Significance
Recently many crystals have been reported to show various mechanical responses when triggered by stimuli (heat, light, and pressure). Such properties are attributed to polymorphic transitions, conformational changes, packing changes, isomerizations, or chemical reactions inside the crystal and the consequent strain developed between parent and daughter phases. The role of molecular chirality in such macroscopic phenomena is yet to be established. In the present study we found that crystals of dipeptide LL undergo right-handed twisting and those of its enantiomer, dipeptide DD, undergo left-handed twisting upon heat-induced topochemical azide–alkyne cycloaddition. This study provides clear evidence for the role of molecular chirality in controlling the direction of macroscopic twisting of crystals.
Keywords: chirality, mechanoresponsive crystals, topochemical reactions, twisting, spontaneous
Abstract
Crystals that show mechanical response against various stimuli are of great interest. These stimuli induce polymorphic transitions, isomerizations, or chemical reactions in the crystal and the strain generated between the daughter and parent domains is transcribed into mechanical response. We observed that the crystals of modified dipeptide LL (N3-l-Ala-l-Val-NHCH2C≡CH) undergo spontaneous twisting to form right-handed twisted crystals not only at room temperature but also at 0 °C over time. Using various spectroscopic techniques, we have established that the twisting is due to the spontaneous topochemical azide–alkyne cycloaddition (TAAC) reaction at room temperature or lower temperatures. The rate of twisting can be increased by heating, exploiting the faster kinetics of the TAAC reaction at higher temperatures. To address the role of molecular chirality in the direction of twisting the enantiomer of dipeptide LL, N3-d-Ala-d-Val-NHCH2C≡CH (DD), was synthesized and topochemical reactivity and mechanoresponse of its crystals were studied. We have found that dipeptide DD not only underwent TAAC reaction, giving 1,4-triazole–linked pseudopolypeptides of d-amino acids, but also underwent twisting with opposite handedness (left-handed twisting), establishing the role of molecular chirality in controlling the direction of mechanoresponse. This paper reports (i) a mechanical response due to a thermal reaction and (ii) a spontaneous mechanical response in crystals and (iii) explains the role of molecular chirality in the handedness of the macroscopic mechanical response.
There is tremendous interest in the study of stimuli-induced molecular level changes leading to macroscopic mechanical motion or changes in crystals. Such mechanically responsive crystals are expected to find use in a variety of practical applications (1–3). Various stimuli such as light, heat, and pressure induce conformational changes, packing changes, isomerizations, or chemical reactions inside the crystal. Such molecular-level responses to stimuli often result in the development of local packing strain between the parent and daughter domains (4, 5). Relaxation of such strain can manifest in a mechanical response at the macroscopic level (whole crystal) (1). There are two pathways of relaxation leading to different kinds of mechanical responses: (i) slow and continuous relaxation of strain, as and when generated, by mechanical reconfiguration of the crystal leading to bending (2, 4–31), twisting (6, 10, 14, 32, 33), coiling (16, 34), shape change (35), or size change (7, 25, 31, 36–38) or (ii) sudden relaxation leading to stimuli-salient effects (2, 9, 12, 39–50) such as jumping, hopping, exploding, displacement, and so on, after accumulation of stress over an induction period, to a critical level. All of the known mechanical responses in crystals are due to (i) photoisomerizations (16, 24–31, 34, 43), (ii) photochemical reactions (2, 5–23, 32, 33, 35–37, 39–42), (iii) pressure-induced change in molecular packing (44, 51–57), or (iv) heat-induced polymorphic transitions (4, 38, 44–50). As many photochemical processes (photochemical reactions or photoisomerizations) are reversible, light-induced mechanical responses in crystals are often reversible. To the best of our knowledge there is no mechanical response reported as a result of thermal reaction. Also, the role of molecular chirality in macroscopic mechanical behavior of a crystal has not been addressed so far. Herein, we report a spontaneous twisting of the crystals due to a thermal cycloaddition reaction between azide and alkyne and also the role of molecular chirality in dictating the direction of twisting.
Results and Discussion
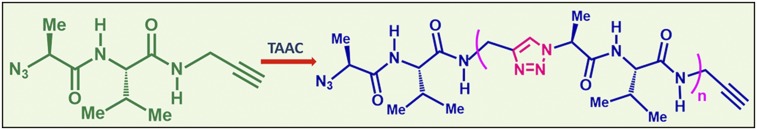
Recently we reported a method for the synthesis of pseudopolypeptides by the topochemical polymerization of a small peptide (58, 59). Dipeptide LL (N3-l-Ala-l-Val-NHCH2C≡CH) has the complimentary reactive motifs azide and alkyne at its termini crystallized in parallel β-sheet packing, which brings the azide and the alkyne motifs of adjacent molecules to proximity. The rectangular plate-like crystals of LL upon heating at 85 °C underwent crystal-to-crystal topochemical azide–alkyne cycloaddition (TAAC) reaction, forming 1,4-triazole–linked pseudoproteins (58) (Fig. 1).
Fig. 1.
Crystal-to-crystal topochemical oligomerization of dipeptide LL to triazole-linked oligopeptides.
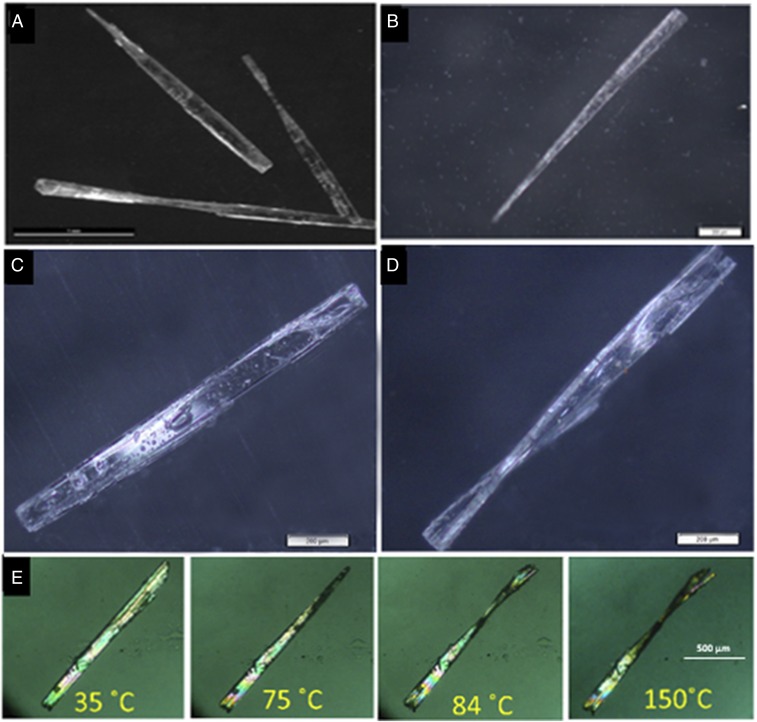
We have serendipitously noticed that the crystals of dipeptide LL stored at room temperature for several weeks showed twisted morphology. However, freshly made crystals of LL were not twisted and showed normal rectangular plate-like morphology. This suggested that the crystals of LL undergo spontaneous twisting at ambient conditions. To confirm this, crystals of freshly crystallized dipeptide LL were kept at room temperature and were monitored regularly. To our surprise, these crystals were slowly transformed to twisted crystals over a period of 1 mo (Fig. 2A). Also, it was observed that the extent of twisting increased with time. More interestingly, the twisting happens even at 0 °C, though after a long time. Thus, crystals of LL kept at 0 °C for 3–4 mo underwent twisting (Fig. 2B). Various spectroscopic analyses and other characterizations [IR, NMR, powder X-ray diffraction (PXRD), and differential scanning calorimetry] revealed that the crystals had undergone a spontaneous topochemical reaction to give oligomers (SI Appendix, Fig. S1) and that the TAAC reaction was the cause of twisting of the crystals.
Fig. 2.
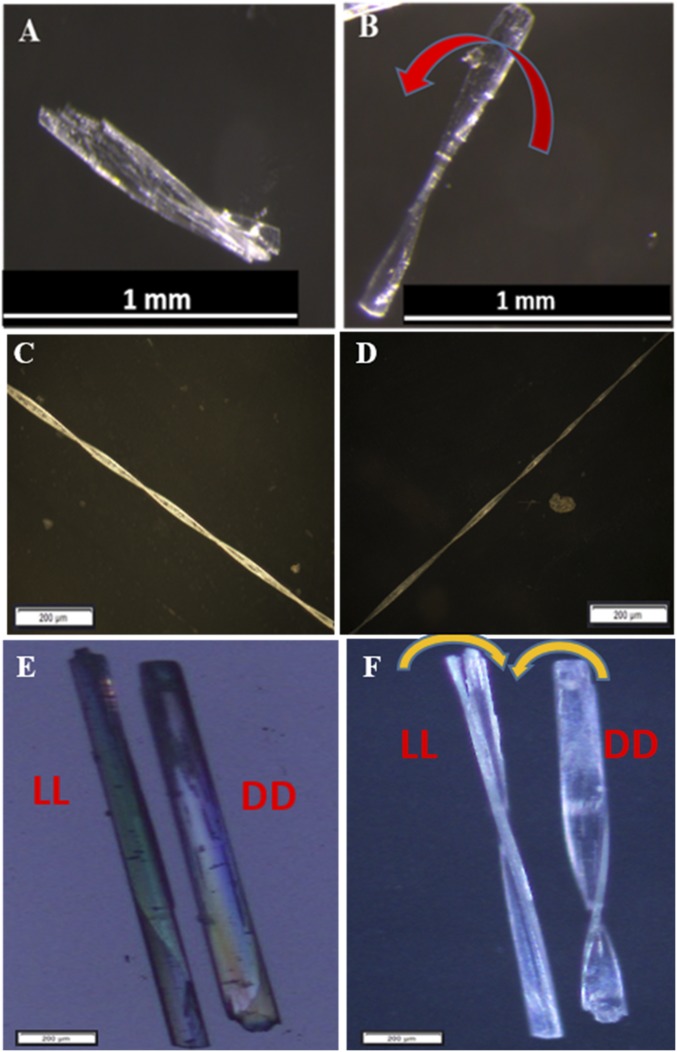
Photographs of crystals of dipeptide LL (A) after storage at rt for 1 mo, showing twisted morphology (scale bar: 1 mm) and (B) after storage at 0 °C for 3 mo (scale bar: 200 µm). Optical microscopic image of the crystals of dipeptide LL (C) before (scale bar: 200 µm) and (D) after TAAC at 85 °C for 3 d (scale bar: 200 µm). (E) Hot-stage polarizing microscopic images of crystals during heating from 35 °C to 150 °C showing gradual twisting. (Scale bar: 500 µm.)
This previously unnoticed, interesting phenomenon of spontaneous TAAC reaction and the consequent twisting at room temperature (rt) motivated us to investigate whether such a mechanical response can happen during the thermally activated TAAC reaction of LL, leading to the formation of higher oligomers. We heated a freshly crystallized sample of the dipeptide LL at 85 °C for 3 d, and these crystals were imaged using optical polarizing microscopy (Fig. 2 C and D). As expected, all of the crystals were twisted without losing their birefringence (SI Appendix, Fig. S2 A–D). This suggests that their crystalline nature is maintained even after twisting.
Hot-stage polarizing microscopic study revealed that the twisting happens gradually (Movie S1). We heated a crystal of LL gradually in a hot-stage polarizing microscope at a heating rate of 10 °C/min (Fig. 2E). The crystal started twisting without losing its birefringence at 75 °C and continued twisting up to 90 °C. Further heating did not result in more twisting, but the birefringence of these crystals completely disappeared at 150 °C, suggesting the loss of crystallinity at this temperature; 1H NMR of these heated crystals showed 10% conversion (reaction). This suggests that the twisting of the crystals occurs as a result of topochemical reaction (SI Appendix, Fig. S4). Cooling of the twisted crystals did not show any untwisting (SI Appendix, section 3C and Fig. S3). This irreversible nature of the mechanical response is not surprising as the TAAC reaction, the basic process responsible for the mechanoresponse, is irreversible. This is in contrast to photoinduced twisting in crystals, which is reversible.
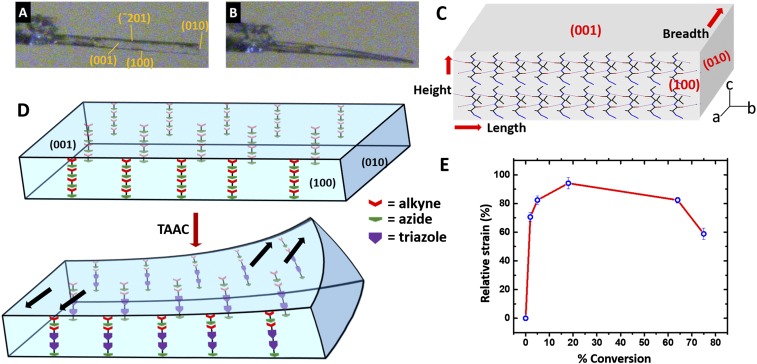
To correlate the direction of the TAAC reaction with twisting, crystal-face indexing was done (SI Appendix, Fig. S7). The (100), (010), (001), and (−¯201) planes were identified as various faces of the crystal (Fig. 3C). A probable mechanism for twisting may be similar to the one proposed by Naumov et al. (1). It is clear from the face indexing that the reaction occurs along the height (c-direction) of the crystal. Since the reaction involves the formation of covalent linkages, there will be a reduction in the thickness of the crystal. Upon heating, molecules at the heat-exposed face of the crystal would react faster, thereby reducing d-spacing between the (001) planes present at the exposed face (shrinking of the crystal along the c-direction or height of the crystal) while the d-spacing at the unexposed (unreacted) part remains intact (Fig. 3D). This asymmetric distribution of product and reactant domains (layers) due to the nonuniform thermal stimuli leads to a layered bimorphic structure and generates interfacial strain between the layers (10). Also, the energy released in the reaction, the reduction in the thickness (height) of the crystal due to the cycloaddition reaction along that dimension, and the change in the crystal volume would contribute to the overall strain. To release this strain the crystal undergoes a gradual relaxation by ribbon twisting along the length of the crystal contemporaneously with the reaction (Fig. 3D). It is noteworthy that in such layered bimorphic structures ribbon twisting is a predicted mode of relaxation (10, 32, 60). As the reactive motifs, azide and alkyne, are not in a reactive orientation in the monomer crystal, for the reaction to happen the monomer molecules have to twist to align the reactive motifs parallely, and this molecular motion (twisting) of the homochiral monomer would influence the direction of the macroscopic twisting. As the polymerization/oligomerization reaction leads to oligomers/polymers of different sizes the heterometry in the crystal would be sustained. This might also account for the irreversibility of the twisting.
Fig. 3.
(A) Photograph of the LL crystal and face indices before twisting of the crystal (0.250 mm × 0.080 mm × 0.030 mm). (B) Appearance of the crystal (0.250 mm × 0.080 mm × 0.030 mm) after heating at 85 °C for 30 min on the goniometer. (C) Representation of a long rectangular plate-like crystal with various planes. Directions of TAAC reaction (c) and β-sheet formation (b) are also shown. (D) Schematic representation of generation of asymmetric distribution of product and reactant domains (layers) due to the nonuniform thermal stimuli and subsequent twisting. The triazole motif is color-coded as a violet pentagon, unreacted azide as a green arrowhead, and alkyne as red arrow tail (arrow indicates plausible direction of strain generated during the reaction). (E) Plot of the change in strain with the progress of the TAAC reaction (percent conversion) at 85 °C for LL (error bar shown in relative strain is obtained by the SD method).
Williamson–Hall strain analysis (61, 62) of the crystals of dipeptide LL revealed that they are inherently strained (SI Appendix, section 9B). Time-dependent strain analysis showed that the strain increases initially with time and later decreases, presumably due to the release of strain through mechanical reconfiguration of the crystal. The inherent strain of the parent crystal could be one of the contributing factors to the spontaneous nature of the TAAC reaction. As the 1,3-dipolar cycloaddition reaction between azide and alkyne is highly exothermic (ΔH0 between −50 and −65 kcal/mol) (63), the energy released in the crystal lattice and the packing strain developed between the product (daughter) phase and unreacted reactant (parent) phase would increase the total strain further. Once a threshold energy (strain) is reached the crystal may undergo the mechanical response (twisting) to release the strain (Fig. 3E).
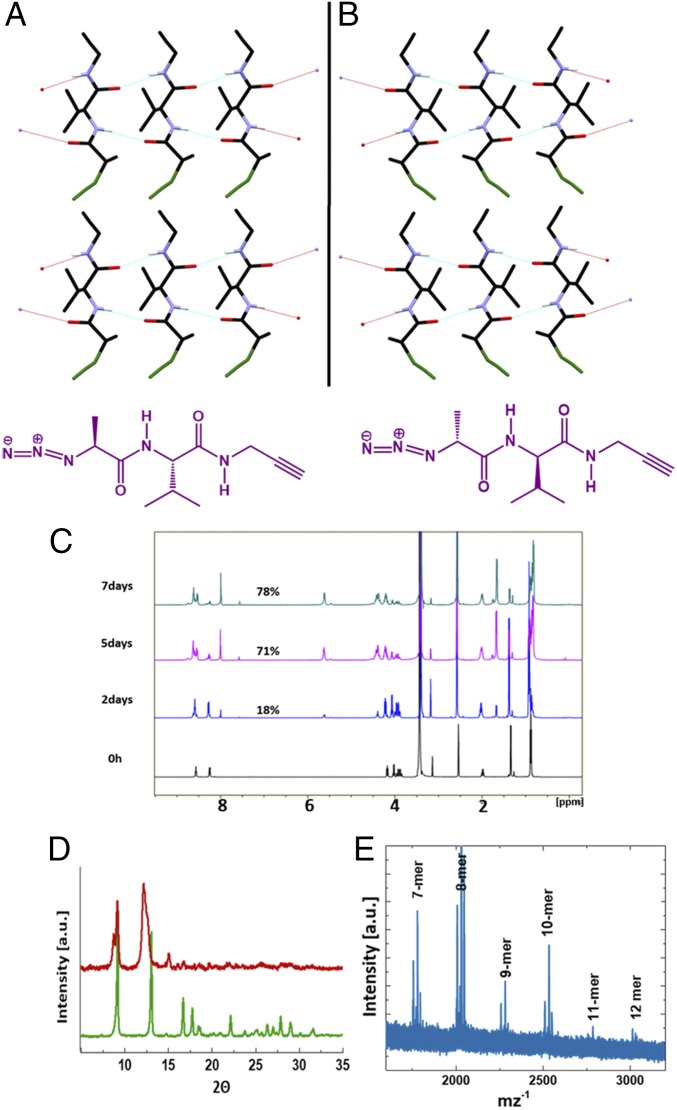
The role of molecular chirality in the macroscopic mechanical response is an interesting but unexplored topic (50). The crystals of dipeptide LL consistently showed right-handed helicity in twisting. To investigate the correlation of molecular chirality with the handedness of twisting we synthesized the dipeptide DD (N3-d-Ala-d-Val-NHCH2C≡CH), the enantiomer of dipeptide LL (SI Appendix). Single-crystal XRD analysis of DD showed a crystal structure which is the mirror image of the crystal structure of LL, as anticipated (Fig. 4 A and B). As expected, the dipeptide DD not only underwent a TAAC reaction, giving oligomers/polymers (up to 12-mers) akin to LL (Fig. 4 C–E and SI Appendix, Figs. S14 and S15), but also its crystals underwent twisting both at rt and even under heating (Fig. 5 A and B and Movie S2) as a consequence of the TAAC reaction.
Fig. 4.
(A) Comparison of crystal packing arrangement of LL and DD, viewed along the bc plane. (B) Chemical structures. (C) Time-dependent 1H NMR spectra of DD crystals undergoing TAAC reaction at 80 °C. (D) PXRD comparison of DD before and after TAAC reaction. (E) MALDI-TOF spectrum of dipeptide DD heated at 80 °C for 7 d.
Fig. 5.
Optical microscopic images of a crystal of dipeptide DD (A) kept at rt for 1 mo; (B) after heating at 85 °C for 1 d, showing the left-handed twisted crystal; and (C and D) after heating longer crystal at 85 °C for more than 2 d, forming four and seven twists, respectively. Optical microscopic images of crystals of dipeptide LL and DD kept together (E) before heating and (F) after heating at 85 °C for 1 d. The arrows indicate the handedness of twist in each case. (Scale bars in C–F: 200 µm.)
Interestingly, the crystals of DD twisted with handedness opposite to that of LL. When a crystal of LL and a crystal of DD were heated together the enantiomeric crystals underwent twisting with opposite handedness (Fig. 5 E and F, Movie S3, and SI Appendix, Figs. S16 and S17). This clearly demonstrates that the molecular chirality dictates the direction of mechanical response. Such a handle to control the direction of mechanical response is of utmost importance for practical applications.
The size (36) and shape (6, 10, 14, 64–68) of crystals are two important bulk parameters which influence the mechanical response of a crystal. To study the effect of crystal size on the mechanical response (twisting) of dipeptide LL we selected two crystals of different sizes, a thin crystal (2.7 mm × 0.07 mm × 0.015 mm) and a thick crystal (2.7 mm × 0.13 mm × 0.036 mm). Both the crystals were heated at 85 °C and imaged through optical microscopy. The thin crystal twisted completely within a day (Fig. 6 A and B), whereas the thick crystal required prolonged heating for 3 d to undergo considerable twisting (Fig. 6 C–E). Although this suggests that the kinetics of twisting depends on the size, it is noteworthy that even large crystals undergo twisting. The influence of crystal length on the twisting was further investigated by monitoring the twisting of several crystals (of DD and LL) of different lengths heated at 85 °C for 5 d. It revealed that longer crystals underwent more twists with time, whereas shorter ones formed a few twists (SI Appendix, Fig. S18 and Table S2). It was also found that the rate of twisting is slow at lower conversion and increases rapidly at higher conversion (SI Appendix, Fig. S19). The energy required to twist the representative sample crystals by 360° was calculated (69) to be 34.5–62.8 kJ/mol (Fig. 6F and SI Appendix). This energy can be easily obtained from the heat released (−250 kJ/mol) due to the TAAC reaction (SI Appendix, Table S3). Also, plots of angle of twist versus the energy required to twist for crystals (of various dimensions) showed the influence of crystal dimensions on the ease of twisting.
Fig. 6.
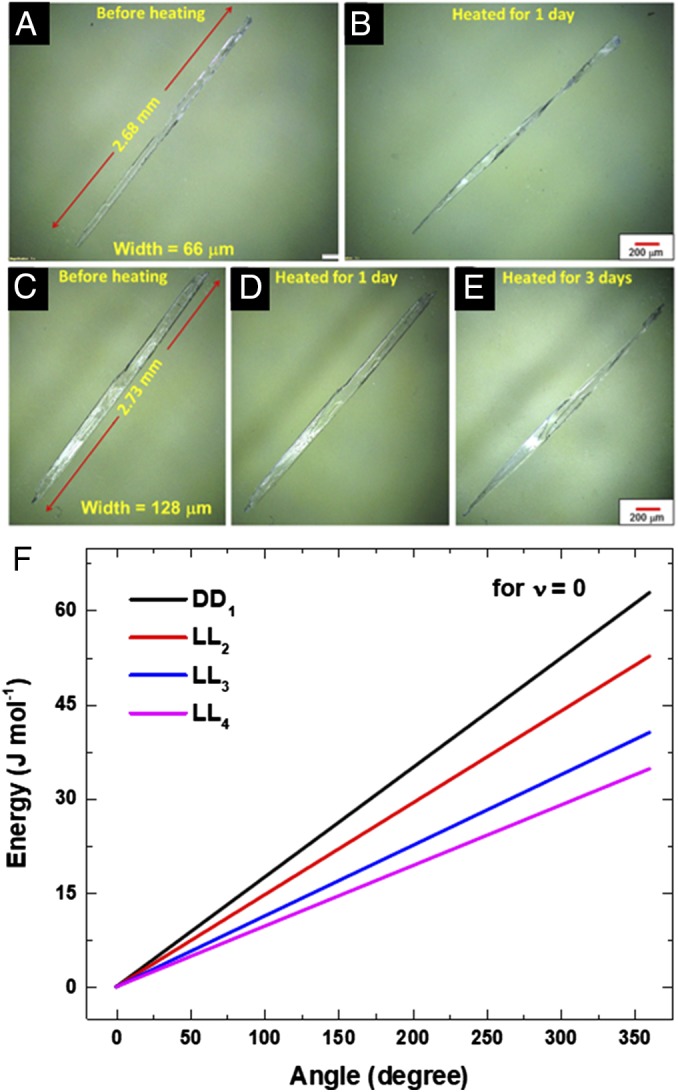
Optical microscopic images of thinner crystal (A) before and (B) after heating at 85 °C for 1 d. Optical microscopic images of thicker crystal (C) before and after heating for (D) 1 d and (E) for 3 d. (F) Plot of energy versus angle of twist of various dipeptide crystals (DD and LL) having different dimensions. (Scale bar: 200 µm.)
Conclusions
We have presented a report of a spontaneous twisting of crystals not only at room temperature but also at 0 °C as a consequence of spontaneous TAAC reaction. The crystals of dipeptide LL undergo right-handed helical twisting, whereas its enantiomer dipeptide, DD, undergoes left-handed twisting. The inherent strain in the crystals triggers the TAAC reaction, and this reaction involves considerable rearrangement of molecules within the crystal lattice, which leads to further packing strain. To release the strain the crystal undergoes twisting in a direction dictated by the molecular chirality. This report shows a mechanical response by a crystal (i) that occurs spontaneously and (ii) is triggered by a thermal topochemical reaction and (iii) whose directionality is controlled by the molecular chirality. This study showing a trigger to initiate mechanoresponse and chirality as a tool to bias the direction of mechanoresponse might be useful for designing smart materials.
Supplementary Material
Acknowledgments
We thank Mr. Alex for his help in crystal-face indexing and the Indian Institute of Space Science and Technology Thiruvananthapuram for the use of the hot-stage microscopy facility. This work was supported by a Department of Science and Technology Ramanujan Fellowship and a Swarnajayanti Fellowship (to K.M.S.) and by a University Grant Commission Fellowship (to B.P.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates have been deposited in the Cambridge Structural Database, Cambridge Crystallographic Data Centre, Cambridge CB2 1EZ, United Kingdom (CSD reference no. CCDC1527669).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1718965115/-/DCSupplemental.
References
- 1.Naumov P, Chizhik S, Panda MK, Nath NK, Boldyreva E. Mechanically responsive molecular crystals. Chem Rev. 2015;115:12440–12490. doi: 10.1021/acs.chemrev.5b00398. [DOI] [PubMed] [Google Scholar]
- 2.Nath NK, Panda MK, Sahoo SC, Naumov P. Thermally induced and photoinduced mechanical effects in molecular single crystals—A revival. CrystEngComm. 2014;16:1850–1858. [Google Scholar]
- 3.Lan T, Chen W. Hybrid nanoscale organic molecular crystals assembly as a photon-controlled actuator. Angew Chem Int Ed Engl. 2013;52:6496–6500. doi: 10.1002/anie.201300856. [DOI] [PubMed] [Google Scholar]
- 4.Shima T, et al. Thermally driven polymorphic transition prompting a naked-eye-detectable bending and straightening motion of single crystals. Angew Chem Int Ed Engl. 2014;53:7173–7178. doi: 10.1002/anie.201402560. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Garibay MA. Molecular crystals on the move: From single-crystal-to-single-crystal photoreactions to molecular machinery. Angew Chem Int Ed Engl. 2007;46:8945–8947. doi: 10.1002/anie.200702443. [DOI] [PubMed] [Google Scholar]
- 6.Kim T, Zhu L, Mueller LJ, Bardeen CJ. Dependence of the solid-state photomechanical response of 4-chlorocinnamic acid on crystal shape and size. CrystEngComm. 2012;14:7792–7799. [Google Scholar]
- 7.Kobatake S, Takami S, Muto H, Ishikawa T, Irie M. Rapid and reversible shape changes of molecular crystals on photoirradiation. Nature. 2007;446:778–781. doi: 10.1038/nature05669. [DOI] [PubMed] [Google Scholar]
- 8.Morimoto M, Irie M. A diarylethene cocrystal that converts light into mechanical work. J Am Chem Soc. 2010;132:14172–14178. doi: 10.1021/ja105356w. [DOI] [PubMed] [Google Scholar]
- 9.Naumov P, et al. Topochemistry and photomechanical effects in crystals of green fluorescent protein-like chromophores: Effects of hydrogen bonding and crystal packing. J Am Chem Soc. 2010;132:5845–5857. doi: 10.1021/ja100844m. [DOI] [PubMed] [Google Scholar]
- 10.Kim T, Zhu L, Mueller LJ, Bardeen CJ. Mechanism of photoinduced bending and twisting in crystalline microneedles and microribbons composed of 9-methylanthracene. J Am Chem Soc. 2014;136:6617–6625. doi: 10.1021/ja412216z. [DOI] [PubMed] [Google Scholar]
- 11.Al-Kaysi RO, Bardeen CJ. Reversible photoinduced shape changes of crystalline organic nanorods. Adv Mater. 2007;19:1276–1280. [Google Scholar]
- 12.Naumov P, Sahoo SC, Zakharov BA, Boldyreva EV. Dynamic single crystals: Kinematic analysis of photoinduced crystal jumping (the photosalient effect) Angew Chem Int Ed Engl. 2013;52:9990–9995. doi: 10.1002/anie.201303757. [DOI] [PubMed] [Google Scholar]
- 13.Sun JK, et al. Photoinduced bending of a large single crystal of a 1,2-bis(4-pyridyl)ethylene-based pyridinium salt powered by a [2+2] cycloaddition. Angew Chem Int Ed Engl. 2013;52:6653–6657. doi: 10.1002/anie.201301207. [DOI] [PubMed] [Google Scholar]
- 14.Zhu L, Al-Kaysi RO, Bardeen CJ. Reversible photoinduced twisting of molecular crystal microribbons. J Am Chem Soc. 2011;133:12569–12575. doi: 10.1021/ja201925p. [DOI] [PubMed] [Google Scholar]
- 15.Terao F, Morimoto M, Irie M. Light-driven molecular-crystal actuators: Rapid and reversible bending of rodlike mixed crystals of diarylethene derivatives. Angew Chem Int Ed Engl. 2012;51:901–904. doi: 10.1002/anie.201105585. [DOI] [PubMed] [Google Scholar]
- 16.Al-Kaysi RO, et al. Chemical reaction method for growing photomechanical organic microcrystals. CrystEngComm. 2015;17:8835–8842. [Google Scholar]
- 17.Nakai H, et al. Photoinduced bending of rod-like millimetre-size crystals of a rhodium dithionite complex with n-pentyl moieties. Chem Commun (Camb) 2016;52:4349–4352. doi: 10.1039/c6cc00059b. [DOI] [PubMed] [Google Scholar]
- 18.Zhu L, et al. Improved solid-state photomechanical materials by fluorine substitution of 9-anthracene carboxylic acid. Chem Mater. 2014;26:6007–6015. [Google Scholar]
- 19.Ohshima S, Morimoto M, Irie M. Light-driven bending of diarylethene mixed crystals. Chem Sci (Camb) 2015;6:5746–5752. doi: 10.1039/c5sc01994j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitagawa D, Tanaka R, Kobatake S. Photoinduced stepwise bending behavior of photochromic diarylethene crystals. CrystEngComm. 2016;18:7236–7240. [Google Scholar]
- 21.Kitagawa D, Kobatake S. Photoreversible current ON/OFF switching by the photoinduced bending of gold-coated diarylethene crystals. Chem Commun (Camb) 2015;51:4421–4424. doi: 10.1039/c5cc00355e. [DOI] [PubMed] [Google Scholar]
- 22.Zhu L, Al-Kaysi RO, Bardeen CJ. Photoinduced ratchet-like rotational motion of branched molecular crystals. Angew Chem Int Ed Engl. 2016;55:7073–7076. doi: 10.1002/anie.201511444. [DOI] [PubMed] [Google Scholar]
- 23.Kitagawa D, Tanaka R, Kobatake S. Dependence of photoinduced bending behavior of diarylethene crystals on irradiation wavelength of ultraviolet light. Phys Chem Chem Phys. 2015;17:27300–27305. doi: 10.1039/c5cp03073k. [DOI] [PubMed] [Google Scholar]
- 24.Nath NK, et al. Model for photoinduced bending of slender molecular crystals. J Am Chem Soc. 2014;136:2757–2766. doi: 10.1021/ja4101497. [DOI] [PubMed] [Google Scholar]
- 25.Koshima H, Ojima N, Uchimoto H. Mechanical motion of azobenzene crystals upon photoirradiation. J Am Chem Soc. 2009;131:6890–6891. doi: 10.1021/ja8098596. [DOI] [PubMed] [Google Scholar]
- 26.Taniguchi T, Fujisawa J, Shiro M, Koshima H, Asahi T. Mechanical motion of chiral azobenzene crystals with twisting upon photoirradiation. Chemistry. 2016;22:7950–7958. doi: 10.1002/chem.201505149. [DOI] [PubMed] [Google Scholar]
- 27.Bushuyev OS, Singleton TA, Barrett CJ. Fast, reversible, and general photomechanical motion in single crystals of various azo compounds using visible light. Adv Mater. 2013;25:1796–1800. doi: 10.1002/adma.201204831. [DOI] [PubMed] [Google Scholar]
- 28.Bushuyev OS, Corkery TC, Barrett CJ, Friscic T. Photo-mechanical azobenzene cocrystals and in situX-ray diffraction monitoring of their optically-induced crystal-to-crystal isomerisation. Chem Sci (Camb) 2014;5:3158–3164. [Google Scholar]
- 29.Bushuyev OS, et al. Azo···phenyl stacking: A persistent self-assembly motif guides the assembly of fluorinated cis-azobenzenes into photo-mechanical needle crystals. Chem Commun (Camb) 2016;52:2103–2106. doi: 10.1039/c5cc08590j. [DOI] [PubMed] [Google Scholar]
- 30.Bushuyev OS, Tomberg A, Friščić T, Barrett CJ. Shaping crystals with light: Crystal-to-crystal isomerization and photomechanical effect in fluorinated azobenzenes. J Am Chem Soc. 2013;135:12556–12559. doi: 10.1021/ja4063019. [DOI] [PubMed] [Google Scholar]
- 31.Irie M. Photochromism of diarylethene single molecules and single crystals. Photochem Photobiol Sci. 2010;9:1535–1542. doi: 10.1039/c0pp00251h. [DOI] [PubMed] [Google Scholar]
- 32.Kitagawa D, Nishi H, Kobatake S. Photoinduced twisting of a photochromic diarylethene crystal. Angew Chem Int Ed Engl. 2013;52:9320–9322. doi: 10.1002/anie.201304670. [DOI] [PubMed] [Google Scholar]
- 33.Shtukenberg AG, Freudenthal J, Kahr B. Reversible twisting during helical hippuric acid crystal growth. J Am Chem Soc. 2010;132:9341–9349. doi: 10.1021/ja101491n. [DOI] [PubMed] [Google Scholar]
- 34.Kim T, Al-Muhanna MK, Al-Suwaidan SD, Al-Kaysi RO, Bardeen CJ. Photoinduced curling of organic molecular crystal nanowires. Angew Chem Int Ed Engl. 2013;52:6889–6893. doi: 10.1002/anie.201302323. [DOI] [PubMed] [Google Scholar]
- 35.Kuroki L, Takami S, Yoza K, Morimoto M, Irie M. Photoinduced shape changes of diarylethene single crystals: Correlation between shape changes and molecular packing. Photochem Photobiol Sci. 2010;9:221–225. doi: 10.1039/b9pp00093c. [DOI] [PubMed] [Google Scholar]
- 36.Al-Kaysi RO, Müller AM, Bardeen CJ. Photochemically driven shape changes of crystalline organic nanorods. J Am Chem Soc. 2006;128:15938–15939. doi: 10.1021/ja064535p. [DOI] [PubMed] [Google Scholar]
- 37.Zhu L, et al. Solid-state photochemical and photomechanical properties of molecular crystal nanorods composed of anthracene ester derivatives. J Mater Chem. 2011;21:6258–6268. [Google Scholar]
- 38.Yao ZS, et al. Molecular motor-driven abrupt anisotropic shape change in a single crystal of a Ni complex. Nat Chem. 2014;6:1079–1083. doi: 10.1038/nchem.2092. [DOI] [PubMed] [Google Scholar]
- 39.Natarajan A, et al. The photoarrangement of α-santonin is a single-crystal-to-single-crystal reaction: A long kept secret in solid-state organic chemistry revealed. J Am Chem Soc. 2007;129:9846–9847. doi: 10.1021/ja073189o. [DOI] [PubMed] [Google Scholar]
- 40.Commins P, et al. Structure-reactivity correlations and mechanistic understanding of the photorearrangement and photosalient effect of α-santonin and its derivatives in solutions, crystals, and nanocrystalline suspensions. Cryst Growth Des. 2015;15:1983–1990. [Google Scholar]
- 41.Medishetty R, et al. Single crystals popping under UV light: A photosalient effect triggered by a [2+2] cycloaddition reaction. Angew Chem Int Ed Engl. 2014;53:5907–5911. doi: 10.1002/anie.201402040. [DOI] [PubMed] [Google Scholar]
- 42.Medishetty R, Sahoo SC, Mulijanto C, Naumov P, Vittal JJ. Photosalient behavior of photoreactive crystals. Chem Mater. 2015;27:1821–1829. [Google Scholar]
- 43.Uchida E, Azumi R, Norikane Y. Light-induced crawling of crystals on a glass surface. Nat Commun. 2015;6:7310–7317. doi: 10.1038/ncomms8310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghosh S, Mishra MK, Ganguly S, Desiraju GR. Dual stress and thermally driven mechanical properties of the same organic crystal: 2,6-Dichlorobenzylidene-4-fluoro-3-nitroaniline. J Am Chem Soc. 2015;137:9912–9921. doi: 10.1021/jacs.5b05324. [DOI] [PubMed] [Google Scholar]
- 45.Skoko Ž, Zamir S, Naumov P, Bernstein J. The thermosalient phenomenon. “Jumping crystals” and crystal chemistry of the anticholinergic agent oxitropium bromide. J Am Chem Soc. 2010;132:14191–14202. doi: 10.1021/ja105508b. [DOI] [PubMed] [Google Scholar]
- 46.Sahoo SC, Panda MK, Nath NK, Naumov P. Biomimetic crystalline actuators: Structure-kinematic aspects of the self-actuation and motility of thermosalient crystals. J Am Chem Soc. 2013;135:12241–12251. doi: 10.1021/ja404192g. [DOI] [PubMed] [Google Scholar]
- 47.Sahoo SC, et al. Kinematic and mechanical profile of the self-actuation of thermosalient crystal twins of 1,2,4,5-tetrabromobenzene: A molecular crystalline analogue of a bimetallic strip. J Am Chem Soc. 2013;135:13843–13850. doi: 10.1021/ja4056323. [DOI] [PubMed] [Google Scholar]
- 48.Lusi M, Bernstein J. On the propulsion mechanism of “jumping” crystals. Chem Commun (Camb) 2013;49:9293–9295. doi: 10.1039/c3cc45646c. [DOI] [PubMed] [Google Scholar]
- 49.Panda MK, et al. Colossal positive and negative thermal expansion and thermosalient effect in a pentamorphic organometallic martensite. Nat Commun. 2014;5:4811–4819. doi: 10.1038/ncomms5811. [DOI] [PubMed] [Google Scholar]
- 50.Panda MK, Runčevski T, Husain A, Dinnebier RE, Naumov P. Perpetually self-propelling chiral single crystals. J Am Chem Soc. 2015;137:1895–1902. doi: 10.1021/ja5111927. [DOI] [PubMed] [Google Scholar]
- 51.Hayashi S, Koizumi T. Elastic organic crystals of a fluorescent π-conjugated molecule. Angew Chem Int Ed Engl. 2016;55:2701–2704. doi: 10.1002/anie.201509319. [DOI] [PubMed] [Google Scholar]
- 52.Ghosh S, Mishra MK, Kadambi SB, Ramamurty U, Desiraju GR. Designing elastic organic crystals: Highly flexible polyhalogenated N-benzylideneanilines. Angew Chem Int Ed Engl. 2015;54:2674–2678. doi: 10.1002/anie.201410730. [DOI] [PubMed] [Google Scholar]
- 53.Ghosh S, Reddy CM. Elastic and bendable caffeine cocrystals: Implications for the design of flexible organic materials. Angew Chem Int Ed Engl. 2012;51:10319–10323. doi: 10.1002/anie.201204604. [DOI] [PubMed] [Google Scholar]
- 54.Panda MK, et al. Spatially resolved analysis of short-range structure perturbations in a plastically bent molecular crystal. Nat Chem. 2015;7:65–72. doi: 10.1038/nchem.2123. [DOI] [PubMed] [Google Scholar]
- 55.Reddy CM, Kirchner MT, Gundakaram RC, Padmanabhan KA, Desiraju GR. Isostructurality, polymorphism and mechanical properties of some hexahalogenated benzenes: The nature of halogen...halogen interactions. Chemistry. 2006;12:2222–2234. doi: 10.1002/chem.200500983. [DOI] [PubMed] [Google Scholar]
- 56.Jiang L, Hu W, Wie Z, Xu W, Meng H. High-performance organic single-crystal transistors and digital inverters of an anthracene derivative. Adv Mater. 2009;21:3649–3653. [Google Scholar]
- 57.Takamizawa S, Miyamoto Y. Superelastic organic crystals. Angew Chem Int Ed Engl. 2014;53:6970–6973. doi: 10.1002/anie.201311014. [DOI] [PubMed] [Google Scholar]
- 58.Krishnan BP, Rai R, Asokan A, Sureshan KM. Crystal-to-crystal synthesis of triazole-linked pseudo-proteins via topochemical azide-alkyne cycloaddition reaction. J Am Chem Soc. 2016;138:14824–14827. doi: 10.1021/jacs.6b07538. [DOI] [PubMed] [Google Scholar]
- 59.Krishnan BP, Sureshan KM. Topochemical azide-alkyne cycloaddition reaction in gels: Size-tunable synthesis of triazole-linked polypeptides. J Am Chem Soc. 2017;139:1584–1589. doi: 10.1021/jacs.6b11549. [DOI] [PubMed] [Google Scholar]
- 60.Chen Z, Majidi M, Srolovitz DJ, Haataja M. Tunable helical ribbons. Appl Phys Lett. 2011;98:011906. [Google Scholar]
- 61.Williamson GK, Hall WH. X-ray line broadening from filed aluminum and wolfram. Acta Metall. 1953;1:22–31. [Google Scholar]
- 62.Zak AK, Majid WHA, Abrishami ME, Yousefi R. X-ray analysis of ZnO nanoparticles by WilliamsoneHall and sizeestrain plot methods. Solid State Sci. 2011;13:251–256. [Google Scholar]
- 63.Hein JE, Fokin VV. Copper-catalyzed azide-alkyne cycloaddition (CuAAC) and beyond: New reactivity of copper(I) acetylides. Chem Soc Rev. 2010;39:1302–1315. doi: 10.1039/b904091a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nath NK, et al. Surface and bulk effects in photochemical reactions and photomechanical effects in dynamic molecular crystals. J Am Chem Soc. 2015;137:13866–13875. doi: 10.1021/jacs.5b07806. [DOI] [PubMed] [Google Scholar]
- 65.Keating AE, GarciaGaribay MA. Photochemical solid-to-solid reactions. In: Ramamurthy V, Schanze KS, editors. Organic and Inorganic Photochemistry. Dekker; New York: 1998. p. 198. [Google Scholar]
- 66.Bucar DK, MacGillivray LR. Preparation and reactivity of nanocrystalline cocrystals formed via sonocrystallization. J Am Chem Soc. 2007;129:32–33. doi: 10.1021/ja0671161. [DOI] [PubMed] [Google Scholar]
- 67.Takahashi S, et al. Single-crystal-to-single-crystal transformation of diolefin derivatives in nanocrystals. J Am Chem Soc. 2002;124:10944–10945. doi: 10.1021/ja026564f. [DOI] [PubMed] [Google Scholar]
- 68.Lange CW, et al. Photomechanical properties of Rhodium(I)-Semiquinone complexes. The structure, spectroscopy, and magnetism of (3,6-Di-tert-butyl-1,2-semiquinato)dicarbonylrhodium(I) J Am Chem Soc. 1992;114:4220–4222. [Google Scholar]
- 69.Saha S, Desiraju GR. Crystal engineering of hand-twisted helical crystals. J Am Chem Soc. 2017;139:1975–1983. doi: 10.1021/jacs.6b11835. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.