Abstract
The epithelial Na+ channel (ENaC) regulates airway surface hydration. In mouse airways, ENaC is composed of three subunits, α, β, and γ, which are differentially expressed (α > β > γ). Airway-targeted overexpression of the β subunit results in Na+ hyperabsorption, causing airway surface dehydration, hyperconcentrated mucus with delayed clearance, lung inflammation, and perinatal mortality. Notably, mice overexpressing the α- or γ-subunit do not exhibit airway Na+ hyperabsorption or lung pathology. To test whether overexpression of multiple ENaC subunits produced Na+ transport and disease severity exceeding that of βENaC-Tg mice, we generated double (αβ, αγ, βγ) and triple (αβγ) transgenic mice and characterized their lung phenotypes. Double αγENaC-Tg mice were indistinguishable from WT littermates. In contrast, double βγENaC-Tg mice exhibited airway Na+ absorption greater than that of βENaC-Tg mice, which was paralleled by worse survival, decreased mucociliary clearance, and more severe lung pathology. Double αβENaC-Tg mice exhibited Na+ transport rates comparable to those of βENaC-Tg littermates. However, αβENaC-Tg mice had poorer survival and developed severe parenchymal consolidation. In situ hybridization (RNAscope) analysis revealed both alveolar and airway αENaC-Tg overexpression. Triple αβγENaC-Tg mice were born in Mendelian proportions but died within the first day of life, and the small sample size prevented analyses of cause(s) of death. Cumulatively, these results indicate that overexpression of βENaC is rate limiting for generation of pathological airway surface dehydration. Notably, airway co-overexpression of β- and γENaC had additive effects on Na+ transport and disease severity, suggesting dose dependency of these two variables.
Keywords: α-, β-, γ-ENaC subunits; CCSP-driven overexpression; epithelial sodium transport; mucus concentration; mucociliary clearance
INTRODUCTION
Proper airway surface hydration is critical to achieve effective mucus clearance and preserve lung health. In normal conditions, airway epithelial cells respond to stimuli that perturb the composition of the mucus layer by regulating the balance between Cl− secretion, via the cystic fibrosis transmembrane conductance regulator (CFTR) and the Ca+-activated Cl− channel (CaCC), vs. Na+ absorption, via the epithelial Na+ channel (ENaC). The net transepithelial flux of ions drives water from the luminal to the basolateral compartment if Na+ absorption dominates or from the basolateral to luminal compartment if Na+ absorption is inhibited and Cl− secretion is activated. Due to its central role in this process, ENaC has been proposed as a key regulator of airway surface hydration, and several reports have linked abnormal ENaC activity with al features of human mucoobstructive lung diseases, including cystic fibrosis, chronic bronchitis, and COPD (2, 13, 23, 26, 33).
In mouse airways, ENaC is composed of three subunits, α, β, and γ, but it does not contain the δ-subunit (16). Quantitative mRNA analysis in the trachea of wild-type (WT) mice indicates that the endogenous transcript level for the α-subunit (1 × 106 transcripts/µg RNA) is much higher than the β-subunit (4 × 104 transcripts/µg RNA), which is higher yet than the γ-subunit (1 × 104 transcripts/µg RNA) (24), mirroring the relative abundance of these three subunits in human lungs (16). The notion that unregulated Na+ absorption would produce airway surface dehydration and hinder mucus clearance was tested experimentally in vivo by targeting transgenic overexpression of the βENaC subunit (βENaC-Tg) in airway club cells under the control of the rat club cell secretory protein (Scgb1a1, secretoglobin, family 1A, member 1) promoter (24). βENaC-Tg mice exhibited upregulation of ENaC function, as measured by increased amiloride-sensitive short-circuit current (Isc), higher percent solid contents of airway mucus, periciliary layer collapse, and decreased mucus clearance compared with WT littermates (24, 25). As predicted, βENaC-Tg overexpression resulted in lung pathology that closely resembles many aspects of human mucoobstructive lung diseases, such as increased mortality, susceptibility to spontaneous bacterial infection, airway mucus obstruction and inflammation, and structural lung damage (10, 20, 22, 26, 34). Notably, overexpression of the βENaC subunit alone appeared to be rate-limiting to generate this phenotype in vivo, as overexpression of either the αENaC or the γENaC subunit alone did not produce any measurable change in Na+ absorption or lung phenotype (24).
To test the hypothesis that overexpression of multiple ENaC subunits would produce a range of Na+ absorption rates and a proportionate range in disease severity, we generated double (αβ, αγ, βγ) and triple (αβγ) ENaC Tg mice. We studied survival, airway bioelectric properties in Ussing chambers, and the severity of lung disease as indexed by histopathology, bronchoalveolar lavage (BAL) differential cell counts, airway mucus hydration, and lung bacterial burden.
MATERIALS AND METHODS
Animals.
Animals were maintained and studied under protocols approved by the University of North Carolina Institutional Animal Care and Use Committee, and experiments were performed according to the principles outlined by the Animal Welfare and the National Institutes of Health guidelines for the care and use of animals in biomedical research. All mice were housed in individually ventilated microisolator cages in a specific pathogen-free facility maintained at the University of North Carolina at Chapel Hill, on a 12-h:12-h day-night cycle. Mice were fed a regular chow diet and given water ad libitum.
The original lines of individual subunit-overexpressing mice, i.e., αENaC-Tg (line 6514), βENaC-Tg (line 6608), and γENaC-Tg (line 6645), were hemizygous (Tg+/−) and were generated in a mixed C3H/HeN:C57BL/6N genetic background (24). Two approaches were undertaken to generate mice overexpressing multiple ENaC subunits. The first was to sequentially breed the original C3H:C57 αENaC-Tg, βENaC-Tg, and γENaC-Tg mice to obtain single, double, and triple Tg mice from the same breeding pair. To this end, we first bred αENaC-Tg with γENaC-Tg mice to generate double αγENaC-Tg mice. αγENaC-Tg mice were then bred with βENaC-Tg mice to generate mice of eight different genotypes, as expected, for transgenes segregating independently: WT (i.e., no transgene overexpression) α, β, γ, αβ, αγ, βγ, and αβγ-Tg mice, all expected to exhibit one-eighth Mendelian distribution. A second approach was adopted to maximize the number of double Tg mice available for further studies and involved crossing of single C3H:C57 Tg mice, e.g., αENaC-Tg with βENaC-Tg (or βENaC-Tg with γENaC-Tg), to generate mice of four different genotypes: wt, α, β, and αβENaC-Tg (or wt, β, γ, and βγENaC-Tg), all expected to exhibit one-fourth Mendelian distribution. Furthermore, we derived congenic C57BL/6N αENaC-Tg and γENaC-Tg lines using the same approach described for congenic βENaC-Tg mice (19) and used the two approaches described above to generate congenic (N > 12) C57BL/6N mice overexpressing multiple ENaC subunits. At postnatal day (PND)1 or 2, pups were toe-clipped for identification and genotyping, as previously described (24). Mice studied were littermates, age-matched, and of both sexes. When used for Ussing chamber experiments, mouse pups were euthanized with an overdose of ketamine-xylazine, and adult mice were euthanized by CO2 inhalation. When used for BAL and histopathology, all mice were euthanized by exsanguination under deep 2,2,2-tribromoethanol (Avertin) anesthesia.
Embryonic studies and post-natal lung wet-to-dry ratio measurements.
Pregnancy was timed based on the presence of vaginal plug. At embryonic day 18 (E18), fetuses were harvested, the tail was collected for genotyping, and the lungs were dissected, blotted on filter paper, and weighed. For postnatal lung wet-to-dry ratio measurements, pregnant dams were monitored regularly starting at E19–20 and allowed to give birth spontaneously, and pups were monitored for survival. Four hours after the birth of the last pup, pups were euthanized and thoracotomy was performed. The left lobe was dissected, blotted on filter paper, placed on preweighted aluminum weight boats, and weighed at four timed intervals (30–40–50–60 s) to allow back-extrapolation for evaporative water loss to initial wet weights (18). Lungs were desiccated for >16 h at 90°C and then reweighed to obtain dry weights and determine wet/dry ratios.
Bioelectric studies.
Mouse pups remained with the dams until time of study. All studies were performed blinded with respect to genotype. Details of the Ussing chamber preparations have been previously published (11). Amiloride (10−4 M apical addition) was used to block electrogenic Na+ absorption. Forskolin (10−5 M apical) and UTP (10−4 M apical) were used to induce anion secretion via an increase in intracellular [cAMP] and [Ca2+]i, respectively. Bumetanide (10−4 M), was added to the basolateral bath to inhibit Cl− entry through the Na-K-2Cl cotransporter (NKCC1). All drugs were purchased from Sigma-Aldrich with the exception of UTP (Amersham Pharmacia Biotech). To generate amiloride dose-response curves, amiloride was added sequentially starting at 10−9 M and increased by log10 units until 10−3 M was reached. The preparations were allowed to stabilize for at least 5 min between doses before addition of the next dose. To determine IC50 and the fractional proportion of low- and high-affinity channels, amiloride dose-response data were fitted with a single- or two-component Hill model as described (25).
BAL and bacteriology.
For 10-day-old or older mice, BAL cell counts, and lung histology were obtained from the same animal, as described (22). Due to their small size, 5-day-old pups were subjected to either whole lung lavage or fixation for histology. Lungs were immersion-fixed in 10% neutral-buffered formalin (NBF) to prevent dislodging of airway luminal contents. For microbiology studies, BAL was performed aseptically and plated, and colony-forming units (CFUs) were enumerated, as previously described (20).
Lung histology and histopathology score.
Formalin-fixed left lung lobes were cut in cross-sections starting at the hilum and 2 mm apart along the main stem bronchus, yielding three slices per lung (called level 1, 2, and 3 from rostral to caudal). All three slices were embedded in paraffin, and 5-μm-thick sections were cut and stained with hematoxylin and eosin (H&E) and Alcian Blue-Periodic Acid Schiff staining (AB-PAS). Lung pathology was graded using a semiquantitative score, as previously described (22). Note that, due to the severity of air space enlargement and airway inflammation in βγENaC-Tg mice, the severity scale had to be increased from four levels (0–3) to five levels (0–4). Tissue blocks received a numerical code at time of embedding, and scoring of the slides was performed by an investigator blinded to specimen genotype.
Agarose gel mucin Western blot.
Secreted pulmonary mucin quantification was performed using a modification of the agarose Western blot protocol described in Ref. 22. Unfractionated BAL samples collected from PND10 mice were solubilized by addition of urea powder to a final 6 M concentration. Samples were reduced with 10 mM DTT and alkylated with 25 mM iodoacetamide. Equal volumes of reduced samples (40 μl) were run on 1% agarose gel using a submerged gel electrophoresis apparatus with Tris acetate EDTA-SDS buffer at 80 V for 90 min. Gels were vacuum-blotted onto nitrocellulose membranes, blocked with Odyssey blocking buffer (OBB; Li-COR Biosciences, Lincoln, NE), and probed with rabbit polyclonal antibodies raised against either Muc5b [UNC223 (35)] or Muc5ac [UNC294 (5)] at 1:1,000 in OBB or OBB+ 0.1% Tween 20 (OBBT), respectively. The secondary antibody was Alexa fluor 680 goat anti-rabbit IgG diluted 1:15,000 in OBB. Detection and analysis of specific signals were performed using the Odyssey Infrared Imaging System (LI-COR Biosciences).
Mucociliary clearance assay.
PND10 mice were anesthetized with 2–3% isoflurane, and a small incision was made through the tracheal ventral wall. Using a fine-bore cannula, 200 nl of PBS containing a known number of fluorescent microspheres (3 µm Molecular Probes FluoSpheres, Nile Red, Invitrogen) was deposited near the tracheal bifurcation. After the cannula was removed and the tracheostomy closed, the anesthetized mouse was allowed to breath spontaneously for 15 min. After this period, the mouse was euthanized, and the lungs and trachea (up to the larynx) were removed and solubilized in KOH, and the beads remaining in the lung were counted. Mucociliary clearance (MCC) was determined as percentage of delivered beads that cleared in 15 min.
Sialic acid/urea filter paper collection.
The set-up consisted in a humidity tent constructed around a dissecting scope and connected to a Holmes nebulizer that maintained the humidity at >85%. Collection paper strips (Kimwipes) were laser cut to 0.8 ×10 mm. To collect the samples, PND12 pups were anesthetized with isoflurane and euthanized by exsanguination, and the trachea was visualized and opened lengthwise. The paper strip was grasped with clean forceps, carefully laid lengthwise in the trachea for 20 s, removed, placed in a 0.5-ml Eppendorf tube, and frozen until mass spectrometry analysis. Blanks were run in which all manipulations of the strips were identical except that they were not placed onto the trachea.
Sialic acid/urea assay for bronchoalveolar lavage fluid and filter paper.
Cell-free, bronchoalveolar lavage fluid (BALF) was processed as previously described (6–8). Slight modifications of this procedure were employed for filter paper samples. Briefly, filters were submerged in a small volume (20 µl) of a solution of 1% formic acid with internal standards of isotopically labeled sialic acid, urea, and other metabolites and then incubated at 80°C for 1 h to release sialic acid residues from mucins. Sialic acid and urea concentrations were measured by mass spectrometry as described (7).
In situ hybridization.
In situ RNA detection was performed using the RNAscope 2-plex detection kit (Advanced Cell Diagnostics, Hayward, CA) on formalin-fixed, paraffin-embedded lungs according to the manufacturer's instructions. Modifications specific for our protocol included the following: 1) lungs were inflation-fixed with 10% NBF at 25 cm of H2O pressure for 24 h at room temperature (RT) and then processed to obtain 5-µm-thick histologic sections; 2) pretreatment 2 was applied for 15 min and pretreatment 3 for 30 min; 3) before mounting, the slides were allowed to dry at 60°C for 30 min. The positive control probes were Ppib (peptidylprolyl isomerase B, moderate-high expression, C1 channel, teal) and Polr2a (RNA polymerase II subunit A, moderate-low expression, C2 channel, magenta), and the negative control probe was DapB (4-hydroxy-tetrahydrodipicolinate reductase from Bacillus subtilis, C1 and C2 channels). Note that the RNAscope 2-plex detection system allows for detection of variations in mRNA abundance of individual genes (labeled either magenta or teal) across samples/conditions and/or colocalization of the two genes. The signal intensity obtained from each probe (magenta or teal) is probe specific, however, so comparisons of the relative abundance of two different mRNAs cannot be made.
Statistics.
Data are shown as means ± SE, with the number of preparations or mice (n). Survival curves were compared using log rank analysis (Mantel-Cox test) and the Gehan-Breslow-Wilconox test for multiple comparisons. For bioelectric studies, a one-way ANOVA was used to determine if there were significant differences among the groups, in which case, the Holm-Sidak test was used for all pairwise comparisons. A nonparametric Kruskall-Wallis test followed by Dunn’s test for multiple comparisons was used to analyze semiquantitative histological scores. ANOVA followed by Dunnett’s test for multiple comparisons was used to determine significant differences among groups for weights, lung wet/dry ratio, BAL cell and CFU counts, mucin content, mucociliary clearance rates, and sialic acid/urea ratios. P < 0.05 was considered statistically significant. To test for outliers in the values of low-affinity channels, relative population across genotypes, Grubbs test (12) with two extreme observations in one-tail test was performed using the R package “outliers”, (https://CRAN.R-project.org/package=outliers) by Lukasz Komsta.
RESULTS
Overexpression of multiple ENaC subunits affects neonatal survival in a combination-specific fashion.
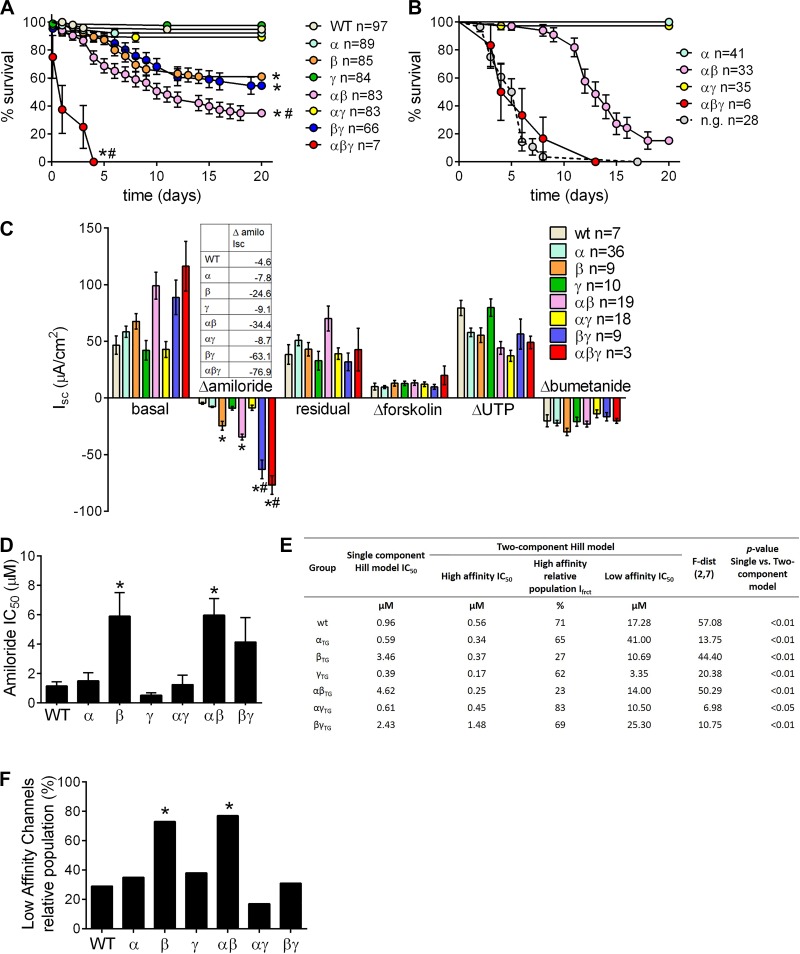
To investigate the effect of multiple ENaC subunits' overexpression in mouse airways, we first crossed mice overexpressing individual ENaC subunits, i.e., α, β, and γ, originally described in Ref. 24, and obtained breeding pairs that produced litters comprising eight different genotypes, i.e., WT (i.e., no transgene overexpression), α-, β-, γ-, αβ-, αγ, βγ-, and αβγENaC-Tg mice, in the expected equal proportions (1/8). Survival analysis revealed that overexpression of βENaC alone, or in combination with either αENaC or γENaC, significantly reduced survival compared with WT littermates (Fig. 1A). αβENaC-Tg mice exhibited significantly lower survival than β- or βγENaC-Tg mice. Of note, overexpression of all three subunits (αβγENaC-Tg) resulted in significant mortality within the first few days of life, so profound that most newborn triple transgenic pups could not be found, and thus genotyped, during routine laboratory operations.
Fig. 1.
Overexpression of multiple epithelial sodium channel (ENaC) subunits affects neonatal survival in a combination-specific fashion and produces graded rates of Na+ absorption. A: survival curves for progeny of the triple transgenic (Tg) cross in the C57:C3H mixed genetic background; n = no. of mice/genotype. Log-rank *P < 0.05 vs. WT mice; #P < 0.05 vs. βENaC-Tg mice. B: survival curves for progeny of the cross between homozygous αENaC-Tg mice with hemizygous βγENaC-Tg+/−, predicted to yield ¼ of αβ and ¼ αβγ pups; n = no. of mice/genotype; n.g. = not genotyped. These mice were detected at birth but cannibalized soon after, so no genotyping could be performed. Log-rank *P < 0.05 vs. WT mice; #P < 0.05 vs. αβENaC-Tg littermates. C: ion transport properties of freshly excised tracheal tissues. “Basal” indicates Isc before drug application. Change in Isc (Δ) after sequential drug addition is shown. “Residual” Isc is the Isc remaining after amiloride application; n = no. of mice/genotype. ANOVA *P < 0.05 vs. WT mice; #P < 0.05 vs. βENaC-Tg mice. Table inset contains Δamiloride for all genotypes vs. WT mice. D: IC50 values derived from analysis of amiloride dose-response curves according to the 1-component Hill model; n = 6–8/genotype. *P < 0.05 vs. WT mice. E: Hill model dose-response fit parameters for ENaC channels in native tracheal epithelia of WT mice and mice overexpressing different ENaC subunits, individually or in combination. F: relative population of low-affinity channels in different genotypes derived from analysis of amiloride dose-response curves according to the 2-component Hill model. *“Outliers” according to Grubbs’ test.
To confirm the severe mortality affecting αβγ and αβ pups by using a different breeding strategy, crosses between homozygous αENaC-Tg mice with hemizygous βγENaC-Tg+/−, predicted to yield one-fourth of αβ and αβγ pups, were performed. The survival of αβENaC-Tg pups averaged ~20% at 20 days (Fig. 1B). The number of αβγENaC-Tg pups that was genotyped at PND2–3 was significantly lower than expected (7 vs. ~30), and none survived post-PND15. The number of pups born but not genotyped due to cannibalism was 28.
Overexpression of multiple ENaC subunits produces graded Na+ hyperabsorption.
Due to the high mortality of mice overexpressing some ENaC subunit combinations, we studied the bioelectric properties of tracheas from pups at PND3 (Fig. 1C). As previously reported, mice overexpressing the β-, but not the α- or γENaC-Tg subunits alone, exhibited airways Na+ hyperabsorption (24), as indexed by the increased amiloride-sensitive short-circuit currents (Isc) in Ussing chambers. Mice overexpressing the αγ combination exhibited bioelectrical properties indistinguishable from those of WT littermates. Overexpression of both the α- and βENaC subunits resulted in rates of Na+ absorption that did not differ significantly from those of the βENaC subunit alone. However, mice overexpressing the β- and γENaC subunit combination produced an amiloride-sensitive Isc that was ~14-fold greater than that of WT compared with an ~5-fold increase vs. WT observed for overexpression of the βENaC subunit alone (Fig. 1C, table inset). The tracheal bioelectrics of the few 3-day-old αβγENaC-Tg pups available were also studied. Tracheas from the αβγENaC-Tg pups exhibited Na+ absorption that was ~16.7-fold greater than that of WT, but due to the small sample size we could not determine whether this response was significantly different from the response of the βγENaC-Tg pups. The post-amiloride residual currents, which we have previously shown to represent Cftr-mediated anion secretion in neonatal mouse tracheas (21, 30), did not systematically differ among genotypes, as well as the responses to forskolin, UTP, and bumetanide.
The sensitivity to the ENaC selective blocker amiloride has been used to define the subunit composition of ENaC (25). Accordingly, amiloride dose-responses of Isc in isolated tracheas from PND10 mice were measured. Analysis of the dose-response curves according to the one-component Hill model revealed that for βENaC-Tg and αβENaC-Tg mice the amiloride half-inhibitory concentration (IC50) was higher compared with WT (Fig. 1D). This finding is consistent with the presence of two populations of ENaC channels, with “high” and “low” affinity for amiloride (25). Further analysis indicated that a two-component model provided a better fit with the experimental data for all genotypes studied (Fig. 1E) and allowed the estimation of the percentage of low-affinity channels relative to the total channel populations (Fig. 1F). The low-affinity population was significantly elevated in βENaC-Tg and αβENaC-Tg tracheas compared with all other genotypes.
Overexpression of βENaC subunit alone or in combination with other ENaC subunits correlates with development of lung pathology in neonatal mice.
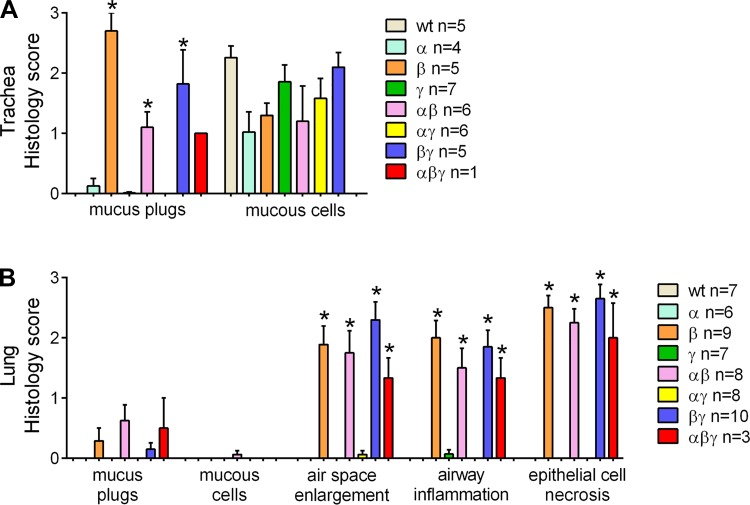
Semiquantitative analysis of lung pathology in 3-day-old pups revealed that all the subunit combinations that exhibited increased airway Na+ absorption and decreased survival compared with WT littermates, e.g., β, αβ, βγ and αβγ, exhibited tracheal mucus plugging but not mucus cell metaplasia (Fig. 2A). With respect to the lung, alveolar space enlargement, airway inflammation, and airway epithelial necrotic degeneration was observed (Fig. 2B). At that early time point, no significant differences in each parameter could be detected among β, αβ, βγ and αβγ genotypes.
Fig. 2.
Overexpression of βENaC subunit alone or in combination with other ENaC subunit correlates with development of lung pathology in neonatal mice. A and B: semiquantitative histopathology scores for 3-day-old pups from the triple transgenic cross in the C57:C3H mixed genetic background. All combinations exhibiting increased airway Na+ absorption and decreased survival compared with WT littermates, also presented with tracheal mucus plugging (A), and signatures of early lung disease in the intrapulmonary compartment (i.e., alveolar space enlargement, airway inflammation, and airway epithelia necrotic degeneration) (B); n = no. of mice/genotype. ANOVA *P < 0.05 vs. WT mice.
To maximize the number of double transgenic mice available to study the relationship between Na+ transport rates and lung disease severity in a homogeneous genetic background (C57BL/6N), we crossed congenic (N > 12) single transgenic mice, e.g., βENaC-Tg with γENaC-Tg (or αENaC-Tg with βENaC-Tg), and generated mice of four different genotypes: WT, β-, γ-, and βγENaC-Tg (or WT, α-, β-, and αβENaC-Tg). Offspring from all these crosses exhibited the expected one-fourth Mendelian distribution (Fig. 3A for β × γENaC-Tg cross and Fig. 7A for α × βENaC-Tg cross). The results obtained from these studies are described in the following paragraphs.
Fig. 3.
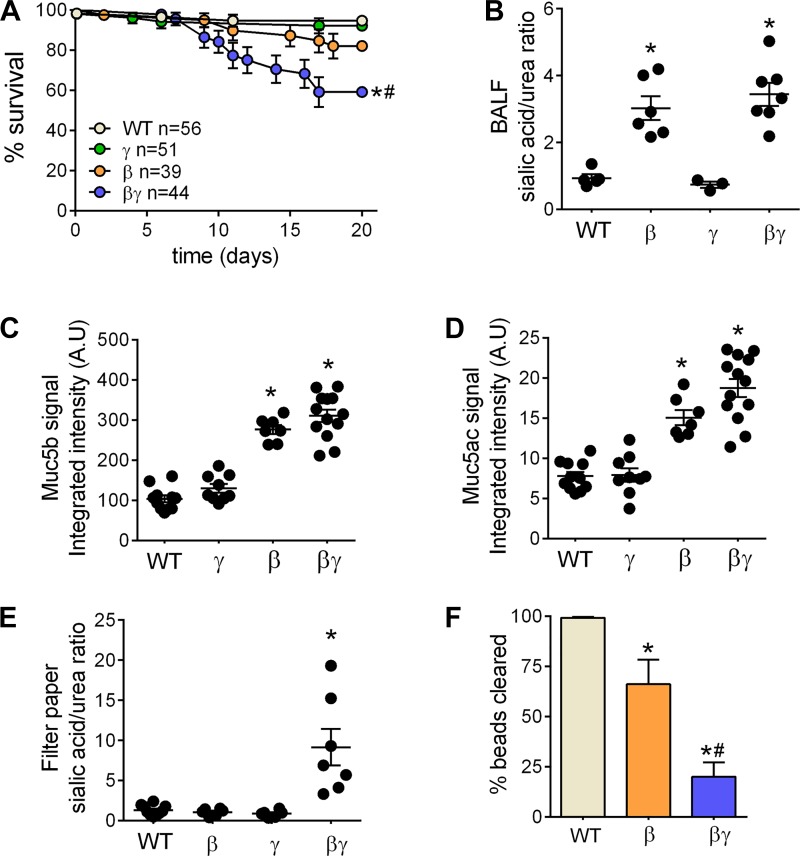
Congenic βγENaC-Tg mice exhibit decreased survival, increased mucus dehydration, and decreased mucociliary clearance (MCC) compared with βENaC-Tg littermates. A: survival curves for progeny of the β × γENaC-Tg cross in the C57BL/6N genetic background; n = no. of mice/ genotype. Log-rank *P < 0.05 vs. WT mice; #P < 0.05 vs. βENaC-Tg mice. B: bronchoalveolar lavage fluid (BALF) sialic acid/urea ratio from postnatal day (PND)10 mice from the congenic β × γENaC-Tg cross; n = 3–7/genotype. Log-rank *P < 0.05 vs. WT mice. C and D: densitometric analysis of BAL mucin agarose Western blots for progeny of the congenic β × γENaC-Tg cross at PND10. Blots were probed with anti-Muc5b (C) or anti-Muc5ac (D) antibodies; n = 7–13 mice/genotype. ANOVA *P < 0.05 vs. WT mice. E: sialic acid/urea ratio in native mucus harvested with filter paper from tracheas of PND10 mice of the congenic β × γENaC-Tg cross; n = 6–10 mice/genotype. ANOVA *P < 0.05 vs. WT mice. F: MCC measurements in progeny of the congenic β × γENaC-Tg cross at PND10; n = 5–8 mice/genotype. ANOVA *P < 0.05 vs. WT mice; #P < 0.05 vs. βENaC-Tg mice.
Fig. 7.
αβENaC-Tg mouse survivors develop obstructive lung disease with progressive parenchymal involvement. A–D: representative photomicrographs of left lobes cut in cross-section at different levels (2 mm apart, starting at the hilum. Level 1 is the rightmost section). PND35 βENaC-Tg mice (A and C) or αβENaC-Tg mice (B–D), illustrating lobar distension, parenchymal consolidation, and more severe interstitial inflammation in αβENaC-Tg mice compared with βENaC-Tg littermates. Scale bars: (A and B) 2 mm, (C and D) 0.2 mm. H&E stain. E: representative photomicrograph of a BAL cytospin preparation for αβENaC-Tg mice illustrating morphologically activated macrophages and significant granulocytic infiltrate. F–I: differential BAL cell counts for mice from the congenic α × βENaC-Tg cross at PND35; F, macrophages; G, neutrophils; H, eosinophils; and I, lymphocytes; n = 3–6 mice/genotype. ANOVA *P < 0.05 vs. WT mice; #P < 0.05 vs. βENaC-Tg mice. J: quantification of CFU in BAL samples from progeny of the congenic α × βENaC-Tg cross at PND35. (Log10+1)-transformed data; n = 1–4 mice/genotype.
Overexpression of β- and γ subunits causes worsening of muco-obstructive phenotype.
Survival analysis of congenic C57BL/6N β × γENaC-Tg crosses revealed that βγENaC-Tg mice exhibited lower survival than βENaC-Tg mice (Fig. 3A). To test whether mucus was more dehydrated, and thus more concentrated, as a function of the rates of Na+ absorption (βγ > β > γ = WT), we measured the sialic acid/urea ratio, a surrogate marker of mucin concentration (7), in cell-free bronchoalveolar lavage fluid (BALF) harvested from the tracheas of WT, γENaC-Tg, βENaC-Tg, and βγENaC-Tg littermates at PND10. The sialic acid/urea ratio was higher in BALF from βENaC-Tg and βγENaC-Tg mice than in WT or γENaC-Tg mice (Fig. 3B), consistent with significant mucus hyperconcentration. Quantification of Muc5b and Muc5ac levels in unfractionated BAL harvested from PND10 mice by agarose Western blot revealed higher Muc5ac and Muc5b content in BAL from βENaC-Tg and βγENaC-Tg mice vs. WT or γENaC-Tg mice. These values were not significantly different for βENaC-Tg vs. βγENaC-Tg mice (Fig. 3, B–D). As a second measure of mucus concentration, native mucus was collected from the tracheas of PND10 mice on filter paper and the sialic acid/urea ratio of the adherent mucus measured. Mucus harvested from βγENaC-Tg mice had a higher sialic acid/urea ratio compared with all other samples (Fig. 3E), indicating significant mucus hyperconcentration and possibly higher adherence to the filter.
Since airway surface dehydration is predicted to produce mucus adhesion and reduce mucociliary clearance (MCC) rates, we next tested whether the hyperconcentration of mucous found in βENaC-Tg and βγENaC-Tg mice correlated with the rate of MCC. At PND10, MCC (%beads cleared from the lungs) was inversely related to the rate of airway Na+ absorption exhibited by each genotype, with the rate of MCC being WT > β > βγ (Fig. 3F).
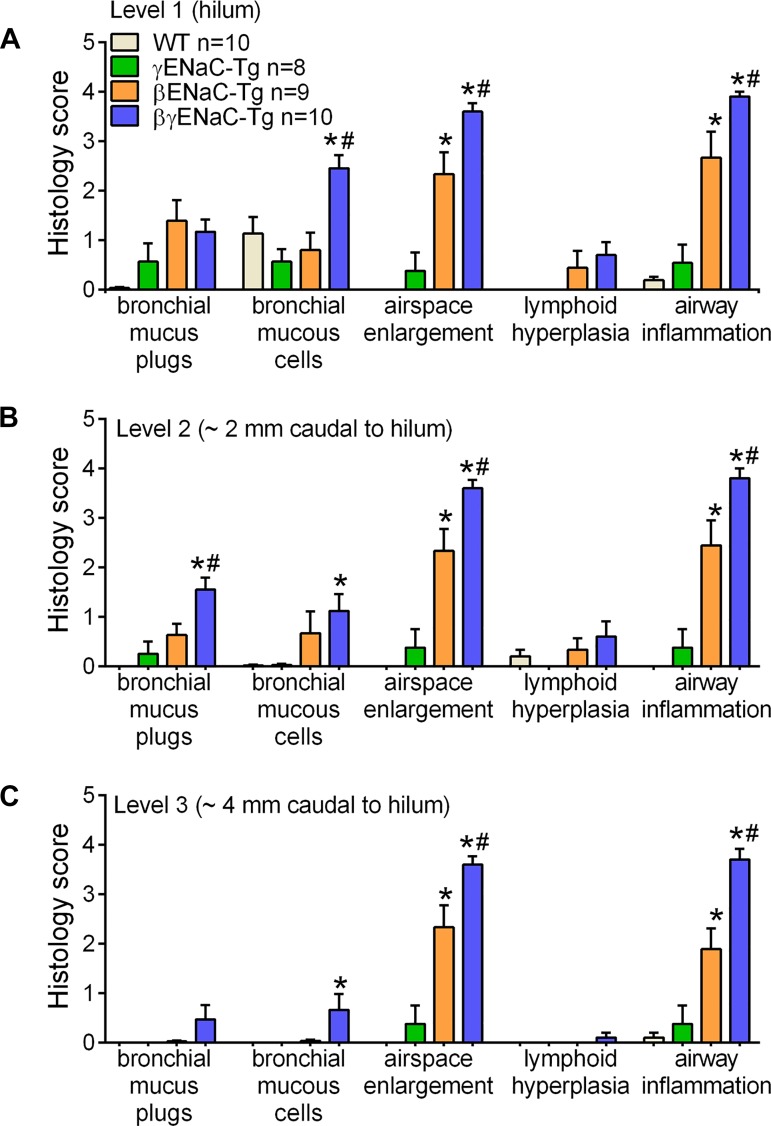
To test whether the reduced MCC defect and increased mucus concentration exhibited by βγENaC-Tg mice was reflected by an increased severity of lung pathology as the mice aged, histopathological assessments of PND35 mice from the congenic β × γENaC-Tg cross were performed using a semiquantitative histology score (22). The pathological features of mucoobstructive lung disease previously described for βENaC-Tg mice, i.e., airway mucus obstruction, mucous cell metaplasia, airspace enlargement, and airway inflammation, were more pronounced in βγENaC-Tg mice than in βENaC-Tg mice (Fig. 4, A–C). Notably, pathological findings were expressed through the more distal portions of the airways in βγENaC-Tg mice compared with βENaC-Tg mice, suggesting a stronger/more widespread insult to the airway surfaces. Moreover, βγENaC-Tg mice exhibited a higher number of BAL neutrophilis, eosinophils, and lymphocytes compared with βENaC-Tg littermates (Fig. 5, A–D). The worsened phenotype of PND35 βγENaC-Tg mice was, however, not associated with a higher level of Muc5b in BAL (supernatant or pellet fraction; Fig. 5E) or increased severity of spontaneous bacterial infection at PND5–7, or incidence at PND35 (Fig. 5, F and G).
Fig. 4.
Overexpression of β- and γ-subunits causes worsening of mucoobstructive lung phenotype. Semiquantitative histopathology scores for mice from the congenic β × γENaC-Tg cross at PND35. At all airway levels probed, i.e., hilum (A, level 1), 2 mm caudal to the hilum (B, level 2), and 4 mm caudal to the hilum (C, level 3), βγENaC-Tg mice exhibited more severe airway mucous cells, air space enlargement, and airway inflammation compared with βENaC-Tg littermates; n = 8–10 mice/genotype. ANOVA *P < 0.05 vs. WT mice; #P < 0.05 vs. βENaC-Tg mice.
Fig. 5.
Overexpression of β- and γ-subunits causes worsening of airway inflammation. Differential BAL cell counts for mice from the congenic β × γENaC-Tg cross at PND35. A: macrophages; B: neutrophils; C: eosinophils; D: lymphocytes; n = 8–10 mice/genotype. ANOVA *P < 0.05 vs. WT mice; #P < 0.05 vs. βENaC-Tg mice. E: densitometric analysis of BAL mucin agarose Western blots for progeny of the congenic β × γENaC-Tg cross at PND35. Supernatant and pellet fractions were analyzed separately. Blots were probed with anti-Muc5b antibody; n = 3–9 mice/genotype. ANOVA *P < 0.05 vs. WT mice. F and G: quantification of colony-forming units (CFU) in BAL samples from progeny of the congenic β × γENaC-Tg cross at PND5–7 (F) and PND35 (G). (Log10+1)-transformed data; n = 2–10 mice/genotype. ANOVA *P < 0.05 vs. WT mice.
Overexpression of α- and β-subunits results in severe neonatal mortality and αβENaC-Tg mouse survivors develop obstructive lung disease with progressive parenchymal involvement due to parenchymal overexpression of the α-subunit.
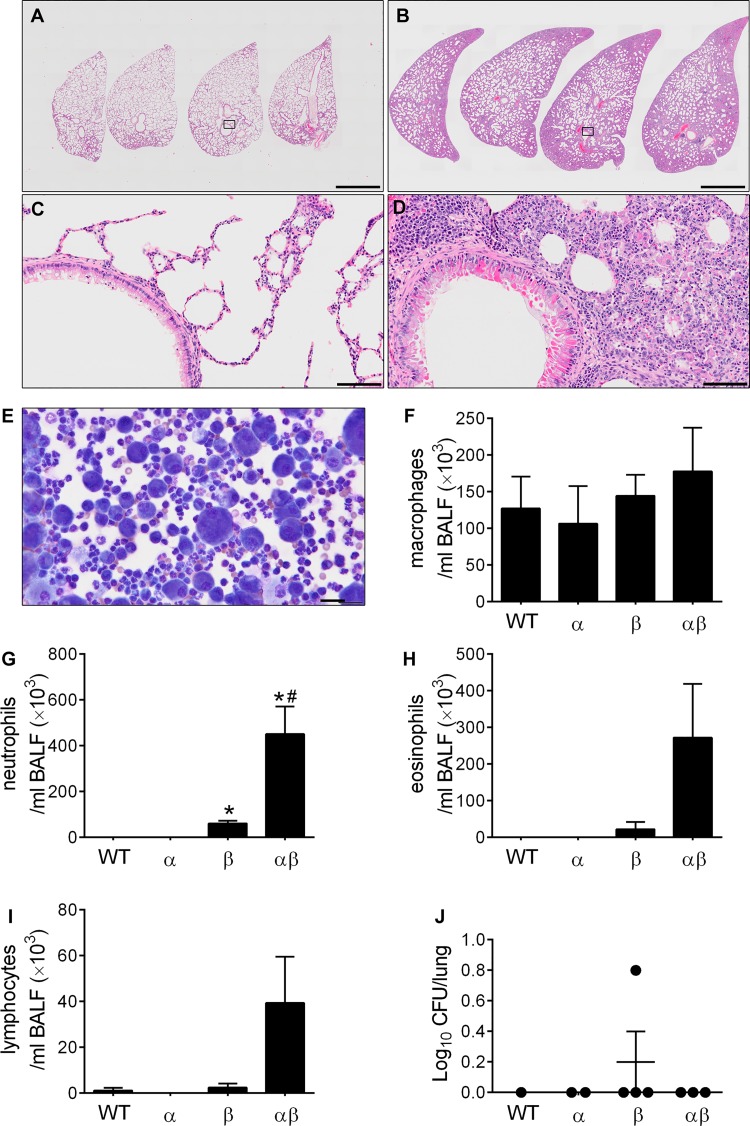
Similar to what observed in the crosses that generated triple and double transgenic mice in the mixed C3H:C57 genetic background (Fig. 1, A and B), congenic C57BL6/N αβENaC-Tg mice exhibited reduced survival compared with both WT and βENaC-Tg littermates (Fig. 6A). Lung histopathological analyses (Fig. 6B), measurements of BAL differential cell counts (Fig. 6, C–E), and microbiological assessment of spontaneous bacterial infection (Fig. 6F) failed to reveal significant differences in the degree of airway pathology, inflammation, and spontaneous infection between αβENaC-Tg mice and βENaC-Tg littermates at PND5–7, which corresponds to the peak of mortality in the αβENaC-Tg line. In contrast, the few αβENaC-Tg mice that survived until adulthood (≥PND35) exhibited a striking lung phenotype characterized by lobar distension, parenchymal consolidation, and severe interstitial inflammation (Fig. 7, B and D) compared with βENaC-Tg littermates (Fig. 7, A and C). In agreement with these findings, BAL differential cell counts revealed that PND35 αβENaC-Tg mice exhibited morphologically activated macrophages (Fig. 7E), higher number of neutrophils (Fig. 7, E and G), and trends toward more eosinophils and lymphocytes (Fig. 7, H and I) compared with βENaC-Tg littermates. Of note, the increase in lung parenchymal pathology and inflammatory indexes in αβENaC-Tg mice were not associated with an increased incidence of spontaneous bacterial infections (Fig. 7J), which occur episodically in adult βENaC-Tg mice (20).
Fig. 6.
Overexpression of α- and β-subunits results in severe neonatal mortality. A: survival curves for progeny of the α × βENaC-Tg cross in the C57BL/6N genetic background; n = no. of mice/genotype. Log-rank *P < 0.05 vs. WT mice. B: representative photomicrographs of airway lumens cut in cross-section proximal to the hilum from PND5–7 mice of indicated genotypes (β = βENaC-Tg and αβ = abENaC-Tg). Stained with H&E illustrating equivalent lung pathology in both genotypes. C–E: differential BAL cell counts for mice from the congenic α × βENaC-Tg cross at PND5–7; C, macrophages; D, neutrophils; E, eosinophils; n = 5–16 mice/genotype. ANOVA *P < 0.05 vs. WT mice. F: quantification of CFU in BAL samples from progeny of the congenic α × βENaC-Tg cross at PND5–7. (Log10+1)-transformed data; n = 3–11 mice/genotype. ANOVA *P < 0.05 vs. WT mice.
The observation that αβENaC-Tg mice exhibited parenchymal consolidation superimposed on the typical airway muco-obstructive phenotype found in βENaC-Tg mice led us to hypothesize that in αβENaC-Tg mice the α subunit might be overexpressed in the alveolar region as well as in the airway epithelium. In the constructs used to generate mice overexpressing individual subunits, the α, β, and γ subunits were under the control of the rat club cell secretory protein (rCCSP or Scgb1a1) promoter (24). In rats, endogenous CCSP expression extends from the trachea to the distal airways and is readily detectable in type II alveolar cells (28), whereas endogenous mouse CCSP expression is more confined to the conducting airways. The mouse CCSP (mCCSP) promoter region shares >73% of homology with the rCCSP sequence. However, differences in the regulatory portions of the two promoters have been found and could account for the different expression patterns observed for rCCSP promoter-driven genes in comparison to the native CCSP in mice, as assayed by in situ hybridization (32).
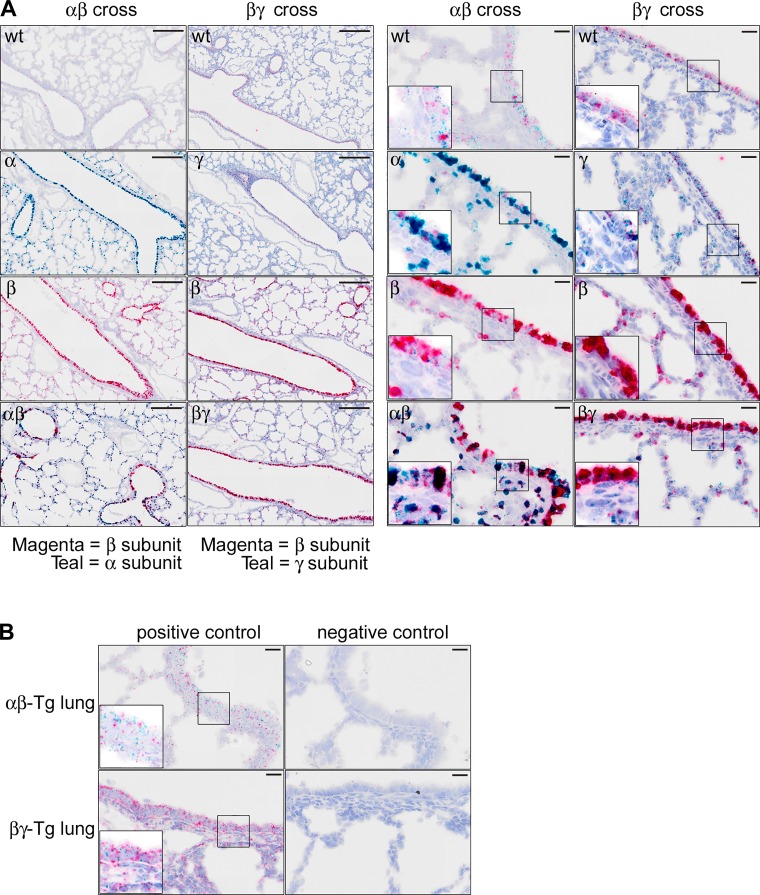
Accordingly, we used a novel in situ hybridization technique (RNA scope) to visualize the expression pattern of the α-, β-, and γ-subunits in the lungs of WT, αβENaC-Tg, and βγENaC-Tg mice. As shown in Fig. 8, A and B, RNAscope analysis revealed that the α- and β-subunits, and to a lesser extent the γ-subunit, were overexpressed in the airway epithelium of α-, β-, and γENaC-Tg mice compared with WT mice. By using the same detection probe (teal), γENaC subunit overexpression was significantly lower than that of αENaC in the corresponding transgenic mice. Of note, the α-subunit was also markedly overexpressed in alveolar cells, whereas this parenchymal overexpression was less prominent for the β- and γ-subunits. The combination of α- and β-subunit overexpression resulted in a strong signal (dark red/black) in both the airways and the parenchyma of αβENaC-Tg mice which significantly differed from the one observed in βENaC-Tg mice, whereas the signal distribution pattern was similar in βγENaC-Tg and βENaC-Tg mice. Overall, these results support the hypothesis that increased parenchymal overexpression of αENaC subunit may contribute to parenchymal pathology and reduced survival of αβENaC-Tg mice.
Fig. 8.
αENaC-Tg and αβENaC-Tg mice exhibit overexpression of the α-subunit in both airway and alveolar compartments. A: representative photomicrographs of main-stem bronchial airways and parenchyma isolated from mice of the α × βENaC-Tg cross (columns 1 and 3) or β × γENaC-Tg cross (columns 2 and 4) at PND5–7 and subjected to RNAscope in situ hybridization. Scale bars: low-magnification panels (columns 1 and 2) = 200 µm; high-magnification panels (columns 3 and 4) = 20 µm. Inset at higher magnification are presented in the bottom left corner of each high magnification panel to appreciate the intracellular distribution of the probes. The probe for the βENaC subunit was labeled with a magenta chromogen in both crosses, and the probe for the αENaC and γENaC subunits were labeled with a teal chromogen. Note that overexpression of the β- and γ-subunits is largely confined to the airways, whereas the α-subunit is highly expressed in both airways and alveolar compartments. B: representative micrographs illustrating typical positive (peptidylprolyl isomerase B, moderate-high expression, C1 channel, teal; and RNA polymerase II subunit A, moderate-low expression, C2 channel, magenta), and negative (4-hydroxy-tetrahydrodipicolinate reductase from Bacillus subtilis, both C1 and C2 channels) control probes staining pattern in αβENaC and βγENaC-Tg mice.
αβγENaC-Tg mice do not die in utero but shortly after birth.
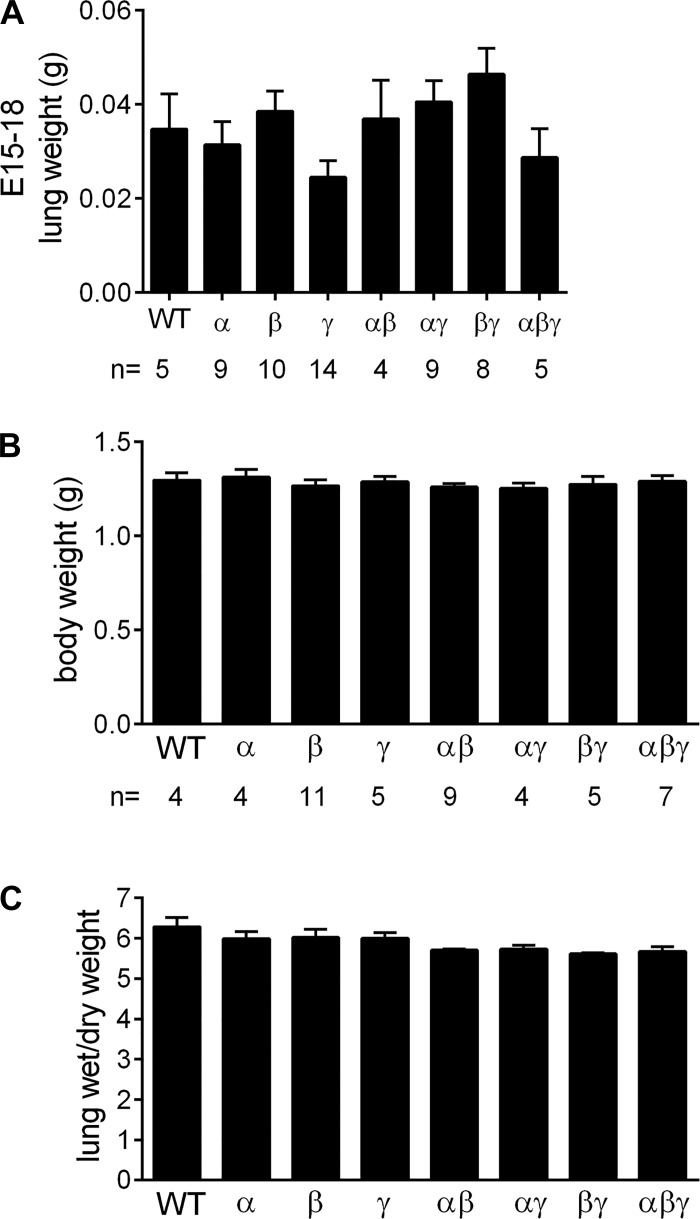
To identify the cause of death of αβγENaC-Tg mice, we first tested whether the triple transgenic combination was embryonically lethal. Analysis of 64 pups harvested at E18 revealed the expected distribution of genotypes (i.e., WT n = 5, α n = 9, β n = 10, γ n = 14, αβ n = 4, αγ n = 9, βγ n = 8, and αβγ n = 5) and no differences in lung weight (Fig. 9A). Further studies were thus conducted to assess the distribution of genotypes at birth and the lung wet/dry ratio, an index of prenatal lung fluid homeostasis. Pregnant dams were monitored hourly around the day of birth, and pups were weighed and monitored for 4 h after birth. No significant differences were observed in the distribution of genotypes or body weight (Fig. 9B). Lung wet/dry weight was assessed 4 h after birth, and no significant differences were detected among the different genotypes (Fig. 9C).
Fig. 9.
αβγENaC-Tg mice do not die in utero but shortly after birth. A: lung weights and Mendelian distribution of genotypes in embryonic days (E)15–18 mice form the triple transgenic cross in the C57:C3H mixed genetic background; n = no. of mice/genotype. B and C: body weight and lung wet/dry weight in mice form the triple transgenic cross in the C57:C3H mixed genetic background as measured 4 h after birth.
Our previous studies indicated that survival of βENaC-Tg mice is greatly ameliorated in the C57BL/6N genetic background (19). Therefore, in an effort to increase the survival of the triple transgenic αβγENaC-Tg mice and study them postnatally, we used congenic C57BL/6N αENaC-Tg, βENaC-Tg, and γENaC-Tg mice to generate mice overexpressing all subunit combinations. Of 137 mice found and genotyped at PND1–2 (i.e., 13 litters), only one pup was a αβγENaC-Tg mouse, indicating that the cause of death for the triple transgenic mice was independent of genetic background and challenging to be determined experimentally.
DISCUSSION
Through combinatory overexpression of multiple subunits of ENaC in airway epithelia, mouse models were generated with different levels of transepithelial Na+ transport. Overexpression of the βENaC subunit was required to generate Na+ hyperabsorption, as all βENaC-overexpressing mice (βENaC-, αβENaC-, βγENaC-, and αβγENaC-Tg) exhibited elevated amiloride-sensitive currents compared with WT littermates. In contrast, overexpression of α- and/or γ-subunits alone or in combination (αENaC-, γENaC-, and αγENaC-Tg) did not produce increases in tracheal Na+ transport rates. Based on our Ussing chamber data and on previous studies describing the relative abundance of endogenous α-, β-, and γ-subunit transcripts in mouse airways (24), we hypothesized that in βENaC-Tg mice the excess of β-subunits led to the generation of both more αβγENaC channels compared with WT, and an increased subpopulation of αβENaC channels. Importantly, αβENaC channels are constitutively open, i.e. do not require activation by endogenous proteases (25), and exhibit a lower affinity for amiloride (Fig. 1D). The rate-limiting factor in the production of αβγENaC channels in βENaC-Tg mice is postulated to be the abundance of the native γ-subunits.
These conditions change upon overexpression of other ENaC subunits in addition to β. For example, overexpression of both the β- and γ-subunits is predicted to increase the number of γ-subunits available to form more αβγENaC channels compared with single βENaC-overexpressing mice. The data describing an increased amiloride-sensitive Isc in βγENaC-Tg mice vs. βENaC mice are consistent with this prediction (Fig. 1B). Furthermore, the absence of an increase in the population of low-affinity channels in βγENaC-Tg mice suggests that overexpression of the γ-subunit indeed promoted the assembly of more high-affinity αβγENaC channels rather than low-affinity αβENaC channels, which occurs upon overexpression of the β-subunit alone (Fig. 1F).
By similar logic, overexpression of the α- and β-subunits should result in the same number of αβγENaC channels obtained by overexpression of the β-subunit alone, because endogenous αENaC is in excess. αβENaC-Tg and βENaC-Tg mice exhibited equivalent amiloride-sensitive Isc and had similar increases in low-affinity channels (Fig. 1, C and D), indicating that the overexpressed β-subunit is rate limiting for the formation of αβENaC channels. Interestingly, analysis of the amiloride dose-response curves indicated that the two-component Hill model provides a better fit for all the experimental data, including WT samples, suggesting that native tracheal tissue also expresses a small percentage of low-affinity, amiloride-sensitive Isc at baseline. Notably, our hypothesis that the airways of ENaC-Tg mice exhibit channels of different subunit composition is based solely on amiloride affinity studies. Although it was expected that overexpression of multiple ENaC subunits would modify the macroscopic characteristics of ENaC currents, i.e., amiloride sensitivity, the precise basis underlying this modification still needs to be confirmed, as multiple factors could be involved in the changes of ENaC affinities observed. First, ENaC stoichiometry is at present not certain, i.e. trimers (4, 31), tetramers (1), or more variable arrangements (9) of ENaC subunits have been proposed. Second, there are reports that ENaC subunits, especially when in excess, can combine with other degenerin family members to form hybrid channels with biophysical characteristics that differ from ENaC (27). Third, genetic manipulation of ENaC subunit expression by siRNAs have produced nonselective cation channels (15). In native airway epithelium cation channels that may contribute to Na+ absorption display a range of single channel conductances, cation selectivity, and amiloride sensitivity (3). Although beyond the scope of the current paper, surface biotinylation studies on cells isolated from the different ENaC-Tg mice could address details about trafficking (29), maturation (14), and assembly (9) of the overexpressed subunits, for which we have demonstrated airway-specific mRNA overexpression (Fig. 8). Finally, whereas mouse airways do not express the low-affinity δENaC subunit (16), we cannot formally exclude contributions of amiloride-sensitive, electrogenic off-target proteins to the low-affinity population measured (e.g., the Na+-Ca2+ exchange mediated by Slc8a1). However, evidence of expression and/or function of this transporter in mouse airway epithelia is lacking.
Importantly, in the βENaC-Tg and βγENaC-Tg mice, the magnitude of Na+ transport rates scaled with the severity of muco-obstructive lung disease. Compared with βENaC-Tg mice, βγENaC-Tg mice exhibited increased mortality, more concentrated mucus, and reduced MCC at a very early age (PND10; Fig. 3), which resulted in more peripheral airway disease and increased inflammatory infiltrates once they reached adulthood (Figs. 4 and 5). Thus, an apparent dose-effect relationship between abnormal Na+ transport rates and disease severity was revealed in the comparison of βENaC-Tg vs. βγENaC-Tg mice. In situ hybridization data suggested that overexpression of both the βENaC and γENaC subunits was mainly localized to the airways. We did not assess their relative expression along airway generations, so we cannot exclude that the more peripheral airway disease in βγENaC-Tg mice reflects more peripheral γENaC expression. Regardless, we propose that this set of reagents, i.e., βENaC-Tg mice and βγENaC-Tg mice, can be used to develop biomarkers to score airway mucus obstruction/burden and corresponding lung disease. In particular, the sialic acid/urea ratio appears to be a sensitive biomarker of airway surface hydration and thus a surrogate marker of mucus concentration, which can be referenced to BAL cellular infiltrates and lung histopathology (7).
In the course of these studies, we also encounter deviations from the linear relationship between tracheal Na+ transport rates and lung disease severity observed in βENaC- and βγENaC-Tg mice. Analyses of transgenic expression of the α- and β-subunits highlighted how differences in regional transgene overexpression may lead to profound phenotypic differences. Although all the lines were generated using the same targeting approach and the same rat CCSP promoter (24), the RNAscope in situ hybridization technique revealed that the transgenes differed markedly in the location of their overexpression. Specifically, whereas the β- and γ-subunits were overexpressed predominantly in the airway epithelium, the α-subunit was overexpressed in both the airways and the alveolar parenchyma. This outcome is not surprising, since transgene expression is influenced by the genetic context of its random insertion and suboptimal regulation by exogenous promoters. Although the resolution of our in situ hybridization approach was not sufficient to confidently assign an identity to the cells overexpressing the α-subunit in the parenchyma, it is likely that αENaC was expressed in alveolar type II cells, a cell type where leaky overexpression of mouse genes driven by the rat CCSP promoter has been previously described (28).
The severe mortality exhibited by neonatal αβENaC-Tg mice, as well as the lung phenotype with predominant parenchymal involvement exhibited by the few αβENaC-Tg mice surviving to adulthood, resembles the phenotype of SPC-Cre Nedd4-2 KO mice. In the Nedd4-2 KO mice, genetic deletion of Nedd4-2, a ubiquitin ligase that regulates endocytosis and lysosomal degradation of ENaC and other ion channels, produces, among other consequences, increased Na+ transport (17). Kimura et al. (17) reported that a proportion of Nedd4-2 mice died briefly after birth from unknown causes, and those that reached adulthood exhibited a lung phenotype closely resembling our αβENaC-Tg mouse survivors. Collectively, these results suggest that hyperactive ENaC both in the airways and in the alveoli is incompatible with life and/or proper lung development and might be a factor contributing to the dismal survival of αβENaC- and αβγENaC-Tg mice.
In summary, overexpression of single or multiple ENaC subunits in mouse airways resulted in different rates of Na+ hyperabsorption. In the case of βENaC- and βγENaC-Tg mice (and perhaps αβγENaC-Tg), increases in Na+ hyperabsorption correlated with lung disease severity, making these two mouse models useful for identification of novel biomarkers underscoring the severity of muco-obstructive airway disease. Moreover, we have found that region-specific increases in Na+ transport resulted in differential phenotypes. Specifically, the results obtained for the α × βENaC-Tg cross suggest that Na+ hyperabsorption in the alveolar region was associated with a unique disease phenotype.
GRANTS
This work was funded by the Cystic Fibrosis Research Development Program Grant RDP CFF R026-CR11 (to W. K. O'Neil), National Institutes of Health (NIH) Grants P30-DK-065988, P50-HL-060280, P50-HL-084934, P50-HL-107168, P01-HL-108808, P01-HL-110873, and UH2-HL-123645 (to R. C. Boucher); NIH Grant R01-HL-116228, and NIH Grant P30-ES-10126 (to C. R. Esrther, Jr); and the German Federal Ministry of Education and Research (82DZL00401 and 82DZL004A1) to M. A. Mall.
DISCLOSURES
Drs. Boucher, Grubb, Mall, and O’Neal are inventors in US Patent US 7,772,458 B2 for the Scnn1b-Tg mouse model. All other authors have no conflict of interest to disclose.
AUTHOR CONTRIBUTIONS
A.L.-B., M.A.M., R.C.B., W.K.O., and B.R.G. conceived and designed research; A.L.-B., K.J.W., A.S.V., R.C.G., T.D.R., K.A.B., C.R.E.J., and B.R.G. performed experiments; A.L.-B., K.J.W., A.S.V., R.C.G., T.D.R., R.A.C., C.R.E.J., and B.R.G. analyzed data; A.L.-B., R.A.C., C.R.E.J., R.C.B., W.K.O., and B.R.G. interpreted results of experiments; A.L.-B. prepared figures; A.L.-B. drafted manuscript; A.L.-B., C.R.E.J., M.A.M., R.C.B., W.K.O., and B.R.G. edited and revised manuscript; A.L.-B., K.J.W., A.S.V., R.C.G., T.D.R., R.A.C., K.A.B., C.R.E.J., M.A.M., R.C.B., W.K.O., and B.R.G. approved final version of manuscript.
AKNOWLEDGMENTS
We thank E. Jane Kelly and Danielle Keys for technical assistance with mouse breeding, phenotyping, and morphometric analyses; Michael Chua for assistance with imaging; Hong Dang for assistance with statistical analyses and expression databases search; and Eric Roe for editorial assistance.
REFERENCES
- 1.Anantharam A, Palmer LG. Determination of epithelial Na+ channel subunit stoichiometry from single-channel conductances. J Gen Physiol 130: 55–70, 2007. doi: 10.1085/jgp.200609716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boucher RC, Stutts MJ, Knowles MR, Cantley L, Gatzy JT. Na+ transport in cystic fibrosis respiratory epithelia. Abnormal basal rate and response to adenylate cyclase activation. J Clin Invest 78: 1245–1252, 1986. doi: 10.1172/JCI112708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chinet TC, Fullton JM, Yankaskas JR, Boucher RC, Stutts MJ. Sodium-permeable channels in the apical membrane of human nasal epithelial cells. Am J Physiol Cell Physiol 265: C1050–C1060, 1993. [DOI] [PubMed] [Google Scholar]
- 4.Collier DM, Snyder PM. Identification of epithelial Na+ channel (ENaC) intersubunit Cl- inhibitory residues suggests a trimeric alpha gamma beta channel architecture. J Biol Chem 286: 6027–6032, 2011. doi: 10.1074/jbc.M110.198127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehre C, Worthington EN, Liesman RM, Grubb BR, Barbier D, O’Neal WK, Sallenave JM, Pickles RJ, Boucher RC. Overexpressing mouse model demonstrates the protective role of Muc5ac in the lungs. Proc Natl Acad Sci USA 109: 16528–16533, 2012. doi: 10.1073/pnas.1206552109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esther CR Jr, Coakley RD, Henderson AG, Zhou YH, Wright FA, Boucher RC. Metabolomic evaluation of neutrophilic airway inflammation in cystic fibrosis. Chest 148: 507–515, 2015. doi: 10.1378/chest.14-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esther CR Jr., Hill DB, Button B, Shi S, Jania CM, Duncan EA, Doerschuk CM, Chen G, Ranganathan S, Stick SM, Boucher RC. The sialic acid to urea ratio as a measure of airway surface hydration. Am J Physiol Lung Cell Mol Physiol 312: L398–L404, 2017. doi: 10.1152/ajplung.00398.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esther CR Jr, Turkovic L, Rosenow T, Muhlebach MS, Boucher RC, Ranganathan S, Stick SM, Arest CF; AREST CF . Metabolomic biomarkers predictive of early structural lung disease in cystic fibrosis. Eur Respir J 48: 1612–1621, 2016. doi: 10.1183/13993003.00524-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frindt G, Gravotta D, Palmer LG. Regulation of ENaC trafficking in rat kidney. J Gen Physiol 147: 217–227, 2016. doi: 10.1085/jgp.201511533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gehrig S, Duerr J, Weitnauer M, Wagner CJ, Graeber SY, Schatterny J, Hirtz S, Belaaouaj A, Dalpke AH, Schultz C, Mall MA. Lack of neutrophil elastase reduces inflammation, mucus hypersecretion, and emphysema, but not mucus obstruction, in mice with cystic fibrosis-like lung disease. Am J Respir Crit Care Med 189: 1082–1092, 2014. doi: 10.1164/rccm.201311-1932OC. [DOI] [PubMed] [Google Scholar]
- 11.Grubb BR, Paradiso AM, Boucher RC. Anomalies in ion transport in CF mouse tracheal epithelium. Am J Physiol Cell Physiol 267: C293–C300, 1994. [DOI] [PubMed] [Google Scholar]
- 12.Grubbs FE. Sample criteria for testing outlying observations. Ann Math Stat 21: 27–58, 1950. doi: 10.1214/aoms/1177729885. [DOI] [Google Scholar]
- 13.Hobbs CA, Da Tan C, Tarran R. Does epithelial sodium channel hyperactivity contribute to cystic fibrosis lung disease? J Physiol 591: 4377–4387, 2013. doi: 10.1113/jphysiol.2012.240861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughey RP, Bruns JB, Kinlough CL, Kleyman TR. Distinct pools of epithelial sodium channels are expressed at the plasma membrane. J Biol Chem 279: 48491–48494, 2004. doi: 10.1074/jbc.C400460200. [DOI] [PubMed] [Google Scholar]
- 15.Jain L, Chen XJ, Malik B, Al-Khalili O, Eaton DC. Antisense oligonucleotides against the alpha-subunit of ENaC decrease lung epithelial cation-channel activity. Am J Physiol Lung Cell Mol Physiol 276: L1046–L1051, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Ji HL, Zhao RZ, Chen ZX, Shetty S, Idell S, Matalon S. δ ENaC: a novel divergent amiloride-inhibitable sodium channel. Am J Physiol Lung Cell Mol Physiol 303: L1013–L1026, 2012. doi: 10.1152/ajplung.00206.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura T, Kawabe H, Jiang C, Zhang W, Xiang YY, Lu C, Salter MW, Brose N, Lu WY, Rotin D. Deletion of the ubiquitin ligase Nedd4L in lung epithelia causes cystic fibrosis-like disease. Proc Natl Acad Sci USA 108: 3216–3221, 2011. doi: 10.1073/pnas.1010334108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knowles MR, Robinson JM, Wood RE, Pue CA, Mentz WM, Wager GC, Gatzy JT, Boucher RC. Ion composition of airway surface liquid of patients with cystic fibrosis as compared with normal and disease-control subjects. J Clin Invest 100: 2588–2595, 1997. doi: 10.1172/JCI119802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livraghi-Butrico A, Grubb BR, Kelly EJ, Wilkinson KJ, Yang H, Geiser M, Randell SH, Boucher RC, O’Neal WK. Genetically determined heterogeneity of lung disease in a mouse model of airway mucus obstruction. Physiol Genomics 44: 470–484, 2012. doi: 10.1152/physiolgenomics.00185.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livraghi-Butrico A, Kelly EJ, Klem ER, Dang H, Wolfgang MC, Boucher RC, Randell SH, O’Neal WK. Mucus clearance, MyD88-dependent and MyD88-independent immunity modulate lung susceptibility to spontaneous bacterial infection and inflammation. Mucosal Immunol 5: 397–408, 2012. doi: 10.1038/mi.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livraghi-Butrico A, Kelly EJ, Wilkinson KJ, Rogers TD, Gilmore RC, Harkema JR, Randell SH, Boucher RC, O’Neal WK, Grubb BR. Loss of Cftr function exacerbates the phenotype of Na(+) hyperabsorption in murine airways. Am J Physiol Lung Cell Mol Physiol 304: L469–L480, 2013. doi: 10.1152/ajplung.00150.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livraghi A, Grubb BR, Hudson EJ, Wilkinson KJ, Sheehan JK, Mall MA, O’Neal WK, Boucher RC, Randell SH. Airway and lung pathology due to mucosal surface dehydration in beta-epithelial Na+ channel-overexpressing mice: role of TNF-alpha and IL-4Ralpha signaling, influence of neonatal development, and limited efficacy of glucocorticoid treatment. J Immunol 182: 4357–4367, 2009. doi: 10.4049/jimmunol.0802557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mall M, Bleich M, Greger R, Schreiber R, Kunzelmann K. The amiloride-inhibitable Na+ conductance is reduced by the cystic fibrosis transmembrane conductance regulator in normal but not in cystic fibrosis airways. J Clin Invest 102: 15–21, 1998. doi: 10.1172/JCI2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mall M, Grubb BR, Harkema JR, O’Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med 10: 487–493, 2004. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 25.Mall MA, Button B, Johannesson B, Zhou Z, Livraghi A, Caldwell RA, Schubert SC, Schultz C, O’Neal WK, Pradervand S, Hummler E, Rossier BC, Grubb BR, Boucher RC. Airway surface liquid volume regulation determines different airway phenotypes in liddle compared with betaENaC-overexpressing mice. J Biol Chem 285: 26945–26955, 2010. doi: 10.1074/jbc.M110.151803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mall MA, Harkema JR, Trojanek JB, Treis D, Livraghi A, Schubert S, Zhou Z, Kreda SM, Tilley SL, Hudson EJ, O’Neal WK, Boucher RC. Development of chronic bronchitis and emphysema in beta-epithelial Na+ channel-overexpressing mice. Am J Respir Crit Care Med 177: 730–742, 2008. doi: 10.1164/rccm.200708-1233OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meltzer RH, Kapoor N, Qadri YJ, Anderson SJ, Fuller CM, Benos DJ. Heteromeric assembly of acid-sensitive ion channel and epithelial sodium channel subunits. J Biol Chem 282: 25548–25559, 2007. doi: 10.1074/jbc.M703825200. [DOI] [PubMed] [Google Scholar]
- 28.Perl AK, Wert SE, Loudy DE, Shan Z, Blair PA, Whitsett JA. Conditional recombination reveals distinct subsets of epithelial cells in trachea, bronchi, and alveoli. Am J Respir Cell Mol Biol 33: 455–462, 2005. doi: 10.1165/rcmb.2005-0180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prince LS, Welsh MJ. Cell surface expression and biosynthesis of epithelial Na+ channels. Biochem J 336: 705–710, 1998. doi: 10.1042/bj3360705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rock JR, O’Neal WK, Gabriel SE, Randell SH, Harfe BD, Boucher RC, Grubb BR. Transmembrane protein 16A (TMEM16A) is a Ca2+-regulated Cl- secretory channel in mouse airways. J Biol Chem 284: 14875–14880, 2009. doi: 10.1074/jbc.C109.000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart AP, Haerteis S, Diakov A, Korbmacher C, Edwardson JM. Atomic force microscopy reveals the architecture of the epithelial sodium channel (ENaC). J Biol Chem 286: 31944–31952, 2011. doi: 10.1074/jbc.M111.275289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stripp BR, Sawaya PL, Luse DS, Wikenheiser KA, Wert SE, Huffman JA, Lattier DL, Singh G, Katyal SL, Whitsett JA. cis-acting elements that confer lung epithelial cell expression of the CC10 gene. J Biol Chem 267: 14703–14712, 1992. [PubMed] [Google Scholar]
- 33.Tarran R, Sabater JR, Clarke TC, Tan CD, Davies CM, Liu J, Yeung A, Garland AL, Stutts MJ, Abraham WM, Phillips G, Baker WR, Wright CD, Wilbert S. Nonantibiotic macrolides prevent human neutrophil elastase-induced mucus stasis and airway surface liquid volume depletion. Am J Physiol Lung Cell Mol Physiol 304: L746–L756, 2013. doi: 10.1152/ajplung.00292.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trojanek JB, Cobos-Correa A, Diemer S, Kormann M, Schubert SC, Zhou-Suckow Z, Agrawal R, Duerr J, Wagner CJ, Schatterny J, Hirtz S, Sommerburg O, Hartl D, Schultz C, Mall MA. Airway mucus obstruction triggers macrophage activation and matrix metalloproteinase 12-dependent emphysema. Am J Respir Cell Mol Biol 51: 709–720, 2014. doi: 10.1165/rcmb.2013-0407OC. [DOI] [PubMed] [Google Scholar]
- 35.Zhu Y, Ehre C, Abdullah LH, Sheehan JK, Roy M, Evans CM, Dickey BF, Davis CW. Munc13-2-/- baseline secretion defect reveals source of oligomeric mucins in mouse airways. J Physiol 586: 1977–1992, 2008. doi: 10.1113/jphysiol.2007.149310. [DOI] [PMC free article] [PubMed] [Google Scholar]