elevated IL-1β production during an acidic infection was dependent on the function of the bacterial T3SS. We previously showed that T3SS activity or production of the Exo proteins is not regulated by pH (75, 76). Therefore, the T3SS dependence of this response is likely due to its requirement to activate the NLRC4 inflammasome and caspase-1 for IL-1β production (22, 46, 47, 52, 71, 76, 79).
Interestingly, IL-1β was not the only cytokine upregulated with an acidic infection. Luminex assay and ELISA demonstrated that a subset of cytokines, including IL-6, CCL2, granulocyte colony-stimulating factor (G-CSF), and CXCL1, were also significantly induced during an acidic infection with P. aeruginosa. Importantly, however, not all cytokines were upregulated. IL-2, IL-10, IL-12p40, and IFNγ levels were comparable between both buffered infections, indicating that acidosis does not lead to a total dysregulation of host immune responses. Primarily cytokines pertinent to an innate response to an acute P. aeruginosa infection were augmented during acidosis (42): the chemokines CXCL1, CCL2, and G-CSF, which mediate recruitment and survival of neutrophils, were upregulated during an infection at acidic pH (3, 5, 26, 78). It is worth noting that the findings of elevated cytokines detected during the in vivo murine P. aeruginosa infections at acidic pH are similar to those reported from human and porcine CF studies. Specifically, elevated levels of IL-1β, IL-8, IL-6, and G-CSF have been reported in the BALF, sputum, and blood of CF patients and are proposed to contribute to CF airway inflammation (10, 14, 27, 33, 58, 65). Thus this study may provide insights into how these cytokines remain elevated by the acidic environment during the course of CF inflammation.
To determine which cytokines were elevated as a direct downstream consequence of the enhanced IL-1 production, we employed IL-1R1−/− mice. Parallel infection of WT and IL-1R1−/− mice revealed that CXCL1, CCL2, and IL-6 were dependent on IL-1R1 signaling during induction by acidosis. However, IL-6 showed some pH dependence, even in the absence of IL-1R signaling, which could be due to inflammatory transcription factor regulation by the acidic pH (7, 25, 81). As a logical consequence of the IL-1R-dependent acidosis-enhanced cytokines, we found that, within 6 h after infection, neutrophils were enriched in the lungs of mice infected with P. aeruginosa at acidic pH compared with those infected at basic pH. This rapid neutrophil recruitment to P. aeruginosa-infected lungs at acidic pH was dependent on IL-1 signaling, since lower levels of neutrophils were detected in IL-1R1-deficient mice at 3 h after infection (79). However, at 12 h after infection, neutrophil recruitment was comparable between pH values in WT mice. These data suggest that even a transient flux to acidic pH is sufficient to durably alter the IL-1β response for as long as 12 h. Correspondingly, a transient buffering at slightly basic pH is sufficient to delay IL-1R-dependent neutrophil influx and activity compared with that following infection during acidic conditions. However, our data suggest that, by 12 h following the pH flux, the neutrophil recruitment delay is lapsed, since neutrophils at the site of infection are equivalent in both pH conditions. Therefore, the levels of the chemokines produced during an infection at basic pH were eventually sufficient for pulmonary neutrophil influx, albeit at delayed kinetics; this was likewise observed during an acidified infection with the T3SS-defective mutant popB.
Ultimately, infection with WT PA14 at acidic pH resulted in an increase in lung damage and bacterial burden compared with infection at basic pH. This is consistent with previous findings that prolonged inflammatory responses and neutrophil activity can interfere with bacterial killing and promote lung injury (68). Additionally, this correlates with our findings of early induction of proinflammatory mediators and subsequent rapid IL-1R1 signaling-driven neutrophil recruitment and activity at acidic pH. These findings help tie together previous independent observations that excessive IL-1R1 signaling is deleterious to the host, can promote bacterial outgrowth, and can contribute to pulmonary pathology (66) and that extracellular acidosis may induce neutrophil functions, including MPO release and reactive oxygen species production, that enhance inflammation and injury (45, 77). However, an infection with the popB mutant of PA14 at acidic pH resulted in significantly reduced lung damage compared with an infection with WT PA14 at the same pH. This suggests that, under these conditions, the acidic buffer itself is not inducing the pulmonary damage and that there is a bacterial T3SS dependence for this response. This supports previous reports that P. aeruginosa strains expressing a functional T3SS are more virulent and are associated with increased disease severity (18, 28, 64). In support of this premise, epithelial cells are more susceptible to cytotoxicity during an acidic infection with P. aeruginosa, and this is dependent on the function of the bacterial T3SS (75). Therefore, it is likely that the increased LDH release is due to T3SS-induced epithelial cell death.
The role of acidic pH in mediating host inflammatory responses has been a recent focus in studies of CF. Relevant to our studies, in a recent report, transient use of tromethamine, which increases the pH in CF airway surface liquid and sputum and can enhance bacterial killing by antimicrobial peptides, was employed (1). Thus there are likely multiple mechanisms by which alterations in pH exert their effects on disease processes. In conclusion, our data provide insights into the impact and extent of pH regulation on the in vivo host immune response during a P. aeruginosa infection and additional and specific immune targets that could be modulated by use of buffered therapeutics during airway disease. We demonstrate that, during an acidic infection, increased production of IL-1β and IL-1-dependent cytokines results in increased pulmonary neutrophil influx. Importantly, we also demonstrate that extracellular acidosis enhances IL-1R1-dependent responses during in vivo P. aeruginosa infections and identify the inflammatory and pathological consequences. These data provide key insights into the specificity of pH-regulated host inflammatory responses and pathogenesis during bacterial infections.
GRANTS
This work was supported by National Institutes of Health Grants P30-RR-032136-01, P30-GM-106394, R21-AI-121820, and T32-AI-007363 (to I. M. Torres and B. Berwin) and Cystic Fibrosis Foundation Research Development Program Grants STANTO19R0 and STANTO11R0 (to B. Berwin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.M.T., Y.R.P., and B.L.B. conceived and designed research; I.M.T. and Y.R.P. performed experiments; I.M.T., Y.R.P., and B.L.B. analyzed data; I.M.T. and B.L.B. interpreted results of experiments; I.M.T. and B.L.B. prepared figures; I.M.T. and B.L.B. drafted manuscript; I.M.T. and B.L.B. edited and revised manuscript; I.M.T., Y.R.P., and B.L.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank George O’Toole, Josh Obar, Sally Demirdjian, Sladjana Skopelja-Gardner, and Alayna Caffrey (Geisel School of Medicine at Dartmouth) for reagents and discussion. This work was facilitated by the Dartmouth Lung Biology Translational Research Core and the Norris Cotton Cancer Center Immune Monitoring Laboratory.
REFERENCES
- 1.Abou Alaiwa MH, Launspach JL, Sheets KA, Rivera JA, Gansemer ND, Taft PJ, Thorne PS, Welsh MJ, Stoltz DA, Zabner J. Repurposing tromethamine as inhaled therapy to treat CF airway disease. JCI Insight 1: e87535, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abou Alaiwa MH, Reznikov LR, Gansemer ND, Sheets KA, Horswill AR, Stoltz DA, Zabner J, Welsh MJ. pH modulates the activity and synergism of the airway surface liquid antimicrobials β-defensin-3 and LL-37. Proc Natl Acad Sci USA 111: 18703–18708, 2014. doi: 10.1073/pnas.1422091112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amano H, Morimoto K, Senba M, Wang H, Ishida Y, Kumatori A, Yoshimine H, Oishi K, Mukaida N, Nagatake T. Essential contribution of monocyte chemoattractant protein-1/C-C chemokine ligand-2 to resolution and repair processes in acute bacterial pneumonia. J Immunol 172: 398–409, 2004. doi: 10.4049/jimmunol.172.1.398. [DOI] [PubMed] [Google Scholar]
- 4.Bakker J, Coffernils M, Leon M, Gris P, Vincent JL. Blood lactate levels are superior to oxygen-derived variables in predicting outcome in human septic shock. Chest 99: 956–962, 1991. doi: 10.1378/chest.99.4.956. [DOI] [PubMed] [Google Scholar]
- 5.Balamayooran G, Batra S, Theivanthiran B, Cai S, Pacher P, Jeyaseelan S. Intrapulmonary G-CSF rescues neutrophil recruitment to the lung and neutrophil release to blood in Gram-negative bacterial infection in MCP-1−/− mice. J Immunol 189: 5849–5859, 2012. doi: 10.4049/jimmunol.1200585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behnen M, Möller S, Brozek A, Klinger M, Laskay T. Extracellular acidification inhibits the ROS-dependent formation of neutrophil extracellular traps. Front Immunol 8: 184, 2017. doi: 10.3389/fimmu.2017.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellocq A, Suberville S, Philippe C, Bertrand F, Perez J, Fouqueray B, Cherqui G, Baud L. Low environmental pH is responsible for the induction of nitric-oxide synthase in macrophages. Evidence for involvement of nuclear factor-κB activation. J Biol Chem 273: 5086–5092, 1998. doi: 10.1074/jbc.273.9.5086. [DOI] [PubMed] [Google Scholar]
- 8.Berkebile AR, McCray PB Jr. Effects of airway surface liquid pH on host defense in cystic fibrosis. Int J Biochem Cell Biol 52: 124–129, 2014. doi: 10.1016/j.biocel.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bodini A, D’Orazio C, Peroni D, Corradi M, Folesani G, Baraldi E, Assael BM, Boner A, Piacentini GL. Biomarkers of neutrophilic inflammation in exhaled air of cystic fibrosis children with bacterial airway infections. Pediatr Pulmonol 40: 494–499, 2005. doi: 10.1002/ppul.20336. [DOI] [PubMed] [Google Scholar]
- 10.Bonfield TL, Panuska JR, Konstan MW, Hilliard KA, Hilliard JB, Ghnaim H, Berger M. Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med 152: 2111–2118, 1995. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]
- 11.Carpagnano GE, Barnes PJ, Francis J, Wilson N, Bush A, Kharitonov SA. Breath condensate pH in children with cystic fibrosis and asthma: a new noninvasive marker of airway inflammation? Chest 125: 2005–2010, 2004. doi: 10.1378/chest.125.6.2005. [DOI] [PubMed] [Google Scholar]
- 12.Coakley RD, Grubb BR, Paradiso AM, Gatzy JT, Johnson LG, Kreda SM, O’Neal WK, Boucher RC. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc Natl Acad Sci USA 100: 16083–16088, 2003. doi: 10.1073/pnas.2634339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen TS, Prince AS. Activation of inflammasome signaling mediates pathology of acute P. aeruginosa pneumonia. J Clin Invest 123: 1630–1637, 2013. doi: 10.1172/JCI66142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Courtney JM, Ennis M, Elborn JS. Cytokines and inflammatory mediators in cystic fibrosis. J Cyst Fibros 3: 223–231, 2004. doi: 10.1016/j.jcf.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Craig A, Mai J, Cai S, Jeyaseelan S. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect Immun 77: 568–575, 2009. doi: 10.1128/IAI.00832-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deshpande GG, Heidemann SM, Sarnaik AP. Heat stress is associated with decreased lactic acidemia in rat sepsis. Crit Care 4: 45–49, 2000. doi: 10.1186/cc649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edye ME, Lopez-Castejon G, Allan SM, Brough D. Acidosis drives damage-associated molecular pattern (DAMP)-induced interleukin-1 secretion via a caspase-1-independent pathway. J Biol Chem 288: 30485–30494, 2013. doi: 10.1074/jbc.M113.478941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Solh AA, Hattemer A, Hauser AR, Alhajhusain A, Vora H. Clinical outcomes of type III Pseudomonas aeruginosa bacteremia. Crit Care Med 40: 1157–1163, 2012. doi: 10.1097/CCM.0b013e3182377906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer H, Widdicombe JH. Mechanisms of acid and base secretion by the airway epithelium. J Membr Biol 211: 139–150, 2006. doi: 10.1007/s00232-006-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer H, Widdicombe JH, Illek B. Acid secretion and proton conductance in human airway epithelium. Am J Physiol Cell Physiol 282: C736–C743, 2002. doi: 10.1152/ajpcell.00369.2001. [DOI] [PubMed] [Google Scholar]
- 21.Folkesson A, Jelsbak L, Yang L, Johansen HK, Ciofu O, Høiby N, Molin S. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: an evolutionary perspective. Nat Rev Microbiol 10: 841–851, 2012. doi: 10.1038/nrmicro2907. [DOI] [PubMed] [Google Scholar]
- 22.Franchi L, Stoolman J, Kanneganti TD, Verma A, Ramphal R, Núñez G. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur J Immunol 37: 3030–3039, 2007. doi: 10.1002/eji.200737532. [DOI] [PubMed] [Google Scholar]
- 23.Garland AL, Walton WG, Coakley RD, Tan CD, Gilmore RC, Hobbs CA, Tripathy A, Clunes LA, Bencharit S, Stutts MJ, Betts L, Redinbo MR, Tarran R. Molecular basis for pH-dependent mucosal dehydration in cystic fibrosis airways. Proc Natl Acad Sci USA 110: 15973–15978, 2013. doi: 10.1073/pnas.1311999110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gellatly SL, Hancock RE. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis 67: 159–173, 2013. doi: 10.1111/2049-632X.12033. [DOI] [PubMed] [Google Scholar]
- 25.Gerry AB, Leake DS. Effect of low extracellular pH on NF-κB activation in macrophages. Atherosclerosis 233: 537–544, 2014. doi: 10.1016/j.atherosclerosis.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregory AD, Hogue LA, Ferkol TW, Link DC. Regulation of systemic and local neutrophil responses by G-CSF during pulmonary Pseudomonas aeruginosa infection. Blood 109: 3235–3243, 2007. doi: 10.1182/blood-2005-01-015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartl D, Gaggar A, Bruscia E, Hector A, Marcos V, Jung A, Greene C, McElvaney G, Mall M, Döring G. Innate immunity in cystic fibrosis lung disease. J Cyst Fibros 11: 363–382, 2012. doi: 10.1016/j.jcf.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Hauser AR, Cobb E, Bodi M, Mariscal D, Vallés J, Engel JN, Rello J. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit Care Med 30: 521–528, 2002. doi: 10.1097/00003246-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Hunt JF, Fang K, Malik R, Snyder A, Malhotra N, Platts-Mills TA, Gaston B. Endogenous airway acidification. Implications for asthma pathophysiology. Am J Respir Crit Care Med 161: 694–699, 2000. doi: 10.1164/ajrccm.161.3.9911005. [DOI] [PubMed] [Google Scholar]
- 30.Jackson AS, Sandrini A, Campbell C, Chow S, Thomas PS, Yates DH. Comparison of biomarkers in exhaled breath condensate and bronchoalveolar lavage. Am J Respir Crit Care Med 175: 222–227, 2007. doi: 10.1164/rccm.200601-107OC. [DOI] [PubMed] [Google Scholar]
- 31.Jancic CC, Cabrini M, Gabelloni ML, Rodríguez Rodrigues C, Salamone G, Trevani AS, Geffner J. Low extracellular pH stimulates the production of IL-1β by human monocytes. Cytokine 57: 258–268, 2012. doi: 10.1016/j.cyto.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Jayaraman S, Song Y, Verkman AS. Airway surface liquid pH in well-differentiated airway epithelial cell cultures and mouse trachea. Am J Physiol Cell Physiol 281: C1504–C1511, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Jensen PO, Moser C, Kharazmi A, Presler T, Koch C, Høiby N. Increased serum concentration of G-CSF in cystic fibrosis patients with chronic Pseudomonas aeruginosa pneumonia. J Cyst Fibros 5: 145–151, 2006. doi: 10.1016/j.jcf.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Jones KM, Randtke EA, Howison CM, Cárdenas-Rodríguez J, Sime PJ, Kottmann MR, Pagel MD. Measuring extracellular pH in a lung fibrosis model with acidoCEST MRI. Mol Imaging Biol 17: 177–184, 2015. doi: 10.1007/s11307-014-0784-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kellum JA, Song M, Li J. Science review: extracellular acidosis and the immune response: clinical and physiologic implications. Crit Care 8: 331–336, 2004. doi: 10.1186/cc2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koh AY, Priebe GP, Ray C, Van Rooijen N, Pier GB. Inescapable need for neutrophils as mediators of cellular innate immunity to acute Pseudomonas aeruginosa pneumonia. Infect Immun 77: 5300–5310, 2009. doi: 10.1128/IAI.00501-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kostikas K, Papatheodorou G, Ganas K, Psathakis K, Panagou P, Loukides S. pH in expired breath condensate of patients with inflammatory airway diseases. Am J Respir Crit Care Med 165: 1364–1370, 2002. doi: 10.1164/rccm.200111-068OC. [DOI] [PubMed] [Google Scholar]
- 38.Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, Cross JR, Jung E, Thompson CB, Jones RG, Pearce EJ. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 115: 4742–4749, 2010. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kyle H, Ward JP, Widdicombe JG. Control of pH of airway surface liquid of the ferret trachea in vitro. J Appl Physiol (1985) 68: 135–140, 1990. [DOI] [PubMed] [Google Scholar]
- 40.Labow M, Shuster D, Zetterstrom M, Nunes P, Terry R, Cullinan EB, Bartfai T, Solorzano C, Moldawer LL, Chizzonite R, McIntyre KW. Absence of IL-1 signaling and reduced inflammatory response in IL-1 type I receptor-deficient mice. J Immunol 159: 2452–2461, 1997. [PubMed] [Google Scholar]
- 41.Lardner A. The effects of extracellular pH on immune function. J Leukoc Biol 69: 522–530, 2001. [PubMed] [Google Scholar]
- 42.Lavoie EG, Wangdi T, Kazmierczak BI. Innate immune responses to Pseudomonas aeruginosa infection. Microbes Infect 13: 1133–1145, 2011. doi: 10.1016/j.micinf.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyczak JB, Cannon CL, Pier GB. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect 2: 1051–1060, 2000. doi: 10.1016/S1286-4579(00)01259-4. [DOI] [PubMed] [Google Scholar]
- 44.Månsson B, Geborek P, Saxne T, Björnsson S. Cytidine deaminase activity in synovial fluid of patients with rheumatoid arthritis: relation to lactoferrin, acidosis, and cartilage proteoglycan release. Ann Rheum Dis 49: 594–597, 1990. doi: 10.1136/ard.49.8.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martínez D, Vermeulen M, Trevani A, Ceballos A, Sabatté J, Gamberale R, Alvarez ME, Salamone G, Tanos T, Coso OA, Geffner J. Extracellular acidosis induces neutrophil activation by a mechanism dependent on activation of phosphatidylinositol 3-kinase/Akt and ERK pathways. J Immunol 176: 1163–1171, 2006. doi: 10.4049/jimmunol.176.2.1163. [DOI] [PubMed] [Google Scholar]
- 46.Miao EA, Ernst RK, Dors M, Mao DP, Aderem A. Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proc Natl Acad Sci USA 105: 2562–2567, 2008. doi: 10.1073/pnas.0712183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miao EA, Mao DP, Yudkovsky N, Bonneau R, Lorang CG, Warren SE, Leaf IA, Aderem A. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci USA 107: 3076–3080, 2010. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 10: 767–777, 2011. doi: 10.1038/nrd3554. [DOI] [PubMed] [Google Scholar]
- 49.Newport S, Amin N, Dozor AJ. Exhaled breath condensate pH and ammonia in cystic fibrosis and response to treatment of acute pulmonary exacerbations. Pediatr Pulmonol 44: 866–872, 2009. doi: 10.1002/ppul.21078. [DOI] [PubMed] [Google Scholar]
- 50.Ng AW, Bidani A, Heming TA. Innate host defense of the lung: effects of lung-lining fluid pH. Lung 182: 297–317, 2004. doi: 10.1007/s00408-004-2511-6. [DOI] [PubMed] [Google Scholar]
- 51.Ojoo JC, Mulrennan SA, Kastelik JA, Morice AH, Redington AE. Exhaled breath condensate pH and exhaled nitric oxide in allergic asthma and in cystic fibrosis. Thorax 60: 22–26, 2005. doi: 10.1136/thx.2003.017327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patankar YR, Lovewell RR, Poynter ME, Jyot J, Kazmierczak BI, Berwin B. Flagellar motility is a key determinant of the magnitude of the inflammasome response to Pseudomonas aeruginosa. Infect Immun 81: 2043–2052, 2013. doi: 10.1128/IAI.00054-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patankar YR, Mabaera R, Berwin B. Differential ASC requirements reveal a key role for neutrophils and a noncanonical IL-1β response to Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 309: L902–L913, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pearlman E, Sun Y, Roy S, Karmakar M, Hise AG, Szczotka-Flynn L, Ghannoum M, Chinnery HR, McMenamin PG, Rietsch A. Host defense at the ocular surface. Int Rev Immunol 32: 4–18, 2013. doi: 10.3109/08830185.2012.749400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pezzulo AA, Tang XX, Hoegger MJ, Abou Alaiwa MH, Ramachandran S, Moninger TO, Karp PH, Wohlford-Lenane CL, Haagsman HP, van Eijk M, Bánfi B, Horswill AR, Stoltz DA, McCray PB Jr, Welsh MJ, Zabner J. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 487: 109–113, 2012. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poulsen JH, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA 91: 5340–5344, 1994. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rajamäki K, Nordström T, Nurmi K, Åkerman KE, Kovanen PT, Öörni K, Eklund KK. Extracellular acidosis is a novel danger signal alerting innate immunity via the NLRP3 inflammasome. J Biol Chem 288: 13410–13419, 2013. doi: 10.1074/jbc.M112.426254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rao S, Wright AK, Montiero W, Ziegler-Heitbrock L, Grigg J. Monocyte chemoattractant chemokines in cystic fibrosis. J Cyst Fibros 8: 97–103, 2009. doi: 10.1016/j.jcf.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 59.Rello J, Díaz E, Rodríguez A. Etiology of ventilator-associated pneumonia. Clin Chest Med 26: 87–95, 2005. doi: 10.1016/j.ccm.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 60.Ricciardolo FL, Gaston B, Hunt J. Acid stress in the pathology of asthma. J Allergy Clin Immunol 113: 610–619, 2004. doi: 10.1016/j.jaci.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 61.Richards MJ, Edwards JR, Culver DH, Gaynes RP; National Nosocomial Infections Surveillance System . Nosocomial infections in medical intensive care units in the United States. Crit Care Med 27: 887–892, 1999. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- 62.Riemann A, Wußling H, Loppnow H, Fu H, Reime S, Thews O. Acidosis differently modulates the inflammatory program in monocytes and macrophages. Biochim Biophys Acta 1862: 72–81, 2016. doi: 10.1016/j.bbadis.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 63.Robroeks CM, Rosias PP, van Vliet D, Jöbsis Q, Yntema JB, Brackel HJ, Damoiseaux JG, den Hartog GM, Wodzig WK, Dompeling E. Biomarkers in exhaled breath condensate indicate presence and severity of cystic fibrosis in children. Pediatr Allergy Immunol 19: 652–659, 2008. [DOI] [PubMed] [Google Scholar]
- 64.Roy-Burman A, Savel RH, Racine S, Swanson BL, Revadigar NS, Fujimoto J, Sawa T, Frank DW, Wiener-Kronish JP. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J Infect Dis 183: 1767–1774, 2001. doi: 10.1086/320737. [DOI] [PubMed] [Google Scholar]
- 65.Sagel SD, Chmiel JF, Konstan MW. Sputum biomarkers of inflammation in cystic fibrosis lung disease. Proc Am Thorac Soc 4: 406–417, 2007. doi: 10.1513/pats.200703-044BR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schultz MJ, Rijneveld AW, Florquin S, Edwards CK, Dinarello CA, van der Poll T. Role of interleukin-1 in the pulmonary immune response during Pseudomonas aeruginosa pneumonia. Am J Physiol Lung Cell Mol Physiol 282: L285–L290, 2002. doi: 10.1152/ajplung.00461.2000. [DOI] [PubMed] [Google Scholar]
- 67.Shah VS, Meyerholz DK, Tang XX, Reznikov L, Abou Alaiwa M, Ernst SE, Karp PH, Wohlford-Lenane CL, Heilmann KP, Leidinger MR, Allen PD, Zabner J, McCray PB Jr, Ostedgaard LS, Stoltz DA, Randak CO, Welsh MJ. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science 351: 503–507, 2016. doi: 10.1126/science.aad5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith JA. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol 56: 672–686, 1994. [DOI] [PubMed] [Google Scholar]
- 69.Song Y, Salinas D, Nielson DW, Verkman AS. Hyperacidity of secreted fluid from submucosal glands in early cystic fibrosis. Am J Physiol Cell Physiol 290: C741–C749, 2006. doi: 10.1152/ajpcell.00379.2005. [DOI] [PubMed] [Google Scholar]
- 70.Song Y, Thiagarajah J, Verkman AS. Sodium and chloride concentrations, pH, and depth of airway surface liquid in distal airways. J Gen Physiol 122: 511–519, 2003. doi: 10.1085/jgp.200308866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med 204: 3235–3245, 2007. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takenouchi T, Iwamaru Y, Sugama S, Tsukimoto M, Fujita M, Sekigawa A, Sekiyama K, Sato M, Kojima S, Conti B, Hashimoto M, Kitani H. The activation of P2X7 receptor induces cathepsin D-dependent production of a 20-kDa form of IL-1β under acidic extracellular pH in LPS-primed microglial cells. J Neurochem 117: 712–723, 2011. [DOI] [PubMed] [Google Scholar]
- 73.Tate S, MacGregor G, Davis M, Innes JA, Greening AP. Airways in cystic fibrosis are acidified: detection by exhaled breath condensate. Thorax 57: 926–929, 2002. doi: 10.1136/thorax.57.11.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tong J, Wu WN, Kong X, Wu PF, Tian L, Du W, Fang M, Zheng F, Chen JG, Tan Z, Gong F. Acid-sensing ion channels contribute to the effect of acidosis on the function of dendritic cells. J Immunol 186: 3686–3692, 2011. doi: 10.4049/jimmunol.1001346. [DOI] [PubMed] [Google Scholar]
- 75.Torres IM, Demirdjian S, Vargas J, Goodale BC, Berwin B. Acidosis increases the susceptibility of respiratory epithelial cells to Pseudomonas aeruginosa-induced cytotoxicity. Am J Physiol Lung Cell Mol Physiol 313: L126–L137, 2017. doi: 10.1152/ajplung.00524.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Torres IM, Patankar YR, Shabaneh TB, Dolben E, Hogan DA, Leib DA, Berwin BL. Acidosis potentiates the host proinflammatory interleukin-1β response to Pseudomonas aeruginosa infection. Infect Immun 82: 4689–4697, 2014. doi: 10.1128/IAI.02024-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Trevani AS, Andonegui G, Giordano M, López DH, Gamberale R, Minucci F, Geffner JR. Extracellular acidification induces human neutrophil activation. J Immunol 162: 4849–4857, 1999. [PubMed] [Google Scholar]
- 78.Tsai WC, Strieter RM, Mehrad B, Newstead MW, Zeng X, Standiford TJ. CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect Immun 68: 4289–4296, 2000. doi: 10.1128/IAI.68.7.4289-4296.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wangdi T, Mijares LA, Kazmierczak BI. In vivo discrimination of type 3 secretion system-positive and -negative Pseudomonas aeruginosa via a caspase-1-dependent pathway. Infect Immun 78: 4744–4753, 2010. doi: 10.1128/IAI.00744-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williams BJ, Dehnbostel J, Blackwell TS. Pseudomonas aeruginosa: host defence in lung diseases. Respirology 15: 1037–1056, 2010. doi: 10.1111/j.1440-1843.2010.01819.x. [DOI] [PubMed] [Google Scholar]
- 81.Xu L, Fukumura D, Jain RK. Acidic extracellular pH induces vascular endothelial growth factor (VEGF) in human glioblastoma cells via ERK1/2 MAPK signaling pathway: mechanism of low pH-induced VEGF. J Biol Chem 277: 11368–11374, 2002. doi: 10.1074/jbc.M108347200. [DOI] [PubMed] [Google Scholar]