Significance
Discovering new targets and novel determinants of metastatic spread is an unmet need in ovarian cancer, which is plagued by high rates of recurrence. Endothelin-1 receptors (ET-1R), belonging to the G-protein–coupled receptor family, represent important targets critically involved in malignant progression. Here we identify a mechanistic link between ET-1R and the actin regulatory protein hMENA/hMENAΔv6 through the specific interaction with the multifunctional protein β-arrestin1 (β-arr1), which initiates signaling cascades as part of the molecular complex crucial for invadopodial maturation and malignant dissemination. Targeting ET-1R by using macitentan, a Food and Drug Administration-approved antipulmonary arterial hypertension drug, can impair the β-arr1–mediated signaling network controlling ovarian cancer progression and therefore represents a therapeutic option for ovarian cancer patients.
Keywords: endothelin receptor, β-arrestin, invadopodia, ovarian cancer, hMENA
Abstract
Aberrant activation of endothelin-1 receptors (ET-1R) elicits pleiotropic effects relevant for tumor progression. The network activated by this receptor might be finely, spatially, and temporarily orchestrated by β-arrestin1 (β-arr1)–driven interactome. Here, we identify hMENA, a member of the actin-regulatory protein ENA/VASP family, as an interacting partner of β-arr1, necessary for invadopodial function downstream of ET-1R in serous ovarian cancer (SOC) progression. ET-1R activation by ET-1 up-regulates expression of hMENA/hMENAΔv6 isoforms through β-arr1, restricted to mesenchymal-like invasive SOC cells. The interaction of β-arr1 with hMENA/hMENAΔv6 triggered by ET-1 leads to activation of RhoC and cortactin, recruitment of membrane type 1-matrix metalloprotease, and invadopodia maturation, thereby enhancing cell plasticity, transendothelial migration, and the resulting spread of invasive cells. The treatment with the ET-1R antagonist macitentan impairs the interaction of β-arr1 with hMENA and inhibits invadopodial maturation and tumor dissemination in SOC orthotopic xenografts. Finally, high ETAR/hMENA/β-arr1 gene expression signature is associated with a poor prognosis in SOC patients. These data define a pivotal function of hMENA/hMENAΔv6 for ET-1/β-arr1–induced invadopodial activity and ovarian cancer progression.
High-grade serous ovarian cancer (HG-SOC) accounts for 70–80% of ovarian cancer (OC) deaths and is associated with aggressive metastasis (1). Invasive cancer cells utilize actin-rich membrane protrusions, invadopodia, to focally degrade the extracellular matrix (ECM) and metastasize (2, 3). These specialized structures, identified by the presence of cortactin and TKS5 proteins, serve as sites of physical convergence of adhesive, cytoskeletal, proteolytic, and membrane-trafficking signaling pathways. The invadopodia life cycle includes the formation of precursors, followed by invadopodium stabilization, maturation, and elongation to form active invasive protrusions, with the recruitment of matrix metalloproteases (MMP), particularly membrane type 1 (MT1)-MMP (2–4). Although environmental cues and cell metabolism act as drivers of invadopodia, inputs from growth factors are now considered key players of the invadopodia life cycle (4). The endothelin-1 (ET-1) receptors (ET-1R) ETAR and ETBR, belonging to the G-protein–coupled receptor family, are well-recognized drivers of tumorigenesis and metastatic progression of human cancers, including SOC (5). Overexpression of ETAR in patients with SOC is associated with a poor prognosis and chemoresistance (5–7). Autocrine activation of ETAR is critically involved in controlling pleiotropic tumor-promoting activities, including epithelial-to-mesenchymal transition (EMT), invasion, and metastasis (5–7). The diversity of intracellular signaling pathways and cellular processes modulated by ET-1R is driven by the multifunctional protein β-arrestin1 (β-arr1), acting as a molecular hub, orchestrating active signaling complexes required for the engagement of several key pathways and transcriptional regulators, to promote persistent invasive signaling (9–14). We recently reported that ET-1 triggers the signaling to form invadopodia in OC cells (15, 16). However, a detailed knowledge of how ET-1 could regulate critical effectors of invadopodia in the invasive process remains to be addressed.
Among the genes of the “invasion signature” up-regulated in metastatic tumor cells, the human enabled homolog (ENAH) encodes for the 570-aa actin regulatory hMENA protein (17). hMENA, belonging to the Ena/VASP family, is involved in cell adhesion and migration (18, 19). The hMENA gene undergoes a splicing process generating multiple isoforms that are expressed in specific tissues, and its pattern of expression has been proposed as a marker of tumor progression (20). Among these isoforms in breast (21), lung (22), and pancreatic cancer (23), it has been demonstrated that the two alternatively expressed splicing isoforms of hMENA—hMENA11a, an epithelial-associated splice variant with an additional exon (exon 11a) (24), and hMENAΔv6, a mesenchymal-associated isoform lacking exon 6 (21)—have opposite roles in cell invasion and migration. hMENAΔv6 is expressed only in the invasive breast, pancreatic, and lung cell lines (21–23). A different splice variant isoform of hMENA, MENAINV, with an additional exon after the EVH1 domain, has been described as a regulator of invadopodial function and cell invasiveness in response to EGF signaling in breast cancer cells (25).
Here, to define how SOC cells acquire the plasticity for invadopodial formation and maturation driven by the ET-1 axis, and considering the role of hMENA in invadopodia, we delineate an important role for hMENA/hMENAΔv6 as a critical regulator of ET-1/β-arr1–induced cytoskeleton architecture changes and invasive protrusion function in malignant progression.
Results
Expression of hMENA in Human Ovarian Cancer.
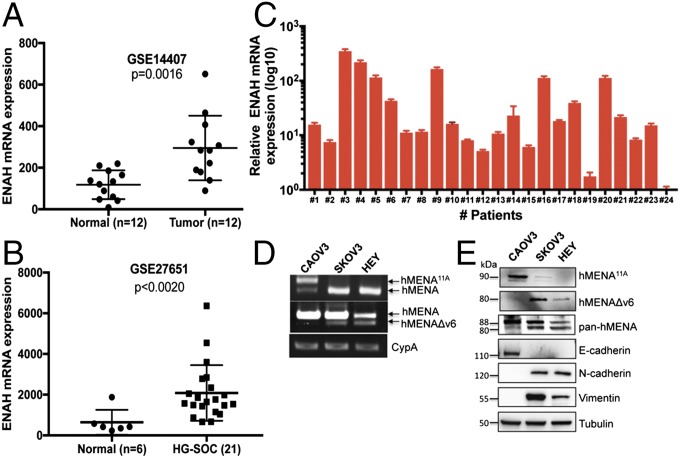
To assess the pathophysiological functions of hMENA (ENAH) in OC, two published mRNA expression profiles, obtained from the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/geo/), have been evaluated (26). ENAH mRNA is significantly up-regulated in OC tissues and, in particular, in HG-SOC tumors, compared with normal tissues (Fig. 1 A and B). Consistently, analysis of a clinically annotated patient cohort of HG-SOC reveals that all 24 patients express ENAH mRNA at different levels (Fig. 1C), supporting the relevance of hMENA in this tumor type. We analyzed the expression of total hMENA and the specific hMENA11a (antiinvasive) and hMENAΔv6 (proinvasive) isoforms (21) in a panel of SOC cell lines, exhibiting a different ability to invade in the transwell Matrigel assay (Fig. S1 A and B). All SOC cells express hMENA, and only the highly invasive cell lines, HEY and SKOV3, also express hMENAΔv6 associated with EMT-related markers, such as N-cadherin and vimentin, consistent with the association of hMENAΔv6 expression with the EMT phenotype. On the contrary, the low invasive cell line CAOV3 expresses hMENA11a along with the epithelial marker E-cadherin (Fig. 1 D and E). Immunofluorescent analysis indicates that hMENA is localized to the periphery of SOC cells in the tips of actin stress fiber-like structures (Fig. S1C).
Fig. 1.
HG-SOC expresses hMENA along with the hMENAΔv6 isoform. (A and B) Expression profiling of ENAH mRNAs in human normal and tumor specimens from two Gene Expression Omnibus profile datasets. (C) Relative ENAH mRNA expression in 24 HG-SOC specimens normalized for GAPDH expression. Error bars: mean ± SD (n = 2). (D) Representative RT-PCR analysis for hMENA, hMENA11a, and hMENAΔv6 mRNA in three different SOC cell lines. (E) Representative Western blotting (WB) analysis of hMENA (pan-hMENA), hMENA11a, hMENAΔv6, E-cadherin, N-cadherin, and vimentin in SOC cell lines. Tubulin was used as a loading control.
ET-1 Is a Regulator of hMENA Expression in SOC Cells.
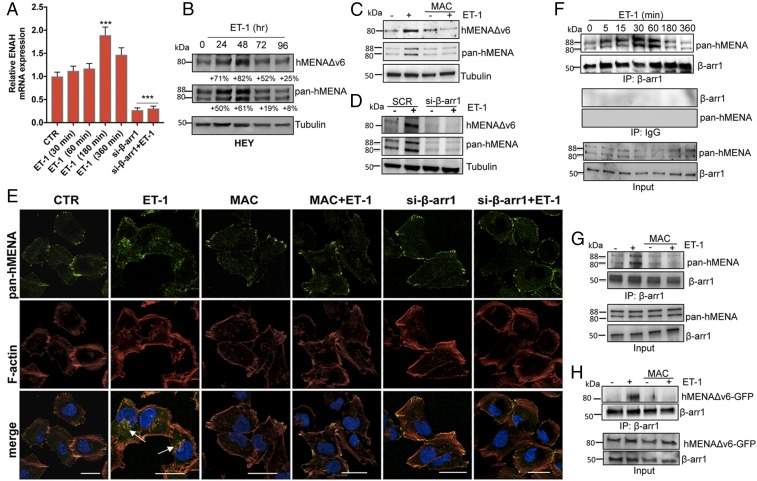
Given that ET-1 is a druggable pathway in OC (7), we explored whether ET-1 signaling is involved in the regulation of hMENA expression and function. In invasive HEY cells, all ENAH transcripts increase upon ET-1 treatment (Fig. 2A). In HEY and SKOV3 cells, hMENAΔv6 and hMENA protein expression enhances upon ET-1 addition in a time-dependent manner with a maximum increase after 48 h (Fig. 2B and Fig. S1D). On the contrary, in the epithelium-like CAOV3 cells, the ET-1 addition leads to a decreased expression of hMENA11a after 72 h (Fig. S1E). These findings strengthen the idea that ET-1 regulates the balance between the mesenchymal-associated hMENAΔv6 and the epithelial-associated hMENA11a isoforms in determining SOC invasive behavior. The treatment with macitentan, a potent dual ETAR/ETBR antagonist, inhibits ET-1–induced hMENA/hMENAΔv6 expression (Fig. 2C). Moreover, considering that SOC cells express both β-arr1 and -2 (8), we evaluated the involvement of β-arr on ET-1–mediated hMENA isoform expression at the mRNA and protein levels. Silencing of β-arr1, but not of β-arr2, inhibits ET-1–induced hMENA/hMENAΔv6 expression (Fig. 2 A and D and Fig. S4 A–C), indicating that ET-1 through β-arr1 up-regulates hMENA/hMENAΔv6 expression at the transcriptional level. In untreated cells, hMENA is distributed to the cell periphery where it appears to be organized with F-actin at the tips of actin filaments (Fig. 2E and Fig. S2 A and B), as shown by confocal scanning laser microscopy (CSLM) analysis. Upon ET-1 addition, hMENA relocalizes from the periphery to the cytosol in a time-dependent manner and, between 30 and 60 min, colocalizes in F-actin/cortactin–enriched dot-like structures in the ventral region of the cells, while treatment with macitentan, as well as silencing of β-arr1, abrogates the ET-1–induced relocalization of hMENA (Fig. 2E and Fig. S2). These data indicate that ET-1/β-arr1 signaling up-regulates hMENA and hMENAΔv6, as well as their recruitment to regions of the F-actin/cortactin–rich dots.
Fig. 2.
ET-1R up-regulates the expression of hMENA and hMENAΔV6 through β-arr1 and promotes the association of hMENA with β-arr1. (A) Expression of hMENA mRNA measured by qRT-PCR in HEY cells stimulated with ET-1 (100 nM) for the indicated times or silenced for β-arr1 and treated with ET-1 for 180 min. ***P ≤ 0.001 vs. CTR or ET-1–stimulated cells. Error bars: mean ± SD. (B) WB analysis of hMENAΔv6 and hMENA (pan-hMENA) expression in cells stimulated or not with ET-1 for different times or (C) treated with macitentan (MAC, 1 μM) alone or in combination with ET-1 for 48 h. For B, percentage of band density compared with unstimulated cells (Lower). (D) WB analysis of hMENAΔv6 and hMENA expression in HEY cells transfected with scramble (SCR)- or β-arr1-siRNA and stimulated with ET-1 for 48 h. (E) Representative immunofluorescent images of HEY cells, transfected with scramble (CTR)- or β-arr1-siRNA, stimulated with ET-1 (60 min) and/or MAC, and stained with phalloidin (to detect F-actin; red), pan-hMENA Ab (green), and DAPI (blue). hMENA/F-actin colocalization: white arrows. (Scale bar: 30 µm.) (F) Protein lysates from HEY cells stimulated with ET-1 for the indicated times were immunoprecipitated with control IgG or anti–β-arr1 Ab. (G) Protein lysates from HEY cells stimulated with ET-1 and/or MAC for 60 min were immunoprecipitated with anti–β-arr1Ab. (H) Protein lysates from HEY cells, transfected with hMENAΔv6-GFP and unstimulated or treated with ET-1 and/or MAC for 60 min were immunoprecipitated with an anti–β-arr1Ab. Input and immunoprecipitation were analyzed by WB for the indicated proteins. For B–H, representative blots are shown.
hMENA Is an Interactor with β-arr1 upon ET-1R Activation.
Given the ability of β-arr1 to directly interact with cytoskeleton remodeling players, and to act as a mediator of ETAR signaling (15, 27, 28), we reasoned that ET-1–dependent hMENA localization could arise from its association with β-arr1. Endogenous β-arr1 and hMENA readily coimmunoprecipitate (co-IP) in ET-1–stimulated HEY cells in a time-dependent manner (Fig. 2F and Fig. S3A). Macitentan treatment significantly impairs the interaction between β-arr1 and endogenous hMENA or exogenously expressed GFP-tagged hMENAΔv6 induced by ET-1 (Fig. 2 G and H). In agreement with these findings, CSLM analysis shows that hMENA colocalizes with β-arr1 in ET-1–stimulated cells, but not upon treatment with macitentan (Fig. S3 B and C). Collectively, these results reveal that hMENA/hMENAΔv6 is a β-arr1 interactor in ETAR-dependent signaling.
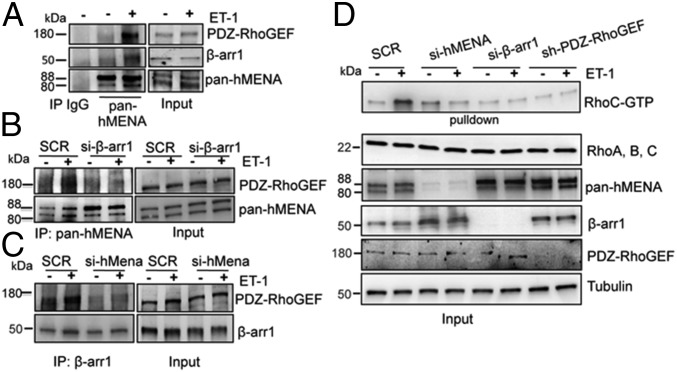
Having demonstrated that the signal-integrating activity of β-arr1 on Rho GTPase is accomplished by its interaction with postsynaptic density protein 95/Disk-Large/Zonula occludens (PDZ)-RhoGEF, a member of the RhoGEF family (15, 16), we tested whether hMENA links the β-arr1/PDZ-RhoGEF complex supporting the ET-1 signaling. ET-1 induces the formation of a trimeric complex with PDZ-RhoGEF, β-arr1, and hMENA, as shown by co-IP analysis (Fig. 3A). The interaction of β-arr1 with PDZ-RhoGEF or PDZ-RhoGEF with hMENA is not detectable when the cells are silenced for hMENA or β-arr1 (Fig. 3 B and C and Fig. S4 A, D, and E), indicating that ET-1R activation might provide β-arr1 for physical recruitment of PDZ-RhoGEF and hMENA, relocalizing hMENA to invadopodia, as evaluated by colocalization of hMENA/F-actin/cortactin (Fig. 2E and Fig. S2).
Fig. 3.
ET-1 induces the association of hMENA with β-arr1 and PDZ-RhoGEF to regulate RhoC activation. (A) Protein lysates from HEY cells stimulated with ET-1 for 60 min were immunoprecipitated with control IgG or pan-hMENA Ab. (B) SCR- and β-arr1-siRNA–transfected HEY cells were stimulated with ET-1 for 60 min. Protein lysates were immunoprecipitated with pan-hMENA Ab. (C) SCR- or hMENA-siRNA transfected HEY cells were stimulated with ET-1 for 60 min. Protein lysates were immunoprecipitated with β-arr1 Ab. For A–C, input and immunoprecipitation were analyzed by WB for the indicated proteins, and representative blots are shown. (D) Rhotekin beads were used to pull down RhoC-GTP from SCR-, hMENA-, β-arr1-siRNA–, or PDZ-RhoGEF-shRNA–transfected HEY cells induced with ET-1 for 5 min. GTP pull-down and input were analyzed by WB for the indicated proteins and representative blots are shown.
hMENA Mediates ET-1R/β-arr1–Induced RhoC Activity.
Among Rho GTPase family members, RhoC activity is spatially confined in the area surrounding the actin core of invadopodia controlling restricted cofilin signaling (29). ET-1–induced RhoC activity significantly decreases in si-hMENA or sh-PDZ-RhoGEF or si-β-arr1–transfected cells (Fig. 3D and Fig. S4F), indicating that hMENA/hMENAΔv6, within the protein complex with β-arr1 and PDZ-RhoGEF, influences the activity of RhoC. Of note, the overexpression of hMENAΔv6 promotes activation of RhoC, which is further potentiated by the addition of ET-1 (Fig. S4G), demonstrating that the formation of a β-arr1/PDZ-RhoGEF/hMENAΔv6 molecular complex elicits ET-1 responses by increasing RhoC signaling. Altogether these findings untangle the interconnected roles of RhoC GTPases, a specific GEF, and hMENA activated by ET-1R/β-arr1 for the formation of invadopodia.
hMENA Is Recruited to Mature Invadopodia in ET-1/β-arr1 Signaling.
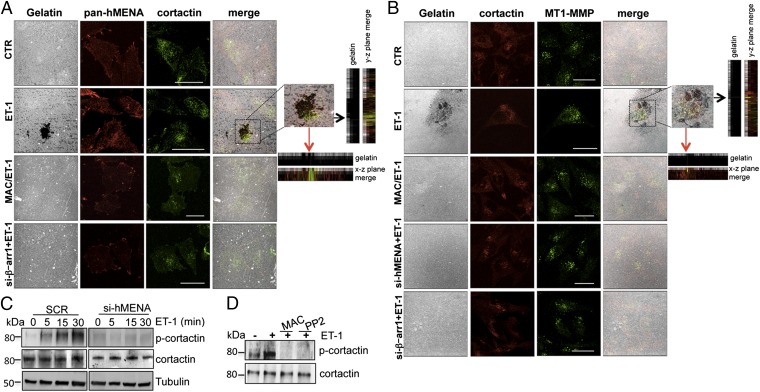
To evaluate whether the recruitment of hMENA/hMENAΔv6 is crucial in ET-1–induced invadopodia and ECM degradation, we plated SOC cells into fluorescently labeled gelatin. Cells stimulated with ET-1 readily degrade the gelatin substrate, and hMENA localizes to a proteolytically degraded area (Fig. S5). Moreover, hMENA colocalizes with cortactin within the degraded area in ET-1–stimulated cells, but not in cells treated with macitentan or silenced for β-arr1 (Fig. 4A), confirming the ET-1R/β-arr1–mediated relocalization of hMENA within active, matrix-degrading invadopodial protrusions. Furthermore, ET-1–treated cells show colocalization of invadopodia markers, as cortactin, MT1-MMP, or TKS5 in the matrix degradation area, which is inhibited by macitentan or by hMENA or β-arr1 silencing (Fig. 4B and Fig. S6), confirming the role of β-arr1/hMENA in ET-1R–promoted maturation of invadopodia, which recruit MT1-MMP to fully degrade ECM.
Fig. 4.
hMENA is recruited to mature invadopodia in ET-1/β-arr1 signaling. (A) CSLM analysis of CTR- or β-arr1-siRNA–transfected HEY cells, plated onto gelatin, and treated with ET-1 and/or MAC for 24 h. Representative images show gelatin (gray), cortactin (green), and hMENA (red). (Scale bar: 30 μm.) (Inset) Higher-power magnification images of the degradation area. Orthogonal views (y–z plane; x–z plane) indicate (arrows) areas of gelatin degradation where hMENA and cortactin are colocalized. (B) CSLM analysis of HEY cells, transfected with SCR or hMENA-, or β-arr1-siRNA, were plated onto gelatin and treated with ET-1 and/or MAC for 24 h. Representative image show gelatin (gray), MT1-MMP (green), and cortactin (red). (Scale bar: 30 µm.) (Inset) Higher-power magnification image of degradation areas. Orthogonal views (y–z plane; x–z plane) indicate (arrows) areas of gelatin degradation where cortactin and MT1-MMP are colocalized. (C) Protein lysates from SCR- or hMENA-siRNA–transfected HEY cells, unstimulated or stimulated with ET-1 for the indicated times, or (D) from HEY cells unstimulated or stimulated with ET-1 and/or MAC and/or PP2 for 30 min, were analyzed by WB by using anti–phospho-cortactin (Y421) and anticortactin Abs. Representative blots are shown.
Cortactin contains two tyrosine phosphorylation sites (Y421, Y466) to regulate invadopodium formation and maturation, triggered also by involvement of Src (30, 31). ET-1 stimulation produces an increase in cortactin phosphorylation (Y421), which is inhibited by treatment with macitentan or with the Src inhibitor PP2 (Fig. 4 C and D). Moreover, a significant inhibition of ET-1–induced, Src-mediated cortactin phosphorylation is evident upon silencing of hMENA (Fig. 4C), indicating that hMENA/hMENAΔv6 is involved in tyrosine phosphorylation of cortactin to regulate invadopodium maturation downstream from ET-1R/β-arr1 signaling.
hMENA Is Required for ET-1R/β-arr1–Dependent Protease Secretion, Cell Plasticity, Transendothelial Migration, and Invasion.
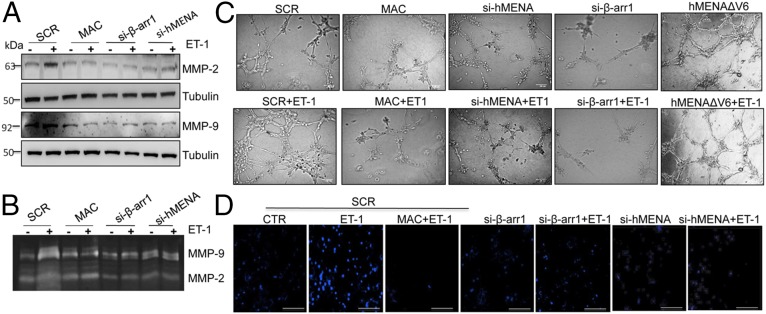
The temporal and spatial control of MMP activity is one of the important functions of invadopodia. Inhibition of ET-1–induced MMP-2/-9 activation and cell invasion is detected upon macitentan treatment, as well as hMENA or β-arr1 silencing, implying a role for ET-1R–dependent β-arr1/hMENA interaction in enhanced proteolytic activity (Fig. 5 A and B) and cell invasion (Fig. S7A).
Fig. 5.
hMENA is involved in ET-1R/β-arr1–induced protease activation, vasculogenic mimicry, and transendothelial migration. SCR-, hMENA-, or β-arr1-siRNA–transfected HEY cells were stimulated with ET-1 and/or MAC for 24 h. (A) Protein lysates were analyzed by WB for indicated proteins. Representative blots are shown. (B) Culture media of cells treated as above reported were analyzed by gelatin zymography to visualize active forms of MMP-9 and MMP-2. Representative gels are shown. (C) Representative tubule-like structure formation in SCR-, hMENA-, β-arr1-siRNA–transfected cells or hMENAΔv6-GFP–expressing SKOV3 cells, unstimulated or stimulated with ET-1 and/or MAC for 16 h. Original magnification: 20×. (Scale bar : 100 µm.) (D) Fluorescence-labeled HEY cells that had migrated through the endothelial layer were photographed. (Scale bar: 30 μM.)
Previous studies described vasculogenic mimicry (VM) as the functional plasticity of aggressive cancer cells that form vascular networks by regulating dynamic formation of actin-based structures (32). SOC cells form connective structures resembling a network-like appearance with long polygonal shape and cellular protrusions after addition of ET-1, as quantified with the number of nodes, junctions, and branches, which are markedly impaired by macitentan as well as by silencing of β-arr1 (Fig. 5C and Fig. S7B). Interestingly, the down-regulation of hMENA significantly disrupts the formation of VM channels, as evidenced by most tubes remaining open and an increase in dissociative cells (Fig. 5C and Fig. S7B). Moreover, hMENAΔv6 overexpression mimics the addition of ET-1, indicating that hMENA/hMENAΔv6 play an important role in VM induced by ET-1R/β-arr1 activation, involved in ECM remodeling and protrusion formation.
To determine how SOC cells extravasate from the blood vessels upon ET-1 stimulus, we evaluated the ET-1-stimulated transendothelial migration capacity of cells, through a monolayer of human umbilical endothelial cells. This capacity is significantly enhanced compared with control cells, and is strongly reduced by macitentan or silencing of hMENA or β-arr1 (Fig. 5D and Fig. S7C). Together, these results imply that the integration with hMENA/hMENAΔv6 is critical in ET-1R/β-arr1–promoted cell plasticity and extravasation through the matrix degradative machinery.
Macitentan Impairs Malignant Progression in Vivo by Interfering with the ET-1R/β-arr1/hMENA Pathway.
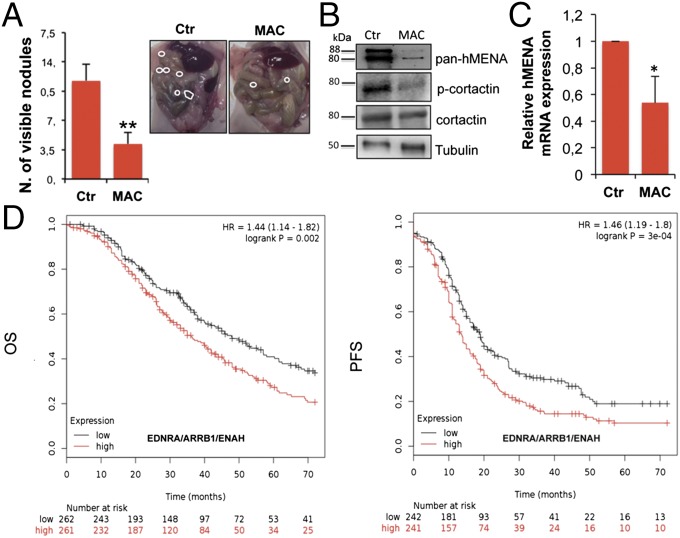
Next, we analyzed the effects of macitentan on SOC invasive behavior and molecular effectors of invadopodia in i.p. orthotopic OC xenografts. All mice in the control group show the spread to the surface of the peritoneum, intestines, omentum, small bowel, mesentery, liver, and spleen and ovaries (Fig. 6A). In mice treated with macitentan, the average of i.p. nodules is significantly reduced, associated with a well-tolerated toxicity profile (no weight loss in the treated mice; Fig. 6A). Consistent with results obtained in vitro in i.p. nodules from macitentan-treated mice, a significant inhibition of hMENA/hMENAΔv6 expression at mRNA and protein levels and reduction of phosphorylation of cortactin are observed (Fig. 6 B and C). These results indicate that the therapeutic efficacy of macitentan to control ET-1R/β-arr1–driven OC progression relies also on its ability to reduce critical invadopodial components, such as hMENA/hMENAΔv6 expression and cortactin activity.
Fig. 6.
ET-1R blockade by macitentan inhibits i.p. spreading and hMENA expression. (A) Female nude mice (eight mice for group) were i.p. injected with SKOV3 cells and treated with vehicle [control (Ctr)] or macitentan (MAC, 30 mg/kg oral daily) for 5 wk. **P ≤ 0.01 (n = 2). Error bars: mean ± SD. Representative i.p. nodules are indicated by white circles (Right). (B) Protein lysate from i.p. nodules of control- or MAC-treated xenografts as in A were analyzed by WB for the indicated proteins. (C) RT-qPCR analysis for the levels of hMENA mRNA from xenografts as in A; *P ≤ 0.05. Error bars: mean ± SD. (D) Combined expression of ETAR, β-arr1, and hMENA as a prognostic signature in SOC patients. Kaplan–Meier curves of OS or PFS in SOC patients with low or high EDNRA/ARRB1/ENAH expression.
Combined Expression of ETAR, β-arr1, and hMENA as a Prognostic Gene Signature in SOC Patients.
To determine the clinical relevance of our experimental data, we assessed the prognostic effect of EDNRA, ARBB1, and ENAH mRNA expression (Affymetrix ID is 204464_at, EDNRA; 218832_at, ARRB1; 222433_at, ENAH) using www.kmplot.com (33). Kaplan–Meier analysis and the log-rank test demonstrate that EDNRA is predictive of overall survival (OS) and progression-free survival (PFS) in SOC patients (Fig. S8). Interestingly, high EDNRA/ARRB1/ENAH gene expression significantly correlates with a worse prognosis for OS [hazard ratio (HR) = 1.44 (1.14–1.82), P = 0.002] and PFS [HR D 1.46 (1.19–1.8), P = 0.0003], with an enhanced HR (Fig. 6D), compared with the expression of each individual biomarker, or even when they are combined (Fig. S8). Overall, these observations indicate that the network-based EDNRA/ENAH/ARRB1 expression could be used as a prognostic signature and potential druggable pathway in SOC.
Discussion
The identification of molecular drivers of OC progression is critical for the development of therapeutic approaches in advanced-stage disease. Here, we identified hMENA/hMENAΔv6 as a signal transducer of ET-1R/β-arr1 signaling to induce SOC cell plasticity, invadopodia function, intravasation, and malignant dissemination. Several studies have demonstrated that ET-1 signaling enables OC cells to acquire the EMT phenotype, thereby increasing cell motility and invasion, and contributes to poor patient outcome (6–8). However, how the actin-cytoskeleton–mediated signaling network driven by ET-1R can regulate tumor progression is still unknown. Here we have identified hMENA/hMENAΔv6 as a binding partner of β-arr1 and as an interconnected regulator of the signaling platform activated in response to ET-1R in SOC cells.
In the present study, we provide an understanding of how ET-1/β-arr1 signals can confer malignant and invasive traits through the modulation of hMENA and the invasive isoform hMENAΔv6 (Fig. S9). Consistent with the opposite role of hMENA isoforms in tumor cell invasion and EMT (21–23), we demonstrate that the proinvasive hMENA/hMENAΔv6 is highly expressed in the most invasive SOC cells, expressing also EMT markers. These isoforms are up-regulated in highly aggressive and metastatic SOC cells by ET-1 signaling, which, conversely, down-regulates the antiinvasive hMENA11a isoform. This is in line with previous data indicating that TGF-β1 up-regulates hMENAΔv6, but not hMENA11a in pancreatic cancer (23), suggesting that the pattern of hMENA isoform expression mediates the functional effects of different proinvasive factors. In agreement, ET-1R/β-arr1 signaling promotes invadopodia maturation through a sequence of events that require the hMENA/hMENAΔv6/RhoC pathway and the recruitment of cortactin, TKS5, and MT1-MMP to invadopodia for pericellular matrix degradation.
Inputs derived from critical growth factors secreted by tumor cells can create an invasive milieu, acting as drivers of invadopodia in the most aggressive human tumors (3, 4). Although the basic components of invadopodia have been characterized, the complex interactome of invadopodia components and the strongest inputs derived upstream from receptors are poorly understood, suggesting that deciphering the regulators is an essential step to fully understanding invadopodial function and to providing novel druggable pathways. The data presented here emphasize a unique mechanism by which ET-1R/β-arr1 increases expression of the invadopodial components hMENA/hMENAΔv6 and engages them in discrete regions at invadopodia, promoting their formation and subsequent ECM degradation. Of note, ET-1R/β-arr1 mobilizes hMENA to actin/cortactin dots, sustaining its presence in the ventral protrusions. These findings support the hypothesis that hMENA/hMENAΔv6 might favor the efficacy of the protrusive force to form the degradative protrusions, conferring also a fitness advantage to OC cells able to breach the endothelial barrier and start the transendothelial migration process. The tyrosine phosphorylation of cortactin at invadopodia is necessary for the release of cofilin from cortactin, supporting cofilin-dependent actin polymerization and the maturation of invadopodia into matrix-degrading structures (30, 31). In this regard, we demonstrated that ET-1 regulates cortactin phosphorylation at tyrosine 421 through hMENA/hMENAΔv6 and Src. It has been recently demonstrated that the MenaINV isoform supports invadopodium maturation by increasing cortactin phosphorylation through suppression of the specific phosphatase PTP1B activity within invadopodia (34, 35). These results likely reflect that different hMENA isoforms concur with nonredundant functional competence in invadopodial maturation. Indeed, our data, uncovering a role for hMENA/hMENAΔv6 in invadopodial maturation, highlight the role of hMENA isoforms at the crossroads of several growth factor signaling receptors, such as ET-1R, to sensitize tumor cells to form invadopodia and to functionally contribute to the invasive process.
Mounting evidence suggests a direct correlation between the propensity of cancer cells to form invadopodia and a poor prognosis in cancer patients (3, 4). By using a public databases, we found that ENAH is overexpressed in OC patients compared with normal tissues. Clinically, in a large cohort of SOC clinical specimens, the integrated high expression levels of EDNRA/ARRB1/ENAH positively correlate with poor prognosis, validating the clinical implication of this druggable pathway and providing a predictive signature in OC progression.
Targeting ET-1R activity by using the small molecule macitentan, which the Food and Drug Administration has approved for nononcological indications, empowering the β-arr1–driven actin-related networks shuts down the expression of hMENA and impairs malignant progression of SOC xenografts. The therapeutic advantage of macitentan is not only to target OC cells expressing ETAR by interfering with the mechanism utilized by β-arr1 to regulate hMENA-signaling networks, but also to target tumor-associated environmental elements, such as vascular, lymphatic, and inflammatory cells and fibroblasts, which all express ETBR (5, 36–38).
Together, this present study highlights a hitherto unappreciated role of the ET-1R/β-arr1 pathway, which integrates hMENA/hMENAΔv6 to regulate invadopodial function and OC progression.
Materials and Methods
Patient Data.
All patients provided written informed consent for their data to be collected and analyzed for the current experimental project; the Catholic University of Rome Institutional Review Board (IRB) approved the current experimental project as well as the related consent procedures. We also obtained ethic committee approval at Regina Elena Institute for the current study.
Cell Lines.
HEY, SKOV3, and CAOV3 cell lines were purchased from the American Type Culture Collection and routinely tested for mycoplasma contaminations. Culture condition, drug treatment, ectopic expression and silencing and siRNA transfection, Rho activation assay, gelatin zimography, RNA isolation and analysis (semiquantitative PCR, quantitative real-time PCR), chemoinvasion assay, transendothelial migration assay, vasculogenic mimicry assay, fluorescent gelatin degradation assay, immunofluorescence, Western blotting, and immunoprecipitation techniques are all described in SI Materials and Methods.
Details of Kaplan–Meyer analysis, statistical analysis, patient data, and in vivo studies are also described in SI Materials and Methods.
In Vivo Assay.
Female athymic (nu+/nu+) mice were injected intraperitoneally (orthotopic site) with viable SKOV3 (2 ± 106) cells. Two weeks after, animals were randomized into two different groups of eight mice undergoing the following treatments for 5 wk: (i) vehicle (control) and (ii) macitentan (30 mg/kg, oral daily). Animals were subjected to experimental protocols approved by the Italian Ministry of Health.
Supplementary Material
Acknowledgments
We thank Aldo Lupo for excellent technical assistance, Maria Vincenza Sarcone for secretarial assistance, and Frank Gertler (Massachusetts Institute of Technology) for providing mouse anti-hMENA antibody. This work was supported by Associazione Italiana Ricerca sul Cancro (AIRC) IG 12852 (to L.R.), IG 14199 (to A.B.), 5x1000 12182, and IG 15224 (to P.N.). L.R. is supported by 5x1000 Ministero della Salute 2011.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 2856.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1715998115/-/DCSupplemental.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: Characteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12:413–426. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eddy RJ, Weidmann MD, Sharma VP, Condeelis JS. Tumor cell invadopodia: Invasive protrusions that orchestrate metastasis. Trends Cell Biol. 2017;27:595–607. doi: 10.1016/j.tcb.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paterson EK, Courtneidge SA. Invadosomes are coming: New insights into function and disease relevance. FEBS J. 2017;285:8–27. doi: 10.1111/febs.14123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosanò L, Spinella F, Bagnato A. Endothelin 1 in cancer: Biological implications and therapeutic opportunities. Nat Rev Cancer. 2013;13:637–651. doi: 10.1038/nrc3546. [DOI] [PubMed] [Google Scholar]
- 6.Rosanò L, et al. Endothelin-1 promotes epithelial-to-mesenchymal transition in human ovarian cancer cells. Cancer Res. 2005;65:11649–11657. doi: 10.1158/0008-5472.CAN-05-2123. [DOI] [PubMed] [Google Scholar]
- 7.Rosanò L, et al. Acquisition of chemoresistance and EMT phenotype is linked with activation of the endothelin A receptor pathway in ovarian carcinoma cells. Clin Cancer Res. 2011;17:2350–2360. doi: 10.1158/1078-0432.CCR-10-2325. [DOI] [PubMed] [Google Scholar]
- 8.Rosanò L, et al. Endothelin A receptor/β-arrestin signaling to the Wnt pathway renders ovarian cancer cells resistant to chemotherapy. Cancer Res. 2014;74:7453–7464. doi: 10.1158/0008-5472.CAN-13-3133. [DOI] [PubMed] [Google Scholar]
- 9.Rosanò L, et al. Beta-arrestin links endothelin A receptor to beta-catenin signaling to induce ovarian cancer cell invasion and metastasis. Proc Natl Acad Sci USA. 2009;106:2806–2811. doi: 10.1073/pnas.0807158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosanò L, Bagnato A. β-arrestin1 at the cross-road of endothelin-1 signaling in cancer. J Exp Clin Cancer Res. 2016;35:121. doi: 10.1186/s13046-016-0401-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peterson YK, Luttrell LM. The diverse roles of arrestin scaffolds in G protein-coupled receptor signalling. Pharmacol Rev. 2017;69:256–297. doi: 10.1124/pr.116.013367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shenoy SK, Lefkowitz RJ. β-arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci. 2011;32:521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cianfrocca R, et al. Nuclear β-arrestin1 is a critical cofactor of hypoxia-inducible factor-1α signaling in endothelin-1-induced ovarian tumor progression. Oncotarget. 2016;7:17790–17804. doi: 10.18632/oncotarget.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosanò L, et al. β-Arrestin-1 is a nuclear transcriptional regulator of endothelin-1-induced β-catenin signaling. Oncogene. 2013;32:5066–5077. doi: 10.1038/onc.2012.527. [DOI] [PubMed] [Google Scholar]
- 15.Semprucci E, et al. Endothelin A receptor drives invadopodia function and cell motility through the β-arrestin/PDZ-RhoGEF pathway in ovarian carcinoma. Oncogene. 2016;35:3432–3442. doi: 10.1038/onc.2015.403. [DOI] [PubMed] [Google Scholar]
- 16.Bagnato A, Rosanò L. Endothelin-1 receptor drives invadopodia: Exploiting how β-arrestin-1 guides the way. Small GTPases. 2016;3:1–5. doi: 10.1080/21541248.2016.1235526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang W, et al. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res. 2004;64:8585–8594. doi: 10.1158/0008-5472.CAN-04-1136. [DOI] [PubMed] [Google Scholar]
- 18.Gertler FB, Niebuhr K, Reinhard M, Wehland J, Soriano P. Mena, a relative of VASP and Drosophila enabled, is implicated in the control of microfilament dynamics. Cell. 1996;87:227–239. doi: 10.1016/s0092-8674(00)81341-0. [DOI] [PubMed] [Google Scholar]
- 19.Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. Ena/VASP proteins: Regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol. 2003;19:541–564. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- 20.Gertler F, Condeelis J. Metastasis: Tumor cells becoming MENAcing. Trends Cell Biol. 2011;21:81–90. doi: 10.1016/j.tcb.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Modugno F, et al. Splicing program of human MENA produces a previously undescribed isoform associated with invasive, mesenchymal-like breast tumors. Proc Natl Acad Sci USA. 2012;109:19280–19285. doi: 10.1073/pnas.1214394109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bria E, et al. Prognostic impact of alternative splicing-derived hMENA isoforms in resected, node-negative, non-small-cell lung cancer. Oncotarget. 2014;5:11054–11063. doi: 10.18632/oncotarget.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melchionna R, et al. The pattern of hMENA isoforms is regulated by TGF-β1 in pancreatic cancer and may predict patient outcome. OncoImmunology. 2016;5:e1221556. doi: 10.1080/2162402X.2016.1221556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Modugno F, et al. Molecular cloning of hMena (ENAH) and its splice variant hMena+11a: Epidermal growth factor increases their expression and stimulates hMena+11a phosphorylation in breast cancer cell lines. Cancer Res. 2007;67:2657–2665. doi: 10.1158/0008-5472.CAN-06-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Philippar U, et al. A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev Cell. 2008;15:813–828. doi: 10.1016/j.devcel.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeFea KA. Arrestins in actin reorganization and cell migration. Prog Mol Biol Transl Sci. 2013;118:205–222. doi: 10.1016/B978-0-12-394440-5.00008-5. [DOI] [PubMed] [Google Scholar]
- 28.Ma X, et al. βArrestin1 regulates the guanine nucleotide exchange factor RasGRF2 expression and the small GTPase Rac-mediated formation of membrane protrusion and cell motility. J Biol Chem. 2014;289:13638–13650. doi: 10.1074/jbc.M113.511360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bravo-Cordero JJ, et al. A novel spatiotemporal RhoC activation pathway locally regulates cofilin activity at invadopodia. Curr Biol. 2011;21:635–644. doi: 10.1016/j.cub.2011.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oser M, et al. Specific tyrosine phosphorylation sites on cortactin regulate Nck1-dependent actin polymerization in invadopodia. J Cell Sci. 2010;123:3662–3673. doi: 10.1242/jcs.068163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelley LC, et al. Oncogenic Src requires a wild-type counterpart to regulate invadopodia maturation. J Cell Sci. 2010;123:3923–3932. doi: 10.1242/jcs.075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumour-cell plasticity: Lessons from melanoma. Nat Rev Cancer. 2003;3:411–421. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- 33.Gyorffy B, Lánczky A, Szállási Z. Implementing an online tool for genome-wide validation of survival-associated biomarkers in ovarian-cancer using microarray data from 1287 patients. Endocr Relat Cancer. 2012;19:197–208. doi: 10.1530/ERC-11-0329. [DOI] [PubMed] [Google Scholar]
- 34.Rajadurai CV, et al. 5′-Inositol phosphatase SHIP2 recruits Mena to stabilize invadopodia for cancer cell invasion. J Cell Biol. 2016;214:719–734. doi: 10.1083/jcb.201501003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weidmann MD, et al. MenaINV dysregulates cortactin phosphorylation to promote invadopodium maturation. Sci Rep. 2016;6:36142. doi: 10.1038/srep36142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buckanovich RJ, et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. 2008;14:28–36. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]
- 37.Kandalaft LE, Facciabene A, Buckanovich RJ, Coukos G. Endothelin B receptor, a new target in cancer immune therapy. Clin Cancer Res. 2009;15:4521–4528. doi: 10.1158/1078-0432.CCR-08-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim SJ, et al. Antivascular therapy for multidrug-resistant ovarian tumors by macitentan, a dual endothelin receptor antagonist. Transl Oncol. 2012;5:39–47. doi: 10.1593/tlo.11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.