Significance
Fruit flies release a previously unreported signal when they consume nutritive food. This signal communicates the presence of nutritive food to other flies and guides them to aggregate around the nutritive food. The nutritional value of food, rather than its taste, is critical for the release of calorie-induced secreted factor (CIF) from the gut end. This signal is not detected by the olfactory system and is not limited to one species in Drosophila. We propose that CIF acts as an ethologically important pheromone that informs nutritional food sources to other flies in the wild.
Keywords: nutritive sugar, aggregation pheromone, CIF, communication, sweet-insensitive mutant
Abstract
Sweet-insensitive Drosophila mutants are unable to readily identify sugar. In presence of wild-type (WT) flies, however, these mutant flies demonstrated a marked increase in their preference for nutritive sugar. Real-time recordings of starved WT flies revealed that these flies discharge a drop from their gut end after consuming nutritive sugars, but not nonnutritive sugars. We proposed that the drop may contain a molecule(s) named calorie-induced secreted factor (CIF), which serves as a signal to inform other flies about its nutritional value. Consistent with this, we observed a robust preference of flies for nutritive sugar containing CIF over nutritive sugar without CIF. Feeding appears to be a prerequisite for the release of CIF, given that fed flies did not produce it. Additionally, correlation analyses and pharmacological approaches suggest that the nutritional value, rather than the taste, of the consumed sugar correlates strongly with the amount (or intensity) of the released CIF. We observed that the release of this attractant signal requires the consumption of macronutrients, specifically nutritive sugars and l-enantiomer essential amino acids (l-eAAs), but it is negligibly released when flies are fed nonnutritive sugars, unnatural d-enantiomer essential amino acids (d-eAAs), fatty acids, alcohol, or salts. Finally, CIF (i) is not detected by the olfactory system, (ii) is not influenced by the sex of the fly, and (iii) is not limited to one species of Drosophila.
Communication among animals is required to mediate behaviors that are essential for survival and reproductive success, including foraging for food and mating. A variety of communication strategies have evolved to support these behaviors. While foraging, parrots, crows, and ravens, for example, follow acoustic signals produced by conspecific birds to identify potential food sources (1). Similarly, honey bees perform a “waggle dance” to indicate the direction and approximate distance of food sources based on their flight trajectory and pattern (2, 3). Other insects produce semiochemicals that guide their conspecifics to appropriate food sources. For example, ants secrete “trail pheromones” to guide other ants from the home colony toward a marked food source (4).
Drosophila melanogaster also displays aggregation behavior that is driven primarily by olfactory cues (5–7). Drosophila species produce cis-vaccenyl acetate (cVA), a pheromone that promotes aggregation when combined with food (5, 8). Additionally, the plume of apple cider vinegar causes male flies to deposit 9-tricosene, a cuticular hydrocarbon that promotes the aggregation of additional flies around food sources (7). Aggregation behavior driven by purely olfactory cues may not depend directly on the nutritional value of food, however. Flies are equipped with olfactory circuits, which could evaluate the quality of a food source [e.g., antioxidants (9), acidic compounds (10), polyamines (11), toxic microbes (12) within it] to select favorable food. However, the nutritional content of food is unlikely to be perceived by olfactory cues alone. It would be more advantageous for flies to have a means of evaluating the caloric content of food and relaying this information to their conspecifics.
Being able to detect sugar is critical for the survival of animals, including Drosophila. Previously, investigations of sugar detection mechanisms in animals focused mainly on taste buds or peripheral sensory neurons capable of facilitating the detection of a sweet taste (13, 14). Animals can respond to the nutritional value of sugar without tasting it, however (15, 16). The use of synthetic l-enantiomer sugars further elucidated the paradigm for distinguishing between gustation and perception of the nutritional value of sugar. More recent studies in Drosophila and rodent models confirmed that internal or postingestive nutrient sensors can detect the caloric content of sugar independent of its taste (15, 16). In particular, Dh44-expressing neurons in the brain are activated directly by nutritive sugars and are essential for mediating behavioral responses to these sugars (17). In this study, we uncovered the role of calorie-induced secreted factor (CIF) that is released from the gut end after consumption of nutritive sugar, which acts as an aggregation pheromone.
Results
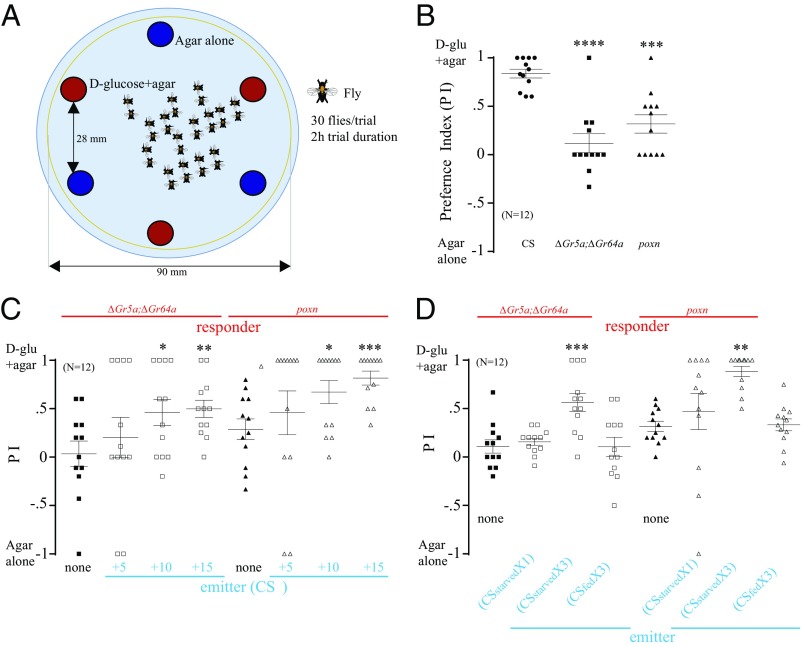
WT Flies Guide Sweet-Insensitive Mutants to Nutritive Sugar.
Gustatory receptors (Grs) are expressed in peripheral taste neurons such as those found in the proboscis, leg tarsi, and wing margin (18, 19). The receptors that detect a sweet taste include Gr5a and Gr64a (14); some of the mutant flies used in this study lack these receptors (∆Gr5a;∆Gr64a flies), and thus are sweet-insensitive (20, 21). Another sweet-insensitive mutant used in this study is poxnΔM22-B5 (22), which retains a limited ability to detect a sweet flavor (23). We compared the preference for d-glucose containing agar (d-glucose+agar) or plain agar in male WT Canton-S (CS) flies versus sweet-insensitive mutants in the two-choice arena (Fig. 1A) for 2 h in the dark at room temperature (∼23 °C) in fed and starved states. Among the flies that had been starved for 24 h, WT flies demonstrated a robust preference for d-glucose+agar, whereas ∆Gr5a;∆Gr64a mutants exhibited no preference for this sugar and poxnΔM22-B5 mutants exhibited a reduced preference for it compared with WT flies (Fig. 1B). When we evaluated these flies after various lengths of starvation, we found that WT flies showed no preference for d-glucose+agar when fed (0 h of starvation) or lightly starved for 5 h, and a strong and significantly increased preference for it after 24 h of starvation (Fig. S1A). By contrast, both ∆Gr5a;∆Gr64a and poxnΔM22-B5 mutants demonstrated no significant preference for d-glucose+agar, even after 24 h of starvation (Fig. S1A). Next, we compared the preference of mutants versus WT flies for another nutritive sugar, d-fructose+agar versus plain agar. Both ∆Gr5a;∆Gr64a and poxnΔM22-B5 mutants showed a significantly reduced preference for d-fructose compared with WT flies (Fig. S1B) and exhibited feeding behaviors similar to those observed in the presence of d-glucose. These results suggest that ∆Gr5a;∆Gr64a and poxnΔM22-B5 mutants have defects in their ability to detect a sweet taste accurately. Their postingestive caloric sensing mechanism appears to be intact, however, given that their preference for d-glucose+agar over plain agar increased significantly when the duration of time they were allowed to forage was increased from 2 h to 5 h (Discussion and Fig. S1C).
Fig. 1.
WT flies guide sweet-insensitive mutants to locate nutritive sugar. (A) Schematic drawing of the two-choice foraging assay: 100 mM d-glucose + 1% agar versus 1% agar alone. Thirty male flies starved for 24 h, unless otherwise stated, were introduced into the arena at room temperature (∼23 °C) in the dark, and their preferences were scored after 2 h. (B) Preference of 24 h-starved ∆Gr5a;∆Gr64a, poxnΔM22-B5 (poxn) mutants, and WT CS flies in the two-choice assay. Asterisks indicate significant differences from WT (one-way ANOVA, followed by a Bonferroni test; n = 12). glu, glucose. (C) Preference of 20 starved ∆Gr5a;∆Gr64a or poxnΔM22-B5 responders when mixed with a varying number of starved WT emitters. Asterisks indicate significant differences from the control groups, where no WT flies were mixed (nonparametric Student’s t test, followed by a Mann–Whitney U test; n = 12). (D) Preference of 30 starved ∆Gr5a;∆Gr64a or poxnΔM22-B5 responders in the two-choice arena: preexposed to starved WT emitters either fed or starved for 24 h. CSX1 and CSX3 indicate one round and three rounds of preexposure, respectively, with starved WT flies in the arena for 2 h. Asterisks indicate significant differences from the control group when the arena was not preexposed to WT flies (one-way ANOVA, followed by a Bonferroni test; n = 12). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Error bars indicate SEM.
To determine whether flies produce a signal that promotes aggregation of other flies around nutritive food sources, we created a mixed population consisting of 10 male WT flies (red eye), which served as “emitters,” and 20 male ∆Gr5a;∆Gr64a or poxnΔM22-B5 mutant flies (white eye), which served as “responders.” After starving these flies for 24 h, we placed them in the arena with a choice of d-glucose+agar versus plain agar. We allowed them to forage for 2 h at room temperature (∼23 °C) in the dark, and then evaluated the preference of the responders. Surprisingly, we found a significantly increased preference for d-glucose+agar in both mutant groups in the presence of emitter flies compared with their preference in the absence of emitters (Fig. 1C). To further investigate whether this signaling phenomenon requires the presence of emitters, we varied the number of emitters over three trials (5, 10, and 15 flies) while keeping the number of responder constant (20 flies). In the presence of five emitter flies, the responder preference did not increase significantly. In the presence of 10 emitters, the preference of both ∆Gr5a;∆Gr64a and poxnΔM22-B5 mutants for d-glucose+agar increased significantly (Fig. 1C). Finally, in the presence of 15 emitters, the preference of the mutant flies for d-glucose+agar over plain agar increased more significantly (Fig. 1C). Furthermore, we carried out similar experiments using a different nutritive sugar, d-fructose, thereby giving the flies a choice between d-fructose+agar and plain agar. We found that both groups of sweet-insensitive mutants demonstrated a significantly greater preference for d-fructose+agar compared with their preference in the absence of emitters (Fig. S1D). We propose that the signal intensity is proportional to the number of emitter flies.
A Signal Emitted by WT Flies Guides Sweet-Insensitive Mutants to Nutritive Sugar.
Having demonstrated that sweet-insensitive mutants can find nutritive sugars in the presence of WT flies, we sought to determine whether emitter flies must be physically present in order for responder flies to aggregate around nutritive sugars or whether they secrete a signal molecule(s) that promotes this aggregation. To investigate this, we introduced 30 starved WT flies into the arena and allowed them to forage in the presence of d-glucose+agar and plain agar for 2 h in the dark at room temperature (∼23 °C) [Canton-S exposure 1 (CSX1)] and referred it as a preexposed arena. We removed these flies and repeated the process in two more trials using a new set of 30 starved WT flies in each successive trial (CSX3). We then introduced 30 starved sweet-insensitive mutants into the same arena and allowed them to forage for 2 h. In essence, WT flies served as emitters and sweet-insensitive mutants served as responders, even though the two groups were never physically present in the arena at the same time. We did not observe a significant increase in preference for the nutritive sugar by responders in the arena that had been preexposed to WT flies one time only (CSX1). After the arena had been preexposed to WT flies three times (CSX3), however, both ∆Gr5a;∆Gr64a and poxnΔM22-B5 mutant responders that were placed in that arena exhibited a robust and significant increase in preference for d-glucose+agar (Fig. 1D). Based on this observation, we conclude that the physical presence of emitter flies is not required for sweet-insensitive mutant responders to aggregate around nutritive sugar. We proposed that a signal(s) or molecule(s) released by the emitter flies was more likely to be responsible for mediating this aggregation behavior rather than the flies themselves.
In the preexposed arena, some parameters needed to be adjusted. We used 100 μL of each food (d-glucose+agar) drop when we mixed sweet-insensitive mutants with WT flies, whereas we used 150 μL of food drop when we preexposed the arena with WT flies to avoid food dehydration due to a longer experimental duration. When we compared preference indices of WT flies with ΔGr5a;ΔGr64a or poxnΔM22-B5 mutants in the two arenas, we found no significant alteration associated with the difference in food volume (Fig. S1E).
Feeding Is a Prerequisite for Signal Release.
To release the signal, emitter flies must consume and evaluate food. We hypothesized that the signal would be released only when flies consumed sufficient amounts of nutritive sugar. Accordingly, the signal would cause a behavioral response in responder flies if they had also been starved. To evaluate the role of both responder and emitter flies according to their physiological state (starved versus fed), we presented a mixed population of starved WT emitters and starved sweet-insensitive mutant responder flies (∆Gr5a;∆Gr64a or poxnΔM22-B5) with a choice of d-glucose+agar versus plain agar. The responders demonstrated a marked increase in their preference for the nutritive sugar (Fig. S1F). When the mixed population consisted of fed WT emitters and starved sweet-insensitive mutant responders, almost no increase in preference was observed (Fig. S1F). When either starved or fed emitters were mixed with fed sweet-insensitive mutant responders, almost no preference for the nutritive sugar was observed in both mutants (Fig. S1F). When placed in the arena that had been preexposed to starved emitter flies (CSStarvedX3), both ∆Gr5a;∆Gr64a and poxnΔM22-B5 mutant flies demonstrated a robust preference for d-glucose+agar. By contrast, when placed in an arena that had been preexposed to fed WT emitter flies (CSFedX3), both mutant groups exhibited no increase in preference for the nutritive sugar (Fig. 1D). These observations suggest that feeding is a prerequisite for the release of the signal and for starved responders to respond to the signal.
Nutrition-Dependent Discharge.
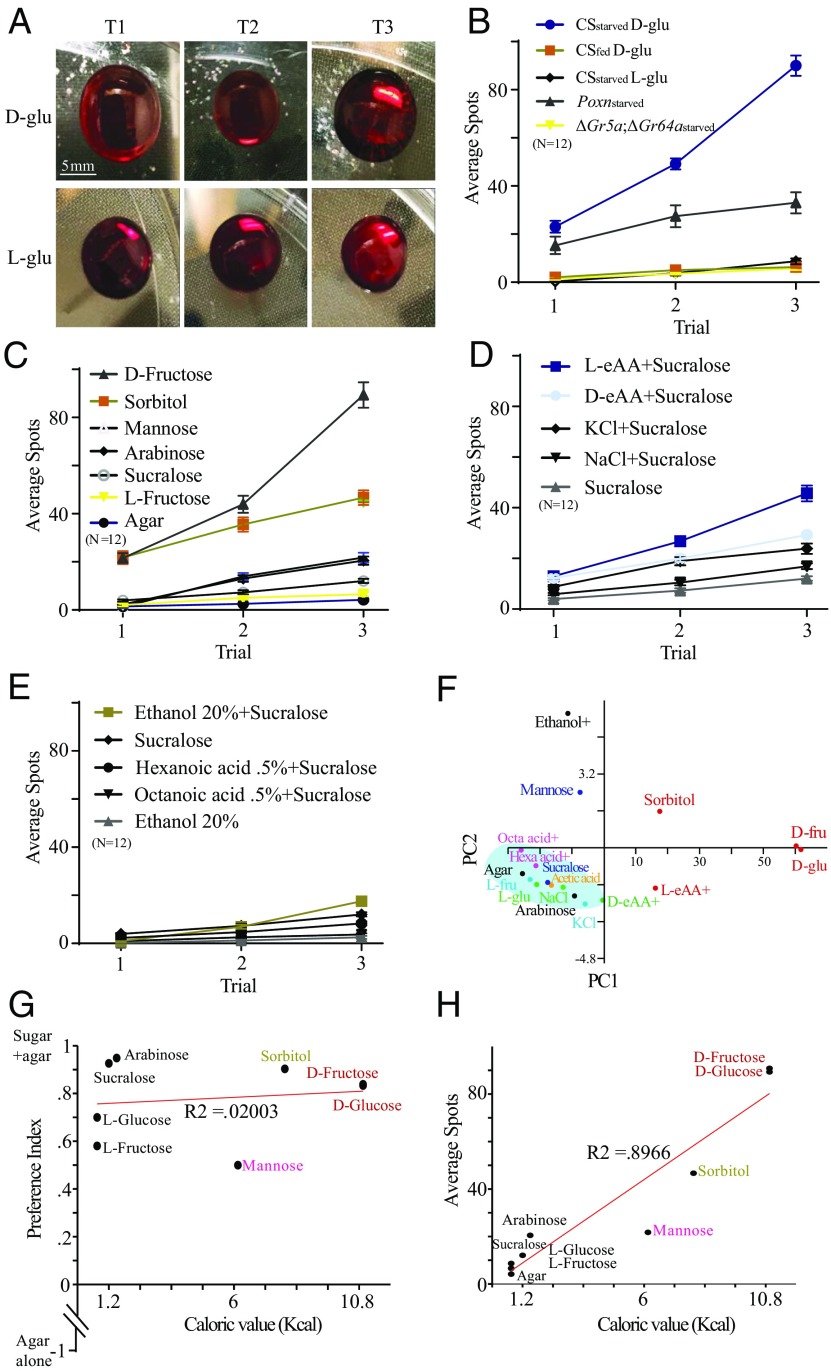
To monitor the release of a signal in real time, we gave WT flies that had been starved for ≥24 h access to 200 mM d-glucose. Within few minutes after consuming the sugar, these flies in the single-fly assay ejected a translucent drop of CIF from the gut end (Movie S1). When we provided another nutritive sugar, d-fructose, we observed a similar phenomenon (Fig. S2A and Movie S2). However, when we provided either 1 mM sucralose (Movie S3), which is similar in sweetness to 200 mM d-glucose; 200 mM l-glucose (Movie S4), which is nonnutritive but similar in sweetness to 200 mM d-glucose; or l-fructose (Movie S5), which is nonnutritive but similar in sweetness to 200 mM d-fructose, the flies did not discharge any drop of CIF (Fig. S2A). We also tested mannose, a sweetless nutritive sugar, and observed that some flies secreted CIF after consumption of the sugar (Fig. S2A and Movie S2). It is possible that less nutritive sugars can affect the amount of food consumed, and thus the release of CIF. We observed, however, that flies drank a bellyful of sucralose, l-glucose, or l-fructose (Movies S3–S5) without discharging a drop during an hour-long video-recording period. Furthermore, we observed that flies occasionally released CIF after drinking smaller amounts of nutritive sugar (either d-glucose or d-fructose) (Movies S1 and S2). Thus, CIF is not necessarily dependent either on the amount of food consumed or the extent to which the abdomen was stretched; rather, it is dependent on the nutritive value of food.
WT Flies Deposit CIF Around Nutritive Sugar.
Having observed in the single-fly assay that a drop was released only after flies had consumed a nutritive sugar (but not a nonnutritive sugar), we closely investigated the two-choice foraging arenas (d-glucose+agar versus agar alone) after 2 h of foraging for any evidence of a discharge. Surprisingly, we found numerous discharged CIF spots (represented by spots) released by WT flies clustered closely around agar containing nutritive d-glucose but almost no spots around agar containing l-glucose (Fig. 2A and Movie S7). The number of spots increased with each successive trial (Fig. 2 A and B). These spots seemed to have been produced specifically after d-glucose was consumed.
Fig. 2.
Release of the signal is specific to sugar and l-eAAs and is highly correlated with their caloric value. (A) Representative photographs of spots produced by 30 starved WT flies after feeding in a two-choice arena: d-glucose (d-glu)+agar or l-glucose(l-glu)+agar versus agar alone for 2 h [trial 1 (T1)]. T2 and T3 refer to second and third additional 2-h exposures to new sets of 30 starved WT flies. (B) Average number of spots produced around agar containing d-glu or l-glu by WT flies, ∆Gr5a;∆Gr64a mutants, and poxnΔM22-B5 mutants, either fed (0 h) or starved (24 h) (n = 12). (C) Average number of spots produced by 30 starved WT flies in a two-choice arena containing one of the following sugars (d-fructose, d-sorbitol, l-fructose, arabinose, mannose, or sucralose)+agar versus agar alone. The concentration of these sugars was 100 mM, except sucralose (0.5 mM) (n = 12). (D) Average number of spots produced by 30 starved WT flies in a two-choice arena containing l-eAAs or d-eAAs+sucralose+agar versus agar alone, and 0.3 M NaCl or KCl+sucralose+agar versus agar alone. As a control, sucralose+agar versus agar alone was used (n = 12). (E) Average number of spots produced by 30 starved WT flies in a two-choice arena containing ethanol+agar+sucralose versus agar, and hexanoic or octanoic acid+agar+sucralose versus agar. Sucralose+agar versus agar alone was used as a control (n = 12). (F) PCA analysis plotting the distribution of spot production around different macronutrients. Clusters are based on the number of spots produced by flies correlated to the caloric value of corresponding macronutrients. PC1, principal component 1. (G) Linear correlation of PI to the caloric value of different sugars used (n = 12). (H) Linear correlation of spots produced to the caloric value of different sugars used (n = 12). Error bars indicate SEM.
We then counted the number of spots that were released around each type of sugar. When d-glucose was used as a food source, WT flies robustly deposited spots around the food, which increased with the successive number of trials carried out in the same arena (Fig. 2 A and B). When l-glucose was used as a food source, however, almost no spots were observed in the arena even after three successive trials (Fig. 2 A and B). When we used fed WT flies, we found no spots around d-glucose either (Fig. 2B). When we used sweet-insensitive ∆Gr5a;∆Gr64a mutants as emitters, we observed almost no preference for d-glucose+agar by these flies (Fig. 2B) and found almost no spots around d-glucose (Fig. 2B), indicating a severe defect in their ability to detect and feed sugar. Similarly, poxnΔM22-B5 mutants showed a reduction in their preference for d-glucose+agar as described (Fig. 1B) and produced fewer spots around d-glucose compared with WT flies (Fig. 2B).
CIF Deposits Around Other Sugars and Macronutrients.
We next sought to evaluate the pattern of the signal release by WT flies following the consumption of each type of sugar according to its palatability and nutritional value, and following the consumption of other macronutrients. After consuming d-fructose, WT flies robustly produced a high number of spots around the food, which increased with each successive trial (Fig. 2C). After consuming l-fructose (which cannot be metabolized), however, no spots were found (Fig. 2C). When we provided d-sorbitol, which contains moderate levels of sweetness and calories compared with d-glucose, flies produced more spots but slightly fewer than seen after they consumed either d-glucose or d-fructose (Fig. 2C). After consuming arabinose (a minimally caloric but sweet sugar), flies produced few spots (Fig. 2C). Following the consumption of sucralose, which is sweet but contains essentially no calories, flies produced nearly no spots. After consuming mannose (a caloric but nonsweet sugar), flies produced a moderate number of spots compared with the number of spots seen after consuming sweet caloric sugars (Fig. 2C).
We tested other macronutrients that were supplemented with sucralose as an incentive for their consumption. When we used natural l-enantiomers of essential amino acids (l-eAAs), flies produced a moderate number of spots. In the presence of unnatural d-enantiomers of essential amino acids (d-eAAs), however, they produced few spots (Fig. 2D). This is consistent with the notion that l-enantiomers of amino acids, but not d-enantiomers, can readily be metabolized. When we added 0.3 M NaCl or KCl to the foraging arena, flies failed to release spots (Fig. 2D). This suggests that the signal release is specific to the nutritional value of sugar and amino acids. However, only a few spots were produced in the presence of fatty acids (particularly hexanoic acid and octanoic acid) (Fig. 2E), and none were observed in the presence of 20% ethanol (Fig. 2E).
Using principal component analysis (PCA) to categorize spotting behavior in response to the consumption of nutritive and nonnutritive sugars, as well as the other macronutrients according to their caloric values (Fig. 2F), we found a distinct pattern of segregation of spotting behavior. Spots associated with nonnutritive sugars were markedly separated from those associated with nutritive sugars. This corroborates that the signal release specific to nutritive sugars or l-eAAs is not associated with nonnutritive sugars or any other macronutrients used in this study (Fig. 2F). Further analyses showed that the taste preference for different sugars did not correlate with the corresponding caloric content of those sugars (Fig. 2G and Fig. S2B). By contrast, the intensity of the signal correlated strongly and proportionately with the caloric content of sugars (Fig. 2H) rather than their taste (Fig. S2C).
In the wild, rotting fruits are the fly’s preferred choice for food, being ideal for mating and oviposition. Because sugars and amino acids are the primary constituents of rotting fruits, one would expect flies to have innate mechanisms for evaluating food quality. Our observation of the signal released around specific nutrients suggests that this phenomenon is an evolutionary trait conferred on flies to enable them to distinguish and mark sugar- and amino acid-rich food sources.
CIF Is Sufficient to Attract Sweet-Insensitive Mutants to Food.
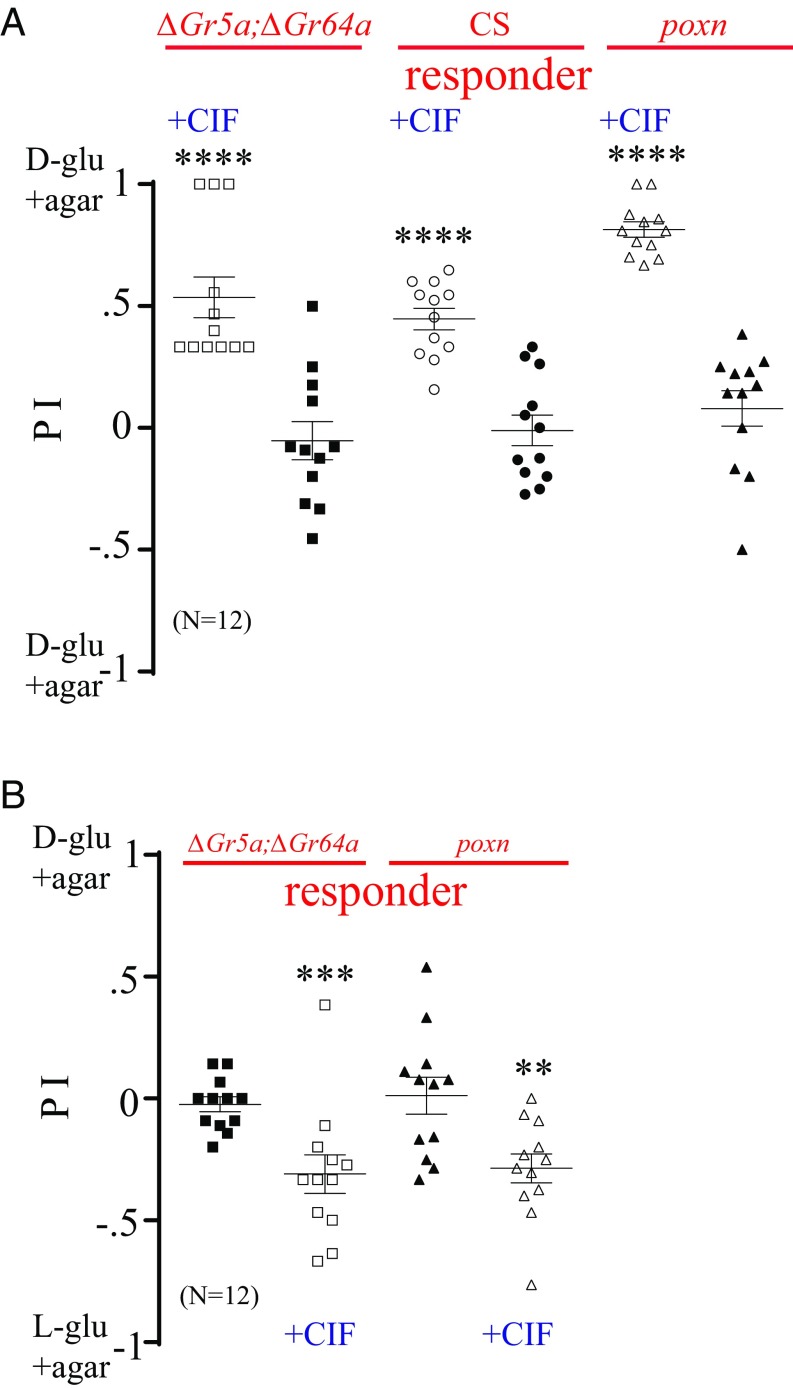
To determine whether the spots around nutritive sugar contain a signal representing its caloric value, we preexposed the arena (CSX3) of d-glucose+agar versus plain agar to WT flies. We then replaced the plain agar with d-glucose+agar, thereby changing the food choices to d-glucose+agar with CIF versus d-glucose+agar without CIF. When we presented this choice to starved ∆Gr5a;∆Gr64a or poxnΔM22-B5 mutants, they preferentially consumed the d-glucose+agar with CIF over the d-glucose+agar without CIF (Fig. 3A). WT flies also exhibited a similar preference (Fig. 3A). This finding supports the hypothesis that the spots contain signaling molecules that attract flies to the particular food. As a control experiment, we presented flies with a choice of d-glucose+agar without CIF versus d-glucose+agar without CIF. Neither the sweet-insensitive mutants nor WT flies showed a preference for either food source (Fig. 3A).
Fig. 3.
Deposition around nutritive sugars triggers an attractive response. (A) Preference of starved ∆Gr5a;∆Gr64a, poxnΔM22-B5 (poxn), and WT responders in a two-choice assay: d-glucose (d-glu)+agar with spots versus d-glu+agar without CIF. As a control, flies were given a choice between d-glu+agar without CIF and d-gluc+agar without CIF. Asterisks indicate significant differences from the control group without CIF (nonparametric Student’s t test, followed by a Mann–Whitney U test; n = 12). (B) Preference of starved ∆Gr5a;∆Gr64a or poxn mutants in a two-choice assay, l-glu+agar with CIF versus d-glu+agar without CIF. Asterisks indicate significant differences from the control group without CIF (nonparametric Student’s t test, followed by a Mann–Whitney U test; n = 12). **P < 0.01; ***P < 0.001; ****P < 0.0001. Error bars indicate SEM.
For further evidence that the spots around nutritive sugar contain CIF rather than the spots deposited directly on the food itself, we used a preexposed arena (CSX3), replaced the old agar containing d-glucose with fresh agar containing d-glucose, and provided a choice of fresh d-glucose+agar with spots versus agar alone. Both mutant groups still showed a greater preference for the new d-glucose+agar surrounded with old spots (Fig. S3A). This suggests that the spots around sugars contain the signal that guides sweet-insensitive mutants to food rather than the signal deposited directly on food. To characterize the spots further, we switched the position of d-glucose+agar with that of plain agar after being exposed to WT flies (CSX3), thereby giving a new set of mutant flies a choice of plain agar with CIF versus d-glucose+agar without CIF. Interestingly, starved ∆Gr5a;∆Gr64a mutants showed a preference for the plain agar with CIF, while starved poxnΔM22-B5 mutants still demonstrated a reduced preference for d-glucose+agar (Fig. S3A). On the other hand, when given a choice between d-glucose+agar without CIF and l-glucose+agar with CIF, both mutant groups demonstrated a significant preference for l-glucose+agar with CIF (Fig. 3B). As a control, we gave these flies the choice of d-glucose+agar versus l-glucose+agar. Both mutant groups seemed to have no preference for either food source in this assay (Fig. 3B). These findings suggest that the presence of spots results in a bias toward the food source by responders.
To further show that CIF is present in the spot, we preexposed the arena using starved ∆Gr5a;∆Gr64a mutants (which had not produced any spot in the arena) as emitters (∆Gr5a;∆Gr64aX3) (Fig. 2B) and then introduced ∆Gr5a;∆Gr64a or poxnΔM22-B5 mutants to the arena as responders. We observed no increased preference for d-glucose+agar by the responders (Fig. S3B). We next used starved poxnΔM22-B5 mutants as emitters (poxnX3), and observed a moderate number of spots around nutritive d-glucose (Fig. 2B) and an increase in preference for d-glucose+agar by both mutant responders (Fig. S3B). This result links the presence of CIF to an increased preference for nutritive sugar.
Nutritional Value Is Critical for the Release of CIF.
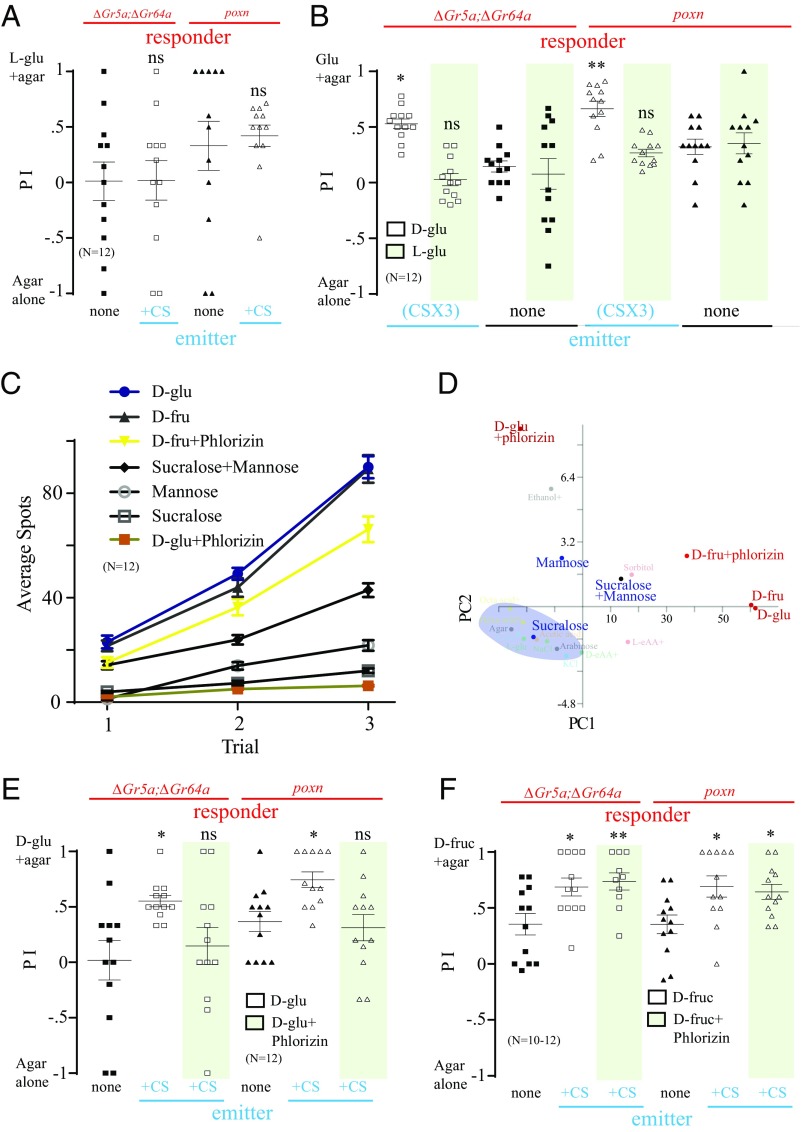
Single-fly and spot-deposition studies revealed that the release of CIF by emitter flies is highly dependent on the caloric value of sugar. When l-glucose was used as a food source, CIF was not released (Fig. 2 A and B, Fig. S2A, and Movie S4). When a mixed population of sweet-insensitive mutants and WT flies was given a choice of l-glucose+agar versus plain agar, the mutant flies did not demonstrate a significant increase in their preference for nonnutritive sugar (Fig. 4A). A significant increase in preference for nutritive sugar was observed when d-glucose+agar was used instead of l-glucose+agar, however (Fig. 4B). When the arena (l-glucose+agar versus plain agar) was preexposed three times to starved WT emitters (CSX3) and starved ∆Gr5a;∆Gr64a or poxnΔM22-B5 responders were then given a choice, we observed an insignificant increase in preference for the nonnutritive sugar (Fig. 4B).
Fig. 4.
Caloric value of sugar is important for signal release. (A) Preference of 20 starved ∆Gr5a;∆Gr64a or poxnΔM22-B5 (poxn) responders when mixed with 10 starved WT emitters in a two-choice assay: l-glucose (l-glu) +agar versus agar alone. ns, nonsignificant differences from the control group, where no WT flies were mixed (nonparametric Student’s t test, followed by a Mann–Whitney U test; n = 12). (B) Preference of 30 starved ∆Gr5a;∆Gr64a or poxn responders in a two-choice assay: d-glu or l-glu (shaded area)+agar versus agar alone. The two-choice arena was previously preexposed to starved WT emitters for 2 h for three rounds or was not preexposed. Asterisks indicate significant differences from the control group when not preexposed to WT emitters (one-way ANOVA, followed by a Bonferroni test; n = 12). (C) Average number of spots produced by 30 starved WT flies in a two-choice arena containing d-glu+agar with or without phlorizin versus agar alone, d-fructose (d-fru)+agar with or without phlorizin versus agar alone, mannose+agar with or without sucralose versus agar alone, and mannose+agar versus agar alone. (D) PCA analysis plotting the distribution after adding phlorizin to d-glu or d-fru and adding mannose to sucralose. Clusters are based on the number of spots produced by flies correlated to the caloric value of consumed sugar. (E) Preference of 20 starved ∆Gr5a;∆Gr64a or poxn responders when mixed with 10 starved WT emitters in a two-choice assay: d-glu+agar with (shaded area) or without phlorizin versus agar alone. Asterisks indicate a significant difference from the control group when no WT flies were mixed (one-way ANOVA, followed by a Bonferroni test; n = 12). (F) Preference of 20 starved ∆Gr5a;∆Gr64a or poxn responders when mixed with 10 starved WT emitters in a two-choice assay: d-fructose (d-fruc)+agar with (shaded area) or without phlorizin versus agar alone. ns, nonsignificant differences from the control group, where no WT flies were mixed. Asterisks indicate significant differences from the preference for d-glu+agar (one-way ANOVA, followed by a Bonferroni test; n = 10–12). *P < 0.05; **P < 0.01. Error bars indicate SEM.
For additional evidence of the influence of the caloric value of sugar on fly preference, we added phlorizin, a drug that blocks glucose transporters in the gut (24, 25), to d-glucose, thereby transforming it into a sweet but nonnutritive sugar. We observed robust spot deposition after the consumption of d-glucose alone as expected (Figs. 2 A and B and 4C). However, only a relatively few spots were seen around agar containing d-glucose+phlorizin (Fig. 4C). Indeed, the amount of CIF released in the presence of phlorizin was similar to that seen following the consumption of nonnutritive sugar (Figs. 2C and 4C). Because phlorizin is known to block glucose transporters but not fructose transporters (26), we performed a control experiment by giving the flies a choice of agar containing d-fructose+phlorizin versus plain agar. Flies deposited spots robustly around the agar containing d-fructose+phlorizin, similarly observed in agar containing d-fructose without phlorizin (Fig. 4C). On the other hand, when we supplemented a sweet nonnutritive sugar (sucralose) with a nutritive nonsweet sugar (mannose), flies demonstrated a fourfold increase in spot production (Fig. 4C, Fig. S2A, and Movie S8). Our PCA analysis had revealed a shift of d-glucose from a signal-producing cluster to a non–signal-producing cluster after phlorizin was added, but no shift in d-fructose was observed after adding phlorizin (Fig. 4D). Conversely, a shift of nonnutritive sucralose from the non–signal-producing cluster to the signal-producing cluster was observed after adding mannose to sucralose (Fig. 4D).
We further tested our hypothesis that CIF release is calorie-dependent and promotes the consumption of CIF-marked food using a mixed population of starved sweet-insensitive mutants and WT flies. Both ∆Gr5a;∆Gr64a and poxnΔM22-B5 mutants showed a statistically insignificant increase in preference for d-glucose+agar with phlorizin over plain agar (Fig. 4E) and a significant increase in preference for d-glucose+agar without phlorizin (Fig. 4E). When we used d-fructose+agar with phlorizin, both mutant groups demonstrated a statistically significant increase in their preference for d-fructose with phlorizin over plain agar (Fig. 4F) similar to that seen for d-fructose+agar without phlorizin (Fig. 4F).
We next preexposed the arena of d-glucose+agar with phlorizin versus plain agar using starved WT flies (CSX3), and then tested ∆Gr5a;∆Gr64a or poxnΔM22-B5 mutant flies. The preference for d-glucose+agar with phlorizin (Fig. S4A) was statistically insignificant but increased significantly in the absence of phlorizin (Fig. S4A). To determine whether this change in preference was due to a change in palatability, we gave WT flies a choice of d-sugar+agar with phlorizin versus agar alone and also gave WT flies a choice of d-sugar+agar without phlorizin versus agar alone. We found that the responses in these two sets of tested WT flies were highly similar (Fig. S4B). These results suggest that the nutritional value of the sugar, rather than its taste, is critical for the release of the signal that could guide sweet-insensitive mutants to caloric food sources.
Olfactory System Is Not Involved in the Detection of CIF.
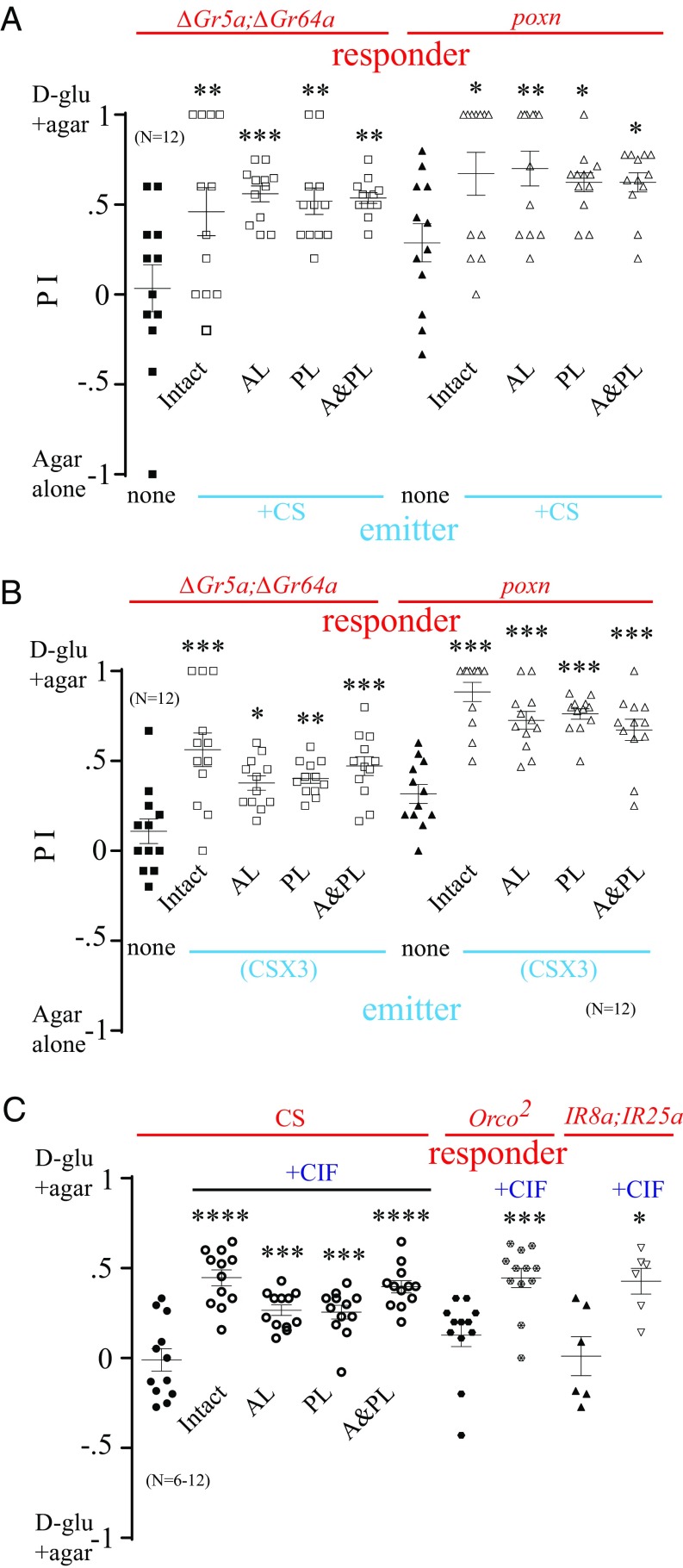
Insects, including Drosophila, are highly dependent on their olfactory system for foraging and other social behaviors. Pheromones reportedly involved in aggregation behavior are primarily perceived by olfactory receptors (ORs). In Drosophila, these include cVA, which is detected by Or67d (27) and Or65a (28), and 9-tricosene, which is reported as a ligand for Or7a (7). To determine whether the signal produced after a nutritive sugar has been consumed is detected through an olfactory pathway or an alternative pathway, we surgically removed the antennae, maxillary palp, or both from starved sweet-insensitive mutants and used them as responders. We then created a mixed population consisting of these surgically amputated mutants as responders and WT flies as emitters, and placed them in the arena with a choice of d-glucose+agar versus plain agar. The preference for d-glucose+agar over plain agar was significant in sweet-insensitive mutants in which the antennae, palp, or both had been surgically amputated (Fig. 5A). We then introduced these flies to an arena that had been preexposed to WT emitters (CSX3). Again, we observed a robust and significant preference for d-glucose+agar in these surgically amputated mutants (Fig. 5B).
Fig. 5.
Olfactory system is not required for signal detection. (A) Preference of 20 starved, ∆Gr5a;∆Gr64a or poxnΔM22-B5 (poxn) responders in which the antennae (AL), palp (PL), or both (A&PL) were removed when mixed with 10 WT emitters in the two-choice assay: d-glucose(d-glu)+agar and agar alone. Asterisks indicate significant differences from the control group, where no WT flies were mixed (one-way ANOVA, followed by a Bonferroni test; n = 12). (B) Preference of 30 starved surgically amputated ∆Gr5a;∆Gr64a or poxn responders in a two-choice arena preexposed to starved WT emitters. Asterisks indicate significant differences from the control group when not preexposed to WT flies (one-way ANOVA, followed by a Bonferroni test; n = 12). (C) Preference of 30 starved surgically amputated WT responders in a two-choice assay: d-glu+agar with CIF versus d-glu+agar without CIF. The preference of Orco2 and IR8a;IR25a mutants as responders in a two-choice assay, d-glu+agar with spots versus d-gluc+agar without spots, is shown. As a control, Orco2 or IR8a;IR25a mutants were given a choice between d-glu+agar without CIF and d-glu+agar without CIF. Asterisks indicate significant differences from the control group without CIF (one-way ANOVA, followed by a Bonferroni test, and nonparametric Student’s t test, followed by a Mann–Whitney U test for Orco2 mutant; n = 6–12). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Error bars indicate SEM.
Furthermore, when WT flies with surgical removal of the antennae, palp, or both were exposed to the choice of d-glucose+agar with CIF versus d-glucose+agar without CIF, these surgically amputated flies showed a significant preference for d-glucose with CIF despite their loss of olfactory appendages (Fig. 5C). We next exposed flies with mutated Orco2, which is a required coreceptor for the functioning of most ORs (29), to the same food sources. As with the surgically amputated flies, Orco2 mutants demonstrated a significant preference for d-glucose+agar with CIF (Fig. 5C). Furthermore, ionotropic receptors (IRs), which are expressed in some olfactory and gustatory neurons (30), may not be important because flies harboring the mutations in IR8a and IR25a, the IR coreceptors (31), responded to CIF (Fig. 5C). These results indicate that olfactory neurons and gustatory neurons expressing IR8a or IR25a are not required for the detection of CIF.
Oenocytes, which are found beneath the Drosophila cuticle, have been reported to mediate the production of cuticular hydrocarbons (32), including 9-tricosene (7). We labeled the oencytes using promE-Gal4 and then ablated the oenocytes in emitter flies using UAS-Hid. We created a mixed population consisting of these oenocyte-ablated flies as emitters and sweet-insensitive mutants as responders. Even after the ablation of oenocytes, a significant increase in preference for d-glucose+agar by both ∆Gr5a;∆Gr64a and poxnΔM22-B5 mutants was observed (Fig. S5A). This suggests that the ablation of oenocytes does not affect the release of CIF around nutritive sugar. Because Or7a was shown to mediate the detection of 9-tricosene, we gave Or7a mutant flies a choice between d-glucose+agar with CIF and d-glucose+agar without CIF. We observed a robust and significant preference for the d-glucose+agar with CIF in these mutants (Fig. S5B). As a control, we gave Or7a mutants a choice of d-glucose+agar versus d-glucose+agar, and observed no biased behavior (Fig. S5B). We also tested Orco2 and Or7a mutants for d-glucose+agar versus agar alone, and observed a robust preference for the sugar similar to WT flies (Fig. S5C). Together, these results provide additional evidence that oenocytes have no role in the release of CIF.
To examine the nature of CIF released after the consumption of nutritive sugar, we preexposed the arena (d-glucose+agar versus agar alone) using WT flies (CSX3), washed it with either a polar solvent (e.g., water) or a nonpolar solvent (e.g., hexane, acetonitrile), and allowed the arena to dry for 30 min. We then placed starved ∆Gr5a;∆Gr64a or poxnΔM22-B5 mutants in the arena that had been washed with a polar or nonpolar solvent. In an arena washed with water, sweet-insensitive mutants demonstrated an increased preference for nutritive sugar (Fig. S5D), whereas in arenas washed with a nonpolar solvent (particularly hexane or acetonitrile) sweet-insensitive mutants showed no significant increase in their preference for d-glucose+agar (Fig. S5D), suggesting that the putative CIF molecules may be hydrophobic in nature. As controls, we placed these mutants and WT flies in fresh (not preexposed) arenas that had been washed with water, hexane, or acetonitrile to confirm that they had not experienced any adverse effects or demonstrated any biased behavior as a result of exposure to these solvents (Fig. S5D). We also measured the rate of CIF degradation following its release after WT flies had consumed nutritive d-glucose. The half-life of the signal was ∼3 d, and CIF is viable for up to 14 d after being released by emitter flies (Fig. S5E).
The CIF Release Is Not Sex-Biased.
Both male and female Drosophila species produce pheromones, but many are released by one sex and not by the other. For example, cVA is produced in an internal male organ (the ejaculatory bulb) and transferred to females during copulation (8, 33) and 9-tricosene is a cuticular hydrocarbon produced by male flies after exposure to certain food odors (e.g., apple cider vinegar) (7). Moreover, most cuticular hydrocarbons are not volatile and are detected through gustatory contact (34).
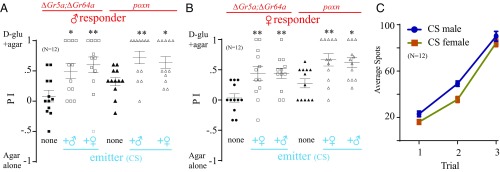
To determine whether the release of CIF is also sex-specific, we used either males or females as emitters and males as responders in one arena (Fig. 6A) and females as responders in another arena (Fig. 6B). In doing so, we observed an increase in preference for d-glucose+agar over plain agar by both male and female ∆Gr5a;∆Gr64a and poxnΔM22-B5 responders (Fig. 6 A and B), regardless of the sex of emitters or responders.
Fig. 6.
Signal is not sex-biased. (A) Preference of 20 starved male ∆Gr5a;∆Gr64a or poxnΔM22-B5 (poxn) responders when mixed with 10 starved WT emitters, either male or female, in the two-choice assay. Asterisks indicate significant differences from the control group, where no WT flies were mixed (one-way ANOVA, followed by a Bonferroni test; n = 12). (B) Preference of 20 starved female ∆Gr5a;∆Gr64a or poxn responders when mixed with 10 starved WT emitters, either female or male, in the two-choice assay. Asterisks indicate significant differences from the control group, where no WT flies were mixed (one-way ANOVA, followed by a Bonferroni test; n = 12). (C) Average number of spots produced by 30 starved WT male or female flies in the two-choice arena. Trial 2 and trial 3 refer to second and third additional 2-h exposures to new sets of 30 starved WT flies (n = 12). *P < 0.05; **P < 0.01. Error bars indicate SEM. d-glu, d-glucose.
We further confirmed that CIF release is not sex-biased by placing male or female flies in d-glucose+agar versus agar alone and counting the number of spots that subsequently appeared around the d-glucose+agar. We observed a large and equal number of spots produced by both male and female WT flies, which increased with each successive trial (Fig. 6C). Single-fly assays also revealed that exposure of both female (Movie S1) and male (Movie S9) flies to nutritive sugar triggers release of the translucent drops. These results suggest a lack of sex bias in the release of CIF. We also observed a strong preference for d-glucose+agar by both male and female WT responders (Fig. S6).
CIF May Not Be Strictly Conspecific.
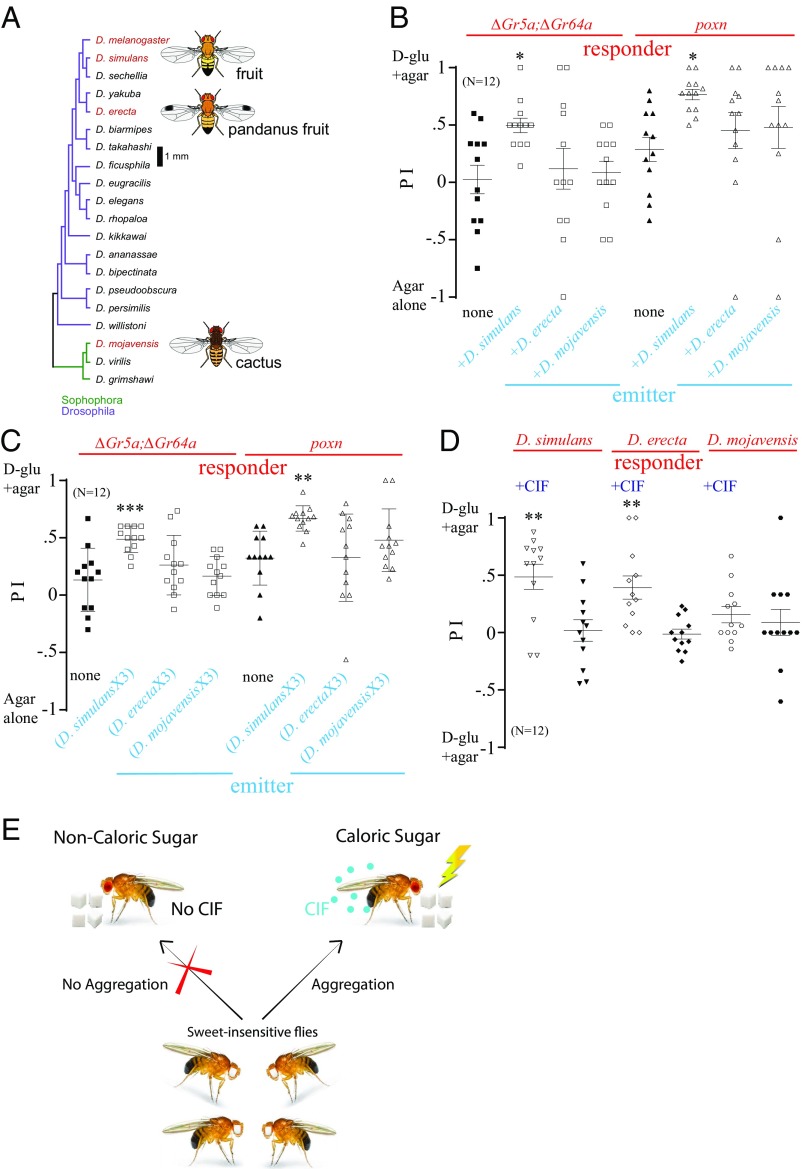
Most of the Drosophila pheromones are conspecific (i.e., they allow members of the same species to aggregate around marked areas). To determine whether CIF produced by flies after consuming nutritive sugar is conspecific or interspecies (i.e., capable of communicating with other phylogenetically related species), we used each of three other Drosophila species (Drosophila simulans, Drosophila erecta, and Drosophila mojavensis) as emitters (Fig. 7A), with ∆Gr5a;∆Gr64a or poxnΔM22-B5 D. melanogaster mutants serving as responders. D. simulans was starved for 24 h, D. erecta was starved for 30 h, and D. mojavensis was starved for 90 h before subjecting them as emitters or responders. Both mutants demonstrated an increased preference for d-glucose+agar over plain agar when mixed with D. simulans, but not when mixed with D. erecta or D. mojavensis (Fig. 7B). When the arena was preexposed to each species and ∆Gr5a;∆Gr64a or poxnΔM22-B5 mutants were added afterward, the mutants again demonstrated an increased preference for d-glucose+agar in the arena that had been preexposed to D. simulans, but not in the arena that had been preexposed to D. erecta or D. mojavensis (Fig. 7C). We observed that a large number of spots were produced by both D. simulans and D. erecta flies, which increased with each successive trial, but few spots were produced by D. mojavensis (Fig. S7A).
Fig. 7.
Signal released by D. melanogaster promotes aggregation in some Drosophila species and vice versa. (A) Phylogenetic relationship of the Drosophila species. (B) Preference of starved ∆Gr5a;∆Gr64a or poxnΔM22-B5 (poxn) responders (20 flies) when mixed with other Drosophila species as emitters (D. simulans, D. erecta, and D. mojavensis; n = 10) in the two-choice assay. Asterisks indicates significant differences from the control group, where no other flies were mixed (one-way ANOVA, followed by a Bonferroni test; n = 12). (C) Preference of 30 starved ∆Gr5a;∆Gr64a or poxn responders in the two-choice arena preexposed to D. simulans, D. erecta, or D. mojavensis emitters for three rounds of 2 h of foraging. Asterisks indicate significant differences from the control group when not preexposed to any emitters (one-way ANOVA, followed by a Bonferroni test; n = 12). (D) Preference of 30 D. simulans, D. erecta, and D. mojavensis responders in a two-choice assay: CIF (produced by D. melanogaster) + d-glu(d-glu)+agar versus d-glu+agar. As a control, flies were given a choice between d-glu+agar without CIF and d-glu+agar without CIF. Asterisks indicate significant differences from the control group when not preexposed to other flies (one-way ANOVA, followed by a Bonferroni test; n = 12). *P < 0.05; **P < 0.01; ***P < 0.001. Error bars indicate SEM. (E) Summary diagram for communication of the nutritional value of food through CIF among flies. Upon the consumption of nutritional food, flies secrete CIF that attracts other flies to the food source.
Notably, D. mojavensis is found in diverse habitats, predominantly in desert environments (35); consequently, it is able to survive more than 100 h without food (Fig. S7B). This is considerably longer than the survival of D. melanogaster, which is ∼30 h. The feeding habits and natural habitat of D. erecta also differ considerably from those of D. melanogaster, with D. erecta eating primarily tropical African screw pines and pandanus fruits in the wild (36, 37). Phylogenetic analyses suggest that D. simulans is much closer to D. melanogaster and that D. mojavensis is the farthest from D. melanogaster in terms of evolutionary divergence (Fig. 7A). Taking all these factors into account, we suggest that consummatory behaviors on d-glucose differ across these species, and therefore could affect the release of the signal.
To further investigate whether the signal produced by Drosophila is interspecies, we used WT D. melanogaster as emitters and other species as responders. The arena was preexposed to WT flies three times (CSX3), and plain agar was then replaced with d-glucose+agar, thereby giving the flies a choice of d-glucose+agar with CIF versus d-glucose+agar without CIF. D. simulans and D. erecta both showed a preference for d-glucose+agar with CIF over d-glucose+agar without spots, whereas D. mojavensis did not show a preference (Fig. 7D). These results suggest that CIF is not strictly conspecific, as it is produced and recognized by other Drosophila species.
Discussion
In this work, we found that fruit flies secrete a signal, CIF, to inform other flies about the nutritional value of consumed food and promote their aggregation around the food sources. Previously, we demonstrated that flies are equipped with a mechanism that allows them to detect the nutritional value of sugar independent of taste (16, 17, 24). In the current study, we found that the production and release of CIF also depend on the nutritional content of food, rather than its orosensory value. Using video-tracking and a linear correlation model, we found additional evidence in support of the hypothesis that the release of the signal correlates with the nutritional value of the consumed food rather than with its palatability (Fig. 7E). A sweet taste, however, serves as an incentive for flies to sample and initiate consummatory behavior when food is found.
Differences in the Two-Choice Behavior Paradigms.
Dus et al. (16) previously showed that ∆Gr5a;∆Gr64a and poxnΔM22-B5 mutants are able to preferentially select nutritive d-glucose over nonnutritive l-glucose within 2 h after 22 h of starvation. In our modified assay, ∆Gr5a;∆Gr64a and poxnΔM22-B5 mutants that had been starved for 24 h did not show a preference for d-glucose+agar over agar alone. The two-choice assay used by Dus et al. (16) was carried out in a 60-well microtiter Terasaki plate in which the food choices were in close proximity, separated by only 2 mm. By contrast, our modified two-choice foraging assay was carried out using a Petri dish (diameter of 90 mm) containing only three drops of each food, with food samples separated by 28 mm. Dus et al. (16) used ∼50 flies in a microtiter plate encompassing an area of 4,400 mm2, whereas we used 30 flies in a Petri dish encompassing an area of 6,361.7 mm2. The increased distance between food sources posed a challenge for foraging flies to effectively identify nutritive sugar. Nonetheless, the mutant flies demonstrated a significant preference for d-glucose+agar over agar alone when they were allowed to forage for 5 h instead of 2 h (Fig. S1C).
Previously Unreported Signal.
Most of the pheromones produced in D. melanogaster have been reported to be sex-biased. For example, the well-known pheromones cVA (5) and 9-tricosene (7) are produced by males; these are involved in mating and aggregation behavior, respectively. By contrast, we found that CIF produced after the consumption of nutritive food is not sex-biased. It was produced and detected by both male and female flies (Fig. 6 A and B) and found to be released by both sexes (Fig. 6C and Movies S1 and S9).
The pheromones (27, 28, 32, 38) and fecal material (frass) (39) identified by other studies are perceived primarily through ORs. We found that surgical removal of the antennae, the maxillary palp, or both has no effect on aggregation behavior, however. This suggests that the main components of the signal may not overlap with those of the pheromones or frass. Furthermore, the facts that the ablation of oenocytes, which produce 9-tricosene, has no effect on the secretion of CIF (Fig. S5A) and knockout of Or7a has no effect on the ability of flies to track the signal (Fig. S5B) support the view that this signal is likely a compound(s) that has not been previously identified.
Finally, our degradation experiment suggests that CIF is relatively stable and has a half-life of ∼3 d, which is consistent with the notion that the compound comprising CIF is not too volatile, and therefore cannot be readily detected through the olfactory system. It is possible that CIF contains a number of active hydrophobic compounds, however. The future challenge is to identify these compounds and investigate the component of the neuroendocrine axis that is activated by nutritive food to mediate the release of CIF.
Materials and Methods
Fly Strains.
Flies were reared in standard cornmeal-molasses medium at 25 °C with 12/12-h light/dark cycles. The standard laboratory line CS was used as the WT control. We obtained poxnΔM22-B5 mutant from Ulrike Heberlein, Janelia Farm, VA, and ∆Gr5a;∆Gr64a mutant from John Carlson, Yale University, New Haven, CT. Or83b2 mutant was obtained from Kathy Nagel, New York University. Or7a knockout, UAS-Hid, and promE-Gal4 flies were provided by Chris Potter, Johns Hopkins School of Medicine, Baltimore. D. simulans, D. erecta, and D. mojavensis flies were obtained from the Drosophila Species Stock Center at the University of California, San Diego.
Two-Choice Foraging Assay.
Male flies (0–2 d old) were collected under CO2 anesthesia and allowed to recover in standard cornmeal food vials for at least 2 d before experiments. Male flies (WT CS flies and other Drosophila species) were then starved for 24 h at room temperature (∼23 °C), unless otherwise noted, in vials containing Kimwipe tissue soaked with ∼2 mL of deionized water (Picosystem plus pump). For the two-choice foraging assay, groups of 30 male flies starved for 24 h were cold-anesthetized, transferred into Petri dishes (Falcon Disposable Petri Dishes, Sterile; Corning), and allowed to forage for 2 h in the dark at room temperature (∼23 °C). In the assay, each Petri dish contained three dots (100 μL) of both choices, arranged radially in alternating sequence. Sugar (100 mM) was added to 1% agar and color-labeled with 1% red McCormick tasteless food dye, while 1% agar without sugar was color-labeled with 1% blue dye (Indigo carmine; Sigma). Preference was then scored by examining the color of the fly’s abdomen. Color-labeled 1% agar without sugar was previously tested (16), and did not produce a proboscis extension reflex response. Flies showed no bias for either dye. Preference index (PI) was calculated as [(number of flies that ate sugar with agar) − (number of flies that ate agar alone)]/(total number of flies that fed). A PI of 0 indicates no preference, whereas a PI of 1 indicates the maximum preference for sugar+agar and a PI of −1 indicates the maximum preference for agar alone. Flies that consumed both food substrates or consumed none were excluded from the calculation. All tested sugars, d-glucose, l-glucose, d-fructose, l-fructose, sucralose, mannose, arabinose, sorbitol, and agar at 99% purity, were purchased from Sigma–Aldrich.
Preexposed Two-Choice Foraging Arena.
To obtain a preexposed arena, we used the two-choice arena with the same configuration as previously described, but each Petri dish contained six dots of at a volume of 150 μL instead of 100 μL to prevent evaporation during longer durations of experiment. We introduced 30 starved WT male flies into the arena three times in successive trials one after another with an allotted foraging time of 2 h each trial. After the third trial, either ∆Gr5a;∆Gr64a or poxnΔM22-B5 mutant flies were introduced into the arena.
Surgical Removal of Antenna and Maxillary Palp.
Male flies (2–4 d old) were collected and anesthetized using CO2 before surgical removal of the third antennal segment, maxillary palp, or both. Removal was done under a microscope (Olympus SZ51) using forceps. Flies were given 2–3 d to recover and then subsequently starved for 24 h at room temperature (∼23 °C) in vials containing Kimwipe tissue soaked with ∼2 mL of deionized water before experiments.
Single-Fly Assay Recording.
Single-fly recordings were performed on WT male and female flies starved for ∼24 h with Kimwipe tissue soaked with deionized water. Flies were anesthetized, fixed to a capillary tube (VWR, Inc.) using transparent, odorless glue, and mounted with clay under a microscope (Olympus SZX7 with an Infinity 2 recorder). Infinity CAPTURE software (Lumenera Corporation) was used for recordings at room temperature (∼23 °C). Before recordings, flies were allowed to recover for 30 min. Solutions containing different sugars and nutrients were presented to a fixed fly using another capillary tube before the start of recording. The concentration used for d-glucose, d-fructose, and l-glucose was 200 mM, and the concentration used for sucralose was 1 mM. Visual inspection of the release of the CIF was obtained during feeding. The recording of flies in the two-choice assay from the bottom of the arena was conducted using Celestron MicroCapture Pro software. Videos were compressed using Infinity CAPTURE software, and playback was adjusted to 8× speed for viewing using Apple iMovie software.
Quantification of Spots Around Sugar and Other Macronutrients.
To quantify spots around agar containing sugar, we used starved 30 male WT flies, unless otherwise noted, that were introduced into the two-choice assay with a choice of agar containing sugar (100 mM) and agar alone for 2 h (trial 1). Another 30 starved male WT flies were introduced into the same two-choice arena for a second time (trial 2) and a third time (trial 3). After each trial, photographs of each plate were taken using an iPhone camera. Adobe Photoshop CS6 software was then used for counting spots. Spots produced around other macronutrients were counted similarly.
Statistics.
GraphPad Prism6 (provided by New York University School of Medicine) and Past software (folk.uio.no/ohammer/past/) were used for all statistical analyses. Figure layouts were designed and organized using Adobe Illustrator CC. One-way ANOVA, followed by a Bonferroni test, was used for most of the multiple comparisons, and a nonparametric Student’s t test, followed by a Mann–Whitney U test, was used for binary comparisons. PCA and linear correlations were done using Past software.
Supplementary Material
Acknowledgments
We thank Dr. Chris Potter, Dr. Ulrike Heberlein, Dr. Kathy Nagel, and Dr. John Carlson for providing the flies used in this study and the G.S.B.S. laboratory for critical reading of the manuscript. This work is supported by NIH R01 Grants NIDCD R01DC01279 and NIDDK R01DK106636 (to G.S.B.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1719827115/-/DCSupplemental.
References
- 1.Caccamise D, Reed L, Stouffer P. Roosting behavior and group territoriality in American crows. Auk. 1997;114:628–637. [Google Scholar]
- 2.Seeley TD, Visscher PK, Passino KM. Group decision making in honey bee swarms. Am Sci. 2006;94:220–229. [Google Scholar]
- 3.Riley JR, Greggers U, Smith AD, Reynolds DR, Menzel R. The flight paths of honeybees recruited by the waggle dance. Nature. 2005;435:205–207. doi: 10.1038/nature03526. [DOI] [PubMed] [Google Scholar]
- 4.Wilson EO. Chemical communication among workers of the fire ant Solenopsis saevissima (Fr. Smith) 3. The experimental induction of social responses. Anim Behav. 1962;10:159–164. [Google Scholar]
- 5.Bartelt RJ, Schaner AM, Jackson LL. cis-Vaccenyl acetate as an aggregation pheromone in Drosophila melanogaster. J Chem Ecol. 1985;11:1747–1756. doi: 10.1007/BF01012124. [DOI] [PubMed] [Google Scholar]
- 6.Wang L, Anderson DJ. Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature. 2010;463:227–231. doi: 10.1038/nature08678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin CC, Prokop-Prigge KA, Preti G, Potter CJ. Food odors trigger Drosophila males to deposit a pheromone that guides aggregation and female oviposition decisions. eLife. 2015;4:e08688. doi: 10.7554/eLife.08688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Everaerts C, Farine JP, Cobb M, Ferveur JF. Drosophila cuticular hydrocarbons revisited: Mating status alters cuticular profiles. PLoS One. 2010;5:e9607. doi: 10.1371/journal.pone.0009607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dweck HK, Ebrahim SA, Farhan A, Hansson BS, Stensmyr MC. Olfactory proxy detection of dietary antioxidants in Drosophila. Curr Biol. 2015;25:455–466. doi: 10.1016/j.cub.2014.11.062. [DOI] [PubMed] [Google Scholar]
- 10.Ai M, et al. Acid sensing by the Drosophila olfactory system. Nature. 2010;468:691–695. doi: 10.1038/nature09537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussain A, et al. Ionotropic chemosensory receptors mediate the taste and smell of polyamines. PLoS Biol. 2016;14:e1002454. doi: 10.1371/journal.pbio.1002454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stensmyr MC, et al. A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell. 2012;151:1345–1357. doi: 10.1016/j.cell.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 13.Ueno K, et al. Trehalose sensitivity in Drosophila correlates with mutations in and expression of the gustatory receptor gene Gr5a. Curr Biol. 2001;11:1451–1455. doi: 10.1016/s0960-9822(01)00450-x. [DOI] [PubMed] [Google Scholar]
- 14.Dahanukar A, Foster K, van der Goes van Naters WM, Carlson JR. A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nat Neurosci. 2001;4:1182–1186. doi: 10.1038/nn765. [DOI] [PubMed] [Google Scholar]
- 15.de Araujo IE, et al. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 16.Dus M, Min S, Keene AC, Lee GY, Suh GS. Taste-independent detection of the caloric content of sugar in Drosophila. Proc Natl Acad Sci USA. 2011;108:11644–11649. doi: 10.1073/pnas.1017096108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dus M, et al. Nutrient sensor in the brain directs the action of the brain-gut axis in Drosophila. Neuron. 2015;87:139–151. doi: 10.1016/j.neuron.2015.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott K, et al. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell. 2001;104:661–673. doi: 10.1016/s0092-8674(01)00263-x. [DOI] [PubMed] [Google Scholar]
- 19.Clyne PJ, Warr CG, Carlson JR. Candidate taste receptors in Drosophila. Science. 2000;287:1830–1834. doi: 10.1126/science.287.5459.1830. [DOI] [PubMed] [Google Scholar]
- 20.Dahanukar A, Lei YT, Kwon JY, Carlson JR. Two Gr genes underlie sugar reception in Drosophila. Neuron. 2007;56:503–516. doi: 10.1016/j.neuron.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiao Y, Moon SJ, Montell C. A Drosophila gustatory receptor required for the responses to sucrose, glucose, and maltose identified by mRNA tagging. Proc Natl Acad Sci USA. 2007;104:14110–14115. doi: 10.1073/pnas.0702421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bopp D, Jamet E, Baumgartner S, Burri M, Noll M. Isolation of two tissue-specific Drosophila paired box genes, pox meso and pox neuro. EMBO J. 1989;8:3447–3457. doi: 10.1002/j.1460-2075.1989.tb08509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Awasaki T, Kimura K. pox-neuro is required for development of chemosensory bristles in Drosophila. J Neurobiol. 1997;32:707–721. doi: 10.1002/(sici)1097-4695(19970620)32:7<707::aid-neu6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 24.Dus M, Ai M, Suh GS. Taste-independent nutrient selection is mediated by a brain-specific Na+/solute co-transporter in Drosophila. Nat Neurosci. 2013;16:526–528. doi: 10.1038/nn.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bogdanove EM, Barker SB. Effect of phlorhizin on intestinal absorption of glucose, galactose, fructose, mannose, and sorbose. Proc Soc Exp Biol Med. 1950;75:77–80. doi: 10.3181/00379727-75-18106. [DOI] [PubMed] [Google Scholar]
- 26.Fridhandler L, Quastel JH. Absorption of sugars from isolated surviving intestine. Arch Biochem Biophys. 1955;56:412–423. doi: 10.1016/0003-9861(55)90262-9. [DOI] [PubMed] [Google Scholar]
- 27.Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- 28.van der Goes van Naters W, Carlson JR. Receptors and neurons for fly odors in Drosophila. Curr Biol. 2007;17:606–612. doi: 10.1016/j.cub.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsson MC, et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 30.Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abuin L, et al. Functional architecture of olfactory ionotropic glutamate receptors. Neuron. 2011;69:44–60. doi: 10.1016/j.neuron.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferveur JF. Cuticular hydrocarbons: Their evolution and roles in Drosophila pheromonal communication. Behav Genet. 2005;35:279–295. doi: 10.1007/s10519-005-3220-5. [DOI] [PubMed] [Google Scholar]
- 33.Brieger G, Butterworth FM. Drosophila melanogaster: Identity of male lipid in reproductive system. Science. 1970;167:1262. doi: 10.1126/science.167.3922.1262. [DOI] [PubMed] [Google Scholar]
- 34.Ferveur JF, et al. Genetic feminization of pheromones and its behavioral consequences in Drosophila males. Science. 1997;276:1555–1558. doi: 10.1126/science.276.5318.1555. [DOI] [PubMed] [Google Scholar]
- 35.Ruiz A, Heed WB, Wasserman M. Evolution of the mojavensis cluster of cactophilic Drosophila with descriptions of two new species. J Hered. 1990;81:30–42. doi: 10.1093/oxfordjournals.jhered.a110922. [DOI] [PubMed] [Google Scholar]
- 36.Lachaise D, Tsacas L. Les drosophilidae des savanes préforestières de la region tropicale de Lamto (Cote-d’lvoire). Le peuplement des fruit de pandanus candelabrum (Pandanacées) Ann Univ d’Abidjan Ser E Ecol. 1974;7:153–192. French. [Google Scholar]
- 37.Rio B, et al. Evolution d’une specialisation saisonniere chez Drosophila erecta (Dipt., Drosophilidae) Ann Soc Entomol. 1983;19:235–248. French. [Google Scholar]
- 38.Ha TS, Smith DP. A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J Neurosci. 2006;26:8727–8733. doi: 10.1523/JNEUROSCI.0876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keesey IW, et al. Adult frass provides a pheromone signature for Drosophila feeding and aggregation. J Chem Ecol. 2016;42:739–747. doi: 10.1007/s10886-016-0737-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.