Abstract
Objective
To examine whether galantamine, a cognitive enhancing medication which is both acetylcholinesterase inhibitor and agonist at nicotinic acetylcholine receptors, is effective at improving cocaine use outcomes and cognitive functioning, alone and in combination with computerized cognitive behavioral therapy.
Methods
Twelve-week, randomized 2X2, factorial trial evaluating galantamine versus placebo (double blind), and computerized cognitive behavioral therapy plus standard methadone treatment versus standard methadone treatment alone in a community based methadone maintenance program (September 2009–April 2015). 120 individuals diagnosed with DSM-IV cocaine use disorder were randomized to either extended-release galantamine (8 mg/day) or matched placebo, supervised at the time of daily methadone dosing. The primary cocaine use outcome was change in percent days of abstinence over time. Number of cocaine-negative urine toxicology screens submitted and cognitive function were secondary outcomes.
Results
Random effect regression analysis indicated significant reductions in frequency of cocaine use over time, with significant treatment by time effects for both galantamine over placebo (F=5.3, p=.02, d=.34) and computerized cognitive behavioral therapy over standard methadone treatment (F=4.2, p=.04, d=.30, but no evidence of significant benefit of the combination over either treatment alone. Pre- to posttreatment comparisons of multiple indices of cognitive functioning, including sustained attention, indicated no benefit of galantamine over placebo.
Conclusions
Findings suggest benefits of galantamine and computerized cognitive behavioral therapy for cocaine use in this sample. While galantamine did not improve measures of cognitive function in this sample, multiple measures of cognitive function were associated with cocaine use outcomes, underlining the significance of cognitive function in cocaine treatment outcomes.
Introduction
Cocaine use within methadone maintenance programs remains an intractable problem associated with significantly poorer outcomes1–3. While there are no approved pharmacotherapies for cocaine use disorder, behavioral approaches such as contingency management and cognitive behavioral therapy (CBT) have been demonstrated to reduce cocaine use in this population4, 5. Computerized CBT also demonstrated efficacy in reducing cocaine use relative to standard methadone maintenance-based counseling6, but there remains substantial room for improvement in outcomes.
CBT is comparatively cognitively demanding, as its emphasis on learning and applying complex concepts calls upon attention, memory, and decision making skills. Cognitive impairment is associated with poorer outcome and higher dropout in CBT among cocaine users7–9. Potential strategies for improving responses to cognitively demanding therapies such as CBT include simplifying treatment for patients with cognitive impairment10 or targeting impairment directly via cognitive training exercises11; both strategies have yielded mixed results to date12–15. A novel strategy is use of cognitive-enhancing agents (e.g., cholinesterase inhibitors) to improve attention and concentration as a means of addressing both cognitive function and substance use11, 16, 17.
The cholinergic system plays an important role in multiple brain functions including attention, working memory, reward and motivation18–20. Evidence from preclinical studies suggest that downregulation of the cholinergic system is a critical part of the neuroadaptations to chronic cocaine use21. Galantamine, a reversible and competitive inhibitor of acetylcholinesterase, elevates synaptic concentrations of acetylcholine which leads to increased stimulation of both nicotinic and muscarinic receptors. Galantamine also directly stimulates the nicotinic alpha7 and alpha4-beta2 receptors, as an allosteric positive modulator. This results in dopamine release in the mesolimbic/mesocortical dopaminergic pathway22, providing an additional mechanism by which galantamine may enhance cognitive function and reduce stimulant use19, 21, 23.
Few studies have evaluated galantamine, either in terms of direct effects on substance use or as a strategy to improve cognitive impairment: Among 114 alcohol-dependent individuals, galantamine was associated with significant reductions in cigarette smoking compared with placebo24.. A trial evaluating effects of galantamine in 149 recently detoxified alcohol-dependent patients reported no significant effects on relapse, but some evidence of reduced drinking among those who relapsed25. In a randomized placebo controlled pilot study with 14 cocaine-dependent methadone-maintained individuals, 16 mg/day galantamine was associated with fewer cocaine positive urine specimens, (45% versus 95%, P=.15) as well as a higher proportion of days of abstinence from cocaine 80% versus 60%, P=.06) relative to placebo, with participants reporting moderate nausea and fatigue26. Differential effects on cognitive functioning were not seen. In a 10-day proof-of-concept trial with 34 abstinent cocaine users, 8/mg/day of galantamine was associated with significant improvement in the Rapid Visual Information Processing task (RVP) of the CANTAB (Cambridge Neurologic Test Battery) compared with placebo27. These two pilot studies by our group suggested evaluation of galantamine on cocaine use and cognitive functioning was warranted in an a full randomized clinical tiral.
Herein we describe outcomes of a 2X2 randomized factorial trial in 120 methadone maintained individuals with cocaine use disorder who were randomized to one of the following conditions: Galantamine plus standard methadone maintenance treatment (treatment as usual, TAU), placebo plus TAU, galantamine plus computerized CBT (computer based training in CBT, or CBT4CBT) plus TAU, or placebo plus CBT4CBT+TAU. We hypothesized a main effect of both galantamine and CBT4CBT on reduction in cocaine use compared to their respective controls and a third hypothesis contrasting the combination of galantamine and CBT4CBT to each condition delivered singly (galantamine plus TAU or placebo plus CBT4CBT). We also hypothesized that galantamine would be more effective in improving cognitive functioning (memory and sustained attention) compared with placebo and explored relationships of cognitive function to cocaine use outcomes.
Methods
Participants
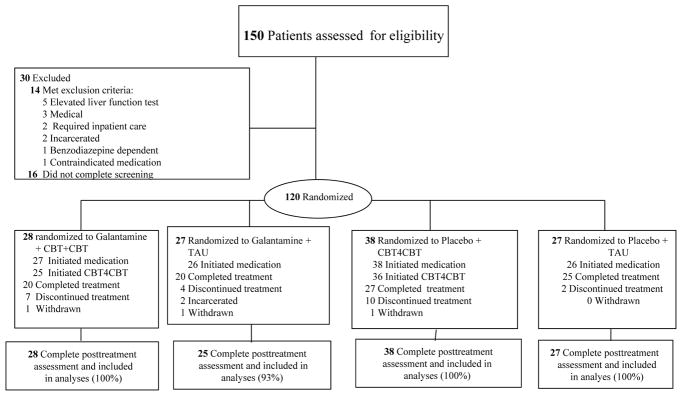
Participants were recruited from individuals stabilized on methadone maintenance at Recovery Network of Programs, a community-based program in Bridgeport, Connecticut between September 2009 and April 2015 (clinicaltrials.gov #NCT0080935. Individuals were included as participants if they were 18 years or older and met DSM-IV-R criteria for current cocaine dependence, as assessed by the Structured Clinical Interview for DSM-IV-R (SCID)28 and provided at least one cocaine-positive urine test during screening. Individuals were excluded if they (1) were currently dependent on another illicit drug or whose principal drug use was not cocaine (n=1), (2) met lifetime DSM-IV-R criteria for a non-substance-induced psychotic or bipolar disorder (n=1), (3) had a current medical condition contraindicating galantamine29 (e.g. asthma, chronic obstructive lung disease, history of or current gastrointestinal ulcer, hepatic or renal impairment, cardiac rhythm disturbance, or pregnancy) (n=3), as assessed by baseline physical examination (EKG, urinalysis and blood work), (4) had a screening liver function test greater than 3 times normal (n=5) (5) used medications including beta-blockers and NSAIDS contraindicated with galantamine (n=1), or (6) were not sufficiently stable for outpatient treatment (n=2). Two individuals were incarcerated prior to randomization and 16 did not complete the screening process (Figure 1).
Figure 1.
CONSORT Flow Diagram of Participants through the Trial
Note : Completing treatment defined as taking at least 1 day of study medication in week 12 .
One hundred twenty of the 150 individuals screened were determined to be eligible, provided written informed consent approved by the Yale School of Medicine IRB and were randomized. A masked, computerized urn randomization program used in previous trials30–33 was used to produce equivalent group size and balance groups with respect to baseline level of cocaine use (more or less than 11 days per month), gender, ethnicity (ethnic minority/non-minority), age (over/under 40), and baseline Shipley34 estimated IQ score.
Treatments
All participants received standard methadone treatment, consisting of daily methadone and weekly individual or group counseling, with access to other program services. Participants met twice weekly with research staff blind to medication condition who collected urine and breath samples and monitored other clinical symptoms. Adverse events and blood pressure were monitored weekly.
Galantamine
Participants assigned to galantamine were prescribed a maximum dose of 8 mg. galantamine extended release (ER), given limited tolerability of the 16 mg/day dose in our pilot study27. Galantamine or matched placebo capsules were dispensed daily at the time of methadone dosing and observed by program nurses. To evaluate the medication blind, participants and the project nurse were asked to guess medication assignment at the end of the trial. Among the 117 participants who initiated medication, 69 (61%) guessed their medication condition correctly. The project nurse guessed no better than chance (56%).
Computerized Cognitive Behavioral Therapy (CBT4CBT)
CBT4CBT is a direct-to-patient computer based version of a CBT manual35 that makes extensive use of video examples to each cognitive and behavioral control skills in 7 modules, each requiring about 30–40 minutes to complete. As described earlier36, the CBT4CBT program uses video vignettes, quizzes and interactive exercises to model effective use of skills and strategies. The vignettes present connected scenes of engaging characters portrayed by professional actors, who first experience a common risky situation or problem and then, after the skill is taught, demonstrate using the targeted skill to successfully negotiate that situation without resorting to drug use. Participants assigned to CBT4CBT worked with the program in a private area at the clinic on a weekly basis, usually at the time they completed study assessments.
Assessments
Participants were assessed before treatment, weekly during treatment (urine and breath samples were collected twice weekly; participants received a gift card worth $10 for each completed assessment), and at the 12-week treatment termination point. In cases where a randomized participant did not initiate (n=3), was withdrawn from treatment (n=3; one for elevated blood pressure, one for suicidal ideation, one for deliberately breaking the medication blind), or dropped out of treatment (n=25), he or she was interviewed at the 12-week point in order to collect data from the intent-to-treat sample, regardless of level of treatment involvement. Thus, complete 12-week self-report data were available for 118 of 120 (98.3%) of the randomized sample, permitting sensitivity analyses by including or excluding data points that were collected after a participant dropped out of treatment37.
The Timeline Follow Back38 method was used to collect detailed day-by-day self-reports of substance use throughout the 84-day treatment period. Self-reports of cocaine were verified through onsite urine toxicology screens (ToxCup™ Drug Screen Cup 5 with adulterant checks, Branan Medical Corporation) obtained twice weekly. Of 1911 urine specimens collected, 1601 (83.8%) were consistent with the participants’ self-reports, 52 (2.7%) tested negative for cocaine although the participant reported recent cocaine use, and 258 (13.5%) were positive for cocaine in cases where the participant denied use in the past 3 days. This rate is consistent with that reported for previous studies of cocaine-dependent samples evaluating the accuracy of self-report data39, 40.
Multiple cognitive tasks, drawn from the CANTAB41 were administered at baseline and end of treatment to evaluate effects of study treatments on indicators of cognitive function. These included potential effects of galantamine on sustained attention (Rapid Visual Information Processing, RVP A`: target sensitivity with higher scores indicating better attention28, 42), and potential effects of CBT4CBT on cognitive flexibility (Intra-Extra Dimensional Set Shifting (IED) total adjusted errors: number of intra- or extradimensional errors, adjusted for trials completed, where lower errors shows faster learning of changing contingencies43, 44). Response inhibition (Stop Signal Reaction Time (SSRT) where lower SSRT indicates better ability to inhibit a pre-potent motor response45, 46) and visual memory (Pattern Recognition Memory, PRM percent correct47) were also evaluated. Working memory was evaluated using Digit Span (longest backward span)48.
Data analyses
The primary outcome measure was self-reported cocaine use (operationalized as percent days of abstinence from cocaine per month), using random effect regression models49 to evaluate change across time, in monthly intervals, with the following contrasts: medication condition (galantamine versus placebo), behavioral condition (CBT4CBT versus TAU), and the combination of galantamine and CBT4CBT versus each intervention delivered singly (galantamine plus CBT4CBT versus galantamine plus TAU or CBT4CBT plus placebo). A logarithmic transformation of time was used to accommodate more rapid change occurring earlier in treatment. Number of cocaine-negative urine toxicology screens by month was included a secondary measure50 due to the likelihood of over-estimation of instances of cocaine use when obtained twice weekly due to carry-over effects 50,51. Repeated measures ANOVAs were used to evaluate changes in cognitive measures over time.
Power calculations utilizing estimates of effect sizes for galantamine (d=.4) and CBT4CBT (d=.5) on cocaine use outcomes based on previous trials6, 27, 36 indicated 35 participants per cell would provide sufficient power (>80%, two-sided). This effect size would be sufficient to detect a large effect (.50 or more) for the interaction of galantamine plus CBT4CBT, as well as for the effect of galantamine on CANTAB RVP A’ 27. Recruitment fell short of this target (averaging 30 per condition), but high rates of data availability permitted analysis of the full intention to treat sample.
Results
Sample characteristics, treatment adherence
Sample characteristics by treatment condition are presented in Table 1; there were no statistically significant differences across groups on multiple demographic and baseline substance use variables. The sample was predominantly male; about half were white, 21% were African-American, and 27% were Latino. Participants reported they used cocaine an average of 14 days of the 28 prior to baseline.
Table 1.
Baseline Characteristics by Treatment Group
| Characteristic | Galantamine + CBT4CBT | Galantamine + TAUb | Placebo + CBT4CBT | Placebo + TAU | F or X2 | p-value |
|---|---|---|---|---|---|---|
| (n= 28) | (n=27) | (n=38) | (n=27) | |||
| Female, No. (%) | 12 (43%) | 11 (41) | 10 (26) | 7 (26) | 3.32 | .35 |
| Race and ethnicity, No. (%) | ||||||
| Caucasian | 12 (42) | 15 (56) | 19 (50) | 16 (60) | 5.34 | .80 |
| African-American | 7 (25) | 6 (22) | 9 (24) | 3 (11) | ||
| Hispanic | 9 (32) | 6 (22) | 9 (24) | 8 (30) | ||
| Multiracial/Other | 0 | 0 | 1 (3) | 0 | ||
| Completed high school, No. (%) | 17 61) | 19 (70) | 29 (77) | 21 (78) | 2.58 | .46 |
| Unemployed, No. (%) | 20 (71) | 21 (78) | 27 (71) | 19 (70) | 0.50 | .92 |
| On public assistance, No. (%) | 23 (83) | 21 (78) | 23 (61) | 18 (67) | 4.55 | .21 |
| Major depression -lifetimea, No. (%) | 1 (4) | 3 (11) | 2 (5) | 4 (15) | 3.06 | .38 |
| Anxiety disorder -lifetime, No. (%) | 3 (11) | 5 (19) | 2 (5) | 5 (19) | 3.69 | .30 |
| Antisocial personality dis., No. (%) | 3 (11) | 3 (11) | 5 (13) | 5 (19) | 0.91 | .82 |
| Alcohol use dis.-lifetime, No. (%) | 15 (54) | 14 (52) | 21 (55) | 16 (59) | 0.33 | .95 |
| Age, mean (SD) | 38.0 (8.7) | 38.7 (10.8) | 39.8 (9.0) | 36.3 (9.4) | 0.75 | .52 |
| Days marijuana use, past 28, mean (SD) | 4.9 (8.9) | 3.3 (7.8) | 2.0 (5.5) | 3.3 (7.4) | 0.80 | .50 |
| Days cocaine use, past 28, mean (SD) | 17.2 (8.3) | 12.4 (8.1) | 13.6 (8.5) | 13.4 (7.9) | 1.80 | .15 |
| Days cigarette use, past 28, mean (SD) | 27.1 (4.9) | 25.4 (7.8) | 27.3 (4.5) | 25.3 (7.9) | 0.81 | .49 |
| Days alcohol use, past 28, mean (SD) | 2.9 (5.3) | 4.6 (7.8) | 1.7 (4.9) | 1.7 (3.1) | 1.79 | .15 |
| Days opiate use, past 28, mean (SD) | 1.6 (2.5) | 3.3 (4.4) | 4.3 (7.9) | 1.5 (2.6) | 2.15 | .10 |
| Days benzodiazepine use, past 28, mean (SD) | 0.5 (1.9) | 0.3 (0.7) | 0.3 (0.8) | 0.3 (1.0) | 0.20 | .90 |
| Age of first cocaine use, mean (SD) | 18.1 (3.5) | 20.2 (6.1) | 20.6 (6.6) | 20.2 (5.0) | 1.27 | .29 |
| Years of regular cocaine use, mean (SD) | 10.2 (8.7) | 12.6 (10.3) | 8.5 (7.1) | 8.5 (8.1) | 1.53 | .21 |
| Lifetime number of arrests, mean (SD) | 6.6 (7.3) | 7.2 (10.9) | 8.9 (14.7) | 5.8 (6.6) | 0.50 | .69 |
| Number of prior outpt drug treatments, mean (SD) | 3.5 (4.6) | 2.3 (2.7) | 2.4 (2.6) | 2.5 (2.2) | 0.97 | .41 |
| Number of prior inpt drug treatments, mean (SD) | 2.6 (2.5) | 3.7 (6.8) | 3.4 (5.7) | 2.4 (3.9) | 0.46 | .71 |
| Estimated IQ from Shipley, mean (SD) | 98.4 (11.7) | 101.6 (12.2) | 101.4 (11.4) | 100.5 (11.9) | 0.45 | .72 |
| Methadone dose, mg/day, mean (SD) | 77.3 (35.7) | 65.3 (25.3) | 72.4 (26.4) | 65.3 (25.6) | 1.15 | .33 |
Abbreviations: GAL=galantamine, PLA=placebo, CBT4CBT=computerized cognitive behavioral therapy, TAU=standard methadone treatment as usual
All psychiatric diagnoses made from SCID interviews for DSM-IV-R
Table 2 indicates there were no differences across treatment group, medication condition, behavioral therapy condition, or their interaction in terms of days retained in the protocol, days receiving methadone, or percent days of compliance with study medication. Participants assigned to the CBT4CBT condition completed an average of about 5 of the 7 modules offered, consistent with prior trials6, 36.
Table 2.
Treatment Process and Adherence by Group
| Variable | Galantamine + CBT4CBT | Galantamine + TAU | Placebo + CBT4CBT | Placebo + TAU | Contrast 1 | Contrast 2 | Contrast 3 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| GAL+CBT4CBT v GAL+TAU and PLA+CBT4CBT | GAL v PLA | CBT4CBT v TAU | ||||||||
| (n=28) | (n=27) | (n=38) | (n=27) | F/X2 | p | F/X2 | p | F/X2 | p | |
| Days in treatment (of 84), mean (SD) | 65.79 (31.06) | 66.96 (30.11) | 70.21 (25.77) | 79.41 (17.6) | .22 | .64 | 2.95 | .09 | 1.12 | .29 |
| Days took study medication, mean (SD) | 62.25 (30.89) | 63.89 (29.55) | 65.45 (26.11) | 77.67 (17.34) | .16 | .69 | 3.01 | .09 | 2.00 | .16 |
| Percent days medication adherent, mean (SD) | 89.85 (20.89) | 91.73 (20.37) | 92.69 (11.14) | 94.22 (19.06) | .34 | .56 | .66 | .42 | .27 | .60 |
| Number CBT4CBT modules completed (of 7), mean (SD) | 4.43 (2.87) | 4.95 (2.31) | .66 | .42 | ||||||
| Total individual tx sessions completed, mean (SD)a | 5.00 (2.33) | 7.50 (4.29) | 5.97 (4.98) | 6.44 (3.65) | 2.48 | .12 | .00 | .96 | 3.04 | .09 |
| Total group tx sessions completed, mean (SD)a | 8.37 (20.66) | 2.70 (4.21) | 13.97 (40.50) | 3.32 (7.84) | .00 | 1.00 | .35 | .56 | 2.41 | .12 |
Abbreviations: GAL=Galantamine, PLA=Placebo, CBT4CBT=computerized cognitive behavioral therapy, TAU=standard methadone treatment as usual
Includes only those participants who initiated treatment
Primary and secondary cocaine outcomes
Random effects regression for effects of study treatments on the primary outcome, days of self-reported cocaine use by month, are presented in Table 3 and illustrated in Figure 2. For the model which included all data collected (that is, including data collected after the point of attrition if the participant dropped out), there was a significant effect of time (F(1,351=152.0, P=.00), medication by time (F(1,351=5.27, P=.02), and behavioral therapy by time (F(1,351=4.22, P=.04), but the third contrast evaluating the interaction effect was not statistically significant. Effects were similar when only those data collected while each participant was still actively enrolled in the treatment protocol were analyzed.
Table 3.
Primary outcomes by time and treatment condition: Random effects model estimates
| Variable | N, obs. | −2RLL | Denominator. df | F | p | Effect size, d |
|---|---|---|---|---|---|---|
| Self-reported percent days abstinent by month | ||||||
| Intercept | 120, 475 | 3100.139 | 215.58 | 517.86 | .00 | |
| Medication Condition (GAL vs PLA) | 215.58 | .45 | .50 | |||
| Behavioral Condition (CBT4CBT vs TAU) | 215.58 | 1.98 | .16 | |||
| Time | 351.07 | 152.04 | .00 | |||
| Contrast 1: Gal + CBT4CBT vs GAL+TAU and PLA+CBT4CBT by time | 351.07 | .03 | .86 | .03 | ||
| Contrast 2: Galantamine vs placebo by time | 351.07 | 5.27 | .02 | .34 | ||
| Contrast 3: CBT4CBT vs TAU by time | 351.07 | 4.22 | .04 | .30 | ||
| Number of cocaine-negative urine specimens submitted by month | ||||||
| Intercept | 120, 367 | 1495.842 | 267.52 | 21.58 | .00 | |
| Medication Condition (GAL vs PLA) | 267.52 | .01 | .91 | |||
| Behavioral Condition (CBT4CBT vs PLA) | 267.52 | .00 | 1.00 | |||
| Time | 286.14 | 32.17 | .00 | |||
| Contrast 1: Gal + CBT4CBT vs GAL+TAU and PLA+CBT4CBT by time | 286.14 | 5.04 | .03 | .75 | ||
| Contrast 2: Galantamine vs placebo by time | 286.14 | 3.46 | .06 | .43 | ||
| Contrast 3: CBT4CBT vs TAU by time | 286.14 | .11 | .74 | .03 | ||
Abbreviations: −2Rll=-2 restricted log likelihood. Gal=Galantamine, PLA=placebo, CBT4CBT=Computerized CBT, TAU=Treatment as Usual
Figure 2.
Primary and Secondary Cocaine Use Outcomes by Group over Time, time, estimates from random effects regression models
Analyses evaluating change in the number of urine specimens collected which were negative for cocaine by month are presented in Table 3: The effect for time was significant, indicating an increase in the frequency of negative urine specimens submitted across time (F(1,286)=32.2, P<.001); however, the effect for medication by time fell short of statistical significance (F(1,286)=3.5 P=.06) and the effect of behavioral therapy by time was not significant (F1,286)=.11, P=.74). Unlike the self-report data, the interaction of medication, behavioral therapy and time was statistically significant (F(1,286)=5.0, P=.03). These effects are presented in Figure 2, which indicates greatest change (improvement) in the number of cocaine-negative urine specimens submitted for the group assigned to galantamine plus TAU, least change in the group assigned to placebo plus TAU, and an intermediate rate of change for those assigned to galantamine plus CBT4CBT or placebo plus CBT4CBT. Post hoc comparisons of the primary and secondary outcomes, summarized across the twelve weeks, by baseline severity are shown in Supplemental Table e1.
Effects of study treatments on cognitive tasks over time
Data from the cognitive task battery are presented in Supplemental Table e2. In general, these showed little change across time, with no evidence of significant medication by time or behavioral therapy by time effects on any of these tasks. A composite score, computed by averaging the standardized scores for the 5 key cognitive tasks and corrected for direction so that higher scores indicate better performance (RVP A’, SSRT, PRM % correct, IED total adjusted errors, and Digit Span backwards), also indicated no significant change over time, nor any evidence of any treatment condition by time effects on the composite score.
While these cognitive indicators did not improve during treatment, they were nevertheless consistently associated with treatment outcome. For example, multiple cognitive measures at baseline were significantly positively correlated with percentage of urine specimens submitted that were negative for all drugs, including the Composite score (r=.25, P=0.01), RVP A’ (r=.20, P=.04), PRM % correct (r=.19, P=.05) and digits backward (r=.26, P=.01). Similar relationships were found for self-reported days of abstinence from cocaine, where better cognitive function was consistently associated with less frequent cocaine use.
Adverse events
The most frequently reported adverse events were nausea/vomiting (reported at least once by 21% of participants), headache (17.7%), loss of appetite (15.9%), fatigue (15%), and diarrhea/constipation (13.3%), but none of these differed significantly by medication condition. Four participants reported significant weight loss; all were in the placebo condition (galantamine versus placebo, X2=3.54, p=.06). Rates of serious adverse events occurred infrequently (8.3% of all those randomized, n=10; 3 for medical reasons, 6 for substance use hospitalization, and 1 for psychiatric reasons) and did not differ by treatment condition.
Discussion
Analyses of primary outcomes in this randomized controlled trial of galantamine and computerized CBT4CBT supported the hypotheses of a main effect of each treatment over time on the primary cocaine use outcome, but there was no evidence of an additive or synergistic effect by combining the two. Contrary to our hypothesis, there was no effect of galantamine relative to placebo over time for the cognitive measures, including sustained attention (RVP A`); moreover, there were few indications of improvement over time for any of these cognitive indicators. Thus, galantamine and CBT4CBT each seemed to contribute to better self-reported cocaine use outcomes; however, as there was no evidence that galantamine improved cognitive functioning in this sample, it was unlikely to have improved response to CBT4CBT by improving participants’ ability to learn CBT skills and strategies.
Galantamine, while not demonstrating efficacy on cognitive function in this sample, was associated with a significant effect on reducing cocaine use. These findings are consistent with our prior pilot study in a cocaine-dependent methadone maintained sample, where galantamine appeared more effective than placebo in reducing cocaine use but did not demonstrate a significant effect on cognitive tasks, including RVP26. The potential for galantamine to have some benefit in treating cocaine use disorder is notable, and consistent with work suggesting a role for the cholinergic system in treating stimulant disorders19, 21. An ongoing RCT is evaluating galantamine versus placebo in a non-methadone sample of individuals with a primary cocaine use disorder (Clinicaltrials.gov #NCT01531153).
While evaluating galantamine in a methadone-maintained sample of cocaine users conferred several advantages from a methodologic point of view, it introduced limitations as well. A key advantage of studying a methadone-maintained sample is that retention and adherence were high via dispensing study medications at the time of daily methadone dosing, also permitting close monitoring of adverse events. In terms of limitations, a sample of individuals maintained on methadone over a long period reduces generalizability and may not have been ideal to detect galantamine effects on cognitive function. Significant problems in cognitive function are well-established in individuals maintained on methadone52, 53 and include broad impairment in domains encompassing attention, memory, cognitive impulsivity and cognitive flexibility in the methadone group54. The level of impairment in this sample who had both cocaine and opioid use disorders may have overwhelmed galantamine’s effects on cognitive enhancement, which tend to be modest, particularly at lower doses55. In addition, cognitive functions may fluctuate depending on recency of methadone dose56, which may have further undercut the ability to detect possible galantamine effects on cognitive function, particularly with the relatively low dose used here.
In summary, this randomized controlled trial included several important design features intended to enhance internal validity, including random assignment to treatment using an urn variable program, relatively high adherence across conditions, twice weekly collection of urine specimens in conjunction with monitored medication ingestion, a well-validated set of assessments to assess cognitive function (CANTAB), and a comparatively complete dataset with few missing data. Although galantamine did not appear to improve cognitive functioning or response to CBT4CBT in this sample, this trial provided evidence for galantamine as a potential therapy for cocaine use disorder, which also proved to be safe and well tolerated in this sample at the dose provided. It also provided confirmatory evidence for the efficacy of CBT4CBT in this challenging sample, which is significant given the relative lack of confirmatory trials of computerized therapies with appropriate control conditions.
Supplementary Material
Clinical points.
There are as yet no approved medications for treating cocaine use disorder among methadone maintained patients
This study suggested the potential of galantamine in this challenging clinical population.
Acknowledgments
Funding/support: This work was supported by grants P50-DA09241 from the National Institute on Drug Abuse.
We gratefully acknowledge the support of the administration and staff of the Kinsella and CHS centers at the Recovery Network of Programs and particularly the individuals who participated in this trial. We especially recognize the highest level of dedication to patient care and Good Clinical Practice by Dorothy Eagan, R.N., M.P.H. and Liz Doohan, M.S.W. of Yale University, the principal research staff for the project. Karen Hunkele, BS. and Joanne Corvino, M.P.H. of Yale University, provided critical support in implementing the trial and data analysis. Ms. Eagan, Doohan, Hunkele, and Corvino have no conflicts of interest to declare.
Footnotes
Potential conflicts of interest: Dr. Carroll is a member in trust of CBT4CBT LLC, which makes CBT4CBT available to qualified clinical providers and organizations on a commercial basis. Dr. Carroll works with Yale University to manage any potential conflicts of interest. The other authors have no conflicts to disclose.
Role of the sponsor: The National Institute on Drug Abuse had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Trial registration: clinicaltrials.gov NCT0080935
References
- 1.Bux DA, Lamb RJ, Iguchi MY. Cocaine use and HIV risk behavior in methadone maintenance patients. Drug Alcohol Dependence. 1995;37(1):29–35. doi: 10.1016/0376-8716(94)01058-s. [DOI] [PubMed] [Google Scholar]
- 2.Condelli WS, Fairbank JA, Dennis ML, Rachal JV. Cocaine use by clients in methadone programs: significance, scope, and behavioral interventions. J Sub Abuse Treat. 1991;8(4):203–212. doi: 10.1016/0740-5472(91)90040-h. [DOI] [PubMed] [Google Scholar]
- 3.Dominguez-Salas S, Diaz-Batanero C, Lozano-Rojas OM, Verdejo-Garcia A. Impact of general cognition and executive function deficits on addiction treatment outcomes: Systematic review and discussion of neurocognitive pathways. Neurosci Biobehavioral Reviews. 2016;71:772–801. doi: 10.1016/j.neubiorev.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 4.Peirce JM, Petry NM, Stitzer ML, et al. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: a National Drug Abuse Treatment Clinical Trials Network study. Arch General Psychiatry. 2006;63(2):201–208. doi: 10.1001/archpsyc.63.2.201. [DOI] [PubMed] [Google Scholar]
- 5.Rawson RA, Huber A, McCann MJ, et al. A comparison of contingency management and cognitive-behavioral approaches during methadone maintenance for cocaine dependence. Arch General Psychiatry. 2002;59(9):817–824. doi: 10.1001/archpsyc.59.9.817. [DOI] [PubMed] [Google Scholar]
- 6.Carroll KM, Kiluk BD, Nich C, et al. Computer-assisted delivery of cognitive-behavioral therapy: efficacy and durability of CBT4CBT among cocaine-dependent individuals maintained on methadone. Am J Psychiatry. 2014;171(4):436–444. doi: 10.1176/appi.ajp.2013.13070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aharonovich E, Nunes EV, Hasin D. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug Alcohol Dependence. 2003;71:207–211. doi: 10.1016/s0376-8716(03)00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Dependence. 2006 Feb 28;81(3):313–322. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Bates ME, Pawlak AP, Tonigan JS, Buckman JF. Cognitive impairment influences drinking outcome by altering therapeutic mechanisms of change. Psych Addictive Behaviors. 2006;20(3):241–253. doi: 10.1037/0893-164X.20.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aharonovich E, Hasin D, Nunes EV, et al. Modified Cognitive Behavioral Therapy (M-CBT) for cocaine dependence: Development of treatment for cognitively impaired users. doi: 10.1037/adb0000398. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology. 2013;64:452–463. doi: 10.1016/j.neuropharm.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bickel WK, Yi R, Landes R, Hill PF, Baxter C. Remembering the future: Working memory training decreases delay discounting among stimulant addicts. Biological Psychiatry. 2011;69:260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rass O, Schacht RL, Buckheit K, Johnson MW, Strain EC, Mintzer MZ. A randomized controlled trial of the effects of working memory training in methadone maintenance patients. Drug Alcohol Dependence. 2015;156:38–46. doi: 10.1016/j.drugalcdep.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bates ME, Buckman JF, Nguyen TT. A role for cognitive rehabilitation in increasing the effectiveness of treatment for alcohol use disorders. Neuropsychology Rev. 2013;23(1):27–47. doi: 10.1007/s11065-013-9228-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verdejo-Garcia A. Cognitive training for substance use disorders: Neuroscientific mechanisms. Neurosci Biobeh Reviews. 2016;68:270–281. doi: 10.1016/j.neubiorev.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 16.Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive function as a trans-diagnostic treatment target in stimulant use disorders. J Dual Diagnosis. 2016;12(1):90–106. doi: 10.1080/15504263.2016.1146383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brady KT, Gray KM, Tolliver BK. Cognitive enhancers in the treatment of substance use disorders: clinical evidence. Pharm, Biochem,Beh. 2011;99(2):285–294. doi: 10.1016/j.pbb.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mark GP, Shabani S, Dobbs LK, Hansen ST. Cholinergic modulation of mesolimbic dopamine function and reward. Physiology & Behavior. 2011;104(1):76–81. doi: 10.1016/j.physbeh.2011.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sofuoglu M, Mooney M. Cholinergic functioning in stimulant addiction: implications for medications development. CNS drugs. 2009 Nov;23(11):939–952. doi: 10.2165/11310920-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarter M, Lustig C, Howe WM, Gritton H, Berry AS. Deterministic functions of cortical acetylcholine. Eur J Neuroscience. 2014;39(11):1912–1920. doi: 10.1111/ejn.12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grasing K. A threshold model for opposing actions of acetylcholine on reward behavior: Molecular mechanisms and implications for treatment of substance abuse disorders. Behav Brain Res. 2016;312:148–162. doi: 10.1016/j.bbr.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schilstrom B, Ivanov VB, Wiker C, Svensson TH. Galantamine enhances dopaminergic neurotransmission in vivo via allosteric potentiation of nicotinic acetylcholine receptors. Neuropsychopharmacology. 2007;32(1):43–53. doi: 10.1038/sj.npp.1301087. [DOI] [PubMed] [Google Scholar]
- 23.Diehl A, Nakovics H, BC, Smolka MN, Batra A, Mann K. Galantamine reduces smoking in alcohol-dependent patients: A randomized, placebo controlled trial. International J Clin Pharmacol Ther. 2006;44(12):614–622. doi: 10.5414/cpp44614. [DOI] [PubMed] [Google Scholar]
- 24.Ashare RL, Kimmey BA, Rupprecht LE, Bowers ME, Hayes MR, Schmidt HD. Repeated administration of an acetylcholinesterase inhibitor attenuates nicotine taking in rats and smoking behavior in human smokers. Transl Psychiatry. 2016;6:e713. doi: 10.1038/tp.2015.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mann K, Ackermann K, Diehl A, et al. Galantamine: A cholinergic patch in the treatment of alcoholism: A randomized, placebo-controlled trial. Psychopharmacology. 2006;184:115–121. doi: 10.1007/s00213-005-0243-9. [DOI] [PubMed] [Google Scholar]
- 26.Sofuoglu M, Carroll KM. Effects of galantamine on cocaine use in chronic cocaine users. Am J Addictions. 2011;20(3):302–303. doi: 10.1111/j.1521-0391.2011.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sofuoglu M, Waters AJ, Poling J, Carroll KM. Galantamine improves sustained attention in chronic cocaine users. Exper Clinical Psychopharmacology. 2011;19(1):11–19. doi: 10.1037/a0022213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV, Patient Edition. Washington, D.C: American Psychiatric Press; 1995. [Google Scholar]
- 29.Scott LJ, Goa KL. Galantamine: a review of its use in Alzheimer’s disease. Drugs. 2000;60:1095–1122. doi: 10.2165/00003495-200060050-00008. [DOI] [PubMed] [Google Scholar]
- 30.Carroll KM, Fenton LR, Ball SA, et al. Efficacy of disulfiram and cognitive-behavioral therapy in cocaine-dependent outpatients: A randomized placebo controlled trial. Arch Gen Psychiatry. 2004;64:264–272. doi: 10.1001/archpsyc.61.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carroll KM, Martino S, Suarez-Morales L, et al. A multisite randomized effectiveness study of motivational enhancement therapy for Spanish-speaking substance abusers. J Consult Clin Psychology. 2009;77:993–999. doi: 10.1037/a0016489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ball SA, Martino S, Nich C, et al. Site matters: multisite randomized trial of motivational enhancement therapy in community drug abuse clinics. J Consult Clin Psychology. 2007;75(4):556–567. doi: 10.1037/0022-006X.75.4.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stout RL, Wirtz PW, Carbonari JP, DelBoca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol. 1994;(Suppl 12):70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- 34.Zachary RA. The Manual of the Shipley Institute of Living Scale. Los Angeles: Western Psychological Services; 1991. [Google Scholar]
- 35.Carroll KM. A Cognitive-Behavioral Approach: Treating Cocaine Addiction. Rockville, Maryland: NIDA; 1998. [Google Scholar]
- 36.Carroll KM, Ball SA, Martino S, et al. Computer-assisted cognitive-behavioral therapy for addiction. A randomized clinical trial of 'CBT4CBT'. Am J Psychiatry. 2008;165(7):881–888. doi: 10.1176/appi.ajp.2008.07111835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nich C, Carroll KM. Intention-to-treat meets missing data: implications of alternate strategies for analyzing clinical trials data. Drug Alcohol Dependence. 2002;68(2):121–130. doi: 10.1016/s0376-8716(02)00111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol Addictive Behav. 2014;28(1):154–162. doi: 10.1037/a0030992. [DOI] [PubMed] [Google Scholar]
- 39.Zanis DA, McLellan AT, Randall M. Can you trust patient self-reports of drug use during treatment? Drug Alcohol Dependence. 1994;35:127–132. doi: 10.1016/0376-8716(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 40.Babor TF, Steinberg K, Anton RF, Del Boca FK. Talk is cheap: Measuring drinking outcomes in clinical trials. J Stud Alcohol. 2000;61:55–63. doi: 10.15288/jsa.2000.61.55. [DOI] [PubMed] [Google Scholar]
- 41.Robbins TW, James M, Owen AM, et al. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: implications for theories of executive functioning and cognitive aging. Cambridge Neuropsychological Test Automated Battery. J Int Neuropsychological Soc. 1998;4(5):474–490. doi: 10.1017/s1355617798455073. [DOI] [PubMed] [Google Scholar]
- 42.Sahakian B, Jones G, Levy R, Gray J, Warburton D. The effects of nicotine on attention, information processing, and short-term memory in patients with dementia of the Alzheimer type. Brit J Psychiatry. 1989;154:797–800. doi: 10.1192/bjp.154.6.797. [DOI] [PubMed] [Google Scholar]
- 43.Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1991;29(10):993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- 44.Soar K, Mason C, Potton A, Dawkins L. Neuropsychological effects associated with recreational cocaine use. Psychopharmacology. 2012;222(4):633–643. doi: 10.1007/s00213-012-2666-4. [DOI] [PubMed] [Google Scholar]
- 45.Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6(2):115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- 46.Filmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Dependence. 2002;66(3):265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- 47.Kopera M, Wojnar M, Brower K, et al. Cognitive functions in abstinent alcohol-dependent patients. Alcohol. 2012;46(7):665–671. doi: 10.1016/j.alcohol.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Bowden SC, Petrauskas VM, Bardenhagen FJ, Meade CE, Simpson LC. Exploring the dimensionality of digit span. Assessment. 2013;20(2):188–198. doi: 10.1177/1073191112457016. [DOI] [PubMed] [Google Scholar]
- 49.Bryk AS, Raudenbush SW. Application of hierarchical linear models to assessing change. Psychological Bull. 1987;101:147–158. [Google Scholar]
- 50.Preston KL, Silverman K, Schuster CR, Cone EJ. Assessment of cocaine use with quantitative urinalysis and estimation of new uses. Addiction. 1997;92:717–727. [PubMed] [Google Scholar]
- 51.Kiluk BD, Carroll KM, Duhig A, et al. Measures of outcome for stimulant trials: ACTTION recommendations and research agenda. Drug Alcohol Dependence. 2016;158:1–7. doi: 10.1016/j.drugalcdep.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ersche KD, Clark L, London M, Robbins T, Sahakian BJ. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology. 2006;31:1036–1047. doi: 10.1038/sj.npp.1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mintzer MZ, Stitzer ML. Cognitive impairment in methadone maintenance patients. Drug Alcohol Dependence. 2002;67(1):41–51. doi: 10.1016/s0376-8716(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 54.Baldacchino A, Armanyous M, Balfour DJK, Humphris G, Matthews K. Neuropsychological functioning and chronic methadone use: A systematic review and meta-analysis. Neuroscience Biobehavioral Rev/ 2017;73:23–38. doi: 10.1016/j.neubiorev.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 55.Takeda A, Loveman E, Clegg A, et al. A systematic review of the clinical effectiveness of donepezil, rivastigmine and galantamine on cognition, quality of life and adverse events in Alzheimer's disease. Int J Geriatr Psychiatry. 2006 Jan;21(1):17–28. doi: 10.1002/gps.1402. [DOI] [PubMed] [Google Scholar]
- 56.Rass O, Kleykamp BA, Vandrey RG, et al. Cognitive performance in methadone maintenance patients: effects of time relative to dosing and maintenance dose level. Exp Clin Psychopharmacology. 2014;22(3):248–256. doi: 10.1037/a0035712. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.