Significance
The wide variety of functional trait combinations among the world’s coral faunas can be represented by just a few dimensions of variation. The diversity of coral traits among these dimensions is consistently high along Pacific and Indian Ocean diversity gradients, despite a threefold decline in species richness (from approximately 600 to 200 species). Functional redundancy, defined as multiple species sharing similar arrays of traits, is highest in the central Indo-Pacific biodiversity hotspot. While these Indo-Pacific provinces are globally important reserves of coral reef resilience and function, peripheral species-poor regions are potentially more vulnerable to functional collapse, as indicated by a critical lack of redundancy among species and the reduced capacity for similar species to respond differently to chronic or acute stressors.
Keywords: species richness, functional diversity, functional redundancy, biogeography, resilience
Abstract
Corals are major contributors to a range of key ecosystem functions on tropical reefs, including calcification, photosynthesis, nutrient cycling, and the provision of habitat structure. The abundance of corals is declining at multiple scales, and the species composition of assemblages is responding to escalating human pressures, including anthropogenic global warming. An urgent challenge is to understand the functional consequences of these shifts in abundance and composition in different biogeographical contexts. While global patterns of coral species richness are well known, the biogeography of coral functions in provinces and domains with high and low redundancy is poorly understood. Here, we quantify the functional traits of all currently recognized zooxanthellate coral species (n = 821) in both the Indo-Pacific and Atlantic domains to examine the relationships between species richness and the diversity and redundancy of functional trait space. We find that trait diversity is remarkably conserved (>75% of the global total) along latitudinal and longitudinal gradients in species richness, falling away only in species-poor provinces (n < 200), such as the Persian Gulf (52% of the global total), Hawaii (37%), the Caribbean (26%), and the East-Pacific (20%), where redundancy is also diminished. In the more species-poor provinces, large and ecologically important areas of trait space are empty, or occupied by just a few, highly distinctive species. These striking biogeographical differences in redundancy could affect the resilience of critical reef functions and highlight the vulnerability of relatively depauperate, peripheral locations, which are often a low priority for targeted conservation efforts.
The species composition and biodiversity of ecosystems are increasingly responding to human activity (1), highlighting the urgent need to manage and preserve ecosystem functions (2, 3). A key challenge is to understand the components of biodiversity that contribute to essential functions, and that support their resilience to chronic and acute stressors (4). The influence of biodiversity on ecosystem function is underpinned not by species richness per se but by the diversity of functional roles among species, as measured by their characteristics or traits (2, 5, 6). A diverse range of functional roles (functional diversity) is critical for maintaining multiple functions (2, 6) and for the sustainable provision of ecosystem services to people (7).
Loss of functional diversity can cause major shifts in ecosystem function (7–9). This loss of function can be avoided, however, if each functional role is supported by multiple species, each with different responses to anthropogenic or natural change (10). Groups of species with overlapping functional roles generate functional redundancy and provide a chance for declining species to be replaced by other similar species, thereby maintaining certain functions (10, 11). The stabilizing effect of functional redundancy is often described as ecosystem reliability (12), the insurance effect (13), or the portfolio effect (14), and it has been documented in a wide range of ecosystems (5, 15, 16). On coral reefs, for example, herbivory is shared among a diverse range of species, including some that are susceptible to overfishing (e.g., parrotfish) and others that are not heavily targeted (e.g., sea urchins). In the Caribbean, the decline of herbivory due to overfishing was ameliorated because a redundant species, the sea urchin Diadema antillarum, maintained this key process (15), thereby providing a “reservoir of resilience” (5). For this source of resilience to take effect, redundant species must show different tolerances to environmental stressors or different regeneration rates after perturbation, a phenomenon often referred to as response diversity (17). However, overreliance on a smaller group of tolerant species can reduce resilience, a scenario that occurred on many Caribbean reefs when D. antillarum populations were drastically reduced by disease (15).
Coral reef assemblages differ in species richness at global and provincial scales (18–20). Their biogeography is characterized by a global biodiversity hotspot in the central Indo-Pacific (the Coral Triangle); by decreasing diversity with increasing latitudinal and longitudinal distance from this hotspot; and by a secondary, less diverse hotspot in the Caribbean (18–20). Such gradients in species richness can alter biogeographical pools of functional trait diversity and redundancy, and therefore potentially influence the resilience of different provinces (11). Quantifying the diversity of functional traits along gradients of increasing species richness can reveal the extent to which additional species provide new functions or increase the number of redundant species supporting the same functions (21–23). Furthermore, although functional redundancy is likely to be common in tropical ecosystems, a critical question is whether redundancy is restricted to a subset of functions, leaving other functions supported by just one, or a few, unique species (9, 24).
Reef-building corals (in the order Scleractinia) are often dominant contributors to a range of ecological (25, 26), biogeochemical (27), structural (28), and geological (29) functions on coral reefs. This complex interplay of functions influences some of the defining features of coral reefs, such as reef growth and development, productivity and nutrient recycling, and the provision of habitat to other reef-associated species. As coral assemblages respond to escalating human stressors (3, 11), critical reef functions are being impaired, including calcification (30) and the provision of three-dimensional reef structure (31). Understanding the potential functional roles of corals is an essential task that must consider a large number of species and a large number of functionally relevant traits. In addition, since many traits, including morphological dimensions and physiological rates, fall along a continuum, quantifying the diversity and importance of species’ functional roles must move beyond categorical groups, and instead use quantitative estimates of trait-based dissimilarity (32).
In this study, we provide a comprehensive analysis of the functional traits of all extant reef-building coral species. Our aim is to quantify how the functional diversity and redundancy of corals change with species richness across 12 biogeographical provinces in both the Indo-Pacific and Atlantic domains. Using seven quantitative traits that facilitate key ecological functions, we generate a 7D trait space in which species are positioned according to their functional dissimilarity (32). In this trait space, we examine the global patterns of functional diversity (the range of unique trait combinations) and functional redundancy (the number of species sharing similar sets of traits), testing the relationship of each with species richness. Our analysis focuses on functional redundancy at multiple levels of trait-based dissimilarly, allowing us to identify locations and functions where redundancy is critically lacking. Finally, we conduct an analysis of specific traits that influence dispersal and regeneration, and of additional traits that influence reef productivity and growth, providing insights into the potential for the occurrence of response diversity among functionally similar species.
Results
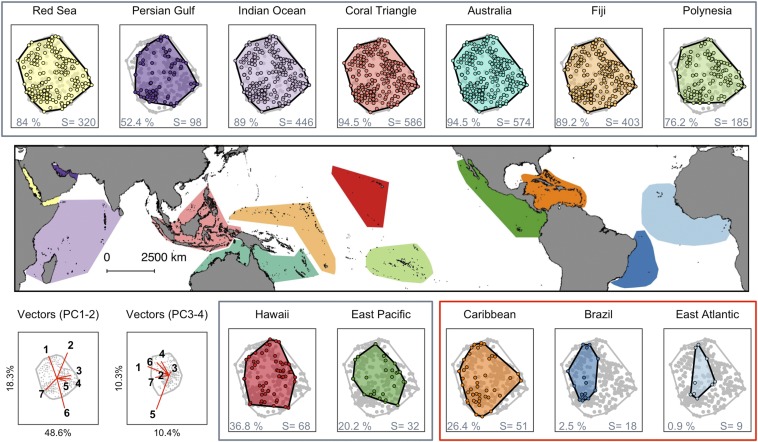
A principal component analysis (PCA) of the global trait space for 821 species of corals, based on seven functional traits (Table S1), reveals four significant axes of correlated trait variation, with 67% of variation expressed in just two dimensions and 88% in four dimensions. Along PCA axis 1 (48.6% of variation explained), coral species are positioned from small, slow-growing taxa with large corallites and dome-shaped morphologies to large fast-growing taxa with small corallites and complex morphologies. Along PCA axis 2 (18.3% of variation explained), coral species are positioned from short taxa with large surface areas and high skeletal densities to tall taxa with small surface areas and low skeletal densities (vector plot axes 1 and 2 in Fig. 1). Skeletal densities and maximum colony sizes also load heavily onto PCA axes 3 and 4, respectively (vector plot axes 3 and 4 in Fig. 1). The periphery of the four dimensions is occupied by taxa with rare or extreme trait values, and individual species (points in Fig. 1 and Fig. S1) are dispersed widely between these outer boundaries, leaving few areas of the trait space unoccupied. Thus, our analysis shows that a wide variety of unique trait combinations in corals can be condensed into just a few axes of variation representing major dimensions of coral functional diversity.
Fig. 1.
PCA of the trait space of corals across biogeographically distinct provinces. In each panel, the global multidimensional trait space for all corals is presented in gray, with each provincial trait space overlaid in color. Colored points represent the positions of species along PCA axis 1 [principal component 1 (PC1) and and PCA axis 2 (PC2). Values in each panel indicate the percentage of occupancy of the 4D global trait space and species richness (S) for each province. (Bottom Left) Seven traits used to construct the PCA and the four axes making up the trait space are shown in the two panels along with their percentage of explained variance. The trait vectors are (1) skeletal density, (2) surface area-to-volume ratio, (3) growth rate, (4) interstitial space size, (5) maximum colony size, (6) colony height, and (7) corallite width.
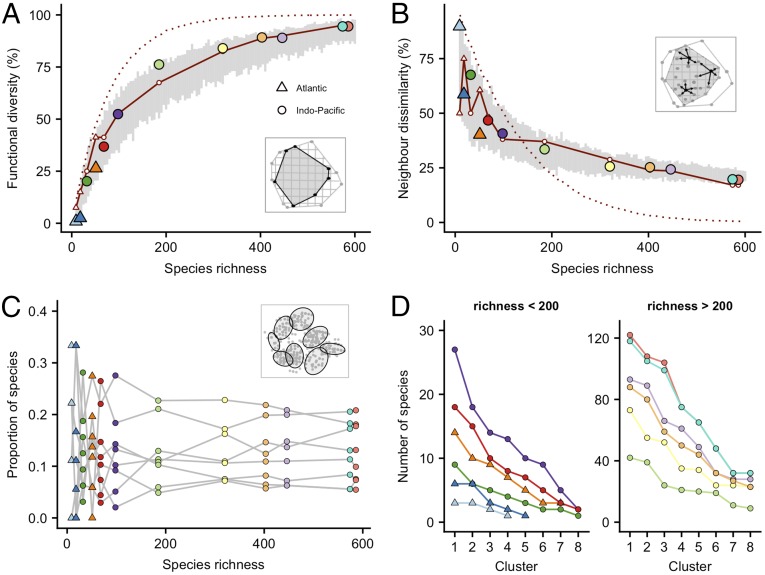
The percentage occupancy of the global coral trait space by the species pool in the Atlantic is small, even in the Caribbean hotspot, where only 26% of the trait space is occupied. The Brazilian and West African provinces occupy only 3% and 1% of trait space, respectively. In the Indo-Pacific, trait diversity is greatest in the Coral Triangle, where 95% of the global range of traits occurs. Trait diversity is largely maintained across five other provinces stretching from the Red Sea and western Indian Ocean, and eastward to Polynesia in the central Pacific (Fig. 1 and Fig. S1). These five provinces each contain >75% of the global functional diversity, despite a decrease from 586 coral species in the Coral Triangle to 320 westward across the Indian Ocean and a drop to 185 species eastward across the Pacific Ocean (Figs. 1 and 2A). In contrast, as species richness declines further in more peripheral Indo-Pacific provinces, functional diversity diminishes sharply, indicating that smaller regional species pools support a relatively depauperate mix of functional traits (Figs. 1 and 2A). Consequently, our analysis reveals an asymptotic relationship between species richness and trait diversity (Fig. 2A), consistent with a null model in which provincial species pools are randomly assembled from the global species pool (gray bootstrapped confidence limits in Fig. 2A).
Fig. 2.
Relationship between species richness and functional diversity (A), species dissimilarity (B), imbalances in trait space (C), and redundancy in trait space (D) across major biogeographical provinces. In A and B, colored points represent 12 provinces with the corresponding color code from Fig. 1. Functional diversity is measured as the percentage of occupancy of the global trait space hypervolume in each province. Species dissimilarity is measured as the average distance between species and their closest neighbors in trait space (a visual representation of each metric is shown in gray boxes). Gray bars show a null model of random species allocation for each species richness value, indicating the mean and 95% confidence intervals of 100 iterations. Solid red lines indicate an alternative analysis of 80 fine-scale clusters of species (or functional entities), indicating the proportion of clusters occupied by species (A) and the proportion of occupied clusters that contain only one species (B). Dotted red lines indicate the same metrics under a null model of random assignment of species to clusters. In C and D, colored points represent eight coarse-scale clusters of species in trait space, colored by province (the positioning of clusters in trait space is shown in the gray box). Imbalances in trait space are indicated by the proportional representation of each major cluster in the species pool of each province, with gray lines linking the same clusters across provinces. Redundancy in trait space is indicated by the number of species occupying major clusters in each provincial trait space (colored lines), plotted in rank-descending order along the x axis from high to low redundancy.
The average distance between neighboring species in multidimensional trait space is markedly lower in provinces with higher species richness, signifying a closer degree of similarity, or more redundancy, between species (Fig. 2B). These differences in average species similarity are heavily influenced by clustering in trait space. For the global species pool, we identified 80 fine-scale clusters of highly similar species, or “functional entities.” The number of clusters occurring in each province increases asymptotically with species richness (solid red line, Fig. 2A). In addition, the proportion of clusters in each province represented by just one species decreases with species richness (solid red line, Fig. 2B). In the Caribbean, for example, 38% (30 of 80) of the clusters are represented. Furthermore, 65% of the clusters in the Caribbean are represented by just one unique species (i.e., the remaining 35% have redundancy), while only 18% of the clusters in the Coral Triangle are represented by a single species (82% exhibit redundancy) (Fig. 2B). Thus, redundant species in species-rich provinces are spread widely throughout multidimensional trait space, whereas in depauperate regions, limited levels of redundancy are necessarily more restricted to smaller portions of the total trait space (Fig. 2B). Notably, the number of occupied clusters and the proportion of single-species clusters in each province differ from a null model in which species are randomly assigned to clusters (dotted red lines, Fig. 2 A and B). This disparity between our observed results and null expectations is highest in the Indo-Pacific, where species are confined to smaller areas of trait space (Fig. 2A) and packed into fewer clusters in trait space [i.e., there are more single-species clusters (Fig. 2B) than expected if species-cluster associations are randomized].
A broader cluster analysis of trait space reveals eight distinct clusters and shows that major disparities exist in the density of species across large areas of trait space (Figs. 2 C and D and 3). These imbalances are most prominent in species-poor provinces (n < 200), where large clusters in trait space are heavily underrepresented (Fig. 2C) and supported by just one or a few species (Fig. 2D). In contrast, in species-rich provinces (n > 200), imbalances across trait space are less extreme due to higher levels of redundancy in all clusters. These larger areas of trait space represent key morphological types, including mound-like corals of various sizes; two-dimensional corals, including solitary and nonattached species; plate-like or foliose corals; digitate or tabular corals; branching corals; and tall, complex mounds or columnar corals (Fig. 3). The proportional representation in provincial species pools of each of the major clusters is constrained within a narrow range of values across the Indo-Pacific (varying between 0.05 and 0.25, depending on the cluster) (Fig. 2C), where each major cluster is supported by tens or even hundreds of species (Fig. 2D). Furthermore, the proportion of the species pool represented in each cluster is remarkably constant across a very broad gradient in species richness (Fig. 2C).
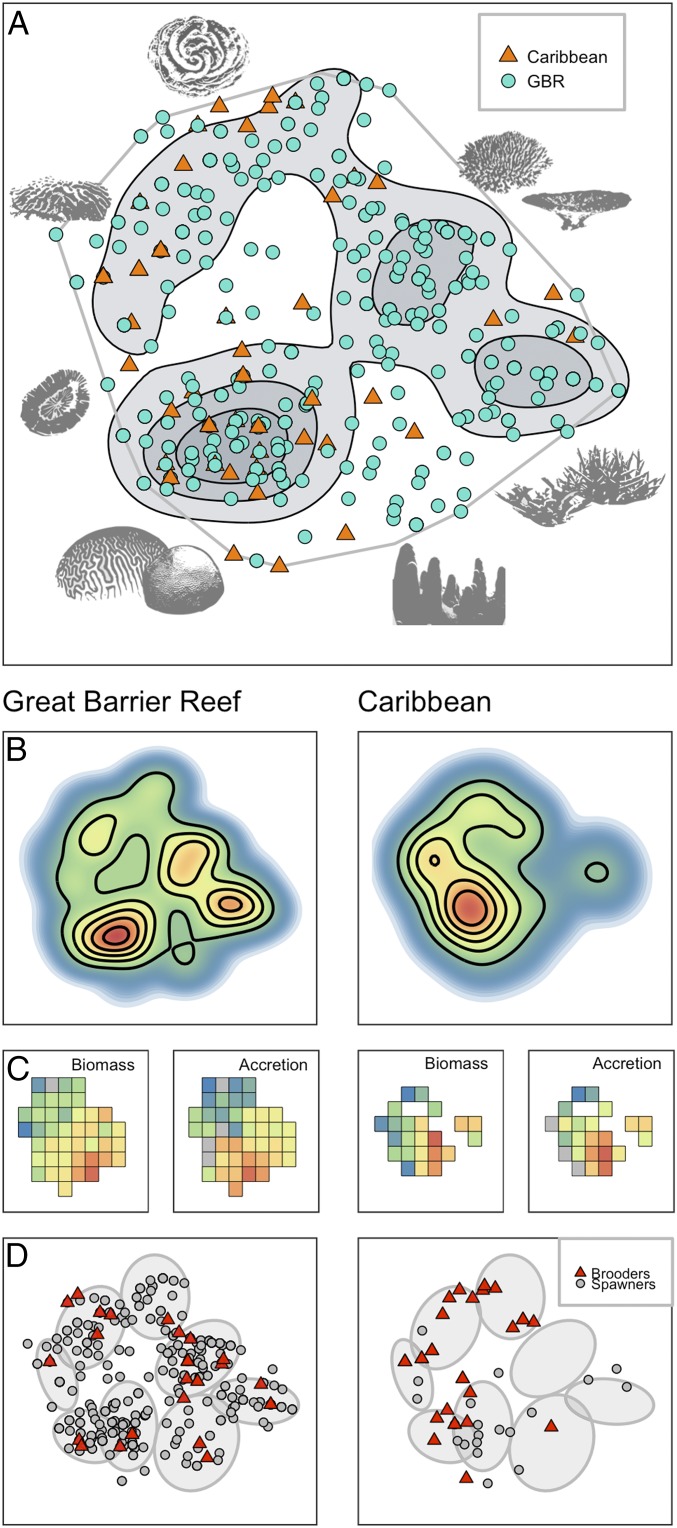
Fig. 3.
Diversity and redundancy of trait space on the Great Barrier Reef and in the Caribbean. (A) Coral trait space, with species (points) colored blue for the Great Barrier Reef and orange for the Caribbean, showing the overlap in trait space between the two domains, and contours of high species density shown in gray. (B) Heat map of species density in the global trait space for each domain, with contours indicating peaks of richness and similarity. (C) Heat map for each domain of two functional traits, the total tissue biomass and skeletal accretion rates of colonies. The squares represent the portion of 80 fine-scale species clusters or functional entities that are occupied in each province (gray indicates deficient data). (D) Distribution of reproductive modes in trait space in both domains. The ellipses illustrate eight coarse-scale species clusters.
The occupancy of trait space is strikingly different between the two major domains, as exemplified by the disparity between the Caribbean and the Great Barrier Reef (Fig. 3). The two domains have no native species in common (18), yet they share a range of functional roles, because Caribbean species occupy similar areas of trait space to those on the Great Barrier Reef, despite an 11-fold difference in species richness (Figs. 1 and 3A). Contours of high redundancy in trait space (based on kernel density estimation) for the Great Barrier Reef indicate that the highest redundancy occurs in three major clusters of species that are predominantly mound-shaped, tabular, or digitate, and branching (Fig. 3 A and B). These clusters represent a broad range of traits for >200 species with a wide range of taxonomic affiliations. In contrast, the Caribbean trait space is heavily depleted, with only one high-redundancy cluster of massive and submassive corals, no fast-growing digitate, bushy, or tabular corals, and only three species of branching corals (Acropora palmata, Acropora cervicornis, and Acropora prolifera) (Fig. 3 A and B). The clustering of species in the Caribbean trait space (Fig. 2 B–D) therefore leaves large portions of trait space unoccupied or populated by just one or a few functionally distinctive species.
The distinct biogeographical patterns of diversity and redundancy in coral trait space can have major consequences for the dynamics of coral assemblage functions in different provinces. In particular, critical attributes underlying reef resilience and function are poorly represented in the Caribbean trait space (Fig. 3 C and D). For example, two fundamental traits, the tissue biomass and skeletal growth rates of whole colonies, reach their highest overall value among species with complex morphologies and fast-growth rates, or with very large mound-like or columnar skeletons (Fig. 3C). In the Caribbean, corals with these attributes (e.g., Acropora, Dendrogyra, Orbicella) are poorly represented in trait space (Fig. 3 A–C). In contrast, on the Great Barrier Reef, corals with these attributes comprise a diverse mix of taxa (e.g., Acropora, Porites, Pavona, Diploastrea) and are widely distributed across trait space (Fig. 3 A–C). Furthermore, a key trait, the mode of larval development (i.e., brooders, spawners), is distributed differently in trait space between the two domains (Fig. 3D). Although there are proportionally more brooding species in the Caribbean, they are limited to a subset of functional types and devoid of large, branching or columnar species. In contrast, both brooding and spawning strategies are widely distributed across the Great Barrier Reef trait space, indicating that a diversity of dispersal and recruitment patterns occurs across a broad range of coral functional types in this region (Fig. 3).
Discussion
This study reveals that a diverse variety of coral functional trait combinations can be represented along the same few axes of correlated trait variation. The boundaries of these axes differ between the two Indo-Pacific and Atlantic domains, and along regional-scale gradients in reef biodiversity. Provinces with higher species richness exhibit a greater range of traits and trait combinations, in addition to a greater similarity, or redundancy, among species. Moreover, the proportion of coral species in different hotspots of trait space is remarkably consistent across the major provinces of the Indo-Pacific, consistent with their high species richness and their uniform taxonomic composition at the family level (19). Like reef fish (24), all Indo-Pacific provinces contain a mix of high-redundancy clusters of species with similar traits, alongside an unexpectedly high number of distinctive species that are relatively isolated in trait space. For corals, however, peripheral provinces with comparatively low species richness are particularly low in redundancy, because major functional roles are supported by just a few, unique species occupying large areas of trait space. This lack of functional redundancy is critical, because it can reduce the collective potential of groups of similar species to resist or recover from a variety of stressors (5, 11).
The broader functional roles of corals, as measured here by numerous morphological and life history traits, correspond to previously used groupings based on colony shape (11, 33) (Fig. 3A), highlighting the functional relevance of colony morphology and the intrinsic association of numerous morphological and physiological traits (34–38). By analyzing a more comprehensive range of traits, however, we reveal a greater dissimilarity between species, creating a more accurate and quantitative depiction of the potential functional roles of species. Trait diversity is high in most Indo-Pacific provinces, making them even greater hotspots of functional diversity than previously assumed (11). Some Indo-Pacific species break the rules of conventional trait associations, and therefore occupy remote corners of trait space where there are fewer species than expected by chance. For example, they are species with unusually low skeletal densities, large corallites on branches, or enormous colony sizes. In depauperate provinces, the most unique species are often the sole representatives of large areas of trait space, thereby upholding critical functions, such as reef productivity, carbonate accretion, and habitat complexity. For example, A. palmata, a species that was once abundant but is now considered to be in danger, is the tallest three-dimensional coral in the Caribbean. Its decline has dramatically reduced rates of reef accretion and the provision of habitat on shallow reefs (30, 31). Declines in such unique and essential species can severely compromise reef function with little hope of compensation by other, dissimilar species.
How biogeographical differences in trait diversity and redundancy translate into differences in ecosystem function depends on how regional pools of species assemble at local scales, accounting for the abundance and trait variability of individual species, in addition to noncoral taxa with similar functional roles (e.g., calcifying algae). Clearly, coral reefs can develop and flourish even in depauperate provinces with low functional diversity. For example, Clipperton Atoll, in the remote East Pacific, has only seven species of corals, and assemblages there are dominated by mound-shaped Porites (39), highlighting the ability of just a few species to maintain a broad array of functions sufficient to sustain a coral reef. In addition, reef growth and development in the Atlantic has persisted independent of a 10-fold variation in coral species richness over the past 28 million years (40). Nevertheless, many studies highlight the importance of high coral functional diversity, for example, to maintain complex variations in microhabitat (41) and for the provision of habitats and resources used by different fish and invertebrate species (42). Measuring redundancy therefore requires a careful consideration of the functions of interest, and of the resolution of a trait-based analysis necessary to distinguish functionally important and redundant species. Our analysis measures the similarity between species at two scales: fine-scale clustering of 80 aggregates and coarser scale similarities within eight hotspots of trait similarity. At both scales, redundancy is substantially lower in depauperate regions, highlighting their vulnerability to major shifts in function, in addition to more intricate shifts that may otherwise go unrecognized.
For redundancy to enhance the resilience of high-richness regions compared with depauperate ones, redundant species must exhibit response diversity (i.e., have different tolerances to environmental change) or have different regeneration capacities after a perturbation (17). Numerous traits influence species responses, including resistance to stress, reproductive capacity, dispersal ability, and growth rate. However, low redundancy in key groups can reduce the diversity of these traits and limit the extent of response diversity among species (4, 43). Indeed, the poor representation of key reproductive modes in large areas of Caribbean trait space may be a liability, since it may reduce the potential for response diversity when only one reproductive mode (brooding or broadcast spawning) is dominant. Low morphological diversity in large areas of Caribbean trait space may also limit the diversity of tolerances to mechanical disturbances (e.g., storms) among functionally similar species (38). Response diversity is common in highly redundant marine and terrestrial ecosystems (5, 15, 16, 33). Nevertheless, the stabilizing influence of response diversity becomes weaker as the severity of multiple stressors increases. For example, response diversity within similar guilds of terrestrial plants (44) and tropical birds (45) diminishes under land-use intensification. Thus, a key challenge for coral research is to understand the role of response diversity among functionally similar species, especially in the context of multiple chronic and acute stressors, and to identify the traits that enhance the resistance or recovery of assemblages.
Coral reefs face an uncertain future, and, already, the goal of returning degraded reefs to their original state is no longer an option in many cases. Instead, the global challenge in the face of climate change is to maintain reefs in a way that preserves their ecological functions, recognizing that the species composition is already changing rapidly (3). Across the world’s reefs’ provinces, there is an increasing prevalence of heavily impacted coral assemblages, where more tolerant or regenerative species are favored (46). In many regions, the extent of shifts in ecosystem functions or the prospects of returning to a normal functioning state are unknown. Ultimately, the degree of functional transition by reefs depends on shifts in the abundance of corals and on the level of similarity between persistent and declining species. The critical task of understanding and preserving reef function rests on our comprehension of these phenomena, including the wide range of functional traits among species and the vulnerability of reef functions within and across biogeographical regions.
Materials and Methods
Coral Trait Space.
Seven traits were selected for their functional importance: growth rate, skeletal density, corallite width, maximum colony size, colony height, interstitial space size, and surface area-to-volume ratio (Table S1). Mean trait scores for every zooxanthella coral species (n = 876) were obtained from the Coral Trait Database (47), and subsequently placed into numerical (range: 1–5) categories (Table S1). Three ordinal morphological traits (colony height, interstitial space size, and surface area-to-volume ratio) were assigned to species based on their morphological types and a simplified model of coral geometry (37) (Table S2). The Coral Trait Database includes most of the empirical data from the literature. However, deficiencies in the data remain for certain traits. To ensure that nearly every known reef-building coral species was included in the analysis, a regression approach (37) was used to fill in missing data for four traits: growth rates (131 empirical values), colony sizes (348 empirical values), skeletal densities (54 empirical values), and corallite width (842 empirical values). Using log-transformed trait values, a linear model was run for each trait against predictor variables using the lm function in R (R Development Core Team 2017). Since each of these traits is known to be phylogenetically (37) and morphologically (34, 36) conserved, the predictor variables chosen were molecular family and growth form for growth rates and skeletal densities and molecular family and growth rates for corallite widths and maximum colony sizes. The predictive functions were then used to return model estimates for each species. The strength of the predictive functions is demonstrated by their ability to accurately predict known empirical values from the trait database (Fig. S2). Combinations that were not predicted by the function were based on the taxonomy and growth form of a species. Unknown reproductive modes (brooding or broadcast spawning) were also predicted, as reproductive modes in corals are generally conserved among congeners, except for well-known exceptions such as Porites and Pocillopora. After infilling was completed, taxa that are not associated with reef habitats were subsequently removed from the dataset, leaving 821 species.
Occurrence Data.
Species pools for distinct Indo-Pacific provinces separated by faunal boundaries were based on the system used by Keith et al. (48). Atlantic provinces were based on the system used by Veron (18).
Functional Trait Diversity and Redundancy.
Trait diversity was measured as the 4D convex hull volume of trait space, signifying the outer boundary of trait space, or the most extreme trait values (49). Four PCA dimensions were used to maximize the amount of variation explained by functional diversity metrics (88%). Provincial values were divided by the global convex hull to get a percentage occupancy of trait space. Neighbor similarity is based on the sum of nearest neighbor distances for the nearest five species, calculated using the R package “FNN” (R Development Core Team 2017). This metric was then averaged over all species and presented for each province. Assigning different numbers of neighbors did not change the results. Fine-scale clustering of species in trait space was calculated by binning species coordinates in the global trait space, with a consistent bin width of 0.6 (resulting in 80 global clusters). The proportion of clusters with only one species was then quantified (24). Broader aggregations of species in trait space were derived from a clustering analysis, in which the optimal number of clusters (k = 8) was determined from the Bayesian information criterion for a k-means clustering algorithm. For each analysis, a null model was created to compare observed provincial values with a random sample from the global species pool. Samples were taken without replacement, with species richness fixed at a specific level (each increment of five species from 0 to 600). Analyses were repeated on each sample 100 times before presenting the mean and 95% confidence interval for the replicate samples.
Tissue Biomass and Skeletal Accretion.
Absolute values for these two key traits at the colony level were estimated using a simple model of colony geometry (Table S2), standardized by empirical values of maximum size, growth rates, and skeletal density for Caribbean and Great Barrier Reef species. Tissue biomass (in grams) was measured by multiplying estimates of tissue biomass (per square centimeter) by maximum colony area (Table S2). Whole-colony skeletal accretion rates (in grams per year) were estimated by finding the difference in maximum colony volume after 1 y of uninterrupted growth and multiplying by skeletal density. Initial colony volumes were calculated using models of colony geometry (Table S2) calibrated by maximum colony diameter minus 1 y of linear growth (estimated using empirical growth rates in millimeters per year). Final colony volumes were calculated using the same models of colony geometry calibrated by maximum colony diameter.
Supplementary Material
Acknowledgments
We thank Tom C. Bridge for assistance with data compilation and mapping, and two anonymous reviewers for their helpful comments and suggestions. This study received support from the Australian Research Council’s Centre of Excellence Program and a Laureate Fellowship (to T.P.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1716643115/-/DCSupplemental.
References
- 1.McGill BJ, Dornelas M, Gotelli NJ, Magurran AE. Fifteen forms of biodiversity trend in the Anthropocene. Trends Ecol Evol. 2015;30:104–113. doi: 10.1016/j.tree.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Cardinale BJ, et al. Biodiversity loss and its impact on humanity. Nature. 2012;486:59–67, and erratum (2012) 489:326. doi: 10.1038/nature11148. [DOI] [PubMed] [Google Scholar]
- 3.Hughes TP, et al. Coral reefs in the Anthropocene. Nature. 2017;546:82–90. doi: 10.1038/nature22901. [DOI] [PubMed] [Google Scholar]
- 4.Oliver TH, et al. Biodiversity and resilience of ecosystem functions. Trends Ecol Evol. 2015;30:673–684. doi: 10.1016/j.tree.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Walker B, Kinzig A, Langridge J. Original articles: Plant attribute diversity, resilience, and ecosystem function: The nature and significance of dominant and minor species. Ecosystems. 1999;2:95–113. [Google Scholar]
- 6.Reiss J, Bridle JR, Montoya JM, Woodward G. Emerging horizons in biodiversity and ecosystem functioning research. Trends Ecol Evol. 2009;24:505–514. doi: 10.1016/j.tree.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Díaz S, et al. Incorporating plant functional diversity effects in ecosystem service assessments. Proc Natl Acad Sci USA. 2007;104:20684–20689. doi: 10.1073/pnas.0704716104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Symstad AJ, Tilman D, Willson J, Knops JMH. Species loss and ecosystem functioning: Effects of species identity and community composition. Oikos. 1998;81:389–397. [Google Scholar]
- 9.Bellwood DR, Hoey AS, Choat JH. Limited functional redundancy in high diversity systems: Resilience and ecosystem function on coral reefs. Ecol Lett. 2003;6:281–285. [Google Scholar]
- 10.Walker B. Conserving biological diversity through ecosystem resilience. Conserv Biol. 1995;9:747–752. [Google Scholar]
- 11.Bellwood DR, Hughes TP, Folke C, Nyström M. Confronting the coral reef crisis. Nature. 2004;429:827–833. doi: 10.1038/nature02691. [DOI] [PubMed] [Google Scholar]
- 12.Naeem S. Species redundancy and ecosystem reliability. Conserv Biol. 1998;12:39–45. [Google Scholar]
- 13.Yachi S, Loreau M. Biodiversity and ecosystem productivity in a fluctuating environment: The insurance hypothesis. Proc Natl Acad Sci USA. 1999;96:1463–1468. doi: 10.1073/pnas.96.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tilman D, Reich PB, Knops JMH. Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature. 2006;441:629–632. doi: 10.1038/nature04742. [DOI] [PubMed] [Google Scholar]
- 15.Hughes TP. Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science. 1994;265:1547–1551. doi: 10.1126/science.265.5178.1547. [DOI] [PubMed] [Google Scholar]
- 16.Steneck RS, et al. Kelp forest ecosystems: Biodiversity, stability, resilience and future. Environ Conserv. 2002;29:436–459. [Google Scholar]
- 17.Elmqvist T, et al. Response diversity, ecosystem change, and resilience. Front Ecol Environ. 2003;1:488–494. [Google Scholar]
- 18.Veron JEN. Corals in Space and Time: The Biogeography and Evolution of the Scleractinia. Cornell Univ Press; Ithaca, NY: 1995. [Google Scholar]
- 19.Bellwood DR, Hughes TP. Regional-scale assembly rules and biodiversity of coral reefs. Science. 2001;292:1532–1535. doi: 10.1126/science.1058635. [DOI] [PubMed] [Google Scholar]
- 20.Stehli FG, Wells JW. Diversity and age patterns in hermatypic corals. Syst Biol. 1971;20:115–126. [Google Scholar]
- 21.Ricklefs RE. Species richness and morphological diversity of passerine birds. Proc Natl Acad Sci USA. 2012;109:14482–14487. doi: 10.1073/pnas.1212079109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swenson NG, et al. Constancy in functional space across a species richness anomaly. Am Nat. 2016;187:E83–E92. doi: 10.1086/685083. [DOI] [PubMed] [Google Scholar]
- 23.Lamanna C, et al. Functional trait space and the latitudinal diversity gradient. Proc Natl Acad Sci USA. 2014;111:13745–13750. doi: 10.1073/pnas.1317722111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mouillot D, et al. Functional over-redundancy and high functional vulnerability in global fish faunas on tropical reefs. Proc Natl Acad Sci USA. 2014;111:13757–13762. doi: 10.1073/pnas.1317625111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buss L, Jackson JBC. Competitive networks: Nontransitive competitive relationships in cryptic coral reef environments. Am Nat. 1979;113:223–234. [Google Scholar]
- 26.Jackson JBC. Adaptation and diversity of reef corals. Bioscience. 1991;41:475–482. [Google Scholar]
- 27.Done TJ, Ogden JC, Wiebe WJ, Rosen BR. Functional Roles of Biodiversity: A Global Perspective. John Wiley; New York: 1996. Biodiversity and ecosystem function on coral reefs. [Google Scholar]
- 28.Lawton JH. What do species do in ecosystems? Oikos. 1994;71:367–374. [Google Scholar]
- 29.Stoddart DR. Ecology and morphology of recent coral reefs. Biol Rev Camb Philos Soc. 1969;44:433–498. [Google Scholar]
- 30.Perry CT, et al. Caribbean-wide decline in carbonate production threatens coral reef growth. Nat Commun. 2013;4:1402. doi: 10.1038/ncomms2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alvarez‐Filip L, Cote IM, Gill JA, Watkinson AR, Dulvy NK. Region‐wide temporal and spatial variation in Caribbean reef architecture: Is coral cover the whole story? Glob Change Biol. 2011;17:2470–2477. [Google Scholar]
- 32.Petchey OL, Gaston KJ. Functional diversity: Back to basics and looking forward. Ecol Lett. 2006;9:741–758. doi: 10.1111/j.1461-0248.2006.00924.x. [DOI] [PubMed] [Google Scholar]
- 33.Denis V, Ribas-Deulofeu L, Sturaro N, Kuo CY, Chen CA. A functional approach to the structural complexity of coral assemblages based on colony morphological features. Sci Rep. 2017;7:9849. doi: 10.1038/s41598-017-10334-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hughes TP. Skeletal density and growth form of corals. Mar Ecol Prog Ser. 1987;35:259–266. [Google Scholar]
- 35.Hoogenboom M, Rottier C, Sikorski S, Ferrier-Pagès C. Among-species variation in the energy budgets of reef-building corals: Scaling from coral polyps to communities. J Exp Biol. 2015;218:3866–3877. doi: 10.1242/jeb.124396. [DOI] [PubMed] [Google Scholar]
- 36.Pratchett MS, et al. Spatial, temporal and taxonomic variation in coral growth—Implications for the structure and function of coral reef ecosystems. Oceanogr Mar Biol. 2015;53:215–295. [Google Scholar]
- 37.Madin JS, et al. A trait-based approach to advance coral reef science. Trends Ecol Evol. 2016;31:419–428. doi: 10.1016/j.tree.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Madin JS, Connolly SR. Ecological consequences of major hydrodynamic disturbances on coral reefs. Nature. 2006;444:477–480. doi: 10.1038/nature05328. [DOI] [PubMed] [Google Scholar]
- 39.Glynn PW, Veron JEN, Wellington GM. Clipperton Atoll (eastern Pacific): Oceanography, geomorphology, reef-building coral ecology and biogeography. Coral Reefs. 1996;15:71–99. [Google Scholar]
- 40.Johnson KG, Jackson JBC, Budd AF. Caribbean reef development was independent of coral diversity over 28 million years. Science. 2008;319:1521–1523. doi: 10.1126/science.1152197. [DOI] [PubMed] [Google Scholar]
- 41.Vytopil E, Willis B. Epifaunal community structure in Acropora spp. (Scleractinia) on the Great Barrier Reef: Implications of coral morphology and habitat complexity. Coral Reefs. 2001;20:281–288. [Google Scholar]
- 42.Shirayama Y, Horikoshi M. A new method of classifying the growth form of corals and its application to a field survey of coral-associated animals in Kabira Cove, Ishigaki Island. J Oceanogr Soc Jpn. 1982;38:193–207. [Google Scholar]
- 43.Suding KN, et al. Scaling environmental change through the community‐level: A trait‐based response‐and‐effect framework for plants. Glob Change Biol. 2008;14:1125–1140. [Google Scholar]
- 44.Laliberté E, et al. Land-use intensification reduces functional redundancy and response diversity in plant communities. Ecol Lett. 2010;13:76–86. doi: 10.1111/j.1461-0248.2009.01403.x. [DOI] [PubMed] [Google Scholar]
- 45.Karp DS, Ziv G, Zook J, Ehrlich PR, Daily GC. Resilience and stability in bird guilds across tropical countryside. Proc Natl Acad Sci USA. 2011;108:21134–21139. doi: 10.1073/pnas.1118276108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Darling ES, McClanahan TR, Côté IM. Life histories predict coral community disassembly under multiple stressors. Glob Change Biol. 2013;19:1930–1940. doi: 10.1111/gcb.12191. [DOI] [PubMed] [Google Scholar]
- 47.Madin JS, et al. The Coral Trait Database, a curated database of trait information for coral species from the global oceans. Sci Data. 2016;3:160017, and erratum (2017) 4:170174. doi: 10.1038/sdata.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keith SA, Baird AH, Hughes TP, Madin JS, Connolly SR. Faunal breaks and species composition of Indo-Pacific corals: The role of plate tectonics, environment and habitat distribution. Proc Biol Sci. 2013;280:20130818. doi: 10.1098/rspb.2013.0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cornwell WK, Schwilk LD, Ackerly DD. A trait-based test for habitat filtering: Convex hull volume. Ecology. 2006;87:1465–1471. doi: 10.1890/0012-9658(2006)87[1465:attfhf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.